Abstract
Background
The incidence of non-alcoholic fatty liver disease (NAFLD) is rapidly increasing worldwide parallel to the global obesity epidemic. NAFLD encompasses a range of liver pathologies and most often originates from metabolically driven accumulation of fat in the liver, or non-alcoholic fatty liver (NAFL). In a subset of NAFL patients, the disease can progress to non-alcoholic steatohepatitis (NASH), which is a more severe form of liver disease characterized by hepatocyte injury, inflammation, and fibrosis. Significant progress has been made over the past decade in our understanding of NASH pathogenesis, but gaps remain in our mechanistic knowledge of the precise metabolic triggers for disease worsening.
Scope of review
The transition from NAFL to NASH likely involves a complex constellation of multiple factors intrinsic and extrinsic to the liver. This review focuses on early metabolic events in the establishment of NAFL and initial stages of NASH. We discuss the association of NAFL with obesity as well as the role of adipose tissue in disease progression and highlight early metabolic drivers implicated in the pathological transition from hepatic fat accumulation to steatohepatitis.
Major conclusions
The close association of NAFL with features of metabolic syndrome highlight plausible mechanistic roles for adipose tissue health and the release of lipotoxic lipids, hepatic de novo lipogenesis (DNL), and disruption of the intestinal barrier in not only the initial establishment of hepatic steatosis, but also in mediating disease progression. Human genetic variants linked to NASH risk to date are heavily biased toward genes involved in the regulation of lipid metabolism, providing compelling support for the hypothesis that NASH is fundamentally a metabolic disease.
Keywords: NAFLD, NASH, Obesity, Adipose tissue, Lipotoxicity, Fructose
Highlights
-
•
NAFLD is fundamentally a disease of altered systemic metabolism.
-
•
Transition from NAFL to NASH involves a complex interaction of multiple factors.
-
•
Genetic variants linked to NASH are largely genes involved in lipid metabolism.
-
•
Adipose dysfunction and gut barrier integrity likely influence progression to NASH.
Abbreviations
- ACC
acetyl-CoA carboxylase
- BMI
body mass index
- ChREBP
carbohydrate regulatory element-binding protein
- CVD
cardiovascular disease
- DNL
de novo lipogenesis
- FA
fatty acid
- FAS
fatty acid synthase
- FFA
free fatty acid
- GCKR
glucokinase regulatory protein
- GLP-1
glucagon-like peptide 1
- HCC
hepatocellular carcinoma
- HFD
high-fat diet
- HGP
hepatic glucose production
- HSCs
hepatic stellate cells
- HSD17b13
hydroxysteroid 17-beta dehydrogenase 13
- IEC
intestinal epithelial cells
- KHK
ketohexokinase
- LCFA
long-chain fatty acids
- LDLR
low-density lipoprotein receptor
- MARC1
mitochondrial amidoxime reducing component 1
- MBOAT7
membrane-bound O-acyltransferase domain-containing protein 7
- NAFL
non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- oxLDL
oxidized LDL
- oxPL
oxidized phospholipid
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- PUFA
polyunsaturated fatty acids
- ROS
reactive oxygen species
- SREBP1c
sterol regulatory element-binding protein 1c
- T2D
type 2 diabetes
- TG
triglyceride
- TM6SF2
transmembrane 6 superfamily member 2
- TJP
tight junction protein
- VLDL
very low-density lipoprotein
- WC
waist circumference
1. Introduction
NAFLD comprises a continuum of liver pathologies ranging from NAFL, defined by the presence of fat accumulation in ≥5% of hepatocytes by histological assessment or non-invasive imaging [1], to a more serious and progressive form of the disease, NASH. The definitive diagnosis of NASH is established by liver biopsy and histologically characterized by the presence of hepatocyte injury and inflammation and typically pericellular fibrosis [2]. Patients with NASH are at a substantially greater risk of progression to cirrhosis and hepatocellular carcinoma (HCC) than those with hepatic steatosis alone and at a greater risk of mortality, with fibrosis being the most predictive histological feature [3,4]. A scarcity of robust longitudinal data in NASH patients and a lack of stage-specific circulating biomarkers continues to create impediments for understanding who might be at risk for disease progression. Moreover, reliance on a liver biopsy as the gold standard for NASH diagnosis creates challenges as accuracy and reproducibility can be diminished by sampling variability and subjectivity of pathological grading.
The prevalence of NAFLD is escalating globally at an unprecedented rate, paralleling the increase in obesity and features of metabolic syndrome. In the US alone, the incidence of NAFLD is estimated at ∼25% of the population and the prevalence of NASH is estimated to be 3–4% [5], with a projected economic burden of more than $100 billion in NAFLD-related US health care costs [6]. Notably, NASH is associated with an increased risk of cardiovascular disease (CVD), a leading cause of mortality in patients with the disease [7,8]. Despite these staggering numbers and a renewed focus on the etiology of this liver disease, gaps remain in our collective understanding of the specific molecular and biochemical events underlying the transition from fatty liver to NASH.
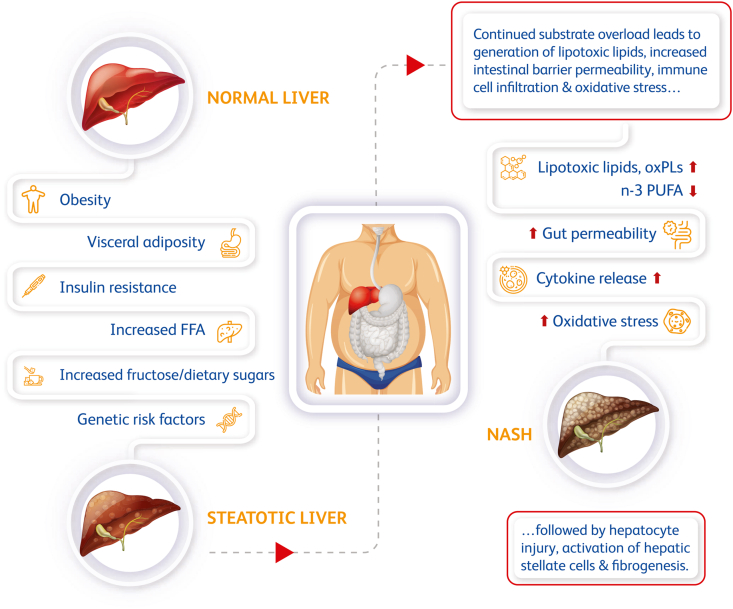
The journey along the disease spectrum from NAFL to NASH is heterogenous and a complex collective integration of metabolic comorbidities, genetic variations, age, ethnicity, and diet [9]. At a high level, the disease trajectory of NAFLD can be described in three phenotypic stages: NAFL, NASH (with or without fibrosis), and ultimately cirrhosis and/or HCC. Early metabolic features driving NAFL include overnutrition, insulin resistance, and obesity. Multiple insults have been posited to contribute to worsening of the disease subsequent or in parallel to excess hepatic fat accumulation, including lipotoxicity, increased oxidative stress, inflammatory cytokine release, and intestinal barrier disruption (Figure 1). In later disease stages, pathogenic factors promote inflammation and fibrosis in the liver via activation of resident tissue Kupffer cells, recruitment of circulating immune cells, and subsequent activation of hepatic stellate cells (HSCs) ultimately leading to fibrogenesis. In concordance, later stages of NASH are associated with significant histological evidence of inflammation and fibrosis, highlighting an important role of inflammatory cells and activation of HSCs later in the course of disease (reviewed extensively elsewhere, including in [[10], [11], [12], [13]]). Understanding the molecular basis for progression from hepatic steatosis to NASH, a critical first step for effective diagnosis and treatment of this disease, has proven challenging given the complex and heterogeneous disease trajectory. In this review, we focus on the metabolic mechanisms that contribute to the initial deposition of fat in the liver and metabolic insults that may subsequently influence progression from NAFL to early stages of NASH.
Figure 1.
Risk factors associated with progression from fatty liver to NASH. Obesity, insulin resistance, and excess visceral adiposity are associated with the development of NAFL. Hepatic steatosis can be further exacerbated by overnutrition and consumption of dietary sugars and may also be influenced by genetic risk. FFA flux to the liver combined with increased DNL results in substrate overload and an inability of the liver to properly metabolize lipids. Many factors are hypothesized to play a role in the transition to NASH, including a build-up of lipotoxic or oxidized lipids, disrupted intestinal barrier function, increased immune cell activation, secretion of inflammatory cytokines, and elevated hepatic oxidative stress.
2. Hepatic steatosis and adipose tissue insulin resistance
Fat can accumulate in the liver via several mechanisms; the major sources in humans are flux of free fatty acids (FFAs) from adipose tissue due to increased lipolysis, increased DNL, and dietary fat intake [14,15]. Human isotope-labeling studies demonstrate that (1) DNL is significantly upregulated in NAFLD patients and (2) the major component of hepatic fat in NAFLD comes from excess circulating FFAs [[16], [17], [18], [19]]. Under normal conditions, plasma FFA concentrations rise during fasting to supply substrate for tissues in the body and decline in response to feeding due to insulin-induced suppression of lipolysis. In the context of insulin resistance, however, unrestrained lipolysis leads to increased levels of circulating FFAs and deposition of fat in the liver, highlighting adipocyte insulin resistance as a major contributor to NAFLD [20]. Consistent with this hypothesis, plasma FFA levels were suppressed and liver triglyceride (TG) levels were significantly decreased in high-fat diet (HFD)-fed mice upon chronic suppression of lipolysis via activation of adipocyte Gi signaling [21]. Recent studies also pointed to an important link between a failure of insulin to suppress lipolysis in adipose and the regulation of hepatic glucose production (HGP). Using in vivo metabolomics in rats, Perry et al. showed that the ability of insulin to suppress lipolysis is critical for suppression of HGP via reducing hepatic acetyl CoA levels and pyruvate carboxylase activity, which regulates the first step of gluconeogenesis in the liver [22]. Titchenell et al. utilized mouse loss-of-function models to demonstrate that excess circulating FFAs, rather than direct hepatocyte mediators of insulin signaling, are the major factor promoting HGP in the context of obesity and insulin resistance [23]. These mechanistic studies are consistent with the fact that most significant comorbid conditions associated with NAFLD are prominent features of insulin resistance, including hypertriglyceridemia, type 2 diabetes (T2D), and obesity [24].
