Abstract
Significance: Iron is an essential element required for sustaining a normal healthy life. However, an excess amount of iron in the bloodstream and tissue generates toxic hydroxyl radicals through Fenton reactions. Henceforth, a balance in iron concentration is extremely important to maintain cellular homeostasis in both normal hematopoiesis and erythropoiesis. Iron deficiency or iron overload can impact hematopoiesis and is associated with many hematological diseases.
Recent Advances: The mechanisms of action of key iron regulators such as erythroferrone and the discovery of new drugs, such as ACE-536/luspatercept, are of potential interest to treat hematological disorders, such as β-thalassemia. New therapies targeting inflammation-induced ineffective erythropoiesis are also in progress. Furthermore, emerging evidences support differential interactions between iron and its cellular antioxidant responses of hematopoietic and neighboring stromal cells. Both iron and its systemic regulator, such as hepcidin, play a significant role in regulating erythropoiesis.
Critical Issues: Significant pre-clinical studies are on the way and new drugs targeting iron metabolism have been recently approved or are undergoing clinical trials to treat pathological conditions with impaired erythropoiesis such as myelodysplastic syndromes or β-thalassemia.
Future Directions: Future studies should explore how iron regulates hematopoiesis in both benign and malignant conditions. Antioxid. Redox Signal. 35, 415–432.
Keywords: iron, hematopoiesis, erythropoiesis, oxidative stress
Introduction
The unique properties of iron are essential to many physiological phenomena responsible for sustaining life. Iron can readily accept or donate an electron to participate in oxidation–reduction reactions, such as those that occur in cellular respiration, nucleic acid synthesis, metabolic reactions, and oxygen transport. Iron is also crucial for mitochondrial biogenesis, heme synthesis, and the formation of iron–sulfur clusters, which are essential electron-transfer proteins (98, 119). However, the same properties that give iron its versatility in orchestrating a wide array of diverse physiological processes present a danger. In the human body, iron uptake and its concentration are stringently regulated as there are no mechanisms for excreting iron from the body once absorbed. Therefore, the balance between iron uptake, transport, utilization, and storage must be regulated in a very precise and orderly manner.
Excess iron is toxic and can form free radicals leading to oxidative stress, DNA, and tissue damage. Failure to properly regulate systemic iron levels can lead to impairment of many biological processes, giving rise to a wide range of pathological conditions, including anemia and iron overload-related disorders. Iron accumulation has been shown in genetic disorders and hematological and neurodegenerative diseases (61). In this review, we discuss the different regulatory pathways involved in iron metabolism as it relates to hematopoiesis. We have also emphasized the perturbations of iron homeostasis in hematological disorders such as hemochromatosis, β-thalassemia, and anemia of inflammation (AI). Finally, we summarize novel therapeutic interventions that can restore iron homeostasis in patients suffering from iron deficiency- or iron overload-related hematological disorders.
Systemic Iron Homeostasis
Uptake, export, and recycling of iron
Iron enters the body from the diet. Approximately 2 mg of iron are absorbed by enterocytes every day after being reduced from ferric (Fe3+) to ferrous state (Fe2+) by duodenal cytochrome B-reductase also known as duodenal cytochrome b (113). Divalent metal transporter 1, an iron importer with 12 transmembrane domains localized at the apical membrane of enterocytes, transports Fe2+ into the intracellular space (66). The excess iron is stored in ferritin, the universal storehouse of iron. Ferritin is a highly conserved globular protein consisting of 24 subunits and forms a central cavity where iron is stored (20). During iron deficiency, iron is released from ferritin. A selective nuclear receptor coactivator 4 (NCOA4) mediating the release of iron was recently identified (15). The process by which NCOA4 releases iron from ferritin is known as ferritinophagy. Ncoa4 knockout (KO) mice fed an iron-rich diet died prematurely due to iron overload and exhibited signs of severe liver damage. In contrast, Ncoa4-KO mice receiving a low-iron diet developed microcytic hypochromic anemia due to inefficient mobilizing of iron from ferritin stores (15).
Ferrous iron (Fe2+) is highly reactive and toxic and is therefore oxidized back to the ferric state (Fe3+) by the ferroxidase hephaestin (169). It enters circulation through ferroportin (FPN), the only known exporter of elemental iron in the cell (43, 124, 128). Fe3+ iron in the bloodstream is then transported by the carrier protein, transferrin (Tf). The majority of this iron is used in the synthesis of heme and iron–sulfur clusters in the mitochondria. Tf binds to its receptors, transferrin receptor 1 (TFR1) and receptor 2 (TFR2). Tfr1−/− mice are embryonically lethal at E12.5 due to severe anemia (100). However, mice lacking TFR1 only in hepatocytes are viable although showing relatively high levels of hepcidin (when normalized to liver iron content), modest hypoferremia, and microcytosis. This indicates that TFR1 is redundant for basal hepatocellular iron supply but essential for fine-tuning hepcidin responses, most likely through its interaction with HFE (47, 149).
TFR1 is also known to be highly expressed in erythroid cells, brain, skeletal muscle cells, gut cells, and cardiomyocytes, whereas TFR2 is known to be only expressed in the brain, kidney, liver, colon, and testes (13, 36, 175). Recent studies have shown a novel iron-sensing function of TFR2 in erythropoiesis (123). TFR2 binds to and stabilizes the erythropoietin (EPO) receptor (EPOR) in erythroid precursors (50). Studies have also identified another molecular pathway involving the scaffold protein scribble that links the iron-sensing function of TFR2 to EPOR expression at the cell membrane of erythroid precursors (91). Under low iron conditions, unbound TFR2 traffics to lysosomes and TFR2-scribble complexes are catabolized. As a consequence, the levels of scribble decrease and EPOR is not efficiently stabilized for surface presentation. Mouse chimeras with TFR2-deficient hematopoietic cells have erythrocytosis, increased EPO sensitivity, and reduced apoptosis of late erythroblasts (123).
Tf is a bilobed glycoprotein that binds one iron ion on each lobe. Tf and iron can associate in four distinct ways: (i) no iron bound (apo-Tf), (ii) iron bound on the lobe closest to the amino-terminal (Tf-N), (iii) iron bound on the lobe closest to the carboxyl-terminal (Tf-C) (both are mono-Tf), or (iv) iron bound to both lobes (holo-Tf). The two lobes have distinct affinities for iron (1, 71). Tf can be taken up by the cell via clathrin-mediated endocytosis after binding to TFR1 or TFR2. However, holo-Tf is thought to be the primary supplier of iron to differentiating erythroid cells. It has been also indicated that holo-Tf stabilizes TFR2 (86, 170). A new study shows that the different lobes of Tf mediate distinct signaling events within cells (129). In this study, two mouse models of mono-Tf were utilized. One model harbored a knockin mutation that inhibited iron binding on the N-terminal (Tf-N) lobe, while the other model had an analogous mutation, blocking iron binding to the C-terminal (Tf-C) lobe. It was observed that iron entering the cell via the Tf-N or Tf-C lobes affects EPO sensitivity distinctly.
In both models, animals were mildly anemic; however, the Tf-N mutants were unresponsive to exogenous EPO and had higher baseline levels of EPO in circulation. In contrast, Tf-C mutants had normal EPO levels and responded to exogenous EPO with greater sensitivity. Treatment of Tf-C mice with exogenous EPO produced more red blood cells (RBCs) compared with EPO-treated wild-type mice. To understand the molecular underpinnings of this finding, expression of protein kinase B/Akt was assessed, since this pathway is implicated in EPO responsiveness. Tf-N mutant mice had lower pAKT levels, suggesting that EPO responsiveness can be modulated by Tf forms. It is yet to be determined if these different sensitivities to EPO arise from Tf interactions with TFR1 or TFR2, as TFR2 is also found on early progenitors and has been implicated in EPOR stabilization at the membrane (50). Interestingly, both models exhibit a hemochromatosis phenotype despite having normal serum iron levels. The Tf-N mutant appeared mostly affected, showing higher liver iron loading and lower serum hepcidin compared with the C lobe mutant. This work highlights the importance of functionally distinct mono-Tf forms.
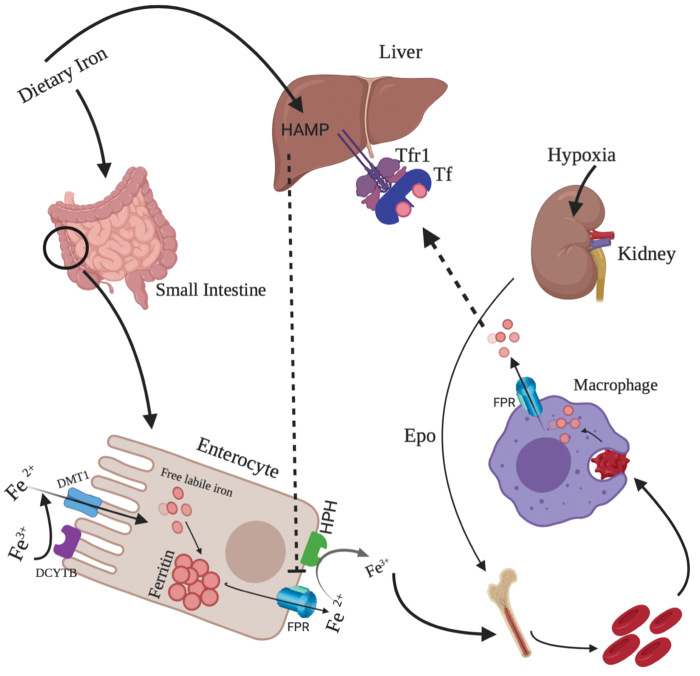
An illustration depicting iron intake, export, and recycling is shown in Figure 1.
FIG. 1.
Uptake, export, and recycling of iron. Dietary iron after being absorbed by intestinal cells is stored and then exported to the blood stream. Majority of the iron is utilized in the synthesis of RBC production under the stimulation of erythropoietin (a kidney hormone). Dead and senescent RBCs are phagocytosed by macrophages, resulting in the production of iron, which is again utilized for the heme production. RBC, red blood cell. Color images are available online.
In other models, under conditions of iron overload where the Tf saturation is high, excess free iron in the form of nontransferrin bound iron (NTBI) is present. NTBI is highly oxidative and leads to the formation of reactive hydroxyl radicals by Fenton reactions, resulting in oxidative stress (104). Cellular importers of NTBI during pathological conditions include zinc transporter proteins such as ZIP14 in hepatocytes and pancreatic acinar cells (78). This transporter together with ZIP8 has been identified as transmembrane proteins likely to regulate cellular uptake of divalent metal ions such as zinc, iron, manganese, and cadmium (79). Studies have shown that deficiency of Zip14 in mouse models can attenuate the conditions of iron overload in the liver and pancreas (78).
Regulation of iron homeostasis
One of the most important regulators of iron homeostasis is hepcidin, a peptide synthesized by the liver that degrades FPN (43, 124, 128). The pathways that regulate the hepcidin gene (HAMP) are activated when iron rises to levels outside of homeostatic range, and are suppressed when iron concentrations fall (59, 61). Mutations in hepcidin and FPN are associated with hereditary hemochromatosis type 2b and type 4, respectively (125, 133, 145) (discussed in the section “Steady-State Erythropoiesis”). Higher levels of hepcidin are implicated in several conditions, including AI (127). Significant players regulating hepcidin include the hemochromatosis-associated protein (HFE), hemojuvelin (HJV), bone morphogenetic proteins (BMPs), matriptase-2 (TMPRSS6), erythroferrone (ERFE), and interleukin 6 (IL-6). HJV is a coreceptor of BMPs (9, 10, 173).
