Abstract
Purpose
To assess whether anti-Müllerian hormone (AMH) can predict response to ovulation induction (OI) with clomiphene citrate (CC), letrozole (LET), or follicle-stimulating hormone (FSH) in women with polycystic ovary syndrome (PCOS) undergoing OI/intrauterine inseminations (IUI).
Methods
A total of 738 OI/IUI cycles from 242 patients at an academic center were stratified in three groups by medication: CC (n = 295), LET (n = 180), and FSH (n = 263), in a retrospective fashion. Ovarian response to treatment (RT, development of at least one dominant follicle) was assessed using mixed effects logistic regression models.
Results
Overall, RT cycles had lower AMH levels compared to no-RT cycles (p < 0.001). This finding persisted when analysis was limited to oral agents but attenuated in FSH cycles. For CC and LET cycles, the predicted probability (PProb) for RT decreased as AMH levels increased (PProb (95%CI): 97% (93–100), 79% (70–88), and 75% (61–89); 85% (78–93), 75% (67–83), and 73% (63–86) for AMH pct.: ≤ 25th, ≥ 50th, and ≥ 75th, for CC and LET, respectively)). However, RT was noted in 98.5% of FSH/IUI cycles regardless of AMH. For CC cycles, those with AMH ≥ 75th pct. had lower odds for RT over cycles with AMH < 75th pct. (OR 0.2, 95%CI 0.04–0.8, p = 0.02). Similarly, lower odds for RT were observed in LET cycles with AMH ≥ 75th pct. (0.6, 0.3–1.4, p = 0.25).
Conclusion
In PCOS, increasing serum AMH levels are associated with lower probability of RT to oral agents. Our findings constitute a valuable tool for the clinician when counseling PCOS patients and designing a personalized ovulation induction treatment strategy.
Keywords: PCOS, Ovulation induction, Treatment response, AMH
Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent cause of oligo-anovulation, commonly seen among reproductive-aged women [1], and couples with infertility [2, 3]. Among the latter group, ovulation induction (OI) is often the first line of treatment and a few OI options are available, including clomiphene citrate (CC), letrozole (LET), and/or gonadotropins [4].
Since its introduction as an OI agent in 1961, CC became a popular treatment mainly because of its affordable cost, excellent safety profile, and ease of use [5, 6]. However, its overall low chance of success (measured both as ovulatory response and live birth rates) and its associated risk of multiple pregnancy (3–8%) and cyst formation [7], as well as its anti-estrogenic effects on the endometrium [4, 8–10], necessitated alternative treatment options. In recent years, aromatase inhibitors emerged as an alternative, and letrozole (LET) was initially utilized in cases of CC resistance. LET became a preferred treatment for OI in PCOS patients due to a growing body of evidence suggesting that stimulation with LET, as opposed to CC, is associated with higher rates of ovulation, pregnancy, and cumulative live birth, and a lower risk of multiple pregnancy [11–15]. Alternatively, gonadotropins may successfully stimulate follicular growth in PCOS patients, albeit their use is associated with increased cost and more frequent monitoring. Among patients with idiopathic infertility, on the other hand, gonadotropins are associated with higher rates of live birth at the expense of a higher rate of multiples when compared to CC and LET [16]. At the present time, establishing personalized treatment plans for ovulation induction remains challenging mostly because specific patient characteristics that best predict response to treatment have not been elucidated [17–22].
In the recent years, anti-Müllerian hormone (AMH), a dimeric glycoprotein which is usually elevated in women with PCOS [23, 24], emerged as a candidate marker with possible value in predicting OI outcomes among PCOS patients [25–27]. Among women undergoing assisted reproductive technology treatment, it correlates positively with ovarian response, and at substantially higher levels it also correlates with the risk for ovarian hyperstimulation syndrome [28, 29]. A negative association between high AMH levels and response to OI with either CC or LET has also been speculated [25–27, 30]. However, given the absence of convincing evidence regarding the association between AMH and response to OI agents, serum AMH levels are not taken into consideration in the selection of OI treatment protocols.
The objective of this study was to evaluate the association, if any, between pre-treatment serum AMH levels and follicular response to different OI regimens among women with PCOS. We hypothesized that in cycles characterized by higher pre-treatment serum AMH levels, response to treatment might differ between OI regimens (CC, LET, and FSH).
