Abstract
Clasmatodendrosis derives from the Greek for fragment (klasma), tree (dendron), and condition (- osis). Cajal first used the term in 1913: he observed disintegration of the distal cell processes of astrocytes, along with a fragmentation or beading of proximal processes closer to the astrocyte cell body. In contemporary clinical and experimental reports, clasmatodendrosis has been observed in models of cerebral ischemia and seizures (including status epilepticus), in elderly brains, in white matter disease, in hippocampal models and cell cultures associated with amyloid plaques, in head trauma, toxic exposures, demyelinating diseases, encephalitides and infection-associated encephalopathies, and in the treatment of cancer using immune effector cells. We examine evidence to support a claim that clasmatodendrotic astrocyte cell processes overtly bead (truncate) as a morphological sign of ongoing damage premortem. In grey and white matter and often in relationship to vascular lumina, beading becomes apparent with immunohistochemical staining of glial fibrillary acidic protein when specimens are examined at reasonably high magnification, but demonstration of distal astrocytic loss of processes may require additional marker study and imaging. Proposed mechanisms for clasmatodendrotic change have examined hypoxic-ischemic, osmotic-demyelinating, and autophagic models. In these models as well as in neuropathological reports, parenchymal swelling, vessel-wall leakage, or disturbed clearance of toxins can occur in association with clasmatodendrosis. Clasmatodendrotic features may serve as a marker for gliovascular dysregulation either acutely or chronically. We review correlative evidence for blood-brain barrier (BBB) dysfunction associated with astrocytic structural change, with attention to interactions between endothelial cells, pericytes, and astrocytic endfeet.
Keywords: Clasmatodendrosis, Astrocyte, Endfoot, Blood-brain barrier, Endothelial cell, Pericyte
Clasmatodendrosis, Astrocyte, Endfoot, Blood-brain barrier, Endothelial cell, Pericyte
1. Introduction
In a recent consensus statement [1] regarding the many names used to describe astrocyte reactions in health and disease, a word of warning emerged specifically regarding clasmatodendrosis: astrocytes may be damaged by cleavage of membrane and cytoskeletal proteins (such as glial fibrillary acidic protein, GFAP), but the phenomenon of clasmatodendrosis could be an artifact without pathophysiological bearing. What is clasmatodendrosis? What is its relationship, if indeed there is any relationship, to ongoing pathophysiology in the central nervous system?
Etymologically, clasmatodendrosis derives from the Greek for fragment (klasma), tree (dendron), and condition (-osis), and was first used by Cajal (1913) in his study of astrocytes using a gold sublimate stain [2]. Cajal observed disintegration of the distal astrocytic cell processes together with a fragmentation or beading of proximal processes closer to the astrocyte cell body (Figure 1). Three years before Cajal's introduction of the term, Alzheimer described similar changes not only in an astrocyte in relationship to a capillary lumen, but also in oligodendroglia and microglia in epilepsy, syphilis, and his eponymic dementia [3]. After Cajal, Rosental [4] and Del Río-Hortega [5] corroborated Alzheimer's and Cajal's observations in various glial cells and diseases. Rosental speculated that the underlying process or processes responsible for clasmatodendrosis could be reversible; Cone, based on his work with Wilder Penfield, corroborated that early view [6].
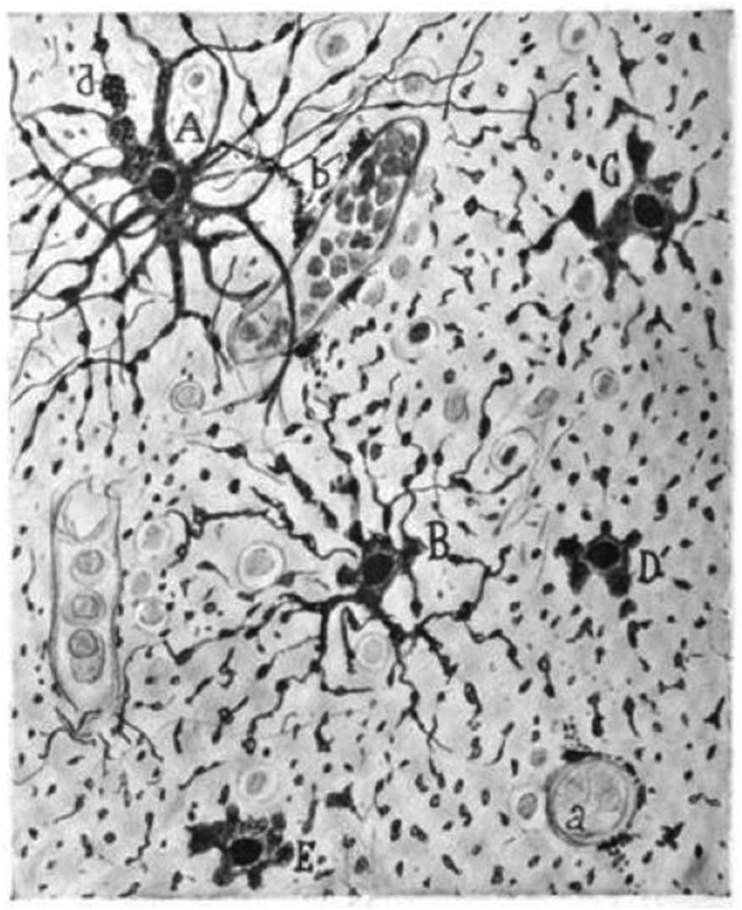
Figure 1.
Autolytic phenomena in the white matter of the brain of a female adult autopsied two hours after death (Ramón y Cajal 1913, fig. 18, public domain. [2]). A. Cell with preserved processes. B. Astrocyte with fragmentations. C, D, E. Astrocyte with disrupted cytoplasmic expansions, but with preservation of perikaryon. a. capillary. b. disaggregated end feet.
Cajal maintained that, if not examined in very fresh specimens after death, astrocytes would undergo autolytic, post-mortem fragmentation, klasmatodendrosis autolítica, reminiscent of what Alzheimer called amoeboid cells. In subsequent reports from 1928 and following, clasmatodendrosis has been characterized as a reaction during life associated with astrocytes—“reaction” meaning some engagement by those glia in biochemical, morphological, metabolic, and/or physiological remodeling resulting in gain or loss of function, as defined by the recent consensus statement [1].
In this review, we examine evidence to support a claim that clasmatodendrotic GFAP-positive astrocytic cell processes in cortex or white matter overtly bead (truncate) as a major morphological sign of ongoing damage pre mortem. Beading becomes apparent with GFAP immunohistochemical (IHC) staining examined by light microscopy at reasonably high magnification (Figure 2), but demonstration of distal astrocytic loss of processes may require additional imaging and marker study, as we will describe. Since we contend that clasmatodendrotic features may serve as a marker for gliovascular dysregulation either acutely or chronically, we review correlative evidence for blood-brain barrier (BBB) dysfunction associated with astrocytic structural change.
