Abstract
Background and Objectives:
Minimally invasive surgery for renal masses is complex and relies on two-dimensional (2D) computer tomography (CT) and magnetic resonance imaging (MRI) scans for surgical planning. We sought to determine if three-dimensional (3D) virtual reality (VR) models generated from imaging of patients undergoing robotic partial nephrectomy influenced presurgical planning approaches when compared to routine planning.
Methods:
The initial 15 patients underwent robotic assisted laparoscopic partial nephrectomy performed by one urologic surgeon. All patients pre-operatively underwent a CT and/or MRI scan. A pre-operative surgical plan was then recorded. 3D VR models were generated from these scans and reviewed. A second surgical plan was developed based on the 3D VR images. A comparison was made between the two studies prior to surgical intervention. All final surgical plans were implemented based on the 3D VR imaging studies.
Results:
Six surgical approaches were changed based on the 3D VR images. Two surgical approaches were changed from a transperitoneal to a retroperitoneal approach and two from a retroperitoneal to a transperitoneal approach. Two patients had distinctive renal vasculature related to the renal cancers which were not appreciated on routine scans but were well delineated by VR imaging studies. As a result, the surgical approach for two patients was altered to accommodate the new findings.
Conclusion:
Operative planning is paramount when performing robotic partial nephrectomy and developing a 3D surgical approach from 2D imaging can be difficult. Three-dimensional VR models affords the surgeon a 3D view prior to and during surgery and can ensure the selection of the appropriate surgical approach.
Keywords: Carcinoma, Renal cell, Virtual reality, Surgical procedures, Robotic, Surgical planning
INTRODUCTION
Kidney cancer affected over 73,000 individuals in the U.S. in 2019.1 Of these, more than 2 in 3 cases remain localized to the kidney. The primary treatment for renal cancer today remains surgical intervention, either open, laparoscopic, or robotic operations. Based on the American Urological Association guidelines and outcomes of partial versus radical nephrectomies, the number of partial nephrectomies performed in the U.S. for small, organ-confined lesions is increasing each year.2,3
Historically, urologists perform surgical planning by reconstruction of magnetic resonance imaging (MRI) and computerized tomography (CT) scans into a mental 3-dimensional (3D) image. Visualizing 3D objects from 2-dimensional (2D) cross sectional images can create mental errors when attempting to translate these images into in-situ situations.4,5 In addition, spatial relationships can be difficult to interpret. Three-dimensional printed renal cancer models have been shown to influence surgical decision making for robotic partial nephrectomies.6 However, total 3D printing time and material costs range from 29 to 55 hours and approximately 700 – 1700 US dollars per case.7 Surgical outcomes of robotic partial nephrectomy are improved with respect to operative time, warm ischemic time, estimated blood loss, and patient length-of-stay when using 3D virtual reality (VR) models as opposed to using standard pre-operative imaging.8 The reasons for these improvements are likely related to better surgeon understanding of patient anatomy from the 3D VR models, but also from the changes in surgical planning implemented as a result of this understanding.
In this context, we identified an initial 15 patients undergoing robotic assisted partial nephrectomies performed by a single surgeon and using 3D VR models generated from their CT or MRI scans for surgical planning. We sought to determine the number and characteristics of the changes made by the surgeon based on review of the 3D VR models.
MATERIALS AND METHODS
A prospective, single arm, single site, single surgeon, longitudinal study was created with patients at the time of consultation and scheduling for robotic partial nephrectomy. This pilot study was exempt from institutional review board oversight. This is a review of our first 15 patients.
The initial operative plan was recorded in an Excel spreadsheet that included the patient’s identifying number, age, sex, and body mass index (BMI). The CT and/or MRI scan(s) were then reviewed for the laterality of the tumor, location (anterior, posterior, or lateral), approximate size and number of renal arteries. A final determination of the surgical approach (transperitoneal or retroperitoneal) was completed at that time.
The 3D VR models were then created from the scans by the surgical planning company (Ceevra, Inc). Once the 3D models were created, usually within three to four business days, the images were viewed using the surgeon’s smartphone and an off the shelf VR headset. A second surgical plan was then devised and also documented in the spreadsheet. Those changes in the surgical approach performed are noted in Table 3. The operation proceeded following the second round of surgical planning using the second plan developed from the 3D VR model.
