Abstract
Stem cells are the central element of regenerative medicine (RM). However, in many clinical applications, the use of scaffolds fabricated with biomaterials is required. In this sense, mesoporous bioactive glasses (MBGs) are going to play an important role in bone regeneration because of their striking textural properties, quick bioactive response, and biocompatibility. As other bioactive glasses, MBGs are mainly formed by silicon, calcium, and phosphorus oxides whose ions play an important role in cell proliferation as well as in homeostasis and bone remodeling process. A common improvement of bioactive glasses for RM is by adding small amounts of oxides of elements that confer them additional biological capacities, including osteogenic, angiogenic, antibacterial, anti-inflammatory, hemostatic, or anticancer properties. Moreover, MBGs are versatile in terms of the different ways in which they can be processed, such as scaffolds, fibers, coatings, or nanoparticles. MBGs are unique because their textural properties are so high that they still exhibit outstanding bioactive responses even after adding extra inorganic ions or being processed as scaffolds or nanoparticles. Moreover, they can be further improved by loading with biomolecules, drugs, and stem cells. This article reviews the state of the art and future perspectives of MBGs in the field of RM of hard tissues.
Keywords: Bioactive ceramics, Tissue engineering, Bone regeneration, Therapeutic inorganic ions, Drugs release, Stem cells
Graphical abstract
1. Introduction
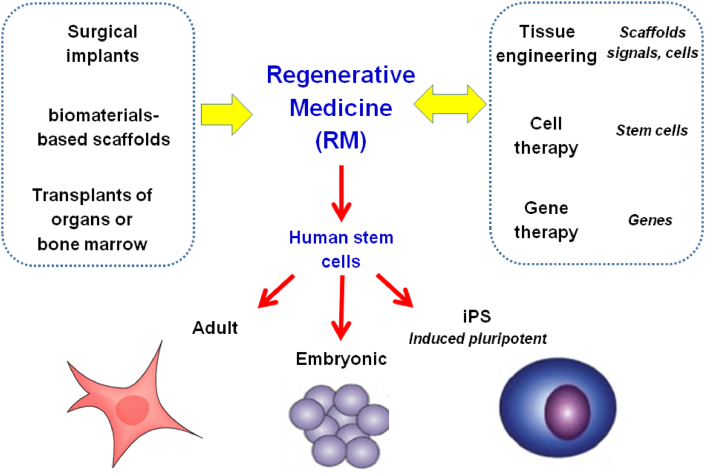
Regenerative medicine (RM) emerged from clinical practices, such as the design of surgical implants, the use of biomaterials-based scaffolds, or the transplant of organs or bone marrow, and is closely related to tissue engineering [[1], [2], [3]]. Said practices have limitations, such as the loss of prostheses with time, the inflammatory process induced by the scaffolds, the contamination of the bone marrow aspirate, or the need to take immunosuppressive drugs after organ transplantation. The bases of RM are human stem cells [[4], [5], [6]] that can be of adult or embryo-derived origin and also they can be the so-called induced pluripotent stem (iPS) cells obtained by reprogramming adult cells. Human embryos are not the ideal source. Therefore, obtaining iPS cells is an attractive approach, as it involves the transfer of genes to human cells, which brings RM close to gene and cell therapies (Fig. 1).
Fig. 1.
Foundations of Regenerative Medicine and its relationships with other advanced therapies.
Researchers specializing in cells generally try to use the minimum possible amount of synthetic biomaterials. However, biomedical industry of tissue regeneration is combining cellular therapies with others based on genes, biomaterials, and drugs. A successful RM focused on human stem cells could replace molecular pharmaceuticals and biomedical prostheses. For example, RM seeking cartilage regeneration restoration can give way to a rational development of prosthetics. Moreover, there is a growing link between gene therapy and RM. Cell therapy seeks to place genes in cells to implant them. The current interest in reprogramming adult cells into iPS cells is driving the linkage between genes and cells in the use of a genetic approach to several therapies.
Typically, regeneration describes the process by which lost specialized tissue is replaced by the proliferation of specialized cells. In humans, the process is limited to a few tissues, such as bone [7,8]. In this sense, the goal of RM is to regenerate mainly by supplying cells, particularly stem cells, that can stimulate a broader regeneration. Similarly, repair is the replacement of lost tissue with granulation tissue that matures to form scar tissue. Organ regeneration is different from organ repair after an injury [9]. Repair leads to the restoration by synthesis of scar tissue without restoration of normal tissue. As the ultimate goal of RM is to return the patient to a healthy state, repair can be considered to fall within technologies, such as surgery. In most cases, the goal of regeneration is to restore a deteriorated function.
RM includes tissue engineering, genetic engineering, and molecular activators and is an interdisciplinary research field focused on the repair, replacement or regeneration of cells, tissues, or organs to restore the deterioration of function resulting from birth defects, diseases, trauma, or aging [10]. RM uses several approaches that move it beyond traditional transplant and replacement therapies that may include the use of biomolecules, gene therapy, stem cells transplantation, tissue engineering, and reprogramming of cell and tissue types [3,11].
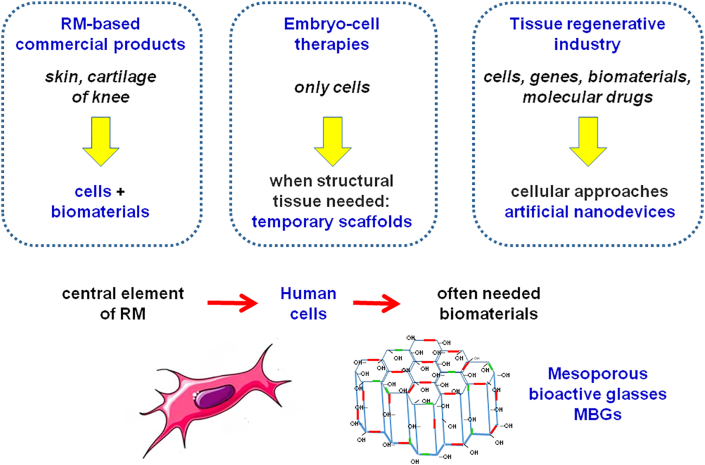
At present, there are commercial products based on RM to treat skin ulcers or knee cartilage injuries (Fig. 2). These therapies often include a scaffold fabricated with biomaterials. More therapies involving embryonic stem cells and temporary scaffolding are expected to appear but, in the situations where structural tissue is needed, it is difficult for cells alone to succeed. Therefore, although stem cells are the central element of RM, many clinical applications need the use of scaffolds, often fabricated with bioceramics [12]. In bone regeneration, autografts, the current gold standard, have many limitations, including morbidity or a limited amount of material available [[13], [14], [15]]. In this area, synthetic grafts obtained with RM approaches using mesoporous bioactive glasses (MBGs) [[16], [17], [18], [19], [20]] are going to play a very important role, as is described in subsequent sections.
Fig. 2.
Many applications of RM require the use of biomaterials behaving as scaffolds of the stem cells as are the MBGs for hard tissues regeneration.
2. Mesoporous glasses in the context of bioactive glasses
Bioactive glasses (BGs) are known for 50 years when the first melt-prepared bioactive glass (MPG) belonging to the SiO2–Na2O–CaO–P2O5 system was reported by Hench et al. [21]. These materials, prepared by the traditional method of quenching of a melt, are dense materials, which exhibit a particular surface reactivity when in contact with aqueous biological fluids, leading to the formation of a mechanically strong bond between the biomaterial and living bone. This unusual property, denoted as bioactivity in the field of biomaterials, is greatly sought in the development of new biomaterials for regenerate bone. Other bioactive materials, most of them bioceramics, were reported, including calcium phosphates, such as hydroxyapatite [22], or glass ceramics, such as apatite/wollastonite glass ceramic [23]. However, none of the biomaterials described so far has exhibited a bioactive response as quick as BGs, which, as a result of their partial solubility in contact with physiological fluids, release significant amounts of the ions that make them up into the surrounding medium. In particular, the Si (IV) ions, the major component of BGs, that benefit the presence of extracellular events, including angiogenesis and the Ca2+ ions that contribute to cell proliferation and exhibit osteogenic activity, as well as play a very important role in gene transfection [24,25].
MPGs showed room for improvement, considering the expected increase of glasses reactivity if obtained as porous materials with high specific surface areas and a greater number of silanol groups (Si–OH) on their surface. Thus, in the 1990s, Li et al. proposed the synthesis of porous BGs by sol-gel, a wet chemistry method [26]. Glasses so obtained are denoted as ‘gel glasses’ or sol-gel glasses (SGGs). These glasses exhibit high surface areas and nanometric pores in a great diversity of sizes. The wide distribution of pores sizes of SGGs does not allow an optimal control of the release of biomolecules and drugs if these glasses are used as matrices for drug delivery systems (DDS). The sol-gel method does not allow controlling the SGGs nanostructure, but the microstructure and, consequently, their in vitro and in vivo behaviors can be controlled with different synthesis parameters such as type and concentration of catalyst used for the tetraethyl orthosilicate (the SiO2 source) hydrolysis, the proportion of water, or the glass composition [[27], [28], [29]]. Moreover, sol-gel method allows processing BGs in complex forms such as fibers or coatings.
To improve the SGGs capabilities, in 2004, Yan et al. [30] described the synthesis of so-called MBGs. The synthesis method is based on the sol-gel chemistry and supramolecular chemistry principles. A surfactant that acts as a template to control the glass nanostructure is added. The surfactants used to synthesize MBG are amphiphilic molecules capable of self-assembling in aqueous solutions when a certain concentration – called critical micellar concentration – is reached. At that point, mesophases formed to guide the obtaining of ordered arrangements of mesoporous channels with virtually identical pore diameter.
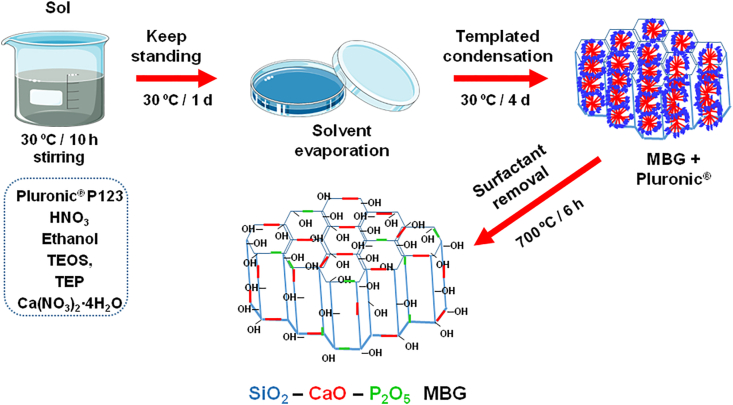
Fig. 3 shows the method of synthesis of MBGs called Evaporation-Induced Self Assembly, proposed by Brinker et al. [31]. As observed, after the surfactant removal, in this case, Pluronic 123, glasses exhibiting highly ordered arrangements of mesopores with diameters around 5 nm in a very narrow pores size distribution are obtained [17,32,33]. MBGs can be considered as intermediate materials between SGGs and pure silica mesoporous materials, such as MCM-41, that were described in 1992 by Kresge et al. [34] for catalysis applications, which, in 2001, were proposed by Vallet-Regi et al. as matrices for drug release systems [35]. Their very narrow mesopore size distribution and highly ordered mesopore structure convert MBGs in materials that allow a high control of the processes of load and release of biomolecules and drugs. Therefore, they are candidates to be used in DDS. Moreover, because of their excellent textural properties, MBGs exhibit, as will be described below, faster bioactive responses than MPGs and SGGs.
Fig. 3.
Schematic representation of the Evaporation-Induced Self Assembly method of synthesis used to obtain MBGs.
