Abstract
The relationship between two common movement disorders, dystonia and tremor, is controversial. Both deficits have correlates in the network that includes connections between the cerebellum and the basal ganglia. In order to assess the physiological relationship between tremor and dystonia, we measured the activity of 727 pallidal single-neurons during deep brain stimulation surgery in patients with cervical dystonia without head oscillations, cervical dystonia plus jerky oscillations, and cervical dystonia with sinusoidal oscillations. Cluster analyses of spike-train recordings allowed classification of the pallidal activity into burst, pause, and tonic. Burst neurons were more common, and number of spikes within spike and inter-burst intervals was shorter in pure dystonia and jerky oscillation groups compared to the sinusoidal oscillation group. Pause neurons were more common and irregular in pure tremor group compared to pure dystonia and jerky oscillation groups. There was bihemispheric asymmetry in spontaneous firing discharge in pure dystonia and jerky oscillation groups, but not in sinusoidal oscillation group. These results demonstrate that the physiology of pallidal neurons in patients with pure cervical dystonia is similar to those who have cervical dystonia combined with jerky oscillations, but different from those who have cervical dystonia combined with sinusoidal oscillations. These results imply distinct mechanistic underpinnings for different types of head oscillations in cervical dystonia.
Keywords: Dystonic tremor, Essential tremor, Dystonia, Globus pallidus
Introduction
The dystonias are a group of disorders characterized by excessive muscle contractions leading to involuntary postures or movements that have a repetitive or twisting quality [1, 2]. Tremors are defined by regular oscillations of a body region, typically with a sinusoidal pattern [3, 4]. Although dystonia and tremor are viewed as distinct disorders, they share many relationships. For example, both dystonia and tremor are considered disorders of network involving the cerebellum, thalamus, basal ganglia, midbrain, and the cerebral cortex; but there are substantial differences in the network connectivity patterns between these two phenomenologies [5–30].
Cervical dystonia (CD) is the most common form of focal dystonia, and it is typically characterized by abnormal postures of the head. Sometimes CD can be associated with jerky head oscillations [31]. On some occasions, the head oscillations in CD can be sinusoidal [32–41]. The coexistence of two forms of oscillations (jerky versus sinusoidal) often results in disagreements regarding fundamental diagnostic process, leading to variability in therapeutic interventions and efficacy [4, 42, 43]. The physiological relationship between pure CD without any forms of head oscillations, CD with jerky oscillations, and CD with sinusoidal oscillations remains unclear. Contemporary literature utilizing the experimental strategies spanning across diverse disciplines such as neurophysiology, imaging, pathology, and clinical neuroscience highlighted the role of cerebellum. Patients with essential tremor who had presented with the involvement of the head also had reduced cerebellar gray matter density, while Purkinje cell loss is a known finding in patients with dystonia [13, 24]. Resting-state functional MRI revealed correlation of head tremor with cerebello-cortical areas [30]. The patients with head tremor had specific phenotype that highlights cerebellar involvement [9]. A phenotype of cervical dystonia that presents with early onset of head tremor had prominent ataxia compared to dystonia [20].
We set out to examine the physiological association between pure CD, CD with jerky head oscillations, and sinusoidal head oscillations. We analyzed the differences in the neuronal discharge patterns of the globus pallidus in three groups of patients – those with neck dystonia without any head oscillations (CD group), those with neck dystonia combined with jerky head oscillations (CD-J group), and those with neck dystonia combined with sinusoidal oscillations (CD-S group). We selected globus pallidus because it is accessible to measure single-unit physiology during standard-of-care deep brain stimulation surgery; and it is also a critical part of the network that is connected with the cerebellum. We predict: (1) If the two types of head oscillations in CD were both merely reflections of the underlying biology of dystonia, then we expect no differences among these three patient groups. (2) If the two types of head oscillations in CD reflected different biological processes, then we expect differences among all three patient groups. (3) If pure CD and CD-J groups were biologically related, then we expect to see their comparable physiological patterns that would be different compared to the CD-S group. (4) If pure CD and CD-S groups were biologically related, then we expect to see their comparable physiological patterns that would be different compared to CD-J group. (5) If pure CD-J and CD-S groups were biologically related, then we expect to see their comparable physiological patterns that would be different compared to pure CD group.
Methods
Subject Recruitment
Institutional ethics committee at N.N.Burdenko Neurosurgical Institute approved the study protocol. The subjects provided written consent prior to deep brain stimulation surgery. Thirteen idiopathic CD patients (7 men, age range of 31–60 years and 6 women age range 22–68 years), refractory to medical therapy, opted for the deep brain stimulation surgery of the bilateral globus pallidus. The average duration of symptoms was 6.86 ± 4.9 years (range 2–17 years). None of the patients had the diagnosis of Parkinsonism, Parkinson’s disease, history of stroke or other structural abnormalities affecting the basal ganglia or the cerebellum, exposure to dopamine receptor blocking agents, active psychiatric illness or dementia, and generalized dystonia. Table 1 depicts the clinical summary recorded immediately prior to the surgery.
Table 1.
