Highlight
-
•
HPI, DPI, PSP, and PKH were attached by active phenolic compounds.
-
•
The added peptides have considerable antioxidant and antimicrobial activity.
-
•
M-PSP has extended shelf-life and good quality properties.
Keywords: White egg protein isolates, Pepper seed protein, Phenolic compounds, Antioxidant activity, Antimicrobial activity, Milk cold preservation
Abstract
This study aimed to prolong the raw buffalo milk handling and cold storage period by controlling the microbes, enhancing sensory properties and their functionality after supplementing bioactive peptides. The additions included hen and duck egg white protein isolates (HPI and DPI), pepper seed protein (PSP), and pepsin-kidney bean protein hydrolysate (PKH). Five milk treatments were prepared and evaluated as non-supplemented milk (M- Control), hen egg white protein isolate-supplemented milk (M-HPI), duck egg white protein isolate-supplemented milk (M-DPI), pepper seeds protein-supplemented milk (M-PSP), and kidney bean hydrolysate-supplemented milk (M-PKH). Pyrogallol, protocatechuic, catechin, benzoic and caffeine were the main phenolic compounds, Apignin-6-arabinose, naringin, hesperidin, naringenin, kaempferol 3–2-p-comaroyl were the dominant flavonoids in milk samples based on HPLC profile. During 30 days of cold storage, the antioxidant potential of peptides-supplemented milk samples was significantly decreased (p ≤ 0.05) as decrement of phenolic compounds and flavonoids; the pH was nearly stable, the titratable acidity and total soluble solids (TTS) were (p ≤ 0.05) raised. PSP and PKH were inhibited (p ≤ 0.05) the decay of sugars in M-PSP, and M-PKH by reducing 45% of bacterial load as compared to other milk samples. PSP was significantly (p ≤ 0.05) scavenged 87% of DPPḢ compared to other peptides. Besides, PSP followed by PKH reduced considerably (p ≤ 0.05) the growth of tested bacteria, molds, and yeasts. The PSP has significantly increased the whiteness of M-PSP as compared to other milk samples. M-PSP had the highest score in color, taste, and flavor, followed by M-PKH.
1. Introduction
Milk is one of the most vital and nutritional foods for humans, as it contains valuable nutrients (Nicolaou and Goodacre, 2008). Milk is almost sterile when secreted from a vital udder (Gershom and Edward, 2017). Lactoferrin and lactoperoxidase are naturally found in raw milk, inhibiting the microflora growth for 3–4 h after milking at the ambient temperature, and cooling at 4 °C maintains the milk quality. Refrigeration was considered a problem in developing countries because of the operating costs and the electricity supply deficiency, which can partially be controlled by boiling milk after milking or plunging milk containers in cold water. In the milk collection point, the expert people use the lactoperoxidase system to preserve raw milk from 7 to 8 h at 30 °C and overnight at 20 °C, this system didn’t replace pasteurization, and it can be induced by chemical preservative, e.g., H2O2 (Alemu and Girma, 2018). Pasteurized milk was maintained for 7–14 days if stored under recommended conditions, but the differences were based on areas and seasons. Consumers check the best before date, but milk can deteriorate before the validation date recorded on the package (Lu et al., 2013). The microbiological standard for milk varies from one region to another, i.e., in Egypt, Staphylococcus aureus should be less than 100 CFU/mL in pasteurized milk, and Escherichia coli should not exist (Egyptian Standard, 2005). Identifying and characterizing chemical or natural additives and their use methods can be essential in developing appropriate techniques for handling milk and maintaining it quality. Chemical and natural additives are antimicrobials, antioxidants, anti-browning, texturizing, flavoring, coloring, and miscellaneous agents and they enhanced milk properties and maintained quality (Branen et al., 2001, Dickson-Spillmann et al., 2011, Randhawa and Bahna, 2009, Wilson and Bahna, 2005). The over-limit usage of chemical preservatives causes adverse damages, i.e., respiratory, dermatological, gastrointestinal, and neural damages (Carocho et al., 2014). However, the supplementation of raw milk with hydrogen peroxide-induced antioxidant and antimicrobial activity of the initial lactoperoxidase enzyme in milk (Arefin et al., 2017), also, potassium sorbate is one of the safer and lower toxicity preservatives used in the food industry as an antimicrobial agent (González-Fandos and Dominguez, 2007, Karabulut et al., 2001, Liu et al., 2014). Antioxidants, e.g., polyphenols, carotenoids, peptides, and vitamins present in plants and by-products, are considering natural additives that can be substituted the synthetic ones (Saad et al, 2021). The phenolic compounds were considered more relevant natural compounds to be used as food preservatives and active ingredients (Baines and Seal, 2012, Caleja et al., 2015a, Caleja et al., 2015b, Carocho and Ferreira, 2013, Carocho et al., 2015, Saad et al., 2020). Bioactive peptides are the main defense in most animals. They release the oxidative stress caused by different environmental conditions. They can be used as natural preservatives because of their solubility, stability, and activity in foods (Abdelnour et al., 2020, Thery et al., 2019). Esterified legume protein isolates were reduced the bacterial load and titratable acidity in raw and pasteurized milk and improved the preservation quality (Sitohy and Osman, 2011). Osman et al. (2013) used 11S soybean protein subunit in milk preservation for 30 days; it was exhibited considerable antibacterial activity. Several peptides with biological activity were applied in food products (Meinert et al., 2016, Meshginfar et al., 2017). Also, Saad et al. (2020) studied the impact of pepsin-white kidney bean protein hydrolysate on minced beef quality, lifetime, and safety. Furthermore, Wan and Xu, (2018) evaluated the physical–chemical and sensory properties of a beverage supplemented with whey protein isolate. Moreover, Rachman et al. (2019) investigated the effects of egg white protein isolate administration on the quality of banana pasta, and they found that quality properties of pasta enhanced than control, and El-Saadony et al. (2020) enhanced the sensory properties and lifetime of cucumber juice with peptides isolated from vegetable and animal sources.
No studies tried to preserve raw milk with HPI, DPI, PSP, and PKH. Therefore, in this work: i) the radical-scavenging and antimicrobial activity of the HPI, DPI, PSP, and PKH were estimated ii) the effect of HPI, DPI, PSP, and PKH on raw milk preservation at cold condition 4 °C for 30 days was investigated, iii) polyphenols and flavonoids profile of HPI, DPI, PSP, and PKH were analyzed by HPLC, iv) the changes of the microbial count, color parameters, and sensory properties of milk were monitored during the storage period for the month.
2. Materials and methods
2.1. Materials
White kidney bean (Phaseolus vulgaris L.) seeds, chili pepper (Capsicum annuum L.) pods, hen and duck eggs were acquired from the local market in Zagazig (Egypt). Raw buffalo milk was directly obtained after milking from a private farm in Abu-Hammad (Sharkia, Egypt), then transferred in a sterilized container to the laboratory. Pepsin enzyme, DPPḢ, Muller Hinton agar (MHA), Muller Hinton broth (MHB), Sabouraud dextrose agar (SDA), MacConkey agar and Plate count ager (PCA) were obtained from Oxoid Ltd (Basingstroke, Hampshire, UK). The milk pathogenic bacterial strains (Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Yersinia enterocolitica, Escherichia coli, and Campylobacter jejuni) and pathogenic yeasts and molds (Candida apis, Candida blankii, Candida glabrata, Candida glaebosa, Candida rugosa, Candida stellata, Penicillium solitum, Penicillium crustosum, Aspergillus niger, Aspergillus flavus, Fusarium solani, and Fusarium oxysporum) were used in this work.
2.2. Methods
2.2.1. Protein isolates’ preparation
2.2.1.1. PKH preparation
The kidney beans were ground to flour, the flour (10 g) was homogenized in hexane (300 mL) and stirred for 2 h, the solvent was discarded and the precipitate was dried. Total protein was isolated from defatted flour (5 g) according to Johnson and Brekke (1983) with some modifications then lyophilized. The total protein was mixed with pepsin (1:200 w/w), then dissolved in phosphate buffer (pH 2) and incubated at 37 °C for 3 h. The enzyme was inactivated in boiling water. The hydrolysate was centrifuged at 4000 xg for 30 min to obtain the lyophilized supernatant, then kept.
2.2.1.2. DPI and HPI preparation
The duck and hen egg-white were dissolved in water (1:3 v/v), and homogenized for 30 min and then centrifuged at 14,000 undercooling for 20 min. The supernatant was precipitated by polyethylene glycol (10%) then was centrifuged under same conditions. The precipitate was dissolved in Tris-Ca buffer pH 7.8 and kept overnight at 4 °C. The mixture was centrifuged under the same conditions, and the precipitates were washed with Tris-Ca, then mixed with Tris-EDTA, pH 7.8 and kept for 30 min at 4 °C. The supernatant was obtained by centrifugation at 1400g undercooling for 20 min. Then pH was adjusted to 5. The supernatant was dialyzed against Tris buffer pH 8, then was fractionated on the Q Sepharose column by the gradual addition of NaCl concentrations (0.1–0.6 M). HPI and DPI were eluted with NaCl concentrations (0.35–0.45 M) and then lyophilized according to Yoo et al. (2012).