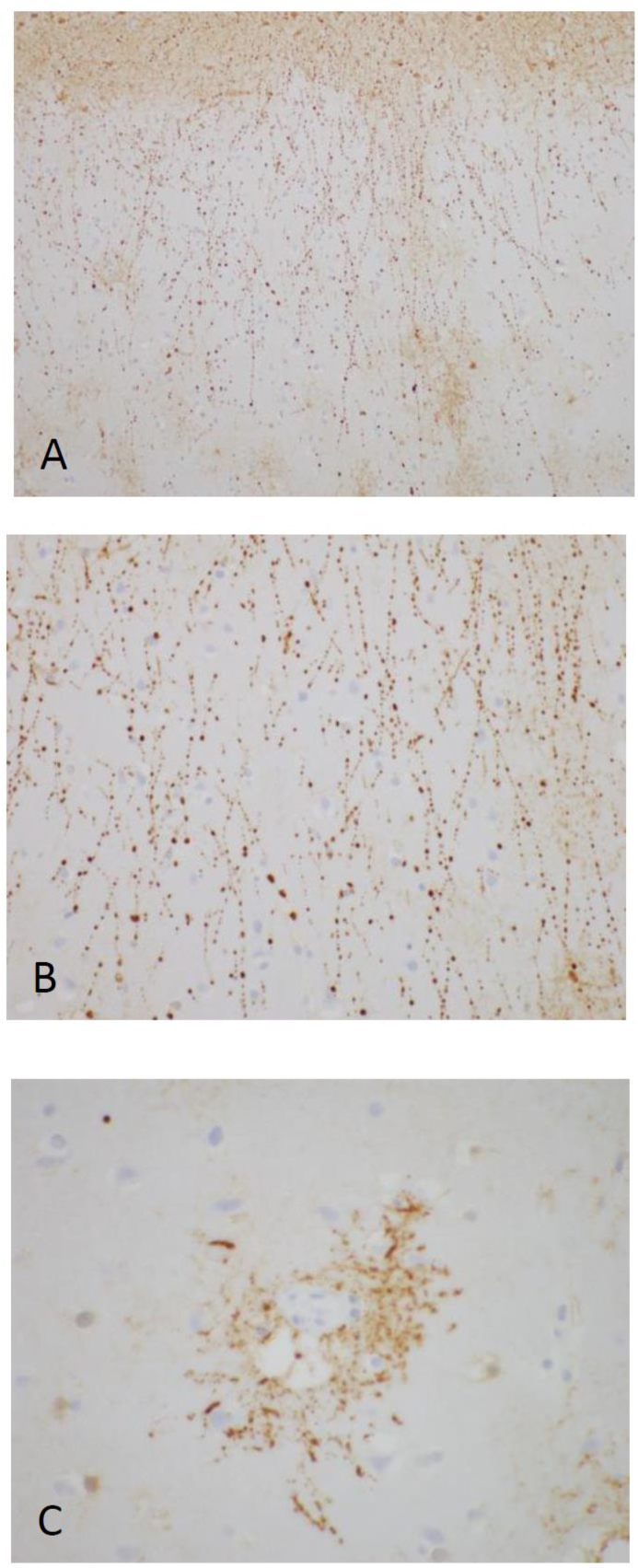
Figure 2.
Postmortem neuropathologic evaluation showing prominent clasmatodendrosis in a patient with a history of acute lymphoblastic leukemia treated with anti-CD19 CAR T-cell therapy complicated by fulminant cerebral edema. Beading and fragmentation of astrocytic processes (highlighted by GFAP immunostains) were seen in sections of cortex (A, 200x; B, 400x) and were accentuated around blood vessels (C, 600x).
In contemporary clinical and experimental reports, clasmatodendrosis has been observed in white matter disease with or without dementia [7, 8, 9, 10, 11], models of interaction between astrocytes and beta-amyloid plaques [12, 13], in hippocampal models and cell cultures [14, 15, 16, 17], head trauma [18, 19, 20, 21], models of cerebral ischemia [22, 23, 24, 25, 26], models of status epilepticus [27, 28, 29, 30, 31], in toxic exposures [32, 33, 34, 35], demyelinating diseases [36, 37, 38], in osmotic-induced demyelination and an inherited condition with cerebral edema [39, 40], in association with autophagy and/or apoptosis [24, 27, 41, 42, 43, 44], encephalitides or infection-associated encephalopathies [45, 46, 47, 48], and in chimeric antigen receptor (CAR) T-cell therapy [49, 50]. Parenchymal swelling, vessel-wall leakage, or disturbed clearance of toxins commonly occurs in association with clasmatodendrosis in pathological and in vivo reports; in cell-culture studies and some seizure or Alzheimer models, astroglial changes are reported without comment about non-astrocytic edema [12, 13, 15, 16, 29, 30, 31, 35, 42]. Table 1 summarizes how clasmatodendrosis has been defined, visualized, and characterized in the above contexts.
Table 1.
Studies in clasmatodendrosis.
| First Author Reference [reference number in brackets] | Definition of clasmatodendrosis | Staining | Microscopy used | Location | Magnification | Context |
|---|---|---|---|---|---|---|
| Cone 1928 [6] | fragmentation of cell expansions with little or no swelling | gold sublimate | light | not specified | 2000x | not specified |
| Friede 1961 [52] | not formally defined; described as shortened and swollen processes with tendency to disintegrate, forming small corpuscles | Hortega silver carbonate | light | white matter | 260x | post-mortem rats |
| Kraig 1990 [53] | not defined. Described as isolation of distal processes and disintegration of branches | horseradish peroxidase. Astrocytes confirmed by high membrane potential and absence of injury spontaneous discharges via single-barrel microelectrode intracellular recordings | light | not specified | not specified. 50 micrometer scale bar | acidosis and ischemia in rats |
| Rafalowska 1992 [38] | fragmentation of perivascular glial fibers | perioxidase-antiperioxidase method to visualize GFAP | light | white matter | not available | young and senile multiple sclerosis plaques |
| Cavanagh 1993 [35] | loss of glial filaments, hydropic swelling of astrocyte perikaryal cytoplasm and nearby processes | GFAP IHC | light, electron microscopy (EM) |
inferior colliculus, but, at high dose expo-sure to toxin (100 mg/kg/day), all areas of brain and spinal cord exhibited changes | x35 | mice and rats exposed to neurotoxin, alpha-chlorohydrin |
| Tomimoto 1996 [26] | swelling of astroglia, decreased GFAP immunoreactivity, intracytoplasmic vacuolization, beading |
Klüver-Barrera stain of white matter, anti-fibrinogen, anti-HLA-DR, GFAP IHC, anti-immuno-globulin, anti-amyloid precursor protein (all IHC) | light | tissue blocks anterior caudate including cingulate, superior, middle, and inferior frontal gyri | not specified. 50 micrometer scale bars | ischemic cerebro-vascular disease, Alzheimer's disease, 6 controls |
| Tomimoto 1997 [10] | swelling, vacuolation of white matter astroglia; disintegrated and beaded processes; condensed nuclear chromatin; large, membrane-bound osmiophilic cytoplasmic inclusions corresponding to lipophilic granules visualized by light microscopy | IgG, IgM, fibrinogen, C3, C1q, C3d, vimentin, alpha-B crystallin, ApoE, laminin, leukocyte common antigen, anti-HLA-DR; double labeling with GFAP (all IHC) | light; EM | deep and periventricu-lar white matter | not specified. 50 micrometer scale bars | human post-mortem in cerebro-vascular and Alzheimer's diseases |
| Hulse 2001 [15] | loss of distal processes, formation of filling bodies | gold sublimate, GFAP-GFP | differential interference contrast; brightfield | hippocampal organ tissue culture, neocortical polygonal astrocytes in primary culture | 20x and 50x objectives used. 100 micrometer scale bars | simulated ischemia using ischemic Ringer's solution |
| Sahlas 2002 [9] | cytoplasmic swelling and vacuolation with beading of their dendrites | H&E, GFAP immune-staining | light | periventri-cular white matter | x40-100 | case report of mixed dementia |
| Simpson 2007 [11] | swollen, vacuolated cell bodies. Disintegrated processes | double-labeled GFAP and Cluster of differentiation 68 (CD68) IHC, fibrinogen IHC | brightfield | periventri-cular and subcortical white matter | x20 and x40 objectives used but not specified if for clasmatodendrosis. 100 micrometerscale bars | older adults |
| Gelot 2009 [25] | loss of distal processes (short rigid and broken and/or beaded), chromatin condensation | vimentin IHC, GFAP IHC. some double labeled TUNEL and GFAP IHC | Light; fluorescence | cerebral cortex and different depths of white matter | x20 and x40 objectives used for cell counts, but not specified if used for judging clasmato-dendrosis. 20–50 micrometer scale bars |
rats, neonates |
| Kim 2011 [28] | swelling and vacuolization of somata; disintegrated/beaded processes | GFAP, GFAP IHC | Confocal microscopy (CONFOC) | hippocampus | not specified. 30 micrometer scale bar | status epilepticus in rats |
| Qin 2010 [41] | not mentioned, but they do mention fragmentation of astrocyte “indicative of dying cells” | GFAP, GFAP IHC, LAMP-1, microtubule associated protein 1A/1B-light chain 3- phosphatidyl-ethanolamine conjugate (LC3-II), Beclin 1 |
Light; fluor-escence; transmission EM | possibly white matter (not specified in text) | 200–400x. 50 micrometer scale bars | focal ischemia, glucose and oxygen deprivation in rats |
| Ryu 2011 [42] | swelling and vacuolization of somata. disintegrated and beaded processes | GFAP IHC. Pyridoxyl 5-prime phosphate phosphatase (PLPP)/chrono-trophin (CIN), LAMP-1, LC3-II stained, but not used to define clasmato-dendrosis. | CONFOC | hippocampus | not specified. 25–50 micrometer scale bars | status epilepticus in rats |
| Ryu 2011 [44] | irreversible astroglial degenerative change, swelling and vacuolization of cell bodies; short, blunt, beaded, and disintegrated and beaded processes; GFAP tangles in the cytoplasm and nuclear dissolution | GFAP. Beclin-1, LAMP-1, LC3-II staining and p65/RelA-Ser529 phosphory-lation (subunit of NFkappaB) positive, but not used to define clasmato-dendrosis | fluorescence | hippocampus | 40x objective used for cell count, but unclear if used for judging clasmatodendrosis. 5–10 micrometer scale bars | status epilepticus in rats |
| Misu 2013 [36] | cytoplasmic swelling and vacuolation, beading and dissolution of their processes and nuclear alterations resembling apoptosis | GFAP IHC. Compared with AQP4, AQP1, IgG (all IHC) which all showed granular internalization | light | Medulla | x1100 | neuromyelitis optica |
