Abstract
There is mounting evidence which suggests the involvement of gut microbiota dysbiosis in the pathogenesis of various cardiovascular diseases (CVD) and associated risk states such as hypertension, type 2 diabetes, obesity and dyslipidaemia, atherosclerosis, heart failure and atrial fibrillation. The current review comprehensively summarizes the various pathogenetic mechanisms of dysbiosis in these conditions and discusses the key therapeutic implications. Further deeper understanding of the pathogenetic links between CVD and gut microbiota dysbiosis can aid in the development of novel microbiota-based targets for the management of CVDs.
Keywords: Gut microbiota, Cardiovascular diseases, Hypertension, Diabetes, Obesity, Short-chain fatty acids
1. Introduction
The term ‘microbiome’ was coined by Lederberg and McCray to denote the group of commensal, symbiotic and pathogenic microorganisms (including majorly bacteria along with viruses, protozoa and fungi) dwelling in the human body.1,2 According to the latest estimates, the ratio of the number of bacterial to human cells in humans has been revised from 10:1 to 1:1.3 The majority of these microorganisms are found in the human gastrointestinal tract and are collectively termed as ‘gut microbiota’; their collective genome is referred to as ‘gut microbiome’.2
The adult gut microbiota is diverse and majorly consists of bacteria from the phyla, Bacteroidetes and Firmicutes. The other less abundant phyla include Proteobacteria, Verrucomicrobia, Actinobacteria, and Fusobacteria.4,5 The composition of the gut microbiota undergoes transition from an infant to adult and may vary depending on several factors and health conditions,5,6 which may in turn, have wide therapeutic implications. The advent of newer molecular techniques such as deep 16 S rRNA sequencing has enabled microbial profiling and research into the role of the gut microbiota in human health and diseases.5
Several factors may potentially alter the gut microbial composition. The type of diet one follow significantly impacts the gut microbiota. While vegetarian diet has been found to be associated with healthy and diverse gut microbiota, non-vegetarian diet has been found to be associated with a decrease in overall gut microbiota and beneficial species. The mode of delivery also impacts the gut microbiota. While newborns delivered vaginally have vaginal microbiota in excess, new-borns delivered via caesarean derive microbiota from the skin. The primary microbiota after birth evolves over time and stabilizes at the age of three. Physiological changes and weakened immune activity affect the microbial composition among the elderly. Besides, exposure to pathogens and usage of antibiotics can alter he gut microbial composition.7
The evolving significance of gut microbiota in maintaining homeostasis and pathogenesis of several diseases is being increasingly appreciated. Gut microbiota has been found to play a vital role in the maturation and regulation of the immune system of the host.8 It also modulates various metabolic pathways in the host.9,10 Further, alterations in the composition of gut microbiota have been found to have significant implications on cardiovascular health in humans.11,12
Cardiovascular disease (CVD) is the leading cause of mortality, worldwide.13 Therefore, considering the accumulating evidence on the role of gut microbiota alterations in the pathogenesis of CVD, we conducted this narrative review, to evaluate and discuss the plausible association between gut microbiota and CVD and its risk factors and the therapeutic implications that this association may have, on the cardiovascular health in humans. The role of gut microbiota and derived uremic toxins in chronic kidney disease (CKD) has also been described in brief as some of the risk factors for CVD and CKD overlap.
2. Gut microbiota and risk factors for cardiovascular diseases
Perturbations in the composition of the gut microbiota is known as ‘dysbiosis’. Dysbiosis has been linked to the development and progression of various CVDs. Gut microbiota plays a central role in regulating the energy harvesting process and metabolism in the host. Alteration in microbial flora along with changes in the gut-microbiota-derived metabolites have been shown to have a significant association with several risk factors for CVD.
2.1. Gut microbiota and diabetes mellitus
2.1.1. Pathogenetic mechanisms
Type 2 diabetes mellitus (T2DM) is one the major risk factors for CVD. Recent observational studies and systematic reviews on the association between gut microbiota and T2DM have revealed key differences in the gut microbiota composition between people with T2DM and healthy controls. While, the genera of bacteria with a protective role in glucose metabolism and T2DM such as Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia were found to be less abundant in individuals with T2DM; the phylum, Firmicutes and the genera of bacteria such as Ruminococcus, Fusobacterium, and Blautia were positively associated with T2DM.14,15 The genus, Lactobacillus with the highest number of operational taxonomic units (OTUs) in the human gut, was noted to be diverse with discrepant distribution patterns.14
Multiple mechanisms have been postulated to explain the potential association between altered gut microbiota and T2DM, including modulation of inflammation, gut permeability, glucose and fatty acid metabolism and insulin sensitivity. Specific species of bacteria from the genera, Roseburia, Bacteroides, Lactobacillus and Akkermansia have been observed to induce the production of anti-inflammatory cytokines and chemokines such as interleukin (IL)-10 and 22, and transforming growth factor (TGF)-β; and inhibit pro-inflammatory cytokines and chemokines such as IL-1β, monocyte chemoattractant protein-1, intercellular adhesion molecule-1, IL-8, IL-16, IL-17, CD36, nuclear factor-kappa B (NK-κB), interferon (IFN)-γ and C-reactive protein, thus preventing inflammation and enhancing insulin sensitivity. On the contrary, bacteria from the genera, Ruminococcus, Fusobacterium have been reported to augment the production of cytokines that promote inflammation.13 Further, reduction in gut permeability by enhancing the expression of tight junction genes through activation of adenosine monophosphate kinase (AMPK) by Bacteroides and Akkermansia genera has been noted to prevent metabolic endotoxemia.14 Another important mechanism is through modulation of fatty acid oxidation in adipose tissue. The action of gut microbiota on indigestible carbohydrates and proteins results in the formation of short-chain fatty acids (SCFAs) which regulate several endocrine pathways in humans. They trigger the release of postprandial plasma peptide YY (PYY) and glucagon-like-peptide-1 (GLP-1) by binding to G protein-coupled receptors, GPR41 and GPR43.14,16,17 Additionally, SCFAs also activate AMPK, a key regulator of metabolic homeostasis.18 Further, SCFAs and SCFA-producing bacteria such as Bacteroides, Akkermansia and Lactobacillus have been found to increase fatty acid oxidation and energy expenditure and reduce the synthesis of fatty acids, which may help improve T2DM.14 Of note, the gut-microbiota of T2DM patients has been found to have lower levels of bacteria that produce SCFAs such as butyrate.19
2.1.2. Therapeutic implications
