Abstract
Background
Approximately 40% of human pregnancies are unintended, indicating a need for more acceptable effective contraception methods. New antibody production systems make it possible to manufacture reagent-grade human monoclonal antibodies (mAbs) for clinical use. We used the Nicotiana platform to produce a human antisperm mAb and tested its efficacy for on-demand topical contraception.
Methods
Heavy and light chain variable region DNA sequences of a human IgM antisperm antibody derived from an infertile woman were inserted with human IgG1 constant region sequences into an agrobacterium and transfected into Nicotiana benthamiana. The product, an IgG1 mAb [“Human Contraception Antibody” (HCA)], was purified on Protein A columns, and QC was performed using the LabChip GXII Touch protein characterization system and SEC-HPLC. HCA was tested for antigen specificity by immunofluorescence and western blot assays, antisperm activity by sperm agglutination and complement dependent sperm immobilization assays, and safety in a human vaginal tissue (EpiVaginal™) model.
Findings
HCA was obtained at concentrations ranging from 0.4 to 4 mg/ml and consisted of > 90% IgG monomers. The mAb specifically reacted with a glycan epitope on CD52g, a glycoprotein produced in the male reproductive tract and found in abundance on sperm. HCA potently agglutinated sperm under a variety of relevant physiological conditions at concentrations ≥ 6.25 µg/ml, and mediated complement-dependent sperm immobilization at concentrations ≥ 1 µg/ml. HCA and its immune complexes did not induce inflammation in EpiVaginal™ tissue.
Interpretation
HCA, an IgG1 mAb with potent sperm agglutination and immobilization activity and a good safety profile, is a promising candidate for female contraception.
Funding
This research was supported by grants R01 HD095630 and P50HD096957 from the National Institutes of Health.
Keywords: Contraception; Non-hormonal, Monoclonal antibody; Antisperm antibody; Nicotiana; Sperm
Research in context.
Evidence before this study
We chose to pursue an antisperm monoclonal antibody for contraception because there is an extensive literature about antisperm antibodies and infertility. A substantial percentage of infertile men and women have antisperm antibodies in genital secretions that affect sperm function, and for many years infertility patients were screened for these antibodies. The World Health Organization convened a Contraceptive Vaccine Task Force in 1985, and in conjunction with this program a number of scientists developed antisperm monoclonal antibodies (mAbs) to characterize sperm antigens with potential for use in contraceptive vaccines. A leading antisperm mAb, H6-3C4, was made by a Japanese group by hybridizing lymphocytes from an infertile woman with mouse hybridoma cells. The WHO's contraceptive vaccine project was discontinued following criticism that the efficacy of an antisperm vaccine might vary depending on the strength of the immune response in vaccinated women, and that it could be difficult to reverse a strong infertility effect.
Added value of this study
Passive immunization with preformed antisperm antibodies would circumvent the concerns raised over active immunization with sperm for contraception. Over the past 10 years, the field of passive immunization has gained traction due to improved cloning techniques for human mAbs, and new production platforms. In this translational research project we transfected the variable region gene sequence from the H6-3C4 mAb into a modern platform, Nicotiana benthamina, used to make clinical grade mAbs, to produce a human antisperm mAb suitable for contraception. We demonstrate that the IgG1 mAb is specific, agglutinates sperm within 15 seconds, does not cause irritation, and remains active in vaginal conditions (low pH, diverse microflora) for up to 24 hours.
Implications of all the available evidence
This antibody appears to be suitable for contraceptive use. It could be administered vaginally in a film for a woman-controlled on-demand method, and could be combined with other antibodies such as anti-HIV and anti-HSV antibodies for a multipurpose prevention technology. Such products may be acceptable to women who do not use currently available contraception methods, and could have a significant impact on global health as unintended pregnancies constitute 40% of all pregnancies and often adversely affect maternal health and economic well-being.
Alt-text: Unlabelled box
1. Introduction
Over the past 200 years the human population has grown from under 1 billion to over 7.7 billion individuals, and is on track to exceed 10 billion by the end of this century [1, 2]. Many population experts have voiced concern that this rapid rate of population growth exerts dangerous pressure on planetary resources and is unsustainable. Despite the availability of numerous effective birth control methods, over 40% of pregnancies worldwide are unintended. These pregnancies are significantly contributing to population growth, and unintended pregnancies can have pronounced adverse effects on maternal physical, mental, and economic wellbeing [1]. There is an urgent need for new contraception methods to address the problem of unintended pregnancies. The majority of effective female contraceptive methods require medical visits and/or procedures which can introduce economic and accessibility barriers. Among the most widely used reversible contraceptives, hormonal methods are frequently discontinued due to side effects, and barrier methods often fail due to inconsistent or improper use [3], [4], [5], [6]. New non-hormonal, woman-controlled methods that are safe, accessible, inexpensive, and discrete could provide an important contribution to the contraception field. Recent surveys indicate that women find two approaches highly desirable: on-demand contraception, and multipurpose prevention technology (MPT) products that provide dual protection against unintended pregnancy and sexually transmitted infections (STIs) [7, 8]. Such products could increase the popularity and use of contraceptive products.
Human monoclonal antibodies (mAbs), once used primarily as research and diagnostic tools, are now employed in a variety of clinical applications due to recent advances in cloning, genetic engineering, and improved mAb production platforms [9]. Over 100 mAbs have been approved for human therapeutic use, mostly in the fields of oncology, autoimmunity, and inflammatory disease [10]. A human anti-HIV mAb, VRC01, was recently tested in two large Phase 3 clinical trials for HIV prevention [11]. Most mAbs are administered systemically through intravenous or subcutaneous injection, but nonhuman primate studies and Phase 1 human clinical trials have also demonstrated the feasibility of topical intravaginal mAb administration to prevent the transmission of HIV-1 and other STIs [9]. Our group recently completed a Phase 1 clinical trial that assessed the safety and pharmacokinetics of a topical vaginal film containing human mAbs against HIV-1 and Herpes Simplex Viruses (HSV)-1 and 2 made in Nicotiana benthamiana, a species of tobacco plant. The Nicotiana platform is fast and versatile, and produces a very clean antibody product free from potential contamination with mammalian pathogens. The trial demonstrated that mAbs made in Nicotiana were safe, and that effective concentrations of neutralizing antibodies could be detected in vaginal secretions for at least 24 h after film administration [12]. This study provides proof-of-concept that combinations of pathogen-specific mAbs administered intravaginally could safely protect women against a variety of STIs. The addition of mAbs against reproductive targets, such as sperm, could also confer contraceptive protection and constitute a unique non-hormonal MPT product.
Antisperm antibodies are often detected in the sera and genital secretions of infertility patients. Antibodies reactive with sperm surface antigens, most frequently detected in the clinical laboratory by immunobead assay, can affect a number of sperm functions. They appear to have a primary effect on endocervical mucus penetration by inducing sperm agglutination, complement-mediated sperm immobilization, or by trapping sperm through charge interactions between the antibody Fc region and mucus fibrils [13], [14], [15]. One such human antisperm mAb, H6-3C4, has shown promise as a contraceptive antibody. The mAb is directed against an N-linked carbohydrate epitope on a male reproductive tract (MRT)-specific glycoprotein, CD52g, which is expressed and secreted primarily by epithelial cells in the human cauda epididymis [16]. CD52g has a GPI anchor that mediates its insertion into the plasma membrane of sperm and other cells found in the lumen of the MRT. The unique carbohydrate epitope differentiates MRT-CD52g from CD52 which is highly expressed on lymphocytes [17]. H6-3C4 was an IgM subclass antibody originally cloned from B-cells from a woman with infertility and a high titer of sperm-immobilizing antibodies [18].
Our research team used variable region sequences from H6-3C4 [19] to manufacture a human antisperm IgG1 mAb in Nicotiana benthamiana plants, a versatile, cost-effective platform that has been used to produce a variety of human mAbs and other proteins for clinical applications [20], [21], [22]. We have designated this antibody “Human Contraception Antibody (HCA)”. In this report, we describe the production of HCA, its specificity, and its performance in sperm function and safety assays.
