Abstract
Objective:
The goal of this study was to determine whether the acute analgesic effects of alcohol intake are moderated by acute alcohol tolerance, characterized by differing subjective and neurobehavioral effects of a given blood alcohol concentration (BAC) depending on whether BAC is rising or falling.
Method:
Twenty-nine healthy drinkers (20 women) completed two laboratory sessions in which they consumed a study beverage: active alcohol (target BAC= .08 g/dl) and placebo. Acute alcohol tolerance was assessed by examining the main and interactive effects of beverage condition and assessment limb (ascending vs. descending) on quantitative sensory testing measures collected using slowly ramping heat stimuli and perceived relief ratings at comparable breath alcohol concentrations on the ascending and descending limbs.
Results:
BAC limb moderated the effect of condition on pain threshold, such that the threshold was significantly elevated in the alcohol condition on the ascending limb. The alcohol condition produced greater ratings of perceived pain relief than the placebo condition, and pain relief ratings were greater on the ascending versus descending limb of the BAC curve. Alcohol intake did not significantly affect pain tolerance or aftersensation ratings on either BAC limb.
Conclusions:
This study provides initial experimental evidence that alcohol's analgesic and pain-relieving effects are subject to acute tolerance following acute alcohol intake. These findings suggest that self-medicating pain via alcohol intake may be associated with high-risk drinking topography, increasing the risk for alcohol-related consequences. Further research is needed to determine if these effects extend to the context of clinical and chronic pain.
People experiencing pain, whether acute or chronic, engage in various behaviors to manage pain, including using prescription and over-the-counter pain medication, physical therapy, and a variety of complementary and alternative medicine treatments (Institute of Medicine, 2011). The majority of people experiencing acute pain engage in self-management with over-the-counter analgesics (Moore et al., 2015), which are considered standard of care for acute pain, including postoperative pain resulting from dental and orthopedic procedures (Hersch et al., 2019; Sraj 2019; Wong et al., 2016). However, many patients with acute dental pain exceed the maximum daily dose of overthe-counter analgesics, presumably in an attempt to achieve sufficient pain relief (Hommez et al., 2018). Similarly, frontline pharmaceutical approaches for treating chronic pain (i.e., opioid analgesics) have low therapeutic efficacy (Busse et al., 2018) and rarely meet patient criteria for successful treatment (Robinson et al., 2005). Thus, both acute and chronic pain patients are likely to engage in additional pain self-management strategies, including potentially maladaptive or risky approaches.
Self-medication of pain with alcohol, although potentially maladaptive, is common (Riley & King, 2009). Studies suggest that pain is a significant predictor of negative affect (Paulus et al., 2018), and negative affect is associated with an increased urge and intention to consume alcohol (Moskal et al., 2018). Crucially, alcohol has been shown to reduce pain sensitivity in experimental settings. These effects may be dose dependent, suggesting that individuals self-medicating pain with alcohol may engage in risky drinking patterns (Thompson et al., 2017). Studies have found associations of self-medication of pain with alcohol with poorer health, hazardous drinking patterns (Brennan et al., 2005), greater pain-related dysfunction (Gilson et al., 2014; Zale et al., 2015), and greater pain frequency (Riley & King, 2009).
Following alcohol consumption, blood alcohol concentration (BAC) rapidly rises, peaks, and gradually falls, forming a curve characterized by two “limbs”: ascending and descending. Acute tolerance to alcohol is the well-established phenomenon that a given BAC is associated with differential neurobehavioral and subjective effects depending on the limb during which it is assessed. For example, subjective intoxication also tends to be higher on the ascending than descending limb (Fillmore & Weafer, 2012), and the ascending limb has been associated with impairments in response time during cognitive task performance (Schweizer et al., 2006). Studies have associated the descending limb with performance errors during cognitive tasks, even after processing speed normalizes (Schweizer & Vogel-Sprott, 2008); underestimating one's level of intoxication and degree of impairment (Holland & Ferner, 2017); poorer inhibitory control and impaired perceptions of the dangerousness of driving while intoxicated (Marczinski & Fillmore, 2009; Weafer & Fillmore, 2012); and greater willingness to drive (Amlung et al., 2014; Morris et al., 2014).
Studies of acute alcohol tolerance have primarily focused on its relation to subjective response, impaired driving, and performance on cognitive/behavioral psychomotor tasks (Amlung et al., 2014; Marczinski & Fillmore, 2009; Vogel-Sprout et al., 1989; Weafer & Fillmore, 2012). At the same time, studies of acute alcohol analgesia have not examined the phenomenon in the context of acute tolerance. Thus, the purpose of this study was to investigate whether the effects of alcohol on heat pain sensitivity exhibit acute tolerance in a laboratory setting. Based on previous research, we hypothesized alcohol-related changes in pain sensitivity and endurance (Thompson et al., 2017) such that alcohol administration would be associated with reduced pain sensitivity, greater ability to tolerate painful stimuli, and greater ratings of pain relief relative to a placebo beverage. Although not previously assessed in studies of alcohol analgesia, we also considered aftersensation, which refers to a lingering sensation of pain after stimulation. Aftersensation has been linked to individual differences in endogenous pain modulation (Staud et al., 2006) and is thought to reflect compromise in endogenous pain modulation in chronic pain populations, including people with fibromyalgia (Staud et al., 2007). Consistent with patterns previously noted for subjective intoxication and perceived impairment (e.g., Amlung et al., 2014; Holland & Ferner, 2017), we also predicted a weaker analgesic effect on the descending limb compared with the ascending limb. If the analgesic effects of alcohol are indeed stronger on the ascending than descending limb, individuals may drink more intensely during a given drinking session to reduce pain and/or associated negative affect (Farber et al., 1980; Sher et al., 2007; Uhart & Wand, 2009). Taken together, this behavior could lead to increased risk of hazardous drinking patterns and potential development of alcohol use disorder.