Demographic information of recruited subjects
| N | Gender | Age | Duration | Dystonia phenomenology | Oscillations | Cells |
|---|---|---|---|---|---|---|
| 1 | m | 42 | 3 | Right torticollis Left laterocollis |
Pure dystonia | 46 |
| 2 | f | 53 | 6 | Left torticollis | Dystonic tremor | 49 |
| 3 | m | 44 | 2 | Left torticollis Right taterocollis |
Sinusoidal tremor | 51 |
| 4 | f | 22 | 17 | Right torticollis Right taterocollis |
Pure dystonia | 22 |
| 5 | m | 42 | 2 | Left torticollis Right taterocollis |
Dystonic tremor | 76 |
| 6 | m | 60 | 7 | Right torticollis | Pure dystonia | 51 |
| 7 | m | 41 | 2 | Right torticollis Right taterocollis |
Pure dystonia | 109 |
| 8 | f | 54 | 12 | Right torticollis Left laterocollis |
Dystonic tremor | 64 |
| 9 | m | 31 | 5 | Left torticollis | Pure dystonia | 41 |
| 10 | f | 68 | 6 | Right torticollis Left laterocollis |
Sinusoidal tremor | 66 |
| 11 | f | 63 | 6 | Right torticollis Left laterocollis |
Sinusoidal tremor | 61 |
| 12 | f | 40 | 15 | Right torticollis | Dystonic tremor | 46 |
| 13 | m | 52 | 6 | Right torticollis | Dystonic tremor | 45 |
Subject Classification
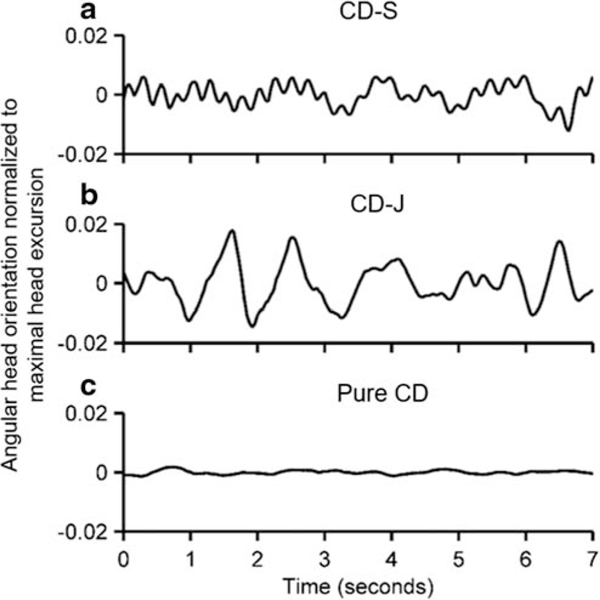
On clinical examination, five patients had pure dystonia of the neck without any clinically visible head oscillations (CD), five patients had jerky head oscillations in addition to neck dystonia (CD-J), and three patients had sinusoidal head oscillations in addition to neck dystonia (CD-S). In order to account for substantial inter-rater variability in calling oscillations sinusoidal versus jerky, we quantitatively verified the subjective classification by measuring the head position trajectory with a video-based face tracking algorithm. An example of sinusoidal head oscillations is depicted in Fig. 1a, while Fig. 1b depicts jerky oscillations. The trace depicted in Fig. 1c, an example of pure dystonia (i.e., CD group), has no oscillations. In all three traces in Fig. 1, the offset of head position is removed in order to facilitate better visualization of oscillations. The oscillation shape (in CD-J and CD-S groups, Fig. 1a, b) was further quantified by measuring total harmonic distortion – a measure of the ratio of the sum of the powers of all harmonic components to the power of the fundamental frequency of the time series. A smaller value of total harmonic distortion corresponds to a more sinusoidal nature, while any form of irregularity in the time series (in this case jerky oscillation) leads to higher values of total harmonic distortion. The value of total harmonic distortion of the sinusoidal oscillation group was 2.4 ± 0.3, while it was 5.9 ± 2.2 in those who had jerky oscillations; the difference was statistically significant (p < 0.01). The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) revealed an average severity score of 20.3 ± 3.8 (range 15–30).
Fig. 1.
Examples of three patients, one with (A) CD-S (B) CD-J and (C) pure CD. Angular head orientation normalized to maximal excursion are plotted on y-axis while corresponding timestamp is plotted on x-axis
Surgical Procedure and Physiological Data Collection
As part of clinical standard-of-care, the microelectrodes were advanced in an obliquely directed trajectory toward globus pallidum internus (GPi) through intervening cerebral cortex. The typical electrode track passed through the putamen and the globus pallidum externus (GPe). Characteristic electrophysiological signatures were utilized to identify the pallidal nuclei. For example, the GPe neurons were characterized by their typical discharge pattern that was associated with an increase in the background noise and numerous cells with high discharge rate. Further advancement of the electrode through internal lamina between GPe and GPi found fewer units with much lower but regular firing rate. Finally, the entrance of the track in GPi was evident by numerous neurons with high firing rate [44, 45].
Spike Sorting and Classification
Spontaneous single-unit activity (in the absence of voluntary muscle activation) was recorded as the electrode advanced through GPe and GPi. Isolated single-unit responses were saved on computer hard disk for post hoc analyses. Each cell was recorded for at least 20 s. Recording that contained more than 200 spikes was included in the analysis. The signals were pre processed and analyzed with Spike 2 (CED, Cambridge, UK). The signal was band-pass filtered (300–5000 Hz) and then aligned for subsequent spike sorting. Amplitude threshold, at the value of 4 times standard deviation, was used to isolate the spikes. The threshold was unique for each recorded neuron. Once identified, the single units were separated by manual cluster selection in principal component analysis (PCA) feature space based on several waveform parameters. The cell that was recorded for at least 20 s with > 200 spikes was considered for further analyses. The spike-trains were clustered based on the histograms for neural activity recordings (spike density histograms SDHs); the analysis revealed parameters unique to the neuronal firing pattern [46]. For grouping spike-trains into the patterns, we used hierarchical clustering approach using the Ward’s method for merging the branches. Jensen-Shannon Divergence (JSD) was used as a distance metrics. The elbow method was used to determine the optimal number of clusters.
We used the Poisson Surprise (PS) algorithm for burst detection [47]. The PS algorithm assumes that baseline neural discharge follows a Poisson process. Therefore the PS statistic, defined as S = − log(p) where “p” is the probability of more or equal as “N” spikes occurring in interval, could be used to maximize the PS statistic with a surprise maximization algorithm [48]. We disregarded the bursts with PS lower than chosen threshold. Reliably detected bursts for each isolated neuron were further used to determine parameters for the burst activity such as burst percent (ratio of spikes in burst to the total number of spikes) and inter-burst intervals. For burst, pause, and tonic neurons, we also analyzed instantaneous firing rate, coefficient of variance of interspike interval (ISI), and asymmetry index (median ISI/mean ISI) quantifying the skewness of irregularity in firing discharge.
Statistical analysis was performed in Matlab Statistics Toolbox (Mathworks, Natick, MA, USA); in some instances, we utilized available Matlab tools to prepare custom statistical software for the analysis. For variables that conformed to a normal distribution (Shapiro–Wilk’s W test, p > 0.05), we used one-way analysis of variance (ANOVA) for group comparisons. Post hoc analysis was performed with Tukey HSD test.