2. Methods
2.1. Study design
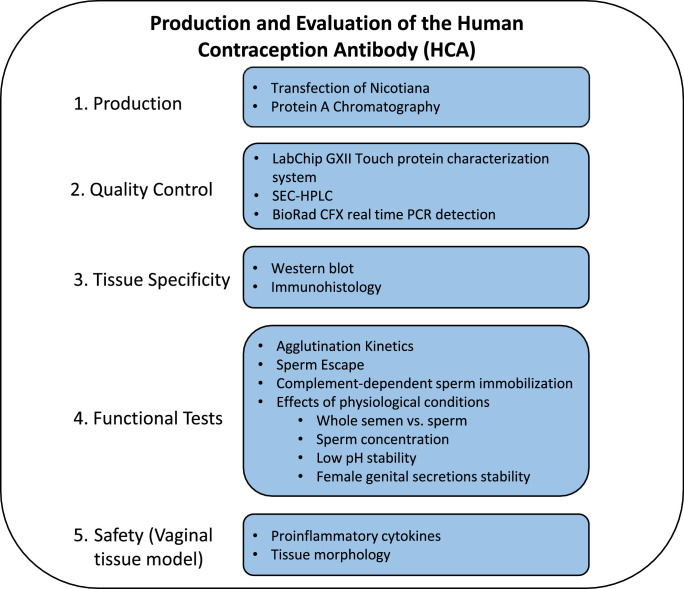
We manufactured HCA in Nicotiana, purified it on Protein A columns, and performed several quality control assays. To evaluate HCA for suitability as a topical contraceptive, we tested its specificity and activity over a wide range of concentrations, and under different physiologically relevant conditions in vitro (see Fig. 1 for study overview). Antibodies were tested at concentrations ranging from 1 µg to 100 µg/ml, which were within the range of mAb concentrations detected in cervicovaginal secretions of women 4–24 h after application of a mAb-containing vaginal film [12]. Three functional tests were performed: the agglutination kinetic assay which measured sperm agglutination efficiency in vitro, the sperm escape assay which determined the percentage of progressively motile sperm that escaped agglutination after mAb treatment, and the immobilization test that assessed sperm immobilization in the presence of complement. Each data point was assessed in triplicate and experiments were replicated a minimum of three times using different sperm donors. All semen samples met WHO fertile semen parameters [23]. Percent sperm agglutination was assessed at time points up to 2 min after the addition of HCA, which was used as a cutoff because it is less than the length of time it takes sperm to reach the endocervical canal (estimated to occur 3–5 min post-insemination [24], [25], [26]). Two blinded, trained laboratory personnel independently observed agglutination to avoid bias. Safety assessments included mAb specificity testing by immunofluorescence and western blot assays, and evaluation of vaginal tissue for proinflammatory cytokines following treatment with HCA and HCA immunocomplexes. These assays were repeated at least twice to confirm the results. S19, a commercially available mouse anti-CD52g mAb, was used as a positive control, and CAMPATH, a commercially available rat anti-human CD52 mAb, and VRC01-N, a human anti-HIV IgG1 mAb manufactured in Nicotiana, were used as negative controls.
Fig. 1.
Study overview.
2.2. Antibodies
2.2.1. Human contraception antibody (HCA)
The human anti-CD52g mAb, HCA, was produced in Nicotiana benthamiana by Mapp Biopharmaceutical Inc. (San Diego, CA, USA). Genes containing the variable region sequences of H6-3C4 [16, 19] were synthesized by Life Technologies (San Diego, CA, USA) and cloned into TMV and PVX plant expression vectors [27] containing codon-optimized human lambda and human IgG1 constant regions. The vectors were then transformed into Agrobacterium tumefaciens strain ICF320 (Icon Genetics; Halle/Saale, Germany) which was used to transfect 4-week old Nicotiana plants by vacuum infiltration as previously described [22]. Seven days post-infiltration, antibody was extracted from the leaf tissue and purified by Protein A chromatography as previously described [22, 28]. The Nicotiana plants used for this study were a transgenic strain in which fucosyl- and xylosyl- transferases were knocked down with RNAi so that the antibodies they produced had a humanized glycosylation pattern [21, 29]. HCA QC was performed using multiple instruments/assays including the LabChip GXII Touch protein characterization system (quantification, purity, molecular weight sizing), SEC-HPLC, BioRad CFX real time PCR detection (thermal melt), and a Wyatt DynaPro Plate Reader II (DLS).
2.2.2. S19
Mouse anti-CD52g mAbs were obtained from the supernatant of S19 hybridoma cells originally established by John Herr (ATCC Cat# HB-12144, Manassas, VA, USA) [30], [31], [32]. S19 hybridoma cells were grown in complete Isocove's Modified Dulbecco's Medium (IMDM) (ATCC Manassas, VA, USA) with 10% fetal bovine serum (FBS), 1% Penicillin Streptomycin, and 1% L-Glutamine 200mM (Gibco, ThermoFisher Scientific, Waltham, MA, USA). For antibody harvesting, hybridomas were seeded at confluency in IMDM without FBS and cultured until most cells died [33]. The supernatant was collected and mAbs were concentrated with a 15mL, 50-kDa centrifuge filter (UFC905096, Millipore Sigma, Burlington, MA, US) at 3,000 x g for 40 min. S19 antibody was resuspended in PBS and quantified by nanodrop. Antibody was stored in Multipurpose Handling Media (MHM; FUJIFILM Irvine Scientific; Santa Ana, CA, USA) at 4°C.
2.2.3. CAMPATH-1
CAMPATH-1 is a commercially available rat mAb, clone YTH34.5, directed against a peptide epitope on CD52 (ThermoFisher Scientific Cat# MA5-16999, Waltham, MA, USA, RRID: AB_2538471).
2.3. Tests of antibody specificity
2.3.1. Immunofluorescence assay
Anti-CD52g mAbs were tested for specificity on washed human spermatozoa and human peripheral blood mononuclear cells (PBMCs, negative control). Cells were smeared on glass slides, air dried and fixed in acetone, and incubated with 25, 50 or 100 µg/mL of HCA, S19 or CAMPATH mAbs. VRC01-N, a human IgG1 mAb produced in Nicotiana and directed against the HIV-1 envelope glycoprotein gp120, was used as an isotype-matched negative control antibody for HCA. A mouse mAb directed against CD3, a prominent T lymphocyte surface protein (Santa Cruz Biotechnology Cat# sc-20047, Santa Cruz, CA, USA, RRID: AB_627014) was used as an isotype-matched control antibody for S19. Primary antibodies were detected with a 1:200 dilution of fluorophore-conjugated secondary antibodies against mouse (Jackson ImmunoResearch Cat# 715-165-150, RRID: AB_2340813), human (Jackson ImmunoResearch Cat# 709-166-149, RRID: AB_2340538) or rat Igs (Jackson ImmunoResearch Cat# 712-165-153, RRID: AB_2340667) (All from Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA).
2.3.2. Immunohistology
Antibodies were tested for reactivity on a variety of human reproductive tract tissues (cauda epididymis, vas deferens, vagina) and tonsil. Paraffin-embedded tissues were sectioned at 5 µm, de-waxed, hydrated, and subjected to antigen retrieval which was carried out by immersing the sections in a citrate buffer, pH 6, and then placing the tissues in a pressure cooker that was heated to 125°C for 30 seconds (Biocare Medical, Concord, CA). The sections were incubated with Cy3-labeled HCA or VRC01 (conjugation kit: Abcam, Cambridge, MA, USA) at a 1:20 dilution overnight at 4°C. Finally, the sections were mounted in AntiFade Mounting Medium with DAPI. All sections were examined under an Olympus microscope (Olympus America Inc. Melville, NY) fitted with epifluorescence imaging and images were captured with a digital camera.
2.3.3. Scanning electron microscopy
Sperm that had been treated with HCA or PBS (control) were air dried on coverslips and fixed in 0.5 strength Karnovsky's fixative for 48 h. Coverslips were then rinsed in PBS and dehydrated in a series of ethanol concentrations- 35, 50, 70, 95% (1x for 10 min) and 100% (3x for 10 min) before critical point drying in a Tousimis semi-automatic Critical Point Dryer. Coverslips were mounted on aluminum stubs and coated with chromium in a GATAN Ion Beam Coater. Images were taken with a JEOL 7401F Field Emission Scanning Electron Microscope.