These plans were compared on three key surgical parameters: if the surgical approach changed based on the 3D VR images, if the renal vasculature was similar between standard and 3D VR imaging; and when the surgical plan changed based on the 3D VR models, if the surgeon agreed with those changes after the operation was complete.
The surgical procedure was performed robotically via a transperitoneal or retroperitoneal approach. The patient was placed in the lateral position for the retroperitoneal approach or semilateral for the transperitoneal approach. Bulldog clamps were used for arterial and in some cases venous clamping, and warm ischemic time was monitored as per standard of care. Renorrhaphy was performed in a 2-layer closure with some exceptions, with the use of 3-0 V-loc and 2-0 vicryl sutures. A Jackson-Pratt drain was placed at the end of each case.
Prospectively collected data included baseline patient sex, age, and BMI, and surgical data (tumor size, location, and nephrometry score).9–11 Survey data regarding changes made after review of the 3D VR model and understanding of the vascular anatomy were collected for each case. An additional survey question was used in cases where the surgical plan was changed due to the 3D VR imaging (Supplement).
As an analytic step, we compared baseline characteristics between cases where the surgical plan was changed after review of the 3D VR imaging versus those where the plan remained unchanged. All statistical tests were 2-sided and carried out at the 5% significance level.
RESULTS
Our first 15 patients utilizing 3D VR models were consecutively entered into a prospective study by a single surgeon at Advent Health Celebration Hospital from September 1, 2019 through January 31, 2020. Baseline characteristics are shown in Table 1. Patients were predominately male (67%), obese (average BMI 34), and had a mass on the left kidney (67%). Masses were complex, with a mean nephrometry score of 7.3. Fourteen patients completed successful robotic assisted laparoscopic partial nephrectomy. One patient had prior microwave thermotherapy and tumor recurrence greater than 6.5 cm in size. A robotic radical nephrectomy was performed due to the size of the mass and difficulty in distinguishing surgical planes. In six cases, the surgical plan was changed based on the 3D VR images. There were no differences in baseline or surgical characteristics between cases in which the surgical plan was modified and in those where it was not (P < .05) (Table 2).
Table 1.
Baseline Characteristics of Patients
| Patient Number | Gender | Location | Body Mass Index | Nephrometry Score | T Stage | Margin Status | Pathology |
|---|---|---|---|---|---|---|---|
| 1 | F | R | 35 | 4a | T1b | N | ccRCC |
| 2 | F | L | 23 | 9x | T1a | N | ccRCC |
| 3 | M | L | 33 | 6a | T1a | N | ccRCC |
| 4 | F | R | 25 | 10ah | T1a | N | Chromo |
| 5 | M | L | 30 | 7p | T1b | N | ccRCC |
| 6 | F | L | 32 | 9p | T1a | N | ccRCC |
| 7 | M | L | 27 | 8p | T1b | N | ccRCC |
| 8 | M | L | 37 | 4p | T1a | N | ccRCC |
| 9 | M | R | 34 | 8a | T1a | N | ccRCC |
| 10 | M | R | 38 | 7p | T1a | N | ccRCC |
| 11 | M | L | 30 | 6a | T1a | N | Muc |
| 12 | M | L | 26 | 7a | T3a | N | Pap |
| 13 | F | L | 62 | 8a | T1a | N | ccRCC |
| 14 | M | R | 47 | 9a | T3a | N | ccRCC |
| 15 | M | L | 35 | 7p | T1a | N | ccRCC |
F, female; M, male; N, negative; P, positive; ccRCC, clear cell renal cell carcinoma; Chromo, chromophobe renal cell carcinoma, Pap, papillary renal cell carcinoma; Muc, mucinous tubular and spindle cell renal cell carcinoma.
Table 2.
Comparing Patients with Changes in Surgical Plan after Reviewing Three-dimensional Virtual Reality Model to Patients Without Changes in Surgical Plan
| No Change in Plan (n = 9) | Change in Plan (n = 6) | P | |
|---|---|---|---|
| Sex | |||
| Male | 78% (7) | 50% (3) | 0.6066* |
| Female | 22% (2) | 50% (3) | |
| Body mass index, mean (SD) | 35.56 (10.7) | 29.4 (8.8) | 0.2625 |
| Laterality | |||
| Right | 33% (3) | 33% (2) | 1.0000* |
| Left | 67% (6) | 67% (4) | |
| Nephrometry score, mean (SD) | 6.8 (1.3) | 8.0 (2.2) | 0.2842 |
| T Stage | |||
| T1a | 66% (6) | 66% (4) | 0.9999* |
| T1b | 22% (2) | 17% (1) | |
| T3b | 11% (1) | 17% (1) |
*, Fisher’s exact test; SD, standard deviation.