Results
Physiological Differences in Neuronal Discharge Patterns in Three Subgroups of CD
Our study addresses the controversial relationship between two common movement disorders, dystonia and tremor. Our overarching goal was to delineate whether there are biological differences in the pallidal activity in CD patients who have pure dystonia with or without phenomenologically distinct (i.e., jerky versus sinusoidal) forms of head oscillations. We studied 13 CD patients who opted for deep brain stimulation surgery, a clinical standard-of-care for refractory neck dystonia. In this population, five patients had CD with objectively confirmed (see methods and Fig. 1 for details) jerky head oscillations (CD-J group), five had objectively confirmed pure CD without clinically overt head oscillations (pure CD group), and three patients had CD with objectively confirmed sinusoidal head oscillations (CD-S group).
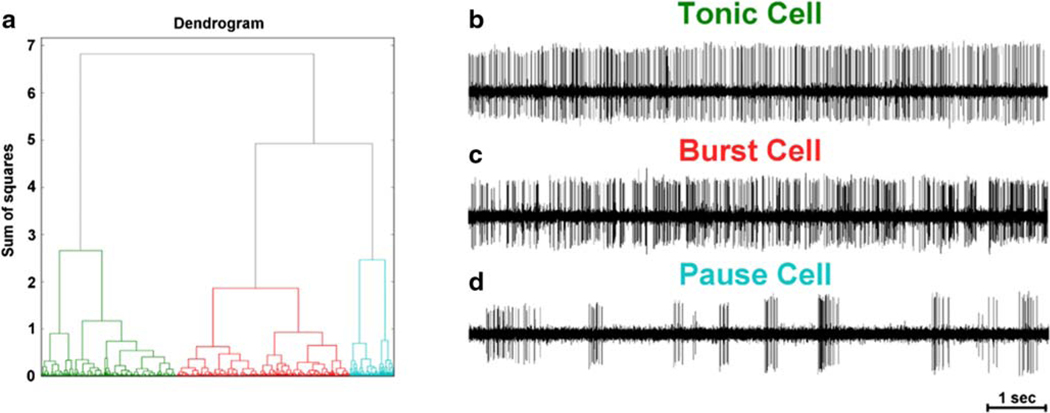
We analyzed responses of 727 pallidal neurons (398 GPi and 329 GPe) recorded from 13 CD patients (26 hemispheres). Further classification of the neurons using unsupervised clustering revealed specific characteristic that further allowed their labeling as burst, pause, and tonic neurons (Fig. 2a). The tonic neurons were classified when the cell had continuous firing activity without intervals of silence (Fig. 2b). The burst neurons had the periods of regular burst and pause activity (Fig. 2c). The pause neurons had recurrent periods of high frequency discharges separated by relatively long interval of silence sometimes lasting for up to several seconds (Fig. 2d).
Fig. 2.
(A) Dendrogram depicting clustering of spike train in three subtypes each with distinct firing characteristics – such as tonic neuron (B), burst neuron (C), and pause neuron (D)
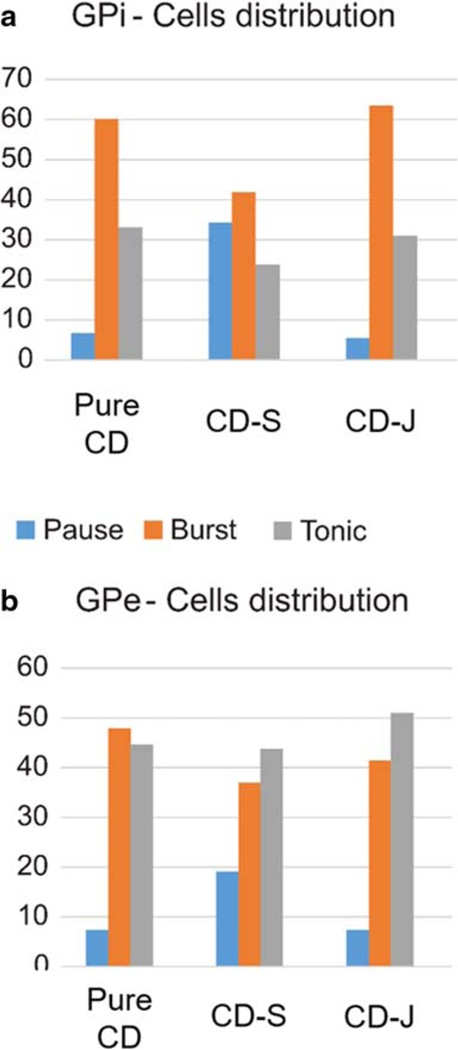
The survey of different types of neuronal patterns in GPi revealed that the patients in the CD group and the CD-J group had a smaller proportion of pause neurons as compared to those in the CD-S group. The burst neurons were highest in prevalence in the pure CD or CD-J groups. The results contrasted with the patients in the CD-S group, who had about the equal proportion of burst and pause neurons. The proportion of tonic neurons was much smaller in patients with pure CD or CD-J as compared to those with CD-S. This disparity in distribution of various cell types was significant in both GPi (X2 test, Fig. 3a, p < 0.001) and GPe (Fig. 3b, X2 test GPe, p = 0.04). In all three patient groups, the GPe had the least proportion of the pause neurons, followed by burst and tonic neurons.
Fig. 3.
