Abstract
Aims:
Theory-driven, exploratory study to: (1) identify a reward drinking phenotype in young adults, (2) evaluate this phenotype as a predictor of naltrexone response, and (3) examine mechanisms of naltrexone in reward drinkers.
Design:
Secondary analysis of a randomized controlled trial.
Setting:
USA.
Participants:
128 young adult (ages 18–25) heavy drinkers.
Interventions:
Naltrexone versus placebo.
Measurements:
Daily surveys assessed affect, urge, drinking, and context. The Drinking Motives Questionnaire was used to identify phenotypes based on reward (enhancement motives) and relief (coping motives) drinking.
Findings:
We identified 3 profiles: “Low reward/Low relief” (14.1%; low enhancement/low coping motives); “Reward drinkers” (62.2%; high enhancement/low coping motives); and “High reward/High relief” (22.7%; high enhancement/high coping motives). Among reward drinkers (versus low profile), naltrexone significantly reduced percent days drinking to intoxication (BAC ≥.08) (PDI) [d=.56; 95% CI(.17,.96)] and percent high intensity drinking days (PHID) (8/10 drinks for women/men) [d=.32; 95% CI(.01,.68)]. Among the high reward/high relief profile drinkers (versus low profile), naltrexone reduced PHID [d=.69; 95% CI(.02,1.50)]. Using profile-informed cutoffs and observed scores (for clinical applicability): (1) among cutoff-derived reward drinkers, we found a medium-to-large [d=.66; 95% CI(.24,1.16)] and small effect [d=.28; 95% CI(.04,.72)] of naltrexone in reducing PDI and PHID, respectively; and (2) among the cutoff-derived high reward/high relief subgroup, we found a medium-to-large effect [d=.63; 95% CI(.05,1.1)] of naltrexone in reducing PHID. Among reward drinkers (not other profiles), naltrexone reduced drinking on days a drinking event occurred by weakening the within-day association between positive affect and urges (ps<.05).
Conclusions:
Naltrexone has pronounced effects in reducing risky drinking among young adult reward drinkers (high reward/low relief) by reducing urges on days when individuals have higher positive affect and are exposed to a drinking event. Naltrexone also appears to reduce risky drinking among young adult high reward/high relief drinkers, but not via the same mechanism.
Keywords: Reward drinker, naltrexone, heavy drinking, young adults, precision medicine
Introduction
Heavy drinking and alcohol use disorder (AUD) are prevalent (1) and have serious negative consequences (2, 3). Although effective pharmacotherapies exist, they generally provide modest benefit (4). A precision medicine approach, which matches interventions to phenotypes, may enhance the impact of pharmacotherapy (5–7).
Reward and relief drinking phenotypes are central to AUD and may advance AUD precision medicine (8–13). A reward drinking phenotype (“reward drinker”) is characterized by alcohol use that is primarily driven by the positive, rewarding effects of alcohol. A relief drinking phenotype (“relief drinker”) is characterized by alcohol use that is primarily driven by the distress-relieving effects of alcohol. Several studies have identified and validated these phenotypes (14–16).
The reward drinker–naltrexone response hypothesis (8–13) states that reward drinkers will respond particularly well to naltrexone, an opioid antagonist and FDA-approved medication for AUD. The efficacy of naltrexone in reducing drinking is supported by a large evidence base, though the magnitude of average effects is small (4, 17). Naltrexone may reduce drinking by dampening the positive, rewarding effects of alcohol and the subjective desire to drink in response to alcohol cues (18–20).
Importantly, in two placebo-controlled studies (14, 16), naltrexone had significant and large effects in reducing drinking among high reward/low relief drinkers, identified using the Inventory of Drinking Situations (IDS) (21). Yet, one secondary analysis did not find a significant reward drinker-by-naltrexone interaction (15) when reward drinkers were identified with the Alcohol Abstinence Self-Efficacy Scale (AASE) (22).
Evaluations of the reward drinker–naltrexone response hypothesis have primarily been among middle-aged adults, with no studies among young adults, among whom reward drinking is prevalent (23). Here, we conducted a secondary analysis of a randomized clinical trial among young adult heavy drinkers (24) to achieve three aims: (1) Identify a reward drinking phenotype in young adults; (2) Evaluate this phenotype as a predictor of naltrexone response; (3) Examine mechanisms of naltrexone response in reward drinkers. We hypothesized that we would identify a reward drinker phenotype that predicted naltrexone response. Based on prior research (16), we hypothesized that reductions in average daily urges would mediate the effect of naltrexone among reward drinkers. Given preliminary research that naltrexone may reduce drinking by weakening the link between positive affect and urge (25–27), we hypothesized that, among young adult reward drinkers, naltrexone (vs. placebo) would weaken the within-person effect of positive affect on drinking via urges on a given day, particularly on drinking days and days when individuals were exposed to drinking events.
Because no previous studies have evaluated the reward drinker phenotype as a predictor of naltrexone response and the underlying mechanisms of this effect in a young adult sample, we adopted a theory-driven, exploratory approach grounded in theory. Because we conducted multiple statistical tests without correction for multiple comparisons, study findings are preliminary.
Method
Design
We conducted a secondary analysis of an 8-week randomized, placebo-controlled trial of naltrexone among young adult heavy drinkers (24). Individuals were randomly assigned to either naltrexone (25 mg daily dose + 25 mg targeted dose on drinking days) or placebo (daily and targeted doses). For the targeted dose, participants were instructed to take a single dose of medication at least 2 hours prior to anticipated drinking situations. All participants received a brief intervention (28). Participants completed web-based daily diary assessments (in the morning after waking) during treatment, with automated emails sent daily as reminders to complete the diary assessments.
Participants
A total of 140 young adults were randomized to receive naltrexone or placebo. Eligibility criteria included: (a) age 18–25; (b) at least 4 heavy drinking days (4/5 drinks in a day for women/men) in the past 4 weeks; (c) able to read English; and (d) no significant cognitive impairments. Exclusion criteria included presence of a serious psychiatric disorder or physical disease, a history of clinically significant withdrawal, drug dependence (other than nicotine), pregnancy, or breastfeeding. One-hundred twenty-eight individuals attended the first session and received treatment (naltrexone=61; placebo=67), and were included in the analyses of the parent trial (24) and this study. Table 1 provides sample characteristics. See Supplementary materials for details on the sample.
Table 1.