The protein expression of HJV is detected in many tissues such as the liver, brain, heart, and skeletal muscle (144). Mutations in HJV lead to a severe iron-overload condition known as hemochromatosis type 2a (93, 99). Under high iron conditions, BMP2 and BMP6 in the liver initiate heterodimerization between BMP type I (ALK2/ALK3) and type II receptors, BMPRII/Act RIIA. This interaction results in the activation of the SMAD1/5/8 complex and SMAD4, which increases hepcidin expression (9). Liver sinusoidal endothelial cells are the primary source of BMP6 (28). Lack of BMP6 in mouse models leads to iron overload (3, 116). Furthermore, interaction of HFE with TFR2 stimulates hepcidin production (54).
The other important negative modulator of hepcidin is matriptase-2, also known as transmembrane II protease serine 6 (TMPRSS6). TMPRSS6 cleaves HJV and decreases hepcidin expression (154). In contrast, TMPRSS6 deficiency leads to increased hepcidin expression and, as a consequence, to iron refractory iron-deficiency anemia (48). However, in mice affected by β-thalassemia, TMPRSS6 deficiency improves iron overload and ineffective erythropoiesis (157). Another significant regulator of hepcidin is ERFE. It is secreted by erythroid progenitor cells under EPO stimulation and downregulates hepcidin in the liver (85). ERFE is also highly expressed in β-thalassemic mice. Mechanistically, it was proposed that the N-terminus of ERFE acts as a ligand trap for BMP6 and presumably downregulates hepcidin by sequestering BMP6 (5). A more recent study has shown that ERFE lowers hepcidin by sequestering a BMP2 and BMP6 heterodimer and prevents their binding to BMP type 1 ALK3 receptor (171). A receptor that binds ERFE has not yet been found.
In addition to the modulators described, hepcidin is also regulated by proinflammatory cytokines, namely IL-6, during inflammatory conditions such as autoimmune disorders, chronic infections, and cancer, characterized by AI. IL-6 stimulates hepcidin via the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway and limits plasma iron availability thereby promoting hypoferremia (52, 53). Additional studies have shown a regulatory role of BMP signaling in mediating IL6-JAK2-STAT5-mediated hepcidin induction (29). Furthermore, studies from our laboratory have shown a role of both IL6 and hepcidin in AI (57). We are currently investigating the plausible reasons for how Il6−/− mice exhibit a better recovery in erythropoietic profile in the bone marrow (BM) from stress erythropoiesis when infected by a pathogen known as Brucella abortus (a mouse model of AI) (article in preparation by Sinha et al.).
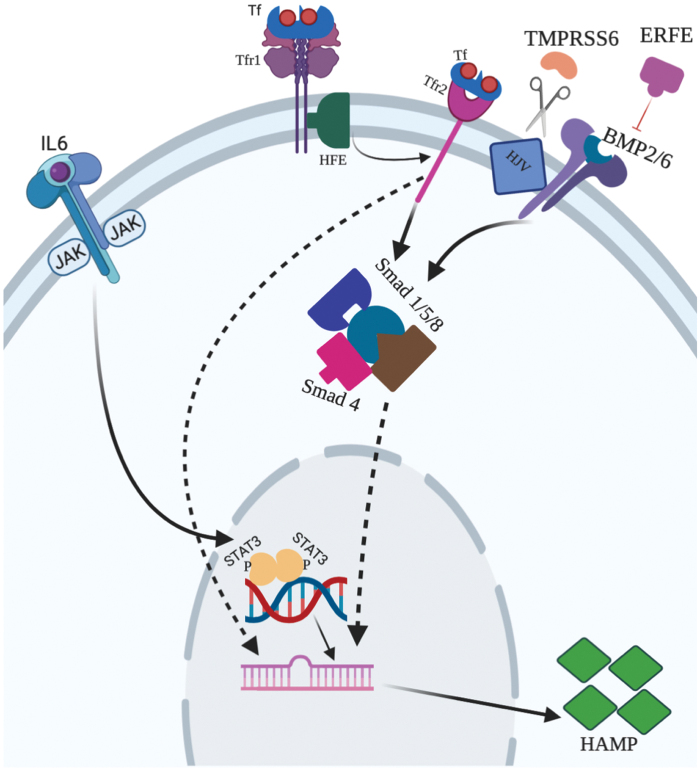
The different regulatory pathways mediating iron homeostasis are shown in Figure 2.
FIG. 2.
Different regulatory pathways in the liver. Hepcidin, the master regulatory protein that regulates iron homeostasis, is modulated by different signals. Hepcidin is regulated by TFR1, TFR2, IL-6, and BMP signaling pathways. The negative regulators of this protein are TMPRSS6 and ERFE. TMPRSS6 is known to cleave HJV, whereas ERFE is known to act as a trap ligand of BMP. BMP, bone morphogenetic protein; ERFE, erythroferrone; HJV, hemojuvelin; IL-6, interleukin 6; TFR1, transferrin receptor 1; TFR2, transferrin receptor 2; TMPRSS6, matriptase-2. Color images are available online.
Iron in Hematopoiesis
Iron and hematopoietic stem cells
Under physiological conditions, mitochondrial respiration represents the main source of reactive oxygen species (ROS), along with the actions of different enzymes such as NADPH oxidases. In iron-overload conditions, an increase in labile cellular iron leads to the generation of free radicals, culminating in cell damage or death, with consequent tissue damage. This typically occurs when Tf saturation exceeds 60%–70%, leading to the presence of NTBI in serum. NTBI ultimately leads to oxidative stress.
Recent studies suggest that hematopoietic stem cells (HSCs) residing in the BM are susceptible to iron overload, due to the generation of ROS that have a harmful impact on HSCs and their niche (Fig. 3). It has been proposed that patients with iron overload and impaired hematopoietic function may benefit from iron chelation (106). Tanaka et al. reported that in vitro iron treatment leads to ROS generation and ROS-mediated injury of both HSCs and differentiated hematopoietic cells (162). Remarkably, HFE hemochromatosis patients do not display a significant impairment in hematopoiesis despite the continuous iron accumulation throughout their lifetime (136). This is probably due to HSC protection within the BM in vivo, which is not replicated in an artificial in vitro coculture system with stromal cell lines.
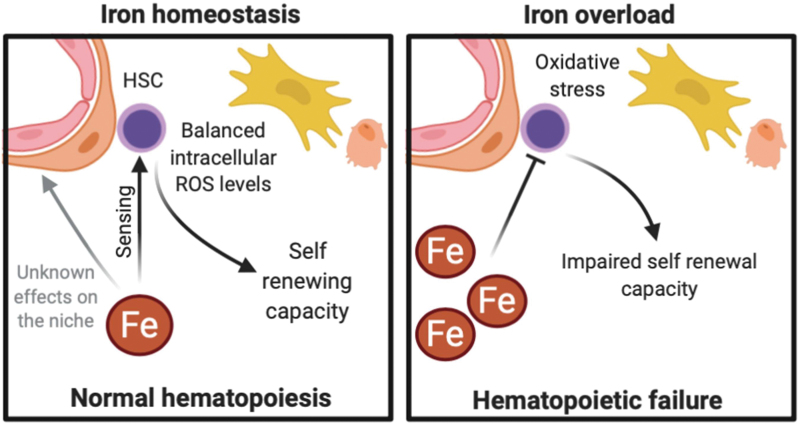
FIG. 3.
The effect of iron on HSCs and their niche. While balanced intracellular oxidative stress levels are important in maintaining HSC function, iron overload increases ROS levels in HSCs and impairs their self-renewal. Iron is also thought to damage the HSC niche and impair HSC transplantation but their effect on the microenvironment is poorly studied. HSC, hematopoietic stem cell; ROS, reactive oxygen species. Color images are available online.
Nevertheless, several in vivo studies using different mouse models suggest that the regulation of cellular iron is important for hematopoiesis. Mice injected with iron dextran have increased production of cellular ROS and impaired hematopoiesis (34). The F-box and leucine-rich repeat protein 5 (FBXL5) regulates cellular iron levels through the ubiquitination of iron regulatory protein 2 (IRP2). It was shown that Fbxl5-deficient HSCs have cellular iron overload that results in reduced cell numbers, impaired self-renewal, and stem cell exhaustion. Interestingly, HSCs from myelodysplastic syndrome (MDS) patients have reduced FBXL5 expression (122). The feline leukemia virus, subgroup C, receptor 1 (FLVCR1) is the only known heme exporter. It was shown that hematopoietic Flvcr1-deficient mice have blocked erythroid maturation and anemia, suggesting that erythroid progenitors require heme iron export for survival and differentiation (81, 87). Interestingly, a study demonstrated direct toxicity of iron and ROS on HSCs of mice with RUNX1-S291fs-induced MDS and iron overload (81). It should be noted, however, that extremely low levels of ROS can also hamper HSC function, causing defects in their differentiation and repopulation capacity (82). Altogether, it appears that finely tuned levels of iron are required to maintain HSC and hematopoietic homeostasis (180).
The impact of iron on hematopoiesis is also clinically demonstrated in patients undergoing hematopoietic stem cell transplantation (HSCT) who often develop iron overload as a consequence of frequent RBC transfusions. Postmortem studies of patients dying shortly after HSCT revealed levels of hepatic and BM iron comparable with the levels observed in patients with HFE hemochromatosis (158). Iron overload in HSCT may cause heart and pancreas damage and promote microbial infection (69, 95). It may also directly affect the BM niche and impair hematopoietic reconstitution, as suggested by the negative prognostic impact of pretransplantation elevated serum ferritin levels in HSCT outcomes (7).
Iron and the HSC niche
The BM microenvironment or niche comprises many different cell types and factors but are broadly characterized by perivascular and endosteal sites where self-renewing HSCs reside (132). Iron and ROS levels are important for both HSC and niche functions (107). Low ROS levels are key for maintenance of HSC quiescence, but increasing ROS are necessary for HSC differentiation (180). The interplay between ROS and the niche is well illustrated by the study of Itkin et al. (73). The authors demonstrated that HSCs localizing next to less permeable arterioles were immotile and in a quiescent state, while HSCs located close to more permeable sinusoids were migratory and differentiated (73). The importance of local iron differences within the BM and the role of iron exported by recycling macrophages in the regulation of ROS levels in HSCs remain to be explored. In the case of excessive oxidative stress, stem cell function is compromised as discussed and HSCs are biased toward a myeloid differentiation (75, 77). Ultimately, excessive ROS levels may lead to HSC exhaustion and apoptosis (74).
The cell-extrinsic role of iron overload and ROS in the BM niche is, however, poorly explored. Evidence from MDS studies suggests that iron overload may inhibit osteoblasts and increase osteoclasts' numbers and activity (24, 132). Using an acute myeloid leukemia (AML) mouse model, we have previously observed that the iron-chelator deferoxamine (DFO) protects the bone-lining endosteal vasculature and HSCs from leukemia-induced damage (44). This observation suggests that increased local iron may play a role in the remodeling of the niche in AML. Future studies should explore how iron affects HSC niche function.
NRF2 and hematopoiesis
The transcription factor nuclear factor (erythroid-derived 2)-like 2 (NFE2l2 or NRF2) is the master regulator of the cellular antioxidant response (92, 109) and regulates the expression of detoxifying enzymes. NRF2 activity is controlled by the Kelch-like-ECH-associated protein 1 (KEAP1) that forms a complex with the Cullin-3-based E3 ubiquitin ligase and targets NRF2 for degradation through the ubiquitin/proteasome pathway. Upon activation, NRF2 protects cells from the consequences of oxidative stress, such as DNA damage and apoptosis (89, 139, 165). The NRF2-mediated cellular response to iron, including its cytoprotective role, has previously been demonstrated by our group. NRF2 regulates the response of hepatocytes against acute iron toxicity (153), prevents the development of liver fibrosis in iron-overloaded Hfe-KO mice (45), and regulates endothelial BMP6 secretion in response to iron overload (103).