| Sakai 2013 [18] | beading and fragmentation astrocytic processes, cytoplasmic swelling and vacuolation of somata | GFAP, GFAP IHC, ubiquitin and lysine 48-linked polyubiquitin chains (K48), K48 IHC. Double immunostaining with GFAP and p62-K48 | Light; CONFOC laser | cerebral cortex | not specified. 10–20 micrometer scale bars | head trauma in humans |
| Nara 2015 [47] | disruption of astrocytic projections | H&E | light | cerebrum, not otherwise specified | x400 | Cytokine storm-derived influenza associated encephalo-pathy |
| Chen 2016 [8] | morphology of irreversibly injured astrocytes; cytoplasmic swelling, somatic vacuolation, beading and fragmentation of dendritic processes | GFAP and ALDH1L1 IHC | brightfield | white matter | not specified. 10–20 micrometer scale bars | aging, post-stroke dementia |
| Daschil 2016 [12] | loss of distal processes; isolated fluorescent bodies | GFAP-GFP | three-dimensional CONFOC | cerebral cortex | 63x objective. 5–40 micrometer scale bars | mouse model of Alzheimer's |
| Ko 2016 [31] | round, edematous soma; short, blunt processes; loss of distal processes | GFAP, Lysosome associated membrane protein 1 (LAMP-1) for lysosomal vacuolization | Immunofluorescence | hippocampus | not specified. 3.75–30 micrometer scale bars | status epilepticus in rats |
| Lana 2016 [17] | beading and disintegration of distal cell processes; cytoplasmic vacuolization and swelling | GFAP | CONFOC laser scanning | hippocampus | 63x objective. 10 micrometer scale bars | aged rats |
| Mercatelli 2016 [14] | beading and disintegration of astrocyte projections | GFAP | three-dimensional CONFOC | hippocampus | 40x and 63x objectives. 90 micrometer scale bars | rats given lipopoly-saccharide (LPS) with induction of amyloid deposition |
| Nishiyama 2016 [37] | not specified. mentions findings of shrinkage of processes and spherical change in soma | GFAP, AQP4 IHC | Light and fluorescence | astrocytes in culture (CC-2565, Lonza Japan, Tokyo, Japan) | not specified. 20–100 μm scale bars | human astrocyte culture; complement-dependent and complement-independent astrocytopathy may operate in NMO |
| Wang 2016 [48] | beading of astrocytic processes | H&E; cleaved capsase 3, AQP4, GFAP (all IHC) | light | Brainstem | not specified. Scale bars not provided for images of clasmatodendrosis | post-mortem hand-foot-mouth disease |
| Canchi 2017 [20] | voids in cytoplasm, rounded somata, disintegrating processes | GFAP | CONFOC laser | forebrain | 63x objective. 25 micrometer scale bars | blast TBI in rats |
| Eltony 2017 [33] | loss of processes, hypertrophy of soma | PTAH | light | optic nerve | x1000 | chronic sildenafil exposure in rats |
| Hase 2017[24] | damaged astrocytes with enlarged somata, loss of processes | GFAP IHC, GFAP-AQP4 double immune-fluorescent staining (AQP4 not used to define clasmatoden-drosis) | brightfield; CONFOC | corpus callosum | brightfield: 20x objective; CONFOC: not specified. 10 micrometer scale bars | chronic cerebral hypoperfusion in mice |
| Hayashi 2017 [34] | not specified | GFAP IHC | light | occipital white matter | not specified. 20 micrometer scale bars | single case autopsy |
| Kim 2017 [27] |
swollen vacuolized cell bodies; disintegrated/beaded processes | GFAP | fluorescence | hippocampus | not specified. 25–50 micrometer scale bars | status epilepticus in rats |
| Miller 2017 [21] | beading, dissolution of astrocytic processes | GFAP to define clasmatodendrosis. Cells double-labeled with GFAP against propium iodide (PI), glial glutamate transporter-1 (GLT-1), and Annexin V | CONFOC laser scanning | hippocampus | not specified. 20 micrometer scale bars | blast TBI in rats |
| Shimoda 2017 [40] | irreversible, “necrobiotic” change of astrocytes; swelling and vacuolization of soma; disintegration/beading of the processes | GFAP IHC | light | periventricular and cerebellar white matter | not specified. 20–100 micrometer scale bars | ataxia-telangiectasia |
| Zhang 2017 [32] | irreversible astroglial degenerative change; swelling and vacuolization of soma; disintegrated and beaded processes | GFAP IHC | light | cerebral cortex | not specified. 50–500 micrometer scale bars | Methamphetamine use in humans |
| Hase 2018 [7] | cytoplasmic swelling and somatic vacuolation; beading and fragmentation of dendritic processes | GFAP and AQP4 double-staining | fluorescence | white matter | not specified. 10 micrometer scale bars | dementia in humans |
| Kim 2018 [43] | astroglial autophagy, swollen, vacuolized soma and disintegrated/beaded processes | GFAP, LAMP-1, TUNEL, HSPB1 | fluorescence | hippocampus | not specified. 25 micrometer scale bars used | status epilepticus in mice |
| Torre 2018 [49] | beading of glial fibrillary acidic protein consistent with astrocyte injury | GFAP IHC | light | superficial cortex | 400x and 1000x | ICANS in one patient |
| Bouchat 2019 [39] | “distal extensions removal,” beading, and irreversible astrocyte injury. Autophagocytosis | GFAP | automated fluorescence; EM | thalamus | not specified. 1 micrometer scale bars for fluorescence; 200 nm scale bars used for electron micrographs | osmotic demyelin-ation in mice |
| Tachibana 2019 [45] | disintegration of distal processes, somatic swelling and vacuolation | GFAP and GFAP IHC to define clasmatodendrosis. AQP4, TUNEL, MAP2, SMI31, synaptophysin tested | CONFOC laser scanning microsopy; transmission EM | cerebellum, thalamus, splenium, corpus callosum | not specified. 50 micrometer scale bars for confocal; 0.2–1 micrometer scale bars for electron | influenza-associated encephalopathy |
| Early 2020 [19] | beading, diminishment of astrocyte projections; vacuolization and swelling of cytoplasm | GFAP, vimentin, S100beta, immune-fluorescent co-labeling. AQP4 | CONFOC | hippocampus | not specified. 25–100 micrometer scale bars | traumatic brain injury in mice |
| Lee 2020 [30] | astroglial autophagy; vacuolization, disintegrated/beaded processes | double-labeled GFAP and HSP25, GFAP and LAMP1, and GFAP and p-focal adhesion kinase (FAK)-Y397 | fluorescence | hippocampus | not specified. 6.25 micrometer scale bars | status epilepticus in mice; kainic acid model |
| Stevenson 2020 [22] | loss of processes; swollen, round soma | GFAP IHC | brightfield | hippocampus | 20x objective. 10 and 50 micrometer scale bars | carotid stenosis in mice |
2. Clasmatodendrosis as an astrocytic reaction in vitro and in vivo
Andriezen [51] drew first attention to morbid structural alteration of glia in the human brain, and he observed different prominent glial populations in grey and white matter–protoplasmic glia in the former and stellate fiber cells in the latter. He described that with brain injury or disease, protoplasmic glia extend a surfeit of fibrils in their immediate vicinity, many directed towards vessels, as the glial cell body eventually assumes a ghost-like, empty appearance.
In a further characterization of astrocytic reaction in time, now with particular attention to clasmatodendrosis, Frieden and Houten [52] examined rat astrocytes in hippocampal cell cultures under conditions of abolished oxidative metabolism. Anaerobic glycolysis in astrocytes can persist for a while after anoxic insult, depending on local carbohydrate reserves, with acidity rising as a function of accumulating lactic acid. With ongoing anaerobic glycolysis, astrocyte cell processes shortened, swelled, and tended to disintegrate or to form corpuscles, consistent with clasmatodendrotic beading. When the glycolytic pathway was blocked experimentally, clasmatodendrosis was “eliminated.”
