Abstract
Adaptive functioning, or the suite of skills essential for real-world, day-to-day functioning, includes daily living, communication, and socialization abilities. Even in the absence of co-occurring intellectual disability (IQ<70), difficulties in adaptive functioning are prominent in autism spectrum disorder (ASD). Further, cognitively-able ASD individuals demonstrate a gap between IQ and adaptive functioning, which widens with age. Existing studies of IQ-adaptive functioning discrepancies have characterized predominantly male ASD samples; thus, whether the gap is demonstrated in ASD females is unknown. To probe sex- versus diagnosis-specific differences in adaptive functioning in ASD, we characterized adaptive functioning using the Vineland Adaptive Behavior Scales, Second Edition in 177 cognitively-able (IQ>70) ASD (females=75, males=102), and 178 TD (females=87, males=91) youth, aged 8–17 years. We examined whether each group evidenced a gap between full-scale IQ and adaptive skills, and its associations with age. ASD youth evinced significantly lower adaptive skills and a significantly greater IQ-adaptive functioning gap than their same-sex TD peers. In this cross-sectional sample, the increase in the IQ-adaptive functioning gap with age was of similar magnitude for ASD males and females, but only reached statistical significance in males. We discuss unique implications the profound IQ-socialization skills gap in particular may have for ASD females.
Keywords: adaptive functioning, autism, sex differences, IQ
Adaptive functioning encompasses those skills essential for real-world, everyday functioning that generally fall within the broad areas of daily living skills (e.g., self-care), socialization (e.g., interpersonal skills), and communication (e.g., the ability to convey your wants and needs). Deficits in adaptive functioning are a foundational criterion for a diagnosis of intellectual disability (ID), along with an intellectual impairment (e.g., IQ<70). In general, co-occurrence of ID in autism spectrum disorder (ASD) is a predictor of poorer prognosis (Matson & Shoemaker, 2009), and a childhood IQ<70 is associated with poorer adult outcomes (Howlin et al., 2013). More narrowly, among individuals diagnosed with ASD and ID, lower IQ is associated with poorer adaptive functioning (Kanne et al., 2011; Matson & Shoemaker, 2009). In ASD individuals1 with ID, daily living skills are a relative strength, followed by communication, with the lowest levels of functioning in socialization (Carter et al., 1998; Kraijer, 2000). This autism profile of adaptive skills (i.e., Communication > Daily Living Skills > Socialization) is distinct from what is seen in other ID populations (e.g., Down Syndrome, where socialization is a relative strength and daily living skills are most impaired) (Loveland & Kelley, 1991; Schatz & Hamdan-Allen, 1995; Volkmar et al., 1987).
Studies in ASD individuals with co-occurring ID, suggest low IQ is a key factor in poor adaptive functioning and adult outcomes. However, research indicates that the converse is not true; cognitively-able ASD individuals still display adaptive skills far below what would be expected given their intellectual potential (Bradshaw et al., 2019; Chatham et al., 2018; Klin et al., 2007). Indeed, a profound discrepancy between cognitive ability and adaptive functioning characterizes ASD individuals with IQ>70 (Alvares et al., 2019; Bölte & Poustka, 2002). The profile of adaptive functioning difficulties demonstrated in those with ID is also present in cognitively-able ASD individuals: relative to daily living and communication skills, these individuals show the greatest impairments in socialization, and these difficulties endure throughout the lifespan (Bradshaw et al., 2019; Chatham et al., 2018; Kraper et al., 2017; Pugliese et al., 2015).
The IQ-adaptive functioning gap in ASD without ID begins as early as toddlerhood (Bradshaw et al., 2019), continues through adolescence (Kanne et al., 2011; Klin et al., 2007; Pugliese et al., 2015), and persists into young adulthood (Kraper et al., 2017). Further, the discrepancy widens with age during childhood, adolescence, and young adulthood due to lack of expected age-normative gains in adaptive abilities, but stable IQ scores (Klin et al., 2007; Pugliese et al., 2015), and cognitively-able ASD adults demonstrate levels of independent living, employment, and social connectedness well below what would be expected given their intellectual functioning (Hofvander et al., 2009).
Existing studies of the IQ-adaptive functioning gap have examined exclusively male (Klin et al., 2007) or male-predominant (~71–90% male) (Alvares et al., 2019; Bölte & Poustka, 2002; Bradshaw et al., 2019; Duncan & Bishop, 2015; Kanne et al., 2011; Kraper et al., 2017; Pugliese et al., 2015; Schatz & Hamdan-Allen, 1995) ASD samples; therefore, whether the IQ-adaptive functioning gap that characterizes cognitively-able males also characterizes cognitively-able ASD females is unknown. Females, particularly at the higher ends of the IQ continuum, may present a unique profile of ASD features (Bargiela et al., 2016; Gould, 2017; Kirkovski et al., 2013; Lai et al., 2011; Tierney et al., 2016; Trubanova et al., 2014; Young et al., 2018). In particular, relative to their ASD male peers, cognitively-able ASD females may demonstrate behavior within social contexts that superficially suggests better social skills, but that upon closer inspection conceal their autism-related challenges. These behaviors include appearing more socially motivated (Head et al., 2014), demonstrating more fluent use of verbal and non-verbal pragmatic markers (Parish-Morris et al., 2017) and showing greater facility in modulating behavior based on context (Hiller et al., 2014), along with showing a strong desire to be liked by others (Hiller et al., 2016), developing intense attachments to particular friends (Bargiela et al., 2016), and interacting with the same sex peer group in ways that give the superficial impression of ‘fitting in’ (Dean et al., 2014). This behavioral profile, along with potentially greater ‘masking’ of autism-related social difficulties (Allely, 2019; Lai et al., 2017; Schuck et al., 2019; Wood-Downie et al., 2020), is associated with increased negative outcomes in ASD females compared to their ASD male peers, among these, elevated risk for co-occurring mental health challenges, including depressive symptomatology (Oswald et al., 2016) and self-harm (Cassidy et al., 2018; Hirvikoski et al., 2016; Maddox et al., 2017).
To address these open questions in our understanding of the IQ-adaptive functioning gap, we examined a unique, sex-balanced sample of ASD youth (aged 8–17 years). In order to disentangle the possibility of typical sex effects in adaptive behavior from effects that may be specific to ASD females versus ASD males (Messinger et al., 2015), we compared ASD females and males to a group of their same-aged TD peers. Although TD individuals are expected to have adaptive skills commensurate with their intellectual functioning, (to our knowledge) no study has directly compared adaptive functioning of cognitively-able ASD youth to that of their TD peers. Direct comparison of ASD females and males with their same-sex peers is necessary to illuminate diagnosis- versus sex-specific differences in adaptive functioning profiles. It is important to note that analyses concerning sex differences are based on parent-reported sex assigned at birth, and data were not collected concerning gender identity, a point we return to in the Discussion.
We characterized adaptive functioning in three domains (Daily Living Skills, Communication and Socialization) utilizing the Vineland Adaptive Behavior Scales, Second Edition (Sparrow et al., 2005). We examined whether each of the four groups (ASD females (ASDf), ASD males (ASDm), TD females (TDf) and TD males (TDm)) evince a gap between intellectual functioning (full-scale IQ) and adaptive skills in each of the three domains, using a proportion score in order to take into account differences in IQ between ASD and TD groups. We additionally explored associations between age and all of the following: IQ, adaptive functioning, and the IQ-adaptive functioning discrepancy. We used FSIQ (rather than verbal or non-verbal IQ), in part, to allow for comparison of the present study with previous literature that has also examined the gap between cognitive abilities, also indexed by FSIQ (and not IQ subscores), and adaptive functioning (Alvares et al., 2019; Bölte & Poustka, 2002; Duncan & Bishop, 2015; Kanne et al., 2011; Kraper et al., 2017; Pugliese et al., 2015) within cognitively-able samples.
We sought to replicate existing work characterizing ASD males by determining 1) whether the IQ-adaptive functioning discrepancy (reported in mostly or exclusively male ASD samples) also holds for ASDm in our sample; and 2) whether the association between adaptive functioning and age reported in the literature is found in our ASDm sample. We sought to extend the literature by answering the following questions: 1) does the IQ-adaptive functioning gap also characterize cognitively-able ASDf; and 2) is there an association between age and both adaptive functioning and the IQ-adaptive functioning discrepancy in ASDf. An exploratory aim of the study was to examine sex differences in the IQ-adaptive functioning gap. To this end, we compared ASDf and ASDm to their same-sex TD peers.
