Abstract
Background
We evaluated deficit accumulation and how deficits affected cognition and physical activity among breast cancer survivors and non-cancer controls.
Methods
Newly diagnosed nonmetastatic survivors (n = 353) and matched non-cancer controls (n = 355) ages 60-98 years without neurological impairments were assessed presystemic therapy (or at enrollment for controls) from August 2010 to December 2016 and followed for 36 months. Scores on a 42-item index were analyzed in growth-mixture models to determine deficit accumulation trajectories separately and combined for survivors and controls. Multilevel models tested associations between trajectory and cognition (FACT-Cog and neuropsychological tests) and physical activity (IPAQ-SF) for survivors and controls.
Results
Deficit accumulation scores were in the robust range, but survivors had higher scores (95% confidence intervals [CI]) than controls at 36 months (0.18, 95% CI = 0.16 to 0.19, vs 0.16, 95% CI = 0.14 to 0.17; P = .001), and averages included diverse deficit trajectories. Survivors who were robust but became frailer (8.8%) had similar baseline characteristics to those remaining robust (76.2%) but experienced a 9.6-point decline self-reported cognition (decline of 9.6 vs 3.2 points; P = .04) and a 769 MET minutes per week decline in physical activity (P < .001). Survivors who started and remained prefrail (15.0%) had self-reported and objective cognitive problems. At baseline, frail controls (9.5%) differed from robust controls (83.7%) on deficits and self-reported cognition (P < .001). Within combined trajectories, frail survivors had more sleep disturbances than frail controls (48.6% [SD = 17.4%] vs 25.0% [SD = 8.2%]; P = .05).
Conclusions
Most survivors and controls remained robust, and there were similar proportions on a frail trajectory. However, there were differences in deficit patterns between survivors and controls. Survivor deficit accumulation trajectory was associated with patient-reported outcomes. Additional research is needed to understand how breast cancer and its treatments affect deficit accumulation.
A growing body of evidence suggests that cancer and its treatments may be “disease drivers” of aging (1‐7). However, aging is difficult to measure in oncology settings, and geriatric syndromes such as frailty, which are thought to reflect aging, are not always clinically apparent (8‐11). Frailty is generally measured using 1 of 2 types of indices: phenotypic, focused on system failure (eg, loss of muscle strength) (12), and deficit accumulation, focused on comorbidities and self-reported functional deficits (10,13,14). Both approaches predict mortality in general populations (10,15).
Deficit accumulation indices can be useful in oncology practice because they utilize readily available data (9,16,17) and predict chemotherapy toxicity, medication adherence and hospitalizations (18,19), cognitive decline (20), quality of life, and all-cause mortality (16). Older cancer survivors are in the age range where deficits are expected. In the initial treatment period, older survivors may have acute deficits such as depression and fatigue. However, there are limited longitudinal data on the accumulation of deficits and even less information about how accumulation of deficits impacts long-term function of older survivors compared with non-cancer populations (2).
The Thinking and Living with Cancer study is a prospective study that provides unique data to examine whether cancer and its treatments affect deficit accumulation. We compared data from older survivors and frequency-matched non-cancer controls to 1) describe deficit accumulation over 36 months; 2) determine deficit accumulation trajectory groups and compare patterns and characteristics of survivors and controls within groups; and 3) test if deficit accumulation trajectories were associated with longitudinal cognition and physical activity levels, and if the pattern of effects differed for survivors vs controls. The results are intended to guide future research to inform care for older survivors.
Methods
Setting, Population, and Data Collection
We included participants recruited between August 1, 2010, and December 31, 2016; the study is ongoing. Eligible survivors were aged 60 years or older, newly diagnosed with primary nonmetastatic breast cancer, and English speaking. We excluded those with neurological disorders or hearing or vision impairment that precluded assessment. Survivors with a history of other cancers were excluded if active treatment was recent (<5 years) or included systemic therapy. Survivor consent rates were 76% across 4 sites; 1 tertiary center had a lower rate (15%). Non-cancer controls met the same eligibility criteria as survivors and were friends of survivors or community controls; 92% consented and were frequency matched by site, age (5 years), education, and race.
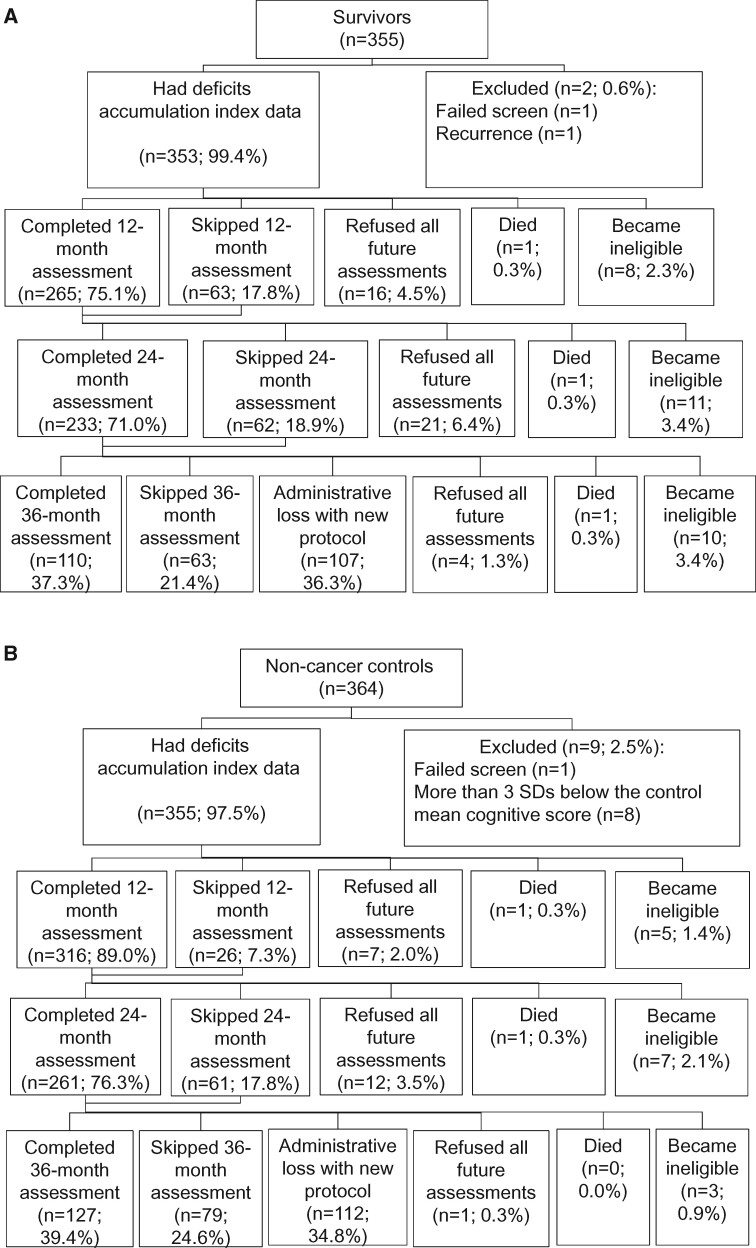
Participants were screened using the Mini-Mental State Examination (21) and the Wide Range Achievement Test, 4th edition (WRAT-4) Word Reading subtest (22); those with scores of less than 24 or lower than 3rd-grade equivalent reading level, respectively, were ineligible (1 survivor, 1 control). Controls scoring more than 3 standard deviations below the control mean baseline neuropsychological scores for their age and education group were ineligible (n = 8) per protocol. Data for survivors who experienced a recurrence were excluded for the data points after recurrence. The analytic sample included 353 survivors and 355 controls (see Figure 1).