Characteristics of the analysis sample (n=128)
| Study Variable | N (%) or Mean (Standard Deviation) |
|---|---|
| Female | 40 (31%) |
| Race | |
| Black/African American | 10 (8%) |
| White | 99 (77%) |
| Asian | 4 (3%) |
| Native American | 1 (0.07%) |
| Multiple | 6 (5%) |
| Other | 8 (6%) |
| Age | 21.5 (2.15) |
| Highest level of education | |
| High School or less | 18 (14%) |
| Some college | 72 (56%) |
| College degree | 38 (30%) |
| Currently enrolled as a college student | 91 (71%) |
| Smoke cigarettes at least weekly | 38 (30%) |
| Family history positive for AUD | 49 (38.3%) |
| PDA | |
| Baseline | 46.5 (18.6) |
| During Treatment | 59.7 (19.4) |
| PHD | |
| Baseline | 33.8 (15.1) |
| During Treatment | 22.3 (14.5) |
| DPDD | |
| Baseline | 6.7 (2.6) |
| During Treatment | 5.4 (2.4) |
| PDI | |
| Baseline | N/A |
| During Treatment | 40.7 (27.9) |
| eBAC | |
| Baseline | N/A |
| During Treatment | .09 (.04) |
| PHID | |
| Baseline | 13.8 (14.1) |
| During Treatment | 6.6 (9.1) |
| Coping Motives | 8.4 (5.0) |
| Enhancement Motives | 12.6 (4.4) |
Note. AUD = alcohol use disorder; PDA = percent days abstinent; PHD = percent heavy drinking days (4/5 drinks per day for women/men); DPDD = drinks per drinking day; PDD = percent days drinking to intoxication; eBAC = estimated blood alcohol concentration per drinking day; PHID = percent high intensity drinking days (8/10 drinks per day for women/men).
Measures
Alcohol use.
The Timeline Follow-back (TLFB) (29) was used to assess alcohol use. During treatment, participants also completed daily diaries and reported the number of standard drinks they consumed during the previous day. Consistent with the parent trial (24), daily diaries were used to measure alcohol outcomes. Estimated blood alcohol concentration (eBAC) was based on number of standard drinks reported, duration of drinking, and total body water (based on sex, age, height, weight) (30).
Reward and relief drinking.
Five items from the coping and enhancement motives subscales (see Appendix) of the Drinking Motives Questionnaire (DMQ) (31, 32), a self-report measure of motives for drinking, were used to assess reward and relief drinking. The DMQ was administered at baseline. Response options range from 0=almost never to 4=almost always. Internal consistency of the coping (α=.86) and enhancement subscales (α=.81) was good.
Family history of AUD.
The alcohol portion of the Family History Assessment Module (33) was used to assess family history of AUD.
Demographics and other characteristics.
A baseline questionnaire was used to measure age, gender, education status, and smoking behavior.
Daily diary assessments.
Participants reported current positive affect at the time of the survey (in the morning after waking) with 3 items rated from 1 (not at all) to 7 (extremely): “enthusiastic,” “excited,” and “happy.” These items were averaged to create a total daily rating of positive affect (α=.92). Urge was measured with three items rated from 1 (strongly disagree) to 9 (strongly agree): “I felt like I could have really used a drink,” “The idea of drinking was appealing,” and “I really didn’t feel like drinking” (reverse-scored). Participants reported on their urge during the previous day. Scores were averaged to create a composite rating of urge (α=.86). Exposure to drinking events was measured by asking whether individuals “were exposed to a situation in which drinking occurred” on the prior day. Medication use was measured by asking whether participants took a daily dose and targeted dose during the previous day and was coded as 0=none, 1=took daily or targeted dose, 2=took both daily and targeted doses. See Supplementary materials for details on medication adherence.
Statistical Analyses
SPSS Version 26 was used for descriptive analyses and ANOVAs, and Mplus Version 8 (34) for all other analyses. With respect to missing data, 80.9% of daily diary assessments were completed. Consistent with the parent trial (24), we used data from the TLFB to replace missing data on alcohol use during treatment; thus drinking data were available for >90% of treatment days. Full information maximum likelihood estimation, which uses all available data, was used to accommodate missing data and estimate model parameters.
Latent profile analysis
To identify phenotypes, we used latent profile analysis, with 10 items from the DMQ as indicators. The optimal model was chosen based on fit statistics, the Lo-Mendell-Rubin (LMR) test, and the theoretical clarity of the profiles (35). Using the estimated posterior probabilities, we saved most likely profile assignment as an observed variable. We also tested construct validity of the final latent profile solution (see Supplementary materials).
Profile by treatment condition interactions.
We used moderated regression models to evaluate phenotype as a predictor of naltrexone response. Similar to the parent trial (24), we examined the following alcohol outcomes during the 8-week treatment period: percent days abstinent (PDA), percent heavy drinking days (PHD; 4/5 drinks in a day for women/men), drinks per drinking day (DPDD), percent days drinking to intoxication (eBAC ≥ .08) (PDI), and eBAC per drinking day. Given work on the negative impact of high-intensity drinking (8/10 drinks for women/men) (36–38), we also examined percent high intensity drinking days (PHID). Moderated regression and follow-up analyses controlled for sex, family history of AUD, baseline PDA (24), and smoking status (39–41). Models with PHD and PHID as the outcomes controlled for baseline PHD and baseline PHID, respectively. Models with DPDD, PDI, and estimated BAC per drinking day as outcomes controlled for baseline DPDD. Significant omnibus moderation effects were explored by testing effects of naltrexone within each profile. Cohen’s d effect sizes were computed.
Consistent with prior work (14, 16), we also identified phenotypes using ±1.5 standard deviation (SD) as a cutoff criterion (see Appendix). We then evaluated the effect of naltrexone on outcomes among subgroups identified by observed cutoff scores.
Testing mechanisms of change via mediation models
All tests of mediation used the products of coefficients approach (42).
Reduction in average daily urge as a mechanism.