The role of the NRF2-iron interaction in cell intrinsic and extrinsic (i.e., niche) regulation of HSCs, however, remains to be explored. Nevertheless, several studies reveal the antioxidant transcription factor NRF2 as a significant player in hematopoiesis and in endothelial cells. Kim et al. showed that Nrf2 loss impairs the ability of hematopoietic stem and progenitor cell (HSPC) to repopulate, after BM transplantation (92). Nrf2−/− HSPC are functionally compromised, possibly due to impairment of the antioxidant defenses (92, 166). Furthermore, Nrf2−/− mice are more susceptible to radiation-induced cell damage after lethal irradiation (92). Keap1-KO mice, characterized by constitutive activation of Nrf2, have unaffected numbers of overall long-term HSCs (121). However, persistent activation of Nrf2 promotes cell cycle entry and further differentiation of these cells, leading to cell exhaustion and impairment of both HSC quiescent state and self-renewal capacity (121).
These results further demonstrate the importance of NRF2 regulation in maintaining HSCs. By having an impact on the differentiation of HSPCs, NRF2 activation enhances the granulocyte/monocyte lineage, suppressing erythroid differentiation (120). Studies conducted using Nrf2−/− mice showed that Nrf2-deficient HSCs are more susceptible to oxidative stress and that their function could not be retrieved with N-acetyl cysteine, suggesting that increased ROS alone are not responsible for defective HSC function in Nrf2-KO mice (114). On the contrary, Nrf2 activation increases HSPC function and mitigates irradiation-induced BM damage (92, 166). NRF2 has also been described as a key player in some hematological diseases, such as sickle cell disease (SCD). In a recent study, Keleku-Lukwete et al. used Keap1-KO mice to show that activation of Nrf2 results in a milder SCD phenotype, possibly due to downregulation of heme-driven cell activation (88).
Steady-State Erythropoiesis
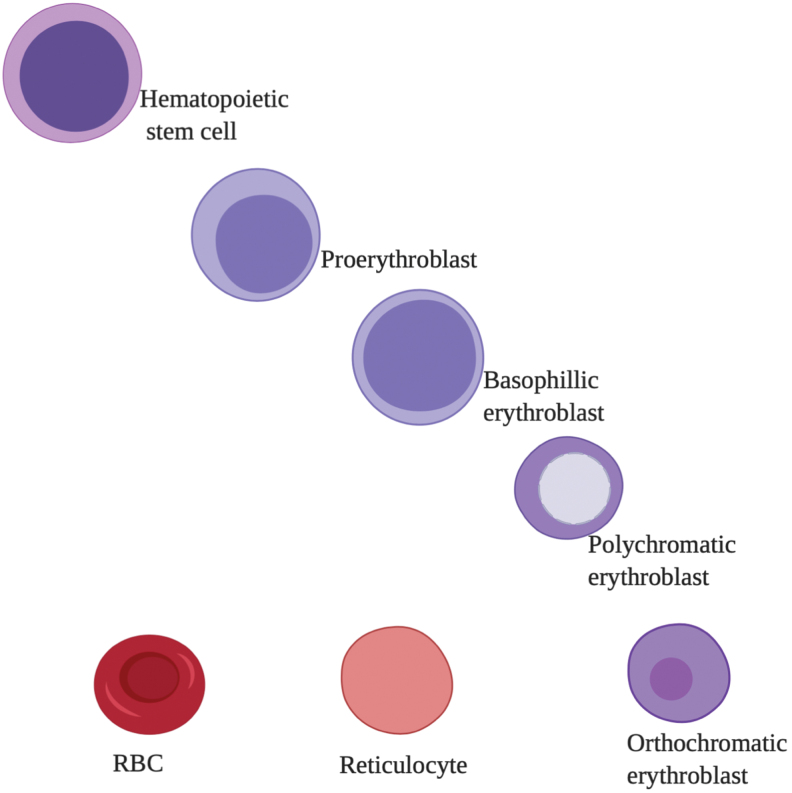
Erythropoiesis is a highly regulated process, ensuring a constant supply of RBCs and is the largest consumer of iron in the body. Humans produce 1 million new RBCs per second throughout their life. RBCs arise from HSCs residing in the BM. HSCs differentiate into burst-forming units, which differentiate into colony forming units (CFUs) that trigger subsequent stages of differentiation (63). Formation of proerythroblasts marks the initiation of the third stage. The proerythroblast (or pronormoblast) divides to sequentially form the basophilic normoblast, polychromatic normoblast, orthochromatic normoblast, and the reticulocyte followed by enucleation and membrane remodeling (58). The later stages of differentiation take place in the circulation where reticulocytes undergo further maturation and are ultimately converted to mature RBCs (62). These steps are illustrated in Figure 4.
FIG. 4.
Different stages of erythropoiesis. Erythropoiesis starts with the formation of hematopoietic stem cells and differentiates into proerythroblasts, which differentiate into basophilic, polychromatic, and orthochromatic followed by enucleation, resulting in the formation of reticulocytes and ultimately the RBCs. Color images are available online.
There are special niches in the BM known as erythroblastic islands that facilitate the enucleation and maturation of erythroblasts. Typically, an erythroblastic island comprises a central macrophage surrounded by erythroblasts (16, 35). A recent study has revealed expression of EPOR in central macrophages, further implicating their role in supporting erythropoiesis (102). Apart from supporting normal erythropoiesis, macrophages also play a role in regulating erythroid differentiation and proliferation in pathological conditions. Interestingly, preclinical studies have shown that treatment with clodronate, a first-generation bisphosphonate that has also been used to treat osteoporosis, can deplete macrophages and reverse the diseased phenotype of β-thalassemia and polycythemia vera (PV) (138). Moreover, macrophages also play a predominant role in recycling iron by scavenging senescent erythrocytes and hence support iron homeostasis (51).
Master regulators of erythropoiesis
The kidney is an efficient oxygen sensor, and under conditions of hypoxia, it produces EPO (25, 76). Binding of EPO to its cognate hypoxic-inducible receptor EPOR initiates an intercellular signaling cascade, culminating in the onset of the first phase of erythropoiesis. The EPO–EPOR interaction activates Janus kinase 2 (JAK2), a cytoplasmic tyrosine kinase and its downstream substrates, STAT5 and STAT3, phosphoinositide 3-kinases (PI3K), and mitogen-activated protein kinase (MAPK), that promote the differentiation and proliferation of erythroid precursors (25, 141). Another erythroid transcription factor that regulates the expression of EPOR is GATA1 (64, 68). Gata1-KO mice die at E10.5–11.5 due to severe anemia. The conditional erythroid KO of this gene shows symptoms of aplastic anemia.
Apart from regulating heme synthesis, GATA1 also promotes early-stage proliferation and maturation by promoting the activation of survival genes such as BCL2 and BCL-xL. GATA1 is highly expressed at the CFU stage and is known to induce EPOR gene expression and its downstream partners MAPK/PI3K, which in turn phosphorylate GATA1 (68). Matured erythroid cells developing in an EPO-independent environment activate Fas death receptors in immature erythroid precursors, leading to caspase 3-mediated cleavage of GATA1 and apoptosis (68). In contrast, in early stages of erythropoiesis during EPO-dependent conditions, GATA1 is protected from caspase-mediated cleavage by the chaperone protein HSP70 to promote early erythroid maturation and differentiation. Apart from regulating steady-state erythropoiesis, GATA1 is also shown to be downregulated in several hematological disorders, such as β-thalassemia, MDS, and Diamond–Blackfan anemia. Inflammatory cytokines have been implicated in downregulating GATA1 expression, resulting in ineffective erythropoiesis (68).
Iron and Platelets
Clinically, iron deficiency is often accompanied by anemia as previously discussed. However, an intriguing common observation is the increased number of platelets, or thrombocytosis in patients with iron deficiency anemia. It has been suggested that megakaryocytes are stimulated by EPO, but the mechanism remained elusive until recently. Xavier-Ferrucio et al. elegantly demonstrated that iron deficiency directly affects megakaryocytic erythroid progenitors (MEPs). The authors showed that low microenvironment iron content reduces ERK signaling and cycling of bipotent MEPs, modifies their metabolism, and biases their commitment toward megakaryocytes (172).
Iron overload and ROS were also shown to affect platelet function (40), as illustrated by hemochromatosis patients who present low thrombin-induced platelet aggregation (135). MDS patients with iron overload also have platelet dysfunction and an increased risk of hemorrhage. Increased iron levels have been correlated with the inhibition of γ-thrombin-induced platelet aggregation, through direct-binding effects (108). Popov et al. (135) described a correlation between ROS levels, leukopenia, and the degree of anemia. Moreover, ROS production led to a decreased platelet aggregation function, and therefore, to impaired platelet function as observed in patients with MDS (135). Furthermore, iron chelation therapy in MDS patients was able to recover platelet numbers in 78% of treated patients (80).
The recent discovery that platelets express proteins involved in iron metabolism, namely HFE and TFR2, suggested that platelets sense Tf saturation and play a role in iron metabolism (11, 69). Interestingly, Barale et al. (11) described that patients with iron overload have reduced response to aggregation stimuli and decreased surface expression of activation markers. Moreover, through ex vivo evaluation of platelet activation, they suggest that Tf saturation is inversely correlated with platelet reactivity (11).
Diseases That Arise from Dysregulated Erythropoiesis and Hematopoiesis and Novel Therapeutics for Restoring Iron Metabolism
HFE hemochromatosis
Hemochromatosis is characterized by dysregulation of systemic iron homeostasis. It can result from mutations in HFE (type 1) (OMIM #235200) (46) or mutations in other genes such as HAMP (type 2b) and HJV (type 2a) (OMIM #602390), FPN (SLC40A1) (type 4) and TFR2 (type 3) (OMIM #604250) (21) (26, 49, 93, 99, 136). Hemochromatosis is characterized by an excess of plasma NTBI, or labile iron (22). This excess iron leads to tissue toxicity and maximum iron retention in the liver, heart, and macrophages resulting in severe diseases such as hepatic carcinoma, cirrhosis, arrhythmia, cardiac failure, and diabetes (21). Increase in cellular iron stores can also hamper mitochondrial function (168). Current treatments include phlebotomy and the use of iron chelators such as deferasirox and desferrioxamine to manage iron levels (83, 130, 133). However, both treatments can result in attenuation of hepcidin, thereby increasing the rate of iron uptake. Moreover, iron chelators have other side effects and can impair auditory, renal, and ocular functions (94).
To mitigate these risks, several preclinical studies are currently testing the use of small interfering antisense oligonucleotides or siRNAs to target TMPRSS6 activity (2, 30). Casu et al. showed that the use of compounds targeting TMPRSS6 led to a reduction in hepatic iron content and an improvement of the iron-overload phenotype in a mouse model of β-thalassemia (30). Recent attention has also focused on the use of polyphenols as a dietary supplement, which can inhibit iron absorption by forming insoluble complexes with ferrous iron and hence are able to mitigate iron overload (https://clinicaltrials.gov/ct2/show/NCT03990181).
Furthermore, other preclinical discoveries such as minihepcidins/PR65 are shown to be effective in hepcidin KO models (137). Recent studies have also shown that thiazolidinones mitigate iron overload in hemochromatosis models by inducing hepcidin expression through activation of SMAD1/5/8 signaling (105). Targeting of the NTBI transporter ZIP14 in hemochromatosis mouse models was also shown to reduce iron overload in the liver and pancreas (78).