Figure 1.
Sample for evaluation of deficits accumulation in older breast cancer survivors and matched non-cancer controls. The percent consenting and refusing was calculated among those alive and eligible to continue the study at each timepoint. Eligibility for continuing in the study was the same as enrollment eligibility and included development of a neurological disease (eg, stroke, Parkinson disease) and being diagnosed with cancer. Data for survivors who were diagnosed with a breast cancer recurrence were excluded starting from 6 months prior to the diagnosis of recurrence. Participants may have skipped a follow-up assessment at 1 timepoint but completed later assessments. Most participants completed 3-4 assessments; 14.8% (17.0% of survivors and 12.6% of controls) completed 2 assessments, and 14.0% (19.5% of survivors and 8.5% of controls) completed baseline only. Analytic models used all data available.
Assessments were conducted by trained staff postsurgery, presystemic therapy for survivors and at enrollment for controls, and annually through 36 months using a structured survey and neuropsychological testing (20,23).
This institutional review board–approved study (ClinicalTrials.gov Identifier: NCT03451383) has been reported previously (20,23) and was conducted at 5 US sites.
Measures
We used a 42-item deficit accumulation index (9,10,13) measuring comorbidities, polypharmacy, activities of daily living and instrumental activities of daily living (24), a timed get-up-and-go score (25), social support and marital status, family history of dementia, nutritional status (body mass index), functional status (SF-12 physical, social, role, and emotional function scales) (26), clinical depression [scores >16 on the Center for Epidemiologic Studies Depression Scale (27)], anxiety (State-Trait Anxiety Inventory) (28), and fatigue (FACT-Fatigue scale) (Supplementary Table 1, available online) (29). Scoring requires 90% or more of items are non-missing (13). Scores were calculated by summing all nonmissing items, each scored from 0 to 1, and dividing by the total number of nonmissing items. A change in score of 0.02 is considered a small, clinically meaningful difference and 0.06 a large difference (30). Scores can also be categorized as robust (0 to <0.2), prefrail (0.2 to <0.35), or frail (>0.35) (10,13).
We examined longitudinal cognition and physical activity. Subjective cognition was measured using the FACT-Cog v.3 (Cronbach alpha = .96); declines of 5%-7% or 7-10 points are clinically meaningful (31‐33). Objective cognitive function was based on standardized z scores for tests for attention, processing speed, and executive functioning (6 tests), and verbal learning and memory (5 tests) (20,23); results were standardized to baseline scores for age- and education-group matched non-cancer controls. The IPAQ-SF estimated self-reported physical activity in MET minutes per week (34); 600 MET minutes per week is the recommended level (35,36). Because there is overreporting of activity, participants with scores more than 3 SD above the mean were assigned a score equal to 3 SD above the mean to limit the impact of extreme values (34).
We considered several potential covariates, including radiotherapy, surgery, and systemic treatment (chemotherapy with or without hormonal therapy, hormonal therapy only), age, race (White vs non-White), cognitive reserve (WRAT-4 score), FACT-G physical well-being (37), and presence of a sleep disturbance at baseline (yes or no) (38).
Statistical Analysis
Prior to analysis, we examined missing data due to dropout or death. The majority of missing data were due to administrative losses, with only 0.7% and 8.6% of participants dying or dropping out, respectively. Missing data did not vary by trajectory group or study outcomes (Supplementary Methods and Supplementary Tables 2 and 3, available online). Our analyses methods allow for missing-at-random data, including dropouts, and did not require participants to contribute complete data to be included (39).
To describe heterogeneity in deficits accumulation, we used stacked percentage plots and multistate models of transition probabilities for moving between categories of deficit accumulation (40,41). Multilevel models (42) were used to estimate the adjusted mean deficit accumulation scores for survivors and controls over 36 months. Based on our prior research (20,38,43), final models included age, race, WRAT score, systemic therapy, sleep, and recruitment site; other covariates were not statistically significant and were not retained.
Deficit accumulation frailty trajectories were defined based on unadjusted deficits accumulation scores over time using growth mixture modeling (4,44). This method identified latent group membership on the basis of differences in scores at baseline and longitudinal changes over time. The number of trajectory groups was determined by statistical fit indices (likelihood ratio tests, the smallest Bayesian information criterion) and practical considerations (having at least 2% of participants in a group) (44). Trajectories were determined separately for survivors and controls; secondary analysis combined survivors and controls in 1 model and used bivariate tests to compare survivor and control characteristics within trajectories.
Finally, we used linear mixed-effects models (42) to determine the association of trajectory group with longitudinal cognitive and physical activity separately for survivors and controls. Final models included age, race, WRAT-4 score, site, and systemic treatment as fixed effects (20,38,43); other covariates did not have statistically significant effects and were not retained in final models. Items included in the deficit accumulation index (eg, anxiety, depression, family history of dementia) were not included as covariates.
