Significance
Herpes simplex virus infections cause painful lesions, blindness, and viral encephalitis. At the cellular level, infection causes a dramatic shutdown of host gene expression, allowing the virus to monopolize the transcriptional and translational machinery. One of the viral proteins responsible is ICP27, which disrupts cellular RNA processing, leading to nuclear accumulation and accelerated turnover of aberrant pre-mRNAs. ICP27 also facilitates nucleocytoplasmic export of viral messenger RNAs (mRNAs). Recent studies establish that the RNA modification N6-methyladenosine (m6A) also regulates mRNA biogenesis, export, stability, and translation. Here, we show that the m6A pathway becomes progressively less important for viral gene expression as the infection cycle progresses and that ICP27 disrupts the m6A pathway through redistribution of nuclear methyltransferase components into the cytoplasm.
Keywords: herpes simplex virus, RNA modification, direct RNA sequencing, nanopore, N6-methyladenosine
Abstract
N6-methyladenosine (m6A) is the most abundant internal messenger RNA (mRNA) modification, contributing to the processing, stability, and function of methylated RNAs. Methylation occurs in the nucleus during pre-mRNA synthesis and requires a core methyltransferase complex consisting of METTL3, METTL14, and WTAP. During herpes simplex virus (HSV-1) infection, cellular gene expression is profoundly suppressed, allowing the virus to monopolize the host transcription and translation apparatus and antagonize antiviral responses. The extent to which HSV-1 uses or manipulates the m6A pathway is not known. Here, we show that, in primary fibroblasts, HSV-1 orchestrates a striking redistribution of the nuclear m6A machinery that progresses through the infection cycle. METTL3 and METTL14 are dispersed into the cytoplasm, whereas WTAP remains nuclear. Other regulatory subunits of the methyltransferase complex, along with the nuclear m6A-modified RNA binding protein YTHDC1 and nuclear demethylase ALKBH5, are similarly redistributed. These changes require ICP27, a viral regulator of host mRNA processing that mediates the nucleocytoplasmic export of viral late mRNAs. Viral gene expression is initially reduced by small interfering RNA (siRNA)-mediated inactivation of the m6A methyltransferase but becomes less impacted as the infection advances. Redistribution of the nuclear m6A machinery is accompanied by a wide-scale reduction in the installation of m6A and other RNA modifications on both host and viral mRNAs. These results reveal a far-reaching mechanism by which HSV-1 subverts host gene expression to favor viral replication.
Methylation at the N6-position of adenosine (m6A) is the most abundant and best studied internal modification of coding and long noncoding RNAs (lncRNAs) in mammalian cells, influencing the splicing and polyadenylation of precursor RNAs as well as the stability, export, localization, and translation of mature messenger RNAs (mRNAs) (1). These functions are conferred through binding of effector proteins with an affinity for RNA containing the modified base (2) by reducing the formation of RNA–RNA and RNA–DNA hybrids (3, 4) or by regulating the ability of mRNAs to participate in phase separation (5). The majority of m6A sites fall within a degenerate RRAmCH (where R = A or G, Am = methylated A, and H = A, C, or U) sequence of which only a fraction are modified in vivo (6–8). Installation occurs during transcription by RNA polymerase II (RNAPII) and is completed before the RNA is released from the chromosomal template (9–11).
Catalysis is carried out by a methyltransferase complex (shown schematically in Fig. 1A) organized around three core components: METTL3 (methyltransferase-like protein 3), METTL14, and WTAP (Wilms’ tumor 1–associated protein) (12–14). METTL3 is the catalytic subunit, while METTL14, which forms a stable heterodimer with METTL3, contributes to substrate RNA binding and METTL3 stability (15). WTAP lacks catalytic activity but is required to localize METTL3/METTL14 to nuclear speckles, sites of pre-mRNA processing, and for biological activity of the methyltransferase complex (13). Inactivation of either METTL3 or METTL14 in murine embryonic stem (ES) cells eliminates more than 99% of m6A in the polyadenylated fraction (16). Additional RNA-binding subunits of the methyltransferase complex include VIRMA (protein virilizer homolog/KIAA1429), ZC3H13 (zinc finger CCCH domain–containing protein 13/KIAA0853), RBM15 (RNA-binding motif protein 15), and its paralogue RBM15B, which likely determine the substrate RNAs and/or the precise sites of catalysis (17–19). Mutations in the Drosophila orthologs of WTAP, VIRMA, Z3CH13, and RBM15/15B impact alternative splicing, consistent with a functional linkage between m6A deposition and spliceosome activity (17). In addition to the methyltransferase complex, a predominantly nuclear m6A demethylase, AlkB homolog 5 (ALKBH5), has been identified (20). Additionally, there are a number of RNA-binding proteins that recognize m6A-modified RNAs. Of these, YTH domain–containing protein 1 (YTHDC1) acts primarily in the nucleus (21), whereas YTHDF1, YTHDF2, and YTHDF3 are predominantly cytoplasmic (22). Regulated installation, removal, and recognition of m6A by this suite of host proteins leads to changes in gene expression that allow cells to respond to physiological stresses including viral infection.
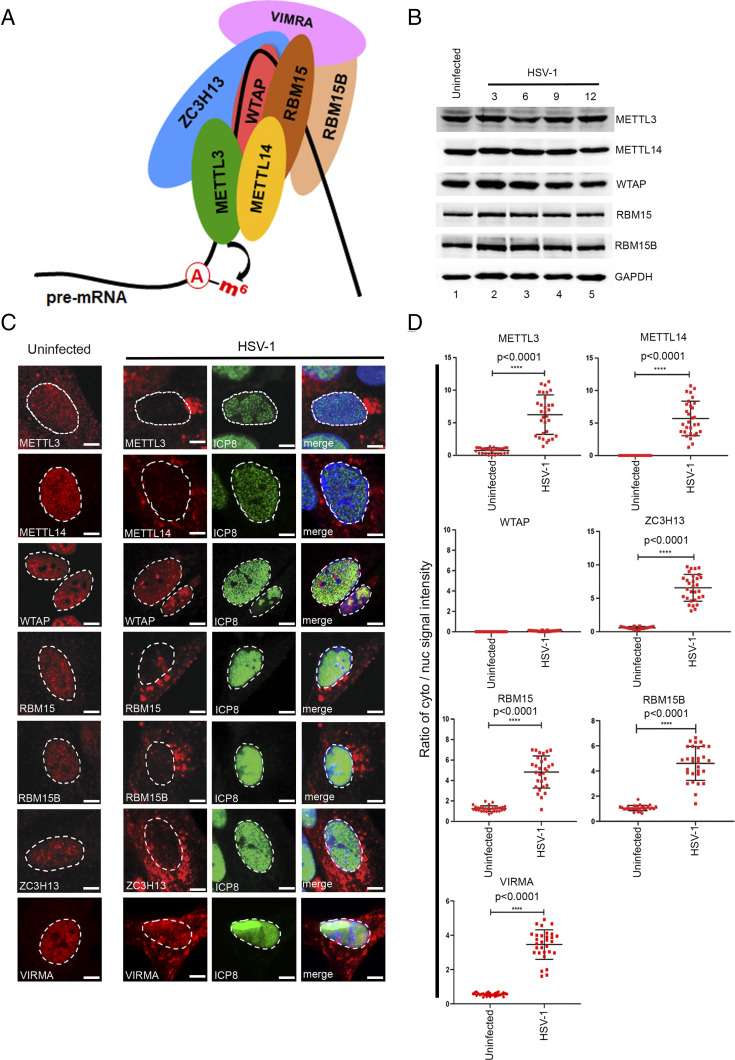
Fig. 1.