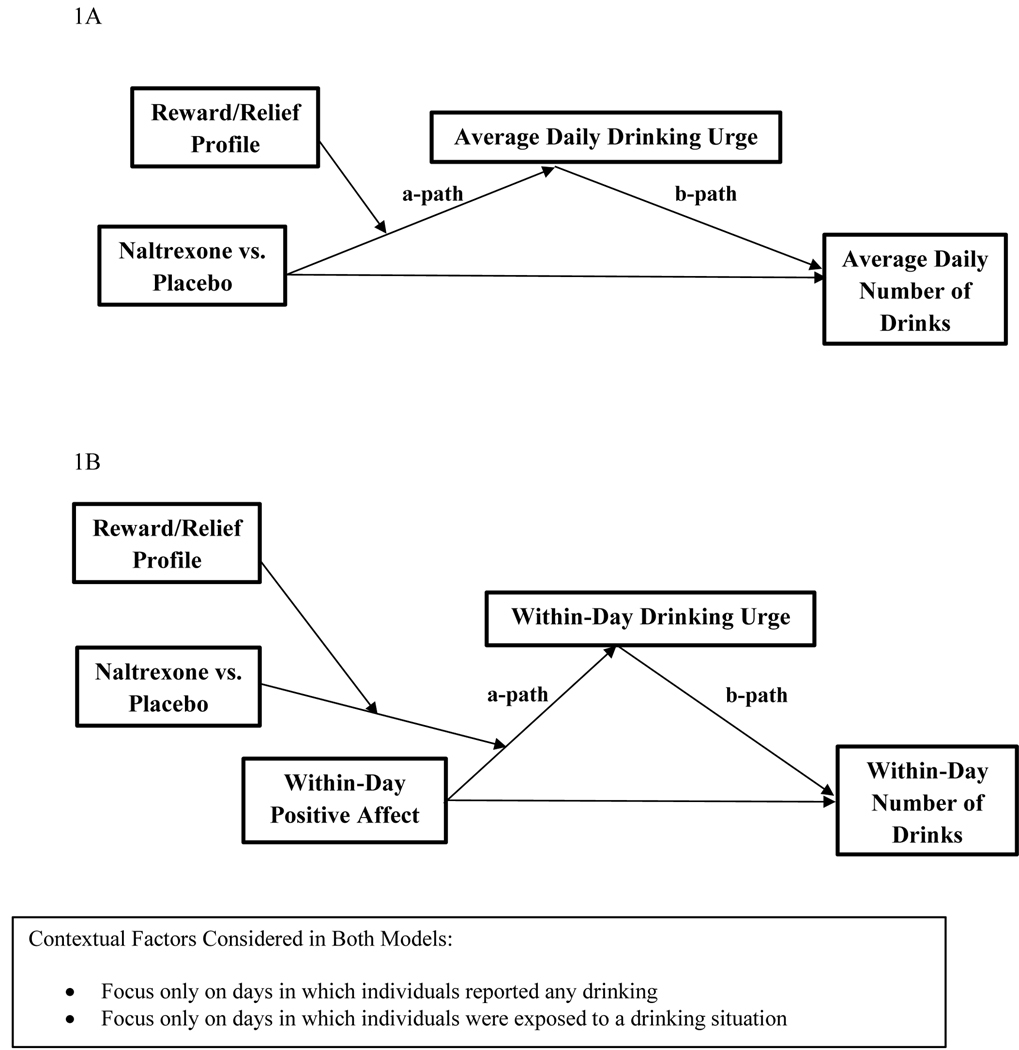
Figure 1A shows the hypothesized moderated mediated effect with reduction in average daily urge as the mechanism of change. We conducted multilevel moderated mediation models (with a random intercept). At the between-person level, we tested whether latent profile membership moderated the mediated effect of naltrexone on average drinks per day via average daily urge, controlling for sex, family history of AUD, baseline PDA, baseline DPDD, and smoking status. At the within-person level, time (i.e., treatment day 1–56) was included as a predictor of urge and total drinks, and urge was included as a predictor of total drinks on a given day. In consideration of context, we implemented models with (1) all treatment days, (2) only days when drinking was reported, and (3) only days when individuals were exposed to drinking events.
Figures 1A and 1B.
Conceptual depictions of the moderated mediation models.
Attenuation of the positive affect-urge-drinking pathway as a mechanism.
Figure 1B shows the hypothesized moderated mediated effect in which latent profile membership moderates the degree to which naltrexone weakens the within-person mediated effect of drinking via urges on a given day. We conducted a series of multilevel moderated mediation models based on context (as noted above). Lagged analyses examined positive affect reported at the time of the survey (in the morning after waking) predicting subsequent urges and drinking later in the same day (see Supplementary materials). The models included random intercepts and slopes for positive affect predicting urge. At the within-person level, positive affect, time, and medication dose were included as predictors of urge and drinks, and urge was included as a predictor of drinks on a given day. At the between-person level, we included average daily positive affect and covariates as predictors of average daily urge, average daily drinks, and the within-person positive affect-urge association (latent slope, i.e., cross-level interaction). The moderated mediation effect of interest was the “a path” (treatment condition x profile predicting the positive affect-urge slope) multiplied by the “b path” (within-person effect of urge on drinks on a given day). We explored significant moderated mediation effects by testing whether naltrexone moderated the within-person mediated effect of (1) positive affect on drinking via urges; and (2) also within subgroups defined by both profile and treatment condition.
Results
Latent profile analysis
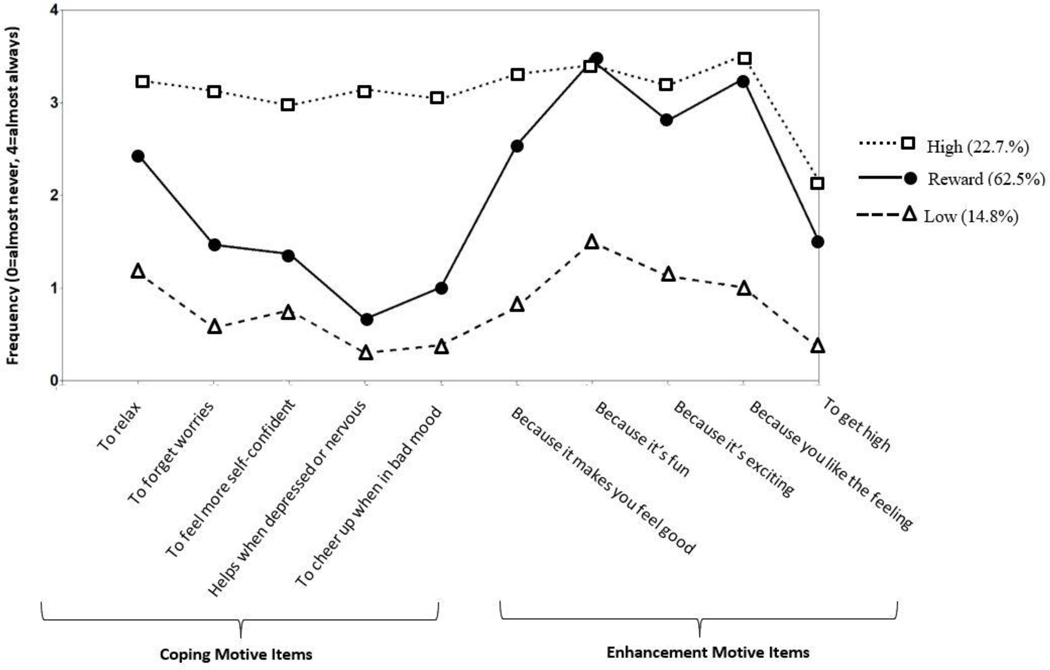
We selected the 3-profile model, which fit significantly better than the 2- and 1-profile models, and the 4-profile model did not fit significantly better than the 3-profile model (Table 2). Classification precision was excellent (entropy=.936). The profiles (Figure 2) could be characterized as a “low reward/relief profile” (n=19; 14.8%) with low coping and enhancement motives, a “reward drinker profile” (n=80; 62.5%) with high enhancement motives but low coping motives, and a “high reward/high relief profile” (n=29; 22.7%) with both high coping and enhancement motives. Latent profiles had good construct validity (see Supplementary Table 1).