β-Thalassemia
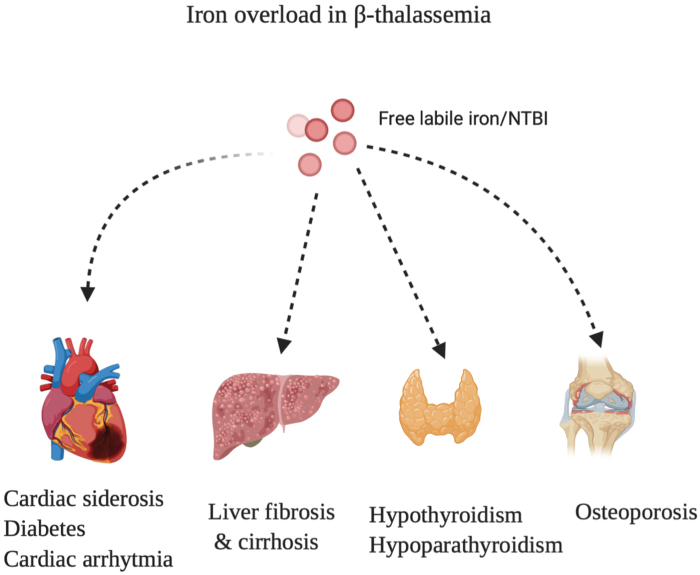
β-Thalassemia is an autosomal recessive disorder that results from a mutation in the β globin gene of the hemoglobin molecule (142, 143). Consequently, there is increased production of α chains leading to an imbalance between the proportion of α and β chains. This leads to the formation of hemichromes, which damage RBC membranes and shorten their life span (142, 161). There are two types of thalassemia: (i) thalassemia major (TM) also known as transfusion-dependent thalassemia and (ii) thalassemia intermedia (TI) also known as nontransfusion-dependent thalassemia (161). TM patients require blood transfusions and iron chelation therapies (126). On the contrary, patients with TI do not require regular transfusions at an early stage, but develop anemia at a later stage and eventually require transfusions. A common manifestation of the disease is an increased rate of iron absorption that results in iron overload and thereby leads to the overproduction of ROS and oxidative stress. This causes cardiac siderosis, liver cirrhosis, osteoporosis, and skeletal deformities, as illustrated in Figure 5.
FIG. 5.
Organs affected by iron overload. An illustration showing the different diseases that are caused due to iron overload in the liver, heart, bone, and thyroid glands. Color images are available online.
In β-thalassemia, excessive generation of ROS leads to apoptosis of a subset of polychromatophilic erythroid cells (112), thereby leading to ineffective erythropoiesis. On the contrary, an excess of α globins also binds to HSP70. HSP70 is known to protect GATA1, the major erythroid transcription factor. As a result, GATA1 is more prone to cleavage by caspase-3, which hampers erythroid maturation and differentiation and leads to inefficient erythropoiesis. Other studies have also shown that HSP70 sequestration can lead to ineffective erythropoiesis in β-thalassemia (6).
Current treatments of β-thalassemia include blood transfusions, hydroxyurea, iron chelators (DFO and deferasirox), HSCT, and splenectomy. Unfortunately, these treatments are often associated with severe adverse effects (94). To specifically target ineffective erythropoiesis, recent studies explored ACE-536, also known as Reblozyl/luspatercept-aamt, in thalassemia. ACE-536/luspatercept is a fusion protein with a modified extracellular domain of activin receptor IIB (ACVR2B) that competes with ACVR2B to bind members of the transforming growth factor (TGF) β superfamily (146) and is now a Food and Drug Administration-approved treatment for β-thalassemia (https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-reblozyl). Originally, luspatercept was thought to function as a trap ligand that binds growth differentiation factor 11 (GDF11), a member of the TGF-β family (159). It was therefore originally considered that luspatercept prevented the negative effect of TGF-β superfamily signaling in erythropoiesis.
However, in a recent study using genetic mouse models, Guerra et al. showed that animals do not show improved recovery from erythropoiesis by conditionally deleting Gdf11 in a mouse model of thalassemia (65). Furthermore, the authors challenged Gdf11-deficient mice with the murine analogue of ACE-536 (RAP-536) and observed an improvement in hematological parameters of thalassemic mice (65). Altogether, this supported the notion that the GDF11 pathway is not underlying the benefit of ACE-536 in β-thalassemia. This work has been further supported by evidence that GDF11 does not play a significant role in regulating hematopoiesis (60). Moreover, recent studies propose that ACE-536 restores GATA1 expression in erythroid precursors and transcription intermediary factor 1γ (TIFγ) in the erythroblasts of β-thalassemic mice (111).
Other promising therapies are also being explored in β-thalassemia. Tf therapy was shown to be effective in preclinical studies, as the mice showed improved erythropoiesis and anemia (101). Additional studies have shown that the JAK2 inhibitor ruxolitinib can reverse splenomegaly in β-thalassemic mouse models and a Phase II clinical trial has been initiated based on these results (32). Although β-thalassemic patients receiving ruxolitinib showed a reduction of spleen size, overall, there were no changes observed in serum iron, ferritin levels, or pretransfusion hemoglobin levels (160). Due to these reasons, a Phase III trial with ruxolitinib was not initiated. Preclinical studies also explored the use of the FPN inhibitor VIT-2763 (140). VIT-2763 has been shown to ameliorate oxidative stress, improve erythropoiesis by reducing the aggregation of α chains hence mitigating iron overload in thalassemic mice (110). Iron chelators combined with amlodipine, an L-type calcium channel blocker, were also used in a recent randomized clinical trial in pediatric patients (90).
Minihepcidins were also tested in preclinical studies to reduce iron overload in β-thalassemic mouse models (31, 65). Based on these observations, two Phase II clinical trials were started to test LJPC-401 and PTG-300. Other modulators of hepcidin have been also shown to be effective in preclinical studies in treating β-thalassemia. Using antisense nucleotides and lipid nanoparticle siRNAs targeting TMPRSS6, researchers were able to mitigate iron overload and improve ineffective erythropoiesis in β-thalassemic mouse models (30, 56, 67, 150). Moreover, several new additional compounds targeting TMPRSS6 have been identified (14). A clinical trial will be also starting soon based on these results. Recent clinical trials are shown in Table 1.
Table 1.
Interventions and Undergoing Clinical Trials for β-Thalassemia
| Intervention | Stage |
|---|---|
| Reblozyl (luspatercept-aamt) | FDA approved |
| VIT 2763 (ferroportin inhibitor) | Phase I |
| LJPC-401 (minihepcidin) | Phase II—NCT03381833 |
| PTG-300 | Phase II—NCT03802201 |
| IONIS TMPRSS6-LRx/TMPRR6 inhibitors | Phase II—NCT04059406 |
FDA, Food and Drug Administration.
Lastly, studies have also shown that monoclonal antibodies targeting ERFE are efficient in reducing serum iron levels and decreasing splenomegaly in thalassemic mouse models. ERFE has been known to reduce hepcidin expression upon stimulation by EPO and this therapeutic intervention will be extremely beneficial in treating iron-overload disorders (4). The different treatment approaches are illustrated in Figure 6.
FIG. 6.
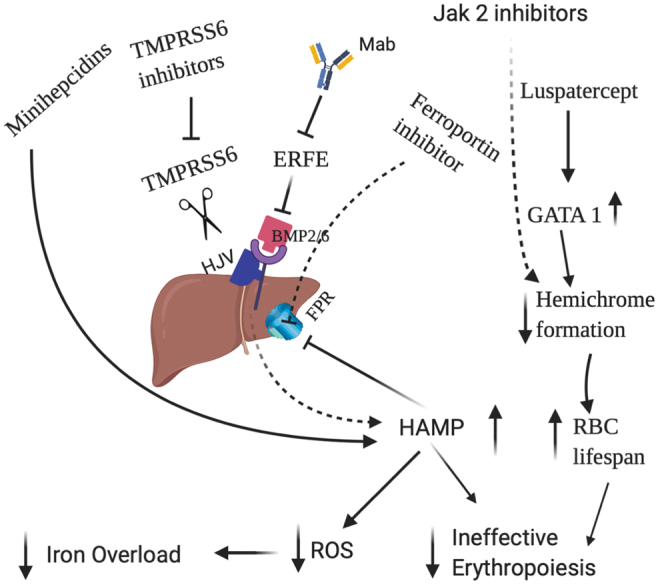
Different therapies that are known to mitigate the iron overload and ineffective erythropoiesis in β-thalassemia. Iron restriction is perturbed by different hepcidin agonists, inhibitor of ferroportin, the use of TMPRSS6 inhibitors and monoclonal antibodies targeting ERFE, whereas improvement of erythropoiesis is modulated by luspatercept and JAK2 inhibitors by increasing the differentiation of erythroid progenitors and decreasing the formation of hemichromes. JAK2, Janus kinase 2. Color images are available online.
Anemia of inflammation
In several pathological conditions such as aging, hematologic and solid malignancies, autoimmune disorders, and chronic infections, normal erythropoiesis is severely compromised and myelopoiesis and lymphopoiesis are stimulated due to host-defense mechanisms (52). This condition is known as AI or anemia of chronic disease, which is characterized by an inflammatory state and chronically elevated cytokine levels. In the immediate innate phase, cytokines including IL-6 and tumor necrosis factor upregulate the transcription factor PU.1 and downregulate GATA1, thereby inhibiting the proliferation of erythroid precursors, which results in anemia. Administration of iron is not beneficial as it contributes to the generation of ROS and oxidative stress, as shown in patients with chronic kidney disease (39). In addition, inflammation also blunts the BM responsiveness to EPO (118).
IL-6 is known to be one of the predominant cytokines causing AI. It stimulates hepcidin, the master regulator of iron homeostasis, thereby leading to hypoferremia and limiting microbial proliferation. Consistently, patients with anemia of chronic disease have increased serum IL-6 and hepcidin levels (177). The hepcidin-induced decrease in iron bioavailability is a major contributor to anemia. Symptoms include dyspnea, fatigue, exercise intolerance, and headache. Patients with underlying health conditions such as diabetes and rheumatoid arthritis are more often anemic and symptomatic. Current therapies in AI include erythropoietic stimulating agents, RBC transfusions, and intravenous iron injections. As discussed, administration of iron may be detrimental in this setting, particularly in patients who are more susceptible to oxidative stress such as patients with cardiovascular disease. Iron may also increase the rate of infections. Several compounds under investigation for the treatment of AI are listed in Table 2.
Table 2.
Anemia of Inflammation Interventions, Targets, and Clinical Trials
| Intervention | Target | Status |
|---|---|---|
| Tocilizumab (Mab) | IL6-receptor inhibitor | Phase II (72) |
| Etanercept, adalimumab anf Infliximab | TNF-alpha receptor antagonists | Phase II (38) |
| Heparin | Hepcidin antagonist | Preclinical (134) |
| Hemojuvelin-Fc, LDN193189 | BMP antagonist | Preclinical (164) |
| Spiegelmer lexaptepid pegol | Hepcidin inhibitor | Phase 1 (19) |
| PRS-080-PEG30 | Hepcidin antagonist | Preclinical (70) |
| LY2928057/Mab | Targeting ferroportin | Phase 1 (152) |
| LY3113593/Mab | Targeting BMP6 | Phase 1 (152) |
| LY2787106/Mab | Targeting HAMP | Preclinical (147) |
| Phase 1 (167) | ||
| Monoclonal antibody | Targeting hemojuvelin | Preclinical (97) |
| Momelotinib | ACVR1/Jak inhibitor | Preclinical (8) |
| Phase III (115) | ||
| FG-4592 | Prolyl hydroxylase inhibitor | Preclinical (12) |
| Phase II (37) |
BMP, bone morphogenetic protein; IL-6, interleukin 6; TNF, tumor necrosis factor.