Evolving understanding of the role of gut microbiota dysbiosis in the pathogenesis of T2DM has paved way to various strategies for ameliorating this disease. Recently, faecal microbial transplantation (FMT) in experimental models has been shown to be effective in alleviating the symptoms associated with T2DM. This treatment strategy lowered the insulin resistance and markers of inflammation in the pancreas and reversed the increased serum insulin level associated with T2DM. There was an improvement in the pancreatic β-cell function and the high levels of pro-inflammatory cytokines were also normalized with FMT. FMT also resulted in decreased expression of cleaved Capsase- 3 and Bax and reduced apoptosis of pancreatic cells.20 Another strategy that is being elucidated for T2DM treatment is the administration of SCFAs. Administration of SCFAs such as acetate in rats elicited an increase in the levels of plasma GLP-1 and PYY and reduction in inflammatory markers such as tumour necrosis factor-α (TNF-α).21 A randomized trial in 60 patients showed that the SCFA, propionate, significantly increased the PYY and GLP-1 levels by 1.8 and 2.4 folds compared to basal secretion. Propionate supplementation also improved insulin sensitivity and resulted in a beneficial change in body weight and composition.22 Probiotics and prebiotics have also been found to be promising for ameliorating T2DM. Treating diabetic rats with Lactobacillus paracasei NL 41 has been shown to remarkably improve blood glucose levels and insulin sensitivity. The probiotic treatment also reduced oxidative stress and conferred β-cell protection.23 In a separate study, eight weeks of Lactobacillus casei 01 supplementation in T2DM patients showed decreased carbohydrate and fat intake, and improved glycaemic control.24
While the aforementioned treatment strategies targeting the microbiota are in the early stages of development, several current established T2DM treatments have also been reported to impact the gut microbiota, independent of their effects on glycemic control. For example, the anti-diabetic drug, metformin has been found to be a potent AMPK activator.25 Treatment with acarbose, an α-glucosidase inhibitor has been evidenced to alter the composition of gut microbiota, with an increase in the gut content of Bifidobacterium and decrease in the concentrations of inflammatory cytokines, regardless of its glucose-lowering effects.26 Further, the dipeptidyl peptidase-4 inhibitors, sitagliptin and vildagliptin have been found to increase the abundance of SCFA-producing bacteria in the gut microbiota and reduce the ratio of the phyla Firmicutes/Bacteroides in experimental models.27,28
2.2. Gut microbiota and hypertension
2.2.1. Pathogenetic mechanisms
Hypertension is another major and well-established risk factor for CVD. Studies have shown that hypertension is associated with an alteration in the composition of gut microbiota. In a research study elucidating the link between gut microbiota dysbiosis and hypertension, spontaneously hypertensive rats (SHR) were found to have a significantly different microbiome when compared to Wistar Kyoto rats (WKY). SHR had a 5-fold increase in Firmicutes to Bacteroides ratio compared with WKY. While WKY had an abundance of butyrate-producing bacteria including Coprococcus and Pseudobutyrivibrio; SHR had higher levels of lactate-producing bacteria, Streptococcus and Turicibacter. Furthermore, WKY had higher levels of two uncultured genera OTUs (109 and 177) (belonging to the Bacteroidetes phylum), which were absent in SHR. The gut microbiome of SHR was also characterized by higher levels of other uncultured and incertae sedis bacteria genera OTUs (2761 and 3955) belonging to the Firmicutes phylum. In the same study, a 4-week course of the broad-spectrum antibiotic, minocycline was found to lower the mean arterial pressure significantly in an angiotensin II infusion model of hypertension. The reduction in blood pressure was accompanied by a decrease in the gut bacterial load. Treatment with minocycline decreased the Firmicutes to Bacteroidetes ratio. It also resulted in enrichment of acetate-producing bacteria such as Akkermansia, Bacteroides, Enterorhabdus and Marvinbryantia, thus highlighting the role of alteration of gut microbiota composition in the pathophysiology of hypertension.29 Similarly, another experimental study by Adnan et al also proved an association between gut microbiota dysbiosis and systolic blood pressure (SBP). In this study, a significant increase in the Firmicutes to Bacteroidetes ratio was observed in hypertensive versus normotensive rats, and a negative correlation was noted between the abundance of the butyrate-producing family Clostridiaceae and acetate-producing genera Holdemania and Coprobacillus and SBP. The authors proposed that alteration of the gut microbiota composition may present a new strategy for the treatment of hypertension.30 Other experimental and clinical studies have also confirmed similar associations between microbial content and blood pressure (BP) regulation.31,32 A very recent study conducted in Brazil involving 48 hypertensive and 32 normotensive subjects showed a significantly increased Firmicutes to Bacteroidetes ratio in hypertensive individuals. More than half of the hypertensive individuals had a high inflammatory score. Further, it was observed that the hypertensive individuals had a higher TNF-α/IFN-γ ratio compared to the normotensive group.33
Several mechanisms have been elucidated to explain the association between alteration in gut microbiota composition and BP regulation, including the role of SCFAs. SCFAs exert a plethora of actions via G protein-coupled receptors such as vascular olfactory receptor 78 (Olfr78) and G protein-coupled receptor 41 (GPR41). While GPR41 activation by SCFAs such as propionate causes vasodilation and reduction in BP; at higher concentrations of SCFAs, Olfr78 activation results in elevation of renin levels and a counter increase in BP to avoid hypotensive effects, thus facilitating BP regulation.34 In addition to direct effects on vasodilation, SCFAs have also been linked to BP regulation indirectly through plasminogen activator inhibitor-1 (PAI-1). PAI-1 levels have been positively correlated with BP and inflammation and a negative correlation has been noted between PAI-1 levels and abundance of butyrate-producing microbiota, which explains an indirect effect of gut microbiota alteration in BP regulation.32
2.2.2. Therapeutic implications
Several studies have explored the usefulness of drug interventions altering the gut microbiota composition and/or their metabolites on BP modulation. Gomez-Arango et al evaluated the effect of probiotic administration in SHR. Treatment with Lactobacillus fermentum CECT5716 (LC40) or a 1:1 mixture of L. coryniformis CECT5711 and L. gasseri CECT5714 (K8/LC9) for 5 weeks resulted in a significant increase in the counts of Lactobacillus spp. These effects were more intense in those animals treated with K8/LC9 compared to LC 40. Probiotic treatment also reduced the vascular levels of reactive oxygen species, vascular inflammation and improved endothelial function in the SHR group.35 In another experimental study, the intake of milk fermented using probiotic bacterial strains such as Lactobacillus casei TMC0409 and Streptococcus thermophilus TMC1543 for 8 weeks was associated with a significant lowering of BP compared to treatment with placebo.36 TMC1543 antibiotic administration has also been shown to lower arterial BP in a case report of a 69-year old lady with resistant hypertension. The patient's BP was controlled for two weeks while she was taking antibiotics and was off antihypertensives. An additional six months of satisfactory BP control was obtained with the use of just one antihypertensive medication.37
2.3. Gut microbiota and dyslipidaemia/obesity
2.3.1. Pathogenetic mechanisms
The association between dyslipidaemia, obesity and CVD is well-established in the literature. Several studies have elucidated the association between gut microbiota and alterations in lipid metabolism/obesity.