2.2.1.3. PSP preparation
The seeds of pepper were separated, dried, and powdered. The pepper seed flour was defatted with hexane (1:3 w/v), then dried. The PSP was isolated from seeds flour according to Terras et al. (1992) with mild modifications, the seeds flour (50 g) was stirred for 1 h with tenfold of phosphate buffer pH 5.4 (10 mM Na2HPO4, 15 mM NaH2PO4, 100 mM KCl, 1.5% EDTA) and kept overnight at 4 °C. The supernatant was precipitated by ammonium sulfate (90%) at 80 °C for 15 min. The obtained suspension was centrifuged at (10,000g for 10 min) and the supernatant was dialyzed, lyophilized and kept.
2.2.2. Milk preservation experiment
Raw milk (200 mL) was transferred to sterile screw bottles supplemented with 0.2% (w/v) of obtained peptides. The supplementations were included distilled sterilized water (M-control), hen egg white protein isolate (M-HPI), duck egg white protein isolate (M-DPI), pepper seed protein (M-PSP), and pepsin kidney bean protein hydrolysate (M-PKH). All milk samples were kept at 4 °C for 30 days. Chemical and microbiological analyses were conducted at an interval of (0, 15, and 30) days.
2.2.3. Chemical analysis
2.2.3.1. Chemical parameters assessment
Titratable acidity was calculated as % lactic acid according to standard method 942.15, pH was measured by pH meter, and total soluble solids (TSS) was estimated by Abbe Refractometer (Model 8987, Puji Kuki Ltd., Tokyo, Japan) according to AOAC (2005). Total sugars were estimated as per Chaplin and Kennedy (1994), hydrolysate milk samples or glucose standard (200 µL) were added to 200 μL phenol (5%) and 1 mL sulfuric acid (conc.) and incubated for 30 min, the color absorbance was measured at 490 nm. It was applied in (standard curve: y = 0.0055x − 0.0187) to obtain total sugars concentration (µg/mL).
2.2.3.2. Total phenolic compounds (TPC)
Polyphenols were assessed in peptides-supplemented milk samples and calculated as µg GAE/ mL, following the Folin-Ciocalteu method (Škerget et al., 2005), the absorbance was measured at 750 nm. It was applied in standard Gallic acid linear (equation: y = 0.005x + 0.1379).
2.2.3.3. Total flavonoids
One mL of each milk sample was added to 3 mL of ethanoic AlCl3 then incubated in dark for 1 h as per Ordonez et al. (2006). The absorbance was measured at 430 nm and then applied in the standard quercetin linear (equation: y = 0.0033x − 0.0045) to obtain total flavonoid concentration as µg QE/mL.
2.2.3.4. HPLC identification of polyphenols and flavonoids
The phenolic and flavonoids compounds of milk samples were identified by the HPLC Shimadzu series (Shimadzu-prominence-20A, Japan.), the separation column (Gemini, C18, 4.6 × 150 mm × 5um) with 2 mL/min flow rate. The phenolic and flavonoids content in milk samples was conducted asper Goupy et al., 1999, Mattila et al., 2000, and Hassanin et al. (2020) .
2.2.3.5. DPPḢ radical scavenging activity
The anti-radical activity of peptides suspension or milk samples was assessed using Hatano et al. (1988) with few modifications. One mL of DPPḢ in ethanol was added to 100 µL of each sample and kept in the dark for 30 min at room conditions (Gülçin et al., 2004). The absorbance was measured at 517 nm against the control and applied in the following equation to obtain DPPḢ antiradical activity (%).
| 100 |
where A control is the absorbance of the control and A sample is sample absorbance.
2.2.4. Microbial analysis
2.2.4.1. Antibacterial activity
Antibacterial activity was evaluated by disc diffusion assay as per Akl et al., 2020, El-Saadony et al., 2021. 100 µL of each pathogenic bacterium was spread at the surface of Muller Hinton agar plates (MHA), and filter paper discs (6 mm) were saturated with each HPI, DPI, PSP, and PKH suspension concentration (200, 400, and 800 µg/mL) then were placed on the Muller Hinton agar surface. The MHA plates were incubated for 24 h at 37 °C. The obtained zones of inhibition (mm) were manually measured using a ruler. The least concentration was inhibited the bacterial growth was minimum inhibitory concentration (MIC) and determined as follow, 500 μL of pathogenic bacteria was added to tubes of 9 mL MHB supplemented with 500 μL of each HPI, DPI, PSP, and PKH suspension concentration (200, 400, and 800 µg/mL), the tubes were incubated for 24 h at 37 °C and the turbidity was measured every 6 h at 600 nm. The least concentration was killed 100% of bacteria was minimum bactericidal concentration (MBC) and was determined as follow 100 μL of each peptide MIC was spread on the surface of MHA plates and were incubated at 37 °C for 24 h and observed the bacterial growth (Janakat et al., 2015).
2.2.4.2. Bacterial count
Microbial count of milk samples supplemented with HPI, DPI, PSP, and PKH 0.2% (w/v) was performed at an interval of (0, 15, and 30 days) of cold preservation at 4 °C (APHA, 1992). Ten mL of each sample was homogenized with 90 mL of saline peptone buffer (0.1% Peptone Water + 0.85% Salt) in a scraw bottle and stirred for 5 min at 25 °C to prepare 10-1 dilution. One ml of the previous suspension was added to 9 mL buffer peptone tube to obtain 10-2 dilution, further serial dilution to 10-7. One mL of each dilution was placed in sterile one-use petri-dishes. The plate count ager (PCA) medium was added to the plate and mixed well, the PCA plates were incubated at 30 °C for 48 h to count (mesophilic bacteria) (Ashour et al., 2020, Reda et al., 2020, Sheiha et al., 2020), and at 7 °C for ten days to enumerate (psychrophilic bacteria) (Lee, 2009). 1 mL of each dilution was placed in sterile one-use Petri-dishes, then the Violet Red Bile Agar was added to the plate and mixed well. The plates were incubated at 37 °C for 24 h to count coliforms. All bacterial counts were converted to logarithms (CFU/mL) (Reda et al., 2021).
2.2.4.3. Antifungal activity
The antifungal activities of HPI, DPI, PSP, and PKH were tested against twelve Candida and fungi species and evaluated by the agar disc diffusion assay (Elgorban et al., 2016). The tested fungi were cultivated on sabouraud dextrose agar plates (SDA) (Lab M Ltd., Heywood, Lancashire, UK) and the plates were incubated at 37 °C at two days (Candida spp.) and 30 °C at 5 days (fungi species). The tested Candida were grown on SD broth medium for two days at 37 °C until getting Candida inoculum concentration approximately 105 CFU ml−1, fungal species were grown on SDA medium for 5 days at 30 °C, and 6 mm mycelia discs were obtained. 100 µL of Candida inoculum was spread over SDA plates, and fungal mycelia discs were put in the center of SDA plates. Discs (6 mm diam.) were saturated with each concentration of HPI, DPI, PSP, and PKH (200, 400, and 800 µg/mL) then was put on the sides of seeded SDA plates, the plates were then incubated at 37 °C for 2 days (Candida) and 30 °C for 5 days (Fungi). The antifungal activity was estimated by determining the diameters of inhibition zone around the discs in mm. The minimum inhibitory concentration (MIC) of HPI, DPI, PSP, and PKH was evaluated using the the micro broth dilution method (Alizadeh et al., 2014, El-Saadony et al., 2019). 500 µL of each HPI, DPI, PSP, and PKH concentration (200, 400, and 800 µg/mL) was added to tubes containing 9 mL of sabouraud dextrose broth and inoculated with 500 µL of Candida (~105 cfu ml−1) or fungal inoculum (~103 cfu ml−1). Tubes were incubated at 37 °C for 2 day (Candida) or 30 °C for 5 days (fungi). The resultant turbidity was measured at 600 nm using a spectrophotometer (Shimadzu Corporation, Analytical Instruments Division, Kyoto, Japan). The lowest concentration of HPI, DPI, PSP, and PKH, which inhibited the fungal growth, was recorded as the MIC. In addition, the lowest concentration of HPI, DPI, PSP, and PKH which kills 100% of fungi was considered to be the minimum fungicidal concentration (MFC) (Alizadeh et al., 2014). The MFC was estimated by sub-culturing the MIC levels of HPI, DPI, PSP, and PKH onto sterile SDA plates. The plates were incubated at 37 °C for 2 day (for tests with Candida) or 30 °C for 3–5 days (for tests with fungi).