Polycythemia vera
PV is a myeloproliferative neoplasm that is mainly due to JAK2 V617F activating mutations. The common symptoms are fatigue, pruritus, night sweats, bone pain, and weight loss. Overactivation of the JAK2 signaling pathway leads to constitutive activation of the downstream pathways, leading to uncontrolled proliferation and hematopoietic output. Ongoing preclinical and clinical studies include the use of pegylated interferon alfa-2a (156), use of the JAK2 inhibitor ruxolitinib, pegylated interferon alfa-2a (Phase II) (155, 176), and of the HDAC inhibitor or givinostat (Phase II Clinical trial: NCT03287245) and minihepcidins (31) (https://clinicaltrials.gov/ct2/show/NCT04057040).
MDS and leukemia
MDS is a heterogeneous group of clonal hematological diseases characterized by impaired hematopoietic differentiation and cytopenias, including anemia, with different degrees of severity (84, 131). The risk of MDS patients to progress to secondary AML can be stratified based on hematological and karyotypic features. Anemia is a major problem in MDS patients and the traditional approach relies on repeated RBC transfusions. Iron overload is a common side effect of RBC transfusions (151). Interestingly, patients with MDS with ring sideroblast have increased systemic iron concentration even before receiving RBC transfusions. These patients are characterized by ineffective erythropoiesis and mutations in the splicing gene SF3B1. A recent study elegantly demonstrated that SF3B1-mutated erythroblasts express higher levels of ERFE, which in turn suppresses hepcidin and leads to iron accumulation (17).
Iron overload in MDS is associated with comorbidities and increased mortality (151). Oxidative stress is linked to atherosclerosis, and iron overload is a risk factor for cardiovascular disease. Transfusion-dependent MDS patients are twice as susceptible to infection-related complications (41). Indeed, increased ferritin levels promote the proliferation, survival, and evolution of fungi, viruses, and bacteria (96, 117). In addition, cellular iron amount and phagocytic activity are inversely correlated (23). Monocytes have impaired activity against bacteria, and CD4+ T lymphocytes have decreased proliferation and activity upon iron overload (148, 170). Transfusion-dependent MDS patients are also more likely to transform to AML, according to retrospective studies. (33). Altogether, these observations support the use of iron chelators in low- or intermediate-low-risk MDS patients, which is also thought to protect nonmalignant hematopoiesis from iron overload and reduce cytopenias (163).
Ferroptosis and malignant hematopoiesis
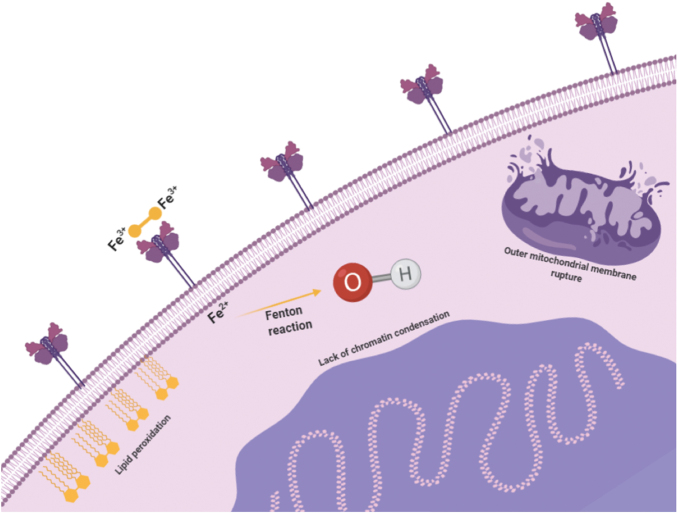
In 2012, Dixon et al. described ferroptosis, a new form of cell death induced by erastin that is morphologically, biochemically, and genetically different from apoptosis, necrosis, necroptosis, and autophagy (42) (Fig. 7). Ferroptosis is characterized by lipid peroxidation and may result from toxic accumulation of iron, leading to an increase in lipid ROS levels. Although other enzymes may also play a role, ROS are fundamental in ferroptosis, as indicated by the absence of ferroptosis induction under anoxic conditions (42). Ferroptosis has been associated with several conditions, including ischemic heart disease, kidney injury, and cancer, in which it has been shown to promote tumoral growth through inactivation of p53 activity (55, 174).
FIG. 7.
The ferroptotic process. Increased ROS caused by iron toxicity trigger the onset of ferroptosis, leading to the activation of MAPKs and NAPDH oxidation. Several morphological features distinguish ferroptosis from other types of cell death, including mitochondrial membrane rupture, the lack of blebbing of the plasma membrane, and absence of chromatin condensation. MAPK, mitogen-activated protein kinase. Color images are available online.
At a morphological level, ferroptosis is characterized by the rupture of the outer mitochondria membrane, lack of nuclear chromatin condensation, and absence of plasma membrane blebbing. Aside from the previously mentioned iron and ROS accumulation, ferroptosis is also characterized by the activation of MAPKs, glutathione (GSH) depletion, and increased NAPDH oxidation, also creating a proinflammatory niche through the release of damage-associated molecular patterns (174). Furthermore, the importance of iron metabolism in ferroptosis is highlighted by the sensitization of erastin-induced cell death after silencing of FBXL5, which ubiquitinates and negatively controls IREB2/IRP2, a master regulator of several iron transport and storage genes that recognizes iron-responsive elements (42, 179). Even though excessive iron can mediate ferroptosis through the generation of ROS, other sources can increase the intracellular oxidative stress with the same outcome, such as NADPH-dependent lipid peroxidation and GSH depletion.
Yang et al. evaluated the effect of erastin-triggered ferroptosis in several suspension cell lines, including diffuse large B cell lymphoma (DLBCL), AML, and multiple myeloma (MM) cells. DLBCL cell lines were more susceptible to ferroptotic cell death, which could be rescued by antioxidants, such as vitamin E (178). Susceptibility to iron toxicity is being tested as a potential adjuvant therapy for MM. MM is a malignant clonal condition characterized by the uncontrolled proliferation of plasma cells (PCs) in the BM, accompanied by high levels of circulating immunoglobulins and organ damage (27). PCs contain high levels of hydrogen peroxide thus making them constitutively vulnerable to iron toxicity. Bordini et al. observed that in the Vk*MYC mouse model of MM, the combination of iron with bortezomib resulted in a synergistic effect and over 80% of the treated mice had partial or complete response and no disease progression (18).
Campanella et al. described similar results, demonstrating that the concomitant treatment with bortezomib and iron resulted in accelerated cell death also through apoptosis (27). In another study (181), tested the susceptibility of AML cells to chemotherapy (cytarabine and doxorubicin) in combination with erastin. They found that erastin was able to enhance the efficacy of the treatment, through the induction of ferroptosis (181). Altogether, ferroptosis inducers may be useful as adjuvant therapy in different hematological malignancies.
Conclusions
Iron homeostasis is maintained by a complex systemic regulation of iron intake, absorption, and recycling. Dysregulation in these pathways may result in iron deficiency or overload. Experimental and observational evidence demonstrates the importance of iron in the regulation of normal and malignant hematopoiesis. Many studies have explored the key role of iron in erythropoiesis. Several promising new therapies have emerged from these studies and are currently in use or under clinical trials for conditions such as SCD, AI, or PV. Future studies addressing the active (and not just the bystander) role of iron in HSC maintenance, niche homeostasis, and in hematological malignancies such as AML and MDS are welcomed.
Acknowledgments
We acknowledge support from the Commonwealth Universal Research Enhancement (C.U.R.E.) Program Pennsylvania Department of Health and National Institute of Diabetes and Digestive and Kidney Diseases Institute of the National Institutes of Health (R01 DK090554, R01 DK095112) to S.R. We also thank the support from the EHA Research Grant award granted by the European Hematology Association to D.D.
Abbreviations Used
- ACVR2B
activin receptor IIB
- AI
anemia of inflammation
- AML
acute myeloid leukemia
- BM
bone marrow
- BMP
bone morphogenetic protein
- CFU
colony forming unit
- DFO
deferoxamine
- DLBCL
diffuse large B cell lymphoma
- E
embryonic day
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- ERFE
erythroferrone
- FBXL5
F-box and leucine-rich repeat protein 5
- FDA
Food and Drug Administration
- FLVCR1
feline leukemia virus, subgroup C, receptor 1
- FPN
ferroportin
- GDF11
growth differentiation factor 11
- HAMP
hepcidin gene
- HFE
hemochromatosis-associated protein
- HJV
hemojuvelin
- HSC
hematopoietic stem cell
- HSCT
hematopoietic stem cell transplantation
- IL-6
interleukin 6
- IRP2
iron regulatory protein 2
- JAK2
Janus kinase 2
- KEAP1
Kelch-like-ECH-associated protein 1
- LCI
labile cellular iron
- MAPK
mitogen-activated protein kinase
- MDS
myelodysplastic syndrome
- MEP
megakaryocytic erythroid progenitors
- MM
multiple myeloma
- NCOA4
nuclear receptor coactivator 4
- Nfe2l2 or Nrf2
nuclear factor (erythroid-derived 2)-like 2
- NTBI
nontransferrin bound iron
- PC
plasma cell
- PI3K
phosphoinositide 3-kinases
- PV
polycythemia vera
- RBC
red blood cell
- ROS
reactive oxygen species
- SCD
sickle cell disease
- STAT
signal transducer and activator of transcription
- Tf
transferrin
- TFR1
transferrin receptor 1
- TFR2
transferrin receptor 2
- TGF
transforming growth factor
- TI
thalassemia intermedia
- TIFγ
transcription intermediary factor 1γ
- TM
thalassemia major
- TMPRSS6
matriptase-2
- TNF
tumor necrosis factor
Author Disclosure Statement
S.R. is a member of the scientific advisory board of Ionis Pharmaceuticals, MeiraGTx, and Disc Medicine and owns stock options from Disc Medicine and MeiraGTx. S.R. has been or is a consultant for Cambridge Healthcare Research, Celgene Corporation, First Manhattan Co., FORMA Therapeutics, Incyte Corp, Ghost Tree Capital, Keros Therapeutics, Inc., Noble insight, Protagonist Therapeutics, Sanofi Aventis U.S., Inc., Slingshot Insight, Techspert.io and venBio Select LLC, and Disc Medicine.
Funding Information
Funding for this project was provided in part by an EHA Research Award granted by the European Hematology Association. D.D. also receives funding from the Portuguese Society of Hematology, Bolsa D. Manuel de Mello from Fundação Amélia de Mello and the National Blood Foundation.