2.3.4. Western blot
Human sperm, seminal plasma, and VK2/E6E7 cells (vaginal epithelial cell line used as a negative control [34]) (VK2/E6E7, ATCC Cat# CRL-2616, RRID: CVCL-6471) were lysed in radioimmunoprecipitation assay (RIPA) buffer, denatured with SDS and used as antigen targets in the western blot assay. 20–30 µg of protein were loaded onto a 4–12% gradient polyacrylamide gel, and after electrophoresis, proteins were transferred to PVDF membranes. The blots were blocked with 5% powdered milk and washed with 0.1% TBS-T. All primary antibodies were used at a concentration of 10 µg/mL; anti-mouse (Santa Cruz Cat# sc-2005, RRID: AB_631736, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-rat (Abcam Cat# ab97057, RRID: AB_10680316, Cambridge, MA, USA) secondary antibodies were used at a 1:20,000 dilution, and the anti-human secondary antibody (Abcam Cat# ab6858, RRID: AB_955433) was used at a 1:5,000 dilution. Blots were developed in ECL substrate (ThermoFisher Scientific, Waltham, MA, USA). To cleave off the N-linked glycan epitope, sperm cell lysates were treated with PNGase F according to the manufacturer's protocol (New England Biolabs, Ipswich, MA, USA).
2.4. Tests of antibody function
2.4.1. Semen analysis and sperm preparation
Healthy men between the ages of 18–45, with semen samples meeting the WHO criteria for fertility [23], provided fresh semen samples for this study. The samples were processed within one hour of collection, after liquefaction. Live sperm were obtained by passage of semen through 90% ISolate density gradient medium (FUJIFILM Irvine Scientific; Santa Ana, CA, USA). Briefly, whole semen was layered over an equivalent volume of ISolate. The sample was centrifuged at 300xg for 20 min, and the pellet containing live, motile sperm was resuspended in MHM. Sperm count and motility were assessed by Computer-Assisted Sperm Analysis (CASA; Human Motility II software, CEROS II, Hamilton Thorne, Beverly, MA, USA) utilizing the recommended settings by the manufacturer. Both whole semen and washed motile sperm were used for the functional assays described below.
2.4.2. Agglutination kinetics assay
Two microliters of sperm suspension were pipetted onto a 9mm well microscope slide (ThermoScientific, Waltham, MA, USA), and an equivalent amount of antibody in MHM was added. Antibody and sperm were mixed by quickly pipetting the mixture up and down three times, and sperm agglutination was observed in real-time on an Olympus inverted microscope at 10X. The time it took for sperm to become completely (100%) agglutinated was recorded by two trained independent readers blinded to experimental variables. For most of the tests a sperm concentration of 20 to 40 × 106/ml was used; antibody concentrations ranged from 1 to 100 µg/ml. A minimum of three replicates was conducted for each data point. The assay was repeated a minimum of three times using samples from different donors.
2.4.3. Modified sperm swim-up (“sperm escape”) assay
The “Sperm Escape Assay” was developed by us to assess the percentage of progressively motile free-swimming sperm that remained in the supernatant following treatment with HCA. MAbs were diluted in MHM, and 30 µL were added to upright 0.2mL PCR tubes. Thirty µL of motile sperm suspension or whole semen were then added to the antibody and the contents were mixed three times by pipetting. Tubes were placed at a 45° angle and the mixture was incubated for 5 min at room temperature to allow agglutinated and immotile sperm to settle to the bottom of the tube. A 2.5 µL sample was taken from the top millimeter of the mixture and placed in a 4-chambered slide (Microcell 15424, Vitrolife, San Diego, CA, USA). The sample was analyzed by CASA to determine the total sperm concentration and the number of progressively motile sperm that had escaped agglutination.
2.4.5. Complement-dependent sperm immobilization assay
Washed sperm with an initial motility > 90% were adjusted to a concentration of 25–40 × 106/mL in MHM. Human serum, separated from fresh whole blood by centrifugation, was used as the complement source. Heat-inactivated (HI) complement (heated to 56 °C for 30 min) was used as the control. Five µL of complement or HI-complement were incubated with 2.5 µL of sperm suspension for 5 min at room temperature. Next, 25 µL of HCA were added in a series of two-fold dilutions (3.125–0.20 µg/mL) and mixed; 3 µL of the suspension were transferred to 4-chambered slides in duplicate and the slides were incubated in a humidified chamber at 32 °C for 1 h. Sperm motility was assessed using the CEROS II CASA. A minimum of three trials was performed using different semen donors.
2.5. Testing HCA activity under various physiological conditions
2.5.1. Whole semen vs. washed sperm
To compare the ability of HCA to agglutinate sperm in whole semen and in washed sperm suspensions, the semen samples were divided into two fractions: (1) whole unprocessed semen, and (2) ISolate- prepared washed motile sperm resuspended in MHM to the same concentration as that of the original semen sample. Semen from two to three donors were pooled for these experiments. Agglutination Kinetics and Sperm Escape Assays were performed as described above.
2.5.2. Effect of sperm concentration
Motile sperm were isolated from semen using ISolate, and resuspended in MHM at concentrations ranging from 2 to 100 × 106/mL.
2.5.3. Effect of low pH
The pH of MHM was lowered to 3.5 using D/L-lactic acid (Sigma Aldrich, MA, USA). HCA was incubated in acidic (pH 3.5) or neutral (pH 7.0) MHM for 0, 2, 4, and 24 h at 37 °C. Following incubation, the low pH MHM was neutralized by addition of HEPES Buffer (Invitrogen) and neat seminal plasma. The antibody was then used in the Agglutination Kinetics Assays.
2.5.4. Effects of female genital secretions
HCA was incubated in cervicovaginal lavage (CVL) supernatant (1:1 vol) for up to 24 h at 37 °C. CVLs from healthy women without bacterial vaginosis (BV−; i.e., Nugent scores between 0–3) and CVLs from women with bacterial vaginosis (BV+; Nugent scores above 8) were obtained as previously described [12]. Pooled samples from three women with each condition were used for this experiment. After incubation with CVL, HCA was diluted in MHM for use in the Agglutination Kinetics Assay.
2.6. Preclinical safety assessment
2.6.1. Vaginal tissue cytokine response assay
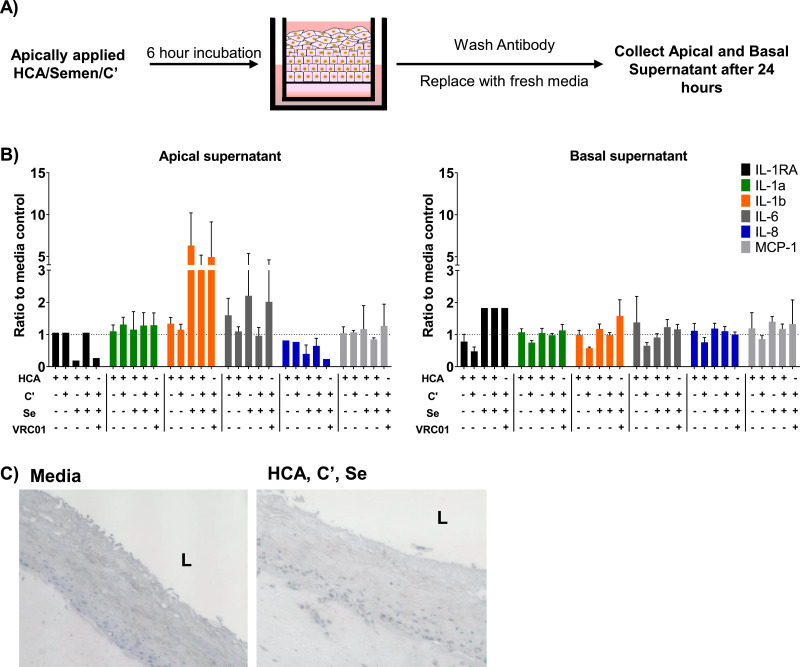
The EpiVaginal™ tissue model (MatTek Cat# VEC-100FT, MatTek Corporation, Ashland, MA, USA) was used to study potential proinflammatory effects of HCA and HCA immune complexes. This tissue model has been validated for vaginal irritation studies [35]. A total of 100 µL of different combinations of HCA (100 µg/mL), semen and complement (C’) were added to the upper chamber (apical side) of the EpiVaginal™ tissue. Tissues were incubated at 37 °C for 6 h after which the antibody/sperm/complement suspensions were washed off, and 80 µL of MatTek EpiVaginal Tissue Media was added to the upper chamber. After 24 h, apical and basal supernatants were collected and cytokines were measured using a custom 6-plex ProcartaPlex Luminex kit (Invitrogen, ThermoFisher Cat# PPX-06, ThermoFisher Scientific, Waltham, MA, USA).