3. Obesity and NAFLD
Epidemiological studies convincingly show that obesity is a major risk factor for developing NAFLD [5,25]. In adults, overweight is defined as a body mass index (BMI) between 25.0 and 29.9 kg/m2 and obesity is defined as a BMI ≥ 30 kg/m2. Recent data showed that more than 100 million people in the US and ∼1.5 billion people worldwide are overweight or obese, with this trend continuing to rise [26,27]. Given the widespread global epidemic of obesity and associated metabolic dysfunction, the burden of NAFLD on the health care system continues to grow [29]. Obesity also continues to affect the health of children and adolescents [30], putting them at increased risk of developing NAFLD [[31], [32], [33]]. A recent meta-analysis confirmed that overweight or obese children have greater odds of developing NAFLD as adults than normal weight children [34]. The risk of having NAFLD and elevated alanine aminotransferase levels was mitigated in subjects who were obese in childhood but non-obese as adults, supporting the idea that at least some features of NAFLD may be reversible with normalization of body weight [35]. Indeed, compelling data in support of weight gain and insulin resistance as major etiological factors in the development of NASH as well as attractive therapeutic targets lies in bariatric surgery outcomes [36,37]. Bariatric surgery leads to significant weight loss and improvements in metabolic regulation resulting in reduced hepatic steatosis and in many cases, resolution of NASH. A meta-analysis of bariatric surgery studies demonstrated improvements in hepatic steatosis in over 90% of patients and improved steatohepatitis in more than 80% of patients with NASH [38]. The efficacy of weight loss in the reversal of NASH is independent of the means of weight reduction, as intensive nutritional interventions also resulted in reduced steatosis and improvements in NASH [[39], [40], [41]].
Weight loss in the ≥10% range is recognized as the most compelling treatment modality to date for meaningful histological improvements in most NASH patients [24,41,42]. These data have spurred the evaluation of the glucagon-like peptide-1 (GLP-1) class of obesity and T2D drugs for treating NASH. In recently reported results of a 72-week, randomized, double-blind placebo-controlled phase 2 study examining the treatment of NASH patients with the GLP-1 receptor agonist semaglutide (NCT02970942), 59% of patients who received a 0.4 mg daily dose of subcutaneous semaglutide had NASH resolution without worsening of fibrosis compared to 17% of subjects on placebo [43]. While it remains to be seen whether this mechanism will lead to improvements in fibrosis in larger biopsy studies, these results are consistent with the promise of GLP-1 therapies for preventing disease worsening and potentially reversing the course of the disease. It is likely that the predominant mechanism by which GLP-1 improves NASH is via weight loss and long-term reduction in insulin resistance, although direct effects on the liver have also been proposed [44].
4. Visceral adiposity and adipose tissue dysfunction
In the context of obesity, regional adiposity influences the risk of metabolic complications. Nielson et al. utilized elegant tracer and computational methods in humans to demonstrate that the delivery of FFAs derived from visceral fat to the portal circulation increases coordinately with the elevated visceral fat mass seen in obesity [45,46]. Expansion of visceral adipose in particular is linked to systemic inflammation [[47], [48], [49]]. Visceral obesity is associated with increased production of many proinflammatory cytokines, including interleukin 6, tumor necrosis factor alpha, and C-reactive protein, and a reduction in protective adipokines such as adiponectin and is thus postulated to have a more toxic profile in the context of metabolic dysfunction compared to subcutaneous fat [48,50,51]. In addition to being more metabolically active in secreting cytokines, visceral fat is more insulin resistant and lipolytic than subcutaneous fat [20,52].
Although it is widely reported that increased visceral adiposity is a risk factor for NAFLD (see Table 1 in Reference [53]), is associated with liver inflammation and fibrosis independent of hepatic steatosis [54], and is predictive of NASH with significant fibrosis [55], the specific contribution of visceral fat to the establishment of NAFL vs progression to NASH remains controversial. Fabbrini et al. suggested that intrahepatic triglyceride content, rather than visceral adipose tissue, may be a better indicator of metabolic complications associated with obesity [56]. Using waist circumference (WC) as a surrogate marker of visceral adiposity, Francanzani et al. reported that patients with normal WC can have NASH and are at a risk of progression to fibrosis, suggesting that visceral adiposity may be less important in the progression to severe liver disease [57]. It is likely that some of these discrepancies in the literature are a result of different measures used to assess visceral fat, including WC, waist-hip ratio, visceral adiposity index, and imaging, some of which cannot distinguish between visceral and subcutaneous fat compartments [58,59]. Additionally, it may be the “health” of the visceral adipose compartment that is relevant to NAFLD pathogenesis rather than the fat mass per se.
Table 1.
Human genes associated with NAFLD and NASH identified from exome- and/or genome-wide association studies [[181], [182], [183], [184],206] and links to metabolism.
| Gene | Protein name | Principal SNP and variant | NAFLD/NASH risk | Biochemical function | Reference |
|---|---|---|---|---|---|
| PNPLA3 | Patatin-like phospholipase domain-containing protein 3 or adiponutrin | rs738409; p.I148M | Increased | Lipid droplet protein with triglyceride hydrolase activity and retinyl-palmitate lipase activity; remodeling of hepatic fatty acids | [185] |
| TM6SF2 | Transmembrane 6 superfamily member 2 | rs58542926; p.E167K | Increased | Involved in hepatic VLDL secretion and lipoprotein metabolism | [186] |
| MBOAT7 | Membrane-bound O-acyltransferase domain-containing protein 7 | rs641738; p.G17E | Increased | Phospholipid remodeling; acyltransferase that catalyzes the acylation of lysophosphatidylinositol with arachidonoyl-CoA | [187] |
| GCKR | Glucokinase regulatory protein | rs1260326; p.P446L | Increased | Inhibitor of glucokinase activity; regulator of hepatic de novo lipogenesis | [188] |
| HSD17β13 | Hydroxysteroid 17-beta dehydrogenase 13 | rs72613567:TA splice variant | Decreased | Hepatic LD protein with retinol dehydrogenase activity; other family members linked to steroid and fatty acid metabolism | [192,193] |
| MARC1 | Mitochondrial amidoxime-reducing component 1 | rs2642438; missense p.A165T | Decreased | Molybdenum-containing enzyme capable of reducing N-hydroxylated compounds; associated with the outer mitochondrial membrane | [194] |
5. Lipotoxicity: are all lipids created equal?
Adipose tissue acts as the body's main reservoir for lipid storage. When this storage capacity is overloaded in the context of obesity and overnutrition, ectopic fat accumulates in the liver and organs that usually only store relatively small amounts of fat, including the skeletal muscle and heart, inducing lipotoxicity. Lipotoxicity is characterized by the accumulation of harmful lipid species, leading to chronic inflammation and progression from hepatic steatosis to NASH [60]. TG is the predominant lipid that accumulates in the liver in NAFL. However, studies suggest that increased TG, due to either increased de novo synthesis [61] or reduced export [62], is not necessarily pathogenic in isolation and may even be protective against liver injury [63]; rather, it is likely that the delivery of excess FFA to the liver or generation of other lipotoxic species within the liver leads to lipotoxicity and subsequent hepatocyte dysfunction [64,65]. Remarkably, under some experimental conditions, DNL can actually be preventative against lipotoxicity [66]. Fatty acids taken up by hepatocytes serve as substrates for lipid synthesis or mitochondrial beta-oxidation [15]. Some lipid classes may be protective in the context of hepatic injury, including n-3 polyunsaturated fatty acids (PUFAs). Levels of n-3 PUFAs are reportedly decreased in NASH patients [67] and an elevated n-6:n-3 PUFA ratio has been associated with the severity of NAFLD [68]. Consistent with the role of n-3 PUFAs in reducing hepatic lipid, meta-analyses demonstrated consistent reductions in steatosis with n-3 supplementation but no effect on NASH activity scores or fibrosis, suggesting the beneficial effects of n-3 PUFAs may be limited to reductions in steatosis or possibly that more long-term benefits exist but were not captured in the endpoints of these studies [69,70].