Materials and methods
Study design
The study was conducted at Massachusetts General Hospital (MGH) and was approved by the Partners Healthcare Institutional Review Board. Data from 13,588 OI/intrauterine insemination cycles, occurring between 4/2003 and 8/2019 at the MGH Fertility Center, were reviewed and patients with the diagnosis of PCOS and available pre-treatment serum levels of AMH were included in the current analysis. The diagnosis of PCOS was based on the Rotterdam criteria [31]. Cycles from women without documented pre-treatment serum AMH levels were excluded from the analysis (most performed prior to 2013).
Ovulation induction protocols
Starting dose for most CC and LET cycles was 50 and 2.5 mg, respectively, with instructions to take for 5 days starting on cycle days 3–5 (post spontaneous menstruation or progesterone withdrawal bleed). Response was monitored via transvaginal ultrasonography with baseline ultrasound performed on cycle day 3 and the first follicular phase ultrasound performed on cycle days 10–12. Monitoring frequency after that was individualized until the dominant follicle reached pre-ovulatory measurements. Similarly, in cycles utilizing gonadotropins, injections were started on the 3rd cycle day. Initial dose was determined by the patient’s fertility physician, who took into consideration the patient’s age, BMI, ovarian reserve biomarkers, and prior response, when available. Follicular development was then monitored by transvaginal ultrasonography and serum estradiol (E2) measurements, performed at regular intervals. The dose of gonadotropins was then adjusted accordingly. Ovulation was triggered with 250 μg of recombinant human chorionic gonadotropin (hCG) (Ovidrel; Serono Laboratories, Norwell, MA), when at least one dominant follicle (≥ 16mm) was identified. Cycles with development of at least one dominant follicle consisted the study’s “response to treatment” group, whereas cycles with no follicular response were canceled and consisted the study’s “no response to treatment” group. In the latter group, the treatment dose was usually increased in subsequent cycles in a “step-up” fashion (100 or 150 mg CC, 5 or 7.5 mg letrozole), unless there was either a reason to believe that the patient will benefit from another OI agent or at the patient’s request. In a smaller portion of cycles that failed to respond to oral agents, gonadotropins were used in a subsequent cycle. The selection of gonadotropins was triggered by various factors (patient’s preference, restricted insurance coverage, side effects from the original OI, or negative impact on the endometrium, etc.).
As previously reported, prior to treatment initiation, all couples underwent a standard infertility evaluation [32], identifying at least one open fallopian tube and post-processing total motile sperm counts ≥ 1 million. All recorded values, related to patient’s characteristics, were abstracted from the patients’ electronic record, and measured within the year preceding the initiation of each treatment cycle. Prior to January 2018, AMH levels were measured at Mayo Clinic Department of Laboratory Medicine and Pathology (Rochester, MN) by the Ansh Labs ultra-sensitive AMH/Müllerian-inhibiting substance enzyme-linked immunosorbent assay (ELISA) (package insert: Ansh Labs Ultra-Sensitive AMH; document AL-105; revision no.04), and later at the Brigham and Women’s Hospital Laboratory of Pathology of Boston, using the Elecsys AMH immunoassay by Roche Diagnostics (Roche Diagnostics GmbH). Seventy-one (71.4%) of the cycles occurred prior to January 2018, and the remaining 28.6% after this date.
Primary outcome measures included ovarian response to each OI regimen.
Statistics
For each treatment type, differences in serum AMH levels in cycles with response to treatment versus cycles with no response were evaluated using p values that were generated from a mixed effects logistic regression model of AMH on treatment response, accounting for patients contributing more than one cycle to any particular treatment type. The following AMH percentiles (pct.), derived from our total study population (25th (5.4 ng/ml), 50th (9.3 ng/ml), 75th (14.0 ng/ml), 90th (19.0 ng/ml)), were used as cutoffs to determine the predicted probabilities (PProb) of response to treatment. For the latter, we used mixed effects logistic regression modeling, adjusting for age and BMI, and controlling for the potential of more than one cycle per woman. Since gonadotropin cycles were characterized by an almost universal response to treatment, it was impossible to calculate PProb and odds ratio (OR) for treatment response among patients in this group. The level of statistical significance was set at 0.05. All statistical analyses were performed in Stata version 14.0.
