Abstract
Significance: While atherosclerosis is an almost inevitable consequence of aging, food preferences, lack of exercise, and other aspects of the lifestyle in many countries, the identification of new risk factors is of increasing importance to tackle a disease, which has become a major health burden for billions of people. Iron has long been suspected to promote the development of atherosclerosis, but data have been conflicting, and the contribution of iron is still debated controversially.
Recent Advances: Several experimental and clinical studies have been recently published about this longstanding controversial problem, highlighting the critical need to unravel the complexity behind this topic.
Critical Issues: The aim of the current review is to provide an overview of the current knowledge about the proatherosclerotic impact of iron, and discuss the emerging role of non-transferrin-bound iron (NTBI) as driver of vasculotoxicity and atherosclerosis. Finally, I will provide detailed mechanistic insights on the cellular processes and molecular pathways underlying iron-exacerbated atherosclerosis. Overall, this review highlights a complex framework where NTBI acts at multiple levels in atherosclerosis by altering the serum and vascular microenvironment in a proatherogenic and proinflammatory manner, affecting the functionality and survival of vascular cells, promoting foam cell formation and inducing angiogenesis, calcification, and plaque destabilization.
Future Directions: The use of additional iron markers (e.g., NTBI) may help adequately predict predisposition to cardiovascular disease. Clinical studies are needed in the aging population to address the atherogenic role of iron fluctuations within physiological limits and the therapeutic value of iron restriction approaches. Antioxid. Redox Signal. 35, 387–414.
Keywords: cardiovascular disease, atherosclerosis, iron, nontransferrin-bound iron (NTBI), iron-aggravated atherosclerosis, reactive oxygen species (ROS), intraplaque macrophages, inflammation, endothelial dysfunction, iron-loaded VSMC, calcification, oxidized LDL
Introduction
Atherosclerosis, one of the leading causes of morbidity and mortality in Western countries, is a complex pathological process affecting arterial walls. Oxidative stress plays a crucial role in atherosclerosis, since free radicals promote lipoprotein (low-density lipoprotein [LDL]) oxidation and therefore accelerate its progression. In this context, iron can be detrimental through the generation of free radicals and reactive oxygen species (ROS) (231). In 1981, Sullivan proposed the “iron hypothesis,” according to which iron overload promotes cardiovascular disease (CVD) and, on the contrary, iron deficiency exerts a protective action (209–213). Multiple experimental and clinical observations provided evidence of a key role of iron in atherogenesis, while others provided conflicting data in this regard. Especially, the absence of increased incidence of atherosclerosis in patients with hereditary hemochromatosis (HH), afflicted by serious life-long iron overload, was perceived as a “paradox” and considered the most persuasive evidence against the iron hypothesis (214, 231). In this review, I provide an overview of the most recent findings and studies, which addressed this longstanding controversial aspect. Importantly, I will show how novel experimental approaches and animal models helped in the last decade to uncover mechanistically the contribution of iron overload to atherosclerosis. While here I will describe predominantly recent findings, a more comprehensive overview of older studies is summarized in a previously published review on this topic (231).
Regulatory Mechanisms of Iron Homeostasis
Systemic iron homeostasis
Due to its potential toxicity, iron levels are tightly regulated in the body. Iron is absorbed by duodenal enterocytes from the diet and recycled by reticuloendothelial macrophages from senescent red blood cells (RBCs) through the membrane iron exporter ferroportin (FPN). Systemic iron fluxes are controlled by a major regulatory system, the hepcidin–FPN axis. The peptide hormone hepcidin, by binding and mediating FPN occlusion, internalization and degradation, controls FPN-mediated iron export (96, 147). Hepcidin levels are regulated by several stimuli: while iron levels and inflammation induce hepcidin synthesis, erythropoiesis and hypoxia exert a negative regulation of the hormone. Therefore, in condition of iron deficiency and stimulated erythropoiesis, hepatic hepcidin expression is decreased to promote dietary iron influx into the circulation through FPN. When body iron levels are elevated or an infection occurs, hepcidin expression is increased to reduce FPN-mediated systemic iron influx and thus overall body iron as well as iron availability for microorganism growth. Once released systemically, iron is bound to the iron carrier transferrin (Tf) and delivered through transferrin receptor 1 (TfR1)-mediated endocytosis to cells throughout the body where it is utilized as cofactor in heme and nonheme enzymes. Erythropoiesis is the process that uses most iron, whereby erythroblasts acquire up to 30 mg iron per day to synthesize hemoglobin (Hb) and provide de novo RBCs (96, 147).
Non-transferrin-bound iron and non-hemopexin-bound heme
Upon iron overload conditions, either inherited (e.g., HH) or acquired (e.g., transfusion dependence), serum iron levels and Tf saturation (>50%) are elevated, which lead to the formation of “free” nontransferrin-bound iron (NTBI). Within the NTBI pool, the labile plasma iron (LPI) represents the toxic reactive fraction, which is loosely bound to plasma proteins and highly pro-oxidant. NTBI and LPI can accumulate intracellularly through the metal transporter ZIP14 (147). Therefore, tissues expressing high levels of ZIP14 and/or low levels of FPN are prone to develop iron overload in presence of NTBI. Similar to NTBI, Hb and heme released upon hemolysis become toxic when their circulating levels override the binding capacity of the Hb scavenger haptoglobin (Hp) and heme scavenger hemopexin (Hx), leading to the formation of non-Hp bound Hb (NHBHB) and non-Hx bound heme (NHBH). While the Hb–Hp and heme–Hx complexes are selectively taken up by macrophages and hepatocytes, respectively, NHBHB and NHBH freely enter cells in a nonspecific manner, causing heme overload and tissue damage (46, 193, 219, 229, 230). In iron overload or hemolytic conditions, the vasculature might be exposed to elevated NTBI, NHBHB, and NHBH. Accumulation of unshielded iron, Hb, and heme in vascular cells elicits an adaptive response, whereby the cytoprotective enzyme heme oxygenase 1 (HO-1) is induced. Pro-oxidant stimuli as well as the substrate heme are able to trigger HO-1 induction. HO-1 exerts a cytoprotective and antioxidant action by promoting carbon monoxide and biliverdin release as a result of heme catabolism (19, 20). Heme-derived iron is oxidized from its toxic ferrous (Fe2+) to nontoxic ferric (Fe3+) form by the ferroxidase of the ferritin heavy chain (H-Ft) and then safely stored into ferritin light chain (18, 19).
Cell iron homeostasis
Cellular iron homeostasis is controlled post-transcriptionally by the iron regulatory protein (IRP)/iron regulatory element (IRE) system. The RNA-binding IRPs 1 and 2 (IRP1, IRP2) interact with conserved cis-regulatory hairpin structures termed IREs, which are located in the 5′ or 3′ untranslated regions (UTRs) of target mRNA transcripts (96, 147). IRP binding to the 5′ UTR IREs of mRNAs encoding for iron storage and export molecules such as ferritin and FPN inhibits their translation, whereas their binding to the 3′ UTR IREs of TFR1 mRNA prevents TfR1 degradation. Therefore, in iron-depleted cells, the IRE–IRP system reduces the expression of molecules involved in iron export and storage, and increases the stability of the main receptor responsible for cell iron uptake, TfR1 (96, 147).
Overall, a fine tuning of both systemic and cellular iron levels orchestrated by the hepcidin/FPN axis and the IRE/IRP system is key to the maintenance of iron homeostasis. The pathological formation of free forms of heme and iron, NHBH and NTBI, eventually leads to systemic and cellular iron overload, causing oxidative stress, cell damage, and organ dysfunction.
Novel Evidence on the Vasculotoxic and Atherogenic Impact of Iron
What we have learned from human studies
Association between iron status and atherosclerotic CVD
While a clear association exists between systemic iron deficiency and CVD, including coronary artery disease (CAD) and myocardial infarction (MI), more complex and less clear is the association between iron overload and CVD emerging from trials and clinical studies. Some studies support a role for iron in atherosclerosis and found a link between body iron status and CVD, whereas others failed to observe any association (231). The Brunick study indicates serum ferritin as one of the strongest predictors of carotid artery disease (119). Likewise, a positive association between iron status measured as serum ferritin, iron levels or Tf saturation, and risk of cardiovascular events has been described in several studies (99, 125, 140, 145, 170, 172, 186, 217, 221, 246). Specifically, a 10 mg/L increase in serum ferritin levels raises by 3% the probability of having at least two atherosclerotic plaques (4). Indeed, patients with severe CAD show higher serum iron levels compared with those with normal, mild, and moderate CAD (13).
Electron paramagnetic resonance spectroscopy and inductively coupled plasma mass spectroscopy analyses revealed that iron accumulates in human arteries and carotid lesions, and its levels positively correlate with cholesterol deposition (206). Iron accumulation in arterial tissue is elevated in subjects with high body iron stores (132). Moreover, plaque iron loading positively correlates with plaque vulnerability and propensity to rupture: advanced plaques in patients with carotid atherosclerosis show in fact higher iron content together with signs of cap rupture and increased number of inflammatory macrophages (91, 248). The association between serum ferritin and atherosclerosis is potentiated by hyperlipidemia, smoking, inflammation, and gender, suggesting a synergistic effect of iron with other classical factors predisposing to CVD (145, 186).
Iron may have cardiovascular implications for pathological conditions that show an altered iron status and a propensity to CVD as a result of disease complication, ranging from diabetes and metabolic syndrome to transfusion-dependent anemia and chronic kidney disease (155). Diseases associated with both heme and iron overload, such as β-thalassemia and sickle cell disease, show underlying cardiovascular alterations. These individuals develop systemic Hb and heme overload consequent to hemolysis, and iron overload consequent to ineffective erythropoiesis, hepcidin downregulation and chronic transfusion requirement (181, 233). Together with NTBI, unshielded Hb and heme might exert vasculotoxic, proatherogenic, and prothrombotic effects due to their pro-oxidant and proinflammatory action (193, 231, 233). This is currently believed to be a major mechanism promoting vascular alteration and vasculopathy in sickle cell disease and thalassemia and underlying complications, including vaso-occlusive crisis, acute chest syndrome, increased blood pressure, hypertension, altered cardiac function, and stroke (113–115). A major cause of death in β-thal patients is congestive heart failure due to transfusional iron overload. While patients with hemoglobinopathies develop to some extent vascular complications, such as arterial and venous thromboembolic events, stroke (2, 43, 45, 90, 93, 94, 198), their atherosclerotic mortality is somehow limited. This is likely explained by the constant exposure of these patients to iron chelation therapy and their favorable lipid profile, with low cholesterol levels (8, 178, 179, 196). Premature atherosclerosis is more frequent in children who often show dyslipidemia associated with high triglycerides (29, 118, 198).
Among the studies that do not support the iron hypothesis, the iron and atherosclerosis study (FeAST) trial failed to demonstrate a beneficial effect of reduced body iron stores on peripheral arterial disease-related mortality and MI/stroke (249, 250). However, in a subset of patients who died during the FeAST study a correlation between levels of circulating ferritin, inflammatory biomarkers, and mortality has been observed (59). A recent study suggested that increased iron status is protective against some forms of atherosclerotic disease and cardiovascular mortality, whereas it promotes the risk of venous thromboembolism (83).
The hemochromatosis paradox and potential explanations
A major challenge to the iron paradigm was the observation that individuals affected by HH, a life-long iron overload disease, do not apparently show increased risk of cardiovascular events. In HH, the hepcidin/FPN regulatory axis is disrupted due to mutations in hepcidin regulators (high Fe2+ protein [HFE], transferrin receptor 2 [TfR2], hemojuvelin), in hepcidin itself or FPN. This results in inadequate hepcidin levels and constant FPN-mediated systemic iron influx, leading to iron overload (147). Whereas some clinical studies showed an increased risk of MI, and cerebrovascular or cardiovascular mortality in HFE-mutated patients (175, 183, 220), most of them showed no association between parameters of iron status and atherosclerotic CVD (65, 67, 143, 148, 161, 215, 224). A matter of debate is the cholesterol level in HH patients, which was found increased (232) and decreased in different studies (156). While decreased cholesterol might explain the lack of increased CVD predisposition, these patients present clear iron-aggravated markers of vascular dysfunction and oxidative stress, which are rescued by therapeutic phlebotomy (232). HH patients bear in fact elevated oxidized lipids and proteins, increased soluble adhesion molecules and cytokine levels, structural alterations in arterial muscle media layer, reduced nitric oxide (NO), and impaired endothelium-dependent vasodilation (70, 77, 111, 232). These alterations might accelerate CVD only in combination with an unfavorable lipid profile in HH subjects, suggesting that iron is a “modifier” rather than per se a trigger of atherosclerosis.
Another explanation of incoherent findings in HH comes from the altered tissue iron distribution in these patients. Low hepcidin in HH induces macrophage iron depletion by enhancing cell iron efflux. Iron-depleted macrophages likely show antiatherogenic and anti-inflammatory properties. Therefore, reduced macrophage iron content has been proposed as mechanism counteracting the atherosclerotic action of elevated systemic iron and the key for the lack of CVD predisposition in HH (214, 215). Mechanistic insights on the atheroprotective role of iron-depleted macrophages are described below in the section “Iron and Macrophages”. This “adjusted hypothesis” therefore implies that tissue and cell iron distribution has a role in iron-aggravated atherosclerosis, whereby macrophage iron retention due to elevated hepcidin represents a risk factor for CVD (215). Indeed, some studies found a positive correlation between serum hepcidin levels and all-cause and cardiovascular mortality in patients with CAD (130) and plaque instability in patients with MI (78, 79, 190). However, to exclusively rely on hepcidin as marker for the association between iron and CVD is susceptible of misleading conclusion, as hepcidin could reflect the iron status (high hepcidin = iron deficiency; low hepcidin = iron overload) as well as an adaptation to the current iron status (high hepcidin = iron overload triggers hepcidin induction; low hepcidin = iron deficiency blocks hepcidin synthesis). This level of complexity helps explain why other studies found no or opposite association between hepcidin and CVD (87, 159, 180). Finally, the observation of a continuous and inverse correlation between hepcidin levels and CAD mortality suggests a dominant role of elevated systemic iron over macrophage iron in promoting CVD (87).
Spectrum and parameters of iron status in CVD
While most of the clinical studies to assess the validity of the iron hypothesis have been performed in patients with CVD or altered iron status, a nuanced understanding of how small fluctuations of iron parameters within the physiological limits predispose to atherosclerosis in the healthy population is still lacking. A recent human population study reported for the first time a correlation between circulating ferritin levels and carotid-intima media thickness in a large healthy cohort of 692 children (171). Importantly, a J-shaped association of systemic iron status and cardiovascular mortality has been recently described in CAD patients (87, 163). The observation that CAD patients at increased risk fall into the two opposite extremes of the iron spectrum points toward the notion that iron-associated cardiovascular risk results from a misbalance in iron homeostasis—either iron overload or deficiency (Table 1). This helps explain the conflicting results obtained from clinical studies, where the definition of a “protected window” of iron levels across studies is difficult to determine.
Table 1.
Range of Parameters of Iron Status and Risk of Cardiovascular Disease Mortality
| |
Iron status |
Risk of CVD mortality/disease severity |
||||
|---|---|---|---|---|---|---|
| Iron parameter | Normal range | Iron deficiency | Iron overload | No risk | Increased | References |
| Serum iron | 65–170 μg/dL | <50 μg/dL | >170 μg/dL | 90–130 μg/dL | <90; >130 μg/dL | (87) |
| Tf saturation | 20%–55% | <20% | >55% | 25%–40% | <25; >40% | (87) |
| Serum ferritin | 20–300 ng/mL | <20 ng/mL | >1000 ng/mL | 150–350 ng/mL | <150; >350 ng/mL | (87) |
| NTBI | <1 μM | Undetectable | >0.5 μM (Thal 0.5 to >10 μM; HH 0.1–5 μM) | <1 μM | Undetectable; >1 μM | (232) |
| LPI | <0.2 μM | Undetectable | >0.2 μM (Thal 0.5–10 μM; HH 0.2–2 μM) | <0.1 μM | Undetectable; >0.1 μM | (232) |
The range of iron parameters, including serum iron, Tf saturation, serum ferritin, NTBI, and LPI, is reported for individuals with a normal iron status, iron deficiency, and iron overload. The range of iron parameters for individuals with no risk and increased risk of CVD mortality is indicated according to the J-shaped association by Grammer et al. (87). Values of NTBI and LPI are extrapolated from the preclinical study by Vinchi et al. (232) as no clinical studies are available. Being NTBI and LPI detectable almost exclusively in iron overload conditions, their association with CVD has to be considered upon iron overload only.
CVD, cardiovascular disease; HH, hereditary hemochromatosis; LPI, labile plasma iron; NTBI, nontransferrin-bound iron; Tf, transferrin.
The discrepancy of epidemiological studies may also derive from the scarce reliability of parameters of iron status, their variability and fluctuations due to multiple modifiers—for example, age, gender, diet—and the limited consideration of treatment regimen. The use of markers of iron status which poorly reflect the systemic and tissue iron burden may have biased the conclusions of several studies. While serum ferritin is considered a marker of tissue iron content, its levels are highly affected by inflammation, and do not reflect the iron saturation of Tf or the amount of NTBI or catalytically active LPI (108, 135). Additional markers may be required, such as NTBI or vascular iron levels, to adequately predict predisposition to CVD (Table 1). The treatment regimen of iron-loaded patients (e.g., anti-inflammatory drugs, iron depletion strategies) should be critically considered as it may mask the cardiovascular risk imposed by iron. As an example, most of HH patients are on iron depletion therapies, the underestimation of which might have biased the outcome of several studies.
