Abstract
Background
Poor mental health has consistently been associated with substance use (smoking, alcohol drinking, cannabis use, and consumption of caffeinated drinks). To properly inform public health policy it is crucial to understand the mechanisms underlying these associations, and most importantly, whether or not they are causal.
Methods
In this pre-registered systematic review, we assessed the evidence for causal relationships between mental health and substance use from Mendelian randomization (MR) studies, following PRISMA. We rated the quality of included studies using a scoring system that incorporates important indices of quality, such as the quality of phenotype measurement, instrument strength, and use of sensitivity methods.
Results
Sixty-three studies were included for qualitative synthesis. The final quality rating was ‘−’ for 16 studies, ‘– +’ for 37 studies, and ‘+’for 10 studies. There was robust evidence that higher educational attainment decreases smoking and that there is a bi-directional, increasing relationship between smoking and (symptoms of) mental disorders. Another robust finding was that higher educational attainment increases alcohol use frequency, but decreases binge-drinking and alcohol use problems, and that mental disorders causally lead to more alcohol drinking without evidence for the reverse.
Conclusions
The current MR literature increases our understanding of the relationship between mental health and substance use. Bi-directional causal relationships are indicated, especially for smoking, providing further incentive to strengthen public health efforts to decrease substance use. Future MR studies should make use of large(r) samples in combination with detailed phenotypes, a wide range of sensitivity methods, and triangulate with other research methods.
Key words: Systematic review, Mendelian randomization, substance use, smoking, alcohol, cannabis, caffeine, mental disorders, cognitive functioning
Introduction
Mental disorders have consistently been associated with substance use – in particular cigarette smoking, alcohol drinking, cannabis use, and consumption of caffeinated drinks. Compared to the general population, individuals diagnosed with a mental disorder – or subclinical symptoms – are more likely to smoke (Garey et al., 2020), drink alcohol excessively (Stephen Rich & Martin, 2014), and use cannabis (Satre, Bahorik, Zaman, & Ramo, 2018). For caffeine, there are conflicting findings with high(er) consumption being associated with a lower odds of depression (Grosso, Micek, Castellano, Pajak, & Galvano, 2016) but a higher odds of schizophrenia (Williams & Gandhi, 2008). A key factor in mental disorders is cognitive functioning, the majority of patients suffering from deficits in attention, learning and/or memory (Nieman et al., 2020). In non-clinical populations, poor cognitive functioning has been associated with increased smoking (Campos, Serebrisky, & Castaldelli-Maia, 2016), alcohol drinking (Topiwala & Ebmeier, 2018), and cannabis use (Curran et al., 2016), although for impaired response inhibition specifically there are contradicting findings (Liu et al., 2019b). Although caffeine is often thought to have acute beneficial effects on cognition (Irwin, Khalesi, Desbrow, & McCartney, 2020), there is evidence that contests this (Galindo, Navarro, & Cavas, 2020; Weibel et al., 2020) and its long(er) term effects remain unclear (Cornelis, Weintraub, & Morris, 2020; Panza et al., 2015).
To properly inform public health policy it is crucial to understand the mechanisms underlying associations between poor mental health and substance use. Typically, a distinction is made between three, not mutually exclusive, mechanisms: (1) shared risk factors, (2) causal effects where poor mental health increases substance use, and (3) causal effects where substance use negatively affects mental health. As for mechanism 1, important non-genetic shared risk factors are the death of a loved one (Keyes et al., 2014) or (other) childhood trauma (Setién-Suero et al., 2020). Although note that these seemingly environmental factors might have a heritable component (Sallis et al., 2020). Poor mental health and substance use are substantially heritable and there is evidence for considerable genetic correlation (Abdellaoui, Smit, van den Brink, Denys, & Verweij, 2020; Vink & Schellekens, 2018). However, genetic correlations can also reflect causal relationships. If trait 1 causally affects trait 2, then genetic variants predictive of trait 1 will, indirectly, also predict trait 2 (Kraft, Chen, & Lindström, 2020).
We review evidence from studies that applied ‘Mendelian randomization’ (MR) (Davies, Holmes, & Davey Smith, 2018b; Lawlor, Harbord, Sterne, Timpson, & Davey Smith, 2008) to assess causal effects between poor mental health and substance use. When we talk about a true causal effect (e.g. A is causal for B), we imply that if A were to be altered this would lead B to change accordingly. To some extent, MR is analogous to a randomized controlled trial (RCT). Instead of participants being assigned to experimental conditions, MR compares subgroups in the population which are at differing levels of genetic risk for a proposed risk factor. We include MR studies that look at cigarette smoking, alcohol drinking, cannabis use, and/or caffeine consumption in relation to (symptoms of) a mental health disorder or cognitive functioning. Below, we briefly discuss epidemiological and (human) experimental evidence on these relationships and then introduce MR.
Epidemiological evidence
Causal inference can be attempted by looking at the temporal nature of relationships. For smoking, there is extensive longitudinal evidence that depression (Audrain-McGovern, Leventhal, & Strong, 2015; Mathew, Hogarth, Leventhal, Cook, & Hitsman, 2017) and attention-deficit hyperactivity disorder (ADHD) (van Amsterdam, van der Velde, Schulte, & van den Brink, 2018) are associated with increased odds of smoking initiation and persistence. In the other direction – from smoking to mental health – a systematic review study including 26 studies with a follow-up of between seven weeks and nine years concluded that smoking cessation is followed by reduced depression, anxiety, and stress (Taylor et al., 2014b). Smoking has also been associated with poorer cognitive performance, which improved after cessation (Vermeulen et al., 2018).
For alcohol, a review of 37 longitudinal studies found that (symptoms of) mental disorders in childhood predict an increased odds of alcohol dependence later on in life (Groenman, Janssen, & Oosterlaan, 2017). In the other direction, alcohol dependence and heavy drinking predicted subsequent increases in depressive symptoms, but for heavy drinking, this association did not persist after adjustment for confounders (Li et al., 2020). A systematic review of alcohol interventions reported that alcohol reduction led to a lower prevalence of psychiatric episodes, and improvement of anxiety and depressive symptoms, self-confidence, and mental quality of life (Charlet & Heinz, 2017).
For cannabis use, the few available studies are smaller and the evidence is mixed. A 10-year prospective cohort study in 1395 adolescents found that symptoms of mental disorders (depression, bipolar, and anxiety disorder) increase the odds of cannabis initiation and cannabis use disorder (Wittchen et al., 2007). There was no indication that cannabis causes elevated anxiety symptoms (Twomey, 2017), but substantial evidence to support that it increases the risk of manic symptoms (Gibbs et al., 2015) and psychosis (Gage, Hickman, & Zammit, 2016a). Another study found evidence that cannabis can be beneficial for post-traumatic stress disorder but is associated with short-term cognitive deficits (Walsh et al., 2017).
For caffeine, research has focussed predominately on cognitive functioning or sleep. The largest available systematic review, including 28 studies, concluded that there is some evidence that caffeine is protective against cognitive decline (Panza et al., 2015). Despite the fact that caffeine has stimulating properties which are thought to interfere with sleep acutely, a cohort study in 26 305 adolescents with a follow-up of 4 years found no association between average daily caffeine consumption and sleep duration (Patte, Qian, & Leatherdale, 2018).
Combined, the current epidemiological literature points topotential bi-directional effects between mental health and substance use. However, there are important methodological limitations to consider. First, there may be bias from confounders that were not included in the analysis or measured with considerable error (Gage, Munafò, & Davey Smith, 2016b). Second, reverse causality, where the outcome variable or a precursor of the outcome variable has affected the exposure, can induce spurious associations (Gage et al., 2016).
Family-based studies are better suited for causal inference. Most notable are twin methods. Because monozygotic and dizygotic twins share 100% of their family environment and 100% or 50% of their genetic make-up, respectively, causality can be inferred by looking at within-twin pair differences. For instance, differences in ADHD symptoms were associated with differential progression to daily smoking, cigarettes per day and nicotine dependence in female monozygotic twin pairs, indicating that ADHD causally impacts smoking (Elkins et al., 2018). A study that identified monozygotic twin pairs who were discordant for smoking (one smoked, the other did not), found evidence suggesting that smoking can also causally increase ADHD symptoms (Treur et al., 2015). However, twin methods also have important limitations – there may be bias from confounders that led twins to differ on the exposure as well as on the outcome of interest, and reverse causation cannot be ruled out (McGue, Osler, & Christensen, 2010).
Experimental evidence from human studies
Experimentally induced stress increased the perceived value of cigarettes in smokers with depressive symptoms (Dahne, Murphy, & MacPherson, 2017). Similarly, when tested after overnight sleep deprivation smokers were more inclined to pick cigarettes over money than when they were tested after a normal night's sleep (Hamidovic & de Wit, 2009). In the other direction, a meta-analysis of 35 clinical trials concluded that participants who were randomly assigned to use nicotine patches to quit smoking experienced more sleep problems than participants assigned not to use them (Greenland, Satterfield, & Lanes, 1998). After randomly assigning 31 smokers to continue smoking and 33 smokers to quit, anxiety and depressive symptoms decreased (more) in the latter group during 3 months follow-up (Dawkins, Powell, Pickering, Powell, & West, 2009).
Among 540 participants randomly assigned to receive different types of treatment for depression there were significant treatment effects on depressive symptoms, but no changes in alcohol consumption (Strid, Hallgren, Forsell, Kraepelien, & Öjehagen, 2019). A considerable amount of work has focussed on cognitive behavioral therapy (CBT) to reduce alcohol consumption. A systematic review including eight RCTs concluded that CBT reduced alcohol use and depressive and/or anxiety symptoms, even when CBT targeted alcohol only (Baker, Thornton, Hiles, Hides, & Lubman, 2012). This could mean that decreases in alcohol use led to improvements in mental health, or that, though not targeted to it specifically, CBT affected depressive/anxiety symptoms.
As reflected in the work described here, only a limited number of causal questions can be answered with experimental designs. Moreover, these questions mostly relate to (relatively) short-term effects. Longer-term effects – for instance, potential effects of prolonged smoking on being diagnosed with a mental disorder, or the impact of lifetime alcohol use on the cognitive decline – cannot be investigated. There are also obvious ethical restrictions; it would not be acceptable to randomize people to initiate or increase their use of an addictive substance.
Mendelian randomization
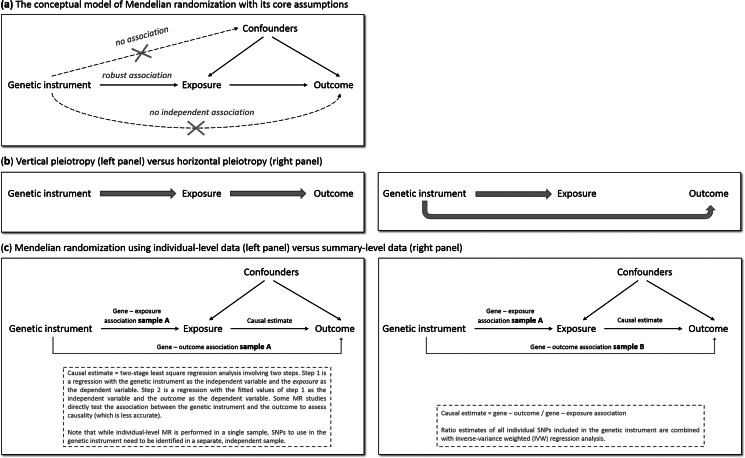
MR has the potential to overcome (some of) the limitations of traditional epidemiological and experimental methods. We will explain MR's rationale by using one specific research question: does smoking (the ‘exposure’ of interest) causally impact depressive symptoms (the ‘outcome’ of interest)? As is the case for practically all human traits (Polderman et al., 2015), individual differences in smoking can partly be explained by genetic differences (Vink, Willemsen, & Boomsma, 2005). Genetic variants robustly associated with smoking have been identified through genome-wide association studies (GWAS) – the most notable variants in nicotinic receptor genes (Liu et al., 2019a). Because the transmission of genetic variants from parents to offspring occurs randomly (Mendel's second law – ‘The law of independent assortment’), there should be minimal bias from confounders and subgroups of differing genetic risk can be thought of as RCT treatment groups. To determine whether smoking causally affects depression, we take genetic variants robustly associated with smoking and test if these also predict higher levels of depressive symptoms. The genetic variants act as proxies for measured smoking behavior, or instrumental variables (Davies et al., 2018b). The most commonly used genetic variants are Single Nucleotide polymorphisms (SNPs). MR provides unbiased results if three assumptions are met: (1) the SNPs used as instrumental variables – together referred to as the ‘genetic instrument’ – are robustly associated with the exposure, (2) the genetic instrument is not directly associated with confounders and (3) the genetic instrument is not directly associated with the outcome, apart from any causal effect running through the exposure variable (Fig. 1a).
Fig. 1.
The main principles of Mendelian randomization: (a) the conceptual model indicating the three core assumptions, (b) an illustration of vertical pleiotropy, that which causal inference is based on in a Mendelian randomization analysis, versus horizontal pleiotropy, which biases a Mendelian randomization analysis, and (c) an illustration of the framework and methods of Mendelian randomization using individual-level data versus summary-level data.
Since the second and third assumptions cannot be known or (exhaustively) tested, sensitivity analyses that assess the robustness of a causal finding are crucial. An important source of bias is pleiotropy, where a genetic variant affects multiple traits. Vertical pleiotropy (sometimes called mediated pleiotropy) occurs when a genetic variant affects the exposure and because of that indirectly also affects the outcome. This is not problematic and in fact is what an MR analysis aims to detect. Horizontal pleiotropy (sometimes referred to as biological pleiotropy) occurs when a genetic variant affects the outcome independently, not mediated through its effect on the exposure (Fig. 1b). This is problematic and could lead to bias.
There are two MR approaches: using individual-level data and using summary-level data from GWAS. Although MR using individual-level data requires a single data set of individuals with genotype data and information on both the exposure and outcome, MR using summary-level data takes summary estimates (i.e. the mean effect size for the genetic variants of interest) from separate GWAS for the exposure and the outcome. The two approaches use different methodology to estimate the causal effect (Burgess, Scott, Timpson, Davey Smith, & Thompson, 2015; Burgess, Small, & Thompson, 2017) (Fig. 1c). MR using summary-level data has been the predominant method in recent years and currently has the most (powerful) sensitivity methods.
Methods
This study was pre-registered at PROSPERO (CRD42019133182; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=133182). We performed a literature search of Medline, EMBASE, PsycINFO, and Web of Science for published, peer-reviewed papers describing MR of one or more type(s) of substance use in combination with mental health (including diagnoses, subclinical symptoms, and cognitive functioning). We also performed a search of pre-print servers (bioRxiv, medRxiv, and arXiv). We restricted our search to English-language publications (search terms provided in Supplementary Methods). Similar to a recent, high-impact review (Firth et al., 2020), we designed our search to pick up studies that performed analyses referred to as ‘Mendelian randomization’ (or (very) closely related methods such as ‘genetic instrumental variable regression’ (DiPrete, Burik, & Koellinger, 2018)). The final search was performed on 27 February 2020, and a final update of all papers (to incorporate transitions from pre-print to a newer pre-print version or published paper) on 12 April 2021.
We followed PRISMA guidelines in extracting and selecting the data and used a flowchart to document the stages of screening. After a deduplication step, two of the co-authors independently selected potentially eligible studies based on title and abstract, and if necessary in the following step, based on the full text. In case of disagreement between the two main reviewers, this was resolved through discussion with a third co-author.
Qualitative synthesis
The studies included in this review use a wide range of genetic instruments, phenotypes, and methods. This precluded us from formally combining effect estimates through meta-analysis. Instead, we extracted the most important information from each study, judged the quality based on an extensive set of predetermined criteria, and summarized our findings stratified on the addictive substance.
We developed a scoring system incorporating the factors most important to the validity of an MR study (Supplementary Table S1), based on our collective knowledge of MR and cross-checked with the most recent (still evolving) MR guidelines (Davey Smith et al., 2019). Important indices of quality are phenotype measurement (sample size and quality of the exposure and outcome measurements) and instrument strength (p value threshold used to select genetic variants, number of genetic variants included, biological knowledge, F-statistic for instrument strength, % variance that the instrument explains). Taking all quality indices into consideration, each study was given a total score of ‘–’, ‘– +’or‘+’. We considered the total score based on a few key indicators that needed to be satisfied in order for the study to be considered sufficient (– + ), most notably: sufficient sample size and sufficient main analytical methods. When, on top of that, a study had used particularly extensive (sensitivity) methods, a total score of (+) was given. Two co-authors scored all studies independently and blind from each other, after which they compared their scores. In case of disagreement, a third co-author was consulted and together, all agreed on the final score.
