Abstract
Neuromodulation is becoming one of the clinical tools for treating chronic neuropathic pain by transmitting controlled physical energy to the pre-identified neural targets in the central nervous system. Its nature of drug-free, non-addictive and improved targeting have attracted increasing attention among neuroscience research and clinical practices. This article provides a brief overview of the neuropathic pain and pharmacological routines for treatment, summarizes both the invasive and non-invasive neuromodulation modalities for pain management, and highlights an emerging brain stimulation technology, transcranial focused ultrasound (tFUS) with a focus on ultrasound transducer devices and the achieved neuromodulation effects and applications on pain management. Practical considerations of spatial guidance for tFUS are discussed for clinical applications. The safety of transcranial ultrasound neuromodulation and its future prospectives on pain management are also discussed.
Keywords: Chronic neuropathic pain, Neuromodulation, Transcranial neuromodulation, Transcranial focused ultrasound, Ultrasound transducer
1. Introduction
Chronic neuropathic pain is a widespread condition that affects 7-8% of the total population. Current treatment options default to pharmacological methods due to cost and ease of use. However, pharmacological agents do not have sustained pain reduction effects and can lead to serious side effects such as addiction, over-dose and even death. While patients fail to respond to pharmacological treatments, invasive neuromodulation techniques are employed such as spinal cord stimulation, deep brain stimulation and motor cortex stimulation. However, invasive stimulations depend on identifying the correct location of intervention targets and are at risk of infections and complications, not to mention, significantly high costs. Recent advancements in non-invasive neuromodulation have led to emerging tools for treatment of chronic neuropathic pain. Current non-invasive stimulation techniques, such as transcranial magnetic stimulation and transcranial current stimulation, have shown promising pain reduction effects, despite their limited spatial focality and depth penetration. While high-intensity focused ultrasound mainly achieves its effectiveness through thermal induction or non-thermal ablation, and accomplishes a wide range of clinical applications[1], low intensity transcranial focused ultrasound (tFUS) has recently emerged as a safe, non-invasive neuromodulation technique with high spatial resolution and focality with capability of accessing the deep brain. Recent efforts on ultrasound neuromodulatory and neurosurgical development have been reviewed with respect to pain diagnosis and treatment mainly at the peripheral nervous system[2]. Here we review the tFUS neuromodulation techniques by focusing on brain stimulation devices, capabilities and targeting measures, especially its emerging roles in chronic neuropathic pain treatment.
2. A Brief Overview of Pain
Pain is a warning signal from the body for immediate or eminent damage to the tissue. As defined by the International Association for the Study of Pain (IASP), pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage. The type of pain can be classified into different categories based on the origin of the pain. Neuropathic pain, affecting 7-8% world-wide adult population chronically according to IASP, is defined as pain that originates as a direct consequence of lesion or diseases affecting the somatosensory system. Depending on the location of the injury, neuropathic pain can be further classified into central and peripheral neuropathic pain. As opposed to neuropathic pain, nociceptive pain is the one that arises from damage to non-neural tissue[3]. Regardless of cause, pain that lasts or recurs for more than three months is defined as chronic pain. In May 2019, the World Health Organization casted a new categorization of chronic pain into chronic primary and secondary pain syndromes[4]. Chronic primary pain syndrome is categorized as chronic pain associated with significant emotional distress or functional disability that cannot be better accounted for by another chronic pain condition and is subdivided into categories of chronic widespread pain (e.g. fibromyalgia), complex regional pain syndromes, chronic primary headache and orofacial pain (e.g. chronic migraine or temporomandibular disorder), chronic primary visceral pain (e.g. irritable bowel syndrome), and chronic primary musculoskeletal pain (e.g. nonspecific low-back pain)[5]. Chronic secondary pain syndromes are linked to other diseases as the underlying cause, for which pain may initially be regarded as a symptom, and subdivided into categories of chronic cancer related pain (including cancer treatment and therapies), chronic postsurgical or post-traumatic pain, chronic neuropathic pain, chronic secondary headache or orofacial pain, chronic secondary visceral pain, chronic secondary musculoskeletal pain[5].
Clinically, chronic pathological pain has become one of the most frequent causes of hospital visits[6] and the leading cause of suffering and disability[7]. Therefore, the effective quantification and treatment of chronic pain is a public health priority. Although subjective, self-reports and questionnaires remain the gold standard of chronic pain diagnosis[8]. The quantification of chronic pain is divided in a few domains, sensory and affective qualities, temporal characteristics, pain location, and behavioral measurements of pain[9]. Sensory and affective qualities of current pain are mainly assessed through numerical rating scales (NRS)[10, 11], visual analog scales (VAS)[11] and verbal descriptor scales[12]. For children and populations with limited verbal capabilities, graphical representation based assessments such as the faces pain scale[13] and the pain thermometer[14] may be used. In order to incorporate the patient history, the brief pain inventory[15] and graded chronic pain scale[16] take the patient’s worst ever pain into account. Temporal qualities of pain are mainly based on retrospective subject report or subject diaries, both on paper or electronically, taking into account the duration, temporal patterns, chronicity, and variability of pain[17]. Pain locations are evaluated by the subject reported pain drawing[11], which shows the physical location and distribution of perceived pain areas. The location information is especially important in pain categorization. Clinical use of behavioral evaluations of pain is case specific based on limitations in movement, performance on behavior task (e.g. straight leg raise tasks in back pain), or sensitivity to palpation and pressure[18, 19].
Other methods for quantifying and identifying pain mechanism have been emerging from many research efforts. Quantitative sensory testing uses a series of controlled somatosensory heat and mechanical stimuli to systematically assess the function or dysfunction of somatosensory perception[20, 21]. However quantitative sensory testing lacks consistency in reliability and reproducibility of measurements and feasibility to be applied as a routine clinical test. Other methods such as brain structural imaging[22], genotyping[23] and pharmacological phenotyping[24] have been applied with limited clinical utility. A promising new method to identify pain mechanism is using magnetic resonance imaging for functional neuroimaging to identifying abnormal neural activity, functional connectivity or neural chemical signaling[22, 25-27]. However, clinical expense and lack of specificity remain the challenge to integrate neuroimaging to clinical utility. In order to translate functional neuroimaging to be easily accessed in clinical settings, ongoing research are using electroencephalography (EEG) based imaging and biomarkers to find quantitative measures of pain[28, 29].
3. Current Treatment Options
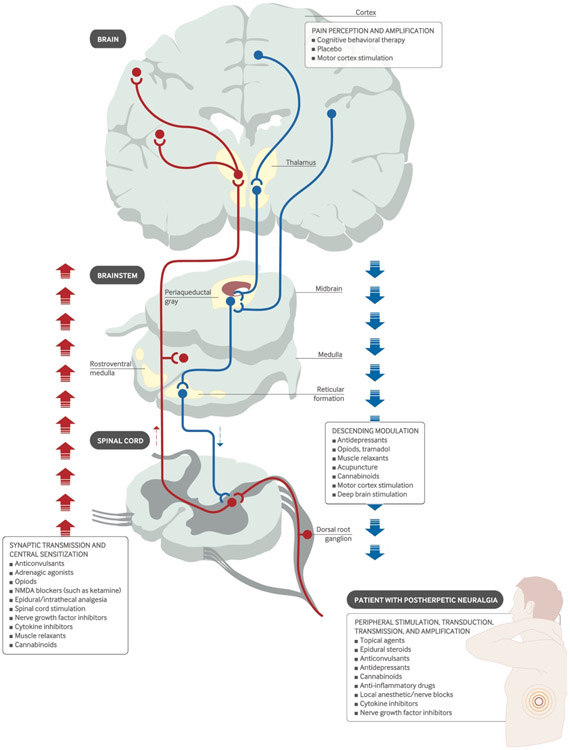
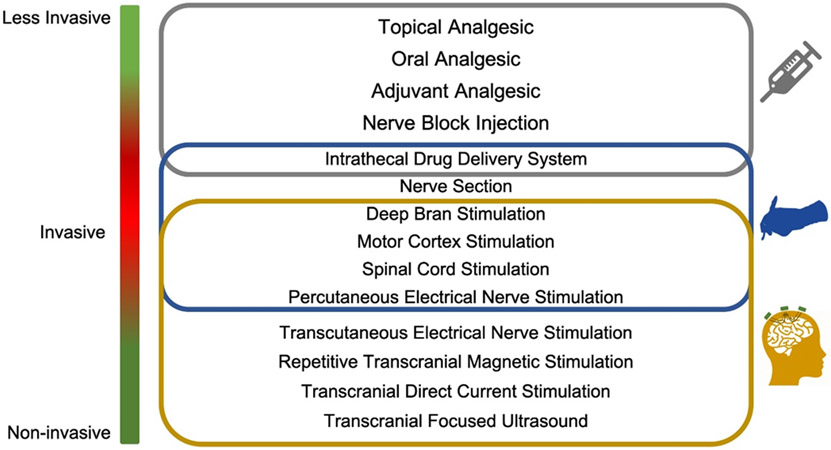
As illustrated in Figure 1, there are four categorical action sites in order to achieve analgesic effects for different types of pain. The neural circuits involved in the pain perception and amplification in the brain, the synaptic transmission and central sensitization in the ascending and descending pathways, and the peripheral stimulation, transduction, transmission and amplification can be treated to modulate and suppress the pain[30]. In this illustration, both pharmacological and physical neuromodulation solutions are listed for various action sites[30]. More generally, the chronic pain treatment options include biomedical and biopsychosocial approaches; we will focus on the first category in this review. Figure 2 depicts the available procedures and tools from pharmacology, surgery, electrical, magnetic, and acoustic stimulation approaches presented with invasiveness scale for each.
Figure 1.
The treatment targets to achieve various analgesics (Figure is adapted from [30]). The pharmacological treatment, e.g. opiods, NMDA blockers, antidepressants, and the physical neuromodulation approaches, e.g. motor cortex stimulation, spinal cord stimulation, deep brain stimulation are included as candidate methods for pain treatment at corresponding action sites.
Figure 2.
Biomedical approaches for treating chronic pain (adapted and revised from[31]). The gray frame includes the pharmacological options, the blue frame lists the treatments with surgical procedures, and the brown frame contains the stimulation methods with electrical, magnetic and acoustic energy.
3.1. Pharmacological Treatment of Pain
Conventional analgesic medications are cheap and fast acting and routinely prescribed for acute pain[32]. Since the 1980’s, growing concern have been raised about the use of opioids in the treatment of pain in an effort to combat opioid addition as a public health concern[33, 34]. The U.S. has declared the overuse of opioids and opioid related deaths an epidemic[35]. Opioids provide relief to nociceptive pain when compared to placebo. Strong opioids, e.g. morphine and oxycodone were significantly more effected compared to non-opioid drugs while weak opioids, e.g. propoxyphene, tramadol and codeine, showed no significant difference in pain reduction compared to non-steroid anti-inflammatory drugs (NSAIDs)[36]. However, in cases of chronic neuropathic pain, side effects and adverse events are prone to occur due to the difficulty to control the dosing and use. Responsiveness to opioid was also found to be individual specific and non-consistent[37]. Furthermore, studies have shown particular mechanisms of chronic pain such as glial cell activation, spinal NMDA receptor activation can contribute to increased opioid tolerance and paradoxically hence sensitivity to pain[38, 39].
In order to avoid opioid side effects, a type of commonly used non-opioid analgesic are NSAIDs, which reduces inflammation or the production of inflammatory factor to provide pain relief[31]. NSAIDs function through the inhibition of the cyclooxygenase enzymes COX-1 and COX-2, enzymes play major roles in vasodilatation, vascular permeability, sensitization of nociceptors, gastric acid secretion and platelet aggregation[40]. Due to the complex involvement of COX-1 and COX-2, clinical trial show risk of serious thrombotic cardiovascular events[41, 42]. Hence, NSAIDs are not recommended in at-risk populations with history of cardiovascular diseases and stroke, and high dose of NSAIDs are not recommended for chronic use due to increased risks of renal and gastric side effects.
Additional lipophilic analgesic can be applied topically for local release of analgesic effects while minimizing systemic side effects. The transdermal application of these analgesic such as fentanyl and buprenorphine have shown effectiveness for treating superficial localized pain regions such as peripheral neuropathic pain[43, 44]. Other adjuvants such as tricyclic antidepressants and serotonin and noradrenaline reuptake inhibitors have been shown to exhibit analgesic effects in neuropathic pain patient populations that show no response to opioids. These antidepressants are understood to modulate pain from the central nervous system modulating the endogenous serotonergic antinociceptive pathways and descending noradrenergic inhibition pathways[45, 46].
