Abstract
Brain-computer interfaces (BCIs) have been explored in the field of neuroengineering to investigate how the brain can use these systems to control external devices. We review the principles and approaches we have taken to develop a sensorimotor rhythm EEG based brain-computer interface (BCI). The methods include developing BCI systems incorporating the control of physical devices to increase user engagement, improving BCI systems by inversely mapping scalp-recorded EEG signals to the cortical source domain, integrating BCI with noninvasive neuromodulation strategies to improve learning, and incorporating mind-body awareness training to enhance BCI learning and performance. The challenges and merits of these strategies are discussed, together with recent findings. Our work indicates that the sensorimotor-rhythm-based noninvasive BCI has the potential to provide communication and control capabilities as an alternative to physiological motor pathways.
Keywords: Brain-computer interface, BCI, EEG, sensorimotor rhythm, motor imagery, neural interface, brain-machine interface, BMI
I. Introduction
Using thought alone to communicate with others and interact with the environment around us has been a theme mentioned frequently in science fiction. Recent scientific findings and emerging technologies over the last two decades have begun to make these ideas a reality. In particular, advances in neuroscience and signal processing have enabled thought-control of external devices.
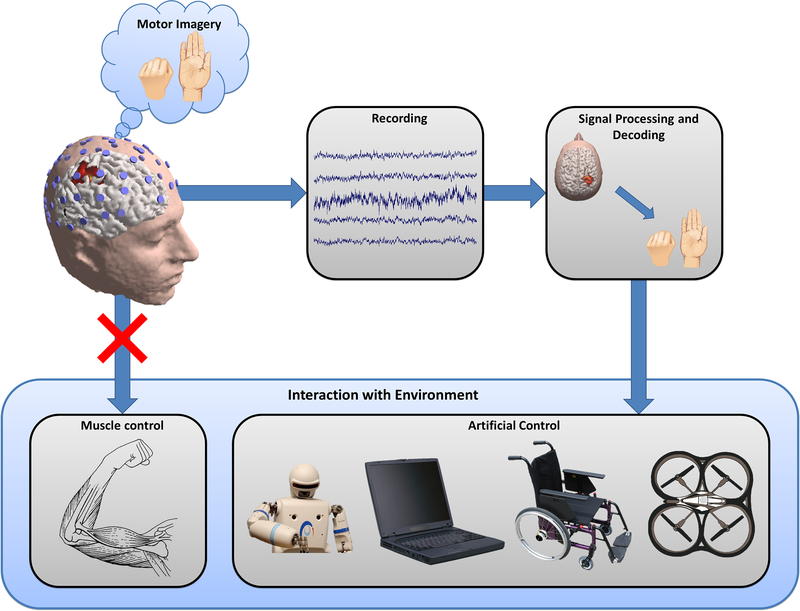
Brain-computer interfacing is an emerging technology that connects our natural brain with man-made devices, providing a new output channel for brain signals to communicate or control external devices without using the natural neuromuscular pathways [1–7]. A brain-computer interface (BCI) recognizes the intent of the user through electrophysiological or other signals from the brain, decodes the ongoing neural activity, and translates it into output commands that accomplish the user’s goal. BCI technology has the potential to restore lost or impaired functions of people severely disabled by various devastating neuromuscular disorders or spinal cord damage, and to enhance or supplement functions in healthy individuals. Figure 1 illustrates the general concept of a BCI system, based upon the principles of sensorimotor rhythms generated via motor imagery (MI) tasks, a framework that we will discuss in this paper.
Figure 1.
General concept diagram of a motor-imagery based brain-computer interface (BCI). A user imagines some motor action, but performs no actual physical movement. The imagery produces a measureable signal that can be recorded with EEG, filtered, and decoded to determine the user’s intent. Once an estimate of user intent is obtained, a variety of physical devices can be controlled as an artificial substitute or replacement for the user’s natural motor movement.
Various kinds of brain signals have been used as the basis for decoding user intent in BCI research. Direct neuronal recordings using implanted sensors have led to precise control and fast learning in animals, and recently also in a few severely paralyzed human subjects [5,8–11]. Similarly, noninvasive BCIs have been widely pursued with the hope of developing a BCI system that can decode and interpret users’ intentions without requiring invasive surgical procedures and implantation, such that the system can be used in daily life [12–25]. Electroencephalography (EEG) has been widely used for this purpose due to its noninvasiveness, ease of use, and low cost. EEG-based BCI signal types include stimuli-evoked potentials, slow cortical potentials, and sensorimotor rhythms (SMRs). Of these, while the steady-state visual evoked potential (SSVEP) based BCI can provide many commands [26], the SMR-based BCI offers a high level of control in terms of degrees of freedom as initiated by the intent of users [7]. SMRs are readily detectable in healthy [6,27] as well as disabled individuals with neuromuscular diseases or injuries, including spinal-cord injury, amyotrophic lateral sclerosis (ALS), and stroke (see [7] for review). SMR signals can be modulated through MI tasks, which have been shown to provide a robust paradigm for generating noninvasively detectable and usable EEG signals.
In this article, we describe an SMR-based BCI approach, focusing on our efforts with this paradigm at the University of Minnesota. Compared with other approaches, a unique feature of our approach is to leverage neuroscience knowledge to maximize the performance of BCIs. With respect to this, we have investigated BCIs for controlling an external device in virtual and physical spaces to engage human subjects in a highly interactive manner, developed the source-analysis-based BCI technique to improve the spatial resolution of scalp-recorded EEG signals, integrated transcranial direct current stimulation (tDCS) with MI to improve BCI performance, and pursued mind-body awareness training (MBAT) to improve the learning rate and applicability of BCI to a greater population of human subjects. This body of work reflects our approach of focusing on the brain aspect of the brain-computer interface, including user engagement, brain source imaging, brain stimulation, and mind-body awareness training. We will review research efforts in these four thrust areas, including our latest findings, and then discuss the challenges and future perspectives to establish a noninvasive BCI system for broad use in daily life.
II. BCI Control of Physical Devices
BCIs have the potential to provide two key benefits to disabled users: an alternate means of communication, and the ability to independently move around in and interact with their environment. Past BCI research has primarily focused on the communication aspect with spelling systems utilizing P300 responses [28–31], SSVEPs [32–35], and sensorimotor-rhythms (SMRs) [19,36,37]. However, there is great potential for BCI systems to provide a means of physical interaction that can restore critical aspects of autonomy for disabled users, and eventually to offer alternate output pathways for healthy users [6].
The bulk of BCI research has focused on simplified tasks confined to a two-dimensional (2-D) computer monitor, such as text entry or movement of a virtual cursor [18]. However, in recent years an increasing amount of effort has been devoted to using BCIs to interface with physical devices, real or virtual. This includes BCI control of virtual helicopters [25,38], physical quadcopters [15], wheelchairs [39–42], limb orthoses [43], and telepresence robots [44].
The common paradigm of a paced, cued virtual cursor task is suited for characterizing fundamental BCI characteristics such as classification accuracy and subject performance, but its practical utility in assisting users with activities of daily living is limited. BCI control of physical devices encourages translational research, advancing technologies that can be useful in real-life conditions. BCI experiments with physical devices are important for discovering practical issues that these systems will face in real-life environments [45], and can better inform how to design rigorous and useful virtual experiments in the future [15]. Additionally, BCI tasks involving physical device control have been observed to increase research subject motivation [25,38], which may influence BCI performance [46].
a. Control Paradigms
A variety of input signals, classification methods, and task paradigms are available for BCI systems. There are several key considerations when using a BCI to control a physical device.
1). Continuous control
The system must be able to respond quickly and smoothly to changes in both dynamic neural processing as well as the external environment. Discrete selection methods and synchronous (cued) trial structures may not be adequate for attaining fast response times in some applications. Some work has been done examining P300 and SSVEP-based humanoid robots [47], robotic arms [48], and wheelchairs [34,49]. However, the P300 response does not allow for continuous control, and both P300 and SSVEP require focusing of visual attention on a presented stimulus, which may distract a user from observing their surroundings. Distraction in an SSVEP paradigm could be minimized by incorporating flashing stimuli into a virtual environment while still allowing for continuous control [50,51], however this is not likely to be feasible in non-virtual settings. For these reasons, BCIs based on SMRs have been pursued for control of physical devices [6,15,40,52]. SMR-based BCIs allow for continuous, asynchronous control paradigms, although challenges still exist for the limited information transfer rates associated with these systems.
2). Multidimensionality
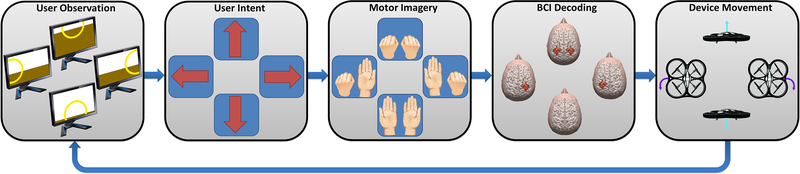
A single dimension of control, or a single degree of freedom, is typically not sufficient for complex real-time physical interaction with the environment. Therefore, most BCI systems controlling physical devices employ methods to interpret multiple simultaneous control signals from the user. For example, in [15] users performed MI of left or right hands individually to rotate left or right, imagery of both hands to move up, and rest to move down, as illustrated in Figure 2. Users could modulate these control signals independently and use them to simultaneously induce movement in multiple dimensions.
Figure 2.
Schematic diagram of an example control paradigm. As described in [15] a user controls a wireless quadcopter to fly through target hoops in three-dimensional space by imagining movement of left or right hands to turn left or right respectively, both hands to move up, or resting to move down.
3). Level of control
A system designer must carefully consider how outputs will be controlled by user input. At the lowest level, a user’s filtered SMR signal may control the position, velocity, or acceleration of the physical device. Alternatively, users could provide higher-level, abstract instructions and rely on an intelligent semi-autonomous system to manage the details of low-level control.
Absolute control of position may make sense for a manipulator such as a robotic hand, in which a user performs MI to open the hand to a desired position, and rests to return the hand to a closed position. However, with a noisy input signal, this control paradigm may produce abrupt movements, and could make it difficult to hold a device in a given position. Additionally, non-stationarities in the control signal [53] may make it difficult to consistently map a user’s intent to absolute positions or orientations over extended periods of time.
Because of these difficulties, velocity control is more common. With this paradigm, a user performs MI to change the position of an object, whereas the position is held when the user rests. This control method has been used widely in the traditional virtual cursor task [18], and is relatively well established. Studies show that SMR EEG signals can be used to decode users’ intention of velocity in a MI paradigm [54], which could help to make BCI velocity control more intuitive. However, velocity control can produce some unrealistic movement commands (e.g., sudden changes in speed) that are not physically feasible [15].
Acceleration control, or force control, can provide a more intuitive experience [15]. For these paradigms, a user performs MI to change an object’s velocity, resulting in relatively smooth changes in position over time. By coupling a user’s intent to applied force (or change in velocity), objects appear to move according to more natural physical principles. Typically some damping force is applied to counteract the user’s “applied” force, bringing the device to a halt gradually when the user rests.
A noninvasive SMR-BCI effectively bypasses much of the closed-loop neural circuitry responsible for smooth motor actions (e.g. proprioceptive sensory feedback, cerebellar circuitry, etc.), and instead solely bases movement off of a gross increase or decrease in energy of specific neural oscillations. As a result, the hardware and software of the BCI system must provide a significant amount of smoothing and other intelligent processing to convert user-generated signals into functional and safe actuator outputs. Some simpler safety measures are to limit maximum acceleration and maximum velocity. However, there are many more possibilities for safeguards with more advanced systems. For example, a quadcopter may perform automatic takeoff and landing [15], or a wheelchair may perform automatic obstacle avoidance [52].
As these systems become more complex, the concept of shared control arises. In these setups, the human user is no longer the only entity providing input. An “intelligent” automated controller is also employed to provide input or some cooperative assistance to help the user accomplish their desired task [38,55,56]. The level of assistance provided by this intelligent controller can vary: a novice, untrained user may require significant support, while an experienced user may be allowed more dexterous manual control of the system. At the highest level, a user may select a predetermined action (e.g., “go to kitchen”) and allow the system to carry out all the necessary steps to complete that action automatically (navigate through several rooms, open any doors, etc.). Because these higher level commands require only sporadic input from the user, they may reduce fatigue associated with long-term continuous control tasks [57]. Additionally, if a system can operate with infrequent discrete commands from the user, a smaller number of robust control signals can be utilized with a decision tree structure to select from many high-level commands [58].
4). Non-control state
During the practical use of a BCI system, the user needs to be able to focus on activities other than controlling the device. There are several ways to implement robust non-control states in which the BCI system does not respond to the user’s stray control signals. The most straightforward approach is to choose strong control signals that are easily discriminated from a rest condition, such as foot or hand MI [40]. If the rest state can be reliably recognized, it can facilitate self-paced operation of the BCI, in which the system can immediately respond to new input from the user while also tolerating periods of inactivity [59]. However, this method does not lend itself to rejecting user input for long periods of time due to noise in the control signal and minimal time needed to trigger an action. A more practical method is to implement some type of brain-controlled “switch” that activates or deactivates physical device control [60–62]. This switch does not need as fast of a response time as the primary BCI control method because it will be toggled infrequently. A longer response time allows a system designer to choose a much more robust signal which will have a low false positive rate [63]. For example, a pre-specified sequence of mental tasks (MI or others) could be used to enable and disable system control [64]. Alternatively, a hybrid BCI could employ other signal types, such as SSVEPs, P300 responses, heart rate [65], or even a different modality such as near-infrared spectroscopy (NIRS) [66], to enable and disable full device control.
b. Training
Unlike other paradigms such as P300 and SSVEP-based BCIs that require minimal training, SMR-based BCIs typically require much longer training periods. In order to attain high levels of performance, the user and the BCI system both need time to adapt to each other. The user is not expected to be able to achieve competent control immediately upon interacting with the device. Instead, a prescribed training process is often employed, both to introduce the user to the system and to provide calibration data for the system’s classifier(s).