Distribution of pause, burst and tonic neurons in globus pallidum interna (GPi) and externa (GPe). Number of cells in each category is plotted on y-axis, the x-axis depicts the patient category, color of bar plot depicts cell type
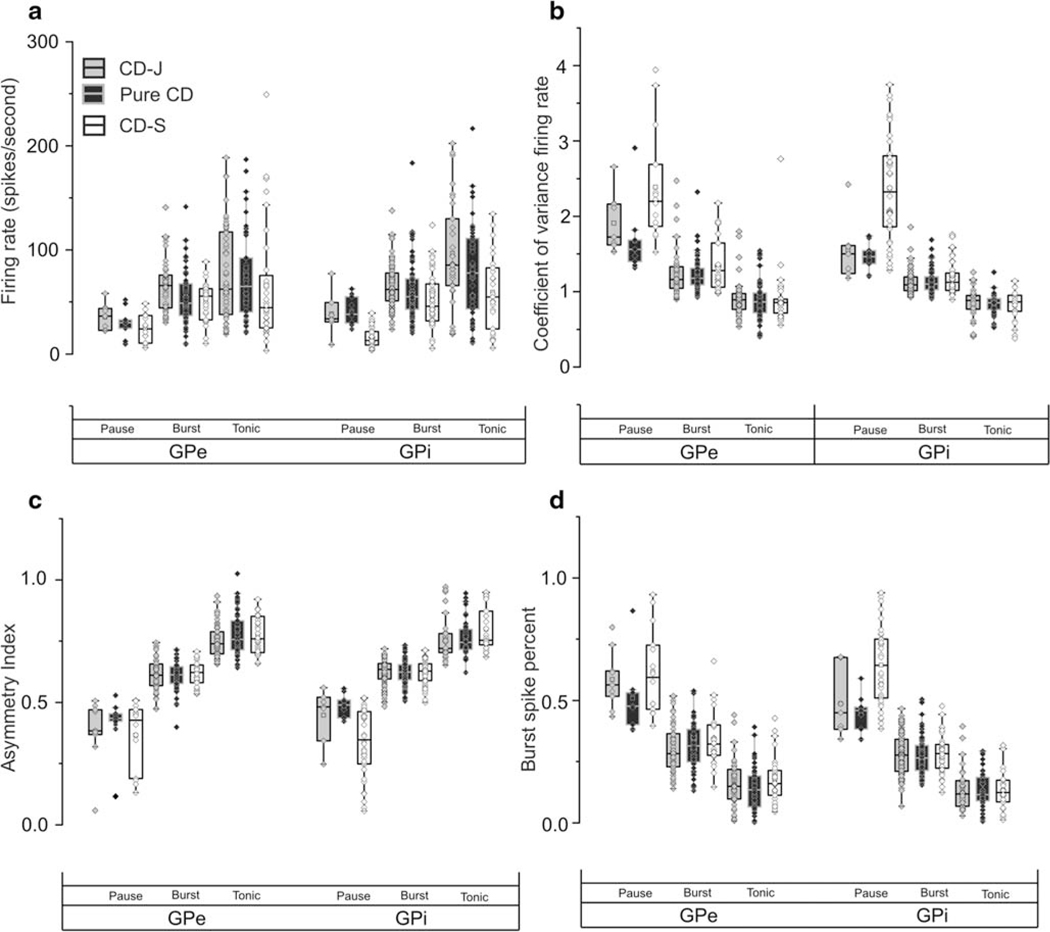
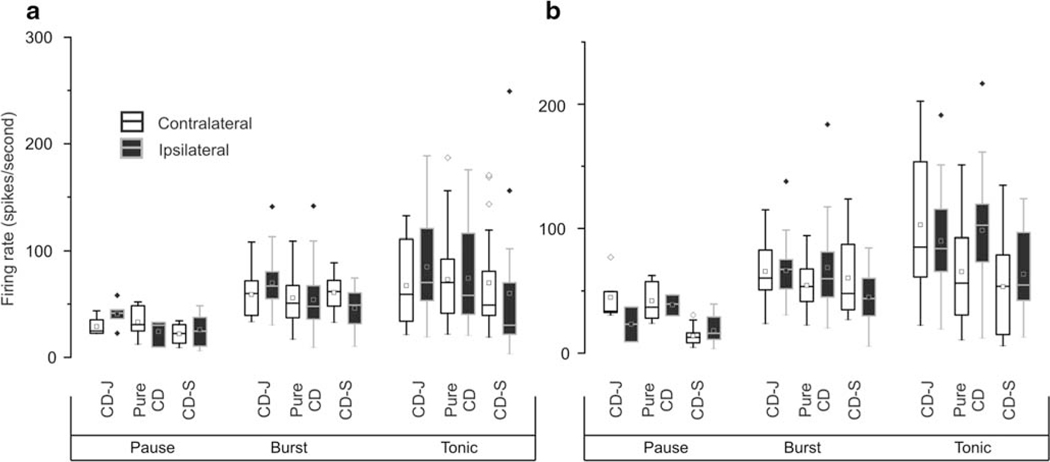
In subsequent analyses, we measured whether the presence of head oscillations and its classification as jerky or sinusoidal is correlated with the firing rate of GPi and GPe neurons. Figure 4a provides the overview. The firing rate of GPi pause neurons was significantly higher in the pure CD group (median:38 (range:29–55) spikes/s) or CD-J group (median:33 (range:31–43) spikes/s) compared to the CD-S (median:13 (range:8–21) spikes/s) (ANOVA: p < 0.001; Tukey: p = 0.0001 and p = 0.0002). The firing rate of GPi burst neuron in the CD-J group (median:61 (range:51–78) spikes/s) was also higher compared to the CD-S group (median:46 (range:32–67) spikes/s) (ANOVA, p = 0.007; Tukey: p = 0,006). The tonic cells had the highest firing rate among all three classes of GPi neurons. The CD-J group had the highest firing rate among tonic neurons (median:102 (range:72–135) spikes/s). The median firing rate of tonic neurons in the pure CD group was 78 spikes/s (range:43–111 spikes/s) and in CD-S group it was 54 spikes/s (range:24–82 spikes/s) (ANOVA: p = 0.0006; Tukey: p = 0.0005). Such differences were also seen in GPe, but they were not statistically significant (ANOVA, p > 0.05). In summary, the group that had CD combined with sinusoidal head oscillations featured lowest firing rate among all three subtypes of GPi neurons when compared with the corresponding neuronal subtype in pure CD or CD plus jerky oscillations groups. There were no significant differences in the firing rates for pure CD compared with CD plus jerky oscillations.
Fig. 4.