2.2.5. Color measurement
Hunter Lab colorimeter (Color Flex EZ, 45°/0°, USA) was used to estimate the milk samples color, the color parameters values were assessed: L* was expressed as [white (100) /black (0)], a* was expressed as [red (+)/green (-)], b* was expressed as [yellow (+)/bleu (-)], C* (Chroma), WI (whiteness index), h° (Hue angle), and differences (distance between two colors) and these values were calculated using standard equations according to Hunter (1975).
2.2.6. Sensory properties
Sixty members of the Food Science department, Faculty of Agriculture, Zagazig University, Egypt staff, and students have evaluated milk samples' sensory traits as per Aderinola and Abaire (2019). Five attributes were estimated (color, odor, flavor, taste, and overall acceptability) using a 9-point Hedonic scale (9 = like extremely, and, 1 = dislike extremely). Water was applied for each panelist for mouth rinsing after testing each product to avoid the carry-over effect.
2.2.7. Statistical analysis
All experiments were in triplicate. The Two-way ANOVA at p ≤ 0.05 level and least significant difference (LSD) tests were used to statistically analyze the means of triplicate data using SPSS (V.20).
3. Results
3.1. Antioxidant activity of bioactive peptides
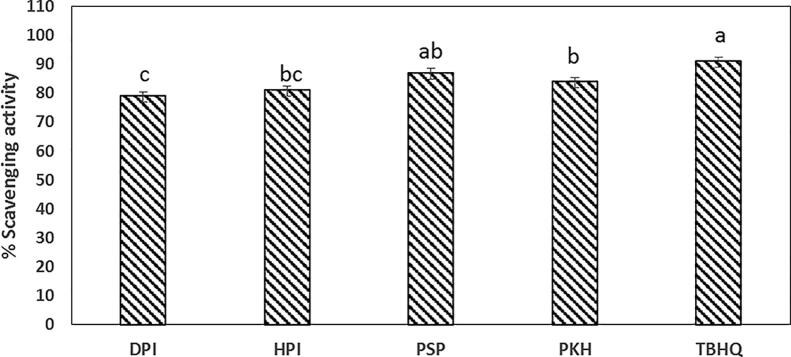
Fig. 1 represented the antiradical activity of HPI, DPI, PSP, and PKH peptides. PSP and PKH were significantly higher antioxidant activity than HPI and DPI peptides. The PSP was exhibited 87% followed by PKH (84%) compared to TBHQ at 91%.
Fig. 1.
DPPḢ radical scavenging activity of isolated proteins (DPI, duck egg protein isolate; HPI, hen egg protein isolate; PSP, pepper seed protein isolate; PKH, pepsin kidney bean protein hydrolysate) compared to Tert-Butylhydroquinone (TBHQ) synthetic antioxidant. Data presented means ± SE, different lowercase letters indicate significant differences (p ≤ 0.05).
3.2. Antimicrobial activity of bioactive peptides
3.2.1. Antibacterial activity of protein isolates against tested bacteria
The antibacterial activity of DPI, HPI, PSP, and PKH on the pathogenic bacteria (B. cereus, L. monocytogenes, S. aureus, Y. enterocolitica, E. coli, and C. jejuni), expressed as inhibition zones diameter (mm) in Table 1. PSP concentrations (200, 400, and 800) have the highest inhibition zones diameters (IZD) were in the range of 15–28 mm against tested bacteria, followed by PKH and HPI, e.g., (12–27 mm) and (10–25 mm), respectively. Based on the IDZs values in Table 1, S. aureus was the most sensitive bacteria intolerance to added peptides; on the other hand, C. jejuni was the most resistant bacteria.
Table 1.
Antibacterial activity of proteins isolates levels (200, 400, and 800 µg/mL) against dairy pathogenic bacteria expressed with inhibition zones (mm/24 h) at 37 °C, MIC, and MBC
| Pathogenic bacteria |
Protein isolates |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DPI |
HPI |
PSP |
KBH |
|||||||||
| 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 | |
| B. cereus | 14ab | 17ab | 21b | 16b | 19b | 22b | 17b | 23b | 26b | 17b | 21ab | 24b |
| L. monocytogenes | 13b | 16b | 20b | 15b | 18b | 24ab | 16b | 21c | 25b | 16b | 20b | 23b |
| S. aureus | 15a | 19a | 25a | 18a | 21a | 25a | 19a | 25a | 29a | 19a | 22a | 28a |
| Y. enterocolitica | 11 cd | 14 cd | 17d | 13 cd | 16 cd | 20c | 14d | 19d | 22d | 14 cd | 18c | 21c |
| E. coli | 12c | 15c | 18c | 14c | 17c | 21bc | 15c | 20c | 24c | 15c | 19b | 22c |
| C. jejuni | 10 cd | 13 cd | 16d | 10d | 15d | 19 cd | 14d | 17de | 21d | 12d | 17 cd | 20d |
| Bacteria | MIC | MBC | ||||||||||
| DPI | HPI | PSP | KBH | DPI | HPI | PSP | KBH | |||||
| B. cereus | 120e | 110e | 100e | 105e | 230e | 200e | 180e | 190e | ||||
| L. monocytogenes | 130d | 120d | 110d | 115d | 250d | 230d | 200d | 210d | ||||
| S. aureus | 100f | 95f | 85f | 90f | 190f | 185f | 160f | 175f | ||||
| Y. enterocolitica | 170b | 160b | 145b | 150b | 320b | 300b | 270b | 280b | ||||
| E. coli | 150c | 140c | 130c | 135c | 290c | 260c | 240c | 250c | ||||
| C. jejuni | 180a | 170a | 150a | 160a | 350a | 320a | 280a | 300a | ||||
DPI, duck egg protein isolate; HPI, hen egg protein isolate; PSP, pepper seed protein isolate; PKH, pepsin kidney bean protein hydrolysate; MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; Different lowercase letters in same column indicate significant differences (p ≤ 0.05).
The MIC and MBC values of DPI, HPI, PSP, and PKH were observed in Table 1, the lowest values of MIC and MBC were detected in PSP, the MIC values in the range of 85–150 µg/ml, and MBC values were ranged 160–280 µg/mL as compared to PKH, HPI and DPI.
3.2.2. Antifungal activity
Table 2 showed the IDZs (mm) of DPI, HPI, PSP, and PKH peptides against the tested Candida and fungi. The IDZ values of PSP were in the range of 13–25 mm against Candida species and they were ranged 16–27 mm in fungal species compared to PKH, DPI, and HPI. The most resistant Candida and fungi were C. glabrata and A. niger, but C. stellate and P. solitum was the most sensitive species, respectively. The MIC and MFC values were the lowest in PSP against tested Candida and fungi species.
Table 2.
Antifungal activity of proteins isolates levels (200, 400, and 800 µg/mL) against dairy pathogenic yeasts and fungi expressed with inhibition zones (mm/4days), MIC, and MFC
| Yeasts |
Protein isolates |
MIC |
MFC |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
DPI |
HPI |
PSP |
KBH |
|||||||||||||||||
| 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 | 200 | 400 | 800 | DPI | HPI | PSP | KBH | DPI | HPI | PSP | KBH | |
| C. apis | 14c | 16c | 19b | 15b | 17b | 20b | 16c | 19d | 22c | 15c | 17d | 19c | 120c | 110c | 90c | 100c | 210c | 200c | 170c | 190c |
| C. blankii | 13d | 15c | 18c | 14c | 16b | 19b | 15d | 17e | 21c | 16b | 18c | 20b | 150b | 130b | 100b | 120b | 280b | 250b | 190b | 220b |
| C. glabrata | 12d | 13d | 16d | 13c | 15c | 18c | 13e | 16e | 20d | 15c | 16d | 18c | 180a | 160a | 130a | 140a | 300a | 285a | 250a | 260a |
| C. glaebosa | 15b | 17b | 19b | 16a | 18a | 21a | 18b | 20c | 23b | 16b | 18c | 20b | 100d | 90d | 80d | 85d | 200d | 170d | 160d | 168d |
| C. rugosa | 16a | 18b | 21a | 17a | 19a | 21a | 19a | 21b | 24a | 17a | 19b | 22a | 80e | 75e | 60e | 70e | 150e | 140e | 110e | 125e |
| C. stellata | 16a | 19a | 20a | 17a | 20a | 22a | 19a | 22a | 25a | 17a | 20a | 23a | 80e | 75e | 60e | 70e | 145e | 135e | 105e | 130e |
| Fungi | Inhibition zones (mm) | Concentration µg/mL | ||||||||||||||||||
| A. niger | 14d | 16d | 19d | 15d | 17c | 20d | 16d | 19d | 23c | 15d | 17d | 20c | 170a | 150a | 130a | 140a | 320a | 300a | 230a | 260a |
| A. flavus | 15d | 17c | 20c | 16c | 19b | 22c | 17c | 22c | 25b | 17c | 18d | 22b | 150b | 130b | 110b | 120b | 290b | 250b | 200b | 220b |
| F. solani | 17b | 18b | 22b | 16c | 18c | 24b | 19b | 25a | 28a | 19b | 20b | 24a | 100e | 95e | 80e | 90e | 190e | 170e | 150e | 165e |
| F. oxysporum | 16c | 17c | 21c | 16c | 17c | 23c | 18c | 23b | 27a | 17c | 19c | 23b | 140c | 125c | 115c | 120c | 260c | 240c | 210c | 225c |
| P. solitum | 18a | 20a | 25a | 19a | 22a | 26a | 20a | 23b | 27a | 20a | 22a | 25a | 120d | 110d | 95d | 100d | 220d | 210d | 180d | 190d |
| P. crustosum | 17b | 18b | 23b | 17b | 20b | 24b | 19b | 20d | 23c | 18b | 20b | 22b | 90e | 85e | 75e | 80e | 170e | 165e | 140e | 150e |
DPI, duck egg protein isolate; HPI, hen egg protein isolate; PSP, pepper seed protein isolate; PKH, pepsin kidney bean protein hydrolysate; MIC, minimum inhibitory concentration; MFC, minimum fungal concentration. Different lowercase letters in same column indicate significant differences (p ≤ 0.05).
