Abstract
The mature embryos of rice seeds contain translatable mRNAs required for the initial phase of germination. To clarify the relationship between seed longevity and RNA integrity in embryos, germinability and stability of embryonic RNAs were analyzed using the seeds of japonica rice cultivars subjected to controlled deterioration treatment (CDT) or long periods of storage. Degradation of RNA from embryos of a japonica rice cultivar “Nipponbare” was induced by CDT before the decline of the germination rate and we observed a positive relationship between seed germinability and integrity of embryonic RNAs. Moreover, this relationship was confirmed in the experiments using aged seeds from the “Nipponbare”, “Sasanishiki” and “Koshihikari” rice cultivars. In addition, the RNA integrity number (RIN) values, calculated using electrophoresis data and Agilent Bioanalyzer software, had a positive correlation with germinability (R2=0.75). Therefore, the stability of embryonic RNAs required for germination is involved in maintaining seed longevity over time and RIN values can serve as a quantitative indicator to evaluate germinability in rice.
Keywords: embryonic RNA stability, Rice, RNA integrity number (RIN), seed longevity
Seed longevity is defined as the ability of a seed to remain viable during storage. Over time, seed viability gradually decreases because of aging or deterioration events, which eventually result in poor seedling establishment (Adsul et al. 2018). Seeds with extended longevity are intact and produce vigorous seedlings, which ultimately ensures a high yield. Therefore, seed longevity is an important trait not only for the seed industry but also for seed storage and conservation of genetic resources (Clerkx et al. 2004; Sano et al. 2016). This prominent agronomic trait is partially affected by genetic factors but is also influenced by the environmental conditions experienced by the mother plant during seed maturation, and conditions imposed during the post-harvest and storage periods (Agacka-Mołdoch et al. 2016; Wiebach et al. 2020). Genetic control of seed longevity has been reported in Arabidopsis thaliana (Bentsink et al. 2000), barley (Nagel et al. 2009), maize (Revilla et al. 2009), wheat (Landjeva et al. 2010), lettuce (Schwember and Bradford 2010), oilseed rape (Nagel et al. 2011) and tobacco (Agacka-Mołdoch et al. 2015).
In various crops, it has been revealed that the RNA stability in dry seeds is related to seed longevity. Bray and Chow (1976) showed that increased levels of RNA fragmentation were observed in dead seeds of peas as compared with unaged living seeds. Reuzeau and Cavalie (1997) reported observing a strong correlation between seed germinability and total RNA content of dry seeds of sunflower (Helianthus annuus L.). Also, in dry seeds of Nicotiana species, a correlation has been observed between the germination rate and the integrity of rRNA (Brocklehurst and Fraser 1980). In addition, loss of germination capacity by artificial aging treatment was accompanied by a reduction in total RNA content and RNA integrity in the seeds of garden pea (Pisum sativum) and mung bean (Vigna radiata L.) (Kranner et al. 2011; Sharma et al. 2018). These reports support the view that a positive and significant relationship exists between seed longevity and RNA stability in dry seeds.
Electrophoresis of total RNA followed by calculation of RNA integrity number (RIN), which evaluates RNA fragment size distribution, is a useful method for examining the integrity of RNA (Schroeder et al. 2006). In general, the 25S and 18S rRNA, the most abundant RNAs in plant cells, can be clearly detected as the major bands in an electrophoretic analysis of total RNA from plant tissues. When degradation of RNAs has occurred, smears or additional bands can be observed. A RIN value can be calculated using Agilent Bioanalyzer software. RIN values are based on the ratio and height of the peaks corresponding to the 25S and 18S rRNA bands, and the presence of peaks of additional bands in inter peak regions between rRNAs. The RIN, which can range in value from one (fully degraded RNA) to 10 (intact RNA), is widely used as an index to evaluate RNA integrity in the analysis of gene expression, such as RNA-seq (Schroeder et al. 2006). In addition, Fleming et al. (2017, 2019) have reported that in the dry seeds of various crops, such as soybean, pea, carrot, crimson clover, lettuce, onion, safflower, sesame and sorghum, the RIN value is significantly and positively correlated with germination potential. Thus, the integrity of embryonic RNA is important for maintaining the seed germinability and the RIN value can serve as an index to evaluate the longevity potential of seeds. However, the mechanism of decline in germinability mediated by degradation of embryonic RNAs remains unidentified. For elucidation of this mechanism, the useful analytical materials will be required.
The molecular mechanisms of various phenomena in plant cells have been elucidated using model plants such as rice and Arabidopsis. In rice, a genome-wide association study (GWAS) of seed longevity was possible because materials useful for genetic analyses such as GWAS have been maintained (Lee et al. 2019). We have also reported that mature dry seeds contain a great number of stored mRNAs (long-lived mRNAs) that accumulate during seed maturation and that protein synthesis during the initial phase of germination occurs from these stored mRNAs (Sano et al. 2012, 2015, 2019, 2020). In addition, we have analyzed RNA-binding proteins, which are known to govern many aspects of RNA metabolism including stability and decay of RNAs. By applying proteomic methods to rice seeds, we were able to detect seed-specific and abundant RNA-binding proteins that are up-regulated during the desiccation stage of rice seed formation (Sano et al. 2013). Therefore, rice seeds are useful materials for elucidation of the mechanism of decline in germinability as mediated by degradation of embryonic RNAs. However, a correlation between stability of embryonic RNAs and seed longevity in rice seeds has not yet been analyzed.