Summary of firing rate (A), coefficient of variance of the firing rate (B), asymmetry index (C), and burst spike percent (D) in pause, burst and tonic neurons recorded from GPi and GPe. The values of corresponding parameter are plotted on y-axis, while axis is category. Box length is interquartile interval, the dashed line in the center of the box is median value, while whisker is the range, symbols depict each single-unit
Then we measured whether the presence of different types of head oscillations were associated with the firing regularity of pallidal neurons. Figure 4 b provides the overview by comparing the firing rate coefficient of variance (CV). The CVof the GPi pause neurons was significantly lower in the pure CD (median:1.46 (range:1.37–1.54), Tukey: p = 0.0004) (Fig. 4b) or CD-J (median:1.55 (range:1.27–1.84), Tukey: p = 0.007) groups compared to the CD-S group (median:2.32 (range:1.86–2.80)). On the contrary, we did not find a significant difference in the CV of burst or tonic neurons in the three groups of patients (burst: CD-J median:1.09 (range:1.01–1.19), pure CD group median:1.10 (range:1.02–1.19), CD-S median:1.12 (range:1.07–1.25), ANOVA p = 0.42;. tonic: CD-J group median:0.87 (range:0.77–0.93), pure CD group median:0.85 (range:0.76–0.91), CD-S group median:0.86 (range:0.74–0.95), ANOVA, p = 0.87) (Fig. 4b). The CV of GPe pause neurons was significantly lower (ANOVA, p = 0.03; Tukey: p = 0.03) in patients from pure CD group (median:1.56 (range:1.39–1.68)) compared to those from the CD-S group (median:2.20 (range:1.87–2.69)) (Fig. 4b). However, we did not find significant difference in the CV of GPe burst or tonic neurons among three groups of patients (burst: CD-J group median:1.16 (range:1.04–1.34), pure CD group median:1.18 (range:1.09–1.31), CD-S group median:1.27 (range:1.06–1.64), ANOVA p = 0.21; tonic: CD-J group median:0.90 (range:0.76–0.98), pure CD group median:0.86 (range:0.72–0.98), CD-S group median:0.85 (range:0.72–0.90), ANOVA, p = 0.73) (Fig. 4b).
In subsequent analysis, we asked whether the presence of different types of head oscillations lead to the skewness in the irregularity of the neuronal discharge. Asymmetry index, the ratio of median interspike interval to mean interspike interval, was measured for each neuron to quantify the skewness of irregularity. Higher asymmetry index suggests more regular firing, as expected in tonic neurons compared to burst and pause neurons (Fig. 4c). The GPi pause neurons in patients with CD-J or pure CD had significantly higher asymmetry index compared to CD-S (CD-J median:0.48 (range:0.41–0.51), pure CD median:0.49 (range:0.43–0.51), CD-S median:0.34 (range:0.25–0.46), ANOVA p < 0.005; Tukey: p = 0.04 and p = 0.003). No significant differences in asymmetry index in patient subtypes were noticeable in GPi burst or tonic neurons, or any GPe neurons (Fig. 4c). These results confirmed that irregularity in the firing rate is higher in pause neurons. We also found that pause neurons in the CD-S group are more irregular and skewed compared to those in CD-J or pure CD groups. There were no differences in the pause neuron irregularities or skewness among CD-J and pure CD groups.
Additional analysis, looking at bursting behavior, focused on burst and pause neurons. We computed burst spike percent – a measure of portion of total spikes that are part of the burst (Fig. 4d). GPi pause neurons had significantly lower burst spike percent in CD-J or pure CD groups (CD-J group (median:0.47 (range:0.38–0.60); pure CD group (median:0.45 (range:0.38–0.47)) compared to CD-S (median:0.64 (range:0.51–0.75), ANOVA: p < 0.001; Tukey: p = 0.003 and p = 0.0001). InGPe pause neurons, there was significantly lower burst spike percent in pure CD group (median:0.47 (range:0.40–0.53)) compared to the CD-S group (median:0.59 (range:0.46–0.72), Tukey: p = 0.0008, ANOVA p < 0.001) or CD-J group (median:0.58 (range:0.51–0.73), Tukey p = 0.02, ANOVA p < 0.001). Then we measured inter-burst interval – the time duration between two adjacent bursts. The inter-burst interval in GP pause neurons in CD-J (GPi: median:0.52 (range:0.34–0.66); GPe: median:0.64 (range:0.53–0.83)) and pure CD (GPi: median:0.42 (range:0.35–0.58); GPe: median:0.56(range:0.47–0.;73)) was significantly shorter compared to CD-S (GPi: median:1.05 (range:0.76–1.33); GPe: median:1.01 (range:0.75–1.59), ANOVA: p < 0.001; Tukey: p < 0.005). The inter-burst interval of the burst neurons measured in CD-J (GPi: median:0.48 (range:0.39–0.57); GPe: median:0.53 (range:0.45–0.67)) or pure CD groups (GPi: median:0.52 (range:0.39–0.67); GPe: median:0.57 (range:0.46–0.87) was significantly shorter compared to CD-S group (GPi: median:0.65 (range:0.47–0.93); GPe: median:0.78 (range:0.73–0.97), ANOVA: p < 0.01; Tukey: p < 0.005).
Lateralized Asymmetry in Different Subtypes of Cervical Dystonia
Asymmetric pallidal activity is a feature of CD [27, 49, 50]. Such asymmetry is evident in GPi, but lateralized differences in GPe are insignificant; the asymmetry is much more robust in GPi tonic neurons [27]. Here we asked whether pallidal asymmetry is influenced by the presence of jerky or sinusoidal oscillations. We compared interhemispheric differences in the pallidal burst, pause, and tonic neurons in all three patient groups. The Gpi tonic neurons had robust firing rate asymmetry in pure CD group; the neurons recorded from the GPi ipsilateral to the direction of torticollis (i.e., contralateral to the dystonic sternocleidomastoid) had significantly higher firing rate (ipsilateral: median:103 (range:73–119); contralateral: median:56 (range:31–93); ANOVA, p < 0.01). Such asymmetry of tonic neuron activity was not statistically significant in GPe tonic neurons (ipsilateral: median:58 (range:40–115); contralateral: median:70 (range:41–93); ANOVA, p > 0.05). In the CD-J group, there was interhemispheric disparity in tonic GPi neuron activity, but the difference was not statistically significant (ipsilateral: median:86 (range:65–124); contralateral: median:113 (range:75–152); ANOVA, p = 0.26). The summary of these differences is graphically described in Fig. 5. In the CD-S group, there was no interhemispheric disparity in instantaneous firing rate in any type (burst, pause, or tonic) of GPi or GPe neurons (Fig. 5). Likewise, burst and pause neuron firing rates were not significantly different between two hemispheres in patients who had pure CD or CD-J (Fig. 5). Coefficient of variance, asymmetry index, burst percent, and inter-burst interval was also not significantly different between two hemispheres in all three groups of patients (Table 2).
