Abstract
Background:
This study explores the potential diagnostic utility of soluble Human Leukocyte Antigen (sHLA) molecules differentially released by lung adenocarcinoma and benign lung lesions.
Methods:
Conditioned media from the NSCLC cell lines H358 and H1703 were immunoblotted for soluble isoforms of major histocompatibility complex (MHC) class I (ABC) and II (DRB1, DMB, and DQ) antigens. Sera from 25 patients with benign and 25 patients with malignant lesions were similarly evaluated to appraise the potential diagnostic value.
Results:
Higher concentrations of soluble HLA class I molecules were observed in conditioned medium for the highly-invasive H1703 cell line, relative to the more indolent H358 cells. Evaluation of these markers against a cohort of 50 cases demonstrated that patients with malignant lesions possess higher levels of HLA class I and II molecules relative to those with benign lesions (p < 0.05), with exception to the primary isoform, DQA1, which was suppressed in malignancies. An analysis of biomarker performance via ROC analysis revealed promising performance (AUC > 0.75) for DMB and the 26 kDa isoform of DQ in distinguishing lesion pathology.
Conclusions:
Soluble HLA molecules may have diagnostic value for early-stage NSCLC. Validation studies are currently underway using sera from a lung cancer screening cohort.
Keywords: NSCLC, HLA, Biomarker, Screening, Nodule
1. Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide [1]. According to the American Cancer Society, approximately 220,000 patients will be diagnosed with lung cancer and 160,000 will die of the disease in 2017 [1]. This has led to a variety of efforts to identify early-stage lung cancer, prime among which has been low-dose computed tomography (LDCT) lung cancer screening. Although the National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual LDCT, there was a high rate of false positive screens [1,2]. Accordingly, the International Association for the Study of Lung Cancer (IASLC) and the Strategic CT Screening Advisory Committee has called for the increased use of blood-based diagnostics to augment LDCT screening [3].
Human leukocyte antigens (HLA) are a key immune mechanism through which self- versus non-self-recognition occurs [4,5]. There are limited reports describing decreased expression of major histocompatibility complex (MHC) class I or class II antigens on the surface of tumor cells [6–8]. It has been proposed that shedding of these HLA molecules into the peripheral circulation may be a key mechanism by which tumor cells circumvent host immune surveillance [6–8]. That is, if the HLA molecules are not present on the tumor cell surface, the host immune system will fail to recognize the tumor cell as foreign and mount an immune response [9]. We hypothesized that patients with malignant and benign lung lesions could be differentiated by comparing serum concentrations of the ‘secretome’ of soluble HLA molecules.
2. Materials and methods
2.1. Cell lines and media
Human adenocarcinoma cell lines H358 and H1703 were cultured at 37 °C in a humidified CO2 incubator in RPMI-1640 containing 10% fetal bovine serum (FBS), 0.3 g/L glutamine, and antibiotics (100 units/mL penicillin/100 units/mL streptomycin/10 μg/mL gentamicin sulfate). Cultures were primary acquisitions from ATCC and all experiments performed within 10 passages of acquisition.
2.2. Collection of conditioned media
Cultures of the H358 and H1703 cells were individually grown to near-confluence (85–90%) and incubated for 3 days in RPMI-1640 containing 2.5% FBS, 0.3 g/L glutamine, and 100 units/mL penicillin and streptomycin, and 10 μg/mL gentamicin sulfate. After 3 days, the cell-conditioned media was collected and centrifuged for 10 min at 1,000 RCF. The resulting supernatant was albumin depleted using the Albumin Depletion Kit (Pierce Thermo Scientific), per manufacturer recommended protocols. The resulting supernatant was stored at −80 °C until further processing.
2.3. Patient specimens
A total of 50 serum specimens (25 patients with a benign nodule and 25 patients limited to T1–2N0M0 adenocarcinoma (7th edition staging [10])) were obtained from the Rush University Biorepository Core. Patients were selected to fit the ‘high risk’ criteria for lung cancer screening (55–80 years old and a minimum 30 pack year smoking history and are either active smokers or had quit within 15 years of evaluation) and were approximately matched in terms of age, gender, and smoking history. All specimens were obtained with full written informed consent under a protocol approved by our Institutional Review Board (IRB). All samples were coded with only basic demographic and clinical parameters provided to the study personnel for the purposes of this study. All serum specimens were albumin in the same manner as mentioned in the previous section.
2.4. Protein concentration
All albumin-depleted samples (serum or cell-condition media) were concentrated using the ProteoExtract Protein Precipitation kit (EMD Millipore). Following resuspension in PBS containing 50 μL/mL Mammalian Protease Inhibitor Cocktail (Sigma), protein concentrations were determined through standard BCA assays (Thermo Scientific).