Results
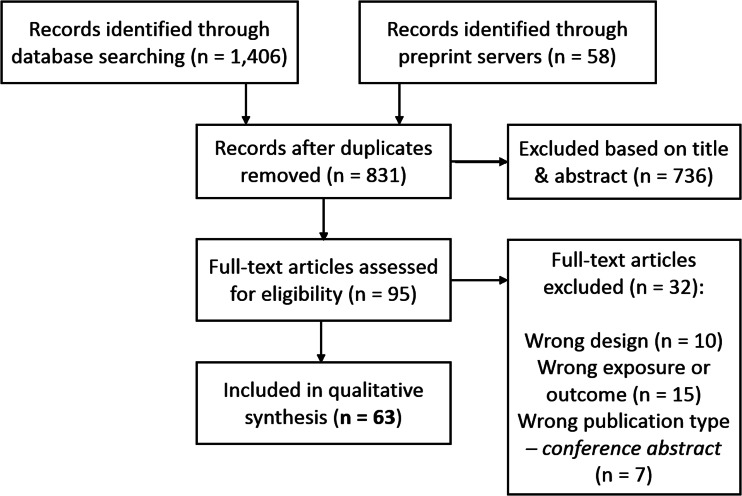
We identified 1464 potentially relevant records, of which 831 unique (Fig. 2). Of the final 63 studies included in qualitative synthesis, 40 investigated smoking, 24 investigated alcohol, 8 investigated cannabis, and 6 investigated caffeine (some investigated multiple substances; Table 1). The final quality rating was – for 16 studies, – + for 37 studies, and + for 10 studies (Supplementary Tables S2 and S3 for MR using individual-level and summary-level data, respectively). Note that some summary-level studies obtained genetic estimates from partly/largely the same data sets, either for the exposure alone or for both exposure and outcome. This is inherent to MR, as it requires robust, replicated estimates from the largest available GWAS. However, this means that the causal findings presented should not be regarded as (completely) independent. The importance of a particular study and its findings is determined not only on the basis of the data used, but also the quality of the analysis and, importantly, sensitivity methods. If two studies use (almost) exactly the same data sets for exposure and outcome, this is indicated in the text. For a more detailed comparison of data sets see Table 2.
Fig. 2.
PRISMA flow chart demonstrating the selection of articles to be included for qualitative synthesis.
Table 1.
All Mendelian randomization (MR) studies included for qualitative synthesis, with their identifying information, description of the exposure and outcome variable(s), whether the study used individual-level and/or summary-level data, the total quality rating, and a brief summary of their findings
| ID | First author (Year) | Type of substance | Individual- /summary-level data | Exposure variable(s) | Outcome variable(s) | Quality | Finding(s) |
|---|---|---|---|---|---|---|---|
| 1 | Zhou et al. (2019a) | Smoking | Summary level | Educational attainment (self-report) | Smoking initiation, cigarettes per day, smoking cessation, age at smoking initiation (all self-report) | –a | Evidence for causal, decreasing effects of educational attainment on smoking initiation and cigarettes per day. Evidence for causal, increasing effects of educational attainment on smoking cessation and age at smoking initiation |
| 2 | Zeng et al. (2019) | Smoking | Summary level | Educational attainment (self-report) | Smoking initiation (self-report) | – + | Evidence for causal, decreasing effects of educational attainment on smoking initiation. This effect was only modestly attenuated when adjusting for years spent in school |
| 3 | Gage et al. (2018) | Smoking | Summary level | Educational attainment (self-report) | Smoking initiation (self-report), cigarettes per day (self-report), smoking cessation (self-report), cotinine levels (measured in the blood) | – + | Evidence for causal, decreasing effects of education on smoking initiation and cigarettes per day. Evidence for causal, increasing effects of education on smoking cessation. Weak evidence for causal, decreasing effects of education on cotinine levels |
| 4 | Tillmann et al. (2017) | Smoking | Summary level | Educational attainment (self-report) | Smoking initiation (self-report) | – + | Evidence for causal, decreasing effects of education on smoking initiation |
| 5 | Carter et al. (2019) | Smoking | Both | Educational attainment (self-report) | Lifetime smoking (self-report) | – + | Evidence for causal, decreasing effects of education on lifetime smoking |
| 6 | Sanderson et al. (2019) | Smoking | Both | Educational attainment (self-report), cognitive functioning (multivariable MR) | Smoking initiation, smoking cessation (all self-report) | + | Evidence for causal, decreasing effects of education on smoking initiation, and evidence for causal, increasing effects of education on smoking cessation. These effects of education are independent of cognitive functioning |
| 7 | Gage et al. (2020) | Smoking | Summary level | Smoking initiation (self-report), lifetime smoking (self-report) | Cognitive functioning (fluid intelligence), educational attainment (self-report) | + | Evidence for causal, decreasing effects of smoking initiation and lifetime smoking on educational attainment and cognitive functioning. Results for educational attainment were more robust than results for cognitive functioning |
| 8b | Fu et al. (2019) | Smoking | Individual level | Smoking initiation (self-report) | Cognitive functioning (a composite measure of cognitive tests) | – | Evidence for causal, decreasing effects of current smoking on cognitive functioning |
| 9 | North et al. (2015) | Smoking | Individual level | Cigarettes per day (self-report) | Cognitive functioning (a general fluid cognitive ability score, derived from a range of different cognitive functioning tests), cognitive decline (% change in continuous cognitive measures from baseline to last available wave) | – + | Overall no consistent evidence for causal effects. Very weak evidence for causal, increasing effects of cigarettes per day on cognitive decline (higher odds of being in the top 25% of cognitive decliners). In never smokers, weak evidence for causal, decreasing effects of smoking on search speed |
| 10b | Adams (2019) | Smoking | Summary level | Lifetime smoking (self-report), cognitive functioning (fluid intelligence), neuroticism (self-report) | Lifetime smoking (self-report), cognitive functioning (fluid intelligence), neuroticism (self-report) | – + | Evidence for causal, increasing effects of lifetime smoking on neuroticism. Evidence for causal, decreasing effects of cognitive functioning on lifetime smoking |
| 11 | Østergaard et al. (2015) | Smoking | Summary level | Smoking initiation, cigarettes per day (all self-report) | Alzheimer's disease (diagnosis) | – + | Evidence for causal, decreasing effects cigarettes per day on Alzheimer's disease. No clear evidence for causal effects of smoking initiation on Alzheimer's disease |
| 12 | Gibson et al. (2019) | Smoking | Both | Smoking initiation, cigarettes per day, smoking cessation, sleep duration, chronotype, insomnia (all self-report) | Smoking initiation, cigarettes per day, smoking cessation; sleep duration, chronotype, insomnia (all self-report) | – + | Evidence for causal, decreasing effects of cigarettes per day on the odds of being a morning person. Weak evidence for causal, increasing effects of insomnia on cigarettes per day, and weak evidence for causal, decreasing effects of insomnia on smoking cessation. No clear evidence for causal effects in any of the other tested relationships |
| 13 | Millard et al. (2019) | Smoking | Individual level | Cigarettes per day (self-report) | Chronotype (self-report) | – + | Evidence for causal, decreasing effects of cigarettes per day on the odds of being a morning person |
| 14 | Jansen et al. (2019) | Smoking | Summary level | Insomnia (self-report), cigarettes per day (self-report) | Insomnia (self-report), cigarettes per day (self-report) | – + | Evidence for causal, increasing effects of insomnia on cigarettes per day. No clear evidence for causal effects of cigarettes per day on insomnia |
| 15 | Lane et al. (2019) | Smoking | Summary level | Insomnia (self-report) | Smoking initiation, age at smoking initiation, cigarettes per day, smoking cessation (all self-report) | – + | No clear evidence for causal effects |
| 16 | Bjorngaard et al. (2013) | Smoking | Individual level | Current smoking, cigarettes per day (all self-report) | Anxiety and depression (self-reported Hospital Anxiety and Depression Scale) | – + | Conflicting findings: in the whole sample evidence for causal, increasing effects of smoking on anxiety, but when stratified, effects were very weak in smokers (stronger in former and never smokers). No clear evidence for causal effects of smoking on depression |
| 17 | Lewis et al. (2011) | Smoking | Individual level | Smoking status (current, former, never smoker), cigarettes per day (all self-report) | Postnatal depression (self-reported Edinburgh Postnatal Depression Scale) | – | Weak evidence for causal, decreasing effects of smoking status and cigarettes per day on depressed mood during pregnancy |
| 18 | Taylor et al. (2014a) | Smoking | Individual level | Cigarettes per day (self-report) | Psychological distress (composite score derived from a range of self-report or symptom scale or diagnosis measures) | – + | No clear evidence for causal effects |
| 19 | Skov-Ettrup et al. (2017) | Smoking | Individual level | Cigarettes per day, pack years of cigarettes (all self-report) | Psychological distress (3 questions on stress, fatigue & hopelessness – all self-report) | – + | No clear evidence for causal effects |
| 20 | Wootton et al. (2019) | Smoking | Summary level | Lifetime smoking (self-report), smoking initiation (self-report); schizophrenia (diagnosis); major depression (diagnosis) | Lifetime smoking (self-report), smoking initiation (self-report), schizophrenia (diagnosis), major depression (diagnosis) | + | Evidence for causal, increasing effects of smoking initiation and of lifetime smoking on schizophrenia and on depression. Evidence (less strong) for causal, increasing effects of depression on smoking initiation and lifetime smoking, and of schizophrenia on lifetime smoking |
| 21 | Vermeulen et al. (2019) | Smoking | Summary level | Lifetime smoking (self-report), smoking initiation (self-report), bipolar disorder (diagnosis) | Lifetime smoking (self-report), smoking initiation (self-report), cigarettes per day (self-report), smoking cessation (self-report); bipolar disorder (diagnosis) | + | Evidence for causal, increasing effects of smoking initiation and of lifetime smoking on bipolar disorder. No clear evidence for causal effects of bipolar disorder on smoking |
| 22b | Barkhuizen et al. (2020) | Smoking | Summary level | Smoking initiation (self-report), psychotic experiences (self-report), schizophrenia (diagnosis), major depression (diagnosis), bipolar disorder (diagnosis) | Smoking initiation (self-report); psychotic experiences (self-report), schizophrenia (diagnosis); major depression (diagnosis); bipolar disorder (diagnosis) | – + | Evidence for causal, increasing effects of smoking initiation on major depression, bipolar disorder and cognitive disorganization. Weak evidence for causal, increasing effects of smoking initiation on schizophrenia and on negative symptoms. Weak evidence for causal, increasing effects of schizophrenia on smoking initiation. No clear evidence for causal effects in any of the other tested relationships |
| 23 | Wium-Andersen et al. (2015a) | Smoking | Individual level | Cigarettes per day (self-report) | Antipsychotic medication use (national health records), schizophrenia (diagnosis); antidepressant use (national health records), major depression (diagnosis) | – + | Weak evidence for causal, increasing effects of cigarettes per day on antipsychotic medication use and schizophrenia, but no clear evidence for causal effects on depression |
| 24 | Byrne et al. (2019) | Smoking | Summary level | Cigarettes per day (self-report) | Schizophrenia (diagnosis) | – + | Evidence for causal, increasing effects of cigarettes per day on schizophrenia |
| 25 | Gage et al. (2017b) | Smoking | Summary level | Smoking initiation (self-report), schizophrenia (diagnosis) | Schizophrenia (diagnosis); smoking initiation (self-report), cigarettes per day (self-report), smoking cessation (self-report) | – + | Evidence for causal, increasing effects of smoking initiation on schizophrenia. No clear evidence for causal effects of schizophrenia on smoking |
| 26 | Fluharty et al. (2018) | Smoking | Summary level | Childhood aggression (parental report; meta-analysis of continuous study-specific scales) and attention-deficit hyperactivity disorder (diagnosis) | Smoking initiation, age at onset smoking (all self-report) | – + | Evidence for causal, increasing effects of attention-deficit hyperactivity disorder on smoking initiation. No clear evidence for causal effects of attention-deficit hyperactivity disorder on age at onset smoking nor of aggression on smoking |
| 27 | Sallis et al. (2019) | Smoking | Both | Extraversion, neuroticism, smoking initiation, cigarettes per day, smoking cessation (all self-report) | Extraversion, neuroticism; smoking initiation, cigarettes per day, smoking cessation (all self-report) | – + | Evidence for causal, increasing effects of neuroticism on cigarettes per day and for causal, increasing effects of extraversion on smoking initiation No clear evidence for causal effects of smoking on extraversion or neuroticism |
| 28b | Leppert et al. (2019) | Smoking | Summary level | attention-deficit hyperactivity disorder (diagnosis) | Lifetime smoking (self-report) | –a | Evidence for causal, increasing effects of attention-deficit hyperactivity disorder on lifetime smoking |
| 29 | Harrison et al. (2020a) | Smoking | Both | Smoking initiation, cigarettes per day, lifetime smoking (all self-report) | Suicidal ideation (self-report) | – + | No clear evidence for causal effects |
| 30 | Rosoff et al. (2019) | Alcohol | Summary level | Educational attainment (self-report); frequency of alcohol use (self-report), alcohol drinks per week (self-report), specific alcohol types in drinks per week (self-report), problematic alcohol use (self-reported alcohol use disorders identification test), alcohol use disorder (diagnosis), individual alcohol use disorder symptoms (self-report) | Educational attainment (self-report); frequency of alcohol use (self-report), alcohol drinks per week (self-report), specific alcohol types in drinks per week (self-report), problematic alcohol use (self-reported alcohol use disorders identification test), alcohol use disorder (diagnosis), individual alcohol use disorder symptoms (self-report) | – + | Evidence for causal, decreasing effects of education on total drinks per drinking day, weekly spirits intake, binge drinking, and alcohol use disorder. Evidence for causal, increasing effects of education on alcohol intake frequency, weekly wine intake. In the other direction, evidence for causal, increasing effects of weekly wine and champagne intake and frequency of alcohol use on education, and evidence for causal, decreasing effects of weekly beer and cider intake on education |
| 31 | Zhou et al. (2019b) | Alcohol | Summary level | Years of education (self-report) | Alcohol use frequency (self-report), frequency of different types of alcohol use (self-report) | – + | Evidence for causal, increasing effects of educational attainment on alcohol use frequency, frequency of red wine use, and frequency of white wine/champagne use. Evidence for causal, decreasing effects of educational attainment on frequency of beer/cider and spirits |
| 32 | Kumari et al. (2014) | Alcohol | Individual level | Alcohol initiation, frequency of alcohol use (all self-report) | Cognitive functioning (word recall, verbal fluency, processing speed tasks) | – | No clear evidence for causal effects |
| 33 | Almeida et al. (2014a) | Alcohol | Individual level | Frequency of alcohol use (self-report) | Cognitive impairment (in-person mini-mental state examination) | – | No clear evidence for causal effects |
| 34 | Ritchie et al. (2014) | Alcohol | Individual level | Alcohol use in gram per day (self-report) | Cognitive functioning (in-person Moray House Test No. 12) | – | No clear evidence for causal effects of alcohol on cognitive functioning, However, there was an interaction such that individuals with higher genetic ability to process alcohol showed relative improvements in cognitive ability with more consumption, whereas those with low processing capacity showed a negative relationship |
| 35 | Au Yeung et al. (2012) | Alcohol | Individual level | Alcohol drinks per day (in-person interview) | Cognitive functioning (10-word list learning task + in-person mini-mental state examination) | – | No clear evidence for causal effects |
| 36 | Mahedy et al. (2020) | Alcohol | Summary level | Alcohol drinks per week (self-report) | Working memory, response inhibition, emotion recognition (all in-clinic test assessments) | – | No clear evidence for causal effects |
| 37 | Andrews et al. (2020) | Alcohol | Summary level | Alcohol drinks per week (self-report), problematic alcohol use (self-reported Alcohol Use Disorders Identification Test), alcohol use disorder (diagnosis) | Alzheimer's disease (diagnosis), Alzheimer's disease age of onset (diagnosis) | – + | No clear evidence for causal effects of alcohol on Alzheimer's disease diagnosis. Evidence that higher number of alcohol drinks per week causes earlier Alzheimer's disease onset. Contradicting, there was evidence that alcohol use disorder caused later disease onset |
| 38 | Nishiyama et al. (2019) | Alcohol | Individual level | Alcohol drinking days per week; cups of coffee per day (all self-report) | Hours of sleep per night (self-report) | – | Evidence that alcohol causes longer sleep duration, no clear evidence for causal effects of coffee on sleep |
| 39 | Almeida et al. (2014b) | Alcohol | Individual level | Frequency of alcohol use (self-report) | Depression (self-report on receiving treatment for or being diagnosed with depression; for a subgroup diagnosis obtained from national health records) | – | No clear evidence for causal effects |
| 40 | Wium-Andersen et al. (2015b) | Alcohol | Individual level | Alcohol drinks per week (self-report) | Depression (diagnosis obtained from national health records), psychological distress (self-report) | – + | No clear evidence for causal effects |
| 41 | Polimanti et al. (2019) | Alcohol | Summary level | Major depression (diagnosis); alcohol use disorder (diagnosis), alcohol use frequency (self-report), alcohol drinks per week (self-report) | Major depression (diagnosis); alcohol use disorder (diagnosis), alcohol use frequency (self-report), alcohol drinks per week (self-report) | + | Evidence for causal, increasing effects of major depression on alcohol use disorder. No clear evidence for causal effects of major depression on the other alcohol use variables, nor for causal effects in the other direction |
| 42 | Zhou et al. (2020) | Alcohol | Summary level | Major depression (diagnosis), schizophrenia (diagnosis), bipolar disorder (diagnosis), depressed effect (self-report), neuroticism (self-report), worrying (self-report), insomnia (self-report), cognitive functioning (fluid intelligence), educational attainment (self-report), alcohol use disorder (diagnosis) | Major depression (diagnosis), schizophrenia (diagnosis), bipolar disorder (diagnosis), depressed affect (self-report), neuroticism (self-report), worrying (self-report), insomnia (self-report), cognitive functioning (fluid intelligence), educational attainment (self-report), alcohol use disorder (diagnosis) | + | Evidence for causal, increasing effects of worrying and neuroticism on alcohol use disorder. Evidence for causal, decreasing effects of cognitive functioning and educational attainment on alcohol use disorder. Evidence for causal, decreasing effects of alcohol use disorder and education. No clear evidence for causal effects in any of the other tested relationships |
