Abstract
Membrane potential (Vmem) is a fundamental biophysical signal present in all cells. Vmem signals range in time from milliseconds to days, and they span lengths from microns to centimeters. Vmem affects many cellular processes, ranging from neurotransmitter release to cell cycle control to tissue patterning. However, existing tools are not suitable for Vmem quantification in many of these areas. In this review, we outline the diverse biology of Vmem, drafting a wish list of features for a Vmem sensing platform. We then use these guidelines to discuss electrode-based and optical platforms for interrogating Vmem. On the one hand, electrode-based strategies exhibit excellent quantification but are most effective in short-term, cellular recordings. On the other hand, optical strategies provide easier access to diverse samples but generally only detect relative changes in Vmem. By combining the respective strengths of these technologies, recent advances in optical quantification of absolute Vmem enable new inquiries into Vmem biology.
Keywords: microscopy, fluorescence, membrane potential, electrophysiology, fluorescence lifetime, quantitative imaging
1. INTRODUCTION
Membrane potential (Vmem), or voltage across a lipid bilayer, is ubiquitous in biology. In electrically excitable cells such as neurons and cardiomyocytes, millisecond Vmem fluctuations cause release of neurotransmitters and contraction. Both excitable and nonexcitable cells exhibit Vmem signals, which vary over timespans of seconds to days. Furthermore, an estimated 10–50% of the cellular energy budget goes to maintain Vmem, even in nonexcitable cells (96). Given this large energy expenditure, the functions of Vmem span far beyond excitable electrical activity in the brain or heart (2); recent work has linked Vmem to cell proliferation (26, 42), differentiation (25, 104), and tissue patterning (64).
Membrane potential (Vmem):
a voltage across a membrane, arising from differences in ion concentration on either side
Vmem results from differences in ion concentration across a semipermeable membrane. Although this phenomenon is best studied with respect to the plasma membrane of mammalian cells, any selectively permeable membrane can maintain a voltage. For example, voltages in bacterial communities (58, 60, 89) and across organelle membranes (20, 124) also have signaling roles. Unless otherwise indicated, we use the term Vmem to indicate the plasma membrane potential between the cytosol and the extracellular space. In addition, we use absolute Vmem to indicate the value of the membrane potential in millivolts, rather than a relative measure of changes in Vmem.
Absolute Vmem:
the value of Vmem in mV units; this term is used to differentiate between Vmem recording techniques that can quantify Vmem and those that can only track relative changes
Vmem is inherently a system-level property, determined by an array of ion channels and pumps and sensed by diverse cellular components. In mammalian cells, the primary ions involved in generating Vmem are Na+ and K+ (Figure 1a), but many other species (including Cl−, Ca2+, H+, and organic anions) play a role. Within the cellular Vmem response, voltage-gated ion channels are the best-documented class of voltage-sensitive proteins; these channels transduce Vmem changes into additional ionic currents or fluctuations in the second messenger Ca2+. Nevertheless, all membrane-localized proteins experience Vmem, and more of these proteins may be sensitive to Vmem than was previously thought. Evidence of Vmem sensitivity exists for various membrane components (121), including phosphatases (80), G protein–coupled receptors (92, 93), and the membrane organization itself (123). For most systems, we have an incomplete understanding of both the determinants of Vmem and the factors that respond to Vmem.
Figure 1.
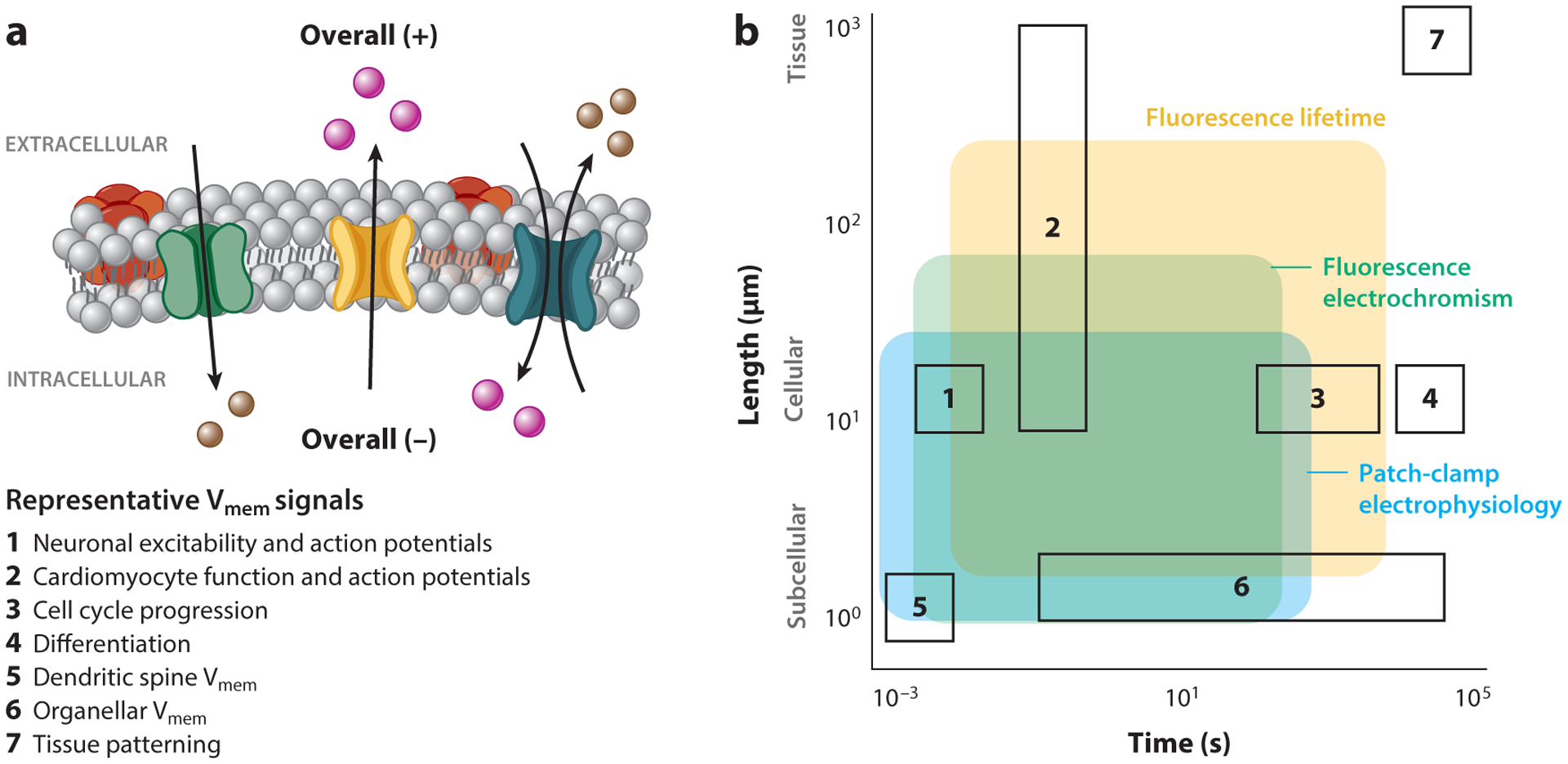
Membrane potential (Vmem) signals and measurement techniques across time and space. (a) A schematic of cellular Vmem, with examples of seven biological processes where Vmem signaling plays a role. The resistance to ionic flow between compartments sets the length scale of Vmem signals; Vmem can scale across tissues or be compartmentalized within organelles or dendritic spines. The properties of ion channels and pumps that determine Vmem, as well as ion diffusion between compartments, dictate the time duration of a Vmem response. (b) Biological space accessible to the three most common strategies for absolute Vmem measurement (shaded regions), overlaid with example biological process of interest (outlined boxes, numbered as in panel a). While many techniques can report cellular Vmem on the scale of milliseconds or seconds, longer recordings or recordings across large areas are difficult to access. Shaded areas indicate regions where single trial recordings retain voltage resolution on the scale of biological Vmem changes (≤20 mV) and minimally alter the biological sample. Recordings with fluorescence electrochromic dyes require recalibration with an electrode on each cell, whereas fluorescence lifetime and patch-clamp electrophysiology can be applied as stand-alone measurements that are comparable between cells. Shaded areas are restricted to demonstrated application space for each technique and do not indicate the full extent of its possible use.
These gaps in our understanding are largely attributable to limitations in the existing Vmem recording toolkit, as most Vmem recording strategies are only tractable in a small subset of biological samples. In particular, strategies to record absolute Vmem and absolute Vmem changes (i.e., to document that Vmem depolarized by 20 mV) are unavailable in many systems (Figure 1b). To better understand necessary features of Vmem recording strategies, we first summarize known Vmem signaling pathways and build a wish list of features for an optimized Vmem reporter. We then turn our focus to the two primary approaches for recording Vmem: electrodes and fluorescent sensors. We review recent progress in Vmem sensing platforms and highlight areas where development of new tools would facilitate discovery.
2. MEMBRANE POTENTIAL SIGNALING ACROSS TIME AND SPACE
Vmem signaling is most extensively studied in excitable tissue, where rapid changes in cellular Vmem transmit information. However, important Vmem signals also occur across minutes, hours, and days(2). Similarly, as a spatial signal, Vmem is canonically treated as cell autonomous and uniform in spherical model cells. Nevertheless, Vmem can be compartmentalized subcellularly (20, 30, 120, 124) and delocalized across tissues (21, 69, 76, 95). In this section, we break down Vmem signals into three length scales: cellular, subcellular, and tissue. From there, we enumerate the unique challenges that each scale brings for Vmem measurement.