3.2. Neuromodulation
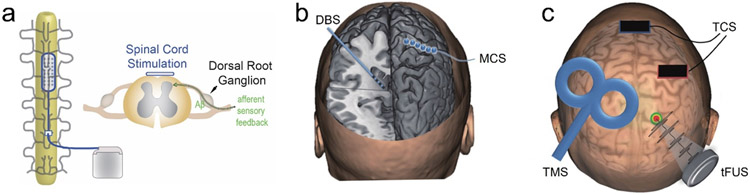
Neuromodulation is a fast-growing field of neurotechnology that offers modulative effects on central or peripheral nervous systems by delivering physical energy into the body. It has a wide range of applications for understanding the brain and managing brain disorders[47, 48]. The brain neuromodulation technologies, via invasive or non-invasive approaches, provide a means to alter irregular activity by stimulating the brain injecting electrical, magnetic, optical, or acoustic energy to intervene with neural activation, which effectively realizing suppression of a certain diseased state and stabilizing the system back to a healthy state[49]. The technologies can be used to excite, inhibit, or disrupt brain network dynamics in a controlled way, depending on the stimulation parameters and applications[50]. When used as a treatment, neuromodulation offers higher specificity than medication, and non-invasive neuromodulation further provides more reversibility than surgical alternatives listed in Figure 2. Neuromodulation offers a spatially specific, direct way to interact with the nervous system that fills in unaddressed needs left by pharmacological treatment plans. As described in Figure 1, neuromodulation for pain can be separated into a few different categories, peripheral (PNS) vs. central nervous system (CNS) modulation, and invasive vs. non-invasive modes of interface. Given the level of risk and high initial cost, invasive neuromodulation is typically considered after pharmacological treatments have been deemed unviable. However, with the development of reliable non-invasive neuromodulation techniques, neuromodulation can become a promising non-addictive alternative to opioids. Currently, such treatment options are limited for patients who do not respond to pharmacological treatments or have pre-existing complications that renders pharmacological treatment risks. Figure 3 provides a summary of invasive and non-invasive neuromodulation modalities for central nervous system.
Figure 3.
Physical neuromodulation technologies. a) A diagram illustration for spinal cord stimulation (SCS). b) Illustration of invasive techniques including deep brain stimulation (DBS) and motor cortical stimulation (MCS). Figure is adapted and revised from [51]. c) Illustration of non-invasive modalities including transcranial current stimulation (TCS), transcranial magnetic stimulation (TMS) and transcranial focused ultrasound stimulation (tFUS). Figure is adapted and revised from [51].
3.2.1. Invasive Neuromodulation: Spinal Cord Stimulation
A myriad of mechanisms of pharmacological treatment involve the inhibition of spinal cord signaling with the CNS. Spinal cord stimulation (SCS) is proposed to perform the same inhibition in the spinal cord through a gating mechanism modulating the cortical and subcortical brain[52]. SCS uses subdermal implanted electrodes to deliver an electric field to the dorsal horn and dorsal column axons (Figure 3a), which inhibits pain signaling in the spinothalamic tract[53, 54]. Although mechanisms remain unclear, SCS is hypothesized to inhibit Aβ fibers in the superficial layers of the dorsal horn, disrupting the afferent sensory input from the dorsal root ganglia and releasing inhibitory neurotransmitters at the spinal cord and CNS[55]. Main stimulation targets include dorsal root ganglion for peripheral pain[56], vagus nerve for headaches and inflammation mediated pain, and trigeminal nerves[57]. Traditionally stimulation frequency at a tonic 40-100 Hz stimulation frequency show a significant decrease in pain score in neuropathic pain patients[58, 59]. Pain reduction can be further improved in select patient groups by applying high frequency stimulation[60, 61], or burst stimulation[62]. However, long term efficacy in pain reduction is not consistent due to potential foreign body response encapsulation of electrodes or therapy tolerance. Nearly half of the patients experience greater than 50% decrease in pain reduction[58, 63], but the therapeutic effect decays over time in more than 13% patients[64].
3.2.2. Invasive Neuromodulation: Motor Cortex Stimulation
In cases of neuropathic pain resulting in loss of pain-related afferent information, known as deafferentation, motor cortex stimulation (MCS), as the conceptual diagram illustrated in Figure 3b, has been shown to reduce pain in patients who show no response to pharmacological treatments. These pain types include post-stroke pain[65], multiple sclerosis[66], phantom limb pain[67], spinal cord injury[68]. Epidural stimulation electrodes are placed on the motor cortex through localized craniotomies, stimulation frequencies are typically around 50 Hz[69]. Mechanism on the MCS are unknow. Deafferentation pain are theorized to be due to reorganization of somatosensory and motor cortex at the level of deafferentation or higher levels. Therefore, stimulation at the motor cortex disrupts the abnormal organization and provides pain relief[65]. The disadvantage of MCS is the difficulty to identify patient population that will respond to treatment, especially considering the risks and irreversibility of invasive treatment. Later sections in this review will discuss non-invasive techniques to help identify patients responsive to MCS.
3.2.3. Invasive Neuromodulation: Deep Brain Stimulation
In the central nervous system, deep brain stimulation (DBS), firstly introduced by Hassler et al. in the middle of last century[70], offers high specificity and treatment efficiency by implanting electrodes into deep brain (Figure 3b). Chronic DBS has been reported in several studies to elicit analgesia in animal and human studies at targets of sensory thalamus lateral and medial nuclei[71-74], internal capsule[75], periaqueductal/periventricular gray matter (PAG/PVG)[71, 75, 76] for the sensory component of pain, and anterior cingulate cortex (ACC) for the perception of pain[77, 78]. More specifically, two clinical trial studies in the 1990’s using Medtronic Model 3380 and 3387 (Medtronic Inc., Minneapolis, MN) were conducted examining the effect of DBS on chronic pain measured by percent pain relief (PPR) and VAS scales. The pain categories for the Model 3387 trial explored were > 70% predominantly neuropathic pain, 20% nociceptive pain, and 8% unidentified. Model 3380 trial was unreported. For these clinical trials, patients who did not report analgesic results during testing period did not internalize DBS treatment, and were excluded from continuation in the study. Furthermore, many patients opted to withdrawal or discontinue follow up from the study, this withdrawal population accounted for 70-73% of the total population. This large withdrawal rate largely confounded study results. When withdrawal population are excluded from efficacy calculations, Model 3380 trial reported 66% of population with > 50% PPR 3 month after internalization and 60% at the 24th month, and Model 3387 reported 38% population with > 50% PPR 3 month after internalization and 50% at the 24th month. However, when withdrawals are taken into account, Model 3380 trial success rate dropped to 53% at 3 month and 17% at the 24th month, similarly Model 3387 trial success rate dropped to 22% at 3 month and 14 % at the 24 month[79, 80]. The studies did not have systematic criteria for DBS target selection and stimulation parameter, whereas the patient stimulation setup was based on surgeon’s preference, the results of pharmacologic tests, or the patient’s symptoms and responses. The drop-off in success rate over time is clearly observed, treatment tolerance mechanisms remain unexplained.
The main components of the latest DBS system include a thin DBS lead implanted to the targeted brain region unilaterally or bilaterally, an implantable pulse generator (IPG), connector wires from the DBS leads to the IPG, and a patient programmer. The patient programmer allows physicians to interface with the IPG wirelessly, through radio frequency or Bluetooth, to adjust DBS stimulation parameters. Latest generations of FDA approved DBS leads in the U.S. market all consist of platinum/iridium electrode sites and polyurethane sheath[81-83].
Adverse events related to implanted DBS can arise from implantation, hardware failure, and stimulation induced damage. Implantation adverse factors involve infection risks at all levels of the implanted device, and the adverse events occur in about 2.6-5% of patients, depending on study location, within the first year of implantation[84, 85] despite pairing with antibiotic treatments orally and locally at implantation site. Hardware failures and IPG material erosion of the IPG over time occurs in 5.5% of the treated patients[84]. Better biocompatibility and reduction of inflammatory response in both material sciences and engineering, and implantation techniques would be greatly beneficial to the patient population.
When selecting DBS lead designs and materials, a few areas need to be considered. The primary factor is the safety of material and stimulation parameters. In the early 1990’s Shannon characterized the safety threshold of stimulation parameter space with respect to charge density and charge per phase[86] based on studies from McCreery et al.[87] These studies demonstrated the difference of charge injection mechanisms and safety based on stimulating material types. Considerations for safety arise based on the chemical reactions that occur at the electrode tissue interface. These chemical reactions can be due to faradaic oxidation and reduction at the electrode surface layer or through electrolytic and electrostatic capacitive charging[88]. Capacitive charge injection mechanisms are preferred over faradaic reactions due to the lack of need to generate or consume new chemical ionic species. Capacitive electrode materials include titanium nitride and tantalum oxide, where the capabilities of capacitive charge injection depend on material surface area. Faradaic electrode materials include platinum, iridium oxide, silver based and tungsten-based electrodes. Macroelectrodes used in commercial DBS systems are almost exclusively platinum based, chosen for the ability to induce faradaic reactions and double layer capacitive charging and stability during chronic implantation. Novel materials have recently emerged as alternative materials for neural stimulation to enhance biocompatibility over time. Electronically conductive polymer PEDOT (Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate)) shows promising ability to reduce electrode impedance and improve charge injection capability[89, 90]. Another aspect to improve biocompatibility of stimulating electrodes is to use soft electrodes for reducing damage due to the presence of chronically implanted stiff electrodes. Soft implantable electrodes have been achieved with carbon nanotubes[91, 92], polymer-based electrodes[89, 93] and graphene[94, 95].
Side effect concurrence of infections, broken leads, surgery related neuropathic pain, and rare seizures have been observed[80]. The described clinical trials were closed without application for market approval. In 1996, FDA approved the use of DBS in movement disorders, and the introduction of DBS to market allowed physicians to use DBS to treat chronic pain on an off-label treatment basis. Current studies show the importance of stimulation target on long term pain treatment outcome. A metanalysis study showed PAG/PVG stimulation produced good to excellent results in 79% of patients and the addition of sensory thalamic or internal capsule stimulation increased the success rate to 87%[96-99]. Moreover, DBS also carries risk of inflammation, gliosis, cell death[100], and requires irreversible surgical implantation procedures. Based on these results, there exists a need for better, safer stimulation target selection for the treatment of pain.
Non-invasive neuromodulation approaches have been developed to enable the modulation of neural tissue without necessitating invasive surgical procedures as demonstrated in Figure 3c, including transcranial magnetic stimulation (TMS), transcranial current stimulation (TCS), and tFUS. Despite the relatively limited spatial resolution compared with invasive approaches, non-invasive neuromodulation techniques carry much lower overall risks due to their non-invasive nature and have the potential to be used in many applications.
3.2.4. Non-invasive Neuromodulation: Transcranial Magnetic Stimulation
TMS employs a coil of wire to generate rapidly changing magnetic fields, leading to electromagnetic induction and thus eddy currents within the brain, which thus elicit synchronous brain activities[47, 101-109] at the cortical brain regions with a few centimeters scale resolution according to Faraday’s law[110]. As shown in Figure 3c, the figure-eight coil is a typical configuration for delivering the focal and pulsed magnetic stimulation. While TMS has been found effective in treating disorders such as pain[111-114], depression[115], stroke and Parkinson’s disease[116], the focality and penetrating depth of TMS remains to be improved in order to have more targeted effects in managing brain conditions. Due to the inability of TMS to focally stimulate deep brain regions, motor cortex stimulation is mainly targeted for pain relief, where the reduction of pain sustains for days to weeks after daily stimulation due to presence of plasticity[114]. As described before, invasive MCS helped pave way for the target selection at the motor cortex. One pilot study of using 20 Hz repetitive TMS (rTMS) targeting at motor cortex for 20 minutes is reported to induce analgesic effects on half of the 12 therapy-resistant chronic pain subjects, but lacks significant difference between active and sham rTMS[117]. The positive outcome of 20 Hz rTMS was also validated by another double-blind study[111], in which the placebo effects were concluded as non-trivial and discussed to be controlled for rigorous implication.
Later, multi-day rTMS applied at 5 Hz or higher frequencies using figure-eight coils, has been reported to induce long-lasting brain plasticity and explored in its efficiency to treat chronic neuropathic pain. In a 60-patient study, Lefaucheur et al. showed rTMS at M1 in right-handed subjects, efficacy in pain reduction varied significantly based on type of pain, with greatest reduction in VAS score in trigeminal nerve lesion subjects. Overall, in all pain types, rTMS significantly reduced pain score in 65% of the patients compared to sham stimulation[118]. Long term reduction of VAS pain score from rTMS has been demonstrated to last 2 weeks after daily rTMS for 5 consecutive days in both peripheral and central neuropathic pain compared to sham controls[119]. In more recent studies, by applying rTMS at contralateral motor cortex to pain, 58% of subjects were found to be responders to treatment, and showed significant decrease in VAS pain score after 9 stimulations, and this reduction in pain score is not only maintained but further reduced at 6 weeks after stimulation, at a mean reduction of 4.59 points out of 10. Non-responders showed no significant changes to baseline pain score at all time points[46]. TMS is hypothesized to lead to depolarizations in the neural tissue, at low intensities TMS seems to mostly stimulate low-threshold inhibitory interneurons, whereas higher intensities excite projection neurons. When pulsed at physiologically relevant frequencies during rTMS, local neural plasticity is hypothesized to account for sustained long term changes in pain perception[110]. The above results show promising, safe use of non-invasive neuromodulation technique in the treatment of specific types of pain[120].