2.5. Immunoblotting of soluble antigens
Protein samples were resolved using standard denaturing polyacrylamide gel electrophoresis (SDS-PAGE) using Criterion 4–20% Tris-HCl gels (BioRad). Proteins were then transferred onto a nitrocellulose membrane using standard overnight “wet” transfer protocols. After being blocked for 1 h in 2% bovine serum albumin (Sigma), membranes were probed with the following monoclonal antibodies against HLA gene products: class I: HLA-ABC [clone EMR8-5; 33 and 41 kDa]; class II: HLA-DRB1 [clone EPR6148; 30 kDa], HLA-DQ [clone TAL 4.1; 28 kDa], HLA-DMB [clone EPR7982; 29 kDa], HLA-DQA1 [clone EPR7300; 28 kDa and 33–35 kDa] were obtained from Abcam (Cambridge, MA, USA). The resulting immunoblots were developed using ECL Prime Western Blotting Detection Reagent (GE Healthcare Amersham) and visualized/images captured using a Versadoc MP 4000 imaging system (BioRad). Densitometry was performed with global background subtraction using the Quantity One software. Representative images of blots for each of the soluble HLA gene products tested are provided online as Supplemental Materials or at https://figshare.com/s/1d858cd5563acb58d381 (DOI https://doi.org//10.6084/m9.figshare.5998574).
2.6. Statistical methods
Mann-Whitney Rank Sum (2-sided) test was performed between the average protein density of HLA-ABC, HLA-DQ, and HLA-DRB1 bands detected in conditioned media of both non-invasive and invasive cell lines. For serum samples from patients with benign and T1–2N0M0 (stage I) NSCLC samples, pixel densities were compared using the sum for the two distinct bands detected for HLA-ABC, HLA–DQA1 or the single detected band for HLA-DRB1, HLA-DQ and HLA-DMB. Replicates for these evaluations were performed in no less than triplicate measures with only sets possessing ≤20% CV permitted to be contrasted in this manner. Distributions of the study findings for the patient samples were plotted as box and whisker plots with significance indicated. Classifications via receiver operator curve (ROC) analysis were conducted in context of pathologically-confirmed benign or T1–2N0M0 (stage I) NSCLC disease. All analyses were accomplished using SPSS v11.0 (IBM).
3. Results
3.1. Soluble HLA molecules in conditioned media from NSCLC cell lines
All HLA-specific protein bands were originally optimized using commercial antibodies (Abcam) with conditioned media from the H358 and H1703 cell lines. For HLA-ABC, the commercial antibodies were found to detect two HLA-ABC specific bands at 33 and 41 kDa. Densitometry values of these two HLA-ABC specific bands for the indolent (H358) and invasive (H1703) cell lines from two experiments were measured and compared, with representative immunoblots illustrated in Fig. 1. Increased levels of class I HLA were observed in the conditioned media from the H1703 cultures (approximately 4- and 7-fold increase for 41 kDa protein, approximately 3- & 8-fold increase for 33 kDa protein) in separate experiments. Mann-Whitney Rank sum tests were performed on the densities of the HLA-ABC proteins detected in the conditioned media of H358 and H1703 cells. Please note that these initial investigations were intended to help us appreciate the size of the soluble HLA molecules to search for in the patient specimens and approximate the sorts of differences we would expect to find with changing tumor phenotype. Therefore, we did not rigorously examine these findings with statistical tests as we do in the remainder of the study.
Fig. 1.