In particular, NTBI together with its reactive fraction LPI likely represent more reliable measures of the circulating iron fraction capable of proatherogenic action. Importantly, NTBI is increased not only in iron overload conditions but also in other diseases such as diabetes and metabolic syndromes. Therefore, the use of these parameters may help identify those patients and link iron dyshomeostasis to accelerated CVD also in nonprimarily iron overload conditions (6). Along this line, an association between the frequency of hemochromatosis mutations in HFE and an increased risk of CAD was observed in diabetic patients, suggesting that elevated systemic iron exacerbates the CVD risk imposed by diabetes (189). Overall, clinical studies specifically monitoring NTBI and LPI modulation and their connection with CVD are urgently needed to assess the validity of these parameters of iron status as better predictor of cardiovascular events (6, 184). The lack of coherence among human studies mirrors the complex relationship existing between the iron status and cardiovascular functionality, where the two extremes of the iron spectrum imply an impairment of the cardiovascular system and translate into propensity to CVD. Further investigations are required to specifically address the association of alternative parameters of iron status—NTBI and LPI—with cardiovascular events in iron-loaded individuals and patients with CVD as well as in the healthy populations. Considering the implications of NTBI and LPI in CVD emerging from animal studies, the assessment of these iron markers in clinical studies will provide new indications on the more relevant iron forms with vasculotoxic and atherosclerotic properties.
What We Have Learned from Animal Models
Experimental evidence on the role of iron in atherosclerosis mainly derives from the analysis of “artificial models” of iron overload, whereby animals have been experimentally overloaded with iron through infusion or supplemented diet. By contrast, for decades, atherosclerosis has remained understudied in pathophysiological models of iron overload, including animal models of genetic iron overload (e.g., HH mouse models). Recent works addressed the development of atherosclerosis in mouse models of hemochromatosis, providing new mechanistic insight into the proatherogenic cellular and molecular mode of action of iron. In this section, I will review the major findings obtained from the analysis of mouse models of iron overload, achieved either by genetic manipulation or by iron treatment.
Lesson from animal models of genetic iron overload
Mouse models with disrupted hepcidin/FPN regulatory axis have been recently analyzed to dissect the relationship between iron overload and atherosclerosis. These models include the following: (i) FPNwt/C326S knock-in mice—a mouse model of type IV HH; (ii) hepcidin knockout mice—a mouse model of type II HH; and (iii) flatiron mice—a mouse model of classical FPN disease (Table 2).
Table 2.
Lessons from Animal Models
| Model | Atherosclerosis phenotype | Iron-related features | Similarities and differences | Human disease | References |
|---|---|---|---|---|---|
| FPNwt/C326S (heterozygous C326S gain of function FPN mutation) | Aggravated Vascular dysfunction and elevated intraplaque inflammatory macrophages |
Hepcidin resistance Constant FPN-mediated iron export High Tf sat. and NTBI tissue iron overload |
ApoE-null background Standard diet High hepcidin and NTBI levels Residual macrophage iron content Increased cholesterol (6M) Arterial iron deposition (VSMCs) Increased plaque macrophages, inflammation, lipid content, endothelial dysfunction, calcification |
Type IV HH | (7, 232) |
| Hamp-null (hepcidin knockout) | Reduced Anti-inflammatory macrophages with improved cholesterol efflux capacity |
Hepcidin deficiency Constant FPN-mediated iron export High Tf sat. and NTBI Tissue iron overload |
Ldlr-null background Western diet Hepcidin deficiency, high NTBI Macrophage iron depletion Reduced cholesterol Unknown arterial iron content |
Type II HH | (134) |
| Flatiron (ffe) (heterozygous H32R missense FPN mutation) | Unaltered/slightly reduced No relevant effect of iron-loaded macrophages |
Suppressed FPN-mediated iron export Macrophage iron accumulation High serum Ft, low Tf sat. |
ApoE-null background High-fat diet Macrophage iron overload |
Classical FPN disease | (116, 260) |
| High-iron diet | (i) Aggravated Elevated intraplaque inflammatory macrophages (ii) Reduced |
(i) High serum iron (ii) High serum iron and LPI |
(i) ApoE-null background, 25 g/kg carbonyl iron diet, elevated inflammation, and plaque macrophages (ii) ApoE-null background, 2% carbonyl iron diet, slightly increased cholesterol levels |
Dietary iron overload | (i) (242) (ii) (121) |
| Iron infusion | Aggravated Endothelial dysfunction |
(i) Iron dextran (ip, 10 mg/mouse/day, five times/week for 4 weeks) (ii) Nephrectomy model, iron sucrose (ip, 2 mg/mouse/weekly per 12 weeks); endothelial dysfunction (iii) Rabbits (intramuscular, 50 mg/every 3 days for a total of 1.5 g iron dextran/rabbit) |
(i) ApoE-null genetic background, standard diet, increased serum cholesterol, arterial iron deposition, endothelial dysfunction (ii) ApoE-null genetic background, standard diet, endothelial dysfunction, and oxidative stress (iii) Cholesterol-enriched diet, arterial iron deposition, increased tissue lipoperoxides |
Systemic iron overload | (i) (137) (ii) (124) (iii) (10) |
| Hx-null (hemopexin knockout) | Aggravated Proinflammatory macrophages with impaired cholesterol efflux capacity |
Elevated systemic heme levels | ApoE-null genetic background Standard diet Elevated cholesterol levels Endothelial dysfunction |
Hx consumption associated with hemolysis (as in SCD, thal) | (139, 228, 229) |
| Hp 2-2 polymorphism (mice bearing Hp1-1 vs. Hp 2-2 pulymorphysm) | Aggravated Elevated intraplaque macrophages |
Decreased ability of the Hp 2 allele to inhibit Hb pro-oxidant and proinflammatory action | ApoE-null background Standard diet Increased intraplaque iron deposition, lipid peroxidation, and macrophages in Hp2-2 compared with Hp1-1 mice |
Different Hp polymorphisms | (109, 128) |
| HO-null/HO inhibition | Aggravated | (i) HO-null (ii) HO-1 inhibition by zinc protoporphyrin (iii) HO-1-null bone marrow reconstitution |
(i) ApoE-null genetic background, Western diet, increased plaque lipid content, macrophages, and VSMCs (ii) Model of vulnerable plaque by carotid cast, ApoE-null background, Western diet, decreased cap thickness and VSMC area, increased necrotic core/intima ratio and plaque lipids (iii) Ldlr-null background, Western diet, increased plaque macrophages in HO-1-null vs. wild-type bone marrow-reconstituted mice |
HO deficiency | (i) (224) (ii) (44) (iii) (153) |
| Hbbth3 | Aggravated Intraplaque inflammation |
Elevated systemic heme levels, Hp/Hx depletion | Knockdown of Ldlr by AAV-PCSK9 High-fat diet, angiotensin II administration, increased plaque inflammation, and macrophages |
Thalassemia intermedia | Hurtado et al. (Abstract 350, ATVB 2020;40:A350) |
Atherosclerosis phenotype, hallmarks, similarities and differences, and corresponding human disease of animal models used to study the impact of iron overload in CVD.
ApoE, apolipoprotein E; FPN, ferroportin; Ft, ferritin; Hb, hemoglobin; HO, heme oxygenase; Hp, haptoglobin; Hx, hemopexin; ip, intraperitoneal; SCD, sickle cell disease; VSMC, vascular smooth muscle cell.
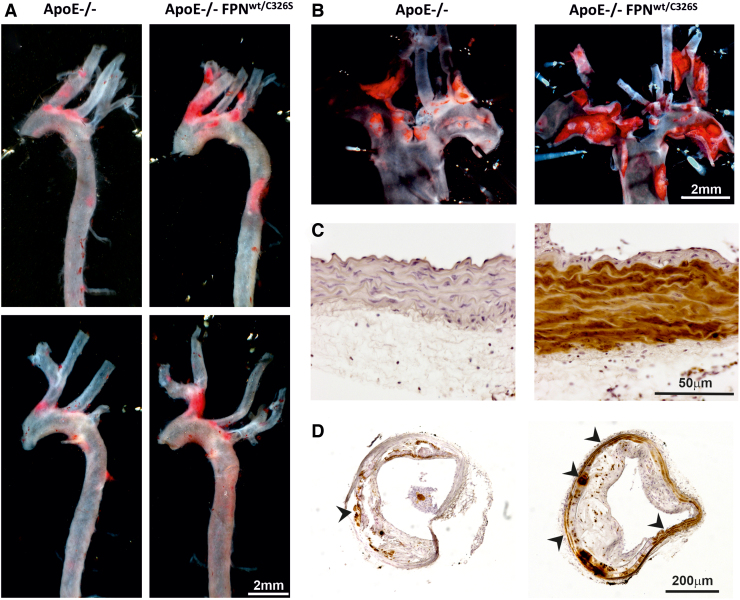
Vinchi et al. have recently assessed the role of iron overload in atherosclerosis by analyzing a mouse model carrying a heterozygous gain-of-function mutation of FPN (FPNC326S) (232). This dominant mutation confers resistance to hepcidin binding and leads to the disruption of the hepcidin–FPN axis by preventing hepcidin-mediated FPN internalization and degradation. The disruption of this regulatory mechanisms results in constitutive iron efflux from macrophages and increased iron uptake by duodenal enterocytes. Indeed, this model is hallmarked by elevated Tf saturation, NTBI formation, and tissue iron overload (7). The presence of the FPNC326S mutation in the apolipoprotein E (ApoE)-null genetic background (ApoE-null FPNC326S mice) results in exacerbated atherosclerosis, whereby atherosclerotic lesion area and numbers are significantly higher compared with ApoE-null control animals (Fig. 1A, B). A hallmark of this model is the massive iron deposition in major arteries (e.g., aorta), consequent to NTBI uptake (Fig. 1C, D). While this work does not speak about the role of iron within physiological limits, it documents the relevance of NTBI in atherogenesis, and its contribution to pro-oxidant and proinflammatory mechanisms with atherogenic effect (232).
FIG. 1.
NTBI accumulates in arteries and exacerbates atherosclerosis. (A, B) Representative images of entire aorta and en-face aortic arch of ApoE-null and ApoE-null FPNC326S mice stained with the lipophilic dye Sudan IV. Plaques are visible in bright red. More and bigger plaques can be observed in ApoE-null FPNC326S mice. (C, D) Representative images of (C) innominate artery and (D) common carotid artery sections of ApoE-null and ApoE-null FPNC326S mice stained for iron with diaminobenzidine-enhanced Perls' staining. An enlarged picture shows iron deposition in brown in the smooth muscle layer of the artery in ApoE-null FPNC326S mice (C). While iron accumulates predominantly in the media layer (see arrowheads), the atherosclerotic plaque is almost completely iron spared (D). ApoE, apolipoprotein E; FPN, ferroportin; NTBI, nontransferrin-bound iron. Color images are available online.
By contrast, Malhotra et al. recently showed that a different model of hemochromatosis, the hepcidin-null (Hamp-null) mouse on low-density lipoprotein receptor (Ldlr)-null genetic background, presents with less severe atherosclerosis than the Ldlr-null control mouse (89, 134). The antiatherogenic effect of hepcidin deletion was mostly attributed to the reduced proinflammatory activation of intraplaque iron-depleted macrophages.
Interestingly, FPNC326S and Hamp-null mice would have been expected to phenocopy in regard to atherosclerosis development, based on the considerations that both models (i) have elevated NTBI, (ii) show tissue iron accumulation, and (iii) bear macrophages with reduced intracellular iron content (88, 232). However, the opposite phenotypes observed should be considered in light of major differences between these models: (i) while FPNC326S mice have been analyzed on the ApoE-null genetic background, which spontaneously promotes plaque development, Hamp-null mice required a high-fat diet to develop atherosclerotic plaques in the Ldlr-null background. In this second model, the diet might contribute to atherosclerosis in a synergistic way together with iron; (ii) LDL levels are different in the two models, being reduced in Ldlr-null Hamp-null mice and slightly increased in ApoE-null FPNC326S mice at 6 months of age. Lower LDL levels might be due to increased cell cholesterol efflux from iron-depleted macrophages but also to different intake from the high-fat diet in Ldlr-null Hamp-null mice (which was not explored in the study). Understanding why LDL levels are different in these models might uncover novel mechanisms whereby iron controls cholesterol levels; (iii) NTBI levels and arterial iron deposition have not been directly compared in these mouse models. Hypothetically, different levels of NTBI and arterial iron content between ApoE-null FPNC326S and Ldlr-null Hamp-null mice would explain the opposite atherosclerotic phenotypes (e.g., if we assume a higher NTBI in ApoE-null FPNC326S mice, the antiatherogenic benefit of macrophage iron depletion might be masked by NTBI detrimental effects); (iv) these models strongly differ in the circulating levels of hepcidin. While the Ldlr-null Hamp-null mouse is hepcidin deficient, the ApoE-null FPNC326S mouse has high levels of hepcidin, which, despite inactive on FPN, is still induced by iron overload. The different levels of hepcidin in these models likely call for extra functions of hepcidin, independent from its FPN-related action and potentially proatherosclerotic; (v) while the Ldlr-null Hamp-null model is completely deficient for hepcidin, therefore having the lowest intracellular iron content possible in macrophages, the ApoE-null FPNC326S mouse may still have residual macrophage iron due to the presence of the FPNC326S mutation in heterozygosity. The presence of residual intracellular iron in macrophages may limit their protective effect in atherosclerosis; (6) finally, the effect of low macrophage iron may be “time dependent,” providing an antiatherogenic action in the early stages of atherosclerosis through anti-inflammatory macrophage phenotypic switching, and a detrimental action in later stages of the disease, whereby hypoxia-triggered vascular endothelial growth factor (VEGF) production by iron-depleted macrophages promotes angiogenesis, vasopermeabilization, and intraplaque inflammatory cell recruitment (88, 169). Despite constantly elevated systemic iron levels, ApoE-null FPNC326S mice show aggravated atherosclerosis at 6 months of age with no obvious differences at earlier age (232). We speculate that the presence of iron-depleted macrophages might provide a certain degree of protection in ApoE-null FPNC326S mice at earlier stages of the disease, until a more advanced stage whereby NTBI toxicity becomes dominant. At the same time, these mice show intraplaque angiogenesis and hemorrhages at older ages (12–18 months), compatible with a detrimental effect of iron-depleted macrophages in advanced atherosclerosis. Whether this duality holds true in Ldlr-null Hamp-null mice requires further exploration as atherosclerosis was not analyzed beyond 5 months of age in this model.
Overall, these models highlight the complexity of the interplay existing between circulating NTBI and intracellular macrophage iron in regard to atherosclerosis progression. Along this line, the two hemochromatosis models are hallmarked by high NTBI and macrophage depletion, which renders difficult the dissection of the contribution of the single components to atherosclerosis. To overcome this issue, Kautz et al. (116) have analyzed atherosclerosis in the flatiron (ffe) mouse, a model of classical FPN disease, carrying the heterozygous H32R missense mutation of FPN (FPNH32R), which causes a dominant negative mistrafficking of the iron exporter and results in macrophage iron overload (260). This model on ApoE-null background allowed one to assess the specific role of elevated macrophage iron content in atherosclerosis. Contrarily to other observations, this study failed to show aggravated atherosclerosis in ApoE-null ffe mice (116). These findings argue against a significant detrimental action of iron-loaded macrophages in atherosclerosis and are seemingly in line with other studies, which rather support a critical role of systemic iron in disease progression (232). Nevertheless, the possibility that the detrimental effects triggered by macrophage iron overload are overall less relevant compared to the beneficial effects induced by macrophage iron depletion cannot be ruled out.
Lesson from animal models of exogenous iron overload
Animal models of experimentally induced iron overload have been used to study the role of iron in atherosclerosis (Table 2). The administration of exogenous iron or iron-enriched diet in experimental animals generates iron overload in a nonspecific manner, likely affecting both the vasculature and macrophages, and thus generally addressing the role of NTBI in atherosclerosis. Whereas an earlier study reported diminished atherosclerosis in ApoE-null mice challenged with dietary iron overload (121), Hu et al. recently showed that an iron-enriched diet aggravates atherosclerosis in ApoE-null mice (101). Likewise, iron infusion (either iron dextran or iron sucrose) promotes the development of atherosclerosis in rabbits and ApoE-null mice (10, 54, 124, 137). A single study showed opposite results with iron dextran in rabbits (55). Although the majority of these studies support a proatherosclerotic role of iron, conflicting data may result from variable parameters and different experimental approaches, including the extent and length of iron treatment, the amount of iron and fat present in animal diet, the age of the animal at the moment of treatment and plaque analysis.
Lesson from animal models of iron restriction
Iron restriction by low-iron diet and iron chelators
Several studies examined the effect of iron restriction achieved by low-iron diet or iron chelation on atherogenesis (Table 3). In this regard, mice fed a low-iron diet developed smaller atherosclerotic lesions than control littermates (127). Dietary iron restriction likely increases plaque stability via elevated collagen and reduces matrix metalloproteinase-9 expressions in the lesion (126). Consistently, iron chelation by deferoxamine and desferricoprogen limits atherosclerotic lesion development in cholesterol-fed rabbit (144) as well as ApoE-null mice (256). Recently, low-iron diet and iron chelation by deferasirox were found to significantly prevent atherosclerosis progression in iron-loaded ApoE-null FPNC326 mice (232), by lowering arterial iron content, endothelial activation, and inflammation.
Table 3.