The material and design of the TMS coil play important roles in the efficacy of treatment. The capabilities of the TMS coil depends directly on the charge delivered to the tissue, focality of induced electric fields and depth of electric field penetration. The amount of charge delivered to the tissue is mainly determined by the capacitance of the stimulation coil. Therefore, the design of the TMS coil, the coil core material and stimulation pulse have a large influence on TMS performance. TMS coil core materials have employed air-cores[107], or ferromagnetic materials such as iron-cores[121-123] and steel-cores[124] to achieve high magnetization and more practically coil geometries. Recently modeling[125] of electromagnetic properties has enabled the field to examine characteristics of the emitted electric field, highlighting the tradeoff between spatial focality and depth of stimulation penetration[107, 126]. Further notable new TMS coil designs include fMRI integrated TMS[127-129] to ensure accuracy of stimulation target in patients.
Disadvantages of rTMS include limited spatial resolution and depth of penetration as restrained by the conductivity and permeability of the magnetic field. The potential discomfort at the stimulation site and possible headache are among the disadvantages of such a transcranial intervention tool[130]. Another safety concern of TMS for vulnerable populations, e.g. children, senior subjects or tinnitus patients is the loud, repetitive clicking sound accompanying the magnetic stimulation pulses[131]. One recent effort of reducing such undesirable sound and remitting the safety concern is the development of a quiet TMS (qTMS) which is featured with ultra-brief pulse for shifting the transmitted energy towards inaudible, high frequency range[131]. The improved coil design of qTMS consists of stiff winding block, high-stiffness epoxy-based polymer bedding, a bitumen-based polymer-modified-asphalt compound for a viscoelastic layer, elastic silicone for a decoupling layer, and polyurethane for casing. As an outcome, the qTMS was demonstrated to reach 9 times quieter than conventional TMS device[131].
3.2.5. Non-invasive Neuromodulation: Transcranial Current Stimulation
TCS is a non-invasive neuromodulation technique[132] that applies low levels of current to the scalp through rectangular or ring electrodes to modulate cortical excitability (Figure 3c). Transcranial direct current stimulation (tDCS)[116, 133-139] uses weak, direct currents to elicit changes in cortical excitability and spontaneous neural activity, while transcranial alternating current stimulation (tACS) uses currents with alternating polarities to similarly alter spontaneous activity and potentially entrain neural oscillations. Applying anodal tDCS to motor cortex was shown to reduce pain in patients with neuropathic pain due to traumatic spinal cord injury[140]; and alpha-tACS was shown to relieve chronic low back pain by enhancing the alpha oscillations in the somatosensory brain region[141]. Similar to TMS, TCS’s focality and depth penetration remain to be improved due to the volume conduction effect, despite of the recent development of high-definition tDCS for improved focality and intensity[139, 142]. Additionally, like TMS, applying direct electrical stimulation through the scalp can lead to unpleasant scalp sensations and potential safety hazards, which limits the amount of current that can be delivered via TCS and thus, the potential desired effects as well. Therefore, there is still an unmet need to further develop the non-invasive neuromodulation technologies by improving the spatial specificity, neuromodulatory effectiveness and safety.
4. Transcranial Focused Ultrasound and Its Application for Pain Management
4.1. An Overview of Ultrasound Neuromodulation
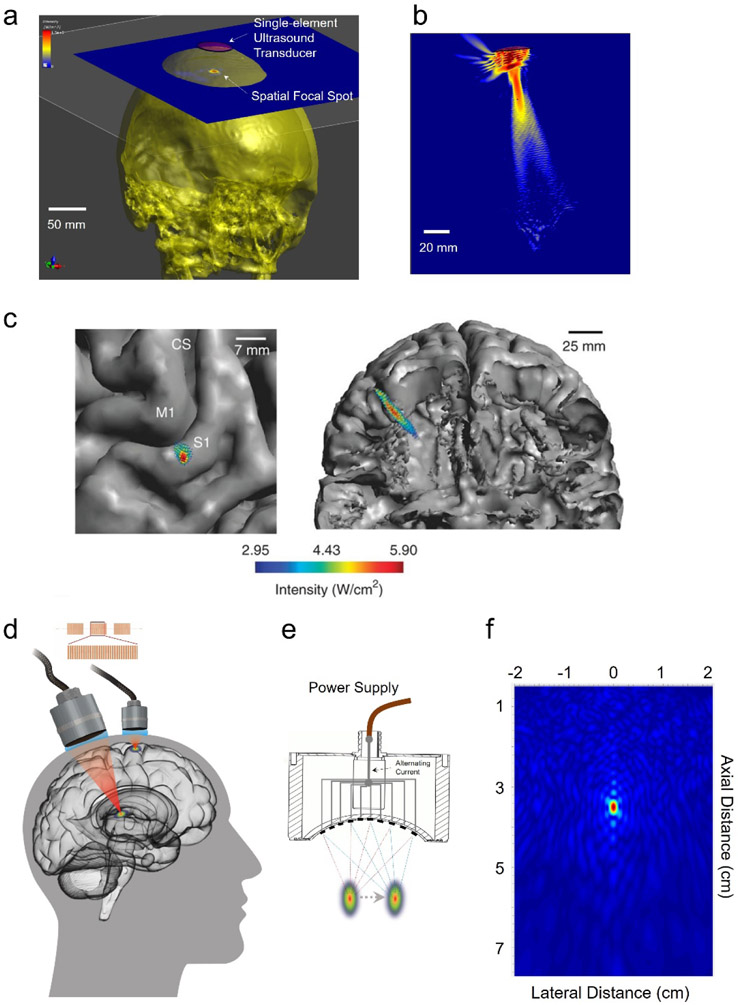
Despite the proven efficacy of TMS and TCS for inducing transient changes non-invasively in the human cortex, thus for treating pain, these technologies are facing challenges from a depth and focality tradeoff, poor spatial resolution even at the cortical surface, and significant attenuation at depth, thus lack of spatio-temporal precision. Unlike using high intensity focused ultrasound to ablate the diseased brain target[143], low-intensity tFUS is a promising non-surgical, low-energy, and portable technique for inducing changes in spontaneous neural activity with high spatial precision, adjustable focus, and relatively low tissue attenuation. Despite that investigations for elucidating mechanisms of ultrasound neuromodulation are still ongoing[144], mounting evidence in multiple preparations has demonstrated that ultrasound has a robust effect upon neural tissue in central and/or peripheral nervous system[144-146]. tFUS can be applied in numerous neuromodulation applications due to its high spatial focality and non-invasive nature as illustrated in Figure 3c and Figure 4a-d. Among a myriad of experimental demonstrations, one pilot study using low-frequency and low-intensity tFUS was able to achieve robust neuromodulation effects at the primary somatosensory cortex[147] with the spatial specificity demonstrated in Figure 4c; the single-element transducer can also achieve high lateral specificity when the ultrasound source is targeted at primary motor cortex (M1) (Figure 4a). The acoustic-evoked potential at the M1 can be localized and imaged using EEG-based source imaging for guidance and objective feedback (see details in Sections 4.4 and 4.5). The forward-looking concepts of tFUS for multi-site brain stimulation[148] (Figure 4d) is proposed along with the technological development in the transducer device (Figure 4d-e) and advanced focusing technology (Figure 4f). The multi-element ultrasound array is able to greatly improve the axial specificity (Figure 4f in the comparison of Figure 4b), steerability of the ultrasound focus, and even multifocal stimulation[149] (Figure 4e). To improve the focal specificity, the side-lobe pattern can also be further suppressed once using a random array design (Figure 4f).
Figure 4.
The current capability of tFUS, the developing focused ultrasound technique and a prospective view of multi-site ultrasound neuromodulation. a) illustrates the outstanding lateral specificity of tFUS among the non-invasive neuromodulation approaches when the 500 kHz ultrasound is targeted at primary motor cortex (M1) to evoke an acoustic-related potential. b) The axial profile of the 500 kHz single-element ultrasound beam is in a “cigar” shape within the skull cavity, which may limit the neuromodulation specificity in the axial direction. c) illustrates the spatial specificity of 500 kHz tFUS when the ultrasound is targeted at primary somatosensory cortex (S1) to modulate the sensory-evoked potential and sensing capabilities[147]. The transmitted ultrasound field is coregistered with the human brain model. (Figure is adapted from [147]) d) Multiple brain regions at cortex or at depth can be targeted by the tFUS using ultrasound refocusing, with the clinical implications highlighted in [148]. e) The focal spot can be spatially steered using multi-element ultrasound array, through which the possible error from mechanical targeting can be avoided. f) The much improved focus of 2-mm lateral and 4-mm axial specificity can be achieved using 256-element random ultrasound array distributed over a hemispherical aperture. Such an improved spatial focus is produced by 0.7 MHz ultrasound for a low acoustic attenuation from the skull.
During tFUS neuromodulation, a highly controllable, pulsed mechanical energy is transmitted though the skull with high spatial selectivity, which can be steered and utilized to elicit activation or inhibition through parameter tuning. tFUS has been used safely and effectively for intact neural stimulation in mice[150-153], rats[154, 155], rabbits[156], sheep[157, 158], pigs[159, 160] and monkeys[161-165]. Recent studies have highlighted its promise for non-invasive neuromodulation with high spatial resolution and targeting capability comparing to other non-invasive techniques, given the controlled focal energy delivering[147, 166, 167]. The work by Legon et al.[147, 168, 169] demonstrated the feasibility of transmitting 500-kHz ultrasound (US) through human cranium with minimal insertion loss and beam deformation and with high spatial resolution, validating US as an efficacious form of highly focal transient stimulation for use in humans. A recent study on using ultrasonic thalamic stimulation through a single-element transducer working at 650 kHz demonstrated that this low-intensity, non-invasive tool may assist a patient to recover consciousness with reliable communication after traumatic brain injury[170].
4.2. Ultrasound Transducers for Brain Neuromodulation
The core of tFUS technology is the transducer device, which is built upon a type of piezoelectric material, transforming applied electric input to mechanical vibrations, i.e. the inverse piezoelectric effect, which was firstly verified by the Curie brothers[171]. The applied electric field is driven by a controlled electrical input through a matching circuit. As one of conventional piezoelectric materials, lead zirconate titanate, also known as PZT is a type of inorganic piezoceramics compound. The PZT ceramics, PZT-4 (density: 7600 kg/m3) and PZT-5H (density: 7500 kg/m3) outperform barium titanate and lead metaniobate in terms of piezoelectric coupling factor, a quantitative indicator for the efficiency of the electro-mechanical energy transformation, which enable the PZT family as outstanding candidates for manufacturing ultrasound transducers. However, the PZT ceramics usually have limited mechanical flexibility. Polyvinylidene fluoride (PVdF, density: 1780 kg/m3), a type of piezoelectric polymer was discovered in 1969, demonstrates much improved mechanical flexibility, strong piezoelectric response, and thus allows the appearance of light-weight, flexible and miniature ultrasound sensors, like the needle hydrophones[172] for ultrasound field mapping. Capacitive micromachined ultrasonic transducer (CMUT)[173] is a relatively new type of ultrasound transducers which is built upon a silicon substrate cavity micromachined with a suspending thin membrane layer (e.g. Si3N4, doped poly-silicon[174] or polymer[175]) on the top for transmitting or receiving ultrasonic waves[176]. This novel transducer technology enables easy constructions of ultrasound array and integrations with driving or sensing circuits[173]. It also allows wide bandwidth and high frequency ultrasound[173].