| 43 | Irons et al. (2007) | Alcohol | Individual level | Alcohol initiation, past year use of alcohol, past year drinking index, past year drunkenness index (all self-report), alcohol use disorder (clinical, in-person interview) | Antisocial personality disorder, delinquent behavior inventory (all clinical, in-person interview), exposure to bad peer models (self-report) | – | No clear evidence for causal effects |
| 44 | Chao et al. (2017) | Alcohol | Individual level | Alcohol use frequency, alcohol drinks per typical drinking occasion, desire to drink (all self-report) | Externalizing problems (Youth Self-Report), internalizing problems (self-report on Children's Depression Inventory and State-trait Anxiety Inventory) | – | Evidence for causal, increasing effects of alcohol on aggression and attention problems but no clear evidence for effects on delinquency, anxiety, or depression |
| 45 | Hodgson et al. (2020) | Cannabis | Summary level | Cannabis initiation (self-report), major depression (diagnosis) | Cannabis initiation (self-report), major depression (diagnosis) | – + | No clear evidence for causal effects |
| 46 | Soler Artigas et al. (2019) | Cannabis | Summary level | attention-deficit hyperactivity disorder (diagnosis), cannabis initiation (self-report) | Attention-deficit hyperactivity disorder (diagnosis), cannabis initiation (self-report) | – + | Evidence for causal, increasing effects of attention-deficit hyperactivity disorder on cannabis initiation. No clear evidence causal effects of cannabis initiation on attention-deficit hyperactivity disorder |
| 47 | Pasman et al. (2018) | Cannabis | Summary level | Cannabis initiation (self-report), schizophrenia (diagnosis) | Cannabis initiation (self-report), schizophrenia (diagnosis) | – + | Evidence for causal, increasing effects of schizophrenia on cannabis initiation. Weak evidence for causal, increasing effects of cannabis initiation on schizophrenia |
| 48 | Vaucher et al. (2018) | Cannabis | Summary level | Cannabis initiation (self-report) | Schizophrenia (diagnosis) | – + | Evidence for causal, increasing effects of cannabis initiation on schizophrenia |
| 49 | Gage et al. (2017a) | Cannabis | Summary level | Cannabis initiation (self-report); schizophrenia (diagnosis) | Cannabis initiation (self-report); schizophrenia (diagnosis) | – + | Evidence for causal, increasing effects of schizophrenia on cannabis initiation. Weak evidence for causal, increasing effects of cannabis initiation on schizophrenia |
| 50 | Zhou et al. (2018) | Coffee | ndividual level | Cups of coffee per day (self-report) | Cognitive functioning (composite global cognition & memory scores, derived from a range of different cognitive functioning tests) | – + | No clear evidence for causal effects |
| 51 | Treur et al. (2018) | Coffee | Summary level | Cups of coffee (self-report), plasma caffeine (measured in blood), caffeine metabolic ratio (measured in blood), sleep duration (self-report), chronotype (self-report), insomnia (self-report) | Cups of coffee (self-report), plasma caffeine (measured in blood), caffeine metabolic ratio (measured in blood); sleep duration (self-report), chronotype (self-report), insomnia (self-report) | – + | Weak evidence for causal, decreasing effects of higher plasma caffeine levels on the odds of being a morning person. No clear evidence for causal effects in any of the other tested relationships |
| 52 | Kwok et al. (2016) | Coffee | Summary level | Cups of coffee per day (self-report) | Major depression (diagnosis), Alzheimer's disease (diagnosis) | – + | No clear evidence for causal effects |
| 53 | Ding et al. (2019) | Multiple: smoking alcohol | Individual level | Years of education (self-report) | Current smoking (self-report); alcohol drinking days per week (self-report) | – | Evidence for causal, decreasing effects of educational attainment on current smoking. No clear evidence for causal effects of educational attainment on alcohol drinking days per week |
| 54b | Yuan et al. (2020a) | Multiple: smoking alcohol : | Summary level | Educational attainment (self-report); cognitive functioning (fluid intelligence) | Age at smoking initiation, cigarettes smoked per day, alcohol drinks per week (all self-report) | –a | Evidence for causal, increasing effects of educational attainment on age at onset smoking and decreasing effect on cigarettes per day, the effect remained the same when adjusted for cognitive functioning. Evidence for causal, increasing effects of cognitive functioning on age at onset smoking and decreasing effect on cigarettes per day, but when adjusted for educational attainment, the effect was largely attenuated Evidence for causal, increasing effects of educational attainment on alcohol drinks per week, when adjusted for cognitive functioning, this effect was attenuated. Evidence for causal, increasing effects of cognitive functioning on alcohol drinks per week, the effect remained the same when adjusted for educational attainment |
| 55 | Davies et al. (2019) | Multiple: smoking alcohol | Both | Years of school (self-report), cognitive functioning (fluid intelligence) | Smoking initiation, current smoking; alcohol use frequency (all self-report) | + | Evidence for causal, increasing effects of cognitive functioning on alcohol use frequency. Evidence for causal, decreasing effects of years of school on smoking initiation and current smoking |
| 56b | Davies et al. (2018a) | Multiple: smoking alcohol | Both | Years of education (self-report) | Alcohol use frequency; smoking initiation, current smoking (all self-report) | + | Evidence for causal, increasing effects of more years of education on alcohol use frequency, and evidence for causal, decreasing effects of more years of education on smoking initiation and current smoking |
| 57 | Harrison et al. (2020b) | Multiple: smoking alcohol | Both | Alcohol drinks per week; smoking initiation, lifetime smoking (all self-report) | Education (self-reported university degree status), loneliness (self-report) | – + | Evidence for causal, decreasing effects of lifetime smoking and smoking initiation on education. No clear evidence for causal effects in any of the other tested relationships |
| 58 | Mahedy et al. (2021) | Multiple: smoking cannabis | Both | Smoking initiation (self-report); cannabis initiation (self-report) | Working memory, response inhibition, emotion recognition (all in-clinic test assessments) | – | No clear evidence for causal effects |
| 59 | Andrews et al. (2021) | Multiple: smoking alcohol | Summary level | Alcohol drinks per week (self-report), problematic alcohol use (self-reported alcohol use disorders identification test); smoking initiation (self-report), cigarettes per day (self-report) | Alzheimer's disease (diagnosis), Alzheimer's disease age at onset (disorder) | + | No clear evidence for causal effects |
| 60 | Larsson et al. (2017) | Multiple: smoking alcohol coffee | Summary level | Smoking initiation, cigarettes per day, smoking cessation; alcohol drinks per week; cups of coffee per day (all self-report) | Alzheimer's disease (diagnosis) | – + | Weak evidence for a causal, decreasing effect of cigarettes smoked per day on Alzheimer's disease. Weak evidence for a causal, increasing effect of coffee on Alzheimer's disease. No clear evidence for causal effects of smoking initiation, smoking cessation or alcohol |
| 61 | Wootton et al. (2020) | Multiple: smoking alcohol | Summary level | Alcohol drinks per week (self-report), alcohol use disorder (diagnosis), smoking initiation (self-report), loneliness (self-report) | Alcohol drinks per week (self-report), alcohol use disorder (diagnosis), smoking initiation (self-report), cigarettes per day (self-report), smoking cessation (self-report); loneliness (self-report) | – + | Weak evidence for causal, increasing effects of loneliness on smoking initiation and cigarettes per day, weak evidence for causal, decreasing effects of loneliness on smoking cessation. Strong evidence for an effect such that smoking initiation increases loneliness. No clear evidence for causal effects in any of the other tested relationships |
| 62 | Lim et al. (2020) | Multiple: alcohol cannabis | Summary level | Cannabis initiation (self-report), alcohol use disorder (diagnosis) | Non-suicidal self-harm (NSSH), suicidal self-harm (SSH) (self-report) | – + | No clear evidence for causal effects |
| 63 | Treur et al. (2019) | Multiple: smoking alcohol cannabis coffee | Summary level | Smoking initiation (self-report); alcohol drinks per week (self-report), problematic alcohol use (self-reported Alcohol Use Disorders Identification Test), alcohol use disorder (diagnosis); cannabis initiation (self-report); cups of coffee per day (self-report), attention-deficit hyperactivity disorder (diagnosis) | Smoking initiation (self-report), cigarettes per day (self-report) smoking cessation (self-report), lifetime smoking (self-report), alcohol drinks per week (self-report), problematic alcohol use (self-reported alcohol use disorders identification test), alcohol use disorder (diagnosis), cannabis initiation (self-report), cups of coffee per day (self-report), attention-deficit hyperactivity disorder (diagnosis in adulthood) | + | Evidence for causal, increasing effects of attention-deficit hyperactivity disorder on smoking initiation, cigarettes per day, smoking cessation and cannabis initiation. Weak evidence for causal, increasing effects of attention-deficit hyperactivity disorder on alcohol use disorder. No clear evidence for causal effects of attention-deficit hyperactivity disorder on the other alcohol measures nor on cups of coffee per day In the other direction, weak evidence for causal, increasing effects of smoking initiation on attention-deficit hyperactivity disorder risk |
This score pertains to the relationship that is of interest to the current systematic review, and not necessarily the whole study. For instance, it may be that in the study as a whole (more) extensive MR sensitivity methods were performed but for the causal estimate of interest no sensitivity methods were applied (e.g. when smoking is merely used as a mediator in a multivariable MR study).
Pre-print publication (not peer-reviewed) obtained from bioRxiv.org, medRxiv.org or arXiv.org.
Note that the quality rating is based on a number of key indices, the most important being: phenotype measurement (sample size, quality of the exposure measurement, quality of the outcome measurement), instrument strength (p value threshold used to select genetic variants, number of genetic variants included, biological knowledge, F statistic for instrument strength, % variance that the instrument explains), and analytical factors (type of main analysis, whether or not basic sensitivity analyses were applied, whether or not additional sensitivity analyses were applied). Combined, these indices were weighted to come to a complete quality score (see Supplementary Table S1). A few important notes regarding this weighting of the evidence: (1) where absolute thresholds were used to judge the quality of a particular aspect of the study (e.g. sample size), it should be noted that these are somewhat arbitrary and were merely used to provide an indication of quality. (2) With regard to ‘phenotype measurement,’ a very well measured phenotype in a moderate sample size may be just as powerful as a more superficially measured phenotype in a very large sample. However, in case of very small sample sizes (e.g. n = 180 such as in the study by Irons et al., 2007) even an extremely thoroughly measured phenotype will not lead to a high total score. (3) With regard to ‘instrument strength,’ when a study uses a single genetic variant that explains a relatively large amount of the variance and for which there is good biological knowledge, the fact that only one SNP was used is not necessarily problematic. For example, this is the case for SNP rs1051730 in the nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster – each additional risk allele increases smoking heaviness with one additional cigarette smoked per day (Katikireddi, Green, Taylor, Davey Smith, and Munafò, 2018).
Table 2.
All Mendelian randomization (MR) studies included for qualitative synthesis, with their identifying information, description of the data samples used for exposure and outcome variable(s), ancestry of those samples, the independence of the include SNPs, whether or not proxies were used, and whether or not a correction for multiple testing was applied
| ID | Author year | GWAS sample exposure variable(s) | Ancestry exposure sample | GWAS sample outcome variable(s) | Ancestry outcome sample | Independence of the SNPs (LD threshold or otherwise) | Proxies used, and if so, LD | Correction multiple testing |
|---|---|---|---|---|---|---|---|---|
| 1 | Zhou et al. (2019a) | Okbay et al. (2016), N = 293 723 | European | Thorgeirsson et al. (2010) (Tobacco and Genetics (TAG) consortium), smoking initiation effective-N = 72 710, smoking cessation effective-N = 41 278 | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 > 0.8 | None |
| 2 | Zeng et al. (2019) | Okbay et al. (2016), N = 293 723; Lee et al. (2018), N = 1 131 881 | European | Thorgeirsson et al. (2010) (TAG consortium) effective-N = 72 710 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 3 | Gage et al. (2018) | Okbay et al. (2016), N = 305 072 (Discovery and replication sample, without 23andme) | European | Thorgeirsson et al. (2010) (TAG consortium), smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278; Ware et al. (2016) – N = 4548 | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 > 0.9 | None |
| 4 | Tillmann et al. (2017) | Okbay et al. (2016), N = 349 306 | European | Thorgeirsson et al., 2010 (TAG consortium) effective-N = 72 710 | European | LD r2 < 0.1 | Yes, LD r2 > 0.8 | None |
| 5 | Carter et al. (2019) | Individual-level MR: UKB (UK Biobank), N = 318 147; Summary-level MR: Lee et al. (2018), N = 1 131 881 | European | Individual-level MR: UKB, N = 318 147; Summary-level MR: Wootton et al. (2019), N = 462 690 | European | LD r2 < 0.1 education; LD r2 < 0.001 smoking | No | None |
| 6 | Sanderson et al. (2019) | Individual-level MR: UKB, N = 120 050; Summary-level MR: Lee et al. (2018), N = 1 131 881 | European | Individual-level MR: UKB, N = 120 050; Summary-level MR: Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710; smoking cessation effective-N = 41 278 | European | LD r2 < 0.001 | No | None |
| 7 | Gage et al. (2020) | Liu et al. (2019a (GSCAN consortium) without UK Biobank (since it is not explicitly mentioned that these were excluded, it is assumed that 23andme data were included), smoking initiation, N = 848 460; Wootton et al. (2019), N = 462 690 | European | Okbay et al. (2016), N = 293 723; Cognitive functioning UKB (Neale lab GWAS: http://www.nealelab.is/uk-biobank), N = 117 131 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 8a | Fu et al. (2019) | The Health and Retirement Cohort, N = 11 246 | European | The Health and Retirement Cohort, N = 11 246 | European | – | – | None |
| 9 | North et al. (2015) | Healthy Aging across the Life Course (HALCyon) consortium, N = 22 329 | European | HALCyon consortium, N = 22 329 | European | n.a. (1 SNP) | No | Bonferroni |
| 10a | Adams (2019) | Wootton et al., 2019, N = 462 690; Cognitive functioning UKB, N = 149 051; Okbay et al. (2016), N = 170 911 | European | Wootton et al. (2019), N = 462 690; Cognitive functioning UKB, N = 149 051; Okbay et al. (2016), N = 170 911 | European | LD r2 < 0.01 | No | False Discovery Rate (FDR) |
| 11 | Østergaard et al. (2015) | Thorgeirsson et al. (2010) (TAG consortium), smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181 | European | Lambert et al. (2013) (International Genomics of Alzheimer's disease's Project (IGAP)) effective-N = 46 668 | European | LD r2 < 0.01 | Yes, LD r2 > 0.8 | Bonferroni |
| 12 | Gibson et al. (2019) | Summary-level MR: Jones et al. (2016) – N = 128 266; Hammerschlag et al. (2017), effective-N = 92 415; Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278; Individual-level MR: UKB – N = 335 708 participants (computed as the biggest sample from Table 1: 184 184 + 118 181 + 33 343) | European | Summary-level MR: Jones et al. (2016), N = 128 266; Hammerschlag et al. (2017), effective-N = 92 415; Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278; Individual-level MR: UKB – N = 335 708 participants (computed as the biggest sample from Table 1: 184 184 + 118 181 + 33 343) | European | LD r2 < 0.001 | Yes, LD r2 ⩾ 0.8 | None |
| 13 | Millard et al. (2019) | UKB, N = 182 961, never smokers; N = 150 831, ever smokers | European | UKB, N = 182 961, never smokers; N = 150 831, ever smokers | European | n.a. (1 SNP) | No | Bonferonni & FDR (note that this is a PHEWAS) |
| 14 | Jansen et al. (2019) | Jansen et al. (2019), N = 1.3 million; Thorgeirsson et al. (2010) (TAG consortium), N = 38 181 | European | Jansen et al. (2019), N = 1.3 million; Thorgeirsson et al. (2010) (TAG consortium), N = 38 181 | European | LD r2 < 0.1 | No | Bonferroni |
| 15 | Lane et al. (2019) | Frequent insomnia symptoms UKB effective-N = 235 787; Any insomnia symptoms UKB effective-N = 329 839 | European | Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278, age at initiation – N = 24 114 | European | LD r2 > 0.8 | No | Bonferroni |
| 16 | Bjorngaard et al. (2013) | The Trøndelag Health Study (HUNT) Cohort, N = 53 601 | European | HUNT cohort, N = 53 601 | European | n.a. (1 SNP) | No | None |
| 17 | Lewis et al. (2011) | The Avon Longitudinal Study of Parents and Children (ALSPAC) Cohort, N = 6.294 | European | ALSPAC cohort, N = 6.294 | European | n.a. (1 SNP) | No | None |
| 18 | Taylor et al. (2014a) | The Causal Analysis Research in Tobacco and Alcohol (CARTA) consortium, N = 127 632 (58 176 never smokers, 37 428 former smokers, 32 028 current smokers) | European | CARTA consortium, N = 127 632 (58 176 never smokers, 37 428 former smokers, 32 028 current smokers) | European | n.a. (1 SNP) | Yes, rs16 969968 or proxy rs1051730 | None |
| 19 | Skov-Ettrup et al. (2017) | The Copenhagen General Population study (CGP), N = 90 108 | European | The Copenhagen General Population study (CGP), N = 90 108 | European | n.a. (1 SNP) | No | None |