2.1. Cellular Membrane Potentials: Action Potentials, Cell Cycle Control, Etc.
The many ion gradients across the plasma membrane create a cellular Vmem, which is generally assumed to be uniform across a cell due to free diffusion of ions within the cytosol. Many cellular Vmem events are short-lived; their quantification requires both high temporal resolution and high voltage resolution. An important fast Vmem signal is the action potential (AP) in excitable cells. APs in mammalian neurons typically last a few milliseconds, involving a rapid depolarization of approximately 100 mV followed by rapid hyperpolarization back to the resting Vmem (8). Many aspects of APs have important cellular effects, including waveform, frequency, and Vmem (AP amplitude, as well as initial and final absolute Vmem) (8). Vmem recording platforms have been engineered with the goal of recapitulating AP waveform and frequency, and modern versions of both electrode-based and optical strategies reflect excellent progress toward this end (14, 57, 77, 115, 116). However, options for recording AP amplitude or absolute Vmem are more limited (see below).
Action potential (AP):
a rapid, all-or-nothing Vmem signal in excitable cells that results from the activity of Vmem-sensitive ion channels
To extend recordings from milliseconds to minutes, hours, or days, two new requirements emerge. First, the Vmem recording technique must be stable over the relevant timeframe, displaying no artefactual, Vmem-independent changes in signal. Second, it must be noninvasive, with cellular processes continuing normally despite chronic observation. With existing tools, Vmem recordings on timescales longer than minutes are challenging and are infrequently attempted. As a result, we have an incomplete picture of what Vmem changes occur and little clarity regarding their effects. Nevertheless, it is evident that slower changes in Vmem are pleiotropic signals with important cellular roles. In neuroscience, for example, the resting Vmem of neurons influences the propensity to fire APs. Furthermore, neuronal resting Vmem changes in association with—and perhaps also regulates—diverse processes, including cellular metabolism (97) and circadian rhythm (63). In addition, Vmem appears to hyperpolarize during neuronal development and differentiation, although the magnitude of the observed changes varies drastically with the measurement technique used (48, 75, 105).
Furthermore, non-excitable cells also show Vmem changes on long timescales, often broadly related to growth. For example, Vmem changes associated with progression from the G1 to the S phase of the cell cycle have been documented in various cell lines (6, 108, 111), and acute epidermal growth factor receptor activation can be accompanied by a Vmem signal (59, 71, 86). In a disease context, cancer cells may be generally more depolarized than their nontransformed counterparts, implying that Vmem modulation may offer selective growth advantages (117). Nevertheless, because current techniques cannot monitor absolute Vmem on the same cell over hours to days, we lack a road map of Vmem throughout the cell cycle or in growth signaling.
2.2. Subcellular Membrane Potentials: Organelles and Membrane Compartmentalization
Because Vmem originates from ion concentration gradients, subcellular Vmem differences can only exist in areas of electrical compartmentalization, where diffusion is regionally restricted. Partial compartmentalization produces transient or metastable local differences in Vmem. To study subcellular Vmem differences, a technique requires exquisite spatial resolution, as well as access to subcellular structures without damaging them. Isolating the Vmem of a subcellular compartment from the cellular Vmem (which may be larger in magnitude and certainly occupies a larger region of space) is particularly challenging. However, direct evidence for subcellular Vmem differences exists, along with a preponderance of indirect evidence that Vmem is locally regulated in membrane-bound compartments.
Electrical compartmentalization:
a restriction on ionic flow that allows for local differences in ion concentration and Vmem
Electrical compartmentalization occurs where the plasma membrane forms compartmentalized processes, and the longevity of localized Vmem signals is determined by the resistance to ion flow in and out of the compartment. Transient subcellular Vmem signals occur in neurons, as voltage waveforms are transmitted and processed by dendrites (102) and the axon initial segment (53). Because these electrical compartments have nearly free ionic flow with other parts of the cell, the signals are fleeting. Visualization of dendritic computation and AP propagation requires exceptional temporal resolution, with recording rates often exceeding 10 kHz (9). Longer-lived subcellular differences likely exist in the micron-scale dendritic spines, insulated by high-resistance bottlenecks between the spine and the dendrite. Mounting evidence suggests that spine Vmem can be electrically distinct from the rest of the neuron, and its role in learning and memory is an area of active study (37, 120). In non-excitable cells, Vmem compartmentalization at the plasma membrane has yet to be conclusively shown. Nevertheless, the subcellular enrichment of particular ion channels in primary cilia (30, 99) and filopodia (44) suggests that nonuniform plasma membrane Vmem is likely.
Organellar membranes also offer an opportunity for ionic, and therefore Vmem, compartmentalization. Organellar electrode-based recordings are both invasive and challenging, as they often involve rupturing the plasma membrane. Nevertheless, through labor-intensive experimental efforts, we have some direct evidence for Vmem signals in organelles. For example, the mitochondrial Vmem (and H+ gradient) is required for successful oxidative phosphorylation (124), and depolarized mitochondrial Vmem, as well as defective oxidative phosphorylation, is associated with neurodegenerative diseases (47). The lysosome contains excitable channels (20), and the endoplasmic reticulum Vmem has been reported to respond to cellular Vmem (90). Furthermore, where direct recordings have not been made, proteomic and transcriptomic studies of organellar channels and transporters imply complex physiology (114), and improved methods tailored to the unique constraints of these subcellular systems are of great interest.
2.3. Membrane Potentials in Tissue: Long-Range Interactions and Delocalization
At the other extreme of the length scale, Vmem carries information via long-range interactions in tissues. Direct experimental interrogation of Vmem in tissue requires throughput and multiplexed measurement across many cells simultaneously, as well as the ability to measure in situ. Electrical communication over distances is facilitated by synaptic transmission, as well as by gap junctions. In the brain, neurons do not act in isolation, but instead form circuits (5, 13); understanding behavior requires simultaneous Vmem mapping across many cells. Beyond neural circuits, Vmem can be delocalized across tissues via gap junctions, which allow free flow of ions and many small molecules between cells. Early studies of electrical coupling and gap junctions were performed in the Drosophila salivary gland, where resistance to current flow between cells is only slightly higher than the resistance to flow within cells (69). Since then, gap junctions have been found to be ubiquitous, with dysfunction connected to various diseases (109). Gap junctions also enable rapid exchange of Vmem information across tissues, as in the synchronous electrical signaling in the heart (95).
Electrical coupling via gap junctions not only enables transmission of Vmem signals across a population of cells, but also inextricably links Vmem between coupled cells. Despite this, many observations about cellular Vmem signaling are made on isolated cells in culture. For epithelial cells that exist in vivo as a tightly coupled tissue, this is an artificial electrical state. What happens to the surrounding tissue when an individual cell changes its Vmem throughout the cell cycle or as a response to growth factor signaling? It remains unclear how and to what extent cell-autonomous Vmem signals discovered in isolated cells will translate into tissues. Theoretical studies suggest that, in some conditions, stable and metastable electrical patterning is possible, but it is highly dependent on the electrical coupling in the tissue (22, 23). These results imply that Vmem signals are accompanied—or at least modulated—by dynamic regulation of cell–cell coupling. Although some progress has been made toward direct visualization of Vmem distributions in tissue (21, 76), such measurements are largely unexplored.
Taken together, diverse biological questions necessitate a noninvasive, multiplexed, in situ Vmem platform with excellent spatiotemporal and voltage resolution. Throughput and technical ease of use of the platform are also important, as they enable top-down profiling experiments that can help elucidate Vmem signaling networks. Of the array of methods available today, no single approach possesses all of these characteristics (Tables 1 and 2). Indeed, more realistically, a toolkit of recording strategies will be required to fully interrogate Vmem. Below, we discuss the performance of existing Vmem measurement techniques, with an eye toward their ability to report Vmem across space and time.
Table 1.
Comparison of electrode-based Vmem recording strategies
| Performance attribute | Whole-cell patch clampa | Cell attachedb | Perforated patchc | Nanopipettesd | Nanopillarse | Planar patch clampf |
|---|---|---|---|---|---|---|
| Spatial resolution | − (single point) |
− (single point) |
− (single point) |
− (single point) |
++ (grid of points) |
− (single point) |
| Temporal resolution | +++ (μs–ms) |
+++ (μs–ms) |
+++ (μs–ms) |
+++ (μs–ms) |
+++ (μs–ms) |
+++ (μs–ms) |
| Voltage resolution | +++ (1 mV) |
+ (variable) |
+++ (1 mV) |
+++ (1 mV) |
+++ (1 mV) |
+++ (1 mV) |
| Stability | + (mins–1 h) |
+ (mins–1 h) |
++ (~1 h) |
+ (mins–1 h) |
+++ (days) |
+ (mins–1 h) |
| Noninvasiveness | − | ++ | + | + | + | − |
| In situ capabilities | ++ | ++ | ++ | ++ | − | − |
| Access to subcellular structures | + | + | + | +++ | ++ | − |
| Throughput and multiplexing | − | − | − | − | ++ | +++ |
| Ease of use | − | − | − | − | + | ++ |
Technique performance in each category is ranked as follows: −, poor; +, fair; ++, good; +++, excellent. Abbreviation: Vmem, membrane potential.
Table 2.
Comparison of optical Vmem recording strategies
| Performance attribute | Single-color fluorescence intensitya | Single-color fluorescence lifetimeb | FRET-basedc | Electrochromic (ratio-based)d | GEVI temporal dynamicse | Stimulated Raman scatteringf |
|---|---|---|---|---|---|---|
| Spatial resolutiong | +++ (<1 μm) |
++ (~1 μm)h |
+++ (<1 μm) |
+++ (<1 μm) |
++ (~1 μm)h |
+ (>1 μm)h |
| Temporal resolution | ++ (1 ms) |
+ (5 ms)h |
+ (1 ms or >10 ms)i |
++ (1 ms) |
− (s)h |
++ (1 ms)h |
| Voltage resolutionj | − (≫00 mV) |
++ (VF: 20 mV, GEVI: >100 mV) |
ND | + (~100 mV) |
++ (10 mV) |
ND |
| Stability | + (minutes) |
++ (hours) |
++ (hours) |
+ (minutes) |
ND | ND |
| Noninvasiveness | +++ | +++ | +++ | +++ | +++ | +++ |
| In situ capabilities | +++ | +++ | +++ | +++ | +++ | ++ |
| Access to subcellular structuresk | ++ | ++ | ++ | ++ | ++ | ++ |
| Throughput and multiplexing | +++ | ++ | +++ | +++ | + | ++ |
| Ease of use | +++ | + | ++ | ++ | − | − |
Many single-color fluorescence intensity Vmem sensors have been designed, although they are seldom used for absolute Vmem quantification. Data are compiled from References 57, 77, 115, and 116.