References
- 1. Aisen P, Leibman A, and Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 253: 1930–1937, 1978 [PubMed] [Google Scholar]
- 2. Altamura S, Schaeper U, Dames S, Loffler K, Eisermann M, Frauendorf C, Mudder K, Neves J, and Muckenthaler MU. SLN124, a GalNAc-siRNA conjugate targeting TMPRSS6, efficiently prevents iron overload in hereditary haemochromatosis type 1. Hemasphere 3: e301, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andriopoulos B Jr., Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, and Babitt JL.. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41: 482–487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arezes J, Foy N, McHugh K, Quinkert D, Benard S, Sawant A, Frost JN, Armitage AE, Pasricha SR, Lim PJ, Tam MS, Lavallie E, Pittman DD, Cunningham O, Lambert M, Murphy JE, Draper SJ, Jasuja R, and Drakesmith H. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood 135: 547–557, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, Brinth A, Tam M, LaVallie ER, Taylor S, Armitage AE, Pasricha SR, Cunningham O, Lambert M, Draper SJ, Jasuja R, and Drakesmith H. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood 132: 1473–1477, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arlet JB, Ribeil JA, Guillem F, Negre O, Hazoume A, Marcion G, Beuzard Y, Dussiot M, Moura IC, Demarest S, de Beauchene IC, Belaid-Choucair Z, Sevin M, Maciel TT, Auclair C, Leboulch P, Chretien S, Tchertanov L, Baudin-Creuza V, Seigneuric R, Fontenay M, Garrido C, Hermine O, and Courtois G. HSP70 sequestration by free alpha-globin promotes ineffective erythropoiesis in beta-thalassaemia. Nature 514: 242–246, 2014 [DOI] [PubMed] [Google Scholar]
- 7. Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, Soiffer RJ, and Antin JH. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 109: 4586–4588, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, Posch W, Nairz M, Maciejewski P, Fowles P, Burns CJ, Smith G, Wagner KU, Weiss G, Whitney JA, and Theurl I. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood 129: 1823–1830, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, and Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38: 531–539, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, and Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest 117: 1933–1939, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barale C, Senkeev R, Napoli F, De Gobbi M, Guerrasio A, Morotti A, and Russo I. Transferrin saturation inversely correlates with platelet function. Thromb Haemost 119: 766–778, 2019 [DOI] [PubMed] [Google Scholar]
- 12. Barrett TD, Palomino HL, Brondstetter TI, Kanelakis KC, Wu X, Yan W, Merton KP, Schoetens F, Ma JY, Skaptason J, Gao J, Tran DT, Venkatesan H, Rosen MD, Shankley NP, and Rabinowitz MH. Prolyl hydroxylase inhibition corrects functional iron deficiency and inflammation-induced anaemia in rats. Br J Pharmacol 172: 4078–4088, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrientos T, Laothamatas I, Koves TR, Soderblom EJ, Bryan M, Moseley MA, Muoio DM, and Andrews NC. Metabolic catastrophe in mice lacking transferrin receptor in muscle. EBioMedicine 2: 1705–1717, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beliveau F, Tarkar A, Dion SP, Desilets A, Ghinet MG, Boudreault PL, St-Georges C, Marsault E, Paone D, Collins J, Macphee CH, Campobasso N, Groy A, Cottom J, Ouellette M, Pope AJ, and Leduc R. Discovery and development of TMPRSS6 inhibitors modulating hepcidin levels in human hepatocytes. Cell Chem Biol 26: 1559–1572 e9, 2019 [DOI] [PubMed] [Google Scholar]
- 15. Bellelli R, Federico G, Matte A, Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L, and Carlomagno F. NCOA4 deficiency impairs systemic iron homeostasis. Cell Rep 14: 411–421, 2016 [DOI] [PubMed] [Google Scholar]
- 16. Bessis M. [Erythroblastic island, functional unity of bone marrow]. Rev Hematol 13: 8–11, 1958. (Article in French) [PubMed] [Google Scholar]
- 17. Bondu S, Alary AS, Lefevre C, Houy A, Jung G, Lefebvre T, Rombaut D, Boussaid I, Bousta A, Guillonneau F, Perrier P, Alsafadi S, Wassef M, Margueron R, Rousseau A, Droin N, Cagnard N, Kaltenbach S, Winter S, Kubasch AS, Bouscary D, Santini V, Toma A, Hunault M, Stamatoullas A, Gyan E, Cluzeau T, Platzbecker U, Ades L, Puy H, Stern MH, Karim Z, Mayeux P, Nemeth E, Park S, Ganz T, Kautz L, Kosmider O, and Fontenay M. A variant erythroferrone disrupts iron homeostasis in SF3B1-mutated myelodysplastic syndrome. Sci Transl Med 11: eaav5467, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bordini J, Galvan S, Ponzoni M, Bertilaccio MTS, Chesi M, Bergsagel PL, Camaschella C, and Campanella A. Induction of iron excess restricts malignant plasma cells expansion and potentiates bortezomib effect in models of multiple myeloma. Leukemia 31: 967–970, 2017 [DOI] [PubMed] [Google Scholar]
- 19. Boyce M, Warrington S, Cortezi B, Zollner S, Vauleon S, Swinkels DW, Summo L, Schwoebel F, and Riecke K. Safety, pharmacokinetics and pharmacodynamics of the anti-hepcidin Spiegelmer lexaptepid pegol in healthy subjects. Br J Pharmacol 173: 1580–1588, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bradley JM, Le Brun NE, and Moore GR. Ferritins: furnishing proteins with iron. J Biol Inorg Chem 21: 13–28, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, and Loreal O. Haemochromatosis. Nat Rev Dis Primers 4: 18016, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brissot P, Ropert M, Le Lan C, and Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820: 403–410, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Bullen JJ, Rogers HJ, Spalding PB, and Ward CG. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 43: 325–330, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Bulycheva E, Rauner M, Medyouf H, Theurl I, Bornhauser M, Hofbauer LC, and Platzbecker U. Myelodysplasia is in the niche: novel concepts and emerging therapies. Leukemia 29: 259–268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med 3: a011619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camaschella C, Roetto A, Cali A, De Gobbi M, Garozzo G, Carella M, Majorano N, Totaro A, and Gasparini P. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet 25: 14–15, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Campanella A, Santambrogio P, Fontana F, Frenquelli M, Cenci S, Marcatti M, Sitia R, Tonon G, and Camaschella C. Iron increases the susceptibility of multiple myeloma cells to bortezomib. Haematologica 98: 971–979, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canali S, Zumbrennen-Bullough KB, Core AB, Wang CY, Nairz M, Bouley R, Swirski FK, and Babitt JL. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 129: 405–414, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, and Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 87: 471–480, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Casu C, Aghajan M, Oikonomidou PR, Guo S, Monia BP, and Rivella S. Combination of Tmprss6-ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica 101: e8–e11, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casu C, Oikonomidou PR, Chen H, Nandi V, Ginzburg Y, Prasad P, Fleming RE, Shah YM, Valore EV, Nemeth E, Ganz T, MacDonald B, and Rivella S. Minihepcidin peptides as disease modifiers in mice affected by beta-thalassemia and polycythemia vera. Blood 128: 265–276, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casu C, Presti VL, Oikonomidou PR, Melchiori L, Abdulmalik O, Ramos P, and Rivella S. Short-term administration of JAK2 inhibitors reduces splenomegaly in mouse models of beta-thalassemia intermedia and major. Haematologica 103: e46–e49, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cazzola M, Della Porta MG, and Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program 166–175, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Chai X, Li D, Cao X, Zhang Y, Mu J, Lu W, Xiao X, Li C, Meng J, Chen J, Li Q, Wang J, Meng A, and Zhao M. ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci Rep 5: 10181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chasis JA and Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood 112: 470–478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen AC, Donovan A, Ned-Sykes R, and Andrews NC. Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis. Proc Natl Acad Sci U S A 112: 11714–11719, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, and Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant 32: 1373–1386, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corrado A, Di Bello V, d'Onofrio F, Maruotti N, and Cantatore FP. Anti-TNF-alpha effects on anemia in rheumatoid and psoriatic arthritis. Int J Immunopathol Pharmacol 30: 302–307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, and Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol 34: 975–991, 2019 [DOI] [PubMed] [Google Scholar]
- 40. Dahi AA, Hanafy E, and Al Pakra M. Iron overload and platelet function defects: possible correlation. J Investig Med High Impact Case Rep 4: 2324709616675645, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dayyani F, Conley AP, Strom SS, Stevenson W, Cortes JE, Borthakur G, Faderl S, O'Brien S, Pierce S, Kantarjian H, and Garcia-Manero G. Cause of death in patients with lower-risk myelodysplastic syndrome. Cancer 116: 2174–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, and Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drakesmith H, Nemeth E, and Ganz T. Ironing out ferroportin. Cell Metab 22: 777–787, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duarte D, Hawkins ED, Akinduro O, Ang H, De Filippo K, Kong IY, Haltalli M, Ruivo N, Straszkowski L, Vervoort SJ, McLean C, Weber TS, Khorshed R, Pirillo C, Wei A, Ramasamy SK, Kusumbe AP, Duffy K, Adams RH, Purton LE, Carlin LM, and Lo Celso C. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell 22: 64–77.e6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duarte TL, Caldas C, Santos AG, Silva-Gomes S, Santos-Goncalves A, Martins MJ, Porto G, and Lopes JM. Genetic disruption of NRF2 promotes the development of necroinflammation and liver fibrosis in a mouse model of HFE-hereditary hemochromatosis. Redox Biol 11: 157–169, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R Jr., Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, and Wolff RK.. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13: 399–408, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Fillebeen C, Charlebois E, Wagner J, Katsarou A, Mui J, Vali H, Garcia-Santos D, Ponka P, Presley J, and Pantopoulos K. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood 133: 344–355, 2019 [DOI] [PubMed] [Google Scholar]
- 48. Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, Mayo MM, Samuel SM, Strouse JJ, Markianos K, Andrews NC, and Fleming MD. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet 40: 569–571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, and Sly WS. Targeted mutagenesis of the murine transferrin receptor-2 gene produces hemochromatosis. Proc Natl Acad Sci U S A 99: 10653–10658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forejtnikova H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, Gaudry M, Camaschella C, Lacombe C, Roetto A, Mayeux P, and Verdier F. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood 116: 5357–5367, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Ganz T. Macrophages and iron metabolism. Microbiol Spectr 4 [Epub ahead of print]; DOI: 10.1128/microbiolspec.MCHD-0037-2016, 2016 [DOI] [PubMed] [Google Scholar]
- 52. Ganz T. Anemia of inflammation. N Engl J Med 381: 1148–1157, 2019 [DOI] [PubMed] [Google Scholar]
- 53. Ganz T and Nemethe E.. Iron sequestration and anemia of inflammation. Semin Hematol 46: 387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, and Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab 9: 217–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, and Jiang X. Role of mitochondria in ferroptosis. Mol Cell 73: 354–363.e3, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gardenghi S, Ramos P, Marongiu MF, Melchiori L, Breda L, Guy E, Muirhead K, Rao N, Roy CN, Andrews NC, Nemeth E, Follenzi A, An X, Mohandas N, Ginzburg Y, Rachmilewitz EA, Giardina PJ, Grady RW, and Rivella S. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in beta-thalassemic mice. J Clin Invest 120: 4466–4477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gardenghi S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM, Greenberg-Kushnir N, Sasu BJ, Cooke KS, and Rivella S. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 123: 1137–1145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, and Bitensky MW. A detailed study of time-dependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br J Haematol 135: 395–404, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Girelli D, Nemeth E, and Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood 127: 2809–2813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goldstein JM, Sengul H, Messemer KA, Fernandez-Alfara M, Garbern JC, Kristl AC, Lee RT, and Wagers AJ. Steady-state and regenerative hematopoiesis occurs normally in mice in the absence of GDF11. Blood 134: 1712–1716, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gozzelino R and Arosio P. Iron homeostasis in health and disease. Int J Mol Sci 17: 130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Granick S and Levere RD. Heme synthesis in erythroid cells. Prog Hematol 4: 1–47, 1964 [PubMed] [Google Scholar]