We predicted that both ASDm and ASDf in our sample would show a significant discrepancy between IQ and adaptive functioning, and that this discrepancy would be inversely associated with age (i.e., a widening IQ-adaptive functioning gap with increasing age). We predicted main effects for group (ASD vs. TD), with the TD group having higher adaptive functioning scores than the ASD group, and the ASD group having a greater IQ-adaptive functioning gap than the TD group.
Methods
Participants
Participants were youth aged 8–17 years recruited from the community for inclusion in ASD and TD groups as part of an NIH-funded Autism Centers of Excellence (ACE) network study (the ‘GENDAAR’ [Gender Exploration of Neurogenetics and Development to Advance Autism Research] project). The ACE network included four data collection sites (Yale University, University of Washington, Boston Children’s Hospital/Harvard Medical School, and University of California, Los Angeles), a data coordinating center at the University of Southern California, and a data analytic and study coordination center at the University of Virginia. Our sample included 355 youth (ASDf=75, ASDm=102, TDf=87, TDm=91).
Expert clinicians confirmed DSM-based ASD diagnoses using the diagnostic algorithm for either module 3 or module 4 of the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012), and the Autism Diagnostic Interview-Revised (ADI-R; Rutter, Le Couteur, & Lord, 2008). Scores on the ADI-R were required to meet the measure’s algorithm cut-off for each of the three domains (10 for Social Interaction; 8 for Communication; and 3 for Restricted, Repetitive Behaviors & Interests) (Lord et al., 1994).
Study exclusionary criteria for both groups of participants included full-scale IQ (FSIQ)≤70; twin status; active seizures within the past year; or current use of any benzodiazepine, barbiturate, or anti-epileptic medication. Dosage of any other medication must have been stable for >6 weeks. Additional exclusionary criteria for the ASD group included significant pre- or perinatal injury (specifically, birth <36 weeks and birth weight <2000g; or hospital stay >3 days in neonatal intensive care); neonatal brain injury; neurological disorder involving pathology above the brainstem, aside from a history of non-focal, uncomplicated epilepsy; known genetic condition (e.g., Fragile X); medical conditions that are likely to be etiological (e.g., focal epilepsy); any known environmental factor that could account for the clinical presentation of ASD (e.g., severe malnutrition or psychological deprivation); clinically significant auditory or visual impairments after correction; or sensorimotor difficulties precluding valid use of diagnostic instrumentation.
Further exclusionary criteria for the TD group included diagnosed, referred, or suspected ASD; a first- or second-degree biological relative with diagnosed ASD; scores on measures of autism features suggestive of ASD or the broader autism phenotype (i.e., total T-score >60 on the Social Responsiveness Scale, Second Edition (Constantino & Gruber, 2012) or a raw score >11 on the Social Communication Questionnaire, Lifetime version (Lord & Rutter, 2003)); or clinical impression of ASD, the broader autism phenotype, or a developmental disorder. TD participants were also excluded if the parent reported referred or suspected psychiatric, learning, or other neurodevelopmental disorder; or if, based on clinician interpretation of measures that probe behavioral concerns related to DSM psychiatric disorders (Child and Adolescent Symptom Inventory (Gadow & Sprafkin, 2005), Child Behavior Checklist (Achenbach, 1991) a significant psychiatric disorder (i.e., psychiatric disorder that would prevent participation in daily activities (e.g., schizophrenia, bipolar disorder)) was suspected.
Parents provided written informed consent, and youth provided written assent. Procedures were conducted in compliance with ethical standards set forth by the universities’ Institutional Review Boards and the Declaration of Helsinki.
Community Involvement
The PI of this ACE Network is a parent of autistic children. As such, he serves at the request of the Secretary of Health and Human Services as a public member on the Interagency Autism Coordinating Committee.
Measures
Adaptive Functioning
Adaptive functioning was measured using the Vineland Adaptive Behavior Scales, 2nd edition [VABS-II] (Sparrow, Cicchetti, & Balla, 2005). Parents of ASD and TD participants were administered the semi-structured interview VABS-II, which is an age- (but not sex-) normed measure appropriate for individuals from birth to 90+ years. Three adaptive behavior domains were assessed: Communication (Receptive, Expressive, and Written subdomains), Daily Living Skills (Personal, Domestic, and Community subdomains), and Socialization (Interpersonal, Play and Leisure Time, and Coping Skills). Age-normed standard scores for VABS domains are calculated and range from 20–160, with a mean of 100 and a standard deviation of 15; higher scores are indicative of better adaptive functioning.
Full-Scale IQ
Full-scale IQ (FSIQ) was estimated using participants’ General Conceptual Ability Standard Score on the Differential Ability Scale, Second edition [DAS-II] (Elliott, 1990). The DAS-II provides a comprehensive assessment of cognitive functioning in youth ranging from 7.0–17.11 years. Age-normed standardized scores range from 30–170, with a mean of 100 and a standard deviation of 15.
FSIQ-Adaptive Functioning Gap Proportion Scores
Proportion scores of the difference between FSIQ and the VABS-II Daily Living Skills, Communication and Socialization domain scores were calculated as follows: IQ-adaptive functioning gap proportion score=(IQ - Domain Score) / FSIQ to index the IQ-adaptive functioning gap. Rather than a simple difference score, a proportion score was used in analyses testing for group and sex differences in order to take into account the higher FSIQ of the TD groups.
Autistic Traits
Parent-report on the Social Responsiveness Scale, Second Edition [SRS-2] (Constantino & Gruber, 2012) was utilized as a continuous measure of autistic traits. Raw scores from the aggregate domains of Social Communication and Interaction (SCI) and Restricted Interests and Repetitive Behavior (RRB) were used to characterize participants. Higher SRS scores reflect greater autistic traits.
Data Analysis
Statistical analyses were conducted in R v.3.4.1. Prior to analyses, continuous variables were inspected to ensure assumptions of parametric statistical tests were met. If assumptions were not met within acceptable limits of skewness and kurtosis, non-parametric tests were used. Comparisons were conducted by group (ASD vs. TD) and by sex (m vs. f) on the following demographic variables: age, maternal highest level of education, race, and ethnicity. These comparisons were also conducted for FSIQ and VABS domain scores as well as SRS raw SCI and RRB aggregate scores. Within the ASD group, females and males were compared on ADI-R Reciprocal Social Behavior, Communication and Language, and Restricted, Repetitive Behaviors and Interests, and on ADOS-2 CSS scores.
IQ versus Adaptive Functioning
Paired-samples t-tests, separately for each of the four groups (ASDf, ASDm, TDf, TDm), were conducted to determine if FSIQ differed significantly from adaptive functioning in each of the VABS-II domains.
IQ, Adaptive Functioning, and IQ-Adaptive Functioning Gap Proportion Scores: Correlations with Age
Given that previous studies have shown that age-normed adaptive functioning scores do not keep pace with age-based expectations among cognitively-able ASD individuals, we examined associations between age and VABS-II composite domain scores, as well as between age and IQ, and between age and the IQ-adaptive functioning gap proportion scores.
Group and Sex Differences in the IQ-Adaptive Functioning Gap Proportion Scores
Group and sex differences in the discrepancy between IQ and adaptive functioning were probed for the VABS-II domains (Communication, Daily Living Skills, and Socialization). To determine whether a model with an interaction of group × sex × age was appropriate, MANOVA models with and without these interactions were compared. As noted in the Introduction, participants were assigned female or male labels based on parent-reported biological sex at birth. No measure of gender identity was collected.
Results
Participant Characteristics
ASD and TD groups did not differ in their sex ratios (χ2(1, N=355)=1.5, p=.22). For age and maternal level of education, neither significant group nor sex effects were found (see Table 1). Due to small cell sizes for race, categories were collapsed. There were no differences for proportion of participants who identified as Caucasian. Further, comparisons revealed similar proportions for participants who identified as being of Latinx descent. ASD youth showed significantly lower FSIQ compared to TD youth. Within the ASD group, ASDf and ASDm did not differ for IQ; however, within the TD group TDm had significantly higher FSIQ than TDf. Relative to their same-sex TD peers, ASD youth showed significantly lower adaptive functioning scores across all domains (see Table 1, Figure 1). Further, 26.7–44% of ASD youth fell below the cut-off of 70 on the VABS domain scores (see Table 1).
Table 1.