The specific lipid species responsible for promoting hepatocyte injury and NASH pathogenesis is of considerable debate [71]. FFAs have most commonly been implicated as possible drivers of hepatic lipotoxicity. Some studies highlighted a more severe pathogenic role for saturated fatty acids (FAs), such as palmitic acid, compared to unsaturated FAs; however, most of these studies were performed in vitro and/or in rodent NASH models, and there is less evidence pointing to a definitive role of saturated FAs in driving NASH progression in NAFLD patients ([67]; reviewed in [60,71,72]). The lack of an animal model that faithfully mimics the histological progression of NASH in humans has severely hampered such mechanistic studies. In the context of metabolically driven NAFLD, the lipid composition of the liver is predominated by saturated and mono-unsaturated TGs and ceramides [73,74]. In contrast, however, in carriers of the patatin-like phospholipase domain-containing protein 3 (PNPLA3)-148M NASH risk allele, hepatic TGs are enriched in PUFAs compared to PNPLA3-148I carriers [75,76], demonstrating that there is much more to learn about liver TG remodeling, the metabolism of PUFAs in the liver, and implications int the risk of progression to NASH. A lipidomic analysis of human liver biopsies comparing normal, NAFLD, and NASH livers identified a lipid signature that could discriminate NASH livers: NASH livers were found to have a lipidomic profile interpreted as indicative of decreased of fatty acid desaturase 1 and elongation of very long-chain fatty acid 6 activity, leading to an increase in long-chain fatty acids (LCFA) [77], oxidation of which can lead to elevated reactive oxygen species (ROS) in the liver [78]. Other candidate lipotoxic species implicated in NASH progression include ceramides, diacylglycerols, cholesterol, and lysophosphatidylcholine (reviewed in detail elsewhere [65,71,74,[79], [80], [81], [82], [83], [84]]); however, whether these lipid intermediates actually contribute to severity of NASH in humans remains uncertain. Adding to the complexity is the uncertainty of how well the circulating lipidome reflects the hepatic milieu.
6. Hepatic lipid metabolism and NAFLD
The endogenous synthesis of lipids from dietary substrates, or DNL, is driven by increases in consumption of a high-carbohydrate diet. Elegant tracer studies have shown that hepatic DNL is increased in individuals with NAFLD and is a significant contributor to elevation in liver fat [[17], [18], [19]]. Collectively these studies were very well aligned and showed that the contribution of DNL to liver TG synthesis in the fed state is in the range of ∼25–38% in obese NAFLD subjects. Increased DNL in the context of NAFLD may be at least partially explained by dysregulated transcriptional regulation of lipogenesis in the liver. Two key transcription factors regulate lipogenesis in the liver: sterol regulatory element-binding protein 1c (SREBP1c), which is activated by insulin and the liver X receptor, and carbohydrate regulatory element-binding protein (ChREBP), which is activated by products of carbohydrate metabolism [[85], [86], [87], [88]]. SREBP1c activity and, as a consequence, DNL is increased in the liver in the context of NAFLD due to signaling driven by hyperinsulinemia, despite sustained HGP, a metabolic state that has been called selective insulin resistance [[89], [90], [91]]. SREBP1c also potently induces the transcription of PNPLA3, further exacerbating hepatic steatosis [92]. Similarly, hyperglycemia provides a stimulus for increased transcriptional activity of ChREBP, which, in cooperation with SREBP1c, leads to increased expression of lipogenic genes that include acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). ACC catalyzes the first committed step in fatty acid synthesis, the production of malonyl-CoA, which is an allosteric inhibitor of carnitine palmitoyltransferase and reduces fatty acid oxidation [93], further exacerbating hepatic steatosis. As expected, inhibition of ACC results in potent reduction in DNL and increased fatty acid oxidation, reversing steatosis and resolving multiple features of NASH in rodents and humans. ACC inhibition also results in an adaptive increase in SREBP1 activity, however, and a subsequent elevation in circulating TGs [[94], [95], [96], [97], [98], [99], [100], [101]].
Although increased DNL clearly contributes to hepatic steatosis, whether it specifically drives progression to NASH is unclear. DNL is associated with elevated levels of saturated fatty acids and ceramides, which can lead to increased hepatocyte injury and inflammation [8,102]. In parallel, defects in hepatic mitochondrial fatty acid beta-oxidation have been suggested to contribute to the development of liver steatosis and NASH progression. The data are mixed, however, on whether fatty acid oxidation is altered in NAFLD and NASH patients. Recent measurements of hepatic fluxes using stable isotope tracers in individuals with NAFLD revealed increases in acetyl-CoA oxidation in the tricarboxylic acid cycle despite reductions in ketogenesis [103]. These data indicate that the fate of oxidized fatty acids is important to driving increased glucose production during insulin-resistant states. Further, partitioning of LCFA to peroxisomes for oxidation increases levels of ROS and toxic lipid species. As outlined herein, many lipid species are altered in the context of NAFLD, thus it is likely that the overall composition of the hepatic fatty acid “pool” ultimately determines the tipping point from steatosis to more severe liver injury.
7. Lipid metabolism and oxidative stress
In addition to hepatic steatosis and aberrant lipid metabolism, oxidative stress is a prominent feature associated with progression to NASH. The generation of ROS is a major source of hepatocyte and liver injury and is closely linked to the accumulation of hepatic FFA [104,105]. Excess delivery of FFA can upregulate beta-oxidation and lead to elevation in mitochondrial ROS production, particularly in the context of an environment with impaired anti-oxidant function as occurs in NAFLD [106]. ROS act as normal signaling mediators within cells as well as damaging oxidizing agents for DNA, proteins, and lipids. The intersection of lipid metabolism and oxidative stress has led to a focus on a potential role for oxidized lipids in promoting liver inflammation and worsening of NASH. Oxidized LDL (oxLDL), for example, is a well-known atherogenic factor that has also been linked to inflammation in rodent NASH [[107], [108], [109]]. The uptake of oxLDL by macrophages is pro-inflammatory and promotes the formation of foam cells in the context of atherosclerosis. Similarly, it has been hypothesized that hepatic inflammation in NASH is triggered by the recognition of oxLDL by Kupffer cells. In support, HFD-fed mice administered oxLDL rapidly develop significant hepatic inflammation and cellular injury [110], and strategies to neutralize oxLDL in a rodent model of NASH blunts immune cell infiltration and hepatic inflammation [111]. Oxidized phospholipids (oxPLs) form by lipid peroxidation or chemical modification of phospholipids. OxPLs accumulate in oxLDL and lipoprotein(a), plasma lipoproteins that are linked to cardiovascular disease, and are released by injured or apoptotic cells [[112], [113], [114]]. Recent work by Witztum et al. highlighted the potential relevance of oxPLs in promoting oxidative stress and inflammation in the context of atherosclerosis and NASH. Mice engineered to produce an antibody that binds to oxPL and prevents oxPL-containing LDL particles from being taken up by macrophages displayed reduced systemic inflammation and less atherosclerosis [115]. Similarly, neutralization of oxPL in Ldlr−/− mice fed a NASH-promoting diet enriched in trans-fat, fructose, and cholesterol significantly reduces liver TG, markers of inflammation and fibrosis, and restores mitochondrial function in the liver, although it is important to note that body weight is also reduced in these mice [116]. Given the links between disordered lipid metabolism, NASH, and CVD [8], it is intriguing to consider elevation of oxidized lipids and lipoproteins as a possible causative trigger in both NAFL progression to NASH and CVD in some people. More studies are needed to demonstrate whether neutralization of these inflammatory oxidized lipid species can reverse and/or prevent progression of the disease. Further, although some studies have reported evidence to support altered oxidized lipids or lipoproteins in NAFLD or NASH patients [[116], [117], [118], [119], [120]], circulating and liver levels of oxidized lipids and lipoproteins have not been fully evaluated in large cohorts of NASH patients by disease stage, making even correlative assessments difficult.
8. Fructose and NAFLD
Overconsumption of sugar-sweetened beverages containing sucrose (which is broken down into fructose and glucose) or high-fructose corn syrup (a mixture of fructose and glucose) is linked to increased visceral adiposity, hepatic steatosis, and NASH [[121], [122], [123], [124], [125], [126], [127], [128]]. Excess intake of calories in the form of dietary sugars, particularly simple sugars, potently promotes fatty acid synthesis in humans [129,130]. The ability of moderate amounts of fructose ingestion to promote metabolic dysfunction in people is a point of controversy. Some studies either found no associations with fructose and metabolic dysfunction in cross-sectional studies or attributed any deleterious effects of fructose simply to excess caloric intake [[131], [132], [133]]. In contrast, fructose has been shown to be particularly detrimental in those with even acute fructose consumption, leading to rapid elevations in DNL, very low-density lipoprotein (VLDL) TG secretion, and hepatic steatosis [122,123,[134], [135], [136]].
Unique features associated with fructose metabolism may help explain why it appears to be particularly metabolically harmful compared to glucose [126,[137], [138], [139]]. While glucose metabolism in the liver is tightly regulated, fructose is extracted from the portal circulation and delivered to the liver where it undergoes rapid and uninhibited phosphorylation by ketohexokinase (KHK) to form fructose-1-phosphate, generating substrates for fatty acid synthesis [[140], [141], [142]]. In response to a high fructose load, the metabolism of fructose can deplete ATP in the liver, increasing uric acid production and hepatocyte stress [143]. However, the major mechanism by which fructose promotes DNL is by increasing the intracellular concentration of fructose-1-phosphate, a powerful allosteric activator of glucokinase. In cooperation with increased concentrations of circulating glucose, enhanced glycolytic flux activates ChREBP, which as previously described promotes the transcription of genes that encode lipogenic enzymes [126,138,144].