Fig. 5.
Summary of comparison of ipsilateral versus contralateral firing rate of GPe(A) and GPi (B) neurons. Laterality is determined by the direction of the dystonia
Table 2.
Bihemispheric (ipsilateral (ipsi) versus contralateral (contra)) comparison of burst parameters
| No tremor | Tremor | Jerky | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ipsi | Contra | p | Ipsi | Contra | p | Ipsi | Contra | p | |
| GPi | |||||||||
| Firing rate | 58.64 | 53.14 | 0.06 | 30.70 | 31.05 | 0.87 | 65.52 | 59.47 | 0.61 |
| Coefficient of Variance | 1.13 | 1.10 | 0.65 | 1.44 | 1.40 | 0.93 | 1.12 | 1.10 | 0.54 |
| Asymmetry Index | 0.60 | 0.62 | 0.91 | 0.55 | 0.52 | 0.5 | 0.62 | 0.63 | 0.86 |
| Burst spike percent | 0.27 | 0.28 | 0.91 | 0.38 | 0.39 | 0.78 | 0.31 | 0.27 | 0.14 |
| Inter-burst interval | 0.46 | 0.56 | 0.01 | 0.75 | 0.98 | 0.68 | 0.48 | 0.48 | 0.62 |
| GPe | |||||||||
| Firing rate | 46.77 | 48.30 | 0.66 | 40.10 | 48.07 | 0.28 | 65.66 | 48.55 | 0.05 |
| Coefficient of variance | 1.19 | 1.25 | 0.32 | 1.36 | 1.70 | 0.74 | 1.20 | 1.29 | 0.48 |
| Asymmetry index | 0.61 | 0.59 | 0.39 | 0.59 | 0.56 | 0.54 | 0.60 | 0.59 | 0.86 |
| Burst spike percent | 0.33 | 0.34 | 0.59 | 0.39 | 0.40 | 0.90 | 0.31 | 0.32 | 0.73 |
| Inter-burst interval | 0.54 | 0.63 | 0.63 | 0.81 | 0.85 | 0.66 | 0.55 | 0.54 | 0.79 |
Discussion
Two common movement disorders dystonia and tremor have correlates in abnormal neural network that comprises of the cerebellum, basal ganglia, thalamus, midbrain, and the cerebral cortex. We utilized our ability to measure single-unit activity from the basal ganglia (GPi and GPe) to distinguish physiological differences between CD patients who had pure neck dystonia, with or without jerky or sinusoidal head oscillations. We examined the physiological association between different types of head oscillations in CD. We expect if the two different types of head oscillations in CD are both merely reflections of the underlying biology of dystonia, then there will be no differences among these three groups of patients. If the two different types of head oscillations in CD reflect different biological processes, then we expect differences among all three groups of patients. We found that CD and CD-J groups have comparable physiological patterns that are different compared to CD-S group supporting biological relationship between pure CD and CD-J. Contemporary literature over the last one decade has increasingly supported the role of mesencephalic neural integrator in CD [26, 28, 51]. According to the network model in CD, the impairment anywhere in the network, even outside of the integrator, can lead to deficits in neural integration that is feedback dependent. There is increasing evidence for involvement of cerebellum in the network model for dystonia [5–30]. The cerebellar role in dystonia would be even more supported when the oscillations coexisting with dystonia have sinusoidal features as seen in the essential tremor that is also thought to be related to cerebellar deficits [25, 41]. Our results depicting distinct physiological characteristics of CD-J and pure CD, especially when compared with CD-S, suggest that the latter may have independent physiological correlate.
Consistent with previous studies of other types of dystonia, we found abnormal burst neurons in the GPi of CD patients [44,45, 52–54].There was a higher prevalence of burst neurons and lower prevalence of pause neurons in patients with CD or CD combined with jerky oscillations, compared with CD plus sinusoidal oscillations. Pause neurons were more irregular in the group with CD combined with sinusoidal oscillations. The number of spikes were higher (i.e., the bursts were denser) in the pause neurons in the group with sinusoidal oscillations compared to the other groups. Inter-burst interval measured in burst and pause neurons from the group with sinusoidal oscillations was significantly longer. These results suggest that physiology and classification of neuronal subtypes in the pallidum in patients with jerky oscillations were comparable to the patients with pure dystonia. However, those who had neck dystonia with sinusoidal oscillations were significantly different from the patients with pure dystonia or dystonia plus jerky oscillations. In other words, jerky oscillations appear to be a forme fruste of dystonia with no significant differences in the pallidal physiology; but a distinct biological dysfunction leads to dystonia with sinusoidal oscillations.
Previous studies quantifying the single-unit properties of GPi neurons in dystonia reported mean instantaneous firing rate of 55.3 ± 1.3 spikes/s [53]. Our study, had a large variability in the instantaneous firing rate ranging between the median (confidence interval) value of 13 (range: 8–21) spikes/s in pause neurons in the CD-S group and median of 102 (range: 72–135) spikes/s in tonic cells of CD-J group. The disparity could be due to the fact that previous studies combined findings from different types of dystonia (idiopathic CD, generalized dystonia, tardive dystonia, and secondary dystonia). We also emphasize that we found comparable firing rate of burst neurons as reported in the previous studies that also found burst neurons as predominant subtype in their dystonic patients [53]. Another study reported GPe pause cell firing rate of 54 ± 24 spikes/s in isolated dystonia [52], while we found GPe pause cell firing rate of 24–38 spikes/s. The differences should be interpreted with the caveat that we only studied isolated CD, while dystonia in the other study included patients who had DYT1 or multifocal/segmental dystonia [52].