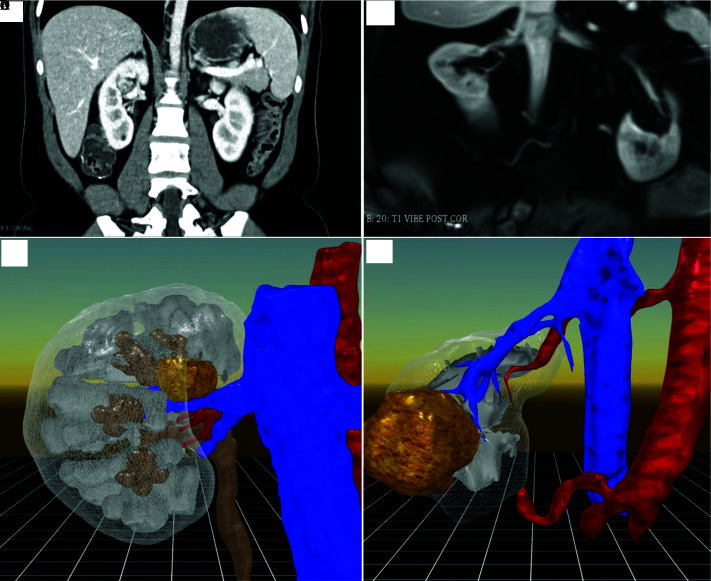
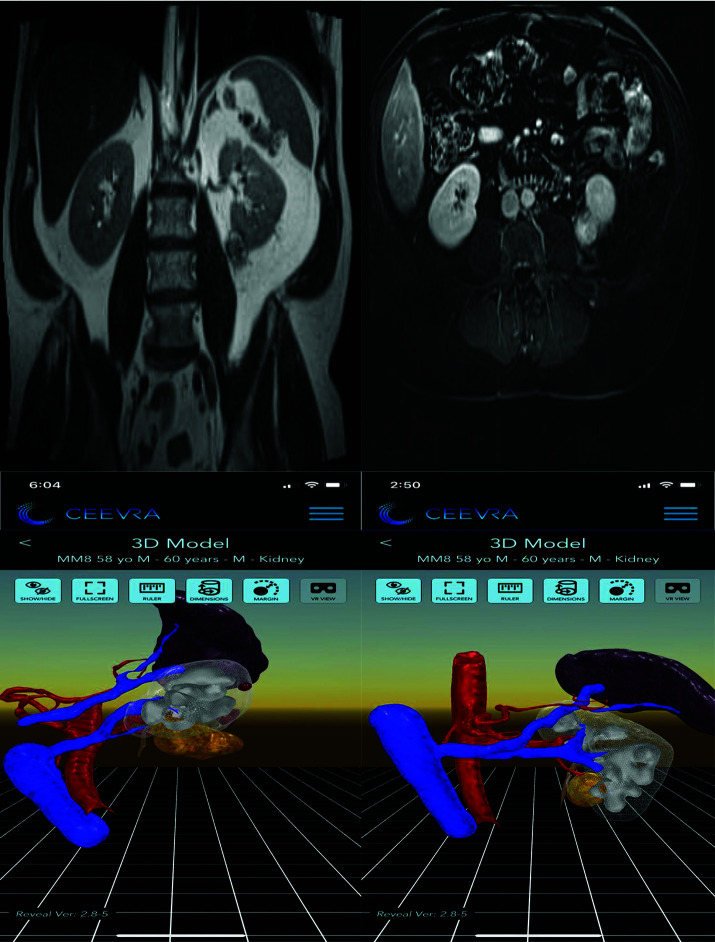
Two cases were changed from a transperitoneal to a retroperitoneal approach. An additional two cases were changed from a retroperitoneal to a transperitoneal approach. Finally, two patients had vascular findings not appreciated on standard presurgical imaging. The first (Patient Number 4) had a renal tumor appreciated in the 3D VR image as superior and anterior to the right renal vein. CT, MRI, and TeraRecon™ images had suggested this tumor was anterior to the renal artery. In the second patient, a lower pole renal artery was appreciated on 3D VR images that was not recognized on initial MR imaging review. Examples of these imaging findings in the CT or MRI scan as compared to the 3D VR models are shown in Figure 1. In Patient Number 8, initial images are seen from the MRI, and reported by radiology as “a lower pole, medial tumor approximately 3 cm in size”. In comparison the 3D VR model clearly shows a posterior tumor with a retroperitoneal approach being advantageous in this case (Figure 2).