Single-color fluorescence lifetime recordings have been shown for genetically encoded voltage indicators (16) and for small-molecule VFs (59).
FRET-based systems may use conformational changes of the indicator (4), changes in the absorption spectrum of a rhodopsin derivative (3, 125), or translocation of charged groups in the membrane (15, 35, 72) to report Vmem. Many properties are similar across these architectures; metrics in this case represent all types of FRET-based strategies unless otherwise noted.
Demonstrated in Reference 41.
Spatial resolution is determined by the diffraction limit, as well as optics used in the microscope. Techniques rated ++ rather than +++ generally require moderate spatial binning to obtain adequate signal.
For fluorescence lifetime, GEVI temporal dynamics, and stimulated Raman scattering measurements, there is a trade-off between spatial and temporal resolution, and one may be sacrificed to improve the other. We list the best values of either spatial or temporal resolution shown in the literature or that we have demonstrated in our laboratory.
FRET sensors based on protein conformational changes or dye translocation exhibit temporal resolution of >10 ms, whereas electrochromic FRET-based sensors can resolve millisecond-time Vmem events.
Voltage resolution listed is for applying a calibration determined on one cell onto another individual cell; more precise Vmem measurements can be obtained if measurements from multiple cells are averaged. Most techniques have substantially better resolution for quantifying the magnitude of a Vmem change on an individual cell (e.g., VF-FLIM has 5-mV resolution for quantifying Vmem changes on a single cell versus 20-mV resolution for quantifying absolute Vmem alone).
Limited by delivery of sensor to intracellular structures and/or the ability to isolate signal from only the subcellular membrane structure of interest when all membrane structures are stained.
Technique performance in each category is ranked as follows: −, poor; +, fair; ++, good; +++, excellent. Abbreviations: FLIM, fluorescence lifetime imaging; FRET, Förster resonance energy transfer; GEVI, genetically encoded voltage indicator; ND, not determined; VF, VoltageFluor; Vmem, membrane potential.
3. ELECTRODE-BASED APPROACHES FOR RECORDING ABSOLUTE MEMBRANE POTENTIAL
Electrode-based techniques are the gold standard for recording Vmem, ion channel properties, and electrical currents in cells. Three primary configurations are used in cellular Vmem recordings: whole-cell patch clamp, cell attached, and perforated patch (Figure 2). All three can quantify absolute Vmem with excellent temporal resolution, but they suffer from high invasiveness, instability over time, low throughput, and poor spatial resolution (Table 1).
Figure 2.
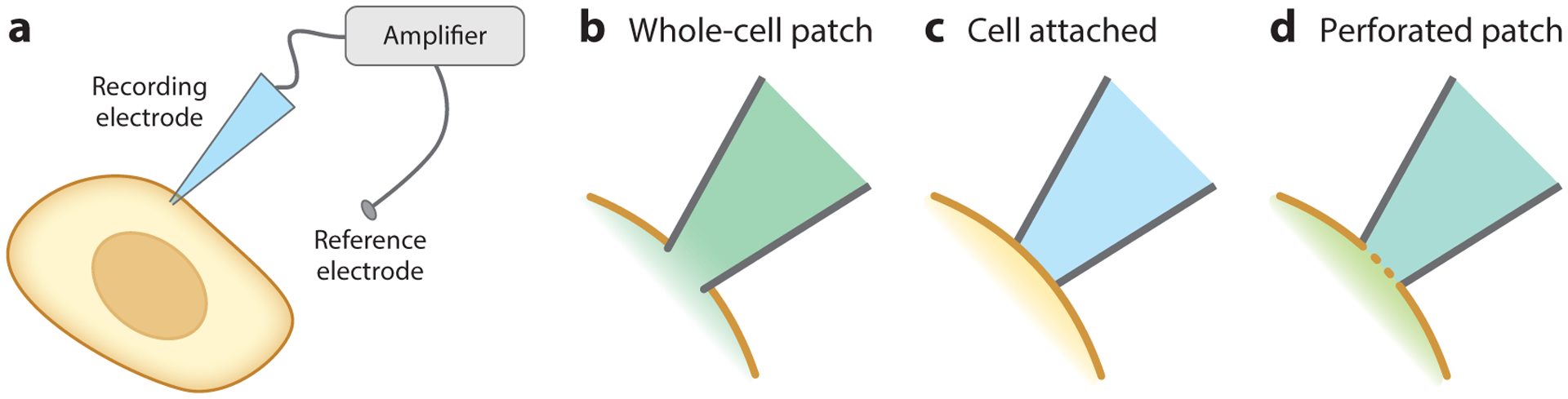
Electrophysiological configurations for Vmem recording. (a) General schematic for electrode-based Vmem recordings, in which a cell comes into direct contact with an electrode. Voltage is measured as the difference between the recording electrode and a reference electrode in the bath solution. (b–d) Close-up of the interface between the membrane and the electrode in different electrophysiology configurations. (b) In whole-cell patch-clamp electrophysiology, the plasma membrane is ruptured, and the cytosol mixes with the recording electrode solution. (c) In the cell-attached configuration, the plasma membrane is left intact. (d) In perforated patch, the membrane is not ruptured, but ionophores introduced into the recording solution allow ionic exchange across the membrane.
Whole-cell patch clamp:
a direct, electrode-based technique for recording Vmem in which physical contact is established between an electrode and the inside of a cell
3.1. Intracellular Recordings of Absolute Membrane Potential
In intracellular recording, Vmem is determined from the difference between a recording electrode in the cytosol and a reference electrode in the extracellular solution. More detailed treatment of the many capabilities of intracellular recording can be found elsewhere (14); we focus only on Vmem measurement. Two main types of intracellular recordings exist: sharp electrode and whole-cell recordings. Sharp microelectrodes possess very fine tips and high tip resistances, which produce a variable tip potential and a Vmem artifact on the order of tens of millivolts (14, 65). Furthermore, they create a leak in the cell of interest, producing artificially depolarized apparent Vmem and altered input resistance (65, 101). Although sharp electrode recordings are still in use in certain in vivo preparations, they have largely been replaced by intracellular recordings in the whole-cell configuration. Whole-cell intracellular recordings use much larger patch electrodes with lower tip potentials; such electrodes can be sealed onto the cell with less damage than sharp electrodes (100). Whole-cell recordings can also provide higher-resolution information about the particular ions and types of channels involved in setting Vmem.
Intracellular recording provides a direct, in situ measurement of Vmem with sub-mV precision and excellent temporal resolution, but it suffers from a variety of drawbacks. First, the whole-cell patch is highly invasive and disrupts the very processes under investigation. By dialyzing the cytosol with electrode internal solution, whole-cell patch clamp washes out soluble signaling factors (14, 49, 73). In addition, physical contact with the electrode can activate mechanosensitive channels and signal transduction pathways (70, 112), introducing further artifacts. With respect to the measurement itself, leaks in the seal between the recording electrode and the membrane can produce errors in recorded Vmem (105). More subtly, an electrode has poor spatial resolution, reporting Vmem at a point of contact that may or may not reflect other parts of the cell or tissue. For complex membrane structures or electrically coupled tissues, this space clamp error renders the effective recording area unknown (7, 110). For subcellular recordings, electrode-based strategies struggle to physically access subcellular compartments, as the size of the electrode tip provides a lower bound on the size of structures that can be interrogated (30, 37). Furthermore, intracellular recordings are challenging to execute. Attempting each whole-cell recording takes an expert researcher or specialized robot 5–10 minutes, with a 10–30% success rate for these efforts (38, 52, 103). While in situ automated patch systems have reduced the need for human expertise and labor, they still do not achieve high throughput or multiplexing. Therefore, recording across many cells in the same tissue is nearly impossible, as is cataloging Vmem subpopulations over many cells.
3.2. Reducing Invasiveness: Cell-Attached and Perforated Patch Configurations
To mitigate the washout of soluble factors associated with whole-cell recording, Vmem must be measured without disrupting the plasma membrane. This is possible in the cell-attached configuration, in which an extracellular patch electrode is sealed onto the cell without rupturing the membrane. If the membrane resistance is approximately 100-fold lower than the seal resistance, then the voltage across the small membrane region under the pipette will reflect cellular Vmem with reasonable accuracy (87). Alternatively, if the channel composition and electrical properties are known in the cells of interest, then the reversal potential of these channels (determined in the cell-attached configuration) can also be used to infer Vmem. Such techniques have been demonstrated with the K+ reversal potential in hippocampal interneurons (106), as well as the NMDA reversal potential in hippocampal CA3 neurons (105). However, this strategy is difficult to extend, as it requires detailed knowledge of the inventory and behavior of channels in the specific system of interest.
Cell-attached configuration:
an electrophysiological configuration where an electrode is sealed onto the membrane of a cell but the membrane is not ruptured
Perforated patch recordings offer a more general solution to reduce invasiveness of whole-cell recordings (40, 66). In perforated patch, an ionophore placed in the pipette internal solution allows ions to cross the membrane without completely disrupting it, thereby electrically connecting the cytosol with the pipette solution. Because the membrane remains impermeable to the diffusion of larger molecules, perforated patch recording can last over an hour without run-down of relevant cytosolic factors (40, 54).
Ionophore:
a molecule that can bind specific ions and diffuse across membranes, equalizing the electrochemical gradient for that ion
3.3. Engineering Electrodes for Improved Performance
Efforts to improve electrode-based recordings have also turned to the electrodes themselves, where recent developments have reduced invasiveness and improved throughput of electrode-based recordings. Below, we highlight two advances in electrode design: planar patch clamp and nanoscale electrodes.