Experimental Therapeutic Approach Targeting Iron in Cardiovascular Disease
| Approach | Atherosclerosis phenotype | Treatment or model | Similarities and Differences | References |
|---|---|---|---|---|
| Iron restriction by reduced dietary iron | Reduced Increased plaque stability |
(i) Low-iron diet (3 months), reduced serum Ft (ii) Low-iron diet (4/8 months) in iron-loaded ApoE-nullFPNwtC/326S mice, reduced Tf sat |
(i, ii) ApoE-null background, elevated plaque collagen and reduced MMP9, reduced arterial and plaque iron deposition and lipid core (iii) ApoE-null background, reduced arterial iron, plaque inflammation, and endothelial dysfunction |
(i) (126) (ii) (127) (iii) (232) |
| Iron restriction by chelation | Reduced Decreased inflammation and vascular dysfunction |
(i) Deferoxamine (ip, 100 mg/kg, 10 weeks) (ii) Deferoxamine (ip, 72 mg/kg/day, 5 days/week, 12 weeks) (iii) Desferricoprogen (ip, 160 mg/kg/every 2 days, 8 weeks) (iv) Deferasirox (oral, 150 mg/kg/day, 4/8 months) in iron-loaded ApoE-nullFPNwtC/326S mice, reduced Tf sat |
(i) ApoE-null background, Western diet, reduced macrophages, and arterial inflammatory cytokines (ii) Rabbits, high cholesterol diet, reduced lesion size (iii) ApoE-null background, high-fat diet, reduced lipid oxidation, foam cells, and endothelial activation (iv) ApoE-null background, standard diet, reduced arterial iron, plaque inflammation, and endothelial dysfunction |
(i) (256) (ii) (144) (iii) (168) (iv) (232) |
| Hepcidin inhibition | Reduced Improved plaque macrophage phenotype |
(i) LDN-193189 (ip, 10 mg/kg, 10 weeks) (ii) BMP ligand trap ALK3-Fc (ip, 2 mg/kg/every 2 days, 6 weeks) (iii) Transgenic mouse model overexpressing BMP signaling antagonist MGP |
(i) ApoE-null background, high-fat diet, decreased foam cell formation, improved macrophage cholesterol efflux (ii) Ldlr-null background, Western diet, reduced plaque macrophages (iii) ApoE-null background, standard and high-fat diet, reduced calcification, and inflammation |
(i) (185) (ii) (60) (iii) (243) |
| HO-1 induction | Reduced | (i) Bach-1-null mice (ii) HO-1 overexpression by intraventricular Adv-HO-1 (iii) HO-1 induction by cobalt protoporphyrin |
(i) ApoE-null background, high-fat diet, vascular HO-1 upregulation (ii) ApoE-null background, standard diet, reduced arterial iron (iii) Model of vulnerable plaque by carotid cast, ApoE-null background, Western diet, reduced necrotic core and plaque lipid, increased VSMCs and cap thickness |
(i) (237) (ii) (105) (iii) (44) |
Approach, atherosclerosis phenotype, treatment or genetic manipulation, similarities and differences of animal models used to study the therapeutic benefit of iron targeting in CVD.
BMP, bone morphogenetic protein; MGP, matrix Gla protein.
Cellular iron depletion by hepcidin modulation
Hepcidin induction is critically regulated by the activation of the bone morphogenetic protein (BMP)/small mother against decapentaplegic (SMAD) signaling pathway. Therefore, inhibitors of hepcidin production such as the small molecule BMP type I receptor inhibitor LDN-193189 have been applied in animal models to reduce macrophage iron content (Table 3). Treatment of ApoE-null mice with LDN-193189 results in significant reduction of macrophage iron and decreased atherosclerotic lesion formation (185). LDN-193189 as well as a soluble receptor–antibody fusion protein acting as BMP ligand trap, ALK3-Fc, reduces vascular calcification, inflammation, and atheroma formation in Ldlr-null mice (60). The overexpression of another BMP signaling antagonist, matrix Gla protein (MGP), similarly decreases atherosclerosis severity and vascular calcification (243). Besides the key role in hepcidin induction, the BMP pathway is a critical mediator of vascular media calcification through vascular smooth muscle cells (VSMCs) osteogenic differentiation (133). Moreover, BMP signaling plays a role in cholesterol homeostasis. LDN-193189 in fact has been found to affect lipoprotein biosynthesis, thus reducing total cholesterol and LDL serum levels (60). Therefore, BMP modulators likely target multiple mechanisms in atherosclerosis, which are not restricted to hepcidin suppression and the modulation of iron levels. In addition, BMP signaling is crucial for the maintenance of bone and cartilage homeostasis, and its inhibition for antiatherosclerotic purposes might trigger a broad range of adverse events (21, 112, 235). Future studies testing drugs that reduce hepcidin expression without interfering with the BMP signaling or that directly counteract hepcidin activity are needed to examine the antiatherosclerotic benefit of hepcidin suppression.
Lesson from animal models of heme overload
Animal models with altered heme homeostasis have been used to better understand the proatherogenic role of heme (Table 2). A recent work showed that Hx deficiency in ApoE-null mice increases systemic free heme, ROS, and proinflammatory high-density lipoprotein, and aggravates atherosclerosis and macrophage infiltration (139). The proatherogenic action of Hb and heme is also demonstrated by studies on Hp polymorphisms, which give rise to the Hp 1-1, Hp 2-2, and Hp 2-1 genotypes. Mice bearing the Hp 2-2 allele show increased intraplaque iron deposition and lipid peroxidation as well as macrophage accumulation, typical features of advanced atherosclerosis (128). These effects are explained by the decreased ability of the Hp 2 allele to inhibit the pro-oxidant and proinflammatory action of Hb (12, 109). Indeed, in humans the Hp 2-2 genotype is associated with an increased risk of CVD and its sequelae such as acute MI (128, 231). Deficiency of the transcriptional repressor Bach1 in mice reduces atherosclerosis burden by upregulating HO-1, further demonstrating heme proatherosclerotic action and the importance of its catabolism (237) (Table 3). Consistent with these observations, HO-1 deficiency promotes while its induction prevents atherosclerosis progression in atherosclerosis-prone ApoE-null or LDLR-null mice (44, 105, 244). HO-1 expression in macrophages seems to be crucial to provide an atheroprotective effect (153) (Tables 2 and 3). Interestingly, the antiatherogenic action of HO-1 is also suggested by the observation that statins, the mainstay treatment for lowering cholesterol, are capable of triggering HO-1 expression both in endothelial cells (ECs) and macrophages through increased promoter activity and HO-1 transcription (5, 42, 146). Statin treatment has been also associated with reduced ferritin levels and hepcidin modulation (9, 40). Overall, these observations suggest that the beneficial role of statins in CVD can be in part mediated by their effects on heme and iron homeostasis.
Current Molecular Mechanisms of Iron-Mediated Vasculotoxicity
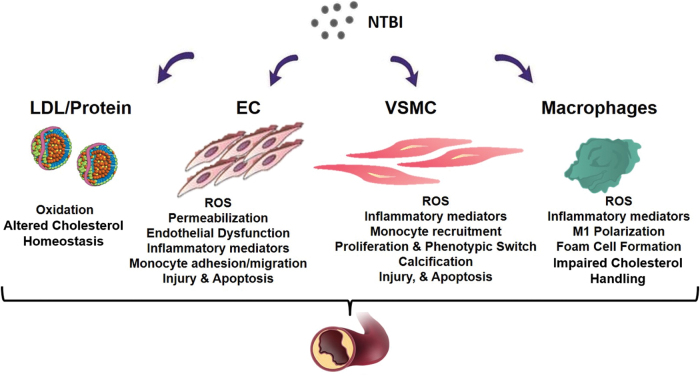
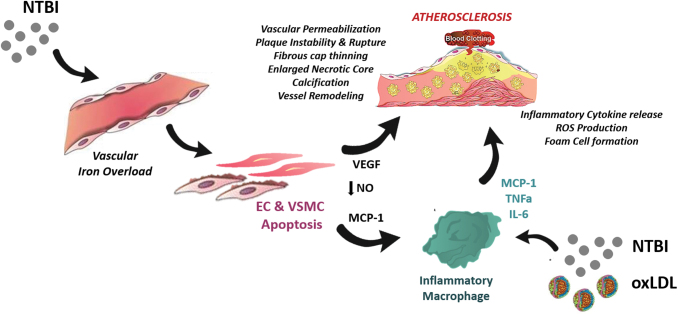
In the following section, I will illustrate how NTBI contributes to atherosclerosis at multiple levels by (i) altering the composition of the serum in a proinflammatory manner; (ii) affecting functionality and survival of endothelial and VSMCs; (iii) promoting foam cell formation; and (iv) inducing angiogenesis, calcification, and plaque destabilization (Fig. 2).
FIG. 2.
Proatherosclerotic action of NTBI. NTBI exerts a complex multifactorial action in atherosclerosis by (i) altering the composition of the serum in terms of cholesterol amount and oxidation of lipids and proteins; (ii) causing oxidative stress, activation, and apoptosis of ECs, which trigger vascular permeability and monocyte adhesion; (iii) affecting the functional phenotype and survival of VSMCs, which induce intraplaque monocyte recruitment and calcification formation; (iv) inducing macrophage shift toward a proinflammatory phenotype with impaired cholesterol handling properties, which facilitates evolution into foam cells. EC, endothelial cell; VSMC, vascular smooth muscle cell. Color images are available online.
Iron, cholesterol, and oxidative potential
Alterations in iron homeostasis could impact lipid metabolism. However, no consensus exists on whether iron overload increases or lowers cholesterol levels. Recent research, using a plethora of model organisms and data from clinical studies, has revealed novel links between iron homeostasis and lipid-related pathways (Fig. 2). Iron overload increases cholesterol levels by triggering the upregulation of key enzymes involved in the cholesterol biosynthetic pathway (86). The expression of cholesterol 7 α-hydroxylase (Cyp7a1), a major player of cholesterol homeostasis, is modulated by iron through a functional noncanonical IRE in the 3′-UTR of the transcript. Increased hepatic iron content results in cholesterol accumulation through IRE-mediated Cyp7a1 downregulation. Iron modulation of Cyp7a1 unravels a clear implication of iron metabolism in the regulation of cholesterol homeostasis through the IRE/IRP regulatory system (131). In addition, elevated iron levels increase the expression but reduce the secretion of a key lipid carrier, ApoE, potentially resulting in impaired cholesterol transport (33, 57, 182, 242).
Elevated iron levels exacerbate hyperlipidemia and hyperglycemia in animal models, including zebrafish, rats, mice as well as humans (35, 120, 137, 164, 232). Likewise, iron-loaded patients with HH show elevated cholesterol and hypertriglyceridemia (141, 204, 232), which can be improved by therapeutic phlebotomy (37, 232). Conversely, iron deficiency is associated with significantly lower hepatic cholesterol content and reduced serum total cholesterol, LDL, and triglyceride levels in rodents and men (47, 51, 138, 207, 227). The synergistic association of serum ferritin and LDL cholesterol with incident CVD supports the concept of a critical interaction between iron and lipids in CVD progression (119). By contrast, other studies showed an opposite modulation of cholesterol and lipid levels by iron in rodents and men as well as hemochromatotic patients (1, 82, 134, 156, 201). Conflicting findings in hemochromatosis may be explained by altered levels of the liver-specific microRNA 122 (miR-122), which regulate both iron and cholesterol metabolism (38). Decreased miR-122 expression in HFE-null mice and HH patients triggers hepcidin upregulation, and acts as a compensatory response to limit iron uptake and counteract iron overload. In addition, low miR-122 is implicated in the reduction of systemic cholesterol levels by targeting yet unidentified genes (66, 68). Therefore, the degree of iron overload in HH patients might determine the levels of microRNA miR-122, resulting in variable cholesterol levels and explaining the conflicting evidence from studies on hemochromatosis cohorts. The regulatory role of miR-122 provides an additional indication of the close interconnection between the homeostatic pathways of iron and cholesterol. This link is supported by a recent genome-wide association study, showing a significant overlap between genes or loci affecting iron biomarkers and known loci affecting plasma lipids or lipoproteins (28).
As serum lipid profile is affected by many factors, the variations of lipid concentration in the presence of elevated or reduced iron levels may not be related to iron overload/deficiency by itself. Patients with β-thalassemia, who are anemic but show transfusional iron overload, have reduced cholesterol levels, suggesting a further level of complexity in the interaction between anemia, iron overload, and cholesterol biosynthesis/utilization (8, 178, 179). Indeed, anemia is associated with low cholesterol level. Hypocholesterolemia is eventually induced by high erythropoietic activity due to the increased cholesterol demand by proliferating erythroid cells (196).
As additional consideration, iron excess exacerbates hypercholesterolemia in ApoE-null mice but only mildly in wild-type mice (137). Thus, aggravated hypercholesterolemia might result from the synergistic action of an already altered lipid state with iron overload (137, 232). Finally, how either dietary or body iron excess interferes with lipid absorption, and vice versa, is understudied. The observation of reduced LDL levels in Ldlr-null Hamp-null mice receiving a high-fat diet points in this direction, whereby fat intake might be modulated by iron levels (134, 154).
In addition to lipid profile alteration, an extent of NTBI and NHBH proatherogenic action lies in their ability to induce lipid and protein oxidation in cells and serum (15, 34, 76, 137, 232, 247) (Fig. 2). Free labile ferrous iron (Fe2+) gets easily engaged and participates in the Fenton and Haber-Weiss reactions, which ultimately lead to the formation of ROS, including free radical species, superoxide, hydroxyl radicals, and hydrogen peroxide (157). Directly through these reactions or indirectly through ROS, NTBI can induce the peroxidation of polyunsaturated fatty acid, and the resulting by-products, such as reactive aldehydes and gamma-keto aldehydes, likely contribute to the vasculotoxic and proinflammatory effects of NTBI (157). Through similar mechanisms, heme, once liberated from Hb after its oxidation to methemoglobin (17), catalyzes free radical reactions in cell lipid domains (16) and lipoproteins (15), thus triggering a pro-oxidant and cytotoxic environment in the vasculature (104) and atherosclerotic plaques (149). Vascular cells respond to such an insult by upregulating the antioxidant HOs and ferritins (3, 14, 17).
Intriguingly, the appearance of oxidized LDLs (oxLDLs) in ApoE−/− FPNwt/C326S mice precedes the elevation of LDL levels, suggesting that LDL oxidation is an early, if not the first, iron-triggered proatherogenic mechanism (232). The use of the iron chelator desferricoprogen inhibits plaque formation in ApoE-null mice by lowering oxLDL levels and limiting plaque lipid oxidation and deposition oxLDL-induced foam cell formation in the vessel wall (168). Moreover, desferricoprogen preserves endothelial integrity and prevents the upregulation of adhesion molecules, thus blunting monocyte–EC adhesion and inflammation.
To date, two mechanisms of iron-mediated alteration of cholesterol homeostasis have been suggested as proatherosclerotic: the alteration of the lipid profile and the systemic and cellular oxidation of lipids. The multifactorial interaction between iron and cholesterol homeostasis (e.g., iron-mediated regulation of cholesterol enzymes, mir122, ineffective erythropoiesis), and variables that influence cholesterol levels (e.g., diet, exercise, malabsorption, antioxidant systems, gender, ethnicity) add an inevitable complexity to the interpretation of experimental and clinical studies.
Iron and ECs
Iron contributes to the aggravation of atherosclerosis by triggering the proinflammatory activation of the vascular endothelium. This early step in atherogenosis facilitates intraplaque accumulation of LDL and recruitment of circulating monocytes through increased vascular permeability and monocyte–EC adhesion (Fig. 2).
Recently, mechanistic studies showed the involvement of iron-triggered endothelial dysfunction in cardiovascular complications. Endothelial activation and damage are features of iron-loaded ApoE-null FPNC326S mice (232). Exposure of a monolayer of ECs to NTBI-like iron sources (e.g., ferric ammonium citrate, iron nitrilotriacetate) triggers increased expression of adhesion molecules (e.g., vascular adhesion molecule 1 [VCAM1], intercellular adhesion molecule 1 [ICAM-1], E-selectin, P-selectin), secretion of chemoattractants (monocyte chemoattractant protein 1 [MCP-1]), intracellular ROS formation, and cell death (Fig. 3). In vivo this results in vasopermeabilization and increased monocyte adhesion, which likely promote LDL infiltration into the subendothelial space and intraplaque monocyte recruitment followed by foam cell formation (231, 232) (Fig. 2). In addition, iron impairs endothelium-dependent vasorelaxation, and triggers arterial stiffness by inducing ROS formation and decreasing NO bioavailability through reduced endothelial NO synthase expression/activation and/or enhanced NO oxidative consumption (61, 66, 95, 110, 137, 258). NTBI has also the ability to increase VEGF expression in ECs and VSMCs (13). Elevated VEGF has been implicated in atherosclerosis due to its proinflammatory and permeabilizing action on the vascular endothelium (39, 100).
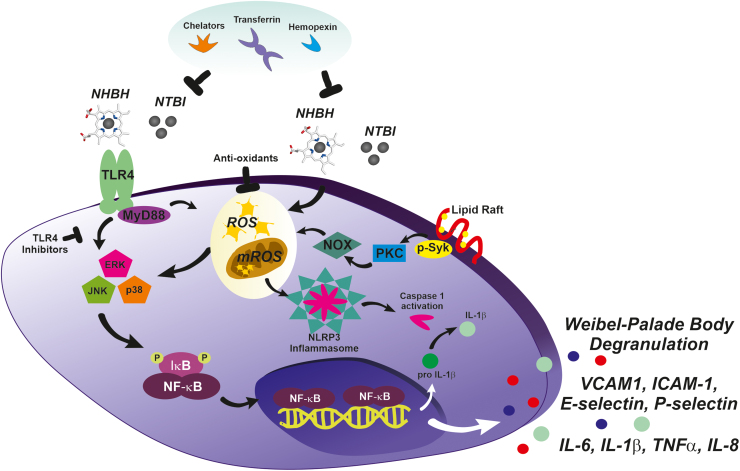
FIG. 3.