Many years would be needed to evaluate seed germinability under natural storage conditions. To circumvent this problem, an artificial aging technique known as controlled deterioration treatment (CDT) has been widely applied to accelerate the agiing process with high temperature and relative humidity to easily and quickly estimate seed viability (Hu et al. 2012; Min et al. 2017; Rajjou et al. 2008). Moreover, analysis of natural aging can be performed in parallel using old seeds stored for long periods, providing data that can be compared with results found using seeds subjected to CDT (Hang et al. 2015; Rajjou et al. 2008).
In this study, we examined whether there is a correlation between seed longevity and the integrity of RNAs in embryos from the japonica rice cultivar “Nipponbare” that have been subjected to CDT or long periods of storage at 4°C, focusing on the RIN value as an analysis method. Furthermore, we analyzed germinability and stability of embryonic RNAs in aged seeds from each of two distinct japonica rice cultivars, “Sasanishiki” and “Koshihikari”.
Rice plants (Oryza sativa L.) of three Japanese cultivars (Nipponbare, Sasanishiki and Koshihikari) were grown during the rice cultivation season (May to September) under natural conditions at the Toyama Prefectural Agricultural Forestry and Fisheries Research Center in 2007–2017 and the harvested seeds were stored at 4°C and ∼35% relative humidity until use. The seeds of the Nipponbare cultivar, which were harvested in 2014, were exposed to CDT after storage for four years. The seeds of Nipponbare, Sasanishiki and Koshihikari harvested in 2010, 2009 and 2007, respectively, and analyzed in 2018 as “aged seeds” in this study. Seeds from these three cultivars harvested in 2017 were used as “fresh seeds”.
CDT is an artificial seed-aging technique performed by increasing the temperature and relative humidity. In the present study, seed samples were subjected to CDT for 8 weeks by storing them at 36°C and 80% relative humidity in a sealed box with an open petri dish containing saturated KCl solution, as previously described (Hu et al. 2012).
Germination assays were carried out in triplicate using 50 seeds from each sample. The seeds were incubated in distilled water at 28°C for 10 days under dark conditions and the water was replaced with fresh water every other day. The number of germinated seeds was counted 10 days after imbibition (DAI).
Dry embryos were separated from dehulled seeds using a surgical blade. Total RNA was extracted from 20 embryos using Fruit-mate for RNA purification and RNAiso Plus (TaKaRa Bio), according to the manufacturer’s protocol. The concentration of extracted RNA was assessed at 230, 260 and 280 nm using a Nano Drop 1000 spectrophotometer (Thermo Fisher Scientific). The extracted total RNAs (200 ng) were analyzed by electrophoresis using an Agilent 2100 Bioanalyzer system (Agilent Technologies), and then RIN values were calculated using Agilent 2100 Bioanalyzer software.
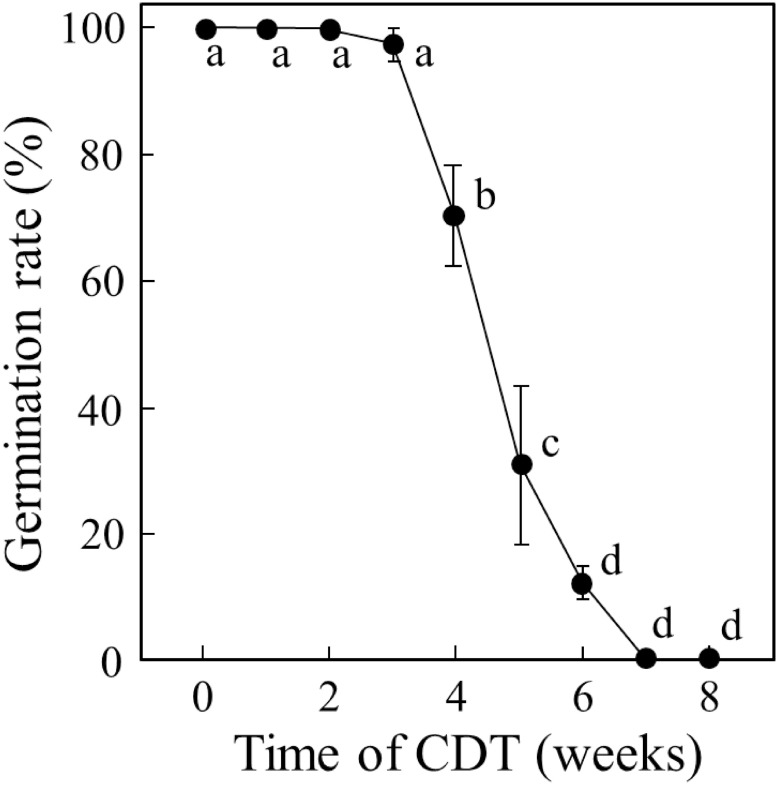
To examine germinability, we first performed a germination assay of seeds from the cultivar “Nipponbare” harvested in 2014 and stored at 4°C for 4 years. After imbibition at 28°C for 10 days under dark conditions, the germination rate of these seeds was almost 100%. Next, these seeds were exposed to CDT at 36°C and 80% relative humidity. Although the germination rate of these seeds remained over 90% after 3 weeks of CDT, we observed a sudden decrease after 4 weeks of treatment (Figure 1), indicating that a decline in germinability was induced before 4 weeks of CDT. At 5 weeks of CDT, the germination rate dropped to 30% and by 8 weeks of CDT, the germinability was completely lost.