Gut microbiota has been found to have a strong influence on the levels of plasma cholesterol. Bacteria belonging to the class Betaproteobacteria, and Bacteriodales and phylum, Firmicutes have been found to be significantly high in experimental high-cholesterol models.38 Further, a study by Ley et al in obese subjects showed that Firmicutes dominate the gut microbiota in obese people, while Bacteroidetes remain the abundant bacterial group in lean subjects. Also, diets restricted in carbohydrates and fats were shown to increase the proportion of the genus, Bacteroidetes in this study.39
Several mechanisms have been postulated to explain the effects of gut microbiota on lipid metabolism and obesity, that involve production of SCFAs, and regulation of secondary bile acids, trimethylamine (TMA)/trimethylamine N-oxide (TMAO) and pro-inflammatory mediators such as lipopolysaccharides:
-
•
Activation of GPR43 by SCFAs has been proposed to prevent fat accumulation.17 Further, SCFAs have also been shown to activate peroxisome proliferator (PPAR)-γ,18,40 and increase plasma PYY and GLP-1 levels,22 resulting in increased energy expenditure, and reduced body weight and accumulation of triglycerides.
-
•
Gut microbiota has also been found to influence the deconjugation and excretion of bile acids that regulate lipid metabolism through farnesoid X receptor (FXR) and Takeda G-protein coupled bile acid receptor (TGR5) signalling.41,42
-
•
Paradoxically, TMA and TMAO generated by specific microbiota may promote an increased risk of atherosclerosis and CVD through mechanisms involving lipid metabolism and inflammation.41
-
•
Reduced gut permeability by specific gut microbiota may prevent the translocation of endotoxins such as lipopolysaccharides into the blood and prevent associated inflammation. It may be pertinent to mention here that lipopolysaccharides have been proven to be associated with the development of atherosclerosis and CVD.41
2.3.2. Therapeutic implications
Several studies have translated the pathophysiological association between gut microbiota and lipid metabolism/obesity for the development of therapeutic strategies addressing these disease states. Co-supplementation with Lactobacillus fermentum MTCC: 5898-fermented milk in a rodent model was found to improve the lipid profile, atherogenic index, coronary artery disease (CAD) risk index and the serum levels of various pro-inflammatory cytokines.43 Further, a meta-analysis by Wu et al also highlighted a beneficial role of the probiotic Lactobacillus in significantly reducing total cholesterol and low-density lipoprotein cholesterol.44 In addition to probiotics, existing therapeutic strategies for dyslipidaemia and obesity have also been explored for association of their efficacy with mechanisms involving the gut microbiota. Lai et al proved that the beneficial effects of diet and exercise in controlling body weight are transmissible through FMT, thus highlighting a plausible role of gut microbiota in the effects of these interventions on obesity.45 In an experimental study, statins significantly increased the abundance of genera Bacteroides, Butyricimonas and Mucispirillum, and this gut microbiota alteration correlated with metabolic improvements.46 Further, in a systematic review, a considerable alteration in the gut microbiota after bariatric surgery was noted with an increase in bacteria belonging to four phyla, including Bacteroidetes, Fusobacteria, Verrucomicrobia and Proteobacteria, thus suggesting a possible link between gut microbiota and metabolic alterations in humans.47
3. Gut microbiota and cardiovascular diseases
In addition to indirect effects on various CVD risk factors such as T2DM, hypertension, dyslipidaemia and obesity, as elaborated above, gut microbiota dysbiosis has been found to have direct implications on cardiovascular health. Factors such as oxidative stress, systemic and vascular inflammation contribute to the genesis and progression of CVD. These factors have been found to have a strong link with gut microbiota dysbiosis. The role of gut microbiota dysbiosis in atherosclerosis and CAD, chronic heart failure (CHF) and atrial fibrillation (AF) and associated therapeutic implications have been discussed in detail below.
3.1. Role of gut microbiota in atherosclerosis and CAD
3.1.1. Pathogenetic mechanisms
Several studies have highlighted the presence of an altered gut microbiota in patients with atherosclerosis and CAD. A study by Cui et al that involved high-throughput screening of stool samples of patients with coronary heart disease (CHD) and healthy controls revealed that CHD patients had a significantly greater -diversity than that of controls. Patients with CHD had an abundance of the phyla, Firmicutes compared to the controls, while Bacteroidetes was the most prominent group in controls. Clostridia was the most prominent in the phylum Firmicutes and was higher in CHD patients when compared to controls. Further, CHD patients also showed a decrease in Proteobacteria and an increase in Fusobacteria compared with the control group.48 In a separate experimental study by Chan et al elucidating the effect of probiotics and telmisartan in improving high-fat induced atherosclerosis, it was noted that high-fat diet resulted in an increase the Firmicutes to Bacteroidetes ratio. High-fat diet also lowered the abundance of Eubacterium, Anaeroplasma, Oscillospira, Roseburia and Dehalobacterium, and increased the levels of Allobaculum, Clostridum, Lactobacillus and Bifidobacteria. Further, a negative correlation was observed between the reduction in: (1) Eubacterium and increase in inflammatory cytokines such as matrix metalloproteinase −9 (MMP-9) and E-selectin; (2) Dehaobacterium and adipocyte fatty acid binding protein (A-FABP); and (3) Roseburia and MMP-9, thus confirming an association between gut microbiota dysbiosis and atherosclerosis.49
