Abstract
Background:
Cerebral small vessel disease is relevant to hypertension. We tried to figure out whether antihypertensive treatment is beneficial for this disease.
Methods:
We systematically searched PubMed, Embase, and Cochrane electronic databases for randomized controlled trials about white matter hyperintensities (WMH), brain atrophy, microbleeds, and lacunar infarcts with antihypertensive treatment and performed a meta-analysis.
Results:
We identified 7 trials on white matter hyperintensities and brain atrophy with antihypertensive treatment. Pooled analysis showed antihypertensive treatment performed positively in the progression of WMH (standardized mean difference, −0.22; 95% CI, −0.36 to −0.07, I^2 = 52%). And in the subgroup meta-analysis, only lower SBP controlled level (110–129 mm Hg) had effect on the progression of WMH (standardized mean difference, −0.37; 95% CI, −0.54 to −0.29, I^2 =0). The meta-regression showed larger difference of SBP in treatment groups having a smaller WMH progression. Antihypertensive treatment is not significant in the progression of brain atrophy (standardized mean difference, −0.02; 95% CI, −0.26 to 0.30, I^2 = 85%). Only 1 trial reported the new patients of lacunar infarcts in the follow-up, no association with antihypertensive treatment (odds ratio, 2.2; 95% CI, 0.4–12.1; P = .36).
Conclusions:
Antihypertensive treatment is beneficial for cerebral small vessel disease on white matter hyperintensities progression, but no impact on brain atrophy. And lower SBP level is more effective on the progression of WMH. There is not enough evidence to prove the relationship between antihypertensive treatment and lacunar stroke, microbleeds.
Keywords: antihypertensive treatment, brain atrophy, cerebral small vessel disease, meta-analysis, white matter hyperintensities
1. Introduction
Cerebral small vessel disease (cSVD) is a disorder of small cerebral vessels which can cause white matter hyperintensities (WMH), brain atrophy, lacunar infarcts, and cerebral microbleeds (CMBs).[1,2] These lesions are associated with cognitive decline and stroke.[1]
Hypertension is an important risk factor for cSVD by producing arteriolosclerosis.[3] And several observational studies have described associations between blood pressure control and cSVD.[4–6] But the outcome of antihypertensive treatment therapy in cSVD is inconsistent.
We aimed to discuss and study the relationship between antihypertensive treatment and progression of cSVD. The randomized controlled trial (RCT) is the most effective way to determine whether an intervention is helpful.[7] So we systematically reviewed and meta-analyzed all RCT evidence on the relation of cSVD progression with antihypertensive treatment. The systematic review and meta-analysis were performed based on based on the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guideline medicine framework population, intervention, comparators, outcomes, study design[8]: Did patients with WMH (population) received antihypertensive treatment (intervention) accompanied with no antihypertensive treatment (comparators) have a small progression (outcome) in RCT (study design)?
2. Methods
There is no review protocol.
2.1. Search strategy
We systematically searched PubMed, Embase, and Cochrane Library for all records published up to September 20, 2018, using the keywords “Antihypertensive,” “hypertension,” “magnetic resonance imaging (MRI),”“ computed tomography (CT),” “white matter,” “infarct,” “lacunes,” “microbleed,” “small vessel,” “brain atrophy.” We also further searched selected publications for relevant complement and contacted with authors to complement the electronic searches. Two independent reviewers assessed all abstracts and full texts, then extracting data from useful articles.
2.2. Selection criteria
For the review, we used the following inclusion criteria: all studies had to be RCT of human; cSVD marker (white matter, lacunar infarcts, microbleeds, and brain atrophy) had to be performed on MRI or CT. All studies had to have investigated the association of antihypertensive treatment and progression of cSVD. Interventions may include medications to reduce blood pressure or goals of lowering blood pressure; the control measures were matching placebo or blood pressure control targets. At least 2 years were followed up. We specifically note that we chose WMH and brain atrophy as quantitative outcome measure, given that the changing of lesions can be defined with high precision studies and is less dependent on different assessment criteria. But the lacunar infarcts were measured as qualitative data. To provide additional insight into the association of cSVD with antihypertensive treatment, we also highlighted the associations with difference of SBP in treatment groups as a meta-regression. We restricted our inclusion to original articles that were RCT trails and excluded review articles, case reports, clinical conference papers, and editorials.
2.3. Data extraction
We made predefined a data form in which we collected information on characteristics of the study population (age, sex, and inclusion criteria); study design; intervention and control methods; blood pressure levels (baseline, follow-up and change) (Table 1). We also extracted cSVD measurement methods (WMH/brain atrophy/lacunar infarcts, MRI/computed tomography) (Table 2). Outcome assessment and the effect estimate are collected in Table 3.