Analyses of growth mixture models were conducted in M-Plus 8.3; R version 3.5.3 was used for the multistate modeling; and the remainder of the analyses were conducted using SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Participants
Participants were aged 60 to 98 years old and well educated (Table 1). Survivors and controls were well matched. Compared with controls, survivors were more likely to have higher baseline anxiety, depression, fatigue, and lower physical well-being (all P < .01) and to have more diabetes (P = .06), hypertension (P = .009), and obesity (P = .04).
Table 1.
Baseline characteristics of older breast cancer survivors and frequency-matched non-cancer controls
| Characteristic | Control | Survivor | P |
|---|---|---|---|
| (n = 355)a | (n = 353)a | ||
| Mean age (SD), y | 67.9 (7.1) | 68.2 (6.0) | .61 |
| Race, % (No.) | |||
| White, non-Hispanic | 79.9 (283) | 79.6 (281) | .91 |
| Non-White | 20.1 (71) | 20.4 (72) | |
| Marital status, % (No.) | .001 | ||
| Married | 49.0 (174) | 60.9(215) | |
| Other | 51.0 (181) | 39.1 (138) | |
| Education, mean (SD), y | 15.4 (2.3) | 15.3 (2.2) | .47 |
| Mean (SD) WRAT-4 score | 112.0 (16.0) | 111.9 (15.3) | .94 |
| Family history of dementia, % (No.) | |||
| Yes | 35.2 (125) | 30.6 (108) | .19 |
| No | 64.8 (230) | 69.4 (245) | |
| Mean (SD) neuropsychological test z scoresb | |||
| APE | −0.07 (0.65) | −0.11 (0.67) | .45 |
| LM | −0.03 (0.82) | −0.03 (0.83) | .99 |
| Mean (SD) self-report cognition scorec | 129.7 (16.0) | 129.6 (17.7) | .92 |
| Mean (SD) depression scored | 4.8 (5.5) | 6.9 (7.8) | <.001 |
| Mean (SD) anxiety scoree | 26.7 (5.6) | 29.0 (7.8) | <.001 |
| Mean (SD) fatigue scoref | 46.3 (5.7) | 43.2 (8.5) | <.001 |
| Mean (SD) physical activity, MET minutes/weekg | 2037 (1992) | 1289 (1343) | <.001 |
| Sleep disturbance, % (No.) | 25.4 (90) | 35.7 (126) | <.001 |
| Physical well-being at enrollment,h mean (SD) | 22.0 (2.4) | 20.0 (3.9) | <.001 |
| Physical function pr-enrollment,i mean (SD) | 52.1 (7.0) | 51.8 (7.1) | .58 |
| Diabetes, % (No.) | 6.8 (24) | 10.8 (38) | .06 |
| Hypertension, % (No.) | 36.8 (131) | 46.5 (164) | .009 |
| Obesity (BMI ≥ 30 kg/m2) | 25.8 (92) | 33.0 (117) | .04 |
| AJCC stage, % (No.) | |||
| 0 | — | 10.8 (38) | |
| 1 | — | 56.1 (198) | |
| 2 | — | 28.0 (99) | |
| 3 | — | 5.1 (18) | |
| Surgery type, % (No.) | |||
| BCS+/-RT | — | 57.8 (203) | — |
| Mastectomy | — | 42.2 (148) | |
| ER status, % (No.) | |||
| Positive | — | 88.1 (311) | — |
| Negative | — | 11.9 (42) | |
| HER2/ERBB2 status, % (No.) | |||
| Positive | — | 9.0 (29) | — |
| Negative | — | 91.0 (293) | |
| Deficits accumulation score,j mean (SD) | 0.13 (0.07) | 0.15 (0.08) | .03 |
| Deficits accumulation trajectory,k % (No.) | |||
| Stays robust | 83.7 (297) | 76.2 (269) | .002 |
| Starts robust, becomes frailer | 9.6 (34) | 8.8 (31) | |
| Starts and remains prefrail | 6.8 (24) | 15.0 (53) |
Some numbers may not add to 100% because of missing data for item; 13 survivors were missing systemic therapy data. Non-White includes Black, Hispanic, and Asian American/Pacific Islander; 1 survivor is missing race data. P values are based on 2-sided χ2 or t tests. AJCC = American Joint Committee on Cancer; BCS = breast-conserving surgery; BMI = body mass index; ER = estrogen receptor; RT = radiotherapy; WRAT-4 = Wide Range Achievement Test, 4th edition, Word Reading Test Standard Score.
Neuropsychological test scores by domain, where APE = attention, processing speed, and executive function and LM = learning and memory. Cognitive scores were standardized using the sample mean and standard deviation of age- and education-group matched baseline controls. Hence, a score of zero indicates a score at the mean of the control group; scores less than zero indicate lower scores than the mean of the control group, and positive scores indicate scores higher than the control mean.
Based on the FACT-Cog. Scores range from 0 to 148, with higher scores indicating better cognition; declines of 5%-7%, or 7-10 points, on this 148-point scale, are considered clinically meaningful.
Based on the Center for Epidemiologic Studies Depression Scale (CES-D). Depression defined by score above the cut point of 16 on the CES-D. Depression is included in the deficit accumulation index.
Based on the State-Trait Anxiety Inventory. Scores range from 20 to 80, with higher scores reflecting more anxiety. Anxiety is included in the deficit accumulation index.
Based on the FACT-Fatigue. Scores range from 0 to 52; higher scores reflect less fatigue. Fatigue is included in the deficit accumulation index.
Based on scores on the IPAQ-SF. Scores >3SD from the mean were capped at 3 SD.
Based on the FACT-G subscale. Scores range from 0 to 24; higher scores reflect better function. Physical well-being is included in the deficit accumulation index.
Based on SF-12 physical components scale asking about function in the 2 months prior to enrollment/diagnoses. Scores range from 0 to 100, with population means of 50 (SD = 10).
Based on scores for baseline deficits accumulation scores. Excludes cognitive function. Scores could not be calculated if more than 10% of items were missing; missing rates were similar for survivors and controls, and approximately 5% were missing at 12 and 24 months and 7%-9% at 36 months. Marital status, BMI, anxiety, depression, fatigue, comorbidities, including diabetes and so forth, were included in the deficit accumulation scores.