Figure 1. Decline in germinability of Nipponbare seeds under controlled deterioration treatment (CDT). Seeds of rice cultivar Nipponbare harvested in 2014 were stored at 4°C for four years and treated with CDT at 36°C and 80% RH for 8 weeks. Values of germination rate are means of three replicates±SE Different letters indicate significant differences according to Tukey–Kramer multiple range test (p<0.05).
To clarify the relationship between germinability and RNA stability in rice seeds, total RNA extracted from the embryos of Nipponbare cultivar used in the experiment shown in Figure 1 under CDT for 2, 4, 6 and 8 weeks or without CDT were analyzed by electrophoresis using the Agilent 2100 Bioanalyzer system. The results of electrophoresis show that 25S and 18S rRNA were detected as prominent bands and other bands were not identified clearly in the fraction of total RNAs from embryos before they were subjected to CDT (Figure 2A). However, bands of RNA fragments of small molecular size were detected after exposure to CDT. At 2 weeks of CDT, a few smeared bands were observed under the position of the 25S and 18S rRNA. Moreover, multiple bands of RNA fragments with lower molecular size than rRNAs were readily detected in fractions from embryos subjected to CDT for 4, 6, or 8 weeks. In general, the presence of additional bands below the rRNAs is an indication of RNA degradation. Therefore, these results suggest that RNA degradation was induced by CDT in embryos of Nipponbare seeds.
Figure 2. Electrophoresis patterns (A) and RIN values (B) of total RNA extracted from embryos of rice (Oryza sativa L. cv. Nipponbare) seeds (n=20) harvested in 2014, stored at 4°C for four years, and treated with CDT at 36°C and 80% RH for 8 weeks. (A) The extracted total RNAs (each 200 ng) were analyzed by electrophoresis using an Agilent 2100 Bioanalyzer system. (B) The RIN values are means of three replicates±SE. Different letters indicate significant differences according to Tukey–Kramer multiple range test (p<0.05).
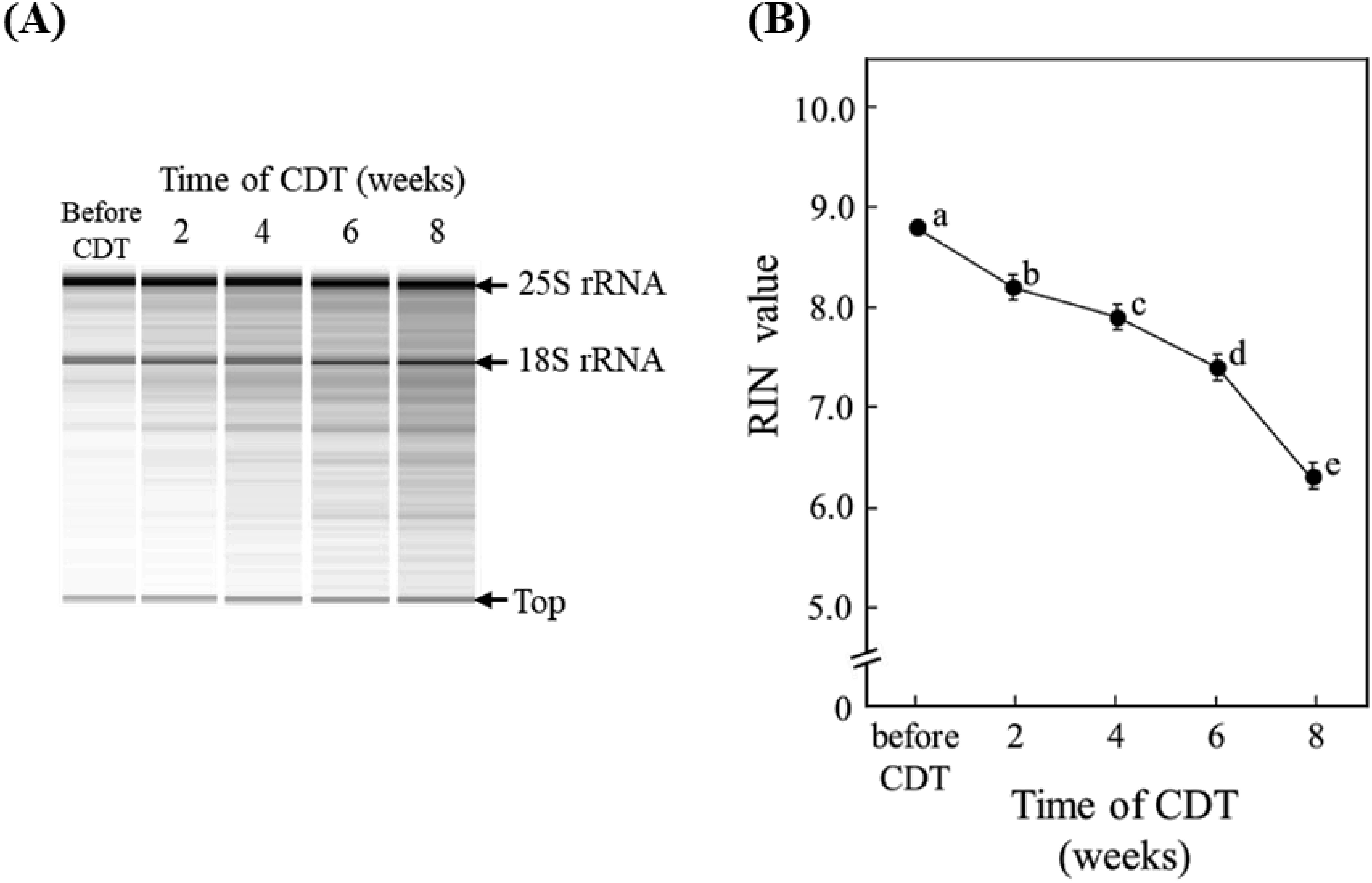
The results of the electrophoretic analysis shown in Figure 2A were used to calculate RIN values using Agilent Bioanalyzer software. The mean RIN value for total RNA from embryos not subjected to CDT was 8.8, and this value was significantly decreased (to 8.2) in embryos from seeds subjected to 2 weeks of CDT (Figure 2B). After 4 or 6 weeks of CDTs, the mean RIN values were markedly reduced to 7.8 and 7.4, respectively, and ultimately, the value decreased to 6.3 for total RNA from embryo of seeds with no germinability under 8 weeks of CDT. Thus, overall, the data indicate that RIN values decline incrementally with reducing germinability in seeds under CDT, and that the process of RNA degradation was induced before 2 weeks of CDT.