Multiple mechanisms have been proposed to explain the link between gut microbiota dysbiosis and the pathogenesis of atherosclerosis and CAD, including induction of systemic inflammation, generation of harmful metabolites that may exert proatherogenic effects, alteration of lipid metabolism and infection. The key molecules that mediate these mechanisms include cytokines, toll-like receptors (TLR), SCFAs, bile acids, lipopolysaccharides and TMAO.50 In homeostatic conditions, TLRs present on the gut epithelial and dendritic cells recognize microbe-associated molecular patterns (MAMPs) and stimulate cytokine production. This, in addition to maintenance of tight barrier function of the epithelium by selected microbiota and prevention of leakage of lipopolysaccharides into the blood help regulate innate immune processes.50 Further, SCFAs produced by specific microbiota, through inhibition of NK-κB exhibit strong anti-inflammatory properties.51 Dysbiosis in the gut microbiota may interfere with these pathways and influence the risk of CVD. An increase in lipopolysaccharides due to dysbiosis may induce TLR4 signalling and result in myocarditis.50,52 In experimental studies, certain strains of Lactobacillus have been found to inhibit the TLR4 pathway.53 Further, a negative correlation has been identified between C–C chemokine receptor type 2 (CCR2) expressed on monocytes and increase in Bifidobacterium54; and CCR2 has a positive association with inflammation in the arterial wall.55
Paradoxically, gut microbial metabolites such as TMAO might play an important role in the formation of atherosclerotic plaques. In a study involving 1876 stable subjects, it was observed that increased levels of choline TMAO and betaine showed dose-dependent associations with the presence of CAD and myocardial infarction. The study also demonstrated a positive correlation between plasma levels of TMAO and atherosclerotic plaque size. The levels of FMO3, an enzyme involved in TMAO production were inversely correlated with the plasma levels of the protective high-density lipoprotein (HDL) cholesterol levels. Inhibition of gut microbiota was shown to inhibit macrophage foam cell formation induced by choline.56 However, the prevalence of TMA-producing gut microbiota has been found to be low and may be confined to specific strains of Clostridia, Eubacteria and Escherichia coli.57
3.1.2. Therapeutic implications
Intake of probiotics has shown to be effective in the management of atherosclerosis and CAD. Kawase et al demonstrated that administration of fermented milk with both Lactobacillus casei TMC0409 and Streptococcus thermophilus TMC1543 in rodents resulted in a significant decrease in the atherogenic index compared with the control group. It was also observed that the intake of fermented milk was associated with a significantly lower levels of serum total cholesterol than the control group.36 A recent study involving administration of Lactobacillus rhamnosus GG supplements in patients with CAD for 12 weeks resulted in a significant reduction in the TMAO and pro-inflammatory cytokine levels.58 In the study by Chan et al in which apolipoprotein E knockout mice were fed with high fat diet to induce atherosclerosis, while treatment with both Lactobacillus rhamnosus GG and telmisartan was effective in reducing the size of atherosclerotic plaques, telmisartan was more effective than Lactobacillus in lowering the Firmicutes to Bacteroidetes ratio.49
3.2. Role of gut microbiota in chronic heart failure
3.2.1. Pathogenetic mechanisms
Chronic heart failure is another important CVD that has been investigated for association with gut microbiota. In a comparative study involving 60 patients with both mild (New York Heart Association [NYHA] functional class I to II) and moderate to severe (NYHA functional class III to IV) CHF, the gut microbiota of CHF patients was found to be significantly different from that of the healthy controls. Patients with severe CHF exhibited increased levels of Candida, Campylobacter, and Shigella species in faecal samples. However, there were no major differences for saprophytic microorganisms and commensal strains between the control group and CHF patients, and between patients with mild and moderate to severe CHF. The intestine permeability was found to be higher in patients with moderate to severe CHF compared with those with mild CHF.59 Another study involving 53 CHF patients and 41 healthy controls reported that CHF patients were characterized by increased levels of Ruminococcus gnavus, Streptococcus sp. and Veillonella sp, while controls had an abundance of Faecalibacterium prausnitzii, Oscillibacter sp. and Sutterella wadsworthensis. The gut microbiota in CHF patients also expressed higher levels of microbial genes for lipopolysaccharide biosynthesis, tryptophan and lipid metabolism, especially TMAO production. Further, the proportion of bacteria involved in SCFA metabolism was lesser in CHF patients.60
Multiple mechanisms and mediators have been proposed to explain the link between gut microbiota and CHF. Heart failure patients with pedal oedema have been found to have higher concentrations of endotoxins and lipopolysaccharide/log lipopolysaccharide-binding proteins (LBP) ratio compared with those without oedema. The plasma concentrations of C-reactive protein, TNFα, soluble TNF receptor-1 and receptor-2, interleukin-6, and soluble CD14 have also been noted to be higher in oedematous heart failure patients compared with non-oedematous patients.61 Tang et al demonstrated that patients with CHF had a higher median level of TMAO and BNP compared to controls; increased TMAO levels were found to be associated with increased 5-year mortality rates. A modest significant correlation was observed between TMA and BNP. High fasting TMAO levels were associated with a 2.2-fold increase in mortality risk even after adjusting for traditional risk factors and BNP and a 1.80-fold increase in mortality risk even after adjusting for traditional risk factors and BNP plus estimated glomerular filtration rate (eGFR).62 Another mechanism is related to increased systemic inflammation in CHF patients. Heart failure is characterized by reduced cardiac output which compromises the blood flow to the intestinal wall,63 thus disrupting the integrity of the gut wall allowing the gut contents to leak into the blood stream, which in turn triggers low-grade systemic inflammation.28,64
3.2.2. Therapeutic implications
Diuretics and probiotics have been studied for microbiota-related effects in CHF patients. Short-term diuretic therapy has been found to be associated with a decrease in endotoxin levels while the cytokine levels remained unaltered.61 The ability of probiotic strains to boost the gut wall integrity and inhibit inflammation might be helpful in preventing these events. Further, probiotic therapy with Lactobacillus rhamnosus has been shown to decrease systemic inflammation and the levels of TMAO,58 which might be beneficial in CHF patients.