To better understand the metabolic fate of fructose, Jang et al. utilized isotope tracers and mass spectrometry to demonstrate that when physiological levels of glucose and fructose are delivered in a 1:1 ratio in mice, ∼90% of fructose is metabolized in the small intestine [145]. Administration of higher doses of fructose results in saturation of small intestine fructose clearance and a significant amount of fructose reaching the liver. These studies provide the framework that fructose metabolism by the small intestine serves to protect the liver from the deleterious effects of fructose. Consistently, deletion of Khk-C in the intestine in mice results in increased delivery of fructose to the liver and enhanced hepatic steatosis [146]. In mice fed a HFD, consumption of fructose-sweetened water was associated with more pronounced weight gain, increased expression of hepatic Srebp1c and Chrebp-β, insulin resistance, and augmented fatty acid synthesis compared to mice fed a HFD and given glucose-sweetened water [137]. Suppression of KHK in the liver improved hepatic steatosis and glucose tolerance in the fructose-supplemented mice. Consistent with this study, mice with genetic deletion of Khk-A/C were protected from fructose-induced metabolic dysfunction [147] and mice with liver-selective Khk-A/C-deficiency were protected from fructose-mediated hepatic steatosis and insulin resistance [141]. The relevance of these preclinical studies to human is an active area of investigation (reviewed in [148]); however, in totality the evidence supports the concept that strategies aimed at limiting fructose metabolism (such as KHK inhibition [149,150]) or reducing fructose in the diet ([121,151,152]) may represent attractive therapeutic approaches for NAFLD and NASH. Indeed, a recent randomized, double-blind placebo-controlled, Phase 2a study (NCT03256526) assessed the effects of the reversible KHK inhibitor PF-06835919 on metabolic parameters in participants with NAFLD. Participants receiving a once-daily oral 300 mg dose of PF-06835919 for 6 weeks displayed a significant reduction in whole liver fat by magnetic resonance imaging-proton density fat fraction (difference of −18.73%; p = 0.04 compared to placebo; no differences were observed at a lower 75 mg dose) [207].
9. Gut microbiome and barrier function in NAFLD
There is a growing body of literature on the “gut–liver axis” highlighting a possible role for the contribution of the gut microbiome to the progression of NAFLD. NAFLD has been linked to intestinal bacterial overgrowth and dysbiosis [[153], [154], [155]], consistent with higher endotoxin levels in the serum of NAFLD patients compared to controls [156]. Numerous studies have reported altered composition of the bacterial microbiome in NAFLD subjects (reviewed in [157]). Collectively, however, cross-sectional studies have not convincingly identified a consistent microbial signature associated with NAFLD in humans. The reported data are often correlative and inconsistent (reviewed in [158,159]), which is not surprising given that the gut microbiome is dynamic, responsive to diet and environment, and influenced by age, sex, bile acids, use of medications, and other factors, making it particularly challenging to study [154]. Further, it is often difficult to determine whether associations between altered gut microbiome and NAFLD are primarily obesity-driven rather than specific to NAFLD. It is currently unclear whether a particular gut microbiome composition can worsen NASH in humans; however, in mice it was reported that co-housing of NASH-susceptible inflammasome-deficient mice with wild-type mice could exacerbate features of NASH in wild-type cage mates on a methionine-choline deficient diet, suggesting the possibility of an autonomous contribution of the gut microbiome to the induction of metabolic dysfunction and liver disease [160].
While challenges remain for studying the precise microbiota composition in NAFLD, more compelling support is emerging for the concept of increased gut permeability influencing progression of liver disease. The gut barrier, consisting of a mucus layer, intestinal epithelial cells (IECs), and the underlying lamina propria, separates the contents of the gut lumen from the circulation. IECs play an active role in responding to gut luminal signals by selective uptake of nutrients and responding to numerous metabolic signals, but they also play an important role in regulating the passive permeability of luminal contents through the expression of tight junction proteins (TJPs) between adjacent cells [161]. Dysbiosis-induced impairment of TJPs leads to increased gut permeability and subsequent transport of bacterial endotoxins to the portal circulation, exposing the liver to mediators of hepatic inflammation [162]. In a meta-analysis, NAFLD and NASH patients had increased rates of intestinal permeability compared to healthy subjects [163].
Macronutrients in obesogenic diets can also induce gut barrier dysfunction. Fructose ingestion promotes bacterial overgrowth in the intestine and induces disruption of the intestinal barrier in rodents, non-human primates, and people, leading to elevations in gut-derived endotoxins entering the portal circulation [[164], [165], [166], [167]], providing a possible mechanistic link between fructose and worsening of liver disease. In a recent report, Todoric et al. described a distinct pathway whereby fructose-induced endotoxemia and gut barrier disruption leads to activated Toll-like receptor signaling in liver macrophages and downstream induction of ACC1, FAS, and hepatic steatosis [168]. This study suggested that inflammation associated with fructose-induced intestinal barrier disruption contributes to fructose-induced DNL in the liver and provides a rationale to improve intestinal barrier function as a part of NASH therapy. Supporting this concept, daily treatment of NAFLD subjects for 12 weeks with lubiprostone, a bicyclic fatty acid compound derivative previously shown to maintain tight junction barrier function, resulted in a modest reduction in liver enzyme levels and liver fat compared to placebo [169,170]. In summary, more studies are needed to understand the timing of altered gut permeability in the course of disease progression from steatosis to NASH to understand if it is causative or correlative and the specific mechanistic pathways by which improving inflammation in the gut and liver leads to a reduction in hepatic steatosis.
10. Lean NAFLD
While most patients with fatty liver are obese, it is important to note that NAFLD can be present in lean individuals, leading to the terms “lean NAFLD” or “non-obese NAFLD” [171,172]. Lean NAFLD was originally described in Asian countries where obesity rates and average BMI are lower, but has since been documented in other populations [5,173,174]. However, population studies such as these are subject to misclassification artifacts introduced by ethnic variations in body fat distribution and definitions of “obesity” based on the Western, Caucasian body habitus [175]. Some studies suggest that lean NAFLD patients are at risk for more severe and progressive liver disease and metabolic abnormalities compared to patients with NAFLD and higher BMI [[176], [177], [178]] and reviewed in [171,172], whereas another study recently showed lower rates of cirrhosis and cardiovascular disease in a lean NAFLD cohort compared to non-lean study participants [179]. Although there are many non-metabolic factors that may contribute to NAFLD in the absence of having a BMI that is classified as overweight/obese, including lipodystrophy, certain genetic mutations, hepatitis C infection, inflammatory disease of the gut, and drug-induced liver toxicity, the most common comorbidities with lean NAFLD are insulin resistance and increased visceral adiposity [172,178]. Indeed, many patients identified with lean NAFLD have often increased waist circumferences and one or more features of metabolic syndrome, raising the possibility that this subset of NAFLD patients has metabolically driven ectopic fat deposition despite normal BMI [180]. Thus, it is likely that adipose tissue dysfunction, particularly in the visceral adipose compartment, contributes to disease pathogenesis in this context.
Despite the classical paradigm described in this review, it is important to consider that there is not always evidence of elevated liver fat preceding NASH. Indeed, in a smaller subset of individuals, NASH can develop independently of hepatic steatosis [171,174]. The origins of NASH in these subjects is not well-characterized, likely to vary, and may include underreported alcohol or medication use, infections, genetic mutations, and altered bile acid profiles and gut microbiome [171,174,180]. Although the histological disease end points may be similar in NASH patients without a prior history of NAFL, it is likely that the disease etiology and trajectory are mechanistically distinct. More longitudinal studies are needed on this subset of NASH patients to understand the risk factors underlying the phenotype of NASH independent of NAFL.
11. Metabolic links to NASH: human genetic evidence
Perhaps the most compelling clue to the molecular underpinnings of NAFLD pathogenesis comes from human genetics. The genes identified to date from exome- and genome-wide association studies with significant odds ratios for susceptibility to or protection from NAFLD and increased (or decreased) risk of progression to NASH are largely genes encoding proteins with known or suspected roles in hepatic and/or extra-hepatic lipid metabolism. Many of these genes encode proteins with an interesting localization pattern to lipid droplets (Table 1) [9,[181], [182], [183], [184]]. For example, mutations in the lipid metabolism genes PNPLA3, transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7), and glucokinase regulatory protein (GCKR) confer increased risk of developing NAFLD and NASH [[185], [186], [187], [188]]. Possessing more than one risk allele may increase the severity of liver disease, as has been shown for PNPLA3 and TM6SF2 [189], leading to efforts aimed at defining a “NASH genetic risk” score [190,191]. Mutations in the hydroxysteroid 17-beta dehydrogenase 13 (HSD17b13) gene, which encodes a hepatic lipid droplet-associated protein possibly involved in steroid or fatty acid metabolism, are associated with a decreased risk of progression to NASH [192,193]. Similarly, a common missense variant in the mitochondrial amidoxime-reducing component 1 (MARC1) gene has recently been shown to confer protection from fatty liver and all-cause cirrhosis [194]. MARC1 is a molybdenum-containing enzyme capable of reducing N-hydroxylated compounds and is associated with the outer mitochondrial membrane [195]. The physiological role of MARC1 in liver disease is unclear. Carriers of the MARC1 variant (n = 53) were recently reported to have higher concentrations of hepatic polyunsaturated phosphatidylcholines than non-carriers (n = 65); however, the significance of this finding in relation to protection from NASH remains uncertain [196]. The functions of these proteins in the liver and other tissues are being actively examined, but overall we still have more to learn about how they influence NAFLD pathogenesis.
12. Conclusions
Many factors associated with dysregulated systemic metabolism have been implicated in some way with the progression from hepatic steatosis to NASH, including altered lipid metabolism, mitochondrial dysfunction, alterations in gut microbiome, oxidative stress, inflammatory cytokines, immune response, and others; however, it remains unclear which combination of these mechanisms are the key drivers of the disease. Similar to other metabolic diseases, the progression to more serious pathologic states is likely driven by several risk factors previously listed and manifests slightly differently in each individual depending on genetic risk, presence of other metabolic comorbidities (obesity and/or diabetes), lifestyle, diet, and environment.