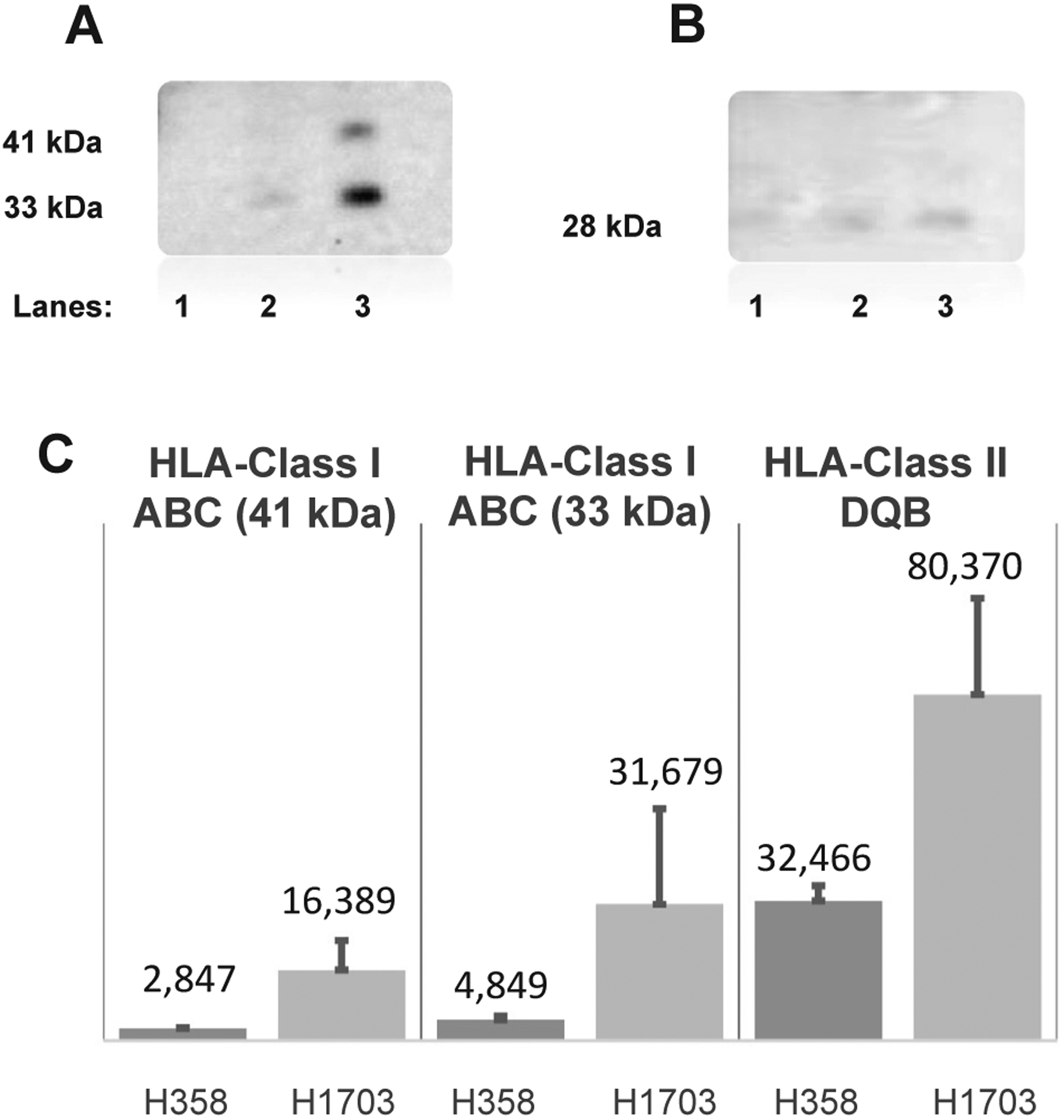
Detection of HLA-ABC and HLA-DQ Antigen in Conditioned Media Obtained from Non-Invasive and Invasive Lung Carcinoma Cell Lines. Human adenocarcinoma cell lines H358 and H1703 were cultured in RPMI-1640 + 10% FBS and antibiotics, as defined in Methods. Protein concentration of 10 mL of cell-conditioned media from each cell line was determined via BCA and equal amounts of total protein were loaded on to a SDS-PAGE gel. The proteins were transferred on to nitrocellulose membrane, immunoblotted with antibodies specific to HLA-ABC and HLA-DQ and developed using ECL reagents. Panel A shows a membrane immunoprobed with HLA-ABC antibody, whereas Panel B shows a membrane probed with the HLA-DQ antibody. Lane 1: control medium; Lane 2: conditioned medium from non-invasive H358 cells and Lane 3: conditioned medium from invasive H1703 cells. Panel C shows the densitometry analysis from the QuantityOne software (BioRad), with global background subtraction.
The conditioned media were also tested for two class II HLA proteins, namely HLA-DQ and HLA-DRB1. The levels of 28 kDa protein representing HLA-DQ in the conditioned media of H358 and H1703 cells were compared by densitometry. There was a 1.9- and 3-fold increase in HLA-DQ expression in the conditioned medium of invasive cell line H1703 compared to that of indolent cell line in two separate experiments. The HLA-DRB western blot data was unfit to be analyzed due to high background in repeated experiments.
3.2. Clinical-demographic parameters for the patient cohorts
All demographic parameters are provided in Table 1 for the patients comprising the cohort tested in this study.
Table 1.
