Abstract
Aureobasidium pullulans is an extremotolerant, cosmopolitan yeast-like fungus that successfully colonises vastly different ecological niches. The species is widely used in biotechnology and successfully applied as a commercial biocontrol agent against postharvest diseases and fireblight. However, the exact mechanisms that are responsible for its antagonistic activity against diverse plant pathogens are not known at the molecular level. Thus, it is difficult to optimise and improve the biocontrol applications of this species. As a foundation for elucidating biocontrol mechanisms, we have de novo assembled a high-quality reference genome of a strongly antagonistic A. pullulans strain, performed dual RNA-seq experiments, and analysed proteins secreted during the interaction with the plant pathogen Fusarium oxysporum. Based on the genome annotation, potential biocontrol genes were predicted to encode secreted hydrolases or to be part of secondary metabolite clusters (e.g., NRPS-like, NRPS, T1PKS, terpene, and β-lactone clusters). Transcriptome and secretome analyses defined a subset of 79 A. pullulans genes (among the 10,925 annotated genes) that were transcriptionally upregulated or exclusively detected at the protein level during the competition with F. oxysporum. These potential biocontrol genes comprised predicted secreted hydrolases such as glycosylases, esterases, and proteases, as well as genes encoding enzymes, which are predicted to be involved in the synthesis of secondary metabolites. This study highlights the value of a sequential approach starting with genome mining and consecutive transcriptome and secretome analyses in order to identify a limited number of potential target genes for detailed, functional analyses.
Keywords: antagonism, Aureobasidium, biocontrol, Fusarium, genome, proteome, secretome, transcriptome, yeast
INTRODUCTION
Aureobasidium pullulans belongs to the Dothideales, not the classical yeast order Saccharomycetales, and exhibits both yeast-like and hyphal growth morphology [1]. Nevertheless, the species is often referred to as a yeast, black yeast, or yeast-like fungus and biocontrol species. Fungi of the genus Aureobasidium, and in particular A. pullulans, are frequently isolated from phyllosphere and soil samples worldwide. Their phenotypic plasticity and tolerance of harsh environmental conditions are likely the reason for the competitiveness of these ascomycetes in a wide range of ecological niches [1, 2]. The genomes of 50 A. pullulans strains from different environments and geographical regions revealed a homogenous population structure and limited genome variability. It has thus been concluded that A. pullulans is an efficiently spreading and recombining fungus [3].
Different strains of A. pullulans produce a wide array of well-characterized metabolites (e.g., pullulan, lanthipeptides, liamocin, aureobasidin), extracellular enzymes, siderophores and toxins [4–10]. Selected enzymes with proteinase, cellulase, lipase, glucanase, mannanase and chitinase activities, among others, have been characterized [9, 11] (and references therein). The diverse collection of metabolites and enzymatic functions could be an explanation for the ecological versatility and antagonistic activity of A. pullulans. The metabolites and enzymatic activities are also the underlying reason for significant biotechnological interest. Aureobasidium species are studied, for example, as sources of proteins for food supplements and enzymes and used for the production of pullulan [4, 12], a polysaccharide used as a food additive. The applications in agriculture and biotechnology also provoked and justified substantial interest in more fundamental molecular studies. Transformation protocols and CRISPR/Cas 9 approaches have been established for some strains [13]. Genome sequences of different Aureobasidium species have also been analysed and are available as a valuable basis for functional and applied studies. The genomes of four Aureobasidium species, A. melanogenum, A. namibiae, A. pullulans, and A. subglaciale, were analysed by the same pipeline used here, revealing interesting features, including numerous secondary metabolite biosynthesis clusters (i.e., 37 for A. melanogenum and A. subglaciale), an unexpectedly large number of aquaporin-like genes (11-12 genes in each species), and an enrichment of sugar transporters [14].
The species A. pullulans is also known as an effective biocontrol agent that is thought to employ a range of mechanisms to suppress the growth of other microorganisms [9]. These mechanisms include direct competition [15–17], the secretion of enzymes and secondary metabolites [4–8, 18, 19], the production of volatile compounds [20–25], as well as the induction of resistance in crop plants [26, 27]. Many Aureobasidium isolates show strong biocontrol activity against destructive plant diseases such as moulds, rots and blights [28–35]. The biocontrol products BlossomProtect™ and Botector® are based on two A. pullulans strains and registered to control fireblight and grey mould, respectively. However, with respect to biocontrol mechanisms, A. pullulans is surprisingly under-studied at the molecular level [9]. An alkaline serine protease from the A. pullulans strain PL5 was identified and biochemically characterised [18, 19]. The purified enzyme reduced the conidia germination rate and the germ tube length of different plant pathogens such as Alternaria alternata, Botrytis cinerea, Penicillium expansum, Monilinia fructicola and Monilinia laxa. However, the corresponding gene has not been deleted or overexpressed to further study its contribution to biocontrol activity. An alkaline protease was also identified in the marine A. pullulans strain HN2-3 [36]. Expressing its encoding gene in Yarrowia lipolytica conferred protease activity and the ability to generate bioactive peptides from different protein sources, but biocontrol activity has not been studied.
In the work presented here, we studied the A. pullulans isolate NBB 7.2.1, which was one of the strongest biocontrol organisms in an in vitro screen [37]. As a basis for functional studies, we de novo assembled a high-quality reference genome using long and short sequencing reads and used comparative genomics to evaluate the metabolic potential of this soil isolate of A. pullulans. We employed transcriptome and proteome analyses to identify potential biocontrol factors in confrontation assays with Fusarium oxysporum. The analyses focused on the putative secreted proteome, degrading enzymes, and on the production of secondary metabolites. Noteworthy was a large number of genes and gene clusters encoding secreted enzymes, biosynthetic proteins of antimicrobial compounds and peptides. Many genes were significantly upregulated by A. pullulans in the presence of F. oxysporum. A differential analysis of the secreted proteome also identified a subset of proteins that was upregulated or only detected during the interaction with F. oxysporum. This comprehensive study of the A. pullulans NBB 7.2.1 genome and the subsequent transcriptome and secretome analyses thus provided a subset of potential biocontrol genes. Detailed studies at the molecular level will determine the contribution of these genes to the biocontrol phenotype of A. pullulans NBB 7.2.1 and antagonistic yeasts in general.
RESULTS
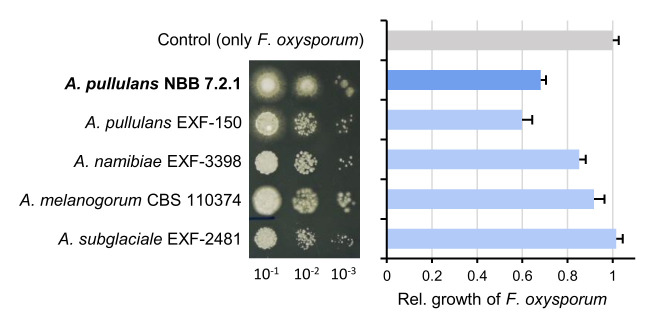
The species A. pullulans and the A. pullulans strain NBB 7.2.1 are strongly antagonistic against several plant pathogens and highly competitive against other yeasts on apple fruits [9, 37, 38]. Here, A. pullulans NBB 7.2.1 exhibited comparable growth and antagonistic activity as A. pullulans EXF-150, but inhibited F. oxysporum NRRL 26381/CL57 more strongly than A. melanogenum CBS 110374, A. namibiae CBS 147.97, and A. subglaciale EXF-2481 (Figure 1). These differences were revealed when using diluted Aureobasidium inocula (i.e., with low colony forming units). As a foundation for mechanistic studies and to elucidate biocontrol factors, we first created a high-quality reference genome and compared it to previously sequenced Aureobasidium species that are not widely described as biocontrol organisms. Subsequently, we performed transcriptome analyses and identified secreted proteins allowing to further prioritise the potential biocontrol genes.
Figure 1. FIGURE 1: Comparison of five Aureobasidium species with respect to growth on agar plates and antagonistic activity.
The different Aureobasidium species grow at comparable rates in spot assays, but show differences in antagonistic activity against the plant pathogenic fungus F. oxysporum NRRL 26381/CL57. Overnight cultures of the five strains were diluted to OD600=0.1 and three serial 1:10 dilutions were spotted on PDB agar (left). Competition assays were performed with highly diluted Aureobasidium samples (OD600 = 0.001) and the area of F. oxysporum growth was measured to reveal the differences in their antagonistic activity. The relative growth of F. oxysporum was reduced in competition with both A. pullulans strains (NBB 7.2.1 and EXF-150), while the other Aureobasidium species were less antagonistic.
Genome analysis of A. pullulans NBB 7.2.1
We sequenced the genome of A. pullulans NBB 7.2.1 using PacBio Sequel long read (mean read length 10,126 bp, 35x-207x coverage, 98.61% of reads mapped to final assembly) and Illumina short read sequencing technologies (2x300 bp, 29x coverage, 100% of reads mapped to final assembly) (Supplementary Table 1). The final A. pullulans NBB 7.2.1 genome assembly had a size of 28.41 Mbp and consisted of twelve contigs, presumably chromosomes, and a circular, complete mitogenome (Supplementary Table 2). Annotation of this contiguous, high quality genome by JGI predicted 10,925 genes (with an average gene and protein length of 1,569 bp and 479 aa, respectively; on average 2.6 introns/gene; https://mycocosm.jgi.doe.gov/AurpulNBB1/AurpulNBB1.info.html) (Supplementary Table 3).