When working with multidimensional BCIs, users may begin with independent single dimension control, then progress to more dimensions as they achieve competency [15,18,25,38,67]. This approach has several benefits. Single dimension tasks are generally perceived to be easier, which helps to maintain subject motivation during the early learning stages, whereas a multidimensional task may excessively frustrate new users. Initial training in a single dimension may also give the user clearer or more obvious feedback on individual control signals. In one training paradigm, subjects begin with simple one-dimensional (1-D) and 2-D cursor tasks, progress to a virtual task mimicking real physical control, and then progress to an actual physical control task [15].
In contrast, it is also possible to have new users begin immediately with full multidimensional control [68]. These systems typically rely on an initial calibration of a data-driven machine learning algorithm with minimal user training required. However, starting untrained users with a more difficult task with higher failure rates risks increasing frustration and limiting learning [69]. A variety of methods for optimizing training have been described in the literature [69]. User-centered training approaches may be employed in which control tasks and signal processing algorithms are highly customized to individual users [70]. Alternate forms of feedback can provide users with supplementary or redundant information to aid learning [71,72]. Motivation can be enhanced by biasing feedback [73] or adaptively adjusting task difficulty [74] to reduce user frustration. Novel or entertaining tasks can help to maintain user interest. In particular, game-like structures and virtual environments can have a significant impact on user learning and performance [63,75–78].
Control of devices within a virtual reality (VR) environment plays an important part in training users to control physical devices. A virtual environment is not subject to the physical, mechanical, or economic constraints of real devices; this freedom allows simulation of a greater range of experimental protocols, and facilitates the exploration of new types of hardware without added expense or design work. Importantly, a virtual environment does not risk compromising the safety of a subject controlling a prosthetic or wheelchair system [40,79]. Virtual experiments can be much more rigorously controlled, allowing for repeated trials with highly consistent conditions in a virtual environment. Additionally, VR facilitates measurements of user and system performance. For instance, a simulated quadcopter’s position, orientation, and velocity can be easily measured in a virtual environment, whereas complex specialized hardware would be required to make similar measurements in reality. Despite these advantages, VR experiments cannot fully capture all of the practical issues that will be encountered in real-world use. For example, a wheelchair simulation cannot provide the user any proprioceptive sensation of acceleration in a way that mimics actual wheelchair movement.
c. Measuring Performance
Robust performance metrics are critical in assessing the performance of online BCI systems. Task-specific metrics can be used for quantitative comparisons within subjects over time and between subjects within a study. More general metrics, often founded in information theory, can facilitate comparison of performances between studies with slightly different conditions or even drastically different task structures.
Simple task-specific metrics can provide some practical information on user performance. Metrics such as percent valid correct (PVC) and percent total correct (PTC) are used in BCI tasks with fixed targets. PVC compares the number of correct target hits to total number of target hits (correct or incorrect). PTC compares the number of correct target hits to the total number of trials, including timeouts or aborts where no target was hit. While these metrics are easy to compute and provide intuitive measures of user performance, they do not provide meaningful quantitative comparisons between different tasks.
Many conventional BCI performance metrics, such as information transfer rate (ITR) [80,81], have been established in the context of very controlled, simple fixed choice tasks such as 1-D cursor control. With physical device control and other complex BCI paradigms, these metrics are often no longer suitable for characterizing system performance [74,82,83]. Targets may not have equal probabilities of being hit, trial length may not be highly controlled, or there may not even be any distinct trials built in to the task paradigm.
Another common performance metric, particularly suited for cursor movement or similar tasks, is the index of difficulty (ID) based on Fitts’ law, initially developed to characterize human motor movement [84]
| (1) |
Where D is net movement distance from starting position to the target, and W is the width of the target [85]. This can be converted to an approximation of ITR simply by dividing ID by the time taken to reach the target [15]. However, as described in detail in [74], there are several limitations to this metric, particularly with regard to its inability to facilitate quantitative comparisons between different tasks.
One common method for characterizing complex BCI system performance is to compare user BCI control to random chance control and “ideal” (manual) control conditions [15,25,38,52,74,86]. The random control condition provides a baseline measure of worst-case performance, in which a subject has no influence on the experiment outcome. The manual control condition, often implemented as keyboard or joystick control, provides a measure of the best-case performance. It is also possible to perform an additional reference measurement to characterize how much a given signal processing pipeline degrades system performance, using manual control to generate a pseudo-BCI signal [74].
In general, it is difficult to apply generalizable performance metrics to a given task without making assumptions about various statistical parameters of that task (e.g. that all targets are presented with equal probabilities). Because of this, it is important to choose a suitable performance metric early and incorporate its constraints into the experimental task design.
d. Future Directions
BCIs have great potential for providing assistive solutions to disabled individuals, and eventually also providing general-purpose interfaces for healthy users. However, there remains a significant amount of work to be done before BCI physical device control systems will be practical for widespread real-world use.
Higher-dimensional control is critical for complex physical interaction with the environment. One possible way to increase dimensionality is to decode additional MI tasks, such as using an inverse-solution-based BCI to discriminate between imagery of different hand gestures [87]. This high-resolution decoding could potentially allow for more naturalistic MI tasks, such as using finger MI for dexterous control of the fingers of a prosthetic hand. Conceivably, naturalistic MI would be more intuitive for users and thus require less training time.
If these systems are going to be used in the real world as assistive devices, they will need to be much more than just single-purpose systems. A multifunctional BCI system would provide integrated control of multiple devices (e.g. wheelchair and robotic arm) and allow for dynamic switching between output tasks (e.g. slower fine movement control for grasping a utensil, and faster gross control for opening a door).
Future advances in robotics, including autonomous navigation and environmental interaction, will drive a transition towards control of more intelligent systems with higher-level user input. Users may not need to dexterously control individual joints in a robotic hand, but instead robustly communicate their intent to grasp a specific object and allow an intelligent BCI-controlled system to perform the task.
Finally, a major future aim is to improve the quality of the MI signal generated by users. There are many possible methods for enhancing a user’s control capability. We will discuss several approaches in the following sections.
III. Source-Analysis-based BCI
EEG is well known to have excellent temporal resolution in tracking neural dynamics across the scalp, but suffers from the drawback of limited spatial resolution. The low spatial resolution of EEG is attributed to the smearing of electrical signals as they travel from the brain, through the skull and meningeal layers, to the scalp. This phenomenon, referred to as the volume conduction effect, results in the detection of mixed signals at individual electrodes on the scalp [88]. Historically, a number of efforts have been made to correct for the volume conduction effect of EEG by solving the so called “inverse problem” which projects the scalp EEG back into the source domain, over the brain surface or within the brain [88,89]. In such source analysis, the relationship between neural sources and the scalp EEG is established by a forward model which leads to a transfer matrix that maps brain electrical activity to the scalp EEG [90,91]. Neural activation, in terms of membrane excitation of neurons, results in transmembrane currents, which generate current flow within the brain. The resulting electrical potential, as sensed by electrodes, is the observed scalp EEG. The activity of a single neuron, or the random firing patterns of multiple neurons, is too weak to be detectable at the scalp. Rather, only synchronized neural networks involving a large number of neurons generate strong enough signals to be detected by the EEG electrodes. Since brain function is encoded by networks involving a number of neurons firing in a synchronized manner, EEG provides a noninvasive manifestation of the underlying synchronized neural networks’ activities, thus revealing brain function or dysfunctions. The superposition of neurons firing simultaneously and synchronously amplifies the signal produced by a local population and can be modeled by a single dipole on the macroscopic scale. The scalp EEG can then be considered as the result of a distribution of equivalent dipoles located within the head volume conductor.
The forward solution determines the contribution of each dipole within the brain model to each electrode located on the scalp. The forward conduction from equivalent current dipoles to the scalp potential has been modeled using spherical models, realistic geometry boundary element models [90,91], and finite element models [92,93]. The forward conduction from the dipole distribution to the scalp electrodes can be represented by a transfer matrix, A. The system equation of EEG generation can then be written as
| (2) |
where x is the source activity, b the EEG measurements, and n the measurement noise.
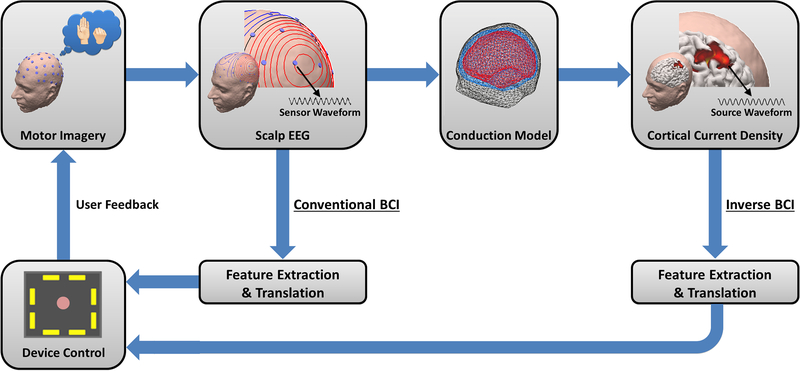
The source analysis entails solving (2) seeking x, given EEG measurement b, and anatomical information A, which can usually be obtained from subjects’ structural MR images. The concept of source-analysis-based BCI is illustrated in Figure 3, in comparison with the traditional sensor-based BCI approach. Since source signals more closely reflect neural activation, we hypothesized that the use of source analysis will improve the performance of BCI and have demonstrated its merits in classifying MI tasks [94–96].
Figure 3.
Concept of EEG source imaging based BCI. Source signals can be estimated from scalp EEG measurements in conjunction with the head conduction model and used to control a computer cursor.
a. Source Analysis and Brain-Computer Interfaces
Sensor-based SMR BCIs take advantage of the event-related phenomena observed at specific electrodes located along the motor cortex. Large numbers of neurons in the motor cortex maintain an idling firing rate in the alpha/mu band (8–13 Hz) and synchronize within focal regions based on the type of task being performed. Upon executing or imagining movement, the cortical processing of neurons encoded for different movements disrupts that idle state and results in a desynchronization of certain local populations. These phenomena are termed event-related synchronization (ERS) and event-related desynchronization (ERD) respectively [27] and are visualized in Figure 4. Because of the brain’s contralateral motor control, when an individual executes a motor movement or motor imagination, ERD is usually observed in the contralateral hemisphere, whereas ERS is often observed in the ipsilateral hemisphere and/or along the brain’s midline. SMR BCIs exploit this neurophysiological phenomenon by detecting different spatio-temporal patterns of increased or decreased activity to determine which motor task a user is performing; however, traditional BCIs rely only on those patterns recognized from signals collected from a limited number of electrodes on the scalp.
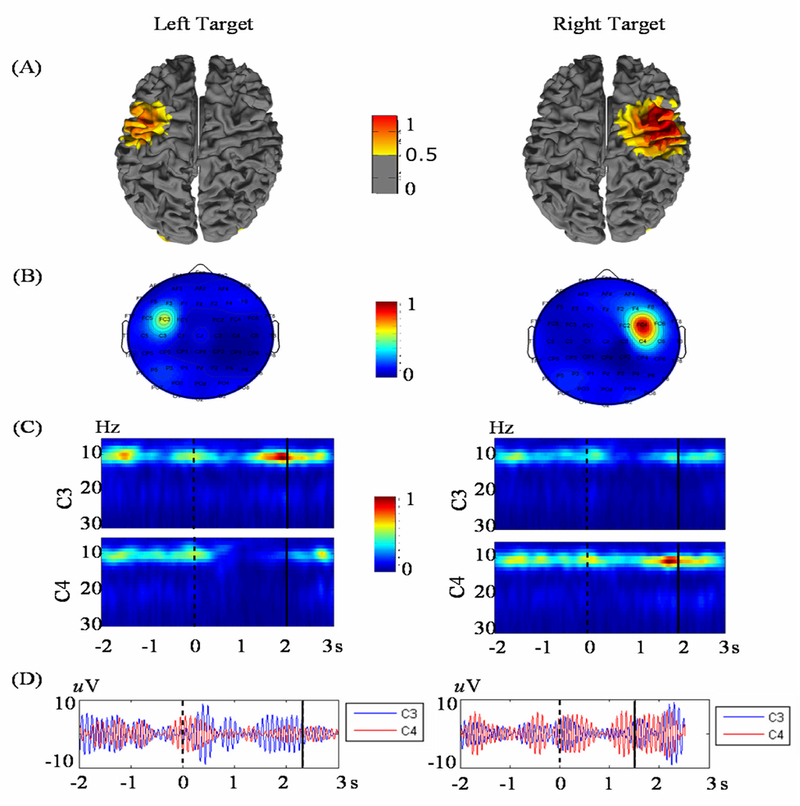
Figure 4.
Source imaging of right and left hand MI tasks in source space (a) and sensor space (b). (c) Time-frequency representation of the C3 and C4 electrode waveforms capture the ERD and ERS phenomena occurring during these two tasks. Localization of this event-related activity to the motor cortex indicates that neural processes responsible for the BCI control signal originate in the sensorimotor cortex [96].
EEG source analysis, on the other hand, has revealed additional information regarding the source generators of these fundamental control signals. Yuan et al. [96] applied linear inverse methods to pre-recorded 1-D BCI data to show not only that the ERD/ERS in response to right and left hand MI tasks have unique anatomical traces within the sensorimotor cortex (Figure 4a), but also that the event-related activity from the source domain was better correlated with the BCI task than that from the scalp electrodes.
Qin et al. [94] first reported the connection between MI tasks and equivalent dipole modeling by separating, in an offline setting, left and right MI tasks on a trial-by-trial basis. Equivalent dipole modeling has been shown to localize neuronal sources with high accuracy [90,97,98]. In this approach, multiple signal processing techniques, in addition to data-driven signal separation algorithms, including independent component analysis (ICA) [99,100], were used to isolate the signal generated in response to MI. Components accounting for high levels of variance within the EEG envelope are thought to represent dynamic on-off processes relative to a specific task, which in this case is the activation or deactivation of cortical regions responsible for performing MI. By fitting a single equivalent dipole to selected component maps at time points of maximal activation, a simple classification scheme was derived. Each trial was classified as a right hand MI trial if the dipole localized to the right hemisphere and was classified as a left hand MI task if the dipole localized to the left hemisphere. This work was soon after explored with a two equivalent dipole analysis in a similar fashion; however, both dipoles were required to localize to one hemisphere in order to classify the trial as either left or right hand MI [95]. In both of these studies, classification rates of >80% were achieved, making these source analysis techniques highly competitive with sensor-based methods.