Demographic Parameters for the Patient Cohorts.
| Benign | Adenocarcinoma | ||
|---|---|---|---|
| Gender | Male | 12 | 10 |
| Female | 13 | 14 | |
| Age | Median | 61 | 64.5 |
| Range | 52–83 | 47–82 | |
| Smoking History | Median | 38 | 38.75 |
| Range | 7–129 | 0–105 | |
| Nodule Size | Median | 0.4 | 2.15 |
| Range | 0.2–1.7 | 0.9–13.9 | |
| Race | |||
| Asian | 1 | 2 | |
| African American | 4 | 4 | |
| Caucasian | 16 | 17 | |
| Other | 4 | 1 | |
| TNM (7th edition) | |||
| T0N0M0 | 25 | 0 | |
| T1aN0M0 | 11 | ||
| T1bN0M0 | 6 | ||
| T2aN0M0 | 5 | ||
| T2bN0M0 | 0 | ||
| T3N0M0 | 2 |
3.3. Increased presence of circulating MHC class I (ABC) antigens in sera of patients with lung adenocarcinoma
HLA class I antigens were mostly detected as two discrete protein isoforms in the serum (33 and 41 kDa) with some other very low abundance isoforms also observed. Both of the MHC class I ABC monoclonal antibody reactive proteins (i.e. 33 and 41 kDa) were considered for analysis. The density of the 41 kDa bands on the western blot showed higher levels of HLA-ABC in the sera from lung adenocarcinoma patients relative to those sera from patients with indolent nodules (p = 0.049; Table 2; Fig. 2 panel A). Further, differences were also observed between these groups for the 33 kDa immunoreactive band (p = 0.028; Table 2; Fig. 2 panel B). These finding are reflective of the observation with the cell culture experiment results, described earlier, where the association of tumor cell malignancy and shed HLA-ABC molecules were noted.
Table 2.
Findings from the Western Blot analysis.
| ABC (33 kDa) | ABC (41 kDa) | DQA1 (28 kDa) | DQA1 (33–35 kDa) | DQ (28 kDa) | DQ (26 kDa) | DRB1 | DMB | ||
|---|---|---|---|---|---|---|---|---|---|
| Benign | Median (pix/mm2) | 138,690 | 154,722 | 144,894 | 313,445 | 315,439 | 298,717 | 316,144 | 162,175 |
| Minimum (pix/mm2) | 8300 | 122,976 | 106,295 | 153,537 | 179,442 | 258,098 | 171,170 | 124,321 | |
| Maximum (pix/mm2) | 164,131 | 196,667 | 208,171 | 385,141 | 350,720 | 337,522 | 375,452 | 211,612 | |
| AdCA | Median (pix/mm2) | 150,129 | 161,265 | 172,248 | 212,372 | 313,208 | 320,524 | 330,966 | 172,210 |
| Minimum (pix/mm2) | 1381 | 127,518 | 155,549 | 106,274 | 220,236 | 256,626 | 175,963 | 125,784 | |
| Maximum (pix/mm2) | 174,871 | 367,017 | 331,529 | 394,352 | 362,882 | 365,965 | 398,730 | 324,979 | |
| Mann-Whitney p-value | 0.028 | 0.049 | < 0.001 | 0.003 | 0.562 | 0.039 | 0.029 | 0.037 | |
| AUC | 0.652 | 0.659 | 0.689 | 0.583 | 0.606 | 0.788 | 0.606 | 0.795 |
Fig. 2.
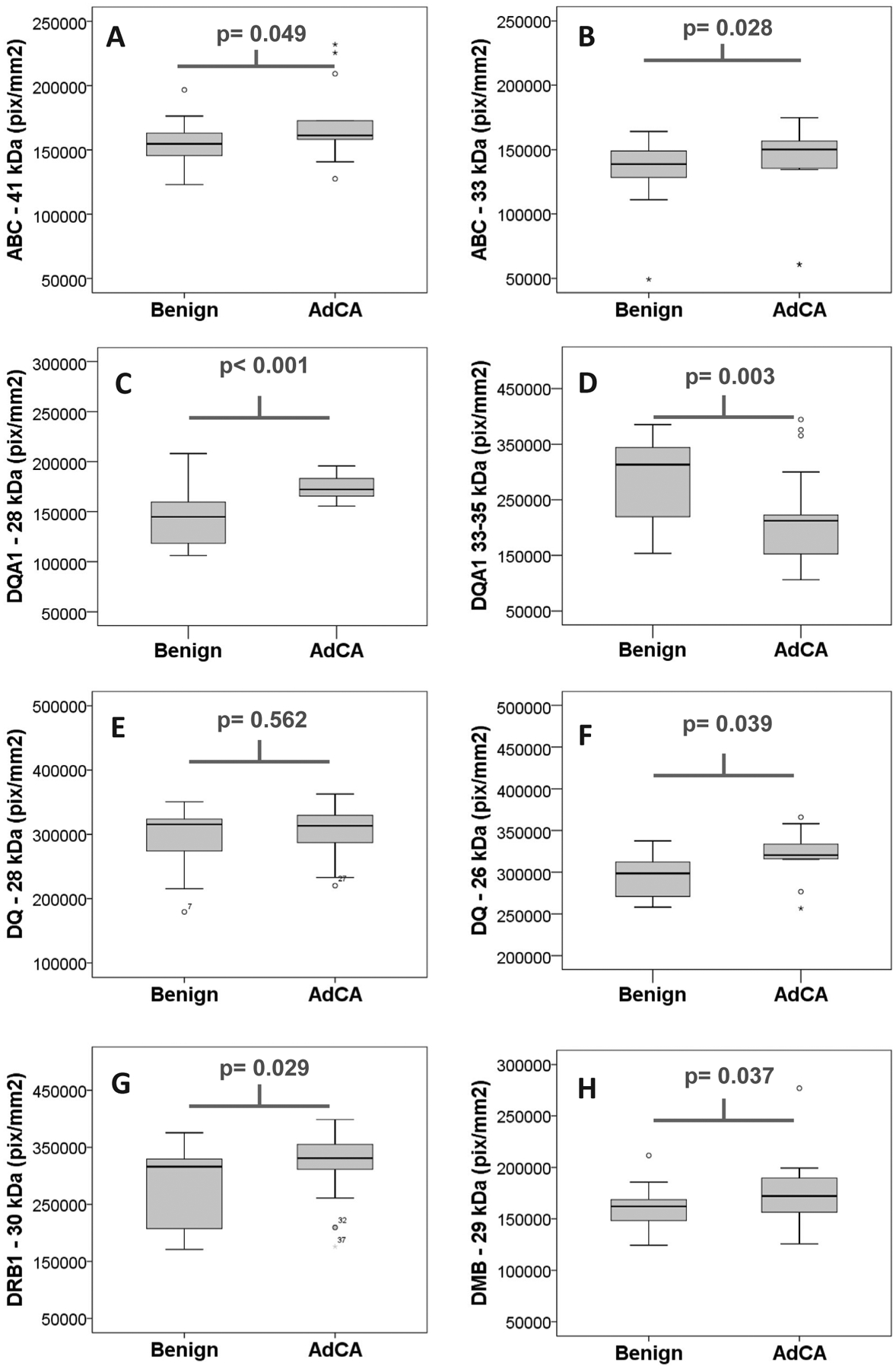