Method
Participant eligibility and recruitment
Healthy social drinkers (N = 29; 20 women) between 25 and 45 years of age were recruited using flyers and by word of mouth. On contacting the laboratory, interested individuals were informed of the basic inclusionary criteria, including (a) being between ages 25 and 45; (b) being a nonsmoker; (c) having no history of chronic pain, major psychiatric disorder, neurological disease, or any chronic medical condition that may affect pain perception; (d) having no history of drug or alcohol dependence; (e) and consuming at least one alcoholic beverage per week, on average, for the past 6 months. If eligible, interested individuals were scheduled for a screening session in the laboratory. The present report is a secondary analysis derived from a larger parent study investigating the neurobehavioral mechanisms of alcohol analgesia using functional magnetic resonance imaging. Aspects of the methods (e.g., participant inclusion/exclusion criteria, laboratory session procedures) have been previously reported (Boissoneault et al., 2020; Sevel et al., 2020). All procedures were approved by the University of Florida Institutional Review Board. Participants provided written informed consent before participating in the study.
Screening
During the screening session, participants completed a battery of self-report questionnaires to assess demographic information, health status, depressive symptomatology (Beck Depression Inventory-II [BDI-II]; Beck et al., 1996), problematic drinking behaviors (Alcohol Use Disorders Identification Test [AUDIT]; Saunders et al., 1993), and alcohol use histories (Alcohol Use Questionnaire [AUQ]; Cahalan et al., 1969). A BDI-II score of at least 20 or AUDIT score of at least 8, indicating hazardous drinking patterns, were exclusionary. Qualifying participants completed the quantitative sensory testing (QST) procedures (described below) and were scheduled for two laboratory sessions.
Laboratory sessions
Before laboratory sessions, participants fasted for at least 4 hours, abstained from consuming alcohol for at least 24 hours, and refrained from using medications that contraindicate alcohol use (e.g., allergy medicine) on the day of testing. Participants were provided a light breakfast (~250 kcal) on arrival. Alcohol administration occurred 1 hour after breakfast consumption. Before alcohol administration, urinebased drug and pregnancy tests were conducted. Baseline breath alcohol concentration (BrAC) measurement was also obtained. A positive result on any of these tests resulted in exclusion. Laboratory sessions occurred a minimum of 48 hours apart, with an average of 18 days (range: 2–66 days).
Alcohol administration
Each participant completed two laboratory sessions: active alcohol (.08 g/dl target BAC) or placebo (.00 g/dl target BAC). Participants were informed they would receive either an alcohol-containing beverage or a placebo in each session and were misled to believe that they could receive each condition once or repeat the same condition twice. Session order was randomized and counterbalanced among all participants. In active alcohol sessions, a modified version of the Widmark formula was used to calculate the amount of medical-grade alcohol (95% ethanol) required to achieve .08 g/dl BAC. Alcohol was mixed in a 1:3 ratio with cold, sugarfree lemon-lime soda and split into two servings. In placebo sessions, the beverage contained only soda. To enhance placebo effectiveness, glasses were misted with alcohol and a small amount was dropped on the rim and surface of the drink. Participants were instructed to consume both beverages within 5 minutes and rinse their mouths thoroughly with water after consumption. To maintain the double blind, volumes of alcohol and soda were calculated, verified, and prepared by researchers uninvolved with QST procedures. Research staff conducting QST procedures were not present for beverage administration. No suggestions regarding potential pain-relieving effects of alcohol were provided at any point to avoid influencing the participant's expectations of alcohol analgesia.
Quantitative sensory testing procedure
QST was conducted in a private exam room using a computer-controlled Q-Sense device (Medoc, Ramat Yishai, Israel). Participants underwent three runs of a slow-ramping thermal stimuli during the ascending limb and again during the descending limb. For each run, a 3 cm × 3 cm thermode was applied to the glabrous skin of the foot and increased in temperature from 32 °C to a maximum of 50 °C at a rate of 0.5 °C/s. Participants indicated the moment (i.e., temperature) when the sensation transitioned from warmth to pain (threshold) and became intolerable (tolerance). Fifteen seconds after stimulus removal, participants rated any lingering pain (i.e., aftersensation) using a 100 mm visual analog scale (VAS) anchored from “no pain” to “most intense pain imaginable.” After each run, participants rated their perceived relief from pain as a result of consuming their beverage from “no relief ” to “most profound relief imaginable” on a 100 mm VAS. QST measures and relief ratings from each of the three runs were averaged for analyses. During the screening session, QST procedures occurred a single time. During each laboratory session, QST occurred twice: 15 minutes and ~85 minutes after beverage administration.
Breath and saliva alcohol concentration measures and placebo manipulation check
Following alcohol administration, BrAC was collected at 10-minute intervals using a standard breath alcohol analyzer (CMI Inc., Owensboro, KY). In between the two bouts of QST, salivary alcohol concentration was obtained using Q.E.D. A150 tests (OraSure Technologies, Inc., Bethlehem, PA), which yields similar results to that of the breath alcohol analyzer (Bates & Martin, 1997). To assess placebo credibility, at the end of each laboratory session, participants completed a manipulation check questionnaire in which they answered “yes” or “no” to the following question: “Do you believe that the beverage you received today contained alcohol?” Participants were transported home when their BrAC reached .02 g/dl or lower (Brown et al., 2014).