Despite the promising results using equivalent dipole analysis for classifying MI tasks, such parametric methods need to solve a nonlinear inverse problem and thus may not be suitable for real time implementation of online BCI. Non-parametric solutions on the other hand not only provide more distributed activation patterns, but also have the capability of being applied in real time using simple matrix algebra [101].
Non-parametric inverse solutions can offer additional information over parametric methods in the sense that these techniques estimate the current density over the entire cortex. These methods involve minimizing the norm of the residual between the model-predicted and recorded scalp EEG distributions, as shown by the first term in (3). For this discussion, only the L2 norm will be discussed although in principle other norms may be applied as well.
Non-parametric solutions involve many more equivalent dipoles than the scalp electrodes, and are thus ill-posed inverse solutions which require regularization [88,102]. In the conventional minimum norm solution, in addition to minimizing the residual (first term of (3)), constraints are applied to minimize the norm of the solution (second term of (3)) in a minimal energy sense. To balance the influence of these two terms, a regularization parameter, λ, is introduced to control the impact of each term on the final solution. The optimal value of λ can be found using various techniques including the generalized cross-validation and L-curve methods [103,104].
| (3) |
When the penalizing term in (3) is quadratic, this formulation is known as Tikhonov regularization and allows (3) to be solved analytically in the form of (4), where I is the identity matrix [105].
| (4) |
This solution, termed the minimum norm estimate (MNE), can be further generalized into forms where a priori knowledge of source and sensor correlation can be integrated into the solution [88].
The MNE and its variations have become much more popular than parametric inverse solutions in the BCI community in order to quickly localize and image brain activation patterns that are responsible for controlling these systems. For many of these studies, the inverse solution was used to map the scalp data onto a higher dimensional cortical space so that the activity of specific dipoles, rather than signals recorded by scalp electrodes, could be examined for MI task classification. Figure 4 illustrates the general concept of an EEG inverse BCI, the framework of which can be utilized in both online and offline applications. Common statistical measures, including information theoretic metrics, were used to identify regions of interest (ROIs) on the cortex that contained dipoles which best separated MI tasks [106,107]. Single-trial signal dynamics from the pre-selected ROIs were then broken down into time-frequency features, in a similar fashion to [108] and fed into a linear classifier. A simulation study with similar ROI selection methods compared the classification between source and sensor space using both time-frequency and phase-locking features from multiple ROIs [109]. In this study, sensor clusters on the scalp were selected to resemble those ROIs on the cortex; with the same features taken from both the electrode groups and cortex ROIs, reconstructed source activity yielded binary classification results up to 12% higher than classification from scalp activity. In particular, this study found that the phase-locking value between different cortical ROIs produced better accuracies than time-frequency features. This result indicates that information from multiple brain regions can significantly improve the identification of different brain states, and that this information can be more precisely extracted in the source domain than in the sensor domain.
It should be noted that various other spatial filtering techniques have been implemented for the classification of MI tasks with comparable results to EEG inverse solutions. Of these, the common spatial pattern (CSP) algorithm is widely accepted as a technique known to yield successful separation of different MI tasks [110–112]. Despite the fact that EEG inverse solutions and CSP are both used to generate spatial filters that are then applied to the scalp recorded data, the two techniques have key differences. Where the EEG inverse solutions derive the filter based on the geometric constraints introduced by the head’s anatomy to remedy the distortions introduced by the physical process of volume conduction, CSP is a data-driven approach that produces sensor weights based on statistical measures that best separate the different MI tasks. Even though some studies have implemented CSP-based classification with more than two tasks [68,113], this technique is difficult to implement for a multi-class MI-based BCI and thus limits the dimensionality of sensor-based BCIs. Furthermore, since the CSP and inverse solutions are based upon different principles, these two methods can be used in combination – that is, CSP can be applied in the source domain.
A study conducted by Congedo et al. [114] attempted to combine these two methods for a two-class MI paradigm by projecting the scalp potentials onto a template brain and applying CSP in different frequency bands. This study found that the combination of these two methods yielded results similar to those previously described and greater than that of the tradition sensor-only paradigms. Of greater importance though is the fact that this study further supports the idea that by working in the source domain, more spatially specific information can be extracted to better determine a user’s motor intent. Additionally, the improvement of distributed non-parametric solutions over parametric methods verifies the idea that current density source imaging provides increased information related to the distinct cortical patterns generated by different MI tasks and is more suited for possible online paradigms.
b. Online Applications and Future Directions
The previous discussion has focused solely on offline classification of different MI tasks; however, most of these studies include information from limited time points to test their methods. Nevertheless, the activity generated in response to MI tasks is a dynamic process which fluctuates over time. Therefore, even though BCIs utilize MI tasks, a subject must be able to sustain focus in order to generate distinct patterns and control an output device. Studies [115] and [116] were conducted to determine if inverse solutions could be used in an online paradigm to gain better control of a BCI than when using sensor data from the scalp. In these studies, a linear inverse solution was integrated into the BCI2000 [117] platform’s filtering module with ROIs selected from those regions dedicated to control of different body parts [118]. Waveforms from multiple dipoles within these ROIs were then used to control the BCI rather than the waveforms from selected electrodes. These studies found that control signals arising from the EEG inverse solution were better correlated with the motor intent of the subjects and therefore provided improved control for the user. This finding was corroborated by the offline analysis in [96] comparing the source and sensor signal correlations with the BCI task, and the comparative study of EEG source imaging and BOLD functional MRI for movement and MI tasks [119].
With the recent development of additional real-time source imaging platforms, various online paradigms have utilized linear inverse methods to investigate the ability of subjects to modulate signals generated in specific regions of the brain for training of BCI control [120–122]. The aforementioned offline analysis of cursor control indicates that the underlying control signal for these systems is directly based on the event-related activity generated in the sensorimotor cortex during different MI tasks. Therefore, the strength of the control signal for these systems depends on the ability of a user to voluntarily modulate activity within specific populations of neurons. By estimating cortical activity during these tasks, the information subspace expands from tens of electrodes to thousands of dipoles, which better represent the geometry of the brain and its neural processes. By incorporating this anatomical information, the inverse solution provides a means to better constrain the information used for interpretation of motor intent. When in the form of ROIs, these constraints can provide subjects direct neurofeedback regarding their performance of a MI task and can strengthen the control signal needed for BCI control [123,124]. Using source-level neurofeedback, it can be seen that the neural response to MI tasks converges to focal regions on the cortex, indicating that sensor space may be a limiting factor in BCI control [125]. In the larger information subspace of the inverse solution these regions can be better isolated for interpretation, whereas the signals collected in sensor space contain mixed information from expanded cortical regions, incorporating noise or activity that is irrelevant to the task being performed.
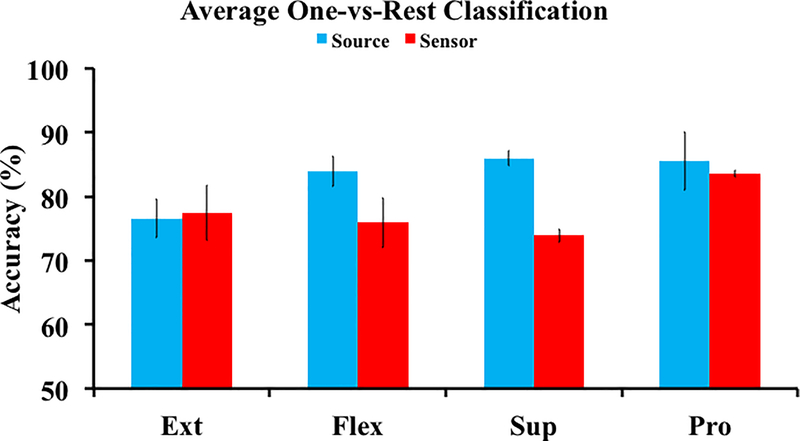
We have recently begun to extend the source level ideology, which we have proposed since 2004 [94], by investigating the unique dynamics, on the cortical level, of more downstream MI tasks of the hand [87]. We have investigated the discriminability of MI tasks of right hand flexion, extension, supination, and pronation in three human subjects. After an inverse transform, an ROI located over the left motor cortex was selected as the estimated right hand dedicated cortical region similar to [116]. Using time-frequency features from dipoles within this ROI, we aimed to determine how distinct the traces of each MI task were by performing four one-vs-all classifications. The most discriminable features in source and sensor space were separated using a linear classifier. The source analysis yielded >85% classification accuracy for two of the four tasks, whereas the sensor analysis was not able to achieve >85% for any of the tasks (Figure 5). Furthermore, the source analysis improved the classification accuracy by up to 15% over the sensor analysis across the four tasks. When considering a chance level of 50%, this advancement represents a noteworthy upgrade in the ability to independently decode the different MI tasks. These results further suggest that a source level feature space provides more discriminable information, in terms of motor intent, than the sensor domain and warrants further investigation.
Figure 5.
Average one-vs-all classification results from three subjects comparing the source (ROI) and sensor data for the different MI tasks (Ext – Extension, Flex – Flexion, Sup – Supination, Pro – Pronation).
IV. tDCS and SMR-based BCI
The use of inverse source imaging allows us to spatially and temporally locate the regions on the cortex involved in MI-based BCI and with this information we can turn our attention towards the use of emerging neuromodulation technologies to alter the activity at these sites. Such noninvasive neuromodulation technologies are being increasingly investigated for targeting learning and behavior [126]. Of particular interest are reports that applying anodal transcranial direct current stimulation (tDCS) during motor tasks results in improved learning and performance [127]. As MI and motor execution proceed from similar neural correlates [119,128], it may be possible to combine tDCS with BCI to improve BCI learning and performance by directly modulating the brain.
a. tDCS, Motor Learning, and Motor Imagery
tDCS, a noninvasive neuromodulation technology, was first investigated in its modern form less than two decades ago [129,130]. tDCS consists of a current source connected to the scalp via electrodes, through which a low level of current is applied and passed into the brain [131]. The amplitude of tDCS stimulation is generally set between 0.5–2 mA for between 5–30 minutes; this current is gradually increased over 5–30 seconds to a constant current which is held for the duration of stimulation, and then ramped down at the end of stimulation. tDCS does not result in direct neuronal firing but rather modulates the membrane potentials of affected neurons to increase or decrease excitability. tDCS suffers from the fact that the skull is much less conductive than the skin and cerebrospinal fluid, which reduces the focality of stimulation. However, it is inexpensive, mobile, easy to apply, and has been suggested to be functionally targeted [132] due to its effect on neuronal excitability and influence on ongoing task-specific neural activity.
Traditional tDCS consists of two electrodes: an anode, which generally increases the excitability of underlying cortical tissue, and a cathode, which generally decreases the excitability of underlying cortical tissue. In high-definition systems, multiple electrodes are used in combination as anodes or cathodes to allow for controlled current during the stimulation. Initial evaluation of tDCS effects were performed by delivering anodal stimulation over the primary motor cortex and using transcranial magnetic stimulation (TMS) to induce a motor evoked potential (MEP), the activation of a peripheral motor neuron by stimulation of descending motor neurons in the brain. Following anodal tDCS over the motor cortex, the MEP was increased, suggesting greater cortical excitability following the stimulation, while cathodal stimulation decreased MEP amplitude [133]. This modulation has been found to last up to an hour following stimulation and improvements in task learning can remain up to 3 months post stimulation [134].
The mechanism of action of tDCS lies in altering the membrane potential across all areas of affected neurons, including dendrites, cell bodies, and axons. The distribution, connectivity, and geometry of these neuronal elements have been characterized and the effects of tDCS have been investigated in vitro [135,136], but the effects within the human cortex are more difficult to determine due to the lack of in vivo imaging of human tissue. Based on in vitro and computational modeling studies, tDCS either depolarizes or hyperpolarizes the membrane of neurons, but the current flow and voltage changes induced by tDCS are complex due to head anatomy and tissue geometry, including cortical sulci and gyri and the neurons’ orientation within these macrostructures [137,138]. The resulting effects on membrane potentials can thus be different in sign and magnitude in a spatially localized area. Additionally, different cortical layers can receive different polarity stimulation simultaneously based on cortical and neural geometry and thus the actual behavioral results are difficult to predict [139,140].
tDCS has been used in humans to safely modulate neural tissues for over a decade [129,130,141]. There have been a plethora of proposed targets for stimulation from learning, in realms such as mathematics, and mental health conditions such as schizophrenia, depression, attention deficit hyperactivity disorder, and obsessive compulsive disorder [131]. Of specific interest to the BCI field is the work on motor learning to evaluate the behavioral effects of tDCS both acutely and with respect to motor learning over time. Anodal stimulation over the motor cortex has resulted in a faster learning rate for implicit [142] and explicit [143] motor learning as well as retention of the learned paradigm [134,144]. With cathodal stimulation, Nitsche and colleagues and Stagg and colleagues also found the opposite or no effect in using the same motor learning paradigms. These studies, and others [139,145], suggest that anodal tDCS can improve behavioral motor learning across implicit and explicit motor tasks. The timing of tDCS application in relation to task learning is of utmost importance. Pharmacological and experimental evidence suggest tDCS application during, as compared to prior to or after, learning of a new motor task results in a faster learning rate and an increased performance for up to six months post stimulation when compared to controls [145].
The networks underlying MI overlap with those that underlie motor execution [128], particularly within the premotor and motor areas, but the effect of tDCS on MI ability is not clear. Increased MI desynchronization has been found in both healthy [146] and stroke [147] subjects. With anodal stimulation of the primary motor cortex, both studies found an increase in the ERD of the stimulated hemisphere, suggesting that there is an increase in excitability during MI following tDCS. More recently, Lapenta and colleagues [148] investigated MI by following tDCS stimulation of 2 mA for 20 minutes with MI and motor observation and found an effect opposite of the initial MI studies: that anodal stimulation decreased the ERD in the same hemisphere as stimulation for both MI and during motor observation. A recent study combining 64-channel EEG recording of MI before and after high-definition anodal tDCS found similar results: a decrease in beta band ERD in the stimulated hemisphere [149]. With these differing results being reported, further work needs to be done to clarify how tDCS affects MI ability acutely following stimulation.