- 63. Gregory CJ and Eaves AC. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood 51: 527–537, 1978 [PubMed] [Google Scholar]
- 64. Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, and Weiss MJ. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood 94: 87–96, 1999 [PubMed] [Google Scholar]
- 65. Guerra A, Oikonomidou PR, Sinha S, Zhang J, Lo Presti V, Hamilton CR, Breda L, Casu C, La P, Martins AC, Sendamarai AK, Fleming M, and Rivella S. Lack of Gdf11 does not improve anemia or prevent the activity of RAP-536 in a mouse model of beta-thalassemia. Blood 134: 568–572, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, and Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388: 482–488, 1997 [DOI] [PubMed] [Google Scholar]
- 67. Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, Peralta R, Watt A, Freier S, Monia BP, and Rivella S. Reducing TMPRSS6 ameliorates hemochromatosis and beta-thalassemia in mice. J Clin Invest 123: 1531–1541, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gutierrez L, Caballero N, Fernandez-Calleja L, Karkoulia E, and Strouboulis J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life 72: 89–105, 2020 [DOI] [PubMed] [Google Scholar]
- 69. Hannuksela J, Parkkila S, Waheed A, Britton RS, Fleming RE, Bacon BR, and Sly WS. Human platelets express hemochromatosis protein (HFE) and transferrin receptor 2. Eur J Haematol 70: 201–206, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Hohlbaum AM, Gille H, Trentmann S, Kolodziejczyk M, Rattenstetter B, Laarakkers CM, Katzmann G, Christian HJ, Andersen N, Allersdorfer A, Olwill SA, Meibohm B, Audoly LP, Swinkels DW, and van Swelm RPL. Sustained plasma hepcidin suppression and iron elevation by Anticalin-derived hepcidin antagonist in cynomolgus monkey. Br J Pharmacol 175: 1054–1065, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huebers HA, Csiba E, Huebers E, and Finch CA. Competitive advantage of diferric transferrin in delivering iron to reticulocytes. Proc Natl Acad Sci U S A 80: 300–304, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Isaacs JD, Harari O, Kobold U, Lee JS, and Bernasconi C. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther 15: R204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I, Poulos MG, Kalinkovich A, Ludin A, Kollet O, Shakhar G, Butler JM, Rafii S, Adams RH, Scadden DT, Lin CP, and Lapidot T. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532: 323–328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, and Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 75. Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, and Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Jacobson LO, Goldwasser E, Fried W, and Plzak L. Role of the kidney in erythropoiesis. Nature 179: 633–634, 1957 [DOI] [PubMed] [Google Scholar]
- 77. Jang YY and Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jenkitkasemwong S, Wang CY, Coffey R, Zhang W, Chan A, Biel T, Kim JS, Hojyo S, Fukada T, and Knutson MD. SLC39A14 is required for the development of hepatocellular iron overload in murine models of hereditary hemochromatosis. Cell Metab 22: 138–150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jenkitkasemwong S, Wang CY, Mackenzie B, and Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25: 643–655, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jensen PD, Heickendorff L, Pedersen B, Bendix-Hansen K, Jensen FT, Christensen T, Boesen AM, and Ellegaard J. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol 94: 288–299, 1996 [DOI] [PubMed] [Google Scholar]
- 81. Jin X, He X, Cao X, Xu P, Xing Y, Sui S, Wang L, Meng J, Lu W, Cui R, Ni H, and Zhao M. Iron overload impairs normal hematopoietic stem and progenitor cells through reactive oxygen species and shortens survival in myelodysplastic syndrome mice. Haematologica 103: 1627–1634, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, and Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115: 4030–4038, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kanwar P and Kowdley KV. Diagnosis and treatment of hereditary hemochromatosis: an update. Expert Rev Gastroenterol Hepatol 7: 517–530, 2013 [DOI] [PubMed] [Google Scholar]
- 84. Kaphan E, Laurin D, Lafeuillade B, Drillat P, and Park S. Impact of transfusion on survival in patients with myelodysplastic syndromes: current knowledge, new insights and transfusion clinical practice. Blood Rev 41: 100649, 2019 [DOI] [PubMed] [Google Scholar]
- 85. Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, and Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 46: 678–684, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kawabata H. Transferrin and transferrin receptors update. Free Radic Biol Med 133: 46–54, 2019 [DOI] [PubMed] [Google Scholar]
- 87. Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, and Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 319: 825–828, 2008 [DOI] [PubMed] [Google Scholar]
- 88. Keleku-Lukwete N, Suzuki M, Panda H, Otsuki A, Katsuoka F, Saito R, Saigusa D, Uruno A, and Yamamoto M. Nrf2 activation in myeloid cells and endothelial cells differentially mitigates sickle cell disease pathology in mice. Blood Adv 3: 1285–1297, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kensler TW, Wakabayashi N, and Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007 [DOI] [PubMed] [Google Scholar]
- 90. Khaled A, Salem HA, Ezzat DA, Seif HM, and Rabee H. A randomized controlled trial evaluating the effects of amlodipine on myocardial iron deposition in pediatric patients with thalassemia major. Drug Des Devel Ther 13: 2427–2436, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Khalil S, Delehanty L, Grado S, Holy M, White Z, 3rd, Freeman K, Kurita R, Nakamura Y, Bullock G, and Goldfarb A. Iron modulation of erythropoiesis is associated with Scribble-mediated control of the erythropoietin receptor. J Exp Med 215: 661–679, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T, Malhotra SV, and Biswal S. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest 124: 730–741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kong X, Xie L, Zhu H, Song L, Xing X, Yang W, and Chen X. Genotypic and phenotypic spectra of hemojuvelin mutations in primary hemochromatosis patients: a systematic review. Orphanet J Rare Dis 14: 171, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kontoghiorghes GJ. Deferasirox: uncertain future following renal failure fatalities, agranulocytosis and other toxicities. Expert Opin Drug Saf 6: 235–239, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Kontoghiorghes GJ. How to manage iron toxicity in post-allogeneic hematopoietic stem cell transplantation? Expert Rev Hematol 13: 299–302, 2020 [DOI] [PubMed] [Google Scholar]
- 96. Kontoyiannis DP, Chamilos G, Lewis RE, Giralt S, Cortes J, Raad, II, Manning JT, and Han X. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer 110: 1303–1306, 2007 [DOI] [PubMed] [Google Scholar]
- 97. Kovac S, Boser P, Cui Y, Ferring-Appel D, Casarrubea D, Huang L, Fung E, Popp A, Mueller BK, and Hentze MW. Anti-hemojuvelin antibody corrects anemia caused by inappropriately high hepcidin levels. Haematologica 101: e173–e176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. La P, Oved JH, Ghiaccio V, and Rivella S. Mitochondria biogenesis modulates iron-sulfur cluster synthesis to increase cellular iron uptake. DNA Cell Biol 39: 756–765, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lanzara C, Roetto A, Daraio F, Rivard S, Ficarella R, Simard H, Cox TM, Cazzola M, Piperno A, Gimenez-Roqueplo AP, Grammatico P, Volinia S, Gasparini P, and Camaschella C. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood 103: 4317–4321, 2004 [DOI] [PubMed] [Google Scholar]
- 100. Levy JE, Jin O, Fujiwara Y, Kuo F, and Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet 21: 396–399, 1999 [DOI] [PubMed] [Google Scholar]
- 101. Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, Cabantchik ZI, Bouhassira EE, Fabry ME, and Ginzburg YZ. Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med 16: 177–182, 2010 [DOI] [PubMed] [Google Scholar]
- 102. Li W, Wang Y, Zhao H, Zhang H, Xu Y, Wang S, Guo X, Huang Y, Zhang S, Han Y, Wu X, Rice CM, Huang G, Gallagher PG, Mendelson A, Yazdanbakhsh K, Liu J, Chen L, and An X. Identification and transcriptome analysis of erythroblastic island macrophages. Blood 134: 480–491, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lim PJ, Duarte TL, Arezes J, Garcia-Santos D, Hamdi A, Pasricha SR, Armitage AE, Mehta H, Wideman S, Santos AG, Santos-Goncalves A, Morovat A, Hughes JR, Soilleux E, Wang CY, Bayer AL, Klenerman P, Willberg CB, Hartley RC, Murphy MP, Babitt JL, Ponka P, Porto G, and Drakesmith H. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat Metab 1: 519–531, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liochev SI and Fridovich I. The Haber-Weiss cycle—70 years later: an alternative view. Redox Rep 7: 55–57; author reply 59–60, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Liu J, Liu W, Liu Y, Miao Y, Guo Y, Song H, Wang F, Zhou H, Ganz T, Yan B, and Liu S. New thiazolidinones reduce iron overload in mouse models of hereditary hemochromatosis and beta-thalassemia. Haematologica 104: 1768–1781, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu W, Zhao M, Rajbhandary S, Xie F, Chai X, Mu J, Meng J, Liu Y, Jiang Y, Xu X, and Meng A. Free iron catalyzes oxidative damage to hematopoietic cells/mesenchymal stem cells in vitro and suppresses hematopoiesis in iron overload patients. Eur J Haematol 91: 249–261, 2013 [DOI] [PubMed] [Google Scholar]
- 107. Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O, and Lapidot T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal 21: 1605–1619, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lynch S and Soslau G. Iron levels found in hemochromatosis patients inhibit gamma-thrombin-induced platelet aggregation. Platelets 23: 611–616, 2012 [DOI] [PubMed] [Google Scholar]
- 109. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Manolova V, Nyffenegger N, Flace A, Altermatt P, Varol A, Doucerain C, Sundstrom H, and Durrenberger F. Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of beta-thalassemia. J Clin Invest 130: 491–506, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Martinez PA, Li R, Ramanathan HN, Bhasin M, Pearsall RS, Kumar R, and Suragani R. Smad2/3-pathway ligand trap luspatercept enhances erythroid differentiation in murine beta-thalassaemia by increasing GATA-1 availability. J Cell Mol Med 24: 6162–6177, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, and Malik P. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol 28: 1343–1353, 2000 [DOI] [PubMed] [Google Scholar]
- 113. McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, and Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291: 1755–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 114. Merchant AA, Singh A, Matsui W, and Biswal S. The redox-sensitive transcription factor Nrf2 regulates murine hematopoietic stem cell survival independently of ROS levels. Blood 118: 6572–6579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, McLornan D, Shimoda K, Winton EF, Deng W, Dubowy RL, Maltzman JD, Cervantes F, and Gotlib J. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol 35: 3844–3850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, and Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 41: 478–481, 2009 [DOI] [PubMed] [Google Scholar]
- 117. Miceli MH, Dong L, Grazziutti ML, Fassas A, Thertulien R, Van Rhee F, Barlogie B, and Anaissie EJ. Iron overload is a major risk factor for severe infection after autologous stem cell transplantation: a study of 367 myeloma patients. Bone Marrow Transplant 37: 857–864, 2006 [DOI] [PubMed] [Google Scholar]
- 118. Miller CB, Jones RJ, Piantadosi S, Abeloff MD, and Spivak JL. Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med 322: 1689–1692, 1990 [DOI] [PubMed] [Google Scholar]
- 119. Muckenthaler MU, Rivella S, Hentze MW, and Galy B. A red carpet for iron metabolism. Cell 168: 344–361, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Murakami S, Shimizu R, Romeo PH, Yamamoto M, and Motohashi H. Keap1-Nrf2 system regulates cell fate determination of hematopoietic stem cells. Genes Cells 19: 239–253, 2014 [DOI] [PubMed] [Google Scholar]