In agreement with the importance of metabolic drivers of NAFLD pathogenesis outlined in this review, recent comprehensive surveys of NASH therapies currently in development and showing preliminary signs of efficacy reveal a bias toward targeting pathways involved in hepatic or systemic metabolism, with relatively fewer drugs directly targeting inflammation or fibrosis alone [2,197]. Additionally, many NASH monotherapies aimed at targeting inflammatory pathways, cell death pathways, or fibrosis have failed to show robust efficacy in clinical trials, including the apoptosis signal-regulating kinase 1 inhibitor selonsertib [198], lysyl-oxidase-like-2 antibody simtuzumab [199], phosphodiesterase-4 inhibitor ASP9831 [200], pan-caspase inhibitor emricasan [201], galectin-3 inhibitor belapectin [202], and dual chemokine receptor type 2/5 antagonist cenicriviroc [203].
The ability to identify a specific “trigger” that accelerates the transition from simple hepatic steatosis to NASH is understandably desirable, since this would allow the identification of individuals at risk and would enable improved monitoring for therapeutic interventions. However, the reality is that the progression from hepatic steatosis to inflammation and fibrosis is heterogeneous across the patient population, can range from relatively quick progression to decades, and as previously mentioned is likely influenced by multiple factors. Much past attention focused on a possible “two-hit” theory that suggests that insulin resistance and hepatic steatosis alone are insufficient to promote NASH, and a second insult such as oxidative stress is required to trigger disease progression [204]. As outlined herein and elsewhere, it is much more likely that the disease trajectory is continuous, defined by an accumulation of both metabolic and inflammatory insults and modified by genetic risk and diet [2,205].
A consistent theme emerging from the considerable body of research is the probable relevance of the substrate overload model of NAFLD, whereby continuous exposure of the liver to excess glucose, fructose, and fatty acids results in partitioning of metabolites and fatty acids into pathways that promote lipogenesis and liver injury. Further, as we learn more about the importance of interorgan communication in driving NASH pathogenesis, it is clear that this is a disease of altered systemic metabolism rather than a liver disease in isolation. In obesity-driven NAFLD, the health of the adipose tissue appears to play an important role in determining the hepatic lipid environment, while in lean NAFLD, the gut microbiome and barrier integrity may play a prominent role. Indeed, examination of intestinal permeability in lean NAFLD provides support for the notion that altered gut microbiome and intestinal permeability may play a pathogenic role in NAFLD independent of obesity; a recent study of lean NAFLD subjects reported an altered gut microbiota profile in addition to higher serum bile acid levels compared to non-lean NAFLD subjects [171]. Learning more about the role of adipose-to-liver communication and the gut–liver axis should shed light on the intersection of these organs in liver disease [10]. Additionally, as we learn more about the function of the metabolic proteins encoded by NASH risk and protective alleles, we should gain critical insight into additional cellular mechanisms and pathways influencing progression to NASH.
Author contributions
KKB and MJB contributed to the review article's overall concept, reviewed the literature, and drafted, edited, and approved the final manuscript.
Acknowledgments
This work was supported by Pfizer, Inc.
Conflict of interest
The authors are employees and shareholders of Pfizer, Inc.
References
- 1.Reeder S.B., Sirlin C.B. Quantification of liver fat with magnetic resonance imaging. Magnetic Resonance Imaging Clinics of North America. 2010;18(3):337–357. doi: 10.1016/j.mric.2010.08.013. [ix] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature Reviews Gastroenterology & Hepatology. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 4.Simon T.G., Roelstraete B., Khalili H., Hagstrom H., Ludvigsson J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322786. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Blissett D., Blissett R., Henry L., Stepanova M., Younossi Y. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A., Scorletti E., Mosca A., Alisi A., Byrne C.D., Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170. doi: 10.1016/j.metabol.2020.154170. [DOI] [PubMed] [Google Scholar]
- 8.Deprince A., Haas J.T., Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Molecular Metabolism. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eslam M., George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nature Reviews Gastroenterology & Hepatology. 2020;17(1):40–52. doi: 10.1038/s41575-019-0212-0. [DOI] [PubMed] [Google Scholar]
- 10.Hart K.M., Fabre T., Sciurba J.C., Gieseck R.L., 3rd, Borthwick L.A., Vannella K.M. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-beta. Science Translational Medicine. 2017;9(396) doi: 10.1126/scitranslmed.aal3694. [DOI] [PubMed] [Google Scholar]
- 11.Heymann F., Tacke F. Immunology in the liver--from homeostasis to disease. Nature Reviews Gastroenterology & Hepatology. 2016;13(2):88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 12.Kazankov K., Jorgensen S.M.D., Thomsen K.L., Moller H.J., Vilstrup H., George J. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nature Reviews Gastroenterology & Hepatology. 2019;16(3):145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nature Reviews Gastroenterology & Hepatology. 2017;14(7):397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 14.Canbay A., Bechmann L., Gerken G. Lipid metabolism in the liver. Zeitschrift für Gastroenterologie. 2007;45(1):35–41. doi: 10.1055/s-2006-927368. [DOI] [PubMed] [Google Scholar]
- 15.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Comparative Physiology. 2017;8(1):1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacome-Sosa M.M., Parks E.J. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Current Opinion in Lipidology. 2014;25(3):213–220. doi: 10.1097/MOL.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 17.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Pydi S.P., Zhu L., Barella L.F., Cui Y., Gavrilova O. Adipocyte Gi signaling is essential for maintaining whole-body glucose homeostasis and insulin sensitivity. Nature Communications. 2020;11(1):2995. doi: 10.1038/s41467-020-16756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry R.J., Camporez J.G., Kursawe R., Titchenell P.M., Zhang D., Perry C.J. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titchenell P.M., Chu Q., Monks B.R., Birnbaum M.J. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nature Communications. 2015;6:7078. doi: 10.1038/ncomms8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. Journal of the American Medical Association. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 25.Loomis A.K., Kabadi S., Preiss D., Hyde C., Bonato V., St Louis M. Body mass index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. Journal of Clinical Endocrinology & Metabolism. 2016;101(3):945–952. doi: 10.1210/jc.2015-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. Journal of the American Medical Association. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bray G.A., Heisel W.E., Afshin A., Jensen M.D., Dietz W.H., Long M. The science of obesity management: an endocrine society scientific statement. Endocrine Reviews. 2018;39(2):79–132. doi: 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 30.Ogden C.L., Carroll M.D., Lawman H.G., Fryar C.D., Kruszon-Moran D., Kit B.K. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. Journal of the American Medical Association. 2016;vol. 315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahima R.S. The natural history of nonalcoholic fatty liver disease: insights from children and mice. Gastroenterology. 2008;135(6):1860–1862. doi: 10.1053/j.gastro.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Mann J.P., Valenti L., Scorletti E., Byrne C.D., Nobili V. Nonalcoholic fatty liver disease in children. Seminars in Liver Disease. 2018;38(1):1–13. doi: 10.1055/s-0038-1627456. [DOI] [PubMed] [Google Scholar]
- 33.Nobili V., Alisi A., Valenti L., Miele L., Feldstein A.E., Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nature Reviews Gastroenterology & Hepatology. 2019;16(9):517–530. doi: 10.1038/s41575-019-0169-z. [DOI] [PubMed] [Google Scholar]
- 34.Anderson E.L., Howe L.D., Jones H.E., Higgins J.P., Lawlor D.A., Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Y., Hou D., Zhao X., Liu J., Cheng H., Wang Y. Childhood adiposity and nonalcoholic fatty liver disease in adulthood. Pediatrics. 2017;139(4) doi: 10.1542/peds.2016-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klebanoff M.J., Corey K.E., Chhatwal J., Kaplan L.M., Chung R.T., Hur C. Bariatric surgery for nonalcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology. 2017;65(4):1156–1164. doi: 10.1002/hep.28958. [DOI] [PubMed] [Google Scholar]
- 37.Hannah W.N., Jr., Harrison S.A. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clinics in Liver Disease. 2016;20(2):339–350. doi: 10.1016/j.cld.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Mummadi R.R., Kasturi K.S., Chennareddygari S., Sood G.K. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2008;6(12):1396–1402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Huang M.A., Greenson J.K., Chao C., Anderson L., Peterman D., Jacobson J. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. American Journal of Gastroenterology. 2005;100(5):1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 40.Hsu C.C., Ness E., Kowdley K.V. Nutritional approaches to achieve weight loss in nonalcoholic fatty liver disease. Advance Nutrition. 2017;8(2):253–265. doi: 10.3945/an.116.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005. e365; quiz e314-365. [DOI] [PubMed] [Google Scholar]
- 42.Hydes T.J., Ravi S., Loomba R., M E.G. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clinical and Molecular Hepatology. 2020;26(4):383–400. doi: 10.3350/cmh.2020.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. New England Journal of Medicine. 2020:13. doi: 10.1056/NEJMoa2028395. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Gupta N.A., Mells J., Dunham R.M., Grakoui A., Handy J., Saxena N.K. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51(5):1584–1592. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen S., Guo Z., Johnson C.M., Hensrud D.D., Jensen M.D. Splanchnic lipolysis in human obesity. Journal of Clinical Investigation. 2004;113(11):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein S. The case of visceral fat: argument for the defense. Journal of Clinical Investigation. 2004;113(11):1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 48.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 49.Li C., Spallanzani R.G., Mathis D. Visceral adipose tissue Tregs and the cells that nurture them. Immunological Reviews. 2020;295(1):114–125. doi: 10.1111/imr.12850. [DOI] [PubMed] [Google Scholar]