3.3.1. Planar patch clamp.
With the development of low-noise arrays of patch electrodes (32), commercial planar patch clamp systems can now perform simultaneous, automated electrophysiological experiments in 384- or 768-well arrayed formats (82). These systems are increasingly used to screen compound libraries against ion channel targets, expediting the drug discovery process. However, the system requires a suspension of dissociated cells, which precludes recording in situ and over extended time courses. As a result, planar patch clamp is more suited for high-throughput screening of ion channel pharmacology than for analysis of cellular or tissue-level voltage signaling.
3.3.2. Nanopipettes and nanopillar electrodes.
The development of nanopipettes and nanopillar electrodes has enabled electrode-based access to broader length scales. Nanopipettes are a smaller version of sharp microelectrodes; their reduced size brings flexibility and access to small cellular compartments. Nanopipettes have been used to record from micron-scale dendritic spines, providing some of the first direct evidence for functional voltage compartmentalization in these structures (45). In addition, the flexibility of the nanopipette can stabilize in vivo recordings during animal behavior (46). As with sharp electrodes, the high tip resistances of the nanopipettes filter the signal and create a shunt that changes the observed Vmem, so the output signal must be processed to obtain accurate results. Nanopillar electrode arrays enable simultaneous intracellular recordings from many cells in a culture via a grid of nanoscale electrodes (94, 113). These platforms facilitate mapping of electrical activity across neuronal circuits or beating cardiomyocytes. However, the cells of interest must be cultured directly onto the electrode grid; nanopillar electrode arrays are not yet capable of in situ mapping of tissue Vmem. Overall, electrode engineering offers a promising avenue for increasing the spatial reach of electrophysiology.
4. CALIBRATED OPTICAL SIGNALS FOR RECORDING ABSOLUTE MEMBRANE POTENTIAL
A variety of optical Vmem measurement strategies use fluorescence as a proxy for Vmem, employing small-molecule or protein-based sensors engineered to display Vmem-sensitive fluorescence (Table 2). Optical platforms generally enjoy excellent spatial resolution, low invasiveness, and medium throughput. However, achieving the requisite temporal resolution, voltage resolution, and signal-to-noise ratio has required more engineering. Optical strategies fall into four categories (single-color intensity, ratio based, pump probe, and single-color lifetime; Figure 3). In all cases, rigorous calibration must be performed to relate the optical signal to the underlying absolute Vmem, which is most effectively achieved by performing the optical recording while Vmem is tuned across a range of known values. As we discuss in this section, a key difference among the various fluorescence-based strategies is voltage resolution, which is a result of the reproducibility of this calibration over time and between cells.
Figure 3.
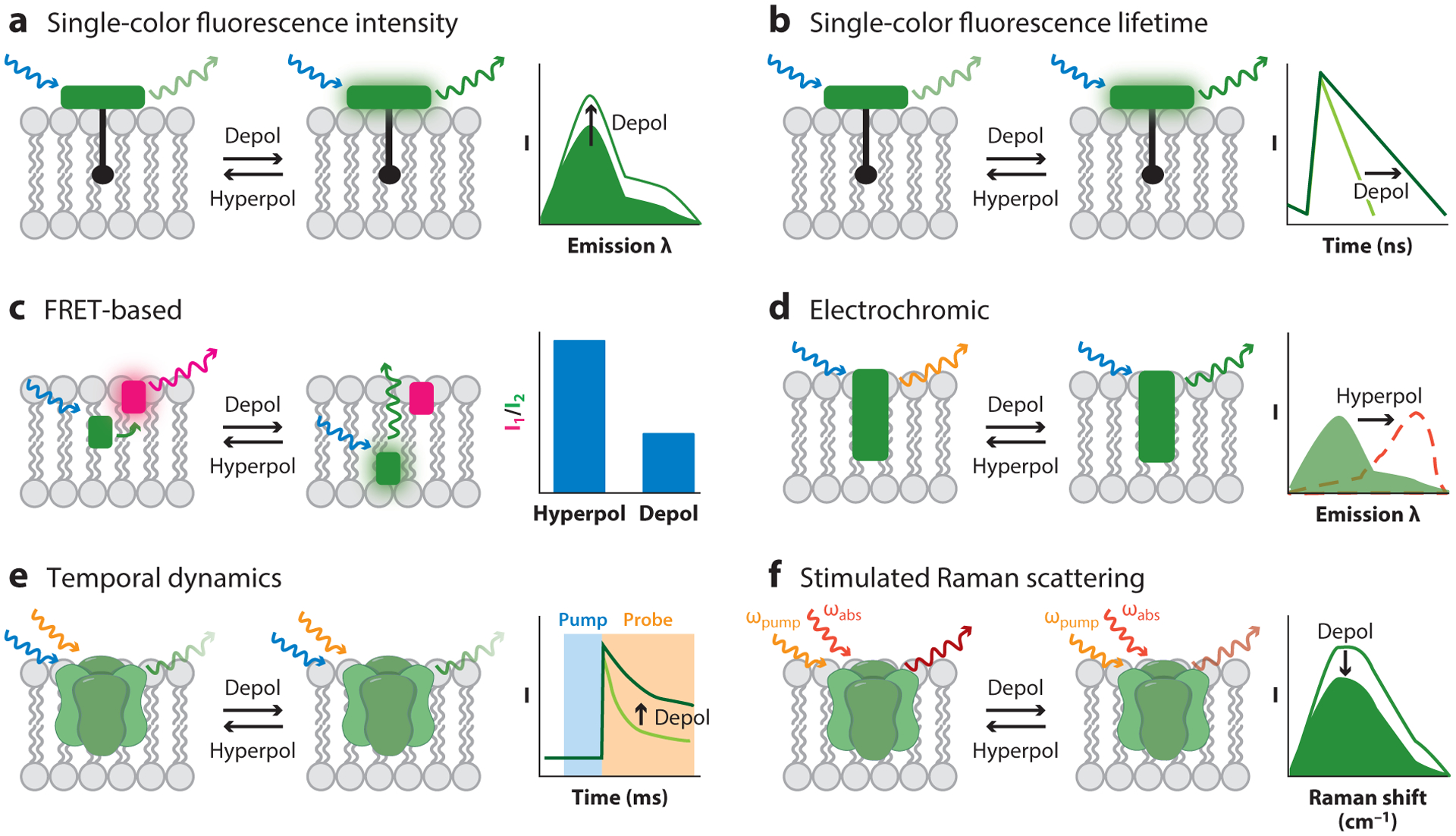
Optical strategies for reporting absolute Vmem. (a) Single-color fluorescence intensity recordings detect changes in Vmem by changes in a sensor’s fluorescence quantum yield (shown), extinction coefficient, or cellular concentration. This technique generally cannot report absolute Vmem optically, although estimates can be made if calibrations are performed for every cell of interest. (b) Single-color fluorescence lifetime can report absolute Vmem, particularly if the Vmem sensor operates via photoinduced electron transfer.(c) FRET-based sensors show differences in the FRET ratios of two fluorophores, often resulting from Vmem-dependent changes in the distance between the two. The diagram shows a FRET-oxonol system. (d) The excitation and/or emission spectra of electrochromic dyes depend on the electric field in the plasma membrane. (e) Certain GEVIs show Vmem-related differences in the temporal dynamics of the absorption of their excited state. (f) SRS imaging reports changes in Vmem-dependent vibrational frequencies. Abbreviations:λ, wavelength; depol, depolarization; FRET, Förster resonance energy transfer; GEVI, genetically encoded voltage indicator; hyperpol, hyperpolarization; SRS, stimulated Raman scattering; Vmem, membrane potential.
4.1. Strategies for Calibration of Optical Membrane Potential Reporters
To calibrate an optical Vmem recording platform, one must be able to measure the optical response across a range of Vmem values. By fitting this measured relationship to a function, one can then convert the optically determined parameter into Vmem values and, equally importantly, estimate the accuracy of the optical Vmem determination. For example, for a linear relationship, one would determine the slope and the y-intercept and use those values to relate the optically recorded parameter to Vmem. To this end, there are three common strategies for setting cellular Vmem: electrode-based, pharmacological, and optogenetic.
4.1.1. Electrode-based calibration.
The most accurate method for calibrating fluorescence with respect to Vmem is whole-cell voltage clamp electrophysiology, which uses an intracellular recording configuration to inject current until the cell reaches the desired Vmem. The response across a range of precisely set Vmem can then be evaluated, determining the relationship between the optical readout and the underlying voltage. However, this approach is best when performed in isolated, single cells, and it suffers from the limitations of electrophysiology discussed above.
4.1.2. Pharmacological calibration.
If the system under study is inaccessible to electrode-based strategies, either pharmacology or intrinsic Vmem signals can be used to approximate the relationship between the optical signal and absolute Vmem. Reference values of Vmem have been established using ionophores for the primary ion establishing Vmem (39, 51, 74, 81), high extracellular K+ (120–150 mM) (55, 74), and cell death or fixation with paraformaldehyde (PFA) (51). PFA and ionophores produce a more reliable 0-mV point than do high K+ treatments, but they interact with the plasma membrane itself, which can change the fluorescence properties of the voltage sensor (79). Furthermore, there is no reliable way to access the sensitivity of the sensor (i.e., slope of signal versus Vmem) from a single pharmacological set point. However, in samples with well-described intrinsic electrical responses, a Vmem event of known magnitude can provide a standard for approximate determination of absolute Vmem sensitivity. Demonstrated reference Vmem signals include sustained hyperpolarization in Purkinje cells (19), depolarization resulting from glutamate uncaging (107), and back-propagating APs in dendrites (85). Such strategies are noninvasive but are limited to the small subset of samples where a useful and consistent Vmem signal can be identified.