3.3. Role of gut microbiota in atrial fibrillation
3.3.1. Pathogenetic mechanisms
Another important CVD with evolving recent research related to gut microbiota is AF. Zuo et al compared the gut microbiome of patients with AF with that of healthy controls. They found that the gut microbiota of patients with AF was characterized by overgrowth of harmful bacteria. There was dramatic difference in 574 genera between the AF patients and healthy controls. Patients with AF had higher proportions of Streptococcus, Enterococcus, Blautia, Dorea, Veillonella and Eubacterium. Bacterial groups such as Bifidobacterium, Roseburia and Ruminococcus were also high AF patients compared with healthy controls. Escherichia coli was the most abundant pathogenic bacterial species found in AF patients. The levels of butyrate-producing species such as Faecalibacterium prausnitzii was low in patients with AF. There was a dramatic decrease in the abundance of Fecalibacterium, Prevotella, Alistipes, Oscillibacter (genus level), and Sutterella in AF patients compared with controls. A similar shift was observed for Butyricicoccus, Flavonifractor, and Bilophila as well. Alterations were also observed in 27 metabolites in the serum and stool samples of patients with AF; 14 metabolites including cholic acid, oleic acid, linoleic acid, and α-linolenic acid were found to be reduced in AF patients.65
Another study conducted on 12 patients with persistent AF >12 months (Pers >12 months) and 8 patients with persistent AF < 12 months (Pers <12 months) showed a remarkable difference in the gut microbiota of the two groups. While the levels of Butyricicoccus and Paraprevotella were lower in patients with longer persistent AF duration, the levels of Blautia, Dorea, and Coprococcus were found to be higher in these patients. Pers >12 months group also showed increased levels of Thermosinus, Anaeroarcus, Clostridium bolteae, and Enterococcus faecium. The abundance of genera such as Faecalibacterium and Corynebacterium, and species like Faecalibacterium prausnitzii, and Eubacterium sp. CAG 581 were increased in the Pers <12 months group. Further, it was found that prolonged AF duration significantly reduced the levels of choline. Choline also displayed a negative association with Enterococcus faecium. Metabolites such as phosphohydroxy pyruvic acid and 3-indoleacetic acid were decreased in Pers>12months and negatively correlated with persistent AF duration. The Pers <12months-enriched genus Anaeroarcus showed a positive correlation with the genus Mycobacterium decreased in the Pers<12months group.66
Recently another comparative study was carried to elucidate the difference in gut microbiota in patients with paroxysmal atrial fibrillation (PAF), persistent atrial fibrillation (psAF) and healthy controls. It was observed that the PAF and psAF groups had a striking similarity in the gut microbial profile. Both PAF and psAF patients were also associated with a higher Firmicutes/Bacteroidetes (F/B) ratio independent of factors such as age, BMI, hypertension, or T2DM. It was also observed that the relative abundance of Firmicutes and Bacteroidetes were similar in PAF and psAF groups but were significantly different from the control group. The study also revealed that PAF- and psAF-enriched metabolites, such as chenodeoxycholic acid (CDCA), were positively correlated with AF-enriched bacterial genera such as Ruminococcus and Streptococcus. A positive correlation was seen between the psAF-enriched serum choline and psAF-enriched family Holosporaceae and genus Holospora, genus Methylovulum and species Methylovulum miyakonense. Additionally, it was observed that the atrial diameter parameters in the PAF and psAF groups showed a significant correlation with one family (Holosporaceae), two genera (Methylovulum and Holospora), and five other bacterial species.67 All these studies clearly indicate a role of gut microbiota in the pathogenesis and persistence of AF.
3.3.2. Therapeutic implications
TMAO plays an important role in the pathogenesis of CVD including AF. TMAO has shown to exacerbate autonomic activity and release of inflammatory cytokine in animal models of AF.68 These effects may be inhibited by probiotics. However, there is lack of studies which have evaluated the effect of modulation of gut microbiota by treatments used for AF.
3.4. Gut microbiota and chronic kidney disease
3.4.1. Pathogenetic mechanisms
Gut microbiota is also altered in patients with CKD. Evidence suggests decreased intake of dietary fibre, constipation, impaired protein metabolism and medication as the major contributing factors of dysbiosis in CKD.69 In CKD, structural and functional modifications of the gut-microbiota and impaired gut barrier function result in gut microbiome dysbiosis. Subsequently, this leads to the production of excessive quantities of uremic toxins which may get retained due to decreased urinary excretion in CKD. These toxins are derived from the unbalanced metabolism of nitrogen compounds mostly in association with the nondigestible carbohydrates, such as p-cresyl sulphate and indoxyl sulphate. They get translocated into systemic circulation via impaired gut barrier and may worsen CKD. They are also implicated in the development of CVD and risk of death in CKD patients.70,71
Indoles are intestinal bacterial metabolites of tryptophan, which are metabolized into indole indoxyl sulphate (IS) and indole-3-acetic acid (IAA). They activate nuclear factor (NF)-Kb and plasminogen activator inhibitor type1 and are culprits in tubulointerstitial fibrosis, aortic and vascular calcification, endothelial lining damage and decreased production of erythropoietin. P-Cresyl sulphate (PCS), another uremic toxin, is derived from the bacterial breakdown of tyrosine and phenylalanine that are subsequently sulphonated into PCS in the liver. They tend to cause renal fibrosis, oxidative stress and generation of inflammatory mediators.
Overall evidence indicates that increased levels of IS, IAA and PCS are associated with all-cause mortality and increased risk of cardiovascular events.71 Trimethylamine N.
Oxide (TMAO) is generated via choline metabolism to trimethylamine gas that subsequently oxidates into TMAO in the liver. They are dependent on renal elimination, and hence, the serum levels of TMAO are elevated in CKD. Similar to aforementioned uremic toxins, TMAO has been implicated in the progression of renal insufficiency and increased mortality in patients with CKD.71,72 Other uremic toxins, such as amines polyamines, d-amino acids, endotoxins, blood urea nitrogen (BUN) urea and their products, are also reported to be nephrotoxic and are implicated in the progression of CKD or CVD.71
3.4.2. Therapeutic implications