| 20 | Wootton et al. (2019) | Wootton et al. (2019) UKB, N = 462 690; Liu et al. (2019a) (GSCAN consortium) smoking initiation, N = 1.2 million; Schizophrenia working group PGC, 2014 effective-N = 111 486; Wray et al. (2018) effective-N = 374 559 | Predominantly European (small Asian cohorts in schiz GWAS) | Wootton et al. (2019) UKB, N = 462 690; Liu et al. (2019a) (GSCAN consortium); smoking initiation, N = 599 289; Schizophrenia working group PGC, 2014 effective-N = 111 486; Wray et al. (2018) effective-N = 141 380 | Predominantly European (small Asian cohorts in schiz GWAS) | LD r2 < 0.001 | Yes, LD r2 ⩾ 0.8 | None |
| 21 | Vermeulen et al. (2019) | Liu et al. (2019a) (GSCAN consortium) smoking initiation, N = 1.2 million; Wootton et al. (2019), N = 462 690; Stahl et al. (2019) effective-N = 49 367 | European | Liu et al. (2019a) (GSCAN consortium) smoking initiation, N = 1.2 million, cigarettes per day, N = 263 954; smoking cessation, N = 312 821; Wootton et al. (2019), N = 462 690; Stahl et al. (2019) effective-N = 49 367 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 22a | Barkhuizen et al. (2020) | Liu et al. (2019a) (GSCAN consortium) without 23andMe, N = 632 802; Pain et al. (2018), N = 6297–10 098; Ortega-Alonso et al. (2017), N = 3967–4057; UKB (Neale lab: http://www.nealelab.is/uk-biobank), N = 157 397; Schizophrenia working group of PGC, 2014 effective-N = 111 487; Wray et al. (2018) excluding 23andMe effective-N = 156 582; Stahl et al. (2019) effective-N = 49 367 | Predominantly European (small Asian cohorts in schiz GWAS) | Liu et al. (2019a) (GSCAN consortium) without 23andMe, N = 632 802; Pain et al. (2018), N = 6297–10 098; Ortega-Alonso et al. (2017), N = 3967–4057; UKB (Neale lab: http://www.nealelab.is/uk-biobank), N = 157 397; Schizophrenia working group of PGC, 2014 effective-N = 111 487; Wray et al. (2018) excluding 23andMe effective-N = 156 582; Stahl et al. (2019) effective-N = 49 367 | Predominantly European (small Asian cohorts in schiz GWAS) | Independent SNPs as reported in exposure GWAS were selected or LD r2 < 0.05 | No | None for MR (the genetic correlations are corrected for multiple testing) |
| 23 | Wium-Andersen et al. (2015a) | Copenhagen General Population Study (CGPS) and Copenhagen City Heart Study (CCHS) cohorts, N = 63 296 (23 282 never smokers and 40 014 ever smokers) | European | Copenhagen General Population Study (CGPS) and Copenhagen City Heart Study (CCHS) cohorts, N = 63 296 (23 282 never smokers and 40 014 ever smokers) | European | n.a. (1 SNP) | No | None |
| 24 | Byrne et al. (2019) | UKB, N = 32 510 | European | Schizophrenia working group of PGC, 2014 effective-N = 99 863 (40 675 cases and 64 643 controls) (note that the PGC schizophrenia working group is referenced but the sample size does not match that in the 2014 PGC publication) | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 25 | Gage et al. (2017b) | Schizophrenia working group of PGC, 2014 effective-N = 111 487; Thorgeirsson et al. (2010) (TAG consortium) effective-N = 72 710 | Predominantly European (small Asian cohorts in schiz GWAS) | Schizophrenia working group of PGC, 2014 effective-N = 111 487; Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278 | Predominantly European (small Asian cohorts in schiz GWAS) | LD r2 < 0.9 | Yes, LD r2 > 0.9 | None |
| 26 | Fluharty et al. (2018) | Demontis et al. (2019) effective-N = 51 205 (note that the reported sample size implies that a small Asian cohort was included); Pappa et al. (2016) (Early Life Epidemiology consortium (EAGLE)) – N = 18 988 | Predominantly European (small Asian cohort in attention-deficit hyperactivity disorder GWAS) | Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710; age at initiation – N = 24 114 | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 ≥ 0.9 | None |
| 27 | Sallis et al. (2019) | Individual-level MR: UKB, N = 273 516; summary-level MR: Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278; Okbay et al. (2016) – N = 170 911; Lo et al. (2017) – N = 122 886 | European | Individual-level MR: UKB – N = 273 516; summary-level MR: Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day – N = 38 181, smoking cessation effective-N = 41 278; Okbay et al. (2016) – N = 170 911; Lo et al. (2017) – N = 122 886 | European | Independent SNPs as reported in exposure GWAS were selected | Yes LD r2 ≥ 0.8 | None |
| 28a | Leppert et al. (2019) | Demontis et al. (2019) effective-N = 49 017 | European | Wootton et al. (2019), N = 462 690 | European | LD r2 < 0.001 | Yes, LD r2 ≥ 0.9 | None |
| 29 | Harrison et al. (2020a) | Individual-level MR: UKB, N = 463 033/2 (split-sample analyses); Summary-level MR: Liu et al. (2019a) (GSCAN consortium) without UKB and 23andMe – N = 249 171; Wootton et al. (2019) – N = 463 033 | European | Individual-level MR: UKB – N = 463 033/2 (split-sample analyses); Summary-level MR: UKB – N = effective-N = 9661 (2433 cases and 334 766 controls) | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 ≥ 0.8 | None |
| 30 | Rosoff et al. (2019) | Okbay et al. (2016) – N = 293 723; Elsworth et al. (2017) alcohol intake frequency – N = 462 346, weekly intake – N = 326 801; Karlsson Linnér et al. (2019) – N = 414 343; Walters et al. (2018) – N = 28 657; Sanchez-Roige et al. (2019) – N = 121 604 | European | Okbay et al. (2016) – N = 293 723; Elsworth et al. (2017) alcohol intake frequency – N = 462 346, weekly intake – N = 326 801; Karlsson Linnér et al. (2019) – N = 414 343; Walters et al. (2018) – N = 28 657; Sanchez-Roige et al. (2019) – N = 121 604 | European | LD r2 = 0.001 | It is mentioned a proxy was used for one SNP but not the LD | Bonferroni |
| 31 | Zhou et al. (2019b) | Lee et al. (2018) – N = 1 131 881 | European | UKB, N = 334 507 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 32 | Kumari et al. (2014) | English Longitudinal Study of Aging (ELSA) + Whitehall II study + Health, Alcohol and Psychosocial factors in Easter Europe Study (HAPIEE) combined, N = 34 452 | European | ELSA + Whitehall II study + HAPIEE combined, N = 34 452 | European | n.a. (1 SNP) | No | None |
| 33 | Almeida et al. (2014a) | The Health in Men Study (HIMS) Cohort, N = 3542 | Predominantly European | HIMS Cohort, N = 3542 | Predominantly European | n.a. (1 SNP) | No | None |
| 34 | Ritchie et al. (2014) | The Lothian Birth Cohort 1936, N = 777 | European | The Lothian Birth Cohort 1936, N = 777 | European | Four SNPs as previously reported in candidate-gene literature | No | None |
| 35 | Au Yeung et al. (2012) | The Guangzhou Biobank Cohort Study (GBCS), N = 4707 | Chinese | GBCS, N = 4707 | Chinese | n.a. (1 SNP) | No | None |
| 36 | Mahedy et al. (2020) | Liu et al. (2019a) (GSCAN consortium), N = 941 280 | European | ALSPAC Cohort 2500 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 37 | Andrews et al. (2020) | Liu et al. (2019a) (GSCAN consortium), N = 537 349; Sanchez-Roige et al. (2019), N = 121 604; Walters et al. (2018) effective-N = 34 780 | European | Lambert et al. (2013) (IGAP) effective-N = 46 668; Huang et al. (2017) effective-N = 37 002 | European | LD r2 < 0.001 | Yes, LD r2 > 0.8 | None |
| 38 | Nishiyama et al. (2019) | Wakai et al. (2011) (The Japan Multi-Institutional Collaborative Cohort (J-MICC) Study), N = 13 618 | Japanese | Wakai et al. (2011) (The Japan Multi-Institutional Collaborative Cohort (J-MICC) Study), N = 13 618 | Japanese | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 39 | Almeida et al. (2014b) | HIMS Cohort, N = 3873 | Predominantly European | HIMS cohort, N = 3873 | Predominantly European | n.a. (1 SNP) | No | None |
| 40 | Wium-Andersen et al. (2015a) | The Copenhagen General Population Study (CGPS) Cohort, N = 78 154 | European | CGPS Cohort, N = 78 154 | European | LD r2 < 0.01 | No | Bonferroni |
| 41 | Polimanti et al. (2019) | Wray et al. (2018) effective-N = 389 039; Walters et al. (2018) effective-N = 30 053 (note that only unrelated individuals were selected); UKB alcohol use frequency – N = 438 308, alcohol use quantity – N = 307 098 | European | Wray et al. (2018) effective-N = 389 039; Walters et al. (2018) effective-N = 30 053 (note that only unrelated individuals were selected); UKB alcohol use frequency – N = 438 308, alcohol use quantity – N = 307 098 | European | LD r2 < 0.01 | No | Bonferroni |
| 42 | Zhou et al. (2020) | Howard et al. (2019) effective-N = 684 817; Schizophrenia working group of PGC, 2014 effective-N = 111 487; Stahl et al. (2019) effective-N = 49 367; Nagel et al. (2018) Neuroticism – N = 449 484, depressed affect, – N = 357 957, worry – N = 348 219; Jansen et al. (2019) – N = 1.3 million; Lee et al. (2018) without UKB effective-N = 179 185 (based on the first paragraph of the results section MVP phase1, effective-N = 114 847 + MVP phase 2, effective-N = 37 485 + PGC effective-N = 26 853) | Predominantly European (small Asian cohorts in schiz GWAS) | Howard et al. (2019) effective-N = 684 817; Schizophrenia working group of PGC, 2014 effective-N = 111 487; Stahl et al. (2019) effective-N = 49 367; Nagel et al. (2018) Neuroticism – N = 449 484, depressed affect – N = 357 957, worry – N = 348 219; Jansen et al. (2019) – N = 1.3 million; Lee et al. (2018) without UKB effective-N = 179 185 (based on the first paragraph of the results section MVP phase1, effective-N = 114 847 + MVP phase2, effective-N = 37 485 + PGC effective-N = 26 853) | Predominantly European (small Asian cohorts in schiz GWAS) | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 > 0.8 | Bonferonni |
| 43 | Irons et al. (2007) | McGue et al. (2007) (The Sibling Interaction and Behavior Study), N = 180 | East Asian (Korean) | McGue et al. (2007) (The Sibling Interaction and Behavior Study), N = 180 | East Asian (Korean) | n.a. (1 SNP) | No | None |
| 44 | Chao et al. (2017) | The BeTwiSt project (adolescents from Beijing), N = 1608 | Chinese | The BeTwiSt project (adolescents from Beijing), N = 1608 | Chinese | n.a. (1 SNP) | No | None |
| 45 | Hodgson et al. (2020) | Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933; Wray et al. (2018) effective-N = 374 559 | European | Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933; Wray et al. (2018) effective-N = 374 559 | European | LD r2 < 0.1 | No | p < 0.01 considered significant |
| 46 | Soler Artigas et al. (2019) | Demontis et al. (2019 effective-N = 49 017; Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933 | European | Demontis et al. (2019 effective-N = 49 017; Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933 | European | LD r2 < 0.05 | No | None |
| 47 | Pasman et al. (2018) | Schizophrenia working group of PGC, 2014 effective-N = 111 486; International Cannabis Consortium effective-N = 180 934 | Predominantly European (small Asian cohorts in schiz GWAS) | Schizophrenia working group of PGC, 2014 effective-N = 111 486; International Cannabis Consortium effective-N = 180 934 | Predominantly European (small Asian cohorts in schiz GWAS) | LD r2 < 0.001 | Yes, LD r2 ≥ 0.8 | None |
| 48 | Vaucher et al. (2018) | Stringer et al., 2016 (International Cannabis Consortium) effective-N = 31 933 | European | Schizophrenia working group of PGC, 2014 effective-N = 78 227 (note that this sample size is lower than that of the original GWAS, and it was not stated how this subsample was selected) | European (unclear whether the Asian cohorts were included) | 10 leading SNPs (not genome-wide significant) from the exposure GWAS, no criteria for independence stated | No | None |
| 49 | Gage et al. (2017a) | Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933; Schizophrenia working group of PGC 2014 effective-N = 111 486 | Predominantly European (small Asian cohorts in schiz GWAS) | Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933; Schizophrenia working group of PGC 2014 effective-N = 111 486 | Predominantly European (small Asian cohorts in schiz GWAS) | r2 < 0.9 (LD was corrected for with correlation matrix) | Yes, LD r2 > 0.9 | None |
| 50 | Zhou et al. (2018) | Meta-analysis of 10 European cohorts (the 1958 British birth cohort (1958BC), UKB, Mothers of Avon Longitudinal Study of Parents and Children (ALSPAC-M), Northern Finland Birth Cohorts 1966 (NFBC1966), Cardiovascular Risk in Young Finns Study (YFS), Helsinki Birth Cohort Study (HBCS), Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS), Uppsala Longitudinal Study of Adult Men (ULSAM), Swedish twin registry (STR), and TwinGene), N = 415 530 (of which 300 760 coffee consumers) | European | Meta-analysis of 10 European cohorts (1958BC, ALSPAC-M, NFBC1966, YFS, HBCS, PIVUS, ULSAM, STR, and TwinGene), N = 415 530 (of which 300 760 coffee consumers) | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 1.0 and 0.97 for two SNPs | None |
| 51 | Treur et al. (2018) | Cornelis et al. (2015) (Caffeine Genetics Consortium) – N = 91 462; Cornelis et al. (2016) – N = 9876; Jones et al. (2016) – N = 128 266; Hammerschlag et al. (2017) effective-N = 92 415 | European | Cornelis et al. (2015) (Caffeine Genetics Consortium), N = 91 462; Cornelis et al. (2016), N = 9876; Jones et al. (2016), N = 128 266; Hammerschlag et al. (2017) effective-N = 92 415 | European | LD r2 < 0.001 | Yes, LD r ≥ 0.8 | None |
| 52 | Kwok et al. (2016) | Cornelis et al. (2015) (Caffeine Genetics Consortium) – N = 129 788 (note that the reported sample size implies that the trans-ethnic data was used) | Predominantly European (~6% African American) | Major Depressive Disorder Working Group of PGC, 2013 effective-N = 18 755; Lambert et al. (2013) effective-N = 46 668 | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 > 0.8 | Bonferroni |
| 53 | Ding et al. (2019) | Health and Retirement Study (HRS) – N = 3935 | Not clearly indicated (representative of >50 year-olds in the US) | Health and Retirement Study (HRS) N = 3935 | Not clearly indicated (representative of >50 year-olds in the US) | Independent SNPs as reported in exposure GWAS were selected, after which some were excluded to prevent horizontal pleiotropy | No | None |
| 54a | Yuan et al. (2020a) | Lee et al. (2018), N = 1 131 881; Savage et al. (2018), N = 269 867 | European | Liu et al. (2019a) (GSCAN consortium) alcohol drinks per week, N = 941 280; age at onset smoking, N = 341 427; cigarettes per day, N = 337 334 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 55 | Davies et al. (2019) | Individual-level MR: UKB, N = 93 135; Summary-level MR: Okbay et al. (2016), N = 293 723; Hill et al. (2019), N = 248 723 | European | Individual-level MR: UKB, N = 93 135 Summary-level MR: UKB smoking initiation effective-N = 136 760, current smoking effective-N = 46 573 | European | Independent SNPs as reported in exposure GWAS were selected, further clumped with LD r2 < 0.01 | No | None |
| 56a | Davies et al. (2018a) | Individual-level MR: UKB – N = 315 436; Summary-level MR: Okbay et al. (2016) – N = 293 723 | European | Individual-level MR: UKB – N = 315 436; Summary-level MR: not clear from the manuscript, note that the summary-level analyses were used as a sensitivity analysis to check for pleiotropy and were not the main aim | European | LD r2 < 0.001 | Yes, LD r2 = 1 | None |
| 57 | Harrison et al. (2020b) | Individual-level MR: UKB N = 336 997 [N = 336 997/2 (split sample) for lifetime smoking]; Summary-level MR: Liu et al. (2019a) (GSCAN consortium) without UKB and 23andme smoking initiation – N = 249 171, alcohol drinks per week – N = 226 223; UKB Lifetime smoking – N = 336 997 (N = 336 997/2 (split sample)) | European | Individual-level MR: UKB – N = 336 997; Summary-level MR: UKB Lifetime smoking – N = 336 997 [N = 336 997/2 (split sample)] | European | Independent SNPs as reported in exposure GWAS were selected | Yes, LD r2 > 0.8 | Bonferonni |
| 58 | Mahedy et al. (2021) | Liu et al. (2019a) (GSCAN consortium) effective-N = 1 220 901; Pasman et al. (2018) (International Cannabis Consortium) – N = 184 765 | European | ALSPAC Cohort, N = 3232 | European | Independent SNPs as reported in exposure GWAS were selected | No | None |
| 59 | Andrews et al. (2021) | Sanchez-Roige et al. (2019) – N = 141 932; Liu et al. (2019a) (GSCAN consortium) smoking initiation effective-N = 1 220 901, alcohol drinks per week – N = 941 280, cigarettes per day – N = 337 334 | European | Lambert et al. (2013) effective-N = 46 670; Kunkle et al. (2019) effective-N = 57 692; Huang et al. (2017) – N = 40 255 | European | LD r2 < 0.001 | Yes, LD r2 ⩾ 0.8 | FDR |
| 60 | Larsson et al. (2017) | Thorgeirsson et al. (2010) (TAG consortium) smoking initiation effective-N = 72 710, cigarettes per day N = 38 181, smoking cessation effective-N = 41 278;. Jorgenson et al. (2017), N = 71 071; Cornelis et al. (2015), N = 91 462 | European | Lambert et al. (2013) effective-N = 46 668 | European | LD r2 < 0.2 | Yes, LD r2 > 0.9 | Bonferroni (0.05/24 test = 0.002), <0.05 was reported as ‘suggestive evidence’) |
| 61 | Wootton et al. (2020) | Abdellaoui et al. (2019) – N = 511 280; Liu et al. (2019a, 2019b) (GSCAN consortium) without UKB and without 23 and me smoking initiation effective-N = 244 920, alcohol drinks per week – N = 226 223; Walters et al. (2018) effective-N = 34 780 | European | Abdellaoui et al. (2019) – N = 511 280; Liu et al. (2019a, 2019b) (GSCAN consortium) without UKB and without 23andme smoking initiation effective-N = 244 920, cigarettes per day – N = 249 171, smoking cessation effective-N = 142 612, alcohol drinks per week – N = 226 223; Walters et al. (2018) effective-N = 34 780 | European | Independent SNPs as reported in exposure GWAS were selected; for instruments at threshold p < 1 × 10−5, LD r2 < 0.01 | Yes, LD r2 ≥ 0.8 | None |
| 62 | Lim et al. (2020) | Stringer et al. (2016) (International Cannabis Consortium) effective-N = 31 933; Walters et al. (2018), effective-N = 34 780 | European | UKB – N = 125 742 | European | LD r2 < 0.001 | No | None |
| 63 | Treur et al. (2019) | Liu et al. (2019a) (GSCAN consortium) smoking initiation effective-N = 1 220 901, alcohol drinks per week – N = 941 280; Sanchez-Roige et al. (2019) – N = 121 604; Walters et al. (2018) effective-N = 34 779; Pasman et al. (2018) (International Cannabis Consortium) effective-N = 180 934; Demontis et al. (2019) effective-N = 49 017; Cornelis et al. (2016) – N = 91 462 | European | Liu et al. (2019a) (GSCAN consortium) excluding 23andme smoking initiation effective-N = 632 783, cigarettes per day – N = 263 954, smoking cessation – N = 312 821, alcohol drinks per week – N = 537 341; Sanchez-Roige et al. (2019) – N = 121 604; Walters et al. (2018) effective-N = 34 779; Pasman et al. (2018) (International Cannabis Consortium) effective-N = 180 934; Demontis et al. (2019) – N = 15 548 (only adults included); Cornelis et al. (2016) – N = 91 462 | European | Independent SNPs as reported in exposure GWAS were selected | No | None (note the authors explain how they define strength of evidence) |
Pre-print publication (not peer-reviewed) obtained from bioRxiv.org, medRxiv.org or arXiv.org.