Table 2.
Fit statistics for class solutions 1 through 6 for the latent profile models
| Number of Classes | ||||||
|---|---|---|---|---|---|---|
| Fit Statistics | 1 | 2 | 3 | 4 | 5 | 6 |
| AIC | 4162.49 | 3895.57 | 3726.51 | 3645.78 | 3581.00 | 3536.18 |
| BIC | 4219.53 | 3983.99 | 3846.30 | 3796.94 | 3763.53 | 3750.09 |
| Adjusted BIC | 4156.28 | 3885.95 | 3713.47 | 3629.32 | 3561.13 | 3512.89 |
| Lo-Mendell-Rubin test | __ | p=.13 | p=.03 | p=.17 | p=.08 | p =.27 |
| Entropy | __ | .852 | .936 | .924 | .914 | .929 |
Note. Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), sample size adjusted BIC (adjusted BIC). Lower values of AIC, BIC, and Adjusted BIC indicate a better fitting model. A p value below .05 for the Lo-Mendell-Rubin test indicates that the k class solutions fits the data significantly better than the k-1 class solution. The entropy value ranges from 0 to 1, with values closer to 1 indicating better classification precision.
Figure 2.
Conditional means on the DMQ items for low, reward, and high reward/high relief profiles.
Profile by treatment condition interaction effects
There were significant interactions between the reward profile (vs. low profile) and treatment condition in the prediction of PDI and PHID, and a significant interaction between the high reward/high relief profile (vs. low profile) and treatment condition in the prediction of PHID (Table 3). Specifically, there was a significant, medium effect [d=.56; 95% CI(.17,.96)] of naltrexone vs. placebo in reducing PDI for the reward profile, but no significant effect of naltrexone vs. placebo on PDI for the low or high reward/high relief profiles (Figure 3). There was also a significant, small effect [d=.32; 95% CI(.01,.68)] of naltrexone vs. placebo in reducing PHID for the reward profile, as well as a significant, medium-to-large effect [d=.69; 95% CI(.02,1.50)] of naltrexone vs. placebo in reducing PHID for the high reward/high relief profile, but no significant effect of naltrexone vs. placebo on PHID for the low profile.
Table 3.
Summary of results across models of profile x treatment condition predicting drinking outcome (low as reference group)
| PDA | PHD | DPDD | PDI | eBAC | PHID | |
|---|---|---|---|---|---|---|
| Predictor | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| Reward Drinker X Treatment Condition | −3.27 (8.37) 95% CI (−19.68, 13.13) p=.69 | −5.64 (6.22) 95% CI (−17.84, 6.55) p=.36 | −.65 (.82) 95% CI (−2.27, .97) p=.43 | −23.03 (11.6) 95% CI (−45.81, −.26) p=.04 | −.03 (.017) 95% CI (−.06, .005) p=.09 | −8.52 (3.69) 95% CI (−15.76, −1.28) p=.02 |
| High X Treatment Condition | 7.39 (9.72) 95% CI (−11.63, 26.45) p=.44 | −10.59 (7.20) 95% CI (−24.14, 3.52) p=.14 | −.52 (.95) 95% CI (−2.39, 1.35) p=.58 | −12.83 (13.4) 95% CI (−39.13, 13.46) p=.33 | −.02 (.02) 95% CI (−.06, .017) p=.27 | −12.94 (4.27) 95% CI (−21.32, −4.56) p=.002 |
Note. PDA = percent days abstinent; PHD = percent heavy drinking days (4/5 drinks per day for women/men); DPDD = drinks per drinking day; PDI = percent days drinking to intoxication; eBAC = estimated blood alcohol concentration per drinking day; PHID = percent high intensity drinking days (8/10 drinks per day for women/men).Treatment condition (1=naltrexone, 0=placebo). All models controlled for gender, family history of AUD, baseline PDA, and smoking status. The model with PHD and PHID as the outcomes additionally controlled for baseline PHD and baseline PHID, respectively. The models with DPDD, PDI, and eBAC as the outcomes additionally controlled for baseline DPDD. Significant interaction effects are bolded for clarity.
Figure 3.
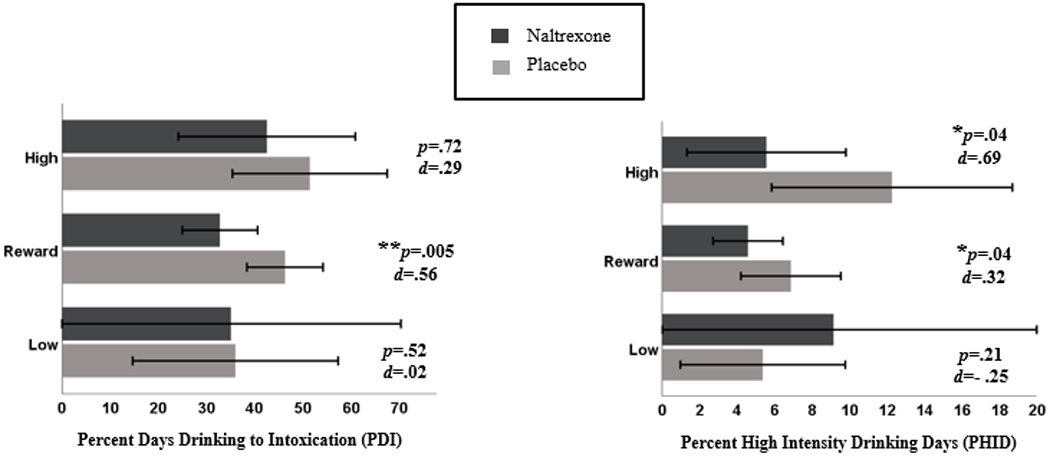
Effects of naltrexone vs. placebo on drinking outcomes by latent profile. Error bars show the 95% confidence interval.