HSV-1 infection leads to a dramatic redistribution of the nuclear m6A methyltransferase subunits. (A) Schematic showing the principal subunits of the m6A methyltransferase complex. Catalysis is provided by METTL3, which forms a stable heterodimer with METTL14. Association with WTAP is required for localization of METTL3/METTL14 to nuclear speckles. Additional subunits ZC3H13, VIRMA, and RBM15/15B are thought to confer substrate specificity. Adenosine residues are methylated during pre-mRNA synthesis prior to release of the RNA (thick black line) from the DNA template. (B) Immunoblots showing the abundance of METTL3, METTL14, WTAP, RBM15, and RMB15B over a time course of HSV-1 (strain KOS) infection. GAPDH provides a loading control. (C) Indirect immunofluorescence detection of the core (METTL3, METTL14, and WTAP) and accessory (RBM15, RBM15B, and ZC3H13) methyltransferase complex subunits in NHDFs after 12 h of HSV-1 (strain KOS) infection. DAPI was used to define the boundary between the nucleus and cytoplasm as indicated with a dashed line. (Scale bar, 10 µm.) (D) Quantification of the ratio of the cytoplasmic/nuclear signal using ImageJ from 30 different images from each condition. Statistical significance was determined using Student’s t test. The mean ± SEM are shown along with the P value for the difference between uninfected and infected cultures.
Herpes simplex virus type 1 (HSV-1) mRNAs were first shown to be m6A-modified in the 1970s (23), but their relevance to viral replication is not yet fully understood (24). Recent studies of other DNA and RNA viruses have uncovered roles for m6A in regulation of alternative RNA splicing (25) and in control of host antiviral responses (24, 26–28). The double-stranded DNA genome of HSV-1 codes for more than 80 viral proteins and a number of noncoding RNAs. Viral genes are arranged in a dense, often overlapping, configuration, and viral RNAs are transcribed by host RNAPII (29) as a cascade beginning with the five immediate-early (IE) genes whose products direct the expression of a larger number of early (E) genes, most of which are required for viral DNA polymerase–dependent replication of the viral genome. The onset of DNA replication allows high-level expression of the late (L) genes, which encode proteins required to assemble and export new infectious particles. A hallmark of HSV-1 infection is a profound reduction in cellular gene expression known as host shutoff, allowing the virus to monopolize the biosynthetic resources of the cells and counteract antiviral responses (30). Shutoff is achieved through a combination of accelerated RNA turnover (31–33), reduced transcription of host genes by RNAPII (34–36), and inefficient transcription termination to produce unstable read-through transcripts (37).
HSV-1 pre-mRNAs are capped and polyadenylated by the nuclear RNA processing machinery, but transcripts from only five genes are consistently spliced (38). Splicing is a prerequisite for nucleocytoplasmic export of most cellular RNAs, but the predominantly unspliced HSV-1 transcripts rely instead on the IE protein ICP27, encoded by UL54, to recruit the transcription–export (TREX) complex and the nucleoporin-interacting export receptor (TAP/NXF1) for transport into the cytoplasm (39). ICP27 is synthesized at the onset of the infection cycle and initially accumulates in the nucleus but at later times actively shuttles between the nucleus and cytoplasm, facilitating the transport of intron-less viral mRNAs (40). The contribution of ICP27 to HSV-1 replication is multifaceted, and, in addition to its export function, ICP27 modifies host and viral splicing patterns (41). When ICP27 is absent, many viral pre-mRNAs undergo patterns of splicing that are not seen with the wild-type (WT) virus, implying that ICP27 suppresses illegitimate alternative splicing (38). In addition to direct interactions with RNA, ALYREF, TAP/NXF1, and various splicing factors, ICP27 also binds to the carboxyl-terminal domain (CTD) of RNAPII regardless of its phosphorylation state (42, 43). This promotes the association of RNAPII with viral genomes in the viral replication compartments (vRCs), sites of viral DNA amplification. ICP27 expression is also required for disruption of transcription termination (DoTT) that predominantly impacts host genes producing unstable transcripts and transcription read-through into downstream genes (44).
Here, we show that HSV-1 infection leads to a wide-scale redistribution of the nuclear RNA-modification machinery. Multiple m6A factors, including METTL3, move from the nucleus into the cytoplasm, and this is accompanied by a global reduction in the installation of m6A on both viral and host polyadenylated RNAs. Redistribution increases as the HSV-1 replication cycle progresses, correlating with the reduced impact of depletion of methyltransferase subunits on viral gene expression. Importantly, redistribution of host m6A factors was not observed in cells infected with a viral mutant lacking functional ICP27, and expression of ICP27 alone was found to be sufficient to induce the cytoplasmic localization of METTL14. A screen of ICP27 mutant viruses identified the conserved CTD as critical for this activity. This is an example of viral effector protein that can bring about widespread changes to a major RNA-modification pathway and thereby globally reshape the landscape of m6A modifications on both viral and host-cell mRNAs.
Results
HSV-1 Infection Redistributes the Nuclear m6A Machinery.
The cellular m6A methyltransferase complex (Fig. 1A) is composed of three evolutionarily conserved core components—METTL3, METTL14, and WTAP—and a number of less-conserved accessory subunits including VIRMA, ZC3H13, RBM15, and RBM15B (1). To examine the impact of HSV-1 infection on the expression and localization of these important host proteins, normal human dermal fibroblasts (NHDFs) were infected at a sufficient multiplicity of infection (MOI) = 3 to ensure the presence of replicating virus in all cells. At 12 h after infection (hpi), cultures were either lysed in sodium dodecyl sulfate (SDS) sample buffer and probed by immunoblotting (Fig. 1B) or fixed and processed for indirect immunofluorescence microscopy (Fig. 1C). Immunoblotting showed that the overall abundance of five methyltransferase subunits was not detectably changed by HSV-1 infection after 3 to 12 hpi (Fig. 1B). As has been reported in other cell types, immunofluorescence analysis of uninfected NHDFs showed that all seven factors were predominantly localized as fine speckles in the nucleoplasm (Fig. 1C), although some signals generated by the METTL3, RBM15, RBM15B, and VIRMA antibodies were also detected in the cytoplasm (Fig. 1D). Unexpectedly, the nuclear signal for METTL3, METTL14, VIRMA, ZC3H13, RBM15, and RBM15B was greatly reduced in the HSV-1–infected cells with a corresponding increase in cytoplasmic signal (Fig. 1 C and D). Only the localization of WTAP appeared not to be changed by infection, which was confirmed using an antibody to the viral nuclear protein ICP8. The nuclear m6A demethylase ALKBH5, as well as the nuclear m6A reader YTHDC1, was similarly found to be redistributed into the cytoplasm of infected NHDFs (SI Appendix, Fig. S1). However, the localization of other factors involved in nuclear processes, such as transcription (RNAPII large subunit RBP1), pre-mRNA splicing (SC35/SRSF1), and nuclear poly(A) binding protein (PABP2), remained unchanged by HSV-1 infection (SI Appendix, Fig. S2). Thus, HSV-1 infection of primary fibroblasts results in a striking redistribution of nuclear m6A methyltransferase subunits and m6A-recognition proteins from the nucleus into the cytoplasm.