2.7. Statistical analysis
GraphPad Prism (Version 7.03; GraphPad Software Inc.; San Diego, CA, USA) was used for statistical analysis and graphing of data. Data were determined to be normally distributed by the Kolmogorov-Smirnov test. Data were subjected to analysis of variance (ANOVA). A significant ANOVA was followed by post hoc Tukey multiple comparison tests. For the Sperm Immobilization test, a t-test with a correction for multiple comparisons, was used. Differences were considered to be statistically significant when p < 0.05.
2.8. Ethics statement
This study was approved by the Boston University Medical Campus Institutional Review Board (Human Subjects Protocol #H-36843), and all participants provided written informed consent.
2.9. Role of the funding source
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; R01 HD095630 and P50 HD096957). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
3. Results
3.1. HCA production and characterization
3.1.1. HCA production and yield
HCA was isolated from leaf extracts on Protein A columns. HCA quality control (QC) was performed using multiple assays to determine quantity, purity, molecular weight sizing, and stability (Table 1). Antibody concentrations ranged from 0.4 to 4 mg/mL.
Table 1.
Quality control parameters for HCA production and purification.
| Test Parameter | Test Method | Specification |
|---|---|---|
| General Characteristics | Visible Appearance | Clear liquid, no visible particulates |
| pH | 6.5 ± 0.5 | |
| Osmolality | 10–100 mOsm/kg | |
| Protein Concentration | UV Absorbance (A280) | 15–25 mg/mL |
| Identity | IEX-HPLC | Chromatogram Conforms to Standard |
| Purity | Size Exclusion HPLC | > 90% Monomer < 5% HMW Species |
| SDS-PAGE Reduced, Coomassie Stained | > 95% (Sum of Heavy and Light Chains) | |
| SDS-PAGE Non-Reduced, Coomassie Stained | > 90% Main Band | |
| Safety | Endotoxin | < 5 EU/mg |
| Bioburden | ≤ 1 CFU/mL | |
| Impurities | Residual Host Cell DNA | < 50 pg/mg |
| Residual Protein A ELISA | < 100 ng/mg | |
| Residual Host Cell Protein ELISA | < 100 ng/mg | |
HMW = High Molecular Weight
3.1.2. HCA specificity
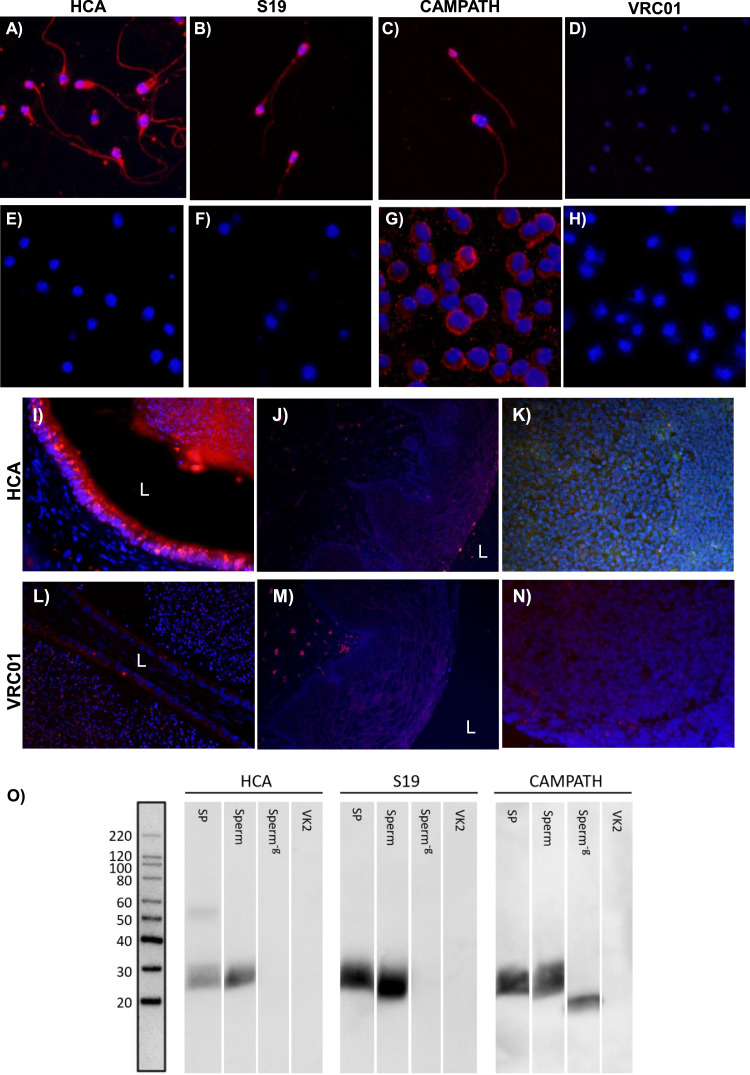
HCA antigen specificity was confirmed by immunohistology and western blot. HCA and control antibodies were tested by indirect immunofluorescence assay on acetone-fixed washed human sperm and peripheral blood mononuclear cells (PBMCs, negative control). HCA, S19, and CAMPATH reacted with the entire sperm surface, whereas VRC01 showed no reactivity (Fig. 2A–D, respectively). HCA, S19, and VRC01 did not react with PBMCs, whereas CAMPATH did as expected [32] (Fig. 2 E–G). Tissue immunohistology was also used to demonstrate HCA specificity. Cy3-conjugated HCA reacted with epithelial cells in the human cauda epididymis and its luminal contents containing sperm (Fig. 2I), but did not react with human vaginal or tonsil tissue (Fig. 2J,K). Cy3-conjugated VRC01 (isotype control) did not react with any of the tissues (Fig. 2L–N). In the western blot assay, HCA detected a ~15–25 kDa band in seminal plasma and sperm lysates, the expected size of heavily glycosylated CD52g. The band was undetectable following treatment of sperm lysates with PNGase F, an enzyme that cleaves N-linked glycans (Fig. 2O, left panel). CAMPATH, which targets the peptide backbone on CD52g, also reacted with the ~15–25 kDa band in sperm lysates; after PNGase F treatment of sperm, CAMPATH detected a lower MW band representing CD52g without N-glycans (Fig. 2O, right panel). None of the antibodies reacted with lysates of VK2 cells, an immortalized human vaginal epithelial cell line [34] used for negative control.
Fig. 2.
Specificity testing of HCA. Indirect immunofluorescence of sperm with (A) HCA, (B) S19, (C) CAMPATH, and (D) VRC01 [isotype control for HCA]. Indirect immunofluorescence of PBMCs with (E) HCA, (F) S19, (G) CAMPATH, and (H) VRC01. Reactivity of Cy3-labeled HCA in (I) human cauda epididymis, (J) vaginal, and (K) tonsil tissue. (L-N) Lack of reactivity of Cy3-labeled VRC01 (isotype control) with the same tissues. L = Lumen. All images 20x magnification. (O) Western blots of HCA, S19 and CAMPATH antibodies tested on electrophoresed lysates of human seminal plasma (SP), sperm, PNGaseF-treated sperm (Sperm−g), and VK2 cells (negative control).