We hypothesized that simultaneous anodal tDCS over the primary motor cortex can improve motor learning and result in an enhanced outcome of MI-based BCIs. Despite a number of efforts, SMR-based noninvasive BCIs face challenges including long training time and the inability of 20% of healthy subjects to learn to self-modulate SMR-based BCIs [150,151]. As motor learning can be improved with specific tDCS paradigms, the integration of tDCS and SMR-based BCIs promises to improve BCI learning through similar MI-based pathways.
b. tDCS and Online BCI
A key factor in the combination of tDCS with learning is the timing of the stimulation with respect to the performance of the task [145]. An initial study of anodal tDCS followed by BCI has reported to increase ERD over the stimulated motor cortex during BCI performance following 15 minutes of 1 mA sponge electrode stimulation, but this did not result in an increase in performance within a single session [152]. With this setup, the sponge electrodes interfere with the EEG recording electrodes due to their relatively large size, and online BCI could not be performed simultaneously with stimulation. More recently, Soekadar et al. utilized trained SMR-BCI subjects to investigate acute BCI performance under stimulation conditions [153]. All subjects initially underwent sham stimulation, and on a second day, half of the subjects underwent sham and the other half underwent anodal stimulation while performing SMR-BCI acutely. There was no change in performance for those who received anodal stimulation compared to the sham stimulated group. This work parallels the mixed results that have been found in the application of tDCS followed by MI, but does not address the ways in which tDCS could affect learning over time.
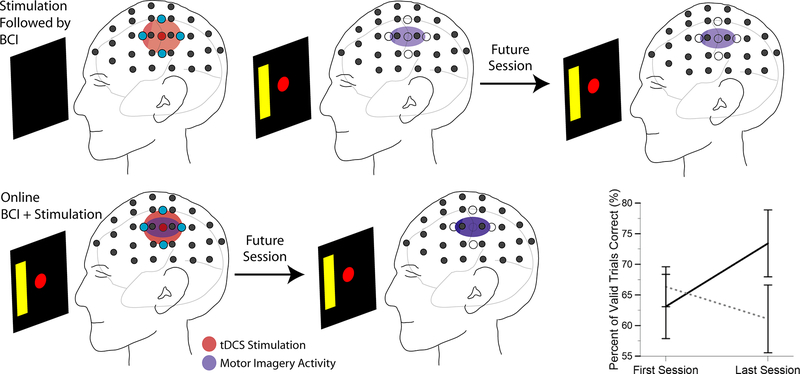
Recently, we have begun to evaluate changes in neural activity and performance during BCI learning induced by simultaneous BCI-tDCS in naïve subjects. As previous motor learning work suggests, simultaneous stimulation and task performance optimizes the effect on task learning (Figure 6). We utilized high-definition tDCS combined with 64-channel EEG to evaluate the effect of simultaneous anodal stimulation on BCI learning. Naïve subjects were recruited and randomly assigned to either sham or anodal stimulation for 20 minutes at 2 mA. Stimulation was delivered equidistant between C3 and CP3 with return electrodes located at a radius of 3.5 cm from the center electrode. Subjects performed 1-D left/right MI to control the BCI within the BCI2000 environment. Each session was divided into four blocks: before stimulation (72 trials), during stimulation (90–108 trials), immediate post-stimulation (72 trials), and delayed post-stimulation (72 trials). The immediate post-stimulation occurred from 0–12 minutes post-stimulation and the delayed post-stimulation occurred from 25–37 minutes post-stimulation, with a visual oddball task between the immediate and delayed post-stimulation blocks. Power in the 11–13 Hz range at C3/C4 was used to control the cursor, when possible. During anodal stimulation, this was not possible on all experimental days; therefore one of the 9 surrounding electrodes was used instead of C3 to minimize the stimulation artifact. We evaluated the percent valid correct for each group of subjects at the beginning of the experiment (start) compared to the result after stimulation during the third experimental session (end). With our initial group of subjects, anodal subjects (n=7) demonstrated a trend for increased performance with an increase in performance from 63% to 73% resulting in an effect size (Hedges’ g) of 0.63. The sham subjects (n=6) showed no change in performance. These pilot results suggest that simultaneous tDCS and BCI leads to an increased learning rate over 3 sessions of 1-D left/right BCI training (Figure 6). These initial results show promise towards utilizing tDCS combined with online BCI to improve subject learning of the MI task and to improve the learning rate for this BCI setup, though more subjects are needed to better understand the learning changes resulting from tDCS application. In addition, future work will target subjects showing BCI illiteracy to evaluate if increasing the excitability of the motor cortex with tDCS during BCI performance will allow subjects who could not previously learn to control their SMR, to begin to control them.
Figure 6.
Overview of conventional approach of stimulation followed by performance with online approach of simultaneous stimulation and brain-computer interface learning. (bottom right) Subject performance change between beginning of first session and end of last session (Session 3) for sham (n=6) (dotted line) and anodal stimulation (n=7) (solid line) subjects. Error bars represent standard error.
c. Issues and approaches to combining tDCS and EEG
Motor evoked potentials have been used to initially evaluate the effect of tDCS, and many groups have utilized behavioral measures to examine the effect as well, but simultaneous electrophysiological recordings in humans is under-investigated due to issues with signal artifacts. Recently, the electrophysiological network effects of tDCS have begun to be evaluated using both simultaneous EEG and MEG. These methods can record rapid oscillations and neural activity altered by tDCS on the millisecond scale, where these changes underlie reported behavioral changes for a variety of tasks [126].
With recently developed high-definition tDCS systems [154], electrodes are the same size as conventional EEG electrodes and can be placed on a conventional electrode cap adjacent to the EEG electrodes, which allows for online recording of the EEG during stimulation [149,154]. In addition, these allow a more localized stimulation area, reducing current flow to a limited area and reducing potential side effects to non-targeted areas. As anodal and cathodal stimulation are predicted to affect neural tissue in opposite ways and can improve or decrease performance in directed tasks [155], it is important to understand and limit current flow to areas of interest. Using noninvasive electrophysiological recordings during stimulation is a promising way of further understanding the effects of tDCS stimulation on brain networks, as without these online recordings, we cannot understand the underlying physiological changes that result in the vast number of behavioral changes reported in the literature [126].
Multiple investigators have now examined neural activity simultaneously with stimulation including high-definition tDCS with EEG [149] and conventional tDCS with MEG [156]. Both have examined stimulation activity with phantom models and healthy human subjects. Roy et al. [149] identified tDCS artifacts utilizing phantom experimental data and cleaned simultaneous data utilizing maps of independent components. Soekadar et al. [156] found wideband noise in MEG sensors up to 8 cm from the stimulation electrodes, though at a greater distance the noise was primarily below 20 Hz and, in more recent work using EEG, found most of the induced noise to be below 8 Hz [156]. Soekadar et al. [153] also found no difference in frequency spectra with or without stimulation in the source space for task or baseline data and no frequency shift in the recorded frequency in the phantom due to stimulation.
As the tDCS system is maintained at a constant current, any changes in impedance result in a voltage change that can interfere with the EEG signals recorded by electrodes adjacent to the stimulation electrodes. In order to examine the recorded signal from the brain, these artifacts must be removed. Multiple techniques have been used and suggested from independent component analysis [149] to frequency-based filtering or high-pass filtering [153]. This also yields problems online; while low pass filtering can be implemented in a straightforward manner, there are residual power increases that can affect the ability of the subject to utilize an affected electrode for BCI control. Future progress for developing inter-compatible tDCS and EEG devices should aim at reducing or accounting for the noise induced by the tDCS system.
The increasing investigation and integration of noninvasive neuromodulation and brain-computer interfaces is beginning to yield new tools for the delivery of modulation based on brain states. This concept has recently been introduced in the field of invasive deep-brain stimulation in order to improve the delivery of therapeutic electrical stimulation to deep brain structures [157]. In all BCI techniques we are measuring neural activity, be it invasive or noninvasive, direct or indirect, and we can exploit this information to improve our targeting of neuromodulation and the BCI tasks. As the technical difficulties of EEG recording during the delivery of electrical stimulation are addressed with online filtering and artifact removal strategies, we can begin to develop systems to deliver stimulation at an optimal time and amplitude to improve the targeted outcome in the subject or patient. This closed-loop control will also allow us to better understand the ability of stimulation technologies to modulate brain activity as well as to investigate cognitive and behavioral psychology and neuroscience.
V. Mind-Body Awareness and BCI
In SMR-based BCI, a key factor is the subject’s ability to intentionally modulate the SMR signal. SMR-based BCI performance relies on a user’s concentration level and ability to focus on modulating a SMR that can be detected and translated into features controlling an external device. Literature suggests that there are a portion of subjects who have difficulty modulating their SMR signal to achieve even a minimal level of control with a noninvasive BCI [150,151]. In order to move noninvasive BCIs towards clinical application or daily use by the general population, there is a need to shorten the lengthy training time that is required by users to achieve satisfactory performance, and increase the proportion of BCI users that ever achieve acceptable BCI control, even after training [158,159].
Considerable progress has been made in algorithm, sensor, and system development within the field of BCI. While researchers are dedicating substantial effort into the machine side of BCI utility, little effort has been focused on enhancing the users’ control abilities. As such, there exists a need for user-centered training techniques that focus on the refinement of the mental practices to improve the signal produced by the user. User-centered BCI training approaches have been attempted recently [160,161] focusing on the brain component of BCIs by emphasizing early focus on the users, tasks, and environment with the goal of improving the performance of BCIs. The investigation of both the “brain” and “computer” aspects will be important to further improve BCI performance and contribute to its translation for wide applications, considering that both aspects are necessary for BCI control.
a. Mind-Body Awareness Training
Mind-body awareness training (MBAT), in the form of yoga and meditation, has recently garnered interest due to an increasing awareness of the potential health benefits and improvements in concentration that this training can provide to practitioners. A consistent and reliable EEG pattern may depend on an undistracted mind and sustained attention. Yoga and other meditation practices are considered to be efficient techniques to reduce stress, anxiety, and frustration which may otherwise contribute to an unstable EEG [162].
Meditation is a practice that involves a complex process of self-regulation and inhibition of interfering internal and external stimuli, which can enhance a practitioner’s ability to sustain attention. There are many different types of meditation techniques. In general they can be categorized into concentrative-based meditation or mindfulness-based meditation. Both categories involve a sense of non-judgmental acceptance of single or multiple stimuli [162]. What different meditation techniques have in common is their ability to build concentration. It has been shown that experienced meditators have more distinguishable EEG patterns than untrained subjects during MI performance [163].
b. Mind-Body Awareness Training and BCI
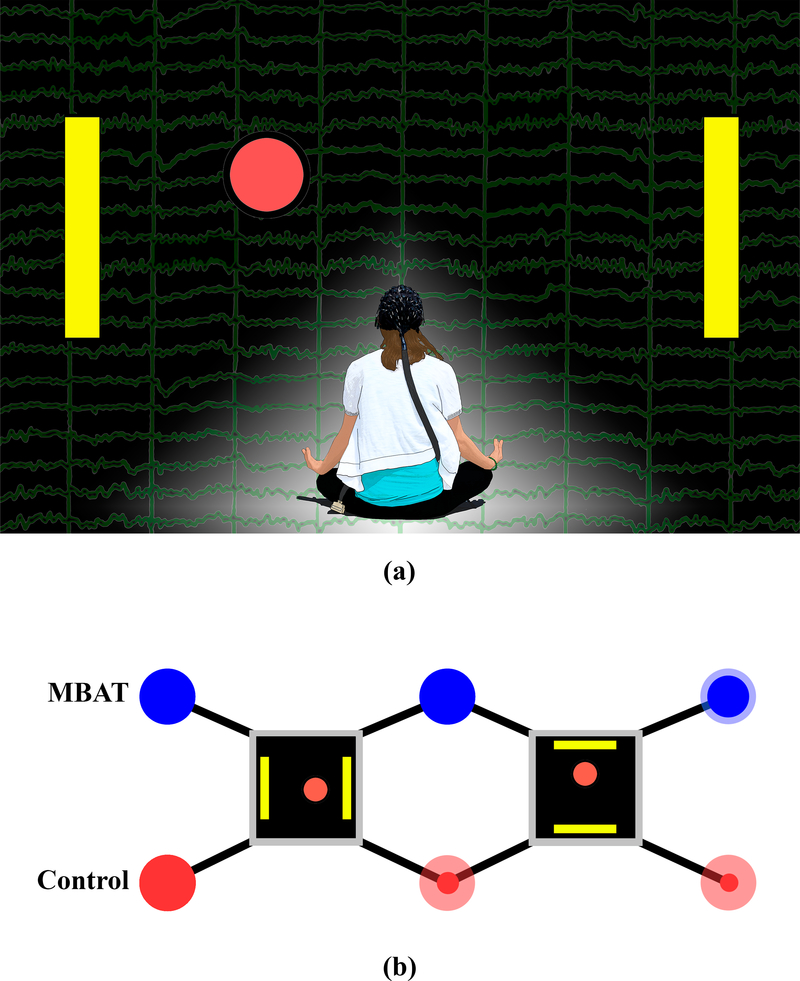
Based upon the observation that some of our best SMR-based BCI performers were meditation practitioners, we hypothesized that MBAT would increase a subject’s learning to control SMR signals and improve the overall performance of noninvasive SMR-based BCI. The role of experience with yoga and/or meditation in the initial learning of an SMR-based BCI has been examined in a group of human subjects [164]. Figure 7a displays a conceptual diagram of the study and the potential role of MBAT in the context of an SMR-based BCI. The study compared the MBAT subject group (12 subjects) and control group (24 subjects; little or no MBAT experience) in early learning of 1-D cursor movement using MI.
Figure 7.