Increased Redistribution of METTL14 as the HSV-1 Replication Cycle Advances.
The HSV-1 productive replication cycle proceeds in a sequential fashion, with the initiation of viral DNA replication acting as a major transition point at which viral gene expression switches from the predominance of IE and E genes to the DNA replication–dependent L genes. To better understand the timing of this transition in NHDFs, we monitored the relative increase in viral genomic DNA using qPCR to amplify a segment of the UL44 gene (Fig. 2A). Although some increase in viral DNA content was evident at 6 hpi, the largest change occurred between 9 and 12 hpi but did not increase further at 15 hpi. No increase in viral DNA was detected in the presence of phosphonoacetic acid (PAA), a selective inhibitor of the viral DNA polymerase, confirming that the increase in detection of HSV-1 DNA was due to viral DNA replication. Next, we performed an infection time course and monitored the subcellular localization of METTL14 (Fig. 2B), which showed the clearest redistribution pattern of the m6A factors tested. A weak METTL14 signal was first detected in the cytoplasm after 6 h of infection, becoming more pronounced at 9 hpi and essentially complete by 12 hpi (Fig. 2B). Progression of the viral replication cycle was visualized by the nuclear accumulation of ICP8 and the formation of vRCs. Subcellular localization of METTL14 was also analyzed by nucleocytoplasmic fractionation and immunoblotting (Fig. 2C). Consistent with the imaging, the majority of METTL14 was present in the nuclear fraction of uninfected NHDFs but, after infection with HSV-1, could be more readily detected in the cytoplasm fraction from 6 hpi (lanes 6 and 8) becoming near complete by 12 hpi (lane 10). Cytoplasmic β-tubulin and nuclear histone H3 provide fractionation controls. Together, these results indicate that redistribution of nuclear METTL14 into the cytoplasm increases over time and tracks with the progression of the HSV-1 replication cycle.
Fig. 2.
METTL14 redistribution increases as the HSV-1 replication cycle progresses. (A) NHDFs were infected with HSV-1 (MOI = 3) either in the presence (+PAA) or absence (−PAA) of 300 µg/mL PAA. Total DNA was collected at 3, 6, 9, 12, and 15 hpi and analyzed by qPCR using primers to the HSV-1 UL44 gene and human ribosomal protein 19 (RPL19) gene for normalization. (B) An equivalent infection time course was processed for an indirect immunofluorescence assay using antibodies to METTL14 or viral ICP8. Nuclear DNA was visualized using DAPI, and the boundary between the nucleus and cytoplasm is indicated by a dashed line. Note the accumulation of ICP8 into discrete vRCs at 6 and 9 hpi. (C) Biochemical fractionation and immunoblotting of HSV-1–infected cells collected over a time course of infection. Cell-equivalent cytoplasmic and nuclear fractions were probed using antibodies to METTL14, cytoplasmic β-tubulin, and nuclear histone H3.
The occupancy of RNAPII on host genes undergoes a substantial reorganization during HSV-1 infection (35). Because m6A catalysis is cotranscriptional, we asked if inhibiting all transcription would elicit a similar redistribution of METTL14 (SI Appendix, Fig. S3A). Uninfected NHDFs were treated with 10 µM actinomycin D, a DNA intercalator that blocks initiation and elongation of RNA polymerase I, II, and III (45). As expected, this abolished the nuclear signal for RNAPII phospho-serine 5 (S5p), a marker for transcriptionally active RNAPII (46). In contrast, the subcellular distribution of METTL14 was indistinguishable from the mock-treated cells, indicating that global inhibition of transcription does not lead to the redistribution of an essential component of the m6A methyltransferase complex. We also tested the TFIIH inhibitor triptolide (47) and observed a similar loss of RNAPII activity without a discernable change in METTL14 distribution (SI Appendix, Fig. S3B). Thus, widespread disruption of RNAPII-mediated transcription during HSV-1 infection does not account for redistribution of nuclear m6A factors.
Redistribution of m6A Factors Requires Viral ICP27.
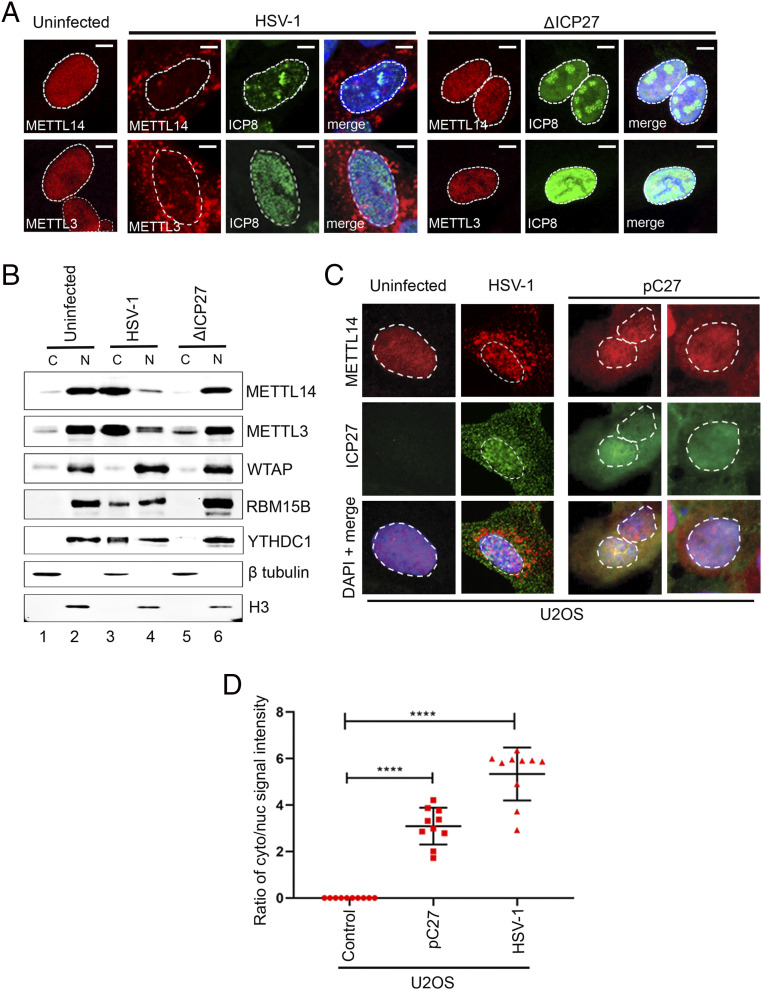
The HSV-1 IE protein ICP27 regulates multiple aspects of RNA processing, including alternative splicing (48), transcription termination (49), and 3′-end processing (44), and also physically interacts with the RNAPII CTD (43). These properties suggest that, within the nucleus, ICP27 will come into close proximity to the nuclear m6A machinery and could potentially influence its activity. Although ICP27 is expressed as an IE gene, it manifests different functions at different stages of the HSV-1 replication cycle. To directly examine the contribution of ICP27 to METTL3 and METTL14 redistribution, NHDFs were infected with either HSV-1 WT or a strain-matched mutant lacking the ICP27 coding region (ΔICP27) and processed for immunofluorescence at 12 hpi (Fig. 3A). Both METTL3 and METTL14 were redistributed into the cytoplasm by HSV-1 WT but not by ΔICP27. The mutant virus was transcriptionally active as demonstrated by the strong signal for the viral E protein ICP8. The inability of HSV-1 ΔICP27 to redistribute the nuclear m6A reader and eraser components into the cytoplasm was confirmed by biochemical fractionation and immunoblotting (Fig. 3B). Lastly, uninfected human U2OS osteosarcoma cells were transfected with a plasmid constitutively expressing ICP27 and probed by immunofluorescence. Expression of ICP27 on its own resulted in the redistribution of endogenous METTL14, although not to the degree observed in infected NHDFs (Fig. 3 C and D). This establishes that expression of ICP27 alone is sufficient to alter the subcellular localization of an essential component of the m6A nuclear methyltransferase. Furthermore, redistribution of METTL14 was not limited to primary fibroblasts but could also be observed in the transformed U2OS cell line.