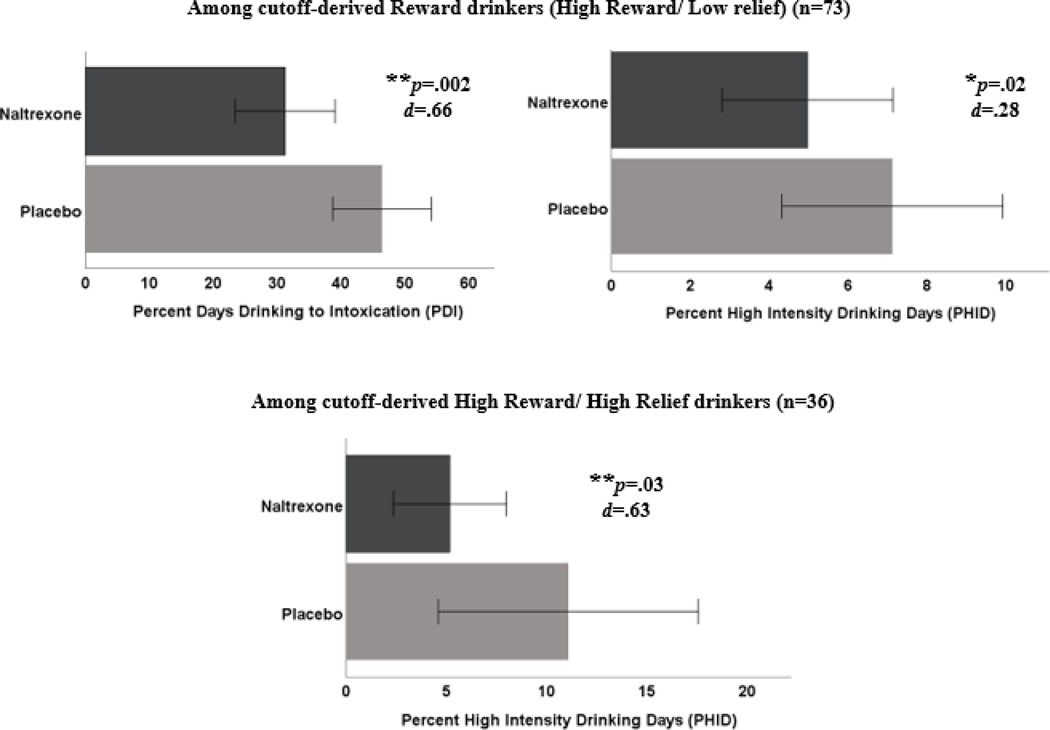
To facilitate clinical utility, we used latent profile-informed cutoffs and observed scores (see Appendix) to identify drinking phenotypes, and found (1) a medium-to-large effect [d=.66; 95% CI(.24,1.16)] of naltrexone on PDI and a small effect [d=.28; 95% CI(.04,.72)] of naltrexone on PHID among cutoff-derived reward drinkers and (2) a medium-to-large effect [d=.63; 95% CI(.05,1.1)] of naltrexone in reducing PHID among the cutoff-derived high subgroup (Figure 4).
Figure 4.
Effects of naltrexone vs. placebo on drinking outcomes among cutoff-derived reward drinkers and high reward/high relief drinkers. Error bars show the 95% confidence interval.
As post-hoc sensitivity analyses, we conducted moderation models with only reward drinking, assessed by the enhancement motives subscale score (rather than profile membership indicated by both reward and relief tendencies). There were no significant enhancement motives-by-treatment interaction effects for PDA (p=.99), PHD (p=.99), DPDD (p=.73), PDI (p=.93), eBAC (p=.74), or PHID (p=.09). There were also no significant differences by profile in baseline drinking (all p’s>.33) (Supplementary Table 2).
Testing mechanisms of change via mediation models
Reduction in average daily urge as a mechanism.
There were no significant moderated mediation effects in which the interaction of profile by treatment condition predicted drinking via average daily urges (Table 4).
Table 4.
Multilevel moderated mediation models with average daily urge as a mediator
| “a-path”‘ Profile x treatment condition → Average daily urge B (SE) | “b-path” Average daily urge → Average drinks per day B (SE) | a*b indirect effect B (SE) | |
|---|---|---|---|
| Including all treatment days | |||
| Reward vs. low contrast | .54 (.56) | .41 (.07)*** | .22 (.23) 95% CI (−.23,.68) |
| High vs. low contrast | .68 (.63) | .41 (.07)*** | .28 (.26) 95% CI (−.23, .80) |
| Including only drinking days | |||
| Reward vs. low contrast | .53 (.56) | .06 (.02)* | .03 (.04) 95% CI (−.04, .11) |
| High vs. low contrast | .68 (.63) | .06 (.02)* | .04 (.04) 95% CI (−.04, .13) |
| Including only days exposed to a drinking event | |||
| Reward vs. low contrast | .53 (.56) | .07 (.02) | .04 (.04) 95% CI (−.04, .11) |
| High vs. low contrast | .68 (.63) | .07 (.02) | .04 (.04) 95% CI (−.04, .13) |
Note.
p<.05;
p<.01
p<.001. Any significant indirect (mediated) effects, as indicated by a confidence interval not containing zero, are bolded for clarity.
Attenuation of the positive affect-urge-drinking pathway as a mechanism.
For the model that included only drinking days, there was a significant moderated mediation effect in which the interaction of the reward profile (vs. low) by treatment condition predicted the positive affect-urge slope, and urge predicted number of drinks (Table 5). However, follow-up analyses to explore the moderated-mediation effect within each profile did not indicate significant moderated-mediation effects on drinking days within profiles.
Table 5.
Multilevel moderated mediation models with profile x treatment condition moderating the within-person positive affect → urge → drinking mediated effect
| “a-path”‘ Profile x treatment condition → positive affect-urge slope B (SE) | “b-path” Urge → Number of drinks B (SE) | a*b indirect effect B (SE) | |
|---|---|---|---|
| Including all treatment days | |||
| Reward vs. low contrast | −.31 (.21) | .85 (.06)*** | −.27 (.18) 95% CI (−.63, .09) |
| High vs. low contrast | .07 (.24) | .85 (.06)*** | .06 (.21) 95% CI (−.35, .48) |
| Including only drinking days | |||
| Reward vs. low contrast | − .48 (.21)* | 1.06 (.09)*** | −.51 (.24) 95% CI (−.99, - .03) |
| High vs. low contrast | .07 (.27) | 1.06 (.09)*** | .07 (.30) 95% CI (−.51, .67) |
| Including only days exposed to a drinking event | |||
| Reward vs. low contrast | −.43 (.21)* | 1.09 (.09)*** | −.47 (.24) 95% CI (−.94, −.002) |
| High vs. low contrast | .16 (.28) | 1.09 (.09)*** | .18 (.30) 95% CI (−.42,.78) |
Note.
p<.05;
p<.01
p<.001. Significant indirect (mediated) effects, as indicated by a confidence interval not containing zero, are bolded for clarity.