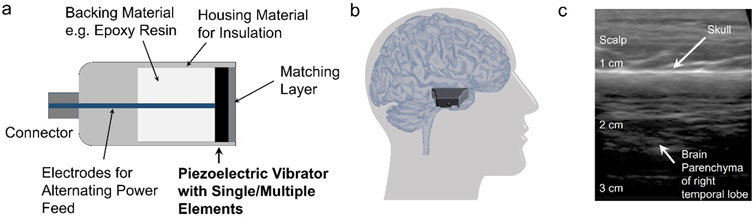
The fundamental mechanical structure of an ultrasound transducer is depicted in Figure 5a, which includes the piezoelectric vibrator, backing material, acoustic impedance matching layer, and housing material for both electrical and acoustic insulations. Miniaturized ultrasound transducer would facilitate brain stimulation research, especially on small animal models. Recently, a miniature, wearable tFUS transducer built on a disk-shape PZT (PbZrxTi(1–x)O3) ceramic was developed to stimulate motor cortex at a 600-kHz fundamental frequency and thus lead to awake behavioral change[177]. In such a wearable transducer design, a pedestal mounted at the anterior skull holds the 6-gram focused transducer, and the feature of ball-and-socket joint endows much degree of freedom for steering the tFUS directionality. As the most exciting capability of the miniaturized transducer, it is demonstrated to apply sonication to the freely moving rat. Another small-size and wearable PZT-8 piezoceramic-based ultrasound transducer working at a higher fundamental frequency of 3.8 MHz was fabricated to stimulate deep brain structures and thus to treat Parkinson’s disease mouse model[178]. Besides the aforementioned efforts in transducer development using the piezoceramics, ferroelectric PMN-PT (Pb(Mg1/3Nb2/3)O3-PbTiO3) single crystal was employed as the piezo vibrator in another work to fabricate a miniaturized transducer actuated at 3 MHz, which became a critical component of a portable ultrasound stimulation system tested on mice’s brain[179]. Thanks to its better piezoelectric properties than those of piezoceramics, as demonstrated, the home-made transducers were able to elicit motor reactions in the in vivo experiment[179]. Later, a lightweight, miniature CMUT ring array equipped with 32 elements was also shown to induce motor responses of awake mice. Thanks to the multi-element circular layout, the CMUT array can still achieve about 2.75 mm lateral resolution even though the array is working at a relatively low fundamental frequency of 183 kHz[180]. Other than observing the motor responses, a very recent effort was to explore the ultrasound effects at primary somatosensory (S1) and visual cortices using an optical imaging platform for mice, while 10 MHz ultrasound was transmitted by soft housing needle transducer in order to achieve a small beam size of 0.68 mm[181]. Such a miniaturized ultrasound transducer for brain stimulation was built upon a thin layer of PZT-5H, which was backed with layers of conductive epoxy materials (E-Solder 3022 and Emcast 501). Its small footprint of the needle transducer (0.25 mm2 aperture size) enabled a seamless integration of ultrasound brain stimulation and optical brain imaging[181]. The home-made transducer is applied not only to animal experiments but also to human study. Recently, a pilot tFUS study for human neuromodulation in fMRI settings adopted a rapid-prototyping fabricated transducer[182], through which the source of tFUS becomes compatible with a 7T MR environment. A statistically increased BOLD volume at human primary motor cortex was observed when low-intensity 500 kHz tFUS was directed to the motor cortex through a 30-mm aperture with a focal length of the same size[183].
Figure 5.
Ultrasound transducer devices. a) Basic mechanical structures of an ultrasound transducer device. b) A hand-held ultrasound imaging probe, iQ is placed over the scalp above the right temporal lobe. This location provides an acoustic window with a minimum skull thickness. c) B-mode ultrasound image produced by the iQ on an axial plane presents the scalp, skull and brain parenchyma.
Researchers also employed commercially available or customized ultrasound transducers by industry manufacturers for studying the transcranial effects by administering a broader range of focused ultrasound. The strategy of harnessing the broader range of ultrasound transducers is to accommodate various application scenarios, i.e. for human, large or small animal models, not only because of the different brain sizes, but also due to the differences in the skull properties. Large ultrasound transmission aperture is usually preferred using either single-element transducer or ultrasound array. A tFUS study on human S1 was practiced using a customized Blatek single-element transducer working at 500 kHz with a 30 mm diameter and a 30 mm focal length. In this study, Legon and colleagues discovered the sensory modulation effects of tFUS in the concurrent median nerve stimulation evidenced in sensor-level electrophysiological recordings[147]. Moreover, the sensory detection thresholds in both two-point and frequency discrimination tasks were lowered and thus was able to provide direct evidence of tFUS neuromodulation effects through behavioral output[147]. A further investigation on human S1 was pursued with a piezoceramic transducer having double-sized aperture of 60 mm and a segmented-sphere radius of 70 mm. The transducer was working at a fundamental frequency of 250 kHz. Transient tactile sensations were reported by healthy human subjects when they were administered with tFUS only, and such subjective feedback was validated through the sonication-specific evoked potentials, which provided the first evidence of tFUS per se inducing sensation and electrophysiological readout[167]. Such a direct neural effect was also observed through functional imaging-guided tFUS study on human primary visual cortex. In this study, a slightly higher fundamental frequency of 270 kHz with a focal length of 30 mm tFUS transducer was integrated to the magnetic resonance imaging setup[184]. Later, when the ultrasound target was moved from cortical areas to deep structures of human brain, a larger aperture of 63 mm fabricated by Ultran Group with a longer focal length of 70.92 mm was employed; such features allow tFUS to target at unilateral sensory thalamus in depth. An EEG biomarker, P14 sensory evoked potential was found to be inhibited by the administered dose of tFUS[169].
Another 64-mm single element transducer, H115 made by Sonic Concepts with a geometrical focal length of 63 mm was employed to target at the left frontal eye field in the premotor cortex of awake monkeys using a fundamental frequency of 320 kHz. The applied low tFUS temporal-average intensity was still demonstrated as effective evidenced through a significantly slowed ipsilateral mean anti-saccade latencies[161]. Such a commercially available transducer was also used to further investigate single neuronal unit activity in response to the low-intensity tFUS administered at frontal eye field[185]. In this study, it was found that not all neurons in supplementary eye field, which is connected with the acoustic-targeted frontal eye field, are activated instantaneously by the ultrasound energy observed through intracranial electrophysiological recordings using tungsten microelectrodes. This study emphasizes the usage of tFUS in changing brain network activities[185]. Recently, an MR-compatible version of H115 single-element transducer was further developed in order to monitor the whole brain responses to the input tFUS. Such a transducer was used to target a specific primary somatosensory cortical region, 3a/3b area in monkey brains with 250 kHz, 300 msec pulses. It was found that the transcranial ultrasound can elicit similar hemodynamic signal changes at the target as those evoked by natural tactile stimulation, but the ultrasound-induced activities are featured with distinct spatial and temporal profiles[163]. This MR-compatible device was also used to manipulate subcortical and deep primate brain activities, i.e. amygdala and ACC, respectively on a monkey model. By mapping the whole-brain activities using BOLD responses in fMRI, it was found that 250 kHz, 30 msec bursting tFUS is able to reduce the coupling among brain networks, specifically at the sonication targets[186]. Beyond the elucidation of how tFUS would modulate the neural activity from a neuroscience perspective, this work may also shed substantial light on how researchers can further develop this non-invasive technology for translational significance in clinical settings by manipulating connections among brain circuits. Such efforts include the development of an MR-compatible 128-channel random phased ultrasound array working at 0.65 MHz (geometrical radius of 72 mm, opening diameter of 103 mm) in order to achieve better focal and electronically steering performances in the transcranial applications[187]. The design was fabricated by Imasonic into a spherical geometry based on piezocomposite material, and was expected to enable aberration correction, electronically focal repositioning and simultaneous multi-target stimulations[187].
The rapid growth of tFUS neuromodulation is also attributed to the widely available ultrasound facilities. For an instance, an 80-element, broadband plane sector ultrasound imaging probe and its connected system by Phillips with a large acoustics aperture size of 203 mm working at a fundamental frequency of 2.32 MHz was translated to modulate the TMS-induced motor evoked potentials (MEPs) on 66 healthy human subjects[188]. The B-mode ultrasound-induced plasticity change at motor cortex is able to reach more than 30% amplitude increase by at least 6 minutes after the sonication[188]. Another very recent example is applying repeated diagnostic ultrasound (DU, with fundamental frequency close to 6 MHz) to human visual cortex[189]. The transcranial DU transmitted from a linear ultrasound array L25x by FUJI Sonosite was able to elicit visual perception at 7 out of 10 participating subjects[189]. Most recently, a hand-held ultrasound imaging device, the Butterfly iQ, has been introduced to the market. The iQ device is working with mobile phone platform and is using a single silicon chip that replaces the traditional multiple piezoelectric element array for imaging. By placing the iQ ultrasound probe at the scalp over right temporal lobe (Figure 5b), one transcranial image is presented in Figure 5c with some details about parenchyma of the imaged temporal lobe. Although the iQ device is only supporting the imaging modes at its current stage, it may provide a low-cost, portable platform and thus offer great opportunities for popularizing ultrasound neuromodulation in clinical and home healthcare settings in spite of potential technical challenges to the silicon chip once it is powered with increased current for neuromodulation.
4.3. Ultrasound Neuromodulation for Pain Management
In concert with the neuroscience investigations, the focused ultrasound has also emerged to become the next generation tool for non-invasive pain management, including several pilot explorations on diagnostic and therapeutic significances. For examples, one fifth second intense focused ultrasound (iFU) pulses at a 1.15 MHz fundamental frequency shows its diagnostic value in differentiating neuropathic tissues with partial sciatic nerve ligation from normal ones in a Sprague-Dawley model[190]. The iFU probe delivered the ultrasound energy to rat’s plantar surface at the paw. The ultrasound transmission gel was used to fill the space within the cage mesh grid, and to couple the ultrasound wave onto the targeted periphery. The iFU with 2 MHz frequency is further demonstrated its diagnostic value in patients whose shoulders have rotator cuff tears or tendinopathy[191]; moreover, the image-guided iFU technology show its potential to help physicians identify deep, tender tissue in patients who report experiencing pain[192].
On human patients, transcranial ultrasound energy, applied over the posterior frontal cortex contralateral to the maximal pain in a non-focused, standard B mode for 15 seconds, demonstrated its long-lasting effect for at least 40 minutes[193]. The possible acoustic windows for ultrasound neuromodulation were summarized with a transcranial ultrasound image presented the scalp, skull and brain parenchyma structures, which is shown to confirm the transcranial ultrasound reaching at the brain. This first transcranial US-mediated cognitive test demonstrated a large potential of transcranial ultrasound in suppressing chronic pain and improving subjects’ mood and global affect[193]. These promising results from chronic back pain patients have laid important research and clinical foundations for tFUS to become an efficient non-invasive modality for pain management.
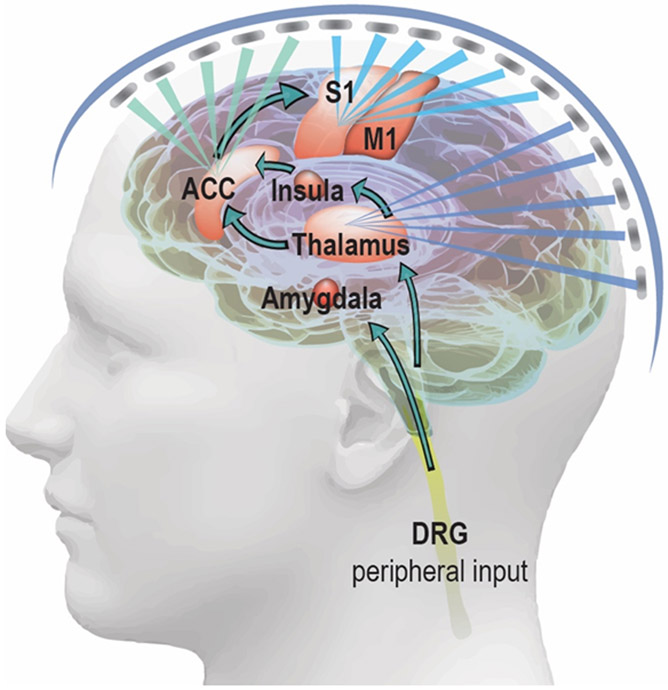
Pain is one type of personal experience that involves different functional brain circuits for processing. To reduce such personal feeling of pain, one can inhibit the relevant brain areas or excite circuits that suppress downstream brain functions[2]. As overviewed in Sections 4.1 and 4.2, tFUS modulation has been shown to induce excitatory and inhibitory effects in the brain depending on the stimulation parameters and paradigm. It is important to distinguish tFUS parameters necessary to generate excitation vs. inhibition. A recent computer simulation work has related the ultrasound parametric sub-spaces to those functional outcomes[195]. Further experimental validations regarding such a relationship between ultrasonic parameter sets and excitatory or inhibitory effects on animal models would become highly useful. Such validations would translate the tFUS neuromodulation technology to be applied on chronic pain management in clinical or home healthcare settings in the near future. The sickle cell disease (SCD) pain is an important model of chronic neuropathic pain syndrome in the CNS due to the widespread and non-specific location of pain caused by inflammation and sensitization, and the availability of animal models to study this disease model. The SCD patients associated with high pain intensity were found to have greater connectivity to pronociceptive areas such as ACC, S1 and insula[196], while the most consistent regions associated with nociception across studies include the ACC, S1, insula and thalamus[197-201]. More specifically, the S1 is involved in processing the sensory aspect of the pain; ACC and insula process the emotional or attention aspect. As shown in Figure 7, the tFUS can be delivered to S1, ACC and thalamus with customized ultrasound parameters, e.g. intensity, pulse repetition frequency and duty cycle, and with designed temporal sequences across these brain regions to suppress the sensory input of SCD pain at S1 with inhibitory parameter set, while facilitate the emotional output at the ACC using an excitatory ultrasound paradigm. Such a whole-head ultrasound array may be constructed using flexible electronics, e.g. a recent stretchable ultrasound array based on CMUTs[202]. Wavy serpentine shaped metal traces provide stretchable interconnects between the transducer elements. A 3D optical-based transducer mapping system can be employed to accurately digitize the position of each transducer element. By further converting the element spatial positions distributed over a subject’s head to a subject-specific time-delay profile of the flexible phased array, one can precisely refocus and electronically steer the ultrasound beam after being transmitted through the skull.