Fig. 3.
ICP27 is necessary and sufficient to redistribute nuclear m6A factors. (A) NHDFs were infected with either HSV-1 WT or HSV-1 ΔICP27 viruses (both strain KOS, MOI = 3) and processed for indirect immunofluorescence analysis using antibodies to METTL14, METTL3, and HSV-1 ICP8 at 12 hpi. Nuclear DNA was stained with DAPI. (Scale bar, 10 μm.) (B) Nucleocytoplasmic fractionation of uninfected NHDFs or NHDFs infected with either HSV-1 WT or HSV-1 ΔICP27 and harvested at 12 hpi. Equivalent proportions of soluble cytoplasmic extract or nuclear lysate were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotted using antibodies to m6A methyltransferase subunits METTL14, METTL3, WTAP, and RBM15B or nuclear m6A RNA-binding protein YTHDC1. Antibodies to cytoplasmic β-actin and nuclear histone H3 serve as fractionation controls. (C) Indirect immunofluorescence analysis of human U2OS osteosarcoma cells transfected with an expression plasmid (pC27) encoding full-length HSV-1 ICP27. Two different fields are shown. Untransfected U2OS cells that were either mock-infected or infected with HSV-1 WT serve as controls. After fixation, cells were probed for antibodies against METTL14, and the nuclei were stained with DAPI; the nuclear-cytoplasmic boundary is indicated by dashed lines. (D) Quantitation of the cytoplasmic/nuclear METTL14 signal from the experiment shown in C. For each condition (n = 3), 100 cells were quantified, and each dot represents the average from 10 cells. Statistical significance was determined by Student’s t test, where ****P < 0.0001.
Functions Associated with the ICP27 Carboxyl Terminus Are Required for m6A Factor Redistribution.
The organization of functional domains within ICP27 has been studied extensively (38). The carboxyl terminus adopts a globular structure and shares both sequence and structural homology with regulators of RNA processing encoded by other herpes viruses and mediates homo-oligomerization. To identify the domains required for the redistribution of nuclear m6A machinery, we tested a selection of ICP27 mutant viruses (Fig. 4A). Of those impacting the N terminus, only ICP27dLeu, which removes the leucine-rich nuclear export signal (residues 5 to 15), prevented METTL14 redistribution (Fig. 4B). Interestingly, removal of the ALY/REF-binding sequence (ICP27d2-3) or the RNA-binding RGG box (ICP27d4-5) did not detectably prevent METTL14 redistribution (Fig. 4B), implying that disruption of the m6A pathway is distinct from the export of unspliced viral mRNAs. Deletions that removed all or part of the carboxyl terminus (n263 and n406, respectively) abolished METTL14 redistribution, suggesting that oligomer formation and/or carboxyl terminus–specific interactions are required. We also tested three single (ICP27M16) or double (ICP27M11 and ICP27M15) missense mutations with the ICP27 homology domain. All three mutants effectively abolished METTL14 redistribution. Immunoblotting (Fig. 4C) showed that expression of the E protein ICP8 was relatively unaffected except for ΔICP27 (lanes 3 and 10). As expected, the steady-state levels of true-late proteins, here represented by US11, were essentially undetectable in the absence of ICP27 expression (lanes 3 and 10) and greatly reduced in ICP27dLeu (lane 4), ICP27d4-5 (lane 7), and in all of the carboxyl-terminal mutants (lanes 11 to 15).
Fig. 4.
The globular carboxyl terminus of ICP27 is required for METTL14 redistribution. (A) Schematic depictions of the ICP27 mutants used in this study. Key functional domains are highlighted on the WT polypeptide, including the leucine-rich NES (residues 5 to 15), a sequence required for interaction with the ALY/REF export adaptor (residues 103 to 112), the arginine-rich RGG box (residues 138 to 152), and the globular CTD (residues 242 to 512), which shares extensive sequence homology with ICP27-like proteins encoded by other herpesviruses. The ability (+) or failure (−) of viruses expressing WT or mutant ICP27 to redistribute nuclear METTL14 into the cytoplasm is summarized. (B) Representative indirect immunofluorescence images showing the subcellular localization of METTL14 in NHDFs infected with HSV-1 (MOI of 3) expressing either WT or mutant versions of ICP27 and fixed at 15 hpi. (C) Immunoblots of cell lysates prepared at 15 hpi from NHDFs infected with WT or ICP27 mutant HSV-1 viruses and probed with antibodies to viral proteins ICP27 (IE), ICP8 (E), US11 (L), and to cellular GAPDH. In B and C, α-ICP27 antibody H1113 was used to detect ICP27dLeu because this mutant lacks the epitope for α-ICP27 antibody P1119.