Participant characteristics.
| ASDf n=75 | ASDm n=102 | TDf n=87 | TDm n=91 | ASD vs. TD test statistics, p-values, effect sizes | f vs. m test statistics, p-values, effect sizes | |||
|---|---|---|---|---|---|---|---|---|
| ASDf vs. TDf | ASDm vs. TDm | ASDf vs. ASDm | TDf vs. TDm | |||||
| Age (years) | t(160)=−1.14, | t(191)=−1.5, | t(175)=−0.63, | t(176)=−0.84, | ||||
| Mean(SD) | 12.3 (2.76) | 12.6 (3.0) | 12.9 (3.18) | 13.2 (2.72) | p=.26, d=0.18 | p=.13, d=0.22 | p=.53, d=0.09 | p=.40, d=0.13 |
| Range | 8.0–17.6 | 8.0–17.9 | 8.1–17.9 | 8.3–17.8 | ||||
| Race, N(%) | χ2(1)=2.8, | χ2(1)=2.3, | χ2(1)=3.7, | χ2(1)=1.5, | ||||
| African American | 2 (2.66%) | 6 (5.88%) | 11 (12.64%) | 2 (2.19%) | p=.09, V=0.12 | p=.13, V=0.10 | p=.053, | p=.22, V=0.08 |
| Asian | 2 (2.66%) | 1 (0.98%) | 5 (5.75%) | 7 (7.69%) | V=0.13 | |||
| Caucasian | 60 (80%) | 72 (70.58%) | 62 (71.26%) | 72 (79.12%) | ||||
| More than one | 8 (10.66%) | 22 (21.57%) | 8 (9.19%) | 9 (9.89%) | ||||
| Native American | 0 (0%) | 1 (0.98%) | 0 (0%) | 0 (0%) | ||||
| Undeclared | 3 (4.0%) | 0 (0%) | 1 (1.15%) | 1 (1.09%) | ||||
| Ethnicity, N(%) | χ2(1)=3.6, | χ2(1)=0.06, | χ2(1)=0.68, | χ2(1)=0.94, | ||||
| Latinx descent | 10 (13.3%) | 19 (18.6%) | 22 (25.3%) | 18 (19.8%) | p=.06, V=0.14 | p=.81, | p=.41, V=0.05 | p=.33, V=0.06 |
| Not of Latinx descent | 62 (82.7%) | 83 (81.4%) | 62 (71.3%) | 72 (79.1%) | V=0.004 | |||
| Undeclared | 3 (4.0%) | 0 (0%) | 3 (3.4%) | 1 (1.1%) | ||||
| Maternal Education | n=66 | n=91 | n=71 | n=81 | χ2(6)=1.07, | χ2(6)=6.2, | χ2(6)=2.49, | χ2(6)=2.97, |
| Graduate degree | 19 | 24 | 22 | 27 | p=.98, V=0.09 | p=.40, V=0.19 | p=.87, V=0.13 | p=.81, V=0.14 |
| Partial graduate | 5 | 5 | 6 | 5 | ||||
| Bachelor’s degree | 20 | 23 | 20 | 28 | ||||
| Associate’s degree | 3 | 7 | 5 | 3 | ||||
| Partial college | 12 | 22 | 13 | 12 | ||||
| High school diploma | 6 | 7 | 4 | 3 | ||||
| Partial high school | 1 | 3 | 1 | 3 | ||||
| FSIQ | t(160)=−3.4, | t(191)=−6.0, | t(175)=−0.14 | t(176)=−2.3 | ||||
| Mean(SD) | 100.7 (19.3) | 101.1 (17.2) | 109.9 (15.2) | 115.3 (15.2) | p=.0008**, | p<.0001†, | p=.89, d=0.02 | p=.02*, |
| Range | 71–162 | 71–160 | 79–147 | 79–149 | d=0.53 | d=0.87 | d=0.35 | |
| VABS-II | t(160)=−10.5, | t(190)=−12.9, | t(174)=1.9, | t(176)=2.0, | ||||
| Communication | n=101 | p<.0001†, | p<.0001†, | p=.056, | p=.04*, | |||
| Mean(SD) | 78.4 (12.7) | 75 (10.7) | 101 (14.3) | 96.8 (12.7) | d=1.7 | d=1.86 | d=−0.29 | d=−0.30 |
| Median (Range) | 77 (52–111) | 74 (57–122) | 101 (72–135) | 97 (67–132) | ||||
| ≤70, N(%) | 22 (29.3%) | 37 (36.6%) | 0 (0%) | 2 (2.2%) | -- | -- | -- | -- |
| Daily Living Skills | t(160)=−9.4, | t(191)=−10.4, | t(175)=1.87, | t(176)=2.4, | ||||
| Mean(SD) | 78.8 (14.0) | 75.0 (13.1) | 99.8 (14.2) | 94.9 (13.6) | p<.0001†, | p<.0001†, | p=.06, | p=.02*, |
| Median(Range) | 77 (54–112) | 73.5 (51–119) | 100 (58–130) | 93 (69–129) | d=1.49 | d=1.49 | d=−0.28 | d=−0.35 |
| ≤70, N(%) | 20 (26.7%) | 40 (39.2%) | 2 (2.3) | 1 (1.1%) | -- | -- | -- | -- |
| Socialization | t(160)=−13.7, | t(191)=−18.0, | t(175)=0.62, | t(176)=0.50, | ||||
| Mean(SD) | 73 (12.7) | 71.9 (10.9) | 101 (13.5) | 100 (11.1) | p<.0001†, | p<.0001†, | p=.54, | p=.62, |
| Median(Range) | 73 (46–118) | 73 (43–107) | 101 (68–145) | 100 (76–127) | d=2.2 | d=2.59 | d=−0.09 | d=−0.07 |
| ≤70, N(%) | 33 (44%) | 43 (42.2%) | 1 (1.2%) | 0 (0%) | -- | -- | -- | -- |
| SRS-2 raw scores | n=69 | n=89 | n=81 | n=89 | ||||
| SCI | ||||||||
| Mean(SD) | 76.3 (20.0) | 74.2 (22.3) | 16.5 (11.4) | 16.1 (13.7) | U=10.5, | U=10.9, | t(156)=0.64, | U=0.85, p=.39, |
| Median(Range) | 75 (39–135) | 71 (5–118) | 13 (0–50) | 12 (1–71) | p<.0001†, | p<.0001†, | p=.53, d=−0.10 | r=.06 |
| r=.86 | r=.82 | |||||||
| RRB | n=69 | n=90 | n=81 | n=85 | ||||
| Mean(SD) | 16.8 (6.4) | 17.5 (6.6) | 1.9 (2.8) | 2.3 (2.8) | U=10.3 | U=10.9 | t(157)= | U=−1.3, p=.19, |
| Median(Range) | 17 (4–33) | 18 (0–32) | 1 (0–20) | 1 (0–16) | p<.0001†, | p<.0001†, | −0.64, | r=−.10 |
| r=.84 | r=.83 | p=.52, d=0.10 | ||||||
| ADI-R, Mean(SD) | ||||||||
| Social | 18.5 (5.4) | 19.4 (5.3) | -- | -- | -- | t(175)=−1.1, p=.29, d=0.16 | ||
| Communication | 15.6 (4.0) | 16.2 (4.4) | t(175)=−0.88, p=.38, d=0.13 | |||||
| RRBIs | 5.6 (2.6) | 6.3 (2.7) | t(175)=−1.7, p=.10, d=0.26 | |||||
| ADOS-2-CSS, | ||||||||
| Mean(SD) | 6.59 (1.85) | 7.38 (1.87) | -- | -- | t(175)=−2.8, p=.005**, d=0.43 | |||
SD: standard deviation; FSIQ: full scale IQ; VABS-II: Vineland Adaptive Behavior Scales, 2nd edition; SRS-2: Social Responsiveness Scale, 2nd edition; SCI: Social Communication and Interaction; RRB: restricted and repetitive behaviors; ADI-R: Autism Diagnostic Interview-Revised; RRBIs: Restricted, Repetitive Behaviors & Interests; ADOS-2: Autism Diagnostic Observation Schedule, Second Edition; CSS: Calibrated Severity Score; ≤70, N(%): number and proportion of individuals who meet the VABS-II cut-off for ‘low’ adaptive functioning; d=Cohen’s d; V=Cramer’s V; r=effect size for non-parametric Mann-Whitney U-test. VABS-II domain scores are reported as standard scores (M=100, SD=15).
p < .05
p<.01
p<.0001.