As measured by pH-sensitive microelectrodes inserted into astrocytes in live rats, an intracellular increase in acidity under anoxic and hyperglycemic conditions has been documented [53]. The authors also noted increases in astrocytic surface membrane resistance related, they argued, to progressive loss of astrocytic arbors (reduced overall astrocytic cell surface area, as occurs in clasmatodendrosis), reduced potassium conductance due to acid sequestration within astrocytes, and reduced coupling via gap junctions between neighboring astrocytes, perhaps especially at the level of astrocytic endfeet at their interface with cerebral vessels. Corroborating the in vivo report, reduction in the length of astrocytic cytoplasmic processes, with distal ends showing the earliest change, occurred ~15 min after reduction in pH combined with mitochondrial inhibition in a hippocampal cell culture [15]. Temporal and spatial changes in morphology were determined both by GFAP immunostaining (thinning, then shortening or frank truncation of astrocytic process length visualized by way of three-dimensional optics) and by transfection of cultured astrocytes with green fluorescent protein (GFP). The latter allowed the authors to visualize by a different means the progressive reduction in process diameter over minutes, followed by a disruption and loss of process fluorescence distally.
In permanent middle cerebral artery occlusion in rats and a parallel in-vitro study of cultured astrocytes undergoing oxygen and glucose deprivation, structural changes occurred in both settings. Shortening and fragmentation of astrocytic cell processes occurred. But astrocytic cytoplasm also contained numerous multimembrane vesicles described as typical for autophagosomes, which eventually fused with lysosomes in the cytoplasm [41]. Autophagosomal markers (see Table 1 for specific molecules) increased and levels of cytoprotective B-cell-lymphoma 2 protein (Bcl-2) decreased. The changes were mitigated, but not entirely reversed, by pharmacological inhibition of autophagy.
In a rat model of status epilepticus, an interesting mechanism for clasmatodendrosis specifically associated with autophagy has been advanced [44], although a number of autophagic or apoptotic pathways have been studied [24, 27, 43]. Tumor necrosis factor alpha (TNF-alpha), expressed at low levels in normal brain, is upregulated in status epilepticus, and the increase is associated with selective phosphorylation of a particular subunit of nuclear factor-kappa-B (NFkappaB) in astrocytes. The specific phosphorylation and ensuing autophagic (vacuolar/cytoplasmic) and clasmatodendrotic changes were reversed by neutralization of TNF-alpha.
A general autophagic mechanism for clasmatodendrosis mediated by TNF-alpha remains an open issue, however, in light of an autopsy series examining influenza-associated encephalopathy, a condition thought to be mediated by inflammatory cytokines such as interleukin 6 (IL-6) and TNF-alpha rather than by viral invasion into brain [45]. Compared to control brains, clasmatodendrosis, characterized as GFAP-positive bead strings (and other marker positivity, see Table 1), was present in all examined regions of influenza-encephalopathy brains. Aquaporin 4 (AQP4) distributions differed between control and disease groups. Intense AQP4 staining occurred at astrocytic perivascular endfeet in control brains. AQP4 staining in the area of GFAP-positive bead strings was observed along with a decrease in AQP4 staining in the vicinity of blood vessels in encephalopathy brains. In addition, autophagic markers in astrocytes were notably absent, and electron microscopy revealed neither autophagosomes nor vacuolization. The authors surmised that autophagy may not be a mechanism for clasmatodendrocyte formation in this virus-associated encephalopathy. All the brains in the encephalopathic group were heavy and exhibited diffuse edema (mean brain weight at autopsy was 1,318 g in encephalopathic brains compared to 1,023 g in the control group; mean ages for the two groups were 4.5 years and 5.8 years, respectively).
The significance of AQP4 redistribution associated with diffuse brain edema merits consideration of a relevant model.
3. Clasmatodendrosis and the BBB in vivo: the case of osmotic demyelination
Clasmatodendrosis figures prominently in the timing of glial changes associated with osmotic demyelination in mice. Building on in vitro observations that astrocytic GFAP expression downregulates after exposure to pro-inflammatory cytokines, particularly interleukin-1-beta (IL-1beta) [54], Nicaise et al. [55] found that astrocytes of demyelination-prone regions underwent profound astrocytic endfoot change and fragmentation of astrocyte processes, with swelling of perinuclear areas, associated with loss of astrocyte cell markers such as AQP4 and aldehyde dehydrogenase 1 family, member L1 (ALDH1L1). Loss of AQP4 immunoreactivity happened prior to demyelination in susceptible regions and before GFAP downregulation. Scalisi et al. [56] have also observed “massive” breach of the BBB on the order of 48 h after correction of hyponatremia and after oligodendrocyte and astrocyte loss. The above studies hint that BBB leakiness could be secondary to astrocyte reaction: also at 48 h post-correction, brain endothelial cell changes reached their apogee at those places where astrocyte endfeet were most disrupted.
In the aggregate, the above observations regarding morphological change in astrocytes and a single model of BBB leakiness suggest that clasmatodendrosis could be characterized by either, but how do we clarify how morphological change leads to variable BBB permeability? What locales might be most vulnerable to edema associated with clasmodendrotic beading in grey and white matter?
4. Astrocytes, their endfeet, and vascular lumina
Astrocytic processes interface with vessels in ways that have been examined with increasing sophistication. Ultrastructural differences between vessels located at the pia-lined cortical surface and those downstream in the arteriovenous axis have been examined using single-cell RNA sequencing [57]. The transcriptome as reported in the rat could be highly evolutionarily conserved, and certain observations are relevant to the distal astrocytic process in humans. In comparison to step-like, discrete transitions in gene expression from one type of smooth muscle cell to another, endothelial cells lining vascular lumina exhibit gradual, not punctuated, changes in gene expression from arterial to capillary to venous endothelium. At a lumen, the distal astrocytic process widens into an endfoot to form, along with other astrocytic endfeet or the ends of a single endfoot, a continuous sheath that possess water channels (AQP4) and potassium channels (ATP-dependent inwardly rectifying potassium channel 4.1, Kir4.1) facing an abluminal glycoprotein- and collagen-rich matrix [58, 59, 60]. An endothelial cell (or endothelial cells) line/s the lumen; abluminal pericytes (without smooth muscle cells, at the capillary level) loosely embed themselves in the matrix underneath the astrocytic endfoot [57, 58].
Dating to tracer leakage assays in the 1960's, tight junctions, where leaflets of endothelial cell membranes appose each other, have been understood as a sine qua non of the BBB [61]. Tight junctions, among other barriers, restrict the entry of hydrophilic molecules into brain parenchyma. Brain endothelial cells themselves exhibit less transcellular transport compared to peripheral endothelium, and their endocytosis and transcytosis are highly selective processes [62, 63]. Tight junctions restrict movement of small ions such that transendothelial electrical resistance (TEER) is orders of magnitude greater across brain endothelium compared to endothelial TEER elsewhere in the body (1,000 Ω.cm2 vs. ~10 Ω.cm2) [62]. Nevertheless, the BBB is as variable as it is static, and much attention has been paid to modulatory influences of astrocytes, pericytes, and endothelial cells–the three populations that comprise the distal extent of the neurovascular unit (NVU) [64, 65].
5. Astrocytes, pericytes, endothelial cells
Just beyond the distal NVU, cells in brain parenchyma may interact with endothelial cells, for example, after vascular injury [66], but we concentrate on three cell populations.
In development, the interaction between astrocytes, pericytes, and endothelial cells is constitutive not only of a barrier, but also of a lumen. Co-culture studies indicate that astrocytes contribute to the orientation of endothelial cells and pericytes into capillary-like structures [67]. Astrocyte precursor cells mitogenically induce the development of endothelial cells, and endothelial cells drive, by way of a cytokine (leukemia inhibitory factor), the differentiation of astrocyte precursor cells into astrocytes [68]. Endothelial cells secrete vascular endothelial growth factor (VEGF) and insulin growth factor 1 (IGF-1), both considered important in developmental neurovascular patterning [69]: VEGF disrupts tight junctions [70], but knockout mice without endothelial expression of IGF-1 receptors do not exhibit changes in BBB permeability in life [71].