As mentioned above, HSV-1 infection of fibroblasts results in widespread DoTT and abnormal 3′-end processing of pre-mRNAs (37, 44). This antihost activity requires ICP27, which physically interacts with the host 3′ processing factor cleavage and polyadenylation specificity factor (CPSF). Indeed, three of the ICP27 CTD mutants we tested (ICP27M15, ICP27M16, and ICP27n406), when expressed as glutathione S-transferase fusions, fail to coprecipitate CPSF subunits FIP1L1 and CPSF3/CPSF73 (44). To ask whether ICP27-mediated DoTT and redistribution of nuclear m6A factors are facets of the same process, we used siRNA to deplete three essential CPSF subunits, FIP1L1, CPSF3/CPSF73, and CPSF4/CPSF30. The knockdown efficiency of two different siRNAs for each factor was assessed as the reduction in the corresponding mRNA and was more complete for CPSF3 and CPSF4 (SI Appendix, Fig. S4A). When the localization of METTL14 was examined by indirect immunofluorescence of siRNA-treated cells, we found no obvious difference compared to the nonsilencing siRNA control (SI Appendix, Fig. S4B). Likewise, when siRNA-treated cells were subsequently infected with HSV-1 WT, we still observed a strong redistribution of the METTL14 signal into the cytoplasm irrespective of the knockdowns (SI Appendix, Fig. S4C). Nuclear accumulation of ICP27 was also unchanged by the depletions. These results show that, despite the overlap in domain requirements, redistribution of nuclear m6A factors is not phenocopied by the depletion of key CPSF subunits and that inactivation of CPSF does not prevent ICP27-mediated redistribution of METTL14 into the cytoplasm.
WTAP and ZC3H13 are thought to anchor other components of the m6A methyltransferase complex within the nucleus and specifically to nuclear speckles (13, 50). Depletion of ZC3H13 in murine ES cells results in the redistribution of METTL3/METTL14 and other methyltransferase complex components from the nucleoplasm, even though subcomplexes of WTAP-METTL3 and METTL14 or WTAP-HAKAI and VIRMA remained intact (51). To test whether this also applies in NHDFs and might recapitulate the activity of ICP27, we individually depleted WTAP or ZC3H13 (SI Appendix, Fig. S5); however, individual depletion of either factor resulted in a discernable change in METTL14 localization. This indicates that redistribution of nuclear m6A factors in HSV-1–infected cells does not simply reflect disassembly of the methyltransferase complex or disassociation from nuclear speckles but a more active relocalization function conferred by ICP27 itself.
ICP27-Dependent Suppression of m6A and Other RNA Modifications.
To detect changes in the distribution and relative abundance of RNA modifications on viral and host mRNAs in HSV-1–infected cells, we performed Nanopore direct RNA sequencing (dRNA-Seq) using total RNA isolated from NHDFs infected with either HSV-1 WT or HSV-1 ΔICP27 and harvested at 3, 6, and 12 hpi (Fig. 5). In this long-read sequencing approach, individual polyadenylated RNA molecules are drawn through membrane-embedded nanopores. Changes in electrical current across individual pores are measured, and any deviations in the signal can be used to infer the sequence of that individual RNA (52). Changes in the abundance of a given RNA modification manifests as change in the base-call error rate around the modified base. An increased error rate in a control (modification present) versus treatment (modification depleted/absent) analysis can be marked by a statistically significant G-test score and an odds ratio >1.5 as is calculated by the DRUMMER software package (53) (Fig. 5A). Conversely, a statistically significant G-test score and an odds ratio <0.667 would reflect the presence/accumulation of a modification in the treatment sample relative to the control. Thus, comparison of modified and unmodified versions of the same RNA sequence identifies bases that are putatively chemically modified.
Fig. 5.
Viral expression of ICP27 results in a global loss of m6A and other RNA modifications. (A) Schematic showing the RNA samples and comparisons used in this analysis. In addition to RNA isolated from HSV-1–infected NHDFs, a synthetic transcript devoid of base modifications corresponding to the first 1,500 nucleotides of the major ACGT1 mRNA isoform was generated by IVT. Hypothetical modifications are shown as blue- or red-filled lollipops that produce base-call errors during dRNA-Seq and can be detected and mapped using the DRUMMER software. (B) Graphical summary of a DRUMMER analysis using dRNA-Seq data for the predominant isoform of human γ-actin (ACTG1) mRNA from HSV-1 WT–infected NHDFs collected at 3, 6, and 12 hpi and compared to reads from the IVT transcript. Dots indicate the positions of candidate modifications. Sites that are depleted are shown as red dots (negative G-test score), and sites that are enriched (positive G-test score) are shown as blue dots. (C) A similar analysis to B, comparing ACGT1 in NHDFs infected with either HSV-1 WT or HSV-1 ΔICP27 at 3, 6, or 12 hpi. (D) DRUMMER analysis of HSV-1 polyadenylated RNAs mapped against the 152-kbp HSV-1 genome. Read depth or dRNA-Seq coverage is shown in gray (HSV-1 WT) or black (HSV-1 ΔICP27). Boundaries of the gene-rich unique long (UL) and unique short (US) regions, gene-poor internal repeats (IRL and IRS), and the terminal repeat sequences (TRL and TRS) are indicated. Dots represent candidate RNA-modification sites that are either depleted (red) or enhanced (blue) in HSV-1 WT compared to HSV-1 ΔICP27, with the darker colors referring to mRNAs that align to the top strand of the genome and the lighter colors to the bottom strand. Distance above or below the central line indicates the G-test score, a proxy for the degree of change as detected by DRUMMER. The inset shows the sequence motif with the highest P value identified by Hypergeometric Optimization of Motif EnRichment (HOMER) analysis of the 1,455 candidate modification sites that are depleted in cells infected with HSV-1 WT compared to HSV-1 ΔICP27. A black line highlights the five bases that match the consensus RRACH m6A motif.
To understand the impact of HSV-1 infection on m6A modification of a cellular transcript, we assembled reads corresponding to the human cytoplasmic γ-actin mRNA (ACTG1), which has been shown to contain m6A at multiple sites (54). Through comparison of ACTG1 from HSV-1 WT–infected cells with an unmodified version of the first 1,500 nucleotides generated by in vitro transcription (IVT) from a PCR-amplified template, we identified a number of candidate modifications that were depleted (red dots) and distributed broadly across the ACTG1 mRNA that were effectively absent in the IVT sample (Fig. 5B). When we compared the HSV-1 WT– and ΔICP27-infected samples, there were few detectable differences at 3 hpi, but this increased at 6 and 12 hpi (Fig. 5C). The majority of the changes corresponded to candidate modifications that were depleted in the WT infection (red dots). Lastly, we compared base-call error frequencies across the HSV-1 transcriptome using RNA from NHDFs infected with WT and ΔICP27 viruses and harvested at 12 hpi (Fig. 5C and Dataset S1). Overall, the transcriptome profiles of the two viruses were very similar (see Fig. 5D, dRNA-Seq coverage plot), except that the abundance of polyadenylated RNAs generated by the mutant virus at this time point is greatly reduced. Nonetheless, we achieved sufficient read depth to perform a DRUMMER analysis and identified 1,455 candidate modifications that were depleted in the WT sample. A further 185 candidates appeared to be enriched, although the G-test scores were low, suggesting the difference between WT and ΔICP27 at these sites was relatively small. A sequence motif analysis using the 1,455 depleted candidates identified a handful of motifs, of which the top-ranked (P value = 1 × 10−25) matched the m6A RRACH consensus sequence, which was present at 19% of the candidate sites. No sequence motifs were enriched in association with the accumulation sites. In summary, this analysis demonstrates that expression of ICP27 brings about widespread and time-dependent changes in the viral and host epitranscriptomes, broadly impacting the installation of m6A but also affecting other less well-characterized internal RNA modifications.