Table 1.
Study characteristics of randomized controlled trials investigating relation of antihypertension with cerebral small vessel disease.
| Study name | ACCORD-MIND | SPRINT-MIND | PROGRESS CT substudy | PreDIVA | PROGRESS MRI substudy | SCOPE | PRoFESS |
| Year of publication | 2014 | 2019 | 2004 | 2017 | 2005 | 2007 | 2012 |
| Places of participant | North America | North America | Asia | Europe | Europe | Europe, North America, Asia | North and South America, Australia, Asia, Europe |
| Study design | 2 × 2 factorial | Parallel-group | Parallel-group | Parallel-group | Parallel-group | Parallel-group | 2 × 2 factorial |
| intervention | Intensive therapy (SBP <120 mm Hg) | Intensive treatment (SBP <120 mm Hg) | Perindopril 4 mg and indapamide 2 mg | Vascular care (mean ≥2 vascular care visits/yr) | Perindopril 4 mg and indapamide 2.5 mg | Participants aged 70–89 yrs, SBP 160–179 mm Hg and/or DBP 90–99 mm Hg, untreated or thiazidetreated | Telmisartan 80 mg |
| Control | Standard therapy (SBP <140 mm Hg) | Standard treatment (SBP <140 mm Hg) | Matching placebo | Standard care (mean <2 cross-over vascular care visits/yr) | Placebo | Placebo | Placebo |
| Inclusion criteria | T2DM at high risk for cardiovascular events, SBP ranging from 130 to 180 mm Hg and taking 3 or fewer antihypertensives | 50 yr or older with SBP between 130 and 180 mm Hg at the screening visit and had increased cardiovascular risk | TIA or stroke within the past 5 yrs (excluded subarachnoid hemorrhage) | SBP≥140 mm Hg | TIA or stroke within the past 5 yrs (excluded subarachnoid hemorrhage) | Participants aged 70–89 yrs with SBP 160–179 mm Hg and/or DBP 90–99 mm Hg, untreated or thiazide- treated | An ischemic stroke within the previous 90 d, ≥55 yrs, SBP <180 mm Hg and DBP <110 mm Hg |
| Time of follow-up (mo) | Mean 40 | Median 48 (range 34–57) | Mean 46.8 (sd 1) | Mean 36 | Median 36 (range 24–49) | 47.3 (sd 0.2) | 27.9 (SD 7.6) |
| Number of participants | 314 | 449 | 667 | 126 | 192 | 92 | 771 |
| Age (yrs) | Mean 62.0 (sd 5.4) | Mean 67.1 (sd 7.8) | Mean 64 (sd 9) | Mean 77.2 (sd 8.9) | Mean 60.8 (sd 12.1) | Mean 77 (sd 4) | Mean 65.4 (sd 8.1) |
| Sex (female) | 167 (53.2%) | 167 (37.2%) | 178 (26.7%) | 67 (53.2%) | 46 (24.0%) | 50 (54.3%) | 275 (35.7%) |
| SBP, intervention | |||||||
| • baseline | 138.7 (sd17.5) | 136.0 (sd17.0) | 143 (sd 17) | 162 (sd 16) | 144.3 (sd 20.0) | 167 (sd 8) | 146.0 (sd 16.3) |
| • follow-up | 118.0 (sd12.0) | 122.1 (sd nr) | 138 (sd nr) | 152 (sd 16) | 131.8 (sd nr) | 141 (sd 11) | 134.9 (sd 20.5) |
| • change | −20.7 | −13.9 | −5 | −10 | −12.5 | −26 | −11.1 |
| SBP, control | |||||||
| • baseline | 139.3 (sd16.9) | 138.2 (sd15.8) | 143 (sd 17) | 160 (sd 14) | 142.2 (sd 19.7) | 167 (sd 8) | 145.5 (sd 16.3) |
| • follow-up | 133.2 (sd14.6) | 136.1 nr | 140.4 (sd nr) | 156 (sd 15) | 140.9 (sd nr) | 147 (sd 12) | 137.4 (sd 18.2) |
| • change | −6.1 | −2.1 | −2.6 | −4 | −1.3 | −20 | −8.1 |
| Funding | American Heart Association Scientist Development Grant, NIH-NINDS grants | NIH, including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke | Daiichi Parmaceutical | Dutch Ministry of Health, Innovatiefonds Zorverzekeraars, the Netherlands Organisation for Health Research and Development, Internationale Stichting Alzheimer Onderzoek, | Servier, the Health Research Council of New Zealand and the National Health and Medical Research Council of Australia | AstraZeneca International and Astra Research Foundation UK | Boehringer Ingelheim |
Table 2.