4.1.3. Efforts toward optogenetic calibration.
In many cases, this limited set of calibration options is inadequate. Recently developed optical strategies for Vmem control may eventually provide an alternative to the above approaches, although they cannot yet tune Vmem to defined voltages. Engineering efforts for optical Vmem actuators have focused on channelrhodopsins, proteins that pass current in response to light. For setting Vmem optically, step-function opsins may be particularly useful, as they continue to pass current for minutes after initial activation (12, 119). Recently, light sensitivity was engineered into other ion channels via domain insertion, allowing diverse channels to serve as optogenetic actuators (27). These strategies could eventually allow precise setting of Vmem without an electrode.
4.2. Single-Color Fluorescence Intensity Recordings Are Difficult to Relate to Absolute Membrane Potential
In single-color fluorescence intensity–based recordings, Vmem affects the quantum yield or absorption coefficient of a sensor, producing changes in its fluorescence emission. Fluorescence intensity of a voltage sensor in a membrane is recorded over seconds or minutes, enabling detection of Vmem changes and characterization of Vmem waveforms. A variety of sensor architectures have been documented (57, 77, 115, 116), including both small-molecule dyes and genetically encoded voltage indicators (GEVIs). Many sensors display sufficiently fast temporal resolution to follow millisecond-time APs. State-of-the-art systems can achieve cell-resolved recording of single APs in vivo from superficial brain regions, often with subcellular detail (1, 3, 88). However, single-color fluorescence intensity is best suited for Vmem event detection and generally cannot report absolute Vmem. Methods that rely solely on fluorescence intensity to monitor Vmem display large differences in baseline signal between cells and over minutes to hours. These variations are Vmem independent, arising from variable sensor loading or trafficking, cell morphology, sensor photobleaching, fluorescence quenching, and illumination intensity. As a result, calibrations made on one cell at a given time cannot be extended to other cells or even to the same cell hours later.
Fluorescence intensity:
the total amount of fluorescence signal from a sample; for fluorescent sensors, intensity depends on the amount of sensor present, the amount of light absorbed, and the quantum yield
Voltage indicator:
a small molecule or protein fluorophore that exhibits Vmem dependence in some or all of its fluorescent properties (e.g., intensity, wavelength, lifetime)
Some attempts have been made to calibrate fluorescence intensity to known Vmem at each cell or structure of interest, but caution must be employed in interpreting these results. Electrophysiological calibration of each individual cell is often challenging or impractical, so fluorescence intensity calibrations are generally performed with less accurate pharmacological strategies. In some cases, this approach is the only viable way to interrogate Vmem—for example, gramicidin-based calibrations and the Vmem indicator Archaerhodopsin (Arch) revealed subcellular differences in the AP waveform (39). However, pharmacological calibration of a single-color Vmem sensor is prone to artifacts. When calibrating from a single pharmacological set point, rather than a range of Vmem set by an electrode, the researcher must assume that the fractional change in fluorescence (%ΔF/F) per mV of the indicator is the same for all samples. In reality, the ΔF/F response to a given ΔVmem will depend on the ratio of productively engaged sensor to background sensor, which may vary in space with trafficking or loading of the sensor. Furthermore, even with electrode-based calibration, many voltage sensors display sensitivity to membrane composition (36), which varies not only between cells but also within cells with complex membrane structures (10).
4.3. Ratio-Based Fluorescent Sensors Improve Reproducibility
Ratio-based voltage sensors show changes in the relative intensities of two fluorescence channels. The primary strategies for ratio-based fluorescent Vmem sensors rely on either Förster resonance energy transfer (FRET)-based indicators or electrochromic dyes. If the stoichiometry between the two signals is known, then the second channel can be used to correct for variability arising from cell morphology and dye loading. Ideally, the corrected signal would then be a stable proxy for absolute Vmem over time and across many cells without recalibration. In practice, ratio-based sensors achieve better absolute Vmem resolution than single-color intensity-based techniques, but they generally do not have sufficient resolution to quantify the biologically relevant Vmem differences between cells (~10–20 mV).
Ratio-based:
expressed as a ratio between two components; in ratio-based fluorescent imaging, the ratio of signal at two different wavelengths changes in response to the phenomenon of interest
4.3.1. Förster resonance energy transfer–based membrane potential sensors.
FRET-based Vmem sensors exhibit Vmem-dependent energy transfer from a donor to an acceptor fluorophore. Because the fluorescence intensities of the donor and acceptor are voltage sensitive in opposite directions, the ratio of the two can provide better fractional responses and a higher signal-to-noise ratio than a single color alone. Although a variety of FRET-based Vmem sensors exist, we are not aware of their use to document absolute Vmem, largely due to variable stoichiometry between the donor and acceptor. For genetically encoded systems (3, 4, 125), different rates of photobleaching, folding, and productive trafficking to the plasma membrane lead to variability in FRET ratios between cells. For small-molecule FRET-oxonol systems (15, 35), differences in loading between the two lipophilic indicators lead to variability in the %ΔF/F (72). As a result, FRET ratio measurements are not extensible between cells. Furthermore, the FRET-based reporters that undergo conformational changes to sense Vmem suffer from reduced temporal resolution and toxicity from capacitive load. In practice, these probes are primarily used to detect APs, with the second color serving to improve the signal-to-noise ratio or reduce motion artifacts.
4.3.2. Electrochromic ratio-based membrane potential sensors.
Electrochromic dyes, such as the ANEPPS (68) and ANNINE (34) classes, show Vmem-dependent excitation and/or emission spectra. A ratio signal is obtained by comparing the emission in a fixed band produced by excitation at two different wavelengths (or vice versa with a fixed excitation and two emission bands). Because electrochromic dyes are based on a charge shift mechanism, they have submillisecond temporal responses but display low sensitivity (~10% change per 100 mV) (33, 79). Normalized ANEPPS fluorescence ratios are accurate to approximately 5 mV for quantifying Vmem changes on an individual cell (17, 122). In combination with random access microscopy, di-8-ANEPPS ratios can report the AP waveform in absolute Vmem optically with excellent spatiotemporal resolution (exposure times of 0.5 ms and spatial resolution of 2 μm) (17). However, the Vmem calibration does not translate between cells and must be performed with an electrode on each cell of interest to achieve this accuracy. The origin of the variability of the absolute ratio between cells is unclear; it likely depends on a complex combination of temperature, membrane composition, and probe loading.
4.3.3. Molecular beacons in ratio-based membrane potential recording.
A recent, promising architecture for ratio-based Vmem sensors uses a molecular beacon, where the sensor contains one fluorophore that is voltage sensitive and a second fluorophore that is a voltage-independent reference. This strategy was tested with the GEVI Arch(D95N) fused to green fluorescent protein (GFP), but absolute Vmem quantification was not possible because of differential photobleaching of GFP and Arch(D95N) (41). More recently, the molecular beacon system was implemented with small-molecule Vmem sensors in Voltair (98), which comprises the VoltageFluor (VF) RVF5 (56) and the reference fluorophore ATTO647N on a DNA chassis. By normalizing the RVF5 signal to ATTO647N, Voltair was able to estimate organellar Vmem optically based on a pharmacological calibration, but absolute Vmem resolution of this technique has not yet been determined.
4.4. Pump-Probe Recordings: Accurate, with Complex Instrumentation
The temporal dynamics and vibrational signature of a probe do not depend on probe concentration; therefore, they can be used as a reproducible proxy for absolute Vmem. The temporal dynamics of the GEVI Arch(D95H)-eGFP can report absolute Vmem between cells with 10-mV resolution (41), the best yet reported for an optical system. However, access to and interpretation of such data is challenging for most laboratories, limiting this optical system’s utility as a general Vmem sensing platform. Stimulated Raman scattering (SRS) has also been investigated as a strategy for absolute Vmem recordings, but its Vmem resolution has yet to be determined. Recently, the SRS signal of near-infrared opsins was shown to be sensitive to bulk depolarization in Escherichia coli membranes (61). Moreover, label-free detection of neuronal APs with SRS has been reported, although the mechanism of such Vmem sensitivity remains unclear (62). Further investigations of temporal dynamics and SRS as Vmem reporting platforms are of great interest, especially with the aim of translating them into more widely available systems.
4.5. Fluorescence Lifetime Offers Accessible Absolute Optical Membrane Potential Recordings
Fluorescence lifetime (τfl), or the amount of time that probe molecules remain in the fluorescent excited state, has recently garnered attention for its ability to report cellular properties (e.g., Ca2+ concentration or Vmem) absolutely using a single fluorescence channel (118). τfl is largely independent of fluorophore concentration, cellular morphology, and photobleaching, although it displays sensitivity to parameters such as temperature and viscosity. The primary downside to fluorescence lifetime imaging (FLIM) is its poor temporal resolution, with most acquisition times on the order of seconds.
Fluorescence lifetime (τfl):
the amount of time that a fluorophore remains in the fluorescent excited state before returning to the ground state, via either fluorescence ora nonradiative pathway
Fluorescence lifetime imaging (FLIM):
a microscopy technique in which both fluorescence intensity and τfl are recorded at each pixel of an image
4.5.1. Fluorescence lifetime of genetically encoded voltage indicators.
τfl-based Vmem recordings can be performed if τfl of a sensor changes reproducibly with Vmem. Many GEVIs sense Vmem through complex photocycles or multiple conformational states; this leads to low sensitivity in τfl or nonlinear relationships between τfl and Vmem. For example, the rhodopsin derivative Arch(D95H) was first evaluated in FLIM for its absolute Vmem-reporting capabilities, but its τfl–Vmem relationship was difficult to interpret (41). Comparison of the Vmem sensitivity of three GEVIs in two-photon illuminated FLIM (16) revealed that only CAESR (125) was suitable for absolute Vmem recording. CAESR exhibits 20-mV accuracy for quantifying Vmem changes on a given cell in a one-second bandwidth, but it displays too much variability to extend optical Vmem calibrations between cells (16). This between-cell variability may arise from the complex dynamics of rhodopsin fluorescence, coupled with intracellular fluorescence signal from incorrectly trafficked protein. Vmem-sensitive FLIM has also been demonstrated for FRET-oxonol Vmem sensors(31) and for accumulation-based sensors of mitochondrial potential (83), but no estimation of the absolute Vmem resolution was made in these systems.