Results
Ovulation induction cycles
Ovulation induction cycles from 2003 to 2019 were reviewed for response to treatment. Two thousand three hundred and nine (2309) OI cycles were identified among PCOS patients, but pre-treatment AMH information was available in 738 cycles only (derived from 242 women). Of those in 295 (40.0%), 180 (24.4%), and 263 (35.6%) cycles, either CC, LET, or FSH were used, respectively. In 59.7% (157/263) of FSH cycles, prior treatment with oral agents had been used. For the FSH group, the average (SD) cycle length was 17.3 (6.2) days, and the average (SD) daily dose was 63.0 (30.6) IU. The demographic and cycle characteristics of the study population are shown in Table 1. Age and BMI did not differ significantly among the treatment groups, whereas serum AMH levels did (p = 0.04) (Table 1).
Table 1.
Baseline characteristics of the study population stratified by ovulation induction medication, described by cycle
| All cycles (n = 738) | CC (n = 295) | LET (n = 180) | FSH (n = 263) | |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 32.3 (30.8–34.7) | 32.5 (30.8–34.8) | 32.1 (30.5–34.2) | 32.7 (31.0–34.8) |
| AMH, ng/ml | ||||
| Median (IQR) | 9.3 (5.4–14.0) | 7.6 (4.5–13.0) | 11.0 (7.5–14.0) | 10.0 (5.9–14.0) |
| Percentiles | ||||
| 10th | 3.1 | 2.9 | 4.5 | 2.8 |
| 25th | 5.4 | 4.5 | 7.5 | 5.9 |
| 75th | 14.0 | 13.0 | 14.0 | 14.0 |
| 90th | 19.0 | 19.6 | 19.0 | 19.0 |
| Day 3 FSH, U/L | ||||
| Median (IQR) | 5.8 (4.8–6.7) | 6.0 (5.1–6.7) | 5.8 (4.5–6.8) | 5.5 (4.6–6.8) |
| BMI, kg/m2 | ||||
| Median (IQR) | 24.6 (22.1–30) | 24.6 (22.3–29.4) | 23.5 (21.8–30.6) | 25.7 (22.1–31.1) |
| Race, n (%) | ||||
| Caucasian | 463 (62.7) | 201 (68.1) | 117 (65.0) | 145 (55.1) |
| Asian | 105 (14.2) | 25 (8.5) | 26 (14.4) | 54 (20.5) |
| Hispanic | 44 (6.0) | 19 (6.4) | 11 (6.1) | 14 (5.3) |
| African American | 37 (5.0) | 16 (5.4) | 6 (3.3) | 15 (5.7) |
| Indian | 31 (4.2) | 6 (2.1) | 3 (1.7) | 22 (8.4) |
| Mixed | 44 (6.0) | 16 (5.4) | 16 (8.9) | 12 (4.6) |
| Unknown | 14 (1.9) | 12 (4.1) | 1 (0.6) | 1 (0.4) |
| No. of follicles > 13 mma | ||||
| Median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 1.0 (1.0–2.0) |
| Endometrial thickness, (mm)a | ||||
| Median (IQR) | 8.0 (6.0–9.2) | 7.3 (6.0–9.0) | 6.9 (5.6–8.0) | 9.0 (7.3–10.4) |
CC clomiphene citrate, LET letrozole, FSH follicle-stimulating hormone, AMH anti-Müllerian hormone, BMI body mass index. aThese values were recorded on the last monitoring ultrasound prior to trigger
Treatment response and AMH
Overall, the majority of cycles (660 cycles (89.4%)) demonstrated a response to treatment. In the oral medication cycles, where no response was noted to the initial treatment dose, the dose was increased in a subsequent attempt in 60% of the cycles, and the same OI agent was utilized. In 80% of those, response to treatment was observed. In 15% of the cycles with no response, gonadotropins were utilized in a subsequent attempt, and in all but one cycles response to treatment was observed. Finally, in the remaining oral medication cycles with no response to treatment, either a different oral agent was utilized in a subsequent cycle or the same agent was used without altering the dose, and overall response was observed in 78.6% of them.