Analysis strategy
Data were analyzed using SPSS Statistics Version 24.0 (IBM Corp., Armonk, NY). Repeated-measures analysis of variance was used to examine main and interactive effects of beverage condition (placebo vs. alcohol) and BAC limb (i.e., time) (ascending vs. descending) on threshold temperature, tolerance temperature, and aftersensation ratings, as well as ratings of perceived relief. Two-tailed post hoc t tests were conducted to decompose significant Condition × Limb interactions. Descriptive statistics (M [SD]) are reported for demographic, typical drinking behavior/alcohol use disorder symptomatology, and QST-related measures. A paired t test was performed to compare BrAC measures immediately before testing on the ascending and descending limbs. Effect sizes are reported as or a within-subjects variant of Cohen's d (Morris & DeShon, 2002), as appropriate.
Results
Participant demographics
Twenty-nine community-dwelling adults (69.0% female) participated in this study. Participants averaged 28.97 years of age (SD = 3.84) and 19.83 years of education (SD = 2.75). A total of 89.7% identified as White and 10.3% as Asian. In all, 21.4% of participants identified as Hispanic/Latinx. Detailed self-reported demographic information is listed in Table 1.
Table 1.
Participant demographic characteristics
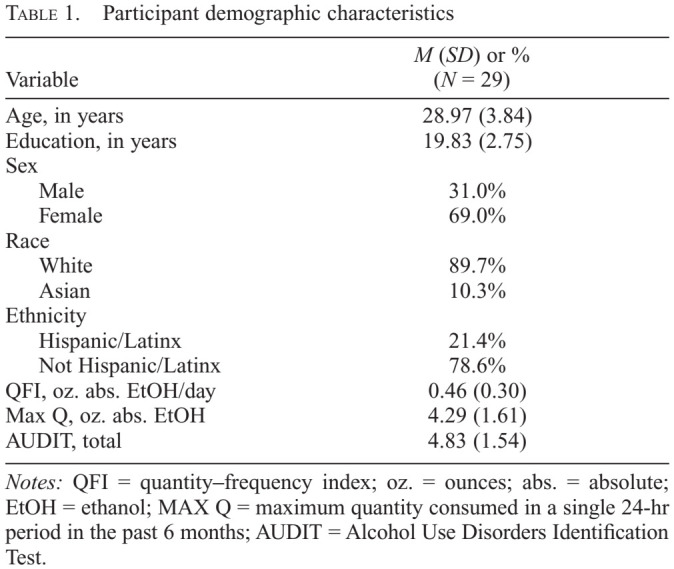
| Variable | M (SD) or %(N = 29) |
|---|---|
| Age, in years | 28.97 (3.84) |
| Education, in years | 19.83 (2.75) Sex |
| Male | 31.0% |
| Female | 69.0% |
| Race | |
| White | 89.7% |
| Asian | 10.3% |
| Ethnicity | |
| Hispanic/Latinx | 21.4% |
| Not Hispanic/Latinx | 78.6% |
| QFI, oz. abs. EtOH/day | 0.46 (0.30) |
| Max Q, oz. abs. EtOH | 4.29 (1.61) |
| AUDIT, total | 4.83 (1.54) |
Notes: QFI = quantity–frequency index; oz. = ounces; abs. = absolute; EtOH = ethanol; MAX Q = maximum quantity consumed in a single 24-hr period in the past 6 months; AUDIT = Alcohol Use Disorders Identification Test.
Typical alcohol use and alcohol use disorder symptomatology
Participants reported an average daily consumption (quantity–frequency index; QFI) of 0.46 oz. (SD = 0.30) of absolute ethanol (~0.8 standard drinks) and a maximum consumption of 4.29 oz. (SD = 1.61) of absolute ethanol within a single 24-hour period (maximum quantity [Max Q]; ~7.2 standard drinks) in the past 6 months. The average score of participants was 4.83 (SD = 1.54) on the AUDIT.A summary of these measures is presented in Table 1.
Breath and saliva alcohol concentration and placebo manipulation measures
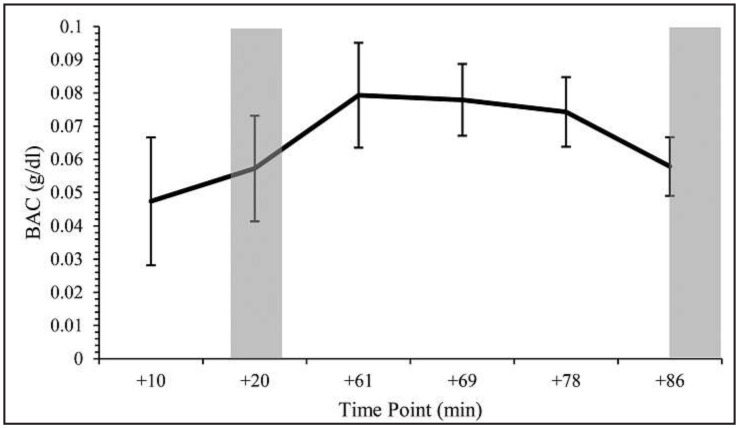
Average measures of BrAC (collected 10, 20, and ~86 minutes after alcohol administration) and salivary alcohol concentration (collected ~61, ~69, and ~78 minutes after alcohol administration) are shown in Figure 1. No differences in BrAC were detected between the ascending limb (M = .0572, SD = .016) and descending limb (M = .0574, SD = .0087, p = .96; d = 0.01). A total of 48.3% of participants believed the placebo beverage contained alcohol, as demonstrated by the placebo manipulation check.
Figure 1.