(a) A conceptual diagram of the study design and the potential role of mind body awareness training (MBAT) in the context of a sensorimotor-rhythm-based BCI. The EEG signal that is produced from motor imaginations is depicted in the background of the figure. The yellow target bars displayed on the left and right sides of the figure, in addition to the red ball in the middle, represent the standard left vs. right cursor task that is used for initial one-dimensional BCI training. (b) Experimental paradigms. Subjects belong to one of two cohorts – MBAT practitioners and controls. All subjects undergo the same task progression starting with a left vs. right cursor task, and later with an up vs. down cursor task. Opaque dots on the figure represent the percentage of subjects (drawn to scale) who have passed each stage of the protocol. Translucent dots represent the original pool of subjects. (The impact of mind-body awareness training on the early learning of a brain-computer interface, K. Cassady, A. You, A. Doud, and B. He, Technology, vol. 2, no. 3, Copyright @ 2014 World Scientific Publishing Co./Imperial College Press)
All subjects were previously naïve to BCI, and participated in three, two-hour BCI experiments. Figure 7b illustrates the experimental paradigms. All subjects underwent the same task progression starting with a left vs. right cursor task, and later with an up vs. down cursor task. Each experiment consisted of ten, three-minute trials using the 1-D cursor movement task controlled by motor imaginations. Subjects were first introduced to and trained in the left vs. right cursor task. All subjects were instructed to use imaginations of either left or right hand movements to move a computer cursor to hit a target on the left or right side of a computer screen respectively. If subjects achieved accuracies of ≥80% over four consecutive three-minute runs or an overall session (ten, three-minute runs) accuracy of ≥80%, subjects progressed to an up vs. down control task. This task consisted of imagining both hands versus a volitional rest state to control the movement of the cursor to targets located at the top or bottom of a computer screen respectively. If subjects achieved accuracies of ≥ 80% over four consecutive three-minute runs or an overall session accuracy of ≥80% for this up vs. down task, subjects were deemed proficient in 1-D BCI control. Figure 7b illustrates the experimental design of subject progression for the MBAT and control groups, and the proportion of subjects completing each of the tasks.
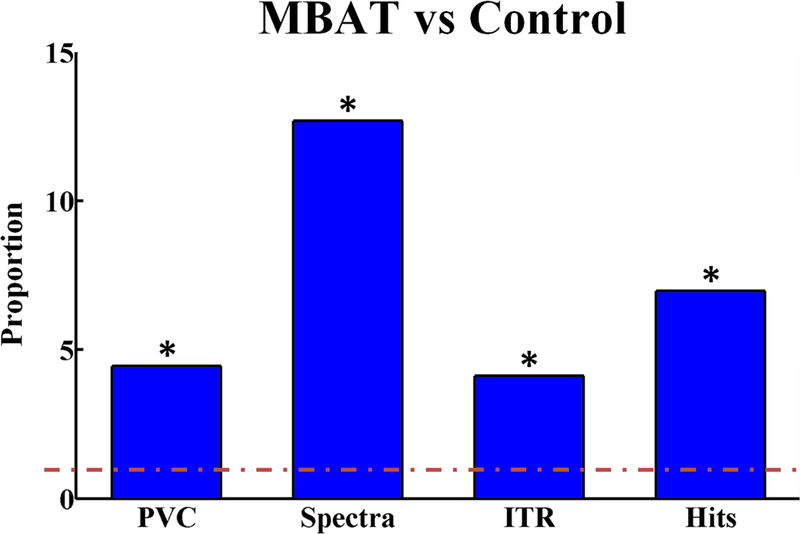
Figure 8 illustrates the ratios of weighted average slope measures for MBAT subject left-right performance as compared to control subject performance. This figure clearly demonstrates the significantly improved performance of the MBAT cohort in early learning of SMR-based BCI as compared with the control cohort. Examination of SMR EEG in all subjects revealed that for both left vs. right and overall 1-D control, the group-weighted average neural power measures were greater for the MBAT cohort compared to the control group. This supports the hypothesis that users with MBAT are able to generate stronger SMR control signals via motor imagination.
Figure 8.
The ratios of weighted average slope measures for MBAT subject left-right performance as compared to control subject performance. The red, dashed line at 1 indicates no difference between the two cohorts evaluated. A star indicates a statistically significant difference between the MBAT and control cohorts. (Based on work from [164])
c. Discussion
Our work so far suggests that experience with MBAT, such as yoga and meditation, can improve learning to control a SMR BCI using the aforementioned MI paradigm. Previously it was suggested that more distinguishable EEG patterns in terms of ERS could be observed in human subjects with meditation experience as compared with controls [163]. This offline study showed that the classification rate in subjects with meditation is higher than that in control subjects, although no online BCI experiments were conducted to directly assess the effects of MBAT on BCI performance. Our online BCI experiments in 36 human subjects show that MBAT subjects not only outperformed control subjects in various measures of BCI control, but that these subjects also demonstrated the ability to learn at a significantly faster rate than controls. Such substantially greater performance in the MBAT group may be due to the process of learning and refining particular mental techniques that provide subjects with the experience and practice of modulating their SMRs prior to even participating in a BCI task. Several forms of yoga and meditative practices utilize such specific mental techniques that intentionally produce increases and decreases in the spectral power of the EEG rhythms during training [165,166]. As can be seen from Figure 8, the SMRs produced in the MBAT cohort are significantly greater than those in the control group.
Meditation involves functional and structural changes of the brain [167]. Concentration is a key element in meditation and depends on the ability to focus. Since EEG represents synchronized neural activity within the brain, the enhanced ability to focus or concentrate may also increase the ability to produce synchronized brain activity that translates into detectable rhythmic activity in the scalp EEG. Through the self-regulation process of meditation, “neural noise” – background non-task related brain activity –- may be filtered out, leading to more stable EEG patterns.
This work suggests that mind-body awareness may be considered a mental skill for human subjects, and MBAT may serve as a means of acquiring such a skill that could be translated to improved SMR BCI performance. Further investigation is needed to explore the subtleties of how mind-body awareness influences BCI performance.
VI. Concluding Remarks
Controlling computers or other devices using our brain is no longer science fiction but is now a reality. Sensorimotor-rhythm-based brain-computer interfaces (BCIs) offer a noninvasive means of communicating and interacting with our environment directly using brain signals based on mental intentions initiated spontaneously and continuously. With ongoing advances in wearable sensors, signal processing algorithms, and “brain”-centered approaches, SMR-based BCIs may soon become more common in our daily lives.
Notwithstanding the rapid progress in BCI development in the past decade, challenges still exist with regard to SMR-based noninvasive BCIs. Lengthy training is still required before most users can use BCIs proficiently, and there remains a portion of users who may not become proficient despite extended training. The approaches reviewed in this article, including integrating noninvasive neuromodulation techniques to improve the learning of BCI control, and mind-body awareness training to enhance early skill acquisition, display great promise towards addressing these issues. Directly targeting a user’s ability to modulate cortical oscillations and attention using mind-body awareness training or targeting a user’s motivation using robotic devices may allow more users to learn to control BCIs faster. Furthermore, there are possible benefits to combining many of these methods to optimize the realization of high-level BCI control. EEG source imaging is a powerful tool that can identify the origins of BCI control signals as well as identify brain regions responsible for motor learning. Creative study designs targeting these regions with noninvasive neuromodulation technologies may unlock doors to overcoming inherent user deficits in regards to obtaining BCI control.
Integrating noninvasive BCIs into clinical and daily use is the ultimate objective of the field [168,169]. The techniques and technologies we have used have the possibility of being integrated into standard BCI training paradigms as well as combined with traditional EEG devices. For the BCI field to progress towards use in everyday applications, continuous innovation in system design and optimized signal detection techniques are needed to increase the speed and utility of noninvasive BCIs. Despite these challenges to the implementation of everyday BCI use, the field continues to move forward in identifying novel approaches to improve user training, understanding the underlying psychological factors influencing learning, and developing tools to address these challenges.
Acknowledgement
This work was supported in part by NSF CBET-1264782, NSF DGE-1069104, NIH EB006433, and NIH EY023101. The authors would like to thank Kaitlin Cassady for useful discussions.
Biography
 Bin He (S’84, M’88, F’04) received his BS in electrical engineering from Zhejiang University, China, in 1982, and PhD in bioelectric engineering from Tokyo Institute of Technology, Japan, in 1988. After completing a postdoctoral fellowship in biomedical engineering at Harvard University – M.I.T., he was a Research Scientist in Harvard – M.I.T. Division of Health Sciences and Technology till 1994. He was on faculty at the University of Illinois at Chicago from 1994–2003 and joined the University of Minnesota since 2004. He is currently a Distinguished McKnight University Professor of Biomedical Engineering, Medtronic-Bakken Endowed Chair for Engineering in Medicine, Director of the Institute for Engineering in Medicine and the Center for Neuroengineering at the University of Minnesota. His research interests cover a broad spectrum in biomedical engineering, mainly in neuroengineering and biomedical imaging. He has published over 200 peer-reviewed journal articles and is the sole editor of the text book entitled Neural Engineering. He has served as a Past President of IEEE Engineering in Medicine and Biology Society (EMBS), International Society for Functional Source Imaging, and the International Society for Bioelectromagnetism. Dr. He has served in a number of editorial boards and is currently the Editor-in-Chief of IEEE Transactions on Biomedical Engineering.
Bin He (S’84, M’88, F’04) received his BS in electrical engineering from Zhejiang University, China, in 1982, and PhD in bioelectric engineering from Tokyo Institute of Technology, Japan, in 1988. After completing a postdoctoral fellowship in biomedical engineering at Harvard University – M.I.T., he was a Research Scientist in Harvard – M.I.T. Division of Health Sciences and Technology till 1994. He was on faculty at the University of Illinois at Chicago from 1994–2003 and joined the University of Minnesota since 2004. He is currently a Distinguished McKnight University Professor of Biomedical Engineering, Medtronic-Bakken Endowed Chair for Engineering in Medicine, Director of the Institute for Engineering in Medicine and the Center for Neuroengineering at the University of Minnesota. His research interests cover a broad spectrum in biomedical engineering, mainly in neuroengineering and biomedical imaging. He has published over 200 peer-reviewed journal articles and is the sole editor of the text book entitled Neural Engineering. He has served as a Past President of IEEE Engineering in Medicine and Biology Society (EMBS), International Society for Functional Source Imaging, and the International Society for Bioelectromagnetism. Dr. He has served in a number of editorial boards and is currently the Editor-in-Chief of IEEE Transactions on Biomedical Engineering.
Bryan Baxter (S’13) received the B.S. degree in biomedical engineering in 2006 from the University of Wisconsin, Madison, WI, USA. From 2007–2009 he was at Brandeis University, Waltham, MA. From 2009–2011 he was with the Laboratory of Neural Systems at The Rockefeller University, New York, NY. In 2013, he was an intern in the Neuromodulation Division of Medtronic, Inc, Minneapolis, MN. He is currently pursuing a PhD degree in biomedical engineering at the University of Minnesota, Minneapolis, MN, USA, investigating the intersection of neuromodulation and brain-computer interfaces.
 Bradley Edelman (S’14) received the B.S. degree in mechanical engineering and biomedical engineering (with honors) from Carnegie Mellon University, Pittsburgh, Pennsylvania, USA, in 2012. He is currently working towards the Ph.D. degree in the department of biomedical engineering at the University of Minnesota, Minneapolis, MN, USA. His current research interests include brain-computer interface and neuroimaging. Mr. Edelman won the “BRAIN Young Investigator Award” at the IEEE EMBS BRAIN Grand Challenges Conference, Washington D.C, 2014.
Bradley Edelman (S’14) received the B.S. degree in mechanical engineering and biomedical engineering (with honors) from Carnegie Mellon University, Pittsburgh, Pennsylvania, USA, in 2012. He is currently working towards the Ph.D. degree in the department of biomedical engineering at the University of Minnesota, Minneapolis, MN, USA. His current research interests include brain-computer interface and neuroimaging. Mr. Edelman won the “BRAIN Young Investigator Award” at the IEEE EMBS BRAIN Grand Challenges Conference, Washington D.C, 2014.
 Christopher C. Cline (S’14) received the B.S. degree in electrical engineering from the University of Wisconsin, Madison, WI, USA, in 2013. He is currently working toward the Ph.D. in biomedical engineering at the University of Minnesota, Minneapolis, MN, USA. His research interests include brain-computer interfaces, transcranial magnetic stimulation, and neuroimaging.
Christopher C. Cline (S’14) received the B.S. degree in electrical engineering from the University of Wisconsin, Madison, WI, USA, in 2013. He is currently working toward the Ph.D. in biomedical engineering at the University of Minnesota, Minneapolis, MN, USA. His research interests include brain-computer interfaces, transcranial magnetic stimulation, and neuroimaging.
 Wenjing W. Ye (Member, IEEE) received the B.S. degree in Biomedical Engineering from Zhejiang University, Hangzhou, China, in 1985 and the M.S. degree in Bioelectrical Engineering from Tokyo Institute of Technology, Tokyo, Japan, in 1990. From 1990 to 1991, she was a Biomedical Engineer at the VA Medical Center, Harvard Medical School, West Roxbury, MA. From 1993 to 1997, she joined Global Prior Art Inc. Boston, MA where she was engaged in patent litigation support. From 1997 to 2003, she was a Member of Technical Staff at Lucent Technologies, Naperville, IL. From 2005 to 2012, she was a principal specialist, at Neuromodulation Department, Medtronic, Minneapolis, MN. She is currently the managing editor of IEEE Transactions on Biomedical Engineering and the managing director at Nexgen Medtech, St. Paul, MN.
Wenjing W. Ye (Member, IEEE) received the B.S. degree in Biomedical Engineering from Zhejiang University, Hangzhou, China, in 1985 and the M.S. degree in Bioelectrical Engineering from Tokyo Institute of Technology, Tokyo, Japan, in 1990. From 1990 to 1991, she was a Biomedical Engineer at the VA Medical Center, Harvard Medical School, West Roxbury, MA. From 1993 to 1997, she joined Global Prior Art Inc. Boston, MA where she was engaged in patent litigation support. From 1997 to 2003, she was a Member of Technical Staff at Lucent Technologies, Naperville, IL. From 2005 to 2012, she was a principal specialist, at Neuromodulation Department, Medtronic, Minneapolis, MN. She is currently the managing editor of IEEE Transactions on Biomedical Engineering and the managing director at Nexgen Medtech, St. Paul, MN.