3.3. Chemical changes in milk samples during cold preservation
3.3.1. Antioxidant activity, polyphenols, and flavonoids changes during cold preservation
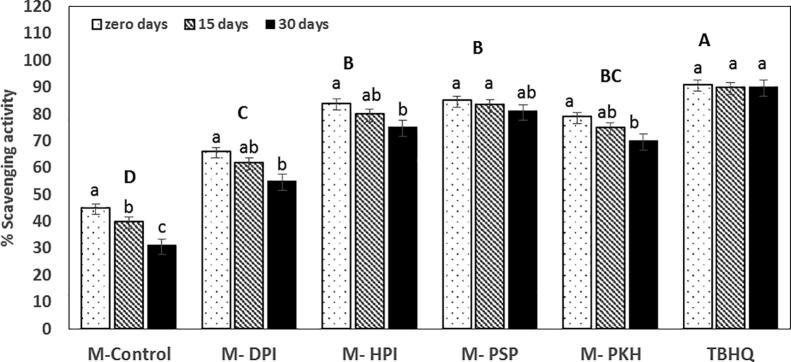
The antioxidant activity of milk was significantly p ≤ 0.05 incremented due to phenolics and flavonoids in added peptides. Still, the content of antioxidants significantly p ≤ 0.05 decreased during storage, as shown in Fig. 2. Table 3 showed the changes in antioxidant activity, phenolic compounds, and flavonoids in peptides supplemented-milk (M-HPI, M-DPI, M-PSP, and M-PKH) as compared to non-supplemented milk (M-control) during cold preservation at 4 °C for 30 days. The antiradical potential of M-PSP significantly p ≤ 0.05 decreased from 85% to 81% as decrement of total phenolic compounds content from 2797 to 932 GAE µg/mL, and the total flavonoids content significantly decremented from 419.4 at zero-day to 186.4 QE µg/mL after 30 days.
Fig. 2.
Changes in the antioxidant activity in peptides-supplemented-milk during storage period of (0, 15, and 30 days) at 4 °C as compared to synthetic antioxidant Tert-Butylhydroquinone (TBHQ), M- Control = non-supplemented milk, M-DPI = duck protein isolate-supplemented milk, M-HPI = hen protein isolate-supplemented milk, M-PSP = pepper seeds protein-supplemented milk, M-PKH = kidney bean hydrolysate-supplemented milk. Different lowercase letters indicate significant differences during storage, uppercase letters indicate significant differences between scavenging activity of milk samples (p ≤ 0.05).
Table 3.
Changes in phenolic and flavonoids content in peptides supplemented-milk during storage period (0, 15, and 30 days) at 4 °C.
|
Storage period (day) |
||||||
|---|---|---|---|---|---|---|
| Flavonoids | Phenolic compounds | |||||
| Sample | 0 | 15 | 30 | 0 | 15 | 30 |
| M−Control | 112.15 ± 0.9dA | 107.00 ± 0.31 dB | 49.85 ± 0.2dC | 1442.67 ± 0.33cA | 1320.10 ± 0.8cB | 447.56 ± 0.2eC |
| M- HPI | 259.12 ± 0.8cA | 247.30 ± 0.15cB | 115.16 ± 0.3cC | 1760.39 ± 0.45bA | 1076.10 ± 0.5eB | 586.80 ± 0.5cC |
| M- DPI | 300.64 ± 0.2bA | 287.00 ± 0.52bB | 133.62 ± 0.6bC | 1494.46 ± 0.3cA | 1201.10 ± 0.45 dB | 498.15 ± 0.6dC |
| M- PSP | 419.42 ± 0.3aA | 399.12 ± 0.15aB | 186.41 ± 0.5aC | 2797.39 ± 0.5aA | 2274.10 ± 1.2aB | 932.46 ± 0.9aC |
| M- PKH | 258.82 ± 0.5cA | 247.00 ± 0.23cB | 115.03 ± 0.7cC | 1917.03 ± 0.4bA | 1549.10 ± 0.9bB | 639.01 ± 0.7bC |
Data are presented mean ± SD; Mean in the same column with different lowercase letters are significantly
different, different uppercase letters in the same raw indicate significant differences between storage period
p ≤ 0.05; M- Control = non-supplemented milk, M−DPI = duck protein isolate-supplemented milk,
M−HPI = hen protein isolate-supplemented milk, M−PSP = pepper seeds protein-supplemented milk,
M−PKH = kidney bean hydrolysate-supplemented milk.
Table 4 presents the HPLC profile of the phenolic compounds and flavonoids in peptides supplemented-milk compared to non-supplemented milk. The results indicated significant differences p ≤ 0.05 between milk samples, where M-PSP significantly higher in the phenolic compounds content than other milk samples; pyrogallol, protocatechuic, catechol, catechin, and caffeine value were (1.1, 0.13, 0.06, 0.06, and 0.05 mg/mL, respectively), and pyrogallol, protocatechuic, and catechin were in high contents in M- HPI, M- DPI, and M- PKH, other phenolic compounds were in lower contents. The flavonoids with the highest contents occurred in M-PSP. Apignin-6-arabinose, naringin, luteolin-7-glucose, hesperidin, and kaempferol 3–2-p-comaroyl values were (1.2, 0.3, 0.08, 0.7, and 0.07 mg/mL), and other flavonoids in lower contents.
Table 4.
Phenolic and flavonoids profile in peptides-supplemented milk samples (µg/mL)
| Phenolic compound | M−control | M−DPI | M−HPI | M−PSP | M−PKH |
|---|---|---|---|---|---|
| Pyrogallol | 125c | 99.1d | 100d | 1118.21a | 326.68b |
| Gallic | 30.2b | 9.65c | 8.99c | 52.91a | 29.01b |
| Protocatchuic | 27.5d | 35.48c | 40.2c | 138.55a | 26.6d |
| Catechol | 33b | 27.26c | 30.55b | 62.87a | 32.37b |
| 4-Aminobenzoic | 4.5b | 2.48d | 4.28b | 6.28a | 3.39c |
| Catechein | 42.22b | 44.55b | 46.6b | 63.55a | 43.09b |
| Chlorogenic | 17.55b | 6.76e | 12.5d | 32.1a | 16.45c |
| p-OH-Benzoic | 9d | 22.82a | 20.33b | 16.62c | 8.42d |
| Benzoic | 49.09a | 26.04b | 27.44b | ND | ND |
| Caffeic | 8.77a | 1.36e | 2.4d | 4.12b | 3.49c |
| Vanillic | 17.32a | 16.78b | 12.5d | 13.12c | 12.98d |
| p-Coumaric | 5.12c | 5.64b | 5.77b | 6.15a | ND |
| Caffeine | ND | ND | ND | 50a | 46.7a |
| Ferulic | 6.52c | 10.32b | 9.99b | 13a | 12.81a |
| Iso-ferulic | 14.45a | 3.78d | 8.5c | 10.3b | 9.69b |
| Alpha-coumaric | 1.86e | 6.62a | 5.12b | 2.5c | 2.21d |
| Coumarin | 18.1c | 8.78d | 7.69d | 22.7a | 21.76b |
| 3,4,5-methoxy-cinnamic | 13.63b | 6.95d | 11.25c | 37a | 36a |
| Flavonoids | M−control | M−DPI | M−HPI | M−PSP | M−PKH |
| Apignin-6-arabinose | 539.12c | 140.07d | 150e | 1235.53a | 1103.5b |
| Narengin | 46.31d | 41.32f | 44.2d | 361.59a | 350b |
| Rosmarinic | 5.3a | 4.33b | 5.1a | ND | ND |
| Apigenin-6-rhamnose | ND | 2.32d | 3.2c | 10.6a | 8.5b |
| Luteolin-7-glucose | 36.28c | 11.54d | 12.15d | 82.26a | 79.55b |
| Hesperdine | 109.09d | 111.46d | 120.5c | 746.2a | 722b |
| Rutin | 9.62c | 4.23d | 5.12d | 18.91a | 15.9b |
| Apigenin-7-Glucose | ND | 1.4d | 2.5c | 5.11a | 4.2b |
| Apig-7-O-neohespiroside | 11.05b | 2.62d | 2.9d | 21.69a | 21.5a |
| Quercetrin | 9.62c | 3.05d | 4.05d | 19.12a | 18.5b |
| Narengenin | 53.25a | 11.45d | 13c | 22.61b | 21.55b |
| Quercetin | 24.18a | 5.23d | 5.9d | 11.51b | 10.9c |
| Hespertin | 4.13c | 6.22a | 6.7a | 5.28b | 4.15c |
| kampferol 3–2-p-comaroyl | 38.39b | 5.77d | 5.9d | 70.23a | 69.12a |
| Acacetin-7 neo-rutinoside | 21.36c | 10.17d | 12d | 27.97a | 25.13b |
| Kampferol | 11.46a | 3.97c | 5.5c | 10.61b | 11.5a |
| Apigenin | ND | 5.27b | 5.15b | 9.33a | 8.56a |
ND, not detected; means in the same raw with different lowercase letters are significantly different p ≤ 0.05.