Figure 1.
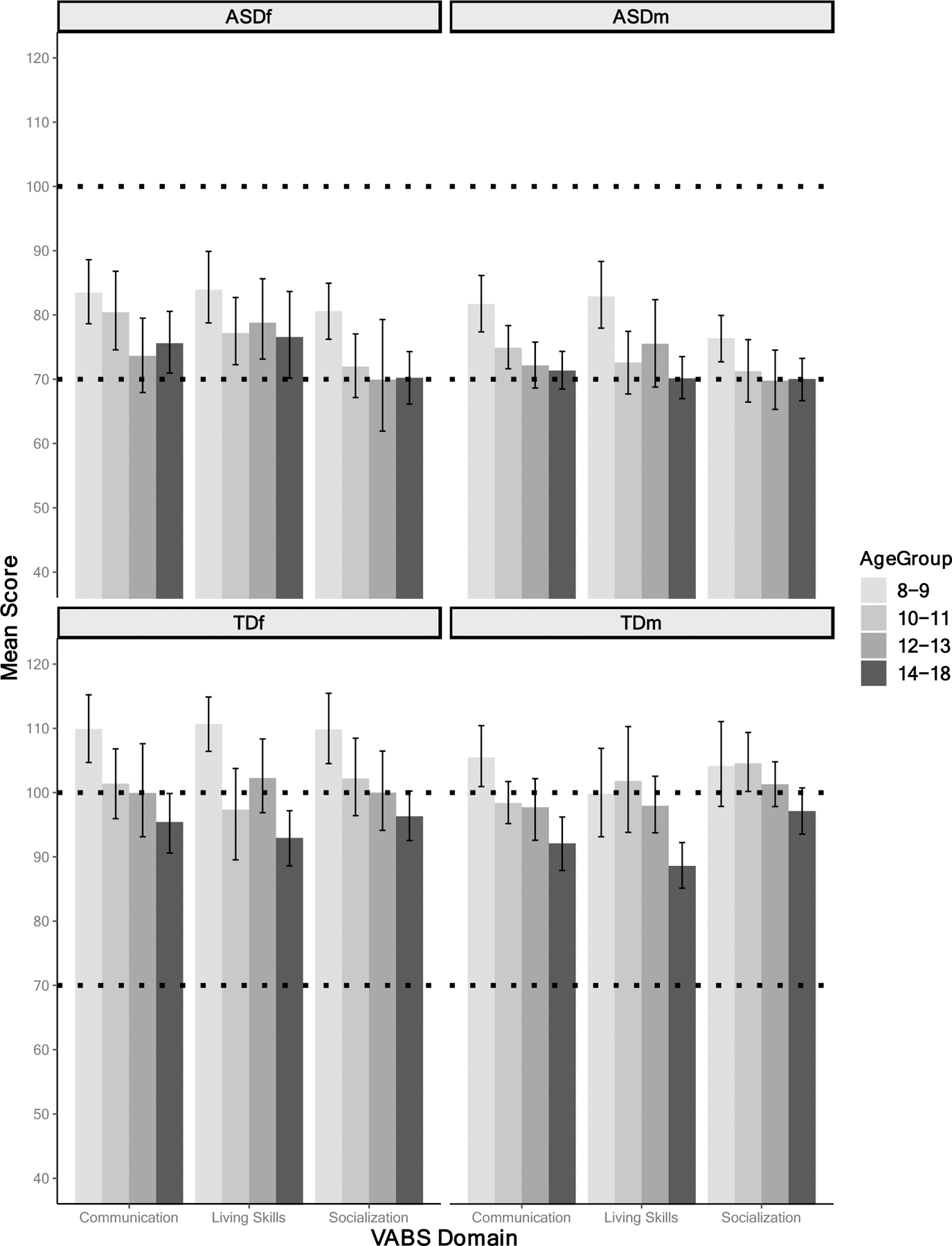
Mean VABS-II domain composite scores, presented by age group. Notes: Ages are binned for visualization purposes only. Dotted lines at 100 and 70 represent standardized mean and clinical cut-off, respectively. Each error bar depicts 95% confidence interval.
ASD youth showed significantly higher autistic trait ratings (SRS-2 SCI and RRBI) compared to TD youth. Within ASD and TD groups, females and males did not significantly differ for SCI or RRBIs. ASDf and ASDm did not differ significantly on ADI-R Communication, Social or RRBI scores. Compared to ASDf, ASDm showed higher ADOS-CSS scores. See Table 1 for details.
IQ versus Adaptive Functioning
A series of paired samples t-tests showed significantly greater FSIQ scores than each of the three VABS-II domain scores in all four groups (ASDf, ASDm, TDf, and TDm) examined separately (see Table 2, Figure 2).
Table 2.
IQ versus adaptive functioning within each group: paired-samples t-test statistics, p-values and effect sizes.
| ASDf n=75 | ASDm n=102 | TDf n=87 | TDm n=91 | |
|---|---|---|---|---|
| Test statistic, p-value, effect size | Test statistic, p-value, effect size | Test statistic, p-value, effect size | Test statistic, p-value, effect size | |
| IQ vs. Communication | t(74)=−10.3, p<.00001†, d=1.19 | t(100)=−14.7, p<.00001†, d=1.46 | t(86)=−4.05, p<.0005**, d=0.44 | t(90)=−10.0, p<.00001†, d=1.05 |
| IQ vs. Daily Living Skills | t(74)=−9.2, p<.00001†, d=1.06 | t(101)=−12.5, p<.00001†, d=1.24 | t(86)=−4.61, p<.00005†, d=0.50 | t(90)=−9.41, p<.00001†, d=0.99 |
| IQ vs. Socialization | t(74)=−11.2, p<.00001†, d=1.30 | t(101)=−14.9, p<.00001†, d=1.48 | t(86)=−3.76, p<.0005**, d=0.41 | t(90)=−7.47, p<.00001†, d=0.79 |
SD: standard deviation
n=101.
p<.01
p<.0001.
Figure 2.
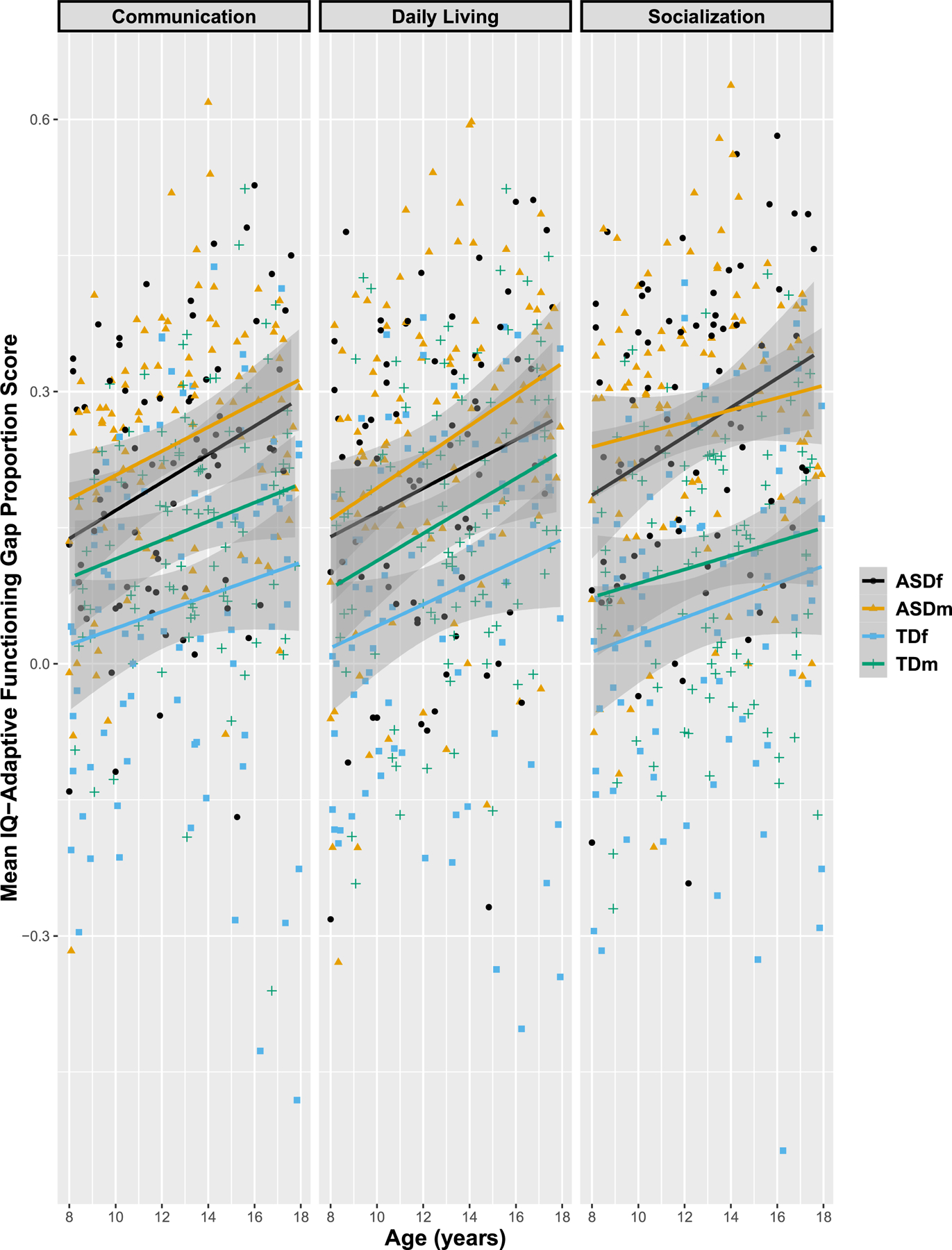
Mean IQ-adaptive functioning gap proportion scores. Shading depicts 95% confidence intervals.