4.5.2. Fluorescence lifetime of VoltageFluor small-molecule dyes.
τfl-based absolute Vmem imaging was developed further in our laboratory, achieving stable calibration between cells through the use of small-molecule VFs as voltage sensors. The voltage-sensing trigger for VFs is photoinduced electron transfer (78), which translates to a rapid, linear Vmem response in both fluorescence intensity (11, 78) and τfl (59). The τfl of VF2.1.Cl (VF-FLIM) can be calibrated to report absolute Vmem with a resolution of 5 mV for quantifying voltage changes on individual cells. These calibrations can be extended from one cell to another while retaining a single-trial Vmem resolution of 20 mV, which is sufficient to detect biologically relevant variation in resting Vmem, especially once multiple measurements are averaged. Because VF-FLIM calibrations are consistent for a given cell line, we were able to extend Vmem calibrations across thousands of cells after an initial electrode-based calibration on only a few cells. We applied VF-FLIM to study epidermal growth factor signaling and found a 15-mV hyperpolarizing response in carcinoma cells, showcasing its potential for elucidating diverse signaling roles of Vmem.
In addition to its improved Vmem resolution, VF-FLIM shows many promising attributes as a Vmem sensing platform. The increased stability of the calibration enhances throughput; an individual researcher using VF-FLIM could perform Vmem recordings at the speed of optical imaging (thousands of cells in an afternoon) rather than electrophysiology (~10 cells per day). Importantly, the temporal and spatial resolution of FLIM-based strategies are related: Acquisition rates as fast as 200 Hz (exposures of 5 ms) can be obtained with reduced spatial resolution, and spatial resolution can be improved from 5 microns (the width of a binned pixel) to approximately 1 micron at the expense of imaging speed (Miller lab, unpublished data). The VF-FLIM technology can be further improved through combination with red-shifted Vmem sensors (28, 43) and targeting of sensors to specific cell populations in thick tissue (29, 67, 84), both of which are ongoing efforts in our lab. Overall, FLIM-based absolute Vmem imaging extends the timescale over which Vmem can be recorded and displays some of the best Vmem resolution to date for optical systems.
5. OUTLOOK
Vmem signals run the gamut from milliseconds to days and from the subcellular to the tissue level (Figure 1). The existing Vmem toolkit can record most cellular Vmem signals and some subcellular ones, with time resolutions ranging from milliseconds to minutes. Vmem signals delocalized across tissues are still challenging to interrogate, especially those occurring over hours to days.
Electrode-based absolute Vmem recordings have defined the field for years and will remain pivotal as engineering efforts continue to improve throughput and reduce invasiveness. However, progress in optical approaches for absolute Vmem recording has already expanded the range of space and time over which Vmem can be recorded. One of the key limitations to the scope of optical, absolute Vmem recording is the need for calibration in the cell or tissue of interest; we excitedly await improved optical actuators to facilitate Vmem calibrations in diverse systems. Further improvements in voltage-sensitive dyes and proteins stand to increase the absolute Vmem resolution of existing optical approaches. Improvements to photon-counting FLIM hardware, as well as fast frequency-domain FLIM strategies (91), are promising avenues for faster lifetime recordings. Optical Vmem recording in vivo can be multiplexed across more cells and larger areas by combining it with lattice light-sheet imaging (24) and other fast volumetric imaging strategies (50).
With these technological advances, we are constructing a picture of Vmem across biological time and space scales. Nevertheless, our atlas remains far from complete. In many cases, the information carried by Vmem is unknown, and the molecular mechanisms of Vmem response remain obfuscated. Each newly observed Vmem signal raises additional, exciting questions: How does the cell bring about these Vmem changes? Which molecules respond to Vmem? How are these signals transduced to downstream cellular processes? Uncovering the signaling networks surrounding Vmem will require platforms that interface with other cell profiling and omics techniques, such as the recently pioneered Patch-Seq system (18). With such efforts, we move ever closer to a systems-level understanding of Vmem in all of its diversity.
SUMMARY POINTS.
Vmem influences cellular processes on timescales ranging from seconds to days and across length scales from microns to centimeters. Beyond excitable cell APs, Vmem also contributes to cell growth, differentiation, and organellar function.
Vmem recording strategies generally fall into one of two categories: electrode-based or optical recordings.
Electrode-based Vmem recordings have high temporal resolution and voltage resolution, but they are invasive, have low throughput, and only record Vmem at a single point.
Recent advances in electrodes and electrode arrays have allowed for electrophysiology of smaller cellular structures, as well as improved throughput and multiplexing.
Optical Vmem recordings can track Vmem noninvasively across many cells at once, but most optical strategies cannot quantify biologically relevant Vmem signals (i.e., measure absolute Vmem).
Recent improvements in certain optical Vmem recording platforms have improved Vmem resolution, thereby enabling quantification of absolute Vmem. In particular, lifetime or temporal dynamics of a sensor’s fluorescence can quantify Vmem across longer space and timescales.
Optical recordings are indirect measurements of Vmem, so they always require calibration to report absolute Vmem. Various optical Vmem recording architectures show differences in the reproducibility of this calibration; this is usually the limiting factor in absolute Vmem resolution.
FUTURE ISSUES.
Many areas of Vmem biology are understudied, especially Vmem changes occurring over long periods of time and in very small or very large structures. Continued efforts toward developing platforms for absolute Vmem recordings are necessary before we can fully document or understand Vmem signaling in these areas.
Electrical signaling in vivo is likely delocalized across multiple cells by gap junctions. As tools tailored to measure Vmem in tissue are developed, further investigations into cell–cell coupling and long-range Vmem signals are needed.
Absolute Vmem recording platforms with signals that are stable over days are needed. Although nanopillar electrode arrays are a major step forward, electrode-based platforms are generally too invasive for chronic recordings. A key challenge for optical platforms is maintaining stable levels of Vmem sensor in the sample during extended recordings.
Standard metrics for—and routine determination of—absolute Vmem resolution should be incorporated into the evaluation and presentation of optical platforms for quantitative Vmem recordings (ratio-based recordings, lifetime-based recordings, SRS, etc.).
More generalizable strategies for calibration of Vmem are needed. In systems that are accessible optically but not with electrodes, it is difficult to determine the accuracy of optical absolute Vmem measurements.
Although FLIM and temporal dynamics–based strategies have accessed resolutions in the range of 10–20 mV, many interesting Vmem changes are on the scale of 5–10 mV. Further engineering of these optical sensors and platforms could enable detection of subtle Vmem changes.
ACKNOWLEDGMENTS
The authors thank Holly Aaron and members of the Miller lab for helpful discussions. J.R.L.-D. was supported in part by a National Science Foundation Graduate Research Fellowship. A.M.M.G. was supported in part by a training grant from the National Institute of General Medical Sciences (NIGMS) (grant T32GM066698). E.W.M. acknowledges funding support from NIGMS (grant R35GM119855).
DISCLOSURE STATEMENT
E.W.M. is listed on a patent, owned by the Regents of the University of California, describing voltage-sensitive fluorophores. The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Abdelfattah AS, Kawashima T, Singh A, Novak O, Liu H, et al. 2019. Bright and photostable chemigenetic indicators for extended in vivo voltage imaging. Science 365(6454):699–704 [DOI] [PubMed] [Google Scholar]
- 2.Abdul Kadir L, Stacey M, Barrett-Jolley R. 2018. Emerging roles of the membrane potential: action beyond the action potential. Front. Physiol 9:1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam Y, Kim JJ, Lou S, Zhao Y, Xie ME, et al. 2019. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569:413–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knopfel T. 2012. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol 108(8):2323–37 [DOI] [PubMed] [Google Scholar]
- 5.Alcamí P, Pereda AE. 2019. Beyond plasticity: the dynamic impact of electrical synapses on neural circuits. Nat. Rev. Neurosci 20(5):253–71 [DOI] [PubMed] [Google Scholar]
- 6.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, et al. 1995. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J. Physiol 489(2):455–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong CM, Gilly WF. 1992. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 207:100–22 [DOI] [PubMed] [Google Scholar]
- 8.Bean BP. 2007. The action potential in mammalian central neurons. Nat. Rev. Neurosci 8(6):451–65 [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, et al. 2018. Enhanced dendritic compartmentalization in human cortical neurons. Cell 175(3):643–51.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedlack RS Jr., Wei MD, Fox SH, Gross E, Loew LM. 1994. Distinct electric potentials in soma and neurite membranes. Neuron 13(5):1187–93 [DOI] [PubMed] [Google Scholar]
- 11.Beier HT, Roth CC, Bixler JN, Sedelnikova AV, Ibey BL. 2019. Visualization of dynamic sub-microsecond changes in membrane potential. Biophys. J 116(1):120–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. 2009. Bi-stable neural state switches. Nat. Neurosci 12(2):229–34 [DOI] [PubMed] [Google Scholar]
- 13.Bi G, Poo M. 2001. Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu. Rev. Neurosci 24:139–66 [DOI] [PubMed] [Google Scholar]
- 14.Brette R, Destexhe A. 2012. Intracellular recording. In Handbook of Neural Activity Measurement, ed. Brette R, pp. 44–91. Cambridge, UK: Cambridge Univ. Press [Google Scholar]
- 15.Briggman KL, Kristan WB, González JE, Kleinfeld D, Tsien RY. 2010. Monitoring integrated activity of individual neurons using FRET-based voltage-sensitive dyes. In Membrane Potential Imaging in the Nervous System: Methods and Applications, ed. Canepari M, Zecevic D, pp. 61–70. Berlin: Springer [Google Scholar]
- 16.Brinks D, Klein AJ, Cohen AE. 2015. Two-photon lifetime imaging of voltage indicating proteins as a probe of absolute membrane voltage. Biophys. J 109(5):914–21 [DOI] [PMC free article] [PubMed] [Google Scholar]; Compared the performance of three GEVIs in reporting absolute Vmem under two-photon illumination.