When the response rates were compared among the three treatment groups, significant differences were observed (p < 0.001). Nearly all FSH cycles (98.5%) responded to treatment, while lower response rates were noted among CC (87.8%) and LET (78.9%) cycles. In our total population, cycles characterized by response to treatment when compared to those with no response had significantly lower AMH levels. This difference persisted even when the comparison was limited within each of the oral OI regimen groups, where cycles with treatment response had lower AMH levels than the ones with no response (for both CC and LET groups). The difference in AMH levels was attenuated within the FSH group (Table 2).
Table 2.
Response to treatment in relation to serum anti-Müllerian hormone (AMH) levels, described by cycle
| Overall treatment | Clomiphene citrate | Letrozole | FSH | |||||
|---|---|---|---|---|---|---|---|---|
| AMH (ng/ml) | No-RT (n = 78) | RT (n = 660) | No-RT (n = 36) | RT (n = 259) | No-RT (n = 38) | RT (n = 142) | No-RT (n = 4) | RT (n = 259) |
| Mean (SD) | 13.7 (7.1) | 10.1 (6.6) | 13.8 (7.9) | 9.2 (7.2) | 13.1 (6.0) | 10.7 (5.4) | 18.3 (9.3) | 10.7 (6.4) |
| Median (IQR) | 12.0 (8.6–19.0) | 8.8 (5.0–13.0) | 11.5 (7.8–19.0) | 7.3 (3.9–12.0) | 11.7 (8.7–15.0) | 11.0 (6.8–14.0) | 16.5 (10.5–28.0) | 10.0 (5.8–14.0) |
| p value* | < 0.001 | 0.017 | 0.019 | < 0.001** | ||||
No-RT no response to treatment, RT response to treatment, FSH follicle-stimulating hormone
*p values generated from mixed effects logistic regression of AMH on treatment response for each treatment type, to account for patients contributing more than one cycle to any particular treatment type
**For the FSH group, p value results should be interpreted with extreme caution since 98.5% of cycles showed response to treatment and only 4 cycles comprise the no-RT group
When cycles were stratified by serum AMH pct., all of those with serum AMH ≤ 10th pct. (3.1 ng/ml) were characterized by response to medication. Overall, after adjusting for age and BMI, there was a trend for lower PProb for response to treatment with higher serum AMH levels (p < 0.001) (Table 3). Similarly, this trend persisted when analysis was restricted within each treatment group for CC and LET cycles (p < 0.001 and p = 0.075, respectively) (Table 3), but response to OI was noted in nearly all FSH cycles regardless of serum AMH levels.
Table 3.
Predicted probability of response to treatment (PProb-RT)
| AMH percentile (pct.) (ng/ml) | All treatments (N = 738) PProb-RT (95%CI) |
Clomiphene citrate (N = 295) PProb-RT (95%CI) |
Letrozole (N = 180) PProb-RT (95%CI) |
|---|---|---|---|
| ≤ 25th pct. (5.4) | 97% (95–99%) | 97% (93–100%) | 95% (85–105%) |
| ≥ 50th pct. (9.3) | 87% (83–90%) | 79% (70–88%) | 75% (67–83%) |
| ≥ 75th pct. (14.0) | 85% (79–90% | 75% (61–89%) | 73% (60–86%) |
| ≥ 90th pct. (19.0) | 82% (73–90%) | 75% (58–92%) | 69% (49–90%) |
| P for trend | < 0.001 | < 0.001 | 0.075 |
AMH anti-Müllerian hormone. Reported results are adjusted for mean age and body mass index of the study population
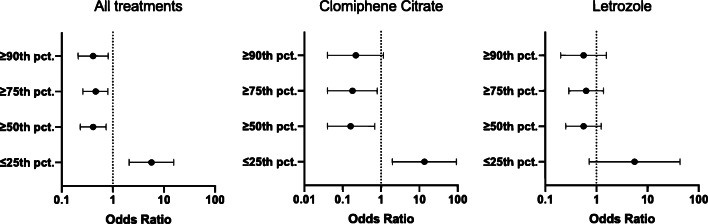
Overall, cycles with AMH ≤ 25th pct. (5.4 ng/ml) were almost 6 times more likely to respond to any treatment over the ones with AMH > 25th pct. (OR 5.7, 95%CI 2.1–15.5, p < 0.001). Similarly, when limiting the analysis in CC cycles only, the odds of treatment response was 13.5 times higher in cycles with AMH ≤ 25th pct. compared to cycles with AMH > 25th pct. (OR 13.5, 95%CI 2.0–92.1, p = 0.01), whereas cycles with AMH ≥ 75th pct. (14.0 ng/ml) had significantly lower odds to respond to CC over those with AMH < 75th pct. (OR 0.2, 95%CI 0.04–0.8, p = 0.02). Although this trend was maintained in LET cycles as well, the difference did not reach statistical significance: neither for LET cycles with AMH ≤ 25th pct. compared to cycles with AMH > 25th pct. (OR 5.6, 95%CI 0.7–43.4, p = 0.10) nor for those with AMH ≥ 75th pct. compared to cycles with AMH < 75th pct. (OR 0.6, 95%CI 0.3–1.4, p = 0.25) (Fig. 1).