Cerebral small vessel disease determination methods of trails.
| Trial | Scan method | Sequences | Field strength | Thickness of slices (mm) | Scan compar- ability | Method of WMH measurement | Method of brain atrophy measurement | Method of lacunar infarction measurement |
| ACCORD-MIND | MRI | T1, T2, FLAIR, 3D FSPGR | 1.5T | 1.5–3 | Yes | Automatic volumetric measurement | Automatic volumetric measurement | None |
| SPRINT-MIND | MRI | T1, T2, FLAIR | 3T | 1 | Yes | Lesion segmentation algorithm | Multiatlas label fusion method | None |
| PROGRESS CT Substudy | CT | None | None | nr | Yes | None | None | Identified by a trained rater on fluid-attenuated inversion recov- ery scans |
| PreDIVA | MRI | T1,T2 | 3T | 1.2 | Yes | k-nearest neighbor algorithm | Adding gray and white matter volumes | nr |
| PROGRESS MRI Substudy | MRI | T1,T2 | 1.0T or 1.5T | 1.4–5 | Yes | A modified version of a validated scale | None | None |
| SCOPE | MRI | T1, T2, FLAIR | 1.5T | 1.7–5 | Yes | Automated procedure in SPM99 | Semiautomated MIDAS | None |
| PRoFESS | MRI | T1, T2, FLAIR, DWI | nr | nr | nr | Semiquantitative Rotterdam Scan Study scale | nr | None |
Table 3.
Original outcomes of randomized controlled trials included in meta-analysis.
| Brain atrophy | WMH | Lacunar infarction | |||||||||||||||||||
| Baseline | Follow-up | Change | Baseline | Follow-up | Change | Baseline | Follow-up | Change | |||||||||||||
| Trail | Intervention | Control | Intervention | Control | Intervention | Control | Unit | Intervention | Control | Intervention | Control | Intervention | Control | Unit | Intervention | Control | Intervention | Control | Intervention | Control | Unit |
| ACCORD-MIND | 923.7 (sd98.6) | 919.3 (sd99.4) | 900.7 (sd96.9) | 904.9 (sd98.7) | −18.6 (sd16.1) | −14.4 (sd16.6) | cm3 (TBV) | 2.04 (sd 2.85) | 1.80 (sd 2.22) | 2.97 (sd 2.77) | 2.71 (sd 3.06) | 0.67 (sd 0.95) | 1.16 (sd 1.13) | cm3 | None | ||||||
| SPRINT-MIND | 1134.5 (95%CI 1125.1 to 1144.0) | 1134.0 (95%CI 1124.4 to 1143.6) | 1104.0 (95%CI 1094.5 to 1113.4) | 1107.1 (95%CI 1097.4 to 1116.8) | −30.6 (95%CI −32.3 to −28.8) | −26.9 (95%CI −28.8 to −24.9) | cm3 (TBV) | 4.57 (95%CI 4.00 to 5.14) | 4.40 (95%CI 3.80 to 5.00) | 5.49 (95%CI 4.91 to 6.07) | 5.85 (95%CI 5.23 to 6.47) | 0.92 (95%CI 0.69 to 1.14) | 1.45 (95%CI 1.21 to 1.70) | cm3 | None | ||||||
| PROGRESS CT Substudy | 28 (sd 4) | 27 (sd 4) | 28 (sd 4) | 28 (sd 5) | 0 | 1 | (cella media index)% of TBV | None | 178 | 169 | nr | nr | nr | nr | patient | ||||||
| 33 (sd 4) | 33 (sd 5) | 33 (sd 5) | 33 (sd 6) | 0 | 0 | (frontal horn index) % of TBV | |||||||||||||||
