Abstract
Transient receptor potential vanilloid 4 (TRPV4) plays an important role in chondrocytes via Ca2+ signaling. However, its role in the progression of osteoarthritis is unclear. This study aimed to evaluate the effects of TRPV4 activation on articular cartilage and chondrocytes stimulated with interleukin (IL)-1β. Bovine and human articular chondrocytes were stimulated with various agents, including IL-1β, GSK1016790A (GSK101; a TRPV4 agonist), Compound C (an AMP-activated protein kinase (AMPK) inhibitor), and STO-609 (a calmodulin-dependent protein kinase kinase (CaMKK) inhibitor), and were processed for Western blot analysis and real-time PCR. The dimethylmethylene blue (DMMB) assay and Safranin O staining were also performed. GSK101 reversed the IL-1β-induced increase in expression of matrix metalloproteinase (MMP)-13 and decrease in expression of aggrecan. GSK101 also decreased proteoglycan release in the DMMB assay and retained Safranin O staining of articular cartilage tissue. Furthermore, GSK101 increased AMPK phosphorylation and decreased IL-1β-induced nuclear factor kappa B (NF-κB) phosphorylation. Compound C and STO-609 reversed the suppressive effects of GSK101 on NF-κB activation and MMP-13 expression. In conclusion, TRPV4 activation had chondroprotective effects on articular cartilage stimulated with IL-1β by activating CaMKK/AMPK and suppressing the NF-κB pathway. TRPV4 activators may offer a promising therapeutic option for preventing the progression of osteoarthritis.
Subject terms: Biochemistry, Molecular biology, Rheumatology
Introduction
Transient receptor potential vanilloid 4 (TRPV4), an osmotically active ion channel associated with Ca2+ intake, plays an important role in mechano-transduction pathways of chondrocytes via Ca2+ signaling1–3. However, the role of TRPV4 in the progression of osteoarthritis (OA) is controversial. For instance, previous studies have shown that TRPV4 activation induced both catabolic and anabolic responses in chondrocytes in vitro3–5. Similarly, inconsistent results have been reported in TRPV4-knockout mice in vivo, with one study reporting progression of OA and another reporting a reduction of OA in these mice6,7.
We previously reported that TRPV4 stimulated with GSK101 plays a role in chondrogenesis by inducing the expression of chondrogenic markers including sex-determining region Y-box transcription factor (SOX9) and aggrecan (AGC)8, whereas signaling pathways of up-regulation of SOX9 and AGC via activation of TRPV4 was not well shown. It has been reported that Ca2+ intake activates AMP-activated protein kinase (AMPK), an evolutionarily conserved fuel and stress-sensing enzyme that can be activated by calmodulin-dependent protein kinase kinase-2 (CAMKK2) and that AMPK activation suppresses matrix degradation responses to interleukin (IL-)1β in chondrocytes9,10. Consistent with this, some drugs have been reported to attenuate cartilage degeneration by activating AMPK11–14. Although regulation of the CaMKK/AMPK/NF-κB signaling pathway inhibits inflammation, which plays a role in modern chronic diseases such as diabetes and cancer15, the role of this pathway in articular cartilage degradation and OA progression is unknown.
Based on the mechanistic findings discussed above, we hypothesized that TRPV4 may have a chondroprotective effect against arthritis caused by IL-1β stimulation. To test this, the present study aimed to determine whether TRPV4 activation in chondrocytes protects articular cartilage from degradation and inhibits the progression of OA via the CaMKK/AMPK/NF-κB signaling pathway.