References
- [1].Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, and Vaughan TM, “Brain-computer interfaces for communication and control,” Clin. Neurophysiol, vol. 113, no. 6, pp. 767–91, June. 2002. [DOI] [PubMed] [Google Scholar]
- [2].Taylor DM, Tillery SIH, and Schwartz AB, “Direct cortical control of 3D neuroprosthetic devices,” Science, vol. 296, no. 5574, pp. 1829–32, June. 2002. [DOI] [PubMed] [Google Scholar]
- [3].Vallabhaneni A, Wang T, and He B, “Brain-Computer Interface,” In Neural Engineering, ed. He B, Kruwer Academic Publishers, 2005, pp. 85–121. [Google Scholar]
- [4].Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, and Donoghue JP, “Neuronal ensemble control of prosthetic devices by a human with tetraplegia,” Nature, vol. 442, no. 7099, pp. 164–71, July. 2006. [DOI] [PubMed] [Google Scholar]
- [5].Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJC, Velliste M, Boninger ML, and Schwartz AB, “High-performance neuroprosthetic control by an individual with tetraplegia,” Lancet, vol. 381, pp. 557–64, February. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yuan H and He B, “Brain-computer interfaces using sensorimotor rhythms: current state and future perspectives,” IEEE Trans. Biomed. Eng, vol. 61, no. 5, pp. 1425–35, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].He B, Gao S, Yuan H, and Wolpaw JR, “Brain-Computer Interface,” In Neural Engineering, 2nd Ed., ed. He B, Boston, MA: Springer US, 2013, pp. 87–151. [Google Scholar]
- [8].Georgopoulos A, Schwartz A, and Kettner R, “Neuronal population coding of movement direction,” Science, vol. 233, no. 4771, pp. 1416–9, September. 1986. [DOI] [PubMed] [Google Scholar]
- [9].Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, and Nicolelis MA, “Real-time prediction of hand trajectory by ensembles of cortical neurons in primates,” Nature, vol. 408, pp. 361–5, November. 2000. [DOI] [PubMed] [Google Scholar]
- [10].Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, and Donoghue JP, “Reach and grasp by people with tetraplegia using a neurally controlled robotic arm,” Nature, vol. 485, pp. 372–5, May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, and Moran DW, “A brain-computer interface using electrocorticographic signals in humans,” J. Neural Eng, vol. 1, no. 2, pp. 63–71, June. 2004. [DOI] [PubMed] [Google Scholar]
- [12].Birbaumer N, Kubler A, Ghanayim N, Hinterberger T, Perelmouter J, Kaiser J, Iversen I, Kotchoubey B, Neumann N, and Flor H, “The thought translation device (TTD) for completely paralyzed patients,” IEEE Trans. Rehabil. Eng, vol. 8, no. 2, pp. 190–3, June. 2000. [DOI] [PubMed] [Google Scholar]
- [13].Blankertz B, Curio G, and Müller K-R, “Classifying single trial EEG: Towards brain computer interfacing,” Adv. Neural Inf. Process. Syst, vol. 1, pp. 157–64, 2002. [Google Scholar]
- [14].Donchin E, Spencer KM, and Wijesinghe R, “The mental prosthesis: assessing the speed of a P300-based brain-computer interface,” IEEE Trans. Rehabil. Eng, vol. 8, no. 2, pp. 174–9, June. 2000. [DOI] [PubMed] [Google Scholar]
- [15].LaFleur K, Cassady K, Doud A, Shades K, Rogin E, and He B, “Quadcopter control in three-dimensional space using a noninvasive motor imagery-based brain-computer interface,” J. Neural Eng, vol. 10, no. 4, August. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pfurtscheller G, Neuper C, Flotzinger D, and Pregenzer M, “EEG-based discrimination between imagination of right and left hand movement,” Electroencephalogr. Clin. Neurophysiol, vol. 103, no. 6, pp. 642–51, December. 1997. [DOI] [PubMed] [Google Scholar]
- [17].Wang T, Deng J, and He B, “Classifying EEG-based motor imagery tasks by means of time-frequency synthesized spatial patterns,” Clin. Neurophysiol, vol. 115, no. 12, pp. 2744–53, December. 2004. [DOI] [PubMed] [Google Scholar]
- [18].Wolpaw JR and McFarland DJ, “Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans.,” Proc. Natl. Acad. Sci. U. S. A, vol. 101, no. 51, pp. 17849–54, December. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolpaw JR, McFarland DJ, Neat GW, and Forneris CA, “An EEG-based brain-computer interface for cursor control,” Electroencephalogr. Clin. Neurophysiol, vol. 78, no. 3, pp. 252–9, March. 1991. [DOI] [PubMed] [Google Scholar]
- [20].Xu R, Jiang N, Mrachacz-Kersting N, Lin C, Asín Prieto G, Moreno JC, Pons JL, Dremstrup K, and Farina D, “A closed-loop brain-computer interface triggering an active ankle-foot orthosis for inducing cortical neural plasticity,” IEEE Trans. Biomed. Eng, vol. 61, no. 7, pp. 2092–101, July. 2014. [DOI] [PubMed] [Google Scholar]
- [21].Robinson N, Vinod AP, Ang KK, Tee KP, and Guan CT, “EEG-based classification of fast and slow hand movements using Wavelet-CSP algorithm,” IEEE Trans. Biomed. Eng, vol. 60, no. 8, pp. 2123–32, August. 2013. [DOI] [PubMed] [Google Scholar]
- [22].Tomita Y, Vialatte F-B, Dreyfus G, Mitsukura Y, Bakardjian H, and Cichocki A, “Bimodal BCI using simultaneously NIRS and EEG,” IEEE Trans. Biomed. Eng, vol. 61, no. 4, pp. 1274–84, April. 2014. [DOI] [PubMed] [Google Scholar]
- [23].Gao S, Wang Y, Gao X, and Hong B, “Visual and auditory brain-computer interfaces,” IEEE Trans. Biomed. Eng, vol. 61, no. 5, pp. 1436–47, May 2014. [DOI] [PubMed] [Google Scholar]
- [24].Yao L, Meng J, Zhang D, Sheng X, and Zhu X, “Combining motor imagery with selective sensation toward a hybrid-modality BCI,” IEEE Trans. Biomed. Eng, vol. 61, no. 8, pp. 2304–12, August. 2014. [DOI] [PubMed] [Google Scholar]
- [25].Doud AJ, Lucas JP, Pisansky MT, and He B, “Continuous three-dimensional control of a virtual helicopter using a motor imagery based brain-computer interface,” PLoS One, vol. 6, no. 10, January. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao X, Xu D, Cheng M, and Gao S, “A BCI-Based Environmental Controller for the Motion-Disabled,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 11, no. 2, pp. 137–40, 2003. [DOI] [PubMed] [Google Scholar]
- [27].Pfurtscheller G and Da Silva L, “Event-related EEG/ MEG synchronization and desynchronization: basic principles,” Clin. Neurophysiol, vol. 110, no. 11, pp. 1842–57, 1999. [DOI] [PubMed] [Google Scholar]
- [28].Farwell LA and Donchin E, “Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials,” Electroencephalogr. Clin. Neurophysiol, vol. 70, no. 6, pp. 510–23, December. 1988. [DOI] [PubMed] [Google Scholar]
- [29].Kindermans P-J, Verschore H, and Schrauwen B, “A unified probabilistic approach to improve spelling in an event-related potential-based brain-computer interface,” IEEE Trans. Biomed. Eng, vol. 60, no. 10, pp. 2696–705, October. 2013. [DOI] [PubMed] [Google Scholar]
- [30].Gu Z, Yu Z, Shen Z, and Li Y, “An online semi-supervised brain-computer interface,” IEEE Trans. Biomed. Eng, vol. 60, no. 9, pp. 2614–23, 2013. [DOI] [PubMed] [Google Scholar]
- [31].Postelnicu C-C and Talaba D, “P300-based brain-neuronal computer interaction for spelling applications,” IEEE Trans. Biomed. Eng, vol. 60, no. 2, pp. 534–43, February. 2013. [DOI] [PubMed] [Google Scholar]
- [32].Middendorf M, McMillan G, Calhoun G, and Jones KS, “Brain-computer interfaces based on the steady-state visual-evoked response,” IEEE Trans. Rehabil. Eng, vol. 8, no. 2, pp. 211–4, June. 2000. [DOI] [PubMed] [Google Scholar]
- [33].Kimura Y, Tanaka T, Higashi H, and Morikawa N, “SSVEP-based brain-computer interfaces using FSK-modulated visual stimuli,” IEEE Trans. Biomed. Eng, vol. 60, no. 10, pp. 2831–8, October. 2013. [DOI] [PubMed] [Google Scholar]
- [34].Li Y, Pan J, Wang F, and Yu Z, “A hybrid BCI system combining P300 and SSVEP and its application to wheelchair control,” IEEE Trans. Biomed. Eng, vol. 60, no. 11, pp. 3156–66, November. 2013. [DOI] [PubMed] [Google Scholar]
- [35].Yin E, Zhou Z, Jiang J, Chen F, Liu Y, and Hu D, “A speedy hybrid BCI spelling approach combining P300 and SSVEP,” IEEE Trans. Biomed. Eng, vol. 61, no. 2, pp. 473–83, February. 2014. [DOI] [PubMed] [Google Scholar]
- [36].Obermaier B, Müller GR, and Pfurtscheller G, “‘Virtual Keyboard’ controlled by spontaneous EEG activity,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 11, no. 4, pp. 422–6, 2003. [DOI] [PubMed] [Google Scholar]
- [37].Perdikis S, Leeb R, Williamson J, Ramsay A, Tavella M, Desideri L, Hoogerwerf E-J, Al-Khodairy A, Murray-Smith R, and Millán JDR, “Clinical evaluation of BrainTree, a motor imagery hybrid BCI speller,” J. Neural Eng, vol. 11, no. 3, June. 2014. [DOI] [PubMed] [Google Scholar]
- [38].Royer A, Doud A, Rose ML, and He B, “EEG control of a virtual helicopter in 3-dimensional space using intelligent control strategies,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 18, no. 6, pp. 581–9, December. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tanaka K, Matsunaga K, and Wang HO, “Electroencephalogram-based control of an electric wheelchair,” IEEE Trans. Robot, vol. 21, no. 4, pp. 762–6, August. 2005. [Google Scholar]
- [40].Leeb R, Friedman D, Müller-Putz GR, Scherer R, Slater M, and Pfurtscheller G, “Self-paced (asynchronous) BCI control of a wheelchair in virtual environments: A case study with a tetraplegic,” Comput. Intell. Neurosci, vol. 2007, January. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Galán F, Nuttin M, Lew E, Ferrez PW, Vanacker G, Philips J, and Millán J. del R, “A brain-actuated wheelchair: Asynchronous and non-invasive brain-computer interfaces for continuous control of robots,” Clin. Neurophysiol, vol. 119, no. 9, pp. 2159–69, September. 2008. [DOI] [PubMed] [Google Scholar]
- [42].Carlson T and del R J. Millan, “Brain-controlled wheelchairs: A robotic architecture,” IEEE Robot. Autom. Mag, vol. 20, no. 1, pp. 65–73, March. 2013. [Google Scholar]
- [43].Pfurtscheller G, Guger C, Müller G, Krausz G, and Neuper C, “Brain oscillations control hand orthosis in a tetraplegic,” Neurosci. Lett, vol. 292, no. 3, pp. 211–4, October. 2000. [DOI] [PubMed] [Google Scholar]
- [44].Carlson T, Tonin L, Perdikis S, Leeb R, and del R Millán J, “A hybrid BCI for enhanced control of a telepresence robot,” In Proceedings of the 35th Annual International IEEE EMBS Conference, 2013, pp. 3097–100. [DOI] [PubMed] [Google Scholar]
- [45].Scherer R and Pfurtscheller G, “Thought-based interaction with the physical world,” Trends Cogn. Sci, vol. 17, no. 10, pp. 490–2, October. 2013. [DOI] [PubMed] [Google Scholar]
- [46].Nijboer F, Birbaumer N, and Kübler A, “The influence of psychological state and motivation on brain-computer interface performance in patients with amyotrophic lateral sclerosis - a longitudinal study,” Front. Neurosci, vol. 4, no. July, January. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bell CJ, Shenoy P, Chalodhorn R, and Rao RPN, “Control of a humanoid robot by a noninvasive brain-computer interface in humans,” J. Neural Eng, vol. 5, no. 2, pp. 214–20, June. 2008. [DOI] [PubMed] [Google Scholar]
- [48].Müller-Putz GR and Pfurtscheller G, “Control of an electrical prosthesis with an SSVEP-based BCI,” IEEE Trans. Biomed. Eng, vol. 55, no. 1, pp. 361–4, January. 2008. [DOI] [PubMed] [Google Scholar]
- [49].Iturrate I, Antelis J, and Minguez J, “Synchronous EEG brain-actuated wheelchair with automated navigation,” In 2009 IEEE International Conference on Robotics and Automation, 2009, pp. 2318–25. [Google Scholar]
- [50].Legeny J, Abad RV, and Lecuyer A, “Navigating in virtual worlds using a self-paced SSVEP-based brain-computer interface with integrated stimulation and real-time feedback,” Presence, vol. 20, no. 6, pp. 529–44, 2011. [Google Scholar]
- [51].Martinez P, Bakardjian H, and Cichocki A, “Fully online multicommand brain-computer interface with visual neurofeedback using SSVEP paradigm,” Comput. Intell. Neurosci, vol. 2007, January. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Millán J. del R., Renkens F, Mouriño J, and Gerstner W, “Noninvasive brain-actuated control of a mobile robot by human EEG,” IEEE Trans. Biomed. Eng, vol. 51, no. 6, pp. 1026–33, June. 2004. [DOI] [PubMed] [Google Scholar]
- [53].Shenoy P, Krauledat M, Blankertz B, Rao RPN, and Müller K-R, “Towards adaptive classification for BCI,” J. Neural Eng, vol. 3, no. 1, pp. R13–23, March. 2006. [DOI] [PubMed] [Google Scholar]
- [54].Yuan H, Perdoni C, and He B, “Relationship between speed and EEG activity during imagined and executed hand movements.,” J. Neural Eng, vol. 7, no. 2, April. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vanacker G, del R Millán J, Lew E, Ferrez PW, Moles FG, Philips J, Van Brussel H, and Nuttin M, “Context-based filtering for assisted brain-actuated wheelchair driving,” Comput. Intell. Neurosci, January. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li T, Hong J, Zhang J, and Guo F, “Brain-machine interface control of a manipulator using small-world neural network and shared control strategy,” J. Neurosci. Methods, vol. 224, pp. 26–38, March. 2014. [DOI] [PubMed] [Google Scholar]
- [57].Rebsamen B, Guan C, Haihong Z, Chuanchu W, Cheeleong T, Marcelo HA Jr., and Burdet E, “A brain controlled wheelchair to navigate in familiar environments,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 18, no. 6, pp. 590–8, 2010. [DOI] [PubMed] [Google Scholar]
