Abstract
Background
The increased risk of cardiovascular morbidity and mortality in chronic kidney disease (CKD) and end-stage renal disease (ESRD) seems particularly pronounced in patients with concomitant aortic and mitral valvular calcifications. Valvular calcification (VC) is accelerated in patients with CKD and even more so with ESRD and haemodialysis (HD) due to premature endothelial cell dysfunction. Mineral and bone disorder (CKD-MBD) is a common complication of CKD/ESRD and may play a pivotal role in VC.
Case summary
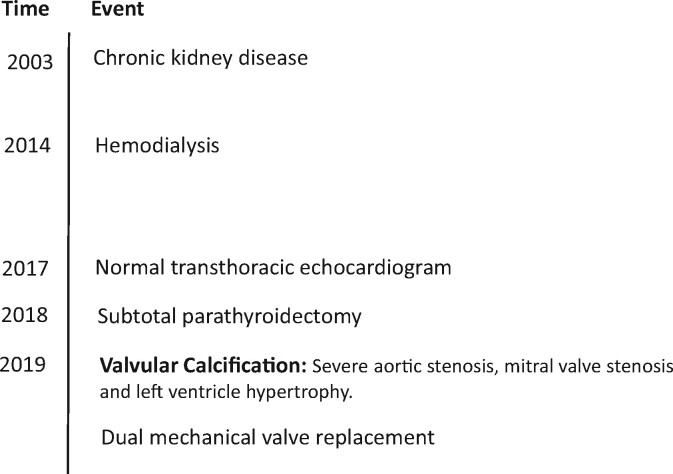
A 25-year-old woman with congenital hypoplastic kidneys and ESRD on HD from the age of 19 was admitted to the emergency department suffering from chest pain and dyspnoea. Transthoracic echocardiogram (TTE) revealed critical aortic stenosis (AS) with indexed aortic valve area 0.4 cm2/m2, a mean gradient 58 mmHg and a moderate mitral stenosis with a mean gradient 6–8 mmHg developed over the course of 2 years, as a normal TTE was performed at that time. During HD, the patient had longstanding alterations in calcium and phosphate metabolism including secondary hyperparathyroidism that eventually progressed into tertiary hyperparathyroidism. Efforts were made to treat CKD-MBD but patient compliance was low. Subtotal parathyroidectomy was performed 6 months prior to admission. The patient had dual mechanical valve replacement.
Discussion
Valvular calcification is common in patients with CKD/ESRD and in particular in patients on HD. Rapid progression of valve disease in this case may be related to the combination of low patient adherence and sustained disturbed calcium and phosphate metabolism with tertiary hyperparathyroidism. Transthoracic echocardiogram should be performed in patients on HD even with minor suspicion of VC and in patients with low adherence and disturbance of calcium and phosphate metabolism.
Keywords: Valvular calcification, Aortic stenosis, Chronic kidney disease, End-stage renal disease, CKD-MBD, Hyperparathyroidism
Learning points
Valvular calcification is common in chronic kidney disease (CKD)/end-stage renal disease.
Disturbed bone and mineral disorder (CKD-BMD) accelerate the calcification process.
Introduction
Valvular calcification (VC) is most often a slow progressive age-related condition caused by calcification of the left-sided heart valves (aortic and mitral valves) with subsequent obstruction of blood flow to and from the left ventricle (LV) with or without regurgitation.1 Dyspnoea is the typical symptom in VC with stenosis and regurgitation. The aetiology of VC is complex and not fully clarified. Endothelial cell dysfunction (ECD) is a ‘culprit’ of cardiovascular morbidity and develops in chronic kidney disease (CKD) with a higher frequency. Endothelial cell dysfunction facilitates deposition of lipoprotein, chronic inflammation, and leaflet calcification with bone formation, all factors involved in the calcification process.2,3 Valvular calcification occurs 10–20 years earlier in patients with CKD compared with the general population and the prevalence of VC is eight times higher in patients treated with haemodialysis (HD) compared with the general population.3,4 In patients on HD, the reported prevalence is 55% for aortic valve calcification (AVC) and 59% for mitral valve calcification. Haemodynamically significant aortic stenosis (AS) was observed in 6–13%.2,4 Numerous factors predispose to development of VC in CKD in particular disorders of mineral and bone metabolism (CKD-MBD). Valvular calcification in CKD and end-stage renal disease (ESRD) is associated with increased rates of clinical complications and mortality.2–4
Timeline
|
Case presentation
A 25-year-old woman with a history of congenital hypoplastic kidneys and ESRD on HD was admitted to the emergency department with sudden onset of chest pain and dyspnoea during HD. Chronic kidney disease was recognized and treated from the age of 8 years old. The patient had been on HD since the age of 19 and was at the time of admission undergoing supplementary investigations aiming for renal transplantation. The patient had a non-supportive childhood, persistent adherence issues that worsened in adolescence, and intermittent cannabis abuse. However, she managed to attend to weekly, but not to all scheduled HD except in periods of heavy cannabis abuse. Six months earlier, she underwent subtotal parathyroidectomy due to tertiary hyperparathyroidism with sustained elevation of parathyroid hormone (PTH) between 134 and 191 pmol/L (reference range 1.7–9.2 pmol/L). During surgery, a total of 4777 mg hyperplastic parathyroid tissue was removed. Multiple efforts were continuously applied to treat the CKD-MBD including treatment with cholecalciferol for vitamin D deficiency, both calcium-free and calcium-containing phosphate binders for hyperphosphataemia, alfacalcidol for hypocalcaemia, and cinacalcet for secondary hyperparathyroidism. Success was, however, limited in part since patient adherence to both medical treatments and dietary restrictions was low despite numerous efforts to improve patient’s self-care. In the aftermath of parathyroidectomy, the PTH level was well controlled; however, patient adherence did not improve and phosphate was persistently elevated.