Mean breath alcohol concentrations (BrACs) during the active alcohol condition. The 10, 20, and ~86 minutes samples were collected via a standard breath alcohol analyzer, and the ~61, ~69, ~78 minutes samples via saliva alcohol tests. On the ascending limb, quantitative sensory testing (QST) was conducted beginning 15 minutes after alcohol administration. QST on the descending limb was conducted an average of 86 minutes (SD = 9.72; range: 65–119 minutes) after alcohol administration. Shaded areas indicate periods of QST testing on the ascending and descending limbs. Error bars represent standard deviation. BAC = blood alcohol concentration; min = minutes.
Quantitative sensory testing
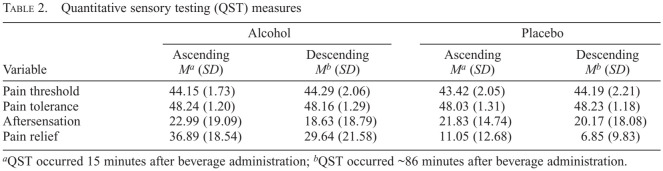
Pain threshold temperatures, pain tolerance temperatures, aftersensation ratings, and pain relief ratings are shown in Table 2. The effects of BAC Limb (time) × Condition interaction on pain sensitivity and relief measures are detailed below and illustrated in Figure 2a–d.
Table 2.
Quantitative sensory testing (QST) measures
| Variable | Alcohol | Placebo | ||
|---|---|---|---|---|
| Ascending Ma (SD) | Descending Mb (SD) | Ascending Ma (SD) | Descending Mb (SD) | |
| Pain threshold | 44.15 (1.73) | 44.29 (2.06) | 43.42 (2.05) | 44.19 (2.21) |
| Pain tolerance | 48.24 (1.20) | 48.16 (1.29) | 48.03 (1.31) | 48.23 (1.18) |
| Aftersensation | 22.99 (19.09) | 18.63 (18.79) | 21.83 (14.74) | 20.17 (18.08) |
| Pain relief | 36.89 (18.54) | 29.64 (21.58) | 11.05 (12.68) | 6.85 (9.83) |
QST occurred 15 minutes after beverage administration;
QST occurred ~86 minutes after beverage administration.
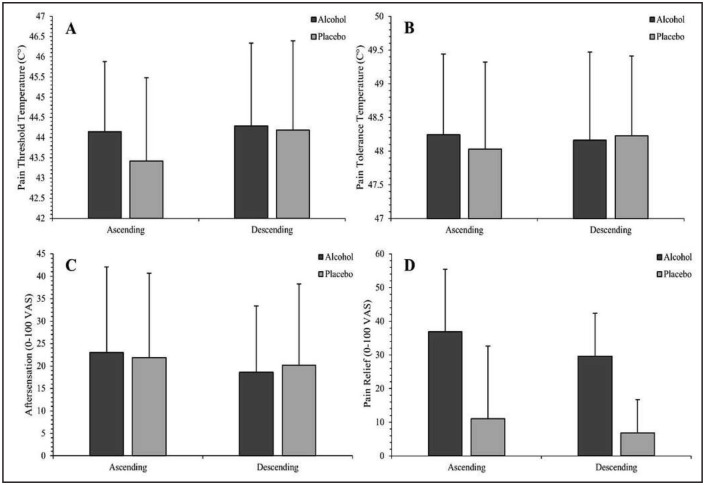
Figure 2.
Effects of blood alcohol concentration (BAC) limb (time) and beverage condition on pain threshold (A), pain tolerance (B), aftersensation (C), and pain relief (D). Vertical bars represent standard deviation. Detailed statistics regarding reported effects are included in text. (A) In the ascending limb, pain threshold was significantly higher in the alcohol condition than the placebo condition. No significant difference in pain threshold was found in the descending limb. (B) A Limb × Condition interaction on pain tolerance approached, but did not achieve, significance. (C) A small, but statistically significant, reduction in aftersensation intensity was noted from the ascending to descending limb. No condition or Condition × Limb interactions were apparent. (D) Pain relief was significantly greater in the alcohol condition than the placebo condition and declined from the ascending to descending limb in both conditions.
Pain threshold
A significant interactive effect of BAC limb and beverage condition was observed for pain threshold, F(1, 27) = 4.44, p = .045, (Figure 2a). De-composition indicated that pain threshold was significantly higher in the alcohol than placebo condition on the ascending limb (p = .020, d = 0.46), but not the descending limb (p =.733, d = 0.06). Within the placebo condition, pain threshold was significantly greater on the descending limb compared with the ascending limb (p = .006, d = 0.55). No significant difference in pain threshold was found between BAC limbs in the alcohol condition (p = .732, d = 0.06). BAC limb (time) had a significant effect on pain threshold temperature, F(1, 27) = 5.46, p = .027, , d = 0.42, such that a higher threshold temperature was reached on the descending limb (M = 44.28, SD = 1.99) than on the ascending limb (M = 43.85, SD = 1.72). No significant main effect of condition on threshold was detected, F(1, 27) = 2.51, p =.125, .
Pain tolerance
A BAC Limb × Condition interaction for pain tolerance did not achieve significance, F(1, 27) = 3.11, p = .089, (Figure 2b). Differences in pain tolerance between the alcohol and placebo condition were nonsignificant on the ascending (p = .259, d = 0.22) and descending limbs (p = .258, d = 0.21). Analysis identified no significant main effects of condition, F(1, 27) = .219, p = .644, , or BAC limb, F(1, 27) = 1.75, p = .197, , on pain tolerance.