Fig. 1.
Association between anti-Müllerian hormone levels (expressed in percentiles, pct.) and treatment response (odds ratio, 95% confidence intervals) for the different treatment groups, after adjusting for mean age and body mass index of the study population
Discussion
Ovulation induction is often the first line of treatment among infertile women with PCOS and the selection of the proper medication is important in achieving ovulation and ultimately pregnancy in such patients. Personalized medicine is still emerging in reproductive sciences, although it has advanced immensely in other areas, such as oncology. New biomarkers are being described and the significance of already established markers is reassessed [33]. Given the availability of multiple regimens for OI, tailored patient-specific ovarian stimulation protocols may allow for optimal treatment response rates.
Our data suggest that among subpopulations of PCOS patients with high serum AMH levels and therefore a higher probability of no response to orally administered ovarian OI agents, there might be a benefit to using gonadotropins as a first-line OI agent (while utilizing a conservative approach to stimulation) or to considering a higher initial dose for the oral agent. In our cohort, nearly all cycles with gonadotropins achieved ovulation regardless of AMH, while significantly lower response rates were noted among cycles with AMH above the 75th or 90th pct. (14.0 or 19.0 ng/ml, respectively) of our population utilizing oral OI agents.
As seen in other studies, serum AMH was significantly lower in cycles with response to CC and LET compared to cycles with no response to these agents [26, 30, 34]. The effect of AMH on outcomes of ovulation induction with gonadotropins is not very well described. Amer et al. [35] and Di Paola et al. [36] suggested that PCOS women with substantially elevated serum AMH levels would have a diminished response to ovulation induction with gonadotropins and recommended stimulation with increased dosage, which, however, might ultimately lead to over-response. Nevertheless, the restricted number of participants (n = 20 and n = 22 PCOS patients, respectively) and the lack of additional information concerning response rates after using increased doses limit the generalizability of these findings. With our population of 263 FSH cycles in PCOS patients and the consistency in the management of patients undergoing ovarian stimulation, the present study adds novel information to the existing literature.
Women with PCOS typically have elevated serum AMH levels, potentially due to the increased number of small antral follicles, the higher AMH production per granulosa cell, and/or a positive correlation with androgens [37]. AMH appears to inhibit the gonadotropin-induced aromatase expression in granulosa cells and, also, to reduce the FSH receptor mRNA expression [38]. While for low AMH levels the probability of response to CC or LET was excellent, our analysis showed that this probability decreased as AMH increased. In our study population, the probability of no response to treatment (for either CC or LET) was over 20% for cycles with serum AMH levels above the median of our population (9.3 ng/ml). In these patients, it might be preferable to skip OI with oral agents and proceed to a conservative gonadotropin stimulation regimen instead, since response to treatment was achieved in nearly all cycles in which FSH was used, while the number of generated pre-ovulatory follicles remained comparable between all groups. We could hypothesize that the effect of CC or LET in the endogenous pathway of gonadotropin production may not be enough to overcome the inhibitory effect of AMH when serum levels are above a certain point. As for exogenous gonadotropin administration, a step-up dosing protocol or increased duration of treatment might outweigh the negative effect of AMH. However, among women with substantially elevated serum AMH values, gonadotropin stimulation requires careful selection of the dose, and closer monitoring with the ultimate goal of mono-ovulatory response, so as to decrease the risk of multiples and ovarian hyperstimulation syndrome, given the positive correlation reported for the latter with serum AMH levels in the in vitro fertilization (IVF) setting [39]. Alternatively, if oral agents are preferred by either the treating physician or the couple, it might be reasonable to consider a higher starting dose.