The m6A Pathway Is Important for HSV-1 Gene Expression at the Beginning of the Replication Cycle, Becoming Dispensable Later.
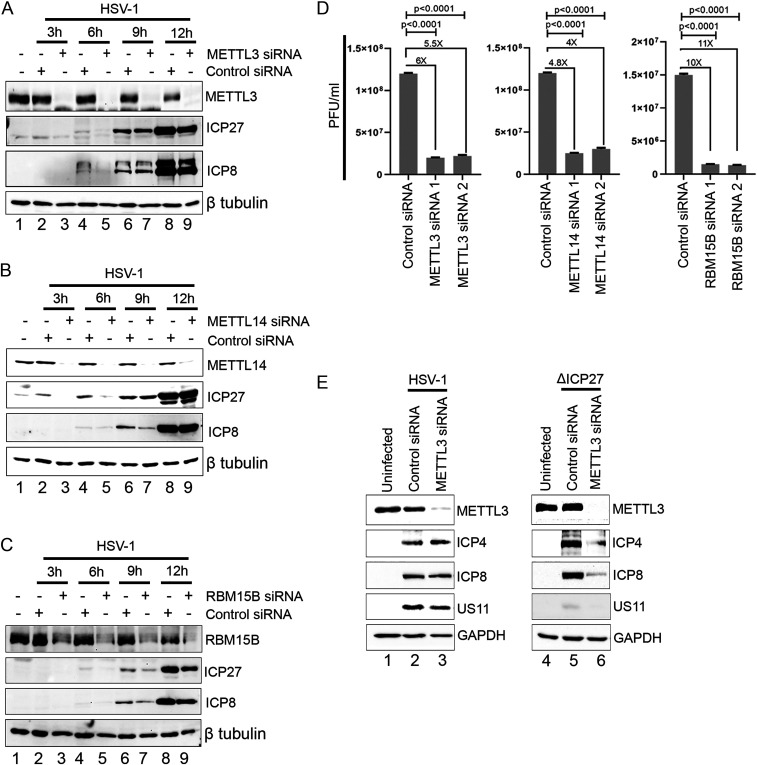
In an earlier study, we did not observe a significant impact on HSV-1 infectious virus production after depleting METTL3 or ALKBH5 in NHDFs (24). However, the impact of depleting METTL3, METTL14, or RBM15B on the accumulation of viral proteins over time was not investigated. To address this, NHDFs were siRNA treated for 72 h, infected with HSV-1 WT, and cell lysates prepared at 3, 6, 9, and 12 hpi (Fig. 6). Depletion of METTL3 (Fig. 6A), METTL14 (Fig. 6B), and RBM15B (Fig. 6C) resulted in a clear reduction in steady-state accumulation of ICP27 (IE) and ICP8 (E) at 3 and 6 hpi (lanes 3 and 5) compared to a nonsilencing control (lanes 2 and 4), and this difference was less apparent at 9 or 12 hpi (lanes 6 and 8 compared to lanes 7 and 9). This indicates that m6A is important for viral gene expression during the first 6 h of the replication cycle, when the nuclear m6A machinery is predominantly nuclear, but becomes dispensable later, when m6A installation is diminished. To determine whether this difference in viral protein synthesis had any impact on overall viral reproduction, we depleted METTL3, METTL14, and RBM15B using two different siRNAs for each factor and determined the titer of infectious viruses at 48 hpi (Fig. 6D). Whereas depletion of METTL3 or METTL14 resulted in modest yet reproducible four- to sixfold reduction, virus yield was reduced 11-fold when RBM15B was depleted. Finally, we examined the impact of METTL3 depletion on viral protein levels at 15 hpi after infection with either HSV-1 WT or ΔICP27 (Fig. 6E). The reduction in METTL3 resulted in almost no change in the steady-state levels of ICP4 (IE), ICP8 (E), and US11 (true-late) in HSV-1 WT–infected cells (compare lanes 2 and 3) but produced a clear decrease in all three gene products in the HSV-1 ΔICP27 infection (compare lanes 5 and 6). This implies that viral gene expression is more dependent on the m6A pathway when ICP27 is absent.
Fig. 6.
Depletion of m6A methyltransferase subunits preferentially impacts viral gene expression at the beginning of the HSV-1 infection cycle. (A). NHDFs were transfected with single siRNAs against METTL3 or a nonsilencing control siRNA and subsequently infected with HSV-1. Cell lysates were prepared at 3, 6, 9, or 12 hpi and analyzed by immunoblotting with antibodies to METTL3, ICP27, ICP8, and β-tubulin. (B) As in A, except that METTL14 was targeted for depletion. (C) As in A, except that RBM15B was targeted for depletion. (D) NHDFs were treated with control siRNA or two different siRNAs targeting METTL3, METTL14, or RBM15B for 72 h before infection with HSV-1 WT at MOI = 0.0001. After a further 48 h, the output of new infectious viruses was determined by plaque assay. Statistical significance (n = 3) was determined by Student’s t test, where ****P < 0.0001. (E) Control siRNA– and METTL3 siRNA–treated NHDFs were infected (MOI = 3) with either HSV-1 WT (Left) or HSV-1 ΔICP27 (Right), and lysates were prepared at 15 hpi and immunoblotted with antibodies to cellular METTL3 and GAPDH and viral ICP4, ICP27, and US11. Note that longer exposures of the ICP4, ICP8, and US11 blots were required for the ΔICP27 samples (lanes 4 to 6) due to the overall reduction in viral gene expression of the mutant.
Discussion
During HSV-1 infection, ICP27 and other viral proteins, including the endonuclease vhs, enforce a profound blockade of host gene expression known as “host shutoff.” This helps the virus to monopolize the transcription and translation capabilities of the infected host cell and limits expression of antiviral defense factors (29). Here, we describe an unexpected function of ICP27 in orchestrating the dismantling of a major internal RNA-modification pathway that potentially impacts the biogenesis, stability, and function of numerous cellular mRNAs and noncoding RNAs. Further studies are needed to fully dissect the mechanism and consequences, but our data suggest that the loss of functional m6A methyltransferase complexes in the nucleus is a pivotal step. Although it is well established that m6A is added cotranscriptionally to pre-mRNAs, there is some uncertainty whether the methyltransferase is physically associated with RNAPII itself or with the underlying chromatin template. Nevertheless, the interaction of ICP27 with either the RNAPII CTD or the nascent ribonucleoprotein (RNP) complexes could destabilize this interaction and prevent catalysis. It is notable that redistribution of nuclear m6A factors did not occur when transcription was inhibited using either actinomycin D or triptolide in uninfected cells, indicating that redistribution is not simply a consequence of the global reduction in RNAPII-dependent transcription associated with HSV-1 infection. Likewise, inactivation of the CPSF complex—another direct target of ICP27—does not induce the redistribution of nuclear m6A factors, nor does it prevent redistribution during infection with WT virus, suggesting these two processes are distinct.