3.2. Functional assays
3.2.1. Sperm agglutination kinetics assay
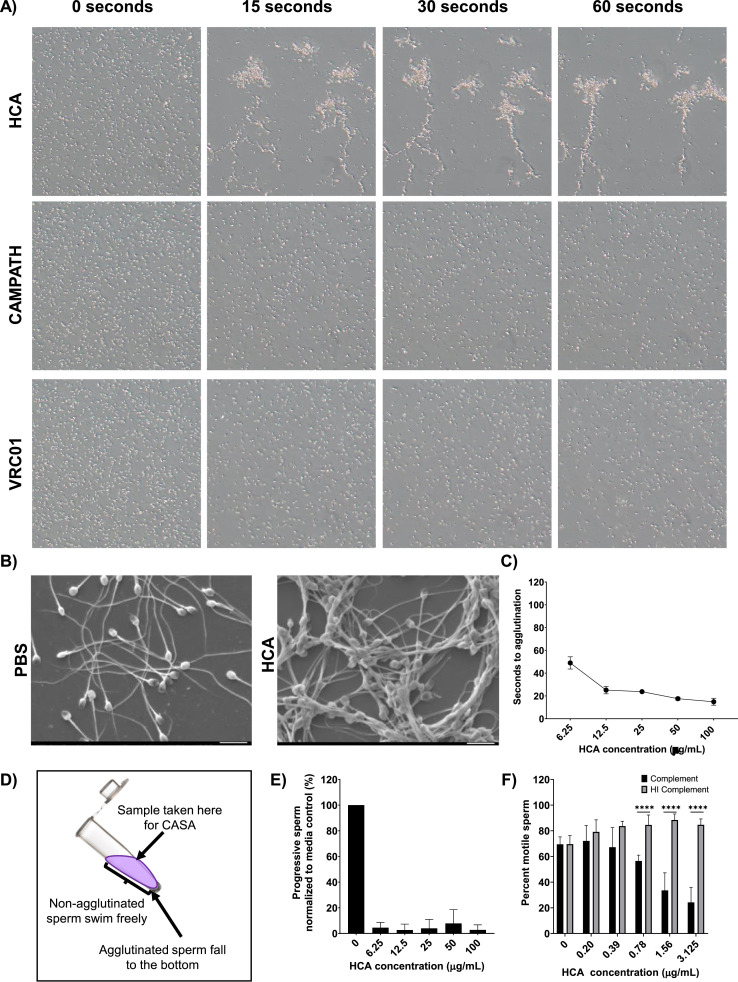
At a concentration of 100 µg/mL, HCA agglutinated 100% of sperm in under 15 s (Figs. 3A and 2C). Scanning electron microscopy of HCA-treated sperm revealed a mixed agglutination pattern: head-to-head, head-to-tail, tail-to-tail (Fig. 3B). Effective agglutination time varied with HCA concentration; at HCA concentrations ≥ 12.5 µg/ml, complete sperm agglutination occurred within 20 s, whereas at an HCA concentration of 6.25 µg/ml complete agglutination occurred in 50 s (Fig. 3C). At a concentration of 1.5 µg/ml, complete agglutination had not occurred by 120 s. CAMPATH and VRC01 did not agglutinate sperm at any concentration (Fig. 3A).
Fig. 3.
HCA effectively agglutinates human sperm (A) Time-elapse photography of 1:1 mixtures of washed sperm (100 × 106/mL) and 100 µg/mL of mAbs (HCA, CAMPATH, VRC01). Sperm agglutination was monitored using an inverted microscope (10x). (B) SEM images of HCA-agglutinated sperm and PBS control. All images at 1500x. (C) Agglutination kinetics assay using ~30 × 106/mL washed sperm and various concentrations of HCA. “Seconds to agglutination” is the time taken for sperm in a 10x field of view to fully agglutinate. Each data point is the average of triplicate reads (Two-way ANOVA with multiple comparisons). (D) Schematic of the sperm escape assay, a modified swim-up assay that measures progressive sperm that escape HCA-induced agglutination. (E) Sperm escape assay using ~30 × 106/mL washed sperm. Y-axis depicts the percentage of progressive sperm present in the supernatant of HCA-treated samples compared to media control. Black bars indicate results with washed sperm and red bars indicated results with whole semen. Each bar indicates the average of triplicate reads (Two-way ANOVA with multiple comparisons). (F) Complement-dependent sperm immobilization test. HI = heat inactivated complement (****p-value < 0.0001). Error bars represent ± SD values.
3.2.2. Sperm escape assay
A modified sperm swim-up assay was used to evaluate the number of progressively motile sperm that escape from sperm agglutinates following exposure to HCA; agglutinated and immotile sperm sink to the bottom of the tube, whereas sperm with progressive motility can be recovered from the upper fraction of the sperm suspension (Fig. 3D). These progressively motile sperm are the antibody “escapees” that could potentially fertilize an ovum. At HCA concentrations ranging from 100 to 12.5 µg/ml, the number of motile sperm in the swim-up fraction was < 2% of the media control; at the lowest HCA concentration tested (6.25 µg/mL), the number of progressively motile sperm in the swim-up fraction was 5% of media control (Fig. 3E).
3.2.3. Complement-dependent sperm immobilization
A modified sperm immobilization test was used to determine whether HCA induces complement (C’)-dependent sperm immobilization, another mechanism that may mediate antibody-mediated infertility [36, 37]. Since HCA potently agglutinates sperm at concentrations ≥ 6.25 µg/mL, HCA concentrations < 6.25µg/mL were used to visualize and quantify individual sperm. With the addition of active human complement, there was a significant decrease in the number of motile sperm (p < 0.0001); significant sperm immobilization was observed at HCA concentrations as low as 0.78 µg/mL (Fig. 3F). HCA did not immobilize sperm in the presence of heat-inactivated complement (negative control).
3.3. HCA activity under physiologically relevant conditions
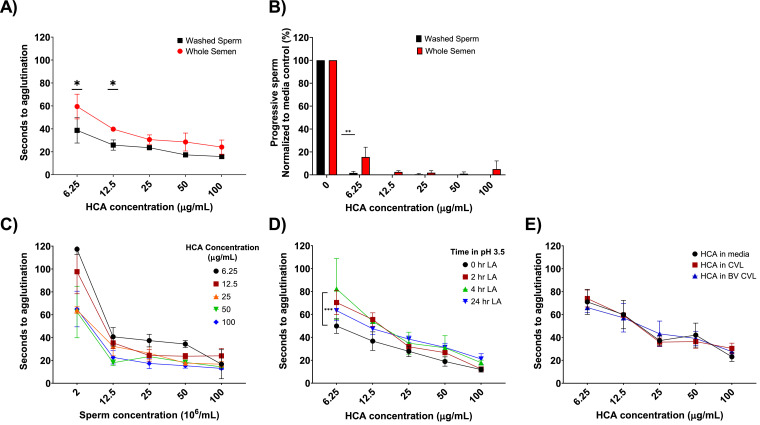
3.3.1. Washed sperm vs. whole semen
We tested the ability of HCA to agglutinate sperm in the presence and absence of seminal fluid because sperm are delivered to the vagina in seminal fluid, but rapidly swim away from the insemination site. We hypothesized that the kinetics could be different since seminal plasma contains high concentrations of soluble CD52g which could compete with CD52g on sperm for HCA binding. At HCA concentrations ≥ 25 µg/ml, the difference between sperm agglutination times in whole semen compared to washed sperm was not significant. At 6.25 and 12.5 µg/ml, the time to agglutination was significantly greater in whole semen compared to washed sperm (p < 0.05, Fig. 4A), but even at these low antibody concentrations, sperm in whole semen were agglutinated in less than 60 s. We also compared the ability of HCA to agglutinate sperm in whole semen versus washed sperm preparations using the sperm escape assay. At HCA concentrations ≥ 12.5 µg/mL, the number of progressively motile sperm in the swim-up fraction was < 5% of media-treated sperm for both whole semen and washed sperm preparations (p > 0.05, NS). At 6.25 µg/mL, the fraction of progressive sperm remained < 5% for washed sperm samples but increased to 15% for whole semen samples (p < 0.01, Fig. 4B).
Fig. 4.