Figure 1.
Figure A (Top Left), Right renal mass, endophytic. Figure B (Bottom Left), Right renal mass clearly seen on renal vein. Figure C (Top Right), Right lower pole renal mass. Figure D (Bottom Right), Accessory lower pole renal artery. Figure A,B same patient. Figure C,D same patient.
Figure 2.
Magnetic resonance imaging “lower pole medial tumor.” Three-dimensional virtual reality showing posterior tumor.
Of the 6 cases where the approach changed due to 3D VR imaging, 2 were based on vascular arterial findings not appreciated on MRI or CT scans. These 2 cases had nephrometry scores of 9A and 10 Ah. Of the 4 cases changed due to the 3D VR images with respect to the surgical approach the nephrometry scores were 4A, 7P, 9P, and 9X (Table 3). In all 6 cases, the surgeon was confident postoperatively that the changes made by reviewing the 3D VR models were appropriate. Operative time, estimated blood loss, and clamp time averaged 158 minutes (standard deviation [SD] ± 29.4), 119 milliliters (SD ± 46.5), and 160.4 minutes (SD ± 3.8), respectively. All surgical margins for patients undergoing partial nephrectomy were negative and no intra-operative complications were encountered (data not shown).
Table 3.
Summary of Changes in Surgical Plans after Reviewing 3D VR Models
| Patient Number | Approach Change | Vascular Change |
|---|---|---|
| 2 | Retroperitoneal to transperitoneal | --------- |
| 4 | ---------- | Tumor anterior to renal vein |
| 5 | Transperitoneal to retroperitoneal | --------- |
| 6 | Retroperitoneal to transperitoneal | --------- |
| 8 | Transperitoneal to retroperitoneal | --------- |
| 14 | --------- | Incidental second renal artery |
DISCUSSION
Cancer care over the last decade has rapidly evolved with the advent of robotic surgery and other minimally invasive techniques.12 However, imaging technology has not kept pace with other aspects of surgical technology and is an integral part of any operation. These new technologies are an important part of improving patient outcomes, but do not come without potential risks and should be evaluated prior to widespread use. Preliminary data around 3D VR models shows significant advantages over traditional sliced based imaging and other techniques such as 3D printing.8
Robotic-assisted partial nephrectomy is a complex operation with varying approaches that are in part determined by the pre-operative planning process.13 For instance, the surgeon can select a transperitoneal or retroperitoneal approach based on the location and size of the mass, can decide which vessels to clamp or ligate based on the vascular configuration, and can determine his depth of resection to ensure negative margins. In this context, 3D VR models may improve the surgeon’s planning process, leading to a higher percentage of optimal surgical approaches and thereby improving surgical outcomes.
In our study we noted a large proportion of cases (6/15) in which the basic surgical approach was changed. This represents one of the earliest decision points in pre-operative planning, and the fact that this was changed so frequently is highly noteworthy. We hypothesize that the location of the tumor in respect to the anterior/posterior plane may be more easily understood using the 3D VR models. Furthermore, tumors in the lower pole of the kidney, or those that are fully posterior to the renal hilar vasculature, may be amenable to a retroperitoneal approach, saving operative time and potential hazard to the patient.
Additionally, the vascular anatomy was better visualized with the 3D VR models in several cases. This anatomy includes small details, such as vessels directly feeding the tumor, and more macroscopic details such as the orientation of the mass in relation to the main hilar vessels. Most notably, accessory vessels were noted, which if ligated unintentionally can lead to perfusion defects in the kidney, and if left unclamped can lead to excessive blood loss. For larger masses, parasitic vessels can be outlined and traced much more easily in 3D than in 2D, allowing the surgeon to deliberately ligate these vessels rather than dealing with them as they proceed through the operative steps.