Currently, MBGs are often obtained as nanoparticles (MBGNs) that, having a smaller, controlled particle size, allow increasing the efficiency of proper transfection and the ability to release biomolecules and genes [[36], [37], [38]]. These nanoparticles contain ordered mesopores with sizes between 5 and 10 nm, and the particle size can be tailored by modifying the solvent and the surfactant concentration. Indeed, the effect of the concentration of surfactant on the characteristics of MBGs has been widely investigated [39,40].
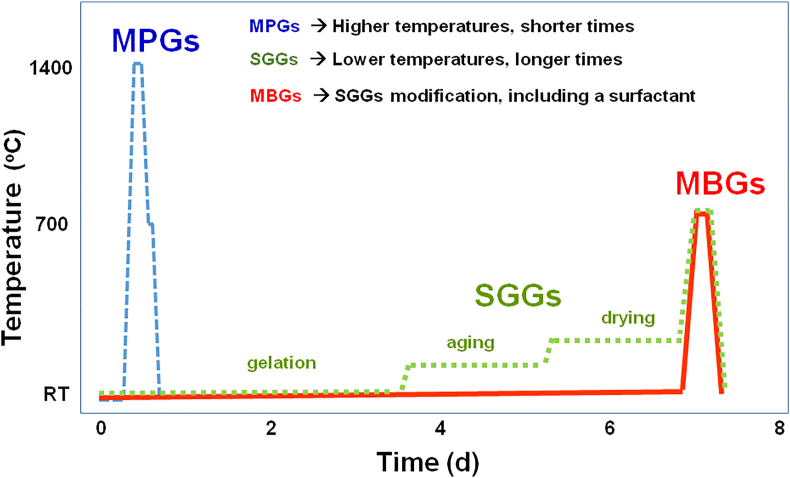
Fig. 4 schematically depicts in a comparative way the time and temperature conditions used for the syntheses of the three families of BGs. As seen, MPGs synthesis requires shorter times, less than 1 day, but much higher temperatures, close to 1,400 °C, which supposes extra energy costs. However, the production of SGGs and MBGs requires around 7 days, but takes place at lower temperatures, for most of the synthesis stages close to room temperature (RT), except for the last one of calcination and stabilization. In both cases, this stage requires thermal treatments close to 700 °C for a few hours. Regarding the first stages of synthesis of SGGs and MBGs, Fig. 4 also shows that in the SGGs synthesis, the stages of aging and drying of the gels (from Days 3–6) are carried out at temperatures somewhat above than RT. In general, gel aging takes place at temperatures around 70 °C, and the drying stage rarely exceeds 150 °C. However, in the MBGs synthesis, the stages of gelation, aging, and drying are performed at a constant temperature close to 30 °C. These lower temperatures used for the synthesis of SGGs and MBGs suppose lower energy costs, although this saving is partially offset because the alkoxides used as sources of SiO2, tetraethyl orthosilicate and P2O5, and triethyl phosphate (TEP) are more expensive than the inorganic precursors used for the synthesis of MPGs. Moreover, the most common CaO source in the three cases is Ca(NO3)2⋅4H2O.
Fig. 4.
Schematic representation of the time vs. temperature conditions used to obtain the three families of BGs.
Therefore, it can be summarized that the most significant distinctive features of the three families of BGs are the higher temperatures required to obtain MPGs and the longer times and more expensive reactants required to synthesize SGGs and MBGs. However, control over the mesostructure in MBGs is significant because it opens up new capabilities for this more recent family of BGs.
3. Textural properties of mesoporous glasses
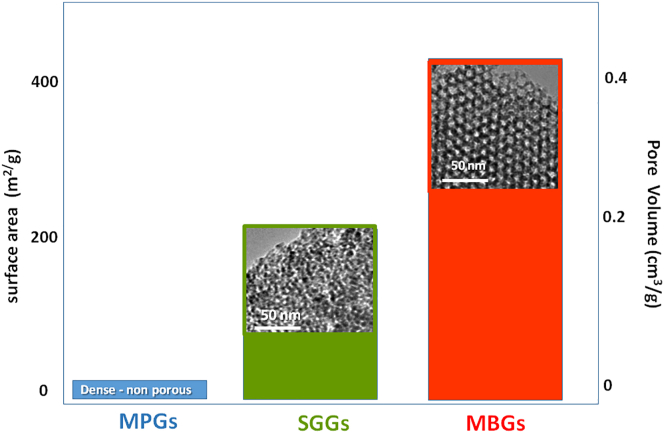
Although the three families of BGs may have a similar or identical chemical composition, MPGs are easily distinguished from the other two types because they are dense materials. Thus, their porosity is null, and their specific surface can be considered negligible when used as monoliths and very small when used as particles. However, SGGs and MBGs are highly porous materials. In addition, MBGs exhibit outstanding structural and textural properties. The synthesis and characterization of several SGGs and MBGs of interest as bioceramics can be found respectively in Refs. [41,[42], [43]]. The most significant textural and mesostructural features of the three families of BGs are shown in Fig. 5 in comparative way.
Fig. 5.
Comparison of the textural properties (surface area, pore volume) typically exhibited by the three families of BGs. High-resolution transmission electron microscopy (HR-TEM) images of the two porous glasses, SGGs and MBGs, are included.
As it is represented in Fig. 5, the pore volume and the surface area in MBGs are approximately double than in SGGs of analogous composition. Furthermore, as can be seen in the high-resolution transmission electron microscopy images, the main difference between the two types of porous glasses is that SGGs exhibit a great diversity of pore sizes, and they are disordered. In MBGs, all mesopores display almost identical sizes, and they are ordered, often in a two-dimensional hexagonal geometry. However, by using the appropriate synthesis parameters, MBGs with three-dimensional cubic mesopores arrangements can be obtained [40]. In short, the three families of BGs exhibit the amorphous structure of glasses at the atomic scale, but MBGs also exhibit an ordered mesopore structure, which confers upon them the outstanding properties that make them optimal materials to use in RM of bone. A recent application of MBGs under investigation, outside of bones and teeth repair, is for soft tissues engineering applications [44].
Therefore, SGGs and MBGs are different at the mesoscale. The remarkably high textural properties of MBGs are close to those of ordered mesoporous materials made of pure silica, such as MCM-41 and SBA-15. However, mesoporous silica materials have a very moderate or null in vitro bioactive response when soaked in simulated body fluid (SBF). This fact demonstrates that the huge textural properties of mesoporous silica materials – surface areas around 1,000 m2/g with pore volumes over 1 cm3/g – and the large number of silanol groups they have on the surface do not guarantee a bioactive response in SBF [45]. Compared with these materials, MBGs have lower textural properties, but still unusually high, and the presence of CaO and P2O5 together with SiO2 give them the optimal reactivity to be coated with hydroxycarbonate apatite (HCA) after being soaked for very short periods in SBF. So-called in vitro bioactivity tests in SBF monitor the time it takes for the HCA layer to form. The shorter the time, the more bioactive a biomaterial is considered to be.
Nitrogen adsorption/adsorption isotherms of MBGs are analogous to SGGs. Both kinds of glasses show type IV isotherms, characteristic of mesoporous materials, as well as type H1 hysteresis loops, characteristic of cylindrical pores open at both ends. However, in SGGs, the pore diameter distribution obtained was broader, and the textural parameters of MBGs are approximately twice than SGGs [32] (Fig. 5).
Another difference between SGGs and MBGs is that the textural properties of SGGs are related to its composition, particularly to the CaO content. When CaO increases, the surface area decreases, and the pore volume increases. However, for MBGs, the variation in the CaO proportion mainly affects the symmetry of the mesopore arrangement, which can evolve from a two-dimensional hexagonal structure (p6mm) to a three-dimensional bicontinuous cubic structure (Ia-3d). This variation in symmetry can also be controlled by the aging temperature used for the synthesis of MBGs [33].
4. Bioactive response of mesoporous glasses
Regarding the in vitro bioactive response, MPGs exhibited bioactivity for SiO2 contents between 45 and 60 mol%. At this point, it must be highlighted the low SiO2 contents of an MPG exhibit a bioactive response if compared with glasses designed for other technological applications, which are close to 70%. Thus, the composition of Hench Bioglass, the first bioactive material, has an unusually low SiO2 content. This yields a glass reactive enough in aqueous media to experience a series of chemical processes that result in the formation of a nanocrystalline HCA layer on the glass surface, which is considered indicative of a bioactive response.
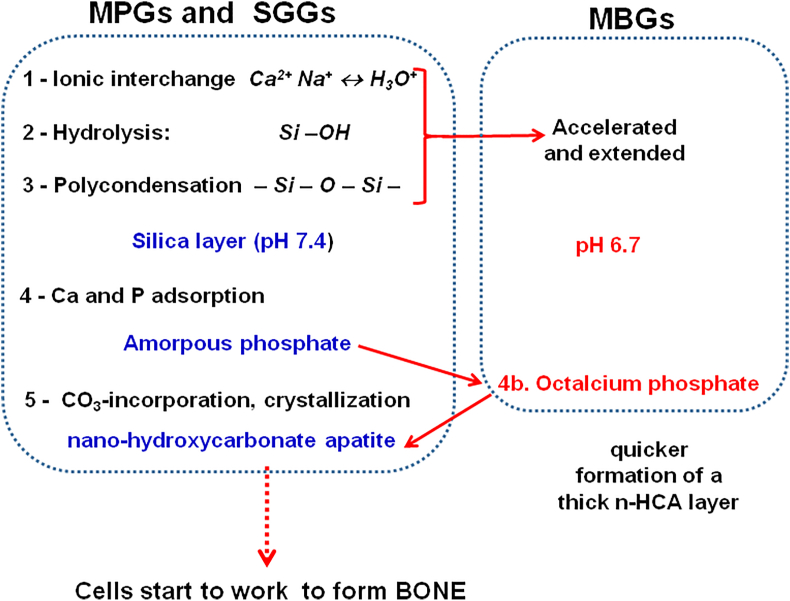
The left part of Fig. 6 shows the first five stages of the Hench mechanism that explains the formation of a strong union between a BG and bone [46]. The full mechanism consists of 11 stages, the last six include the participation of biological entities. However, the first five stages only depend on the intrinsic reactivity of the BG, and they take place both in vivo and in vitro. Therefore, they are the stages that are usually assessed in simulated biological solutions, such as Kokubo’s SBF [47]. It must be considered that although this mechanism was proposed for MPGs, it can be applied to SGGs because the differences in the bioactive kinetics of both types of glasses are minimum. However, there are significant differences with MBGs that justify the extremely rapid bioactive response of these glasses.
Fig. 6.
The first five stages of the Hench mechanism for the in vitro HCA formation on MPGs and SGGs. Variations in this mechanism for the highly bioactive MBGs are displayed at the right.
Most of the SGGs and MBGs investigated as biomaterials belong to the SiO2–CaO–P2O5 system. Our research group reported the influence of P2O5 in the bioactive response of BGs, a component that slightly speeds up the kinetics of formation of the HCA layer but is not an indispensable requisite for bioactivity [48]. For this reason, some compositions of SGGs widely investigated even some ones commercial, are P2O5 free, and belong to the SiO2–CaO system.
Although the high surface area and porosity of SGGs expand the bioactivity window up to SiO2 contents of 90 mol% in SGGs, the bioactivity mechanism is similar for MPGs and SGGs. In both families of BGs, an average period ranging from 3 to 7 days to form the nano-HCA layer is considered appropriate to consider these glasses as potential candidates for bone regeneration. However, MBGs exhibit noticeably quicker in vitro bioactivity results. For instance, some MBG compositions were coated by the HCA layer after only 4 h in SBF. These MBGs are the synthetic materials that display the fastest bioactive response described. The right-hand side of Fig. 6 shows the differences that can explain the extremely quick bioactive response of MBGs. First difference is the lower pH value as a consequence of the stages 1–3 accelerated and extended. The second difference is the formation of octacalcium phosphate, detected in the maturation process of bone but never before observed in the in vitro tests as an intermediate phase that evolves to nano-HCA.