Results
Determination of appropriate GSK101 concentration to inhibit IL-1β-induced cartilage degradation
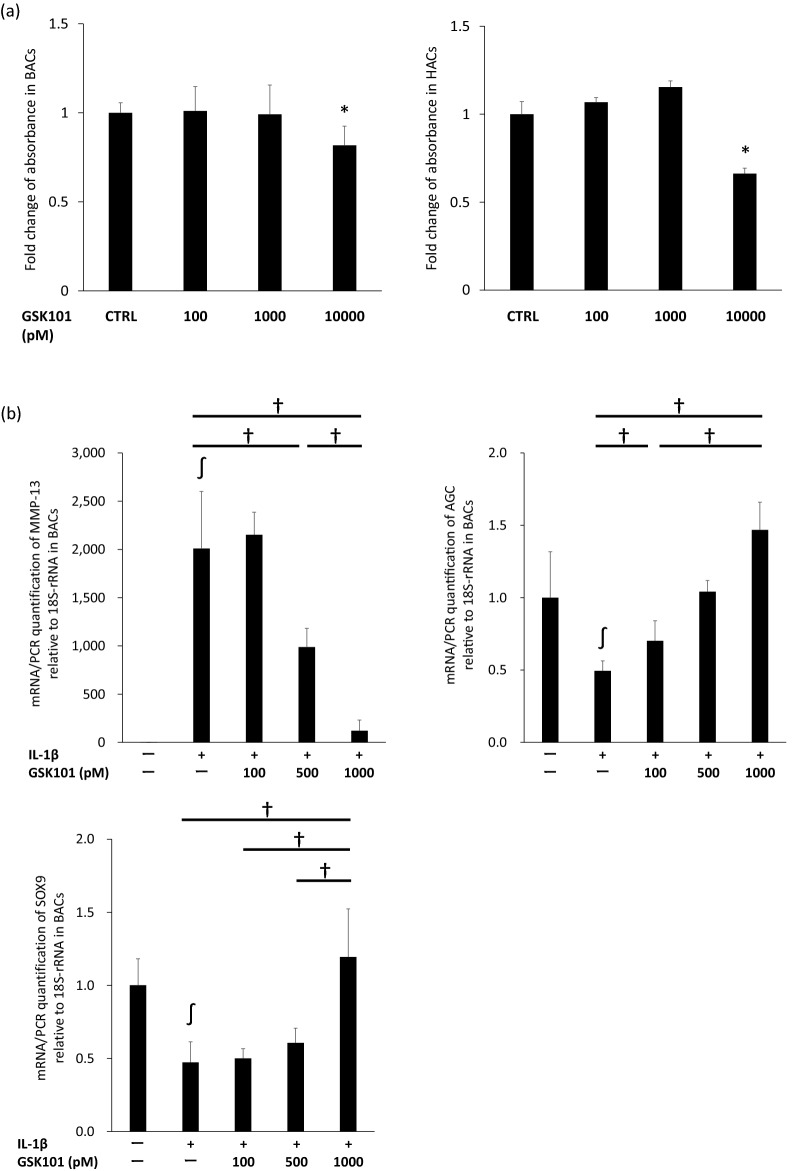
GSK101 was used as a specific TRPV4 agonist to explore the effects of TRPV4 activation on the pro-catabolic phenotype of activated chondrocytes. In an MTS assay, GSK101 was not cytotoxic to bovine articular chondrocytes (BACs) and human articular chondrocytes (HACs) at concentrations of up to 1000 pM, but was cytotoxic at 10,000 pM (Fig. 1a). In BACs, GSK101 significantly reversed the IL-1β-induced increase in expression of matrix metalloproteinase (MMP-)13 mRNA and decrease in expression of AGC and SOX9 mRNA in a dose-dependent manner (Fig. 1b). Since GSK101 was most effective at a concentration of 1000 pM, this concentration was used for subsequent experiments.
Figure 1.
Optimization of GSK101 concentration. The optimal concentration of GSK101 for experiments was determined to be 1000 pM based on 48-h MTS assays in BACs and HACs, and real-time PCR-determined expression levels of MMP-13, aggrecan and SOX9 12 h after treatment in BACs. Experiments were repeated three times. (a) In the MTS assay, GSK101 was not cytotoxic both to BACs and HACs at concentrations of up to 1000 pM, but was cytotoxic at 10,000 pM. (b) In the real-time PCR in BACs, GSK101 significantly reversed the IL-1β-induced increase in expression of MMP-13 mRNA and decrease in expression of AGC and SOX9 mRNA in a dose-dependent manner. *p < 0.05 compared to untreated control, ∫p < 0.05 compared to untreated control, †p < 0.05 using one-way ANOVA with Tukey’s test. BAC: bovine articular cell; CTRL: control; HAC: human articular cell.