Genome analysis and pan-genome clustering
In order to gain a better understanding of the peculiarities of the A. pullulans NBB 7.2.1 genome, a comparative analysis with four other Aureobasidium genomes that were previously annotated by the same pipeline [14] was performed. The genomes of A. melanogenum CBS 110374, A. namibiae CBS 147.97), A. pullulans EXF-150, and A. subglaciale EXF-2481 are of comparable size (26.20 Mbp, 25.43 Mbp, 29.62 Mbp, and 25.80 Mbp, respectively) and contain similar numbers of predicted genes (10,594, 10,266, 11,866, and 10,809, respectively) as A. pullulans NBB 7.2.1 (28.41 Mbp; 10,925 genes; https://mycocosm.jgi.doe.gov/aureobasidium). All five genome annotations comprised a comparable number of KEGG terms (Supplementary Table 3).
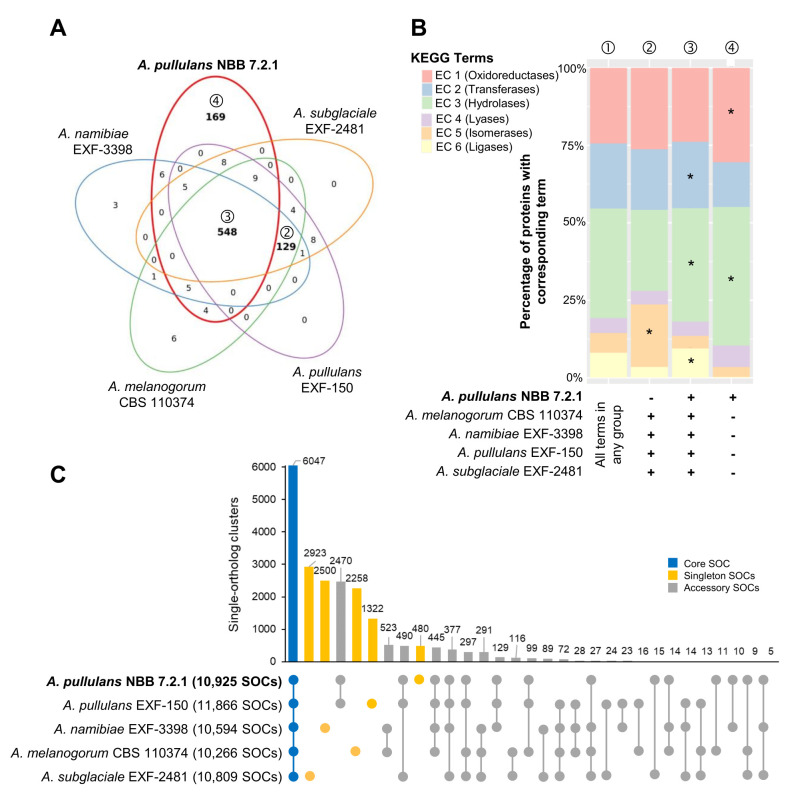
Most of the KEGG terms associated with the five Aureobasidium genomes were shared (548 terms), but a high number (169 terms) was either uniquely found for genes in the A. pullulans NBB 7.2.1 genome or specifically lacking for genes in this genome (129 terms; Figure 2A). Similar analyses for GO, IPR or KOG terms showed comparable results (Supplementary Figure S1). Among the genes with KEGG terms only lacking in A. pullulans NBB 7.2.1 (129 terms), isomerases (EC 5) were significantly enriched (adj. p-value < 0.001) as compared to their frequency among all KEGG terms identified for the five genomes. Among the terms only present in the A. pullulans NBB 7.2.1 genome (169 terms), putative hydrolases (EC 3; adj. p-value < 0.001) and oxidoreductases (EC 1; adj. p-value 0.012) were significantly enriched. Hydrolases (EC 3; adj. p-value < 0.001), in addition to transferases (EC 2; adj. p-value 0.012) and ligases (EC 6; adj. p-value < 0.001), were enriched among the terms shared in all five genomes (548 terms; Figure 2B).
Figure 2. FIGURE 2: Whole genome comparison of five Aureobasidium species.
Gene annotations reveal that the A. pullulans NBB 7.2.1 genome contains more unique KEGG terms than the genomes of four other Aureobasidium strains. (A) KEGG term distribution among annotated genes of five Aureobasidium species. 548 terms were commonly found in all species, but a substantial number of terms (169) was unique for the A. pullulans NBB 7.2.1 genome or specifically lacking in this genome (129 terms; all bold). (B) The relative number of genes annotated to one of the six main enzyme classes (EC 1-6; oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases) for all KEGG terms in the five Aureobasidium species and the subsets described in A. The enzyme classes highlighted with an asterisk (*) are significantly overrepresented (adj. p-value≤0.05) in the respective group (as compared to their frequency among all KEGG terms found in any of the five Aureobasidium genomes (①)). Results are shown for those genes with terms shared among all genomes except A. pullulans NBB 7.2.1 (②), shared among all five genomes (③), or only present in A. pullulans NBB 7.2.1 (④). (C) Pan-genome clustering of five Aureobasidium genomes visualized using an Up Set bar diagram [96]. The A. pullulans NBB 7.2.1 genome contained fewer (480) unique gene models than the four other Aureobasidium genomes.
The Pangloss pan-genome was established using the published workflow and reflects a single-ortholog clustering (SOC), where each cluster contains at most one gene from each species [39, 40]. Comparison to Markov gene families highlights which SOCs are related by multi-copy paralogy, or might be similar to other SOCs, and which pairwise SOCs and singletons truly contain unique sequence. 6,047 SOCs were shared by all five Aureobasidium genomes (out of 10,266-11,866 gene models for the five strains), whereas 15,090 accessory SOCs were only present in some of the five strains (5,603 partial clusters present in two to four strains and 9,483 gene models unique to one of the five strains; Figure 2C). The A. pullulans NBB 7.2.1 genome contained only 480 unique SOCs, as compared to 2,923, 2,500, 2,258, and 1,322 SOCs for A. subglaciale EXF-2481, A. melanogenum CBS 110374, A. namibiae CBS 147.97, and A. pullulans EXF-150, respectively. For A. pullulans NBB 7.2.1, the subset of true singleton SOCs (those without paralogs) was 116, most of which did not have a functional annotation. The strongest pairwise clustering was observed between the A. pullulans NBB 7.2.1 and A. pullulans EXF-150 genomes, which had 2,470 SOCs in common (by far the closest pairwise similarity among any of the five genomes). Of these, 535 SOCs represented pairs of single-copy orthologs, while an additional 55 were found in multi-copy gene families with only A. pullulans NBB 7.2.1 and A. pullulans EXF-150 genes.
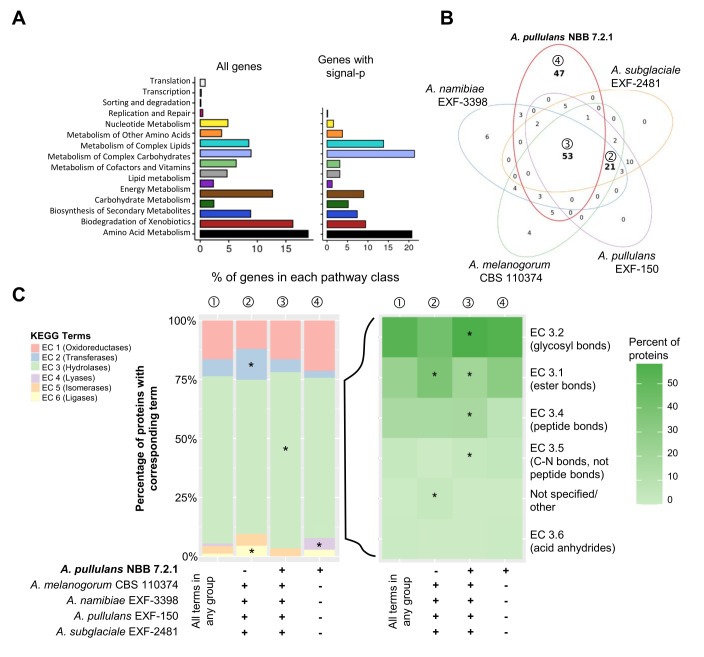
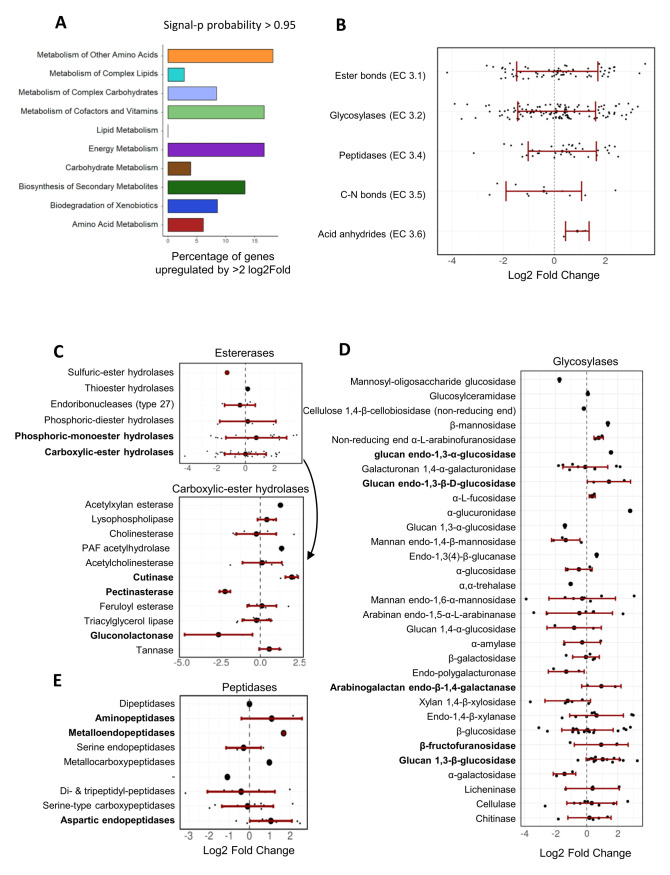
Overall, almost 50% of the A. pullulans NBB 7.2.1 genes with KEGG terms were annotated to function in the metabolism of amino acids, carbohydrates, complex carbohydrates, and complex lipids (Figure 3A). These broad categories were the most abundant in the genome overall, but also among the subset of genes with a predicted signal peptide. Many of these potentially secreted gene products encoded hydrolases. In all five Aureobasidium genomes compared here, they were significantly overrepresented in contrast to hydrolases without a predicted signal peptide. As secreted hydrolases have also been implicated in biocontrol activity, they are discussed in more detail below.