For the model that included only days on which individuals were exposed to a drinking situation, there was a significant moderated mediation effect in which the interaction of the reward profile (vs. low) by treatment condition predicted the positive affect-urge slope, and urge in turn predicted the number of drinks (Table 5). Follow-up analyses (including only days on which individuals were exposed to a drinking event) revealed that: (1) within the reward profile, naltrexone vs. placebo significantly moderated the positive affect-urge-drinking mediated effect [B(SE)= −.27 (.12), 95% CI (−.52, −.01)] and (2) within the low profile [B(SE)= .12 (.11), 95% CI (−.09, .34)] and high reward/high relief profile [B(SE)=.15 (.18), 95% CI (−.20, .51)], naltrexone vs. placebo did not significantly moderate the positive affect-urge-drinking mediated effect.
Follow-up analyses demonstrated that: (1) within the low profile, the positive affect-urge-drinking mediated effect was nonsignificant among individuals who received either placebo or naltrexone; (2) within the reward profile, the positive affect-urge-drinking mediated effect was significant among individuals who received placebo, but nonsignificant among those who received naltrexone; and (3) within the high reward/high relief profile, the positive affect-urge-drinking mediated effect was significant for both individuals who received placebo and those who received naltrexone (see Table 6).
Table 6.
Summary of within-person mediation models (positive affect → urge → drinking) by profile and treatment condition (including only days in which individuals were exposed to a drinking event)
| “a-path”‘ Positive affect → Urge B (SE) | “b-path” Urge → Number of Drinks B (SE) | a*b indirect effect B (SE) | |
|---|---|---|---|
| Low Profile (n=19) | |||
| Received Naltrexone (n=8) | .13 (.18) | .93 (.15)*** | .12 (.18) 95% CI (−.23, .47) |
| Received Placebo (n=11) | .06 (.06) | 1.06 (.30)*** | .06 (.07) 95% CI (−.07, .20) |
| Reward Profile (n=80) | |||
| Received Naltrexone (n=39) | .11 (.08) | .90 (1.26)*** | .10 (.08) 95% CI (−.05, .26) |
| Received Placebo (n=41) | .24 (.07)** | 1.23 (.14)*** | .30 (.10) 95% CI (.09, .50) |
| High reward/high relief profile (n=29) | |||
| Received Naltrexone (n=14) | .30 (.09)** | 1.05 (.16)*** | .31 (.07) 95% CI (.16, .46) |
| Received Placebo (n=15) | .29 (.11)** | 1.16 (.37)*** | .33 (.16) 95% CI (.01, .66) |
Note.
p<.05;
p<.01
p<.001. Significant indirect (mediated) effects, as indicated by a confidence interval not containing zero, are bolded for clarity.
Discussion
Consistent with the reward drinker–naltrexone response hypothesis, we found that naltrexone reduced risky drinking – both percent days drinking to intoxication (BAC ≥.08) and percent high intensity drinking days (8/10 drinks for women/men) – among young adult reward drinkers (high reward/low relief drinking). Further, naltrexone was effective among reward drinkers by reducing urges and drinking on days in which reward drinkers had higher positive affect and were exposed to a drinking event. Hence, in addition to replicating prior findings that naltrexone is particularly effective among reward drinkers (14, 16), the study clarifies mechanisms by which naltrexone works among young adult reward drinkers.
Unexpectedly, we also found that naltrexone had pronounced effects in reducing risky drinking (percent high intensity drinking days only) among high reward/high relief drinkers, inconsistent with prior findings among older adults (14, 16). Young adult heavy drinkers with this high reward/high relief profile, relative to general adult treatment-seeking samples (14, 16), may be more likely to be in the binge/intoxication stage (with emerging relief drinking tendencies), and therefore may still respond well to naltrexone. Interestingly, among the high reward/high relief profile drinkers, naltrexone did not reduce drinking via the mechanism revealed in reward drinkers (i.e., reducing urges and drinking on days when individuals had higher positive affect and were exposed to a drinking event), suggesting that naltrexone may be working via a different mechanism among young adults who are high reward/high relief drinkers. The apparent mechanistic differences between reward and high reward/high relief profile drinkers suggest that differentiating among drinker phenotypes is important, and that more research is needed to elucidate naltrexone’s potentially distinct mechanisms in these subgroups.
Reward drinkers were prevalent (62.5%) in our sample, consistent with findings that enhancement drinking motives are common in young adults (23). The high proportion of reward drinkers and lack of a high relief/low reward profile in this sample is consistent with a neurobiological model of addiction (9) and suggests that it is common for young adult heavy drinkers to be in the “binge/intoxication” stage of the addiction cycle, in which drinking is driven by the rewarding effects of alcohol.
Our study suggests that prescribing naltrexone to young adult heavy drinkers may be clinically useful, and could have a large public health impact (24, 43) given that naltrexone has pronounced effects among reward drinkers and reward drinking is prevalent among young adults. The medium-to-large effect of naltrexone in reducing drinking to intoxication among reward drinkers in this study is notable given most interventions for young adult heavy drinking have small effects (44). Moreover, among reward drinkers, naltrexone reduced drinking to intoxication and high intensity drinking, two drinking indicators that are strongly predictive of serious negative consequences and AUD among young adults (36–38, 45, 46). Given the theory-driven, exploratory approach in which several outcomes were examined here, the findings need to be viewed with caution and replication would enhance confidence in their validity.
This study suggests that naltrexone had effects among reward drinkers by reducing urges and drinking on days when they have higher positive affect and are in drinking contexts, and thus exposed to alcohol cues. This finding is consistent with theories and empirical work on mechanisms of naltrexone (18–20). Prior analysis of the same dataset found that taking a targeted dose of naltrexone on days of higher positive affect and urges reduced the likelihood of drinking to intoxication (25).
Sensitivity analyses suggested that baseline drinking and reward drinking alone (without considering relief drinking), were not driving the moderation effects. Rather, similar to prior work (14, 16), the pattern of reward and relief drinking may be optimal for predicting naltrexone response. This supports the utility of differentiating reward drinkers from high reward/high relief drinkers, and underscores the clinical relevance of this distinction.
Reduction in average daily urge did not significantly mediate the effect of naltrexone on drinking among reward drinkers, which was supported in prior work with adults (16). In addition to different study populations, the prior study focused on current urges (16), and our study used retrospective reports of urges experienced during the previous day.
Of note, it is not known whether young adults in real-world settings will accept and adhere to naltrexone treatment for heavy drinking. It is possible that young adults may be more interested in naltrexone treatment if providers emphasize that naltrexone is helpful to young adults by reducing risky levels of drinking during a drinking occasion, rather than by facilitating abstinence from drinking. Future research is needed on factors that influence young adults’ willingness to take naltrexone, as well as whether providers will prescribe naltrexone for this population.