IQ, Adaptive Functioning, IQ-Adaptive Functioning Gap Proportion Score: Correlations with Age
There were no significant associations between FSIQ and age for any of the four groups (see Table 3). Among ASDf, age was significantly negatively correlated with Socialization, and Communication domain scores on the VABS-II, though not Daily Living Skills. For ASDm, as well as TDf and TDm, Socialization, Daily Living Skills, and Communication domain scores were all significantly negatively correlated with age.
Table 3.
IQ, Adaptive functioning, and IQ-adaptive functioning gap proportion scores: Correlations with age.
| ASDf n=75 | ASDm n=102 | TDf n=87 | TDm n=91 | |
|---|---|---|---|---|
| rs, p-value | rs, p-value | rs, p-value | rs, p-value | |
| FSIQ | 0.02, 0.90 | −0.009, 0.92 | −0.09, 0.40 | −0.08, 0.46 |
| Adaptive Functioning | ||||
| Communication | −0.26, 0.02* | −0.41a, 0.00002† | −0.35, 0.001** | −0.38, 0.0002** |
| Daily Living Skills | −0.18, 0.11 | −0.42, 0.00001† | −0.45, 0.00001† | −0.43, 0.00002† |
| Socialization | −0.27, 0.02* | −0.24, 0.02* | −0.39, 0.0002** | −0.28, 0.006** |
| IQ-Adaptive Functioning Gap | ||||
| Proportion Score | ||||
| IQ-Communication | 0.26, 0.027* | 0.29 a, 0.004* | 0.25, 0.022* | 0.20, 0.06 |
| IQ-Daily Living Skills | 0.20, 0.09 | 0.28, 0.004* | 0.28, 0.008** | 0.22, 0.04* |
| IQ-Socialization | 0.24, 0.035* | 0.12, 0.21 | 0.22, 0.039* | 0.11, 0.28 |
FSIQ: full-scale IQ; rs=Spearman’s rho.
n=101
p<.05
p<.01
p<.0001.
For the IQ-adaptive functioning gap proportion score, among ASDm age was significantly positively correlated with the proportion score (i.e., the proportion score grew with increasing age) between IQ and adaptive functioning in Communication and Daily Living Skills, but not Socialization. Among ASDf, age was significantly positively associated with the proportion score for Communication and Socialization, but not Daily Living Skills. Although only ASDf showed a significant association between age and Socialization, and only for ASDm was age significantly correlated with Daily Living Skills, correlation coefficients for the groups for these measures did not differ significantly (Daily Living: Z=−0.55, p=.29; Socialization: Z=0.80, p=.21). Age was significantly positively correlated with the proportion score for the gap between IQ and adaptive abilities in all three VABS domains among TDf (Table 3). In TDm, positive correlations between age and the proportion score reach significance for only Daily Living Skills.
Group and Sex Differences in IQ-Adaptive Functioning Gap Proportion Scores
The interaction of group × sex × age was examined. There was no significant interaction with age (i.e., there was no sex × age, group × age, or group × sex × age interaction). In analyses reported below, age was included as a covariate. In all analyses of group and sex differences in the IQ-adaptive functioning gap proportion scores, model comparisons revealed no interaction of group × sex (see Figure 2); therefore, models without the interaction are reported.
In the overall MANCOVA model, using Pillai’s trace, there was a significant main effect of group (V=.26, F(348,3)=40.6, p<.0001, ηp2=.26), and sex (V=.052, F(348,3)=6.45, p<.0001, ηp2=.047). In follow-up analyses, univariate ANCOVAs were used and Bonferroni correction with significant threshold was set at 0.05/3=.0167. Separate univariate ANCOVAs (for each of the VABS domains) showed a significant effect of group (F(350,1)=53.7, p<.0001, ηp2=.14) and a main effect of sex (F(350,1)=14.06, p=.0002, ηp2=.034) for the IQ-Communication gap proportion score, with ASD youth demonstrating a greater IQ-Communication skill discrepancy than TD youth, and males demonstrating a greater gap than their female counterparts. Similarly, for the IQ-Daily Living Skills gap proportion score, there were main effects of group (F(350,1)=30.71, p<.0001, ηp2=.087; greater in ASD than TD youth) and sex (F(350,1)=12.12, p=.0006, ηp2=.029; greater in m than f). For the proportion scores of the gap between Socialization and IQ, there was a main effect of group (F(350,1)=101.3, p<.0001, ηp2=.23; greater in ASD than TD youth), but the effect of sex was not significant (F(350,1)=3.79, p=.0522, ηp2=.009).
Discussion
The current study examined group and sex differences in the discrepancy between IQ and adaptive skills among ASD and TD youth, utilizing, for the first time, a proportion score to account for ‘baseline’ group differences in FSIQ (i.e., higher FSIQ in TD youth). The results presented here for ASD and TD youth aged 8–17 years corroborate and extend the literature on the IQ-adaptive functioning gap in cognitively-able ASD individuals. We further show that a gap between IQ and adaptive functioning is attested in TD youth. We discuss these findings in turn, along with the implications the marked gap between IQ and Socialization may have for ASDf, in particular. Results presented for ASDm corroborate the existing literature that has examined predominantly male samples and has demonstrated a discrepancy between cognitive abilities and everyday adaptive functioning (Duncan & Bishop, 2015; Kraper et al., 2017). Extending the literature, we show ASDf evince a discrepancy in IQ and adaptive functioning consistent with the one reported in predominantly ASDm samples.
The current study confirms in a community-recruited cross-sectional sample that ASDm without ID, as a group, show a significant impairment in adaptive functioning across domains compared to their TD peers, and evince the prototypical autistic adaptive functioning profile (i.e., Communication > Daily Living Skills > Socialization). With respect to our main research questions, we corroborate the literature showing that ASDm in our sample: 1) show a discrepancy between cognitive functioning (FSIQ) and each domain of adaptive functioning; and 2) this discrepancy increases with age.
Extending the literature, we show that ASDf, like their same-aged male ASD peers demonstrate lower adaptive functioning compared to their same-sex TD peers (and also show the autistic profile of adaptive skills). In ASDf, as in ASDm, the IQ-adaptive functioning gap proportion score was positively associated with age for Communication; however, whereas ASDf showed a significant association between the proportion score and age for Socialization (but not Daily Living Skills), ASDm showed an association between age and the Daily Living Skills (but not Socialization) proportion score. Importantly, the magnitude of the effect in ASDf and ASDm groups is similar for age-Daily Living Skills correlations and for age-Socialization relationships, and correlation coefficients do not significantly differ. Interestingly, both ASDf and TDf (but neither ASDm nor TDm) show significant associations between the Socialization proportion score and age. In ASD youth, age is associated with adaptive functioning, but not IQ. Our findings are consistent with reports that ASD individuals do not demonstrate age-expected increases in adaptive functioning at the same rate as their TD peers during childhood and adolescence (Klin et al., 2007; Pugliese et al., 2015).