Note that the complete references to the samples listed under ‘GWAS sample exposure variable(s)’ and ‘GWAS sample outcome variable(s)’ can be found in the original publications (1–63).
Cigarette smoking
Cognitive traits
There was consistent evidence that higher educational attainment decreases the odds of initiating smoking (Carter et al., 2019; Davies et al., 2018a; Davies et al., 2019; Ding, Barban, & Mills, 2019; Gage, Bowden, Davey Smith, & Munafo, 2018; Sanderson, Davey Smith, Bowden, & Munafò, 2019; Tillmann et al., 2017; Zeng et al., 2019; Zhou et al., 2019a), increases the age at smoking initiation (Yuan, Xiong, Michaëlsson, Michaëlsson, & Larsson, 2020a; Zhou et al., 2019a), increases smoking heaviness, and decreases the odds of quitting (Gage et al., 2018; Sanderson et al., 2019; Zeng et al., 2019; Zhou et al., 2019a). One study triangulated self-report measures with cotinine (a metabolite of nicotine) in blood samples and found weak evidence that higher educational attainment causes lower cotinine levels (Gage et al., 2018). There was considerable overlap among the data sets used (Table 2). Two studies based their education-to-smoking estimate on the same data sets, one testing whether smoking mediated the effects of education on coronary heart disease, and the other whether smoking mediated the effects of education on lung cancer (Tillmann et al., 2017; Zhou et al., 2019a). There was strong evidence that higher general cognitive ability decreases lifetime smoking (Adams, 2019), but no clear evidence for effects on smoking initiation or cessation (Davies et al., 2019). Two multivariable MR studies found that causal effects of education on smoking were not mediated by cognitive ability (Davies et al., 2019; Sanderson et al., 2019).
Substantially fewer studies looked at causal effects ofsmoking on cognitive functioning. There was consistent evidence that smoking initiation and lifetime smoking decrease educational attainment (Gage et al., 2020; Harrison et al., 2020b), and weaker evidence that they decrease cognitive ability (Gage et al., 2020). Two other studies found no clear evidence for causal effects of smoking initiation on cognitive functioning (Adams, 2019; North et al., 2015), but note that the analysis by Gage et al. (2020) was superior (+ v. – +). There was also no clear evidence that smoking affects working memory, response inhibition, or emotion recognition, but these analyses were likely underpowered (Mahedy et al., 2021). A single analysis (rated –) reported that current smoking increases the odds of cognitive impairment (Fu, Faul, Jin, Ware, & Bakulski, 2019). Two studies found weak evidence that smoking decreases the odds of Alzheimer's disease (Larsson et al., 2017; Østergaard et al., 2015), but a more recent analysis, rated as superior (+ v. – +), found no effects of smoking on Alzheimer's disease (Andrews et al., 2021). The seemingly protective effect of smoking is likely survival bias – smokers who do not die from smoking-related diseases are less prone to diseases making them less likely to develop Alzheimer's disease. Of note, smoking initiation is not an ideal measure – there is accumulating evidence that genetic variants associated with this phenotype are horizontally pleiotropic (Khouja, Wootton, Taylor, Smith, & Munafò, 2020). A conclusion on the causal effects of smoking can more reliably be made by testing the effects of smoking heaviness.
Sleep problems
There was weak evidence that insomnia increases smoking heaviness and decreases cessation from two studies (Gibson, Munafò, Taylor, & Treur, 2019; Jansen et al., 2019), but not from a third (Lane et al., 2019). In contrast, there was no clear evidence that sleep duration impacts smoking (Gibson et al., 2019). There was particularly strong evidence that smoking heaviness impacts chronotype, decreasing the odds of being a morning person (Gibson et al., 2019; Millard, Munafò, Tilling, Wootton, & Davey Smith, 2019), but no clear evidence that smoking influences insomnia risk or sleep duration (Gibson et al., 2019; Jansen et al., 2019).
Internalizing/mood disorders
There was some evidence for causal, increasing effects of depression (Wootton et al., 2019), feelings of loneliness (Wootton et al., 2020), and neuroticism (Sallis, Smith, & Munafo, 2019) on smoking behavior. Adams (2019), using a larger data set for the outcome than Sallis et al. (2019), did not find clear evidence that neuroticism affects smoking. There was no clear evidence for causal effects of depression or bipolar disorder on smoking (Barkhuizen, Dudbridge, & Ronald, 2020; Vermeulen et al., 2019). Most studies also tested effects in the other direction. Earlier studies showed no clear evidence for causal effects (Bjorngaard et al., 2013; Skov-Ettrup, Nordestgaard, Petersen, & Tolstrup, 2017; Taylor et al., 2014a; Wium-Andersen, Orsted, & Nordestgaard, 2015a), with one exception: a small study (n = 6294) of low-rated quality reporting that smoking decreased depression during pregnancy (Lewis et al., 2011). More recent studies, employing much larger samples, found strong evidence for causal, increasing effects of smoking initiation and lifetime smoking on depression and bipolar disorder risk (Barkhuizen et al., 2020; Vermeulen et al., 2019; Wootton et al., 2019). There was weak evidence that smoking initiation increases feelings of loneliness (a phenotype closely related to depression) from one study (Wootton et al., 2020), but no such evidence from another (Harrison et al., 2020b). One study reported weak evidence that smoking initiation decreases neuroticism (Sallis et al., 2019), whereas another, better-powered study found that lifetime smoking increases neuroticism (Adams, 2019). Finally, there was no clear evidence that smoking causally impacts suicidal ideation (Harrison et al., 2020a).
Externalizing disorders
There was strong evidence that ADHD liability increases smoking initiation, smoking heaviness and lifetime smoking, and decreases cessation (Fluharty, Sallis, & Munafò, 2018; Leppert et al., 2019; Sallis et al., 2019; Treur et al., 2019). There was no clear evidence that aggression causally affects smoking, but this analysis was likely underpowered (Fluharty et al., 2018). One study also tested reverse effects, reporting weak evidence that smoking initiation increases ADHD risk, but with important cautionary notes about the pleiotropic nature of the initiation measure (Treur et al., 2019).
Psychotic disorders
Multiple studies reported evidence (ranging from weak to strong) that smoking causally increases schizophrenia risk (Barkhuizen et al., 2020; Byrne et al., 2019; Gage et al., 2017b; Wium-Andersen et al., 2015a, ; Wootton et al., 2019). In the other direction there was no clear evidence for causal effects of liability to schizophrenia on smoking from one study (Gage et al., 2017a, 2017b), and some evidence for such effects from two recent, better-powered studies with largely overlapping samples (Barkhuizen et al., 2020; Wootton et al., 2019).
Alcohol use
Cognitive traits
There was strong evidence that higher educational attainment increases alcohol use frequency (Davies et al., 2018a; Davies et al., 2019; Rosoff et al., 2019; Zhou et al., 2019a, 2019b) and wine intake (Rosoff et al., 2019; Zhou et al., 2019a, 2019b), whereas it decreases beer/cider intake (Zhou et al., 2019a, 2019b), and the risk of binge-drinking and alcohol use disorder (Rosoff et al., 2019; Zhou et al., 2020). Ding et al. (2019) did not find clear evidence for causality from education to alcohol use, but this analysis was likely underpowered. There was also evidence that general cognitive ability increases alcohol use frequency (Davies et al., 2019) and decreases the risk of alcohol use disorder (Zhou et al., 2020). In the other direction, Rosoff et al. (2019) found weak evidence that higher alcohol use decreases educational attainment, whereas another study did not (Harrison et al., 2020b). A third, high-quality rated study found that liability to alcohol use disorder negatively impacts educational attainment (Zhou et al., 2020). Note that GWAS of current alcohol use have largely been performed in adults, reflecting alcohol use after maximum educational attainment occurred for most. Although the genetic instrument may also reflect alcohol use at younger ages, this needs to be taken into account. There was no clear evidence that drinking more alcohol impacts cognition, but this was based on (very) small, low-quality rated studies (Almeida, Hankey, Yeap, Golledge, & Flicker, 2014a, 2014b; Au Yeung et al., 2012; Kumari et al., 2014; Mahedy et al., 2020; Ritchie et al., 2014). There were contradicting findings for Alzheimer's disease, with one study finding no causal effects of alcohol (Larsson et al., 2017) and another finding that while a higher number of drinks caused an earlier onset of Alzheimer's disease, alcohol use disorder caused a later onset (Andrews, Goate, & Anstey, 2020). The latter likely reflects survival bias.
Sleep problems
There was some evidence that drinking more alcohol per week increases sleep duration, but this was based on only one, low-quality rated study (Nishiyama et al., 2019).
Internalizing/mood disorders
A recent, particularly large study reported strong evidence that major depressive disorder (MDD) liability increases alcohol use disorder risk (Polimanti et al., 2019). Similarly, there was evidence that worrying and neuroticism increase alcohol use disorder risk (Zhou et al., 2020). There was no clear evidence that feelings of loneliness affect alcohol use (disorder) (Wootton et al., 2020). In the other direction, there was no clear evidence that alcohol use (disorder) causally impacts internalizing symptoms (Almeida et al., 2014a, 2014b; Chao, Li, & McGue, 2017; Lim et al., 2020; Polimanti et al., 2019; Wium-Andersen, Orsted, Tolstrup, & Nordestgaard, 2015b; Wootton et al., 2020; Zhou et al., 2020).
Externalizing disorders
There was weak evidence that ADHD liability increases alcohol use disorder risk (Treur et al., 2019). In the other direction, there was some evidence that higher alcohol use frequency increases aggression and attention problems from one, small (n = 1608) low-rated analysis (Chao et al., 2017), and no evidence for causal effects on antisocial behavior from another very small (n = 180) low-rated analysis (Irons, McGue, Iacono, & Oetting, 2007).
Psychotic disorders
There was no clear evidence for causal effects, in either direction, between alcohol use disorder and schizophrenia risk (Zhou et al., 2020).
Cannabis use
Cognitive traits
There was no evidence for causal effects from cannabis initiation to cognitive functioning (Mahedy et al., 2021).
Internalizing disorders
There was neither clear evidence for causal effects in either direction between cannabis initiation and MDD (Hodgson et al., 2020), nor was there evidence for causal effects from cannabis initiation to self-harm (Lim et al., 2020).
Externalizing disorders
There was evidence that ADHD liability increases cannabis initiation without clear evidence for the reverse (Soler Artigas et al., 2019; Treur et al., 2019).
Psychotic disorders
Out of eight studies that included cannabis, three looked at schizophrenia. One tested causality from cannabis initiation to schizophrenia risk only, finding evidence for an increasing effect (Vaucher et al., 2018). Two other studies tested causal effects in both directions and found weak evidence that cannabis initiation increases schizophrenia risk and strong evidence that schizophrenia liability increases the odds of cannabis initiation (Gage et al., 2017a, 2017b; Pasman et al., 2018).
Caffeine consumption
Cognitive traits
There was weak evidence that higher coffee consumption increases Alzheimer's risk from one study (Larsson et al., 2017), but no clear evidence from another (Kwok, Leung, & Schooling, 2016). There was also no clear evidence for causal effects of coffee on general cognitive functioning (Zhou et al., 2018).
Sleep problems
There was weak evidence that higher plasma caffeine levels decrease the odds of being a morning person, but no clear evidence for causal effects between self-reported caffeine consumption and sleep duration, insomnia, or chronotype (Treur et al., 2018).
Internalizing disorders
There was no clear evidence for causal effects between caffeine consumption and ADHD, in either direction (Treur et al., 2019),
Externalizing disorders
There was no evidence for causal effects of caffeine consumption on depression (Kwok et al., 2016).
Discussion
We conducted the first systematic review of MR studies investigating causal relationships between mental health and substance use. From a total of 63 studies, we can draw important conclusions regarding if and how mental health and substance use are causally related.
Smoking was the most investigated, resulting in particularly strong evidence that higher educational attainment causally decreases smoking (lower risk of initiating, smoking fewer cigarettes, and more likely to quit). Although smoking prevalence has rapidly decreased in the past two decades, this decline has been most prominent among those with high educational attainment, leading to an increasing (health) gap (Agaku, Odani, Okuyemi, & Armour, 2020). The causal role of education we report is important for policy-makers going forward. Interestingly, causal effects from education are neither mediated by cognitive ability (Sanderson et al., 2019) nor were there clear evidence that cognitive ability by itself affects smoking (Adams, 2019; Davies et al., 2019). The studies included in this review cannot determine exactly why educational attainment affects smoking. Smoking initiation usually occurs during adolescence, at which time the home environment and peer influences are important. Adolescents in lower educational groups tend to experience lower levels of parental involvement, parental monitoring, and self-perceived social competence, factors associated with a higher odds of initiating smoking (Mahabee-Gittens, Xiao, Gordon, & Khoury, 2013; Simons-Morton, 2002). As for smoking heaviness and difficulty quitting, causal mechanisms may involve job opportunities that depend on educational attainment. A lower education often leads to jobs characterized by low skill discretion, high psychological demands and high physical exertion, potentially leading to stress and smoking to cope (Dobson, Gilbert-Ouimet, Mustard, & Smith, 2018a).
Another striking pattern was that of bi-directional, increasing effects between smoking and mental disorders. There was more robust evidence that smoking causally increases the odds of mental disorders than vice versa – most notably for depression, bipolar disorder, and schizophrenia. This concurs with accumulating evidence from longitudinal cohort studies (Taylor et al., 2014b) and animal research (Jobson et al., 2019) indicating neuropsychiatric effects of smoking. A causal mechanism may be that nicotine binds to nicotinic acetylcholine receptors in the brain, given that these are involved in regulating central nervous system pathways relevant to mental disorders (Berk et al., 2011). There is some evidence that repeated nicotine exposure can lead to desensitization of these receptors (Mineur & Picciotto, 2009). Inflammation and oxidative stress induced by toxic compounds from inhaled cigarette smoke is another potential mechanism (Berk et al., 2011). Our conclusion that smoking is detrimental to the brain warrants increased efforts to prevent (heavy) substance use. For individuals with a mental disorder, it implies that smoking cessation may be beneficial to alleviate symptoms. This is an important message given that smokers in this population are not always encouraged to quit (Taylor et al., 2020a). Although not an easy task, it should be communicated to health professionals that there are effective ways to help smokers with a co-morbid mental disorder quit.
A higher education increased alcohol use frequency but decreased the risk of problematic use. Those with higher education tend to drink alcohol more often but spread across multiple drinking occasions, and without developing a dependency. Those with lower education, on the other hand, are at increased odds of developing a problematic relationship with alcohol. This pattern of opposite effects was recently also highlighted in a study that computed genetic correlations and reported high alcohol use frequency to be genetically correlated with higher socio-economic status and lower risk of psychiatric disorders, whereas high alcohol consumption quantity was genetically correlated with lower socio-economic status and higher psychiatric disorder risk (Marees et al., 2019). Similar to smoking, it could be that excessive alcohol use is a way to cope with job stress (Dobson, Ibrahim, Gilbert-Ouimet, Mustard, & Smith, 2018b). There was also consistent evidence that mental disorders increase (problematic) alcohol use, without strong effects in the other direction. The latter implies that observational findings indicating that alcohol use increases mental disorders were due to confounding and/or reverse causality. Indeed, associations between heavy drinking and subsequent increases in depressive symptoms disappeared after adjustment for confounders (Li et al., 2020). It should be noted that this is in contrast to clinical observations where in the short-term, treating alcohol use disorder makes pre-existing depression symptoms disappear (Charlet & Heinz, 2017). This discrepancy may be because MR assesses ‘lifetime’ (longer-term) effects of alcohol on mental health (Labrecque & Swanson, 2019), and the fact that only a small proportion of those with an alcohol use disorder will receive treatment [<9% (Mark, Kassed, Vandivort-Warren, Levit, & Kranzler, 2009)]. In sum, the current MR literature suggests that co-morbidity between poor mental health and alcohol use is primarily the result of alcohol being used as a type of ‘self-medication.’