Deficit accumulation trajectory groups estimated separately for survivors and controls.
Deficits Accumulation Frailty Scores
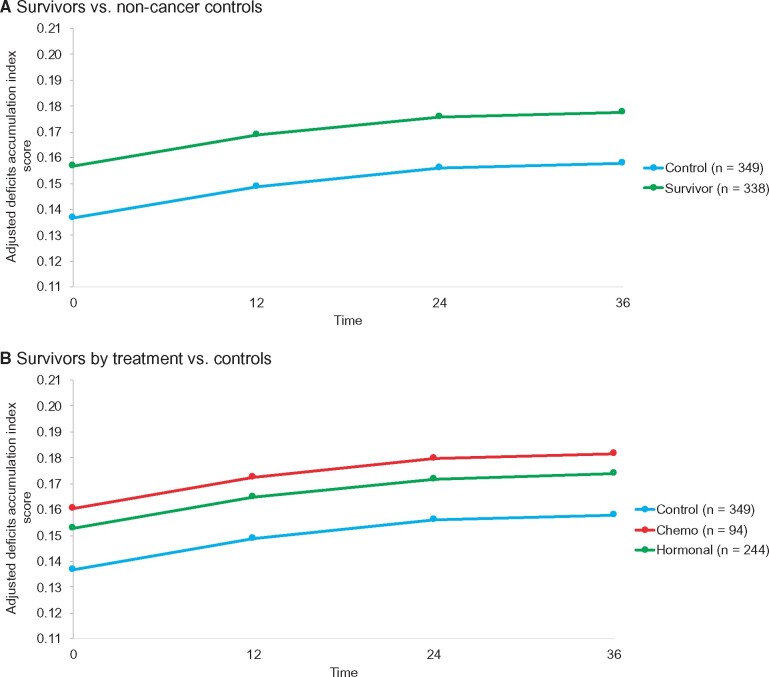
The average adjusted longitudinal deficits accumulation scores were in the robust range but were higher in survivors vs controls (0.18, 95% CI = 0.16 to 0.19, vs 0.16, 95% CI = 0.14 to 0.17 at 36 months; P = .001; Figure 2, A; Supplementary Tables 4 and 5, available online). Survivors receiving chemotherapy (with or without hormonal therapy) (0.18, 95% CI = 0.16 to 0.20; P = .006) or those receiving hormonal treatment alone (0.17, 95% CI = 0.16 to 0.19; P = .01) had higher scores at 36 months than controls (0.16, 95% CI = 0.14 to 0.17), but there was no statistically significant difference between the survivor treatment groups (Figure 2, B; Supplementary Tables 4 and 5, available online). There was heterogeneity in the prevalence of deficits by 36 months, with 23.8% and 25.2% having scores in the prefrail range and 5.0% and 3.4% with scores in the frail range or having died among survivors and controls, respectively (Supplementary Figure 1, available online). There were also transitions between categories, with all participants more likely to move from higher to lower deficit accumulation categories (ie, show recovery) than they were to become frailer over time (P = .05) (Supplementary Figure 2, available online).
Figure 2.
Adjusted mean deficits accumulation scores for older breast cancer survivors and frequency-matched controls. A) Shows adjusted mean deficits accumulation index scores in survivors (green) and controls (blue) from mixed models using least square mean outcome values adjusted for baseline covariates (see Supplementary Tables 4 and 5, available online). B) Adjusted mean deficits accumulation index scores are shown for survivors exposed to chemotherapy with or without hormonal therapy (red), survivors exposed to hormonal therapy only (blue), and controls (green) derived from mixed models using least square mean outcome values adjusted for baseline covariates (see Supplementary Tables 4 and 5, available online). On both panels, the models used data from all women and all timepoints they contributed.
Deficits Accumulation Trajectories
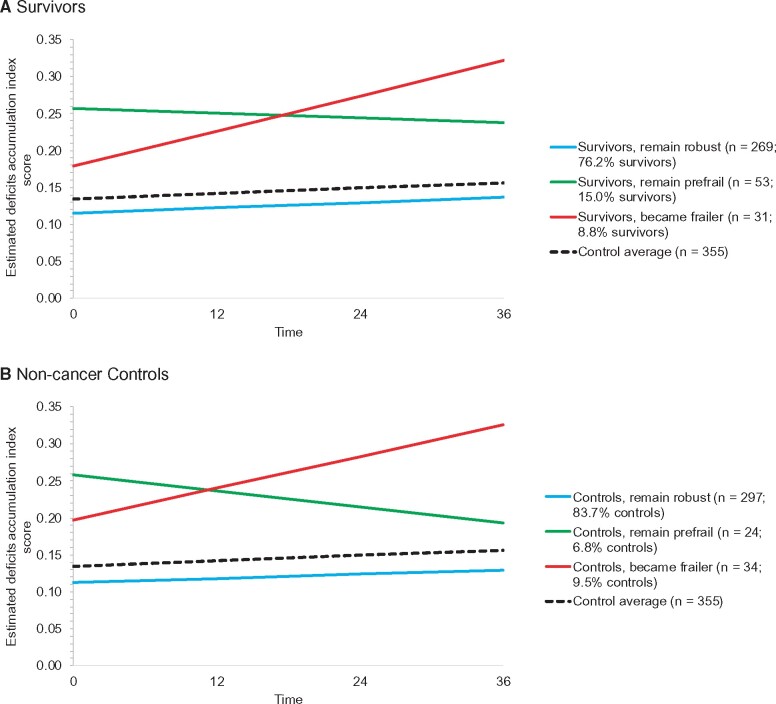
Survivors and controls each had 3 distinct trajectories of deficit accumulation. Most survivors started and remained in the robust range (n = 269, 76.2%); others were prefrail and remained prefrail (n = 53, 15.0%), and a small group (n = 31, 8.8%) started in the robust range presystemic therapy but developed increasing deficits over time (Figure 3, A). Among the controls, 83.7% (n = 297) were in a group that remained robust, 6.8% (n = 24) started and stayed prefrail, and 9.5% (n = 34) started with nearly frail scores and became frailer (Figure 3, B).