| PreDIVA | 0.97 (sd 0.10) | 0.97 (sd 0.10) | nr | nr | nr | nr | L | 6.3 (range 3.5 to 10.9) | 5.7 (range 3.3 to 11.1) | nr | nr | 0.73 (sd 0.84) | 0.70 (sd 0.59) | ml/year | 5 | 4 | nr | nr | 6 | 2 | Patient |
| PROGRESS MRI Substudy | None | nr | nr | nr | nr | 0.4 (se 0.8) | 2.0 (se 0.7) | mm3 | None | ||||||||||||
| SCOPE | nr | nr | nr | nr | 0.46 (sd 0.42) | 0.62 (sd 0.42) | % of TBV | 1.09 (sd 1.23) | 1.16 (sd 1.39) | 1.22 (sd 1.39) | 1.34 (sd 1.59) | 0.13 (sd 0.30) | 0.18 (sd 0.32) | % of TBV | None | ||||||
| PRoFESS | None | 8.17 (sd 6.19) | 7.81 (sd 5.86) | 8.57 (sd 5.51) | 8.71 (sd 6.12) | 0.34 (sd 5.45) | 0.83 (sd 4.79) | mm (subcortical) | None | ||||||||||||
| 2.92 (sd 2.31) | 2.87 (sd 2.29) | 3.48 (sd 2.55) | 3.3 (sd 2.46) | 0.54 (sd 1.89) | 0.40 (sd 1.86) | Score (periventricular) | |||||||||||||||
2.4. Quality assessment
We assessed the quality of the studies using Cochrane risk of bias tool (Table 4). The assessment of cSVD differed considerably across studies, including both qualitative and quantitative measurements and the use of different MRI sequences on which the hyperintensities were quantified. See Table 1 for further details. For the assessment of lacunar infarcts, the criteria were defined by a trained rater on fluid-attenuated inversion recovery scans as round or ovoid, subcortical, fluid-filled (similar signal as cerebral spinal fluid) cavities, between 3 and 15 mm in diameter, regardless of whether these could be linked to any clinical symptoms.[9]
Table 4.
Cochrane risk of bias assessment.
2.5. Statistical analyses
We used R Studio to conduct the meta-analysis of antihypertensive treatment with progression of WMH, brain atrophy. Heterogeneity across studies was defined by an I^2 of more than 50%. In this meta-analysis, pooled progression of markers was calculated and performed by standardized mean difference (SMD). Given that preliminary analyses demonstrated considerable heterogeneity across studies, pooled SMD were calculated using random-effects. We performed subgroup analysis to investigate the effective intervention. We also performed a sensitivity analysis examining the comparative outcomes according to the I^2 of more than 50%.
3. Results
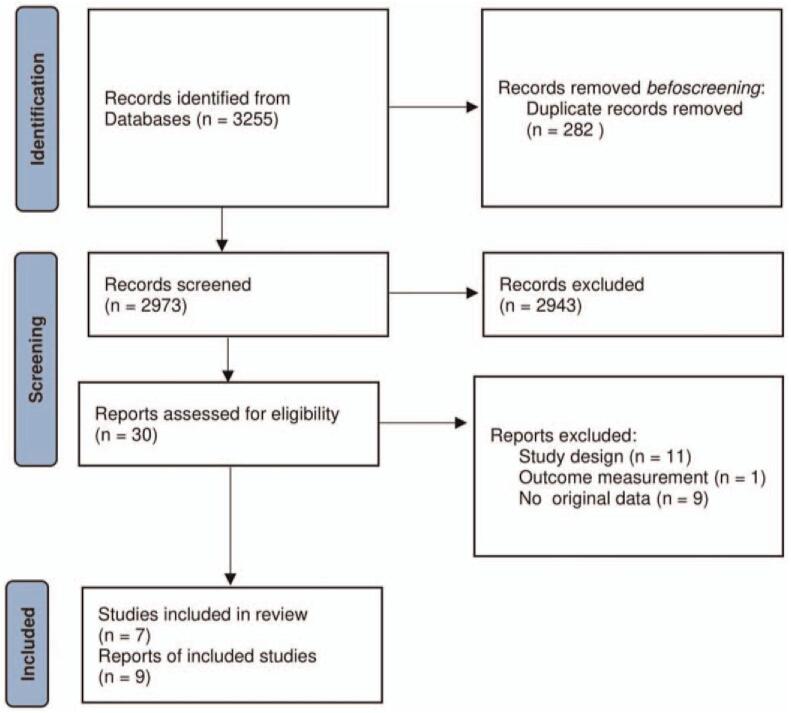
We identified 2973 unique articles with the initial search, of which 7 trials were selected finally for meta-analysis (Fig. 1).[9–17] Six trials contained data on WMH with a similar quantitative assessment.[9–12,14–17] Four trials on brain atrophy[10,12,13,15,17] and 1 study on lacunar infarcts.[9] No one study reported CMBs (Table 3).
Figure 1.