Figure 3. FIGURE 3: Genome mining of Aureobasidium genomes to identify potential biocontrol genes.
Functional annotations of the five Aureobasidium genomes (obtained from the DOE-JGI MycoCosm) identified a plethora of genes and gene clusters that may contribute to biocontrol activity. (A) Relative percentage of A. pullulans NBB 7.2.1 genes (for the 4019 genes with a KEGG annotation or only the subset containing a predicted signal peptide) assigned to the different KEGG pathways. (B) KEGG term distribution among the five Aureobasidium genomes for all annotated genes containing a predicted signal peptide. 53 terms were commonly found in all species (③), while 47 terms were unique for the A. pullulans NBB 7.2.1 genome (④) and 21 were exclusively found in the other genomes (②) (bold numbers in Venn diagram). (C) The relative percentage of genes with a predicted signal peptide annotated to belong to one of the six main enzyme classes (EC 1-6; oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases). Results are shown for genes with terms that were found in any of the five genomes (①) and for those shared in all genomes except A. pullulans NBB 7.2.1 (②), shared among all five genomes (③), or only present in A. pullulans NBB 7.2.1 (④). The enzyme classes highlighted with an asterisk were significantly overrepresented (adj. p-value≤0.05) in the respective group. Among the predicted hydrolase genes with a signal peptide, esterases (EC 3.1), glycosylases (EC 3.2), and peptidases (EC 3.4) were by far the most frequent and significantly overrepresented in genes with terms shared by all five Aureobasidium genomes.
Genes involved in secondary metabolite production
Secondary metabolites are compounds with low molecular weight that are not essential for growth, development and reproduction. They have diverse chemical compositions and biological functions. Many of them exhibit a role in fungal antagonism [8]. We used KEGG annotations and antiSMASH predictions, which rely on different principles to identify genes involved in secondary metabolite production. The KEGG annotation of the A. pullulans NBB 7.2.1 genome predicted 85 genes (2.1% of all genes with a KEGG annotation) to encode proteins involved in the synthesis of secondary metabolites. Of these 85 genes, 33 were predicted to contain a secretion signal (Figure 3A). Additionally, 25 distinct secondary metabolite clusters, comprising 27 biosynthetic genes, were found using the fungal antiSMASH v.5.1.2 online tool [41, 42] (Supplementary Tables 4 and 5). Only three core biosynthetic genes were also predicted by the KEGG annotation. The other four Aureobasidium genomes (A. melanogenum CBS 110374, A. namibiae CBS 147.97, A. pullulans EXF-150, and A. subglaciale EXF-2481) contained 19, 18, 25, and 25 such clusters, respectively. We noted that some of the biosynthesis enzymes identified in this search had no predicted function in the original annotation. The antiSMASH analysis predicted ten non-ribosomal peptide-like synthase clusters (NRPS-like), five polyketide synthase (PKS) clusters, four terpene clusters, three non-ribosomal peptide synthase clusters (NRPS) and one β-lactone cluster (Supplementary Table 5). Additionally, two clusters containing more than one core biosynthetic gene were detected: one with a NRPS and a polyketide synthase and one with a NRPS and a β-lactone synthase. Out of the products from all clusters identified, two had similarity with described biosynthetic enzymes: the polyketide synthase of the A. alternata ACRL toxin cluster, implicated in host-selective pathogenicity, showed 58% amino acid identity with a type 1 PKS in our genome (Protein ID 78529). This cluster is also present in A. pullulans (EXF-150) and in A. subglaciale (EXF-2481) [43]. Similarly, a melanin synthase from Bipolaris oryzae showed 67% amino acid identity with a different type 1 PKS (Protein ID 58443) [44] present in all five strains. The biosynthetic products of the other clusters could not be predicted based on the sequence comparisons.
Genes with a predicted signal peptide
Proteins with a predicted signal peptide likely represent secreted proteins. These are of particular interest, because they may directly mediate antagonistic activity. Among the 10,925 predicted A. pullulans NBB 7.2.1 gene models, 1,044 had a predicted signal peptide and may thus encode secreted proteins (SigP probability > 0.95 according to Mycocosm annotation; Supplementary Table 6). Across the five Aureobasidium genomes, and for the genes comprising a predicted signal peptide, 53 KEGG terms were common to all five genomes, while 47 and 21 were only present in A. pullulans NBB 7.2.1 or specifically lacking in this strain's genome, respectively (Figure 3B). The majority (248) of the 330 proteins with a predicted signal peptide and a KEGG term in A. pullulans NBB 7.2.1 were annotated as hydrolases (EC 3; >70% of all genes). Oxidoreductase (EC 1), transferase (EC 2), lyase (EC 4), isomerase (EC 5), and ligase (EC 6) genes were less frequent. Interestingly, transferase and ligase genes were significantly overrepresented among terms that were lacking in the A. pullulans NBB 7.2.1 genome (adj. p-values 0.006 and 0.002, respectively), while lyase genes were overrepresented among the 47 genes with unique KEGG terms in the A. pullulans NBB 7.2.1 genome (adj. p-value 0.014; Figure 3C). The large group of the predicted hydrolase genes with a signal peptide were significantly enriched among those shared by all five genomes (adj. p-value < 0.001). Enzymes predicted to act on ester (EC 3.1; adj. p-value < 0.001), N-glycosidic (EC 3.2; adj. p-value < 0.001), and peptide bonds (EC 3.4; adj. p-value < 0.001) were by far the most frequent and also significantly overrepresented in genes with terms shared by all genomes (Figure 3C). None of the hydrolase subclasses was uniquely enriched in genes specific for the A. pullulans NBB 7.2.1 genome, but those hydrolases predicted to act on ester bonds (EC 3.1 adj. p-value < 0.001) and unspecified hydrolases were significantly enriched in genes only found in the four other Aureobasidium genomes (adj. p-value < 0.001).
Overall, the genome of A. pullulans NBB 7.2.1 and of all five Aureobasidium species included here are comparable in size and gene model count and harbour a number of genes and gene clusters that may contribute to biocontrol activity. For example, hydrolase genes with a predicted signal peptide, and in particular esterases, glycosylases, and peptidases, were strongly overrepresented. All of these Aureobasidium species thus likely secrete a plethora of hydrolytic enzymes into their surroundings, which might represent one of the mechanisms conferring antagonistic activity to these species. Based on our genome analysis, A. pullulans NBB 7.2.1 is predicted to secrete 248 hydrolytic enzymes (including 39 genes encoding predicted secreted proteases) and to produce yet unidentified secondary metabolites as indicated by the 25 secondary metabolite clusters that were identified and which comprise 366 genes overall.
Transcriptome analysis of A. pullulans NBB 7.2.1 competing with F. oxysporum NRRL 26381/CL57
In order to identify genes that may be responsible for the biocontrol activity detected in A. pullulans NBB 7.2.1, the yeast-like fungus was added to a F. oxysporum NRRL 26381/CL57 liquid culture that had been growing for two days and the transcriptomes were analysed by dual RNA-seq [45]. The addition of A. pullulans strongly inhibited the growth of F. oxysproum, as observed on agar plates. Gene expression was quantified by mapping sequence reads to the high-quality A. pullulans NBB 7.2.1 reference genome and the published F. oxysporum NRRL 26381/CL57 genome [46]. Since it was the aim of this study to identify genes potentially conferring biocontrol activity, we focused this analysis on A. pullulans NBB 7.2.1 genes that were upregulated following the encounter with the plant pathogen F. oxysporum.