5. Clinical applications of the three families of BGs
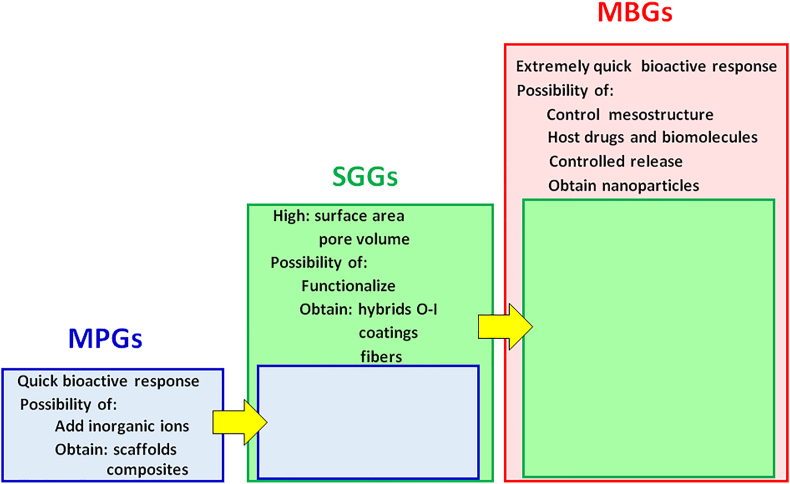
Fig. 7 displays the main clinical applications of the three families of BGs in a comparative way. As is observed, each family exhibits the features of the previous one, increased with additional ones. Thus, MPGs are excellent biomaterials to be used as bone grafts because of their quick bioactive response and possibilities for improvement, such as the inclusion of therapeutic inorganic ions in their composition and the possibility of processing them to obtain scaffolds and composites.
Fig. 7.
Expansion of the biological capabilities of bioactive glasses when going from MPGs to SGGs and MBGs.
In addition to those capabilities, SGGs offer others arising from their wet chemistry processing at low temperatures. First, the considerable expansion of the bioactivity window to include SiO2 contents of up to 90 mol% consequence of the excellent textural properties. However, this fact is of scarce relevance for their clinical application. More significant differences are that SGGs can be functionalized because of their higher concentration of surface silanol groups and that some biocompatible polymer can be added during their syntheses at low temperatures to obtain organic–inorganic hybrid materials [49], also called nanocomposites, with the desired mechanical or degradation properties. Also, by selecting the appropriate moment during the sol to gel transition, it is possible to use this method of synthesis to obtain coatings or fiber meshes of SGGs.
Finally, MBGs, which can be considered an improvement of SGGs, show all the characteristics of MPGs and SGGs but present another new one as a result of its synthesis in the presence of surfactants, which produces a great control over the MBGs mesostructure. As it was told, textural properties of MBGs are more advantageous than those of SGGs, and its in vitro bioactive responses are much faster than of any other BGs family. Moreover, the large volume of monodisperse pores makes MBGs ideal candidates to host drugs and biomolecules, which is essential for their use in bone RM applications and DDS. On the other hand, one of the current most active areas of study in the MBGs field is obtaining as nanoparticles that can be used as nanocarriers of biologically active ions and biomolecules [36]. This field that is subjected to a great expansion is out of the scope of the present review article.
6. MBGs in RM of bone
The remarkable quick bioactive response of MBGs is not the only aspect that should be considered when selecting a BG for RM of bone. The new physical/chemical features and the processing possibilities provided by wet chemistry synthesis methods to obtain coatings and fibers must be also taken into account. Moreover, the great pore volume and control over the morphology and size of pores in MBGs provide the capability to load them with osteogenic biomolecules that, together with their remarkably fast bioactive response, make these materials optimal candidates for use as scaffolds in RM [50,51].
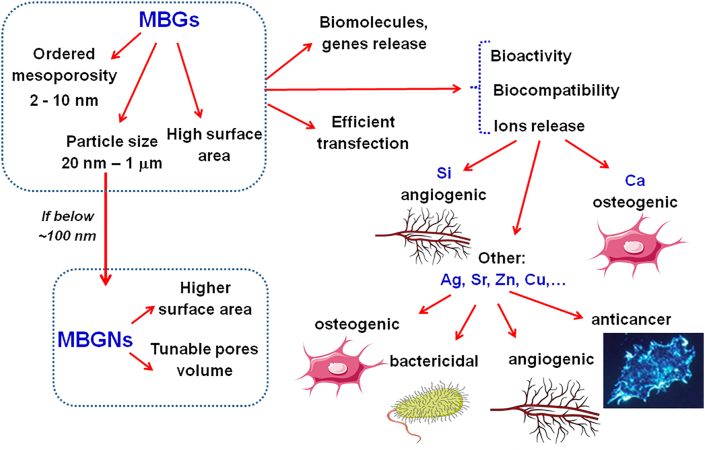
Fig. 8 shows the main capabilities and properties of MBGs, including the potential to obtain as nanoparticles (MBGNs) and also to add additional ions to those that usually make them up like silicon and calcium [[52], [53], [54]]. Thus, Si (IV) ions favor certain cellular events, and the angiogenesis and Ca2+ ions contribute to cell proliferation and the osteogenic activity exerting a crucial role in gene transfection. As can be seen, the addition of certain ions considered therapeutic [55,56], and it is easy to do because the composition of glasses can be easily altered in an almost infinite compositional range bringing additional advantages, including osteogenic, angiogenic, anticancer, or bactericidal properties [[57], [58], [59], [60], [61]].
Fig. 8.
Properties and capabilities exhibited by MBGs in RM of bone tissues.
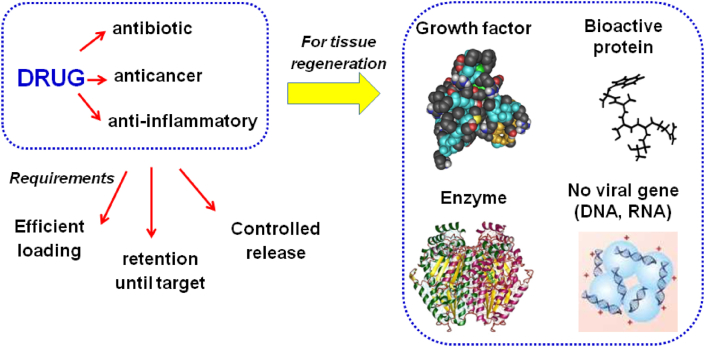
As it was said, another important capability of MBGs in RM is their ability to load and release drugs. However, in this area, the concept of drug, generally used as a substance with antibiotic, anticancer, or anti-inflammatory properties, expands to other types of biomolecules, such as growth factors, bioactive proteins, enzymes, or non-viral genes, such as DNA or RNA (see Fig. 9).
Fig. 9.
The number of substances typically considered as drugs and their requirements is expanded when used for living tissues regeneration.
7. Mesoporous glasses as scaffolds, microparticles, or nanoparticles
In clinical application, MBGs can be used mainly as scaffolds and also as particles, which are considered nanoparticles when they are less than 100 nm in size [62]. However, for practical purposes, nanoparticles are also considered when their dimensions slightly exceed that value. On the other hand, it is also common in the literature to list as nanoparticles of MBGs to particles with sizes of 500 or even 800 nm [36]. Particles of these dimensions may also be useful in bone RM applications, but they should rather be considered as submicrometric particles or as microparticles when exceeding 1,000 nm size.
One of the first attempts to obtain scaffolds tailored to clinical necessities involved the preparation of an injectable paste made of MBGs that was able to set as a calcium phosphate bone cement [63]. However, this material failed in the macroporous architecture required for a scaffold useful in RM because it only exhibited random macroporosity and had poor pore interconnectivity. The incorporation of MBGs in scaffolds to obtain materials with macro- and nano-porosity that could be optimum candidates for bone regeneration was achieved in 2009. As it will be mentioned later, the first method used was that of the sponge of polyurethane that allowed obtaining scaffolds with a predesigned macroporosity.
Keeping in mind the need for three-dimensional hierarchical scaffolds for bone regeneration, several strategies were proposed to design the macroporosity required for functions, such as bone cell ingrowths, nutrient supply, and vascularization, as well as for the adhesion and development of bone cells. These strategies include foaming, freeze drying, fiber bonding, or RP technologies [64]. In all cases, it is very important to confirm that the processing of MBG powders to obtain scaffolds does not eliminate ordered mesoporosity or bioactivity decorating with cells on the MBG surfaces.
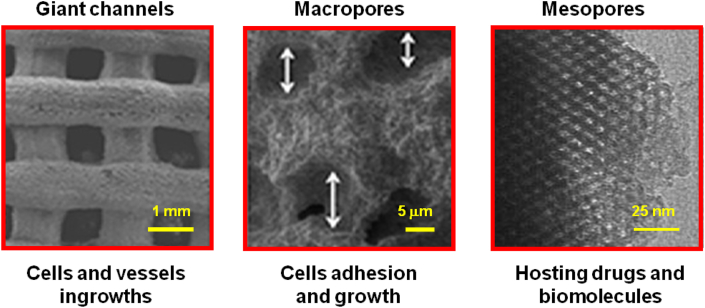
Our research group has widely investigated MBG scaffolds obtained by RP to apply in bone RM [[65], [66], [67]]. With the manufacturing technique used, three-dimensional scaffolds with hierarchical porosity were obtained. Indeed, this method of manufacture allows obtaining biomaterials exhibiting mesopores around 5 nm, which are especially appropriated to host drugs, pores close to 5 μm in size, formed during the evaporation of the solvent used for preparing the printing ink, that are essential for cell adhesion and growth and also pores close to 1 mm that are obtained in the printing process and that allow the scaffolds to be colonized with cells and blood vessels. The micrographs of Fig. 10 show images corresponding to the three categories of pores present in the MBG scaffolds obtained by RP and the main roles that each type of pore plays in bone RM.
Fig. 10.
Three types of pores in MBG scaffolds: giant channels, around 1 mm; macropores, close to 5 μm; and mesopores, around 5 nm. Their biological roles in bone RM are also included.
Other methods of synthesis were successfully used to obtain three-dimensional porous scaffolds with meso-macroporosity based on MBGs, including the polyurethane sponge method [68] or pouring a suspension of MBG powders in polyvinyl alcohol into a negative template of polylactic acid that was subsequently removed by extraction [69].
8. Expanding mesoporous glass properties by adding inorganic ions
A characteristic of glasses, regardless of the method of synthesis used, is that they can be obtained in a practically infinite range of compositions. Indeed, during the synthesis of glasses by quenching a melt to be used in industrial applications, besides the main components, it is common to add small amounts of other oxides, a process often called doping, to confer upon them certain mechanical, optical, electrical, etc. properties. Similarly, in the design of glasses for bone regeneration, many researchers have explored the doping of BGs with oxides of elements with well-known biological activity, for example, osteogenic, angiogenic, or bactericidal properties [55]. When these studies are carried out on the three families of BGs, the limitation on doping is the ability of these extra elements to be integrated into the glass network without eliminating their bioactive response and maintaining biocompatibility, and, in the case of SGGs and MBGs, without an excessive deterioration of their textural properties.
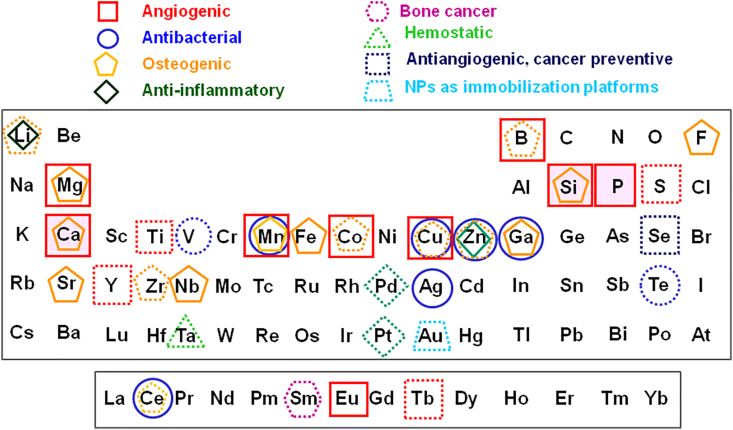
The doping of MBGs with ions of elements with biological activity, often denoted as therapeutic ions, is a subject investigated with great interest in recent years [70,71]. In these studies, the first objective is to determine the maximum amount of the extra oxide that can be incorporated into the MBG network to exert the desired biological action, but maintaining the necessary bioactivity, biocompatibility, and ordered mesoporosity. A comprehensive list of the elements included so far in the MBGs composition and the biological activity sought in each case are shown in Fig. 11.