Molecular mechanisms underlying heme- and iron-driven EC and macrophage activation. NHBH and NTBI mediate the proinflammatory activation of ECs and macrophages by producing ROS and activating the TLR4/MyD88/MAPK/ERK/NFkB and inflammasome signaling pathways. Heme and iron induce ROS generation non-enzymatically, through the Fenton reaction and by converting organic hydroperoxides into free radicals, and enzymatically, through the activation of the p-Syk/PKC/NOX pathway. TLR4 activation by heme/iron promotes MAPKs and NFkB activation, which in turn induce the transcription of inflammation-related genes, including inflammatory cytokines and chemokines (e.g., IL-6, TNFα, IL-1β, IL-8) and adhesion molecules (e.g., E-selectin, P-selectin, ICAM-1, VCAM-1). Although heme and iron trigger ROS formation independently of TLR4 activation, ROS production is required for full MAPK activation and cytokine induction, suggesting a synergistic action of the two pathways. By inducing ROS, heme and iron also contribute to NLRP3/inflammasome activation, leading to the maturation of pro-IL1β to IL1β via caspase-1-mediated cleavage. TLR4 inhibitors and antioxidants by blocking the major heme/iron-induced pathways, and chelators (transferrin, deferoxamine, deferasirox, deferiprone, hemopexin) by scavenging heme/iron prevent the activation of ECs and macrophages in conditions where free heme and iron are elevated. ERK, extracellular-signal-regulated kinase; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response 88; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; NHBH, nonhemopexin-bound iron; NLRP3, NLR Family Pyrin Domain Containing 3; NOX, NADPH oxidase; PKC, protein kinase C; p-Syk = phosphorylated spleen tironase kinase; ROS, reactive oxygen species; TLR4, toll-like receptor 4; TNFα, tumor necrosis factor alpha; VCAM-1, vascular adhesion molecule 1. Color images are available online.
Exposure to free Hb and NHBH similarly impairs the vascular endothelium by inducing toll-like receptor 4 (TLR4)-mediated endothelial dysfunction, NO consumption, and inflammasome activation (24–26, 46, 71, 160, 167, 193, 200, 219, 229, 230, 233) (Fig. 3). Therefore, NHBH likely contributes to atherosclerosis, especially in advanced plaques where hemorrhages occur (103, 149, 231). NHBH- and NTBI-induced EC apoptosis and ROS production are prevented by heme and iron scavenging through Tf or deferoxamine and Hx, respectively (95, 110, 229). ApoE-null FPNC326S mice administered a low-iron diet or receiving a chelation treatment show normalized markers of endothelial activation (e.g., soluble adhesion molecules, nitrotyrosine, VEGF) (232). By reducing cell iron content and oxidative stress, iron chelation as well as a combined iron chelation–antioxidant therapy significantly limits endothelial damage and vascular dysfunction in animal models (102, 205, 231). Interestingly, chelation also prevents EC activation and adhesion molecule expression induced by inflammatory mediators such as tumor necrosis factor α and lipopolysaccharide (255).
Additional processes in which free Hb and heme might play a proatherosclerotic role are neovascularization and atheroma formation. Because of inappropriate angiogenesis, neovessels that form within the lesion are leaky and prone to rupture, leading to intraplaque extravasation of RBCs (103). Intraplaque hemorrhages are considered features of plaque progression and vulnerability and a critical event in atherosclerosis-associated acute clinical symptoms (142). The advanced atheromatous lesion is hallmarked by a pro-oxidant environment whereby hemolytic RBCs release Hb, which is in turn oxidized to ferri- and ferryl-Hb. These Hb forms and liberated heme and iron promote intraplaque lipid oxidation and endothelial activation and cytotoxicity, further contributing to lesion development (149). Essential to counteract heme/iron-oxLDL cytotoxic action on ECs is the ferroxidase activity of intracellular H-Ft and the scavenging function of extracellular Hp, Hx, and Tf (51, 106).
Iron and VSMCs
Major alterations in VSMCs have been recently implicated in iron-aggravated atherosclerosis (Fig. 2). VSMCs accumulate high amount of NTBI without option to export it, due to the lack of FPN expression. This is in line with the observations that (i) iron accumulation occurs in human arterial tissue and is significantly higher in patients with high plasma ferritin (132); (ii) iron heavily deposits in VSMCs of the aortic media layer of iron-loaded ApoE-null FPNC326S mice (232) (Fig. 1C, D). The association between elevated systemic iron and CVD might at least in part ensue from the detrimental effect of NTBI accumulation in VSMCs of the arterial wall, which contributes to vascular dysfunction and plaque formation. When exposed to NTBI, VSMCs develop iron overload, produce ROS, and undergo apoptosis associated with the release of MCP-1 (Figs. 2–4C). Indeed, apoptotic iron-loaded VSMCs stimulate the recruitment of monocytes to the growing plaque, promoting plaque progression (Fig. 4D) (232). Collagen reduction in atherosclerotic lesions of ApoE-null FPNC326S mice suggests decreased production of extracellular matrix by iron-loaded VSMCs and/or increased matrix degradation due to metalloprotease release by VSMCs or macrophages (Fig. 4A) (232). Since iron overload was found to trigger vascular collagen deposition (61), we speculate that while plaque collagen accumulates at early stages of atherosclerosis, reduced collagen production and/or increased matrix degradation likely occur at more advanced stages, leading to a decreased intraplaque collagen amount as observed in ApoE-null FPNC326S mice. One of the mechanisms potentially implicated in reduced matrix deposition at more advanced stage of atherosclerosis is endoplasmic reticulum (ER) stress. Elevated ER stress markers have been observed in VSMCs of complicated lesions with hemorrhage compared with either atheromas or healthy arteries (80). Interestingly, heme triggers ER stress in human VSMCs, which is inhibited by heme scavenging and antioxidants. By suppressing transforming growth factor beta (TGFβ) expression and collagen production, heme-induced ER stress likely contributes to plaque instability and progression (80). Heme regulation of TGFβ levels in VSMCs is hallmarked by an early induction followed by suppression. This biphasic behavior could explain the differential effect of heme/iron on matrix deposition at different stages of atherosclerosis. Overall, the progressive reduction of the extracellular matrix likely results in fibrous plaque thinning and plaque instability.
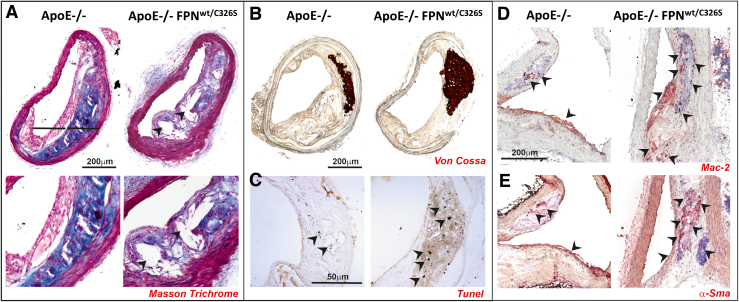
FIG. 4.
NTBI triggers intraplaque lipid accumulation, cell apoptosis, macrophage recruitment, VSMC phenotypic switch, and vascular calcification. (A, B) Representative images of common carotid artery of ApoE-null and ApoE-null FPNC326S mice stained with (A) Masson's trichrome, (B) Von Cossa, and (C) Tunel stains. (D, E) Representative images of innominate arteries of ApoE-null and ApoE-null FPNC326S mice stained with antibodies against (D) Mac-2 and (E) α-Sma. (A) The Masson trichrome stain shows reduced collagen deposition (blue) and increased lipid droplets (white) within the atherosclerotic plaque of ApoE-null FPNC326S compared with ApoE-null control mice. Arrowheads show lipid-filled areas. (B) The Von Cossa stain shows the presence of dark brown-stained intraplaque calcifications, which are bigger and more frequent in ApoE-null FPNC326S mice. (C) The Tunel stain reveals the presence of multiple Tunel-positive dark apoptotic cells within the plaque of the ApoE-null FPNC326S mice, which are almost absent in the ApoE-null mice. Arrowheads show Tunel-positive cells. (D) Mac-2 stains show a higher number of Mac-2-positive macrophages in the plaque of ApoE-null FPNC326S mice compared with ApoE-null mice. Arrowheads point at Mac-2-positive cells. (E) α-Sma stains show α-Sma-positive cells in the plaque of ApoE-null and ApoE-null FPNC326S mice. Arrowheads point at α-Sma-positive cells. The partial overlapping of Mac-2 and α-Sma stain suggests that VSMCs might undergo a phenotypic switching toward macrophage-like cells. αSMA, α smooth muscle actin; Mac2, macrophage 2 protein or Galectin 3. Color images are available online.
After migrating intraplaque, VSMCs might acquire a macrophage-like phenotype, as indicated by the coexpression of both VSMC and macrophage markers (e.g., α smooth muscle actin [α-SMA] and macrophage 2 protein or Galectin 3 [Mac2]) (see atherosclerotic plaques of ApoE-null FPNC326S mice, Fig. 4D, E) (232). Iron-triggered ROS are potentially implicated in VSMC migration. ROS serve in fact as mediators of promigratory signaling pathways, which control VSMC motility through lamellipodia formation, actin cytoskeleton remodeling, focal adhesion turnover, cell body contraction, and matrix remodeling (187). VSMC-derived macrophage-like cells, by behaving as less-differentiated/specialized cells, may directly promote atherosclerosis via inflammation, poor lipid handling, and impaired VSMC-related functions (including matrix deposition) (22, 27).
VSMCs can also undergo transdifferentiation into osteoblasts and chondrocytes. Whereas oxidative stress has been associated with osteogenic differentiation of VSMCs and calcification, iron has been shown to prevent phosphate-induced vascular calcification, through the inhibition of VSMC osteochondrogenic shift (48–50, 195). Ferritin was identified as the major protective molecule behind iron-mediated inhibition of mineralization (23, 252, 253). Moreover, iron might negatively regulate calcification by binding and decreasing phosphate levels in VSMCs (252), and by reducing its systemic levels through the induction of the phosphaturic hormone fibroblast growth factor 23 (FGF23) (194). Therefore, excess iron enhances ROS levels, which in turn trigger molecular mechanisms leading to vascular calcification, whereas iron deposition inhibits this process by inducing protective molecules such as ferritin and HO-1. In vivo, the formation of vascular calcification is likely determined by the balance between these mechanisms, whereby the exhaustion of antioxidant system capacity due to excessive iron-driven ROS production acts as calcification trigger.
Iron contributes to calcification also in an indirect manner, through (i) the oxidation of lipids and proteins (245), (ii) the increased production of BMP-2 (56, 117), and (iii) the induction of cell apoptosis (188, 192). oxLDL and proteins and BMP-2 promote the differentiation of vascular cells to a calcifying osteoblast-like cell type (56, 245). Apoptotic cells induce arterial matrix calcification by releasing calcifying membrane-bound matrix vesicle (62). Recent findings show indeed that iron boosts rather than suppressing VSMC osteogenic differentiation (117, 151). In support of an active role of iron in triggering vascular calcification, iron overload is associated with more frequent and bigger calcifications in ApoE-null FPNC326S mice (232) (Fig. 3B). In this context, iron-driven apoptosis of ECs and VSMCs is likely a major driver of calcification. The contribution of VSMC osteochondrogenic shift to atherosclerosis remains to be elucidated in this and other models of iron overload.
While most NTBI accumulates in the arterial media layer, calcifications are mainly located intraplaque (232) (Fig. 3B). Likewise, a spatial inverse correlation of iron and calcium was shown within the atherosclerotic lesion (173). However, a link between iron and calcium dyshomeostasis is supported by the association between increased ferritin levels and presence of coronary artery calcium in men (216). Not only VSMCs but also recruited macrophages might be implicated in this process, as indicated by the direct association between calcification and macrophage burden in ApoE-null mice (97).
Another relevant atherosclerotic mechanism consists in vascular remodeling. The impact of iron overload on this process still remains understudied. Evidence from iron-overloaded animals shows that iron increases aortic stiffness and induces vascular remodeling associated with collagen deposition (72, 177). Indeed, the reduced distensibility of the aorta from iron-loaded rats is accompanied by collagen deposition, suggesting that changes in the composition of the wall are responsible for iron-induced stiffness. Overall, iron overload increases the vasoconstrictor response of arteries, associated with altered vascular reactivity and the loss of endothelial modulation of the vascular tone (72, 137). Interestingly, inhibition of the renin–angiotensin system limits vascular remodeling in iron-loaded animals, suggesting a critical role of this pathway in iron-triggered aortic stiffness (72). Nonpathological iron accumulation has been shown to drive VSMC proliferation rather than apoptosis, contributing to vascular remodeling and pulmonary hypertension (174). Iron chelation as well as deficiency of the TfR1 inhibits pulmonary artery SMC proliferation and prevents chronic hypoxia-induced pulmonary hypertension (150, 165, 238).
Iron and macrophages
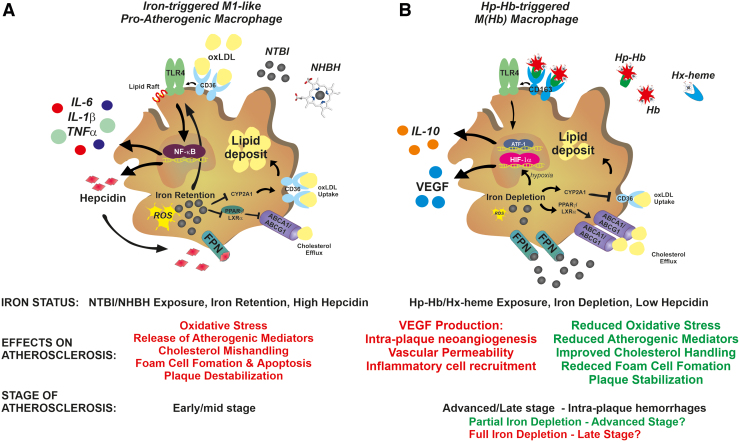
Macrophages are key cells contributing to plaque development through the evolution into lipid-laden foam cells. Whether and how iron content in intraplaque macrophages impacts atherosclerosis is still a matter of debate. Although available evidence is conflicting, the majority suggests that iron accumulation in macrophages has a detrimental role in atherosclerosis (Figs. 2–5). Iron-loaded macrophages show in fact inflammatory properties and impaired cholesterol handling abilities. Intracellular iron content regulates macrophage polarization by triggering macrophage inflammatory activation (98, 123, 176, 202). The exposure of macrophages to NTBI-induces an M1-like proinflammatory phenotypic switching hallmarked by the expression of inflammatory markers and cytokines (53, 98, 101, 123, 202, 218, 228, 251, 254, 259) (Fig. 5A). Likewise, iron accumulation consequent to FPN deficiency increases the expression of inflammatory cytokines in macrophages (257).
FIG. 5.
Role of macrophage iron content in atherosclerosis. (A) In early/mid-stage atherosclerotic plaques, exposure to NHBH and NTBI directs macrophages toward a proinflammatory phenotypic switching, with potential proatherosclerotic action. Iron and heme stimulate the activation of the TLR/NFkB signaling pathway, which is responsible for macrophage inflammatory activation. In a synergistic manner, oxLDL and iron activate the TLR4 pathway, which in turn triggers the autocrine release of hepcidin. This results in the exacerbation of iron accumulation, ROS production, and inflammatory response through a positive feedback loop. In addition, iron retention accelerates foam cell formation by (i) promoting LDL oxidation and (ii) inducing cholesterol mishandling through increased CD36-mediated cholesterol uptake and decreased ABC transporter ABCA1/ABCG1-mediated reverse cholesterol efflux via interference with the CYP27A1/27HC and PPARγ/LXRα signaling, respectively. Iron and lipids therefore show a synergistic action in accelerating foam cell formation. ROS and TLR4 signaling pathways play major roles in iron-driven macrophage inflammatory activation and foam cell formation, which are prevented by chelators, antioxidants, and TLR4 inhibitors. (B) In advanced hemorrhagic plaques, exposure to the Hp–Hb or Hx–heme complexes directs macrophages toward an iron-recycling phenotype characterized by increased ability to take up Hb via the CD163 receptor and export iron via FPN, which lead to reduced intracellular iron and ROS formation. These macrophages, defined as M(Hb) or Mhem macrophages, show reduced lipid retention, decreased production of inflammatory cytokines, and increased secretion of the anti-inflammatory atheroprotective cytokine IL-10. Reduced intracellular iron and ROS lower inflammatory cytokine production, and improve lipid handling by reducing cholesterol loading via CD36 downregulation and increasing reverse cholesterol efflux via ABC transporter upregulation. While these nonfoam M(Hb) macrophages are in principle atheroprotective, the progressive intracellular iron depletion leads to HIF1α stabilization and VEGF secretion. VEGF exerts proatherosclerotic effects by inducing vascular permeabilization, intraplaque neoangiogenesis, and immune cell recruitment. Whether M(Hb) macrophages play an initial protective effect, which turns into a proatherosclerotic one when severe iron depletion is achieved, remains to be determined. Whereas iron depletion and low hepcidin levels are desirable in early/mid-stage atherosclerosis to activate an atheroprotective phenotypic switching of macrophages, iron balance in late-stage atherosclerosis is preferred to prevent VEGF-related atherosclerotic effects. ABC, ATP-binding cassette; Hb, hemoglobin; HIF1α, hypoxia-inducible factor 1α; Hp, haptoglobin; Hx, hemopexin; ox-LDL, oxidized low-density lipoprotein; VEGF, vascular endothelial growth factor. Color images are available online.