Figure 3.
Deficits accumulation index frailty trajectory groups. Deficits accumulation index scores at each assessment were used to derive trajectory groups using growth mixture models. Data were used from all women and all timepoints they contributed. The number of groups was determined by having at least 2% of participants in a group, likelihood ratio tests, the smallest Bayesian information criterion (BIC), and a priori expectation. Based on these criteria, 3 groups best fit the data for each group. The 3 trajectories were statistically significantly different from each other in both the survivor and control models, and the 3 survivor trajectory groups were statistically significantly different than the average of the overall control group (dotted black line) (all 2-sided P < .001 from growth mixture models).
When survivors and non-cancer controls were considered together (n = 708), there were 4 trajectory groups: super robust (12.0%), robust (68.8%), prefrail (9.6%), and became frailer (9.6%) (Supplementary Figure 3, available online). There were some baseline univariate differences between survivors and controls within the combined trajectory group that became frailer: survivors tended to be younger, have a higher rate of baseline sleep disturbances (48.6% [SD = 17.4%] vs 25.0% [SD = 8.2%]; P = .05), and have a higher (better) baseline self-reported cognition than controls (129.5 [SD = 15.0%] vs 118.3 [SD = 16.7%]; P = .01).
Impact of Deficits Accumulation Group on Longitudinal Cognition
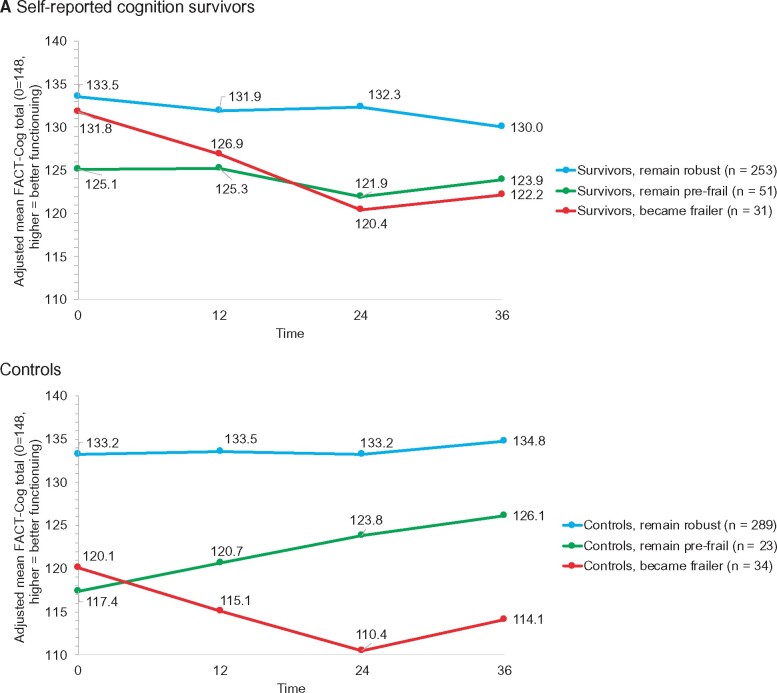
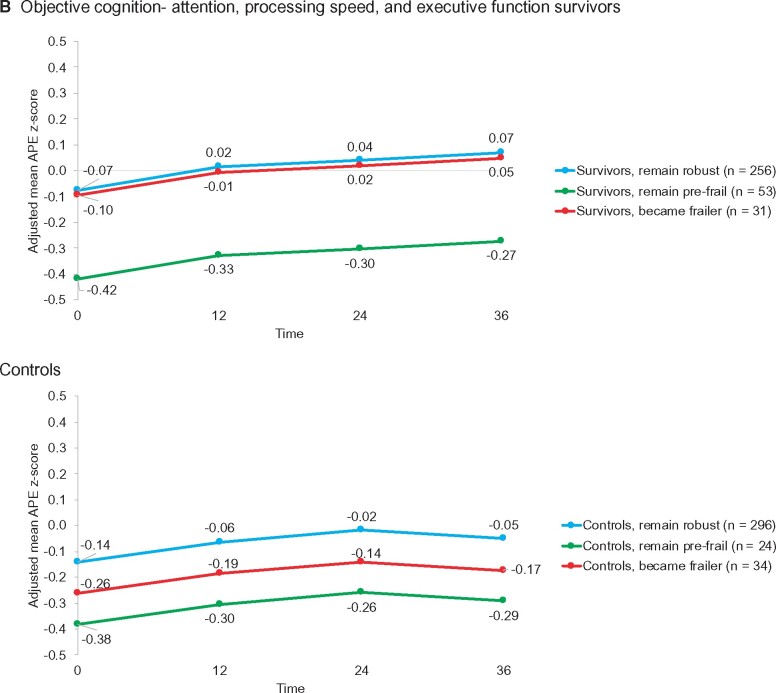
Although starting at similar levels of self-reported cognition, survivors in the group who started robust and became frailer reported greater decline in adjusted self-reported cognition scores from baseline to 36 months than survivors who remained robust (decline of 9.6 vs 3.2 points; P = .04; a meaningful decline is 7-10 points) (Figure 4, A; Supplementary Tables 6 and 7, available online), but both groups had similar scores on objective tests of cognition. The prefrail trajectory group had decline on both self-reported and objective cognition, with statistically significantly lower objective cognitive scores than the other survivor groups (P < .001 for attention, processing speed, and executive function [APE] and .04 for learning and memory [LM]) (Figure 4, B, for APE; Supplementary Tables 8 and 9, available online).
Figure 4.