Both the germinability and RIN values in embryos were reduced by CDT. However, the CDT is an artificial treatment with high temperature and humidity, and the experiments in Figures 1 and 2 were carried out using only one cultivar, Nipponbare. Therefore, we next analyzed the germination rate and RNA stability of aged seeds stored at 4°C for 8–11 years without CDT, using not only Nipponbare but also the Sasanishiki and Koshihikari rice cultivars, so we could compare these results with results obtained using fresh seeds and CDT.
The fresh seeds of Nipponbare harvested in 2017 (one-year-old seeds) exhibited a high germination rate (nearly 100%), whereas aged seeds of the same cultivar harvested in 2010 (eight-year-old seeds) displayed a significantly reduced germination rate of about 36% (Figure 3A). This suggests that long-term dry storage of seeds leads to a significant reduction of seed longevity. To confirm this, seeds from two other rice cultivars, Sasanishiki and Koshihikari, were examined for germinability. Fresh seeds of Sasanishiki harvested in 2017 (one-year-old seeds) germinated at a rate of 100%, whereas aged seeds of this cultivar harvested in 2009 (nine-year-old seeds) germinated at a rate of about 28% (Figure 3A). Also, for Koshihikari, fresh seeds stored only for one year (harvested in 2017) germinated at a rate of 100%, whereas aged seeds stored for about 11 years (harvested in 2007) germinated at a rate of about 22% (Figure 3A). These results show that the germination potential of aged seeds of Nipponbare, Sasanishiki and Koshihikari cultivars was reduced by long-term storage at 4°C without CDT.
Figure 3. Comparison of the germinability (A) and the electrophoresis patterns of total RNA extracted from the embryos (B) of fresh and aged seeds in rice cultivars Nipponbare, Sasanishiki and Koshihikari. The aged seeds of Nipponbare, Sasanishiki and Koshihikari were harvested in 2010, 2009 and 2007, respectively, after cultivation under natural conditions in Toyama Prefecture, Japan. Fresh seeds from three cultivars were harvested in 2017. (A) Fresh (white bars) and aged (black bars) seeds were incubated in petri dishes with distilled water at 28°C for ten days. Values of germination rate are means of three replicates±SE Student’s t-test was used to compare the mean (n=3) germination rate (%) of seeds for each cultivar (**: p<0.01). (B) The extracted total RNAs (each 200 ng) from the embryos of fresh and aged seeds were analyzed by electrophoresis using an Agilent 2100 Bioanalyzer system.