The diversity of functions attributed to ICP27 can seem perplexing but ultimately involve either direct or indirect subversion of key steps in mRNA processing and export (39). The ability of ICP27 mutants lacking residues required for interaction with ALY/REF (ICP27d2-3) or RNA (ICP27d4-5) to redistribute METTL14 implies that disruption of the m6A pathway can be uncoupled from the RNA export function. In contrast, removal of the N-terminal nuclear export signal (NES) (ICP27dLeu) appears to be critical as does loss of the CTD (ICP27n263 and ICP27n406), which mediates higher-order oligomerization and interacts with other cellular proteins including components of the splicing machinery.
It is noteworthy that all of the m6A methyltransferase subunits, with the exception of WTAP, are redistributed into the cytoplasm of HSV-1–infected cells. Depletion studies in HeLa cells have shown that WTAP is required for the localization of METTL3 and METTL14 to SC35-positive nuclear speckles and is required to maintain overall levels of m6A on polyadenylated RNAs (13). Functional nuclear-localization signals have been identified in both METTL3 and WTAP, suggesting they are imported into the nucleus independently. Because METTL3 readily establishes a stable interaction with METTL14, it is likely these core subunits are imported as a preassembled heterodimer (55). In vitro binding studies indicate that that the N-terminal coiled-coil domain of WTAP interacts with the N-terminal leader helix of METTL3. Further independent interactions between WTAP and other subunits of the m6A methyltransferase holoenzyme, including METTL14, have not been reported, although these may exist in the context of the full complex and substrate RNA (51).
As with other IE proteins, the subcellular movements of ICP27 change during the course of the infection cycle. Nucleocytoplasmic shuttling is not initiated until a few hours into the infection cycle and is mediated by direct interaction with the TAP/NXF1 export receptor. Earlier studies (56) showed that the interaction with TAP/NXF1 is disrupted by deletion of the N-terminal NES (ICP27dLeu) and by the carboxyl-terminal truncation (ICP27n406), both of which, as we have shown here, also prevent cytoplasmic redistribution of METTL14. Moreover, the carboxyl-terminal ICP27 mutants ICP27M11, ICP27M15, and ICP27M16, which fail to redistribute METTL14, are either deficient or abrogated in shuttling (40). ICP27dLeu, ICP27M15, and ICP27M16 also prevent interaction with the core nucleoporin Nup62, raising the possibility that importin-dependent reimport of factors cycling in and out of the nucleus could also be blocked by ICP27 (57). The properties of these mutants point to a role for the shuttling function of ICP27 in redistribution of nuclear m6A factors, but, most likely, this does not involve RNA as a bridging factor because METTL14 redistribution is insensitive to deletion of the RNA-binding RGG box (ICP27d4-5). One complicating factor, however, is that ICP27 exists as a head-to-tail oligomer mediated by the same N- and carboxyl-terminal regions (58, 59). As such, it may be that this intramolecular configuration, rather than the direct associations with the export machinery, is required for ICP27 to disperse the nuclear m6A machinery. Additional mutagenesis studies combined with siRNA-mediated depletion of factors involved in nucleocytoplasmic export will distinguish between these possibilities.
In conclusion, we have shown that HSV-1 infection has a strong antagonistic impact on the activity of a major internal RNA-modification pathway, and we have identified ICP27, an essential regulator of RNA processing and export, as the viral activity responsible. In addition to m6A, our dRNA-Seq analysis indicates that other RNA modifications might also be affected, but these are more challenging to characterize at present. In light of the different roles of m6A in regulating key steps in mRNA maturation, stability, and translation, it is conceivable that this function of ICP27 deregulates the expression of host genes that escape suppression by other processes. Although m6A is present on many HSV-1 mRNAs and appears to be important for viral gene expression in the first few hours of infection, it becomes less important over time and is therefore susceptible to disruption by this virus.
Materials and Methods
Cells and Viruses.
Full details of the sources and culture conditions for the human primary dermal fibroblasts and immortalized cell lines used in this study, along with the sources of the HSV-1 viruses and methodologies used to amplify and determine the infectious titer of viral stocks used for the infection studies, are included in SI Appendix, SI Materials and Methods.
Indirect Immunofluorescence Imaging.
The methodology used to fix, probe, and image uninfected and infected cells is detailed in full in SI Appendix, SI Materials and Methods.
Small-Molecule Inhibition and RNA Interference–Depletion Studies.
Specific details of the chemical inhibitors used to block transcription or HSV-1 DNA replication, along with the methodology used for RNA interference–mediated depletion of host proteins, are provided in SI Appendix, SI Materials and Methods.
RNA, DNA, and Protein Analyses.
The methodologies used for quantitative measurements of viral or host proteins by immunoblotting and nucleic acids by qPCR, along with the details of the specific antibodies and oligonucleotide primers used in this study, are provided in SI Appendix, SI Materials and Methods.
Nanopore dRNA-Seq and RNA-Modification Detection.
In short, sequencing libraries were prepared from poly(A) RNA and used for dRNA-Seq on the Oxford Nanopore Technologies MinION platform. Likely sites of RNA modification were identified and mapped onto the HSV-1 and human transcriptomes using a comparative profiling approach that we have developed called DRUMMER. Full details of library preparation, long-read sequencing, base calling, and comparative profiling of direct RNA-Sequencing datasets using the DRUMMER software are included in SI Appendix, SI Materials and Methods.
Statistical Analyses.
Statistical data are presented as the mean ± SEM as described in the corresponding figure legends, and statistical significance was determined using Student’s t test with Prism 8 (GraphPad Software). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to members of the I.M./A.C.W. laboratory for their input during the course of these studies and to Yan Deng from the New York University Langone Microscopy Laboratory (RRID: SCR_017934), a core facility partially supported by Cancer Center Support Grant P30CA016087 at the Laura and Isaac Perlmutter Cancer Center. J.S.A. was supported by the Vilcek Institute of Graduate Biomedical Sciences. This work was supported by NIH Grants AI073898 and GM056927 (I.M.), AI152543-01 (I.M. and D.P.D.), AI130618 and AI147163 (A.C.W.), and AI151617 (S.A.R.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2104805118/-/DCSupplemental.
Data Availability
All sequencing datasets associated with this study are deposited in Nanopore fast5 format at the European Nucleotide Archive under the project accession no. PRJEB43558. All other study data are included in the manuscript and/or supporting information.