Outcomes over 36 months in survivors and controls by deficits accumulation index trajectory group. A) Adjusted mean self-reported cognitive function based on FACT-cog total scores (range = 0-148, higher is better function) by deficits accumulation frailty score trajectory groups from separate mixed models for survivors and controls. Least square mean values for each timepoint for each trajectory group were adjusted for baseline covariates (age, race, Wide Range Achievement Test [WRAT] scores, time, treatment group [survivors only], trajectory group by time interaction, and recruitment site). Among survivors, there were clinically meaningful (7-10 points) and statistically significant differences (P = .002) in cognition scores by trajectory group, but the group by time interaction was not statistically significant (P = .10). Among controls, there were also clinically meaningful (7-10 points) and statistically significant differences (P < .001) in cognition scores by trajectory groups, and the group differences varied over time (group by time interaction P = .001). Detailed results are available in Supplementary Tables 6 and 7 (available online). B) Adjusted objective cognitive test z scores for the attention, processing speed, and executive function (APE) by deficits accumulation frailty score trajectory groups from separate mixed models for survivors and controls. Least square mean values for each timepoint for each trajectory group were adjusted for baseline covariates (age, race, WRAT scores, time, treatment group [survivors only], and recruitment site). Because there were no differences in scores among groups over time, an interaction of group by time was not included in the final models. Among survivors (P < .001) and controls (P = 0.03), the prefrail trajectory group had statistically significantly lower scores over time than the robust groups, and the survivor group that became frailer was not different from the robust group at baseline but was different among controls. Detailed results are available in Supplementary Tables 8 and 9 (available online). C) Adjusted mean MET minutes per week based on IPAQ-SF by deficits accumulation frailty score trajectory groups from separate mixed models for survivors and controls. Least square mean values for each timepoint for each trajectory group were adjusted for baseline covariates (age, race, WRAT scores, time, treatment group [survivors only], trajectory group by time interaction [survivors only; interaction NS in controls], and recruitment site). Survivor trajectory groups differed over time (P < .05), and in post hoc comparisons, the group that started robust and became frailer had statistically significantly lower physical activity levels over time than those in the group that remained robust (P < .001); the prefrail group also had lower physical activity than the robust group (P = .013). Control deficits accumulation frailty trajectory groups were different from each other in mean physical activity levels (P < .001), but physical activity did not change over time for the groups. Detailed results are available in Supplementary Tables 12 and 13 (available online). Data on all panels were derived from all data available for all women at all time points. Blue = remains robust; green = prefrail; red = frail or becomes frailer.
In contrast to survivors, controls in the frail trajectory group had statistically significantly lower self-reported cognition scores at baseline than those in the robust group (120.1 vs 133.2; P < .001) (Figure 4, A). Like the results for survivors, controls in the prefrail group had statistically significantly lower APE (P = .03) and LM (P = .01) scores than the other control groups (Figure 4, B; Supplementary Tables 10 and 11, available online).
Impact of Deficits Accumulation Group on Physical Activity
Survivors who remained robust and those who started robust but became frailer began at similar physical activity levels presystemic therapy, but those remaining robust increased their METs per week from 1470 to 2291 from baseline to 36 months, whereas those who became frailer declined from 1303 to 534 METs per week (a 769 MET minutes per week decline), lower than the recommended level of 600 (P < .001) for group differences at 36 months) (Figure 4, C; Supplementary Tables 12 and 13, available online).
The control group that became frailer was less active at baseline than the control group that remained robust (1135 vs 2138 MET minutes per week; P < .001), but all of the non-cancer control groups had higher physical activity levels than survivors, and none changed physical activity over time (Figure 4, C; Supplementary Tables 12 and 13, available online).
Discussion
The findings of this study add to the cancer and aging literature by describing longitudinal deficits accumulation and their impact on cognitive and physical outcomes in older breast cancer survivors and comparing deficits to matched non-cancer controls. We found that most older survivors and controls remained robust, and only a small proportion of each group had increasing deficits over 36 months. However, survivors had statistically significantly higher deficits accumulation scores than controls over 36 months, and there was variation in the characteristics of survivors vs controls with increasing deficits accumulation. Further, there were clinically meaningful differences between survivors and controls in the relationships of deficits accumulation trajectories to patient-reported cognitive function and physical activity outcomes.
On average, survivors had a small, clinically meaningful increase in deficits accumulation compared with non-cancer controls (30). Although the groups reported similar physical function in the months prior to diagnosis for survivors or before enrollment for non-cancer controls, baseline differences in deficits are not unexpected, because survivors were assessed in a stressful period postsurgery and prior to systemic therapy. We postulated that survivors would also develop more deficits over time than controls because of receipt of treatments that can cause toxicity and physical, psychological, and functional changes. However, we found similar proportions of survivors (8.8%) and controls (9.5%) on a trajectory of becoming frailer. It is possible that comparable rates may still indicate an effect of breast cancer and its therapies because survivors generally have better non-cancer survival than women without cancer (45). It is also possible that 36 months posttreatment was too short a period to detect meaningful changes in deficits accumulation. Alternatively, there may have been important changes in other parameters, such as biological aging markers (4,46), that we did not measure. Other studies with largely younger breast cancer survivors have shown 3-year increases in epigenetic age in less than a year after chemotherapy (47) and more than 10 years of biological aging based on expression of p16INK4a in the 18 months after chemotherapy (6).
One notable difference in the characteristics of survivors and controls that became frailer was that survivors had statistically significantly more baseline sleep disturbances than controls. Poor sleep has been linked to accelerated biological aging in general populations (38,48‐50). Our result suggests that there may be a multiplicative effect of sleep disturbance and cancer on deficits accumulation. If confirmed, improving sleep quality could be a feasible and effective survivorship intervention target (40,51,52).
The overwhelming majority of older breast cancer survivors had deficits accumulation scores in a robust range before systemic therapy, and most remained robust over time, with similar levels of deficits as seen in general populations (53). The survivors who remained robust had no declines in self-reported cognitive function, maintained their performance on neuropsychological tests of cognition, and reported increased physical activity. These observations support the idea that most older individuals could be included in clinical trials and offered indicated systemic therapies.
A small group of older survivors were robust prior to systemic therapy but went on to have increasing deficits accumulation, with large clinically meaningful changes and scores close to the frail range (30). This group was indistinguishable before systemic treatment from survivors who remained robust, whereas non-cancer controls with increasing deficits were statistically significantly different in several characteristics from robust controls at baseline. It is possible that the small group of robust survivors with increasing deficits were those with the most treatment-related side effects. Alternatively, deficit accumulation indices and the geriatric assessment measures that they are based on may not be sufficiently sensitive to predict longitudinal changes in accumulation of deficits. Pretreatment geriatric assessments were initially designed to predict acute chemotoxicity (18) and more recently have been used to predict all-cause mortality (16). Developing measures that can prospectively identify survivors at risk for becoming frailer is important because, in our study, this group had clinically meaningful declines in self-reported cognition and physical activity, 2 health domains important for maintaining independent living (54‐56).