Figure 7.
tFUS is directed to the pain processing circuits in the brain and modulating the functional activity in those brain targets. As illustrated, the primary somatosensory cortex (S1), anterior cingulate cortex (ACC) and thalamus are targeted by a whole-head ultrasound array. The different colors are representing customized ultrasonic parameters for the different brain targets.
Alternative solution is to combine the tFUS with therapeutic agents for pain such that the neuromodulation effects can be directly enhanced by pharmacological molecules while maintain the spatial specificity of tFUS. One example is the ultrasound-enhanced therapeutic delivery based upon the blood-brain barrier (BBB) opening by the expansion of microbubbles[203] or very recently endothelial selective transfection by oscillating the microbubbles without disrupting the BBB [204] driven by controlled ultrasound energy. This would enable the spatially targeted drug or gene delivery[205]. Another successful demonstration is that the low-intensity tFUS is able to uncage and activate potential anesthetic agents, like propofol, ketamine, etc.[206], which is expected to enhance our capability for managing the pain by integrating the pharmacological effects into the ultrasound neuromodulation framework. In fact, cumulating experimental evidence on animal models has shed light upon the tFUS opening the BBB for modulating brain functional dynamics and even changing behavioral outcome once integrating with microbubbles and drug delivery[207].
4.4. Guided Transcranial Focused Ultrasound
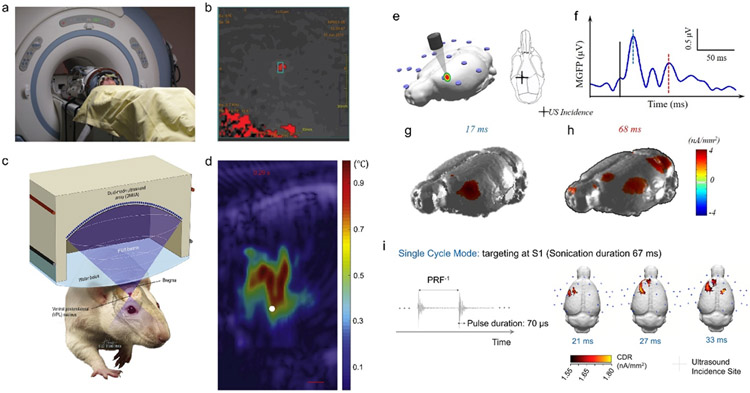
Both diagnostic and therapeutic applications would need a guidance for precise targeting and evaluation feedback. While the B-mode ultrasound image exemplified in Figure 5c provides some initial and rough evidence of the ultrasound targeting, an optical-based image-guided brain navigator is much commonly used to guide the transcranial energy onto wanted brain regions for TMS and tFUS. The spatial navigating performance is majorly determined by the optical tracking camera in terms of 3D root mean square (RMS) volumetric accuracy and repeatability. The quality of surface registration and the stability of the reference trackers during the navigation would both affect the overall accuracy. With an individual MRI brain model, a millimeter spatial accuracy can be achieved. Among such practices, an early effort to quantify the spatial accuracy in guiding the single-element based tFUS with mechanical or MRI-based calibration was reported in 2012[208]. The spatial error using mechanical calibration was further reduced by 57% to 1.9 mm through the MRI-based calibration approach, in which a temperature-sensitive spin-echo MRI sequence was employed[208]. Nowadays, neuro-navigation system has been widely used to guide the ultrasound transducer placement over the head based on brain’s structural information[169, 209]. To enhance the precision of navigation, functional brain maps can also be harnessed in addition to the structural brain landmarks. Strongest BOLD signals in motor representations are able to assist the identification of tFUS target, although to get such functional target information would require an additional pre-session of functional task in fMRI[183]. For large animal models, like on monkeys, such brain navigator was also applied to guide the positioning of tFUS transducer, thus targeting the ultrasound energy to ACC or amygdala[186]. A recent effort to getting rid of the registration process and the expensive optical tracking camera was made by introducing 3D-printed subject-specific helmet based on individual MRI data. 4-7.5 mm spatial accuracies in terms of positioning and direction were achieved using this MR-compatible ultrasound holding/targeting device[210].
The optical-based image-guided brain navigation is mainly for planning the direction of ultrasound focus at the needed brain target. However, the ultrasound aberrations both in amplitude and phase due to the skull are non-trivial. The image-guided approach is not limited to the use of MRI, CT images can provide crucial acoustic properties of the skull, thus serving the accurate refocusing through a time reversal process for a multi-element ultrasound array. Considering the ultrasound beam degradation after penetrating the skull, the focus positioning error using such an electronically targeting manner was able to achieve sub-millimeter level[211]. Furthermore, in order to acquire in vivo knowledge in regard of the transcranial focal location, MR-guided focused ultrasound (MRgFUS) has been developed especially for the application of brain surgery without opening the skull. Two approaches have been usually employed to measure the transcranial focus of ultrasound energy, either by measuring temperature rise using MR thermography[159, 212] or through quantifying the tissue displacement using MR-based acoustic radiation force imaging (MR-ARFI)[213]. Typically, these two technologies would effectively inform the focal spot when relatively high intensity ultrasound is applied using MR-compatible ultrasound transducers. As early as 2009, the MRgFUS-induced thalamotomy was inducted to treat chronic therapy-resistant neuropathic pain[214]. Figure 8a demonstrates the sonication setup using a 1024-element phased array transducer in a 30-cm diameter hemispherical geometry working at 650 kHz by InSightec within a 3-T MR system by GE. Among 11 patients with an overall thermal deposition at 57.5°C, 6 of them received both instantaneous and long-term somatosensory improvements[215]. The thermal spot identified within the blue rectangle in Figure 8b reconstructed from MR imaging and thermometry effectively informs the calculated target of tFUS. Unfortunately, one of the patients suffered bleeding and ischemic symptoms at the targeted thalamic nucleus and related regions due to the high ultrasound input power at a maximum of 1200 W[214, 215]. Very recently, a novel MRI pulse sequence was applied to monitor both temperature rise of 1-2°C at the vicinity of skull and local tissue displacement of 1-2 μm at the ultrasound focus by means of 3T clinical imaging system[216]. In concert with the development of MRgFUS, ultrasound imaging guided focused ultrasound neuromodulation (USgFUS) was also explored. In one of such studies, synthetic aperture (SA) imaging was used to guide and localize the tFUS beam[217]; further, real-time ultrasound temperature imaging[218] with 64-element dual mode ultrasound array (DMUA) working at 3.2 MHz with 50 kHz amplitude modulation was employed to evaluate the thermal-mediated suppression effects to the somatosensory evoked potential (SEP) induced by short tFUS administration as shown in Figure 8c[217]. To get a measurable temperature rise, a relatively high spatial-peak temporal-average intensity greater than 2.5 W/cm2 is dosed. One such example is the “tadpole” shaped heated region as illustrated in Figure 8d, with a white dot indicating the target focus[217]. In order to spatially map the focus of low-intensity tFUS in a natural setting, EEG-based electrophysiological source imaging (ESI)[231] was proposed and validated its feasibility in functionally mapping the ultrasound focus. Furthermore, a recent functional ultrasound (fUS) imaging technology[219] has also attracted tremendous attentions from the community. By taking advantages of improved ultrasound spatial resolution and superior ultrasound frame rate, the fUS has exhibited its scientific power for imaging microvascular dynamics[220] by virtue of high temporal resolution[221, 222] for monitoring responses from small brain models. However, fUS would heavily rely on high frequency energy, e.g. 18.5 MHz employed in Verasonics L22-14v imaging probe, which has factually posed a challenge for a translational application onto large brain models. Nevertheless, the parallel fUS imaging and EEG recordings[223], thus fUS-ESI may enable a multi-scale and multi-modal imaging platform to rigorously evaluate and monitor the tFUS in pain treatment.
Figure 8.
Imaging Guided Transcranial Focused Ultrasound. a)-b) Patient setup with MR guided focused ultrasound, and the thermal spatial mapping for monitoring the focused ultrasound targeting (the panels are adapted from [215]). c)-d) Dual-mode ultrasound array for ultrasound imaging guided focused ultrasound for targeted neuromodulation through thermal effects (the panels are adapted from [217]). e)-h) EEG-based source imaging reconstructing the brain activations in response to low-intensity, non-thermal tFUS with the initial activity at the acoustic administration spot and the observed ancillary activities at other cortical brain areas at a later time. The panels are adapted and revised from [154]. i) The single cycle mode with an ultrasound pulse duration of 70 μs elicited the brain activations at the targeted cortical regions. EEG-source images show cortical activities at 21 ms, 27 ms, and 33 ms after onset of tFUS[224], which is demonstrated to capture the brain dynamics induced by the low-intensity sonication. This panel is adapted from [224].
4.5. Electrophysiological Source Imaging Guided Ultrasound
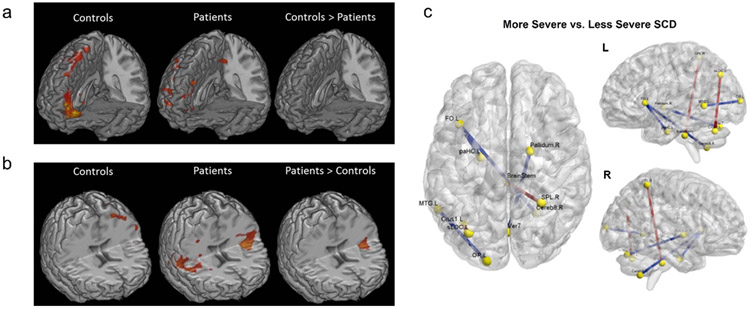
Dynamic and precise functional imaging using scalp EEG has been demonstrated to allow for better understanding the brain network and its connectivity of chronic pain in sickle cell disease (SCD)[27, 194], further differentiating SCD severity levels based on graph theory analyses[194] and assisting an objective assessment of tonic thermal pain[29]. More specifically, the EEG-fMRI spontaneous power analyses have depicted the significant differences of neural activities at color-indicated brain regions in both alpha and low beta bands between the SCD patients and healthy controls, as seen in Figure 6a-b[27]. A follow-up study investigating the SCD severity further identified significant varied functional connectivity strength between the more and less severe diseased states in Figure 6c[194]. Such knowledges of the brain’s functional changes are gradually facilitating the development of closed-loop personalized neuromodulation for treating pain, thus potentially changing the future clinical practices for pain management. At the same time, recent advancement in the electrophysiological source imaging (ESI) from dense array EEG has shown great promises to correct the smearing effect caused by the head volume conductor thus greatly enhance the spatial resolution of imaging brain activation from EEG[225-231]. The EEG-ESI techniques have been widely used for quantifying and imaging dynamic brain activation in healthy subjects, and patients with various neurological and mental disorders[232-242], and are well suited to tracking the dynamic brain response to tFUS. The scalp EEG-based ESI has been employed to guide ultrasound targeting by localizing the initial brain activations using low-intensity focused ultrasound[154] as presented in Figure 8e-h, and evaluate the global sonication-induced brain activation map by comparing the ultrasound parametric dosage[224] with the brain’s response to a single-cycle pulsed mode ultrasound at primary somatosensory cortex (Figure 8i). Whether the auditory responses to the tFUS would exist or not has been reviewed by our previous work[224]. Furthermore, a precise ESI may still require subject-specific MRI-based brain model, and similar to the fMRI-guided tFUS paradigm[183], the functional activation map reconstructed from ESI can be further integrated with the commercially available brain navigation system, thus guiding the tFUS target with increased precision. Combining all the available knowledge, the ESI-guided low-intensity tFUS modulating the pre-identified pain processing circuits in the central nervous system may lead to an effective tool for clinical practices and home healthcare.
Figure 6.
The brain network change observed in sickle cell disease (SCD) patients. a)-b) EEG-fMRI spontaneous power comparison regarding alpha power band (i.e. 8-12 Hz) in a) and beta1 power band (i.e. 13-21 Hz) in b). Significant different brain connectivity at specific locations, e.g. insula, can be observed from the SCD patient against healthy controls[27]. c) A comparison between levels of SCD severity in regard of the functional connectivity strength with significant differences illustrated in colored edges[194].
4.6. The Safety of Ultrasound Neuromodulation
Although it has been very rare to elicit adverse neural effects by administering tFUS through a survey of 33 studies in human and animal models[243] and through a recent review of 54 studies by Blackmore et al.[144], the in vivo guidance and feedback would still be highly needed to ensure the safety of tFUS for translational application. Despite the fact that researchers have been exploring ultrasound parametric space aggressively and propose for a new set of ultrasound safety guideline for neuromodulation purpose, the prevailing standard is still the FDA’s ultrasound energy thresholds for diagnostic ultrasound equipment, i.e. the derated acoustic output at the global maximum is required not to exceed “Pre-amendments acoustic output exposure levels”[244]. To prevent from a significant temperature rise, the derated spatial-peak temporal-average intensity (ISPTA) should not be greater than 720 mW/cm2; in the meanwhile, to avoid potential cavitation due to the sonication, the derated spatial-peak pulse-average intensity (ISPPA) is not allowed to exceed 190 W/cm2 or the mechanical index (MI) is limited by 1.9[244, 245]. For safety validations, on animal models, histological studies have been usually practiced to exam potential microhemorrhages or other micro-scale changes, like the presence of edema, cell necrosis or local inflammatory responses due to the sonication. In addition, post-sonication behavioral monitoring is commonly practiced as a safety measure applied on, e.g. sheep model[157]. The direct feedback, including tingling sensation, itching, heating[188] or even phosphene[184] human subjects through verbal communications or survey, would also an indispensable safety component in the tFUS human study.