This study has several limitations. Because participants reported on urges and drinking during the previous day, we could not evaluate their temporal sequence. The sample was predominantly White and did not have serious psychiatric disorders. Outcomes relied on self-report without biochemical validation. The sample was relatively small and replication with larger, more diverse samples is needed. Finally, although prior work (14–16) has utilized the IDS (21) and the AASE (22) to identify reward drinkers who respond to naltrexone (14, 16), this study used the DMQ (31, 32). The DMQ appears to tap into reward and relief drinking in a similar manner as the IDS and AASE, however further work is needed to clarify which self-report measures are optimal for assessing reward and relief drinking tendencies.
A strength of this study is that we provided cutoffs to identify drinker phenotypes from the 10-item DMQ. Importantly, although neuroimaging and genetics have been useful for identifying AUD phenotypes and naltrexone responders (5, 47, 48), relatively brief self-report measures such as the DMQ are easier to administer and therefore may be particularly useful in applying precision medicine in real-world settings. However, even a 10-item measure may be challenging to implement in real-world settings. Future research is warranted to develop even briefer measures to identify phenotypes that respond differentially to pharmacotherapies.
Altogether, these findings add to a growing literature demonstrating that naltrexone may be particularly effective for reward drinkers (14, 16). Given the prevalence of reward drinking in young adults and the promising preliminary findings reported here, future work is warranted on moderators and mechanisms of naltrexone response among young adult heavy drinkers.
Supplementary Material
Acknowledgments
Funding Sources
This research was supported by NIH grant awards, including support for C.R.R. (T32DA007238–27, and K23AT011342), K.W.B. (K12DA000167), R.F.L. (R21 AA026918, UH3 AA02614, and U24 AA022002), L.M.F. (R34AA026021). Additionally, R.F.L was supported by the Mary F. Lane endowed professorship and the State of Florida.
Appendix
Items for Identifying Drinking Phenotypes among Young Adults - 10 items from the Drinking Motives Questionnaire (DMQ; Cooper et al., 1992)
Listed below are reasons people might be inclined to drink alcoholic beverages. Using the scale below, decide how frequently your own drinking is motivated by each of the reasons listed.
| Almost Never | Some of the Time | Half the Time | Most of the Time | Almost Always | |
|---|---|---|---|---|---|
| 1. To forget your worries | 0 | 1 | 2 | 3 | 4 |
| 2. Because it helps when you feel depressed or nervous | 0 | 1 | 2 | 3 | 4 |
| 3. Because you like the feeling | 0 | 1 | 2 | 3 | 4 |
| 4. To cheer you up when you are in a bad mood | 0 | 1 | 2 | 3 | 4 |
| 5. Because it’s exciting | 0 | 1 | 2 | 3 | 4 |
| 6. To get high | 0 | 1 | 2 | 3 | 4 |
| 7. To relax | 0 | 1 | 2 | 3 | 4 |
| 8. Because you feel more self-confident or sure of yourself | 0 | 1 | 2 | 3 | 4 |
| 9. Because it makes you feel good | 0 | 1 | 2 | 3 | 4 |
| 10. Because it’s fun | 0 | 1 | 2 | 3 | 4 |
Instructions for Identifying Drinking Phenotypes
Calculate reward subscale score by summing items 3, 5, 6, 9, and 10.
- Calculate relief subscale score by summing items 1, 2, 4, 7, and 8.
- An individual is classified as a “Reward Drinker” if reward subscale score is greater than 9 AND relief subscale score is less than 12.
- An individual is classified as a “High reward/high relief drinker” if reward subscale score is greater than 11 AND relief subscale score is greater than 11.
Selection Criteria for Creating Cutoff Scores
The empirically derived latent reward drinker profile had a mean reward subscale score of 13.49 (SD=3.23) and a mean relief subscale score of 6.96 (SD=3.34). Similar to our prior research (Mann et al., 2018; Witkiewitz et al., 2019), we used a relatively flexible 1.5 standard deviation observed cutoff rule to maximize the number of participants classified in a profile given that the subscales scores of a particular individual are in a similar range as the distribution of subscale scores of the reward drinker profile. Therefore, an individual is classified as a reward drinker if the observed reward subscale score is at least greater than 9 (the value at 1.5 SDs below the mean of the latent reward profile) AND the relief subscale score is less than 12 (the value at 1.5 SDs above the mean of the latent reward profile). Among individuals classified as “reward drinkers” with these cut off criteria, naltrexone (as compared to placebo) resulted in a medium-to-large reduction (Cohen’s d= .66) in percent drinking days with intoxication (PDI) (BAC ≥ .08) and a small-sized reduction (Cohen’s d=.28) in percent high intensity drinking days (PHID) (8/10 standard drinks for women/men).
The empirically derived latent high reward/profile had a mean reward subscale score of 15.14 (SD=3.00) and a mean relief subscale score of 15.34 (SD=3.34). Using the same 1.5 standard deviation cut off rule noted above, an individual is classified as a high reward/high relief drinker if the observed reward subscale score is greater than 11 (the value at 1.5 SDs below the mean of the latent high reward/high relief profile) AND the relief subscale score is greater than 11 (the value at 1.5 SDs above the mean of the latent high reward/high relief profile). Among individuals classified as “high reward/high relief drinkers” with these cut off criteria, naltrexone (as compared to placebo) resulted in a medium-to-large-sized reduction (Cohen’s d=.63) in percent PHID.
Footnotes
Conflicts of Interest Declaration
Drs. Witkiewitz, Mann, Kranzler, and O’Malley are members of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative (ACTIVE Group), which over the past three years was supported by Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Dicerna, Ethypharm, Indivior, Lundbeck, Mitsubishi, and Otsuka. Dr. Kranzler is an advisory board member for Dicerna Pharmaceuticals and is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. In the prior 3 years, Dr. O’Malley has been a consultant or advisory board member for Alkermes, Amygdala, Dicerna, Indivior, Mitsubishi Tanabe; has received donated study medications for NIH sponsored research from Astra Zeneca and Novartis; and has been a DSMB Member for NIDA Clinical Trials Network (Emmes). The other authors report no conflicts.