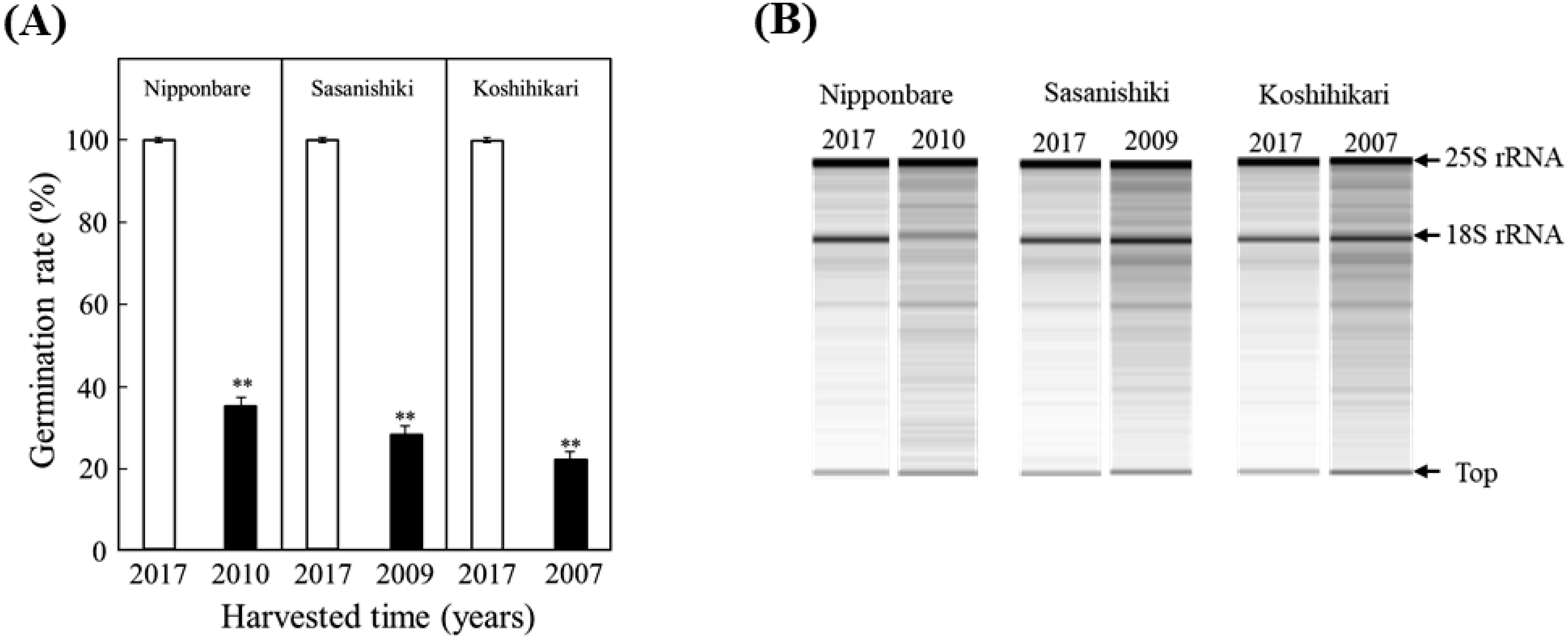
To check the RNA integrity in aged seeds of rice, total RNA extracted from the embryos of Nipponbare, Sasanishiki and Koshihikari seeds stored at 4°C for 8–11 years were analyzed by electrophoresis, followed by calculation of RIN values, using an Agilent 2100 Bioanalyzer system and software. These values were then compared with results obtained using fresh seeds. As shown in Figure 3B, prominent bands corresponding to 25S and 18S rRNA were detected in the fraction of total RNAs from the embryos of fresh seeds from all three cultivars. In contrast, a lot of additional bands (RNA fragments) of a small molecular size were clearly identified below the rRNA bands in total RNAs from aged embryos from these cultivars.
The mean RIN value of total RNA from embryos of fresh seeds (harvested in 2017) of the Nipponbare rice cultivar was 8.0, and the mean RIN value for aged seeds (harvested in 2010) of the same cultivar was significantly reduced (6.6; Table 1), suggesting that long-term dry storage of seeds caused a significant reduction in the RIN value. To confirm this result, RIN values were measured in fresh and aged seeds from two other japonica rice cultivars, Sasanishiki and Koshihikari. Mean RIN values for fresh seeds (harvested in 2017) from both of these two cultivars were 8.0, whereas RIN values for aged seeds were remarkably low. The mean RIN values for aged seeds of the Sasanishiki cultivar (harvested in 2009) were 6.5 and for Koshihikari (2007 harvest) were 6.1. These results indicated that a decline in RIN values in the seeds stored for several years at 4°C without CDT is common to all three japonica rice cultivars, Nipponbare, Sasanishiki and Koshihikari, and further, suggest that RNA degradation had been induced in the aged seeds, which also displayed low germinability.