Our study has a few strengths. Our PCOS population is diverse and includes patients in all BMI and AMH groups. All patients were evaluated and treated in a single academic practice utilizing the same clearly defined protocols. Furthermore, the fact that we did not focus on absolute AMH values but rather on AMH percentiles, as defined by our population, increases the generalizability of our findings, since it might partially account for the variability of the AMH assays between clinics. Our study has certain limitations, as well. A new assay for the measurement of AMH was used towards the later part of the study (affecting 28.6% of the cycles). The performance of the assays and their degree of agreement have been previously evaluated with results suggesting that they provide comparable measurements with a slight discordance mainly in the lower values [40]. Since the vast majority of our patients do not have AMH levels in the low range, where the assays slightly differ, the impact should be minimal if any. The 10th pct. for AMH in our study population is at 3.1 ng/ml; therefore, very few patients, if any, fall in the low range where the assays might differ in performance. Furthermore, the utilization of AMH percentiles, rather than absolute values, might account further for any variability in the assays. Interestingly, the mean (SD) BMI of our population (26.5 (5.7) kg/m2) is lower than that reported among other PCOS cohorts, and the interquartile range was 22.1–30.0 kg/m2. The Fertility Center utilizes a strict BMI cutoff of 40.0 kg/m2 to initiate treatments and this might have contributed to the observed difference. However, given the association between BMI and response to treatment [32], one would not expect patients with BMIs higher than that of our population to respond better. For that reason, one can assume that our leaner population represents the “best-case scenario” regarding the possibility of response. Notwithstanding, approximately 40% of the cycles in the current study derive from obese PCOS patients. Finally, the study is limited by its retrospective design.
In conclusion, we showed that, for patients with PCOS, higher serum AMH levels, as compared to lower levels, are associated with significantly lower probability of response to either CC or LET, whereas nearly all patients responded to FSH irrespective of pre-treatment AMH levels. With AMH values ≤ 25th pct., the vast majority of patients would respond to either treatment, while with AMH values > 75th percentile (> 14.0 ng/ml in our study population) the probability of response decreased for both CC and LET cycles. Our findings constitute a valuable tool for the clinician when counseling PCOS patients and designing a personalized ovulation induction treatment strategy.
Code availability
Not applicable.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azziz R. Introduction: determinants of polycystic ovary syndrome. Fertil Steril. 2016;106(1):4–5. doi: 10.1016/j.fertnstert.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande PS, Gupta AS. Causes and prevalence of factors causing infertility in a public health facility. J Hum Reproduct Sci. 2019;12(4):287–293. doi: 10.4103/jhrs.JHRS_140_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brugo-Olmedo S, Chillik C, Kopelman S. Definition and causes of infertility. Reprod BioMed Online. 2001;2(1):41–53. doi: 10.1016/s1472-6483(10)62187-6. [DOI] [PubMed] [Google Scholar]
- 4.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 5.Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. Preliminary report. Jama. 1961;178:101–104. doi: 10.1001/jama.1961.03040410001001. [DOI] [PubMed] [Google Scholar]