5. Conclusions and Perspectives
As a common healthcare practice nowadays, managing the neuropathic pain is heavily relying on pharmacological approaches, such as opioid medications. Patients often require recurrent hospitalization, long-term use of opioids and live a poor quality of life. Given the side-effects of drug addiction and overdose, there is an urgent but unmet need to develop safe, effective, and non-addictive device-based technologies to treat the chronic neuropathic pain. The neuromodulation, using implantable or, especially, wearable devices to deliver certain controlled physical energy, holds a great promise of changing the current practice for pain treatment. Along with the neuroscientific discoveries on the mechanisms of neuropathic pain, the guided transcranial focused ultrasound stands out as an effective and targeted neuromodulation tool for the central nervous system, rivaling therapeutic advantages of all other current neuromodulation techniques. Clinical trials of treating specific type of pain using the tFUS in optimized dosage control or integrating the tFUS with current clinical approaches would be highly needed as one of the future efforts.
Acknowledgements
This work was supported in part by NIH grants EB029354, MH114233, AT009263, EB021027, and NS096761. X.N. was supported in part by Carnegie Mellon Neuroscience Institute Presidential Fellowship and Liang Ji Dian Graduate Fellowship at Carnegie Mellon University.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Duc NM, Keserci B, Diagn Interv Radiol 2019, DOI: 10.5152/dir.2019.18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bobola MS, Chen L, Ezeokeke CK, Kuznetsova K, Lahti AC, Lou W, Myroniv AN, Schimek NW, Selby ML, Mourad PD, Curr Pain Headache Rep 2018, 22, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].IASP, Classification of Chronic Pain, Second Edition (Revised), https://www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673, accessed: 15 October, 2019. [Google Scholar]
- [4].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, Pain 2019, 160, 19. [DOI] [PubMed] [Google Scholar]
- [5].Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, Cohen M, Evers S, Giamberardino MA, Goebel A, Korwisi B, Perrot S, Svensson P, Wang SJ, Treede RD, I. T. f. t. C. o. C. Pain, Pain 2019, 160, 28. [DOI] [PubMed] [Google Scholar]
- [6].Mantyselka P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamaki H, Halonen P, Takala J, Pain 2001, 89, 175. [DOI] [PubMed] [Google Scholar]
- [7].Goldberg DS, McGee SJ, Bmc Public Health 2011, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dansie EJ, Turk DC, British journal of anaesthesia 2013, 111, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fillingim RB, Loeser JD, Baron R, Edwards RR, J Pain 2016, 17, T10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Price DD, Bush FM, Long S, Harkins SW, Pain 1994, 56, 217. [DOI] [PubMed] [Google Scholar]
- [11].Jensen MP, Karoly P, in Handbook of pain assessment, 2nd ed., The Guilford Press, New York, NY, US: 2001, p. 15. [Google Scholar]
- [12].Gracely RH, Kwilosz DM, Pain 1988, 35, 279. [DOI] [PubMed] [Google Scholar]
- [13].Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB, Pain 1990, 41, 139. [DOI] [PubMed] [Google Scholar]
- [14].Herr K, Spratt KF, Garand L, Pain Med 2007, 8, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cleeland CS, Ryan KM, Ann Acad Med Singapore 1994, 23, 129. [PubMed] [Google Scholar]
- [16].Von Korff M, Dworkin SF, Le Resche L, Pain 1990, 40, 279. [DOI] [PubMed] [Google Scholar]
- [17].Todd N, Zhang Y, Power C, Becerra L, Borsook D, Livingstone M, McDannold N, NeuroImage 2019, 189, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, Mccain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP, Arthritis Rheum-Us 1990, 33, 160. [DOI] [PubMed] [Google Scholar]
- [19].Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, DeLitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK, Int J Ther Massage Bodywork 2015, 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Haanpaa ML, Laippala PA, Nurmikko TJ, Eur J Pain-London 1999, 3, 375. [DOI] [PubMed] [Google Scholar]
- [21].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD, Eur J Pain 2006, 10, 77. [DOI] [PubMed] [Google Scholar]
- [22].Davis KD, Moayedi M, J Neuroimmune Pharm 2013, 8, 518. [DOI] [PubMed] [Google Scholar]
- [23].Mogil JS, Trends Genet 2012, 28, 258. [DOI] [PubMed] [Google Scholar]
- [24].Westermann A, Krumova EK, Pennekamp W, Horch C, Baron R, Maier C, Pain 2012, 153, 1537. [DOI] [PubMed] [Google Scholar]
- [25].DaSilva AF, Nascimento TD, DosSantos MF, Zubieta JK, Curr Pain Headache R 2014, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Napadow V, Harris RE, Arthritis Res Ther 2014, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Case M, Zhang H, Mundahl J, Datta Y, Nelson S, Gupta K, He B, NeuroImage: Clinical 2017, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nir RR, Sinai A, Moont R, Harari E, Yarnitsky D, Clin Neurophysiol 2012, 123, 605. [DOI] [PubMed] [Google Scholar]
- [29].Vijayakumar V, Case M, Shirinpour S, He B, IEEE Trans Biomed Eng 2017, 64, 2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cohen SP, Mao JR, Bmj-Brit Med J 2014, 348. [DOI] [PubMed] [Google Scholar]
- [31].Hylands-White N, Duarte RV, Raphael JH, Rheumatol Int 2017, 37, 29. [DOI] [PubMed] [Google Scholar]
- [32].Dowell D, Haegerich TM, Chou R, MMWR Recomm Rep 2016, 65, 1. [DOI] [PubMed] [Google Scholar]
- [33].Okie S, New Engl J Med 2010, 363, 1981. [DOI] [PubMed] [Google Scholar]
- [34].Ballantyne JC, Anesthesia and analgesia 2017, 125, 1769. [DOI] [PubMed] [Google Scholar]
- [35].Paulozzi L, Baldwin G, Franklin G, Kerlikowske WRG, Jones CM, Popovic T, Jama-J Am Med Assoc 2012, 307, 774. [Google Scholar]
- [36].Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E, CMAJ 2006, 174, 1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kalso E, Edwards JE, Moore RA, McQuay HJ, Pain 2004, 112, 372. [DOI] [PubMed] [Google Scholar]
- [38].Mao JR, Sung BK, Ji RR, Lim G, Journal of Neuroscience 2002, 22, 8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kohno T, Ji RR, Ito N, Allchorne AJ, Befort K, Karchewski LA, Woolf CJ, Pain 2005, 117, 77. [DOI] [PubMed] [Google Scholar]
- [40].Vane JR, Bakhle YS, Botting RM, Annu Rev Pharmacol 1998, 38, 97. [DOI] [PubMed] [Google Scholar]
- [41].Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA, A. T. Investigators, New Engl J Med 2005, 352, 1092. [DOI] [PubMed] [Google Scholar]
- [42].Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, A. S. Investigators, New Engl J Med 2005, 352, 1071. [DOI] [PubMed] [Google Scholar]
- [43].Grape S, Schug SA, Lauer S, Schug BS, Drugs 2010, 70, 57. [DOI] [PubMed] [Google Scholar]
- [44].Pergolizzi J, Aloisi AM, Dahan A, Filitz J, Langford R, Likar R, Mercadante S, Morlion B, Raffa RB, Sabatowski R, Sacerdote P, Torres LM, Weinbroum AA, Pain Practice 2010, 10, 428. [DOI] [PubMed] [Google Scholar]
- [45].Arnold LM, Keck PE Jr., Welge JA, Psychosomatics 2000, 41, 104. [DOI] [PubMed] [Google Scholar]
- [46].McLean AL, Frank S, Zafar N, Waschke A, Kalff R, Reichart R, Neurol Res 2018, 40, 566. [DOI] [PubMed] [Google Scholar]
- [47].Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, He B, Ieee T Bio-Med Eng 2013, 60, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Krames ES, Hunter Peckham P, Rezai AR, Neuromodulation (Second Edition), Academic Press, 2018. [Google Scholar]
- [49].Rajasethupathy P, Ferenczi E, Deisseroth K, Cell 2016, 165, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Harquel S, Bacle T, Beynel L, Marendaz C, Chauvin A, David O, NeuroImage 2016, 135, 115. [DOI] [PubMed] [Google Scholar]
- [51].Edelman BJ, Johnson N, Sohrabpour A, Tong S, Thakor N, He B, Engineering-Prc 2015, 1, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Linderoth B, Foreman RD, Neuromodulation 2017, 20, 525. [DOI] [PubMed] [Google Scholar]
- [53].North RB, Kidd DH, Farrokhi F, Piantadosi SA, Neurosurgery 2005, 56, 98. [DOI] [PubMed] [Google Scholar]
- [54].Shamji MF, De Vos C, Sharan A, Neurosurgery 2017, 80, S108. [DOI] [PubMed] [Google Scholar]
- [55].Rasche D, Siebert S, Stippich C, Kress B, Nennig E, Sartor K, Tronnier VM, Schmerz 2005, 19, 497. [DOI] [PubMed] [Google Scholar]
- [56].Sdrulla AD, Guan Y, Raja SN, Pain practice : the official journal of World Institute of Pain 2018, 18, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fishman MA, Antony A, Esposito M, Deer T, Levy R, Pain Med 2019, 20, S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB, Neurosurgery 2008, 63, 762. [DOI] [PubMed] [Google Scholar]
- [59].Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB, Pain 2007, 132, 179. [DOI] [PubMed] [Google Scholar]
- [60].Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T, Pain Med 2014, 15, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Russo M, Van Buyten JP, Pain Med 2015, 16, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].De Ridder D, Vanneste S, van der Loo E, Plazier M, Menovsky T, van de Heyning P, Journal of neurosurgery 2010, 112, 1289. [DOI] [PubMed] [Google Scholar]
- [63].Cameron T, Journal of neurosurgery 2004, 100, 254. [DOI] [PubMed] [Google Scholar]
- [64].Hayek SM, Veizi E, Hanes M, Neuromodulation 2015, 18, 603. [DOI] [PubMed] [Google Scholar]
- [65].Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S, Journal of neurosurgery 1993, 78, 393. [DOI] [PubMed] [Google Scholar]
- [66].Tanei T, Kajita Y, Wakabayashi T, Neurol Med Chir (Tokyo) 2010, 50, 604. [DOI] [PubMed] [Google Scholar]
- [67].Roux FE, Ibarrola D, Lazorthes Y, Berry I, Neurosurgery 2001, 48, 681. [DOI] [PubMed] [Google Scholar]
- [68].Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier A, Fontaine D, Brugieres P, Pollin B, Feve A, Rostaing S, Cesaro P, Keravel Y, Pain 1999, 82, 245. [DOI] [PubMed] [Google Scholar]
- [69].Ostergard T, Munyon C, Miller JP, Neurosurgery Clinics of North America 2014, 25, 693. [DOI] [PubMed] [Google Scholar]
- [70].Hassler R, Riechert T, Mundinger F, Umbach W, Ganglberger JA, Brain : a journal of neurology 1960, 83, 337. [DOI] [PubMed] [Google Scholar]
- [71].Hamani C, Schwalb JM, Rezai AR, Dostrovsky JO, Davis KD, Lozano AM, Pain 2006, 125, 188. [DOI] [PubMed] [Google Scholar]
- [72].Yamamoto T, Katayama Y, Obuchi T, Kano T, Kobayashi K, Oshima H, Fukaya C, Stereotactic and functional neurosurgery 2006, 84, 180. [DOI] [PubMed] [Google Scholar]
- [73].Marchand S, Kupers RC, Bushnell MC, Duncan GH, Pain 2003, 105, 481. [DOI] [PubMed] [Google Scholar]
- [74].Demierre B, Siegfried J, Neurochirurgie 1985, 31, 281. [PubMed] [Google Scholar]
- [75].Kumar K, Toth C, Nath RK, Neurosurgery 1997, 40, 736. [DOI] [PubMed] [Google Scholar]
- [76].Sims-Williams H, Matthews JC, Talbot PS, Love-Jones S, Brooks JCW, Patel NK, Pickering AE, NeuroImage 2017, 146, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boccard SGJ, Pereira EAC, Moir L, Aziz TZ, Green AL, Neurosurgery 2013, 72, 221. [DOI] [PubMed] [Google Scholar]
- [78].Spooner J, Yu H, Kao C, Sillay K, Konrad P, Journal of neurosurgery 2007, 107, 169. [DOI] [PubMed] [Google Scholar]
- [79].Long DM, Surg Neurol 1998, 49, 142. [DOI] [PubMed] [Google Scholar]
- [80].Coffey RJ, Pain Med 2001, 2, 183. [DOI] [PubMed] [Google Scholar]