Table 1. Comparison of mean RIN values from fresh and aged seeds of rice cultivars Nipponbare, Sasanishiki and Koshihikari.
| Cultivars | Nipponbare | Sasanishiki | Koshihikari | |||
|---|---|---|---|---|---|---|
| 2017 | 2010 | 2017 | 2009 | 2017 | 2007 | |
| Mean RIN values | 8.0±0.4 | 6.6±0.3** | 8.0±0.1 | 6.5±0.3** | 8.0±0.0 | 6.1±0.4** |
Values are means of three replicates±SE Student’s t-test was used to compare the means (n=3) RIN values of the seeds for each cultivar (**: p<0.01).
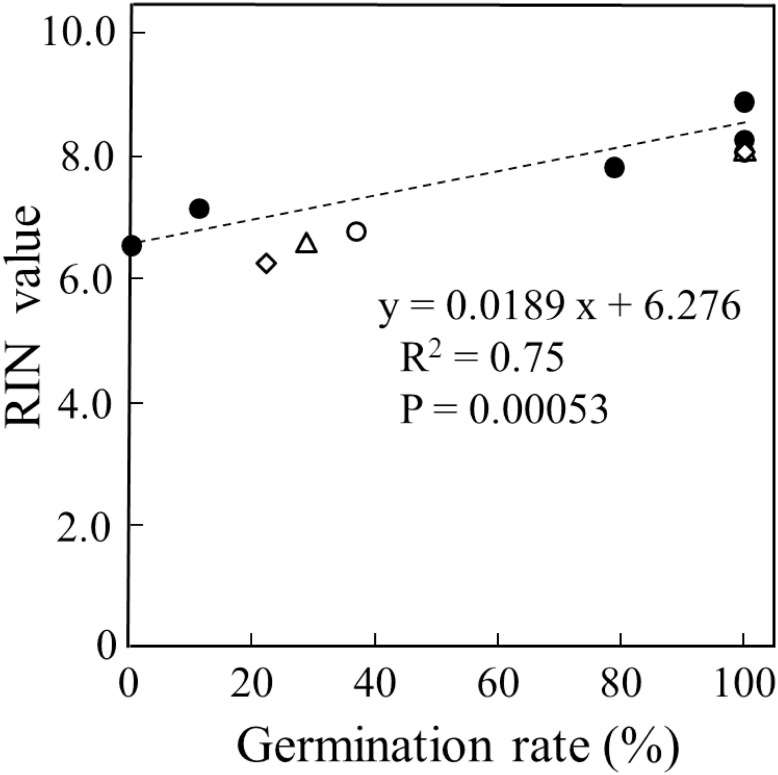
Taking together all of the experimental data for the rice seeds of three cultivars, Nipponbare, Sasanishiki and Koshihikari, that were exposed to CDT and stored for various years in this study, we next examined whether there is a correlation between germinability and RIN values. The germination rates of these seeds have a positive correlation with the corresponding RIN values (R2=0.75) (Figure 4). Given that the RIN value serves as a standard indicator of RNA integrity, these results support the hypothesis that there is a correlation between germinability and RNA integrity in the embryos of rice seeds.
Figure 4. Relationship between germination rate and RIN values of rice seeds in three japonica rice cultivars (Nipponbare. Sasanishiki and Koshihikari). RIN values are taken from Figure 2 and Table 1 and germination data from Figures 1 and 3 are used. Filled circles (●) indicate Nipponbare under CDT, and the open circle (〇) indicates Nipponbare fresh and aged seeds. The open triangle (△) indicates Sasanishiki fresh and aged seeds, and the open diamond (◇) indicates Koshihikari fresh and aged seeds. R2=0.75, and p value=0.00053.
The present study clearly demonstrates that the stability of RNA in embryos has a positive correlation with germinability in seeds of three distinct rice cultivars, Nipponbare, Sasanishiki and Koshihikari, when subjected to CDT or stored for long periods at 4°C. Seed longevity is defined as the total time during which a seed remains viable for germination, suggesting that in rice, integrity of RNA in embryos is involved in maintaining seed longevity for a long time. In addition, the results of this study show that the RIN values of embryonic RNA can serve as a quantitative indicator to evaluate the viability of rice seeds. The embryonic RIN value of Nipponbare seeds was significantly decreased by CDT for two weeks (Figure 2B), although the germination rate of these seeds remained about 100% (Figure 1). These results show that fragmentation of embryonic RNAs had begun before the decline in germination rate of rice seeds and suggest that a loss of seed germinability may be induced by degradation of RNAs in embryo, as reported previously by Fleming et al. (2019) in various crops.
It has been shown that the integrity of embryonic RNA is involved in the maintenance of seed longevity in a lot of crops, such as rye grain (Roberts et al. 1973), the dry seed of Nicotiana tabacum L. (Brocklehurst and Fraser 1980), aged carrot seed (Thompson et al. 1987), dry sunflower seeds (Reuzeau and Cavalie 1997), aged garden pea seeds (Kranner et al. 2011), and mung bean seeds (Sharma et al. 2018). In addition, there was a significant correlation between the germinabilities and RIN values in the dry seeds of soybean, pea, carrot, crimson clover, lettuce, onion, safflower, sesame, and sorghum (Fleming et al. 2017, 2018, 2019). The present study also clarified that in rice, there is a correlation between degradation of embryonic RNAs and loss of seed germinability, suggesting that the importance of embryonic RNA stability for seed longevity is common in Spermatophyta.