The synthesis of a factor in development may or may not impact barrier function in the long term. Yet, in an important in vivo study, pericyte-deficient mouse mutants showed robust increases in BBB water permeability by endothelial transcytosis (the permeability can be reversed by the tyrosine kinase inhibitor imatinib), and pericyte-deficient mice exhibited abnormal channel polarization at the astrocytic endfoot, with redistribution of AQP4 away from the ablumen [72]. The loss of channel polarization at the endfoot or frank loss of the endfoot has implications that we detail below in two clinical contexts associated with clasmatodendrosis.
In maturity, endothelial cells secrete platelet-derived growth factor BB (PDGF-BB), the receptor for which is located on pericytes (a paracrine interaction important for pericyte recruitment); in turn, pericytes express major facilitator superfamily domain-containing protein 2a (MFSD2a) critical to endothelial cell-wall integrity [69]. Pericytes and astrocytes secrete angiopoietin-1 (Ang-1) that acts on a tyrosine kinase/immunoglobulin-like receptor-2 (TIE-2) located on the endothelial cell membrane. Ang-1 at TIE-2 contributes to BBB impermeability [73]. Astrocytes secrete apolipoprotein E 2 and 3 (APOE2 and APOE3), glial cell line-derived neurotrophic factor (GDNF), and fibroblast growth factor-2 (FGF-2), all imputed, based on cell culture studies (and in vivo in the case of APOE3, the most abundant APOE isoform), to induce and maintain the BBB [74]. Astrocytes also secrete Src-suppressed C-kinase substrate (SSeCKS, also known as A-kinase anchor protein-12) that transcriptionally regulates synthesis of tight junctional protein complexes in the endothelial cells [69]. Many other molecular mechanisms of BBB control have been described [65, 74].
Among paracrine signals that contribute to BBB leakiness, three are of note: endothelin-1 (ET-1), tumor necrosis factor-alpha (TNF-alpha, a cytokine known to induce ET-1 synthesis by endothelial cells), and interleukin-1beta (IL-1beta). In a cell-culture study that illustrates the nuances of interaction between endothelial cells and astrocytes [75], ET-1 mRNA expression in endothelial cells increased in the presence of TNF-alpha; both TNF-alpha and ET-1 mediated increased leakiness, but only in the presence of astrocytes; TNF-alpha induced astrocyte synthesis of IL-1beta, as mediated by ET-1; and IL-1beta increased BBB leak. Ultrastructural changes in cells were not reported.
6. Clasmatodendrosis in small-vessel disease and immune-effector-cell associated toxicity
In a neuropathological investigation of sub-populations of persons with manifest small-vessel disease on neuroimaging (but with varying degrees of cognitive impairment by clinical rating scales and clinical histories) [8], double immunofluorescent staining for GFAP and AQP4 revealed overall reduced distal astrocytic cell processes, a morphology Alzheimer and Cajal had observed. In addition, in that study, AQP4 stain was displaced, aggregating at the edges of swollen GFAP-positive cells, in counterpoint to the even, though dotted staining for AQP4 along normal astrocytic endfeet. Punctate staining likely represents normal local clustering of AQP4 in orthogonal arrays of particles at the interface with the abluminal glycoprotein and collagen matrix in the distal NVU. The pathological findings suggest that disruption of normal arrays, with loss of the polarized location of AQP4 at the astrocytic endfoot, could be a marker for gliovascular dysfunction. Further, capillaries in clasmatodendrotic areas were denuded of AQP4 immunoreactive astrocytic endfeet, a finding also found in neuropathologic sampling from a simian model for ischemic encephalopathy reported in the same paper. Loss of antibody staining to aldehyde dehydrogenase 1 family, member L1 (ALDH1L1), a cytoplasmic marker, corroborated cytoplasmic disintegration of astrocytes [8]. Loss or disorganization of astrocytic AQP4 would have effects not only on local BBB permeability, but also on the efficient clearance of toxins (tau in head trauma or in tau-associated neurodegenerations, beta-amyloid, others) from the periendothelial space and from the parenchymal interstitium via a “glymphatic” pathway unique to the brain and dependent on a relatively intact distal NVU in the arterio-venous capillary bed [76, 77, 78].
Not surprisingly, endothelial cells and pericytes themselves exhibit changes in small-vessel disease [79], but the specific mechanisms by which clasmatodendrosis occurs as a consequence of disturbed signaling between the three cell populations remains to be explored fully. Nevertheless, there are clues pointing to at least part of a mechanism, based on experience in a very different clinical context associated with clasmatodendrosis pathologically.
Among biomarkers of endothelial activation in disease, angiopoietins have been studied in infections for some time [80], but, relevant to clasmatodendrosis, we can study the role of two angiopoietins in chimeric antigen receptor (CAR) T-cell immunotherapy for refractory malignancies. We have chosen images (Figure 2) to illustrate clasmatodendrotic beading specifically from our experience with CAR T-cell therapy.
Earlier we described pericytic and astrocytic Ang-1 and its interaction with the endothelial-cell receptor TIE-2. Now we introduce the significance of angiopoietin-2 (Ang-2), which is released from a specialized organelle (the Weibel-Palade body) within endothelial cells [81]. Endothelial Ang-2 binds to endothelial TIE-2; in the presence of Ang-1, Ang-2 is an antagonist at TIE-2 [82]. The ratio of the two ligands at TIE- 2 has contrary downstream effects [73, 82].
In the setting of cytokine release in CAR T-cell therapy and other pro-inflammatory “storms,” [83] endothelial Weibel-Palade bodies exocytose Ang-2 along with the principal cargo of Weibel-Palade bodies, von Willebrand factor (VWF). The two angiopoietins and VWF are measurable in serum, although, as a cautionary point, biomarkers may only partially reflect the complexity of NVU signaling. In a prospective study in pediatric traumatic brain injury, serum increases in Ang-2 were associated with poorer coma scores, as one would expect, but Ang-2 increases were also associated with decreases in ET-1 (a paracrine signal associated with increased BBB permeability, see above), though none of the correlations between biomarkers reached statistical significance in that study [84].
The CAR T-cell therapy experience suggests that Ang-2 expression may, however, be a practical index of gliovascular dysregulation [82]. Ang-2 expression and the ratio Ang-2/Ang-1 are both low in quiescent, normal, mature vessels. Serum Ang-2 and the Ang-2/Ang-1 ratio increased by day 7 after infusion of CAR T cells; serum VWF also increased in that interval–the latter observation consistent with Ang-2 release from endothelial Weibel-Palade bodies. Clinical severity of neurotoxicity tracked with rises in Ang-2 and the Ang-2/Ang-1 ratio; Ang-1 levels did not differ between persons with lesser or no neurotoxicity versus those with severe neurotoxicity and accompanying radiographic evidence for BBB leak. In brief, endothelial Ang 2 to astrocytic and pericytic Ang-1 ratio is telling: low is normal and anti-leak; higher is pro-leak.
The complexity of the cytokine release syndrome (CRS) that typically precedes, but may temporally overlap with immune effector-cell associated neurotoxicity syndrome (ICANS) cannot be done justice in this brief review, but it is important to note that many signals at critical points in CRS evolution are already synthesized by (and have known effects on) endothelial cells, pericytes, and/or astrocytes at the gliovascular interface. Further, CAR T cells in current use are known to produce many of the same cytokines or paracrines synthesized at the distal NVU, though additional CAR T-cell in vitro modification to change a cell's synthetic profile is already being studied [85].
As we have reviewed, some of the critical signals include (the associated effect on BBB permeability follows in parentheses): Ang-1 (less leaky), Ang-2 (leaky), IL-1-beta (leaky), IL-6 (leaky), TNF-alpha (leaky), VEGF (leaky). Complicating the overall picture are conversions that occur among cytokines [83]–for example, from IL-6 to interleukin 17 (IL-17)—elevations of both are associated with ICANS [86]; in addition, interventions targeting, for example, IL-6 (e.g., tocilizumab, an IL-6 inhibitor) may be associated with transiently associated with increases in circulating IL-6 [87]. Nevertheless, an awareness of key signals at the distal NVU has become necessary for both the hospitalist and neurohospitalist in the immunological treatment of cancer. Attention to the short list provided above has practical value in contemplating common therapies to mitigate BBB permeability.
7. NVU salvage, possibly to impede clasmatodendrosis
Treating ICANS mandates vigilance for the possibility of a rapid decline in sensorium, over minutes or hours, associated with radiographic loss of grey-white matter boundaries, effacement of cisterns indicative of intracranial edema, and other worrisome signs. The mainstay of treatment is dexamethasone.