Further advancing our understanding of the IQ-adaptive functioning gap in cognitively-able ASD youth, our comparison of ASD and TD females and males reveals both diagnostic and sex differences in adaptive functioning and in the IQ-adaptive functioning gap. As predicted, ASD youth demonstrated lower adaptive functioning scores than TD youth, and adaptive functioning scores for both ASDf and ASDm were significantly lower than found in the same-sex TD groups. ASDf and ASDm did not significantly differ for adaptive skills in any of the VABS domains.
In examining the IQ-adaptive functioning gap, we further add to the literature, showing that not only ASD but also TD youth demonstrate a discrepancy between intellectual functioning and adaptive skills, and that as in the ASD group, this gap among TD youth increases with age.
These findings are not entirely surprising, given the differences in the psychometric properties of the measures of these two constructs and what we would expect in terms of adaptive functioning within this age group. While IQ captures the full range of intellectual functioning, assessments of adaptive functioning probe for skills needed to execute everyday activities and function independently. Importantly, although we observe an IQ-adaptive functioning gap in TD youth, it is quite small particularly when compared to the profound gap in these scores found in ASDf and ASDm.
Interestingly, a main effect of sex was found, indicating that males showed significantly lower adaptive functioning than females in Communication and Daily Living Skills, but not Socialization. These findings, if replicated, could suggest sex differences do exist in non-standardization TD samples, and this finding would aid the interpretation of sex differences that might be seen in ASD samples. It is important to note that adaptive functioning in TDm, though lower than TDf in these domains, is still solidly average and within the normal range. Thus, although we see similar sex-related patterns in ASD and TD youth, both ASDf and ASDm show marked challenges across adaptive functioning domains.
Although prior studies have not investigated potential sex differences in the IQ-adaptive functioning gap in ASD, they have examined sex differences in adaptive functioning alone. Several studies have shown overall decreased adaptive skills for ASDf relative to ASDm (Frazier et al., 2014; Ratto et al., 2018). Notably differing from our sample, Frazier et al.’s (2014) participants are from the Simons Simplex Collection, which has a low proportion of females with IQ≥80, and the females included had significantly lower IQ than the males. Additionally, Ratto et al. (2018) included participants who met either ADI-R or ADOS diagnostic criteria. When analyzing the effect of diagnostic measure (satisfaction of criteria on only one measure, or on both measures) and its interaction with sex, a main effect of sex and also a trend-level interaction between sex and measure(s) was found: there were greater sex differences among the subgroup that met criteria only on the ADOS, compared to those who met both ADOS and ADI-R criteria (the criterion for inclusion in the ASD sample analyzed here). Thus, results reported here may differ from patterns of results reported in the literature due to important sample-based differences.
For ASD youth, the discrepancy between IQ and adaptive functioning is particularly salient within the socialization domain. When considered alongside the unique presentation of ASD in cognitively-able females, the large IQ-adaptive functioning gap within the Socialization domain may have particularly negative consequences for ASDf. Distinct social challenges are faced by ASDf relative to ASDm (Tierney & Burns, 2017), as well as differing sex-based cultural and societal expectations (Halladay et al., 2015). ASDf are acutely aware of sex-based expectations and report struggling to conform to these expectations (Milner et al., 2019; Sedgewick et al., 2019). Camouflaging, or the masking of ASD-related social difficulties, occurs more often in females relative to their male peers (Dean et al., 2017; Hull et al., 2020; Kenyon, 2014; Lai et al., 2017; Schuck et al., 2019), and potentially reflects increased social motivation and insight into social challenges in ASDf compared to ASDm (Head et al., 2014). Negative outcomes among ASD women appear to be linked to social difficulties (South et al., 2019), and ASD women describe camouflaging as effortful and exhausting (Autistic Science Lady, 2018; Hull et al., 2017), and as having negative impacts on their well-being (Tierney et al., 2016; Tierney & Burns, 2017). Camouflaging appears to be associated with elevated risk for depression (Somerville et al., 2019) and suicidality (Cassidy et al., 2019). Thus, future studies should illuminate how the gap between intellectual ability and Socialization impacts ASDf in particular. More specifically, studies should examine the transitions into adolescence and young adulthood, which mark greater social demands that may outstrip camouflaging resources, along with increased difficulties in social functioning for ASDf (Kanfiszer et al., 2017; Trubanova et al., 2014).
The current study has several limitations. Analyses reported here examine biological sex; therefore, we are unable to determine the influence of gender identity on adaptive functioning and the IQ-adaptive functioning gap. This is an important, open question to address in the future given increased gender variance in ASD (Strang et al., 2014, 2020) and the paucity of research into whether and how findings of sex differences may apply to gender diverse individuals.
Further, the sample investigated here is predominantly Caucasian and lacks desired racial and ethnic diversity (Mandell et al., 2009). This lack of diversity limits the ability to know how the findings reported here may generalize. The examination of group and sex differences in the discrepancy between cognitive ability and adaptive functioning within racially and ethnically representative samples is a critical area for future research.
Further, the sample analyzed is cross-sectional, and primarily composed of participants on the lower end of the age range studied (8–17y). This makes generalizability of the findings here to older adolescents less certain. Age may be a particularly important variable for ASDf, as discussed above, and a study sufficiently powered to detect the interaction of sex with age has reported greater problems in social adaptive functioning with increasing age in ASDf relative to ASDm (Mahendiran et al., 2019). Additionally, given the known male-skewed biases in ASD diagnostic measures (Lai et al., 2015; Loomes et al., 2017), our inclusion criteria using both the ADOS and ADI-R suggests that our ascertainment of participants may also be biased, potentially including those ASDf who more closely resemble the “male” presentation of ASD, while excluding ASDf who are the “best” camouflagers and who may therefore run the risk of being missed by standard diagnostic assessments. Studies examining the IQ-adaptive functioning gap and that implement less stringent criteria for inclusion in an ASD group (e.g., meeting on either the ADOS or ADI; or having a clinically diagnosed ASD) and that therefore may capture individuals with a potentially different profile of ASD features are warranted.
The findings reported here underscore the importance of providing appropriate services to cognitively-able ASD individuals. In particular, it appears that given the distinct findings in the literature for individuals with both ASD and ID as compared to non-ID ASD individuals, intellectual functioning may act as a gatekeeper, where a criterial level of intellectual functioning admits the achievement of a certain level of adaptive functioning, and increases in FSIQ below a certain threshold are associated with improvements in adaptive skills. However, above a certain threshold, and in particular within the “normal” range of IQ, increases in FSIQ are not associated with concomitantly augmented adaptive functioning. Future studies that include both ID and non-ID samples of individuals with ASD can examine this possibility empirically. It is critical, however, to note that in the ASD sample here, in which no FSIQ was less than 71, 27–40% of ASD individuals demonstrated adaptive functioning at or below cut-offs used in the diagnosis of ID and the determination of its severity. As such, evaluation of adaptive functioning and provision of appropriate support services among non-ID ASD individuals is necessary. Further, given that cognitively-able ASDf may present with a profile that obscures some of their social challenges, evaluating adaptive skills and providing services among this group may improve not only adaptive functioning, but also mental health outcomes.
Acknowledgements
We extend our gratitude to all of the participating children and families for their commitment and contributions to this project. Additionally, we would like to thank the clinical and research staff who contributed to recruitment, data collection, and phenotyping assessment. We thank Allison Jack, PhD for providing support in this project.
We thank the members of the GENDAAR Consortium, listed alphabetically: Elizabeth Aylward, Raphael A. Bernier, Susan Y. Bookheimer, Mirella Dapretto, Nadine Gaab, Daniel H. Geschwind, Allison Jack, James C. McPartland, Charles A. Nelson, Kevin A. Pelphrey, John D. Van Horn, Sara J. Webb, Katy Ankenman, Sarah Corrigan, Dianna Depedro-Mercier, Desiree Guilford, Abha R. Gupta, Zachary Jacokes, Shafali Jeste, Cara M. Keifer, Anna Kresse, Erin Libsack, Jennifer K. Lowe, Erin MacDonnell, Nicole McDonald, Adam Naples, Emily Neuhaus, Megha Santhosh, Catherine A. W. Sullivan, Heidi Tsapelas, Carinna M. Torgerson, Pamela Ventola, Olivia Welker, & Julie Wolf.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a National Institute of Mental Health (NIMH) Autism Center of Excellence Network Award (R01 MH100028; PI: K.A.P.), and an Autism Speaks Postdoctoral Fellowship (Grant ID 11808) to G.A.M.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
We adopt identity-first (e.g., ‘autistic individuals’), rather than person-first (e.g., ‘individuals with autism’) language, consistent with practices and preferences among certain autistic individuals and self-advocate groups (Autistic Adults and other Stakeholders Engage Together (AASET), 2017; Brown, 2011; Kenny et al., 2016; Sequenzia, 2013). We recognize, however, that language preferences and practices may differ among those within the autism community (Vivanti, 2020).