Overall results
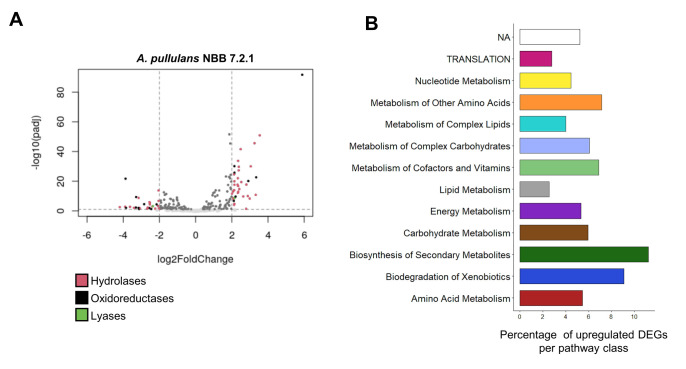
Nearly 25 million raw reads per sample were trimmed and filtered, which resulted in over 93% high quality reads per sample, that were included for further analyses (Supplementary Table 7). More than 96.9% of all reads had a base call accuracy of at least 99% and the respective replicates clustered together in a PCA analysis of the log transformed counts. Between 91 and 93% of the reads in the co-culture treatment were mapped to the genome of F. oxysporum NRRL 2638/CL57, while 7-9% corresponded to expressed A. pullulans NBB 7.2.1 genes. This can be explained by the larger amount of biomass filamentous fungi produce as compared to yeasts and the two-day preculture of F. oxy-sporum in the liquid medium. After controlling for quality (p-value <0.05), 10,842 genes from A. pullulans and 17,466 from F. oxysporum were included in the respective differential expression analyses. Even though fewer reads were mapped to the A. pullulans NBB 7.2.1 genome, this yeast-like fungus showed a strong transcriptional response to the presence of F. oxysporum NRRL 26381/CL57. Overall, 1,337 A. pullulans NBB 7.2.1 genes (12% of all genes) were differentially expressed (log2FoldChange>2, p<0.05; 618 and 719 up- and downregulated genes, respectively) as a response to the interaction with F. oxysporum NRRL 26381/CL57 (Supplementary Figure S2A). Among the 1,044 genes containing a predicted signal peptide, 178 (17%) showed significantly changed expression (92 and 86 up- and downregulated genes, respectively), significantly more than in the genes without a predicted signal peptide (p<0.01; Figure 4A). The A. pullulans NBB 7.2.1 genes most strongly upregulated in the presence of F. oxysporum NRRL 26381/CL57 comprised a large number of uncharacterised genes (annotated as hypothetical genes; i.e., 33 of the 50 most upregulated genes). Among the genes with an annotation, the KEGG categories “biosynthesis of secondary metabolites” (11.2%), “biodegradation of xenobiotics” (9.1%), “metabolism of other amino acids” (7.1 %), and “metabolism of cofactors and vitamins” (6.9 %) comprised the highest frequency of up-regulated genes (Figure 4B). Despite the large number of sequencing reads, we detected only a weak response of F. oxysporum NRRL 26381/CL57 to the competition with A. pullulans NBB 7.2.1 (Figure 2B and 2C). Only 80 genes were differentially expressed: 36 were upregulated and 44 downregulated. Few of these 80 DEGs (differently expressed genes) have a functional description in the F. oxysporum NRRL 26381/CL57 genome annotation as 53 genes were annotated to encode hypothetical proteins. Upregulated F. oxysporum NRRL 26381/CL57 DEGs mainly belonged to the KEGG categories “metabolism of complex lipids”, “lipid metabolism” and “carbohydrate metabolism” (Supplementary Figure 2D). Only two upregulated DEGs, one of which was a putative α-galactosidase, had a predicted signal peptide. An aldehyde dehydrogenase (oxidoreductase), two α-galactosidases, a p-aminomuconate deaminase (hydrolase), and a lactoylglutathione lyase were upregulated as well. Four additional, weakly upregulated genes, a β-N-acetylhexosaminidase (3.4 fold-change (FC)), a chitin deacetylase (2.1 FC), a monophenol monooxygenase (1.5 FC) and an alkaline endopeptidase (Oryzin; 1.3 FC), contained a signal peptide and thus likely represent secreted proteins.
Figure 4. FIGURE 4: Transcriptome analysis of A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57 in pure culture and during competition with each other.
A. pullulans NBB 7.2.1 strongly responds to co-cultivation with F. oxysporum NRRL 26381/CL57 at the transcriptome level. (A) Volcano plot showing expression of the A. pullulans NBB 7.2.1 genes with a signal peptide. In the co-culture, 178 genes exhibited significantly changed expression by at least a factor of four (92 and 86 up- and downregulated genes, respectively) compared to pure culture. (B) Proportion of upregulated DEGs (log2FoldChange > 2) for genes annotated to different KEGG categories. Biosynthesis of secondary metabolites (11.2%), biodegradation of xenobiotics (9.1%), metabolism of other amino acids (7.1 %), and metabolism of cofactors and vitamins (6.9 %) comprised the categories with the highest frequency of upregulated genes.
Genes involved in secondary metabolite production
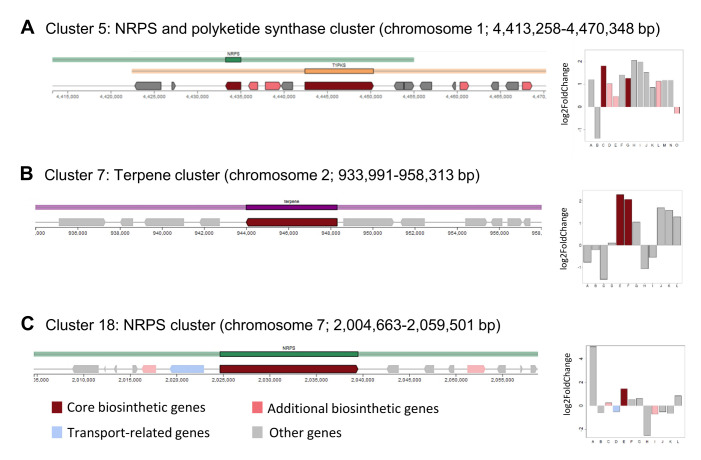
Overall, more than 10% of the genes in the KEGG category “biosynthesis of secondary metabolites” were upregulated (Figure 4B). These 15 DEGs belonged to the flavonoid (8), alkaloid type I (3) and erythromycin biosynthesis (3) pathways. Biosynthetic enzymes from three of the antiSMASH clusters identified in the A. pullulans NBB 7.2.1 genome (cluster 5, 7, and 18; see above) showed increased expression during the competition with F. oxysporum NRRL 26381/CL57. Two gene products from the NRPS (Protein ID 34549) and polyketide synthase (Protein ID 78529) cluster 5 were upregulated with a log2 FC of 1.79 and 1.25, respectively (Figure 5A). The putative core biosynthetic gene in the terpene cluster 7 was predicted as two different genes in our independent genome annotation and subsequent transcriptome analysis (Protein ID 37339 and 39349; Figure 5B). Both of these genes were strongly upregulated (log 2 FC of 2.29 and 2.07). Four other genes in the same cluster were upregulated with a log2 FC above 1. In cluster 18, a NRPS gene (Protein ID 49318) was upregulated by a log2 FC of 1.4 (Figure 5C).
Figure 5. FIGURE 5: Identification of secondary metabolite clusters in the A. pullulans NBB 7.2.1 genome with the fungal antiSMASH v.5.1.2 online tool [41, 42].
Core biosynthetic genes of three A. pullulans NBB 7.2.1 secondary metabolite clusters were upregulated in response to co-cultuvation with F. oxysporum NRRL 26381/CL57. The core biosynthetic genes (dark red colour) of cluster 5 (NRPS and polyketide synthase cluster) (A), 7 (terpene cluster) (B), and 18 (NRPS cluster) (C) were upregulated after one day of interaction with F. oxysporum NRRL 26381/CL57.
Genes with a predicted signal peptide
For the genes with a signal peptide sequence, “metabolism of other amino acids”, “metabolism of cofactors and vitamins”, and “energy metabolism” were the KEGG pathways comprising the highest proportion of upregulated genes (approx. 15% of the genes in each of these three categories; Figure 6A). On average, hydrolase genes were not significantly up- or down-regulated, but in particular those encoding predicted esterases (EC 3.1), glycosylases (EC 3.2) and peptidases (EC 3.4) comprised numerous genes that were strongly upregulated in the presence of F. oxysporum NRRL 26381/CL57 (Figure 6B). All three hydrolase genes predicted to encode enzymes acting on acid anhydrides were up-regulated. Within the esterases, some of the genes annotated to encode phosphoric-monoester hydrolases were the most strongly upregulated, while predicted carboxylic-ester hydrolases (in particular pectinesterases and gluconolactonases) comprised the most strongly downregulated genes (Figure 6C). In contrast, all three genes predicted to encode cutinases (also belonging to the carboxylic-ester hydrolases), were strongly upregulated in the competition with F. oxysporum NRRL 26381/CL57. Among the predicted glycosylase genes, those encoding β-fructofuranidases, glucan endo-1,3-β-D-glucosidases, glucan 1,3-β-glucosidases, glucan endo-1,3-α-glucosidases, and arabinogalactan endo-β-1,4-galactanases were mostly up-regulated, but the majority of these categories only comprised few genes (Figure 6D). The peptidases comprised three categories that showed at least a two-fold upregulation on average; namely the metalloendopeptidases, aspartic endopeptidases, and aminopeptidases (Figure 6E). Finally, lyases with a predicted signal peptide (ten genes) were enriched in the A. pullulans NBB 7.2.1 genome (see Figure 3C). Three of these lyase genes were upregulated, while two were downregulated (Supplementary Figure 2E). The three upreglated genes comprised a carbonic anhydrase, upregulated by over 4-fold, and two pectate lyases that were upregulated by more than 2.5-fold. However, a third pectate lyase gene was strongly downregulated.
Figure 6. FIGURE 6: Transcriptional regulation of A. pullulans NBB 7.2.1 genes with a predicted signal peptide upon co-cultivation with F. oxysporum NRRL 26381/CL57.
Many A. pullulans NBB 7.2.1 hydrolase genes were strongly up- or downregulated during the interaction with F. oxysporum NRRL 26381/CL57. (A) Percentage of upregulated DEGs in each KEGG pathway class for those A. pullulans NBB 7.2.1 genes containing a predicted signal peptide. (B) Variation of the Log2 FC values within each hydrolase enzyme category. Variation of the Log2 FC values of all A. pullulans NBB 7.2.1 DEGs is also shown on a lower level of categorisation for hydrolases acting on ester bonds (C), glycosylases (D), and peptidases (E).