Fig. 11.
Elements included so far in the MBGs and their biological activity. Solid lines indicate well-established properties, whereas the dashed lines correspond to biological effects proposed.
The first point that catches your attention in Fig. 11 is the large number of elements that have been investigated, practically most of those considered non-toxic. In this sense, the absence of elements such as Cr, toxic in certain oxidation states, Ni, which produces an allergic reaction in a growing portion of the population, or Al, which has been related to neurological disorders, can be highlighted. Likewise, the inclusion of elements already present in very large quantities in the human body, such as C or N, and others extremely abundant in extracellular or intracellular fluids, such as Na, K, or Cl, were not investigated either. Moreover, it can be mentioned the small but increasing presence of elements with high atomic numbers whose presence in the human body as essential elements is minimal.
Regarding the chemical elements investigated, we can start with the biological action of the three basic elements of the MBGs, that is, Si, Ca, and P, to which an angiogenic character is attributed and, in the case of the first two, also osteogenic [36]. With regard to the rest of the therapeutic inorganic elements of interest in the present article, we can observe that in addition to the biological actions investigated several years ago, such as angiogenic, antibacterial, and osteogenic, new actions were recently added, such as anti-inflammatory, antitumor, hemostatic, antiangiogenic, cancer preventive, or manufacture nanoparticles to be used as immobilization platforms.
To understand the criteria used in Fig. 11, we must indicate that the solid lines signify well-established properties on which there is a broad consensus on the part of the scientific community, whereas the dashed lines correspond to biological effects more recently proposed or with a minor consensus degree. For instance, Li is considered anti-inflammatory and proposed as osteogenic [72], B is considered angiogenic and proposed as osteogenic [73], Se, is recently proposed as antiangiogenic and cancer preventive [74], Ta, which is gaining much recent notoriety as a hemostatic agent [75], and so on. Likewise, the presence of several lanthanide therapeutic elements investigated to be added to MBGs, such as Ce [76], Sm [77], Eu [78], or Tb [79], must be highlighted. As is observed, many other elements are under investigation, in several cases more than one decade ago, including Ti, V, Mn, Fe, Co, Cu, Zn, Ga, and Sr [61,[80], [81], [82], [83], [84], [85], [86], [87]]. Moreover, studies about new elements added to MBGs are frequently published, for example, the recently investigated antibacterial and antioxidant properties of Te [88].
Up to now, our research group, sometimes in collaboration with other research groups has been deeply interested in investigating the effect of included Co, Cu, Zn, Ga, Sr, and Ce in MBGs with SiO2 contents close to 80% [16,[89], [90], [91], [92], [93], [94], [95]]. Particularly, we have developed MBG scaffolds containing 4% of ZnO, already investigated in three animal models. Finally, to mention that, as is observed in Fig. 11, many chemical elements exhibit osteogenic and angiogenic capabilities and that several of them, such as Zn, Cu, Mn, Ga, and Ce, are very versatile exerting several beneficial biological actions.
On the other hand, when investigating the addition of an extra oxide to an MBG, generally with composition SiO2–CaO or SiO2–CaO–P2O5, the first step is to perform the synthesis with increasing amounts of the new oxide to determine the maximum amount able to be incorporated without eliminating the bioactive response or the mesoporous order. Then, biocompatibility in vitro assays and others must be performed to check that the extra oxide does indeed provide the desired property. Finally, the in vivo evaluation of biocompatibility and the biological activity of this extra element must be assessed.
Therefore, a simple way to improve the biological behavior of any BG is through the addition of therapeutic ions; however, this process will modify the physicochemical properties of the initial glass. In the case of MBGs, a slight decrease in the kinetics of the bioactive response is observed and a decrease of about 40% in the textural properties with additions up to 5% of the extra oxide. However, the starting values for undoped MBGs are so high that substituted MBGs remain useful for their intended use for bone RM and also as matrices for controlled DDS, as will be seen in the following section.
9. Loading mesoporous glasses with biomolecules and drugs
Bone regeneration relies on three basic pillars: stem cells, signal molecules, and scaffolds. In this last pillar, MBGs processed into three-dimensional macroporous scaffolds are considered to be the finest option for bone regeneration for the reasons exposed in previous sections. MBG-based scaffolds designed for RM must exhibit interconnected and hierarchical porosity. Thus, as observed in Fig. 10, they must contain giant pores (channels) and macropores to allow angiogenesis and interaction with cells [60] and pores in the range between 2 and 10 nm such as those in MBGs, optimum to host and release substances with biological activity. Such mesopores allow biomolecules with different biological activities to be included in the scaffolds [96]. Particularly, signal molecules can be included for RM of bone regeneration that induce bone formation, such as bone morphogenetic protein (BMP) [97], growth factors such as vascular endothelial growth factor [98], or different fractions of parathyroid hormone–related peptide (PTHrP) [99]. Table 1 collects a number of biomolecules and drugs which loading into MBGs has been investigated.
Table 1.
Drugs and biomolecules that can be loaded in MBGs and its biological activity.
| Drugs and biomolecules | Main biological action | |
|---|---|---|
| Angiogenic | ||
| DEX, dexamethasone, and QK peptide | QK peptide that mimics the α-helical structure of VEGF | [100] |
| VEGF, vascular endothelial growth factor | Stimulates blood vessels formation | [101] |
| Streptokinase | Dissolves blood clots formed in blood vessels | [102] |
| DMOG, dimethyloxaloylglycine | Used in hypoxia-inducible factor (HIF) activity assays | [103] |
| Antibacterial | ||
| Amoxicillin | Antibiotic semisynthetic (beta-lactam) | [104] |
| Ciprofloxacin | Antibiotic (second-generation fluoroquinolone) | [105] |
| Gentamicin | Antibiotic aminoglycoside | [90] |
| Levofloxacin | Antibiotic synthetic (fluoroquinolone) DNA replication inhibitor | [90] |
| Moxifloxacin | Antibiotic (fluoroquinolone) | [106] |
| Rifampicin | Antibiotic semisynthetic | [90] |
| Teicoplanin | Antibiotic for prophylaxis and treatment gram-positive bacteria | [73] |
| Tetracycline | Antibiotic of broad spectrum | [107] |
| Triclosan | Antibacterial and antifungal agent | [108] |
| Vancomycin | Antibiotic glycopeptides, gram-positive bacteria effective | [90] |
| Vancomycin/tetracycline | Antibiotics | [109] |
| Anticancer | ||
| Isothiocyanate | Cancer-preventive activity | [110] |
| Aflatoxins antibodies | Carcinogens and mutagens produced by molds | [111] |
| Mitomycin C | Chemotherapy agent with antitumor activity | [112] |
| Cisplatin | Chemotherapy agent to treat cancer | [113] |
| DOX, doxorubicin | Chemotherapy medication to treat cancer | [114] |
| DOX/vancomycin | Chemotherapy medication to treat cancer/antibiotic | [109] |
| Osteogenic | ||
| BMP, bone morphogenetic protein | Bone and cartilage formation | [97] |
| Icariin | Osseous fractures repair | [115] |
| Osteostatin | Osteogenic activity, antiresorptive | [93] |
| DEX, dexamethasone | Osteogenic differentiation | [116] |
| Phenamil | Osteogenic, triggers osteoblastic differentiation, and mineralization | [117] |
| rh-BMP-2, recombinant human BMP | Osteoinductive mesenchymal cells to chondroblasts and osteoblasts | [118] |
| Ipriflavone | Osteoporosis: prevention and treatment | [119] |
| Other functions | ||
| ACE, angiotensin-converting enzyme/IBU | High blood pressure and heart failure/anti-inflammatory | [120] |
| Aspirin | Anti-inflammatory non-steroidal | [102] |
| BSA, bovine serum albumin | Cell nutrient and enzymes stabilizer | [121] |
| Chlorhexidine | Disinfectant and antiseptic | [122] |
| Curcumin | Multiple roles: cancer, anti-inflammatory, antioxidant, anti-arthritic | [123] |
| EGF, epidermal growth factor | Cell growth and differentiation stimulation | [124] |
| Fluorescein | Diagnosis | [110] |
| IBU, Ibuprofen | Anti-inflammatory non-steroidal | [125] |
| Metoclopramide | Treatment of heartburn and of ulcers and sores in esophagus | [70] |
| Phenanthrene | To make bile acids, cholesterol, and steroids | [127] |
As can be seen in the Table, a considerable number of drugs and biomolecules have been loaded in MBGs, and very likely the inclusion of more substances will be investigated soon. An inspiration to identify other biologically active substances of interest to be loaded in MBGs can be found in our comprehensive review article regarding drugs and biomolecules loaded in pure silica mesoporous materials. Such materials have similarities with MBGs because both families exhibit nanopores and a great number of surface silanol groups [96].
The great number of drugs and biomolecules included in Table 1 were grouped attending their biological action as angiogenic, antibacterial, anticancer, osteogenic, and other functions, such as anti-inflammatory or disinfectant or various types of treatment or diagnosis. Because the table is already self-explanatory, only a few comments not able to be found in the table will be done here. For instance, the most investigated antibacterial substance so far is gentamicin, which was investigated in more than eight papers, although vancomycin and ciprofloxacin also appear in several articles on the subject. Likewise, doxorubicin was investigated in more than 10 publications as anticancer drug. Moreover, dexamethasone and BMPs for its osteogenic action and ibuprofen for its anti-inflammatory action also aroused a great interest to be loaded in MBGs.
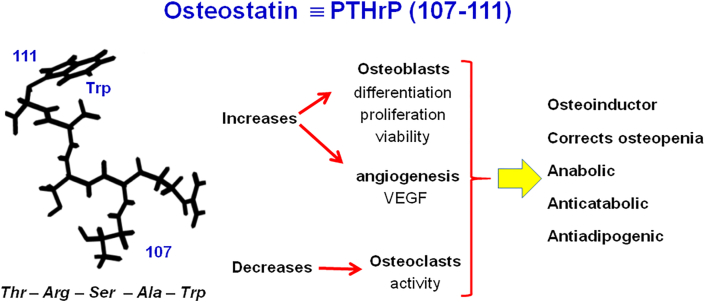
On the other hand, our research group has a wide experience in using the fraction 107–111 of this peptide, that is, PTHrP107-111, which is usually denoted as osteostatin (OST), and sometimes TRSAW, which is represented in Fig. 12. The Figure shows the five amino acids forming this pentapeptide and the beneficial biological effects that make it a promising osteoinductor substance, which some authors propose may be more favorable for this purpose than the well-known and used BMP-2 [50].
Fig. 12.
Osteostatin, a pentapeptide, fragment of parathormone related peptide (PTHrP), with excellent features to be used as an osteoinductor signal in RM of bone.
10. Interaction of stem cells with mesoporous glasses in bone regeneration
Numerous studies have evaluated the biocompatibility of MBGs in the presence of different cell lines. A recent review article by Salètes et al. [127] identified around 100 articles of the period between 2015 and 2021 investigating the interactions of MBGs and living cells. The main results of 63 papers were displayed in a highly comprehensive table. Regarding the MBGs scaffolds, more used compositions were those with SiO2 contents of 58%, 64%, 80%, or 85%. In many cases, adjuvants such as polycaprolactone (PCL), chitosan, or polymethyl methacrylate (PMMA) were used, and often the MBGs were doped with inorganic elements, such as Ga, Cu, Sr, Ce, or nano-Ti to increase the biological capabilities of the MBGs. Several cell lines were used for the studies, including MC3T3-E1 mouse osteoblast cell line, MG-63: human osteoblast-like, human Saos-2, osteoclast-like cells, murine RAW264.7 murine macrophages, human umbilical vein endothelial cells, HUVECs, and others.