3.3.2. Changes in chemical parameters (pH, TAA, and TSS) during milk preservation
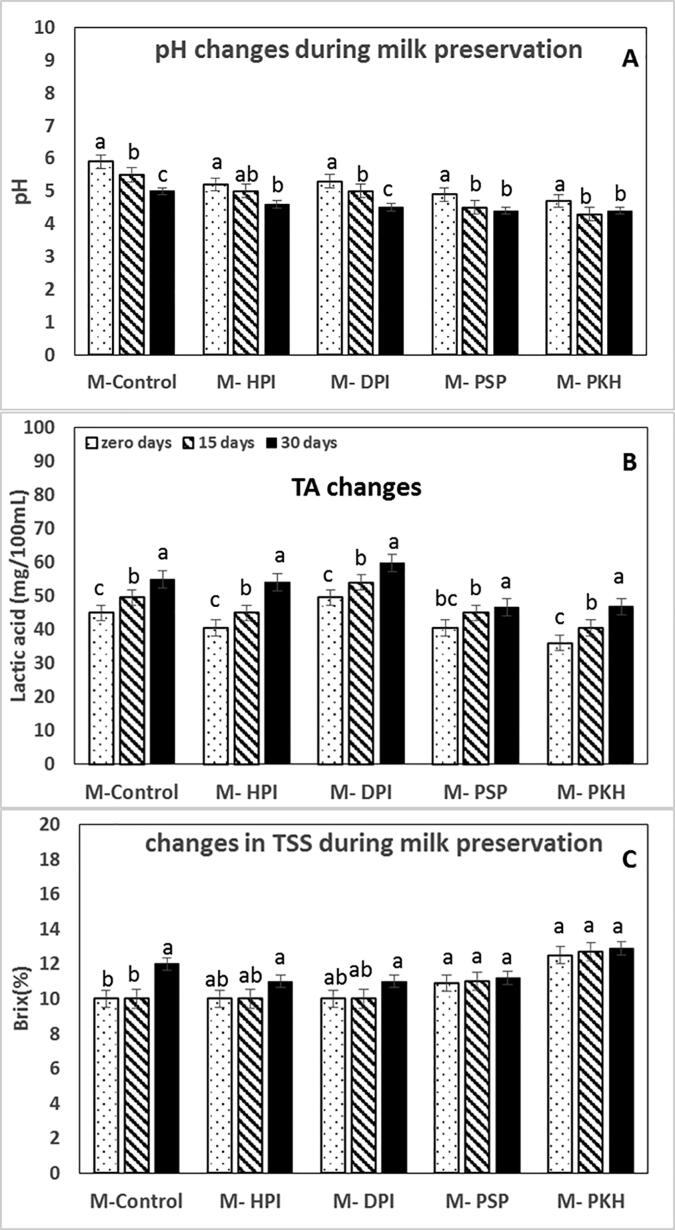
Data in Fig. 3A showed a significant p ≤ 0.05 decrease in pH values of milk samples during storage. PSP and PKH significantly controlled the pH decrement with a relative reduction of about 5–10% in M-PSP and-PKH compared to M-control. At the end of cold storage at 4 °C, the pH of M-control was p ≤ 0.05 decreased from 5.9 to 5, M-HPI from 5.2 to 4.6, M-DPI from 5.3 to 4.5, M-PSP from 4.9 to 4.4, and M-PKH from 4.7 to 4.4. The titratable acidity significantly increased in M-control by 0.14 mg/ml lactic acid. Slower increase in M- HPI, M- DPI, M- PSP, and M- PKH, i.e., 0.11, 0.12, 0.06, and 0.08, respectively Fig. 3B, in the same route, the total soluble solids were increased in Fig. 3C.
Fig. 3.
(A) Changes in pH, (B) in titratable acidity (TA), and (C) in total soluble solids (TSS) of peptides supplemented-milk during storage period of (0, 15, and 30 days) at 4 °C. Different lowercase letters indicate significant differences during storage (p ≤ 0.05).
Table 5 represents the total sugars (µg/mL) changes during milk storage at 4 °C. In M-control, the sugars content significantly (p ≤ 0.05) decremented from 308.4 to 84.09 µg/mL. In comparison, PSP and PKH (p ≤ 0.05) inhibited the decay of sugars 279.3 to 105.9 and 382.5 to 202.9 µg/mL in M-PSP, and M-PKH, respectively. These simple sugars increase the total soluble solids (Brix), as shown in Fig. 3C.
Table 5.
Changes in total sugars content (µg/mL) in peptides-supplemented milk during storage period of 0, 15, and 30 days at 4 °C
| Storage period (day) | |||
|---|---|---|---|
| Sample | 0 | 15 | 30 |
| M−control | 308.43 ± 0.5bA | 129.19 ± 0.11bB | 84.09 ± 0.3cC |
| M−DPI | 278.25 ± 0.2cA | 105.04 ± 0.92cB | 59.94 ± 0.5dC |
| M−HPI | 250.51 ± 0.9dA | 88.81 ± 0.15 dB | 47.68 ± 0.2dC |
| M−PSP | 279.38 ± 0.8eA | 175.60 ± 0.12bB | 105.96 ± 0.9bC |
| M−PKH | 382.58 ± 0.1aA | 216.36 ± 0.31aB | 202.96 ± 0.7aC |
Data are presented mean ± SD; mean in the same column with different lowercases are significantly different between sugars content in milk samples, different uppercase letters indicate significant difference between storage time affecting sugars content p ≤ 0.05.be
3.4. Microbial changes in milk samples during cold preservation
3.4.1. Total viable and coliform bacteria count in milk supplemented with isolated proteins
Data in Table 6 showed that the M-control was acceptable for up to 4–7 days preserved at 4 °C, and the addition of DPI and HPI in M-DPI and M-HPI significantly increased the acceptability to 7–12 days. Moreover, PSP and PKH significantly increased the acceptability up to 18–20 days in M-PSP and M-PKH. The total bacteria and coliform count were significantly affected by DPI, HPI, PSP, and PKH supplementations during the storage period of 0, 15, 30 days at 4 °C. The highest total bacterial count (log CFU/mL) found in M-control during the storage period. The total bacterial count in M-DPI and M-HPI significantly decreased p ≤ 0.05 from 5.2 to 4.7 and 4.6. The total bacterial count in M-PSP was significantly reduced by 45% compared to control. There were no significant differences between the antibacterial activity of the PSP and PKH.
Table 6.