A holosystolic murmur and crackling heard sound was heard on auscultation.
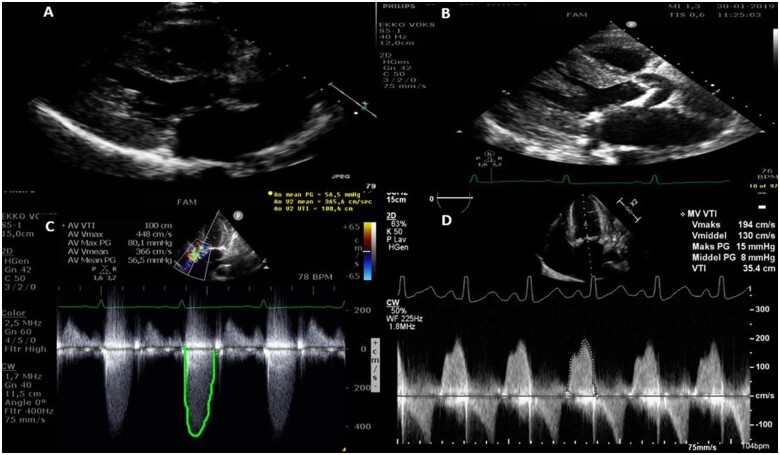
Acute coronary syndrome was excluded with normal electrocardiogram without dynamic rise and fall in high-sensitive troponin TNI 8 (<10 ng/L). The patient further complained of recurrent dyspnoea NYHA class 3 (New York Heart Association Classification), in particular during dialysis and on physical exertion. Oedema and left-sided pleural effusion was discovered by chest X-ray. Transthoracic echocardiogram (TTE) revealed progressive critical AS in a tricuspid valve with indexed aortic valve area of 0.4 cm2/m2 and a mean gradient of 58 mmHg (peak gradient 80 mmHg) in comparison to a normal TTE performed ∼2 years earlier. The mitral valve was moderately stenotic with a mean gradient of 6–8 mmHg but with severe annular calcifications. The LV had become hypertrophic (IVSd 1.5 cm, LVPWd 1.8 cm) with preserved ejection fraction (LVEF 60%) (Figure 1). Infectious endocarditis was excluded with positron emission tomography/computerized tomography without positive fluro-deoxy-D-glucose uptake in relation to valves and absence of bacteraemia and vegetations on transoesophageal echocardiogram (TEE). The patient underwent computed tomography coronary angiography that excluded coronary calcifications and stenosis. Aortic valve calcium Agatston score was 4850.
Figure 1.
Transthoracic echocardiography (A and B): Parasternal long-axis view 2 years before and at admission. (C and D) Modified five-chamber view showed critical aortic stenosis. Apical four-chamber image revealed moderate mitral stenosis.
A multidisciplinary discussion was undertaken involving cardiologists, cardiothoracic surgeons, and cardiothoracic anaesthesiologists and ended up recommending the patient dual mechanical valve replacement. There were concerns in relation to surgery due to a low body mass index of 13 kg/m2 and regarding compliance with future anticoagulation in relation to mechanical valves. At a high volume thoracic centre, a mechanical Master prosthesis 19 mm was inserted in aortic position and a mechanical ATS 27 mm was inserted in mitral position. The postoperative period was complicated with localized pericardial haematoma compromising the right ventricle resulting in acute surgical evacuation.
The patient was treated with parenteral vancomycin and empirical cefuroxime for 6 weeks because of recurrent infections of unknown origin without positive blood cultures. Repeated blood culture tests and TEE did not confirm endocarditis. The patient was discharged without signs of active infection and with normal haemodynamics for usual HD and clinical follow-up with TTE after 1 and 6 months. At evaluation after 1 month, the patient denied shortness of breath or any complaints. Transthoracic echocardiogram revealed functioning mechanical valves. The nutritional status of the patient was poor and there had been difficulties in relation to international ratio (INR) regulation, so the patient received additional fragmin.