References
- 1.He P. C., He C., m6A RNA methylation: From mechanisms to therapeutic potential. EMBO J. 40, e105977 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaccara S., Jaffrey S. R., A unified model for the function of YTHDF proteins in regulating m6A-modified mRNA. Cell 181, 1582–1595.e18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., et al., m6A modification prevents formation of endogenous double-stranded RNAs and deleterious innate immune responses during hematopoietic development. Immunity 52, 1007–1021.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-González B., Aguilera A., Looping the (R) loop in DSB repair via RNA methylation. Mol. Cell 79, 361–362 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Ries R. J., et al., m6A enhances the phase separation potential of mRNA. Nature 571, 424–428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer K. D., et al., Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominissini D., et al., Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Schwartz S., et al., Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 8, 284–296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slobodin B., et al., Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell 169, 326–337.e12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke S., et al., A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 29, 2037–2053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer S., Lavi U., Darnell J. E. Jr., The absolute frequency of labeled N-6-methyladenosine in HeLa cell messenger RNA decreases with label time. J. Mol. Biol. 124, 487–499 (1978). [DOI] [PubMed] [Google Scholar]
- 12.Liu J., et al., A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ping X.-L., et al., Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Śledź P., Jinek M., Structural insights into the molecular mechanism of the m(6)A writer complex. eLife 5, 352 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P., Doxtader K. A., Nam Y., Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell 63, 306–317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geula S., et al., Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Guo J., Tang H.-W., Li J., Perrimon N., Yan D., Xio is a component of the Drosophila sex determination pathway and RNA N6-methyladenosine methyltransferase complex. Proc. Natl. Acad. Sci. U.S.A. 115, 3674–3679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue Y., et al., VIRMA mediates preferential m6A mRNA methylation in 3'UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 4, 10–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akichika S., et al., Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363, eaav0080 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Zheng G., et al., ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao W., et al., Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Patil D. P., Pickering B. F., Jaffrey S. R., Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 28, 113–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss B., Gershowitz A., Stringer J. R., Holland L. E., Wagner E. K., 5′-Terminal and internal methylated nucleosides in herpes simplex virus type 1 mRNA. J. Virol. 23, 234–239 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio R. M., Depledge D. P., Bianco C., Thompson L., Mohr I., RNA m6 A modification enzymes shape innate responses to DNA by regulating interferon β. Genes Dev. 32, 1472–1484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price A. M., et al., Direct RNA sequencing reveals m6A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 11, 10.1038/s41467-020-19787-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler R., et al., m6A modification controls the innate immune response to infection by targeting type I interferons. Nat. Immunol. 20, 173–182 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Zheng Q., Hou J., Zhou Y., Li Z., Cao X., The RNA helicase DDX46 inhibits innate immunity by entrapping m6A-demethylated antiviral transcripts in the nucleus. Nat. Immunol. 18, 1094–1103 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Lichinchi G., et al., Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20, 666–673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dremel S. E., DeLuca N. A., Herpes simplex viral nucleoprotein creates a competitive transcriptional environment facilitating robust viral transcription and host shut off. eLife 8, 2503 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roizman B., Borman G. S., Rousta M. K., Macromolecular synthesis in cells infected with herpes simplex virus. Nature 206, 1374–1375 (1965). [DOI] [PubMed] [Google Scholar]
- 31.Kwong A. D., Frenkel N., Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. U.S.A. 84, 1926–1930 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oroskar A. A., Read G. S., Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63, 1897–1906 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dauber B., Saffran H. A., Smiley J. R., The herpes simplex virus host shutoff (vhs) RNase limits accumulation of double stranded RNA in infected cells: Evidence for accelerated decay of duplex RNA. PLoS Pathog. 15, e1008111 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrisch R. G., Eidem T. M., Yakovchuk P., Kugel J. F., Goodrich J. A., Infection by herpes simplex virus type-1 causes near-complete loss of RNA polymerase II occupancy on the host cell genome. J. Virol. 90, 2503–2513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birkenheuer C. H., Danko C. G., Baines J. D., Herpes simplex virus 1 dramatically alters loading and positioning of RNA polymerase II on host genes early in infection. J. Virol. 92, e02184-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer C. A., Dahmus M. E., Rice S. A., Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71, 2031–2040 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennig T., et al., HSV-1-induced disruption of transcription termination resembles a cellular stress response but selectively increases chromatin accessibility downstream of genes. PLoS Pathog. 14, e1006954 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang S., Patel A., Krause P. R., Hidden regulation of herpes simplex virus 1 pre-mRNA splicing and polyadenylation by virally encoded immediate early gene ICP27. PLoS Pathog. 15, e1007884 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandri-Goldin R. M., The many roles of the highly interactive HSV protein ICP27, a key regulator of infection. Future Microbiol. 6, 1261–1277 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Mears W. E., Rice S. A., The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology 242, 128–137 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Sciabica K. S., Dai Q. J., Sandri-Goldin R. M., ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 22, 1608–1619 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou C., Knipe D. M., Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J. Virol. 76, 5893–5904 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai-Ju J. Q., Li L., Johnson L. A., Sandri-Goldin R. M., ICP27 interacts with the C-terminal domain of RNA polymerase II and facilitates its recruitment to herpes simplex virus 1 transcription sites, where it undergoes proteasomal degradation during infection. J. Virol. 80, 3567–3581 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., et al., Herpes simplex virus blocks host transcription termination via the bimodal activities of ICP27. Nat. Commun. 11, 293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensaude O., Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription 2, 103–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buratowski S., Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Titov D. V., et al., XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 7, 182–188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang S., Patel A., Krause P. R., Herpes simplex virus ICP27 regulates alternative pre-mRNA polyadenylation and splicing in a sequence-dependent manner. Proc. Natl. Acad. Sci. U.S.A. 113, 12256–12261 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rutkowski A. J., et al., Widespread disruption of host transcription termination in HSV-1 infection. Nat. Commun. 6, 7126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horiuchi K., et al., Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 288, 33292–33302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen J., et al., Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 69, 1028–1038.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garalde D. R., et al., Highly parallel direct RNA sequencing on an array of nanopores. Nat. Methods 15, 201–206 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Price A. M., et al., Direct RNA sequencing reveals m6A modifications on adenovirus RNA are necessary for efficient splicing. Nat. Commun. 11, 6016–6017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz S., et al., High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155, 1409–1421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schöller E., et al., Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA 24, 499–512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson L. A., Sandri-Goldin R. M., Efficient nuclear export of herpes simplex virus 1 transcripts requires both RNA binding by ICP27 and ICP27 interaction with TAP/NXF1. J. Virol. 83, 1184–1192 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malik P., et al., Herpes simplex virus ICP27 protein directly interacts with the nuclear pore complex through Nup62, inhibiting host nucleocytoplasmic transport pathways. J. Biol. Chem. 287, 12277–12292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernandez F. P., Sandri-Goldin R. M., Herpes simplex virus 1 regulatory protein ICP27 undergoes a head-to-tail intramolecular interaction. J. Virol. 84, 4124–4135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tunnicliffe R. B., et al., The structure of the folded domain from the signature multifunctional protein ICP27 from herpes simplex virus-1 reveals an intertwined dimer. Sci. Rep. 5, 11234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing datasets associated with this study are deposited in Nanopore fast5 format at the European Nucleotide Archive under the project accession no. PRJEB43558. All other study data are included in the manuscript and/or supporting information.