References
- 1.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74(9):911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet 2009;373(9682):2223–33. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, et al. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res 2014;38(4):1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311(18):1889–900. [DOI] [PubMed] [Google Scholar]
- 5.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res 2015;39(4):579–84. [DOI] [PubMed] [Google Scholar]
- 6.Witkiewitz K, Litten R, Leggio L. Advances in the science and treatment of alcohol use disorder. Sci Adv 2019;5(9):eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Feldstein Ewing SW, et al. The future of translational research on alcohol use disorder. Addict Biol 2020:e12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol and alcoholism (Oxford, Oxfordshire). 1999;34(2):197–222. [DOI] [PubMed] [Google Scholar]
- 9.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skinner MD, Aubin H-J. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev 2010;34(4):606–23. [DOI] [PubMed] [Google Scholar]
- 11.Heinz A, Löber S, Georgi A, Wrase J, Hermann D, Rey E-R, et al. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol 2003;38(1):35–9. [DOI] [PubMed] [Google Scholar]
- 12.Heilig M, Goldman D, Berrettini W, O’brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci 2011;12(11):670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A, et al. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res 2009;33(4):674–83. [DOI] [PubMed] [Google Scholar]
- 14.Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, et al. Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology 2018;43(4):891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos CR, Mann K, Witkiewitz K. Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addict Biol 2017;22(6):1528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkiewitz K, Roos CR, Mann K, Kranzler HR. Advancing precision medicine for alcohol use disorder: Replication and extension of reward drinking as a predictor of naltrexone response. Alcohol Clin Exp Res 2019;43(11):2395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta‐analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 2013;108(2):275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets 2010;9(1):13–22. [DOI] [PubMed] [Google Scholar]
- 19.Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J. Effects of naltrexone on alcohol self‐administration and craving: meta‐analysis of human laboratory studies. Addict Biol 2017;22(6):1515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray LA, Green R, Roche DJ, Magill M, Bujarski S. Naltrexone effects on subjective responses to alcohol in the human laboratory: A systematic review and meta‐analysis. Addict Biol 2019;24(6):1138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annis H, Graham J, Davis C. Inventory of drinking situations. Ontario, Canada: Addiction Research Foundation; 1982. [Google Scholar]
- 22.DiClemente CC, Carbonari JP, Montgomery R, Hughes SO. The alcohol abstinence self-efficacy scale. J Stud Alcohol 1994;55(2):141–8. [DOI] [PubMed] [Google Scholar]
- 23.Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clin Psychol Rev 2005;25(7):841–61. [DOI] [PubMed] [Google Scholar]
- 24.O’Malley SS, Corbin WR, Leeman RF, DeMartini KS, Fucito LM, Ikomi J, et al. Reduction of alcohol drinking in young adults by naltrexone: a double-blind, placebo-controlled, randomized clinical trial of efficacy and safety. J Clin Psychiat 2015;76(2):e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bold KW, Fucito LM, Corbin WR, DeMartini KS, Leeman RF, Kranzler HR, et al. Daily relations among affect, urge, targeted naltrexone, and alcohol use in young adults. Exp Clin Psychopharmacol 2016;24(5):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armeli S, Feinn R, Tennen H, Kranzler HR. The effects of naltrexone on alcohol consumption and affect reactivity to daily interpersonal events among heavy drinkers. Exp Clin Psychopharmacol 2006;14(2):199. [DOI] [PubMed] [Google Scholar]
- 27.Kranzler HR, Armeli S, Feinn R, Tennen H. Targeted naltrexone treatment moderates the relations between mood and drinking behavior among problem drinkers. J Consult Clin Psychol 2004;72(2):317. [DOI] [PubMed] [Google Scholar]
- 28.Dimeff LA. Brief alcohol screening and intervention for college students (BASICS): A harm reduction approach: Guilford Press; 1999. [Google Scholar]
- 29.Sobell LC, Sobell MB. Timeline follow-back. Measuring alcohol consumption: Springer; 1992. p. 41–72. [Google Scholar]
- 30.Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. J Abnorm Psychol 2003;112(3):424. [DOI] [PubMed] [Google Scholar]
- 31.Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychol Assess 1992;4(2):123. [Google Scholar]
- 32.Martin JL, Ferreira JA, Haase RF, Martins J, Coelho M. Validation of the Drinking Motives Questionnaire-Revised across US and Portuguese college students. Addict Behav 2016;60:58–63. [DOI] [PubMed] [Google Scholar]
- 33.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res 1995;19(4):1018–23. [DOI] [PubMed] [Google Scholar]
- 34.Muthén L, Muthén B. Mplus user’s guide. Version 8; 2017. 2018. [Google Scholar]
- 35.Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences: John Wiley & Sons; 2009. [Google Scholar]
- 36.Patrick ME, Terry‐McElrath YM, Kloska DD, Schulenberg JE. High‐intensity drinking among young adults in the United States: Prevalence, frequency, and developmental change. Alcohol Clin Exp Res 2016;40(9):1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrick ME, Terry‐McElrath YM. High‐intensity drinking by underage young adults in the United States. Addiction 2017;112(1):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linden‐Carmichael AN, Vasilenko SA, Lanza ST, Maggs JL. High‐intensity drinking versus heavy episodic drinking: Prevalence rates and relative odds of alcohol use disorder across adulthood. Alcohol Clin Exp Res 2017;41(10):1754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O’Malley SS. Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol Psychiatry 2012;72(10):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton RF, Latham PK, Voronin KE, Randall PK, Book SW, Hoffman M, et al. Nicotine‐use/smoking is associated with the efficacy of naltrexone in the treatment of alcohol dependence. Alcohol Clin Exp Res 2018;42(4):751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, et al. Predictors of naltrexone response in a randomized trial: reward-related brain activation, OPRM1 genotype, and smoking status. Neuropsychopharmacology 2017;42(13):2640–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 2002;7(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMartini KS, Gueorguieva R, Leeman RF, Corbin WR, Fucito LM, Kranzler HR, et al. Longitudinal findings from a randomized clinical trial of naltrexone for young adult heavy drinkers. J Consult Clin Psychol 2016;84(2):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey KB, Scott-Sheldon LA, Carey MP, DeMartini KS. Individual-level interventions to reduce college student drinking: A meta-analytic review. Addict Behav 2007;32(11):2469–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatry 2017;174(11):1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jennison KM. The short‐term effects and unintended long‐term consequences of binge drinking in college: A 10‐year follow‐up study. Am J Drug Alcohol Abuse 2004;30(3):659–84. [DOI] [PubMed] [Google Scholar]
- 47.Spanagel R, Durstewitz D, Hansson A, Heinz A, Kiefer F, Köhr G, et al. A systems medicine research approach for studying alcohol addiction. Addict Biol 2013;18(6):883–96. [DOI] [PubMed] [Google Scholar]
- 48.Mann K, Vollstädt‐Klein S, Reinhard I, Leménager T, Fauth‐Bühler M, Hermann D, et al. Predicting naltrexone response in alcohol‐dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res 2014;38(11):2754–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.