Overall, this dual RNA-seq experiment confirmed the transcription and upregulation of 67 of the 1129 potential A. pullulans NBB 7.2.1 biocontrol genes (i.e., secreted hydrolase genes and genes comprised in secondary metabolite synthesis) during the competition with F. oxysporum NRRL 26381/CL57. The functional category “biosynthesis of secondary metabolites” comprised the highest frequency of upregulated genes and specific groups of putatively secreted hydrolases, such as acid anhydrases, phosphoric-monoester hydrolases, cutinases, specific glucosidases and aminopeptidases and endopeptidases, comprised strongly upregulated genes. We thus hypothesise that these genes contribute to the complex biocontrol phenotype of A. pullulans NBB 7.2.1.
A. pullulans NBB 7.2.1 secretome analysis during competition with F. oxysporum NRRL 26381/CL57
To complement our dual RNA-seq approach and to directly analyse secreted proteins that may mediate biocontrol activity, we collected and enriched proteins secreted by A. pullulans NBB 7.2.1, by F. oxysporum NRRL 26381/CL57, and during the interaction of both fungi under the same experimental conditions used for the transcriptome analysis. Proteins were identified by searching tandem mass spectrometry data against the protein databases based on the reference genomes of A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57, respectively. Overall, and as expected, our differential secretome analysis of A. pullulans NBB 7.2.1 culture supernatants did not correlate well with the transcriptome results. This effect was expected as it had been similarly reported in other systems [47–52]. In general, protein and transcriptome levels do not seem to be highly correlated [53], which is likely due to the additional levels of regulation that determine protein levels (i.e., translation, post-translational modification, formation of complexes, degradation). Importantly, the analytical methods used to quantify transcript and protein levels have vastly different sensitivities and result in different coverage, thus complicating the comparison of the two. Transcriptome and secretome analyses are thus complimentary approaches.
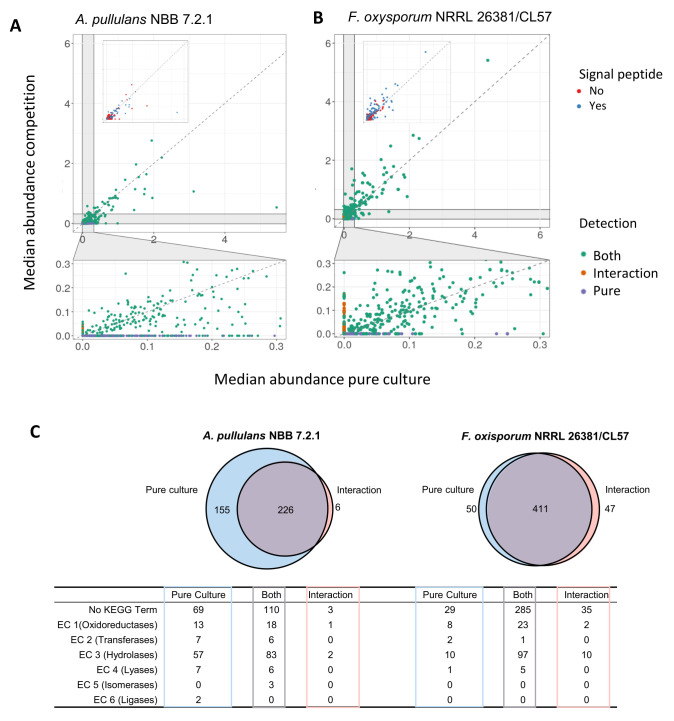
Few A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57 proteins were highly abundant, while the vast majority was detected in comparable amounts and at relatively low abundance (Figures 7A and 7B). The most abundant proteins in the A. pullulans NBB 7.2.1 pure culture and during the competition largely overlapped: 50 proteins were abundant in both treatments, 24 only in the pure culture, and eleven only in the interaction experiment. This was also true for F. oxysporum NRRL 26381/CL57 proteins (110 proteins were abundant in both treatments and 36 and 21 only in the interaction or the pure culture, respectively).
Figure 7. FIGURE 7: Secretome analysis of A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57 pure cultures and a co-culture.
Supernatant from pure and co-cultures of A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57 were filtered with a 0.2 µm membrane and concentrated with > 50 kDa ultrafiltration tubes. These extracts of secreted proteins were analysed with a proteomics pipeline. Median abundance of all proteins detected in pure culture (x-axis) and during the competition (y-axis) for A. pullulans NBB 7.2.1 (A) and F. oxysporum NRRL 26381/CL57 (B). In the upper panel insert, proteins with or without a predicted signal peptide are highlighted in blue and orange color, respectively. The lower panel shows proteins of low abundance and highlights those only detected during the competition (orange) or in pure culture (blue). (C) For A. pullulans NBB 7.2.1, only few proteins were uniquely found during the competition with F. oxysporum NRRL 26381/CL57, while for F. oxysporum NRRL 26381/CL57 almost equal numbers of unique proteins were detected in the pure culture and the competition. KEGG term annotation of the proteins in the different categories is indicated.
Since most proteins had a low abundance and their expression varied among the four replicates, we filtered out proteins detected only in one sample (i.e., we only analysed further those proteins with a median abundance higher than 0). For A. pullulans NBB 7.2.1, this identified a set of 381 and 232 proteins in the pure culture and the interaction, respectively (Figure 7C). Of these proteins, 226 were identified under both conditions, 155 were only detected in the pure culture, while six proteins were found only in the interaction experiment. Among the 381 proteins detected in the supernatant of the pure A. pullulans NBB 7.2.1 culture and in comparison to those proteins not detected in the secreted proteome, sequences containing a predicted signal peptide were significantly overrepresented (p-value<0.001, 45% of all proteins in the secreted proteome, 171 proteins). The 381 proteins were also significantly enriched in hydrolases (EC 3, adj. p-value<0.001, 140 proteins), and lyases (EC 4, adj. p-value<0.008, 13 proteins). Similarly, the 232 A. pullulans NBB 7.2.1 proteins detected during the interaction with F. oxysporum NRRL 26381/CL57 were significantly enriched in proteins with a predicted signal peptide (p-value<0.001, 58% or 134 proteins) and in predicted hydrolases (EC 3, adj. p-value<0.001, 85 proteins). The six proteins detected in the interaction alone comprised two hydrolases (a mannan endo-1,4-β-mannosidase (EC 3.2.1.78) and a thiolester hydrolase (EC 3.1.2)), as well as an oxidoreductase (alcohol dehydrogenase, EC 1.1.1.1), and three proteins without a KEGG annotation. Interestingly, this alcohol dehydrogenase, a predicted GroES-like chaperonin, was upregulated by 1.74 log2 FC in the transcriptome. To detect proteins with a higher abundance in the interaction, we used the set of 226 proteins present in both treatments and calculated the FC of the median. Overall, we found 32 A. pullulans NBB 7.2.1 proteins with a FC higher than 1.5 (Supplementary Table 8). These 32 proteins comprised twelve hydrolases (EC 3), a oxidoreductase (superoxide dismutase, EC 1.15.1.1), and 19 proteins lacking a KEGG annotation. More specifically, the hydrolases comprised two esterases (feruloyl esterase, EC 3.1.1.73; serine/threonine-specific protein phosphatase, EC 3.1.3.16), five glycosylases (α-manno-sidase, EC 3.2.1.24; β-galactosidase, EC 3.2.1.23; α-gluco-sidase, EC 3.2.1.20; and two β-glucosidases, EC 3.2.1.21), two proteases (glutamate carboxypeptidase II, EC 3.4.17.21; aminopeptidase I, EC 3.4.11.22) and three amidases (β-ureidopropionase EC 3.5.1.6; formamidase EC 3.5.1.49; dihydroorotase EC 3.5.2.3).
In the F. oxysporum NRRL 26381/CL57 secretome, 461 and 458 proteins were detected in the pure culture and the interaction, respectively (50 only in the pure culture, 411 under both conditions, and 47 only during the interaction). The latter group was composed of 41 proteins with no KEGG annotation and six hydrolases. These hydrolases included four glycosylases, an α-L-arabinofuranosidase (EC 3.2.1.55), a β-glucosidase (EC 3.2.1.21), a cellulase (EC 3.2.1.4) and a β-fructofuranosidase (EC 3.2.1.26) and two peptidases; deuterolysin (EC 3.4.24.39) and a metallocarboxypeptidase (EC 3.4.17). The interaction with A. pullulans NBB 7.2.1 resulted in the upregulation of 38 genes. Two of these were choline dehydrogenase oxidoreductases (EC 1.1.99.1), one a xyloglucan:xyloglucosyl transferase (EC 2.4.1.207), 16 hydrolases and 18 proteins without a KEGG annotation.
Overall, this secretome analysis identified almost 900 secreted A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381/CL57 proteins. 38 A. pullulans NBB 7.2.1 proteins (32 upregulated and six only detected during the interaction), were identified as interesting targets for further analyses. Among these 38 proteins, predicted hydrolases were enriched within the A. pullulans NBB 7.2.1 secretome. Four of these A. pullulans NBB 7.2.1 hydrolases were only found during the interaction with F. oxysporum NRRL 26381/CL57 or strongly upregulated under this condition, while another eleven had a higher abundance during the interaction, marking these proteins as interesting targets for functional analyses.