HCA agglutinates sperm under various physiologically relevant conditions in vitro. (A) Agglutination kinetics assay comparing agglutination of sperm in whole semen versus washed sperm suspensions. Each data point is the average of triplicate reads (*p-value < 0.05). (B) Sperm escape assay comparing whole semen and washed sperm cell agglutination. Black bars indicate washed sperm data and red bars indicate whole semen data. Each bar indicates the average of triplicate reads (**p-value < 0.01). (C) Agglutination kinetics assay comparing the ability of HCA to agglutinate washed sperm at concentrations ranging from 2 to 100 × 106/mL. Each data point is the average of triplicates. (D) Agglutination kinetics assay comparing the ability of HCA to agglutinate sperm after prolonged incubation in 0.5% D/L lactic acid (pH 3.5) for 0, 2, 4, 24 h. Each data point is the average of triplicate reads (***p-value < 0.001). (E) Agglutination kinetics assay comparing the ability of HCA to agglutinate sperm after 24 h incubation BV+ and BV- cervicovaginal lavage supernatants (pooled samples from 3 donors). Each data point is the average of triplicate reads. Error bars represent ± SD values. All graphs are representative of 2-3 independent experiments and 6 participant samples. (Two-way ANOVA with multiple comparisons)
3.3.2. Effect of sperm concentration
We tested the effect of sperm concentration on antibody-induced sperm agglutination since agglutination is dependent on sperm collisions and antibody bridging. Agglutination could be less efficient at low sperm concentrations where antibody-antigen interactions occur at a lower frequency; at higher sperm concentrations effective agglutination could require higher antibody concentrations due to larger amounts of available antigen. Sperm concentrations in human ejaculates can vary from 0 sperm/mL (azoospermia) to greater than 200 × 106/mL [23]. Men with sperm concentrations ≤ 15 × 106/mL are oligozoospermic, a condition associated with reduced fertility [23, 38, 39], and men with ≤ 1 × 106 sperm/mL have severe oligospermia and a very low fertility rate. Male contraception studies aim to reduce sperm concentrations to ≤ 1 × 106/ml [40]. We tested the ability of HCA to agglutinate sperm over a wide range of physiological sperm concentrations by adjusting the concentration of sperm in ejaculates from normal sperm donors. As shown in Fig. 4C, HCA concentrations ≥ 6.25 µg/mL effectively agglutinated sperm within one minute at sperm concentrations ranging from 12.5 × 106 to 100 × 106/mL, indicating that sperm within the fertile concentration range agglutinate effectively. At the lowest sperm concentration tested, 2 × 106/mL, agglutination occurred within 70 s at HCA concentrations ranging from 25 to 100 µg/mL; at the lowest HCA concentration (6.25 µg/ml), the sperm did not agglutinate within 2 min. Overall, sperm concentrations in the fertile range agglutinated within an acceptable length of time at HCA concentrations ≥ 6.25 µg/ml.
3.3.3. Effect of low pH and female genital secretions
We performed most of the functional assays for this study at neutral pH because the vaginal pH is neutral after intercourse due to the addition of alkaline semen [41]. However, for HCA to successfully be used as a topical product in the vaginal tract, it must withstand physiological conditions in the vagina before intercourse. Vaginal secretions from reproductive-aged women generally have an acidic pH due to the production of lactic acid (LA) by Lactobacillus species that colonize the vagina (average pH ~3.5) [42, 43]. To determine whether HCA can withstand exposure to low pH, we incubated HCA for 0, 2, 4, and 24 h at 37 °C in 0.5% LA, pH 3.5. Since low pH affects sperm viability, we neutralized LA-treated HCA by addition of seminal plasma before conducting the kinetic agglutination assay. LA-treated HCA at concentrations ≥ 12.5 µg/mL effectively agglutinated sperm in under one minute, and there was no significant difference across the various LA-treatment time points. At the lowest antibody concentration tested (6.25 µg/mL), LA-treated HCA was significantly less effective than untreated HCA in the sperm agglutination assay, but still agglutinated sperm within 80 s (p < 0.001, Fig. 4D). In addition to LA, vaginal fluid contains proteases, immunomodulating components, mucins, and other factors that could affect the efficacy of HCA [44], and the composition of vaginal fluid can be affected by bacterial vaginosis (BV) and other infections [45]. To determine whether soluble factors in vaginal secretions affect HCA activity, we incubated the mAb in CVL samples from reproductive aged women with normal vaginal flora or with BV. CVL-treated HCA remained highly active in the sperm agglutination assay indicating that soluble factors in cervicovaginal secretions from healthy women and women with BV do not impair HCA activity (Fig. 4E).
3.4. Preclinical safety test
We tested the effects of HCA and HCA immune complexes in an in vitro model of vaginal integrity and inflammation. Combinations of antibody, semen, and human complement were added to the apical side of MatTek EpiVaginal™ tissues [35] and incubated for 6 h. After washing the apical side of the tissues and allowing cytokines to be secreted into fresh media for 24 h, the apical and basal supernatants were collected and cytokine concentrations were measured by Luminex (Fig. 5A). Semen treatment increased levels of IL-1β and IL-6 in the apical supernatants irrespective of the presence of HCA and/or complement [46, 47] (Fig. 5B). Notably, cultures treated with HCA alone, HCA + C’, and HCA + C’ + semen did not exhibit additional upregulated expression of proinflammatory cytokines by the vaginal epithelial cultures. Histology of the treated tissues showed that tissues were morphologically intact after treatment with HCA immune complexes (Fig. 5C).
Fig. 5.
HCA does not increase proinflammatory cytokines in EpiVaginal tissue. (A) Schematic of MatTek irritation assay where combinations of HCA/Semen/Complement were applied to the apical surface of EpiVaginal tissue. (B) Left panel depicts cytokines secreted into the apical supernatant of tissue normalized to the media control. The right panel depicts cytokines secreted basally by MatTek tissue normalized to the media control. Experiments were performed in duplicate. Error bars represent ± SD values. Graphs are representative of two individual experiments. (C) Hematoxylin staining of MatTek tissue 24 h after exposure to media (control) or immunocomplexes (HCA + C’ + Semen). L = Lumen.
4. Discussion
In this study we engineered an antisperm mAb associated with human infertility [18] for use as a topical contraceptive agent. We demonstrated that it is possible to produce a high-quality antisperm human IgG1 mAb (HCA) using the cost-effective Nicotiana production platform. We next confirmed antibody activity and specificity. HCA was specific for MRT-CD52g; the antibody reacted with sperm but did not bind to peripheral blood mononuclear cells, or tonsil or vaginal tissue. We then assessed antibody antisperm function in two classical assays: the sperm agglutination and complement dependent sperm immobilization assays. HCA was highly effective at agglutinating and immobilizing sperm over a wide concentration range. Studies are currently underway to determine whether HCA prevents sperm penetration through cervical mucus, which could be another protective mechanism [48].
To determine whether the HCA mAb retains antisperm function in the vaginal environment, activity was tested under a variety of physiologically relevant conditions. HCA effectively agglutinated sperm after prolonged exposure to low pH or to cervicovaginal secretions from women with normal and abnormal vaginal microflora, and retained activity in the presence of seminal plasma which contains soluble CD52g. The mAb was also effective over a wide range of sperm concentrations. Pre-clinical safety assessment using the EpiVaginal™ tissue model indicated that the mAb and its immune complexes did not affect vaginal tissue integrity or induce proinflammatory cytokine release; this was an expected outcome since IgG along with IgA isotype antibodies are naturally found in vaginal secretions [49], [50], [51], [52], [53].
Human mAbs are attractive candidates for vaginal contraception and MPT because they are natural mediators of protection at mucosal sites and would enhance existing antibody-mediated immunity in the female genital tract [9, 50]. Our team recently completed a Phase 1 clinical trial in women that tested the safety, pharmacokinetics, and ex vivo efficacy of a topically-applied vaginal film, MB66, containing IgG1 mAbs against HIV-1 and HSV-2 that had been produced in Nicotiana. Daily vaginal application of MB66 film for seven days did not cause notable adverse events, and the concentration of active mAbs present in vaginal secretions 24 h after film application was sufficient to prevent HIV-1 and HSV-2 infection ex vivo (concentration range: 36–700 µg/ml) [12]. The HCA experiments in the present study were conducted using antibody concentrations within the range of mAbs present in vaginal secretions 24 h post-film application. Taken together, the data on HCA specificity, efficacy and safety from the present study, combined with the results from the MB66 clinical trial indicate that HCA is a promising candidate for use as a topical vaginal contraceptive.