Previous studies discovered pallidal asymmetry in CD, and further predicted that such asymmetry is due to the imbalance in the extrinsic feedback to the GPi [27, 49, 50]. However, these studies utilized CD as an entire (unclassified) cohort to examine the asymmetry and its correlation with lateralized severity of dystonia. These results further classified previously published cohort [27] into CD-S, CD-J, and pure CD discovering asymmetric firing rate of tonic neurons in the CD-J and pure CD groups, but lack of such asymmetry in CD-S group. The GPi firing rate ipsilateral to the direction of head turning was higher in pure CD group with statistically significant difference, CD-J group had asymmetry that did not reach statistical significant. We speculate that asymmetry in the feedback to the pallidum may explain such disparity. For example, in patients with pure dystonia (i.e., no head oscillations), the pallidal feedback driven by asymmetric static pull in the direction of head turning leads to asymmetric increase in ipsilateral GPi firing rate. In contrast, in patients with jerky or sinusoidal oscillations, the dynamic feedback due to rapid head oscillations may override the influence of slower head movements.
The interhemispheric differences in the firing rates that were seen in tonic neurons of pure CD or CD-J groups as opposed to the CD-S group is unlikely to explain overall differences in the firing rates that were noted in CD-S group. For example, the firing rates of tonic GPe neurons were also significantly different among patients with CD-S in comparison to pure CD or CD-J; however, there were no interhemispheric differences in the firing rates of GPe tonic neurons. Although the pause neurons in patients with CD-S were more irregular and skewed compared to those in CD-J or pure CD groups, there were no interhemispheric disparities in the asymmetry index and irregularity of these cells. Likewise, GPi pause neurons in CD-S patients had significantly higher burst-spike percent compared to pure CD and CD-J groups; however, the interhemispheric disparity was not present. In all subjects, the data collection was performed under identical protocol, i.e., at the time of single-unit recordings, the subjects were alert (non-anesthetized). Hence, the observed differences in single-unit physiology among different subgroups of neurons could not be attributed to pharmacological effects of the anesthetic agent.
Conclusion
Our results suggest biological differences in the basal ganglia network behavior in pure cervical dystonia or cervical dystonia combined with irregular head oscillations compared with cervical dystonia with sinusoidal oscillations. From the conceptual perspective, if we focus on the pure dystonia group as “benchmark,” then jerky head oscillations are physiologically comparable phenomenon, perhaps forme fruste of pure dystonia; however, a distinct biological process is present when dystonia is associated with sinusoidal head oscillations.
Acknowledgments
Funding Information The study was funded by the Russian Science Foundation project 18–15-00009 (Sedov) for data collection and analysis; The Russian Foundation for Basic Research project 18–315-00202 and 20–015-00438 (Semenova) for estimation of head oscillation; The Career Development Grant from the American Academy of Neurology (Shaikh); George C. Cotzias Memorial Fellowship from American Parkinson’s Disease Association (Shaikh), Network Models in Dystonia grant from the Dystonia Medical Research Foundation (Shaikh), philanthropic funds to the department of neurology at University Hospitals (Shaikh). NIH U54 TR001456 (Jinnah).
References
- 1.Evatt ML, Freeman A, Factor S. Adult-onset dystonia. Handb Clin Neurol. 2011;100:481–511. [DOI] [PubMed] [Google Scholar]
- 2.Jinnah HA, Berardelli A, Comella C, Defazio G, DeLong M, Factor S, et al. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;7:926–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elias WJ, Shah BB. Tremor. JAMA. 2014;311:948–54. [DOI] [PubMed] [Google Scholar]
- 4.Govert F, Deuschl G. Tremor entities and their classification: an update. Curr Opin Neurol. 2015;28:393–9. [DOI] [PubMed] [Google Scholar]
- 5.Avanzino L, Ravaschio A, Lagravinese G, Bonassi G, Abbruzzese G, Pelosin E. Adaptation of feedforward movement control is abnormal in patients with cervical dystonia and tremor. Clin Neurophysiol. 2018;129:319–26. 10.1016/j.clinph.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Bares M, Lungu OV, Husarova I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–35. 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 7.Berman BD, Honce JM, Shelton E, Sillau SH, Nagae LM. Isolated focal dystonia phenotypes are associated with distinct patterns of altered microstructure. Neuroimage Clin. 2018;19:805–12. 10.1016/j.nicl.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bologna M, Berardelli A. Cerebellum: an explanation for dystonia? Cerebellum Ataxias. 2017;4:6. 10.1186/s40673017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bologna M, Berardelli I, Paparella G, Ferrazzano G, Angelini L, Giustini P, et al. Tremor distribution and the variable clinical presentation of essential tremor. Cerebellum. 2019;18:866–72. 10.1007/s12311-019-01070-0. [DOI] [PubMed] [Google Scholar]
- 10.Corp DT, Joutsa J, Darby RR, Delnooz CCS, van de Warrenburg BPC, Cooke D, et al. Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142:1660–74. 10.1093/brain/awz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmaire C, Vidailhet M, Elbaz A, Bourdain F, Bleton JP, Sangla S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology. 2007;69:376–80. 10.1212/01.wnl.0000266591.49624.1a. [DOI] [PubMed] [Google Scholar]
- 12.DeSimone JC, Archer DB, Vaillancourt DE, Wagle SA. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain. 2019;142:1644–59. 10.1093/brain/awz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyke JP, Cameron E, Hernandez N, Dydak U, Louis ED. Gray matter density loss in essential tremor: a lobule by lobule analysis of the cerebellum. Cerebellum Ataxias. 2017;4:10. 10.1186/s40673-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filip P, Gallea C, Lehericy S, Bertasi E, Popa T, Marecek R, et al. Disruption in cerebellar and basal ganglia networks during a visuo-spatial task in cervical dystonia. Mov Disord. 2017;32:757–68. 10.1002/mds.26930. [DOI] [PubMed] [Google Scholar]