- 121. Murakami S, Suzuki T, Harigae H, Romeo PH, Yamamoto M, and Motohashi H. NRF2 activation impairs quiescence and bone marrow reconstitution capacity of hematopoietic stem cells. Mol Cell Biol 37: e00086-17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Muto Y, Nishiyama M, Nita A, Moroishi T, and Nakayama KI. Essential role of FBXL5-mediated cellular iron homeostasis in maintenance of hematopoietic stem cells. Nat Commun 8: 16114, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, Ferrari G, and Camaschella C. The second transferrin receptor regulates red blood cell production in mice. Blood 125: 1170–1179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, and Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 125. Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, and Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A 98: 8780–8785, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Oikonomidou PR and Rivella S. What can we learn from ineffective erythropoiesis in thalassemia? Blood Rev 32: 130–143, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pagani A, Nai A, Silvestri L, and Camaschella C. Hepcidin and anemia: a tight relationship. Front Physiol 10: 1294, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Park CH, Valore EV, Waring AJ, and Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 129. Parrow NL, Li Y, Feola M, Guerra A, Casu C, Prasad P, Mammen L, Ali F, Vaicikauskas E, Rivella S, Ginzburg YZ, and Fleming RE. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood 134: 1373–1384, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Phatak P, Brissot P, Wurster M, Adams PC, Bonkovsky HL, Gross J, Malfertheiner P, McLaren GD, Niederau C, Piperno A, Powell LW, Russo MW, Stoelzel U, Stremmel W, Griffel L, Lynch N, Zhang Y, and Pietrangelo A. A phase 1/2, dose-escalation trial of deferasirox for the treatment of iron overload in HFE-related hereditary hemochromatosis. Hepatology 52: 1671–1779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Picou F, Vignon C, Debeissat C, Lachot S, Kosmider O, Gallay N, Foucault A, Estienne MH, Ravalet N, Bene MC, Domenech J, Gyan E, Fontenay M, and Herault O. Bone marrow oxidative stress and specific antioxidant signatures in myelodysplastic syndromes. Blood Adv 3: 4271–4279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pilo F and Angelucci E. A storm in the niche: iron, oxidative stress and haemopoiesis. Blood Rev 32: 29–35, 2018 [DOI] [PubMed] [Google Scholar]
- 133. Piperno A, Pelucchi S, and Mariani R. Inherited iron overload disorders. Transl Gastroenterol Hepatol 5: 25, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Poli M, Asperti M, Ruzzenenti P, Mandelli L, Campostrini N, Martini G, Di Somma M, Maccarinelli F, Girelli D, Naggi A, and Arosio P. Oversulfated heparins with low anticoagulant activity are strong and fast inhibitors of hepcidin expression in vitro and in vivo. Biochem Pharmacol 92: 467–475, 2014 [DOI] [PubMed] [Google Scholar]
- 135. Popov VM, Vladareanu AM, Bumbea H, Kovacs E, Savopol T, Iordache MM, and Moisescu MG. Hemorrhagic risk due to platelet dysfunction in myelodysplastic patients, correlations with anemia severity and iron overload. Blood Coagul Fibrinolysis 26: 743–749, 2015 [DOI] [PubMed] [Google Scholar]
- 136. Powell LW, Seckington RC, and Deugnier Y. Haemochromatosis. Lancet 388: 706–716, 2016 [DOI] [PubMed] [Google Scholar]
- 137. Ramos E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E, and Ganz T. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood 120: 3829–3836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ramos P, Casu C, Gardenghi S, Breda L, Crielaard BJ, Guy E, Marongiu MF, Gupta R, Levine RL, Abdel-Wahab O, Ebert BL, Van Rooijen N, Ghaffari S, Grady RW, Giardina PJ, and Rivella S. Macrophages support pathological erythropoiesis in polycythemia vera and beta-thalassemia. Nat Med 19: 437–445, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, and Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Richard F, van Lier JJ, Roubert B, Haboubi T, Gohring UM, and Durrenberger F. Oral ferroportin inhibitor VIT-2763: first-in-human, phase 1 study in healthy volunteers. Am J Hematol 95: 68–77, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Richmond TD, Chohan M, and Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol 15: 146–155, 2005 [DOI] [PubMed] [Google Scholar]
- 142. Rivella S. The role of ineffective erythropoiesis in non-transfusion-dependent thalassemia. Blood Rev 26(Suppl 1): S12–S15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rivella S. Iron metabolism under conditions of ineffective erythropoiesis in beta-thalassemia. Blood 133: 51–58, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Rodriguez Martinez A, Niemela O, and Parkkila S. Hepatic and extrahepatic expression of the new iron regulatory protein hemojuvelin. Haematologica 89: 1441–1445, 2004 [PubMed] [Google Scholar]
- 145. Roetto A, Papanikolaou G, Politou M, Alberti F, Girelli D, Christakis J, Loukopoulos D, and Camaschella C. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet 33: 21–22, 2003 [DOI] [PubMed] [Google Scholar]
- 146. Sako D, Grinberg AV, Liu J, Davies MV, Castonguay R, Maniatis S, Andreucci AJ, Pobre EG, Tomkinson KN, Monnell TE, Ucran JA, Martinez-Hackert E, Pearsall RS, Underwood KW, Seehra J, and Kumar R. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J Biol Chem 285: 21037–21048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, and Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 115: 3616–3624, 2010 [DOI] [PubMed] [Google Scholar]
- 148. Schaible UE and Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol 2: 946–953, 2004 [DOI] [PubMed] [Google Scholar]
- 149. Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, and Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab 7: 205–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schmidt PJ, Toudjarska I, Sendamarai AK, Racie T, Milstein S, Bettencourt BR, Hettinger J, Bumcrot D, and Fleming MD. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe(−/−) mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood 121: 1200–1208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Shander A, Cappellini MD, and Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang 97: 185–197, 2009 [DOI] [PubMed] [Google Scholar]
- 152. Sheetz M, Barrington P, Callies S, Berg PH, McColm J, Marbury T, Decker B, Dyas GL, Truhlar SME, Benschop R, Leung D, Berg J, and Witcher DR. Targeting the hepcidin-ferroportin pathway in anaemia of chronic kidney disease. Br J Clin Pharmacol 85: 935–948, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Silva-Gomes S, Santos AG, Caldas C, Silva CM, Neves JV, Lopes J, Carneiro F, Rodrigues PN, and Duarte TL. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J Hepatol 60: 354–361, 2014 [DOI] [PubMed] [Google Scholar]
- 154. Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, and Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab 8: 502–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sorensen AL, Mikkelsen SU, Knudsen TA, Bjorn ME, Andersen CL, Bjerrum OW, Brochmann N, Patel DA, Gjerdrum LMR, El Fassi D, Kruse TA, Larsen TS, Mourits-Andersen HT, Nielsen CH, Ellervik C, Pallisgaard N, Thomassen M, Kjaer L, Skov V, and Hasselbalch HC. Ruxolitinib and interferon-alpha2 combination therapy for patients with polycythemia vera or myelofibrosis: a phase II study. Haematologica 105: 235648, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Spivak JL. How I treat polycythemia vera. Blood 134: 341–352, 2019 [DOI] [PubMed] [Google Scholar]
- 157. Stagg DB, Whittlesey RL, Li X, Lozovatsky L, Gardenghi S, Rivella S, and Finberg KE. Genetic loss of Tmprss6 alters terminal erythroid differentiation in a mouse model of beta-thalassemia intermedia. Haematologica 104: e442–e446, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Strasser SI, Kowdley KV, Sale GE, and McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant 22: 167–173, 1998 [DOI] [PubMed] [Google Scholar]
- 159. Suragani RN, Cawley SM, Li R, Wallner S, Alexander MJ, Mulivor AW, Gardenghi S, Rivella S, Grinberg AV, Pearsall RS, and Kumar R. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine beta-thalassemia. Blood 123: 3864–3872, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Taher AT, Karakas Z, Cassinerio E, Siritanaratkul N, Kattamis A, Maggio A, Rivella S, Hollaender N, Mahuzier B, Gadbaw B, and Aydinok Y. Efficacy and safety of ruxolitinib in regularly transfused patients with thalassemia: results from a phase 2a study. Blood 131: 263–265, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Taher AT, Weatherall DJ, and Cappellini MD. Thalassaemia. Lancet 391: 155–167, 2018 [DOI] [PubMed] [Google Scholar]
- 162. Tanaka H, Espinoza JL, Fujiwara R, Rai S, Morita Y, Ashida T, Kanakura Y, and Matsumura I. Excessive reactive iron impairs hematopoiesis by affecting both immature hematopoietic cells and stromal cells. Cells 8: 226, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tataranni T, Agriesti F, Mazzoccoli C, Ruggieri V, Scrima R, Laurenzana I, D'Auria F, Falzetti F, Di Ianni M, Musto P, Capitanio N, and Piccoli C. The iron chelator deferasirox affects redox signalling in haematopoietic stem/progenitor cells. Br J Haematol 170: 236–246, 2015 [DOI] [PubMed] [Google Scholar]
- 164. Theurl I, Schroll A, Sonnweber T, Nairz M, Theurl M, Willenbacher W, Eller K, Wolf D, Seifert M, Sun CC, Babitt JL, Hong CC, Menhall T, Gearing P, Lin HY, and Weiss G. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood 118: 4977–4984, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, and Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62: 5196–5203, 2002 [PubMed] [Google Scholar]
- 166. Tsai JJ, Dudakov JA, Takahashi K, Shieh JH, Velardi E, Holland AM, Singer NV, West ML, Smith OM, Young LF, Shono Y, Ghosh A, Hanash AM, Tran HT, Moore MA, and van den Brink MR. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol 15: 309–316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vadhan-Raj S, Abonour R, Goldman JW, Smith DA, Slapak CA, Ilaria RL Jr., Tiu RV, Wang X, Callies S, Cox J, Tuttle JL, Lau YK, and Roeland EJ.. A first-in-human phase 1 study of a hepcidin monoclonal antibody, LY2787106, in cancer-associated anemia. J Hematol Oncol 10: 73, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Volani C, Doerrier C, Demetz E, Haschka D, Paglia G, Lavdas AA, Gnaiger E, and Weiss G. Dietary iron loading negatively affects liver mitochondrial function. Metallomics 9: 1634–1644, 2017 [DOI] [PubMed] [Google Scholar]
- 169. Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, and Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 170. Walker EM Jr., and Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci 30: 354–365, 2000 [PubMed] [Google Scholar]
- 171. Wang CY, Xu Y, Traeger L, Dogan DY, Xiao X, Steinbicker AU, and Babitt JL. Erythroferrone lowers hepcidin by sequestering BMP2/6 heterodimer from binding to the BMP type I receptor ALK3. Blood 135: 453–456, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Xavier-Ferrucio J, Scanlon V, Li X, Zhang PX, Lozovatsky L, Ayala-Lopez N, Tebaldi T, Halene S, Cao C, Fleming MD, Finberg KE, and Krause DS. Low iron promotes megakaryocytic commitment of megakaryocytic-erythroid progenitors in humans and mice. Blood 134: 1547–1557, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xia Y, Babitt JL, Sidis Y, Chung RT, and Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood 111: 5195–5204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, and Tang D. Ferroptosis: process and function. Cell Death Differ 23: 369–379, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xu W, Barrientos T, Mao L, Rockman HA, Sauve AA, and Andrews NC. Lethal cardiomyopathy in mice lacking transferrin receptor in the heart. Cell Rep 13: 533–545, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Yacoub A, Mascarenhas J, Kosiorek H, Prchal JT, Berenzon D, Baer MR, Ritchie E, Silver RT, Kessler C, Winton E, Finazzi MC, Rambaldi A, Vannucchi AM, Leibowitz D, Rondelli D, Arcasoy MO, Catchatourian R, Vadakara J, Rosti V, Hexner E, Kremyanskaya M, Sandy L, Tripodi J, Najfeld V, Farnoud N, Papaemmanuil E, Salama M, Singer-Weinberg R, Rampal R, Goldberg JD, Barbui T, Mesa R, Dueck AC, and Hoffman R. Pegylated interferon alfa-2a for polycythemia vera or essential thrombocythemia resistant or intolerant to hydroxyurea. Blood 134: 1498–1509, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Yacoub MF, Ferwiz HF, and Said F. Effect of interleukin and hepcidin in anemia of chronic diseases. Anemia 2020: 3041738, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, and Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell 156: 317–331, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Yang WS and Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15: 234–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ye ZW, Zhang J, Townsend DM, and Tew KD. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta 1850: 1607–1621, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yu Y, Xie Y, Cao L, Yang L, Yang M, Lotze MT, Zeh HJ, Kang R, and Tang D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2: e1054549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]