Furthermore, and of a great interest of interest in the framework of the present article, in 20 of the articles described in Ref. [127], the biocompatibility of MBGs was investigated in the presence of bone mesenchymal stem cells (MSCs), in most cases of rat (rMSCs) or human origins (hMSCs), but also obtained from a donor rabbit. Despite the differences in the MBG compositions, in all cases, the excellent cytocompatibility of MBGs was shown, evidenced by the enhanced proliferation of MSCs and osteogenic differentiation of the MSCs grown on the scaffolds. Moreover, the ionic dissolution products amplified adhesion, proliferation, and the osteogenic differentiation of MSCs and the proliferative and the in vitro angiogenic ability of HUVECs.
The use of MSCs for bone RM still arouses certain controversy [[128], [129], [130]]. Effectively, because of the difficulties inherent in in vivo studies, there are relatively few published results and also a great variability of methods, protocols, and animal models that impedes to compare the results obtained by the different research groups. However, most authors trust that the union of MSCs with scaffolds and signal molecules will be the solution [131], although at this time, it is perceived still far away.
On the other hand, our research group has recently reported the biocompatibility of MBGs in the presence of hMSCs under in vitro [66] and also in vivo conditions, after being implanted in an animal model in a New Zealand rabbit [99]. The MBG scaffolds investigated contained 4% of ZnO because of the osteogenic and bactericidal features of Zn2+ ions and were loaded with OST, an osteoinductive and antiresorptive pentapeptide. The in vitro study [66] showed an excellent internalization of hMSCs cells in the MBG scaffolds and outstanding responses in terms of cell adhesion, growth, and osteogenic differentiation. This system allowed us to disclose, for the first time, a synergistic effect of zinc and OST to enhance hMSCs cell growth and differentiation, suggesting its potential for bone regeneration.
The excellent in vitro results prompted us to carry out in vivo studies [99]. The investigated systems exhibited bone regeneration capability. However, the trabecular bone to volume density values obtained by μCT showed that the good bone healing capability of pristine Zn-MBG was significantly improved by the scaffolds enriched with OST and hMSCs. These in vivo findings suggest the interest of these MBG complete systems is to improve bone repair in the clinical practice. New in vivo studies with different animal models and in one case using rabbit MSCs instead of hMSCs are in progress.
11. Present of mesoporous glasses in bone RM
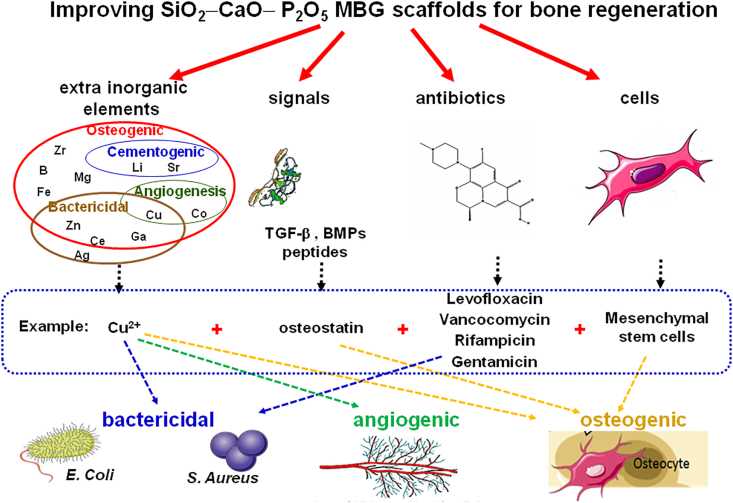
In Fig. 13 are schematically depicted possible improvements of the MBG scaffolds. It must be taken into consideration that MBGs are biocompatible materials exhibiting an extremely quick bioactive response. The scaffolds fabricated from MBGs powders can be improved by adding inorganic ions, biochemical signals, antibiotics, or other molecules with biological activity and stem cells [123,[132], [133], [134], [135]]. Some examples of each element are indicated in Fig. 12. For instance, Cu2+ ions as a therapeutic inorganic ion and OST as biochemical signal. Regarding the drugs able to be hosted and released from the mesopores, four antibiotics were selected and mesenchymal stem cells as cells decorating the surface of the scaffolds. It must be highlighted that the biological properties brought by each of the additional elements are indicated at the bottom of the figure.
Fig. 13.
Strategies used to improve MBG scaffolds adding bactericidal, angiogenic, and osteogenic capabilities.
The two main current trends regarding the use of MBGs in RM applications are based on processing them as three-dimensional scaffolds exhibiting hierarchical porosity or as nanoparticles. The biological capabilities of the starting MBGs of the CaO–P2O5–SiO2 system are extended by adding relatively small amounts of diverse inorganic oxides. Around 30 inorganic elements have been investigated (see Fig. 13) looking for additional biological capabilities as osteogenic, angiogenic, bactericidal, anti-inflammatory, anticancer, or hemostatic.
The processing of MBG powders into scaffolds or nanoparticles and the inclusions of therapeutic inorganic ions produce a decrease in both the textural properties and the bioactive response of the resultant biomaterials. However, one of the main strengths of MBGs is that the original values of these parameters are so high that even after some decrease, they still are enough to be excellent candidates as bone grafts in RM. Furthermore, the processing and the additions of inorganic ions sometimes partially destroy the order of mesopores, but for most clinical applications, this partial order designated as worm-like order is sufficient. BGs exhibiting worm-like order show lower textural properties and bioactivity than well-ordered MBGs but still higher than traditional SGGs.
If MBGs are compared with the other families of BGs, we can observe that MPGs are excellent biomaterials for bone graft substitution because of their quick bioactive response and possibilities to be improved with therapeutic inorganic ions and to be processed in scaffolds and composites. In addition to all the above, SGGs offer new capabilities as the considerable expansion of the bioactivity window up to 90 mol% of SiO2, the possible surface functionalization and obtaining organic–inorganic hybrids with tailored mechanical or degradation properties [49] and the obtaining of coatings or fiber meshes. Finally, MBGs, which can be considered an improvement of SGGs, exhibit controlled nanostructure and huge textural properties because their synthesis in the presence of surfactants and in vitro bioactive responses much faster than the other families of BGs. Moreover, the large volume of monodisperse pores makes MBGs ideal candidates host biomolecules and drugs, an essential feature for their use in applications in bone RM.
12. Future perspective of mesoporous glasses in bone RM
Several issues have to be addressed regarding the use of MBGs in RM, including:
-
➢
the extra elements added to MBGs sometimes bind so strongly with the glass network that the desired biological action is not observed because the inorganic ions are not released to the surrounding medium. For instance, our group investigated an MBG including Ga2O3 because of the bactericidal properties of Ga3+ ions. However, the glass did not exhibit antibacterial properties in the in vitro assays because the Ga3+ ions remained in the glass network without being released to the medium [89]. To solve this drawback, we designed new Ga-MBGs able to release concentrations of Ga3+ within the therapeutic range [91], which now need further investigations.
-
➢
Often, the necessary concentrations of an inorganic element to achieve a biological effect, for instance, bactericidal properties, are so high that the MBG obtained is not biocompatible. In this sense, we investigated MBGs enriched with 4 and 7 mol% ZnO looking for the osteogenic and bactericidal capabilities of Zn2+ ions. The MBG with 7% of ZnO exhibited the highest antibacterial capability. However, this material was not cytocompatible [89]. Therefore, the MBG with 4% of ZnO was selected for our later in vitro and in vivo studies [66,93,99]. The lower bactericidal capability of this MBG was proposed to be improved by adding small amounts of antibiotics [90]. The MBG with 4% of ZnO continues under study as a very promising candidate to be used in bone RM.
-
➢
Sometimes, when the MBG scaffolds or nanoparticles are loaded with biologically active substances such as biomolecules and drugs, the interaction of such substances with the therapeutic ions can substantially modify their release kinetics. Such release kinetics can also be modified by the partial solubility of the MBG in a physiological medium. That way, our group investigated MBGs scaffolds loaded with curcumin, which exhibits numerous positive biological effects, including its possible uses as bactericidal [136], an alternative anticancer drug [137]. We observed that the presence of some inorganic ions in the glass network increased the amount of curcumin able to be uploaded into the MBG scaffold. However, in some cases, the interactions of the drug with the inorganic ions were so strong that the curcumin release was hindered [123]. Thus, we learnt that when several components are included in an MBG, all the possible interactions between them must be investigated because they can influence their biological behavior.
-
➢
Another factor that must be considered arises from the high bioactivity of MBGs-based biomaterials. In some cases, the quick formation of an apatite-like layer coating the MBG surface when it contacts to biological fluids can hinder or slow down the release of the biomolecules, drugs, or therapeutic ions in the MBGs.
-
➢
Other unexpected effect was observed when the MBGs scaffolds were coated with a thin gelatin layer (6 wt%) to increase their mechanical integrity and made easy its handling in the in vitro studies [66] and when they were implanted in vivo [99]. Gelatin layer was slightly swelled with the surrounding fluids improving the fit of the scaffold into the bone defect. Moreover, the gelatin layer scarcely decreased the textural properties of MBGs, but the in vitro studies showed that it substantially improved the interchange of ions, biomolecules, and drugs between the biomaterial and medium [66]. Consequently, MBGs trying to be brought to the clinic must be thoroughly characterized after any small variation in the processing parameters and after the sterilization and packaging processes.
-
➢
When the system becomes more complicated with the addition of therapeutic ions, drugs, signals, and living cells, researchers must consider not only the interactions of each element with the matrix but also the interactions among elements. For instance, when we loaded the MBGs scaffolds with the osteogenic peptide OST, an unexpected synergic effect was observed among the Zn2+ ions and the peptide in terms of increasing the bone regeneration [66]. Similarly, when the MBG scaffolds were implanted, a synergic effect between OST and mesenchymal stem cells was observed [99].
-
➢
One of the current most active areas of study in the MBGs is obtaining them as nanoparticles (MBNs) to be used as nanocarriers of biologically active ions and biomolecules [36]. One of the aspects to clarify is the size of the particles to consider them as true nanoparticles. In general, nanoparticles must be smaller than 100 nm, but for pure silica nanoparticles, particles around 120 nm are admitted in this category. However, in MBGs, particles up to 800 nm were reported as nanoparticles when they rather must be considered as submicrometric particles. This field, which is subjected to a great expansion, ought to produce soon materials with new capabilities for bone RM. However, much research is needed before reaching the clinical use.
-
➢
Other approaches based on conventional routes and also more imaginative ones must be explored. For instance, the enrichment of MBGs with an on with double oxidation state, such as Ce3+/Ce4+, exhibits an interesting behavior because it inhibits oxidative stress by mimicking catalase enzyme activity [76]. Other approaches could be tracing the degradation products from MBGs with isotopic labeling such as 45Ca [54] or the use of molecular modeling [138] to understand the interactions between the components included in the MBGs and with the glass network.
Considerable research work is required before MBGs can be used clinically in humans. However, at the moment, it is clear that three-dimensional scaffolds and nanoparticles based on CaO–P2O5–SiO2 MBGs enhanced with oxides of metals with biological activity, loaded with biological signals, and decorated with stem cells is one of the most promising approaches for RM of bone tissues.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge funding provided by European Research Council, Advanced Grant Verdi-Proposal No. 694160 (ERC-2015-AdG), and the Instituto de Salud Carlos III, grant number PI20/01384, co-funded with European Union FEDER funds.
Contributor Information
M. Vallet-Regi, Email: vallet@ucm.es.
A.J. Salinas, Email: salinas@ucm.es.