TRPV4 activation inhibits IL-1β-induced cartilage degradation
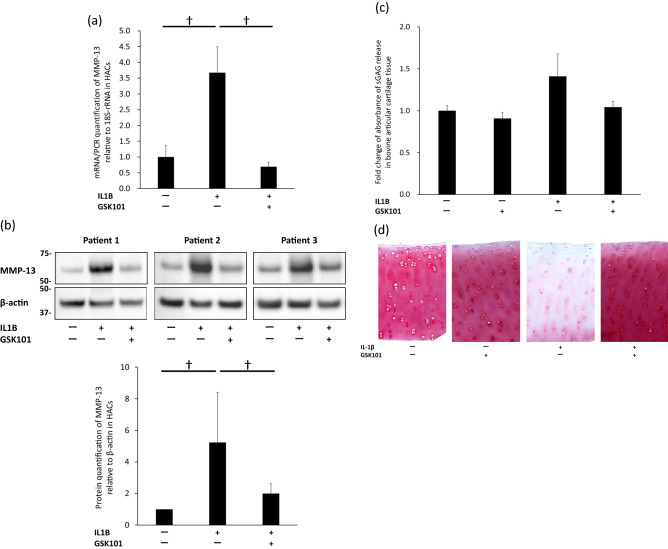
When examined by real-time PCR and Western blot analysis, GSK101 at 1000 pM significantly reduced the expression of MMP13 induced by IL-1β stimulation in HACs (Fig. 2a,b). To determine whether the addition of GSK101 can block proteoglycan release from bovine articular cartilage explants treated with IL-1β ex vivo, a dimethylmethylene blue (DMMB) colorimetric assay of sulfated glycosaminoglycan (sGAG) release after 3 days of IL-1β stimulation and Safranin O/Fast Green staining of bovine articular cartilage explant cores after 7 days of IL-1β stimulation were performed. Three replicates were used in each group both in the DMMB assay and Safranin O/Fast Green staining. In the DMMB assay, stimulating full-thickness 4-mm cores of cartilage explants with IL-1β significantly increased the elution of sGAG into the medium compared to the untreated control. Co-treatment with GSK101 significantly suppressed the IL-1β-induced release of sGAG (Fig. 2c). In Safranin O/Fast Green staining of bovine articular cartilage explant cores after 7 days of IL-1β stimulation, similar tendency among three replicates was observed that substantial amounts of proteoglycans were lost from the explants and that these effects were almost completely rescued by co-treatment with GSK101 (Fig. 2d).
Figure 2.
Reduction of IL-1β-induced cartilage damage by GSK101. (a) Real-time PCR of HAC lysates to examine the expression of MMP-13 after 12 h of treatment with 1000 pM GSK101, and (b) Western blot analysis of HAC lysates to examine the expression of MMP-13 after 48 h of treatment with 1000 pM GSK101. Experiments were repeated three times. Each group of the membrane associated with MMP-13 and the membrane associated with β-actin were different while their protein samples were the same. (a), (b) showed that GSK101 significantly reduced the expression of MMP-13 induced by IL-1β stimulation in HACs. Next, we performed DMMB assay on day 3 of explant culture and Safranin O/Fast Green staining on day 7 of explant culture of bovine articular cartilage tissue. Three replicates were used in each group. (c) In the DMMB assay, stimulating tissues with IL-1β significantly increased the elution of sGAG into the medium compared to the untreated control, while co-treatment with GSK101 significantly suppressed the IL-1β-induced release of sGAG. (d) In Safranin O/Fast Green staining of tissues after 7 days of IL-1β stimulation, similar tendency among three replicates was observed that substantial amounts of proteoglycans were lost from the explants and that these effects were almost completely rescued by co-treatment with GSK101. †p < 0.05 using one-way ANOVA with Tukey’s test. DMMB: dimethylmethylene blue; HAC: human articular cell; sGAG: sulfated glycosaminoglycan.
Pathways involved in chondroprotective effect of TRPV4 activation
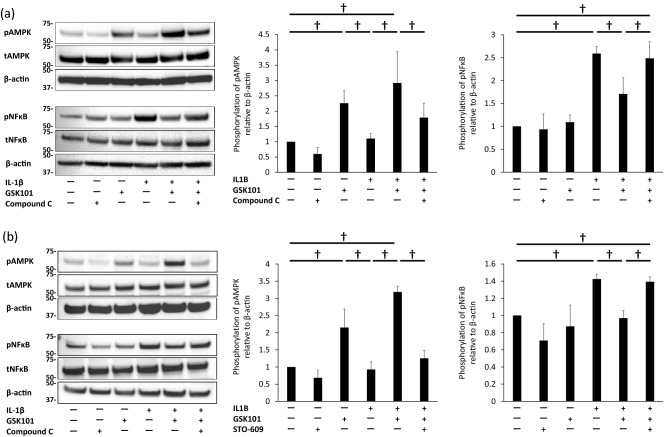
To identify signaling pathways which may be involved in the chondroprotective effect of GSK101, BACs were stimulated with IL-1β in the absence or presence of GSK101. Western blot and band densitometry analyses revealed that stimulation with IL-1β for 30 min increased the levels of phospho-(p)NF-κB (p < 0.001 in Fig. 3a and p = 0.0026 in Fig. 3b,respectively), and GSK101 treatment for 30 min enhanced the levels of pAMPK (p = 0.0028 in Fig. 3a and p = 0.0031 in Fig. 3b, respectively), compared with untreated control samples. Co-treatment with GSK101 significantly suppressed IL-1β-induced NF-κB phosphorylation (p = 0.0147 in Fig. 3a and p = 0.0164 in Fig. 3b, respectively).