There was another small group of survivors who were prefrail at baseline and stayed prefrail. This group may not be readily apparent in clinical encounters, but they reported more cognitive problems and had lower objective performance (with minimal improvement) and had lower activity than prefrail controls. This group of survivors may have been on a trajectory of increasing deficits prior to developing breast cancer because of the effects of diseases and conditions like diabetes and obesity that accelerate aging and increase risk of developing breast cancer (57). Another explanation is that these survivors are the most vulnerable to any aging effects of cancer and cancer therapy. Their results also suggest that self-reported cognitive problems should be included with geriatric assessments because they may be a marker of cognitive aging (58).
Overall, this study is the largest to examine longitudinal deficits accumulation and functional outcomes in older breast cancer survivors and to compare results with a matched, non-cancer control group. Despite these strengths, there are several limitations that should be considered in interpreting our findings. First, this was a secondary, unplanned analysis. Although it will be important to replicate our results in external samples, our results are very consistent with past studies showing increasing deficits in other cancer populations (4,5). Second, the trajectory groups we identified may be specific to our sample and may not be observed in other studies. The proportion of women who became frailer was slightly lower than the 9%-15% seen in longitudinal population aging studies using phenotypic measures (8,40,59) and a 14% cross-sectional rate based on deficits accumulation scores in breast cancer survivors with a mean age of 72 years (16). These differences may reflect the fact that we excluded survivors and controls with neurological disease, dementia, past cancers, and hearing or vision losses that precluded participation. Also, our sample was made up of research volunteers with a mean age of 68 years, so they may have been healthier than other samples, especially given their high self-reported levels of physical activity. Our sample was also well-educated, had limited variability in race, and did not include measures of lifetime stress. All of these factors can affect outcomes but should not have affected frequency-matched survivor-control comparisons (60‐62). Fourth, we only had 2 objective measures in our deficits accumulation index (body mass index and timed-up-and-go). However, we did include self-reported measures of most components of phenotypic frailty indices (exhaustion and/or fatigue, slowness, low physical activity, and physical weakness).
Overall, our findings illustrate that there is clinically important heterogeneity in deficit accumulation among older breast cancer survivors and that these differences are associated with important patient-reported outcomes. Although most women are robust and would be good candidates for clinical trials, women at risk for developing deficits at levels approaching frailty may be indistinguishable prior to systemic therapy. These data suggest that more sensitive and specific measures of deficit trajectories are needed and that related concepts such as biological age may be useful to inform future research, care decisions, and development of supportive care interventions.
Funding
This research was supported by the National Cancer Institute at the National Institutes of Health (NIH) grants R01CA129769 and R35CA197289 to JM. This study was also supported in part by the National Cancer Institute at the NIH grant P30CA51008 to Georgetown-Lombardi Comprehensive Cancer Center for support of the Biostatistics and Bioinformatics Resource and the Non-Therapeutic Shared Resource. The work of AJS and BCM was supported in part by the National Institute of Aging at the NIH grants P30AG10133, R01AG19771, and R01LM01136. TA=A was supported in part by National Cancer Institute at the National Institutes of Health grants R01CA172119 and P30CA008748. The work of JC was supported in part by the American Cancer Society Research Scholars grant 128660-RSG-15-187-01-PCSM and the National Cancer Institute at the National Institutes of Health grant R01CA237535. HJC was supported in part by the National Institute of Aging at the National Institutes of Health grant P30AG028716 for the Duke Pepper Center.
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have declared no conflicts of interest.
Disclaimer: The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Acknowledgements: The work of Paul Jacobsen was done while he was at Moffitt Cancer Center. This work builds on the legacy of our colleague Dr Arti Hurria. We dedicate this research to her memory. We would like to thank the participants in the Thinking and Living with Cancer (TLC) study for their sharing of their time and experiences; without their generosity this study would not have been possible. We are also indebted to Sherri Stahl, Naomi Greenwood, Margery London, and Sue Winarsky, who serve as patient advocates from the Georgetown Breast Cancer Advocates, for their insights and suggestions on study design and methods to recruit and retain participants. We thank the TLC study staff who contributed by ascertaining, enrolling, and interviewing participants.
Author contributions: Jeanne S. Mandelblatt was responsible for conceptualization, investigation, resources, writing and editing, supervision, project administration, and funding acquisition. Xingtao Zhou was responsible for formal analysis, data curation, and writing. Brent J. Small was responsible for supervision, methodology, formal analysis, data curation, and writing. Jaeil Ahn was responsible for supervision, methodology, formal analysis, data curation, and writing. Wanting Zhai was responsible for formal analysis, data curation, and writing, Tim Ahles was responsible for conceptualization, investigation, resources, writing, project administration, and funding acquisition. Martine Extermann was responsible for conceptualization and writing. Deena Graham was responsible for investigation, resources, and writing. Paul B. Jacobsen was responsible for investigation and writing. Heather Jim was responsible for investigation, resources, writing, project administration, and funding acquisition. Brenna C. McDonald was responsible for investigation, resources, writing, project administration, and funding acquisition. Sunita Patel was responsible for investigation, resources, writing, and project administration. James C. Root was responsible for investigation, supervision, and writing. Andrew J. Saykin was responsible for investigation, resources, writing, project administration, and funding acquisition. Harvey Jay Cohen was responsible for conceptualization and writing and editing. Judith E. Carroll was responsible for conceptualization and writing and editing.
Prior presentations: Earlier versions of this study were presented at the International Cognition and Cancer Task Force Annual Meeting in Denver, Colorado, in February 2020.
Data Availability
The data collected for the Thinking and Living with Cancer (TLC) Study used in this publication were supported by funding from the National Institutes of Health. The data are available for sharing under NIH-compliant TLC Study agreements. Please contact the corresponding author for requests.