The bacterial count expressed as (Log CFU/mL) in peptides-supplemented milk samples during storage period (0, 15, and 30 days) at 4 °C.
| Day | 0 | 15 | 30 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | TVC | PBC | CC | TVC | PBC | CC | TVC | PBC | CC |
| M−control | 5.2a ± 0.15 | 4.5a ± 0.12 | 2.7a ± 0.13 | 7.8a ± 0.15 | 5.0a ± 0.21 | 3.53a ± 0.18 | 8.9a ± 0.49 | 6.0a ± 0.31 | 4a ± 0.27 |
| M−DPI | 4.7b ± 0.25 | 4.0b ± 0.14 | 2.3b ± 0.21 | 5.6b ± 0.29 | 4.2b ± 0.25 | 2.84b ± 0.28 | 6.9b ± 0.51 | 5.0b ± 0.34 | 2.9b ± 0.14 |
| M−HPI | 4.6b ± 0.23 | 3.9b ± 0.15 | 1.9c ± 0.14 | 4.9c ± 0.31 | 4.1b ± 0.23 | 2.44c ± 0.19 | 6.2b ± 0.81 | 4.7bc ± 0.35 | 2.7b ± 0.24 |
| M−PSP | 3.0c ± 0.19 | 3.1c ± 0.16 | 1.8c ± 0.12 | 3.2d ± 0.27 | 3.5c ± 0.31 | 2.16d ± 0.28 | 4c ± 0.72 | 3.9c ± 0.36 | 2.1c ± 0.14 |
| M−PKH | 2.9c ± 0.18 | 3.0c ± 0.18 | 1.5d ± 0.15 | 3.1d ± 0.21 | 3.3c ± 0.25 | 1.62d ± 0.18 | 3.8c ± 0.61 | 3.5 cd ± 0.34 | 1.9c ± 0.14 |
Data are presented mean ± SD; mean in the same column with different lowercase letters are significantly different P ≤ 0.05; TVC, total viable count; CC, coliform count, and PBC, psychrophilic bacterial count.
3.5. Color measurement and sensory evaluation
Table 7 presents color parameters of peptides-supplemented milk samples as compared to control during storage at 4 °C. PSP and PKH supplementations were significantly increased p ≤ 0.05, the whiteness of milk, i.e., 88.7 in the control sample to 88.91, and 88.88, in M-PSP and M-PKH, respectively. M-PSP had the significantly highest score in color, taste, and flavor, followed by M-PKH (Table 8).
Table 7.
Color parameters of peptides-supplemented milk samples during cold preservation at 4 °C
| Sample | L* | a* | a* | C* | h° | WI | differences | b/a |
|---|---|---|---|---|---|---|---|---|
| M−control | 88.77 ± 0.7c | −1.99 ± 0.3b | 7.5 ± 0.7b | 7.75 ± 0.1b | −75.1 ± 0.5a | 27.6 ± 0.3a | 0.25 ± 0.02ab | −3.76 ± 0.14c |
| M−DPI | 88.71 ± 0.8d | −2.12 ± 0.7a | 7.96 ± 0.9a | 8.23 ± 0.2a | −75.06 ± 0.6a | 27.1 ± 0.4ab | 0.27 ± 0.01a | −3.75 ± 0.15c |
| M−HPI | 88.76 ± 1.2c | −1.95 ± 0.5b | 7.13 ± 0.3d | 7.39 ± 0.2c | −74.64 ± 0.8b | 23.5 ± 0.6d | 0.26 ± 0.02a | −3.65 ± 0.13d |
| M−PSP | 88.91 ± 0.5a | −1.91 ± 0.19c | 7.5 ± 0.5b | 7.73 ± 0.1b | −75.68 ± 0.5a | 26.3 ± 0.5b | 0.23 ± 0.03b | −3.92 ± 0.12a |
| M−PKH | 88.88 ± 0.25b | −1.92 ± 0.29c | 7.4 ± 0.22c | 7.64 ± 0.4bc | −75.4 ± 0.6a | 25.5 ± 0.4c | 0.24 ± 0.03b | −3.85 ± 0.17b |
Data are presented mean ± SD; Mean in the same column with different lowercase letters are significantly different p ≤ 0.05; L* was expressed
lightness/darkness, a* for redness/ greenness, b* for yellowness/blueness, C* (Chroma), WI (whiteness index), h° (Hue angle),
differences (distance between two colors)
Table 8.
Sensory traits of peptides-supplemented milk samples during cold preservation at 4 °C
| Sample | Color | Taste | Flavor | Over acceptability |
|---|---|---|---|---|
| M−Control | 8.5 ± 0.2c | 8.8 ± 0.1b | 8.5 ± 1.1c | 8.6 ± 0.2b |
| M- DPI | 8.7 ± 0.2b | 7.5 ± 0.5c | 8.3 ± 0.9d | 8.1 ± 0.4c |
| M- HPI | 8.4 ± 0.3d | 7.8 ± 0.5c | 8.2 ± 0.7d | 8.1 ± 0.5c |
| M- PSP | 8.8 ± 0.2a | 8.9 ± 0.1a | 8.8 ± 0.2a | 8.9 ± 0.1a |
| M- PKH | 8.4 ± 0.4d | 8.7 ± 0.2b | 8.8 ± 0.1b | 8.6 ± 0.3b |
Data are presented mean ± SD; Means in the same column with different lowercase letters are significantly different P ≤ 0.05.
4. Discussion
Milk and milk products are sustainable for microbial contamination and chemical deterioration; therefore, the researchers strive to find suitable preservatives to extend the shelf life of milk handling and maintain its quality. Natural additives especially bioactive peptides, are a new trend to achieve this equation. In this study, raw buffalo milk was supplemented with four bioactive peptides: HPI, DPI, PSP, and PKH, then milk samples: M-control, M-HPI, M-DPI, M-PSP, and M-PKH were preserved at 4 °C for 30 days and the chemical, microbial, and sensory changes were noticed during this period. The added bioactive peptides have considerable scavenging activity. Plant-derivated peptides (PSP, and PKH) activity depends on their low molecular weight and amino acids that contain OH and SH residues. Alternatively, poultry-derivated peptides (HPI, and DPI) have high molecular weight. Sarmadi and Ismail (2010) described the antioxidant action of peptides depended on the hydrophobic aromatic amino acids that donate electrons to radicals and make them stable and the own stability kept, the hydrophobicity improve peptides lipophilic action through hydrophobic residues, also, acidic and basic amino acids act as proton donors and metal ion chelators through their amine and carboxylic residues, also, the tested bioactive peptides exhibited antimicrobial activity and that may return to the positive charge or hydrophobicity action, peptides with positive charges were electrostatically bound to negatively charged compounds on the bacterial cell wall, and the cell wall was demolished (Gobbetti et al., 2004, Jenssen et al., 2006, Lei et al., 2019), and the hydrophobic nature of peptide plays a vital role in disquieting the cell wall and membrane of bacteria, besides, the interaction between phenolic compounds and proteins might be alter the cell wall permeability by inhibiting certain enzymes (Upadhyay et al., 2014). The hydrophobicity of polyphenols and flavonoids may increase their interaction with the cell membrane, causing cell content coagulation and inhibiting the DNA synthesis. Besides, the active groups in the polyphenols and flavonoids may account for their antimicrobial activity (Bouarab-Chibane et al., 2019). for these reasons, Samaranayaka and Li-Chan (2011) reported that antioxidants in nutrition improve health and foodstuff quality.
The addition of protein isolates to milk was enhanced the antioxidant activity of milk, prevented lipids oxidation and extended the shelf life (Winata and Lorenz, 1996). The antiradical mechanisms of antioxidant peptides was investigated by Esfandi et al. (2019), who cleared the mechanisms include electron donation or transfer proton, these mechanisms may act together, or one dominated depended on peptides structure, wherein the aromatic amino acids make radicals stable by donating electron besides, acidic and essential amino acids act as metal ion chelators, also, the antioxidant activity of milk may be explained by Jakobek, 2015, Rawel and Rohn, 2010 who mentioned that phenolic compounds might interact with added protein isolates to increase the antioxidant and antibacterial activity, the interaction may occur between OH of phenolic compounds and SH and OH side chains in peptides or protein, protein–phenolic interaction may affect the physicochemical properties of protein and phenolic compounds, the complex may increase the peptide activity through blocking some amino acid side chains, besides, increasing the bioavailability and activity of polyphenols. In a previous study, Cottica et al., 2015, El-Deeb, 2017 reported that the antioxidant potential of milk increased with propolis extract. The presence of phenolic compounds in dairy products protects other antioxidant ingredients as their resistant to thermal treatments.
Protein isolates increased the phenolic and flavonoid compounds in milk samples and that can be attributed to the phenolics-protein complex formation (Jakobek, 2015, Ozdal et al., 2013) that enhanced the antioxidant activity (Gammoh et al., 2017, Guimarães Drummond e Silva et al., 2017), and this is a call for usage of natural alternatives instead of synthetic preservatives in food (Bouarab Chibane et al., 2019, Abd El-Hack et al., 2020), and cosmetic applications (Kočevar Glavač and Lunder, 2018).