Figure 3.
Inhibition of NF-κB phosphorylation by activation of the CaMKK/AMPK pathway. Levels of pAMPK and pNF-κB in BACs treated with (a) IL-1β, GSK101, and Compound C (25 µM), and (b) IL-1β, GSK101, and STO-609 (5 µM) for 30 min, as assessed by Western blot. Experiments were repeated three times. Each group of pNFκB, tNFκB and β-actin was derived from the same membrane. Each group of pAMPK, tAMPK and β-actin was derived from the same membrane. The membrane associated with NFκB and the membrane associated with AMPK were different while their protein samples were the same. (a), (b) showed that stimulation with IL-1β for 30 min increased levels of pNF-κB, and GSK101 treatment for 30 min enhanced levels of pAMPK, compared with untreated control samples. (a) showed that pre-treatment with compound C significantly suppressed phosphorylation of AMPK and increased phosphorylation of NF-κB compared to cells treated with IL-1β and GSK101. (b) showed that pre-treatment with STO-609 for 1 h significantly suppressed phosphorylation of AMPK observed with the combination of GSK101 and IL-1β, and also countered the suppressive effect of GSK101 on IL-1β-induced NF-κB phosphorylation. ∫p < 0.05 compared to untreated control; †p < 0.05 using one-way ANOVA with Tukey’s test. AMPK: AMP-activated protein kinase; BAC: bovine articular cell; NFκB: nuclear factor kappa B; p-: phosphor-; t: total-.
Compound C, a chemical inhibitor of AMPK phosphorylation, was used to determine whether the phosphorylation of AMPK is involved in the GSK101-mediated suppression of IL-1β-induced NF-κB phosphorylation (Fig. 3a). Pre-treatment with compound C significantly suppressed the phosphorylation of AMPK (p = 0.006) and increased the phosphorylation of NF-κB (p = 0.033) compared to cells treated with IL-1β and GSK101.
STO-609, an inhibitor of CaMKK activation, was used to determine whether CaMKK activation cross-talked with the AMPK/NF-κB pathway (Fig. 3b). Pre-treatment with STO-609 for 1 h significantly suppressed the phosphorylation of AMPK observed with the combination of GSK101 and IL-1β (p < 0.001), and also countered the suppressive effect of GSK101 on IL-1β-induced NF-κB phosphorylation (p = 0.026).
These results collectively suggest that TRPV4 activation suppresses IL-1β-induced NF-κB activation by activating the CaMKK/AMPK pathway.
TRPV4-mediated suppression of IL-1β-induced cartilage degradation via the CaMKK/AMPK/NF-κB pathway
As discussed above, the TRPV4/CaMKK/AMPK pathway is involved in suppressing IL-1β-induced NF-κB activation. Thus, we examined whether this mechanism was involved in the chondroprotective effect of TRPV4.
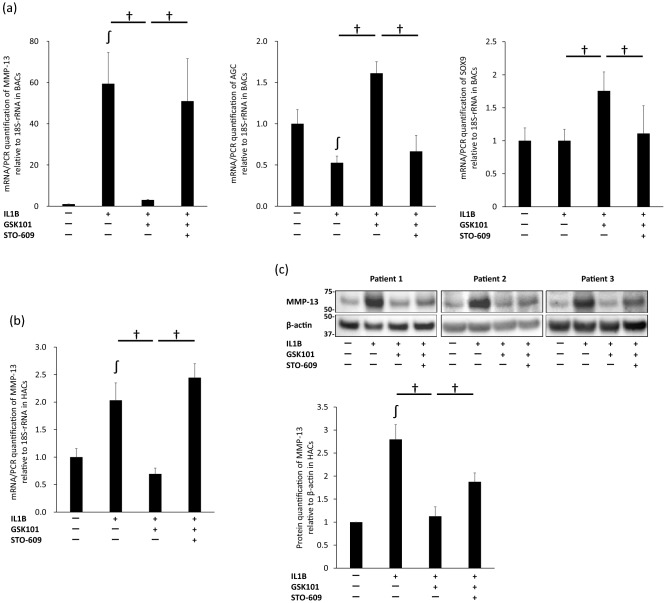
Both MMP-13 mRNA expression in BACs and HACs (n = 3, respectively) and MMP-13 protein expression in HACs (since the anti-MMP-13 antibody did not react with bovine MMP-13) under various conditions were examined. In BACs (Fig. 4a), pre-treatment with STO-609 canceled the suppressive effect of GSK101 on IL-1β-induced up-regulation of MMP-13 mRNA and down-regulation of AGC mRNA (p < 0.001 and p < 0.001, respectively). Pre-treatment with STO-609 also canceled the suppressive effect of GSK101 on up-regulation of SOX9 mRNA in BACs. Effects of STO-609 were also observed on MMP-13 mRNA and protein levels in HACs (p < 0.001 and p = 0.044, respectively; Fig. 4b,c). These results suggest that activation of CaMKK by TRPV4 is involved in the chondroprotective effect of TRPV4 against IL-1β-induced cartilage degradation.