DISCUSSION
The species A. pullulans is highly abundant worldwide and thrives in vastly different types of temperate and extreme environments, which may also be the underlying reason for its success as a biocontrol organism. The study presented here reports a reference genome, transcriptome datasets, and a secretome analysis of the A. pullulans strain NBB 7.2.1, which strongly inhibits the growth of filamentous fungi. This reference biocontrol strain was compared with four other Aureobasidium strains that were studied with respect to their biotechnological potential, stress tolerance, and speciation. Considering the vast number of Aureobasidium strains that are reported to strongly inhibit plant pathogenic fungi, it is not surprising that the five strains compared here all exhibited strong antagonistic activity against F. oxysporum in vitro (Figure 1). Only when diluted, the reproducible and slightly stronger inhibitory activity of A. pullulans EXF-150 and NBB 7.2.1, in comparison to the other three strains (A. melanogenum CBS 110374, A. namibiae CBS 147.97, and A. subglaciale EXF-2481), which are not reported as biocontrol organisms, could be detected. This difference in antagonistic activity was not due to differences in growth or cell density, but growth morphology did differ and may explain the weaker antagonism exhibited by A. subglaciale EXF-2481. The closer similarity of the two A. pullulans isolates was also highlighted by the pangenome analysis, but less apparent based on the functional annotations. This might have been caused by differing KEGG database versions (even though the number of functionally annotated genes was comparable for all five genomes) and the fact that the majority of predicted genes do not have a functional annotation. Overall, these results confirmed the strong biocontrol activity of A. pullulans and were the motivation to identify candidate biocontrol genes that confer this activity.
Secondary metabolites and secreted proteins have biotechnological potential and were proposed to be involved in plant-protection and antagonistic effects of A. pullulans against plant pathogens [8]. As in other Aureobasidium isolates, a large number of secondary metabolite clusters were identified in strain NBB 7.2.1 by the fungal antiSMASH tool. This resource integrates a wealth of different tools for the identification of secondary metabolites and searches a database containing all known secondary metabolite clusters by aligning genome regions [41, 42]. Since antiSMASH searches a database specifically designed for the identification of secondary metabolite clusters, and does not rely on general gene annotation databases (e.g., COG, KOG, KEGG, Pfam, Interpro), this tool identified many more genes potentially involved in the production of secondary metabolites. Interestingly, 15 clusters containing NRPS or NRPS-like synthases were predicted for this isolate, while the genomes of A. melanogenum and A. namibiae encode only seven and nine such clusters, respectively (Supplementary Table 4). NRPS and NRPS-like synthases are large multi-domain enzymes that assemble peptides without mRNAs or ribosomes. In bacteria, these genes often reside in repeats. Comparison of a complete de novo assembled Pseudomonas aeruginosa MPAO1 genome with ten widely used PAO1 lab strains revealed that 50% or more of the NRPS genes could be missed in their fragmented Illumina short read-based assemblies [54]. Though structurally and functionally diverse, many peptides synthesised by NRPS have been described as secreted antimicrobial agents and toxins [55]. Melanin is known to have a protective role for fungi under various stress conditions and is well described as a product of A. pullulans [2]. Neither the melanin PKS cluster, nor other genes predicted to be involved in melanin biosynthesis showed significant expression changes. Thus, the production of melanin does not seem to play a role in the biocontrol activity of A. pullulans, but rather an adaptation to a wide variety of environments. Based on our analyses, we conclude that the A. pullulans NBB 7.2.1 genome harbours diverse secondary metabolite biosynthesis genes and gene clusters that are yet uncharacterised. The strong upregulation of some of these genes during competition with F. oxysporum, in particular of several genes of the terpene cluster, may suggest a role in biocontrol activity, but more detailed molecular studies (e.g., overexpression and deletion, biochemical analyses) are required to determine the exact functions of these genes.
Hydrolases have been repeatedly implicated in yeast biocontrol activity. The best characterised examples are exoglucanases from Candida oleophila that have been studied at the molecular level [56–58]. In the A. pullulans strain PL5, an alkaline serine protease was described and characterised [18, 19]. The corresponding gene in our A. pullulans NBB 7.2.1 genome was slightly and strongly downregulated at the transcriptional and protein level, respectively. However, the comprehensive analysis presented here revealed insights into the large diversity of hydrolytic enzymes A. pullulans can secrete into the environment, including proteinases, glycosylases and esterases. This medley underlines the potential of the genus Aureobasidium for metabolite and enzyme discovery for biotechnological applications. In the A. pullulans NBB 7.2.1 genome, we observed a genomic over-representation of hydrolases potentially involved in the degradation of fungal cell walls and plant products (e.g., glycosidases, proteases, pectate and pectin lyases, as well as cellulases, xylanases, cutinases, and fructofuranosidases). Some of these enzymes were transcriptionally upregulated upon exposure of A. pullulans NBB 7.2.1 to the plant pathogen F. oxy-sporum. Among the enzymes targeting ester bonds, we found a strong upregulation of predicted cutinases. Other plant-degrading enzyme groups, such as cellulases, polygalacturonases or pectinesterases, were downregulated or their transcript levels remained unaffected. Though the upregulation of potentially plant degrading enzymes was unexpected, we believe this result substantiates the epiphytic ecology of the A. pullulans NBB 7.2.1 isolate, as compared to other isolates from glacial and desert habitats, and might be related to the ecological complexity of the plant microenvironment. It should also be emphasised that none of these enzymes have been characterised biochemically. A gene in A. pullulans NBB 7.2.1 may show sequence similarity to plant cuticle targeting enzymes, but the natural target of these enzymes is unknown and could also be a microbial component.
Enzymes targeting peptide bonds are implicated in many microbial interactions and are often identified as virulence factors [59–62]. In A. pullulans, proteinases have already been identified, but their role in biocontrol activity is not clear. Aminopeptidases can also play roles in co- or post-translational modifications, removing residues from the N-terminus, and producing proteoforms that may affect protein activity. Furthermore, they can degrade small peptides or target specific proteins in order to generate active peptides or inactivate inhibitory proteins [63–66]. The genome and transcriptome analysis of A. pullulans NBB 7.2.1 identified specific classes of peptidases that were overrepresented or whose transcripts where up-regulated in A. pullulans cells exposed to the filamentous fungus F. oxysporum. Aspartic peptidases were enriched in the genomes of A. pullulans EXF-150 and NBB 7.2.1 strains. Our transcriptome analysis revealed an increase in the transcription of six aspartic endopeptidases, which use a water molecule activated by two aspartic residues to break the scissile bond. Four enzymes were defined as Aspergillopepsins by our KEGG analyses, while the others were similar to a Candidapepsin and a Yapsin. Aspergillopepsins are known secreted proteinases from the genus Aspergillus and have nutritional relevance in acidic environments and structural targets in a variety of organisms. The metallocarboxypeptidase “Peptidase M28” (ID 49010) was upregulated in the proteome (Supplementary Table 8). Overall, these results identified a plethora of proteinases that are secreted by A. pullulans NBB 7.2.1 under different conditions. The expanded number of serine-dependent protease genes and transcriptional upregulation of some proteinases during competition with F. oxysporum may indicate that proteinases are, at least in part, responsible for the strong biocontrol activity and competitiveness of this yeast-like fungus. The identified protease genes are thus a promising starting point for deciphering biocontrol activity of A. pullulans NBB 7.2.1 at the molecular level.
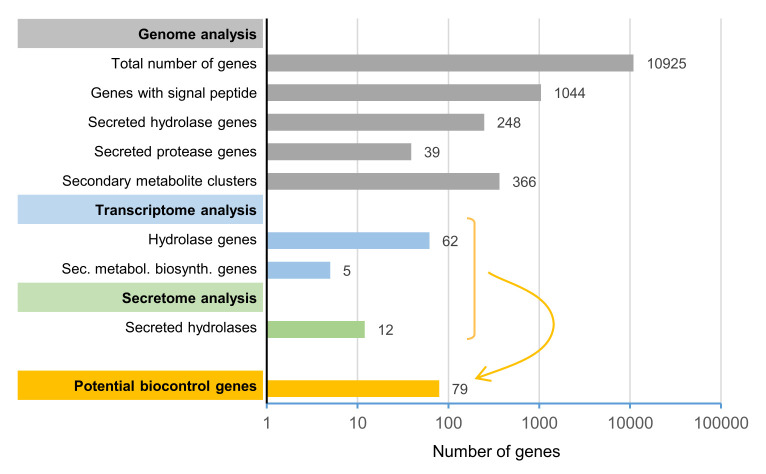
The current study aimed to identify a limited subset of potential biocontrol factors of the yeast-like fungus A. pullulans against F. oxysporum by genome, transcriptome and secretome analyses. Based on our high-quality reference genome, this strain is predicted to produce a wide variety of peptidases, glycosylases and esterases and to have the potential to produce a remarkably diverse range of secondary metabolites. Starting from the high-quality reference genome of A. pullulans NBB 7.2.1 that contained 10,925 predicted genes, we identified 1,044 genes with a predicted signal peptide (Figure 8). 248 of these genes encoded predicted, secreted hydrolases, which included 39 proteases. Furthermore, we identified 25 potential secondary metabolite clusters that comprised, in total, 366 genes. Five biosynthetic genes of three different clusters (in total summing up to 46 genes) were upregulated during the competition. Dual RNA-seq analysis and mapping to our reference genome also identified 62 potentially secreted hydrolases that were upregulated. In the secretome analysis, 38 A. pullulans NBB 7.2.1 proteins were upregulated or only detected during the interaction with F. oxysporum NRRL 26381/CL57, of which twelve encoded hydrolases. Combining these transcriptome and secretome analyses results in a concise list of 79 potential A. pullulans NBB 7.2.1 biocontrol genes encoding predicted hydrolases or secondary metabolite biosynthesis genes that are the main targets for functional characterisation at the molecular level (Figure 8 and Supplementary Table 8). This approach demonstrates how the integration of genome, transcriptome and protein data allows defining a manageable list of top potential biocontrol genes (less than 1% of all annotated genes). Whether or not these genes are indeed involved in the biocontrol phenotype will have to be verified by gene deletions or overexpression analyses. This genome, transcriptome, and secretome analysis thus lays the basis for future molecular studies to decipher the biocontrol mechanisms employed by A. pullulans NBB 7.2.1. We are convinced that the understanding of these mechanisms will allow to optimise and improve biocontrol applications in the future and to explore whether biocontrol mechanisms against different pathogens rely on different or partially overlapping sets of core biocontrol genes.