Due to its activity and safety profile, HCA could address current gaps in the contraception field. However, one of the limitations of antibody therapeutics is their high cost [54]. New production platforms such as Nicotiana, Trichoderma, and second generation Chinese hamster ovary (CHO) systems are projected to bring the cost of mAbs down to approximately $10/gram, making it feasible to use mAbs for contraception and MPT applications [9]. More potent bispecific and multivalent monoclonal antibodies are also being engineered [55] and could further reduce cost as less product would be required for clinical efficacy. Next generation vaginal delivery methods such as long-acting intravaginal rings [56] could further reduce product cost, and increase efficacy and user acceptability. If these projected cost-savings can be achieved, HCA could be accessible to women worldwide [57], [58], [59].
There are other limitations of the current study that will need to be addressed in future research. Only a small number of sperm donors were used for this study (n = 10), and those men were primarily Caucasian. Although, HCA was effective in all of these donors, more research is required to determine whether there is increased variability in the effects of HCA on sperm agglutination among some groups of men, especially in those from different races. Another potential limitation of this research is that HCA could interfere with normal functions of CD52g, which are largely unknown. A report from Flori et al. [60] provided evidence that CD52g anchors sperm to semenogelin in the semen clot that forms minutes after intercourse; it is unknown whether HCA affects this process. A final limitation is that the study was conducted in vitro, and a clinical trial will be required to determine the in vivo contraceptive efficacy of HCA. Indeed, we are currently conducting an exploratory Phase I mechanism-of-action study of HCA.
HCA has the potential to be combined with other antibodies or small molecules, without altering its efficiency. A vaginal MPT product containing a combination of contraceptive and anti-microbial mAbs (e.g., HCA, VRC01, and HSV8) could provide a safe, accessible, and effective solution to address an urgent unmet global need [7, [61], [62], [63]].
Funding
This research was supported by grants R01 HD095630 and P50 HD096957 from the National Institutes of Health.
Data sharing statement
Data supporting the findings of this study are available within the manuscript, and are also available from the authors upon reasonable request.
Declaration of Competing Interest
LZ and KJW own Mapp Biopharmaceutical Inc., a company interested in commercializing contraceptive antibodies. TRM, MB, and MP are employees of Mapp Biopharmaceutical Inc. The other authors (GBV, JGM, JAP, EM, JP, JD, EN, DJA) have no conflict of interest.
Acknowledgments
We thank Drs. Catherine Costello and Suryaram Gummuluru for reading initial drafts of this manuscript and offering constructive feedback.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103478.
Appendix. Supplementary materials
References
- 1.Anderson D.J. Population and the environment-time for another contraception revolution. N Eng J Med. 2019;381(5):397–399. doi: 10.1056/NEJMp1906733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). United Nations 2019.
- 3.Chebet J.J., McMahon S.A., Greenspan J.A., Mosha I.H., Callaghan-Koru J.A., Killewo J. Every method seems to have its problems”-perspectives on side effects of hormonal contraceptives in Morogoro region, Tanzania. BMC Women's Health. 2015;15(1):97. doi: 10.1186/s12905-015-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roach R.E.J., Helmerhorst F.M., Lijfering W.M., Stijnen T., Algra A., Dekkers O.M. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015;(8) doi: 10.1002/14651858.CD011054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skovlund C.W., Mørch L.S., Kessing L.V., Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73(11):1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- 6.Contraception Reproductive health CDC [internet]. 2018. Available from: https://www.cdc.gov/reproductivehealth/contraception/index.htm.
- 7.Hynes J.S., Sales J.M., Sheth A.N., Lathrop E., Haddad L.B. Interest in multipurpose prevention technologies to prevent HIV/STIs and unintended pregnancy among young women in the United States. Contraception. 2017:277–284. doi: 10.1016/j.contraception.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minnis A.M., Browne E.N., Boeri M., Agot K., Straten A., Ahmed K. Young women's stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. 2019:394–403. doi: 10.1097/QAI.0000000000001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson D.J., Politch J.A., Zeitlin L., Hiatt A., Kadasia K., Mayer K.H. Systemic and topical use of monoclonal antibodies to prevent the sexual transmission of HIV. AIDS. 2017;31(11):1505–1517. doi: 10.1097/QAD.0000000000001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.xxx . The Antibody Society; 2021. Antibody therapeutics approved or in regulatory review in the EU or US. [Google Scholar]
- 11.Haynes B.F., Burton D.R., Mascola J.R. Multiple roles for HIV broadly neutralizing antibodies. Sci Transl Med. 2019;11(516):eaaz2686. doi: 10.1126/scitranslmed.aaz2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Politch J.A., Cu-Uvin S., Moench T.R., Tashima K.T., Marathe J.G., Guthrie K.M. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): a phase I randomized trial. PLoS Med. 2021;18(2) doi: 10.1371/journal.pmed.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbonetti A., Castellini C., D'Andrea S., Minaldi E., Totaro M., Francavilla S. Relationship between natural and intrauterine insemination-assisted live births and the degree of sperm autoimmunisation. Hum Reprod. 2020;35(6):1288–1295. doi: 10.1093/humrep/deaa070. [DOI] [PubMed] [Google Scholar]
- 14.Bohring C., Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. the value of proteomic analysis. Hum Reprod. 2003;18(5):915–924. doi: 10.1093/humrep/deg207. [DOI] [PubMed] [Google Scholar]
- 15.Price R.J., Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979;32(1):61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji Y., Clausen H., Nudelman E., Kaizu T., Hakomori S., Isojima S. Human sperm carbohydrate antigens defined by an antisperm human monoclonal antibody derived from an infertile woman bearing antisperm antibodies in her serum. J Exp Med. 1988;168(1):343–356. doi: 10.1084/jem.168.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlo-Stella C., Guidetti A., Di Nicola M., Longoni P., Cleris L., Lavazza C. CD52 antigen expressed by malignant plasma cells can be targeted by alemtuzumab in vivo in NOD/SCID mice. Exp Hematol. 2006;34(6):721–727. doi: 10.1016/j.exphem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Isojima S., Kameda K., Tsuji Y., Shigeta M., Ikeda Y., Koyama K. Establishment and characterization of a human hybridoma secreting monoclonal antibody with high titers of sperm immobilizing and agglutinating activities against human seminal plasma. J Reprod Immunol. 1987;10(1):67–78. doi: 10.1016/0165-0378(87)90051-9. [DOI] [PubMed] [Google Scholar]
- 19.Komori S., Yamasaki N., Shigeta M., Isojima S., Watanabe T. Production of heavy-chain class-switch variants of human monoclonal antibody by recombinant DNA technology. Clin Exp Immunol. 1988;71(3):508–516. [PMC free article] [PubMed] [Google Scholar]
- 20.Klimyuk V., Pogue G., Herz S., Butler J., Haydon H. Production of recombinant antigens and antibodies in nicotiana benthamiana using 'magnifection' technology: GMP-compliant facilities for small-and large-scale manufacturing. Curr Top Microbiol Immunol. 2014;375:127–154. doi: 10.1007/82_2012_212. [DOI] [PubMed] [Google Scholar]
- 21.Strasser R., Stadlmann J., Schähs M., Stiegler G., Quendler H., Mach L. Generation of glyco-engineered nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure: XylT and FucT down-regulation in N. benthamiana. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeitlin L., Bohorov O., Bohorova N., Hiatt A., Kim D.H., Pauly M.H. Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. MAbs. 2013;5(2):263–269. doi: 10.4161/mabs.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.xxx . World Health Organization; Geneva: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 24.Sobrero A.J. Sperm migration in the human female: further clinical observations. J. Sex Res. 1967;3(4):319–322. [Google Scholar]
- 25.Fordney Settlage D.S., Motoshima M., Tredway D.R. Sperm transport from the external cervical Os to the fallopian tubes in women: a time and quantitation study. Fertil. Steril. 1973;24(9):655–661. doi: 10.1016/s0015-0282(16)39908-3. [DOI] [PubMed] [Google Scholar]
- 26.Suarez S.S., Pacey A.A. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12(1):23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- 27.Giritch A., Marillonnet S., Engler C., Eldik G., Botterman J., Klimyuk V. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA. 2006;103(40):14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiatt A., Bohorova N., Bohorov O., Goodman C., Kim D., Pauly M.H. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. PNAS. 2014;111(16):5992–5997. doi: 10.1073/pnas.1402458111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schähs M., Strasser R., Stadlmann J., Kunert R., Rademacher T., Steinkellner H. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol J. 2007;5(5):657–663. doi: 10.1111/j.1467-7652.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 30.Herr J.C., Summers T.A., McGee R.S., Sutherland W.M., Sigman M., Evans R.J. Characterization of a monoclonal antibody to a conserved epitope on human seminal vesicle-specific peptides: a novel probe/marker system for semen identification. Biol Reprod. 1986;35(3):773–784. doi: 10.1095/biolreprod35.3.773. [DOI] [PubMed] [Google Scholar]
- 31.Norton E.J., Diekman A.B., Westbrook V.A., Mullins D.W., Klotz K.L., Gilmer L.L. A male genital tract-specific carbohydrate epitope on human CD52: Implications for immunocontraception. Tissue Antigens. 2002;60(5):354–364. doi: 10.1034/j.1399-0039.2002.600502.x. [DOI] [PubMed] [Google Scholar]
- 32.Diekman A.B., Norton E.J., Westbrook V.A., Klotz K.L., Naaby-Hansen S., Herr J.C. Anti-sperm antibodies from infertile patients and their cognate sperm antigens: a review. identity between SAGA-1, the H6-3C4 antigen, and CD52. Am J Reprod Immunol. 2008;43(3):134–143. doi: 10.1111/j.8755-8920.2000.430302.x. [DOI] [PubMed] [Google Scholar]
- 33.EuroMAbNet. Production of large quantities of monoclonal antibodies protocol. 2021.