We have previously studied long-lived mRNAs stored in embryos during seed maturation and gene expression at the early phase of germination in rice seeds. When rice seeds were exposed to a transcriptional inhibitor, actinomycin D, protein synthesis occurred from these long-lived mRNAs without de novo transcription and seed germination was induced (Sano et al. 2012). In addition, it was determined that accumulation of long-lived mRNAs required for germination is completed in embryos during seed development, between 10–20 days after flowering (Sano et al. 2015). Moreover, it is clear that during seed germination, proteins are translated from both newly synthesized and long-lived mRNAs. Based on a proteomic analysis, a total of 109 proteins that are translated from the long-lived mRNAs related to germination were implicated in energy production, such as glycolysis, or function as nucleotide binding proteins. This shows that long-lived mRNAs, which are produced during seed development and then stored, function in the initial energy production and gene expression of the germination process in rice (Sano et al. 2019). These findings suggest that the degradation of long-lived mRNAs involved in initial energy production and activation of translational machinery influence the germinability of rice seeds.
In addition, Sugimoto et al. (2016) showed that the germinability of rice seeds decreased following exposure to the space environment outside the international space station, i.e., conditions beyond the Earth’s atmosphere. Furthermore, the results of transcriptome analysis on these seeds suggest that the space exposure-induced damage to long-lived mRNA of genes such as glycolysis-related genes that are required for germination delays germination. Taken together, the experimental results presented here and other supporting evidence from previous reports on long-lived mRNA suggest that the stability of mRNAs stored in rice embryos that are required for germination is related to seed longevity. To further understand the mechanisms that maintain seed longevity via maintenance of the stability of long-lived mRNAs in embryos, genetic methods, such as QTL analysis and genome wide association study (GWAS), will be effective, as differences in seed longevity have been observed for different varieties of rice (Hang et al. 2015).
Acknowledgments
We thank Associate Prof. Dr. Mohammad Wali Salari and Dr. Megumi Kashiwagi for their helpful discussions and general support.
Abbreviations
- CDT
controlled deterioration treatment
- RIN
RNA integrity number
- DAI
days after imbibition
References
- Adsul AT, Chimote VP, Deshmukh MP (2018) Inheritance of Seed Longevity and Its Association with Other Seed Related Traits in Soybean (Glycine Max). Agric Res 7: 105–111 [Google Scholar]
- Agacka-Mołdoch M, Nagel M, Doroszewska T, Lewis RS, Börner A (2015) Mapping quantitative trait loci determining seed longevity in tobacco (Nicotiana tabacum L.). Euphytica 202: 479–486 [Google Scholar]
- Agacka-Mołdoch M, Arif MAR, Lohwasser U, Doroszewska T, Qualset CO, Börner A (2016) The inheritance of wheat grain longevity: A comparison between induced and natural ageing. J Appl Genet 57: 477–481 [DOI] [PubMed] [Google Scholar]
- Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M (2000) Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol 124: 1595–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray CM, Chow TY (1976) Lesions in the ribosomes of none-viable pea (Pisum arvense) embryonic axis tissue. Biochim Biophys Acta 442: 14–23 [DOI] [PubMed] [Google Scholar]
- Brocklehurst PA, Fraser RS (1980) Ribosomal RNA integrity and rate of seed germination. Planta 148: 417–421 [DOI] [PubMed] [Google Scholar]
- Clerkx EJM, El-Lithy ME, Vierling E, Ruys GJ, Blankestijn-De Vries H, Groot SPC, Vreugdenhil D, Koornneef M (2004) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135: 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MB, Hill LM, Walters C (2019) The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann Bot 123: 1133–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MB, Patterson EL, Reeves PA, Richards CM, Gaines TA, Walters C (2018) Exploring the fate of mRNA in aging seeds: Protection, destruction, or slow decay? J Exp Bot 69: 4309–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MB, Richards CM, Walters C (2017) Decline in RNA integrity of dry-stored soybean seeds correlates with loss of germination potential. J Exp Bot 68: 2219–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang NT, Lin Q, Liu L, Liu X, Liu S, Wang W, Li L, He N, Liu Z, Jiang L, et al. (2015) Mapping QTLs related to rice seed storability under natural and artificial aging storage conditions. Euphytica 203: 673–681 [Google Scholar]