Figure 8. FIGURE 8: Genome, transcriptome, and secretome analyses were used to reduce the number of target biocontrol genes for functional analyses from 10,925 (total number of A. pullulans NBB 7.2.1 genes) to 79.
As primary candidates for mediating biocontrol activity, genes upregulated during competition, encoding proteins detected in the secretome during the competition, and predicted to encode hydrolase or secondary metabolite biosynthesis genes were defined. The scale of the x-axis is logarithmic.
MATERIALS AND METHODS
Strains and cultivation
A. pullulans ((de Bary) G. Arnaud) (SH1515060.08FU; isolate NBB 7.2.1, CCOS1008 (Culture Collection of Switzerland)) was isolated from agricultural soil samples [37]. A. melanogenum CBS 110374, A. namibiae CBS 147.97, A. pullulans EXF-150, and A. subglaciale EXF-2481 were obtained from the Microbial Culture Collection Ex (http://www.ex-genebank.com) and the CBS-KNAW culture collection (http://www.wi.knaw.nl/collections/). For transcriptome experiments and competition assays, F. oxysporum f. sp. radicis-lycopersici (Schlecht as emended by Snyder and Hansen) NRRL 26381/CL57 (ARS Culture Collection, USDA) was used. All isolates were maintained on potato dextrose agar (PDA; Becton, Dickinson and Company, Le Pont de Claix, France) plates, grown at 22°C, and transferred to fresh plates weekly. For confrontation experiments, liquid cultures that were grown overnight (for yeast-like fungi) or for 4 d (for Fusarium, freshly inoculated from a frozen stock kept at -80°C) at 22°C in potato dextrose broth (PDB; Becton, Dickinson and Company, Le Pont de Claix, France).
Competition assays on agar plates
Binary competition assays were performed as previously described [37, 67]. Aureobasidium cells grown overnight in PDB were collected, washed and resuspended in water (OD600 of 0.001-0.1). Cells were spread (15 μl) on PDA plates, 5 μl of a conidial suspension of F. oxysporum were inoculated in the center of the plates, and the plates were incubated at 22°C. Before F. oxysporum reached the edge of the control plate (plate without A. pullulans), growth was quantified with a planimeter (Planix 5, Tamaya Technics Inc., Tokyo, Japan). The average of the relative growth (growth in presence of yeast/growth on control plate) of four replicates was calculated. All assays were repeated at least twice and showed comparable results.
Genome sequencing and annotation
A. pullulans NBB 7.2.1 cells were collected by centrifugation at 8.000 x g for 10 min from 1.5 mL of PDB over-night culture. The pellet was then exposed to two rounds of 2 min freezing with liquid nitrogen and 1 min 95°C water bath in 200 mL of Harju-buffer (2% Triton X-100, 1%SDS, 100 mM NaCL, 10mM Tris-HCL pH 8.0, 1mM EDTA). Genomic DNA was extracted using a phenol/chloroform protocol and Phase Lock Gel™ tubes (QuantaBio, Beverly, MA, USA). DNA was precipitated from the aqueous phase with 200 μl 5M ammonium acetate and 1.5 ml ice-cold 100% ethanol and by centrifugation for 15 min at 4°C. After two 70% ethanol washes, the DNA pellet was resuspended in Tris-EDTA buffer. The final concentration was measured with the Qubit™ 3 fluorimeter (Fisher Scientific AG, Reinach, Switzerland) and quality was assessed by agarose gel electrophoresis.
PacBio sequencing was carried out on a Sequel machine (1 SMRT cell) with size selected fragments (BluePippin system) with an average subread length of approximately 10 kb (Table 1). Two 2 x 300 bp Illumina paired end libraries were prepared using the Nextera XT DNA kit and sequenced on a MiSeq (Table 1). A subset of the highest quality reads (“LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36”, rq>15) were extracted using trimmomatic (v. 0.36) [68]. Only paired reads were used for further analyses. PloidyNGS (v.3.1.2) [69] was run using the Illumina data to explore the ploidy level of the genome, which was found to be haploid. Computel (v.1.2) [70] was run using the Illumina data to compute the mean telomere length. The number of the telomere pattern “TTAGGG/CCCTAA” was estimated to be 31. PlasmidSpades (v.3.11.1) [71] was run on the Illumina data in order to detect potential evidence for smaller plasmids, which did not give any results. PacBio subreads were filtered with Filtlong (v.0.2.0) and assembled using Flye (v.2.3.3; default parameters, except: estimated genome size of 30 Mb) [72] and with various length cutoffs including 5, 7, 8, 10, 15, 18, 19, 20, 22 and 25 kb. The assemblies resulted in 13-18 contigs each. The filtered subreads were mapped to the polished contigs using graphmap (v.0.5.2) [73] to verify their completeness. All contigs and mapped reads were individually inspected in the Integrative Genomics Viewer (IGV) [74]. The presence of the telomere sequences at both ends of each contig was checked to further verify an end-to-end assembly (i.e., from telomere to telomere). The mitogenome was identified using BLAST [75], the respective contig was start-aligned and reads were mapped to it using graphmap (v.0.5.2) to verify its circularity and completeness, which could be confirmed. The full mitogenome could only be resolved by the assembly using > 5kb subreads and was missed when a higher cutoff was chosen (i.e., all other assemblies). Short contigs were submitted to BLAST [73] and subsequently removed if they appeared spurious. A combination of the assemblies using cutoffs of 7 (for longer contigs) and 25 kb (for resolving repeats) appeared to deliver the best results (plus the >5kb assembly for the complete mitogenome). Both assemblies were individually polished using five Arrow runs. The filtered subreads were mapped to the polished contigs of both assemblies again using graphmap (v.0.5.2) [73] to identify the best result and a combination of contigs from both assemblies was chosen for further processing (Table 2). The contigs were then subjected to another four polishing rounds using Arrow and subreads with a minimum length of 15 kb (in order to allow for a high enough coverage, but maintain resolved repeat regions). The filtered subreads were mapped again to the polished contigs using graphmap (v.0.5.2) [73] to verify their completeness and were individually inspected in the IGV [74]. The number of telomere patterns at both ends of each contig was counted. The contigs were further polished using four Freebayes (v.1.2.0;) [76] runs and the Illumina reads to correct potential small errors (e.g., homopolymer errors). The extensive polishing and manual curation resulted in a total of 12 complete chromosomes and 1 mitogenome. The total genome size is 28,448,966 b (Table 2).
All five Aureobasidium genomes in this study have been annotated using the JGI Annotation pipeline and made available via the JGI fungal genome portal MycoCosm [77–79] (https://mycocosm.jgi.doe.gov). Briefly, the JGI pipeline performs feature prediction, starting with CRISPR elements, then non-coding RNAs, and lastly protein coding gene prediction. The final step is the functional annotation of protein coding genes by comparison with protein families, assignment is performed by searches against COG, KOG, KEGG orthology, Pfam, TIGRfam and Interpro databases. RNAseq data have not yet been used to verify the gene annotations. In addition to precomputed Markov clustering of gene families [80], an “Aureobasidium pangenome view” was constructed by building single-ortholog clusters using the Pangloss workflow [39, 40]. In short, all genes were searched against each other by BLASTP in order to determine core, singleton and accessory SOCs.
Comparative genome analyses
Annotation data for A. pullulans EXF-150, A. melanogenum CBS 110374, A. namibiae CBS 147.97, A. subglaciale EXF-2481 and A. pullulans NBB 7.2.1 was condensed from JGI using R studio (v 4.0.2). Significant enrichment was assessed using Fisher's exact test and p-values were adjusted for multiple testing (method “Benjamini-Hochberg”). Except when stated otherwise, comparisons were made to all genes with terms in any of the five genomes. The packages ggplot2 (v 3.3.1) [81], flextable (v 0.5.10) [82], RColorBrewer (v1.1.2) [83], scales (v 1.1.1) [84], and VennDiagram (v 1.6.20) [85] (e) were used for plotting.
Co-culture of A. pullulans and F. oxysporum for RNA extraction
The co-culture experiments were performed in liquid medium with submerged glass beads as previously described [86]. In short, F. oxysporum f. sp. radicis-lycopersici was precultured in petri dishes containing PDB and a layer of 4 mm glass beads (Fisher Scientific, Reinach, Switzerland). After 2 days, PDB was removed and the plate was washed twice with an indirect stream of sterile water. Independently, overnight liquid cultures of A. pullulans NBB 7.2.1 in PBD were pelleted, and cells were washed twice with sterile water. Cells were resuspended to an OD600 of 5 in salt buffer (comprised of 1 g/L potassium phosphate monobasic, 0.5 g/L magnesium sulphate anhydrous, 0.1 g/L sodium chloride, and 0.1 g/L calcium chloride anhydrous) supplemented with 2% bacteriological peptone. The A. pullulans suspension was applied to the plates with glass beads and F. oxysposum and incubated at 22°C. The corresponding controls with F. oxysporum grown in glass bead plates and overnight cultures of A. pullulans were likewise washed with water and incubated in salt buffer separately. Samples of the interaction between A. pullulans NBB 7.2.1 and F. oxysporum NRRL 26381, as well as their respective pure cultures, were collected after 24 hours and freeze-dried for RNA extraction, keeping the glass beads for mechanical disruption. Culture supernatants were stored for protein concentration and analysis of secreted proteins.