- 50.Wajchenberg B.L., Giannella-Neto D., da Silva M.E., Santos R.F. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Hormone and Metabolic Research. 2002;34(11–12):616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 51.Mirza M.S. Obesity, visceral fat, and NAFLD: querying the role of adipokines in the progression of nonalcoholic fatty liver disease. ISRN Gastroenterology. 2011;2011 doi: 10.5402/2011/592404. Article ID: 592404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Practice & Research Clinical Endocrinology & Metabolism. 2005;19(4):471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Machado M.V., Cortez-Pinto H. No need for a large belly to have NASH. Journal of Hepatology. 2011;54(6):1090–1093. doi: 10.1016/j.jhep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 54.van der Poorten D., Milner K.L., Hui J., Hodge A., Trenell M.I., Kench J.G. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48(2):449–457. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 55.Yu S.J., Kim W., Kim D., Yoon J.H., Lee K., Kim J.H. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2015;94(48):e2159. doi: 10.1097/MD.0000000000002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the U S A. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fracanzani A.L., Valenti L., Bugianesi E., Vanni E., Grieco A., Miele L. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. Journal of Hepatology. 2011;54(6):1244–1249. doi: 10.1016/j.jhep.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 58.Almeida N.S., Rocha R., Cotrim H.P., Daltro C. Anthropometric indicators of visceral adiposity as predictors of non-alcoholic fatty liver disease: a review. World Journal of Hepatology. 2018;10(10):695–701. doi: 10.4254/wjh.v10.i10.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vongsuvanh R., George J., McLeod D., van der Poorten D. Visceral adiposity index is not a predictor of liver histology in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2012;57(2):392–398. doi: 10.1016/j.jhep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 61.Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metabolism. 2007;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Liao W., Hui T.Y., Young S.G., Davis R.A. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. The Journal of Lipid Research. 2003;44(5):978–985. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Alkhouri N., Dixon L.J., Feldstein A.E. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Review of Gastroenterology & Hepatology. 2009;3(4):445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma M., Mitnala S., Vishnubhotla R.K., Mukherjee R., Reddy D.N., Rao P.N. The riddle of nonalcoholic fatty liver disease: progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Journal of Clinical Experiment Hepatology. 2015;5(2):147–158. doi: 10.1016/j.jceh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marra F., Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. Journal of Hepatology. 2018;68(2):280–295. doi: 10.1016/j.jhep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 66.Papazyan R., Sun Z., Kim Y.H., Titchenell P.M., Hill D.A., Lu W. Physiological suppression of lipotoxic liver damage by complementary actions of HDAC3 and SCAP/SREBP. Cell Metabolism. 2016;24(6):863–874. doi: 10.1016/j.cmet.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 68.Araya J., Rodrigo R., Videla L.A., Thielemann L., Orellana M., Pettinelli P. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clinical Science. 2004;106(6):635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 69.Parker H.M., Johnson N.A., Burdon C.A., Cohn J.S., O'Connor H.T., George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Journal of Hepatology. 2012;56(4):944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Sanyal A.J., Abdelmalek M.F., Suzuki A., Cummings O.W., Chojkier M., Group E.-A.S. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147(2):377–384. doi: 10.1053/j.gastro.2014.04.046. e371. [DOI] [PubMed] [Google Scholar]
- 71.Farrell G.C., Haczeyni F., Chitturi S. Pathogenesis of NASH: how metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Advances in Experimental Medicine & Biology. 2018;1061:19–44. doi: 10.1007/978-981-10-8684-7_3. [DOI] [PubMed] [Google Scholar]
- 72.Pierantonelli I., Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103(1):e1–e13. doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 73.Luukkonen P.K., Sadevirta S., Zhou Y., Kayser B., Ali A., Ahonen L. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luukkonen P.K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2016;64(5):1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Luukkonen P.K., Nick A., Holtta-Vuori M., Thiele C., Isokuortti E., Lallukka-Bruck S. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4(16) doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peter A., Kovarova M., Nadalin S., Cermak T., Konigsrainer A., Machicao F. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia. 2014;57(10):2103–2107. doi: 10.1007/s00125-014-3310-0. [DOI] [PubMed] [Google Scholar]
- 77.Chiappini F., Coilly A., Kadar H., Gual P., Tran A., Desterke C. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Scientific Reports. 2017;7:46658. doi: 10.1038/srep46658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bradbury M.W., Berk P.D. Lipid metabolism in hepatic steatosis. Clinics in Liver Disease. 2004;8(3):639–671. doi: 10.1016/j.cld.2004.04.005. [xi] [DOI] [PubMed] [Google Scholar]
- 79.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mauer A.S., Hirsova P., Maiers J.L., Shah V.H., Malhi H. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2017;312(3):G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han M.S., Park S.Y., Shinzawa K., Kim S., Chung K.W., Lee J.H. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. The Journal of Lipid Research. 2008;49(1):84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends in Endocrinology and Metabolism. 2016;27(2):84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. The Journal of Lipid Research. 2015;56(3):722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poss A.M., Summers S.A. Too much of a good thing? An evolutionary theory to explain the role of ceramides in NAFLD. Frontiers in Endocrinology. 2020;11:505. doi: 10.3389/fendo.2020.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimano H., Sato R. SREBP-regulated lipid metabolism: convergent physiology - divergent pathophysiology. Nature Reviews Endocrinology. 2017;13(12):710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 86.Jeon T.I., Osborne T.F. SREBPs: metabolic integrators in physiology and metabolism. Trends in Endocrinology and Metabolism. 2012;23(2):65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metabolism. 2006;4(2):107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Horton J.D., Goldstein J.L., Brown M.S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal of Clinical Investigation. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kohjima M., Higuchi N., Kato M., Kotoh K., Yoshimoto T., Fujino T. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. International Journal of Molecular Medicine. 2008;21(4):507–511. [PubMed] [Google Scholar]
- 90.Brown M.S., Goldstein J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metabolism. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 91.Titchenell P.M., Lazar M.A., Birnbaum M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends in Endocrinology and Metabolism. 2017;28(7):497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang Y., He S., Li J.Z., Seo Y.K., Osborne T.F., Cohen J.C. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proceedings of the National Academy of Sciences of the U S A. 2010;107(17):7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGarry J.D., Leatherman G.F., Foster D.W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. Journal of Biological Chemistry. 1978;253(12):4128–4136. [PubMed] [Google Scholar]
- 94.Griffith D.A., Kung D.W., Esler W.P., Amor P.A., Bagley S.W., Beysen C. Decreasing the rate of metabolic ketone reduction in the discovery of a clinical acetyl-CoA carboxylase inhibitor for the treatment of diabetes. Journal of Medicinal Chemistry. 2014;57(24):10512–10526. doi: 10.1021/jm5016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harwood H.J., Jr., Petras S.F., Shelly L.D., Zaccaro L.M., Perry D.A., Makowski M.R. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. Journal of Biological Chemistry. 2003;278(39):37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- 96.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metabolism. 2017;26(2):394–406. doi: 10.1016/j.cmet.2017.07.009. e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lawitz E.J., Coste A., Poordad F., Alkhouri N., Loo N., McColgan B.J. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 Weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology. 2018;16(12):1983–1991. doi: 10.1016/j.cgh.2018.04.042. e1983. [DOI] [PubMed] [Google Scholar]
- 98.Savage D.B., Choi C.S., Samuel V.T., Liu Z.X., Zhang D., Wang A. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. Journal of Clinical Investigation. 2006;116(3):817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ross T.T., Crowley C., Kelly K.L., Rinaldi A., Beebe D.A., Lech M.P. Acetyl-CoA carboxylase inhibition improves multiple dimensions of NASH pathogenesis in model systems. Cell Molecular Gastroenterology & Hepatology. 2020;10(4):829–851. doi: 10.1016/j.jcmgh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463–1473. doi: 10.1053/j.gastro.2018.07.027. e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68(6):2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roumans K.H.M., Lindeboom L., Veeraiah P., Remie C.M.E., Phielix E., Havekes B. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nature Communications. 2020;11(1):1891. doi: 10.1038/s41467-020-15684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fletcher J.A., Deja S., Satapati S., Fu X., Burgess S.C., Browning J.D. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight. 2019;5 doi: 10.1172/jci.insight.127737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albano E., Mottaran E., Vidali M., Reale E., Saksena S., Occhino G. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54(7):987–993. doi: 10.1136/gut.2004.057968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sumida Y., Niki E., Naito Y., Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radical Research. 2013;47(11):869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 106.Yesilova Z., Yaman H., Oktenli C., Ozcan A., Uygun A., Cakir E. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. American Journal of Gastroenterology. 2005;100(4):850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 107.Houben T., Brandsma E., Walenbergh S.M.A., Hofker M.H., Shiri-Sverdlov R. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(4):416–429. doi: 10.1016/j.bbalip.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 108.Bieghs V., Rensen P.C., Hofker M.H., Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220(2):287–293. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 109.Walenbergh S.M., Koek G.H., Bieghs V., Shiri-Sverdlov R. Non-alcoholic steatohepatitis: the role of oxidized low-density lipoproteins. Journal of Hepatology. 2013;58(4):801–810. doi: 10.1016/j.jhep.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 110.Yimin Furumaki H., Matsuoka S., Sakurai T., Kohanawa M., Zhao S. A novel murine model for non-alcoholic steatohepatitis developed by combination of a high-fat diet and oxidized low-density lipoprotein. Laboratory Investigation. 2012;92(2):265–281. doi: 10.1038/labinvest.2011.159. [DOI] [PubMed] [Google Scholar]