Neuropathologic examination of patients with severe ICANS [49, 50] demonstrates alterations that are in keeping with impaired BBB permeability. Postmortem analysis of one patient who died from fulminant cerebral edema after treatment with CAR T cells [49] showed perivascular fluid extravasation (highlighted by positive fibrin and factor VIIIA staining), aberrant glucose transporter-1 staining (GLUT 1, a marker for endothelial integrity), and beading and fragmentation of the astrocytic processes. Beading and fragmentation were prominent around vessels. In the days preceding death in such cases, the effort to treat vasogenic edema may influence post-mortem observations. For example, reductions in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells may relate to the aggressive use of dexamethasone before a patient's demise [49].
Characterization of dexamethasone's mechanism of action underscores signaling at the distal NVU that either results from or contributes to clasmatodendrosis. Dexamethasone regulates the expression of VEGF, Ang-1, and Ang-2 by way of action at glucocorticoid receptors on astrocytes, pericytes, and endothelial cells. In a cell culture preparation with steroid concentrations comparable to those observed in the treatment of brain tumor-associated edema [70], dexamethasone decreased VEGF and increased Ang-1 secreted by astrocytes and pericytes; Ang-1 is not synthesized in endothelial cells, although pericytes did elaborate Ang-1 in the study. Dexamethasone had no significant effect on endothelial cell synthesis of VEGF or Ang-2. Drug-induced changes were attenuated in the presence of an antagonist to the glucocorticoid receptor. The effect of glucocorticoids may be more complex, since steroids are known to influence the activity of factors (e.g., nuclear factor kappaB, NFkappaB) in prolonged inflammatory states [83], perhaps with ensuing reduced expression of ICAM1, matrix metalloproteinase-9 (MM-9), and VCAM1 [49, 88].
Given a greater understanding of cytokine interactions, therapeutic options have expanded to include inhibition at flash points, although it is not clear that inhibitions target BBB permeability directly so much as they help to interrupt the elaboration of CRS. Infliximab inhibition of TNF-alpha, situximab and tocilizumab inhibition of IL-6, tocilizumab inhibition of the conversion of Il-6 to IL-17, and anakinra inhibition of conversion of IL-1beta to IL-6 in septic-infectious CRS are examples of inhibitors in current use [86, 87]. Based on prior experience with anakinra, the drug was known to cross effectively into brain [86], but the degree to which BBB permeability is already pitched to leakiness in various disease states associated with clasmatodendrosis may be a factor in future trials: if the distal NVU is already breached in some way, as one would expect early in the progression to clasmatodendrosis, does our notion of drug delivery to brain across the BBB change?
In other conditions associated with clasmatodendrosis, interventions will vary depending on the presumed mechanisms of NVU dysfunction–for example, primary and secondary stroke prevention in small-vessel and other cerebrovascular diseases, treatment of infections, etc. It is interesting to consider, however, that permeability changes that either are the result of clasmatodendrosis or which predispose to that change might share common mechanisms. Table 2 and Figure 3 provide summaries of proposed mechanisms addressed in this review.
Table 2.
Summary of Proposed Mechanisms in Clasmatodendrosis (see text for full discussion; references in brackets).
| Experimental Model or Clinical Context | Observations | Cytokine or paracrine mediators | Reversibility by what means? |
|---|---|---|---|
| Hippocampal cell culture, abolished oxidative metabolism [52] | ↓pH as a function of ↑lactic acid | -- | blockade of glycolysis associated with elimination of clasmatodendrosis |
| Rats in vivo, anoxia, hyperglycemia [53] | ↓potassium conductance across astrocytic membrane due to acid sequestration in astrocytes | -- | -- |
| Neuropathologic sampling of small-vessel disease [8] | denuded AQP4 staining of astrocytic endfeet, ↓ALDH1L1 | -- | -- |
| Permanent middle cerebral artery occlusion, rats [41] | appearance of autophagosomes in astrocytes; LAMP-1 immunoreactivity |
↑LC3-II, ↑Beclin-1 (both autophagosome markers); ↓cytoprotective Bcl-2 |
pharmacologic inhibition of autophagy (partial response) |
| Status epilepticus (rats) [44] | selective phosphorylation of NFkappaB | ↑TNF-alpha | neutralization of TNF-alpha |
| Influenza-associated encephalopathy [45] | ↓AQP4 staining of astrocytes in the vicinity of blood vessels | ↑IL-6, ↑TNF-alpha | -- |
| Osmotic demyelination (rats) [56] | ↓AQP4 and ↓ALDH1L1 staining | ↑IL-1beta | -- |
| Pericyte-deficient mice [72] | redistribution of AQP4 away from abluminal astrocytic endfeet | ↓PDGF-BB | tyrosine kinase inhibition (imatinib) |
| Astrocyte and endothelial cell culture [75] | model for ↑BBB permeability | ↑ET-1, ↑TNF-alpha, ↑IL-1beta | -- |
| CAR T-cell immune therapy [82, 83, 86, 87] | signs and symptoms of ICANS | ↑Ang2/Ang1 ratio, ↑IL-1beta, ↑IL- 6, ↑IL-17, ↑TNF-alpha, ↑VEGF | ?IL-6 inhibition (tocilizumab), ?↓IL-1beta to IL-6 conversion (anakinra), dexamethasone (multiple mechanisms of effect, see text) |
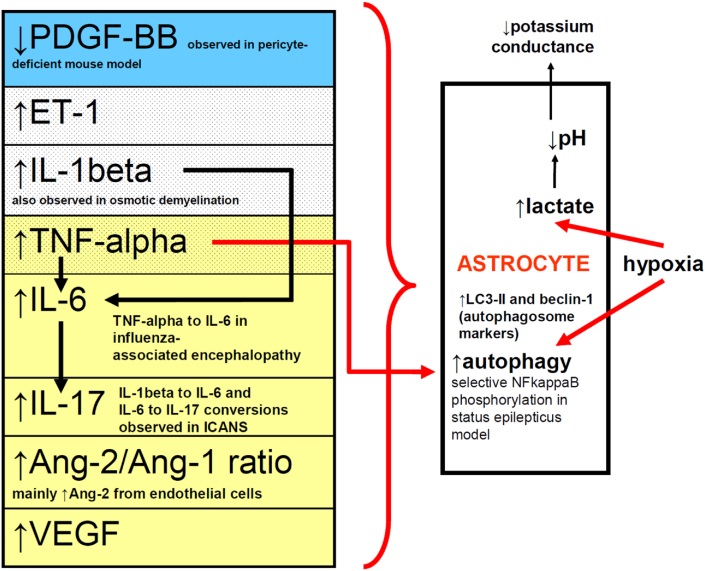
Figure 3.
Schematic illustrating triggers leading to increased BBB permeability associated with astrocytic clasmatodendrosis. Blue row represents findings from a pericyte-deficient mouse model; stippled rows represent findings from co-culture study of astrocytes and endothelial cells; light yellow rows represent findings in ICANS. Abbreviations are those used elsewhere in this paper.
8. Conclusion
Although clasmatodendrosis could be autolytic and artifactual as Cajal suggested in 1913, data are actively accumulating that invite questions about how the GFAP beading in clasmatodendrosis transpires mechanistically and what consequences ensue from the morphological change. Whether clasmatodendrosis is a response to extracellular (paracrine or cytokine) or intracellular cues (induction of autophagic or other genetic mechanisms associated with expression of proteins normally quiescent in astrocytes, such as apolipoprotein E, APOE [45]) or both extra- and intracellular cues remains to be elucidated. A relationship between clasmatodendrosis, astrocytic endfoot changes, and loss of polarity of AQP4 in astrocytes does not allow one to conclude that the astrocytic reaction starts a cascade resulting in increased BBB permeability. It is more likely that interactions especially between endothelial cells and astrocytes, but not excluding pericytes, need to be further elucidated to characterize how the NVU as a whole dysfunctions, especially in capillary beds. The work will be pertinent to further pathophysiological understanding of many disease states now associated with clasmatodendrosis.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Dr. Shamik Bhattacharyya receives personal fees from Alexion Pharmaceuticals and honoraria from UpToDate and Springer. Dr. Matthew Torre is supported by the National Cancer Institute of the National Institutes of Health under award number F32CA257210. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge Umberto DeGirolami, M.D. (Brigham and Women's Hospital, Department of Pathology) for his insight during manuscript preparation and for his translations of Ramòn y Cajal, as well as discussions of this paper's topic with Utkarsh Acharya, D.O. and Douglas Wilcox, M.D., Ph.D., both at Brigham and Women's Hospital and Dana Farber Cancer Institute, Jorg Dietrich, M.D., Ph.D., M.B.A. at Massachusetts General Hospital, and Henrikas Vaitkevicius, M.D. at Marinus Pharmaceuticals, Inc.