A similar effect is exerted by NHBH and free Hb, which trigger cytokine production, inflammasome activation, and cell death in macrophages (53, 63, 64, 73, 75, 152, 218, 228). Preliminary studies suggest that iron induces macrophage inflammatory switch by triggering a metabolic shift toward glycolysis (101). The inflammatory storm triggered by iron-activated macrophages likely contributes to intraplaque inflammation and lesion progression.
oxLDL exposure induces a specific macrophage phenotype, the Mox macrophage, hallmarked by increased expression of nuclear factor erythroid 2-related factor 2 (Nrf2)-induced redox-regulatory genes and oxidized phospholipid-induced genes (107). In response to oxLDL, macrophages upregulate the oxidative stress response enzyme HO-1 and hepcidin, resulting in cell iron accumulation (129, 136). oxLDL-induced macrophage iron retention is mediated by TLR4/nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB) pathway activation, which stimulates the autocrine production of hepcidin (241). Hepcidin-mediated iron accumulation further synergizes with oxLDLs to activate the TLR4 pathway, thus establishing a positive feedback loop. Moreover, macrophage ER stress, which is common in advanced atherosclerosis, plays a role in further increasing hepcidin autocrine production (199, 225). Hepcidin-mediated intracellular iron trapping results in impaired macrophage cholesterol handling, due to enhanced CD36-mediated cholesterol uptake and decreased ABCA1- and ABCG1-mediated reverse cholesterol efflux (Fig. 5). These effects are amplified by exogenous iron sources, such as NTBI-like iron exposure and erythrophagocytosis (122, 234, 241, 256). Iron increases TLR4-dependent macrophage activation by regulating TLR4 trafficking to cell lipid rafts (92). ROS and TLR4 signaling pathway play a critical role in iron-driven inflammatory activation and foam cell formation. Chelators, antioxidants, and TLR4 inhibitors prevent macrophage M1-like phenotypic switching and cholesterol mishandling (129, 218, 228, 241, 257) (Fig. 3).
In vivo, hepcidin overexpression in murine carotid arteries affects plaque composition, increasing intraplaque macrophages and decreasing VSMCs and collagen content (129). This is associated with iron trapping, ROS and cytokine production, and oxLDL accumulation in intraplaque macrophages. Likewise, hepcidin levels and macrophage iron positively correlate with interleukin (IL)-6 and MCP-1 levels and vascular damage in individuals at high risk of vascular disease (223). By contrast, monocytes from HH patients show reduced iron content, and reduced MCP-1 and IL-6 levels (223). Hepcidin deficiency or its pharmacological suppression decreases macrophage iron content and increases cell cholesterol efflux, thus resulting in reduced foam cell formation (134, 185). Reduced macrophage iron content limits ROS formation and increases cholesterol transporter expression, leading to improved cell lipid efflux and reduced foam cell formation.
Overall, these findings indicate that (i) hepcidin is a positive regulator of atherosclerotic plaque destabilization, through the control of macrophage iron content. Elevated hepcidin increases cell iron content, and promotes plaque progression and destabilization by exacerbating macrophage inflammatory cytokine release, intracellular lipid loading, oxidative stress, and cell apoptosis (129, 136, 241). This eventually implies a role not only for systemic but also for local hepcidin production in atherosclerosis, similarly to the local effect that has been recently shown for cardiac hepcidin (226); (ii) iron and lipids act synergistically, and promote foam cell formation by enhancing LDL uptake and impairing iron efflux and cholesterol handling (234, 241).
A different macrophage phenotype has been described within areas of intraplaque hemorrhage and neoangiogenesis, characterized by reduced iron content, resistance to foam cell formation, decreased intracellular ROS formation and inflammatory cytokine expression, and increased levels of FPN and the Hp–Hb scavenger receptor CD163 (30, 31, 74) (Fig. 5B). The so called Mheme or M(Hb) macrophages are triggered by exposure to the Hp–Hb complex, and are considered atheroprotective due to their increased cell iron efflux and cholesterol handling abilities and anti-inflammatory properties. Nevertheless, a proatherogenic function of these macrophages has been recently described, adding complexity to the role of macrophage iron content in atherosclerosis (90). Iron depletion in M(Hb) macrophages leads to the stabilization of the hypoxia-inducible factor 1α and the upregulation of hypoxia-regulated VEGF. VEGF release by macrophages is associated with intraplaque angiogenesis, increased vascular permeability, and inflammatory cell recruitment, leading to severe atherosclerosis (88) (Fig. 5B). These findings are in line with the observation of a proatherosclerotic role of VEGF (39, 100) and the severe atherosclerosis phenotype in iron-loaded ApoE-null FPNC326S mice, which show elevated VEGF levels (232). Genetic ablation of CD163 in ApoE-null mice rescues M(Hb)-associated phenotype. Therefore, despite beneficial features such as improved cholesterol handling, macrophage iron depletion might activate other detrimental mechanisms such as hypoxia-dependent ones. In addition, macrophage iron efflux likely increases iron levels in the plaque microenvironments with potential proatherogenic effects on other cell types (e.g., ECs, VSMCs). Remarkably, VEGF appears as a key mediator of iron-aggravated atherosclerosis, being produced by multiple cell types within the atherosclerotic lesion—ECs, VSMCs, and M(Hb) macrophages (88, 125). Indeed, hemorrhagic areas are likely enriched in M(Hb) macrophages actively pumping iron in the plaque microenvironment and resulting in VEGF production by macrophages, VSMCs, and ECs (66, 88). This points toward VEGF as potential target for antiatherosclerotic treatments in iron overload conditions.
Collectively, these findings indicate that the interactions between hepcidin, retained iron, and accumulated lipids are critical for the atherosclerotic role of macrophages. The dual action of macrophage iron depletion in atherosclerosis suggests a potential beneficial effect at early stages of atherosclerosis—associated with improved lipid handling—and detrimental effect in more advanced stages, when intraplaque neovascularization, hemorrhages, and hypoxia occur (52, 89, 240).
Finally, iron might act on other immune cells within the atherosclerotic plaque. Iron and heme have been proven to affect lymphocyte and neutrophil functions (11, 36, 41, 64, 158, 162, 166, 197, 236), but a direct implication of these mechanisms in atherosclerosis still awaits investigation.
Cardiovascular Health As a Result of Iron Balance
In conclusion, the maintenance of iron balance and Tf saturation within proper limits has a critical role in cardiovascular health. The difficult definition of a risk threshold of iron level within the iron spectrum, whereby both iron deficiency and iron overload promote CVD, likely explains the lack of coherence among studies in this area of investigation. The level of complexity is further increased by the multifactorial contribution of iron to atherosclerosis. Emerging evidence suggests that circulating NTBI, by accumulating in vascular cells and macrophages and altering their functions, play crucial roles in the exacerbation of atherosclerotic disease (Fig. 6).
FIG. 6.
Cascade of events leading to iron-aggravated atherosclerosis. NTBI accumulates in ECs and VSMCs, which eventually undergo apoptosis. Apoptotic ECs and VSMCs produce elevated levels of VEGF and MCP1, which trigger vasopermeabilization and immune cell recruitment, respectively. Increased vascular permeability induces the subendothelial infiltration of LDL, promoting plaque development. Iron-induced NO reduction contributes to proatherogenic mechanisms, including endothelial activation, vascular dysfunction, and arterial stiffness. Under these conditions, monocytes recruited to the plaque differentiate into macrophages and develop a proinflammatory phenotype due to NTBI exposure. This phenotype is associated with poor cholesterol handling ability and foam cell formation. Necrotic core and calcification appear as consequence of NTBI-induced VSMC apoptosis, foam cell development, and collagen loss. These events synergize to promote plaque instability and increased propensity to rupture, with thrombus formation. MCP1, monocyte chemoattractant protein 1; NO, nitric oxide. Color images are available online.
Additional investigations are needed to elucidate what cells within the atherosclerotic plaques and the vessel wall are more sensitive to fluctuations in the catalytic labile iron pool, and better understand at what stage(s) of the disease iron is detrimental. Moreover, the identification of NTBI as a risk factor for atherosclerosis and its use as marker of iron status may help clarify some previously observed controversies, where the distinction between body iron stores and NTBI was not evaluated. Studies are also needed to evaluate the range of LPI which, within the NTBI, is responsible for vasculotoxicity (32, 69).
Despite the potential clinical relevance and the effort made in the last few years to improve available techniques, numerous challenges remain with laboratory standardization and harmonization for the measurement of NTBI and LPI. Nowadays, still few but increasing laboratories have established reproducible and reliable methodology for NTBI and LPI quantification (69, 81, 84, 203). Ten worldwide leading assays—six for NTBI and four for LPI—have been recently compared in the second international round robin for the quantification of serum NTI and LPI (58). All the assays consistently detected elevated NTBI and LPI in patients with untreated HH and β-thalassemia intermedia as well as transfusion-dependent myelodysplastic syndromes and β-thalassemia major. Absolute NTBI and LPI levels differed considerably between assays, with differences lower for LPI than for NTBI measurement (58). Assays showed reproducibility with high between-sample and low within-sample variation. Increased Tf saturation, but not ferritin, correlated and was a good indicator of the presence of circulating NTBI (58). We predict that with methodology improvement, the measurement of these parameters will be more widely adopted in clinical laboratory setting as marker and indicator of iron toxicity, including cardiovascular toxicity.
The use of antioxidants, ranging from vitamin E (alpha-tocopherol), vitamin C, beta-carotene to iron chelation or restriction, has been always considered a potential approach to reduce LDL oxidation and delay atherosclerosis progression. However, this hypothesis has been challenged recently by the failure of most antioxidants to reduce disease progression and clinical events in patients at risk of or with established atherosclerosis (85, 208, 222). An exception to the failure of antioxidants is probucol, a cholesterol lowering drug, which has the benefit to inhibit macrophage accumulation, stimulate re-endothelialization, and prevent VSMC proliferation by inducing HO-1 (191, 239). While HO-1 targeting might represent a valuable approach to elicit antioxidant activity with antiatherosclerotic function, our knowledge about the effect of iron restriction as intervention to limit iron-driven proatherogenic consequences in humans is still limited. Yet, what are the most effective iron restriction approaches and how they could further interact with other antiatherogenic strategies (e.g., statins, anti-inflammatory therapy) remain to be determined. Finally, more studies are required to better address the role of iron fluctuations within physiological limits in atherosclerosis in the healthy population as well as animal models.
Acknowledgments
The author thanks all the coauthors of the article “Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J 2020,” which is extensively reviewed in this work, and especially Martina U. Muckenthaler and Matthias W. Hentze together with whom this research was shared with passion and enthusiasm for several years.
Abbreviations Used
- αSMA
α smooth muscle actin
- ABC
ATP-binding cassette
- ApoE
apolipoprotein E
- BMP
bone morphogenetic protein
- CAD
coronary artery disease
- CVD
cardiovascular disease
- Cyp7a1
cholesterol 7 α-hydroxylase
- EC
endothelial cell
- ER
endoplasmic reticulum
- ERK
extracellular-signal-regulated kinase
- FGF23
fibroblast growth factor 23
- FPN
ferroportin
- Ft
ferritin
- Hamp
hepcidin gene
- Hb
hemoglobin
- HFE
high Fe2+ protein
- H-Ft
ferritin heavy chain
- HH
hereditary hemochromatosis
- HIF1α
hypoxia-inducible factor 1 α
- HO-1
heme oxygenase 1
- Hp
haptoglobin
- Hx
hemopexin
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- ip
intraperitoneal
- IRE
iron regulatory element
- IRP
iron regulatory protein
- JNK
c-Jun N-terminal kinase
- LDL
low-density lipoprotein
- Ldlr
low-density lipoprotein receptor
- LPI
labile plasma iron
- Mac2
macrophage 2 protein or Galectin 3
- MCP-1
monocyte chemoattractant protein 1
- MGP
matrix Gla protein
- MI
myocardial infarction
- miR122
microRNA 122
- mROS
mitochondrial reactive oxygen species
- MyD88
myeloid differentiation primary response 88
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NHBH
nonhemopexin-bound iron
- NHBHB
non-Hp bound Hb
- NLRP3
NLR Family Pyrin Domain Containing 3
- NO
nitric oxide
- NOX
NADPH oxidase
- Nrf2
nuclear factor erythroid 2-related factor 2
- NTBI
nontransferrin-bound iron
- ox-LDL
oxidized low-density lipoprotein
- P38 MAPK
P38 mitogen-activated protein kinase
- PKC
protein kinase C
- p-Syk
phosphorylated spleen tironase kinase
- RBC
red blood cell
- ROS
reactive oxygen species
- SMAD
small mother against decapentaplegic
- Tf
transferrin
- TfR1
transferrin receptor 1
- TfR2
transferrin receptor 2
- TGFβ
transforming growth factor beta
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor α
- UTR
untranslated region
- VCAM-1
vascular adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Adams PC, Pankow JS, Barton JC, Acton RT, Leiendecker-Foster C, McLaren GD, Speechley M, and Eckfeldt JH. HFE C282Y homozygosity is associated with lower total and low-density lipoprotein cholesterol: the hemochromatosis and iron overload screening study. Circ Cardiovasc Genet 2: 34–37, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Aessopos A, Farmakis D, Tsironi M, Diamanti-Kandarakis E, Matzourani M, Fragodimiri C, Hatziliami A, and Karagiorga M. Endothelial function and arterial stiffness in sickle-thalassemia patients. Atherosclerosis 191: 427–432, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Agarwal A, Balla J, Balla G, Croatt AJ, Vercellotti GM, and Nath KA. Renal tubular epithelial cells mimic endothelial cells upon exposure to oxidized LDL. Am J Physiol 271: F814–F823, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Ahluwalia N, Genoux A, Ferrieres J, Perret B, Carayol M, Drouet L, and Ruidavets JB. Iron status is associated with carotid atherosclerotic plaques in middle-aged adults. J Nutr 140: 812–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ali F, Hamdulay SS, Kinderlerer AR, Boyle JJ, Lidington EA, Yamaguchi T, Soares MP, Haskard DO, Randi AM, and Mason JC. Statin-mediated cytoprotection of human vascular endothelial cells: a role for Kruppel-like factor 2-dependent induction of heme oxygenase-1. J Thromb Haemost 5: 2537–2546, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Aljwaid H, White DL, Collard KJ, Moody AJ, and Pinkney JH. Non-transferrin-bound iron is associated with biomarkers of oxidative stress, inflammation and endothelial dysfunction in type 2 diabetes. J Diabetes Complications 29: 943–949, 2015 [DOI] [PubMed] [Google Scholar]
- 7. Altamura S, Kessler R, Grone HJ, Gretz N, Hentze MW, Galy B, and Muckenthaler MU. Resistance of ferroportin to hepcidin binding causes exocrine pancreatic failure and fatal iron overload. Cell Metab 20: 359–367, 2014 [DOI] [PubMed] [Google Scholar]
- 8. Amendola G, Danise P, Todisco N, D'Urzo G, Di Palma A, and Di Concilio R. Lipid profile in beta-thalassemia intermedia patients: correlation with erythroid bone marrow activity. Int J Lab Hematol 29: 172–176, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Arabul M, Gullulu M, Yilmaz Y, Akdag I, Kahvecioglu S, Eren MA, and Dilek K. Effect of fluvastatin on serum prohepcidin levels in patients with end-stage renal disease. Clin Biochem 41: 1055–1058, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Araujo JA, Romano EL, Brito BE, Parthe V, Romano M, Bracho M, Montano RF, and Cardier J. Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler Thromb Vasc Biol 15: 1172–1180, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Arruda MA, Rossi AG, de Freitas MS, Barja-Fidalgo C, and Graca-Souza AV. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J Immunol 173: 2023–2030, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Asleh R, Miller-Lotan R, Aviram M, Hayek T, Yulish M, Levy JE, Miller B, Blum S, Milman U, Shapira C, and Levy AP. Haptoglobin genotype is a regulator of reverse cholesterol transport in diabetes in vitro and in vivo. Circ Res 99: 1419–1425, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Bagheri B, Shokrzadeh M, Mokhberi V, Azizi S, Khalilian A, Akbari N, Habibi V, Yousefnejad K, Tabiban S, and Nabati M. Association between serum iron and the severity of coronary artery disease. Int Cardiovasc Res J 7: 95–98, 2013 [PMC free article] [PubMed] [Google Scholar]
- 14. Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, and Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992 [PubMed] [Google Scholar]
- 15. Balla G, Jacob HS, Eaton JW, Belcher JD, and Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb 11: 1700–1711, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, and Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest 64: 648–655, 1991 [PubMed] [Google Scholar]