Aftersensation
Analyses also revealed a main effect of BAC limb on aftersensation ratings across beverage condition, F(1, 27) = 4.44, p = .045, , d = 0.43 (Figure 2c), such that ratings were lower on the descending limb (M = 18.26, SD = 17.89) compared with the ascending limb (M = 21.49, SD = 15.38). Condition did not significantly affect aftersensation ratings, F(1, 27) = .015, p = .903, , and no BAC limb by condition effect on aftersensation was observed, F(1, 27) = 1.53, p = .227, .
Perceived relief
Beverage condition had a significant main effect on ratings of perceived pain relief, F(1, 27) = 56.07, p < .0001, , d = 1.43 (Figure 2d), with participants reporting greater relief in the alcohol (M = 33.32, SD = 17.71) than placebo condition (M = 9.05, SD =10.25). Significantly greater pain relief, F(1, 27) = 6.03, p = .021, , d = 0.50, was reported on the ascending limb (M = 23.86, SD = 12.71) compared with the descending limb (M = 18.51, SD = 13.47) across beverage types. No significant interactive effect of BAC limb and condition was noted, F(1, 27) = .520, p = .477, .
Discussion
Overview and implications
To our knowledge, the present study is the first investigation of acute tolerance as a potential moderator of alcohol's analgesic effects. Consistent with previous reports (Thompson et al., 2017), pain threshold was significantly greater in the alcohol condition than placebo, but only as BAC was rising. On its face, this finding appears to support our hypothesis that alcohol's analgesic effects may be greatest on the ascending limb of the BAC curve. However, examination of the decomposed interaction suggests that although pain threshold was not elevated in the alcohol condition versus placebo on the descending limb, this appears to be because pain threshold increased between assessments in the placebo condition. No changes in pain threshold from the ascending to descending limb were noted in the alcohol condition. Because this study did not include a no-treatment control condition, we cannot discriminate between potential explanations for this pattern of effects. One possibility is that alcohol has stronger analgesic effects on the ascending than descending limb and that, in the placebo condition, participants’ pain sensitivity declined over the course of testing. Another is that alcohol intake elevates pain threshold on both the ascending and descending limb, and elevations in pain threshold between assessment points in the placebo condition are due to some other factor. Thus, although our results provide initial evidence that acute tolerance may moderate the effects of alcohol on pain threshold, future studies incorporating a no-treatment baseline condition are needed to clarify these effects.
We noted a similar pattern for pain tolerance, but neither main nor interactive effects of beverage condition achieved significance. Detected within-subject effect sizes for the effect of alcohol on pain tolerance on each BAC limb were small. Interestingly, there is only limited prior evidence that acute alcohol intake increases pain tolerance, with a single study showing increased tolerance to a pressure pain stimulus applied to the Achilles tendon in a small sample of healthy women (Woodrow & Eltherington, 1988). Given that we used heat pain stimuli in this study, additional studies are needed to determine whether the effects of acute alcohol intake on pain tolerance depend on stimulus modality or whether other methodological differences between studies might account for this discrepant finding (e.g., the earlier study's inclusion of a financial incentive for tolerating high pressures).
Interestingly, our analysis did not reveal an alcohol-related reduction in aftersensation compared with placebo. In fact, the effect size for the beverage condition main effect on aftersensation ratings was trivial. Although our data suggest that acute alcohol intake does not affect aftersensation in current drinkers without alcohol use disorder or chronic pain, future studies are needed to determine whether alcohol has differential effects in these populations. We also note that our results may be seen as conflicting with a recent study showing that acute alcohol intake was associated with greater conditioned pain modulation than a placebo (Horn-Hofmann et al., 2019), which the authors interpret as evidence that alcohol intake may acutely improve endogenous pain modulation. However, significant methodological differences between studies (i.e., our focus on aftersensation following stimulus removal vs. conditioned pain modulation) complicate direct comparison. Additional systematic research is needed to better characterize alcohol's effects on endogenous pain modulation.
Crucially, our data also suggest that perceived pain relief from consumption of an alcohol-containing beverage is moderated by BAC limb. As expected, participants reported significantly greater pain relief in the alcohol condition than the placebo condition. This effect was statistically large (Cohen's d = 1.43). In fact, it was much larger than the effect of alcohol on pain threshold on the ascending limb (Cohen's d = 0.46), suggesting that the negative reinforcing effects of alcohol may be incompletely captured by changes in pain sensitivity. We also noted a significant decline in relief ratings from the ascending to descending limb (i.e., preto post-test), suggesting that the negatively reinforcing effects of alcohol in the context of pain may be strongest as BAC is rising. Interestingly, data suggested similar declines in perceived relief in the alcohol and placebo conditions. This may be due to reductions in placebo efficacy over the course of a laboratory session. Indeed, many participants receiving a placebo beverage do not remain deceived indefinitely after beverage consumption (Martin & Sayette, 1993).
These findings may have important clinical implications. Stronger analgesic and negative-reinforcing effects of alcohol use on the ascending than descending limb suggest that individuals who self-medicate pain using alcohol may engage in hazardous drinking patterns (i.e., drinking relatively large quantities quickly over extended periods) to maximize and prolong pain relief. Hazardous drinking associated with self-medication of pain, in turn, may result in increased risk of alcohol use disorder and development and/or exacerbation of chronic pain. These considerations may be useful in the design and implementation of interventions designed to prevent and reduce harm associated with self-medication of pain using alcohol.