Figure 4.
Inhibition of IL-1β-induced cartilage damage via the TRPV4/CaMKK pathway. (a) Real-time PCR analysis of relative expression of MMP-13, AGC and SOX9 in BACs treated with IL-1β, GSK101, and STO-609 for 12 h. Pre-treatment with STO-609 canceled the suppressive effect of GSK101 on IL-1β-induced up-regulation of MMP-13 and down-regulation of AGC mRNA. Pre-treatment with STO-609 also canceled the suppressive effect of GSK101 on up-regulation of SOX9 mRNA. (b) Real-time PCR of relative expression of MMP-13 in HACs treated with IL-1β, GSK101, and STO-609 for 12 h, and (c) Western blot analysis of relative expression of MMP-13 in HACs treated with IL-1β, GSK101, and STO-609 for 48 h. (b), (c) showed that pre-treatment with STO-609 canceled the suppressive effect of GSK101 on IL-1β-induced up-regulation of MMP-13 mRNA and protein. Each experiment was repeated three times. Each group of the membrane associated with MMP-13 and the membrane associated with β-actin were different while their protein samples were the same. ∫p < 0.05 compared to untreated control; †p < 0.05 using one-way ANOVA with Tukey’s test. BAC: bovine articular cell; CaMKK: calmodulin-dependent protein kinase kinase; HAC: human articular cell.
Discussion
Although recent studies have found that drugs such as metformin and protectin DX attenuate cartilage damage via the AMPK/NF-κB pathway12–14, the underlying mechanism was unclear. In addition, while the CaMKK/AMPK pathway has been reported to play an important role in myocytes and in circumventricular organs16,17, the role of this pathway in chondrocytes was unknown. The present study is the first to demonstrate that TRPV4 activation inhibits NF-κB phosphorylation by activating the CaMKK/AMPK pathway in chondrocytes in the presence of IL-1β, resulting in a chondroprotective effect.
Jeon et al. previously reported that exercise and contraction induce AMPK activation and inhibit NF-κB activation by increasing the AMP/ATP ratio and/or through the Ca2+/CaMKK signaling pathway in the context of diabetes and cancer15. Moreover, while moderate cyclic tensile strain suppresses IL-1β-induced inflammatory responses in chondrocytes via the AMPK/NF-κB pathway by increasing the AMP/ATP ratio18, no study has reported on the role of the CaMKK/AMPK/NF-κB pathway in chondrocytes. We found that TRPV4 activation down-regulated the expression of MMP-13 by activating CaMKK and AMPK and inactivating NF-κB. TRPV4 activation also up-regulated the expression of cartilage phenotypic genes, including SOX9 and AGC. Given the role of IL-1β in OA progression by inducing catabolic responses and inhibiting anabolic responses19–22, our findings suggest that TRPV4 activation by mechanical stress or chemicals may protect articular cartilage from degeneration and OA progression induced by IL-1β.
The role of TRPV4 in OA has been controversial. In vitro, some studies reported that TRPV4 activation induces catabolic responses in chondrocytes (e.g., increasing the expression of a disintegrin and metalloproteinase domain-containing protein (ADAM)10 and apoptosis of chondrocytes)4,5, while others reported the anabolic effects of TRPV4 activation (e.g., increasing the expression of type 2 collagen and decreasing the expression of disintegrin and metalloproteinase with thrombospondin motifs-5)3. This inconsistency has been noted in vivo as well. For instance, while one study reported that male TRPV4 knockout mice exhibited early and severe development of age-related OA11, another reported that the knockout mice were protected from age-related OA, but not from OA caused by destabilization of the medial meniscus7. In the present study, we observed the anabolic effects of TRPV4 activation, as reflected in the increased expression of AGC and SOX9 and decreased expression of MMP-13, as well as the preservation of proteoglycans in articular cartilage tissue in the IL-1β stimulation model. Given the possibility that the effects of TRPV4 activation may differ by experimental model, further confirmatory studies will be needed.