References
- 1.Escartin C., Galea E., Lakatos A. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramón y., Cajal S. Contribución al conocimiento de la neuroglía del cerebro humano. Trabajos del Laboratorio de Investigaciones Biológicas de la Universidad de Madrid. 1913;11:255–315. [Google Scholar]
- 3.Alzheimer A. Beiträge zur kenntnis der pathologischen neuroglia und ihrer beziehungen zu den abbauvorgangen im nervengewebe. In: Nissl F., Fischer G., editors. Vol. 3. 1910. pp. 410–562. (Histologische und histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtung der pathologischen Anatomie der Geisteskrankheiten). [Google Scholar]
- 4.Rosental V.S. Experimentelle studien über amöboide umwandlung der neuroglia. In: Nissl F., Alzheimer A., editors. Vol. 6. 1913. pp. 89–160. (Histologische und histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtung der pathologischen Anatomie der Geisteskrankheiten). [Google Scholar]
- 5.Del Río-Hortega P. Noticia de un nuevo y fácil método para la coloración de la neuroglía y del tejido conjuntivo. Trabajos del Laboratorio de Investigaciones Biológicas de la Universidad de Madrid. 1917;15:367–378. [Google Scholar]
- 6.Cone W. Acute pathologic changes in neuroglia and in microglia. Arch. Neurol. Psychiatr. 1928;20(1):34–72. [Google Scholar]
- 7.Hase Y., Horsburgh K., Ihara M., Kalaria R.N. White matter degeneration in vascular and other ageing- related dementias. J. Neurochem. 2018;144:617–633. doi: 10.1111/jnc.14271. [DOI] [PubMed] [Google Scholar]
- 8.Chen A., Akinyemi R.O., Hase Y. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-stroke dementia. Brain. 2016;139:242–258. doi: 10.1093/brain/awv328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahlas D.J., Bilbao J.M., Swartz R.H., Black S.E. Clasmatodendrosis correlating with periventricular hyperintensity in mixed dementia. Ann. Neurol. 2002;52:378–381. doi: 10.1002/ana.10310. [DOI] [PubMed] [Google Scholar]
- 10.Tomimoto H., Akiguchi I., Wakita H. Regressive changes of astroglia in white matter lesions in cerebrovascular disease and Alzheimer’s disease. Acta Neuropathol. 1997;94:146–152. doi: 10.1007/s004010050686. [DOI] [PubMed] [Google Scholar]
- 11.Simpson J.E., Fernando M.S., Clark L. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol. Appl. Neurobiol. 2007;33:410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 12.Daschil N., Humpel C. Green-fluorescent protein (+) astrocytes attach to beta-amyloid plaques in an Alzheimer mouse model and are sensitive for clasmatodendrosis. Front. Aging Neurosci. 2016;8 doi: 10.3389/fnagi.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lana D., Ugolini F., Giovannini M.G. Space-dependent glia-neuron interplay in the hippocampus of transgenic models of beta-amyloid deposition. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21249441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercatelli R., Lana D., Bucciantini M. Clasmatodendrosis and beta-amyloidosis in aging hippocampus. Faseb. J. 2016;30:1480–1491. doi: 10.1096/fj.15-275503. [DOI] [PubMed] [Google Scholar]
- 15.Hulse R.E., Winterfield J., Kunkler P.R. Astrocytic clasmatodendrosis in hippocampal organ culture. Glia. 2001;33:169–179. doi: 10.1002/1098-1136(200102)33:2<169::aid-glia1016>3.0.co;2-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lana D., Ugolini F., Giovannini M.G. An overview on the differential interplay among neurons- astrocytes-microglia in CA1 and CA3 hippocampus in hypoxia/ischemia. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.585833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lana D., Iovino L., Nosi D. The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats. Exp. Gerontol. 2016;83:71–88. doi: 10.1016/j.exger.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Sakai K., Fukuda T., Iwadate K. Beading of the astrocytic processes (clasmatodendrosis) following head trauma is associated with protein degradation pathways. Brain Inj. 2013;27:1692–1697. doi: 10.3109/02699052.2013.837198. [DOI] [PubMed] [Google Scholar]
- 19.Early A.N., Gorman A.A., Van Eldik L.J. Effects of advanced age upon astrocyte-specific responses to acute traumatic brain injury in mice. J. Neuroinflammation. 2020;17 doi: 10.1186/s12974-020-01800-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canchi S., Sarntinoranont M., Hong Y. Simulated blast overpressure induces specific astrocyte injury in an ex vivo brain slice model. PloS One. 2017;12 doi: 10.1371/journal.pone.0175396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A.P., Shah A.S., Aperi B.V. Acute death of astrocytes in blast-exposed rat organotypic hippocampal slice cultures. PloS One. 2017;12 doi: 10.1371/journal.pone.0173167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson W., Hase Y., Wilson E. Long-term effects of experimental carotid stenosis on hippocampal infarct pathology, neurons and glia and amelioration by environmental enrichment. Brain Res. Bull. 2020;163:72–83. doi: 10.1016/j.brainresbull.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Gouw A.A., Seewann A., van der Flier W.M. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 24.Hase Y., Craggs L., Hase M. Effects of environmental enrichment on the white matter glial responses in a mouse model of chronic cerebral hypoperfusion. J. Neuroinflammation. 2017;14 doi: 10.1186/s12974-017-0850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gelot A., Villapol S., Billette de Villemeur T. Astrocytic demise in the developing rat and human brain after hypoxic-ischemic damage. Dev. Neurosci. 2009;31:459–470. doi: 10.1159/000232564. [DOI] [PubMed] [Google Scholar]
- 26.Tomimoto H., Akiguchi I., Suenaga T. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.E., Hyun H.W., Min S.J., Kang T.C. Sustained HSP25 expression via ER stress in the rat hippocampus. Front. Cell. Neurosci. 2017;11 doi: 10.3389/fncel.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J.E., Ryu H.J., Yeo S.I., Kang T.C. P2X7 receptor differentially modulates astroglial apoptosis and clasmatodendrosis in the rat brain following status epilepticus. Hippocampus. 2011;21:1318–1333. doi: 10.1002/hipo.20850. [DOI] [PubMed] [Google Scholar]
- 29.Kim J.Y., Ko A.R., Kim J.E. P2X7 receptor-mediated PARP1 activity regulates astroglial death in the rat hippocampus following status epilepticus. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee D.S., Kim J.E. P2X7 receptor inhibits astroglial autophagy via regulating FAK- and PHLPP1/2- mediated AKT-S473 phosphorylation following kainic acid-induced seizures. Int. J. Mol. Sci. 2020:21. doi: 10.3390/ijms21186476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko A.-R., Hyun H.-W., Min S.-J., Kim J.-E. The differential DRP1 phosphorylation and mitochondrial dynamics in the regional specific astroglial death induced by status epilepticus. Front. Cell. Neurosci. 2016;10 doi: 10.3389/fncel.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Gong Q., Feng X. Astrocytic clasmatodendrosis in the cerebral cortex of methamphetamine abusers. Forensic. Sci. Res. 2017;2:139–144. doi: 10.1080/20961790.2017.1280890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eltony S.A., Abdelhameed S.Y. Effect of chronic administration of sildenafil citrate (Viagra) on the histology of the retina and optic nerve of adult male rat. Tissue Cell. 2017;49:323–335. doi: 10.1016/j.tice.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi Y., Kimura A., Nakamura H. Neuropathological findings from an autopsied case showing posterior reversible encephalopathy syndrome-like neuroradiological findings associated with premedication including tacrolimus for autologous peripheral blood stem cell transplantation. J. Neurol. Sci. 2017;375:382–387. doi: 10.1016/j.jns.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Cavanagh J.B., Nolan C.C., Seville M.P. The neurotoxicity of alpha-chlorohydrin in rats and mice: I. Evolution of the cellular changes. Neuropathol. Appl. Neurobiol. 1993;19:240–252. doi: 10.1111/j.1365-2990.1993.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 36.Misu T., Höftberger R., Fujihara K. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013;125:815–827. doi: 10.1007/s00401-013-1116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishiyama S., Misu T., Nuriya M. Complement-dependent and -independent aquaporin 4- antibody-mediated cytotoxicity in human astrocytes: pathogenetic implications in neuromyelitis optica. Biochem. Biophys. Rep. 2016;7:45–51. doi: 10.1016/j.bbrep.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafalowska J., Krajewski S., Doliska E., Dziewulska D. Does damage of perivascular astrocytes participate in blood-brain barrier permeability? Neuropatol. Pol. 1992;30:73–80. [PubMed] [Google Scholar]