There was stronger evidence that liability to schizophrenia increases the odds to initiate cannabis, than that cannabis initiation increases schizophrenia risk, as also indicated recently by others (Gillespie & Kendler, 2021). However, these results should be regarded as tentative, given that the genetic instrument for schizophrenia was more powerful than that for cannabis use, and more insightful analyses, with measures of cannabis use frequency, have not yet been performed. This is an important direction for future MR studies, now that such large-scale cannabis studies are becoming available (Hines, Treur, Jones, Sallis, & Munafò, 2020).
For caffeine, the predominantly studied relationships were with cognitive functioning and sleep. Overall, there was no clear evidence that a high average intake of caffeine (negatively or positively) affects cognitive measures or sleep. This is consistent with recent evidence that average (high) caffeine intake does not necessarily result in changes in alertness or sleep patterns, due to the fact that adaptation occurs after repeated intake (Weibel et al., 2020).
Limitations
Although our scoring system was carefully designed [using the collective experience of the authors and the tentative, developing STROBE-MR ("Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization") guidelines (Davey Smith et al., 2019)] it should be noted that it was not previously validated. As for the included MR studies, while the more recent were sufficiently powered and some included thorough sensitivity methods and triangulation, many earlier studies were low-quality. In the coming years, it will be important to extend and strengthen the current evidence through MR studies that combine better-powered data sets, preferably with more fine-grained phenotypes and extensive sensitivity methods. An important focal point for smoking and cannabis use as exposure variables is to not only investigate initiation, but also the heaviness of use. There is ample evidence that measurements of initiation can introduce bias due to horizontal pleiotropy and reverse causality (Khouja et al., 2020; Li et al., 2020; Treur et al., 2019; Yuan, Yao, & Larsson, 2020b). Another important addition to future work is multivariable MR, which allows the inclusion of multiple exposures to further decrease the risk of horizontal pleiotropy and provide more extensive testing of causal mechanisms. In addition, triangulating with high-quality observational analyses, or as was done by Davies et al. (2018a) with results from policy reform, would be ideal. There are three important sources of potential bias that are not (sufficiently) accounted for in current MR studies; genetic nurturing (genetic variants that are not transmitted from parents to offspring still affecting offspring phenotype), assortative mating (spouses genetically resembling each other more than by chance because they selected each other based on a genetically influenced trait), and geographic genetic clustering (Brumpton et al., 2020). These phenomena may re-introduce bias from potential confounders, shifting the MR estimate towards the observational association. This can be prevented by performing MR with genetic estimates from within-family GWAS, as these will be corrected for all factors shared within families. Finding large enough family samples will be an important challenge in coming years [the first of such efforts recently became available (Howe et al., 2021). Finally, it is important to acknowledge that almost all MR studies were based on cohorts including participants of European descent. Because of the lack of diversity in the field of genetic research, genetic instruments needed to perform MR for other ethnic groups are rarely available. Increasing diversity in genetic research will be pivotal if we want to reach a comprehensive understanding of the genetic etiology of mental health and substance use, as well as the causal nature of their relationship (Abdellaoui & Verweij, 2021).
Conclusion
In this systematic review of MR studies, we found strong evidence that higher educational attainment decreases smoking and that there is a bi-directional, increasing relationship between smoking and (symptoms of) mental disorders (depression, bipolar disorder, and schizophrenia). Another robust finding was that higher educational attainment increases alcohol use frequency, whereas it decreases the risk of binge-drinking and alcohol use problems, and that (symptoms of) mental disorders causally lead to more alcohol drinking without evidence for the reverse. Future work should attempt to tackle important limitations that were highlighted in this review. An approach that is particularly noteworthy, and should be used more routinely, is multivariable MR. The etiology of mental health traits is complex and we have only a limited understanding of the biological pathways from SNP to phenotype. It is, therefore, important to test whether key variables act as confounders (inducing a false-positive causal finding) or mediate the causal relationship (i.e. are part of the causal chain from exposure to the outcome). This is especially relevant for MR studies investigating educational attainment as an exposure (McMartin & Conley, 2020). Multivariable MR allows the modeling of complex networks of genetic effects linking different mental health traits. Finally, triangulation of MR results with other research methods is crucial. This includes comparison to other genetically informative methods such as twin studies, latent causal variable analysis (O'Connor & Price, 2018), or genomic structural equation modeling (Grotzinger et al., 2019), carefully conducted longitudinal analyses of cohort data, and/or instrumental variable methods that use environmental factors (e.g. policy changes) instead of genes as an instrument.
Taken together, the current body of MR studies is a valuable addition to the literature on mental health and substance use. It has provided more robust evidence that substance use (most notably smoking) can cause mental health problems, thereby (further) strengthening the incentive to decrease substance use, particularly among populations with poor mental health.
Acknowledgements
MRM is a member of the MRC Integrative Epidemiology Unit at the University of Bristol (MC_UU_00011/7), and the National Institute for Health Research (NIHR) Biomedical Research Centre at the University Hospitals Bristol National Health Service (NHS) Foundation Trust, and the University of Bristol.
Financial support
JLT was supported by a Veni grant from the Netherlands Organization for Scientific Research (NWO; grant number 016.Veni.195.016). KJHV and JLT were supported by the Foundation Volksbond Rotterdam.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003329172100180X.
click here to view supplementary material
Conflict of interest
None of the authors have any conflicts of interest to declare, financial or otherwise.
References
- Abdellaoui, A., Sanchez-Roige, S., Sealock, J., Treur, J. L., Dennis, J., Fontanillas, P., … Boomsma, D. I.. (2019). Phenome-wide investigation of health outcomes associated with genetic predisposition to loneliness. Human Molecular Genetics, 28(22), 3853–3865. 10.1093/hmg/ddz219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdellaoui, A., Smit, D., van den Brink, W., Denys, D., & Verweij, K. (2020). Genomic relationships across psychiatric disorders including substance use disorders. Drug and Alcohol Dependence, 220, 108535. 10.1101/2020.06.08.20125732. [DOI] [PubMed] [Google Scholar]
- Abdellaoui, A., & Verweij, K. J. H. (2021). Dissecting polygenic signals from genome-wide association studies on human behaviour. Nature Human Behaviour. Online ahead of print. 10.1038/s41562-021-01110-y. [DOI] [PubMed] [Google Scholar]
- Adams, C. (2019). Mendelian randomization analysis of smoking behavior and cognitive ability on the Big five. MedRxiv, 2019.12.11.19014530. 10.1101/2019.12.11.19014530. [DOI] [Google Scholar]
- Agaku, I. T., Odani, S., Okuyemi, K. S., & Armour, B. (2020). Disparities in current cigarette smoking among US adults, 2002–2016. Tobacco Control, 29, 269–276. 10.1136/tobaccocontrol-2019-054948. [DOI] [PubMed] [Google Scholar]
- Almeida, O. P., Hankey, G. J., Yeap, B. B., Golledge, J., & Flicker, L. (2014a). The triangular association of ADH1B genetic polymorphism, alcohol consumption and the risk of depression in older men. Molecular Psychiatry, 19(9), 995–1000. [DOI] [PubMed] [Google Scholar]
- Almeida, O. P., Hankey, G. J., Yeap, B. B., Golledge, J., & Flicker, L. (2014b). Alcohol consumption and cognitive impairment in older men: A mendelian randomization study. Neurology, 82, 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, S. J., Fulton-Howard, B., O'Reilly, P., Marcora, E., Goate, A. M., Farrer, L. A., … Wang, L. S. (2021). Causal associations between modifiable risk factors and the Alzheimer's phenome. Annals of Neurology, 89(1), 54–65. 10.1002/ana.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, S. J., Goate, A., & Anstey, K. J. (2020). Association between alcohol consumption and Alzheimer's disease: A Mendelian randomization study. Alzheimer's and Dementia, 16(2), 345–353. 10.1016/j.jalz.2019.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern, J., Leventhal, A. M., & Strong, D. R.. (2015). The role of depression in the uptake and maintenance of cigarette smoking. International Review of Neurobiology, 124, 209–243. 10.1016/bs.irn.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au Yeung, S. L., Jiang, C. Q., Cheng, K. K., Liu, B., Zhang, W. S., Lam, T. H., … Schooling, C. M. (2012). Evaluation of moderate alcohol use and cognitive function among men using a mendelian randomization design in the Guangzhou biobank cohort study. American Journal of Epidemiology, 175(10), 1021–1028. [DOI] [PubMed] [Google Scholar]
- Baker, A. L., Thornton, L. K., Hiles, S., Hides, L., & Lubman, D. I. (2012). Psychological interventions for alcohol misuse among people with co-occurring depression or anxiety disorders: A systematic review. Journal of Affective Disorders, 139, 217–229. 10.1016/j.jad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Barkhuizen, W., Dudbridge, F., & Ronald, A. (2020). Genetic overlap and causal associations between smoking behaviours and psychiatric traits and disorders in adolescents and adults. MedRxiv, 2020.02.07.20021089. 10.1101/2020.02.07.20021089. [DOI] [Google Scholar]
- Berk, M., Kapczinski, F., Andreazza, A. C., Dean, O. M., Giorlando, F., Maes, M., … Malhi, G. S. (2011). Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience and Biobehavioral Reviews, 35, 804–817. 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bjorngaard, J. H., Gunnell, D., Elvestad, M. B., Smith, G. D., Skorpen, F., Krokan, H., … Romundstad, P. (2013). The causal role of smoking in anxiety and depression: A Mendelian randomization analysis of the HUNT study. Psychological Medicine, 43(4), 711–719. [DOI] [PubMed] [Google Scholar]
- Brumpton, B., Sanderson, E., Hartwig, F. P., Harrison, S., Vie, G. Å., Cho, Y., … Bielak, L. (2020). Within-family studies for Mendelian randomization: Avoiding dynastic, assortative mating, and population stratification biases. Nature Communications, 11, 3519. 10.1101/602516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S., Scott, R. A., Timpson, N. J., Davey Smith, G., & Thompson, S. G., & EPIC- InterAct Consortium. (2015). Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. European Journal of Epidemiology, 30(7), 543–552. 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S., Small, D. S., & Thompson, S. G. (2017). A review of instrumental variable estimators for Mendelian randomization. Statistical Methods in Medical Research, 26(5), 2333–2355. 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, E. M., Ferreira, M. A. R., Xue, A., Lindström, S., Jiang, X., Yang, J., … Chenevix-Trench, G. (2019). Is schizophrenia a risk factor for breast cancer?-evidence from genetic data. Schizophrenia Bulletin, 45(6), 1251–1256. 10.1093/schbul/sby162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos, M. W., Serebrisky, D., & Castaldelli-Maia, J. M. (2016). Smoking and cognition. Current Drug Abuse Reviews, 9(2), 76–79. 10.2174/1874473709666160803101633. [DOI] [PubMed] [Google Scholar]
- Carter, A. R., Gill, D., Davies, N. M., Taylor, A. E., Tillmann, T., Vaucher, J., … Dehghan, A. (2019). Understanding the consequences of education inequality on cardiovascular disease: Mendelian randomisation study. The BMJ, 365, l1855. 10.1136/bmj.l1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, M., Li, X., & McGue, M. (2017). The causal role of alcohol use in adolescent externalizing and internalizing problems: A Mendelian randomization study. Alcoholism: Clinical and Experimental Research, 41(11), 1953–1960. 10.1111/acer.13493. [DOI] [PubMed] [Google Scholar]
- Charlet, K., & Heinz, A. (2017). Harm reduction—a systematic review on effects of alcohol reduction on physical and mental symptoms. Addiction Biology, 22(5), 1119–1159. 10.1111/adb.12414. [DOI] [PubMed] [Google Scholar]
- Cornelis, M. C., Byrne, E. M., Esko, T., Nalls, M. A., Ganna, A., Paynter, N., … Chasman, D. I. (2015). Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Molecular Psychiatry, 20(5), 647–656. 10.1038/mp.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, M., Kacprowski, T., Menni, C., Gustafsson, S., Pivin, E., Adamski, J., … Ingelsson, E. (2016). Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Human Molecular Genetics, 25(24), ddw334. 10.1093/hmg/ddw334. [DOI] [PubMed] [Google Scholar]
- Cornelis, M. C., Weintraub, S., & Morris, M. C. (2020). Caffeinated coffee and tea consumption, genetic variation and cognitive function in the UK biobank. Journal of Nutrition, 150(8), 2164–2174. 10.1093/jn/nxaa147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran, H. V., Freeman, T. P., Mokrysz, C., Lewis, D. A., Morgan, C. J. A., & Parsons, L. H. (2016). Keep off the grass? Cannabis, cognition and addiction. Nature Reviews Neuroscience, 17, 293–306. 10.1038/nrn.2016.28. [DOI] [PubMed] [Google Scholar]
- Dahne, J., Murphy, J. G., & MacPherson, L. (2017). Depressive symptoms and cigarette demand as a function of induced stress. Nicotine and Tobacco Research, 19(1), 49–58. 10.1093/ntr/ntw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith, G., Davies, N. M., Dimou, N., Egger, M., Gallo, V., Golub, R., … Yarmolinsky, J. (2019). STROBE-MR: Guidelines for strengthening the reporting of Mendelian randomization studies. 10.7287/peerj.preprints.27857v1. [DOI]
- Davies, N., Dickson, M., Davey Smith, G., Windmeijer, F., & van den Berg, G. (2018a). The effect of education on adult mortality, health, and income: Triangulating across genetic and policy reforms. BioRxiv, 250068. 10.1101/250068. [DOI] [Google Scholar]
- Davies, NM, Hill, W. D., Anderson, E. L., Sanderson, E., Deary, I. J., & Smith, G. D. (2019). Multivariable two-sample mendelian randomization estimates of the effects of intelligence and education on health. ELife, 8, e43990. 10.7554/eLife.43990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, NM, Holmes, M. V, & Davey Smith, G. (2018b). Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ (Clinical Research Ed.), 362, k601. 10.1136/BMJ.K601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, G., Marioni, R. E., Liewald, D. C., Hill, W. D., Hagenaars, S. P., Harris, S. E., … Deary, I. J. (2016). Genome-wide association study of cognitive functions and educational attainment in UK biobank (N = 112 151). Molecular Psychiatry, 21(6), 758–767. 10.1038/mp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins, L., Powell, J. H., Pickering, A., Powell, J., & West, R. (2009). Patterns of change in withdrawal symptoms, desire to smoke, reward motivation and response inhibition across 3 months of smoking abstinence. Addiction, 104(5), 850–858. 10.1111/j.1360-0443.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis, D., Walters, R. K., Martin, J., Mattheisen, M., Als, T. D., Agerbo, E., … Neale, .B M. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51(1), 63–75. 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X., Barban, N., & Mills, M. C. (2019). Educational attainment and allostatic load in later life: Evidence using genetic markers. Preventive Medicine, 129, 105866. 10.1016/j.ypmed.2019.105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPrete, T. A., Burik, C. A. P., & Koellinger, P. D. (2018). Genetic instrumental variable regression: Explaining socioeconomic and health outcomes in nonexperimental data. Proceedings of the National Academy of Sciences of the United States of America, 115(22), E4970–E4979. 10.1073/pnas.1707388115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, K. G., Gilbert-Ouimet, M., Mustard, C. A., & Smith, P. M. (2018a). Association between dimensions of the psychosocial and physical work environment and latent smoking trajectories: A 16-year cohort study of the Canadian workforce. Occupational and Environmental Medicine, 75(11), 814–821. 10.1136/oemed-2018-105138. [DOI] [PubMed] [Google Scholar]
- Dobson, K. G., Ibrahim, S., Gilbert-Ouimet, M., Mustard, C. A., & Smith, P. M. (2018b). Association between psychosocial work conditions and latent alcohol consumption trajectories among men and women over a 16-year period in a national Canadian sample. Journal of Epidemiology and Community Health, 72(2), 113–120. 10.1136/jech-2017-209691. [DOI] [PubMed] [Google Scholar]
- Elkins, I. J., Saunders, G. R. B., Malone, S. M., Keyes, M. A., Samek, D. R., McGue, M., & Iacono, W. G. (2018). Increased risk of smoking in female adolescents who had childhood ADHD. American Journal of Psychiatry, 175(1), 63–70. 10.1176/appi.ajp.2017.17010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth, B., Mitchell, R., Raistrick, C., Paternoster, L., Hemani, G., & Gaunt, T. (2017). MRC IEU UK Biobank GWAS pipeline version 1. Bristol, UK: University of Bristol. [Google Scholar]
- Firth, J., Solmi, M., Wootton, R. E., Vancampfort, D., Schuch, F. B., Hoare, E., … Stubbs, B. (2020). A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry, 19(3), 360–380. 10.1002/wps.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluharty, M. E., Sallis, H., & Munafò, M. R. (2018). Investigating possible causal effects of externalizing behaviors on tobacco initiation: A Mendelian randomization analysis. Drug and Alcohol Dependence, 191, 338–342. 10.1016/j.drugalcdep.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, M., Faul, J., Jin, Y., Ware, E., & Bakulski, K. (2019). Mendelian randomization of smoking behavior on cognitive status among older Americans. MedRxiv, 2019.12.11.19014522. 10.1101/2019.12.11.19014522. [DOI] [Google Scholar]