The inconsistent effects of TRPV4 activation in chondrocytes discussed above may reflect differences in the mechanism of AMPK activation. Indeed, AMPK is known to be activated by various mechanisms, including canonical and non-canonical pathways. As we have shown here, in one non-canonical pathway, AMPK is activated via a Ca2+/CaMKK2-dependent mechanism. Another non-canonical pathway in which AMPK is activated by glucose starvation was recently reported by Li et al.23. In that study, endoplasmic reticulum-localized TRPVs channels and Ca2+ release were inhibited by fructose-1,6-bisphosphate (FBP)-unoccupied aldolase under low glucose conditions, subsequently leading to the formation, phosphorylation, and activation of an AXIN-LKB1-AMPK complex on the lysosomal membrane. Importantly, GSK101 inhibited AMPK activation under glucose starvation conditions due to an increase in local Ca2+ concentrations, suggesting that the concentration of the TRPV agonist may lead to differing results because high concentrations can induce a bulk, global increase in Ca2+ concentration via a CaMKK2-dependent mechanism23. These results suggest that the effect of TRPV4 activation may depend on various factors, for example, the type of cell, the degree of inflammation, and glucose conditions.
The mechanism underlying the inhibition of NF-κB phosphorylation by AMPK activation remains unclear. Previous studies have reported that activation of AMPK or sirtuin (SIRT)1 inhibited IL-1β-induced inflammatory responses by inhibiting NF-κB activation in chondrocytes11,24,25. The activation of peroxisome proliferator activated receptor γ coactivator (PGC)-1α and Forkhead box O (FOXO)3a by AMPK activation was also reported to inhibit NF-κB activation and inflammatory cytokine-induced catabolic responses in chondrocytes26. Given that AMPK activation was reported to induce PGC-1α activation directly or via SIRT1 activation in myocytes16, the AMPK/SIRT1/PGC-1α pathway may play a role in suppressing IL-1β-induced inflammatory responses by inhibiting NF-κB activation in chondrocytes as well. The link between AMPK activation and suppression of NF-κB may also involve changes in glucose metabolism. In this regard, we recently reported that aerobic respiration switched to glycolysis in IL-1β-stimulated chondrocytes, and that IL-1β reduced the phosphorylation of AMPK, which was rescued by a chemical glycolysis inhibitor10.
In conclusion, the activation of TRPV4 suppressed IL-1β-induced chondro-degenerative changes and inflammatory responses including up-regulation of MMP-13 expression and down-regulation of AGC and SOX9 in chondrocytes by activating CaMKK/AMPK and suppressing the activation of NF-κB. As it has been reported that the Ca2+/CaMKK signaling pathway induces AMPK activation and inhibition of NF-κB activation in some chronic inflammatory diseases15, TRPV4/ CaMKK/AMPK pathway played a key role against IL-1β-induced articular cartilage degradation and OA progression. Although the mechanism underlying the inhibition of NF-κB phosphorylation by AMPK activation remains unclear, it has been reported that some other signaling pathways including SIRT1, PGC-1α and FOXO3a, which are associated with AMPK, inhibits IL-1β-induced inflammatory responses by inhibiting NF-κB activation in chondrocytes11,24–26. Taken together, TRPV4 activators may offer a promising therapeutic option for preventing OA progression.
Methods
Cells and cell culture
BACs were isolated from full-thickness slices of the articular surface of metatarsophalangeal joints of young adult cows (aged 18–24 months) which were obtained from Nagoya City Central Wholesale Market in Japan with institutional approval. No live animals were used in this study. HACs were isolated from slices of knee joints of patients who underwent total knee arthroplasty with institutional IRB approval (Ethics Committee of the Nagoya University Graduate School of Medicine #2020-0146). A written informed consent was obtained from the participants. with the World Medical Association of Helsinki Ethical Principles for Medical Research Involving Human Subjects. Also, these tissues were obtained with no identifying information except age/sex. All methods were carried out in accordance with relevant guidelines and regulations. Slices of bovine and human articular cartilage were digested in 0.2% Pronase (≥ 70,000 proteolytic units/g dry weight, Catalog #: 537088; Merck, Germany) for 1 h at 37 °C and subsequently in 0.025% collagenase P (> 1.5 U/mg lyophilizate; Catalog #: 11213865001; Roche, Germany) overnight at 37 °C27. Isolated cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) low glucose medium with 4% fetal bovine serum (FBS) and 1% antibiotics at 37 °C in a 5% CO2 environment. After 48–72 h in culture, cells were passaged once (P1) and 1–2 × 105 cells/cm2 were cultured on 6- or 12-well plates. After static incubation for 48–72 h in 4% FBS-containing medium, cells were cultured in serum-free medium for 12 h. Subsequently, cells were stimulated in the presence of various agents, including IL-1β (10 ng/ml), various concentrations of GSK101 (a selective TRPV4 agonist), Compound C (an AMPK inhibitor; 25 µM), and STO-609 (a CaMKK inhibitor; 5 µM), under serum-free conditions. Cells were collected after stimulation and processed for Western blot analysis and real-time PCR. As we used primary chondrocytes isolated from bovines and humans whose age, sex and conditions of articular cartilage were different, potential variability due to individual differences among reactivity of chondrocytes to IL-1β might occur in the data from Western blot analysis and real-time PCR.