- 17.Bullen A, Saggau P. 1999. High-speed, random-access fluorescence microscopy: II. Fast quantitative measurements with voltage-sensitive dyes. Biophys. J 76(4):2272–87 [DOI] [PMC free article] [PubMed] [Google Scholar]; Combined random-access imaging with electrochromic dyes to quantify Vmem along neurons with high spatiotemporal resolution.
- 18.Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, et al. 2016. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol 34(2):199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]; Characterized the transcriptomic basis of morphological and electrical signatures of diverse neurons.
- 19.Canepari M, Vogt K, Zecevic D. 2008. Combining voltage and calcium imaging from neuronal dendrites. Cell. Mol. Neurobiol 28(8):1079–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cang C, Bekele B, Ren D. 2014. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol 10(6):463–69 [DOI] [PubMed] [Google Scholar]
- 21.Ceriani F, Mammano F. 2013. A rapid and sensitive assay of intercellular coupling by voltage imaging of gap junction networks. Cell Commun. Signal 11(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervera J, Alcaraz A, Mafe S. 2016. Bioelectrical signals and ion channels in the modeling of multicellular patterns and cancer biophysics. Sci. Rep 6:20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervera J, Meseguer S, Mafe S. 2016. The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles. Sci. Rep 6:35201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BC, Legant WR, Wang K, Shao L, Milkie DE, et al. 2014. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346(6208):1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Becker TM, Koch U, Stauber T. 2019. The LRRC8/VRAC anion channel facilitates myogenic differentiation of murine myoblasts by promoting membrane hyperpolarization. J. Biol. Chem 294:14279–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cone CD, Cone CM. 1976. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science 192(4235):155–58 [DOI] [PubMed] [Google Scholar]
- 27.Coyote-Maestas W, He Y, Myers CL, Schmidt D. 2019. Domain insertion permissibility-guided engineering of allostery in ion channels. Nat. Commun 10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deal PE, Kulkarni RU, Al-Abdullatif SH, Miller EW. 2016. Isomerically pure tetramethylrhodamine voltage reporters. J. Am. Chem. Soc 138(29):9085–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deal PE, Liu P, Al-Abdullatif SH, Muller VR, Shamardani K, et al. 2020. Covalently tethered rhodamine voltage reporters for high speed functional imaging in brain tissue. J. Am. Chem. Soc 142(1):614–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delling M, Decaen PG, Doerner JF, Febvay S, Clapham DE. 2013. Primary cilia are specialized calcium signalling organelles. Nature 504(7479):311–14 [DOI] [PMC free article] [PubMed] [Google Scholar]; Showed that primary cilia are electrically compartmentalized, with distinct Ca2+ levels and Vmem.
- 31.Dumas D, Stoltz JF. 2005. New tool to monitor membrane potential by FRET voltage sensitive dye (FRET-VSD) using spectral and fluorescence lifetime imaging microscopy (FLIM): interest in cell engineering. Clin. Hemorheol. Microcirc 33(3):293–302 [PubMed] [Google Scholar]
- 32.Fertig N, Blick RH, Behrends JC. 2002. Whole cell patch clamp recording performed on a planar glass chip. Biophys. J 82(6):3056–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fluhler E, Burnham VG, Loew LM. 1985. Spectra, membrane binding, and potentiometric responses of new charge shift probes. Biochemistry 24(21):5749–55 [DOI] [PubMed] [Google Scholar]
- 34.Fromherz P, Hübener G, Kuhn B, Hinner MJ. 2008. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur. Biophys. J 37(4):509–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez JE, Tsien RY. 1997. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem. Biol 4(4):269–77 [DOI] [PubMed] [Google Scholar]
- 36.Gross E, Bedlack RS, Loew LM. 1994. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J 67(1):208–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holcman D, Yuste R. 2015. The new nanophysiology: regulation of ionic flow in neuronal subcompartments. Nat. Rev. Neurosci 16(11):685–92 [DOI] [PubMed] [Google Scholar]
- 38.Holst GL, Stoy W, Yang B, Kolb I, Kodandaramaiah SB, et al. 2019. Autonomous patch-clamp robot for functional characterization of neurons in vivo: development and application to mouse visual cortex. J. Neurophysiol 121(6):2341–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppa MB, Gouzer G, Armbruster M, Ryan TA. 2014. Control and plasticity of the presynaptic action potential waveform at small CNS nerve terminals. Neuron 84(4):778–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horn R, Marty A. 1988. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol 92(2):145–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou JH, Venkatachalam V, Cohen AE. 2014. Temporal dynamics of microbial rhodopsin fluorescence reports absolute membrane voltage. Biophys. J 106(3):639–48 [DOI] [PMC free article] [PubMed] [Google Scholar]; Reported absolute Vmem with 10-mV resolution using temporal dynamics of the GEVI Arch(D95H).
- 42.Huang X, Jan LY. 2014. Targeting potassium channels in cancer. J. Cell Biol 206(2):151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YL, Walker AS, Miller EW. 2015. A photostable silicon rhodamine platform for optical voltage sensing. J. Am. Chem. Soc 137(33):10767–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacquemet G, Baghirov H, Georgiadou M, Sihto H, Peuhu E, et al. 2016. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun 7:13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayant K, Hirtz JJ, Plante IJ-L, Tsai DM, De Boer WDAM, et al. 2017. Targeted intracellular voltage recordings from dendritic spines using quantum-dot-coated nanopipettes. Nat. Nanotechnol 12(4):335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]; Developed nanopipettes capable of recording from dendritic spines and demonstrated spine electrical compartmentalization.
- 46.Jayant K, Wenzel M, Bando Y, Hamm JP, Mandriota N, et al. 2019. Flexible nanopipettes for minimally invasive intracellular electrophysiology in vivo. Cell Rep. 26(1):266–78.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johri A, Beal MF. 2012. Mitochondrial dysfunction in neurodegenerative diseases. J. Pharmacol. Exp. Ther 342(3):619–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasper EM, Larkman AU, Lübke J, Blakemore C. 1994. Pyramidal neurons in layer 5 of the rat visual cortex. II. Development of electrophysiological properties. J. Comp. Neurol 339(4):475–94 [DOI] [PubMed] [Google Scholar]
- 49.Kato K, Clifford DB, Zorumski CF. 1993. Long-term potentiation during whole-cell recording in rat hippocampal slices. Neuroscience 53(1):39–47 [DOI] [PubMed] [Google Scholar]
- 50.Kazemipour A, Novak O, Flickinger D, Marvin JS, Abdelfattah AS, et al. 2019. Kilohertz frame-rate two-photon tomography. Nat. Methods 16(8):778–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klapperstück T, Glanz D, Klapperstück M, Wohlrab J. 2009. Methodological aspects of measuring absolute values of membrane potential in human cells by flow cytometry. Cytom. A 75(7):593–608 [DOI] [PubMed] [Google Scholar]
- 52.Kodandaramaiah SB, Franzesi GT, Chow BY, Boyden ES, Forest CR. 2012. Automated whole-cell patch-clamp electrophysiology of neurons in vivo. Nat. Methods 9(6):585–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kole MHP, Stuart GJ. 2012. Signal processing in the axon initial segment. Neuron 73(2):235–47 [DOI] [PubMed] [Google Scholar]
- 54.Korn SJ, Horn R. 1989. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J. Gen. Physiol 94(5):789–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krasznai Z, Márián T, Balkay L, Emri M, Trón L. 1995. Flow cytometric determination of absolute membrane potential of cells. J. Photochem. Photobiol. B 28(1):93–99 [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni RU, Kramer DJ, Pourmandi N, Karbasi K, Bateup HS, Miller EW. 2017. Voltage-sensitive rhodol with enhanced two-photon brightness. PNAS 114(11):2813–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulkarni RU, Miller EW. 2017. Voltage imaging: pitfalls and potential. Biochemistry 56(39):5171–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larkin J, Garcia-Ojalvo J, Prindle A, Liu J, Gabalda-Sagarra M, et al. 2017. Coupling between distant biofilms and emergence of nutrient time-sharing. Science 356(6338):638–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazzari-Dean JR, Gest AMM, Miller EW. 2019. Optical estimation of absolute membrane potential using fluorescence lifetime imaging. eLife 8:e44522. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated that the fluorescence lifetime of VFs reports absolute Vmem with high resolution and improved throughput.