- 34.Fichorova R.N., Rheinwald J.G., Anderson D.J. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57(4):847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 35.Ayehunie S., Cannon C., Lamore S., Kubilus J., Anderson D.J., Pudney J. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol in Vitro. 2006;20(5):689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Hellema H.W., Rümke P. The micro-sperm immobilization test: the use of only motile spermatozoa and studies of complement. Clin Exp Immunol. 1978;31(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 37.Isojima S., Koyama K. Techniques for sperm immobilization test. Arch Androl. 1989;23(3):185–199. doi: 10.3109/01485018908986841. [DOI] [PubMed] [Google Scholar]
- 38.Zyl J.A., Menkveld R., Kotze T.J., Retief A.E., Niekerk W.A. Oligozoospermia: a seven-year survey of the incidence, chromosomal aberrations, treatment and pregnancy rate. Int J Fertil. 1975;20(3):129–132. [PubMed] [Google Scholar]
- 39.Zyl J.A.V., Menkveld R. Oligozoospermia: recent prognosis and the outcome of 73 pregnancies in oligozoospermic couples. Andrologia. 2006;38(3):87–91. doi: 10.1111/j.1439-0272.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 40.Long J.E., Lee M.S., Blithe D.L. Update on novel hormonal and nonhormonal male contraceptive development. J Clin Endocrinol Metab. 2021;106(6) doi: 10.1210/clinem/dgab034. e2381-e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox C.A., Meldrum S.J., Watson B.W. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33(1):69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 42.O'Hanlon D.E., Moench T.R., Cone R.A. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLOS ONE. 2013;8(11):e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldunate M., Srbinovski D., Hearps A.C., Latham C.F., Ramsland P.A., Gugasyan R. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw J.L., Smith C.R., Diamandis E.P. Proteomic analysis of human cervico-vaginal fluid. J Proteom Res. 2007;6(7):2859–2865. doi: 10.1021/pr0701658. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira C.S.T., da Silva M.G., de Pontes L.G., Dos Santos L.D., Marconi C. Protein content of cervicovaginal fluid is altered during bacterial vaginosis. J Low Genit Tract Dis. 2018;22(2):147–151. doi: 10.1097/LGT.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 46.Sharkey D.J., Macpherson A.M., Tremellen K.P., Robertson S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13(7):491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 47.Sharkey D.J., Tremellen K.P., Jasper M.J., Gemzell-Danielsson K., Robertson S.A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 48.Kremer J., Jager S. The significance of antisperm antibodies for sperm-cervical mucus interaction. Hum Reprod. 1992;7(6):781–784. doi: 10.1093/oxfordjournals.humrep.a137737. [DOI] [PubMed] [Google Scholar]
- 49.Berard A.R., Perner M., Mutch S., Zuend C.F., McQueen P., Burgener A.D. Understanding mucosal and microbial functionality of the female reproductive tract by metaproteomics:implications for HIV transmission. Am J Reprod Immunol. 2018;80(2):e12977. doi: 10.1111/aji.12977. [DOI] [PubMed] [Google Scholar]
- 50.Hickey D.K., Patel M.V., Fahey J.V., Wira C.R. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88(2):185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kutteh W.H., Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31(1):40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.K., Kim C.J., Kim D.-J., Kang J.-h. Immune cells in the female reproductive tract. Immune Netw. 2015;15(1):16–26. doi: 10.4110/in.2015.15.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S.K. John Wiley & Sons; Hoboken, NJ: 2018. Diagnostics to pathogenomics of sexually transmitted infections. [Google Scholar]
- 54.Sonfield A., Hasstedt K., Gold R.B. Guttmacher Institute; New York: 2014. Moving forward: family planning in the era of health reform. [Google Scholar]
- 55.Spiess C., Zhai Q., Carter P.J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67:95–106. doi: 10.1016/j.molimm.2015.01.003. (2, Part A) [DOI] [PubMed] [Google Scholar]
- 56.Zhao C., Gunawardana M., Villinger F., Baum M.M., Remedios-Chan M., Moench T.R. Pharmacokinetics and preliminary safety of pod-intravaginal rings delivering the monoclonal antibody VRC01-N for HIV prophylaxis in a macaque model. Antimicrob Agents Chemother. 2017;61(7) doi: 10.1128/AAC.02465-16. e02465-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arcangues C. Worldwide use of intrauterine devices for contraception. Contraception. 2007;75(6):S2–S7. doi: 10.1016/j.contraception.2006.12.024. Supplement. [DOI] [PubMed] [Google Scholar]
- 58.The ESHRE capri workshop group: intrauterine devices and intrauterine systems. Hum Reprod Update. 2008;14(3):197–208. doi: 10.1093/humupd/dmn003. [DOI] [PubMed] [Google Scholar]
- 59.Buhling K.J., Zite N.B., Lotke P., Black K. Worldwide use of intrauterine contraception: a review. Contraception. 2014;89(3):162–173. doi: 10.1016/j.contraception.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Flori F., Ermini L., La Sala G.B., Nicoli A., Capone A., Focarelli R. The GPI-anchored CD52 antigen of the sperm surface interacts with semenogelin and participates in clot formation and liquefaction of human semen. Mol Reprod Dev. 2008;75(2):326–335. doi: 10.1002/mrd.20738. [DOI] [PubMed] [Google Scholar]
- 61.Harrison P.F., Hemmerling A., Romano J., Whaley K.J., Young Holt B. Developing multipurpose reproductive health technologies: an integrated strategy. AIDS Res Treat. 2013;2013:1–15. doi: 10.1155/2013/790154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McConville C., Major I., Devlin B., Brimer A. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Eur J Pharm Biopharm. 2016;104(Supplement C):171–179. doi: 10.1016/j.ejpb.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Guilamo-Ramos V., Reading M., Bowman A.S., Perlman D.C., Barrett S. Multipurpose prevention technologies: a global sexual and reproductive health priority. J Assoc Nurses AIDS Care. 2018;29(1):6–9. doi: 10.1016/j.jana.2017.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.