Cartilage explant cultures
Full-thickness 4-mm cores of bovine articular cartilage were cultured in 1.0 ml DMEM low glucose medium with 4% FBS for 24 h. The medium was then replaced, and tissues were incubated with IL-1β (10 ng/ml), with or without GSK101. On day 3 of culture, aliquots of medium were analyzed by the DMMB colorimetric assay to measure sGAG release. On day 7, the treated explants were fixed with 4% buffered paraformaldehyde overnight at 4 °C for histology; rinsed in 30% sucrose/PBS; and embedded in paraffin. Sections (8 µm) were prepared and stained with Safranin O for the detection of proteoglycans and counterstained with Fast Green10.
Real-time PCR
Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Germany). Reverse transcription (RT) was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Real time RT-PCR was carried out using a Light cycler System with FastStart Master SYBR Green PLUS (Roche, USA)27. Primers for matrix metalloproteinase (MMP)-13, AGC, SOX9, and 18S ribosomal RNA (18S rRNA) in bovine and human were synthesized by Sigma-Aldrich (USA). The following primers were used: bovine MMP-13, forward primer 5′-TCCAGTTTGCAGAGAGCTACCT-3′, reverse primer 5′-CCTGTCAATCACAGAGCTTGCT-3′; bovine AGC, forward primer 5′-AAATATCACTGAGGGTGAAGCCCG-3′, reverse primer 5′-ACTTCAGGGACAAACGTGAAAGGC-3′; bovine SOX9, forward primer 5′-CGACTCCCCACATTCCTCCTC-3′, reverse primer 5′-GGACCCTGAGATTGCCCAGAG-3′; bovine 18S rRNA, forward primer 5′-GTAACCCGTTGAACCCCATT-3′, reverse primer 5′-CCATCCAATCGGTAGTAGCG-3′; human MMP-13, forward primer 5′-CCAGTCTCCGAGGAGAAACA-3′, reverse primer 5′-AAAAACAGCTCCGCATCAAC-3′; and human 18S rRNA, forward primer 5′-CCGATTGGATGGTTTAGTGAG-3′, reverse primer 5′-AGTTCGACCGTCTTCTCAGC-3′.
Western blot analysis
The protein expressions of MMP-13, AGC, AMPK, and NF-κB were evaluated by Western blot analysis using BAC and HAC lysates. Cells cultured on 6-well plates were trypsinized and pelleted by centrifugation. Total protein was extracted from cell pellets with Cell Lysis Buffer (Cell Signaling, USA) containing a protease and phosphatase inhibitor cocktail. Samples were separated by 10% SDS-PAGE under reducing conditions and transferred to a nitrocellulose membrane. Antibodies against MMP-13 (18165-1-AP, Proteintech Group), AGC (ab3778, abcam), pAMPK (2535, Cell Signaling), AMPK (5831, Cell Signaling), pNF-κB (3033, Cell Signaling), NF-κB (8242, Cell Signaling), and beta-actin (4970, Cell Signaling) were used. Band intensities were captured with a digital image scanner and quantified using densitometry software (CS Analyzer 3.0; ATTO, Tokyo, Japan).
Statistical analysis
Values are expressed as mean ± standard deviation (SD). One-way ANOVA with Tukey’s test was performed for comparisons. Statistical significance was defined as p < 0.05. All analyses were performed with BellCurve for Excel version 3.21.
Supplementary Information
Acknowledgements
The work was supported in part by a grant from JSPS KAKENHI JP20K18061, 19K09620 and 19K09619 (KT, NT and TK). We also thank Dr. Tsuyoshi Nishiume, Dr. Yasumori Sobue, Dr. Masataka Maeda and Dr. Daisuke Kihira for the technical supports.