- 60.Lee DD, Galera-Laporta L, Bialecka-Fornal M, Moon EC, Shen Z, et al. 2019. Magnesium flux modulates ribosomes to increase bacterial survival. Cell 177(2):352–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ, Huang K-C, Mei G, Zong C, Mamaeva N, et al. 2019. Electronic preresonance stimulated Raman scattering imaging of red-shifted proteorhodopsins: toward quantitation of the membrane potential. J. Phys. Chem. Lett 10(15):4374–81 [DOI] [PubMed] [Google Scholar]
- 62.Lee HJ, Zhang D, Jiang Y, Wu X, Shih P-Y, et al. 2017. Label-free vibrational spectroscopic imaging of neuronal membrane potential. J. Phys. Chem. Lett 8(9):1932–36 [DOI] [PubMed] [Google Scholar]
- 63.LeSauter J, Silver R, Cloues R, Witkovsky P. 2011. Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. J. Neurophysiol 106(2):576–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin M 2014. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 25(24):3835–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W-C, Soffe SR, Roberts A. 2004. A direct comparison of whole cell patch and sharp electrodes by simultaneous recording from single spinal neurons in frog tadpoles. J. Neurophysiol 92(1):380–86 [DOI] [PubMed] [Google Scholar]
- 66.Linley JE. 2013. Perforated whole-cell patch-clamp recording. In Ion Channels: Methods and Protocols, ed. Gamper N, pp. 149–57. Berlin: Springer; [DOI] [PubMed] [Google Scholar]
- 67.Liu P, Grenier V, Hong W, Muller VR, Miller EW. 2017. Fluorogenic targeting of voltage-sensitive dyes to neurons. J. Am. Chem. Soc 139(48):17334–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loew LM, Scully S, Simpson L, Waggoner AS. 1979. Evidence for a charge-shift electrochromic mechanism in a probe of membrane potential. Nature 281(5731):497–99 [DOI] [PubMed] [Google Scholar]
- 69.Loewenstein WR, Kanno Y. 1964. Studies on an epithelial (gland) cell junction I. Modifications of surface membrane permeability. J. Cell Biol 22:565–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. 2014. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 588(16):2663–70 [DOI] [PubMed] [Google Scholar]
- 71.Magni M, Meldolesi J, Pandiella A. 1991. Ionic events induced by epidermal growth factor: evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J. Biol. Chem 266(10):6329–35 [PubMed] [Google Scholar]
- 72.Maher MP, Wu NT, Ao H. 2007. pH-insensitive FRET voltage dyes. J. Biomol. Screen 12(5):656–67 [DOI] [PubMed] [Google Scholar]
- 73.Malinow R, Tsien RW. 1990. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature 346(5429):177–80 [DOI] [PubMed] [Google Scholar]
- 74.Maric D, Maric I, Smith SV, Serafini R, Hu Q, Barker JL. 1998. Potentiometric study of resting potential, contributing K+ channels and the onset of Na+ channel excitability in embryonic rat cortical cells. Eur. J. Neurosci 10(8):2532–46 [DOI] [PubMed] [Google Scholar]
- 75.McCormick DA, Prince DA. 1987. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J. Physiol 393(1):743–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McNamara HM, Salegame R, Al Tanoury Z, Xu H, Begum S, et al. 2020. Bioelectrical domain walls in homogeneous tissues. Nat. Phys 16(3):357–64 [DOI] [PMC free article] [PubMed] [Google Scholar]; Provided theoretical and direct experimental evidence for stable Vmem patterning in tissue.
- 77.Miller EW. 2016. Small molecule fluorescent voltage indicators for studying membrane potential. Curr. Opin. Chem. Biol 33:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB, Tsien RY. 2012. Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. PNAS 109(6):2114–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montana V, Farkas DL, Loew LM. 1989. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry 28(11):4536–39 [DOI] [PubMed] [Google Scholar]
- 80.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. 2005. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 435(7046):1239–43 [DOI] [PubMed] [Google Scholar]
- 81.Novo D, Perlmutter NG, Hunt RH, Shapiro HM. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35(1):55–63 [DOI] [PubMed] [Google Scholar]
- 82.Obergrussberger A, Brüggemann A, Goetze TA, Rapedius M, Haarmann C, et al. 2016. Automated patch clamp meets high-throughput screening: 384 cells recorded in parallel on a planar patch clamp module. J. Lab. Autom 21(6):779–93 [DOI] [PubMed] [Google Scholar]
- 83.Okkelman IA, Papkovsky DB, Dmitriev RI. 2020. Estimation of the mitochondrial membrane potential using fluorescence lifetime imaging microscopy. Cytom. A 97(5):471–82 [DOI] [PubMed] [Google Scholar]
- 84.Ortiz G, Liu P, Naing SHH, Muller VR, Miller EW. 2019. Synthesis of sulfonated carbofluoresceins for voltage imaging. J. Am. Chem. Soc 141(16):6631–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palmer LM, Stuart GJ. 2009. Membrane potential changes in dendritic spines during action potentials and synaptic input. J. Neurosci 29(21):6897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandiella A, Magni M, Lovisolo D, Meldolesi J. 1989. The effects of epidermal growth factor on membrane potential. J. Biol. Chem 264(22):12914–21 [PubMed] [Google Scholar]
- 87.Perkins KL. 2006. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J. Neurosci. Methods 154(1–2):1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piatkevich KD, Jung EE, Straub C, Linghu C, Park D, et al. 2018. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol 14(4):352–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, et al. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527(7576):59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rad MS, Cohen LB, Braubach O, Baker BJ. 2018. Monitoring voltage fluctuations of intracellular membranes. Sci. Rep 8:6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raspe M, Kedziora KM, van den Broek B, Zhao Q, de Jong S, et al. 2016. siFLIM: Single-image frequency-domain FLIM provides fast and photon-efficient lifetime data. Nat. Methods 13(6):501–4 [DOI] [PubMed] [Google Scholar]
- 92.Rinne A, Birk A, Bünemann M. 2013. Voltage regulates adrenergic receptor function. PNAS 110(4):1536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rinne A, Mobarec JC, Mahaut-Smith M, Kolb P, Bünemann M. 2015. The mode of agonist binding to a G protein-coupled receptor switches the effect that voltage changes have on signaling. Sci. Signal 8(401):ra110. [DOI] [PubMed] [Google Scholar]
- 94.Robinson JT, Jorgolli M, Shalek AK, Yoon MH, Gertner RS, Park H. 2012. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol 7(3):180–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rohr S 2004. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc. Res 62(2):309–22 [DOI] [PubMed] [Google Scholar]
- 96.Rolfe DFS, Brown GC. 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev 77(3):731–58 [DOI] [PubMed] [Google Scholar]
- 97.Sada N, Lee S, Katsu T, Otsuki T, Inoue T. 2015. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347(6228):1362–67 [DOI] [PubMed] [Google Scholar]
- 98.Saminathan A, Devany J, Veetil AT, Suresh B, Pillai KS, et al. 2021. A DNA-based voltmeter for organelles. Nat. Nanotechnol 16(1):96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sánchez A, Urrego D, Pardo LA. 2016. Cyclic expression of the voltage-gated potassium channel KV10.1 promotes disassembly of the primary cilium. EMBO Rep. 17(5):708–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sigworth FJ, Neher E. 1980. Single Na+ channel currents observed in cultured rat muscle cells. Nature 287:447–49 [DOI] [PubMed] [Google Scholar]
- 101.Spruston N, Johnston D. 1992. Perforated patch-clamp analysis of the passive membrane properties of three classes of hippocampal neurons. J. Neurophysiol 67(3):508–29 [DOI] [PubMed] [Google Scholar]
- 102.Stuart GJ, Spruston N. 2015. Dendritic integration: 60 years of progress. Nat. Neurosci 18(12):1713–21 [DOI] [PubMed] [Google Scholar]
- 103.Suk HJ, van Welie I, Kodandaramaiah SB, Allen B, Forest CR, Boyden ES. 2017. Closed-loop real-time imaging enables fully automated cell-targeted patch-clamp neural recording in vivo. Neuron 95(5):1037–47.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsuchiya W, Okada Y. 1982. Membrane potential changes associated with differentiation of enterocytes in the rat intestinal villi in culture. Dev. Biol 94(2):284–90 [DOI] [PubMed] [Google Scholar]
- 105.Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. 2003. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J. Neurophysiol 90:2964–72 [DOI] [PubMed] [Google Scholar]; Revealed discrepancies in Vmem as reported by whole-cell, cell-attached, and perforated-patch recordings.
- 106.Verheugen JA, Fricker D, Miles R. 1999. Noninvasive measurements of the membrane potential and GABAergic action in hippocampal interneurons. J. Neurosci 19(7):2546–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vogt KE, Gerharz S, Graham J, Canepari M. 2011. Combining membrane potential imaging with lglutamate or GABA photorelease. PLOS ONE 6(10):e24911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang SY, Melkoumian Z, Woodfork KA, Cather C, Davidson AG, et al. 1998. Evidence for an early G1 ionic event necessary for cell cycle progression and survival in the MCF-7 human breast carcinoma cell line. J. Cell. Physiol 176(3):456–64 [DOI] [PubMed] [Google Scholar]
- 109.White TW, Paul DL. 1999. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol 61:283–310 [DOI] [PubMed] [Google Scholar]
- 110.Williams SR, Mitchell SJ. 2008. Direct measurement of somatic voltage clamp errors in central neurons. Nat. Neurosci 11(7):790–98 [DOI] [PubMed] [Google Scholar]
- 111.Wonderlin WF, Woodfork KA, Strobl JS. 1995. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J. Cell. Physiol 165(1):177–85 [DOI] [PubMed] [Google Scholar]
- 112.Wu J, Lewis AH, Grandl J. 2017. Touch, tension, and transduction—the function and regulation of piezo ion channels. Trends Biochem. Sci 42(1):57–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xie C, Lin Z, Hanson L, Cui Y, Cui B. 2012. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol 7(3):185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]; Constructed nanopillar electrode arrays for intracellular recording of action potentials from many neurons over days.
- 114.Xu H, Martinoia E, Szabo I. 2015. Organellar channels and transporters. Cell Calcium 58(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Y, Zou P, Cohen AE. 2017. Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol 39:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang HH, St-Pierre F. 2016. Genetically encoded voltage indicators: opportunities and challenges. J. Neurosci 36(39):9977–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang M, Brackenbury WJ. 2013. Membrane potential and cancer progression. Front. Physiol 4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yellen G, Mongeon R. 2015. Quantitative two-photon imaging of fluorescent biosensors. Curr. Opin. Chem. Biol 27:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, et al. 2011. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477(7363):171–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yuste R 2013. Electrical compartmentalization in dendritic spines. Annu. Rev. Neurosci 36:429–49 [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Chen X, Xue Y, Gamper N, Zhang X. 2018. Beyond voltage-gated ion channels: voltage-operated membrane proteins and cellular processes. J. Cell. Physiol 233(10):6377–85 [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Davidson RM, Wei MD, Loew LM. 1998. Membrane electric properties by combined patch clamp and fluorescence ratio imaging in single neurons. Biophys. J 74(1):48–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y, Wong C, Cho K, van der Hoeven D, Liang H, et al. 2015. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science 349(6250):873–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, et al. 2018. Mitochondrial membrane potential. Anal. Biochem 552:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zou P, Zhao Y, Douglass AD, Hochbaum DR, Brinks D, et al. 2014. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat. Commun 5:4625. [DOI] [PMC free article] [PubMed] [Google Scholar]