- [58].Lotte F, Langhenhove AV, Lamarche F, Ernest T, Renard Y, Arnaldi B, and Lécuyer A, “Exploring large virtual environments by thoughts using a brain-computer interface based on motor imagery and high-level commands,” Presence, vol. 19, no. 1, pp. 54–70, 2010. [Google Scholar]
- [59].Mason S, Kronegg J, Huggins J, Fatourechi M, and Schlogl A, “Evaluating the performance of self-paced brain-computer interface technology,” Neil Squire Soc, 2006. [Google Scholar]
- [60].Mason SG and Birch GE, “A brain-controlled switch for asynchronous control applications,” IEEE Trans. Biomed. Eng, vol. 47, no. 10, pp. 1297–307, October. 2000. [DOI] [PubMed] [Google Scholar]
- [61].Pfurtscheller G, Allison BZ, Brunner C, Bauernfeind G, Solis-Escalante T, Scherer R, Zander TO, Mueller-Putz G, Neuper C, and Birbaumer N, “The hybrid BCI,” Front. Neurosci, vol. 4, no. April, January. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Solis-Escalante T, Müller-Putz GR, Pfurtscheller G, and Neuper C, “Cue-induced beta rebound during withholding of overt and covert foot movement,” Clin. Neurophysiol, vol. 123, no. 6, pp. 1182–90, June. 2012. [DOI] [PubMed] [Google Scholar]
- [63].Leeb R, Lancelle M, Kaiser V, Fellner DW, and Pfurtscheller G, “Thinking penguin: multimodal brain-computer interface control of a VR game,” IEEE Trans. Comput. Intell. AI Games, vol. 5, no. 2, pp. 117–28, 2013. [Google Scholar]
- [64].Keirn ZA and Aunon JI, “A new mode of communication between man and his surroundings,” IEEE Trans. Biomed. Eng, vol. 37, no. 12, pp. 1209–14, December. 1990. [DOI] [PubMed] [Google Scholar]
- [65].Scherer R, Müller-Putz GR, and Pfurtscheller G, “Self-initiation of EEG-based brain-computer communication using the heart rate response,” J. Neural Eng, vol. 4, no. 4, pp. L23–9, December. 2007. [DOI] [PubMed] [Google Scholar]
- [66].Coyle SM, Ward TE, and Markham CM, “Brain-computer interface using a simplified functional near-infrared spectroscopy system,” J. Neural Eng, vol. 4, no. 3, pp. 219–26, September. 2007. [DOI] [PubMed] [Google Scholar]
- [67].McFarland DJ, Sarnacki WA, and Wolpaw JR, “Electroencephalographic (EEG) control of three-dimensional movement,” J. Neural Eng, vol. 7, no. 3, June. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Friedrich EVC, Scherer R, and Neuper C, “Long-term evaluation of a 4-class imagery-based brain-computer interface,” Clin. Neurophysiol, vol. 124, no. 5, pp. 916–27, May 2013. [DOI] [PubMed] [Google Scholar]
- [69].Lotte F, Larrue F, and Mühl C, “Flaws in current human training protocols for spontaneous brain-computer interfaces: Lessons learned from instructional design,” Front. Hum. Neurosci, vol. 7, no. September, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Friedrich EVC, Neuper C, and Scherer R, “Whatever works: A systematic user-centered training protocol to optimize brain-computer interfacing individually,” PLoS One, vol. 8, no. 9, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gomez-Rodriguez M, Peterst J, Hint J, Scholkoprt B, and Gharabaghi A, “Closing the sensorimotor loop: haptic feedback facilitates decoding of arm movement imagery,” J. Neural Eng, vol. 8, 2011. [DOI] [PubMed] [Google Scholar]
- [72].Cincotti F, Kauhanen L, Aloise F, Palomäki T, Caporusso N, Jylänki P, Mattia D, Babiloni F, Vanacker G, Nuttin M, Marciani MG, and Del R Millán J, “Vibrotactile feedback for brain-computer interface operation,” Comput. Intell. Neurosci, vol. 2007, January. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Barbero Á and Grosse-wentrup M, “Biased feedback in brain-computer interfaces,” J. Neuroeng. Rehabil, vol. 7, no. 34, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hill NJ, Häuser A-K, and Schalk G, “A general method for assessing brain-computer interface performance and its limitations,” J. Neural Eng, vol. 11, no. 2, April. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Freidman D, Leeb R, Guger C, Steed A, Pfurtscheller G, and Slater M, “Navigating virtual reality by thought: What is it like?,” Presence, vol. 16, no. 1, pp. 100–10, 2007. [Google Scholar]
- [76].Leeb R, Lee F, Keinrath C, Scherer R, Bischof H, and Pfurtscheller G, “Brain-computer communication: motivation, aim, and impact of exploring a virtual apartment,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 15, no. 4, pp. 473–82, 2007. [DOI] [PubMed] [Google Scholar]
- [77].Ron-Angevin R and Díaz-Estrella A, “Brain-computer interface: Changes in performance using virtual reality techniques,” Neurosci. Lett, vol. 449, no. 2, pp. 123–7, January. 2009. [DOI] [PubMed] [Google Scholar]
- [78].Lotte F, Faller J, Guger C, Renard Y, Pfurtscheller G, Anatole L, and Leeb R, “Combining BCI with virtual reality: Towards new applications and improved BCI,” Presence, pp. 197–220, 2013. [Google Scholar]
- [79].Wang PT, King CE, Chui LA, Do AH, and Nenadic Z, “Self-paced brain-computer interface control of ambulation in a virtual reality environment,” J. Neural Eng, vol. 9, no. 5, October. 2012. [DOI] [PubMed] [Google Scholar]
- [80].Wolpaw JR, Ramoser H, McFarland DJ, and Pfurtscheller G, “EEG-based communication: improved accuracy by response verification,” IEEE Trans. Rehabil. Eng, vol. 6, no. 3, pp. 326–33, September. 1998. [DOI] [PubMed] [Google Scholar]
- [81].McFarland DJ, Sarnacki WA, and Wolpaw JR, “Brain–computer interface (BCI) operation: optimizing information transfer rates,” Biol. Psychol, vol. 63, no. 3, pp. 237–51, July. 2003. [DOI] [PubMed] [Google Scholar]
- [82].Yuan P, Gao X, Allison B, Wang Y, Bin G, and Gao S, “A study of the existing problems of estimating the information transfer rate in online brain-computer interfaces,” J. Neural Eng, vol. 10, no. 2, April. 2013. [DOI] [PubMed] [Google Scholar]
- [83].Thomas E, Dyson M, and Clerc M, “An analysis of performance evaluation for motor-imagery based BCI,” J. Neural Eng, vol. 10, no. 3, June. 2013. [DOI] [PubMed] [Google Scholar]
- [84].Fitts PM, “The information capacity of the human motor system in controlling the amplitude of movement,” J. Exp. Psychol, vol. 47, no. 6, pp. 381–91, 1954. [PubMed] [Google Scholar]
- [85].Soukoreff RW and MacKenzie IS, “Towards a standard for pointing device evaluation, perspectives on 27 years of Fitts’ law research in HCI,” Int. J. Hum. Comput. Stud, vol. 61, no. 6, pp. 751–89, December. 2004. [Google Scholar]
- [86].Leeb R, Perdikis S, Tonin L, Biasiucci A, Tavella M, Molina A, Al-Khodairy A, Carlson T, and Millán J. del R, “Transferring brain-computer interfaces beyond the laboratory: Successful application control for motor-disabled users,” Artif. Intell. Med, vol. 59, pp. 121–32, 2013. [DOI] [PubMed] [Google Scholar]
- [87].Edelman B, Baxter B, and He B, “Discriminating hand gesture motor imagery tasks using cortical current density estimation,” In Proceedings of the 37th Annual International IEEE EMBS Conference, 2014, pp. 1314–7. [DOI] [PubMed] [Google Scholar]
- [88].He B and Ding L, “Electrophysiological Neuroimaging,” In Neural Engineering, 2nd Ed., ed. He B, Boston, MA: Springer US, 2013, pp. 499–543. [Google Scholar]
- [89].He B, Yang L, Wilke C, and Yuan H, “Electrophysiological imaging of brain activity and connectivity-challenges and opportunities.,” IEEE Trans. Biomed. Eng, vol. 58, no. 7, pp. 1918–31, July. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].He B, Musha T, Okamoto Y, Homma S, Nakajima Y, and Sato T, “Electric dipole tracing in the brain by means of the boundary element method and its accuracy,” IEEE Trans. Biomed. Eng, vol. 34, no. 6, pp. 406–14, June. 1987. [DOI] [PubMed] [Google Scholar]
- [91].Hämäläinen MS and Sarvas J, “Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data,” IEEE Trans. Biomed. Eng, vol. 36, no. 2, pp. 165–71, February. 1989. [DOI] [PubMed] [Google Scholar]
- [92].Hallez H, Vanrumste B, Grech R, Muscat J, De Clercq W, Vergult A, D’Asseler Y, Camilleri KP, Fabri SG, Van Huffel S, and Lemahieu I, “Review on solving the forward problem in EEG source analysis,” J. Neuroeng. Rehabil, vol. 4, no. 46, January. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zhang YC, Zhu S. a, and He B, “A second-order finite element algorithm for solving the three-dimensional EEG forward problem,” Phys. Med. Biol, vol. 49, no. 13, pp. 2975–87, July. 2004. [DOI] [PubMed] [Google Scholar]
- [94].Qin L, Ding L, and He B, “Motor imagery classification by means of source analysis for brain-computer interface applications,” J. Neural Eng, vol. 1, no. 3, pp. 135–41, September. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kamousi B, Liu Z, and He B, “Classification of motor imagery tasks for brain-computer interface applications by means of two equivalent dipoles analysis,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 13, no. 2, pp. 166–71, June. 2005. [DOI] [PubMed] [Google Scholar]
- [96].Yuan H, Doud A, Gururajan A, and He B, “Cortical imaging of event-related (de) synchronization during online control of brain-computer interface using minimum-norm estimates in frequency domain,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 16, no. 5, pp. 425–31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].He B and Musha T, “Equivalent dipole estimation of spontaneous EEG alpha activity,” Med. Biol. Eng. Comput, vol. 30, no. May, pp. 324–32, 1992. [DOI] [PubMed] [Google Scholar]
- [98].Makeig S, Gramann K, Jung TP, Sejnowski TJ, and Poizner H, “Linking brain, mind and behavior,” Int. J. Psychophysiol, vol. 73, pp. 95–100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Comon P, “Independent component analysis, a new concept?,” Signal Processing, vol. 36, no. 3, pp. 287–314, April. 1994. [Google Scholar]
- [100].Makeig S and Bell A, “Independent component analysis of electroencephalographic data,” Adv. Neural Inf. Process. Syst, pp. 145–51, 1996. [Google Scholar]
- [101].Pascual-Marqui RD, Michel CM, and Lehmann D, “Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain,” Int. J. Psychophysiol, vol. 18, no. 1, pp. 49–65, October. 1994. [DOI] [PubMed] [Google Scholar]
- [102].Phillips C, Rugg MD, and Friston KJ, “Systematic regularization of linear inverse solutions of the EEG source localization problem,” Neuroimage, vol. 17, no. 1, pp. 287–301, September. 2002. [DOI] [PubMed] [Google Scholar]
- [103].Hansen PC, The L-curve and its use in the numerical treatment of inverse problems, vol. 2. IMM, Department of Mathematic Modeling, Technical University of Denmark, 1999. [Google Scholar]
- [104].Hansen PC, “Analysis of discrete ill-posed problems by means of the L-curve,” SIAM Rev, vol. 34, no. 4, pp. 561–80, 1992. [Google Scholar]
- [105].Tikhonov AN and Arsenin VY, “Solutions of Ill-Posed Problems,” Am. Math. Soc., vol. 32, no. 144, pp. 1320–2, 1977. [Google Scholar]
- [106].Noirhomme Q, Kitney RI, and Macq B, “Single-trial EEG source reconstruction for brain-computer interface,” IEEE Trans. Biomed. Eng, vol. 55, no. 5, pp. 1592–601, May 2008. [DOI] [PubMed] [Google Scholar]
- [107].Grave de Peralta Menendez R, Gonzalez Andino S, Perez L, Ferrez PW, and del R. Millan J, “Non-invasive estimation of local field potentials for neuroprosthesis control,” Cogn. Process, vol. 6, no. 1, pp. 59–64, January. 2005. [Google Scholar]
- [108].Qin L and He B, “A wavelet-based time-frequency analysis approach for classification of motor imagery for brain-computer interface applications,” J. Neural Eng, vol. 2, no. 4, pp. 65–72, December. 2005. [DOI] [PubMed] [Google Scholar]
- [109].Laurent F, Besserve M, Garnero L, Philippe M, Florence G, and Martinerie J, “Source reconstruction and synchrony measurements for revealing functional brain networks and classifying mental states,” Int. J. Bifurc. Chaos, vol. 20, no. 06, pp. 1703–21, June. 2010. [Google Scholar]
- [110].Blankertz B, Tomioka R, Lemm S, Kawanabe M, and Müller K-R, “Optimizing spatial filters for robust EEG single-trial analysis,” IEEE Signal Process. Mag, vol. 25, pp. 41–56, 2008. [Google Scholar]
- [111].Ramoser H, Müller-Gerking J, and Pfurtscheller G, “Optimal spatial filtering of single trial EEG during imagined hand movement,” IEEE Trans. Rehabil. Eng, vol. 8, no. 4, pp. 441–6, 2000. [DOI] [PubMed] [Google Scholar]
- [112].Ang KK, Chin ZY, Wang C, Guan C, and Zhang H, “Filter Bank Common Spatial Pattern Algorithm on BCI Competition IV Datasets 2a and 2b,” Front. Neurosci, vol. 6, no. March, January. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Grosse-Wentrup M and Buss M, “Multiclass common spatial patterns and information theoretic feature extraction,” IEEE Trans. Biomed. Eng, vol. 55, no. 8, pp. 1991–2000, August. 2008. [DOI] [PubMed] [Google Scholar]
- [114].Congedo M, Lotte F, and Lécuyer A, “Classification of movement intention by spatially filtered electromagnetic inverse solutions,” Phys. Med. Biol, vol. 51, no. 8, pp. 1971–89, April. 2006. [DOI] [PubMed] [Google Scholar]