Box and Whisker Plots for soluble MHC targets. Serum from a total of 50 cases relevant to lung cancer screening were evaluated in this study and consisted of 25 cases of benign lesions and 25 of stage I lung adenocarcinoma (AdCA). Sera was examined using commercial antibodies (as specified in the ‘Methods’ section) for the presence of soluble HLA class I gene products (ABC), which were observed as a 41 kDa (panel A) and 33 kDa (panel B) isoforms. Further, MHC class II molecules were also evaluated, including DQA1, which was observed as both a 28 kDa (panel C) and 33–35 kDa (panel D) isoforms; DQ, observed as 28 kDa (panel E) and 26 kDa (panel F) isoforms; DRB1, as a single 30 kDa isoform (panel G); and DMB, observed also only as a single 29 kDa isoform (Panel H). All values on the ordinate axis are densitometry values presented as a median pixel density (i.e. pixels/mm2) from the western blots analysis performed in Quantity One software package v4.6.6 (BioRad).
3.4. Increased presence of circulating MHC class II (DQ, DQA1, DRB1, and DMB) antigens in sera of patients with lung adenocarcinoma
Western blot analysis of sera from NSCLC and benign lung nodule patients with monoclonal antibodies against HLA-DQA1, HLA-DQ, HLA-DRB1, and HLA-DMB revealed increased levels of these antigens in the sera obtained from NSCLC patients. Impressive differences were observed in the levels of DQA1 observed at 28 and 33–35 kDa. Significant increases (p < 0.001) were observed in DQA1 28 kDa protein band in NSCLC patients’ sera as compared to the sera from patients with benign lesions (Table 2 and Fig. 2 panel C). Interestingly, the most significant decrease in NSCLC patients’ sera as compared to sera from patients with benign lesions was noted in DQA1 33–35 kDa protein band (Table 2 and Fig. 2 panel D p = 0.003). The functional DQ molecules that are present on the cell surface are heterodimers formed by DQA1 and DQB1 chains. Therefore, lack or deficiency of any one chain could result in non-functional DQ molecule. In this study, we observed no differences between the two patient groups in the primary 28 kDa band for DQ (Table 2; Fig. 2 panel E; p = 0.562), however, a 26 kDa isoform of DQ was observed to be elevated in NSCLC patients relative to cases with benign lesions (Table 2; Fig. 2 panel F; p = 0.04). The levels of another HLA class II antigen, DRB1, a molecule intimately involved in the exogenous antigen peptide presenting molecules for T helper cells, was also found to be at significantly (p = 0.029) higher levels in sera from NSCLC patients relative to sera from patients with benign lesions (Table 2; Fig. 2 panel G).