- 17. Balla J, Jacob HS, Balla G, Nath K, Eaton JW, and Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A 90: 9285–9289, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Eaton JW, and Balla G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res 49: 1030–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, and Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal 9: 2119–2137, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Balla J, Vercellotti GM, Nath K, Yachie A, Nagy E, Eaton JW, and Balla G. Haem, haem oxygenase and ferritin in vascular endothelial cell injury. Nephrol Dial Transplant 18(Suppl 5): v8–v12, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Bandyopadhyay A, Yadav PS, and Prashar P. BMP signaling in development and diseases: a pharmacological perspective. Biochem Pharmacol 85: 857–864, 2013 [DOI] [PubMed] [Google Scholar]
- 22. Basatemur GL, Jorgensen HF, Clarke MCH, Bennett MR, and Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol 16: 727–744, 2019 [DOI] [PubMed] [Google Scholar]
- 23. Becs G, Zarjou A, Agarwal A, Kovacs KE, Becs A, Nyitrai M, Balogh E, Banyai E, Eaton JW, Arosio P, Poli M, Jeney V, Balla J, and Balla G. Pharmacological induction of ferritin prevents osteoblastic transformation of smooth muscle cells. J Cell Mol Med 20: 217–230, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belcher JD, Beckman JD, Balla G, Balla J, and Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, and Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123: 377–390, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Belcher JD, Nath KA, and Vercellotti GM. Vasculotoxic and proinflammatory effects of plasma heme: cell signaling and cytoprotective responses. ISRN Oxidative Med 2013: 831596, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bennett MR, Sinha S, and Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res 118: 692–702, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benyamin B, Esko T, Ried JS, Radhakrishnan A, Vermeulen SH, Traglia M, Gogele M, Anderson D, Broer L, Podmore C, Luan J, Kutalik Z, Sanna S, van der Meer P, Tanaka T, Wang F, Westra HJ, Franke L, Mihailov E, Milani L, Halldin J, Winkelmann J, Meitinger T, Thiery J, Peters A, Waldenberger M, Rendon A, Jolley J, Sambrook J, Kiemeney LA, Sweep FC, Sala CF, Schwienbacher C, Pichler I, Hui J, Demirkan A, Isaacs A, Amin N, Steri M, Waeber G, Verweij N, Powell JE, Nyholt DR, Heath AC, Madden PA, Visscher PM, Wright MJ, Montgomery GW, Martin NG, Hernandez D, Bandinelli S, van der Harst P, Uda M, Vollenweider P, Scott RA, Langenberg C, Wareham NJ, InterAct C, van Duijn C, Beilby J, Pramstaller PP, Hicks AA, Ouwehand WH, Oexle K, Gieger C, Metspalu A, Camaschella C, Toniolo D, Swinkels DW, and Whitfield JB. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun 5: 4926, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boudrahem-Addour N, Izem-Meziane M, Bouguerra K, Nadjem N, Zidani N, Belhani M, and Djerdjouri B. Oxidative status and plasma lipid profile in beta-thalassemia patients. Hemoglobin 39: 36–41, 2015 [DOI] [PubMed] [Google Scholar]
- 30. Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, and Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 174: 1097–1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, and Haskard DO. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 110: 20–33, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, and Cabantchik ZI. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron, used for assessing chelation therapy. Blood 97: 792–798, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Britton LJ, Bridle K, Jaskowski LA, He J, Ng C, Ruelcke JE, Mohamed A, Reiling J, Santrampurwala N, Hill MM, Whitehead JP, Subramaniam VN, and Crawford DHG. Iron inhibits the secretion of apolipoprotein E in cultured human adipocytes. Cell Mol Gastroenterol Hepatol 6: 215–217.e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brouwers A, Langlois M, Delanghe J, Billiet J, De Buyzere M, Vercaemst R, Rietzschel E, Bernard D, and Blaton V. Oxidized low-density lipoprotein, iron stores, and haptoglobin polymorphism. Atherosclerosis 176: 189–195, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Brunet S, Thibault L, Delvin E, Yotov W, Bendayan M, and Levy E. Dietary iron overload and induced lipid peroxidation are associated with impaired plasma lipid transport and hepatic sterol metabolism in rats. Hepatology 29: 1809–1817, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Cantinieaux B, Hariga C, Clumeck N, De Maertelaere E, Glupczynski Y, Magrez P, Reding P, Van Laethem Y, and Fondu P. The role of excess iron in the pathogenesis of disturbed neutrophil functions in cirrhotic patients (neutrophil functions in cirrhotic patients). Acta Clin Belg 42: 153–167, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Casanova-Esteban P, Guiral N, Andres E, Gonzalvo C, Mateo-Gallego R, Giraldo P, Paramo JA, and Civeira F. Effect of phlebotomy on lipid metabolism in subjects with hereditary hemochromatosis. Metabolism 60: 830–834, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Castoldi M, Vujic Spasic M, Altamura S, Elmen J, Lindow M, Kiss J, Stolte J, Sparla R, D'Alessandro LA, Klingmuller U, Fleming RE, Longerich T, Grone HJ, Benes V, Kauppinen S, Hentze MW, and Muckenthaler MU. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J Clin Invest 121: 1386–1396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, and Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 7: 425–429, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Chang CC, Chiu PF, Chen HL, Chang TL, Chang YJ, and Huang CH. Simvastatin downregulates the expression of hepcidin and erythropoietin in HepG2 cells. Hemodial Int 17: 116–121, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Chen G, Zhang D, Fuchs TA, Manwani D, Wagner DD, and Frenette PS. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123: 3818–3827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen JC, Huang KC, and Lin WW. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell Signal 18: 32–39, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Chen YG, Lin CL, Ho CL, Chen YC, and Kao CH. Risk of coronary artery disease in transfusion-naive thalassemia populations: a nationwide population-based retrospective cohort study. Eur J Intern Med 26: 250–254, 2015 [DOI] [PubMed] [Google Scholar]
- 44. Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, and Duckers HJ. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation 119: 3017–3027, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Cheung YF, Chan GC, and Ha SY. Arterial stiffness and endothelial function in patients with beta-thalassemia major. Circulation 106: 2561–2566, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Chiabrando D, Vinchi F, Fiorito V, Mercurio S, and Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol 5: 61, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi JW, Kim SK, and Pai SH. Changes in serum lipid concentrations during iron depletion and after iron supplementation. Ann Clin Lab Sci 31: 151–156, 2001 [PubMed] [Google Scholar]
- 48. Ciceri P, Elli F, Braidotti P, Falleni M, Tosi D, Bulfamante G, Block GA, and Cozzolino M. Iron citrate reduces high phosphate-induced vascular calcification by inhibiting apoptosis. Atherosclerosis 254: 93–101, 2016 [DOI] [PubMed] [Google Scholar]
- 49. Ciceri P, Falleni M, Tosi D, Martinelli C, Bulfamante G, Block GA, Messa P, and Cozzolino M. High-phosphate induced vascular calcification is reduced by iron citrate through inhibition of extracellular matrix osteo-chondrogenic shift in VSMCs. Int J Cardiol 297: 94–103, 2019 [DOI] [PubMed] [Google Scholar]
- 50. Ciceri P, Falleni M, Tosi D, Martinelli C, Cannizzo S, Marchetti G, D'Arminio Monforte A, Bulfamante G, Block GA, Messa P, and Cozzolino M. Therapeutic effect of iron citrate in blocking calcium deposition in high Pi-calcified VSMC: role of autophagy and apoptosis. Int J Mol Sci 20: 5925, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Conigliaro A, Costa V, Amato R, and Mancone C. Data on the effects of low iron diet on serum lipid profile in HCV transgenic mouse model. Data Brief 12: 22–25, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cornelissen A, Guo L, Sakamoto A, Virmani R, and Finn AV. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine 47: 598–606, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM, Meister M, Muley T, Warth A, Platten M, Hentze MW, Cerwenka A, and Muckenthaler MU. Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol 8: 1479, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dabbagh AJ, Mannion T, Lynch SM, and Frei B. The effect of iron overload on rat plasma and liver oxidant status in vivo. Biochem J 300(Pt 3): 799–803, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dabbagh AJ, Shwaery GT, Keaney JF Jr., and Frei B. Effect of iron overload and iron deficiency on atherosclerosis in the hypercholesterolemic rabbit. Arterioscler Thromb Vasc Biol 17: 2638–2645, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Dalfino G, Simone S, Porreca S, Cosola C, Balestra C, Manno C, Schena FP, Grandaliano G, and Pertosa G. Bone morphogenetic protein-2 may represent the molecular link between oxidative stress and vascular stiffness in chronic kidney disease. Atherosclerosis 211: 418–423, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Davignon J. Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler Thromb Vasc Biol 25: 267–269, 2005 [DOI] [PubMed] [Google Scholar]
- 58. de Swart L, Hendriks JC, van der Vorm LN, Cabantchik ZI, Evans PJ, Hod EA, Brittenham GM, Furman Y, Wojczyk B, Janssen MC, Porter JB, Mattijssen VE, Biemond BJ, MacKenzie MA, Origa R, Galanello R, Hider RC, and Swinkels DW. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 101: 38–45, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Depalma RG, Hayes VW, Chow BK, Shamayeva G, May PE, and Zacharski LR. Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: a substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) Trial. J Vasc Surg 51: 1498–1503, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, and Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 613–622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF Jr., and Vita JA. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation 103: 2799–2804, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Durham AL, Speer MY, Scatena M, Giachelli CM, and Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res 114: 590–600, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, and Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A 111: E4110–E4118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dutra FF and Bozza MT. Heme on innate immunity and inflammation. Front Pharmacol 5: 115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ellervik C, Tybjaerg-Hansen A, Grande P, Appleyard M, and Nordestgaard BG. Hereditary hemochromatosis and risk of ischemic heart disease: a prospective study and a case-control study. Circulation 112: 185–193, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, and Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36: 1153–1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Engberink MF, Povel CM, Durga J, Swinkels DW, de Kort WL, Schouten EG, Verhoef P, and Geleijnse JM. Hemochromatosis (HFE) genotype and atherosclerosis: increased susceptibility to iron-induced vascular damage in C282Y carriers? Atherosclerosis 211: 520–525, 2010 [DOI] [PubMed] [Google Scholar]
- 68. Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, and Monia BP. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 3: 87–98, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, and Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 102: 2670–2677, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Failla M, Giannattasio C, Piperno A, Vergani A, Grappiolo A, Gentile G, Meles E, and Mancia G. Radial artery wall alterations in genetic hemochromatosis before and after iron depletion therapy. Hepatology 32: 569–573, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Fejes Z, Czimmerer Z, Szuk T, Poliska S, Horvath A, Balogh E, Jeney V, Varadi J, Fenyvesi F, Balla G, Edes I, Balla J, Kappelmayer J, and Nagy B Jr. Endothelial cell activation is attenuated by everolimus via transcriptional and post-transcriptional regulatory mechanisms after drug-eluting coronary stenting. PLoS One 13: e0197890, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fidelis HG, Mageski JGA, Goes SCE, Botelho T, Marques VB, Avila RA, and Dos Santos L. Blockade of angiotensin AT1 receptors prevents arterial remodelling and stiffening in iron-overloaded rats. Br J Pharmacol 177: 1119–1130, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, and Bozza MT. Characterization of heme as activator of toll-like receptor 4. J Biol Chem 282: 20221–20229, 2007 [DOI] [PubMed] [Google Scholar]
- 74. Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, and Virmani R. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59: 166–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fortes GB, Alves LS, de Oliveira R, Dutra FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher M, Golenbock D, Chan FK, and Bozza MT. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood 119: 2368–2375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fuhrman B, Oiknine J, and Aviram M. Iron induces lipid peroxidation in cultured macrophages, increases their ability to oxidatively modify LDL, and affects their secretory properties. Atherosclerosis 111: 65–78, 1994 [DOI] [PubMed] [Google Scholar]
- 77. Gaenzer H, Marschang P, Sturm W, Neumayr G, Vogel W, Patsch J, and Weiss G. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol 40: 2189–2194, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Galesloot TE, Holewijn S, Kiemeney LA, de Graaf J, Vermeulen SH, and Swinkels DW. Serum hepcidin is associated with presence of plaque in postmenopausal women of a general population. Arterioscler Thromb Vasc Biol 34: 446–456, 2014 [DOI] [PubMed] [Google Scholar]
- 79. Galesloot TE, Janss LL, Burgess S, Kiemeney LA, den Heijer M, de Graaf J, Holewijn S, Benyamin B, Whitfield JB, Swinkels DW, and Vermeulen SH. Iron and hepcidin as risk factors in atherosclerosis: what do the genes say? BMC Genet 16: 79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gall T, Petho D, Nagy A, Hendrik Z, Mehes G, Potor L, Gram M, Akerstrom B, Smith A, Nagy P, Balla G, and Balla J. Heme induces endoplasmic reticulum stress (HIER Stress) in human aortic smooth muscle cells. Front Physiol 9: 1595, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garbowski MW, Ma Y, Fucharoen S, Srichairatanakool S, Hider R, and Porter JB. Clinical and methodological factors affecting non-transferrin-bound iron values using a novel fluorescent bead assay. Transl Res 177: 19–30.e5, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gill D, Benyamin B, Moore LSP, Monori G, Zhou A, Koskeridis F, Evangelou E, Laffan M, Walker AP, Tsilidis KK, Dehghan A, Elliott P, Hypponen E, and Tzoulaki I. Associations of genetically determined iron status across the phenome: a mendelian randomization study. PLoS Med 16: e1002833, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gill D, Brewer CF, Monori G, Tregouet DA, Franceschini N, Giambartolomei C, Consortium I, Tzoulaki I, and Dehghan A. Effects of genetically determined iron status on risk of venous thromboembolism and carotid atherosclerotic disease: a Mendelian randomization study. J Am Heart Assoc 8: e012994, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gosriwatana I, Loreal O, Lu S, Brissot P, Porter J, and Hider RC. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem 273: 212–220, 1999 [DOI] [PubMed] [Google Scholar]
- 85. Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, and Megson IL. Antioxidants in cardiovascular therapy: panacea or false hope? Front Cardiovasc Med 2: 29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Graham RM, Chua AC, Carter KW, Delima RD, Johnstone D, Herbison CE, Firth MJ, O'Leary R, Milward EA, Olynyk JK, and Trinder D. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology 52: 462–471, 2010 [DOI] [PubMed] [Google Scholar]
- 87. Grammer TB, Scharnagl H, Dressel A, Kleber ME, Silbernagel G, Pilz S, Tomaschitz A, Koenig W, Mueller-Myhsok B, Marz W, and Strnad P. Iron metabolism, hepcidin, and mortality (the Ludwigshafen risk and cardiovascular health study). Clin Chem 65: 849–861, 2019 [DOI] [PubMed] [Google Scholar]
- 88. Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, Gupta A, Jenkins AL, Lipinski MJ, Kim J, Chhour P, de Vries PS, Jinnouchi H, Kutys R, Mori H, Kutyna MD, Torii S, Sakamoto A, Choi CU, Cheng Q, Grove ML, Sawan MA, Zhang Y, Cao Y, Kolodgie FD, Cormode DP, Arking DE, Boerwinkle E, Morrison AC, Erdmann J, Sotoodehnia N, Virmani R, and Finn AV. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest 128: 1106–1124, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Guo L, Sakamoto A, Cornelissen A, Hong CC, and Finn AV. Ironing-out the role of hepcidin in atherosclerosis. Arterioscler Thromb Vasc Biol 39: 303–305, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gursel O, Kurekci AE, Tascilar E, Ileri T, Altun D, Tapan S, Kurt I, Kocaoglu M, Aydin A, Okutan V, and Ozcan O. Premature atherosclerosis in children with beta-thalassemia major. J Pediatr Hematol Oncol 34: 630–634, 2012 [DOI] [PubMed] [Google Scholar]
- 91. Gustafsson H, Hallbeck M, Norell M, Lindgren M, Engstrom M, Rosen A, and Zachrisson H. Fe(III) distribution varies substantially within and between atherosclerotic plaques. Magn Reson Med 71: 885–892, 2014 [DOI] [PubMed] [Google Scholar]
- 92. Habib A, Polavarapu R, Karmali V, Guo L, Van Dam R, Cheng Q, Akahori H, Saeed O, Nakano M, Pachura K, Hong CC, Shin E, Kolodgie F, Virmani R, and Finn AV. Hepcidin-ferroportin axis controls toll-like receptor 4 dependent macrophage inflammatory responses in human atherosclerotic plaques. Atherosclerosis 241: 692–700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hahalis G, Kalogeropoulos A, Terzis G, Tselepis AD, Kourakli A, Mylona P, Grapsas N, and Alexopoulos D. Premature atherosclerosis in non-transfusion-dependent beta-thalassemia intermedia. Cardiology 118: 159–163, 2011 [DOI] [PubMed] [Google Scholar]