- 39.Bouchat J., Gilloteaux J., Suain V. Ultrastructural analysis of thalamus damages in a mouse model of osmotic-induced demyelination. Neurotox. Res. 2019;36:144–162. doi: 10.1007/s12640-019-00041-x. [DOI] [PubMed] [Google Scholar]
- 40.Shimoda K., Mimaki M., Fujino S. Brain edema with clasmatodendrosis complicated ataxia telangiectasia. Brain Dev. 2017;39:629–632. doi: 10.1016/j.braindev.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Qin A.-P., Liu C.-F., Qin Y.-Y. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6:738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 42.Ryu H.J., Kim J.E., Yeo S.I. F-actin depolymerization accelerates clasmatodendrosis via activation of lysosome-derived autophagic astroglial death. Brain Res. Bull. 2011;85:368–373. doi: 10.1016/j.brainresbull.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Kim J.E., Ko A.R., Hyun H.W. P2RX7-MAPK1/2-SP1 axis inhibits MTOR independent HSPB-1 mediated astroglial autophagy. Cell Death Dis. 2018;9 doi: 10.1038/s41419-018-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu H.J., Kim J.E., Yeo S.I., Kang T.C. p65/RelA-Ser529 NF-kappaB subunit phosphorylation induces autophagic astroglial death (clasmatodendrosis) following status epilepticus. Cell. Mol. Neurobiol. 2011;31:1071–1078. doi: 10.1007/s10571-011-9706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tachibana M., Mohri I., Hirata I. Clasmatodendrosis is associated with dendritic spines and does not represent autophagic astrocyte death in influenza-associated encephalopathy. Brain Dev. 2019;41:85–95. doi: 10.1016/j.braindev.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Wickel J., Chung H.Y., Kirchhof K. Encephalitis with radial perivascular emphasis: not necessarily associated with GFAP antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nara A., Nagai H., Yamaguchi R. An unusual autopsy case of cytokine storm-derived influenza- associated encephalopathy without typical histopathological findings: autopsy case report. Am. J. Forensic Med. Pathol. 2015;36:3–5. doi: 10.1097/PAF.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z., Nicholls J.M., Liu F. Pulmonary and central nervous system pathology in fatal cases of hand foot and mouth disease caused by enterovirus A71 infection. Pathology. 2016;48:267–274. doi: 10.1016/j.pathol.2015.12.450. [DOI] [PubMed] [Google Scholar]
- 49.Torre M., Solomon I.H., Sutherland C.L. Neuropathology of a case with fatal CAR T-cell- associated cerebral edema. J. Neuropathol. Exp. Neurol. 2018;77:877–882. doi: 10.1093/jnen/nly064. [DOI] [PubMed] [Google Scholar]
- 50.Rubin D.B., Danish H.H., Ali A.B. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142:1334–1348. doi: 10.1093/brain/awz053. [DOI] [PubMed] [Google Scholar]
- 51.Andriezen W.L. The neuroglia elements in the human brain. Br. Med. J. 1893;2:227–230. doi: 10.1136/bmj.2.1700.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friede R.L., van Houten W.H. Relations between post-mortem alterations and glycolytic metabolism in the brain. Exp. Neurol. 1961;4:197–204. doi: 10.1016/0014-4886(61)90041-3. [DOI] [PubMed] [Google Scholar]
- 53.Kraig R.P., Chesler M. Astrocytic acidosis in hyperglycemic and complete ischemia. J. Cerebr. Blood Flow Metabol. 1990;10:104–114. doi: 10.1038/jcbfm.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S.C., Liu W., Dickson D.W., Brosnan C.F. In human fetal astrocytes exposure to interleukin-1 beta stimulates acquisition of the gd3+ phenotype and inhibits cell division. J. Neurochem. 1995;64:1800–1807. doi: 10.1046/j.1471-4159.1995.64041800.x. [DOI] [PubMed] [Google Scholar]
- 55.Nicaise C., Marneffe C., Bouchat J., Gillloteaux J. Osmotic demyelination: from an oligodendrocyte to an astrocyte perspective. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scalisi J., Balau B., Deneyer L. Blood-brain barrier permeability towards small and large tracers in a mouse model of osmotic demyelination syndrome. Neurosci. Lett. 2021 doi: 10.1016/j.neulet.2021.135665. [DOI] [PubMed] [Google Scholar]
- 57.Vanlandewijck M., He L., Mäe M.A. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- 58.Leblond C.P., Inoue S. Structure, composition, and assembly of basement membrane. Am. J. Anat. 1989;185:367–390. doi: 10.1002/aja.1001850403. [DOI] [PubMed] [Google Scholar]
- 59.Chan F., Inoue S. Lamina lucida of basement membrane: an artefact. Microsc. Res. Tech. 1994;28:48–59. doi: 10.1002/jemt.1070280106. [DOI] [PubMed] [Google Scholar]
- 60.Daneman R. The blood-brain barrier in health and disease. Ann. Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 61.Reese T.S., Karnovsky M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbott N.J., Rönnbäck L., Hansson E. Astroctye-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 63.Tian X., Leite D.M., Scarpa E. On the shuttling across the blood-brain barrier via tubule formation: mechanism and cargo avidity bias. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abc4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan L., Chow B.W., Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat. Rev. Neurosci. 2020;21:416–432. doi: 10.1038/s41583-020-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brown N., Pfau S.J., Gu C. Bridging barriers: a comparative look at the blood-brain barrier across organisms. Genes Dev. 2018;32:466–478. doi: 10.1101/gad.309823.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramsauer M., Krause D., Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. Faseb. J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 68.Mi H., Haeberle H., Barres B.A. Induction of astrocyte differentiation by endothelial cells. J. Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim H., Lee J.M., Park J.S. Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood-brain barrier. Biochem. Biophys. Res. Commun. 2008;372:243–248. doi: 10.1016/j.bbrc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 71.Kondo T., Hafezi-Moghadam A., Thomas K. Mice lacking insulin or insulin-like growth factor 1 receptors in vascular endothelial cells maintain normal blood-brain barrier. Biochem. Biophys. Res. Commun. 2004;317:315–320. doi: 10.1016/j.bbrc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 72.Armulik A., Genové G., Mäe M. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 73.Thurston G., Daly C. The complex role of angiopoietin-2 in the angiopoietin-Tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood- brain barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Didier N., Romero I.A., Créminon C. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J. Neurochem. 2003;86:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- 76.Nedergaard M. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iliff J.J., Wang M., Liao Y. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iliff J.J., Chen M.J., Plog B.A. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uemura M.T., Maki T., Ihara M. Brain microvascular pericytes in vascular cognitive impairment and dementia. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Page A.V., Liles W.C. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507–516. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schillemans M., Karampini E., Kat M., Bierings R. Exocytosis of Weibel-Palade bodies: how to unpack a vascular emergency kit. J. Thromb. Hemostat. 2019;17:6–18. doi: 10.1111/jth.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gust J., Hay K.A., Hanafi L.-A. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Canc. Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fajgenbaum D.C., June C.H. Cytokine storm. NEJM. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lele A.V., Alunpipatthanachai B., Qiu Q. Plasma levels, temporal trends and clinical associations between biomarkers of inflammation and vascular homeostasis after pediatric brain injury. Dev. Neurosci. 2019;41:177–192. doi: 10.1159/000502276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salas-McKee J., Kong W., Gladney W.L. CRISPR/Cas9-based genome editing in the era of CAR T cell immunotherapy. Hum. Vaccines Immunother. 2019;15:1126–1132. doi: 10.1080/21645515.2019.1571893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gust J., Ponce R., Liles W.C. Cytokines in CAR T cell-associated neurotoxicity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang J-T., Lee T-H., Lee I-N. Dexamethasone inhibits ICAM-1 and MMP-9 expression and reduces brain edema in intracerebral hemorrhagic rats. Acta Neurochir. 2011;153 doi: 10.1007/s00701-011-1122-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.