- Gage, S. H., Bowden, J., Davey Smith, G., & Munafo, M. R. (2018). Investigating causality in associations between education and smoking: A two-sample Mendelian randomization study. International Journal of Epidemiology, 47(4), 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, S. H., Hickman, M., & Zammit, S. (2016a). Association between cannabis and psychosis: Epidemiologic evidence. Biological Psychiatry, 79, 549–556. 10.1016/j.biopsych.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Gage, S. H., Jones, H. J., Burgess, S., Bowden, J., Davey Smith, G., Zammit, S., & Munafò, M. R. (2017a). Assessing causality in associations between cannabis use and schizophrenia risk: A two-sample Mendelian randomization study. Psychological Medicine, 47(5), 971–980. 10.1017/S0033291716003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, S. H., Jones, H. J., Taylor, A. E., Burgess, S., Zammit, S., & Munafò, M. R. (2017b). Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Scientific Reports, 7(1), 1–8. 10.1038/srep40653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, S. H., Munafò, M. R., & Davey Smith, G. (2016b). Causal inference in Developmental Origins of Health and Disease (DOHaD) research. Annual Review of Psychology, 67(1), 567–585. 10.1146/annurev-psych-122414-033352. [DOI] [PubMed] [Google Scholar]
- Gage, S. H., Sallis, H. M., Lassi, G., Wootton, R. E., Mokrysz, C., Davey Smith, G., … Munafò, M. R. (2020). Does smoking cause lower educational attainment and general cognitive ability? Triangulation of causal evidence using multiple study designs. Psychological Medicine. Online ahead of print. 10.1017/S0033291720003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo, M. N., Navarro, J. F., & Cavas, M. (2020). The influence of placebo effect on craving and cognitive performance in alcohol, caffeine, or nicotine consumers: A systematic review. Frontiers in Psychiatry, 11, 849. 10.3389/fpsyt.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey, L., Olofsson, H., Garza, T., Shepherd, J. M., Smit, T., & Zvolensky, M. J. (2020). The role of anxiety in smoking onset, severity, and cessation-related outcomes: A review of recent literature. Current Psychiatry Reports, 22(8), 38. 10.1007/s11920-020-01160-5. [DOI] [PubMed] [Google Scholar]
- Gibbs, M., Winsper, C., Marwaha, S., Gilbert, E., Broome, M., & Singh, S. P. (2015). Cannabis use and mania symptoms: A systematic review and meta-analysis. Journal of Affective Disorders, 171, 39–47. 10.1016/j.jad.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Gibson, M., Munafò, M. R., Taylor, A. E., & Treur, J. L. (2019). Evidence for genetic correlations and bidirectional, causal effects between smoking and sleep behaviors. Nicotine and Tobacco Research, 21(6), 731–738. 10.1093/ntr/nty230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, N. A., & Kendler, K. S. (2021). Use of genetically informed methods to clarify the nature of the association between Cannabis Use and risk for schizophrenia. JAMA Psychiatry, 78(5), 467–468. 10.1001/jamapsychiatry.2020.3564. [DOI] [PubMed] [Google Scholar]
- Greenland, S., Satterfield, M. H., & Lanes, S. F. (1998). A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Safety, 18(4), 297–308. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9565740. [DOI] [PubMed] [Google Scholar]
- Groenman, A. P., Janssen, T. W. P., & Oosterlaan, J. (2017). Childhood psychiatric disorders as risk factor for subsequent substance abuse: A meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 556–569. 10.1016/j.jaac.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Grosso, G., Micek, A., Castellano, S., Pajak, A., & Galvano, F. (2016). Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Molecular Nutrition and Food Research, 60(1), 223–234. 10.1002/mnfr.201500620. [DOI] [PubMed] [Google Scholar]
- Grotzinger, A. D., Rhemtulla, M., de Vlaming, R., Ritchie, S. J., Mallard, T. T., Hill, W. D., … Tucker-Drob, E. M. (2019). Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nature Human Behaviour, 3(5), 513–525. 10.1038/s41562-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic, A., & de Wit, H. (2009). Sleep deprivation increases cigarette smoking. Pharmacology Biochemistry and Behavior, 93(3), 263–269. 10.1016/j.pbb.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag, A. R., Stringer, S., De Leeuw, C. A., Sniekers, S., Taskesen, E., Watanabe, K., … Posthuma, D. (2017). Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet, 49, 1584–1592. 10.1038/ng.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, S., Davies, A. R., Dickson, M., Tyrrell, J., Green, M. J., Katikireddi, S. V., … Howe, L. D. (2020b). The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK Biobank. International Journal of Epidemiology, 49(5), 1661–1681. 10.1093/ije/dyaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, R., Munafò, M. R., Davey Smith, G., & Wootton, R. E. (2020a). Examining the effect of smoking on suicidal ideation and attempts: Triangulation of epidemiological approaches. The British Journal of Psychiatry, 217(6), 701–707. 10.1192/bjp.2020.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, W. D., Marioni, R. E., Maghzian, O., Ritchie, S. J., Hagenaars, S. P., Mcintosh, A. M., … Deary, I. J. (2019). A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Molecular Psychiatry, 24(2), 169–181. 10.1038/s41380-017-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, L. A., Treur, J. L., Jones, H. J., Sallis, H. M., & Munafò, M. R. (2020). Using genetic information to inform policy on cannabis. The Lancet Psychiatry, 7(12), P1002-1003. 10.1016/S2215-0366(20)30377-1. [DOI] [PubMed] [Google Scholar]
- Hodgson, K., Coleman, J. R. I., Hagenaars, S. P., Purves, K. L., Glanville, K., Choi, S. W., … Lewis, C. M. (2020). Cannabis use, depression and self-harm: Phenotypic and genetic relationships. Addiction, 115(3), 482–492. 10.1111/add.14845. [DOI] [PubMed] [Google Scholar]
- Howard, D. M., Adams, M. J., Clarke, T. K., Hafferty, J. D., Gibson, J., Shirali, M., … Mcintosh, A. M. (2019). Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience, 22(3), 343–352. 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, L. J., Nivard, M. G., Morris, T. T., Hansen, A. F., Rasheed, H., Cho, Y., … Davies, N. M. (2021). Within-sibship GWAS improve estimates of direct genetic effects. BioRxiv, 2021.03.05.433935. 10.1101/2021.03.05.433935. [DOI] [Google Scholar]
- Huang, K. L., Marcora, E., Pimenova, A. A., Di Narzo, A. F., Kapoor, M., Jin, S. C., ... Goate, A. M.. (2017). A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nature Neuroscience, 20(8), 1052–1061. 10.1038/nn.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons, D. E., McGue, M., Iacono, W. G., & Oetting, W. S. (2007). Mendelian randomization: A novel test of the gateway hypothesis and models of gene-environment interplay. Special Issue: Gene-Environment Interaction, 19(4), 1181–1195. [DOI] [PubMed] [Google Scholar]
- Irwin, C., Khalesi, S., Desbrow, B., & McCartney, D. (2020). Effects of acute caffeine consumption following sleep loss on cognitive, physical, occupational and driving performance: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 108, 877–888. 10.1016/j.neubiorev.2019.12.008. [DOI] [PubMed] [Google Scholar]
- Jansen, P. R., Watanabe, K., Stringer, S., Skene, N., Bryois, J., Hammerschlag, A. R., … Posthuma, D. (2019). Genome-wide analysis of insomnia in 1 331 010 individuals identifies new risk loci and functional pathways. Nature Genetics, 51(3), 394–403. 10.1038/s41588-018-0333-3. [DOI] [PubMed] [Google Scholar]
- Jobson, C. L. M., Renard, J., Szkudlarek, H., Rosen, L. G., Pereira, B., Wright, D. J., … Laviolette, S. R. (2019). Adolescent nicotine exposure induces dysregulation of mesocorticolimbic activity states and depressive and anxiety-like prefrontal cortical molecular phenotypes persisting into adulthood. Cerebral Cortex, 29(7), 3140–3153. 10.1093/cercor/bhy179. [DOI] [PubMed] [Google Scholar]
- Jones, S. E., Tyrrell, J., Wood, A. R., Beaumont, R. N., Ruth, K. S., Tuke, M. A., … Weedon, M. N. (2016). Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet, 12(8), e1006125. 10.1371/journal.pgen.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson, E., Thai, K. K., Hoffmann, T. J., Sakoda, L. C., Kvale, M. N., Banda, Y., … Choquet, H. (2017). Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Molecular Psychiatry, 22(9), 1359–1367. 10.1038/mp.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson Linnér, R., Biroli, P., Kong, E., Meddens, S. F. W., Wedow, R., Fontana, M. A., … Wagner, G. G. (2019). Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nature Genetics, 51(2), 245–257. 10.1038/s41588-018-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katikireddi, S. V., Green, M. J., Taylor, A. E., Davey Smith, G., & Munafò, M. R. (2018). Assessing causal relationships using genetic proxies for exposures: An introduction to Mendelian randomization. Addiction, 113(4), 764–774. 10.1111/add.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes, K. M., Pratt, C., Galea, S., McLaughlin, K. A., Koenen, K. C., & Shear, M. K. (2014). The burden of loss: Unexpected death of a loved one and psychiatric disorders across the life course in a national study. American Journal of Psychiatry, 171(8), 864–871. 10.1176/appi.ajp.2014.13081132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouja, J. N., Wootton, R. E., Taylor, A. E., Smith, G. D., & Munafò, M. R. (2020). Association of genetic liability to smoking initiation with e-cigarette use in young adults. MedRxiv, 2020.06.10.20127464. 10.1101/2020.06.10.20127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft, P., Chen, H., & Lindström, S. (2020). The use of genetic correlation and Mendelian randomization studies to increase our understanding of relationships between Complex traits. Current Epidemiology Reports, 7(2), 104–112. 10.1007/s40471-020-00233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, M., Holmes, M. V, Dale, C. E., Hubacek, J. A., Palmer, T. M., Pikhart, H., … Bobak, M. (2014). Alcohol consumption and cognitive performance: A Mendelian randomization study. Addiction, 109(9), 1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle, B. W., Grenier-Boley, B., Sims, R., Bis, J. C., Damotte, V., Naj, A. C., … Pericak-Vance, M. A. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics, 51(3), 414–430. 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok, M. K., Leung, G. M., & Schooling, C. M. (2016). Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer's disease: A Mendelian randomization study. Scientific Reports, 6(101563288), 36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque, J. A., & Swanson, S. A. (2019). Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. American Journal of Epidemiology, 188(1), 231–238. 10.1093/aje/kwy204. [DOI] [PubMed] [Google Scholar]
- Lambert, J. C., Ibrahim-Verbaas, C. A., Harold, D., Naj, A. C., Sims, R., Bellenguez, C., … Seshadri, S. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature Genetics, 45(12), 1452–1458. 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, J. M., Jones, S. E., Dashti, H. S., Wood, A. R., Aragam, K. G., van Hees, V. T., … Saxena, R. (2019). Biological and clinical insights from genetics of insomnia symptoms. Nature Genetics, 51, 387–393. 10.1038/s41588-019-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, S. C., Traylor, M., Malik, R., Dichgans, M., Burgess, S., & Markus, H. S. (2017). Modifiable pathways in Alzheimer's disease: Mendelian randomisation analysis. BMJ: British Medical Journal, 359, j5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N., & Davey Smith, G. (2008). Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Statistics in Medicine, 27(8), 1133–1163. 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- Lee, J. J., Wedow, R., Okbay, A., Kong, E., Maghzian, O., Zacher, M., … Turley, P. (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50(8), 1112–1121. 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert, B., Riglin, L., Dardani, C., Thapar, A., Staley, J., Tilling, K., … Stergiakouli, E. (2019). ADHD Genetic liability and physical health outcomes – A two-sample Mendelian randomization study. BioRxiv, 630467. 10.1101/630467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, S. J., Araya, R., Smith, G. D., Freathy, R., Gunnell, D., Palmer, T., … Munafo, M. (2011). Smoking is associated with, but does not cause, depressed mood in pregnancy-A Mendelian randomization study. PLoS ONE, 6(7), e21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wang, H., Li, M., Shen, Q., Li, X., Zhang, Y., … Peng, Y. (2020). Effect of alcohol use disorders and alcohol intake on the risk of subsequent depressive symptoms: A systematic review and meta-analysis of cohort studies. Addiction, 115, 1224–1243. 10.1111/add.14935. [DOI] [PubMed] [Google Scholar]
- Lim, K. X., Rijsdijk, F., Hagenaars, S. P., Socrates, A., Choi, S. W., Coleman, J. R. I., … Pingault, J. B. (2020). Studying individual risk factors for self-harm in the UK Biobank: A polygenic scoring and Mendelian randomisation study. PLoS Medicine, 17(6), e1003137. 10.1371/journal.pmed.1003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M., Jiang, Y., Wedow, R., Li, Y., Brazel, D. M., Chen, F., … Vrieze, S. (2019a). Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nature Genetics, 51, 237–244. 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., van den Wildenberg, W. P. M., de Graaf, Y., Ames, S. L., Baldacchino, A., Bø, R., … Wiers, R. W. (2019b). Is (poly-) substance use associated with impaired inhibitory control? A mega-analysis controlling for confounders. Neuroscience and Biobehavioral Reviews, 105, 288–304. 10.1016/j.neubiorev.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Lo, M. T., Hinds, D. A., Tung, J. Y., Franz, C., Fan, C. C., Wang, Y., … Chen, C. H. (2017). Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nature Genetics, 49(1), 152–156. 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabee-Gittens, E. M., Xiao, Y., Gordon, J. S., & Khoury, J. C. (2013). The dynamic role of parental influences in preventing adolescent smoking initiation. Addictive Behaviors, 38(4), 1905–1911. 10.1016/j.addbeh.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahedy, L., Suddell, S., Skirrow, C., Fernandes, G. S., Field, M., Heron, J., … Munafò, M. R. (2020). Alcohol use and cognitive functioning in young adults: Improving causal inference. Addiction, 116(2), 292–302. 10.1111/add.15100. [DOI] [PubMed] [Google Scholar]
- Mahedy, L., Wootton, R., Suddell, S., Caroline, S., Heron, J., Hickman, M., … Munafò, M. R. (2021). Testing the association between tobacco and cannabis use and cognitive functioning: Findings from an observational and Mendelian randomization study. Drug and Alcohol Dependence, 221, 108591. 10.1016/j.drugalcdep.2021.108591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marees, A. T., Smit, D. J. A., Ong, J.-S., MacGregor, S., An, J., Denys, D., … van den Brink, W. D. E. (2019). Potential influence of socio-economic status on genetic correlations between alcohol consumption measures and mental health. Psychological Medicine, 15, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, T. L., Kassed, C. A., Vandivort-Warren, R., Levit, K. R., & Kranzler, H. R. (2009). Alcohol and opioid dependence medications: Prescription trends, overall and by physician specialty. Drug and Alcohol Dependence, 99(1–3), 345–349. 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, A. R., Hogarth, L., Leventhal, A. M., Cook, J. W., & Hitsman, B. (2017). Cigarette smoking and depression comorbidity: Systematic review and proposed theoretical model. Addiction, 112, 401–412. 10.1111/add.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue, M., Keyes, M., Sharma, A., Elkins, I, Legrand, L., Johnson, W., … Iacono, W. G.. (2007). The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS). Behavior Genetics, 37(3), 449–462. 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- McGue, M., Osler, M., & Christensen, K. (2010). Causal inference and observational research: The utility of twins. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 5(5), 546–556. 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMartin, A., & Conley, D. (2020). Commentary: Mendelian randomization and education–challenges remain. International Journal of Epidemiology, 49(4), 1193–1206. 10.1093/ije/dyaa160. [DOI] [PubMed] [Google Scholar]
- Millard, L. A. C., Munafò, M. R., Tilling, K., Wootton, R. E., & Davey Smith, G. (2019). MR-pheWAS with stratification and interaction: Searching for the causal effects of smoking heaviness identified an effect on facial aging. PLoS Genetics, 15(10), e1008353. 10.1371/journal.pgen.1008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur, Y. S., & Picciotto, M. R. (2009). Biological basis for the co-morbidity between smoking and mood disorders. Journal of Dual Diagnosis, 5, 122–130. 10.1080/15504260902869964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel, M., Jansen, P. R., Stringer, S., Watanabe, K., De Leeuw, C. A., Bryois, J., … Posthuma, D. (2018). Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nature Genetics, 50(7), 920–927. 10.1038/s41588-018-0151-7. [DOI] [PubMed] [Google Scholar]
- Nieman, D. H., Chavez-Baldini, U., Vulink, N. C., Smit, D. J. A., Van Wingen, G., De Koning, P., … Denys, D. (2020). Protocol across study: Longitudinal transdiagnostic cognitive functioning, psychiatric symptoms, and biological parameters in patients with a psychiatric disorder. BMC Psychiatry, 20(1), 212. 10.1186/s12888-020-02624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama, T., Nakatochi, M., Goto, A., Iwasaki, M., Hachiya, T., Sutoh, Y., … Suzuki, S. (2019). Genome-wide association meta-analysis and Mendelian randomization analysis confirm the influence of ALDH2 on sleep durationin the Japanese population. Sleep, 42(6), zsz046. 10.1093/sleep/zsz046. [DOI] [PubMed] [Google Scholar]
