Immunoglobulin E–mediated food allergy (IgE-FA) can be life-threatening in children, and rates are rising in the United States.1 We now know that early introduction of allergenic foods can reduce the risk of IgE-FA.2 Food protein-induced allergic proctocolitis (FPIAP) (also called cow’s milk protein allergy and/or intolerance or non–IgE-mediated milk allergy) is an early, common form of food allergy presenting with bloody or mucoid stools, often together with fussiness and feeding difficulty.3,4 Not thought to be associated with IgE-FA, FPIAP is treated with avoidance of the trigger antigen, most commonly milk, for the first year of life.3,4 Guidelines recommend an oral challenge after short dietary elimination to confirm the diagnosis,5–7 but this is rarely done in clinical practice.8,9 Given that FPIAP is associated with both eczema8 and diet restriction, we hypothesized that children with FPIAP would be at increased risk for IgE-FA.
Methods
In the Gastrointestinal Microbiome and Allergic Proctocolitis prospective observational healthy-infant cohort study,8 FPIAP was diagnosed by the primary care provider on the basis of symptoms of fussiness, diarrhea, vomiting, poor feeding, and blood and/or mucous in the stools as is commonly done in clinical practice. Our a priori FPIAP case inclusion criteria required documented blood in the stool. IgE-FA likelihood was ranked (“confirmed,” “probable,” “possible,” or “unlikely”) by 2 allergists who independently reviewed all clinical and sensitization data from children whose parents reported suspected reactions. IgE-FA cases were defined as those ranked by both reviewers as “confirmed” or “probable.” A strong clinical reaction history with evidence of sensitization was present in 53 (95%) of the 56 with IgE-FA (Supplemental Fig 2). Eczema was based on parental report on longitudinal surveys with physician chart review for missing data. Univariable and multivariable logistic regression with significance set at α < .05 were done by using R version 3.5.0.10 Only variables meeting significance were included in the multivariable regression. The Gastrointestinal Microbiome and Allergic Proctocolitis study was approved by the Massachusetts General Hospital Institutional Review Board.
Results
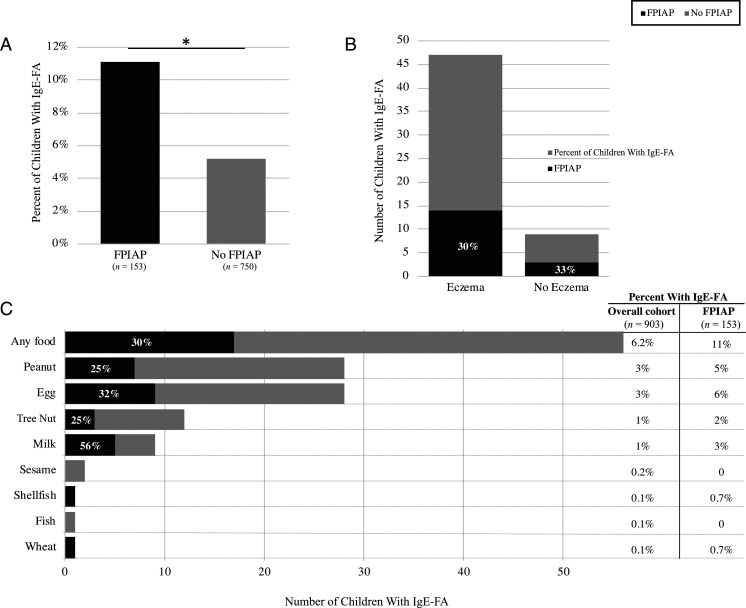
Of 903 infants analyzed (median age: 42 months; range: 24–59), 153 (17%) had FPIAP, and 56 (6%) had IgE-FA (28 peanut, 28 egg, 12 tree nut, and 9 milk) (Fig 1 A and C). As expected, children with IgE-FA were less likely girls (odds ratio [OR] = 0.5; 95% 95% confidence interval [CI]: 0.27–0.87]; P = .02) and more likely to have eczema (OR = 7.6; 95% CI = 3.7–15.7; P < .001) (Fig 1B). In total, 11% of children with FPIAP developed IgE-FA (compared with 5% without FPIAP) (Fig 1A). Adjusting for eczema, children with FPIAP had twice the odds of developing IgE-FA (OR = 1.9; 95% CI = 1.0–3.6; P = .04) (Table 1). Although our study was not powered to detect a relationship with individual foods, FPIAP was most strongly associated with milk IgE-FA (OR = 5.4; 95% CI = 1.4–20.8; P = .01) (Fig 1C, Table 1).
FIGURE 1.
A, Increased rate of IgE-FA in children with FPIAP. B, Children with IgE-FA were more likely to have eczema, but the increased rate of FPIAP was independent of that association. C, Increased rate of IgE-FA in children with FPIAP, most strongly associated with milk. Percentages within the black bars show the percent of children with IgE-FA to that food who also had FPIAP. * P < .05.
TABLE 1.