Limitations and future directions
Although novel, our findings should be interpreted in light of several important limitations. First, our analysis was adequately powered to detect interactive and main effects of assessment point and beverage condition on QST measures. However, we did not have a sufficient sample to characterize other factors that may affect perceptions of pain sensitivity across limbs of the BAC curve. Enrollment for the parent study is ongoing, and we look forward to future analyses examining potential moderators of acute tolerance to alcohol analgesia, including sex, alcohol-related expectancies, subjective response (i.e., feelings of stimulation/sedation), typical drinking pattern, and family history of alcoholism. Of note, positive affect induction has been demonstrated to have analgesic effects and may act as a buffer to nocebo stimuli (Finan & Garland, 2015; Geers et al., 2019; Thong et al., 2017). The association between alcohol-related changes in affect (both positive and negative) and analgesia will be an interesting focus for future studies. Furthermore, although individuals with chronic pain were not included in this study, chronic pain status is another potentially critical moderator of alcohol analgesia deserving of systematic study.
Another limitation of the present study is that our QST protocol used only heat-based stimuli. Although heat pain paradigms have many advantages in a controlled laboratory setting, alternative methods of pain induction, including other common laboratory pain induction modalities (e.g., cold pressor, electric shock, etc.) and those that produce more clinically relevant musculoskeletal pain (e.g., vigorous eccentric exercise), should be investigated in future studies. In addition, because we did not provide a common referent for participants to base their ratings of perceived relief, points of reference may have varied between individuals. Future studies should consider including a referent in the instructions for providing relief ratings (e.g., “Based on your experience with pain sensitivity testing before drinking your beverage, how much relief from pain do you believe your beverage provided?”).
Last, alcohol was mixed with sugar-free soda and administered orally in this study. Although this approach is consistent with our prior work (e.g., Boissoneault et al., 2014; Garcia et al., 2020; Lewis et al., 2016; Sklar et al., 2014), some studies have found that the addition of a sugarfree beverage can increase BAC to a greater extent than with a similar sweetened beverage (Marczinski & Stamates, 2013; Stamates et al., 2015). Crucially, however, participants achieved our target BrAC of .08 g/dl at the group level in the alcohol condition, suggesting that the sugar-free nature of the beverage did not meaningfully affect dosing procedures in this study. Furthermore, although oral alcohol administration has greater ecological validity than alternative methods (i.e., IV administration), use of IV administration in future studies of acute tolerance to alcohol analgesia would provide greater control over participants’ BAC than is possible with oral administration paradigms (providing more consistent trajectories across participants) and enable more robust placebo controls (Cyders et al., 2020). Such an approach would also permit targeting of specific BACs on the ascending and descending limb and facilitate imaging studies of the functional neural correlates of acute tolerance to alcohol analgesia.
Conclusion
This study provides important initial evidence that the analgesic effects of alcohol, particularly perceived relief from pain, may be subject to acute tolerance. Combined with meta-analytic evidence that the analgesic effects of alcohol are dose dependent (at least in laboratory settings), findings suggest that, if replicated, individuals self-treating their pain via alcohol consumption are at high risk for engaging in hazardous drinking patterns characterized by rapid consumption of relatively large quantities of alcohol over extended periods. Future studies are needed to characterize individual factors that may modulate this risk.
Acknowledgment
The authors thank Dr. Ben Lewis for his helpful comments on a prior draft of this manuscript.
Footnotes
Support for this work was provided by National Institute on Alcohol Abuse and Alcoholism award numbers R01AA025337 and R21AA026805. The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors confirm the following contributions to the article—study conception and design: J.B. and M.R.; acquisition of data: B.S. and D.V.; analysis and interpretation of results: E.F., D.V., and M.W.; drafting of the manuscript: J.B., D.V., and M.W; and critical revision: J.B, E.F., B.S., and M.R. All authors read and approved the final manuscript.
References
- Amlung M. T., Morris D. H., McCarthy D. M. Effects of acute alcohol tolerance on perceptions of danger and willingness to drive after drinking. Psychopharmacology. 2014;231:4271–4279. doi: 10.1007/s00213-014-3579-1. doi:10.1007/s00213-014-3579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates M. E., Martin C. S. Immediate, quantitative estimation of blood alcohol concentration from saliva. Journal of Studies on Alcohol. 1997;58:531–538. doi: 10.15288/jsa.1997.58.531. doi:10.15288/jsa.1997.58.531. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. Second Edition. San Antonio, TX: The Psychological Corporation; 1996. Beck Depression Inventory. [Google Scholar]
- Boissoneault J., Sklar A., Prather R., Nixon S. J. Acute effects of moderate alcohol on psychomotor, set shifting, and working memory function in older and younger social drinkers. Journal of Studies on Alcohol and Drugs. 2014;75:870–879. doi: 10.15288/jsad.2014.75.870. doi:10.15288/jsad.2014.75.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissoneault J., Stennett B., Robinson M. E. Acute alcohol intake alters resting state functional connectivity of nucleus accumbens with pain-related corticolimbic structures. Drug and Alcohol Dependence. 2020;207:107811. doi: 10.1016/j.drugalcdep.2019.107811. doi:10.1016/j.drugalcdep.2019.107811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. L., Schutte K. K., Moos R. H. Pain and use of alcohol to manage pain: Prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–786. doi: 10.1111/j.1360-0443.2005.01074.x. doi:10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- Brown S. A., de Wit H., O’Connor S., O’Malley S. S., Ota-Wang V., Palmer L. I., Taylor R. E. Recommended council guidelines on ethyl alcohol administration in human experimentation. 2014 Retrieved from https://www.niaaa.nih.gov/research/guidelines-and-resources/administering-alcohol-human-studies.