Many chemical and microbial changes occurred during raw milk preservation, and natural additives limited these alternations. Protein isolates inhibited lactose decay by fermentative bacteria to glucose and lactic acid, raising the titratable acidity and total soluble solids (Sivakumar, 2017). Also, the protein isolates reduced the bacterial growth and inhibited the oxidation and some enzymatic reactions occurring in milk. In the previous study, an increase in the titratable acidity and a decrease in pH were observed in milk samples enriched with 0.5%, 0.75% and 1% level of tulsi leaves extract (Abbas and Osman, 1998, Sivakumar, 2017), these changes was limited by the addition of antibacterial aqueous extract of tulsi leaves at a concentration of 0.25–1% in raw milk because of phenolic compounds content in extracts. In the same route, Osman et al. (2013) used 11 s soybean protein subunit in milk preservation for 30 days and found the soybean 11S subunit exerted considerable antibacterial actions.
In the present study, the milk supplementation with antimicrobial peptides was significantly reduced the bacterial load to an acceptable level (Sivakumar and Dhanalakshmi, 2016). The antimicrobial mechanism was explained by various models, which occur when peptides attach to the cell membrane of bacteria and it completely disrupts the cell membrane integrity (Berglund et al., 2015). The carpet model, toroidal pore model, brave straw model and aggregate model are well-studied models of antimicrobial peptides (Strempel et al., 2015, Zhao et al., 2015).
Color is an indicator of milk quality, freshness, and food safety. Light reflects by dispersed fat granules and proteins cause milk color, besides natural milk pigments such as riboflavin and carotenoids (Nozière et al., 2006, Solah et al., 2007). Bacterial growth affects the sensory properties of milk, make color, texture, odor, and taste were unacceptable commercially (Ahmed and Abdellatif, 2013, Samet-Bali et al., 2013), because of sensory evaluation of food products is an essential indicator of potential consumer preference, the addition of bioactive peptides was enhanced the color parameters and sensorial traits of milk and that agree with Bakr et al., 2015, Cottica et al., 2015.
5. Conclusion
Microbial contamination and chemical deterioration are the main problems that affected milk and milk products after milking and during handling. Therefore, the need to find suitable preservatives to extend the lifetime of milk handling and maintain its quality. Natural additives, especially bioactive peptides, are a new trend to achieve this equation. The bioactive peptides in this work have considerable antioxidant and antimicrobial activity. The present study was confirmed that the supplementation of raw milk with HPI, DPI, PSP, and PKH as a natural preservative significantly maintained the shelf life of milk after milking and during handling besides enhancing its quality, where the addition of the peptide reduced the microbial load to an acceptable level, and that decrease the sugar decay in milk, consequently, the titratable acidity decreased and that extend the shelf life of milk. The M-PSP showed the highest color and sensory scores compared with the control. This preservation method encourages dairy farming to produce much milk with longer shelf life, which is a prerequisite for increased manufacture of high-quality milk.
Funding
The current work was funded by Taif University, Saudi Arabia, for financial support through its Researchers Supporting Project (TURSP-2020–105).
Declaration of Competing Interest
All authors declare that they do not have any conflicts of interest that could inappropriately influence this manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas F., Osman M. Properties of labneh like products manufactured using acid and acid-rennet coagulation. Annal. Agric. Sci. Moshtohor. 1998;36:401–411. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020;1(164):2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Aderinola T.A., Abaire K.E. Quality Acceptability, Nutritional Composition and Antioxidant Properties of Carrot-Cucumber Juice. Beverages. 2019;5:15. [Google Scholar]
- Ahmed K., Abdellatif N. Quality control of milk in the dairy industry. World J. Dairy Food Sci. 2013;8:18–26. [Google Scholar]
- Akl B., Nader M.M., El-Saadony M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11:1–8. [Google Scholar]
- Alemu T., Girma M. Indigenous knowledge on preservative plants and preservation techniques of milk and milk products in south wollo zone, northern Ethiopia. Int. J Avian & Wildlife Biol. 2018;3:127–131. [Google Scholar]
- Alizadeh H., Rahnema M., Semnani S.N., Ajalli M. Synergistic antifungal effects of quince leaf’s extracts and silver nanoparticles on Aspergillus niger. J. Appl. Biol. Sci. 2014;8:10–13. [Google Scholar]
- AOAC 2005. Official Methods of Analysis 18th Edition (Pub AOAC International Maryland).
- APHA, 1992. American Public Health Association. Compendium of methods for the microbiological examination of foods (3rd Ed.). Washington, DC.
- Arefin S., Sarker M.A.H., Islam M.A., Harun-ur-Rashid M., Islam M.N. Use of Hydrogen Peroxide (H2O2) in raw cow’s milk preservation. J. Adv. Vet. Anim. Res. 2017;4:371–377. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of Green Coffee Powder Supplementation on Growth Performance, Carcass Characteristics, Blood Indices, Meat Quality and Gut Microbial Load in Broilers. Agriculture. 2020;10:457. [Google Scholar]
- Baines, D., Seal, R., 2012. Natural food additives, ingredients and flavourings. Elsevier.
- Bakr I., Mohamed T., Tammam A., El-Gazzar F. Characteristics of bioyoghurt fortified with fennel honey. Int. J. Curr. Microbiol. Appl. Sci. 2015;4:959–970. [Google Scholar]
- Berglund N.A., Piggot T.J., Jefferies D., Sessions R.B., Bond P.J., Khalid S. Interaction of the antimicrobial peptide polymyxin B1 with both membranes of E. coli: a molecular dynamics study. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure–activity relationship) models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouarab Chibane L., Degraeve P., Ferhout H., Bouajila J., Oulahal N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019;99:1457–1474. doi: 10.1002/jsfa.9357. [DOI] [PubMed] [Google Scholar]
- Branen, A.L., Davidson, P.M., Salminen, S., Thorngate, J., 2001. Food additives. CRC Press.
- Caleja C., Barros L., Antonio A.L., Ciric A., Barreira J.C., Sokovic M., Oliveira M.B.P., Santos-Buelga C., Ferreira I.C. Development of a functional dairy food: Exploring bioactive and preservation effects of chamomile (Matricaria recutita L.) J. Funct. Foods. 2015;16:114–124. [Google Scholar]
- Caleja C., Barros L., Antonio A.L., Ciric A., Soković M., Oliveira M.B.P., Santos-Buelga C., Ferreira I.C. Foeniculum vulgare Mill. as natural conservation enhancer and health promoter by incorporation in cottage cheese. J. Funct. Foods. 2015;12:428–438. [Google Scholar]
- Carocho M., Barreiro M.F., Morales P., Ferreira I.C. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Safety. 2014;13:377–399. doi: 10.1111/1541-4337.12065. [DOI] [PubMed] [Google Scholar]
- Carocho M., Ferreira I.C. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Carocho M., Morales P., Ferreira I.C. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015;45:284–295. [Google Scholar]
- Chaplin, M.F., Kennedy, J.F., 1994. Carbohydrate analysis: a practical approach.. ed. 2. IRL Press Ltd.
- Cottica S.M., Sabik H., Bélanger D., Giroux H.J., Visentainer J.V., Britten M. Use of propolis extracts as antioxidant in dairy beverages enriched with conjugated linoleic acid. Eur. Food Res. Technol. 2015;241:543–551. [Google Scholar]
- Dickson-Spillmann M., Siegrist M., Keller C. Attitudes toward chemicals are associated with preference for natural food. Food Qual. Prefer. 2011;22:149–156. [Google Scholar]
- Egyptian Standard, R.M.E., Egyptian Organization for Standardization and Quality Control, Ministry of Industry, Cairo, Egypt. , 2005.
- El-Deeb A.M. Utilization of propolis extract as A natural preservative in raw milk. J. food Dairy Sci. 2017;8:315–321. [Google Scholar]
- El-Saadony M.T., El-Wafai N., El-Fattah H., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of Chemical and Natural Additives on Cucumber Juice’s Quality, Shelf Life, and Safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69(1) [Google Scholar]
- Elgorban A., Aref S., Seham S., Elhindi K., Bahkali A., Sayed S., Manal M. Extracellular synthesis of silver nanoparticles using Aspergillus versicolor and evaluation of their activity on plant pathogenic fungi. Mycosphere. 2016;7:844–852. [Google Scholar]
- Esfandi R., Walters M.E., Tsopmo A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh, S., Alu’datt, M.H., Alhamad, M.N., Rababah, T., Ereifej, K., Almajwal, A., Ammari, Z.A., Al Khateeb, W., Hussein, N.M., 2017. Characterization of phenolic compounds extracted from wheat protein fractions using high-performance liquid chromatography/liquid chromatography mass spectrometry in relation to anti-allergenic, anti-oxidant, anti-hypertension, and anti-diabetic properties. Int. J. Food Prop. 20, 2383-2395.