Discussion
While ESRD and renal replacement therapy usually cause accelerated VC, disease progression is usually slow to moderate. This case report illustrates extremely rapid progression of VC to critical AS in a young patient with ESRD on HD over the course of 2 years. Symptoms of AS had progressed over the course of months leading to acute admission and the diagnosis of critical AS with impaired haemodynamics.
The pathogenesis of VC is multifactorial and shares a number of aetiological factors with vascular calcification including genetic and metabolic factors that ultimately lead to an active inflammatory process in the valvular tissue.2,4 We speculate that the three main causes of rapid progression of VC in this case is related to the following sections.
Severe chronic kidney disease-bone and mineral disorder
Patients with CKD have impairments in a number of processes related to inflammation and bone metabolism that are associated with the development of VC.5 Specifically, the patient reported in this case had longstanding CKD-MBD with hyperphosphataemia and secondary hyperparathyroidism that eventually progressed into tertiary hyperparathyroidism necessitating parathyroidectomy.6,7 Several studies have reported that the magnitude of both hyperphosphataemia and hyperparathyroidism were independently associated with progressive VC.2 In addition, several other metabolic disturbances related to calcium, phosphate, and bone homeostasis are observed in patients with CKD including alterations in the osteoprotegerin/receptor activator of nuclear factor kB ligand (RANKL) ratio, fibroblast growth factor 23, and klotho.4 Such hormonal changes may not only cause deleterious effects in the skeleton but also promote VC potentially through activation of intra-valvular osteo-blast-like cells with calcium-deposition and bone-forming capacity.5
Endothelial cell dysfunction and volume overload
Aortic valve calcification and eventually stenosis induce LV hypertrophy and increase afterload causing a circulus vitiosus for valve disease progression. Duration of HD is also correlated to progression of VC and a possible accelerator of VC with induction of ECD as well as chronic inflammation with persistent elevation in ferritin level, anaemia, and malnutrition. In this case, the patient had been on HD for 5 years.1,3
In addition, pre-existing ECD caused by mechanical stress from high cardiac output related to fistulas and chronic volume overload due to low patient adherence to fluid restrictions and occasionally HD drop-outs likely also contributed to accelerated calcification leading to critical AS.
Low patient adherence
Low or at times non-existing patient adherence to treatments led to a number of changes in haemodynamics with volume overload and severely disturbed calcium and phosphate metabolism that are likely responsible for the rapid disease progression. The preoperative decision regarding choice of prosthetic valves was very difficult. The high bleeding risk with anticoagulation in patients on HD and the patient’s history with low adherence were acknowledged. However, dual mechanical valves were chosen over bio-prosthetics because of native anatomical conditions with a small size of the left ventricle outflow (LVOT) diameter that would increase the risk of post-operative LVOT obstruction in case of a bio-prosthetic valves. In addition, the young age of the patient and the prospect of repeated valve replacement with bio-prosthetic valves in combination with the possibility of future renal transplantation were taken into account. The patient was educated about her condition; she understood the terms and accepted mechanical valve replacement and life-long anticoagulation therapy and agreed to have INR monitored at scheduled HD.
Conclusion
This case illustrates rapid progression of VC resulting in critical AS in a young patient with ESRD on HD and low treatment adherence. A high index of suspicion for performing TTE to detect VC and valvular stenosis should be applied, especially in the case of low treatment adherence or in the presence of symptoms. In addition, if VC is detected follow-up should be scheduled to assess disease progression. Evidence of the effects of medical treatment for preventing progression of VC in patients on HD is limited and warrant future studies.
Lead author biography
Christina Stolzenburg Oxlund, Senior registrar, Ph.D at department of Cardiology at University Hospital of Southwest Jutland, Esbjerg, Denmark.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflicts of interest: none declared.
Funding: none declared.
Supplementary Material
References
- 1. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM. et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urena-Torres P, D’Marco L, Raggi P, Garcia-Moll X, Brandenburg V, Mazzaferro S. et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant 2019;35:2046–2053. [DOI] [PubMed] [Google Scholar]
- 3. Bellasi A, Mangano M, Galassi A, Cozzolino M.. CKD-MBD, cardiovascular involvement and prognosis. G Ital Nefrol 2017;34:150–161. [PubMed] [Google Scholar]
- 4. Ternacle J, Cõté N, Krapf L, Nguyen A, Clavel MA, Pibarot P.. Chronic kidney disease and the pathophysiology of valvular heart disease. Can J Cardiol 2019;35:1195–1207. [DOI] [PubMed] [Google Scholar]
- 5. Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv 2012;5:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberú S, Bakr MA. et al. Summary of Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 2017;101:1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallant KMH, Spiegel DM.. Calcium balance in chronic kidney disease. Curr Osteoporos Rep 2017;15:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.