- 94. Hahalis G, Zacharioglou E, Xanthopoulou I, Koniari I, Kalogeropoulou C, Tsota I, Rigopoulou A, Diamantopoulos A, Gkizas V, Davlouros P, Akinosoglou K, Leopoulou M, Gogos C, and Alexopoulos D. Coronary atherosclerosis burden is not advanced in patients with beta-thalassemia despite premature extracardiac atherosclerosis: a coronary artery calcium score and carotid intima-media thickness study. J Geriatr Cardiol 13: 158–162, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. He H, Qiao Y, Zhou Q, Wang Z, Chen X, Liu D, Yin D, and He M. Iron overload damages the endothelial mitochondria via the ROS/ADMA/DDAHII/eNOS/NO pathway. Oxid Med Cell Longev 2019: 2340392, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hentze MW, Muckenthaler MU, Galy B, and Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 142: 24–38, 2010 [DOI] [PubMed] [Google Scholar]
- 97. Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, and Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J 31: 1975–1984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hoeft K, Bloch DB, Graw JA, Malhotra R, Ichinose F, and Bagchi A. Iron loading exaggerates the inflammatory response to the toll-like receptor 4 ligand lipopolysaccharide by altering mitochondrial homeostasis. Anesthesiology 127: 121–135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Holay MP, Choudhary AA, and Suryawanshi SD. Serum ferritin-a novel risk factor in acute myocardial infarction. Indian Heart J 64: 173–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Holm PW, Slart RH, Zeebregts CJ, Hillebrands JL, and Tio RA. Atherosclerotic plaque development and instability: a dual role for VEGF. Ann Med 41: 257–264, 2009 [DOI] [PubMed] [Google Scholar]
- 101. Hu X, Cai X, Ma R, Fu W, Zhang C, and Du X. Iron-load exacerbates the severity of atherosclerosis via inducing inflammation and enhancing the glycolysis in macrophages. J Cell Physiol 234: 18792–18800, 2019 [DOI] [PubMed] [Google Scholar]
- 102. Ishizaka N, Saito K, Mori I, Matsuzaki G, Ohno M, and Nagai R. Iron chelation suppresses ferritin upregulation and attenuates vascular dysfunction in the aorta of angiotensin II-infused rats. Arterioscler Thromb Vasc Biol 25: 2282–2288, 2005 [DOI] [PubMed] [Google Scholar]
- 103. Jeney V, Balla G, and Balla J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front Physiol 5: 379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, and Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887, 2002 [DOI] [PubMed] [Google Scholar]
- 105. Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, and Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation 104: 1519–1525, 2001 [DOI] [PubMed] [Google Scholar]
- 106. Juckett MB, Balla J, Balla G, Jessurun J, Jacob HS, and Vercellotti GM. Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am J Pathol 147: 782–789, 1995 [PMC free article] [PubMed] [Google Scholar]
- 107. Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, and Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107: 737–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kalantar-Zadeh K, Rodriguez RA, and Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 19: 141–149, 2004 [DOI] [PubMed] [Google Scholar]
- 109. Kalet-Litman S, Moreno PR, and Levy AP. The haptoglobin 2–2 genotype is associated with increased redox active hemoglobin derived iron in the atherosclerotic plaque. Atherosclerosis 209: 28–31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kamanna VS, Ganji SH, Shelkovnikov S, Norris K, and Vaziri ND. Iron sucrose promotes endothelial injury and dysfunction and monocyte adhesion/infiltration. Am J Nephrol 35: 114–119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kartikasari AE, Georgiou NA, Visseren FL, van Kats-Renaud H, van Asbeck BS, and Marx JJ. Endothelial activation and induction of monocyte adhesion by nontransferrin-bound iron present in human sera. FASEB J 20: 353–355, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Katagiri T and Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol 8: a021899, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kato GJ, Gladwin MT, and Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37–47, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kato GJ, Hebbel RP, Steinberg MH, and Gladwin MT. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol 84: 618–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kato GJ, Steinberg MH, and Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127: 750–760, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kautz L, Gabayan V, Wang X, Wu J, Onwuzurike J, Jung G, Qiao B, Lusis AJ, Ganz T, and Nemeth E. Testing the iron hypothesis in a mouse model of atherosclerosis. Cell Rep 5: 1436–1442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kawada S, Nagasawa Y, Kawabe M, Ohyama H, Kida A, Kato-Kogoe N, Nanami M, Hasuike Y, Kuragano T, Kishimoto H, Nakasho K, and Nakanishi T. Iron-induced calcification in human aortic vascular smooth muscle cells through interleukin-24 (IL-24), with/without TNF-alpha. Sci Rep 8: 658, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Khera R, Singh M, Goel G, Gupta P, Singh T, and Dubey AP. Hypertriglyceridemia thalassemia syndrome: a report of 4 cases. Indian J Hematol Blood Transfus 30: 288–291, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kiechl S, Willeit J, Egger G, Poewe W, and Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation 96: 3300–3307, 1997 [DOI] [PubMed] [Google Scholar]
- 120. Kim SH, Yadav D, Kim SJ, Kim JR, and Cho KH. High consumption of iron exacerbates hyperlipidemia, atherosclerosis, and female sterility in zebrafish via acceleration of glycation and degradation of serum lipoproteins. Nutrients 9: 690, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kirk EA, Heinecke JW, and LeBoeuf RC. Iron overload diminishes atherosclerosis in apoE-deficient mice. J Clin Invest 107: 1545–1553, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kraml PJ, Klein RL, Huang Y, Nareika A, and Lopes-Virella MF. Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes. Metabolism 54: 453–459, 2005 [DOI] [PubMed] [Google Scholar]
- 123. Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, and David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83: 1098–1116, 2014 [DOI] [PubMed] [Google Scholar]
- 124. Kuo KL, Hung SC, Lee TS, and Tarng DC. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J Am Soc Nephrol 25: 2596–2606, 2014. 24722448 [Google Scholar]
- 125. Lauffer RB. Iron depletion and coronary disease. Am Heart J 119: 1448–1449, 1990 [DOI] [PubMed] [Google Scholar]
- 126. Lee HT, Chiu LL, Lee TS, Tsai HL, and Chau LY. Dietary iron restriction increases plaque stability in apolipoprotein-e-deficient mice. J Biomed Sci 10: 510–517, 2003 [DOI] [PubMed] [Google Scholar]
- 127. Lee TS, Shiao MS, Pan CC, and Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation 99: 1222–1229, 1999 [DOI] [PubMed] [Google Scholar]
- 128. Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, and Moreno PR. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 27: 134–140, 2007 [DOI] [PubMed] [Google Scholar]
- 129. Li JJ, Meng X, Si HP, Zhang C, Lv HX, Zhao YX, Yang JM, Dong M, Zhang K, Liu SX, Zhao XQ, Gao F, Liu XL, Cui TX, and Zhang Y. Hepcidin destabilizes atherosclerotic plaque via overactivating macrophages after erythrophagocytosis. Arterioscler Thromb Vasc Biol 32: 1158–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 130. Li X, Ding D, Zhang Y, Su D, Wang M, Chen X, Yang Y, Hong C, Hu G, and Ling W. Associations of plasma hepcidin with mortality risk in patients with coronary artery disease. Oncotarget 8: 109497–109508, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liang H, Huang H, Tan PZ, Liu Y, Nie JH, Zhang YT, Zhang KL, Diao Y, He Q, Hou BY, Zhao TT, Li YZ, Lv GX, Lee KY, Gao X, and Zhou LY. Effect of iron on cholesterol 7alpha-hydroxylase expression in alcohol-induced hepatic steatosis in mice. J Lipid Res 58: 1548–1560, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Madsen JB, Pedersen L, Kidholm CL, and Rasmussen LM. Arterial iron content is increased in patients with high plasma ferritin levels. J Vasc Res 53: 301–307, 2016 [DOI] [PubMed] [Google Scholar]
- 133. Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O'Rourke C, Li P, Derwall M, Spagnolli E, Kolodziej SA, Hoeft K, Mayeur C, Jiramongkolchai P, Kumar R, Buys ES, Yu PB, Bloch KD, and Bloch DB. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS One 10: e0117098, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Malhotra R, Wunderer F, Barnes HJ, Bagchi A, Buswell MD, O'Rourke CD, Slocum CL, Ledsky CD, Peneyra KM, Sigurslid H, Corman B, Johansson KB, Rhee DK, Bloch KD, and Bloch DB. Hepcidin deficiency protects against atherosclerosis. Arterioscler Thromb Vasc Biol 39: 178–187, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, Leandro G, Arvaniti V, Germani G, Patch D, Calvaruso V, Mikhailidis DP, Dhillon AP, and Burroughs AK. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int 31: 730–739, 2011 [DOI] [PubMed] [Google Scholar]
- 136. Marques L, Negre-Salvayre A, Costa L, and Canonne-Hergaux F. Iron gene expression profile in atherogenic Mox macrophages. Biochim Biophys Acta 1862: 1137–1146, 2016 [DOI] [PubMed] [Google Scholar]
- 137. Marques VB, Leal MAS, Mageski JGA, Fidelis HG, Nogueira BV, Vasquez EC, Meyrelles SDS, Simoes MR, and Dos Santos L. Chronic iron overload intensifies atherosclerosis in apolipoprotein E deficient mice: role of oxidative stress and endothelial dysfunction. Life Sci 233: 116702, 2019 [DOI] [PubMed] [Google Scholar]
- 138. Marquez-Ibarra A, Huerta M, Villalpando-Hernandez S, Rios-Silva M, Diaz-Reval MI, Cruzblanca H, Mancilla E, and Trujillo X. The effects of dietary iron and capsaicin on hemoglobin, blood glucose, insulin tolerance, cholesterol, and triglycerides, in healthy and diabetic wistar rats. PLoS One 11: e0152625, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mehta NU, Grijalva V, Hama S, Wagner A, Navab M, Fogelman AM, and Reddy ST. Apolipoprotein E−/− mice lacking hemopexin develop increased atherosclerosis via mechanisms that include oxidative stress and altered macrophage function. Arterioscler Thromb Vasc Biol 36: 1152–1163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Menke A, Fernandez-Real JM, Muntner P, and Guallar E. The association of biomarkers of iron status with peripheral arterial disease in US adults. BMC Cardiovasc Disord 9: 34, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Merono T, Brites F, Dauteuille C, Lhomme M, Menafra M, Arteaga A, Castro M, Saez MS, Ballerga EG, Sorroche P, Rey J, Lesnik P, Sorda JA, Chapman MJ, Kontush A, and Daruich J. Metabolic alterations, HFE gene mutations and atherogenic lipoprotein modifications in patients with primary iron overload. Clin Sci (Lond) 128: 609–618, 2015 [DOI] [PubMed] [Google Scholar]
- 142. Michel JB, Virmani R, Arbustini E, and Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J 32: 1977–1985, 1985a, 1985b, 1985c, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Miller M and Hutchins GM. Hemochromatosis, multiorgan hemosiderosis, and coronary artery disease. JAMA 272: 231–233, 1994 [PubMed] [Google Scholar]
- 144. Minqin R, Rajendran R, Pan N, Tan BK, Ong WY, Watt F, and Halliwell B. The iron chelator desferrioxamine inhibits atherosclerotic lesion development and decreases lesion iron concentrations in the cholesterol-fed rabbit. Free Radic Biol Med 38: 1206–1211, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Morrison HI, Semenciw RM, Mao Y, and Wigle DT. Serum iron and risk of fatal acute myocardial infarction. Epidemiology 5: 243–246, 1994 [DOI] [PubMed] [Google Scholar]
- 146. Mrad MF, Mouawad CA, Al-Hariri M, Eid AA, Alam J, and Habib A. Statins modulate transcriptional activity of heme-oxygenase-1 promoter in NIH 3T3 cells. J Cell Biochem 113: 3466–3475, 2012 [DOI] [PubMed] [Google Scholar]
- 147. Muckenthaler MU, Rivella S, Hentze MW, and Galy B. A red carpet for iron metabolism. Cell 168: 344–361, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Munoz-Bravo C, Gutierrez-Bedmar M, Gomez-Aracena J, Garcia-Rodriguez A, and Navajas JF. Iron: protector or risk factor for cardiovascular disease? Still controversial. Nutrients 5: 2384–2404, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklosi J, Mehes G, Csonka T, Smith A, Vercellotti GM, Balla G, and Balla J. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol 30: 1347–1353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Naito Y, Hosokawa M, Sawada H, Oboshi M, Hirotani S, Iwasaku T, Okuhara Y, Morisawa D, Eguchi A, Nishimura K, Soyama Y, Fujii K, Mano T, Ishihara M, Tsujino T, and Masuyama T. Transferrin receptor 1 in chronic hypoxia-induced pulmonary vascular remodeling. Am J Hypertens 29: 713–718, 2016 [DOI] [PubMed] [Google Scholar]
- 151. Neven E, De Schutter TM, Behets GJ, Gupta A, and D'Haese PC. Iron and vascular calcification. Is there a link? Nephrol Dial Transplant 26: 1137–1145, 2011 [DOI] [PubMed] [Google Scholar]
- 152. Nyakundi BB, Toth A, Balogh E, Nagy B, Erdei J, Ryffel B, Paragh G, Cordero MD, and Jeney V. Oxidized hemoglobin forms contribute to NLRP3 inflammasome-driven IL-1beta production upon intravascular hemolysis. Biochim Biophys Acta Mol Basis Dis 1865: 464–475, 2019 [DOI] [PubMed] [Google Scholar]
- 153. Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, Agarwal A, Lusis AJ, and Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res 100: 1703–1711, 2007 [DOI] [PubMed] [Google Scholar]
- 154. Padda RS, Gkouvatsos K, Guido M, Mui J, Vali H, and Pantopoulos K. A high-fat diet modulates iron metabolism but does not promote liver fibrosis in hemochromatotic Hjv(−)/(−) mice. Am J Physiol Gastrointest Liver Physiol 308: G251–G261, 2015 [DOI] [PubMed] [Google Scholar]
- 155. Palanca A, Castelblanco E, Perpinan H, Betriu A, Soldevila B, Valdivielso JM, Bermudez M, Duran X, Fernandez E, Puig-Domingo M, Groop PH, Alonso N, and Mauricio D. Prevalence and progression of subclinical atherosclerosis in patients with chronic kidney disease and diabetes. Atherosclerosis 276: 50–57, 2018 [DOI] [PubMed] [Google Scholar]
- 156. Pankow JS, Boerwinkle E, Adams PC, Guallar E, Leiendecker-Foster C, Rogowski J, and Eckfeldt JH. HFE C282Y homozygotes have reduced low-density lipoprotein cholesterol: the Atherosclerosis Risk in Communities (ARIC) study. Transl Res 152: 3–10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Patel M and Ramavataram DV. Non transferrin bound iron: nature, manifestations and analytical approaches for estimation. Indian J Clin Biochem 27: 322–332, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Patruta SI, Edlinger R, Sunder-Plassmann G, and Horl WH. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol 9: 655–663, 1998 [DOI] [PubMed] [Google Scholar]
- 159. Pechlaner R, Kiechl S, Mayr M, Santer P, Weger S, Haschka D, Bansal SS, Willeit J, and Weiss G. Correlates of serum hepcidin levels and its association with cardiovascular disease in an elderly general population. Clin Chem Lab Med 54: 151–161, 2016 [DOI] [PubMed] [Google Scholar]
- 160. Petrillo S, Chiabrando D, Genova T, Fiorito V, Ingoglia G, Vinchi F, Mussano F, Carossa S, Silengo L, Altruda F, Merlo GR, Munaron L, and Tolosano E. Heme accumulation in endothelial cells impairs angiogenesis by triggering paraptosis. Cell Death Differ 25: 573–588, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Pilling LC, Tamosauskaite J, Jones G, Wood AR, Jones L, Kuo CL, Kuchel GA, Ferrucci L, and Melzer D. Common conditions associated with hereditary haemochromatosis genetic variants: cohort study in UK Biobank. BMJ 364: k5222, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pinto JP, Arezes J, Dias V, Oliveira S, Vieira I, Costa M, Vos M, Carlsson A, Rikers Y, Rangel M, and Porto G. Physiological implications of NTBI uptake by T lymphocytes. Front Pharmacol 5: 24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ponikowska B, Suchocki T, Paleczny B, Olesinska M, Powierza S, Borodulin-Nadzieja L, Reczuch K, von Haehling S, Doehner W, Anker SD, Cleland JG, and Jankowska EA. Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care 36: 4147–4156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Pool GF and van Jaarsveld H. Dietary iron elevates LDL-cholesterol and decreases plasma antioxidant levels: influence of antioxidants. Res Commun Mol Pathol Pharmacol 100: 139–150, 1998 [PubMed] [Google Scholar]
- 165. Porreca E, Ucchino S, Di Febbo C, Di Bartolomeo N, Angelucci D, Napolitano AM, Mezzetti A, and Cuccurullo F. Antiproliferative effect of desferrioxamine on vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb 14: 299–304, 1994 [DOI] [PubMed] [Google Scholar]
- 166. Porto BN, Alves LS, Fernandez PL, Dutra TP, Figueiredo RT, Graca-Souza AV, and Bozza MT. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem 282: 24430–24436, 2007 [DOI] [PubMed] [Google Scholar]
- 167. Potor L, Banyai E, Becs G, Soares MP, Balla G, Balla J, and Jeney V. Atherogenesis may involve the prooxidant and proinflammatory effects of ferryl hemoglobin. Oxid Med Cell Longev 2013: 676425, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Potor L, Eva SK, Hegedus H, Petho D, Szabo Z, Szigeti ZM, Pocsi I, Trencsenyi G, Szikra D, Garai I, Gall T, Combi Z, Kappelmayer J, Balla G, and Balla J. The fungal iron chelator desferricoprogen inhibits atherosclerotic plaque formation. Int J Mol Sci 21: 4746, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pourcet B and Staels B.. Alternative macrophages in atherosclerosis: not always protective! J Clin Invest 128: 910–912, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, and Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler 10: 32–36, 2014 [PMC free article] [PubMed] [Google Scholar]
- 171. Prats-Puig A, Moreno M, Carreras-Badosa G, Bassols J, Ricart W, Lopez-Bermejo A, and Fernandez-Real JM. Serum ferritin relates to carotid intima-media thickness in offspring of fathers with higher serum ferritin levels. Arterioscler Thromb Vasc Biol 36: 174–180, 2016 [DOI] [PubMed] [Google Scholar]
- 172. Rajapurkar MM, Shah SV, Lele SS, Hegde UN, Lensing SY, Gohel K, Mukhopadhyay B, Gang S, and Eigenbrodt ML. Association of catalytic iron with cardiovascular disease. Am J Cardiol 109: 438–442, 2012 [DOI] [PubMed] [Google Scholar]