References
- 1.Mao A.S., Mooney D.J. Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. U. S. A. 2015;112:14452–14459. doi: 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang Chien G.C., Stogicza A. Regenerative medicine. In: Pangarkar S., Pham Q.G., Eapen B.C., editors. Pain Care Essentials and Innovations. 2021. pp. 245–253. [DOI] [Google Scholar]
- 3.Mason C., Dunnill P. A brief definition of regenerative medicine. Regen. Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Rajabzadeh N., Fathi E., Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Invest. 2019;6:19. doi: 10.21037/sci.2019.06.04. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoltz J.-F., de Isla N., Li Y.P., Bensoussan D., Zhang L., Huselstein C., Chen Y., Decot V., Magdalou J., Li N., Reppel L., He Y. Stem Cell. Int. 2015;2:734731. doi: 10.1155/2015/734731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nombela-Arrieta C., Ritz J., Silberstein L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Dimitriou R., Jones E., Mcgonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yannas I.V. Springer; New York: 2001. Tissue and Organ Regeneration in Adults. [DOI] [Google Scholar]
- 10.Greenwood H.L., Singer P.A., Downey G.P., Martin D.K., Thorsteinsdóttir H., Daar A.S. Regenerative medicine and the developing world. PLoS Med. 2006;3:e381. doi: 10.1371/journal.pmed.0030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampogna G., Guraya S.Y., Forgione A. Regenerative medicine: historical roots and potential strategies in modern medicine. J. Microsc. Ultrastruct. 2015;3:101–107. doi: 10.1016/j.jmau.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C., Chang J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today. 2019;24:41–56. doi: 10.1016/j.mattod.2018.07.016. [DOI] [Google Scholar]
- 13.Pape H.C., Evans A., Kobbe P. Autologous bone graft: properties and techniques. J. Orthop. Trauma. 2010;24:S36–S40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 14.Laurencin C., Khan Y., El-Amin S.F. Bone graft substitutes. Expet Rev. Med. Dev. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Roberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8:114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salinas A.J., Vallet-Regi M. Glasses in bone regeneration: a multiscale issue. J. Non-Cryst. Solids. 2016;432:9–14. doi: 10.1016/j.jnoncrysol.2015.03.025. [DOI] [Google Scholar]
- 17.Salinas A.,J. Mesoporous bioactive glasses: state of the art and future prospects. In: Arcos D., Vallet-Regi Maria, editors. Bioactive Glasses: Properties, Composition and Recent Applications. Nova Series: Materials Science and Technologies; NY: 2020. pp. 243–274. [Google Scholar]
- 18.Migneco C., Fiume E., Verné E., Baino F. A guided walk through the world of mesoporous bioactive glasses (MBGs): fundamentals, processing, and applications. Nanomaterials. 2020;10:2571. doi: 10.3390/nano10122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallet-Regí M., Salinas A.J. Mesoporous bioactive glasses in tissue engineering and drug delivery. In: Boccaccini A.R., Brauer D.S., Hupa L., editors. Bioactive Glasses: Fundamentals, Technology and Applications. The Royal Society of Chemistry; London (UK): 2017. pp. 393–419. [Google Scholar]
- 20.Kargozar S., Montazerian M., Hamzehlou S., Kim H.W., Baino F. Mesoporous bioactive glasses: promising platforms for antibacterial strategies. Acta Biomater. 2018;1:1–19. doi: 10.1016/j.actbio.2018.09.052. [DOI] [PubMed] [Google Scholar]
- 21.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;2:117–141. doi: 10.1002/jbm.820050611. [DOI] [Google Scholar]
- 22.Denissen H.W., de Groot K., Makkes P. Ch, van den Hooff A., Klopper P.J. Tissue response to dense apatite implants in rats. J. Biomed. Mater. Res. 1980;14:713–721. doi: 10.1002/jbm.820140603. [DOI] [PubMed] [Google Scholar]
- 23.Kokubo T., Shigematsu M., Nagashima Y., Tashiro M., Nakamura T., Yamamuro T., Higashi S. Apatite- and wollastonite-containing glass-ceramics for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982;60:260–268. http://hdl.handle.net/2433/77000 [Google Scholar]
- 24.Salinas A.J., Vallet-Regi M., Heikkilä J. Use of bioactive glasses as bone substitutes in orthopedics and traumatology. In: Ylanen H., editor. Bioactive Glasses. second ed. Woodhead Publishing; 2018. pp. 337–364. [Google Scholar]
- 25.Montazerian M., Zanotto E.D. A guided walk through Larry Hench's monumental discoveries. J. Mater. Sci. 2017;52:8695–8732. doi: 10.1007/s10853-017-0804-4. [DOI] [Google Scholar]
- 26.Li R., Clark A.E., Hench L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991;2:231–239. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 27.Vallet-Regí M., Ragel C.V., Salinas A.J. Glasses with medical applications. Eur. J. Inorg. Chem. 2003;6:1029–1042. doi: 10.1002/ejic.200390134. [DOI] [Google Scholar]
- 28.Arcos D., Vallet-Regí M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010;6:2874–2888. doi: 10.1016/j.actbio.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Jones J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013;9:4457–4486. doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Yan X., Yu C., Zhou X., Tang J., Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem. Int. Ed. Engl. 2004;43:5980–5984. doi: 10.1002/anie.200460598. [DOI] [PubMed] [Google Scholar]
- 31.Brinker C.J., Lu Y., Sellinger A., Fan H. Evaporation-Induced self-assembly: nanostructures made easy. Adv. Mater. 1999;11:579–585. doi: 10.1002/(SICI)1521-4095(199905)11:7. [DOI] [Google Scholar]
- 32.Izquierdo-Barba I., Salinas A.J., Vallet-Regí M. Bioactive glasses: from macro to nano. Int. J. Appl. Glass Sci. 2013;4:149–161. doi: 10.1111/ijag.12028. [DOI] [Google Scholar]
- 33.López-Noriega A., Arcos D., Izquierdo-Barba I., Sakamoto Y., Terasaki O., Vallet- Regí M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006;18:3137–3144. doi: 10.1021/cm060488o. [DOI] [Google Scholar]
- 34.Kresge C.T., Leonowitz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi: 10.1038/359710a0. [DOI] [Google Scholar]
- 35.Vallet-Regí M., Ramila A., del Real R.P., Perez-Pariente J. A new property of MCM-41: drug delivery system. Chem. Mater. 2001;13:308–311. doi: 10.1021/cm0011559. [DOI] [Google Scholar]
- 36.H. Zhu, K. Zheng, A.R. Boccaccini, Multi-Functional silica-based mesoporous materials as Co-delivery systems for biologically active ions and therapeutic biomolecules. At SSRN: https://ssrn.com/abstract=3770987 or 10.2139/ssrn.3770987. [DOI] [PubMed]
- 37.El-Kady A.M., Farag M.M., El-Rashedi A.M.I. Bioactive glass nanoparticles designed for multiple deliveries of lithium ions and drugs: curative and restorative bone treatment. Eur. J. Pharm. Sci. 2016;91:243–250. doi: 10.1016/j.ejps.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Liang Q., Hu Q., Miao G., Yuan B., Chen X. A facile synthesis of novel mesoporous bioactive glass nanoparticles with various morphologies and tunable mesostructure by sacrificial liquid template method. Mater. Lett. 2015;148:45–49. doi: 10.1016/j.matlet.2015.01.122. [DOI] [Google Scholar]
- 39.Kumar A., Aditya A., Murugavel S. Effect of surfactant concentration on textural characteristics and biomineralization behavior of mesoporous bioactive glasses. Mater. Sci. Eng. C. 2019;96:20–29. doi: 10.1016/j.msec.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Shih C., Chien C.-S., Kung J.-C., Chen J.-C., Chang S.-S., Lu P.-S., Shih C.J. Effect of surfactant concentration on characteristics of mesoporous bioactive glass prepared by evaporation induced self-assembly process. Appl. Surf. Sci. 2013;264:105–110. doi: 10.1016/j.apsusc.2012.09.134. [DOI] [Google Scholar]
- 41.Salinas A.J., Martin A.I., Vallet-Regí M. Bioactivity of three CaO-P2O5-SiO2 sol-gel glasses. J. Biomed. Mater. Res. 2002;61:524–532. doi: 10.1002/jbm.10229. [DOI] [PubMed] [Google Scholar]
- 42.Gómez-Cerezo N., Casarrubios L., Morales I., Feito M.J., Vallet-Regí M., Arcos D., Portolés M.T. Effects of a mesoporous bioactive glass on osteoblasts, osteoclasts and macrophages. J. Colloid Interface Sci. 2018;528:309–320. doi: 10.1016/j.jcis.2018.05.099. [DOI] [PubMed] [Google Scholar]
- 43.Yun H., Kim S., Hyeon Y. Preparation of 3D cubic ordered mesoporous bioactive glasses. Solid State Sci. 2008;10:1083–1092. doi: 10.1016/j.solidstatesciences.2007.11.037. [DOI] [Google Scholar]
- 44.Baino F., Novajra G., Miguez-Pacheco V., Boccaccini A.R., Vitale-Brovarone C. Bioactive glasses: special applications outside the skeletal system. J. Non-Cryst. Solids. 2016;432, A:15–30. doi: 10.1016/j.jnoncrysol.2015.02.015. [DOI] [Google Scholar]
- 45.Vallet-Regí M., Ruiz-González L., Izquierdo-Barba I., González-Calbet J.M. Revisiting silica based ordered mesoporous materials: medical applications. J. Mater. Chem. 2006;16:26–31. doi: 10.1039/B509744D. [DOI] [Google Scholar]
- 46.Hench L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991;74:1487–1510. doi: 10.1111/j.1151-2916.1991.tb07132.x. [DOI] [Google Scholar]
- 47.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Vallet-Regi M., Salinas A.J. Role of the short distance order in glass reactivity materials. Materials. 2018;11:415. doi: 10.3390/ma11030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín A.I., Salinas A.J., Vallet-Regí M. Bioactive and degradable organic-inorganic hybrids. J. Eur. Ceram. Soc. 2005;25:3533–3538. doi: 10.1016/j.jeurceramsoc.2004.09.030. [DOI] [Google Scholar]
- 50.Salinas A.J., Esbrit P., Vallet-Regí M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013;1:40–51. doi: 10.1039/C2BM00071G. [DOI] [PubMed] [Google Scholar]
- 51.Salinas A.J., Vallet Regi M. Bioactive ceramics: from bone grafts to tissue engineering. RSC Adv. 2013;3:11116–11131. doi: 10.1039/c3ra00166k. [DOI] [Google Scholar]
- 52.Li Y., Bastakoti B.P., Yamauchi Y. Smart soft-templating synthesis of hollow mesoporous bioactive glass spheres. Chemistry. 2015;21:8038–8042. doi: 10.1002/chem.201406570. [DOI] [PubMed] [Google Scholar]
- 53.Zheng K., Sui B., Ilyas K., Boccaccini A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: developments, properties and emerging biomedical applications. Mater. Horiz. 2021;8:300–335. doi: 10.1039/D0MH01498B. [DOI] [PubMed] [Google Scholar]
- 54.Sui B., Zhong G., Sun J. Drug-loadable mesoporous bioactive glass nanospheres: biodistribution, clearance, BRL cellular location and systemic risk assessment via 45Ca labelling and histological analysis. Sci. Rep. 2016;6:33443. doi: 10.1038/srep33443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoppe A., Guldel N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Salinas A.J., Shruti S., Malavasi G., Menabue L., Vallet-Regí M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011;7:3452–3458. doi: 10.1016/j.actbio.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 57.Wu C., Chang J., Xiao Y. Mesoporous bioactive glasses for drug delivery and bone tissue regeneration. In: Wu C., Chang J., Xiao Y., editors. Advanced Bioactive Inorganic Materials for Bone Regeneration and Drug Delivery. CRC Press; 2013. pp. 1–24. [Google Scholar]