- 6.Homburg R. Clomiphene citrate--end of an era? A mini-review. Hum Reprod. 2005;20(8):2043–2051. doi: 10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Kunselman AR, Brzyski RG, Casson PR, Diamond MP, Schlaff WD, Christman GM, Coutifaris C, Taylor HS, Eisenberg E, Santoro N, Zhang H, NICHD Reproductive Medicine Network The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial: rationale and design of a double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Contemp Clin Trials. 2012;33(3):470–481. doi: 10.1016/j.cct.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch Petersen K, Pedersen NG, Pedersen AT, Lauritsen MP, la Cour Freiesleben N. Mono-ovulation in women with polycystic ovary syndrome: a clinical review on ovulation induction. Reprod BioMed Online. 2016;32(6):563–583. doi: 10.1016/j.rbmo.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Thessaloniki EA-SPCWG. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H, NICHD Reproductive Medicine Network Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzer H, Casper R, Tulandi T. A new era in ovulation induction. Fertil Steril. 2006;85(2):277–284. doi: 10.1016/j.fertnstert.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 13.Badawy A, Abdel Aal I, Abulatta M. Clomiphene citrate or letrozole for ovulation induction in women with polycystic ovarian syndrome: a prospective randomized trial. Fertil Steril. 2009;92(3):849–852. doi: 10.1016/j.fertnstert.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Du X, Wang R, Li R, Wang H, Luo L, et al. Ovulation induction and intrauterine insemination in infertile women with polycystic ovary syndrome: a comparison of drugs. Eur J Obstet Gynecol Reprod Biol. 2018;231:117–121. doi: 10.1016/j.ejogrb.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34(1):73–76. doi: 10.1177/147323000603400109. [DOI] [PubMed] [Google Scholar]
- 16.Diamond MP, Legro RS, Coutifaris C, Alvero R, Robinson RD, Casson P, Christman GM, Ager J, Huang H, Hansen KR, Baker V, Usadi R, Seungdamrong A, Bates GW, Rosen RM, Haisenleder D, Krawetz SA, Barnhart K, Trussell JC, Ohl D, Jin Y, Santoro N, Eisenberg E, Zhang H, NICHD Reproductive Medicine Network Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373(13):1230–1240. doi: 10.1056/NEJMoa1414827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homburg R, Hendriks ML, Konig TE, Anderson RA, Balen AH, Brincat M, Child T, Davies M, D'Hooghe T, Martinez A, Rajkhowa M, Rueda-Saenz R, Hompes P, Lambalk CB. Clomifene citrate or low-dose FSH for the first-line treatment of infertile women with anovulation associated with polycystic ovary syndrome: a prospective randomized multinational study. Hum Reprod. 2012;27(2):468–473. doi: 10.1093/humrep/der401. [DOI] [PubMed] [Google Scholar]
- 18.Lopez E, Gunby J, Daya S, Parrilla JJ, Abad L, Balasch J. Ovulation induction in women with polycystic ovary syndrome: randomized trial of clomiphene citrate versus low-dose recombinant FSH as first line therapy. Reprod BioMed Online. 2004;9(4):382–390. doi: 10.1016/s1472-6483(10)61273-4. [DOI] [PubMed] [Google Scholar]
- 19.van Santbrink EJ, Eijkemans MJ, Laven JS, Fauser BC. Patient-tailored conventional ovulation induction algorithms in anovulatory infertility. Trends Endocrinol Metab. 2005;16(8):381–389. doi: 10.1016/j.tem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.van Santbrink EJ, Fauser BC. Is there a future for ovulation induction in the current era of assisted reproduction? Hum Reprod. 2003;18(12):2499–2502. doi: 10.1093/humrep/deg515. [DOI] [PubMed] [Google Scholar]
- 21.Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 1998;83(7):2361–2365. doi: 10.1210/jcem.83.7.4919. [DOI] [PubMed] [Google Scholar]
- 22.Palihawadana TS, Wijesinghe PS, Seneviratne HR. Factors associated with nonresponse to ovulation induction using letrozole among women with World Health Organization group II anovulation. J Hum Reproduct Sci. 2015;8(2):75–79. doi: 10.4103/0974-1208.158598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cate RL, Mattaliano RJ, Hession C, Tizard R, Farber NM, Cheung A, Ninfa EG, Frey AZ, Gash DJ, Chow EP, Fisher RA, Bertonis JM, Torres G, Wallner BP, Ramachandran KL, Ragin RC, Manganaro TF, MacLaughlin DT, Donahoe PK. Isolation of the bovine and human genes for Mullerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 24.Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, Grayson K. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28(4):1077–1083. doi: 10.1093/humrep/det015. [DOI] [PubMed] [Google Scholar]