HLA-DMB levels in NSCLC patients’ sera vs benign nodule patients’ sera, HLA-DMB, is normally present in the cell vesicles and are involved in peptide loading of HLA-II (MHC-II) molecules by removing the class II associated invariant chain peptide (CLIP) from the peptide binding site of the class II molecule. Without this mechanism, an altered self (as in tumor and autoimmune diseases) or foreign peptide would not be presented by the class II molecule. With this, it was interesting to note that levels of DMB was slightly elevated in the sera of patients with stage I NSCLC compared to serum of patients with benign lesions (Table 2; Fig. 2 panel H; p = 0.04). Overall our results show that higher levels of HLA both class I and class II HLA antigens are present in conditioned medium of invasive cells derived from cancerous lung tissues compared to non-invasive cells. Reflecting this in-vitro scenario, higher levels of both HLA class I and class II antigens were observed in sera obtained from stage I NSCLC patients as compared to sera obtained from patients with benign nodules.
3.5. Receiver operator characteristics curve values
In addition to the boxplot analysis provided above, the findings from the Western blot analysis were also subjected to an analysis via receiver operator characteristics (ROC) curve. From this analysis we present the maximum area-under-the-curve (AUC) for each protein isoform (see Table 2), with classification efficiency (likelihood of being malignant vs benign) increasing as the number approaches “1”, as a measure of perfect classification efficiency. With this, most of the markers had an average to slightly above average (i.e. AUC 0.583–0.7) performance characteristic for classifying the cases, but DMB and the 26 kDa form of DQ stood out as having strong classification efficiencies (AUCs of 0.795 and 0.788, respectively) and are, therefore, our strongest candidate biomarkers from this study. Based on the cures calculated in SPSS, these would have sensitivity and specificity values of 75% and 75% for DMB (respectively) and 83.3% and 75% for the 26 kDa isoform of DQ.
4. Discussion
The accumulation of genetic mutations that affect or alter normal cellular regulatory functions can lead to malignancy [11]. Such genetic alterations commonly lead to generation of neo-antigens variously called “tumor specific antigens,” cell differentiation antigens, or altered self-antigens. In immune competent individuals, this will lead to the presentation of altered self or neo-antigens to T cells by the MHC/HLA molecules, which will destroy the tumor cells [12,13]. Since MHC/HLA are involved in the presentation of antigens and induction of effector immune cells, down-regulation of these molecules have long been considered as a potential immune escape mechanism. While the loss of heterozygosity (LOH) and other means at the DNA and RNA levels resulting in reduced expression of HLA on tumor cell surface could lead to tumor progression, it is possible that in individuals with developing malignant tumors, shedding HLA molecules from the cell into the circulation could be a tumor immune escapism. Rebmann and colleagues showed decreased levels of circulating (soluble) HLA-DR in melanoma patients, and the authors concluded that the low levels of DR was an indication of the immunocompromised status of their patients [6]. Previous clinical studies have shown the correlation between soluble HLA and active immune status in patients with rheumatoid arthritis [14–16] and in patients with systemic lupus erythematosus [17,18]. Both autoimmunity and cancer immune surveillance escapism are results of altered immune response. However, reports on soluble HLA antigens in circulation for patients with cancer relative to healthy individuals are poorly studied and the few studies published have mixed outcomes in relation to correlation between stages of disease and levels of HLA in circulation and progression of disease [6,19].
In phase I of the present study, western blot analysis using commercial HLA-specific antibodies showed significantly higher levels of HLA-class I antigens in the conditioned media of invasive cells cultured in vitro (See Fig. 1, panels A and C). In phase II of this study, significantly elevated levels of circulating levels of HLA class I and class II antigens were observed in sera obtained from stage I NSCLC patients compared to sera obtained from patients with benign nodules.