PRISMA flow chart of study selection process. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
The total number of participants was 1944, with a mean age ranging from 60 to 78 years at study entry. The inclusions are different, 1 including T2 diabetes patients, [10,11] 1 including individuals aged 50 years older with high cardiovascular risk, [12] 1 including people aged 70 to 89 years,[15] 1 including SBP≥140 mm Hg[9] and 3 including patients with stroke or TIA.[13,14,16] 4 studies used placebo as the control measurement,[13–16] 2 studies compared SBP<120 mm Hg to SBP<140 mm Hg,[10–12] and 1 study compared the standard vascular care to intensive vascular care.[9] More information about cSVD measurement methods in Table 1.
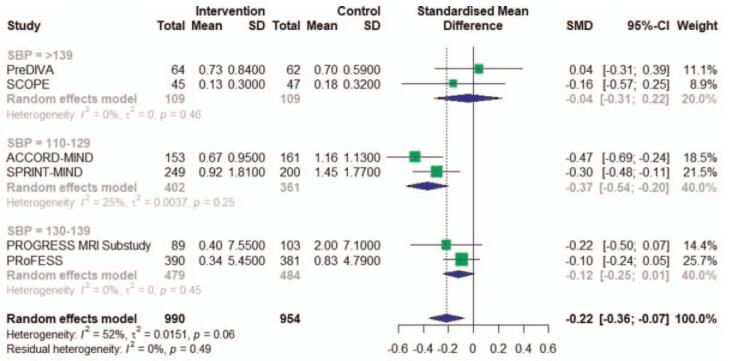
We found a statistically significant difference for the relation between antihypertensive treatment with the progression of WMH of −0.22 (95% CI, −0.36 to −0.07, I^2 = 52%) (Fig. 2A). There obvious heterogeneity between studies (I^2 = 52%), largely accounted for by one single study [11], that despite having the largest SMD, didn’t obey the blinding of participants and treating physicians. Excluding this study in a sensitivity analysis reduced heterogeneity (I^2 = 6%) and resulted in a pooled SMD of −0.16 (95% CI, −0.26 to −0.06) (Fig. 2B). And in the subgroup meta-analysis, the heterogeneity was low in every group defined by SBP levels in treatment groups, so we found the different SBP intervention level was the source of heterogeneity. The subgroup analysis also showed that SBP level at follow-up impacted the antihypertensive treatment effect on WMH progression. In higher SBP level, antihypertensive treatment had no effect on the progression of WMH. Only the group of 110 to 129 mm Hg showed significant relation with antihypertensive treatment of −0.37 (95% CI, −0.54 to −0.29). The group of 130 to 139 mm Hg of −0.12 (95% CI, −0.25 to −0.01) and the group of >139 mm Hg of −0.04 (95% CI, −0.31 to 0.22) showed no association of antihypertensive treatment with WMH (Fig. 3). We also made a meta-regression of WMH volume changes with differences in SBP between intervention and control at follow-up. And we found larger difference of SBP in treatment groups having a smaller WMH progression (Fig. 4).
Figure 2.
A, Meta-analysis of RCT studies investigating the association of antihypertensive treatment and white matter hyperintensity. RCT = randomized controlled trials. The effect sizes (boxes) with 95% confidence intervals (CI) for the quantitative outcomes are plotted. The size of the box is proportional to the weight of the study. The diamond is the result of the random-effect meta-analysis. B, Meta-analysis of RCT studies investigating the association of antihypertensive treatment and white matter hyperintensity after excluding this study. RCT = randomized controlled trials. The effect sizes (boxes) with 95% confidence intervals (CI) for the quantitative outcomes are plotted. The size of the box is proportional to the weight of the study. The diamond is the result of the random-effect meta-analysis.
Figure 3.
Subgroup meta-analysis of RCT studies investigating the association of antihypertensive treatment and white matter hyperintensity. RCT = randomized controlled trials. The grouping factors are systolic blood pressure in intervention groups at follow-up. The effect sizes (boxes) with 95% confidence intervals (CI) for the quantitative outcomes are plotted. The size of the box is proportional to the weight of the study. The diamond is the result of the random-effect meta-analysis.
Figure 4.