- 111.Bieghs V., van Gorp P.J., Walenbergh S.M., Gijbels M.J., Verheyen F., Buurman W.A. Specific immunization strategies against oxidized low-density lipoprotein: a novel way to reduce nonalcoholic steatohepatitis in mice. Hepatology. 2012;56(3):894–903. doi: 10.1002/hep.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang M.K., Binder C.J., Miller Y.I., Subbanagounder G., Silverman G.J., Berliner J.A. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. Journal of Experimental Medicine. 2004;200(11):1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boffa M.B., Koschinsky M.L. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nature Reviews Cardiology. 2019;16(5):305–318. doi: 10.1038/s41569-018-0153-2. [DOI] [PubMed] [Google Scholar]
- 114.Binder C.J., Papac-Milicevic N., Witztum J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nature Reviews Immunology. 2016;16(8):485–497. doi: 10.1038/nri.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Que X., Hung M.Y., Yeang C., Gonen A., Prohaska T.A., Sun X. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558(7709):301–306. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sun X., Seidman J.S., Zhao P., Troutman T.D., Spann N.J., Que X. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metabolism. 2020;31(1):189–206. doi: 10.1016/j.cmet.2019.10.014. e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ampuero J., Ranchal I., Gallego-Duran R., Pareja M.J., Del Campo J.A., Pastor-Ramirez H. Oxidized low-density lipoprotein antibodies/high-density lipoprotein cholesterol ratio is linked to advanced non-alcoholic fatty liver disease lean patients. Journal of Gastroenterology and Hepatology. 2016;31(9):1611–1618. doi: 10.1111/jgh.13335. [DOI] [PubMed] [Google Scholar]
- 118.Ho C.M., Ho S.L., Jeng Y.M., Lai Y.S., Chen Y.H., Lu S.C. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. Journal of Inflammation. 2019;16:7. doi: 10.1186/s12950-019-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ikura Y., Ohsawa M., Suekane T., Fukushima H., Itabe H., Jomura H. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43(3):506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 120.Hendrikx T., Watzenbock M.L., Walenbergh S.M., Amir S., Gruber S., Kozma M.O. Low levels of IgM antibodies recognizing oxidation-specific epitopes are associated with human non-alcoholic fatty liver disease. BMC Medicine. 2016;14(1):107. doi: 10.1186/s12916-016-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stanhope K.L., Schwarz J.M., Keim N.L., Griffen S.C., Bremer A.A., Graham J.L. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. Journal of Clinical Investigation. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarz J.M., Noworolski S.M., Erkin-Cakmak A., Korn N.J., Wen M.J., Tai V.W. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology. 2017;153(3):743–752. doi: 10.1053/j.gastro.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Digestive Diseases and Sciences. 2016;61(5):1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J.L., Diehl A.M. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. Journal of Hepatology. 2008;48(6):993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abid A., Taha O., Nseir W., Farah R., Grosovski M., Assy N. Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. Journal of Hepatology. 2009;51(5):918–924. doi: 10.1016/j.jhep.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 126.Herman M.A., Samuel V.T. The sweet path to metabolic demise: fructose and lipid synthesis. Trends in Endocrinology and Metabolism. 2016;27(10):719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malik V.S., Hu F.B. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. Journal of the American College of Cardiology. 2015;66(14):1615–1624. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goran M.I. Sugar-sweetened beverages, genetic risk, and obesity. New England Journal of Medicine. 2013;368(3):285–286. doi: 10.1056/NEJMc1213563. [DOI] [PubMed] [Google Scholar]
- 129.Parks E.J., Skokan L.E., Timlin M.T., Dingfelder C.S. Dietary sugars stimulate fatty acid synthesis in adults. Journal of Nutrition. 2008;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hudgins L.C., Seidman C.E., Diakun J., Hirsch J. Human fatty acid synthesis is reduced after the substitution of dietary starch for sugar. American Journal of Clinical Nutrition. 1998;67(4):631–639. doi: 10.1093/ajcn/67.4.631. [DOI] [PubMed] [Google Scholar]
- 131.Tappy L., Le K.A., Tran C., Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition. 2010;26(11–12):1044–1049. doi: 10.1016/j.nut.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 132.Sievenpiper J.L., Toronto D.K.S., Clinical Trials U. Fructose: where does the truth lie? Journal of the American College of Nutrition. 2012;31(3):149–151. doi: 10.1080/07315724.2012.10720021. [DOI] [PubMed] [Google Scholar]
- 133.Chiu S., Sievenpiper J.L., de Souza R.J., Cozma A.I., Mirrahimi A., Carleton A.J. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. European Journal of Clinical Nutrition. 2014;68(4):416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hudgins L.C., Parker T.S., Levine D.M., Hellerstein M.K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. Journal of Clinical Endocrinology & Metabolism. 2011;96(3):861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sobrecases H., Le K.A., Bortolotti M., Schneiter P., Ith M., Kreis R. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes & Metabolism. 2010;36(3):244–246. doi: 10.1016/j.diabet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 136.Merino B., Fernandez-Diaz C.M., Cozar-Castellano I., Perdomo G. Intestinal fructose and glucose metabolism in health and disease. Nutrients. 2019;12(1) doi: 10.3390/nu12010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. Journal of Clinical Investigation. 2017;127(11):4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hannou S.A., Haslam D.E., McKeown N.M., Herman M.A. Fructose metabolism and metabolic disease. Journal of Clinical Investigation. 2018;128(2):545–555. doi: 10.1172/JCI96702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jensen T., Abdelmalek M.F., Sullivan S., Nadeau K.J., Green M., Roncal C. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. Journal of Hepatology. 2018;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tesz G.J., Bence K.K. Finding the sweet spot: parsing tissue-specific contributions of fructose metabolism. Cell Metabolism. 2020;32(1):6–8. doi: 10.1016/j.cmet.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 141.Andres-Hernando A., Orlicky D.J., Kuwabara M., Ishimoto T., Nakagawa T., Johnson R.J. Deletion of fructokinase in the liver or in the intestine reveals differential effects on sugar-induced metabolic dysfunction. Cell Metabolism. 2020;32(1):117–127. doi: 10.1016/j.cmet.2020.05.012. e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nature Reviews Gastroenterology & Hepatology. 2010;7(5):251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 143.Abdelmalek M.F., Lazo M., Horska A., Bonekamp S., Lipkin E.W., Balasubramanyam A. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56(3):952–960. doi: 10.1002/hep.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim M.S., Krawczyk S.A., Doridot L., Fowler A.J., Wang J.X., Trauger S.A. ChREBP regulates fructose-induced glucose production independently of insulin signaling. Journal of Clinical Investigation. 2016;126(11):4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G. The small intestine converts dietary fructose into glucose and organic acids. Cell Metabolism. 2018;27(2):351–361. doi: 10.1016/j.cmet.2017.12.016. e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jang C., Wada S., Yang S., Gosis B., Zeng X., Zhang Z. The small intestine shields the liver from fructose-induced steatosis. Nature Metabolism. 2020;2(7):586–593. doi: 10.1038/s42255-020-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ishimoto T., Lanaspa M.A., Le M.T., Garcia G.E., Diggle C.P., Maclean P.S. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proceedings of the National Academy of Sciences of the U S A. 2012;109(11):4320–4325. doi: 10.1073/pnas.1119908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gonzalez J.T., Betts J.A. Dietary fructose metabolism by splanchnic organs: size matters. Cell Metabolism. 2018;27(3):483–485. doi: 10.1016/j.cmet.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 149.Huard K., Ahn K., Amor P., Beebe D.A., Borzilleri K.A., Chrunyk B.A. Discovery of fragment-derived small molecules for in vivo inhibition of ketohexokinase (KHK) Journal of Medicinal Chemistry. 2017;60(18):7835–7849. doi: 10.1021/acs.jmedchem.7b00947. [DOI] [PubMed] [Google Scholar]
- 150.Futatsugi K., Smith A.C., Tu M., Raymer B., Ahn K., Coffey S.B. Discovery of PF-06835919: a potent inhibitor of ketohexokinase (KHK) for the treatment of metabolic disorders driven by the overconsumption of fructose. Journal of Medicinal Chemistry. 2020;63(22):13546–13560. doi: 10.1021/acs.jmedchem.0c00944. [DOI] [PubMed] [Google Scholar]
- 151.Haufe S., Engeli S., Kast P., Bohnke J., Utz W., Haas V. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53(5):1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 152.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Faria Ghetti F., Oliveira D.G., de Oliveira J.M., de Castro Ferreira L., Cesar D.E., Moreira A.P.B. Influence of gut microbiota on the development and progression of nonalcoholic steatohepatitis. European Journal of Nutrition. 2018;57(3):861–876. doi: 10.1007/s00394-017-1524-x. [DOI] [PubMed] [Google Scholar]