- Hu D, Ma G, Wang Q, Yao J, Wang Y, Pritchard HW, Wang X (2012) Spatial and temporal nature of reactive oxygen species production and programmed cell death in elm (Ulmus pumila L.) seeds during controlled deterioration. Plant Cell Environ 35: 2045–2059 [DOI] [PubMed] [Google Scholar]
- Kranner I, Chen H, Pritchard HW, Pearce SR, Birtic S (2011) Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regul 63: 63–72 [Google Scholar]
- Landjeva S, Lohwasser U, Börner A (2010) Genetic mapping within the wheat D genome reveals QTL for germination, seed vigour and longevity, and early seedling growth. Euphytica 171: 129–143 [Google Scholar]
- Lee J-S, Velasco-Punzalan M, Pacleb M, Valdez R, Kretzschmar T, McNally KL, Ismail AM, Cruz PCS, Hamilton NRS, Hay FR (2019) Variation in seed longevity among diverse Indica rice varieties. Ann Bot (Lond) 124: 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min CW, Lee SH, Cheon YE, Han WY, Ko JM, Kang HW, Kim YC, Agrawal GK, Rakwal R, Gupta R, et al. (2017) In-depth proteomic analysis of Glycine max seeds during controlled deterioration treatment reveals a shift in seed metabolism. J Proteomics 169: 125–135 [DOI] [PubMed] [Google Scholar]
- Nagel M, Rosenhauer M, Willner E, Snowdon RJ, Friedt W, Börner A (2011) Seed longevity in oilseed rape (Brassica napus L.) genetic variation and QTL mapping. Plant Genet Resour 9: 260–263 [Google Scholar]
- Nagel M, Vogel H, Landjeva S, Buck-Sorlin G, Lohwasser U, Scholz U, Börner A (2009) Seed conservation in ex situ genebanks: Genetic studies on longevity in barley. Euphytica 170: 5–14 [Google Scholar]
- Rajjou L, Lovigny Y, Groot SP, Belghazi M, Job C, Job D (2008) Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol 148: 620–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuzeau C, Cavalie G (1997) Changes in RNA and protein metabolism associated with alterations in the germination efficiency of sunflower seeds. Ann Bot (Lond) 80: 131–137 [Google Scholar]
- Revilla P, Butrón A, Rodríguez VM, Malvar RA, Ordás A (2009) Identification of genes related to germination in aged maize seed by screening natural variability. J Exp Bot 60: 4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BE, Payne PI, Osborne DJ (1973) Protein synthesis and the viability of rye grains. Loss of activity of protein-synthesizing systems in vitro associated with a loss of viability. Biochem J 131: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwember AR, Bradford KJ (2010) Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. J Exp Bot 61: 4423–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Masaki S, Tanabata T, Yamada T, Hirasawa T, Kashiwagi M, Kanekatsu M (2013) RNA-binding proteins associated with desiccation during seed development in rice. Biotechnol Lett 35: 1945–1952 [DOI] [PubMed] [Google Scholar]
- Sano N, Ono H, Murata K, Yamada T, Hirasawa T, Kanekatsu M (2015) Accumulation of long-lived mRNAs associated with germination in embryos during seed development of rice. J Exp Bot 66: 4035–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Permana H, Kumada R, Shinozaki Y, Tanabata T, Yamada T, Hirasawa T, Kanekatsu M (2012) Proteomic analysis of embryonic proteins synthesized from long-lived mRNAs during germination of rice seeds. Plant Cell Physiol 53: 687–698 [DOI] [PubMed] [Google Scholar]
- Sano N, Rajjou L, North HM, Debeaujon I, Marion-Poll A, Seo M (2016) Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol 57: 660–674 [DOI] [PubMed] [Google Scholar]
- Sano N, Rajjou L, North HM (2020) Lost in translation: Physiological roles of stored mRNAs in seed germination. Plants 9: 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano N, Takebayashi Y, To A, Mhiri C, Rajjou L, Nakagami H, Kanekatsu M (2019) Shotgun proteomic analysis highlights the roles of long-lived mRNAs and de novo transcribed mRNAs in rice seeds upon imbibition. Plant Cell Physiol 60: 2584–2596 [DOI] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T (2006) The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SN, Maheshwari A, Sharma C, Shukla N (2018) Gene expression patterns regulating the seed metabolism in relation to deterioration/ageing of primed mung bean (Vigna radiata L.) seeds. Plant Physiol Biochem 124: 40–49 [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Oono Y, Kawahara Y, Gusev O, Maekawa M, Matsumoto T, Levinskikh M, Sychev V, Novikova N, Grigoriev A (2016) Gene expression of rice seeds surviving 13- and 20-month exposure to space environment. Life Sci Space Res (Amst) 11: 10–17 [DOI] [PubMed] [Google Scholar]
- Thompson S, Bryant JA, Brocklehurst PA (1987) Changes in levels and integrity of ribosomal RNA during seed maturation and germination in carrot (Daucus carota L.). J Exp Bot 38: 1343–1350 [Google Scholar]
- Wiebach J, Nagel M, Börner A, Altmann T, Riewe D (2020) Age dependent loss of seed viability is associated with increased lipid oxidation and hydrolysis. Plant Cell Environ 43: 303–314 [DOI] [PubMed] [Google Scholar]