Intake of prebiotics and probiotics has been shown to be effective in reducing uremic toxin levels and slow the progression of CKD and prolong life. Meijers et al observed that serum concentrations of PCS and IS were reduced post oral intake of p-inulin, a prebiotic in haemodialysis patients.73 Similarly, Evenepoel et al reported reduction in PCS levels post treatment with acarbose in healthy persons.74 In haemodialysis patients, treatment with oral Lactobacillus acidophilus decreased levels of uremic toxins such as serum dimethylamine.75 Several novel therapies such as lubiprostone, a prostaglandin derivative and 3, 3-dimethyl-1-butanol (DMB), a trimethylamine (TMA) inhibitor, have shown beneficial effects in pre-clinical studies. Lubiprostone has been shown to improve the microbiota profile with a rapid increase in the Saccharolytic species and reduce the BUN levels.76 Further, 3, 3-dimethyl-1-butanol that is found in some essential oils has been shown to reduce the levels of TMAO in addition to the inhibition of macrophage foam cell and atherosclerotic lesion development.77
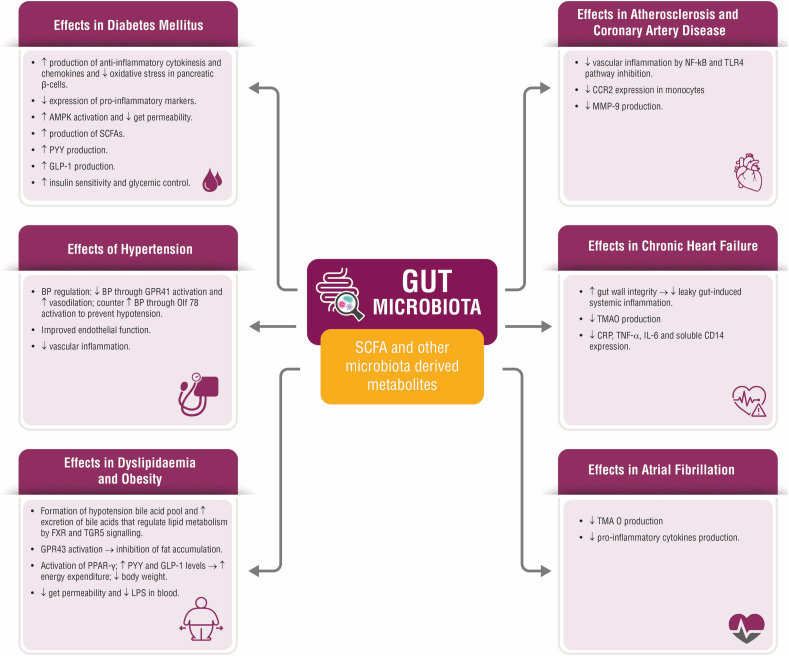
An overview of the various mechanisms of gut microbiota in ameliorating CVD risk factors such as T2DM, hypertension, dyslipidaemia and obesity; and CVDs such as atherosclerosis, CAD, CHF and AF is shown in Fig. 1. With respect to treatment, the European and American guidelines recommend metformin (unless contraindicated or not tolerated) or sodium-glucose cotransporter 2 (SGLT2) inhibitors or glucagon-like peptide 1 (GLP) receptor agonists for glycaemic management in established atherosclerotic CVD or CKD. These medications are also recommended for weight loss along with lifestyle interventions.78 Other drugs for weight loss are endocannabinoids, phentermine in combination with topiramate, liraglutide, and naltrexone in combination with bupropion.79 The latest European Society of Cardiology. ESH: European Society of Hypertension (ESH-ESC) guidelines for hypertension recommended renin–angiotensin system (RAS) blockers and calcium antagonists as the first-line treatment for metabolic syndrome in patients not attaining target BP of less than 140/90 mmHG with lifestyle changes.80 Agents such as telmisartan that act at peroxisome proliferator-activated receptor gamma (PPAR-γ) may have beneficial role in controlling BP and managing metabolic syndrome. Pharmacotherapy, particularly statins, have been effective in the management of dyslipidaemia in metabolic syndrome setting. Both high-dose statins or moderate-intensity statins have been found beneficial in managing dyslipidaemia. Newer drugs including apolipoprotein B may be used as secondary treatment in patients with high triglyceride levels and low HDL-C levels. Alirocumab, a monoclonal antibody, has also been shown to reduce LDL-C levels in patients receiving statins.79 Table 1 summaries the therapeutic intervention related to gut microbiota such as prebiotics, probiotics, diet modification and FMT in each cardiometabolic disease.
Fig. 1.
Summary of the effects of gut microbiota in ameliorating various CVDs and CVD risk factors.
Table 1.
Therapeutic Interventions related to Gut microbiota for Cardiometabolic diseases.
| Diabetes Mellitus | |||
|---|---|---|---|
| Study | Intervention | Patients | Comments |
| Vrieze et al.80 | FMT | Individuals with MS | Improves insulin sensitivity |
| deGroot et al.81 | |||
| RCT | |||
| Chambers et al.22 | SCFA (Propionate) | Overweight individuals | Stimulates glucose metabolism Improves insulin sensitivity |
| Bock et al.82 | Lactobacillus, Bifidobacterium and Streptococcus strains | Individuals with diabetes | Decreased FPG and insulinaemia |
| Meta-analysis | |||
| Hypertension | |||
| Bartolomaeus et al.83 | SCFA (Propionate) | Angiotensin II infused wild-type NMRI mice, | Reduction in BP |
| Khalesi et al.84 | Probiotics of Lactobacillus, Bifidobacterium, Saccharomyces, Enterococcus and Streptococcus strains | Individuals with or without hypertension | Significant reduction in BP, especially improvement in lower baseline BP. Significant decrease with long-term intervention |
| Meta-analysis | |||
| Dyslipidaemia/obesity | |||
| Jung et al.85 | Probiotics of L. gasser | Overweight and obese adults | Significant reduction in BMI, waist and hip circumference |
| RCT | |||
| Pedret et al.86 | Probiotics of B. animalis | Abnormal overweigh adults | Significant reduction in BMI, abdominal visceral fat and hip circumference |
| RCT | |||
| Reimer et al.87 | Prebiotics of Inulin-type fructans | Overweight and obese adults | Patients reported outcomes of subjective hunger rating and subjective satiety rating is increased. Energy and carbohydrate consumption are also reduced |
| RCT | |||
| Ruscica et al.88 | Co-administration of Bifidobacterium/yeast extract | Individuals with moderate hypercholesterolemia | Decrease in total cholesterol, LDL and triglycerides as well as an increase in HDL |
| Meta-analysis | |||
| Atherosclerosis and CAD | |||
| Moludi et al.58 | Weight loss diet along with probiotics supplementation LGG | Individuals with CVD | Improved endotoxemia, by reducing the LPS and TMAO levels. Also improved microbiota profile |
| Kawase et al.36 | L. casei TMC0409 and S. thermophilus TMC1543 | Rodents | Significant decrease in the atherogenic index |
| Chen et al.89 | L.acidophilus | ApoE−/- mice | Decrease in Atherosclerotic burden |
| Chronic Heart failure | |||
| Contstanza et al.90 | Probiotics of S. boulardii | Patients with NYHA II and III, CHF and EF <50% | Improvement in ventricular function |
| RCT | Decrease in inflammatory biomarkers | ||
| Uremic toxins | |||
| Ranganathan et al.91 | S. thermophilus, L. acidophilus, and B. longum | Patients with CKD | Reduction in BUN |
| de Preter et al.92 | Oligofructose-enriched inulin | Healthy individuals | Decrease in p-cresol |
BP: Blood pressure; BMI: Body mass index; BUN: Blood urea nitrogen; CAD: Coronary artery disease; CHF: Congestive heart failure; CKD: Chronic kidney disease CVD: Cardiovascular disease; EF: Ejection fraction; FMT: Fecal Microbiota Transplantation; FPG: Fasting plasma glucose; HDL: High-density lipoproteins; LDL: Low-density lipoproteins; LGG: Lactobacillus rhamnosus GG; LPS: Lipopolysaccharide; MS: Metabolic syndrome; NHYA: New York Heart Association; RCT: Randomized clinical trial.; SCFA: Short chain fatty acids; TAMO: Trimethylamine N-oxide.
4. Conclusion
There is a growing body of evidence, which suggests that there is a clear association between gut-microbiota dysbiosis and CVDs, CVD risk factors and CKD-related complications discussed in this review. Modern molecular techniques have enabled the characterization of gut-microbiota and elucidation of their protective mechanisms in these disease states. Translation of this knowledge into useful therapeutics such as FMT, probiotics, and diet therapies has been proven to be safe and effective for the management of CVDs and associated risk factors and CKD and associated complications. Both animal and human studies evaluating the efficacy of gut-microbiota-modulating therapies have shown promising results. However, implementation of such therapeutic strategies entails a more lucid understanding of the role of the gut-microbiota in the pathogenesis of CVDs and associated risk factors through large-scale, controlled trials in humans.