- North, T.-L., Palmer, T. M., Lewis, S. J., Cooper, R., Power, C., Pattie, A., … Day, I. N. M. (2015). Effect of smoking on physical and cognitive capability in later life: A multicohort study using observational and genetic approaches. BMJ Open, 5(12), e008393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, L. J., & Price, A. L. (2018). Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nature Genetics, 50(12), 1728–1734. 10.1038/s41588-018-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay, A., Beauchamp, J. P., Fontana, M. A., Lee, J. J., Pers, T. H., Rietveld, C. A., … Benjamin, D. J. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533(7604), 539–542. 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alonso, A., Ekelund, J., Sarin, A. P., Miettunen, J., Veijola, J., Järvelin, M. R., … Hennah, W. (2017). Genome-Wide Association Study of Psychosis Proneness in the Finnish Population. Schizophrenia Bulletin, 43(6), 1304–1314. 10.1093/schbul/sbx006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard, S. D., Mukherjee, S., Sharp, S. J., Proitsi, P., Lotta, L. A., Day, F., … Wareham, N. J. (2015). Associations between potentially modifiable risk factors and Alzheimer disease: A Mendelian randomization study. PLoS Medicine, 12(6), e1001841. 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain, O., Dudbridge, F., Cardno, A. G., Freeman, D., Lu, Y., Lundstrom, S., … Ronald, A. (2018). Genome-wide analysis of adolescent psychotic-like experiences shows genetic overlap with psychiatric disorders. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 177(4), 416–425. 10.1002/ajmg.b.32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza, F., Solfrizzi, V., Barulli, M. R., Bonfiglio, C., Guerra, V., Osella, A., … Logroscino, G. (2015). Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. Journal of Nutrition, Health and Aging, 19(3), 313–328. 10.1007/s12603-014-0563-8. [DOI] [PubMed] [Google Scholar]
- Pappa, I., St Pourcain, B., Benke, K., Cavadino, A., Hakulinen, C., Nivard, M. G., … Tiemeier, H. (2016). A genome-wide approach to children’s aggressive behavio: The EAGLE consortium. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 171(5), 562–572. 10.1002/ajmg.b.32333. [DOI] [PubMed] [Google Scholar]
- Pasman, J. A., Verweij, K. J. H., Gerring, Z., Stringer, S., Sanchez-Roige, S., Treur, J. L., … Vink, J. M. (2018). GWAS Of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nature Neuroscience, 21(9), 1161–1170. 10.1038/s41593-018-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte, K. A., Qian, W., & Leatherdale, S. T. (2018). Modifiable predictors of insufficient sleep durations: A longitudinal analysis of youth in the COMPASS study. Preventive Medicine, 106, 164–170. 10.1016/j.ypmed.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Polderman, T. J. C., Benyamin, B., De Leeuw, C. A., Sullivan, P. F., Van Bochoven, A., Visscher, P. M., & Posthuma, D. (2015). Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics, 47(7), 702–709. 10.1038/ng.3285. [DOI] [PubMed] [Google Scholar]
- Polimanti, R., Peterson, R. E., Ong, J. S., MacGregor, S., Edwards, A. C., Clarke, T. K., … Derks, E. M. (2019). Evidence of causal effect of major depression on alcohol dependence: Findings from the psychiatric genomics consortium. Psychological Medicine, 49(7), 1218–1226. 10.1017/S0033291719000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S. J., Bates, T. C., Corley, J., McNeill, G., Davies, G., Liewald, D. C., … Deary, I. J. (2014). Alcohol consumption and lifetime change in cognitive ability: A gene × environment interaction study. Age, 36(3), 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff, D. B., Clarke, T. K., Adams, M. J., McIntosh, A. M., Davey Smith, G., Jung, J., … Lohoff, F. W. (2019). Educational attainment impacts drinking behaviors and risk for alcohol dependence: Results from a two-sample Mendelian randomization study with ~780000 participants. Molecular Psychiatry, 26, 1119–11132. 10.1038/s41380-019-0535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis, H. M., Croft, J., Havdahl, A., Jones, H. J., Dunn, E. C., Davey Smith, G., … Munafò, M. R. (2020). Genetic liability to schizophrenia is associated with exposure to traumatic events in childhood. Psychological Medicine. Online ahead of print. 10.1017/S0033291720000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis, H. M., Smith, G. D., & Munafo, M. R. (2019). Cigarette smoking and personality: Investigating causality using Mendelian randomization. Psychological Medicine, 25, 1–9. 10.1101/246181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige, S., Palmer, A. A., Fontanillas, P., Elson, S. L., Adams, M. J., Howard, D. M., … Wilson, C. H.. (2019). Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. American Journal of Psychiatry, 176(2), 107–118. 10.1176/appi.ajp.2018.18040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson, E., Davey Smith, G., Bowden, J., & Munafò, M. R. (2019). Mendelian randomisation analysis of the effect of educational attainment and cognitive ability on smoking behaviour. Nature Communications, 10(1), 2949. 10.1038/s41467-019-10679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre, D. D., Bahorik, A., Zaman, T., & Ramo, D. (2018). Psychiatric disorders and comorbid Cannabis Use. The Journal of Clinical Psychiatry, 79(5), 18ac12267. 10.4088/JCP.18ac12267. [DOI] [PubMed] [Google Scholar]
- Savage, J. E., Jansen, P. R., Stringer, S., Watanabe, K., Bryois, J., De Leeuw, C. A., … Posthuma, D. (2018). Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nature Genetics, 50(7), 912–919. 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setién-Suero, E., Suárez-Pinilla, P., Ferro, A., Tabarés-Seisdedos, R., Crespo-Facorro, B., & Ayesa-Arriola, R. (2020). Childhood trauma and substance use underlying psychosis: A systematic review. European Journal of Psychotraumatology, 11(1), 1748342. 10.1080/20008198.2020.1748342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons-Morton, B. G. (2002). Prospective analysis of peer and parent influences on smoking initiation among early adolescents. Prevention Science, 3(4), 275–283. 10.1023/A:1020876625045. [DOI] [PubMed] [Google Scholar]
- Skov-Ettrup, L. S., Nordestgaard, B. G., Petersen, C. B., & Tolstrup, J. S. (2017). Does high tobacco consumption cause psychological distress? A Mendelian randomization study. Nicotine & Tobacco Research, 19(1), 32–38. [DOI] [PubMed] [Google Scholar]
- Soler Artigas, M., Sánchez-Mora, C., Rovira, P., Richarte, V., Garcia-Martínez, I., Pagerols, M., … Ribasés, M. (2019). Attention-deficit/hyperactivity disorder and lifetime cannabis use: Genetic overlap and causality. Molecular Psychiatry, 25, 2493–2503. 10.1038/s41380-018-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, E. A., Breen, G., Forstner, A. J., Mcquillin, A., Ripke, S., Trubetskoy, V., … Sklar, P. (2019). Genome-wide association study identifies 30 loci associated with bipolar disorder. Nature Genetics, 51(5), 793–803. 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen Rich, J., & Martin, P. R. (2014). Co-occurring psychiatric disorders and alcoholism. In Sullivan E. V., & Pfefferbaum A. (Eds.), Handbook of clinical neurology (Vol. 125, pp. 573–588). Amsterdam, the Netherlands: Elsevier. 10.1016/B978-0-444-62619-6.00033-1. [DOI] [PubMed] [Google Scholar]
- Strid, C., Hallgren, M., Forsell, Y., Kraepelien, M., & Öjehagen, A. (2019). Changes in alcohol consumption after treatment for depression: A secondary analysis of the Swedish randomised controlled study REGASSA. BMJ Open, 9(11), e028236. 10.1136/bmjopen-2018-028236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer, S., Minică, C. C., Verweij, K. J. H., Mbarek, H., Bernard, M., Derringer, J., … Vink, J. M. (2016). Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Translational Psychiatry, 6(3), e769. 10.1038/tp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, G. M. J., Baker, A. L., Fox, N., Kessler, D. S., Aveyard, P., & Munafò, M. R. (2020a). Addressing concerns about smoking cessation and mental health: Theoretical review and practical guide for healthcare professionals. BJPsych Advances, 27, 85–95. 10.1192/bja.2020.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. E., Fluharty, M. E., Bjørngaard, J. H., Gabrielsen, M. E., Skorpen, F., Marioni, R. E., … Munafò, M. R. (2014a). Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: The CARTA consortium. BMJ Open, 4(10), e006141. 10.1136/bmjopen-2014-006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, G., McNeill, A., Girling, A., Farley, A., Lindson-Hawley, N., & Aveyard, P. (2014b). Change in mental health after smoking cessation: Systematic review and meta-analysis. BMJ, 348, g1151. 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, G. M., Sawyer, K., Kessler, D., Munafo, M., Aveyard, P., & Shaw, A. (2020b). Views about integrating smoking cessation treatment within psychological services for patients with common mental illness: A multi-perspective qualitative study. MedRxiv, 2020.02.18.20024596. 10.1101/2020.02.18.20024596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann, T., Vaucher, J., Okbay, A., Pikhart, H., Peasey, A., Kubinova, R., … Holmes, M. V. (2017). Education and coronary heart disease: Mendelian randomisation study. BMJ (Online), 358, j3542. 10.1136/bmj.j3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco and Genetics Consortium. (2010). Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics, 42(5), 441–447. 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala, A., & Ebmeier, K. P. (2018). Effects of drinking on late-life brain and cognition. Evidence-Based Mental Health, 21(1), 12–15. 10.1136/eb-2017-102820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur, J. L., Demontis, D., Sallis, H., Richardson, T., Wiers, R., Borglum, A., … Munafo, M. (2019). Investigating causal pathways between liability to ADHD and substance use, and liability to substance use and ADHD risk, using Mendelian randomization. Addiction Biology, 26(1), e12849. 10.1111/adb.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur, J. L., Gibson, M., Taylor, A. E., Rogers, P. J., Munafo, M. R., & Munafò, M. R. (2018). Investigating genetic correlations and causal effects between caffeine consumption and sleep behaviours. Journal of Sleep Research, 27(5), e12695. 10.1111/jsr.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treur, J. L., Willemsen, G., Bartels, M., Geels, L. M., van Beek, J. H. D. A., Huppertz, C., … Vink, J. (2015). Smoking during adolescence as a risk factor for attention problems. Biological Psychiatry, 78(9), 656–663. 10.1016/J.BIOPSYCH.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Twomey, C. D. (2017). Association of cannabis use with the development of elevated anxiety symptoms in the general population: A meta-analysis. Journal of Epidemiology and Community Health, 71, 811–816. 10.1136/jech-2016-208145. [DOI] [PubMed] [Google Scholar]
- van Amsterdam, J., van der Velde, B., Schulte, M., & van den Brink, W. (2018). Causal factors of increased smoking in ADHD: A systematic review. Substance Use & Misuse, 53(3), 432–445. 10.1080/10826084.2017.1334066. [DOI] [PubMed] [Google Scholar]
- Vaucher, J., Keating, B. J., Lasserre, A. M., Gan, W., Lyall, D. M., Ward, J., … Holmes, M. V. (2018). Cannabis use and risk of schizophrenia: A Mendelian randomization study. Molecular Psychiatry, 23(5), 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen, J. M., Schirmbeck, F., Blankers, M., Van Tricht, M., Bruggeman, R., Van Den Brink, W., … Van Winkel, R. (2018). Association between smoking behavior and cognitive functioning in patients with psychosis, siblings, and healthy control subjects: Results from a prospective 6-year follow-up study. American Journal of Psychiatry, 175(11), 1121–1128. 10.1176/appi.ajp.2018.18010069. [DOI] [PubMed] [Google Scholar]
- Vermeulen, J. M., Wootton, R. E., Treur, J. L., Sallis, H. M., Jones, H. J., Zammit, S., … Munafò, M. R. (2019). Smoking and the risk for bipolar disorder: Evidence from a bidirectional Mendelian randomisation study. The British Journal of Psychiatry, 218(2), 88–94. 10.1192/bjp.2019.202. [DOI] [PubMed] [Google Scholar]
- Vink, J., & Schellekens, A. (2018). Relating addiction and psychiatric disorders. Science (New York, N.Y.), 361, 1323–1324. 10.1126/science.aav3928. [DOI] [PubMed] [Google Scholar]
- Vink, J. M., Willemsen, G., & Boomsma, D. I. (2005). Heritability of smoking initiation and nicotine dependence. Behavior Genetics, 35(4), 397–406. 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Wakai, K, Hamajima, N, Okada, R, Naito, M, Morita, E, Hishida, A, … Tanaka, H. (2011). Profile of participants and genotype distributions of 108 polymorphisms in a cross-sectional study of associations of genotypes with lifestyle and clinical factors: A project in the Japan Multi-Institutional Collaborative Cohort (J-MICC) study. Journal of Epidemiology, 21(3), 223–235. 10.2188/jea.JE20100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, Z., Gonzalez, R., Crosby, K., S. Thiessen, M., Carroll, C., & Bonn-Miller, M. O. (2017). Medical cannabis and mental health: A guided systematic review. Clinical Psychology Review, 51, 15–29. 10.1016/j.cpr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Walters, R. K., Polimanti, R., Johnson, E. C., Mcclintick, J. N., Adams, M. J., Adkins, A. E., … Agrawal, A. (2018). Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience, 21(12), 1656–1669. 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. J., Chen, X., Vink, J., Loukola, A., Minica, C., Pool, R., … Munafò, M. R. (2016). Genome-wide meta-analysis of cotinine levels in cigarette smokers identifies locus at 4q13.2. Scientific Reports, 6(1), 20092. 10.1038/srep20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel, J., Lin, Y. S., Landolt, H. P., Garbazza, C., Kolodyazhniy, V., Kistler, J., … Reichert, C. F. (2020). Caffeine-dependent changes of sleep-wake regulation: Evidence for adaptation after repeated intake. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 99, 109851. 10.1016/j.pnpbp.2019.109851. [DOI] [PubMed] [Google Scholar]
- Williams, J. M., & Gandhi, K. K. (2008). Use of caffeine and nicotine in people with schizophrenia. Current Drug Abuse Reviews, 1, 155–161. 10.2174/1874473710801020155. [DOI] [PubMed] [Google Scholar]
- Wittchen, H. U., Fröhlich, C., Behrendt, S., Günther, A., Rehm, J., Zimmermann, P., … Perkonigg, A. (2007). Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal community study in adolescents. Drug and Alcohol Dependence, 88(Suppl 1), S60–S70. 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen, M. K., Orsted, D. D., & Nordestgaard, B. G. (2015a). Tobacco smoking is causally associated with antipsychotic medication use and schizophrenia, but not with antidepressant medication use or depression. International Journal of Epidemiology, 44(2), 566–577. 10.1093/ije/dyv090. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen, M. K., Orsted, D. D., Tolstrup, J. S., & Nordestgaard, B. G. (2015b). Increased alcohol consumption as a cause of alcoholism, without similar evidence for depression: A Mendelian randomization study. International Journal of Epidemiology, 44(2), 526–539. [DOI] [PubMed] [Google Scholar]
- Wootton, R. E., Greenstone, H. S. R., Abdellaoui, A., Denys, D., Verweij, K. J. H., Munafò, M. R., … Treur, J. L. (2020). Bidirectional effects between loneliness, smoking and alcohol use: Evidence from a Mendelian randomization study. Addiction, 116(2), 400–406. 10.1111/add.15142. [DOI] [PubMed] [Google Scholar]
- Wootton, R. E., Richmond, R. C., Stuijfzand, B. G., Lawn, R. B., Sallis, H. M., Taylor, G. M. J., … Munafò, M. R. (2019). Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: A Mendelian randomisation study. Psychological Medicine, 50(14), 2435–2443. 10.1017/s0033291719002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray, N. R., Ripke, S., Mattheisen, M., Trzaskowski, M., Byrne, E. M., Abdellaoui, A., … Sullivan, P. F. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50(5), 668–681. 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Tian, F., Zhang, H., Zeng, J., Chen, T., Wang, S., … Gong, Q. (2016). Cortical and subcortical gray matter shrinkage in alcohol-use disorders: A voxel-based meta-analysis. Neuroscience and Biobehavioral Reviews, 66, 92–103. 10.1016/j.neubiorev.2016.03.034. [DOI] [PubMed] [Google Scholar]
- Yuan, S., Xiong, Y., Michaëlsson, M., Michaëlsson, K., & Larsson, S. C. (2020a). Health-related effects of education level: A Mendelian randomization study. MedRxiv, 2020.02.01.20020008. 10.1101/2020.02.01.20020008. [DOI] [Google Scholar]
- Yuan, S., Yao, H., & Larsson, S. C. (2020b). Associations of cigarette smoking with psychiatric disorders: Evidence from a two-sample Mendelian randomization study. Scientific Reports, 10(1), 13807. 10.1038/s41598-020-70458-4.. 10.1038/s41598-020-70458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L., Ntalla, I., Kessler, T., Kastrati, A., Erdmann, J., Danesh, J., … Schunkert, H. (2019). Genetically modulated educational attainment and coronary disease risk. European Heart Journal, 40(29), 2413–2420. 10.1093/eurheartj/ehz328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Sealock, J. M., Sanchez-Roige, S., Clarke, T. K., Levey, D. F., Cheng, Z., … Gelernter, J. (2020). Genome-wide meta-analysis of problematic alcohol use in 435 563 individuals yields insights into biology and relationships with other traits. Nature Neuroscience, 23(7), 809–818. 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., Sun, D., Li, X., Ma, H., Heianza, Y., & Qi, L. (2019b). Educational attainment and drinking behaviors: Mendelian randomization study in UK biobank. Molecular Psychiatry. online ahead of print. 10.1038/s41380-019-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, A., Taylor, A. E., Karhunen, V., Zhan, Y., Rovio, S. P., Lahti, J., … Hypponen, E. (2018). Habitual coffee consumption and cognitive function: A Mendelian randomization meta-analysis in up to 415 530 participants. Scientific Reports, 8(1), 7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H., Zhang, Y., Liu, J., Yang, Y., Fang, W., Hong, S., … Zhang, L. (2019a). Education and lung cancer: A Mendelian randomization study. International Journal of Epidemiology, 48(3), 743–750. 10.1093/ije/dyz121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003329172100180X.
click here to view supplementary material