In this initial pilot study, we focused only on the changes made to the surgical plan. While one would expect that these changes improved the surgery, and as a result, the outcomes, additional data may be needed to confirm this conjecture. Conversely, it would be difficult, if not impossible, to contrast the original planned approach with the modified approach in the same patient, so the surgeon’s postoperative opinion provides a reasonable surrogate. Additionally, this is a single surgeon study with an experienced robotic surgeon, and the results may not translate to other surgeons and settings.
This pilot study has shown a positive impact when using 3D VR as compared to traditional imaging for robotic partial nephrectomy. We examined the reasons why 3D VR changed the surgical approach and its impact on those cases. In this surgeon’s experience, 3D VR provided important additional information and understanding above and beyond standard imaging studies with respect to tumor location, depth, and renal vascularity.
Although 3D VR models represent a significant improvement over slice-based imaging, it may be a steppingstone to further enhanced surgical imaging in robotic surgery. For robotic surgery, where the surgeon’s view of the patient anatomy is already technology-based, these models may be used intraoperatively and correlated with the live patient anatomy. Additionally, 3D VR models may be used in other surgical fields, most notably to aid in resection of tumors in other complex solid organ systems. Future work should focus on these areas to fully transform robotic surgery into an image-guided procedure across all surgical fields.
CONCLUSIONS
Three-dimensional VR models utilized in surgical planning for robotic assisted laparoscopic partial nephrectomy changed the operative plan for a significant number of cases. The 3D VR model provides the surgeon with more information in a format that greatly assists the decision making during presurgical planning. Future work should focus on integrating these models into the surgical planning workflow and ultimately the live operation.
Footnotes
Disclosure: none.
Funding sources: none.
Conflict of interests: Joseph D. Shirk is a consultant for Ceevra. Michael McDonald has none.
Informed consent: Dr. Michael McDonald declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Michael McDonald, Department of Surgery, University of Central Florida, 400 Celebration Pl, Celebration, FL.
Joseph D. Shirk, UCLA Department of Urology, David Geffen School of Medicine at UCLA, 300 Stein Plaza, 3rd Floor, Los Angeles, CA..
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Liss MA, Wang S, Palazzi K, et al. Evaluation of national trends in the utilization of partial nephrectomy in relation to the publication of the American Urologic Association guidelines for the management of clinical T1 renal masses. BMC Urol. 2014;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, Klatzky RL, Stetten G. Visualizing 3D objects from 2D cross sectional images displayed in-situ versus ex-situ. J Exp Psychol Appl. 2010;16(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wake N, Wysock JS, Bjurlin MA, Chandarana H, Huang WC. Pin the tumor on the kidney: an evaluation of how surgeons translate CT and MRI data to 3D models. Urology. 2019;131:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wake N, Rosenkrantz AB, Huang R, et al. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: impact on patient education. 3D Print Med. 2019;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lupulescu C, Sun Z. A systematic review of the clinical value and applications of three-dimensional printing in renal surgery. JCM. 2019;8(7):990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirk JD, Thiel DD, Wallen EM, et al. Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial. JAMA Netw Open. 2019;2(9):e1911598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–853. [DOI] [PubMed] [Google Scholar]
- 10.Ellison JS, Montgomery JS, Hafez KS, et al. Association of RENAL nephrometry score with outcomes of minimally invasive partial nephrectomy. Int J Urol. 2013;20(6):564–570. [DOI] [PubMed] [Google Scholar]
- 11.Kolla SB, Spiess PE, Sexton WJ. Interobserver reliability of the RENAL nephrometry scoring system. Urology. 2011;78(3):592–594. [DOI] [PubMed] [Google Scholar]
- 12.Sivarajan G, Taksler GB, Walter D, et al. The effect of the diffusion of the surgical robot on the hospital-level utilization of partial nephrectomy. Med Care. 2015;53(1):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi L, Challacombe B. Robotic partial nephrectomy for posterior renal tumours: tetro or transperitoneal approach? Eur Urol Focus. 2018;4(5):632–635. [DOI] [PubMed] [Google Scholar]