Source of funding
None.
Declaration of competing interest
None.
Acknowledgment
We thank BioQuest Solutions Pvt Ltd for providing editorial support for the manuscript.
References
- 1.Lederberg J., McCray A.T. Ome SweetOmics--A genealogical treasury of words. Scientist. 2001;15:8. [Google Scholar]
- 2.Saxena R., Sharma V.K. A metagenomic insight into the human microbiome: its implications in health and disease. InMedical and Health Genomics. 2016:107–119. [Google Scholar]
- 3.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoSbiology. 2016;14 doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckburg P.B., Bik E.M., Bernstein C.N. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinninella E., Raoul P., Cintoni M. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7:14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avershina E., Lundgård K., Sekelja M. Transition from infant- to adult-like gut microbiota. Environ Microbiol. 2016;18:2226-36. doi: 10.1111/1462-2920.13248. [DOI] [PubMed] [Google Scholar]
- 7.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7 doi: 10.7717/peerj.7502. Published 2019 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazmanian S.K., Liu C.H., Tzianabos A.O. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Bäckhed F., Ding H., Wang T. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci Unit States Am. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M., Wang B., Zhang M. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci Unit States Am. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida N., Yamashita T., Hirata K.I. Gut microbiome and cardiovascular diseases. Diseases. 2018;6:56. doi: 10.3390/diseases6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emoto T., Yamashita T., Sasaki N. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atherosclerosis Thromb. 2016;23:908-21. doi: 10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardiovascular diseases. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 Available at:
- 14.Gurung M., Li Z., You H. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q., Chang Y., Zhang K. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep. 2020;10:1–8. doi: 10.1038/s41598-020-62224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel B.S., Shaito A., Motoike T. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor. Gpr41. Proceedings of the National Academy of Sciences. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura I., Ozawa K., Inoue D. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1–2. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Besten G., Bleeker A., Gerding A. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 19.Sanna S., van Zuydam N.R., Mahajan A. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019;51:600-05. doi: 10.1038/s41588-019-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Lu Y., Yan Y. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Frontiers in Cellular and Infection Microbiology. 2020;9:455. doi: 10.3389/fcimb.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeland K.R., Wolever T.M. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br J Nutr. 2010;103:460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 22.Chambers E.S., Viardot A., Psichas A. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng Z., Yuan Q., Yu R. Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol Nutr Food Res. 2019;63:1900457. doi: 10.1002/mnfr.201900457. [DOI] [PubMed] [Google Scholar]
- 24.Khalili L., Alipour B., Jafarabadi M.A. Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndrome. 2019;11:5. doi: 10.1186/s13098-019-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Sinnett-Smith J., Kisfalvi K., Kui R. Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun. 2013;430:352–357. doi: 10.1016/j.bbrc.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su B., Liu H., Li J. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J Diabetes. 2015;7:729-39. doi: 10.1111/1753-0407.12232. [DOI] [PubMed] [Google Scholar]
- 27.Yan X., Feng B., Li P. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res. 2016;2016:2093171. doi: 10.1155/2016/2093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q., Xiao X., Li M. Vildagliptin increases butyrate-producing bacteria in the gut of diabetic rats. PloS One. 2017;12 doi: 10.1371/journal.pone.0184735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T., Santisteban M.M., Rodriguez V. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adnan S., Nelson J.W., Ajami N.J. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genom. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mell B., Jala V.R., Mathew A.V. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genom. 2015;47:187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Arango L.F., Barrett H.L., McIntyre H.D. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 33.Silveira-Nunes G., Durso D.F., Cunha E.H. Hypertension is associated with intestinal microbiota dysbiosis and inflammation in a Brazilian population. Front Pharmacol. 2020;11:258. doi: 10.3389/fphar.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microb. 2014;5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez-Guzmán M., Toral M., Romero M. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59:2326–2336. doi: 10.1002/mnfr.201500290. [DOI] [PubMed] [Google Scholar]
- 36.Kawase M., Hashimoto H., Hosoda M. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255–263. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 37.Qi Y., Aranda J.M., Rodriguez V. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension—a case report. Int J Cardiol. 2015;201:157. doi: 10.1016/j.ijcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Roy T., Lécuyer E., Chassaing B. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019;17(1):94. doi: 10.1186/s12915-019-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley R.E., Turnbaugh P.J., Klein S. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 40.Gao Z., Yin J., Zhang J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509-17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20:461-472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayin S.I., Wahlström A., Felin J. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabol. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Yadav R., Khan S.H., Mada S.B. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-fermented milk attenuates dyslipidemia, oxidative stress, and inflammation in male rats fed on cholesterol-enriched diet. Probiotics and antimicrobial proteins. 2019;11:509–518. doi: 10.1007/s12602-018-9429-4. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Zhang Q., Ren Y. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PloS One. 2017;12 doi: 10.1371/journal.pone.0178868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Z.L., Tseng C.H., Ho H.J. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33893-y. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J., Lee H., An J. Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front Microbiol. 2019;10:1947. doi: 10.3389/fmicb.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Y., Huang Z.P., Liu C.Q. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2018;178:43–56. doi: 10.1530/EJE-17-0403. [DOI] [PubMed] [Google Scholar]
- 48.Cui L., Zhao T., Hu H. Association study of gut flora in coronary heart disease through high-throughput sequencing. BioMed Res Int. 2017 doi: 10.1155/2017/3796359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan Y.K., Brar M.S., Kirjavainen P.V. High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamnosus GG (LGG) or telmisartan in ApoE−/− mice. BMC Microbiol. 2016:16264. doi: 10.1186/s12866-016-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsimichas T., Antonopoulos A.S., Katsimichas A. The intestinal microbiota and cardiovascular disease. Cardiovasc Res. 2019;115 doi: 10.1093/cvr/cvz135. 1471-86. [DOI] [PubMed] [Google Scholar]