- Busse J. W., Wang L., Kamaleldin M., Craigie S., Riva J. J., Montoya L., Guyatt G. H. Opioids for chronic noncancer pain: A systematic review and meta-analysis. JAMA. 2018;320:2448–2460. doi: 10.1001/jama.2018.18472. doi:10.1001/jama.2018.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D., Cissin L., Crossley H. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. American drinking practices: A national study of drinking behaviors and attitudes (Monograph No. 6. [Google Scholar]
- Cyders M. A., Plawecki M. H., Corbin W., King A., McCarthy D. M., Ramchandani V. A., … O’Connor S. J.2020To infuse or ingest in human laboratory alcohol research Alcoholism: Clinical and Experimental Research 44764–776.doi:10.1111/acer.14305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber P. D., Khavari K. A., Douglass F. M. A factor analytic study of reasons for drinking: Empirical validation of positive and negative reinforcement dimensions. Journal of Consulting and Clinical Psychology. 1980;48:780–781. doi: 10.1037//0022-006x.48.6.780. doi:10.1037/0022-006X.48.6.780. [DOI] [PubMed] [Google Scholar]
- Fillmore M. T., Weafer J.2012Acute tolerance to alcohol in at-risk binge drinkers Psychology of Addictive Behaviors 26693–702. 0 doi:10.1037/a0026110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan P. H., Garland E. L. The role of positive affect in pain and its treatment. Clinical Journal of Pain. 2015;31:177–187. doi: 10.1097/AJP.0000000000000092. doi:10.1097/AJP.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C. C., Lewis B., Boissoneault J., Nixon S. J. Effects of age and acute moderate alcohol consumption on electrophysiological indices of attention. Journal of Studies on Alcohol and Drugs. 2020;81:372–383. doi: 10.15288/jsad.2020.81.372. doi:10.15288/jsad.2020.81.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A. L., Close S., Caplandies F. C., Vogel C. L., Murray A. B., Pertiwi Y., Vase L. Testing a positive-affect induction to reduce verbally induced nocebo hyperalgesia in an experimental pain paradigm. Pain. 2019;160:2290–2297. doi: 10.1097/j.pain.0000000000001618. doi:10.1097/j.pain.0000000000001618. [DOI] [PubMed] [Google Scholar]
- Gilson K. M., Bryant C., Judd F. The hidden harms of using alcohol for pain relief in older adults. International Psychogeriatrics. 2014;26:1929–1930. doi: 10.1017/S1041610214001513. doi:10.1017/S1041610214001513. [DOI] [PubMed] [Google Scholar]
- Hersch C., Denis C., Sugár D. Frequency, nature and management of patient-reported severe acute pain episodes in the over-thecounter setting: Results of an online survey. Pain Management. 2019;9:379–387. doi: 10.2217/pmt-2018-0092. doi:10.2217/pmt-2018-0092. [DOI] [PubMed] [Google Scholar]
- Holland M. G., Ferner R. E. A systematic review of the evidence for acute tolerance to alcohol - the “Mellanby effect. Clinical Toxicology. 2017;55:545–556. doi: 10.1080/15563650.2017.1296576. doi:10.1080/15563650.2017.1296576. [DOI] [PubMed] [Google Scholar]
- Hommez G., Ongena B., Cauwels R. G. E. C., De Paepe P., Christiaens V., Jacquet W. Analgesia (mis)usage on a dental emergency service: A patient survey. Clinical Oral Investigations. 2018;22:1297–1302. doi: 10.1007/s00784-017-2228-6. doi:10.1007/s00784-017-2228-6. [DOI] [PubMed] [Google Scholar]
- Horn-Hofmann C., Capito E. S., Wolstein J., Lautenbacher S. Acute alcohol effects on conditioned pain modulation, but not temporal summation of pain. Pain. 2019;160:2063–2071. doi: 10.1097/j.pain.0000000000001597. doi:10.1097/j.pain.0000000000001597. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Washington, DC: The National Academies Press; 2011. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. [PubMed] [Google Scholar]
- Lewis B., Boissoneault J., Frazier I., Nixon S. J. Effects of age and acute moderate alcohol administration on neurophysiology during simulated driving. Alcoholism: Clinical and Experimental Research. 2016;40:2519–2527. doi: 10.1111/acer.13243. doi:10.1111/acer.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski C. A., Fillmore M. T. Acute alcohol tolerance on subjective intoxication and simulated driving performance in binge drinkers. Psychology of Addictive Behaviors. 2009;23:238–247. doi: 10.1037/a0014633. doi:10.1037/a0014633. [DOI] [PubMed] [Google Scholar]
- Marczinski C. A., Stamates A. L. Artificial sweeteners versus regular mixers increase breath alcohol concentrations in male and female social drinkers. Alcoholism: Clinical and Experimental Research. 2013;37:696–702. doi: 10.1111/acer.12039. doi:10.1111/acer.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. S., Sayette M. A. Experimental design in alcohol administration research: Limitations and alternatives in the manipulation of dosage-set. Journal of Studies on Alcohol. 1993;54:750–761. doi: 10.15288/jsa.1993.54.750. doi:10.15288/jsa.1993.54.750. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Wiffen P. J., Derry S., Maguire T., Roy Y. M., Tyrrell L. Non-prescription (OTC) oral analgesics for acute pain - an overview of Cochrane reviews. Cochrane Database of Systematic Reviews. 2015. 2015. p. CD010794. doi:10.1002/14651858.CD010794.pub2. [DOI] [PMC free article] [PubMed]
- Morris S. B., DeShon R. P. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. doi:10.1037/1082-989X.7.1.105. [DOI] [PubMed] [Google Scholar]