- 15.Filip P, Lungu OV, Bares M. Dystonia and the cerebellum: a new field of interest in movement disorders? Clin Neurophysiol. 2013;124:1269–76. 10.1016/j.clinph.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Filip P, Lungu OV, Shaw DJ, Kasparek T, Bares M. The mechanisms of movement control and time estimation in cervical dystonia patients. Neural Plast. 2013;2013:908741. 10.1155/2013/908741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgescu EL, Georgescu IA, Zahiu CDM, Steopoaie AR, Morozan VP, Pana AS, et al. Oscillatory cortical activity in an animal model of dystonia caused by cerebellar dysfunction. Front Cell Neurosci. 2018;12:390. 10.3389/fncel.2018.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenka A, Bhalsing KS, Panda R, Jhunjhunwala K, Naduthota RM, Saini J, et al. Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology. 2017;59: 157–68. 10.1007/s00234-016-1771-1. [DOI] [PubMed] [Google Scholar]
- 19.Mantel T, Meindl T, Li Y, Jochim A, Gora-Stahlberg G, Kraenbring J, et al. Network-specific resting-state connectivity changes in the premotor-parietal axis in writer’s cramp. Neuroimage Clin. 2018;17:137–44. 10.1016/j.nicl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merola A, Dwivedi AK, Shaikh AG, Tareen TK, Da Prat GA, Kauffman MA, et al. Head tremor at disease onset: an ataxic phenotype of cervical dystonia. J Neurol. 2019;266:1844–51. 10.1007/s00415-019-09341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–509. 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prudente CN, Pardo CA, Xiao J, Hanfelt J, Hess EJ, Ledoux MS, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241: 95–104. 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quattrone A, Cerasa A, Messina D, Nicoletti G, Hagberg GE, Lemieux L, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29:1692–7. 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sedov A, Popov V, Shabalov V, Raeva S, Jinnah HA, Shaikh AG. Physiology of midbrain head movement neurons in cervical dystonia. Mov Disord. 2017;32:904–12. 10.1002/mds.26948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sedov A, Usova S, Semenova U, Gamaleya A, Tomskiy A, Crawford JD, et al. The role of pallidum in the neural integrator model of cervical dystonia. Neurobiol Dis. 2019;125:45–54. 10.1016/j.nbd.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaikh AG, Zee DS, Crawford JD, Jinnah HA. Cervical dystonia: a neural integrator disorder. Brain. 2016;139:2590–9. 10.1093/brain/aww141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tewari A, Fremont R, Khodakhah K. It’s not just the basal ganglia: cerebellum as a target for dystonia therapeutics. Mov Disord. 2017;32:1537–45. 10.1002/mds.27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Lei D, Suo X, Li N, Lu Z, Li J, et al. Resting-state fMRI study on drug-naive patients of essential tremor with and without head tremor. Sci Rep. 2018;8:10580. 10.1038/s41598-018-28778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahn S. The varied clinical expressions of dystonia. Neurol Clin. 1984;2:541–54. [PubMed] [Google Scholar]
- 32.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–26. [DOI] [PubMed] [Google Scholar]
- 33.Jankovic J, Leader S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–91. [DOI] [PubMed] [Google Scholar]
- 34.Deuschl G, Heinen F, Guschlbauer B, Schneider S, Glocker FX, Lucking CH. Hand tremor in patients with spasmodic torticollis. Mov Disord. 1997;12:547–52. [DOI] [PubMed] [Google Scholar]
- 35.Pal PK, Samii A, Schulzer M, Mak E, Tsui JK. Head tremor in cervical dystonia. Can J Neurol Sci. 2000;27:137–42. [PubMed] [Google Scholar]
- 36.Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–3. [DOI] [PubMed] [Google Scholar]
- 37.Godeiro-Junior C, Felicio AC, Aguiar PC, Borges V, Silva SM, Ferraz HB. Head tremor in patients with cervical dystonia: different outcome? Arq Neuropsiquiatr. 2008;66:805–8. [DOI] [PubMed] [Google Scholar]
- 38.Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL. Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol. 2010;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shatunov A, Sambuughin N, Jankovic J, Elble R, Lee HS, Singleton AB, et al. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain. 2006;129:2318–31. [DOI] [PubMed] [Google Scholar]
- 40.Rana AQ, Kabir A, Dogu O, Patel A, Khondker S. Prevalence of blepharospasm and apraxia of eyelid opening in patients with parkinsonism, cervical dystonia and essential tremor. Eur Neurol. 2012;68:318–21. [DOI] [PubMed] [Google Scholar]
- 41.Louis ED, Hernandez N, Alcalay RN, Tirri DJ, Ottman R, Clark LN. Prevalence and features of unreported dystonia in a family study of “pure” essential tremor. Parkinsonism Relat Disord. 2013;19:359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elble RJ. What is essential tremor? Curr Neurol Neurosci Rep. 2013;13:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor - some controversial aspects. Mov Disord. 2011;26:18–23. 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
- 44.Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. [DOI] [PubMed] [Google Scholar]
- 45.Vitek JL, Delong MR, Starr PA, Hariz MI, Metman LV. Intraoperative neurophysiology in DBS for dystonia. Mov Disord. 2011;26(Suppl 1):S31–6. 10.1002/mds.23619. [DOI] [PubMed] [Google Scholar]
- 46.Myrov V, Sedov A, Belova E. Neural activity clusterization for estimation of firing pattern. J Neurosci Methods. 2019;311:164–9. 10.1016/j.jneumeth.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Cotterill E, Charlesworth P, Thomas CW, Paulsen O, Eglen SJ. A comparison of computational methods for detecting bursts in neuronal spike trains and their application to human stem cell-derived neuronal networks. J Neurophysiol. 2016;116:306–21. 10.1152/jn.00093.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–39. 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 49.Moll CK, Galindo-Leon E, Sharott A, Gulberti A, Buhmann C, Koeppen JA, et al. Asymmetric pallidal neuronal activity in patients with cervical dystonia. Front Syst Neurosci. 2014;8:15. 10.3389/fnsys.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JR, Kiss ZH. Interhemispheric difference of pallidal local field potential activity in cervical dystonia. J Neurol Neurosurg Psychiatry. 2014;85:306–10. 10.1136/jnnp-2013-305476. [DOI] [PubMed] [Google Scholar]
- 51.Shaikh AG, Wong AL, Zee DS, Jinnah HA. Keeping your head on target. J Neurosci. 2013;33:11281–95. 10.1523/JNEUROSCI.3415-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sani S, Ostrem JL, Shimamoto S, Levesque N, Starr PA. Single unit “pauser” characteristics of the globus pallidus pars externa distinguish primary dystonia from secondary dystonia and Parkinson’s disease. Exp Neurol. 2009;216:295–9. 10.1016/j.expneurol.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starr PA, Rau GM, Davis V, Marks WJ Jr, Ostrem JL, Simmons D, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–76. 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 54.Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–62. [DOI] [PubMed] [Google Scholar]