The observed increase in HLA class I (ABC) circulating antigens could reflect active shedding of class I antigens from the tumor cells thereby escaping immune surveillance [20]. This type of shedding may account for the increased presence of HLA antigen expression in the malignant patients’ sera as described above. It is intriguing to note significant differences in HLA class II antigens (especially in one of the DQA1 bands) since normally cells other than those belonging to the immune system involved in antigen presentation do not express HLA class II antigens. An alternative explanation is the shedding of class II antigens from the immune system cells so that these cells can no longer present antigens to invoke an active immune response against the developing tumor, favoring tumor growth and metastasis. However, this is highly speculative at this point. Also, the significant difference in HLA-DMB levels in serum samples from patients with benign and malignant lesions of the lung is interesting. This HLA-DMB molecule is essential for the generation of altered self or foreign peptide loaded HLA by replacing the self-peptide from the HLA molecule in the endoplasmic reticulum (Fig. 2, panel H). HLA-DMB molecule is not directly involved in ‘self’ versus ‘non-self’ recognition. Hence down-regulation of DMB could result in locking presentation of tumor-specific antigens to the immune effector cells. From a therapeutic point of view, Bassani-Sternberg et al. showed that sHLA molecules present in cancer patients’ circulation contains cancer-related peptides. They were able to identify very large sHLA peptidomes by mass spectrometry using plasma and tumor cells of multiple-myeloma and leukemia patients, plasma of healthy controls, and with cultured cancer cells [21]. These peptides could be used for cancer vaccination or for cancer immunotherapy, as described by Kamata and colleagues [22], and also for studying protein synthesis and degradation within tumor cells.
Another avenue is the potential to screen individuals who can really benefit from the modern immunotherapy that augments T cell activation, such as the PD-1/PD-L targeted therapy [23,24]. Because if the tumor cells are not expressing the HLA antigens, this immune augmentation therapy is not likely to work in such patients. Hence quick screening for soluble HLA in circulation and cell surface expression of HLA molecules could be added preliminary tests for immune-augmentation therapy trials. Such studies will be important as we investigate potential predictive and prognostic biomarkers for advanced NSCLC patients receiving PD-1/-L1 directed immunotherapy.
All the HLA antigens detected in the conditioned media and in circulation had more than one specific band by western blot analysis. This could be due to several reasons, which may include products resulting from alternate splicing or post-translational processing. Presence of soluble HLA molecules in the circulation of normal and non-cancerous individuals in low levels could be part of the normal immunoregulatory mechanisms.
If the preliminary data obtained from this study can be confirmed by a more extensive study with larger number of patients and controls, perhaps circulating HLA molecules could be used as an early biomarker for cancer invasiveness along with other clinical markers. This will be facilitated by being able to develop a multiplex assay using microbead array for HLA antibodies in serum using the Luminex platform. Furthermore, there is a limitation to this study that concerns the lack of a means to normalize protein loading beyond the employed ‘total protein loaded’ method, similar to a ‘housekeeping protein’ (e.g. GAPDH, β-tubulin) used in examination of cellular lysates. To promote the quick translation of these findings along with improved technical robustness, our current efforts will be focused on developing a magnetic immunobead assay in the Luminex platform to conveniently evaluating soluble class I and class II HLA molecules in patient serum or plasma.
5. Conclusions
In-vitro studies using non-invasive and invasive cell lines showed higher levels of HLA-class I in conditioned medium, with slightly higher levels observed in medium from an invasive cell line relative to a non-invasive cell line. We also detected significant differences in the serum levels of HLA-ABC, DQA1, HLA-DQ, HLA-DRB1 and HLA-DMB, between patients with benign pulmonary lesions relative to those with stage I adenocarcinoma.
These preliminary data suggest that levels of HLA class I and class II antigens may have value as a potential biomarkers to improve early detection of lung cancer and also have the potential for developing vaccines based on peptides. Additional studies with much larger sample sizes and more accurate analytical methods are required to validate the results of this study.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families for donating biospecimens to the Rush University Biorepository.
Funding
This project was supported by Harriet Blair Borland Chair of Pathology, The Mary and John Bent Chair of Cardiovascular Surgery (ML), and the Rush Lung Cancer Research Fund.
Abbreviations:
- NSCLC
non-small cell lung cancer
- HLA
human leukocyte antigens
- LDCT
low-dose computed tomography
- MHC
major histocompatibility complex
- ATCC
American Type Culture Collection
- RPMI
Roswell Park Memorial Park
- SDS-PAGE
standard denaturing polyacrylamide gel electrophoresis
- LOH
loss of heterozygosity
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.humimm.2018.04.003.