References
- Achenbach TM (1991). Manual for the Child Behavior Checklist/4–18 and 1991 profile.Department of Psychiatry, University of Vermont. [Google Scholar]
- Allely CS (2019). Understanding and recognising the female phenotype of autism spectrum disorder and the “camouflage” hypothesis: a systematic PRISMA review. In Advances in Autism (Vol. 5, Issue 1, pp. 14–37). Emerald Group Publishing Ltd. 10.1108/AIA-09-2018-0036 [DOI] [Google Scholar]
- Alvares GA, Bebbington K, Cleary D, Evans K, Glasson EJ, Maybery MT, Pillar S, Uljarević M, Varcin K, Wray J, & Whitehouse AJO (2019). The misnomer of ‘high functioning autism’: Intelligence is an imprecise predictor of functional abilities at diagnosis. Autism, 136236131985283. 10.1177/1362361319852831 [DOI] [PubMed] [Google Scholar]
- Autistic Adults and other Stakeholders Engage Together (AASET). (2017). Year 1 Meeting Executive Summary.
- Autistic Science Lady. (2018). Autistic masking, late diagnosis, and dissociation: The toll It takes on autistic mental health. https://autisticsciencelady.wordpress.com/2018/05/20/autistic-masking-late-diagnosis-and-dissociation-the-toll-it-takes-on-autistic-mental-health/
- Bargiela S, Steward R, & Mandy W (2016). The experiences of late-diagnosed women with autism spectrum conditions: An investigation of the female autism phenotype. Journal of Autism and Developmental Disorders, 46(10), 3281–3294. 10.1007/s10803-016-2872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S, & Poustka F (2002). The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without co-morbid mental retardation. Child Psychiatry and Human Development, 33(2), 165–172. 10.1023/A:1020734325815 [DOI] [PubMed] [Google Scholar]
- Bradshaw J, Gillespie S, Klaiman C, Klin A, & Saulnier C (2019). Early emergence of discrepancy in adaptive behavior and cognitive skills in toddlers with autism spectrum disorder. Autism, 23(6), 1485–1496. 10.1177/1362361318815662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L (2011). The Significance of Semantics: Person-First Language: Why It Matters. https://www.autistichoya.com/2011/11/identity-and-hypocrisy-second-argument.html
- Carter AS, Volkmar FR, Sparrow SS, Wang JJ, Lord C, Dawson G, Fombonne E, Loveland K, Mesibov G, & Schopler E (1998). The Vineland Adaptive Behavior Scales: Supplementary norms for individuals with autism. Journal of Autism and Developmental Disorders, 28(4), 287–302. 10.1023/A:1026056518470 [DOI] [PubMed] [Google Scholar]
- Cassidy S, Bradley L, Shaw R, & Baron-Cohen S (2018). Risk markers for suicidality in autistic adults. Molecular Autism, 9(1), 42. 10.1186/s13229-018-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S, Gould K, Townsend E, Pelton M, Robertson AE, & Rodgers J (2019). Is camouflaging autistic traits associated with suicidal thoughts and behaviours? Expanding the Interpersonal Psychological Theory of Suicide in an undergraduate student sample. Journal of Autism and Developmental Disorders. 10.1007/s10803-019-04323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Taylor KI, Charman T, Liogier D’ardhuy X, Eule E, Fedele A, Hardan AY, Loth E, Murtagh L, del Valle Rubido M, San Jose Caceres A, Sevigny J, Sikich L, Snyder L, Tillmann JE, Ventola PE, Walton-Bowen KL, Wang PP, Willgoss T, & Bolognani F (2018). Adaptive behavior in autism: Minimal clinically important differences on the Vineland-II. Autism Research, 11(2), 270–283. 10.1002/aur.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale, Second Edition. (Vol. 2). Western Psychological Services. http://www.wpspublish.com/store/p/2994/social-responsiveness-scale-second-edition-srs-2 [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism, 21(6), 678–689. 10.1177/1362361316671845 [DOI] [PubMed] [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, Lord C, Orlich F, King B, & Harwood R (2014). The peer relationships of girls with ASD at school: comparison to boys and girls with and without ASD. Journal of Child Psychology and Psychiatry, 55(11), 1218–1225. 10.1111/jcpp.12242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, & Bishop SL (2015). Understanding the gap between cognitive abilities and daily living skills in adolescents with autism spectrum disorders with average intelligence. Autism, 19(1), 64–72. 10.1177/1362361313510068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (1990). DAS Introductory and Technical Handbook. The Psychological Corporation. [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, & Hardan AY (2014). Behavioral and cognitive characteristics of females and males with autism in the simons simplex collection. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 329. 10.1016/j.jaac.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow K, & Sprafkin J (2005). Child and adolescent symptom inventory-4R. Checkmate Plus. [Google Scholar]
- Gould J (2017). Towards understanding the under-recognition of girls and women on the autism spectrum. Autism, 21(6), 703–705. 10.1177/1362361317706174 [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop SL, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, Singer AT, Taylor JL, & Szatmari P (2015). Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism, 6(1), 36. 10.1186/s13229-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, & Stokes MA (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5(1), 19. 10.1186/2040-2392-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2014). Sex differences in autism spectrum disorder based on DSM-5 Criteria: Evidence from clinician and teacher reporting. Journal of Abnormal Child Psychology, 42(8), 1381–1393. 10.1007/s10802-014-9881-x [DOI] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2016). Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism : The International Journal of Research and Practice, 20(1), 75–84. 10.1177/1362361314568899 [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, & Bölte S (2016). Premature mortality in autism spectrum disorder. British Journal of Psychiatry, 208(03), 232–238. 10.1192/bjp.bp.114.160192 [DOI] [PubMed] [Google Scholar]
- Hofvander B, Delorme R, Chaste P, Nydén A, Wentz E, Ståhlberg O, Herbrecht E, Stopin A, Anckarsäter H, Gillberg C, Råstam M, & Leboyer M (2009). Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry, 9(1), 35. 10.1186/1471-244X-9-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlin P, Moss P, Savage S, & Rutter M (2013). Social outcomes in mid- to later adulthood among individuals diagnosed with autism and average nonverbal IQ as children. Journal of the American Academy of Child and Adolescent Psychiatry, 52(6), 572–581.e1. 10.1016/j.jaac.2013.02.017 [DOI] [PubMed] [Google Scholar]
- Hull L, Lai MC, Baron-Cohen S, Allison C, Smith P, Petrides KV, & Mandy W (2020). Gender differences in self-reported camouflaging in autistic and non-autistic adults. Autism, 24(2), 352–363. 10.1177/1362361319864804 [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides KV, Allison C, Smith P, Baron-Cohen S, Lai M-C, & Mandy W (2017). “Putting on my best normal”: Social camouflaging in adults with autism spectrum conditions. Journal of Autism and Developmental Disorders, 47(8), 2519–2534. 10.1007/s10803-017-3166-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfiszer L, Davies F, & Collins S (2017). ‘I was just so different’: The experiences of women diagnosed with an autism spectrum disorder in adulthood in relation to gender and social relationships. Autism, 21(6), 661–669. 10.1177/1362361316687987 [DOI] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, & Saulnier CA (2011). The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders, 41(8), 1007–1018. 10.1007/s10803-010-1126-4 [DOI] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Kenyon S (2014). Autism in pink: Qualitative research report.