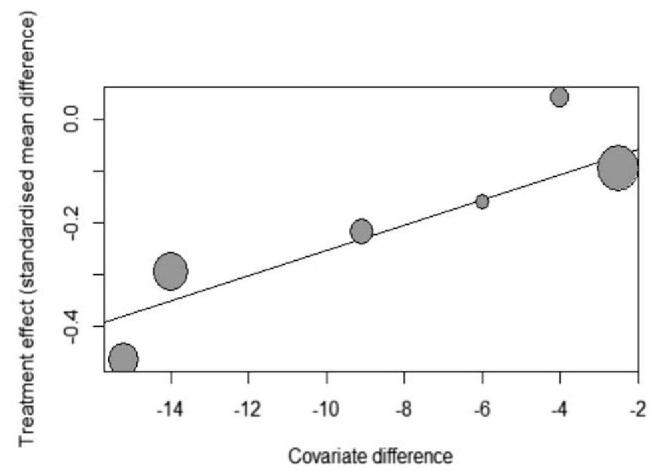
Meta-regression of SBP difference influence on the effect of antihypertensive treatment on WMH progression. Horizontal ordinate means difference of SBP between intervention and control groups; vertical ordinate means WMH progression. SBP = systolic blood pressure, WMH = white matter hyperintensities.
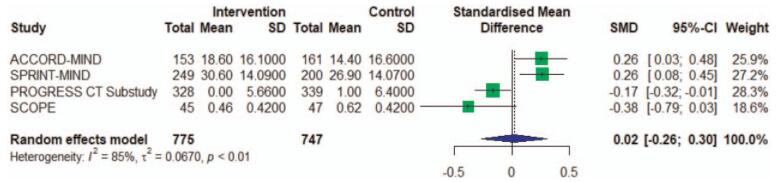
With 4 studies we found no significant difference in antihypertensive treatment and brain atrophy of 0.02 (95% CI, −0.26 to 0.30, I^2 = 85%) (Fig. 5). The substantial heterogeneity between studies (I^2 = 85%) was difficult to interpretate because the number of included studies is small, possibly caused by different study including conditions, and intervention measures.
Figure 5.
Meta-analysis of RCT studies investigating the association of antihypertensive treatment and brain atrophy. RCT = randomized controlled trials. The effect sizes (boxes) with 95% confidence intervals (CI) for the quantitative outcomes are plotted. The size of the box is proportional to the weight of the study. The diamond is the result of the random-effect meta-analysis.
One study indicated antihypertensive treatment was no effect on lacunar infarcts, odds ratio of 2.2 (95% confidence interval, 0.4–12.1, P = .36).[9]
4. Discussion
By means of systematic review and meta-analysis of RCT studies on the role of antihypertensive treatment of cVSD, we found evidence that antihypertensive treatment represents an important indicator of a higher effect of preventing progression of WMH. The lower SBP levels seemed to had better effect on stopping WMH progression. But we found no significant relation between antihypertensive treatment and brain atrophy. Only one study reported antihypertensive treatment therapy with no effect on lacunar infarcts incidence, but numbers were low.
Antihypertensive treatment was associated with a decreased risk of WMH progression in the general old-aged population in mean more than 3 years. We also found 1 study in which no association between antihypertensive treatment and WMH volume was reported, but an association between antihypertensive treatment persons with severe WMH load at baseline, the study proportion of participants initiating antihypertensive medication during study was similar in both treatment arms.[9] And another study indicated the higher WMH volume at baseline, the more effective of antihypertensive treatment.[14] In the subgroup meta-analysis, we found the relation of SBP and progression of WMH. Keeping SBP at low level may is more beneficial for prevent WMH from progressing further. The meta-regression also supported the performance, larger difference between treatment groups producing smaller progression, which means lower SBP level in intervention groups can stop the progression. The resultant loss of myelin and gliosis manifests on MRI as WMH.[18–20] The exact mechanism underlying the association of hypertension and WMH is that small cerebral vessels are key targets of hypertension, resulting in pathological alteration of the vascular wall, impairment of vital hemodynamic responses regulating cerebral perfusion, and disruption of blood brain barrier permeability leading to major alterations in the brain microenvironment,[3] and antihypertensive treatment can slow the pathological progression.
Of the above hypertension treatment studies, the ACCORD-MIND trial and SPRINT-MIND trial reported effect on total brain volume (TBV), the intensive blood pressure treatment group showing greater loss of TBV. But PROGRESS CT study and SCOPE trial reported the opposite result. The relationship of hypertension to TBV is less robust and less well documented, although high blood pressure generally has been associated with decreased brain volumes.[21–23] The evidence of lacunar infarcts with antihypertensive treatment is still not enough. Because the number of participants with new lacunar infarcts was too low to allow adjustment in regression analyses, PreDIVA trial did not perform extensive analyses on the outcome and this findings about lacunar infarcts are inconclusive.[9]
In terms of the use of antihypertensive drugs, the Progress CT Substudy and the Progress CT Substudy combined Perindopril and Indapamide, while the SCOPE study used Candesartan and the PROESS trial used Telmisartan. The remaining 3 trials did not specify the specific drug to be used (Table 1). Regarding progress in WMH, the results of combined and monotherapy antihypertensive therapy were similar, without statistical correlation with the progress of WMH (Fig. 2). About brain atrophy, the effect of the combination was better than that of the single drug (Fig. 5).