- 51.Segain J.P., Raingeard de la Blétière D., Bourreille A. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47 doi: 10.1136/gut.47.3.397. 397–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jenke A., Wilk S., Poller W. Adiponectin protects against Toll-like receptor 4-mediated cardiac inflammation and injury. Cardiovasc Res. 2013;99:422–431. doi: 10.1093/cvr/cvt118. [DOI] [PubMed] [Google Scholar]
- 53.Su X., Yan H., Huang Y. Expression of FABP, adipsin and adiponectin in Paneth cells is modulated by gut Lactobacillus. Sci Rep. 2015;5:18588. doi: 10.1038/srep18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H., Qian L., Lv Q. Change in gut microbiota is correlated with alterations in the surface molecule expression of monocytes after Roux-en-Y gastric bypass surgery in obese type 2 diabetic patients. Am J Transl Res. 2017;9:1243–1254. [PMC free article] [PubMed] [Google Scholar]
- 55.Verweij S.L., Duivenvoorden R., Stiekema L.C.A. CCR2 expression on circulating monocytes is associated with arterial wall inflammation assessed by 18F-FDG PET/CT in patients at risk for cardiovascular disease. Cardiovasc Res. 2018;114:468–475. doi: 10.1093/cvr/cvx224. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z., Klipfell E., Bennett B.J. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rath S., Heidrich B., Pieper D.H. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moludi J, Kafil HS, Gholizadeh P, et al. Effect of Probiotic Supplementation along with Calorie Restriction on Metabolic Endotoxemia, Trimethylamine-N-Oxide, Inflammation, Metabolic Factors, and Gut Microbiota Profile in Coronary Artery Disease Patients: A Double Blind Placebo Controlled Randomized Clinical Trial. [DOI] [PMC free article] [PubMed]
- 59.Pasini E., Aquilani R., Testa C. Pathogenic gut flora in patients with chronic heart failure. JACC (J Am Coll Cardiol): Heart Fail. 2016;4:220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Cui X., Ye L., Li J. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:1–5. doi: 10.1038/s41598-017-18756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niebauer J., Volk H.D., Kemp M. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 62.Tang W.W., Wang Z., Fan Y. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogler G., Rosano G. The heart and the gut. Eur Heart J. 2014;35:426-30. doi: 10.1093/eurheartj/eht271. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y., Huang Z., Li H. Insights into innate immune signalling in controlling cardiac remodelling. Cardiovasc Res. 2017;113:1538–1550. doi: 10.1093/cvr/cvx130. [DOI] [PubMed] [Google Scholar]
- 65.Zuo K., Li J., Li K. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. GigaScience. 2019;8 doi: 10.1093/gigascience/giz058. giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuo K., Li J., Wang P. Duration of persistent atrial fibrillation is associated with alterations in human gut microbiota and metabolic phenotypes. mSystems. 2019;4 doi: 10.1128/mSystems.00422-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo K., Yin X., Li K. Different types of atrial fibrillation share patterns of gut microbiota dysbiosis. mSphere. 2020;5 doi: 10.1128/mSphere.00071-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu L., Meng G., Huang B. A potential relationship between gut microbes and atrial fibrillation: trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 69.Ramezani A., Raj D.S. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25(4):657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armani R.G., Ramezani A., Yasir A., Sharama S., Canziani M.E.F., Raj D.S. Gut microbiome in chronic kidney disease. Curr Hypertens Rep. 2017;19(4):29. doi: 10.1007/s11906-017-0727-0. [DOI] [PubMed] [Google Scholar]
- 71.Nallu A., Sharma S., Ramezani A., Muralidharan J., Raj D. Gut microbiome in chronic kidney disease: challenges and opportunities. Transl Res. 2017;179:24–37. doi: 10.1016/j.trsl.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trøseid M., Andersen G.Ø., Broch K., Hov J.R. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52:102649. doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijers B.K., De Preter V., Verbeke K., Vanrenterghem Y., Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25(1):219–224. doi: 10.1093/ndt/gfp414. [DOI] [PubMed] [Google Scholar]
- 74.Evenepoel P., Bammens B., Verbeke K., Vanrenterghem Y. Acarbose treatment lowers generation and serum concentrations of the protein-bound solute p-cresol: a pilot study. Kidney Int. 2006 Jul;70(1):192–198. doi: 10.1038/sj.ki.5001523. [DOI] [PubMed] [Google Scholar]
- 75.Simenhoff M.L., Dunn S.R., Zollner G.P. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab. 1996;22(1–3):92–96. [PubMed] [Google Scholar]
- 76.Mishima E., Fukuda S., Shima H. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol. 2015 Aug;26(8):1787–1794. doi: 10.1681/ASN.2014060530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z., Roberts A.B., Buffa J.A., Levison B.S. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies M.J., D'Alessio D.A., Fradkin J. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. Management of Hyperglycemia in Type 2 Diabetes. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srikanth S., Deedwania P. Management of dyslipidemia in patients with hypertension, diabetes, and metabolic syndrome. Curr Hypertens Rep. 2016;18(10):76. doi: 10.1007/s11906-016-0683-0. [DOI] [PubMed] [Google Scholar]
- 80.Vrieze A., Van Nood E., Holleman F. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916. doi: 10.1053/j.gastro.2012.06.031. e7. [DOI] [PubMed] [Google Scholar]
- 81.de Groot P., Scheithauer T., Bakker G.J. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut. 2020;69(3):502–512. doi: 10.1136/gutjnl-2019-318320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bock P.M., Telo G.H., Ramalho R. The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2021;64:26–41. doi: 10.1007/s00125-020-05295-1. [DOI] [PubMed] [Google Scholar]
- 83.Bartolomaeus H., Balogh A. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalesi S., Sun J., Buys N., Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64(4):897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 85.Jung S.P., Lee K.M., Kang J.H. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J Fam Med. 2013;34(2):80–89. doi: 10.4082/kjfm.2013.34.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pedret A., Valls R.M., Calderón-Pérez L. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes. 2019 Sep;43(9):1863–1868. doi: 10.1038/s41366-018-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reimer R.A., Willis H.J., Tunnicliffe J.M. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: a randomized controlled trial. Mol Nutr Food Res. 2017;61(11) doi: 10.1002/mnfr.201700484. [DOI] [PubMed] [Google Scholar]
- 88.Ruscica M., Pavanello C., Gandini S. Nutraceutical approach for the management of cardiovascular risk – a combination containing the probiotic Bifidobacterium longum BB536 and red yeast rice extract: results from a randomized, double-blind, placebo-controlled study. Nutr J. 2019;18:13. doi: 10.1186/s12937-019-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen L., Liu W., Li Y., Luo S. Lactobacillus acidophilus ATCC 4356 attenuates the atherosclerotic progression through modulation of oxidative stress and inflammatory process. Int Immunopharm. 2013;17(1):108–115. doi: 10.1016/j.intimp.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 90.Costanza A.C., Moscavitch S.D., Faria Neto H.C., Mesquita E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015 Jan 20;179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 91.Ranganathan N., Ranganathan P., Friedman E.A. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010 Sep;27(9):634–647. doi: 10.1007/s12325-010-0059-9. [DOI] [PubMed] [Google Scholar]
- 92.de Preter V., Vanhoutte T., Huys G., Swings J., Rutgeerts P., Verbeke K. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment Pharmacol Ther. 2008 Mar 15;27(6):504–513. doi: 10.1111/j.1365-2036.2007.03588.x. [DOI] [PubMed] [Google Scholar]