- 25.Gulsen MS, Ulu I, Yildirim Kopuk S, Kiran G. The role of anti-Mullerian hormone in predicting clomiphene citrate resistance in women with polycystic ovarian syndrome. Gynecol Endocrinol. 2019;35(1):86–89. doi: 10.1080/09513590.2018.1499085. [DOI] [PubMed] [Google Scholar]
- 26.Mumford SL, Legro RS, Diamond MP, Coutifaris C, Steiner AZ, Schlaff WD, Alvero R, Christman GM, Casson PR, Huang H, Santoro N, Eisenberg E, Zhang H, Cedars MI. Baseline AMH level associated with ovulation following ovulation induction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101(9):3288–3296. doi: 10.1210/jc.2016-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi W, Yang Y, Mao H, Zhao X, Liu M, Fu S. Circulating anti-Mullerian hormone as predictor of ovarian response to clomiphene citrate in women with polycystic ovary syndrome. J Ovar Res. 2016;9:3. doi: 10.1186/s13048-016-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tal R, Seifer DB, Khanimov M, Malter HE, Grazi RV, Leader B. Characterization of women with elevated anti-Mullerian hormone levels (AMH): correlation of AMH with polycystic ovarian syndrome phenotypes and assisted reproductive technology outcomes. Am J Obstet Gynecol. 2014;211(1):59 e1–59 e8. doi: 10.1016/j.ajog.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum Mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–471. doi: 10.1016/s0015-0282(01)03201-0. [DOI] [PubMed] [Google Scholar]
- 30.Mahran A, Abdelmeged A, El-Adawy AR, Eissa MK, Shaw RW, Amer SA. The predictive value of circulating anti-Mullerian hormone in women with polycystic ovarian syndrome receiving clomiphene citrate: a prospective observational study. J Clin Endocrinol Metab. 2013;98(10):4170–4175. doi: 10.1210/jc.2013-2193. [DOI] [PubMed] [Google Scholar]
- 31.Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Souter I, Baltagi LM, Kuleta D, Meeker JD, Petrozza JC. Women, weight, and fertility: the effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertil Steril. 2011;95(3):1042–1047. doi: 10.1016/j.fertnstert.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 33.Simon C, Sakkas D, Gardner DK, Critchley HO. Biomarkers in reproductive medicine: the quest for new answers. Hum Reprod Update. 2015;21(6):695–697. doi: 10.1093/humupd/dmv043. [DOI] [PubMed] [Google Scholar]
- 34.Ellakwa HE, Sanad ZF, Hamza HA, Emara MA, Elsayed MA. Predictors of patient responses to ovulation induction with clomiphene citrate in patients with polycystic ovary syndrome experiencing infertility. Int J Gynaecol Obstet. 2016;133(1):59–63. doi: 10.1016/j.ijgo.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Amer SA, Mahran A, Abdelmaged A, El-Adawy AR, Eissa MK, Shaw RW. The influence of circulating anti-Mullerian hormone on ovarian responsiveness to ovulation induction with gonadotropins in women with polycystic ovarian syndrome: a pilot study. Reprod Biol Endocrinol RB&E. 2013;11:115. doi: 10.1186/1477-7827-11-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Paola R, Garzon S, Giuliani S, Lagana AS, Noventa M, Parissone F, et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch Gynecol Obstet. 2018;298(5):1029–1035. doi: 10.1007/s00404-018-4906-2. [DOI] [PubMed] [Google Scholar]
- 37.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, Griesinger G, Kelsey TW, la Marca A, Lambalk C, Mason H, Nelson SM, Visser JA, Wallace WH, Anderson RA. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 38.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, Brown K, Simpson ER, Mason HD. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96(5):1246–1251. doi: 10.1016/j.fertnstert.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Sopa N, Larsen EC, Westring Hvidman H, Andersen AN. An AMH-based FSH dosing algorithm for OHSS risk reduction in first cycle antagonist protocol for IVF/ICSI. Eur J Obstet Gynecol Reprod Biol. 2019;237:42–47. doi: 10.1016/j.ejogrb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson JM, Pepin D, Duru C, Matejtschuk P, Donahoe PK, Burns CJ. Towards international standardization of immunoassays for Müllerian inhibiting substance/anti-Müllerian hormone. Reprod BioMed Online. 2018;37(5):631–640. doi: 10.1016/j.rbmo.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.