- Morris D. H., Treloar H. R., Niculete M. E., McCarthy D. M. Perceived danger while intoxicated uniquely contributes to driving after drinking. Alcoholism: Clinical and Experimental Research. 2014;38:521–528. doi: 10.1111/acer.12252. doi:10.1111/acer.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal D., Maisto S. A., De Vita M., Ditre J. W. Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Experimental and Clinical Psychopharmacology. 2018;26:65–76. doi: 10.1037/pha0000170. doi:10.1037/pha0000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus D. J., Garey L., Gallagher M. W., Derrick J. L., Jardin C., Langdon K., Ditre J. W., Zvolensky M. J. Pain severity as a predictor of negative affect following a self-guided quit attempt: An ecological momentary assessment study. American Journal of Drug and Alcohol Abuse. 2018;44:543–550. doi: 10.1080/00952990.2018.1467432. doi:1080/00952990.2018.1467432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J. L., III, & King C.2009Self-report of alcohol use for pain in a multi-ethnic community sample Journal of Pain 10944–952.doi:10.1016/j.jpain.2009.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. E., Brown J. L., George S. Z., Edwards P. S., Atchison J. W., Hirsh A. T., Fillingim R. B. Multidimensional success criteria and expectations for treatment of chronic pain: The patient perspective. Pain Medicine. 2005;6:336–345. doi: 10.1111/j.1526-4637.2005.00059.x. doi:10.1111/j.1526-4637.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Saunders J. B., Aasland O. G., Babor T. F., de la Fuente J. R., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. doi:10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schweizer T. A., Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: A review of acute tolerance and recovery of cognitive performance. Experimental and Clinical Psychopharmacology. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. doi:10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Schweizer T. A., Vogel-Sprott M., Danckert J., Roy E. A., Skakum A., Broderick C. E. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31:1301–1309. doi: 10.1038/sj.npp.1300941. doi:10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- Sevel L., Stennett B., Schneider V., II, Bush N., Nixon S. J., Robinson M., Boissoneault J. Acute alcohol intake produces widespread decreases in cortical resting signal variability in healthy social drinkers. Alcoholism: Clinical and Experimental Research. 2020;44:1410–1419. doi: 10.1111/acer.14381. doi:10.1111/acer.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher K. J., Bartholow B. D., Peuser K., Erickson D. J., Wood M. D. Stress-response-dampening effects of alcohol: Attention as a mediator and moderator. Journal of Abnormal Psychology. 2007;116:362–377. doi: 10.1037/0021-843X.116.2.362. doi:10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar A. L., Boissoneault J., Fillmore M. T., Nixon S. J. Interactions between age and moderate alcohol effects on simulated driving performance. Psychopharmacology. 2014;231:557–566. doi: 10.1007/s00213-013-3269-4. doi:10.1007/s00213-013-3269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sraj S. Narcotic-free, over-the-counter pain management after wideawake hand surgery. Journal of the American Academy of Orthopaedic Surgeons. Global Research & Reviews. 2019;3:e19.00137. doi: 10.5435/JAAOSGlobal-D-19-00137. doi:10.5435/JAAOSGlobal-D-19-00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamates A. L., Maloney S. F., Marczinski C. A. Effects of artificial sweeteners on breath alcohol concentrations in male and female social drinkers. Drug and Alcohol Dependence. 2015;157:197–199. doi: 10.1016/j.drugalcdep.2015.10.015. doi:10.1016/j.drugalcdep.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R., Koo E., Robinson M. E., Price D. D. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain. 2007;130:177–187. doi: 10.1016/j.pain.2007.03.015. doi:10.1016/j.pain.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R., Vierck C. J., Robinson M. E., Price D. D. Overall fibromyalgia pain is predicted by ratings of local pain and pain-related negative affect—possible role of peripheral tissues. Rheumatology. 2006;45:1409–1415. doi: 10.1093/rheumatology/kel121. doi:10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- Thompson T., Oram C., Correll C. U., Tsermentseli S., Stubbs B. Analgesic effects of alcohol: A systematic review and metaanalysis of controlled experimental studies in healthy participants. Journal of Pain. 2017;18:499–510. doi: 10.1016/j.jpain.2016.11.009. doi:10.1016/j.jpain.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Thong I. S. K., Tan G., Jensen M. P. The buffering role of positive affect on the association between pain intensity and pain related outcomes. Scandinavian Journal of Pain. 2017;14:91–97. doi: 10.1016/j.sjpain.2016.09.008. doi:10.1016/j.sjpain.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Uhart M., Wand G. S. Stress, alcohol and drug interaction: an update of human research. Addiction Biology. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. doi:10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Sprott M., Kartechner W., McConnell D. Consequences of behavior influence the effect of alcohol. Journal of Substance Abuse. 1989;1:369–379. [PubMed] [Google Scholar]
- Weafer J., Fillmore M. T. Acute tolerance to alcohol impairment of behavioral and cognitive mechanisms related to driving: Drinking and driving on the descending limb. Psychopharmacology. 2012;220:697–706. doi: 10.1007/s00213-011-2519-6. doi:10.1007/s00213-011-2519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. J., Keenan J., Hudson K., Bryan H., Naftolin F., Thompson V. P., Curro F. A. Opioid, NSAID, and OTC analgesic medications for dental procedures: PEARL Network Findings. Compendium of Continuing Education in Dentistry. 2016;37:710–718. Retrieved from https://www.aegisdentalnetwork.com/cced/2016/11/opioid-nsaid-and-otc-analgesic-medications-for-dental-procedures-pearl-network-findings. [PubMed] [Google Scholar]
- Woodrow K. M., Eltherington L. G. Feeling no pain: Alcohol as an analgesic. Pain. 1988;32:159–163. doi: 10.1016/0304-3959(88)90064-4. doi:10.1016/0304-3959(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Zale E. L., Maisto S. A., Ditre J. W. Interrelations between pain and alcohol: An integrative review. Clinical Psychology Review. 2015;37:57–71. doi: 10.1016/j.cpr.2015.02.005. doi:10.1016/j.cpr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]