- [81].U. S. FDA, Summary of Safety And Effectiveness Data For A Supplemental Premarket Approval Application Activa Parkinson’s Control Therapy, Medtronic Inc., 2002, P960009. [Google Scholar]
- [82].U. S. FDA, Summary of Safety And Effectiveness Data For Vercise™ Deep Brain Stimulation (DBS) System, Boston Scientific Corporation, 2017, P150031. [Google Scholar]
- [83].U. S. FDA, Summary of Safety And Effectiveness Data For Brio Neurostimulation System, St. Jude Medical, 2015, P140009. [Google Scholar]
- [84].Atchley TJ, Laskay NMB, Sherrod BA, Rahman A, Walker HC, Guthrie BL, Journal of neurosurgery 2019, DOI: 10.3171/2019.3.JNS1830231. [DOI] [PubMed] [Google Scholar]
- [85].Hardaway FA, Raslan AM, Burchiel KJ, Neurosurgery 2018, 83, 540. [DOI] [PubMed] [Google Scholar]
- [86].Shannon RV, IEEE Trans Biomed Eng 1992, 39, 424. [DOI] [PubMed] [Google Scholar]
- [87].McCreery DB, Agnew WF, Yuen TGH, Bullara L, Ieee T Bio-Med Eng 1990, 37, 996. [DOI] [PubMed] [Google Scholar]
- [88].Cogan SF, Annual review of biomedical engineering 2008, 10, 275. [DOI] [PubMed] [Google Scholar]
- [89].Castagnola E, Maiolo L, Maggiolini E, Minotti A, Marrani M, Maita F, Pecora A, Angotzi GN, Ansaldo A, Boffini M, Fadiga L, Fortunato G, Ricci D, Ieee T Neur Sys Reh 2015, 23, 342. [DOI] [PubMed] [Google Scholar]
- [90].Schander A, Tessmann T, Strokov S, Stemmann H, Kreiter AK, Lang W, 2016 38th Annual International Conference of the Ieee Engineering in Medicine and Biology Society (Embc) 2016, 6174. [DOI] [PubMed] [Google Scholar]
- [91].Vitale F, Summerson SR, Aazhang B, Kemere C, Pasquali M, Acs Nano 2015, 9, 4465. [DOI] [PubMed] [Google Scholar]
- [92].Castagnola E, Maggiolini E, Ceseracciu L, Ciarpella F, Zucchini E, De Faveri S, Fadiga L, Ricci D, Frontiers in Neuroscience 2016, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Du ZJ, Kolarcik CL, Kozai TDY, Luebben SD, Sapp SA, Zheng XS, Nabity JA, Cui XT, Acta Biomaterialia 2017, 53, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bramini M, Alberini G, Colombo E, Chiacchiaretta M, DiFrancesco ML, Maya-Vetencourt JF, Maragliano L, Benfenati F, Cesca F, Frontiers in Systems Neuroscience 2018, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kostarelos K, Vincent M, Hebert C, Garrido JA, Advanced Materials 2017, 29. [DOI] [PubMed] [Google Scholar]
- [96].Levy R, Deer TR, Henderson J, Pain Physician 2010, 13, 157. [PubMed] [Google Scholar]
- [97].Boccard SGJ, Pereira EAC, Aziz TZ, Journal of Clinical Neuroscience 2015, 22, 1537. [DOI] [PubMed] [Google Scholar]
- [98].Boccard SGJ, Prangnell SJ, Pycroft L, Cheeran B, Moir L, Pereira EAC, Fitzgerald JJ, Green AL, Aziz TZ, World Neurosurgery 2017, 106, 625. [DOI] [PubMed] [Google Scholar]
- [99].Farrell SM, Green A, Aziz T, Brain Sci 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Grill WM, Norman SE, Bellamkonda RV, Annual review of biomedical engineering 2009, 11, 1. [DOI] [PubMed] [Google Scholar]
- [101].Wagner T, Valero-Cabre A, Pascual-Leone A, Annual review of biomedical engineering 2007, 9, 527. [DOI] [PubMed] [Google Scholar]
- [102].Krieg T, Mogul DJ, in Neural Engineering, DOI: 10.1007/978-1-4614-5227-0_9 (Ed: He B), Springer US, Boston, MA: 2013, p. 405. [DOI] [Google Scholar]
- [103].Barker AT, Jalinous R, Freeston IL, Lancet 1985, 1, 1106. [DOI] [PubMed] [Google Scholar]
- [104].Chen M, Mogul DJ, IEEE Trans. Biomed Engr. 2010, 57, 1216. [DOI] [PubMed] [Google Scholar]
- [105].Lisanby SH, Belmaker RH, Depress Anxiety 2000, 12, 178. [DOI] [PubMed] [Google Scholar]
- [106].Hallett M, Nature 2000, 406, 147. [DOI] [PubMed] [Google Scholar]
- [107].Deng ZD, Lisanby SH, Peterchev AV, Brain Stimul 2013, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Johnson NN, Carey J, Edelman BJ, Doud A, Grande A, Lakshminarayan K, He B, Journal of Neural Engineering 2018, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Petrichella S, Johnson N, He B, PloS one 2017, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, Lenz F, Lefaucheur JP, Lang M, Hallett M, Fox M, Cudkowicz M, Costello A, Carr DB, Ayache SS, Oaklander AL, Pain 2015, 156, 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Andre-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L, Clin Neurophysiol 2006, 117, 1536. [DOI] [PubMed] [Google Scholar]
- [112].Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, Rosa M, Rigonatti SP, Camprodon J, Walpoth M, Heaslip J, Grunhaus L, Hausmann A, Pascual-Leone A, Int J Neuropsychoph 2006, 9, 641. [DOI] [PubMed] [Google Scholar]
- [113].Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F, NeuroImage 2014, 85, 1003. [DOI] [PubMed] [Google Scholar]
- [114].Nurmikko T, MacIver K, Bresnahan R, Hird E, Nelson A, Sacco P, Neuromodulation 2016, 19, 669. [DOI] [PubMed] [Google Scholar]
- [115].O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA, Biological psychiatry 2007, 62, 1208. [DOI] [PubMed] [Google Scholar]
- [116].Fregni F, Pascual-Leone A, Nat Clin Pract Neurol 2007, 3, 383. [DOI] [PubMed] [Google Scholar]
- [117].Rollnik JD, Wüstefeld S, Däuper J, Karst M, Fink M, Kossev A, Dengler R, European Neurology 2002, 48, 6. [DOI] [PubMed] [Google Scholar]
- [118].Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Zerah F, Bendib B, Cesaro P, Keravel Y, Nguyen JP, J Neurol Neurosur Ps 2004, 75, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC, J Neurol Neurosur Ps 2005, 76, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lefaucheur JP, Ayache SS, Sorel M, Farhat WH, Zouari HG, de Andrade DC, Ahdab R, Menard-Lefaucheur I, Brugieres P, Goujon C, Eur J Pain 2012, 16, 1403. [DOI] [PubMed] [Google Scholar]
- [121].Epstein CM, Davey KR, Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2002, 19, 376. [DOI] [PubMed] [Google Scholar]
- [122].Epstein C, Wassermann E, Ziemann U, Oxford Handbook of Transcranial Stimulation, 2012. [Google Scholar]
- [123].Yamamoto K, Miyawaki Y, Saitoh Y, Sekino M, Ieee T Magn 2016, 52, 1. [Google Scholar]
- [124].Negahbani E, Stitt IM, Davey M, Doan TT, Dannhauer M, Hoover AC, Peterchev AV, Radtke-Schuller S, Fröhlich F, 2019, DOI: 10.1101/563163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Koponen LM, Nieminen JO, Mutanen TP, Stenroos M, Ilmoniemi RJ, Brain Stimulation 2017, 10, 795. [DOI] [PubMed] [Google Scholar]
- [126].Deng ZD, Lisanby SH, Peterchev AV, Clin Neurophysiol 2014, 125, 1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Cobos Sanchez C, Cabello MR, Olozabal AQ, Pantoja MF, J Neural Eng 2020, 17, 016056. [DOI] [PubMed] [Google Scholar]
- [128].Wang WT, Xu B, Butman JA, Journal of neuroscience methods 2017, 289, 1. [DOI] [PubMed] [Google Scholar]
- [129].de Lara LIN, Tik M, Woletz M, Frass-Kriegl R, Moser E, Laistler E, Windischberger C, NeuroImage 2017, 150, 262. [DOI] [PubMed] [Google Scholar]
- [130].Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS, Eur J Neurol 2007, 14, 952. [DOI] [PubMed] [Google Scholar]
- [131].Peterchev AV, Murphy DL, Goetz SM, Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2015, 2015, 226. [DOI] [PubMed] [Google Scholar]
- [132].Priori A, Berardelli A, Rona S, Accornero N, Manfredi M, Neuroreport 1998, 9, 2257. [DOI] [PubMed] [Google Scholar]
- [133].Nitsche MA, Paulus W, J Physiol 2000, 527 Pt 3, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Nitsche MA, Paulus W, Neurology 2001, 57, 1899. [DOI] [PubMed] [Google Scholar]
- [135].Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F, Brain Stimul 2012, 5, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stagg CJ, Nitsche MA, Neuroscientist 2011, 17, 37. [DOI] [PubMed] [Google Scholar]
- [137].Baxter BS, Edelman BJ, Sohrabpour A, He B, Frontiers in Neuroscience 2017, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Baxter BS, Edelman BJ, Nesbitt N, He B, Brain Stimulation 2016, 9, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Roy A, Baxter B, He B, Ieee T Bio-Med Eng 2014, 61, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI, Neurorehabil Neural Repair 2014, 28, 250. [DOI] [PubMed] [Google Scholar]
- [141].Ahn S, Prim JH, Alexander ML, McCulloch KL, Frohlich F, J Pain 2019, 20, 277 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M, Brain Stimul 2009, 2, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Monteith S, Snell J, Eames M, Kassell NF, Kelly E, Gwinn R, Journal of neurosurgery 2016, 125, 1557. [DOI] [PubMed] [Google Scholar]
- [144].Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO, Ultrasound in medicine & biology 2019, DOI: 10.1016/j.ultrasmedbio.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].di Biase L, Falato E, Di Lazzaro V, Front Neurol 2019, 10, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wang P, Zhang J, Yu J, Smith C, Feng W, Front Neurosci 2019, 13, 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ, Nat Neurosci 2014, 17, 322. [DOI] [PubMed] [Google Scholar]
- [148].Tyler WJ, Lani SW, Hwang GM, Curr Opin Neurobiol 2018, 50, 222. [DOI] [PubMed] [Google Scholar]
- [149].Naor O, Krupa S, Shoham S, J Neural Eng 2016, 13, 031003. [DOI] [PubMed] [Google Scholar]
- [150].Tufail Y, Yoshihiro A, Pati S, Li MM, Tyler WJ, Nat Protoc 2011, 6, 1453. [DOI] [PubMed] [Google Scholar]
- [151].Ye PP, Brown JR, Pauly KB, Ultrasound in medicine & biology 2016, 42, 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Mohammadjavadi M, Ye PP, Xia A, Brown J, Popelka G, Pauly KB, Brain Stimulation 2019, DOI: 10.1016/j.brs.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Yuan Y, Wang Z, Liu M, Shoham S, NeuroImage 2020, 211, 116597. [DOI] [PubMed] [Google Scholar]
- [154].Yu K, Sohrabpour A, He B, IEEE Trans Biomed Eng 2016, 63, 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kim H, Lee SD, Chiu A, Yoo SS, Park S, Neuroreport 2014, 25, 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Yoo SS, Bystritsky A, Lee JH, Zhang Y, Fischer K, Min BK, McDannold NJ, Pascual-Leone A, Jolesz FA, NeuroImage 2011, 56, 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lee W, Lee SD, Park MY, Foley L, Purcell-Estabrook E, Kim H, Fischer K, Maeng LS, Yoo SS, Ultrasound in medicine & biology 2016, 42, 459. [DOI] [PubMed] [Google Scholar]
- [158].Yoon K, Lee W, Lee JE, Xu L, Croce P, Foley L, Yoo SS, PloS one 2019, 14, e0224311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Dallapiazza RF, Timbie KF, Holmberg S, Gatesman J, Lopes MB, Price RJ, Miller GW, Elias WJ, Journal of neurosurgery 2018, 128, 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Daniels D, Sharabi S, Last D, Guez D, Salomon S, Zivli Z, Castel D, Volovick A, Grinfeld J, Rachmilevich I, Amar T, Liraz-Zaltsman S, Sargsyan N, Mardor Y, Harnof S, Ultrasound in medicine & biology 2018, 44, 1022. [DOI] [PubMed] [Google Scholar]
- [161].Deffieux T, Younan Y, Wattiez N, Tanter M, Pouget P, Aubry JF, Curr Biol 2013, 23, 2430. [DOI] [PubMed] [Google Scholar]
- [162].Downs ME, Buch A, Karakatsani ME, Konofagou EE, Ferrera VP, Sci Rep 2015, 5, 15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yang PF, Phipps MA, Newton AT, Chaplin V, Gore JC, Caskey CF, Chen LM, Sci Rep 2018, 8, 7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry J-F, Rushworth MFS, Sallet J, Neuron 2019, DOI: 10.1016/j.neuron.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Verhagen L, Gallea C, Folloni D, Constans C, Jensen DE, Ahnine H, Roumazeilles L, Santin M, Ahmed B, Lehericy S, Klein-Flugge MC, Krug K, Mars RB, Rushworth MF, Pouget P, Aubry JF, Sallet J, Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Mueller J, Legon W, Opitz A, Sato TF, Tyler WJ, Brain Stimul 2014, DOI: 10.1016/j.brs.2014.08.008. [DOI] [PubMed] [Google Scholar]