- 58.Wu C., Chang J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Contr. Release. 2014;193:282–295. doi: 10.1016/j.jconrel.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 59.Romero-Sanchez L.B., Marí-Beffa M., Carrillo P., Medina M.A., Díaz-Cuenca A. Copper-containing mesoporous bioactive glass promotes angiogenesis in an in vivo zebrafish model. Acta Biomater. 2018;68:272–285. doi: 10.1016/j.actbio.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive glasses: sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Kargozar S., Baino F., Hamzehlou S., Hamblin M.R., Mozafari M. Nanotechnology for angiogenesis: opportunities and challenges. Chem. Soc. Rev. 2020;49:5008–5057. doi: 10.1039/C8CS01021H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colilla M., Vallet-Regi M. Targeted stimuli-responsive mesoporous silica nanoparticles for bacterial infection treatment. Int. J. Mol. Sci. 2020;21:8605. doi: 10.3390/ijms21228605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Q.H., Wang J.F., Zhang J.P., Fan J., Stucky G.D. Rapid-setting, mesoporous, bioactive glass cements that induce accelerated in vitro apatite formation. Adv. Mater. 2006;18:1038–1042. doi: 10.1002/adma.200502292. [DOI] [Google Scholar]
- 64.Salgado A.J., Coutinho O.P., Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol. Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 65.Cicuendez M., Portolés M.T., Izquierdo-Barba I., Vallet-Regí M. New nanocomposite system with nanocrystalline apatite embedded into mesoporous bioactive glass. Chem. Mater. 2012;24:1100–1106. doi: 10.1021/cm203416x. [DOI] [Google Scholar]
- 66.Heras C., Sanchez-Salcedo S., Lozano D., Peña J., Esbrit P., Vallet-Regi M., Salinas A.J. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn2+ ions in human mesenchymal stem cells. Acta Biomater. 2019;89:359–371. doi: 10.1016/j.actbio.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shruti S., Salinas A.J., Lusvardi G., Malavasi G., Menabue L., Vallet-Regi M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 2013;9:4836–4844. doi: 10.1016/j.actbio.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y., Kaskel S. Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Microporous Mesoporous Mater. 2009;118:176–182. doi: 10.1016/j.micromeso.2008.08.046. [DOI] [Google Scholar]
- 69.Jiménez-Holguín J., López-Hidalgo A., Sánchez-Salcedo S., Peña J., Vallet-Regí M., Salinas A.J. Strontium-modified scaffolds based on mesoporous bioactive glasses/polyvinyl alcohol composites for bone regeneration. Materials. 2020;13:5526. doi: 10.3390/ma13235526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C., Chang J. Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus. 2012;2:2292–2306. doi: 10.1098/rsfs.2011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bano S., Akhtar M., Yasir M., Salman Maqbool M., Niaz A., Wadood A., Rehman M.-A.-U. Synthesis and characterization of silver-strontium (Ag-Sr)-Doped mesoporous bioactive glass nanoparticles. Gels. 2021;7:34. doi: 10.3390/gels7020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han P., Wu C., Chang J., Xiao Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials. 2012;33:6370–6379. doi: 10.1016/j.biomaterials.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 73.Huang C.-L., Fang W., Huang B.-R., Wang Y.-H., Dong G.-C., Lee T.-M. Bioactive glass as a nanoporous drug delivery system for teicoplanin. Appl. Sci. 2020;10:2595. doi: 10.3390/app10072595. [DOI] [Google Scholar]
- 74.Hu M., Fang J., Zhang Y., Wang X., Zhong W., Zhou Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020;579:654–666. doi: 10.1016/j.jcis.2020.06.122. [DOI] [PubMed] [Google Scholar]
- 75.Nagrath M., Yazdi A.R., Rafferty A., Daly D., Rahman S.U., Gallant R.C., Ni H., Arany P.R., Towler M.R. Tantalum-containing meso-porous glass fibres for hemostatic applications. Mater. Today Commun. 2021;27:102260. doi: 10.1016/j.mtcomm.2021.102260. [DOI] [Google Scholar]
- 76.Varini E., Sánchez-Salcedo S., Malavasi G., Lusvardi G., Vallet-Regí M., Salinas A.J. Cerium (III) and (IV) containing mesoporous glasses/alginate beads for bone regeneration: bioactivity, biocompatibility and reactive oxygen species activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105:109971. doi: 10.1016/j.msec.2019.109971. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Wang X., Su Y., Chen D., Zhong W. A doxorubicin delivery system: samarium/mesoporous bioactive glass/alginate composite microspheres. Mater. Sci. Eng. C. 2016;67:205–213. doi: 10.1016/j.msec.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Hu M., Wang X., Zhou Z., Liu Y. Design and evaluation of Europium containing mesoporous bioactive glass nanospheres: doxorubicin release kinetics and inhibitory effect on osteosarcoma MG 63 Cells. Nanomaterials (Basel) 2018;8:961. doi: 10.3390/nano8110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X., Zhang Y., Lin C., Zhong W. Sol-gel derived terbium-containing mesoporous bioactive glasses nanospheres: in vitro hydroxyapatite formation and drug delivery. Colloids Surf. B Biointerfaces. 2017;160:406–415. doi: 10.1016/j.colsurfb.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 80.Li J., Li X., Li J., Pu X., Wang J., Huang Z., Yin G. Effects of incorporated vanadium and its chemical states on morphology and mesostructure of mesoporous bioactive glass particles. Microporous Mesoporous Mater. 2021;319:111061. doi: 10.1016/j.micromeso.2021.111061. [DOI] [Google Scholar]
- 81.Guduric V., Belton N., Richter R.F., Bernhardt A., Spangenberg J., Wu C., Lode A., Gelinsky M. Tailorable zinc-substituted mesoporous bioactive glass/alginate-methylcellulose composite bioinks. Materials. 2021;14:1225. doi: 10.3390/ma14051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kermani F., Mollazadeh Beidokhti S., Baino F., Gholamzadeh-Virany Z., Mozafari M., Kargozar S. Strontium- and cobalt-doped multicomponent mesoporous bioactive glasses (MBGs) for potential use in bone tissue engineering applications. Materials. 2020;13:1348. doi: 10.3390/ma13061348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barrioni B.R., de Carvalho S.M., Naruphontjirakul P., Norris E., Kelly N.L., Hanna J.V., Jones J.R., Pereira M.M. Cobalt-containing spherical glass nanoparticles for therapeutic ion release. J. Am. Ceram. Soc. 2021 doi: 10.1111/jace.17916. First published: 13 May. [DOI] [Google Scholar]
- 84.Kurtuldu F., Mutlu N., Michálek M., Zheng K., Masar M., Liverani L., Chen S., Galusek D., Boccaccini A.R. Cerium and gallium containing mesoporous bioactive glass nanoparticles for bone regeneration: bioactivity, biocompatibility and antibacterial activity. Mater. Sci. Eng. C. 2021;124:112050. doi: 10.1016/j.msec.2021.112050. [DOI] [PubMed] [Google Scholar]
- 85.Westhauser F., Wilkesmann S., Nawaz Q., Hohenbild F., Rehder F., Saur M., Fellenberg J., Moghaddam A., Ali M.S., Peukert W., Boccaccini A. Aldo. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. 2021;109:1457–1467. doi: 10.1002/jbm.a.37136. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y., Liu Y., Li M., Lu S., Wang J. The effect of iron incorporation on the in vitro bioactivity and drug release of mesoporous bioactive glasses. Ceram. Int. 2013;39:6591–6598. doi: 10.1016/j.ceramint.2013.01.094. [DOI] [Google Scholar]
- 87.Ge F., Yu M., Yu C., Lin J., Weng W., Cheng K., Wang H. Improved RhBMP-2 function on MBG incorporated TiO2 nanorod films. Colloids Surf. B Biointerfaces. 2017;150:153–158. doi: 10.1016/j.colsurfb.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 88.Miola M., Massera J., Cochis A., Kumar A., Rimondini L., Vernè Tellurium E. A new active element for innovative multifunctional bioactive glasses. Mater. Sci. Eng. C, Mater Biol Appl. 2021;123:111957. doi: 10.1016/j.msec.2021.111957. [DOI] [PubMed] [Google Scholar]
- 89.Sanchez-Salcedo S., Shruti S., Salinas A.J., Malavasi G., Menabue L., Vallet-Regi M. In vitro antibacterial capacity and cytocompatibility of SiO2–CaO–P2O5 meso-macroporous glass scaffolds enriched with ZnO. J. Mater. Chem. B. 2014;2:4836–4847. doi: 10.1039/C4TB00403E. [DOI] [PubMed] [Google Scholar]
- 90.Heras C., Jiménez-Holguín J., Doadrio A.L., Vallet-Regí M., Sánchez-Salcedo S., Salinas A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020;114:395–406. doi: 10.1016/j.actbio.2020.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sanchez-Salcedo S., Malavasi G., Salinas A.J., Lusvardi G., Rigamonti L., Menabue L., Vallet-Regi M. Highly-bioreactive silica-based mesoporous bioactive glasses enriched with gallium(III) Materials. 2018;11:367. doi: 10.3390/ma11030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiménez-Holguín J., Sánchez-Salcedo S., Vallet-Regí M., Salinas A. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Microporous Mesoporous Mater. 2020;308:110454. doi: 10.1016/j.micromeso.2020.110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pérez R., Sanchez-Salcedo S., Lozano D., Heras C., Esbrit P., Vallet-Regí M., Salinas A.J. Osteogenic effect of ZnO-mesoporous glasses loaded with osteostatin. Nanomaterials. 2018;8:592. doi: 10.3390/nano8080592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Balasubramanian P., Salinas A.J., Sánchez-Salcedo S., Detsch R., Vallet Regí M., Boccaccini A.R. Induction of VEGF secretion from bone marrow stromal cell line (ST-2) by the dissolution products of mesoporous silica glass particles containing CuO and SrO. J. Non-Cryst. Solids. 2018;500:217–224. doi: 10.1016/j.jnoncrysol.2018.07.073. [DOI] [Google Scholar]
- 95.Philippart A., Gómez-Cerezo N., Arcos D., Salinas A.J., Boccardi E., Vallet-Regi M., Boccaccini A.R. Novel ion-doped mesoporous glasses for bone tissue engineering: study of their structural characteristics influenced by the presence of phosphorous oxide. J. Non-Cryst. Solids. 2017;455:90–97. doi: 10.1016/j.jnoncrysol.2016.10.031. [DOI] [Google Scholar]
- 96.Doadrio A.L., Salinas A.J., Sánchez-Montero J.M., Vallet-Regí M. Drug release from ordered mesoporous silicas. Curr. Pharm. Des. 2015;21:6189–6213. doi: 10.2174/1381612822666151106121419. [DOI] [PubMed] [Google Scholar]
- 97.Berkmann J.C., Herrera Martin A.X., Pontremoli C., Zheng K., Bucher C.H., Ellinghaus A., Boccaccini A.R., Fiorilli S., Vitale Brovarone C., Duda G.N., Schmidt-Bleek K. In vivo validation of spray-dried mesoporous bioactive glass microspheres acting as prolonged local release systems for BMP-2 to induce bone regeneration. Pharmaceutics. 2020;12:823. doi: 10.3390/pharmaceutics12090823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J.-H., Parthiban P., Jin G.-Z., Knowles J.C., Kim H.-W. Materials roles for promoting angiogenesis in tissue regeneration. Prog. Mater. Sci. 2020;117:100732. doi: 10.1016/j.pmatsci.2020.100732. [DOI] [Google Scholar]