Author contributions
K.H., N.T., K.T. and T.K. contributed to the conception and design of the study, or acquisition of data, or analysis and interpretation of data. N.T., Y.O., K.K., Y.Y., M.S., K.T. and T.K. contributed to drafting the article or revising it critically for important intellectual content. N.T., K.T., T.K. and S.I. contributed to final approval of the version to be submitted.
Data availability
All data generated or analyzed during this study are includes in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94938-3.
References
- 1.Mobasheri A, et al. The chondrocyte channelome: A narrative review. Jt. Bone Spine. 2019;86:29–35. doi: 10.1016/j.jbspin.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Lv M, et al. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: Roles of calcium sources and cell membrane ion channels. J. Orthop. Res. 2018;36:730–738. doi: 10.1002/jor.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl .Acad. Sci. USA. 2014;28:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayakawa T, et al. Mechanical stress loading induces CD44 cleavage in human chondrocytic HCS-2/8 cells. Biochem. Biophys. Res. Commun. 2016;23:1230–1235. doi: 10.1016/j.bbrc.2016.08.099. [DOI] [PubMed] [Google Scholar]
- 5.Xu B, et al. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019;1:158–166. doi: 10.1016/j.lfs.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: Age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–2983. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Conor CJ, et al. Cartilage-specific knockout of the mechanosensory ion channel TRPV4 decreases age-related osteoarthritis. Sci. Rep. 2016;8(6):29053. doi: 10.1038/srep29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa Y, et al. Hyaluronan promotes TRPV4-induced chondrogenesis in ATDC5 cells. PLoS ONE. 2019;8:e0219492. doi: 10.1371/journal.pone.0219492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawley SA, et al. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995;10:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 10.Terabe K, et al. Chondroprotective effects of 4-methylumbelliferone and hyaluronan synthase-2 overexpression involve changes in chondrocyte energy metabolism. J. Biol. Chem. 2019;22:17799–17817. doi: 10.1074/jbc.RA119.009556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1β and tumor necrosis factor α. Arthrit. Rheum. 2011;2011(63):1928–1937. doi: 10.1002/art.30333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020;79:635–645. doi: 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng X, et al. Metformin attenuates cartilage degeneration in an experimental osteoarthritis model by regulating AMPK/mTOR. Aging (Albany NY) 2020;16:1087–1103. doi: 10.18632/aging.102635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piao S, et al. Protectin DX attenuates IL-1β-induced inflammation via the AMPK/NF-κB pathway in chondrocytes and ameliorates osteoarthritis progression in a rat model. Int. Immunopharmacol. 2020;78:106043. doi: 10.1016/j.intimp.2019.106043. [DOI] [PubMed] [Google Scholar]
- 15.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;15:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;29:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, et al. Adropin is a key mediator of hypoxia induced anti-dipsogenic effects via TRPV4-CamKK-AMPK signaling in the circumventricular organs of rats. Front. Mol. Neurosci. 2017;20:105. doi: 10.3389/fnmol.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, et al. Mechanical stress protects against osteoarthritis via regulation of the AMPK/NF-κB signaling pathway. J Cell Physiol. 2019;234:9156–9167. doi: 10.1002/jcp.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. 2009;191:325–338. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Speichert S, et al. Role of norepinephrine in IL-1β-induced chondrocyte dedifferentiation under physioxia. Int. J. Mol. Sci. 2019;11:1212. doi: 10.3390/ijms20051212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kour S, et al. IL-3 decreases cartilage degeneration by downregulating matrix metalloproteinases and reduces joint destruction in osteoarthritic mice. J. Immunol. 2016;15:5024–5035. doi: 10.4049/jimmunol.1500907. [DOI] [PubMed] [Google Scholar]
- 23.Li M, et al. Transient receptor potential V channels are essential for glucose sensing by aldolase and AMPK. Cell Metab. 2019;3:508–524. doi: 10.1016/j.cmet.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita T, et al. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J. Orthop. Res. 2013;31:531–537. doi: 10.1002/jor.22268. [DOI] [PubMed] [Google Scholar]
- 25.Lei M, et al. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012;15:73–79. doi: 10.1016/j.ejphar.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, et al. Peroxisome proliferator-activated receptor γ coactivator 1α and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthrit. Rheumatol. 2014;66:3073–3082. doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobue Y, et al. Inhibition of CD44 intracellular domain production suppresses bovine articular chondrocyte de-differentiation induced by excessive mechanical stress loading. Sci. Rep. 2019;17:14901. doi: 10.1038/s41598-019-50166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are includes in this published article.