- [115].Mattiocco M, Babiloni F, Mattia D, Bufalari S, Sergio S, Salinari S, Marciani MG, and Cincotti F, “Neuroelectrical source imaging of mu rhythm control for BCI applications,” In Proceedings of the 28th Annual International IEEE EMBS Conference, 2006, vol. 1, pp. 980–3. [DOI] [PubMed] [Google Scholar]
- [116].Cincotti F, Mattia D, Aloise F, Bufalari S, Astolfi L, De Vico Fallani F, Tocci A, Bianchi L, Marciani MG, Gao S, Millan J, and Babiloni F, “High-resolution EEG techniques for brain-computer interface applications,” J. Neurosci. Methods, vol. 167, no. 1, pp. 31–42, January. 2008. [DOI] [PubMed] [Google Scholar]
- [117].Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, and Wolpaw JR, “BCI2000: A general-purpose brain-computer interface (BCI) system,” IEEE Trans. Biomed. Eng, vol. 51, no. 6, pp. 1034–43, June. 2004. [DOI] [PubMed] [Google Scholar]
- [118].Mattia D, Cincotti F, Mattiocco M, Scivoletto G, Marciani MG, and Babiloni F, “Motor-related cortical dynamics to intact movements in tetraplegics as revealed by high-resolution EEG,” Hum. Brain Mapp, vol. 27, no. 6, pp. 510–9, June. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, and He B, “Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements,” Neuroimage, vol. 49, no. 3, pp. 2596–606, February. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Congedo M, Lubar JF, and Joffe D, “Low-resolution electromagnetic tomography neurofeedback,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 12, no. 4, pp. 387–97, December. 2004. [DOI] [PubMed] [Google Scholar]
- [121].Im C-H, Hwang H-J, Che H, and Lee S, “An EEG-based real-time cortical rhythmic activity monitoring system,” Physiol. Meas, vol. 28, no. 9, pp. 1101–13, September. 2007. [DOI] [PubMed] [Google Scholar]
- [122].Sudre G, Parkkonen L, Bock E, Baillet S, Wang W, and Weber DJ, “rtMEG: A real-time software interface for magnetoencephalography,” Comput. Intell. Neurosci, vol. 2011, January. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Florin E, Bock E, and Baillet S, “Targeted reinforcement of neural oscillatory activity with real-time neuroimaging feedback,” Neuroimage, vol. 88C, pp. 54–60, November. 2013. [DOI] [PubMed] [Google Scholar]
- [124].Hwang H-J, Kwon K, and Im C-H, “Neurofeedback-based motor imagery training for brain-computer interface (BCI),” J. Neurosci. Methods, vol. 179, no. 1, pp. 150–6, April. 2009. [DOI] [PubMed] [Google Scholar]
- [125].Boe S, Gionfriddo A, Kraeutner S, Tremblay A, Little G, and Bardouille T, “Laterality of brain activity during motor imagery is modulated by the provision of source level neurofeedback,” Neuroimage, vol. 101, pp. 159–67, July. 2014. [DOI] [PubMed] [Google Scholar]
- [126].Filmer HL, Dux PE, and Mattingley JB, “Applications of transcranial direct current stimulation for understanding brain function,” Trends Neurosci, vol. 37, no. 12, pp. 742–53, September. 2014. [DOI] [PubMed] [Google Scholar]
- [127].Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik PA, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, and Cohen LG, “Consensus: ‘Can tDCS and TMS enhance motor learning and memory formation?,’” Brain Stimul, vol. 1, no. 4, pp. 363–9, October. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lotze M and Halsband U, “Motor imagery,” J. Physiol. Paris, vol. 99, no. 2006, pp. 386–95, June. 2006. [DOI] [PubMed] [Google Scholar]
- [129].Priori A, Berardelli A, Rona S, Accornero N, and Manfredi M, “Polarization of the human motor cortex through the scalp,” Neuroreport, vol. 9, no. 10, pp. 2257–60, July. 1998. [DOI] [PubMed] [Google Scholar]
- [130].Nitsche MA and Paulus W, “Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation,” J. Physiol, vol. 527, no. 3, pp. 633–9, September. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, and He B, “Neuromodulation for brain disorders: Challenges and opportunities,” IEEE Trans. Biomed. Eng, vol. 60, no. 3, pp. 610–24, March. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Bikson M, Name A, and Rahman A, “Origins of specificity during tDCS: Anatomical, activity-selective, and input-bias mechanisms,” Front. Hum. Neurosci, vol. 7, no. October, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nitsche M and Paulus W, “Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans,” Neurology, vol. 57, no. 10, pp. 1899–901, November. 2001. [DOI] [PubMed] [Google Scholar]
- [134].Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, and Krakauer JW, “Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation,” Proc. Natl. Acad. Sci. U. S. A, vol. 106, no. 5, pp. 1590–5, February. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Radman T, Ramos RL, Brumberg JC, and Bikson M, “Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro,” Brain Stimul, vol. 2, no. 4, pp. 215–28, October. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Purpura DP and McMurtry JG, “Intracellular activities and evoked potential changes during polarization of motor cortex,” J. Neurophysiol, vol. 28, pp. 166–85, January. 1965. [DOI] [PubMed] [Google Scholar]
- [137].de Berker AO, Bikson M, and Bestmann S, “Predicting the behavioral impact of transcranial direct current stimulation: Issues and limitations,” Front. Hum. Neurosci, vol. 7, no. October, p. 613, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, and Pascual-Leone A, “Transcranial direct current stimulation: A computer-based human model study,” Neuroimage, vol. 35, no. 3, pp. 1113–24, April. 2007. [DOI] [PubMed] [Google Scholar]
- [139].Madhavan S and Shah B, “Enhancing motor skill learning with transcranial direct current stimulation - A concise review with applications to stroke,” Front. Psychiatry, vol. 3, no. January, January. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, and M. a. Nitsche, “Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study,” Brain Stimul, vol. 6, no. 4, pp. 644–8, July. 2013. [DOI] [PubMed] [Google Scholar]
- [141].Stagg CJ and Nitsche MA, “Physiological basis of transcranial direct current stimulation,” Neurosci, vol. 17, no. 1, pp. 37–53, February. 2011. [DOI] [PubMed] [Google Scholar]
- [142].Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, and Tergau F, “Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human,” J. Cogn. Neurosci, vol. 15, no. 4, pp. 619–26, May 2003. [DOI] [PubMed] [Google Scholar]
- [143].Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, and Johansen-Berg H, “Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning,” Neuropsychologia, vol. 49, no. 5, pp. 800–4, April. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Galea JM and Celnik P, “Brain polarization enhances the formation and retention of motor memories,” J. Neurophysiol, vol. 102, no. 1, pp. 294–301, July. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Reis J and Fritsch B, “Modulation of motor performance and motor learning by transcranial direct current stimulation,” Curr. Opin. Neurol, vol. 24, no. 6, pp. 590–6, December. 2011. [DOI] [PubMed] [Google Scholar]
- [146].Matsumoto J, Fujiwara T, Takahashi O, Liu M, Kimura A, and Ushiba J, “Modulation of mu rhythm desynchronization during motor imagery by transcranial direct current stimulation,” J. Neuroeng. Rehabil, vol. 7, no. 27, January. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kasashima Y, Fujiwara T, Matsushika Y, Tsuji T, Hase K, Ushiyama J, Ushiba J, and Liu M, “Modulation of event-related desynchronization during motor imagery with transcranial direct current stimulation (tDCS) in patients with chronic hemiparetic stroke,” Exp. Brain Res, vol. 221, no. 3, pp. 263–8, September. 2012. [DOI] [PubMed] [Google Scholar]
- [148].Lapenta OM, Minati L, Fregni F, and Boggio PS, “Je pense donc je fais: transcranial direct current stimulation modulates brain oscillations associated with motor imagery and movement observation,” Front. Hum. Neurosci, vol. 7, no. June, p. 256, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Roy A, Baxter BS, and He B, “High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: A simultaneous tDCS–EEG study,” IEEE Trans. Biomed. Eng, vol. 61, no. 7, pp. 1967–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Blankertz B, Sannelli C, Halder S, Hammer EM, Kübler A, Müller K-R, Curio G, and Dickhaus T, “Neurophysiological predictor of SMR-based BCI performance,” Neuroimage, vol. 51, no. 4, pp. 1303–9, July. 2010. [DOI] [PubMed] [Google Scholar]
- [151].Guger C, Edlinger G, Harkam W, Niedermayer I, and Pfurtscheller G, “How many people are able to operate an EEG-based brain-computer interface (BCI)?,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 11, no. 2, pp. 145–7, June. 2003. [DOI] [PubMed] [Google Scholar]
- [152].Wei P, He W, Zhou Y, and Wang L, “Performance of motor imagery brain-computer interface based on anodal transcranial direct current stimulation modulation,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 21, no. 3, pp. 404–15, May 2013. [DOI] [PubMed] [Google Scholar]
- [153].Soekadar SR, Witkowski M, Cossio EG, Birbaumer N, and Cohen LG, “Learned EEG-based brain self-regulation of motor-related oscillations during application of transcranial electric brain stimulation: Feasibility and limitations,” Front. Behav. Neurosci, vol. 8, no. March, p. 93, January. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, and Bikson M, “Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: A basis for high-definition tDCS,” Neuroimage, vol. 74, pp. 266–75, July. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Iuculano T and Cohen Kadosh R, “The mental cost of cognitive enhancement,” J. Neurosci, vol. 33, no. 10, pp. 4482–6, March. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Soekadar SR, Witkowski M, Cossio EG, Birbaumer N, Robinson SE, and Cohen LG, “In vivo assessment of human brain oscillations during application of transcranial electric currents,” Nat. Commun, vol. 4, no. May, January. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Linde D, Dani S, Lazarewicz M, Cong P, Giftakis J, Stypulkowski P, and Denison T, “A translational platform for prototyping closed-loop neuromodulation systems,” Front. Neural Circuits, vol. 6, no. January, January. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Bradberry TJ, Gentili RJ, and Contreras-Vidal JL, “Fast attainment of computer cursor control with noninvasively acquired brain signals,” J. Neural Eng, vol. 8, no. 3, June. 2011. [DOI] [PubMed] [Google Scholar]
- [159].Tkach D, Reimer J, and Hatsopoulos NG, “Observation-based learning for brain-machine interfaces,” Curr. Opin. Neurobiol, vol. 18, no. 6, pp. 589–94, December. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Allison BZ, Brunner C, Kaiser V, Müller-Putz GR, Neuper C, and Pfurtscheller G, “Toward a hybrid brain-computer interface based on imagined movement and visual attention,” J. Neural Eng, vol. 7, no. 2, April. 2010. [DOI] [PubMed] [Google Scholar]
- [161].Holz EM, Höhne J, Staiger-Sälzer P, Tangermann M, and Kübler A, “Brain-computer interface controlled gaming: Evaluation of usability by severely motor restricted end-users,” Artif. Intell. Med, vol. 59, no. 2, pp. 111–20, October. 2013. [DOI] [PubMed] [Google Scholar]
- [162].Tan L-F, Dienes Z, Jansari A, and Goh S-Y, “Effect of mindfulness meditation on brain-computer interface performance,” Conscious. Cogn, vol. 23, pp. 12–21, January. 2014. [DOI] [PubMed] [Google Scholar]
- [163].Eskandari P and Erfanian A, “Improving the performance of brain-computer interface through meditation practicing,” In Proceedings of the 30th Annual International IEEE EMBS Conference, 2008, pp. 662–5. [DOI] [PubMed] [Google Scholar]
- [164].Cassady K, You A, Doud A, and He B, “The impact of mind-body awareness training on the early learning of a brain-computer interface,” Technology, vol. 2, no. 3, pp. 254–60, September. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Aftanas LI and Golocheikine SA, “Non-linear dynamic complexity of the human EEG during meditation,” Neurosci. Lett, vol. 330, no. 2, pp. 143–6, September. 2002. [DOI] [PubMed] [Google Scholar]
- [166].Hebert R, Lehmann D, Tan G, Travis F, and Arenander A, “Enhanced EEG alpha time-domain phase synchrony during transcendental meditation: Implications for cortical integration theory,” Signal Processing, vol. 85, no. 11, pp. 2213–32, November. 2005. [Google Scholar]
- [167].Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, and Fischl B, “Meditation experience is associated with increased cortical thickness,” Neuroreport, vol. 16, no. 17, pp. 1893–7, November. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].He B, Baird R, Butera R, Datta A, George S, Hecht B, Hero A, Lazzi G, Lee RC, Liang J, Neuman M, Peng GCY, Perreault EJ, Ramasubramanian M, Wang MD, Wikswo J, Yang G-Z, and Zhang Y-T, “Grand challenges in interfacing engineering with life sciences and medicine,” IEEE Trans. Biomed. Eng, vol. 60, no. 3, pp. 589–98, March. 2013. [DOI] [PubMed] [Google Scholar]
- [169].He B, Coleman T, Genin GM, Glover G, Hu X, Johnson N, Liu T, Makeig S, Sajda P, and Ye K, “Grand challenges in mapping the human brain: NSF workshop report,” IEEE Trans. Biomed. Eng, vol. 60, no. 11, pp. 2983–92, November. 2013. [DOI] [PubMed] [Google Scholar]