The progression of cSVD is prevalent in patients with hypertension and involved in cognitive impairing as well as an increased risk of stroke, among other consequences.[24,25] And several observational studies have increasingly suggested that cSVD is associated with cognitive decline and the pathogenesis of Alzheimer disease and related dementias.[26] About the mechanism of WMH and dementia, the WMH showed on imaging represents only a tip of the iceberg of the total underlying brain damage, and the composition of WMH varies greatly, ranging from gliosis to demyelination of white matter tracts.[27–29] We found 2 articles reported there no effect of WMH on cognition impairment.[9,17] But 1 article reported participants with probable dementia exhibited significantly larger increases in WMH volume as well as significantly larger decreases in TBV compared with participants having no cognitive impairment.[12] The difference may be caused by the intervention constancy on blood pressure and selective dropout of cognition impairment participants.
Studies have shown that blood pressure variability (BPV) affects cSVD independently of blood pressure levels, and elevated BPV is associated with a higher risk of cSVD.[30] Endothelial cell and blood-brain barrier damage caused by blood pressure fluctuations and perfusion imbalance can induce microglia overactivation, increase the secretion of proinflammatory cytokines and reactive oxygen species, and up-regulation of the neuroinflammatory environment and reactive glial proliferation are considered to be further causes of neurodegenerative changes.[31] The effect of antihypertensive drugs on BPV may modulate the effect of BPV on cSVD, because antihypertensive medications have different effects on the individual blood pressure fluctuations.[32,33] The calcific channel blockers and diuretics are the most effective options for minimizing the BPV.[34] This will give us more help in the selection of antihypertensive drugs on the basis of antihypertensive treatment.
In our meta-analysis, we reviewed and discussed the articles about imaging of antihypertensive treatment and cSVD, but we didn’t put attention on the mechanism and clinical performance of cSVD. The number of included articles was small, so we didn’t conduct calculating publication bias. Blood pressure threshold for therapy initiation, time of treatment, and the blood pressure reduction to maximize benefits and reduce risks are not certain, but the great benefits for general health afforded by blood pressure control justify early and aggressive intervention. More RCT trials processing will reveal the truth of relation between antihypertensive treatment and cSVD finally.[35,36]
In conclusion, we found that antihypertensive treatment associated with a decreased progression of WMH, in the general population. And lower SBP level is more effective on the progression of WMH. We found that lacunar infarcts had no relation with antihypertensive treatment. In addition, there was no association between antihypertensive treatment and brain atrophy. No study reported CMBs with antihypertensive treatment. Our results also highlight that RCT data on the association of antihypertensive treatment with cSVD remains limited and that further study into their exact role in the therapy of cSVD is warranted.
Author contributions
Conceptualization: Chen Su, Renliang Zhao.
Investigation: Chen Su.
Resources: Chen Su, Hao Wu.
Software: Su Chen, Hao Wu, Xiaoyu Yang.
Supervision: Renliang Zhao.
Validation: Renliang Zhao.
Visualization: Bing Zhao.
Writing – original draft: Chen Su.
Writing – review & editing: Chen Su.
Footnotes
Abbreviations: 95% CI = 95% confidence intervals, BPV = blood pressure variability, CMBs = cerebral microbleeds, cSVD = cerebral small vessel disease, CT = computed tomography, MRI = magnetic resonance imaging, RCT = randomized controlled trials, SBP = systolic blood pressure, WMH = white matter hyperintensities.
How to cite this article: Su C, Wu H, Yang X, Zhao B, Zhao R. The relation between antihypertensive treatment and progression of cerebral small vessel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100:30(e26749).
Access statement: the research is available.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
nr = not reported, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus.
CT = computed tomography, nr = not reported, RI = magnetic resonance imaging, WMH = white matter hyperintensity.
95% CI = 95% confidence interval, nr = not report, TBV = total brain volume, WMH = white matter hyperintensity.
The green plus indicates a low risk of bias; the orange question indicates an unclear risk of bias; the red minus indicates a high risk of bias.