Prospective Association Between FPIAP and IgE-FA
| Unadjusted OR (95% CI) | P | Adjusteda OR (95% CI) | P | |
|---|---|---|---|---|
| Any IgE-FA (n = 56) | 2.3 (1.3–4.2) | .007 | 1.9 (1.0–3.6) | .04 |
| Milk (n = 9) | 6.3 (1.7–23.7) | .007 | 5.4 (1.4–20.8) | .01 |
| Egg (n = 28) | 2.4 (1.1–5.4) | .03 | 2.0 (0.9–4.5) | .11 |
| Peanut (n = 28) | 1.7 (0.7–4.0) | .25 | 1.4 (0.6–3.4) | .44 |
Adjusted for eczema by multivariable logistic regression.
Discussion
In this large healthy-infant cohort, children with FPIAP were at increased risk for IgE-FA. We hypothesize 2 non–mutually exclusive mechanisms to explain this important finding. The first is a shared immunopathogenic food allergic response in both FPIAP and IgE-FA that likely culminates in a specific cluster of differentiation 4 (CD4) effector T helper type 2 (Th2) cell response, predisposing children with FPIAP to also develop IgE-FA. Like eczema, FPIAP may therefore represent the first step of the atopic march for many children. The second hypothesized mechanism is that the antigen elimination diet used to treat FPIAP in the first year of life increases IgE-FA risk. The increased rate of milk IgE-FA that we observed in the FPIAP population supports this hypothesis, given that milk is the most common antigen restricted when FPIAP is diagnosed. Both proposed mechanisms, which are not mutually exclusive, can be evaluated by in-depth immune phenotyping together with intervention in future studies. Study limitations include single-center recruitment and lack of oral challenge IgE-FA confirmation in this observational setting.
Many children diagnosed with FPIAP clinically do not have allergic colitis on rectal biopsy9 or do not react with acute symptoms to a milk challenge.4 Treating FPIAP with empirical dietary elimination was previously thought to be a low-risk intervention, but it is suggested in our findings that this may have lasting consequences in the form of IgE-FA. Given the high rates of clinically diagnosed FPIAP, it may be an important target for allergy prevention. We hence encourage the use of the international interpretation of the Milk Allergy in Primary Care guidelines,5 which uses oral challenges to restrict prolonged milk elimination to only those infants with FPIAP who react to a postelimination challenge. We also recommend earlier attempts at milk reintroduction and consideration of early peanut and egg introduction for infants with FPIAP.2,11
Glossary
- CI
confidence interval
- FPIAP
food protein-induced allergic proctocolitis
- IgE-FA
immunoglobulin E–mediated food allergy
- OR
odds ratio
Footnotes
Drs Martin and Virkud conceptualized and designed the study, coordinated and supervised data collection, conducted statistical analyses, drafted the initial manuscript, and reviewed and revised the final manuscript; Drs Yuan and Shreffler conceptualized and designed the study, coordinated and supervised data collection, supervised data analysis, and critically reviewed the manuscript for important intellectual content; Dr Keet the conceptualized study design, supervised statistical analyses, and critically reviewed the manuscript for important intellectual content; Dr Phadke assisted in data generation and analysis drafting of the initial manuscript and critically reviewed the manuscript for important intellectual content; Drs Atkins and Su and Ms Seay assisted in data generation and analysis and critically reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Provided by the Gerber Foundation (1685-3680), the Demarest Lloyd Jr Foundation (230465), and the Food Allergy Science Initiative (229711). Dr Virkud was supported by a grant from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (K23AI130408).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Jones SM, Burks AW. Food allergy. N Engl J Med. 2017;377(12):1168–1176 [DOI] [PubMed] [Google Scholar]
- 2.lerodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316(11):1181–1192 [DOI] [PubMed] [Google Scholar]
- 3.Odze RD, Wershil BK, Leichtner AM, Antonioli DA. Allergic colitis in infants. J Pediatr. 1995;126(2):163–170 [DOI] [PubMed] [Google Scholar]
- 4.Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(s15):23–28 [DOI] [PubMed] [Google Scholar]
- 5.Venter C, Brown T, Meyer R, et al. Better recognition, diagnosis and management of non-IgE-mediated cow’s milk allergy in infancy: iMAP-an international interpretation of the MAP (Milk Allergy in Primary Care) guideline. [published correction appears in Clin Transl Allergy. 2018;4:4]. Clin Transl Allergy. 2017;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampson HA, Aceves S, Bock SA, et al. ; Joint Task Force on Practice Parameters; Practice Parameter Workgroup . Food allergy: a practice parameter update–2014. J Allergy Clin Immunol. 2014;134(5):1016.e43-1025.e43 [DOI] [PubMed] [Google Scholar]
- 7.Koletzko S, Niggemann B, Arato A, et al. ; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition . Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–229 [DOI] [PubMed] [Google Scholar]
- 8.Martin VM, Virkud YV, Seay H, et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract. 2020;8(5):1692.e1-1699.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005;41(1):16–22 [DOI] [PubMed] [Google Scholar]
- 10.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2018. Available at: https://www.R-project.org/. Accessed January 20, 2020
- 11.Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]