- Kirkovski M, Enticott PG, & Fitzgerald PB (2013). A review of the role of female gender in autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(11), 2584–2603. 10.1007/s10803-013-1811-1 [DOI] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, & Lord C (2007). Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders, 37(4), 748–759. 10.1007/s10803-006-0229-4 [DOI] [PubMed] [Google Scholar]
- Kraijer DW (2000). Review of adaptive behavior studies in mentally retarded persons with autism/pervasive developmental disorder. Journal of Autism and Developmental Disorders, 30(1), 39–47. 10.1023/A:1005460027636 [DOI] [PubMed] [Google Scholar]
- Kraper CK, Kenworthy L, Popal H, Martin A, & Wallace GL (2017). The gap between adaptive behavior and intelligence in autism persists into young adulthood and is linked to psychiatric co-morbidities. Journal of Autism and Developmental Disorders, 47(10), 3007–3017. 10.1007/s10803-017-3213-2 [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Auyeung B, Chakrabarti B, & Baron-Cohen S (2015). Sex/gender differences and autism: Setting the scene for future research. Journal of the American Academy of Child and Adolescent Psychiatry, 54(1), 11–24. 10.1016/j.jaac.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Pasco G, Ruigrok ANV, Wheelwright SJ, Sadek SA, Chakrabarti B, & Baron-Cohen S (2011). A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE, 6(6), e20835. 10.1371/journal.pone.0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Ruigrok AN, Chakrabarti B, Auyeung B, Szatmari P, Happé F, & Baron-Cohen S (2017). Quantifying and exploring camouflaging in men and women with autism. Autism, 21(6), 690–702. 10.1177/1362361316671012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, & Rutter M (2003). Social communication questionnaire (SCQ). Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule–2nd edition (ADOS-2) Manual (Part I): Modules 1–4. Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Loveland KA, & Kelley ML (1991). Development of adaptive behavior in preschoolers with autism or Down syndrome. American Journal on Mental Retardation, 96(1), 13–20. [PubMed] [Google Scholar]
- Maddox BB, Trubanova A, & White SW (2017). Untended wounds: Non-suicidal self-injury in adults with autism spectrum disorder. Autism, 21(4), 412–422. 10.1177/1362361316644731 [DOI] [PubMed] [Google Scholar]
- Mahendiran T, Dupuis A, Crosbie J, Georgiades S, Kelley E, Liu X, Nicolson R, Schachar R, Anagnostou E, & Brian J (2019). Sex differences in social adaptive function in autism spectrum disorder and attention-deficit hyperactivity disorder. Frontiers in Psychiatry, 10, 607. 10.3389/fpsyt.2019.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson JL, & Shoemaker M (2009). Intellectual disability and its relationship to autism spectrum disorders. Research in Developmental Disabilities, 30(6), 1107–1114. 10.1016/j.ridd.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, Carver L, Charman T, Chawarska K, Curtin S, Dobkins K, Hertz-Picciotto I, Hutman T, Iverson JM, Landa R, Nelson CA, Stone WL, Tager-Flusberg H, & Zwaigenbaum L (2015). Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular Autism, 6(1). 10.1186/s13229-015-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner V, McIntosh H, Colvert E, & Happé F (2019). A qualitative exploration of the female experience of autism spectrum disorder (ASD). Journal of Autism and Developmental Disorders, 49(6), 2389–2402. 10.1007/s10803-019-03906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald TM, Winter-Messiers MA, Gibson B, Schmidt AM, Herr CM, & Solomon M (2016). Sex differences in internalizing problems during adolescence in autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(2), 624–636. 10.1007/s10803-015-2608-1 [DOI] [PubMed] [Google Scholar]
- Parish-Morris J, Liberman MY, Cieri C, Herrington JD, Yerys BE, Bateman L, Donaher J, Ferguson E, Pandey J, & Schultz RT (2017). Linguistic camouflage in girls with autism spectrum disorder. Molecular Autism, 8(1), 48. 10.1186/s13229-017-0164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, & Kenworthy L (2015). Increasing adaptive behavior skill deficits from childhood to adolescence in autism spectrum disorder: Role of executive function. Journal of Autism and Developmental Disorders, 45(6), 1579–1587. 10.1007/s10803-014-2309-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratto AB, Kenworthy L, Yerys BE, Bascom J, Wieckowski AT, White SW, Wallace GL, Pugliese C, Schultz RT, Ollendick TH, Scarpa A, Seese S, Register-Brown K, Martin A, & Anthony LG (2018). What about the girls? Sex-based differences in autistic traits and adaptive skills. Journal of Autism and Developmental Disorders, 48(5), 1698–1711. 10.1007/s10803-017-3413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2008). ADI-R. Autism Diagnostic Interview Revised. Manual. Weatern Psychological Services. [Google Scholar]
- Schatz J, & Hamdan-Allen G (1995). Effects of age and IQ on adaptive behavior domains for children with autism. Journal of Autism and Developmental Disorders, 25(1), 51–60. 10.1007/BF02178167 [DOI] [PubMed] [Google Scholar]
- Schuck RK, Flores RE, & Fung LK (2019). Brief report: Sex/gender differences in symptomology and camouflaging in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 49(6), 2597–2604. 10.1007/s10803-019-03998-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, & Pellicano E (2019). “It’s different for girls”: Gender differences in the friendships and conflict of autistic and neurotypical adolescents. Autism, 23(5), 1119–1132. 10.1177/1362361318794930 [DOI] [PubMed] [Google Scholar]
- Sequenzia A (2013). I am autistic. Autistic Women & Nonbinary Network (AWN). https://awnnetwork.org/i-am-autistic/
- Somerville M, MacPherson S, & Fletcher-Watson S (2019). Camouflaging in non-autistic adults is associated with poorer mental health. 10.31234/OSF.IO/MYP4G [DOI] [Google Scholar]
- South M, Beck JS, Lundwall R, Christensen M, Cutrer EA, Gabrielsen TP, Cox JC, & Lundwall RA (2019). Unrelenting depression and suicidality in women with autistic traits. Journal of Autism and Developmental Disorders. 10.1007/s10803-019-04324-2 [DOI] [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Balla D (2005). Vineland adaptive behavior scales, 2nd edition (Vineland II), survey interview form/caregiver rating form. Pearson Assessments. [Google Scholar]
- Strang JF, Kenworthy L, Dominska A, Sokoloff J, Kenealy LE, Berl M, Walsh K, Menvielle E, Slesaransky-Poe G, Kim KE, Luong-Tran C, Meagher H, & Wallace GL (2014). Increased gender variance in autism spectrum disorders and attention deficit hyperactivity disorder. Archives of Sexual Behavior, 43(8), 1525–1533. 10.1007/s10508-014-0285-3 [DOI] [PubMed] [Google Scholar]
- Strang JF, van der Miesen AI, Caplan R, Hughes C, daVanport S, & Lai M-C (2020). Both sex- and gender-related factors should be considered in autism research and clinical practice. Autism, 24(3), 539–543. 10.1177/1362361320913192 [DOI] [PubMed] [Google Scholar]
- Tierney S, & Burns J (2017). Behind the mask: The experience of assessment, diagnosis, and living with autism for girls and young women. In Principles of Gender-Specific Medicine: Gender in the Genomic Era: Third Edition (pp. 203–217). Academic Press. 10.1016/B978-0-12-803506-1.00004-8 [DOI] [Google Scholar]
- Tierney S, Burns J, & Kilbey E (2016). Looking behind the mask: Social coping strategies of girls on the autistic spectrum. Research in Autism Spectrum Disorders, 23, 73–83. 10.1016/J.RASD.2015.11.013 [DOI] [Google Scholar]
- Trubanova A, Donlon K, Kreiser NL, Ollendick TH, & White SW (2014). Underidentification of autism spectrum disorder in females: A case series illustrating the unique presentation of this disorder in young women. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology, 2(2), 66–76. 10.21307/sjcapp-2014-010 [DOI] [Google Scholar]
- Vivanti G (2020). Ask the editor: What is the most appropriate way to talk about individuals with a diagnosis of autism? Journal of Autism and Developmental Disorders, 50(2), 691–693. 10.1007/s10803-019-04280-x [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Sparrow SA, Goudreau D, Cicchetti DV, Paul R, & Cohen DJ (1987). Social deficits in autism: An operational approach using the Vineland Adaptive Behavior Scales. Journal of the American Academy of Child and Adolescent Psychiatry, 26(2), 156–161. 10.1097/00004583-198703000-00005 [DOI] [PubMed] [Google Scholar]
- Wood-Downie H, Wong B, Kovshoff H, Mandy W, Hull L, & Hadwin JA (2020). Sex/Gender differences in camouflaging in children and adolescents with autism. Journal of Autism and Developmental Disorders, 1–12. 10.1007/s10803-020-04615-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H, Oreve MJ, & Speranza M (2018). Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Archives de Pédiatrie, 25(6), 399–403. 10.1016/j.arcped.2018.06.008 [DOI] [PubMed] [Google Scholar]