References
- [1].Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019;18:684–96. [DOI] [PubMed] [Google Scholar]
- [2].Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013;62:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakanishi K, Jin Z, Homma S, et al. Association of blood pressure control level with left ventricular morphology and function and with subclinical cerebrovascular disease. J Am Heart Assoc 2017;6: e006246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scharf EL, Graff-Radford J, Przybelski SA, et al. Cardiometabolic health and longitudinal progression of white matter hyperintensity: The Mayo Clinic Study of Aging. Stroke 2019;50:3037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vlek ALM, Visseren FLJ, Kappelle LJ, et al. Blood pressure and progression of cerebral atrophy in patients with vascular disease. Am J Hypertension 2009;22:1183–9. [DOI] [PubMed] [Google Scholar]
- [7].Devereaux PJ, McKee MD, Yusuf S. Methodologic issues in randomized controlled trials of surgical interventions. Clin Orthop Relat Res 2003;413:25–32. [DOI] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [9].van Dalen JW, van Charante EPM, Caan MWA, et al. Effect of long-term vascular care on progression of cerebrovascular lesions magnetic resonance imaging substudy of the PreDIVA Trial (Prevention of Dementia by Intensive Vascular Care). Stroke 2017;48:1842–8. [DOI] [PubMed] [Google Scholar]
- [10].Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med 2014;174:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology 2019;92:E1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019;322:524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hasegawa Y, Yamaguchi T, Omae T, Woodward M, Chalmers J. Effects of perindopril-based blood pressure lowering and of patient characteristics on the progression of silent brain infarct: the Perindopril Protection against Recurrent Stroke Study (PROGRESS) CT Substudy in Japan. Hypertens Res 2004;27:147–56. [DOI] [PubMed] [Google Scholar]
- [14].Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644–50. [DOI] [PubMed] [Google Scholar]
- [15].Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O’Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol 2007;254:713–21. [DOI] [PubMed] [Google Scholar]
- [16].Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke 2012;43:2336–42. [DOI] [PubMed] [Google Scholar]
- [17].Murray AM, Hsu FC, Williamson JD, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017;60:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang CE, Wong SM, van de Haar HJ, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology 2017;88:426–32. [DOI] [PubMed] [Google Scholar]
- [19].Mestre H, Kostrikov S, Mehta RI, Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin Sci (Lond) 2017;131:2257–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology 2005;64:1704–11. [DOI] [PubMed] [Google Scholar]
- [22].Vlek AL, Visseren FL, Kappelle LJ, et al. Blood pressure and progression of cerebral atrophy in patients with vascular disease. Am J Hypertension 2009;22:1183–9. [DOI] [PubMed] [Google Scholar]
- [23].Jochemsen HM, Muller M, Visseren FL, et al. Blood pressure and progression of brain atrophy: the SMART-MR Study. JAMA Neurol 2013;70:1046–53. [DOI] [PubMed] [Google Scholar]
- [24].Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- [25].Jimenez-Balado J, Riba-Llena I, Abril O, et al. Cognitive impact of cerebral small vessel disease changes in patients with hypertension. Hypertension 2019;73:342–9. [DOI] [PubMed] [Google Scholar]
- [26].Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wardlaw JM, Hernandez MCV, Munoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 2015;4: 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maniega SM, Valdes Hernandez MC, Clayden JD, et al. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol Aging 2015;36:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].de Groot M, Ikram MA, Akoudad S, et al. Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement 2015;11:321–30. [DOI] [PubMed] [Google Scholar]
- [30].Tully PJ, Yano Y, Launer LJ, et al. Association between blood pressure variability and cerebral small-vessel disease: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McKenzie JA, Spielman LJ, Pointer CB, et al. Neuroinflammation as a common mechanism associated with the modifiable risk factors for Alzheimer's and Parkinson's Diseases. Curr Aging Sci 2017;10:158–76. [DOI] [PubMed] [Google Scholar]
- [32].Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis 2017;43:207–13. [DOI] [PubMed] [Google Scholar]
- [33].Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of Angiotensin receptor blockers. Int J Hypertens 2013;2013:941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 2010;375:906–15. [DOI] [PubMed] [Google Scholar]
- [35].Markus H, Egle M, Croall I, et al. Preserve trial: intensive versus standard blood pressure lowering in cerebral small vessel disease assessed by DTI. Conference Abstract Eur Stroke J 2019;4:48. [Google Scholar]
- [36].Gk P, Turner N, Howden E, et al. Results of the reducing pathology in Alzheimer's disease through angiotensin targeting (RADAR) trial. J Prevention Alzheimer's Dis 2019;6:S31–2. [DOI] [PubMed] [Google Scholar]