References
- [1].Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, et al. , Baseline characteristics of participants in the randomized national lung screening trial, J. Natl. Cancer Inst 102 (2010) 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. , Reduced lung-cancer mortality with low-dose computed tomographic screening, N. Engl. J. Med 365 (2011) 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Field JK, Smith RA, Aberle DR, Oudkerk M, Baldwin DR, Yankelevitz D, et al. , International association for the study of lung cancer computed tomography screening workshop 2011 report, J. Thorac. Oncol 7 (2012) 10. [DOI] [PubMed] [Google Scholar]
- [4].Topalian SL, Drake CG, Pardoll DM, Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity, Curr. Opin. Immunol 24 (2012) 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Topalian SL, Drake CG, Pardoll DM, Immune checkpoint blockade: a common denominator approach to cancer therapy, Cancer Cell 27 (2015) 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rebmann V, Ugurel S, Tilgen W, Reinhold U, Grosse-Wilde H, Soluble HLA-DR is a potent predictive indicator of disease progression in serum from early-stage melanoma patients, Int. J. Cancer 100 (2002) 580. [DOI] [PubMed] [Google Scholar]
- [7].Shimura T, Hagihara M, Yamamoto K, Takebe K, Munkhbat B, Ogoshi K, et al. , Quantification of serum-soluble HLA class I antigens in patients with gastric cancer, Hum. Immunol 40 (1994) 183. [DOI] [PubMed] [Google Scholar]
- [8].Yoshii M, Tanaka H, Ohira M, Muguruma K, Sakurai K, Kubo N, et al. , Association of MHC class I expression and lymph node metastasis of gastric carcinoma, Hepatogastroenterology 60 (2013) 611. [DOI] [PubMed] [Google Scholar]
- [9].Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D, et al. , Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6, Clin. Cancer Res 2 (1996) 641. [PubMed] [Google Scholar]
- [10].Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. , International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma, J. Thorac. Oncol.: Off. Publ. Int. Assoc. Study Lung Cancer 6 (2011) 244. [Google Scholar]
- [11].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144 (2011) 646. [DOI] [PubMed] [Google Scholar]
- [12].Nebot-Bral L, Brandao D, Verlingue L, Rouleau E, Caron O, Despras E, et al. , Hypermutated tumours in the era of immunotherapy: the paradigm of personalised medicine, Eur. J. Cancer 84 (2017) 290. [DOI] [PubMed] [Google Scholar]
- [13].Schumacher TN, Hacohen N, Neoantigens encoded in the cancer genome, Curr. Opin. Immunol 41 (2016) 98. [DOI] [PubMed] [Google Scholar]
- [14].Adamashvili IM, McDonald JC, Fraser PA, Milford EL, Pressly TA, Gelder FB, Soluble class I HLA antigens in patients with rheumatoid arthritis and their families, J. Rheumatol 22 (1995) 1025. [PubMed] [Google Scholar]
- [15].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity 39 (2013) 1. [DOI] [PubMed] [Google Scholar]
- [16].Verbruggen LA, Versaen H, Rebmann V, Duquet W, De Cock S, Grosse-Wilde H, et al. , Soluble HLA-DR levels in serum are associated with therapy and genetic factors in rheumatoid arthritis, Hum. Immunol 63 (2002) 758. [DOI] [PubMed] [Google Scholar]
- [17].Brenol CV, Veit TD, Chies JA, Xavier RM, The role of the HLA-G gene and molecule on the clinical expression of rheumatologic diseases, Rev. Bras. Reumatol 52 (2012) 82. [PubMed] [Google Scholar]
- [18].Claus R, Bittorf T, Walzel H, Brock J, Uhde R, Meiske D, et al. , High concentration of soluble HLA-DR in the synovial fluid: generation and significance in “rheumatoid-like” inflammatory joint diseases, Cell. Immunol 206 (2000) 85. [DOI] [PubMed] [Google Scholar]
- [19].Tabayoyong WB, Zavazava N, Soluble HLA revisited, Leuk. Res 31 (2007) 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Campoli M, Ferrone S, Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands, Tissue Antigens 72 (2008) 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bassani-Sternberg M, Barnea E, Beer I, Avivi I, Katz T, Admon A, Soluble plasma HLA peptidome as a potential source for cancer biomarkers, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kamata Y, Kuhara A, Iwamoto T, Hayashi K, Koido S, Kimura T, et al. , Identification of HLA class I-binding peptides derived from unique cancer-associated proteins by mass spectrometric analysis, Anticancer Res 33 (2013) 1853. [PubMed] [Google Scholar]
- [23].Aust S, Felix S, Auer K, Bachmayr-Heyda A, Kenner L, Dekan S, et al. , Absence of PD-L1 on tumor cells is associated with reduced MHC I expression and PD-L1 expression increases in recurrent serous ovarian cancer, Sci. Rep 7 (2017) 42929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smahel M, PD-1/PD-L1 blockade therapy for tumors with downregulated MHC class I expression, Int. J. Mol. Sci 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