- Gershom N., Edward S. Traditional methods of milk processing and preservation by local farmers in kashongi sub county kiruhura district. Am. J. Food Technol. 2017;2(2):62–71. [Google Scholar]
- Gobbetti M., Minervini F., Rizzello C.G. Angiotensin I-converting-enzyme-inhibitory and antimicrobial bioactive peptides. Int. J. Dairy Technol. 2004;57:173–188. [Google Scholar]
- González-Fandos E., Dominguez J. Effect of potassium sorbate washing on the growth of Listeria monocytogenes on fresh poultry. Food Control. 2007;18:842–846. [Google Scholar]
- Goupy P., Hugues M., Boivin P., Amiot M.J. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 1999;79:1625–1634. [Google Scholar]
- Guimarães Drummond e Silva, F., Miralles, B., Hernández-Ledesma, B., Amigo, L., Iglesias, A.H., Reyes Reyes, F.G., Netto, F.M., 2017. Influence of protein–phenolic complex on the antioxidant capacity of flaxseed (Linum usitatissimum L.) products. J. Agric. Food Chem. 65, 800-809. [DOI] [PubMed]
- Gülçin I., Küfrevioǧlu Ö.İ., Oktay M., Büyükokuroǧlu M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J. Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Hassanin A.A., Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J. Agric. Food Chem. 2020;68:10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Hatano T., Kagawa H., Yasuhara T., Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Hunter, R., 1975. Scales for the measurements of color difference. the Measurement of Appearance. John Willy & Sons, New York, 133-140.
- Jakobek L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015;175:556–567. doi: 10.1016/j.foodchem.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Janakat S., Al-Nabulsi A.A.R., Allehdan S., Olaimat A.N., Holley R.A. Antimicrobial activity of amurca (olive oil lees) extract against selected foodborne pathogens. Food Sci. Technol. 2015;35(2):259–265. [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.A., Brekke C. Functional properties of acylated pea protein isolates. J. Food Sci. 1983;48:722–725. [Google Scholar]
- Karabulut O.A., Lurie S., Droby S. Evaluation of the use of sodium bicarbonate, potassium sorbate and yeast antagonists for decreasing postharvest decay of sweet cherries. Postharvest Biol. Technol. 2001;23:233–236. [Google Scholar]
- Kočevar Glavač N., Lunder M. Preservative efficacy of selected antimicrobials of natural origin in a cosmetic emulsion. Int. J. Cosmet. Sci. 2018;40:276–284. doi: 10.1111/ics.12461. [DOI] [PubMed] [Google Scholar]
- Lee P.S. Quantitation of microorganisms. Practical handbook of microbiology. 2009;3:19–38. [Google Scholar]
- Lei J., Sun L., Huang S., Zhu C., Li P., He J., Mackey V., Coy D.H., He Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019;11:3919. [PMC free article] [PubMed] [Google Scholar]
- Liu K., Wang X., Young M. Effect of bentonite/potassium sorbate coatings on the quality of mangos in storage at ambient temperature. J. Food Eng. 2014;137:16–22. [Google Scholar]
- Lu M., Shiau Y., Wong J., Lin R., Kravis H., Blackmon T., Pakzad T., Jen T., Cheng A., Chang J. Milk spoilage: Methods and practices of detecting milk quality. Food Nutr. Sci. 2013;4:113. [Google Scholar]
- Mattila P., Astola J., Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. J. Agric. Food Chem. 2000;48:5834–5841. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- Meinert, L., Broge, E.H.d.L., Bejerholm, C., Jensen, K., 2016. Application of hydrolyzed proteins of animal origin in processed meat. Food Sci. Nutr. 4, 290-297. [DOI] [PMC free article] [PubMed]
- Meshginfar N., Sadeghi Mahoonak A., Ghorbani M., Aalami M. Effects of protein hydrolysate from sheep visceral on oxidative stability of soybean oil and chicken sausage. J. Food Process. Preserv. 2017;41 [Google Scholar]
- Nicolaou N., Goodacre R. Rapid and quantitative detection of the microbial spoilage in milk using Fourier transform infrared spectroscopy and chemometrics. Analyst. 2008;133:1424–1431. doi: 10.1039/b804439b. [DOI] [PubMed] [Google Scholar]
- Nozière P., Graulet B., Lucas A., Martin B., Grolier P., Doreau M. Carotenoids for ruminants: from forages to dairy products. Anim. Feed Sci. Technol. 2006;131:418–450. [Google Scholar]
- Ordonez A., Gomez J., Vattuone M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food chem. 2006;97:452–458. [Google Scholar]
- Osman A.O., Mahgoub S.A., Sitohy M.Z. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25°C. J. Dairy Res. 2013;80:174–183. doi: 10.1017/S0022029913000095. [DOI] [PubMed] [Google Scholar]
- Ozdal T., Capanoglu E., Altay F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013;51:954–970. [Google Scholar]
- Rachman A., Brennan M.A., Morton J., Brennan C.S. Effect of egg white protein and soy protein fortification on physicochemical characteristics of banana pasta. J. Food Process. Preserv. 2019;43 [Google Scholar]
- Randhawa S., Bahna S.L. Hypersensitivity reactions to food additives. Curr. Opin. Allergy Clin. Immunol. 2009;9:278–283. doi: 10.1097/ACI.0b013e32832b2632. [DOI] [PubMed] [Google Scholar]
- Rawel H.M., Rohn S. Nature of hydroxycinnamate-protein interactions. Phytochem. Rev. 2010;9:93–109. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20(1):324–335. [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: characterization of hydrolysates and effect on the quality of minced beef during cold storage. Int. J. Pept. Res. Ther. 2020;26:567–577. [Google Scholar]
- Saad, A. M., El‐Saadony, M. T., Mohamed, A. S., Ahmed, A. I., & Sitohy, M. Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour‐based noodles. Int. J. Food Sci.Technol. 2021.
- Samaranayaka A.G., Li-Chan E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods. 2011;3:229–254. [Google Scholar]
- Samet-Bali O., Felfoul I., Lajnaf R., Attia H., Ayadi M.A. Study of proteolytic and lipolytic activities of Pseudomonas spp. isolated from pasteurized milk in Tunisia. J. Agric. Sci. 2013;5:46. [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitohy M.Z., Osman A.O. Enhancing milk preservation with esterified legume proteins. Probiotics Antimicrob. Proteins. 2011;3:48–56. doi: 10.1007/s12602-010-9060-5. [DOI] [PubMed] [Google Scholar]
- Sivakumar G. Effect of tulsi leaf extract on physico-chemical and microbial quality of raw milk. Int. J. Sci. Environ. Technol. 2017;6:1626–1631. [Google Scholar]
- Sivakumar G., Dhanalakshmi B. Betel leaf extract as shelf life extender of raw milk. Int. J. Sci. Environ. Technol. 2016;5:2832–2836. [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89:191–198. [Google Scholar]
- Solah V., Staines V., Honda S., Limley H. Measurement of milk color and composition: effect of dietary intervention on Western Australian Holstein-Friesian cow's milk quality. J. Food Sci. 2007;72:S560–S566. doi: 10.1111/j.1750-3841.2007.00491.x. [DOI] [PubMed] [Google Scholar]
- Strempel N., Strehmel J., Overhage J. Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr. Pharm. Des. 2015;21:67–84. doi: 10.2174/1381612820666140905124312. [DOI] [PubMed] [Google Scholar]
- Terras F., Schoofs H., De Bolle M., Van Leuven F., Rees S.B., Vanderleyden J., Cammue B., Broekaert W.F. Analysis of two novel classes of plant antifungal proteins from radish (Raphanus sativus L.) seeds. J. Biol. Chem. 1992;267:15301–15309. [PubMed] [Google Scholar]
- Thery T., Lynch K.M., Arendt E.K. Natural Antifungal Peptides/Proteins as Model for Novel Food Preservatives. Compr. Rev. Food Sci. Food Safety. 2019;18:1327–1360. doi: 10.1111/1541-4337.12480. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: a minireview of the mechanistic basis. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W., Xu B. Development of an orange juice beverage formulated with oat beta-glucan and whey protein isolate. J. Sci. Food Agric. 2018;98:4685–4691. doi: 10.1002/jsfa.9002. [DOI] [PubMed] [Google Scholar]
- Wilson B.G., Bahna S.L. Adverse reactions to food additives. Ann. Allergy Asthma. Immunol. 2005;95:499–507. doi: 10.1016/S1081-1206(10)61010-1. [DOI] [PubMed] [Google Scholar]
- Winata A., Lorenz K. Antioxidant potential of 5-n-pentadecylresorcinol. J. Food Process. Preserv. 1996;20:417–429. [Google Scholar]
- Yoo W., Araki T., Saito J., Kurata Y., Tokita K., Kato K., Matsushita M. Isolation and characterization of a novel chicken egg white protein with scavenger receptor cysteine-rich domains. Int. J. Poult. Sci. 2012;50(2):159–163. [Google Scholar]
- Zhao R., Duan G., Yang T., Niu S., Wang Y. Purification, characterization and antibacterial mechanism of bacteriocin from Lactobacillus acidophilus XH1. Trop. J. Pharm. Res. 2015;14:989–995. [Google Scholar]