- [167].Lee W, Kim H, Jung Y, Song IU, Chung YA, Yoo SS, Sci Rep 2015, 5, 8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Legon W, Bansal P, Tyshynsky R, Ai L, Mueller JK, bioRxiv 2018, DOI: 10.1101/234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Legon W, Ai L, Bansal P, Mueller JK, Human brain mapping 2018, DOI: 10.1002/hbm.23981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM, Brain Stimul 2016, DOI: 10.1016/j.brs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- [171].Mason WP, The Journal of the Acoustical Society of America 1981, 70, 1561. [Google Scholar]
- [172].Platte M, Ferroelectrics 1987, 75, 327. [Google Scholar]
- [173].Brenner K, Ergun AS, Firouzi K, Rasmussen MF, Stedman Q, Khuri-Yakub BP, Micromachines (Basel) 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ergun AS, Yongli H, Xuefeng Z, Oralkan O, Yarahoglu GG, Khuri-Yakub BT, IEEE transactions on ultrasonics, ferroelectrics, and frequency control 2005, 52, 2242. [DOI] [PubMed] [Google Scholar]
- [175].Gerardo CD, Cretu E, Rohling R, Microsyst Nanoeng 2018, 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Zhao Y, Zhao L, Li Z, Li J, Yang P, Xu T, Liu Z, Guo S, Wang J, Jiang Z, Journal of Micromechanics and Microengineering 2019, 29. [Google Scholar]
- [177].Lee W, Croce P, Margolin RW, Cammalleri A, Yoon K, Yoo SS, BMC Neurosci 2018, 19, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhou H, Niu L, Meng L, Lin Z, Zou J, Xia X, Huang X, Zhou W, Bian T, Zheng H, Research 2019, 2019, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Qiu W, Zhou J, Chen Y, Su M, Li G, Zhao H, Gu X, Wang Meng, C., Xiao Y, Lam KH, Dai J, Zheng H, IEEE Trans Neural Syst Rehabil Eng 2017, 25, 2509. [DOI] [PubMed] [Google Scholar]
- [180].Kim H, Kim S, Sim NS, Pasquinelli C, Thielscher A, Lee JH, Lee HJ, Brain Stimul 2019, 12, 251. [DOI] [PubMed] [Google Scholar]
- [181].Choi T, Bae S, Suh M, Park J, Ann Biomed Eng 2019, DOI: 10.1007/s10439-019-02431-w. [DOI] [PubMed] [Google Scholar]
- [182].Kim Y, Maxwell AD, Hall TL, Xu Z, Lin KW, Cain CA, IEEE transactions on ultrasonics, ferroelectrics, and frequency control 2014, 61, 1559. [DOI] [PubMed] [Google Scholar]
- [183].Ai L, Bansal P, Mueller JK, Legon W, BMC Neurosci 2018, 19, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lee W, Kim HC, Jung Y, Chung YA, Song IU, Lee JH, Yoo SS, Sci Rep 2016, 6, 34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wattiez N, Constans C, Deffieux T, Daye PM, Tanter M, Aubry JF, Pouget P, Brain Stimul 2017, 10, 1024. [DOI] [PubMed] [Google Scholar]
- [186].Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry J-F, Rushworth MFS, Sallet J, Neuron 2019, 101, 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Chaplin V, Phipps MA, Caskey CF, Physics in medicine and biology 2018, 63, 105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gibson BC, Sanguinetti JL, Badran BW, Yu AB, Klein EP, Abbott CC, Hansberger JT, Clark VP, Front Neurol 2018, 9, 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Schimek N, Burke-Conte Z, Abernethy J, Schimek M, Burke-Conte C, Bobola M, Stocco A, Mourad PD, Front Hum Neurosci 2020, 14, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Tych RE, Gofeld M, Jarvik JG, Kliot M, Loeser JD, McClintic AM, Ollos RJ, Pederson KD, Sparks RE, Terman GW, Mourad PD, Ultrasound in medicine & biology 2013, 39, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Gellhorn AC, Gillenwater C, Mourad PD, Ultrasound in Medicine and Biology 2015, 41, 2412. [DOI] [PubMed] [Google Scholar]
- [192].Mourad PD, Friedly JL, McClintic AM, Olmstead TA, Loeser JD, Pain Med 2018, 19, 541. [DOI] [PubMed] [Google Scholar]
- [193].Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, Lucas A, Amos Q, Buadu A, Badal JJ, Brain Stimul 2013, 6, 409. [DOI] [PubMed] [Google Scholar]
- [194].Case M, Shirinpour S, Vijayakumar V, Zhang H, Datta Y, Nelson S, Pergami P, Darbari DS, Gupta K, He B, Neuroimage Clin 2019, 21, 101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Plaksin M, Kimmel E, Shoham S, eNeuro 2016, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, Clauw DJ, Taylor JG, Harris RE, Journal of Pain 2015, 16, 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Apkarian AV, Hashmi JA, Baliki MN, Pain 2011, 152, S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK, Eur J Pain 2005, 9, 463. [DOI] [PubMed] [Google Scholar]
- [199].Lee MC, Tracey I, British journal of anaesthesia 2013, 111, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Shao SY, Shen KQ, Yu K, Wilder-Smith EPV, Li XP, Clin Neurophysiol 2012, 123, 2042. [DOI] [PubMed] [Google Scholar]
- [201].Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM, Journal of Neuroscience 2002, 22, 2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hu H, Zhu X, Wang C, Zhang L, Li X, Lee S, Huang Z, Chen R, Chen Z, Wang C, Gu Y, Chen Y, Lei Y, Zhang T, Kim N, Guo Y, Teng Y, Zhou W, Li Y, Nomoto A, Sternini S, Zhou Q, Pharr M, di Scalea FL, Xu S, Sci Adv 2018, 4, eaar3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Choi K-H, Kim J-H, Journal of Neurosonology and Neuroimaging 2019, 11, 62. [Google Scholar]
- [204].Gorick CM, Mathew AS, Garrison WJ, Thim EA, Fisher DG, Copeland CA, Song J, Klibanov AL, Miller GW, Price RJ, Proceedings of the National Academy of Sciences of the United States of America 2020, 117, 5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Szablowski JO, Lee-Gosselin A, Lue B, Malounda D, Shapiro MG, Nature Biomedical Engineering 2018, 2, 475. [DOI] [PubMed] [Google Scholar]
- [206].Wang JB, Aryal M, Zhong Q, Vyas DB, Airan RD, Neuron 2018, 100, 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Munoz F, Aurup C, Konofagou EE, Ferrera VP, Current Behavioral Neuroscience Reports 2018, 5, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Kim H, Chiu A, Park S, Yoo SS, International Journal of Imaging Systems and Technology 2012, 22, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Legon W, Bansal P, Tyshynsky R, Ai L, Mueller JK, Sci Rep 2018, 8, 10007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Joe H, Pahk KJ, Park S, Kim H, Computer methods and programs in biomedicine 2019, 176, 105. [DOI] [PubMed] [Google Scholar]
- [211].Marquet F, Pernot M, Aubry JF, Montaldo G, Marsac L, Tanter M, Fink M, Physics in medicine and biology 2009, 54, 2597. [DOI] [PubMed] [Google Scholar]
- [212].Rieke V, Butts Pauly K, Journal of magnetic resonance imaging : JMRI 2008, 27, 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kaye EA, Chen J, Pauly KB, Magnet Reson Med 2011, 65, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B, Annals of neurology 2009, 66, 858. [DOI] [PubMed] [Google Scholar]
- [215].Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, Martin E, Neurosurg Focus 2012, 32, E1. [DOI] [PubMed] [Google Scholar]
- [216].Ozenne V, Constans C, Bour P, Santin MD, Valabregue R, Ahnine H, Pouget P, Lehericy S, Aubry J, Quesson B, NeuroImage 2019, DOI: 10.1016/j.neuroimage.2019.116236116236. [DOI] [PubMed] [Google Scholar]
- [217].Darrow DP, O’Brien P, Richner T, Netoff TI, Ebbini ES, Brain Stimulation 2019, DOI: 10.1016/j.brs.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Ebbini ES, Simon C, Liu D, IEEE Signal Process Mag 2018, 35, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Mace E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M, Nature methods 2011, 8, 662. [DOI] [PubMed] [Google Scholar]
- [220].Mace E, Montaldo G, Trenholm S, Cowan C, Brignall A, Urban A, Roska B, Neuron 2018, 100, 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Dizeux A, Gesnik M, Ahnine H, Blaize K, Arcizet F, Picaud S, Sahel JA, Deffieux T, Pouget P, Tanter M, Nature communications 2019, 10, 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rabut C, Correia M, Finel V, Pezet S, Pernot M, Deffieux T, Tanter M, Nature methods 2019, 16, 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Sieu LA, Bergel A, Tiran E, Deffieux T, Pernot M, Gennisson JL, Tanter M, Cohen I, Nature methods 2015, 12, 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Niu X, Yu K, He B, Current Opinion in Biomedical Engineering 2018, 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Gramfort A, Strohmeier D, Haueisen J, Hamalainen MS, Kowalski M, NeuroImage 2013, 70, 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Bolstad A, Van Veen B, Nowak R, NeuroImage 2009, 46, 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Michel CM, Murray MM, NeuroImage 2012, 61, 371. [DOI] [PubMed] [Google Scholar]
- [228].Haufe S, Nikulin VV, Ziehe A, Muller KR, Nolte G, NeuroImage 2008, 42, 726. [DOI] [PubMed] [Google Scholar]
- [229].He B, Astolfi L, Valdes-Sosa PA, Marinazzo D, Palva S, Benar CG, Michel CM, Koenig T, IEEE Trans Biomed Eng 2019, DOI: 10.1109/TBME.2019.2913928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].He B, Sohrabpour A, Brown E, Liu Z, Annual review of biomedical engineering 2018, DOI: 10.1146/annurev-bioeng-062117-120853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Ding L, Worrell GA, Lagerlund TD, He B, NeuroImage 2007, 34, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Brodbeck V, Spinelli L, Lascano AM, Wissmeier M, Vargas MI, Vulliemoz S, Pollo C, Schaller K, Michel CM, Seeck M, Brain : a journal of neurology 2011, 134, 2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].He B, Ding L, Electrophysiological Mapping and Neuroimaging, Springer Berlin Heidelberg, 2013. [Google Scholar]
- [234].Michel C, He B, in Niedermeyer's Electroencephalography (Eds: Schomer D, Lopes da Silva F), Wolters Kluwer & Lippincott Williams & Wilkins, Philadelphia: 2011, Ch. 55, p. 1179. [Google Scholar]
- [235].He B, Yang L, Wilke C, Yuan H, IEEE Trans Biomed Eng 2011, 58, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].He B, Coleman T, Genin GM, Glover G, Hu X, Johnson N, Liu T, Makeig S, Sajda P, Ye K, IEEE Trans Biomed Eng 2013, 60, 2983. [DOI] [PubMed] [Google Scholar]
- [237].Huishi Zhang C, Sohrabpour A, Lu Y, He B, Human brain mapping 2016, 37, 2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Van de Ville D, Britz J, Michel CM, Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Britz J, Landis T, Michel CM, Cerebral Cortex 2009, 19, 55. [DOI] [PubMed] [Google Scholar]
- [240].Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmidt AM, Dale AM, Hamalainen MS, Marinkovic K, Schacter DL, Rosen BR, Halgren E, Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Sergent C, Baillet S, Dehaene S, Nat Neurosci 2005, 8, 1391. [DOI] [PubMed] [Google Scholar]
- [242].Ding L, Yuan H, Human brain mapping 2013, 34, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Pasquinelli C, Hanson LG, Siebner HR, Lee HJ, Thielscher A, Brain Stimulation 2019, DOI: 10.1016/j.brs.2019.07.024. [DOI] [PubMed] [Google Scholar]
- [244].U. S. FDA, Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers, Center for Devices and Radiological Health, 2008. [Google Scholar]
- [245].Duck FA, Medical Engineering & Physics 2008, 30, 1338. [DOI] [PubMed] [Google Scholar]