Supplementary Material
References
- 1. Armenian SH, Gibson CJ, Rockne RC, et al. Premature aging in young cancer survivors. J Natl Cancer Inst. 2019;111(3):226–232. [DOI] [PubMed] [Google Scholar]
- 2. Alfano CM, Peng J, Andridge RR, et al. Inflammatory cytokines and comorbidity development in breast cancer survivors versus noncancer controls: evidence for accelerated aging? J Clin Oncol. 2017;35(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodes RJ, Sierra F, Austad SN, et al. Disease drivers of aging. Ann NY Acad Sci. 2016;1386(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guida JL, Ahles TA, Belsky D, et al. Measuring aging and identifying aging phenotypes in cancer survivors. J Natl Cancer Inst. 2019;111(12):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ness KK, Wogksch MD.. Frailty and aging in cancer survivors. Transl Res. 2020;221:65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henderson TO, Ness KK, Cohen HJ.. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014;34:e423–e430. [DOI] [PubMed] [Google Scholar]
- 8. Xue QL, Bandeen-Roche K, Varadhan R, et al. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63(9):984–990. [DOI] [PubMed] [Google Scholar]
- 9. Cohen HJ, Smith D, Sun CL, et al. ; for the Cancer and Aging Research Group. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rockwood K, Howlett SE.. Fifteen years of progress in understanding frailty and health in aging. BMC Med. 2018;16(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirkhus L, Šaltytė Benth J, Rostoft S, et al. Geriatric assessment is superior to oncologists’ clinical judgement in identifying frailty. Br J Cancer. 2017;117(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 13. Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockwood K, Howlett SE.. Age-related deficit accumulation and the diseases of ageing. Mech Ageing Dev. 2019;180:107–116. [DOI] [PubMed] [Google Scholar]
- 15. Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. [DOI] [PubMed] [Google Scholar]
- 16. Guerard EJ, Deal AM, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw. 2017;15(7):894–902. [DOI] [PubMed] [Google Scholar]
- 17. Mandelblatt JS, Cai L, Luta G, et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res Treat. 2017;164(1):107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheppard VB, Faul LA, Luta G, et al. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;32(22):2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mandelblatt JS, Small BJ, Luta G, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol. 2018;36(32):3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson GS, Robertson GJ.. Wide Range Achievement Test - Fourth Edition: Professional Manual. Lutz, FL: Psychological Assessment Resources; 2006.
- 23. Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18):1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914–919. [DOI] [PubMed] [Google Scholar]
- 25. Podsiadlo D, Richardson S.. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. [DOI] [PubMed] [Google Scholar]
- 26. Ware J Jr., Kosinski M, Keller SD.. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 27. Radloff L, The CES.. D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 28. Spielberger C. Manual for the State-Trait Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 29. Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. [DOI] [PubMed] [Google Scholar]
- 30. Jang I-Y, Jung H-W, Lee HY, et al. Evaluation of clinically meaningful changes in measures of frailty. J Gerontol Ser A. 2020;75(6):1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67(7):811–820. [DOI] [PubMed] [Google Scholar]
- 32. Costa DSJ, Loh V, Birney DP, et al. The structure of the FACT-Cog v3 in cancer patients, students, and older adults. J Pain Symptom Manage. 2018;55(4):1173–1178. [DOI] [PubMed] [Google Scholar]
- 33. Dyk KV, Crespi CM, Petersen L, et al. Identifying cancer-related cognitive impairment using the fact-cog perceived cognitive impairment. JNCI Cancer Spectr. 2020;4(1):pkz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- 35. Blanchard CM, Courneya KS, Stein K.. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 37. Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. [DOI] [PubMed] [Google Scholar]
- 38. Carroll JE, Small BJ, Tometich DB, et al. ; for the Thinking and Living With Cancer Study. Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: interaction with genotype. Cancer. 2019;125(24):4516–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Little RJA, Rubin DB.. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons, Inc; 1986. [Google Scholar]
- 40. Stenholm S, Ferrucci L, Vahtera J, et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies. J Gerontol A Biol Sci Med Sci. 2019;74(5):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jackson C. Multi-state models for panel data: The msm package for R. J Stat Softw. 2011;38(8):1–29. [Google Scholar]
- 42. Littell RC, Milliken GA, Stroup WW, et al. SAS for Mixed Models ,2nd ed.Cary, NC: SAS Publishing; 2006. [Google Scholar]
- 43. Mandelblatt JS, Jacobsen PB, Ahles T.. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol. 2014;32(24):2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ram N, Grimm KJ.. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev. 2009;33(6):565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho H, Mariotto AB, Mann BS, et al. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2):e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sehl ME, Carroll JE, Horvath S, et al. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. Npj Breast Cancer. 2020;6(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carroll JE, Esquivel S, Goldberg A, et al. Insomnia and telomere length in older adults. Sleep. 2016;39(3):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carroll JE, Irwin MR, Levine M, et al. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative Study. Biol Psychiatry. 2017;81(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carroll JE, Irwin MR, Seeman TE, et al. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2019;42(7):zsz089. doi: 10.1093/sleep/zsz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. [DOI] [PubMed] [Google Scholar]
- 53. Rockwood K, Song X, Mitnitski A.. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ. 2011;183(8):E487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Albala C, Lera L, Sanchez H, et al. Frequency of frailty and its association with cognitive status and survival in older Chileans. Clin Interv Aging. 2017;12:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okura M, Ogita M, Arai H.. Self-reported cognitive frailty predicts adverse health outcomes for community-dwelling older adults based on an analysis of sex and age. J Nutr Health Aging. 2019;23(7):654–664. [DOI] [PubMed] [Google Scholar]
- 56. Patnode CD, Perdue LA, Rossom RC, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(8):764–785. [DOI] [PubMed] [Google Scholar]
- 57. Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15(9):996–997. [DOI] [PubMed] [Google Scholar]
- 58. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. Gerona. 2015;70(11):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rentscher KE, Carroll JE, Repetti RL, et al. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinology. 2019;102:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rentscher KE, Carroll JE, Mitchell C.. Psychosocial stressors and telomere length: a current review of the science. Annu Rev Public Health. 2020;41(1):223–245. [DOI] [PubMed] [Google Scholar]
- 62. Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for the Thinking and Living with Cancer (TLC) Study used in this publication were supported by funding from the National Institutes of Health. The data are available for sharing under NIH-compliant TLC Study agreements. Please contact the corresponding author for requests.