- 99.Lozano D., Gil-Albarova J., Heras C., Sánchez-Salcedo S., Gómez-Palacio V.E., Gómez-Blasco A., Doadrio J.C., Vallet-Regí M., Salinas A.J. ZnO-mesoporous glass scaffolds loaded with osteostatin and mesenchymal cells improve bone healing in a rabbit bone defect. J. Mater. Sci.: Mater. Med. 2020;31:1–11. doi: 10.1007/s10856-020-06439-w. [DOI] [PubMed] [Google Scholar]
- 100.Sun P., Zhang Q., Nie W., Zhou X., Chen L., Du H., Yang S., You Z., He J., He C. Biodegradable mesoporous silica nanocarrier bearing angiogenic QK peptide and dexamethasone for accelerating angiogenesis in bone regeneration. ACS Biomater. Sci. Eng. 2019;5:6766–6778. doi: 10.1021/acsbiomaterials.9b01521. [DOI] [PubMed] [Google Scholar]
- 101.Dashnyam K., Jin G.-Z., Kim J.-H., Perez R., Jang J.-H., Kim H.-W. Promoting angiogenesis with mesoporous microcarriers through a synergistic action of delivered silicon ion and VEGF. Biomaterials. 2017;116:145–157. doi: 10.1016/j.biomaterials.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 102.Bithi K.A., Minami H., Hossain M.K., Rahman M.M., Rahman M.A., Gafur M.A., Ahmad H. Cationic polyelectrolyte grafted mesoporous magnetic silica composite particles for targeted drug delivery and thrombolysis. Materialia. 2020;11:100676. doi: 10.1016/j.mtla.2020.100676. [DOI] [Google Scholar]
- 103.Shi M., Zhou Y., Shao J., Chen Z., Song B., Chang J., Wu C., Xiao Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015;21:1785–1789. doi: 10.1016/j.actbio.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 104.Tabia Z., El Mabrouk K., Bricha M., Nouneh K. Mesoporous bioactive glass nanoparticles doped with magnesium: drug delivery and acellular in vitro bioactivity. RSC Adv. 2019;9:12232–12246. doi: 10.1039/C9RA01133A-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farag M.M., Al-Rashidy Z.M., Ahmed M.M. In vitro drug release behavior of Ce-doped nano-bioactive glass carriers under oxidative stress. J. Mater. Sci. Mater. Med. 2019;30:18. doi: 10.1007/s10856-019-6220-3. [DOI] [PubMed] [Google Scholar]
- 106.Pouroutzidou G.K., Liverani L., Theocharidou A., Tsamesidis I., Lazaridou M., Christodoulou E., Beketova A., Pappa C., Triantafyllidis K.S., Anastasiou A.:D., Papadopoulou L., Bikiaris D.N., Boccaccini A.R., Kontonasaki E. Synthesis and characterization of mesoporous Mg- and Sr-doped nanoparticles for moxifloxacin drug delivery in promising tissue engineering applications. Int. J. Mol. Sci. 2021;22:577. doi: 10.3390/ijms22020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saneei Siavashy O., Nabian N., Rabiee S.M. Titanium dioxide nanotubes incorporated bioactive glass nanocomposites: synthesis, characterization, bioactivity evaluation and drug loading. Int. J. Eng. 2021;34:1–9. doi: 10.5829/ije.2021.34.01a.01. [DOI] [Google Scholar]
- 108.Arcos D., Lopez-Noriega A., Ruiz-Hernandez E., Terasaki O., Vallet-Regi M. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009;21:1000–1009. doi: 10.1021/cm801649z. [DOI] [Google Scholar]
- 109.Garg S., Thakur S., Gupta A., Kaur G., Pandey O.P. Antibacterial and anticancerous drug loading kinetics for (10-x)CuO-xZnO-20CaO-60SiO2-10P2O5 (2 </= x </= 8) mesoporous bioactive glasses. J. Mater. Sci. Mater. Med. 2017;28:11. doi: 10.1007/s10856-016-5827-x. [DOI] [PubMed] [Google Scholar]
- 110.Aslankoohi N., Mequanint K. Poly(ester amide)–bioactive glass hybrid biomaterials for bone regeneration and biomolecule delivery. ACS Appl. Biol. Mater. 2020;3:3621–3630. doi: 10.1021/acsabm.0c00257. [DOI] [PubMed] [Google Scholar]
- 111.Althagafi I.I., Ahmed S.A., El-Said W.A. Colorimetric aflatoxins immunoassay by using silica nanoparticles decorated with gold nanoparticles. Spectrochim. Acta A. 2021;246:118999. doi: 10.1016/j.saa.2020.118999. [DOI] [PubMed] [Google Scholar]
- 112.Rahman M.S.U., Tahir M.A., Noreen S., Yasir M., Ahmad I., Khan M.B., Ali K.W., Shoaib M., Bahadur A., Iqbal S. Magnetic mesoporous bioactive glass for synergetic use in bone regeneration, hyperthermia treatment, and controlled drug delivery. RSC Adv. 2020;10:21413–21419. doi: 10.1039/d0ra08656h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tran V.A., Van Giau Vo K.S., Lee S.-W., An S.S.A. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int. J. Nanomed. 2020;15:7667. doi: 10.2147/IJN.S254344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sui B., Liu X., Sun J. Dual-Functional dendritic mesoporous bioactive glass nanospheres for calcium influx-mediated specific tumor suppression and controlled drug delivery in vivo. ACS Appl. Mater. Interfaces. 2018;10:23548–23559. doi: 10.1021/acsami.8b05616. [DOI] [PubMed] [Google Scholar]
- 115.Mosqueira L., Barrioni B.R., Martins T., Ocarino N.M., Serakides R., Pereira M.M. In vitro effects of the co-release of icariin and strontium from bioactive glass submicron spheres on the reduced osteogenic potential of rat osteoporotic bone marrow mesenchymal stem cells. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab9095. [DOI] [PubMed] [Google Scholar]
- 116.Wu C., Fan W., Gelinsky M., Xiao Y., Simon P., Schulze R., Doert T., Luo Y., Cuniberti G. Bioactive SrO–SiO2 glass with well-ordered mesopores: characterization, physiochemistry and biological properties. Acta Biomater. 2011;7:1797–1806. doi: 10.1016/j.actbio.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 117.Lee J.H., Mandakhbayar N., El-Fiqi A., Kim H.W. Intracellular co-delivery of Sr ion and phenamil drug through mesoporous bioglass nanocarriers synergizes BMP signaling and tissue mineralization. Acta Biomater. 2017;60:93–108. doi: 10.1016/j.actbio.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 118.Xin T., Mao J., Liu L., Tang J., Wu L., Yu X., Gu Y., Cui W., Chen L. Programmed sustained release of recombinant human bone morphogenetic protein-2 and inorganic ion composite hydrogel as artificial periosteum. ACS Appl. Mater. Interfaces. 2020;12:6840–6851. doi: 10.1021/acs.chemmater.0c03018. [DOI] [PubMed] [Google Scholar]
- 119.Casarrubios L., Polo-Montalvo A., Serrano M.C., Feito M.J., Vallet-Regí M., Arcos D., Portolés M.T. Effects of ipriflavone-loaded mesoporous nanospheres on the differentiation of endothelial progenitor cells and their modulation by macrophages. Nanomaterials. 2021;11:1102. doi: 10.3390/nano11051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chitra S., Bargavi P., Balasubramaniam M., Chandran R.R., Balakumar S. Impact of copper on in vitro biomineralization, drug release efficacy and antimicrobial properties of bioactive glasses. Mater. Sci. Eng. C. 2020;109:110598. doi: 10.1016/j.msec.2019.110598. [DOI] [PubMed] [Google Scholar]
- 121.Fu S., Du X., Zhu M., Tian Z., Wei D., Zhu Y. 3D printing of layered mesoporous bioactive glass/sodium alginate-sodium alginate scaffolds with controllable dual-drug release behaviors. Biomed. Mater. 2019;14 doi: 10.1088/1748-605X/ab4166. [DOI] [PubMed] [Google Scholar]
- 122.De Cremer K., Braem A., Gerits E., De Brucker K., Vandamme K., Martens J.A., Michiels J., Vleugels J., Cammue B.P., Thevissen K. Controlled release of chlorhexidine from a mesoporous silica-containing macroporous titanium dental implant prevents microbial biofilm formation. Eur. Cell. Mater. 2017;33:13–27. doi: 10.22203/eCM.v033a02. [DOI] [PubMed] [Google Scholar]
- 123.Shruti S., Salinas A.J., Ferrari E., Malavasi G., Lusvardi G., Doadrio A.L., Menabue L., Vallet-Regi M. Curcumin release from cerium, gallium and zinc containing mesoporous bioactive glasses. Microporous Mesoporous Mater. 2013;180:92–101. doi: 10.1016/j.micromeso.2013.06.014. [DOI] [Google Scholar]
- 124.Matter M.T., Probst S., Läuchli S., Herrmann I.K. Uniting drug and delivery: metal oxide hybrid nanotherapeutics for skin wound care. Pharmaceutics. 2020;12:780. doi: 10.3390/pharmaceutics12080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X., Wang X., Hua Z., Shi J. One-pot synthesis of magnetic and mesoporous bioactive glass composites and their sustained drug release property. Acta Mater. 2008;56:3260–3265. doi: 10.1088/1468-6996/14/2/025004. [DOI] [Google Scholar]
- 127.Salètes M., Vartin M., Mocquot C., Chevalier C., Grosgogeat B., Colon P., Attik N. Mesoporous bioactive glasses cytocompatibility assessment: a review of in vitro studies. Biomimetics (Basel) 2021;6:9. doi: 10.3390/biomimetics6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iaquinta M.R., Mazzoni E., Bononi I., Rotondo J.C., Mazziotta C., Montesi M., Sprio S., Tampieri A., Tognon M., Martini F. Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 2019;7:268. doi: 10.3389/fcell.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oryan A., Kamali A., Moshiri A., Baghaban Eslaminejad M. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs. 2017;204:59–83. doi: 10.1159/000469704. [DOI] [PubMed] [Google Scholar]
- 130.Chaparro O., Linero I. Advanced Techniques in Bone Regeneration. In Tech; 2016. Regenerative medicine: a new paradigm in bone regeneration. [DOI] [Google Scholar]
- 131.Battafarano G., Rossi M., De Martino V., Marampon F., Borro L., Secinaro A., Del Fattore A. Strategies for bone regeneration: from graft to tissue engineering. Int. J. Mol. Sci. 2021;22:1128. doi: 10.3390/ijms22031128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anand A., Das P., Nandi S.K., Kundu B. Development of antibiotic loaded mesoporous bioactive glass and its drug release kinetics. Ceram. Int. 2020;46:5477–5483. doi: 10.1016/j.msec.2019.110180. [DOI] [Google Scholar]
- 133.Lalzawmliana V., Anand A., Roy M., Kundu B., Nandi S.K. Mesoporous bioactive glasses for bone healing and biomolecules delivery. Mater. Sci. Eng.: C. 2020;106:110180. doi: 10.1016/j.msec.2019.110180. [DOI] [PubMed] [Google Scholar]
- 134.Nirmala C., Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- 135.Fiorilli S., Pagani M., Boggio E., Gigliotti C.L., Dianzani C., Gauthier R., Pontremoli C., Montalbano G., Dianzani U., Vitale-Brovarone C. Sr-containing mesoporous bioactive glasses bio-functionalized with recombinant ICOS-Fc: an in vitro study. Nanomaterials. 2021;11:321. doi: 10.3390/nano11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Teow S.Y., Liew K., Ali S.A., Khoo A.S., Peh S.C. Antibacterial action of curcumin against Staphylococcus aureus: a brief review. J. Trop. Med. 2016;2016:2853045. doi: 10.1155/2016/2853045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anand P., Sundaram C., Jhurani S., Kunnumakkare A.B., Aggarwal B.B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Canc. Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 138.Doadrio A.L., Sánchez-Montero J.M., Doadrio J.C., Salinas A.J., Vallet-Regí M. A molecular model to explain the controlled release from SBA-15 functionalized with APTES. Micropor. Mesopor. Mater. 2014;195:43–49. doi: 10.1016/j.micromeso.2014.04.019. [DOI] [Google Scholar]