- 173. Rajendran R, Minqin R, Ronald JA, Rutt BK, Halliwell B, and Watt F. Does iron inhibit calcification during atherosclerosis? Free Radic Biol Med 53: 1675–1679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ramakrishnan L, Pedersen SL, Toe QK, West LE, Mumby S, Casbolt H, Issitt T, Garfield B, Lawrie A, Wort SJ, and Quinlan GJ. The Hepcidin/Ferroportin axis modulates proliferation of pulmonary artery smooth muscle cells. Sci Rep 8: 12972, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rasmussen ML, Folsom AR, Catellier DJ, Tsai MY, Garg U, and Eckfeldt JH. A prospective study of coronary heart disease and the hemochromatosis gene (HFE) C282Y mutation: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 154: 739–746, 2001 [DOI] [PubMed] [Google Scholar]
- 176. Recalcati S, Gammella E, Buratti P, Doni A, Anselmo A, Locati M, and Cairo G. Macrophage ferroportin is essential for stromal cell proliferation in wound healing. Haematologica 104: 47–58, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Ribeiro Junior RF, Marques VB, Nunes DO, Stefanon I, and Dos Santos L. Chronic iron overload induces functional and structural vascular changes in small resistance arteries via NADPH oxidase-dependent O2(−) production. Toxicol Lett 279: 43–52, 2017 [DOI] [PubMed] [Google Scholar]
- 178. Ricchi P, Ammirabile M, Spasiano A, Costantini S, Cinque P, Di Matola T, Pagano L, and Prossomariti L. Combined chelation therapy in thalassemia major with deferiprone and desferrioxamine: a retrospective study. Eur J Haematol 85: 36–42, 2010 [DOI] [PubMed] [Google Scholar]
- 179. Ricchi P, Ammirabile M, Spasiano A, Costantini S, Di Matola T, Cinque P, Pagano L, and Prossomariti L. Hypocholesterolemia in adult patients with thalassemia: a link with the severity of genotype in thalassemia intermedia patients. Eur J Haematol 82: 219–222, 2009 [DOI] [PubMed] [Google Scholar]
- 180. Risko P, Platenik J, Buchal R, Potockova J, and Kraml PJ. Long-term donors versus non-donor men: iron metabolism and the atherosclerotic process. Atherosclerosis 272: 14–20, 2018 [DOI] [PubMed] [Google Scholar]
- 181. Rivella S. Iron metabolism under conditions of ineffective erythropoiesis in beta-thalassemia. Blood 133: 51–58, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rockfield S, Chhabra R, Robertson M, Rehman N, Bisht R, and Nanjundan M. Links between iron and lipids: implications in some major human diseases. Pharmaceuticals (Basel) 11: 113, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Roest M, van der Schouw YT, de Valk B, Marx JJ, Tempelman MJ, de Groot PG, Sixma JJ, and Banga JD. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation 100: 1268–1273, 1999 [DOI] [PubMed] [Google Scholar]
- 184. Roghi A, Poggiali E, Duca L, Mafrici A, Pedrotti P, Paccagnini S, Brenna S, Galli A, Consonni D, and Cappellini MD. Role of non-transferrin-bound iron in the pathogenesis of cardiotoxicity in patients with ST-elevation myocardial infarction assessed by cardiac magnetic resonance imaging. Int J Cardiol 199: 326–332, 2015 [DOI] [PubMed] [Google Scholar]
- 185. Saeed O, Otsuka F, Polavarapu R, Karmali V, Weiss D, Davis T, Rostad B, Pachura K, Adams L, Elliott J, Taylor WR, Narula J, Kolodgie F, Virmani R, Hong CC, and Finn AV. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 299–307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, and Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 86: 803–811, 1992 [DOI] [PubMed] [Google Scholar]
- 187. San Martin A and Griendling KK.. Redox control of vascular smooth muscle migration. Antioxid Redox Signal 12: 625–640, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sandberg EM and Sayeski PP. Jak2 tyrosine kinase mediates oxidative stress-induced apoptosis in vascular smooth muscle cells. J Biol Chem 279: 34547–34552, 2004 [DOI] [PubMed] [Google Scholar]
- 189. Saremi L, Saremi M, Lotfipanah S, Imani S, Fu J, and Zhang T. Correlation between HFE gene polymorphisms and increased risk of coronary artery disease among patients with type 2 diabetes in Iran. Turk J Med Sci 46: 590–596, 2016 [DOI] [PubMed] [Google Scholar]
- 190. Sasai M, Iso Y, Mizukami T, Tomosugi N, Sambe T, Miyazaki A, and Suzuki H. Potential contribution of the hepcidin-macrophage axis to plaque vulnerability in acute myocardial infarction in human. Int J Cardiol 227: 114–121, 2017 [DOI] [PubMed] [Google Scholar]
- 191. Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, and Hayashi J. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol 39: 610–616, 2002 [DOI] [PubMed] [Google Scholar]
- 192. Schaeffer C, Thomassin L, Rochette L, and Connat JL. Apoptosis induced in vascular smooth muscle cells by oxidative stress is partly prevented by pretreatment with CGRP. Ann N Y Acad Sci 1010: 733–737, 2003 [DOI] [PubMed] [Google Scholar]
- 193. Schaer DJ, Vinchi F, Ingoglia G, Tolosano E, and Buehler PW. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol 5: 415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Schouten BJ, Hunt PJ, Livesey JH, Frampton CM, and Soule SG. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J Clin Endocrinol Metab 94: 2332–2337, 2009 [DOI] [PubMed] [Google Scholar]
- 195. Seto T, Hamada C, and Tomino Y. Suppressive effects of iron overloading on vascular calcification in uremic rats. J Nephrol 27: 135–142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Shalev H, Kapelushnik J, Moser A, Knobler H, and Tamary H. Hypocholesterolemia in chronic anemias with increased erythropoietic activity. Am J Hematol 82: 199–202, 2007 [DOI] [PubMed] [Google Scholar]
- 197. Shaw J, Chakraborty A, Nag A, Chattopadyay A, Dasgupta AK, and Bhattacharyya M. Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. Eur J Haematol 99: 399–408, 2017 [DOI] [PubMed] [Google Scholar]
- 198. Sherief LM, Dawood O, Ali A, Sherbiny HS, Kamal NM, Elshanshory M, Alazez OA, Alhady MA, Nour M, and Mokhtar WA. Premature atherosclerosis in children with beta-thalassemia major: new diagnostic marker. BMC Pediatr 17: 69, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Shin DY, Chung J, Joe Y, Pae HO, Chang KC, Cho GJ, Ryter SW, and Chung HT. Pretreatment with CO-releasing molecules suppresses hepcidin expression during inflammation and endoplasmic reticulum stress through inhibition of the STAT3 and CREBH pathways. Blood 119: 2523–2532, 2012 [DOI] [PubMed] [Google Scholar]
- 200. Silva G, Jeney V, Chora A, Larsen R, Balla J, and Soares MP. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J Biol Chem 284: 29582–29595, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Silva M, Silva ME, de Paula H, Carneiro CM, and Pedrosa ML. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr Res 28: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 202. Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, and Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Singh S, Hider RC, and Porter JB. A direct method for quantification of non-transferrin-bound iron. Anal Biochem 186: 320–323, 1990 [DOI] [PubMed] [Google Scholar]
- 204. Solanas-Barca M, Mateo-Gallego R, Calmarza P, Jarauta E, Bea AM, Cenarro A, and Civeira F. Mutations in HFE causing hemochromatosis are associated with primary hypertriglyceridemia. J Clin Endocrinol Metab 94: 4391–4397, 2009 [DOI] [PubMed] [Google Scholar]
- 205. Sripetchwandee J, Pipatpiboon N, Chattipakorn N, and Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One 9: e85115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Stadler N, Lindner RA, and Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol 24: 949–954, 2004 [DOI] [PubMed] [Google Scholar]
- 207. Stangl GI and Kirchgessner M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids 33: 889–895, 1998 [DOI] [PubMed] [Google Scholar]
- 208. Stocker R and Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 209. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet 1: 1293–1294, 1981 [DOI] [PubMed] [Google Scholar]
- 210. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J 117: 1177–1188, 1989 [DOI] [PubMed] [Google Scholar]
- 211. Sullivan JL. Stored iron and ischemic heart disease. Empirical support for a new paradigm. Circulation 86: 1036–1037, 1992 [DOI] [PubMed] [Google Scholar]
- 212. Sullivan JL. Iron versus cholesterol—perspectives on the iron and heart disease debate. J Clin Epidemiol 49: 1345–1352, 1996 [DOI] [PubMed] [Google Scholar]
- 213. Sullivan JL. Are menstruating women protected from heart disease because of, or in spite of, estrogen? Relevance to the iron hypothesis. Am Heart J 145: 190–194, 2003 [DOI] [PubMed] [Google Scholar]
- 214. Sullivan JL. Do hemochromatosis mutations protect against iron-mediated atherogenesis? Circ Cardiovasc Genet 2: 652–657, 2009 [DOI] [PubMed] [Google Scholar]
- 215. Sullivan JL and Zacharski LR. Hereditary haemochromatosis and the hypothesis that iron depletion protects against ischemic heart disease. Eur J Clin Invest 31: 375–377, 2001 [DOI] [PubMed] [Google Scholar]
- 216. Sung KC, Kang SM, Cho EJ, Park JB, Wild SH, and Byrne CD. Ferritin is independently associated with the presence of coronary artery calcium in 12,033 men. Arterioscler Thromb Vasc Biol 32: 2525–2530, 2012 [DOI] [PubMed] [Google Scholar]
- 217. Syrovatka P, Kraml P, Hulikova K, Fialova L, Vejrazka M, Crkovska J, Potockova J, and Andel M. Iron stores are associated with asymptomatic atherosclerosis in healthy men of primary prevention. Eur J Clin Invest 41: 846–853, 2011 [DOI] [PubMed] [Google Scholar]
- 218. Tangudu NK, Alan B, Vinchi F, Worle K, Lai D, Vettorazzi S, Leopold K, and Vujic Spasic M. Scavenging reactive oxygen species production normalizes ferroportin expression and ameliorates cellular and systemic iron disbalances in hemolytic mouse model. Antioxid Redox Signal 29: 484–499, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Tolosano E, Fagoonee S, Morello N, Vinchi F, and Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal 12: 305–320, 2010 [DOI] [PubMed] [Google Scholar]
- 220. Tuomainen TP, Kontula K, Nyyssonen K, Lakka TA, Helio T, and Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation 100: 1274–1279, 1999 [DOI] [PubMed] [Google Scholar]
- 221. Tuomainen TP, Punnonen K, Nyyssonen K, and Salonen JT. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation 97: 1461–1466, 1998 [DOI] [PubMed] [Google Scholar]
- 222. Upston JM, Kritharides L, and Stocker R. The role of vitamin E in atherosclerosis. Prog Lipid Res 42: 405–422, 2003 [DOI] [PubMed] [Google Scholar]
- 223. Valenti L, Dongiovanni P, Motta BM, Swinkels DW, Bonara P, Rametta R, Burdick L, Frugoni C, Fracanzani AL, and Fargion S. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol 31: 683–690, 2011 [DOI] [PubMed] [Google Scholar]
- 224. van der AD, Rovers MM, Grobbee DE, Marx JJ, Waalen J, Ellervik C, Nordestgaard BG, Olynyk JK, Mills PR, Shepherd J, Grandchamp B, Boer JM, Caruso C, Arca M, Meyer BJ, and van der Schouw YT. Mutations in the HFE gene and cardiovascular disease risk: an individual patient data meta-analysis of 53 880 subjects. Circ Cardiovasc Genet 1: 43–50, 2008 [DOI] [PubMed] [Google Scholar]
- 225. Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, and Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science 325: 877–880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Vela D. Balance of cardiac and systemic hepcidin and its role in heart physiology and pathology. Lab Invest 98: 315–326, 2018 [DOI] [PubMed] [Google Scholar]
- 227. Verma U, Shankar N, Madhu SV, Tandon OP, Madan N, and Verma N. Relationship between iron deficiency anaemia and serum lipid levels in Indian adults. J Indian Med Assoc 108: 555–558, 562, 2010 [PubMed] [Google Scholar]
- 228. Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, and Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 127: 473–486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Vinchi F, De Franceschi L, Ghigo A, Townes T, Cimino J, Silengo L, Hirsch E, Altruda F, and Tolosano E. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation 127: 1317–1329, 2013 [DOI] [PubMed] [Google Scholar]
- 230. Vinchi F, Gastaldi S, Silengo L, Altruda F, and Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol 173: 289–299, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Vinchi F, Muckenthaler MU, Da Silva MC, Balla G, Balla J, and Jeney V. Atherogenesis and iron: from epidemiology to cellular level. Front Pharmacol 5: 94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Vinchi F, Porto G, Simmelbauer A, Altamura S, Passos ST, Garbowski M, Silva AMN, Spaich S, Seide SE, Sparla R, Hentze MW, and Muckenthaler MU. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J 41:2681–2695, 2020 [DOI] [PubMed] [Google Scholar]
- 233. Vinchi F and Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: scavenging free heme to preserve vasculature homeostasis. Oxid Med Cell Longev 2013: 396527, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Wang Q, Ji J, Hao S, Zhang M, Li K, and Qiao T. Iron together with lipid downregulates protein levels of ceruloplasmin in macrophages associated with rapid foam cell formation. J Atheroscler Thromb 23: 1201–1211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, and Shi LL. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1: 87–105, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Wang Z, Yin W, Zhu L, Li J, Yao Y, Chen F, Sun M, Zhang J, Shen N, Song Y, and Chang X. Iron drives T helper cell pathogenicity by promoting RNA-binding protein PCBP1-mediated proinflammatory cytokine production. Immunity 49: 80–92.e7, 2018 [DOI] [PubMed] [Google Scholar]
- 237. Watari Y, Yamamoto Y, Brydun A, Ishida T, Mito S, Yoshizumi M, Igarashi K, Chayama K, Ohshima T, and Ozono R. Ablation of the bach1 gene leads to the suppression of atherosclerosis in bach1 and apolipoprotein E double knockout mice. Hypertens Res 31: 783–792, 2008 [DOI] [PubMed] [Google Scholar]
- 238. Wong CM, Preston IR, Hill NS, and Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radic Biol Med 53: 1738–1747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, and Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med 203: 1117–1127, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wunderer F, Traeger L, Sigurslid HH, Meybohm P, Bloch DB, and Malhotra R. The role of hepcidin and iron homeostasis in atherosclerosis. Pharmacol Res 153: 104664, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Xiao L, Luo G, Guo X, Jiang C, Zeng H, Zhou F, Li Y, Yu J, and Yao P. Macrophage iron retention aggravates atherosclerosis: evidence for the role of autocrine formation of hepcidin in plaque macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 1865: 158531, 2020 [DOI] [PubMed] [Google Scholar]
- 242. Xu H, Perreau VM, Dent KA, Bush AI, Finkelstein DI, and Adlard PA. Iron regulates apolipoprotein E expression and secretion in neurons and astrocytes. J Alzheimers Dis 51: 471–487, 2016 [DOI] [PubMed] [Google Scholar]
- 243. Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, and Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res 107: 485–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, and Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J 17: 1759–1761, 2003 [DOI] [PubMed] [Google Scholar]
- 245. You H, Yang H, Zhu Q, Li M, Xue J, Gu Y, Lin S, and Ding F. Advanced oxidation protein products induce vascular calcification by promoting osteoblastic trans-differentiation of smooth muscle cells via oxidative stress and ERK pathway. Ren Fail 31: 313–319, 2009 [DOI] [PubMed] [Google Scholar]
- 246. You SA and Wang Q. Ferritin in atherosclerosis. Clin Chim Acta 357: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 247. Young IS, Trouton TG, Torney JJ, McMaster D, Callender ME, and Trimble ER. Antioxidant status and lipid peroxidation in hereditary haemochromatosis. Free Radic Biol Med 16: 393–397, 1994 [DOI] [PubMed] [Google Scholar]
- 248. Yuan XM, Ward LJ, Forssell C, Siraj N, and Li W. Carotid atheroma from men has significantly higher levels of inflammation and iron metabolism enabled by macrophages. Stroke 49: 419–425, 2018 [DOI] [PubMed] [Google Scholar]
- 249. Zacharski LR, Chow B, Lavori PW, Howes PS, Bell MR, DiTommaso MA, Carnegie NM, Bech F, Amidi M, and Muluk S. The iron (Fe) and atherosclerosis study (FeAST): a pilot study of reduction of body iron stores in atherosclerotic peripheral vascular disease. Am Heart J 139: 337–345, 2000 [DOI] [PubMed] [Google Scholar]
- 250. Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, and Lavori PW. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA 297: 603–610, 2007 [DOI] [PubMed] [Google Scholar]
- 251. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, and Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11: 986–994, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Zarjou A, Jeney V, Arosio P, Poli M, Antal-Szalmas P, Agarwal A, Balla G, and Balla J. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J Am Soc Nephrol 20: 1254–1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, and Balla J. Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res 25: 164–172, 2010 [DOI] [PubMed] [Google Scholar]
- 254. Zhang DL, Ghosh MC, Ollivierre H, Li Y, and Rouault TA. Ferroportin deficiency in erythroid cells causes serum iron deficiency and promotes hemolysis due to oxidative stress. Blood 132: 2078–2087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Zhang WJ and Frei B. Intracellular metal ion chelators inhibit TNFalpha-induced SP-1 activation and adhesion molecule expression in human aortic endothelial cells. Free Radic Biol Med 34: 674–682, 2003 [DOI] [PubMed] [Google Scholar]
- 256. Zhang WJ, Wei H, and Frei B. The iron chelator, desferrioxamine, reduces inflammation and atherosclerotic lesion development in experimental mice. Exp Biol Med (Maywood) 235: 633–641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, Nie G, Knutson MD, Anderson GJ, and Wang F. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118: 1912–1922, 2011 [DOI] [PubMed] [Google Scholar]
- 258. Zheng H, Huang X, Zhang Q, and Katz SD. Iron sucrose augments homocysteine-induced endothelial dysfunction in normal subjects. Kidney Int 69: 679–684, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX, You Y, Gong JP, and Liu ZJ. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med 7: 4012–4022, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Zohn IE, De Domenico I, Pollock A, Ward DM, Goodman JF, Liang X, Sanchez AJ, Niswander L, and Kaplan J. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood 109: 4174–4180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]