- 154.Mehal W.Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nature Reviews Gastroenterology & Hepatology. 2013;10(11):637–644. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 155.Hoyles L., Fernandez-Real J.M., Federici M., Serino M., Abbott J., Charpentier J. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nature Medicine. 2018;24(7):1070–1080. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harte A.L., da Silva N.F., Creely S.J., McGee K.C., Billyard T., Youssef-Elabd E.M. Elevated endotoxin levels in non-alcoholic fatty liver disease. Journal of Inflammation. 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Albhaisi S.A.M., Bajaj J.S., Sanyal A.J. Role of gut microbiota in liver disease. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2020;318(1):G84–G98. doi: 10.1152/ajpgi.00118.2019. [DOI] [PubMed] [Google Scholar]
- 158.Lang S., Schnabl B. Microbiota and fatty liver disease-the known, the unknown, and the future. Cell Host & Microbe. 2020;28(2):233–244. doi: 10.1016/j.chom.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharpton S.R., Yong G.J.M., Terrault N.A., Lynch S.V. Gut microbial metabolism and nonalcoholic fatty liver disease. Hepatology Communications. 2019;3(1):29–43. doi: 10.1002/hep4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Review of Gastroenterology & Hepatology. 2017;11(9):821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferro D., Baratta F., Pastori D., Cocomello N., Colantoni A., Angelico F. New insights into the pathogenesis of non-alcoholic fatty liver disease: gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12(9) doi: 10.3390/nu12092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Luther J., Garber J.J., Khalili H., Dave M., Bale S.S., Jindal R. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Molecular Gastroenterology & Hepatology. 2015;1(2):222–232. doi: 10.1016/j.jcmgh.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thuy S., Ladurner R., Volynets V., Wagner S., Strahl S., Konigsrainer A. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. Journal of Nutrition. 2008;138(8):1452–1455. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 165.Spruss A., Bergheim I. Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. The Journal of Nutritional Biochemistry. 2009;20(9):657–662. doi: 10.1016/j.jnutbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 166.Jin R., Willment A., Patel S.S., Sun X., Song M., Mannery Y.O. Fructose induced endotoxemia in pediatric nonalcoholic Fatty liver disease. International Journal of Hepatology. 2014:560620. doi: 10.1155/2014/560620. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kavanagh K., Wylie A.T., Tucker K.L., Hamp T.J., Gharaibeh R.Z., Fodor A.A. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. American Journal of Clinical Nutrition. 2013;98(2):349–357. doi: 10.3945/ajcn.112.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Todoric J., Di Caro G., Reibe S., Henstridge D.C., Green C.R., Vrbanac A. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nature Metabolism. 2020;2(10):1034–1045. doi: 10.1038/s42255-020-0261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arakawa K., Ishigami T., Nakai-Sugiyama M., Chen L., Doi H., Kino T. Lubiprostone as a potential therapeutic agent to improve intestinal permeability and prevent the development of atherosclerosis in apolipoprotein E-deficient mice. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kessoku T., Imajo K., Kobayashi T., Ozaki A., Iwaki M., Honda Y. Lubiprostone in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancent Gastroenterology & Hepatology. 2020;5(11):996–1007. doi: 10.1016/S2468-1253(20)30216-8. [DOI] [PubMed] [Google Scholar]
- 171.Chen F., Esmaili S., Rogers G.B., Bugianesi E., Petta S., Marchesini G. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71(4):1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 172.Albhaisi S., Chowdhury A., Sanyal A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Report. 2019;1(4):329–341. doi: 10.1016/j.jhepr.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Das K., Das K., Mukherjee P.S., Ghosh A., Ghosh S., Mridha A.R. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 174.Younes R., Bugianesi E. NASH in lean individuals. Seminars in Liver Disease. 2019;39(1):86–95. doi: 10.1055/s-0038-1677517. [DOI] [PubMed] [Google Scholar]
- 175.Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for asian Indians and American diabetes association position statement. Diabetes Technology & Therapeutics. 2015;17(9):667–671. doi: 10.1089/dia.2015.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hagstrom H., Nasr P., Ekstedt M., Hammar U., Stal P., Hultcrantz R. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatology Communications. 2018;2(1):48–57. doi: 10.1002/hep4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dela Cruz A.C., Bugianesi E., George J., Day C., Liaquat H., Charatcharoenwitthaya P. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:S-909. [Google Scholar]
- 178.Sinn D.H., Kang D., Cho S.J., Paik S.W., Guallar E., Cho J. Lean non-alcoholic fatty liver disease and development of diabetes: a cohort study. European Journal of Endocrinology. 2019;181(2):185–192. doi: 10.1530/EJE-19-0143. [DOI] [PubMed] [Google Scholar]
- 179.Weinberg E.M., Trinh H.N., Firpi R.J., Bhamidimarri K.R., Klein S., Durlam J. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and Co-morbid diseases. Clinical Gastroenterology and Hepatology. 2020 doi: 10.1016/j.cgh.2020.06.066. [DOI] [PubMed] [Google Scholar]
- 180.Verdelho Machado M., Cortez-Pinto H. Fatty liver in lean patients: is it a different disease? Annals of Gastroenterology. 2012;25(1):1–2. [PMC free article] [PubMed] [Google Scholar]
- 181.Romeo S., Sanyal A., Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metabolism. 2020;31(1):35–45. doi: 10.1016/j.cmet.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 182.Tavaglione F., Targher G., Valenti L., Romeo S. Human and molecular genetics shed lights on fatty liver disease and diabetes conundrum. Endocrinology Diabetes Metabolism. 2020;3(4) doi: 10.1002/edm2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jonas W., Schurmann A. Genetic and epigenetic factors determining NAFLD risk. Molecular Metabolism. 2020:101111. doi: 10.1016/j.molmet.2020.101111. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mann J.P., Pietzner M., Wittemans L.B., Rolfe E.L., Kerrison N.D., Imamura F. Insights into genetic variants associated with NASH-fibrosis from metabolite profiling. Human Molecular Genetics. 2020;29(20):3451–3463. doi: 10.1093/hmg/ddaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjaerg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genetics. 2011;7(3) doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koo B.K., Joo S.K., Kim D., Bae J.M., Park J.H., Kim J.H. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2018;33(6):1277–1285. doi: 10.1111/jgh.14056. [DOI] [PubMed] [Google Scholar]
- 190.Kawaguchi T., Shima T., Mizuno M., Mitsumoto Y., Umemura A., Kanbara Y. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0185490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Di Costanzo A., Belardinilli F., Bailetti D., Sponziello M., D'Erasmo L., Polimeni L. Evaluation of polygenic determinants of non-alcoholic fatty liver disease (NAFLD) by a candidate genes resequencing strategy. Scientific Reports. 2018;8(1):3702. doi: 10.1038/s41598-018-21939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P. A protein-truncating HSD17B13 variant and protection from chronic liver disease. New England Journal of Medicine. 2018;378(12):1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kozlitina J., Stender S., Hobbs H.H., Cohen J.C. HSD17B13 and chronic liver disease in blacks and hispanics. New England Journal of Medicine. 2018;379(19):1876–1877. doi: 10.1056/NEJMc1804027. [DOI] [PubMed] [Google Scholar]
- 194.Emdin C.A., Haas M.E., Khera A.V., Aragam K., Chaffin M., Klarin D. A missense variant in Mitochondrial Amidoxime Reducing Component 1 gene and protection against liver disease. PLoS Genetics. 2020;16(4) doi: 10.1371/journal.pgen.1008629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Klein J.M., Busch J.D., Potting C., Baker M.J., Langer T., Schwarz G. The mitochondrial amidoxime-reducing component (mARC1) is a novel signal-anchored protein of the outer mitochondrial membrane. Journal of Biological Chemistry. 2012;287(51):42795–42803. doi: 10.1074/jbc.M112.419424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Luukkonen P.K., Juuti A., Sammalkorpi H., Penttila A.K., Oresic M., Hyotylainen T. MARC1 variant rs2642438 increases hepatic phosphatidylcholines and decreases severity of non-alcoholic fatty liver disease in humans. Journal of Hepatology. 2020;73(3):725–726. doi: 10.1016/j.jhep.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 197.Esler W.P., Bence K.K. Metabolic targets in nonalcoholic fatty liver disease. Cell Molecular Gastroenterology & Hepatology. 2019;8(2):247–267. doi: 10.1016/j.jcmgh.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Harrison S.A., Wong V.W., Okanoue T., Bzowej N., Vuppalanchi R., Younes Z. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. Journal of Hepatology. 2020;73(1):26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 199.Harrison S.A., Abdelmalek M.F., Caldwell S., Shiffman M.L., Diehl A.M., Ghalib R. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155(4):1140–1153. doi: 10.1053/j.gastro.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 200.Ratziu V., Bedossa P., Francque S.M., Larrey D., Aithal G.P., Serfaty L. Lack of efficacy of an inhibitor of PDE4 in phase 1 and 2 trials of patients with nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology. 2014;12(10):1724–1730. doi: 10.1016/j.cgh.2014.01.040. e1725. [DOI] [PubMed] [Google Scholar]
- 201.Frenette C.T., Morelli G., Shiffman M.L., Frederick R.T., Rubin R.A., Fallon M.B. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clinical Gastroenterology and Hepatology. 2019;17(4):774–783. doi: 10.1016/j.cgh.2018.06.012. e774. [DOI] [PubMed] [Google Scholar]
- 202.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334–1345. doi: 10.1053/j.gastro.2019.11.296. e1335. [DOI] [PubMed] [Google Scholar]
- 203.Friedman S.L., Ratziu V., Harrison S.A., Abdelmalek M.F., Aithal G.P., Caballeria J. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67(5):1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Day C.P., James O.F. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 205.Sanyal A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nature Reviews Gastroenterology & Hepatology. 2019;16(6):377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 206.Eslam M., Valenti L., Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. Journal of Hepatology. 2018;68(2):268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 207.Kazierad D., Chidsey K., Somayaji V., Bergman A., Birnbaum M.J., Calle R. Inhibition of ketohexokinase in adults with non-alcoholic fatty liver disease reduces liver fat and inflammatory markers: A randomized Phase II trial. Medicine (N Y) 2020 doi: 10.1016/j.medj.2021.04.007. in press. [DOI] [PubMed] [Google Scholar]