Transcriptome sequencing and analysis
Total RNA was extracted with the TRI reagent solution (Fisher Scientific, Reinach, Switzerland) following instructions from the manufacturer. Cells were disrupted mechanically by high-speed agitation with glass beads (2x for 1 min, 1 min on ice in between). Total RNA was quantified with the Qubit system (Fisher Scientific, Reinach, Switzerland) and its quality and purity was assessed by agarose gel electrophoresis. The 2100 Bioanalyzer system was used for quality control to confirm a RIN≥6.5 and a 28S/18S ratio ≥1.0.
RNA sequencing (RNA-seq) was performed using the BGISEQ-500 platform and an oligo-T enriched library at the Beijing Genomics Institute (BGI). All raw reads (2x100 bp) were trimmed, filtered, and adapters were removed using trimmomatic (v.0.39; parameters: SLIDINGWINDOW:4:15 MINLEN:36) [68] (Supplementary Tables 3 and 4). The filtered and paired reads were further processed using sortmerna (v.2.1) [87] in order to remove potential rRNA contamination (parameters: num_alignments: 1, paired_in, ref: all available rRNA databases). The resulting high quality reads (Supplementary Tables 3 and 5, fifth column) were used for further analyses. The A. pullulans JGI gff3 annotation file was reformatted to match the style of Ensembl gtf files using sed commands. The filtered high quality reads were mapped to the indexed reference genome of A. pullulans or the concatenated and indexed reference genomes of A. pullulans and F. oxysporum using hisat2 (v.2.1.0) [88]. The alignment files were subsequently split to contain only reads mapping to one of the genomes (Table 4) for the differential expression analysis. Features were counted in R (v.3.6.2) using the package featureCounts [89] and the feature type “gene” and the string “gene_id”. The RNA-seq data are available at NCBI under the BioProject accession PRJNA702246 (https://www.ncbi.nlm.nih.gov/Traces/study/?acc=PRJNA702246). Differential expression analysis was performed in R using the package DESeq2 [90]. Principal component analysis (PCA) was carried out on the log transformed count table. Pairwise comparisons were performed for all treatments. Fold changes (FC) were calculated and p-values were adjusted for multiple comparisons (method “Benjamini-Hochberg”). Genes with a p-value < 0.05 and a log2FoldChange ratio ≥ 2 were defined as differentially expressed genes (DEGs). ReviGo [91] was used to summarize gene onthology terms. Functional analyses of DEGs was performed based on KEGG pathway terms.
Identification of secreted proteins
Culture supernatant was collected for each sample and sterilized through a 0.2 m filter. Samples were then passed through a 50 kDa cutoff filter (Sartorius AG, Göttingen, Germany) and the protein concentrations were measured with the Qbit protein assay kit (Fisher Scientific AG, Reinach, Switzerland). Peptides were prepared for mass spectrometry using the iST protein digestion and clean-up kit (PreOmics). Briefly, protein samples were denatured in lysis buffer and digested with trypsin for 1 h at 37°C. Resulting peptides were purified and collected as a filtrate. Each resulting sample was dried, resuspended in 3% ACN, 0.1% formic acid and spiked with iRT peptides (Biognosys AG, Schlieren, Switzerland). An aliquot of 2 µL was transferred to autosampler vials for LC-MS/MS analysis on a Q-Exactive HFX mass spectrometer. 12 LC MS/MS samples (supernatants from pure cultures of A. pullulans NBB 7.2.1 or F. oxysporum f. sp. radicis-lycopersici NRRL 26381/CL57, and from the competition of both fungi; four biological replicates each) were processed using our previously published shotgun proteomics data analysis pipeline [92]. Quality control and conversion of raw data was performed using a wrapper function written in R, that executes ThermoRawFileParser [93] and rawDiag [94]. The converted files were then searched against a protein search database of the annotated genomes of the respective organisms: Aureobasidium pullulans NBB 7.2.1(10,925 CDS, https://mycocosm.jgi.doe.gov/AurpulNBB1/AurpulNBB1.home.html) and F. oxysporum f. sp. radicis-lycopersici NRRL 26381/CL57 (12,103 CDS; Genbank acc. AGNB00000000), to each of which 256 sequences of common contaminants were added. Protein searches were carried out with MS-GF+ (v2020.03.14) [88] using a precursor mass accuracy of 10 ppm, cysteine carbamidomethylation as fixed, and oxidation of methionine as variable modifications. The false discovery rate (FDR) was estimated using the target-decoy approach of MS-GF+. The FDR at the peptide-spectrum-matching (PSM) level was set such that the FDR at the protein-level was below 1%. For protein inference, we only considered proteins which were identified by unambiguous peptides as returned by a PeptideClassifier analysis [95] for A. pullulans NBB 7.2.1; for F. oxysporum, several peptides could imply more than one protein. In addition, we required at least two distinct peptides or three PSMs for a protein identification under one condition. Protein abundances were calculated as the percentage of total PSMs and filtered by “median > 0”, to exclude proteins detected in only one of the four replicates. Proteins with a median abundance FoldChange ratio ≥ 1.5 were defined as differentially upregulated proteins. Protein abundances were further analysed and visualized using R studio (v 4.0.2). The secretome data are deposited at MassIVE with the dataset identifier MSV000086991 (https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?accession=MSV000086991). Annotation data for A. pullulans NBB 7.2.1 and Fusarium oxysporum f. sp. radicis-lycopersici NRRL 26381/CL57 was used from JGI. Significant enrichment was assessed using the Fisher's exact test and p-values were adjusted for multiple testing (method “Benjamini-Hochberg”). For plotting, the packages ggplot2 (v 3.3.1) [81], RColorBrewer (v1.1.2) [83] and VennDiagram (v 1.6.20) [85] (e) were used.
SUPPLEMENTAL MATERIAL
All supplemental data for this article are available online at http://www.microbialcell.com/researcharticles/2021a-rueda-mejia-microbial-cell/.
Abbreviations:
- DEG
– differently expressed gene,
- FC
– fold change,
- NRPS
– non-ribosomal peptide synthase,
- PKS
– polyketide synthase,
- SOC
– single-ortholog clustering.
Funding Statement
We thank Daniel Frei and Jürg Frey for generating MiSeq data and Inés Sumann for DNA extraction. Weihong Qi and Paolo Nanni (both from the Functional Genomics Center Zurich, FGCZ) are acknowledged for support regarding the transcriptome and secretome experiments. The work conducted by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. FMF is supported by the Swiss National Science Foundation (SNSF) grant 31003A_175665/1.
REFERENCES
- 1.Slepecky RA, Starmer WT. Phenotypic plasticity in fungi: a review with observations on Aureobasidium pullulans. Mycologia. 2009;101(6):823–832. doi: 10.3852/08-197. [DOI] [PubMed] [Google Scholar]
- 2.Gostincar C, Muggia L, Grube M. Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front Microbiol. 2012;3:390. doi: 10.3389/fmicb.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gostincar C, Turk M, Zajc J, Gunde-Cimerman N. Fifty Aureobasidium pullulans genomes reveal a recombining polyextremotolerant generalist. Env Microbiol. 2019;21(10):3638–3652. doi: 10.1111/1462-2920.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasongsuk S, Lotrakul P, Ali I, Bankeeree W, Punnapayak H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018;63(2):129–140. doi: 10.1007/s12223-017-0561-4. [DOI] [PubMed] [Google Scholar]
- 5.Price NP, Bischoff KM, Leathers TD, Cosse AA, Manitchotpisit P. Polyols, not sugars, determine the structural diversity of anti-streptococcal liamocins produced by Aureobasidium pullulans strain NRRL 50380. J Antibiot. 2017;70(2):136–141. doi: 10.1038/ja.2016.92. [DOI] [PubMed] [Google Scholar]
- 6.Price NP, Manitchotpisit P, Vermillion KE, Bowman MJ, Leathers TD. Structural characterization of novel extracellular liamocins (mannitol oils) produced by Aureobasidium pullulans strain NRRL 50380. Carbohydr Res. 2013;370:24–32. doi: 10.1016/j.carres.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Takesako K, Ikai K, Haruna F, Endo M, Shimanaka K, Sono E, Nakamura T, Kato I, Yamaguchi H. Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. J Antibiot. 1991;44(9):919–924. doi: 10.7164/antibiotics.44.919. [DOI] [PubMed] [Google Scholar]
- 8.Zain M, Awaad A, Razzak A, Maitland D, El-Sayed N, Sakhawy M. Secondary metabolites of Aureobasidium pullulans isolated from Egyptian soil and their biological activity. J Appl Sci Res. 2009;5:1582–1591. [Google Scholar]
- 9.Freimoser FM, Rueda-Mejia MP, Tilocca B, Migheli Q. Biocontrol yeasts: mechanisms and applications. World J Microbiol Biotechnol. 2019;35(10):154. doi: 10.1007/s11274-019-2728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leathers TD, Price NP, Bischoff KM, Manitchotpisit P, Skory CD. Production of novel types of antibacterial liamocins by diverse strains of Aureobasidium pullulans grown on different culture media. Biotechnol Lett. 2015;37(10):2075–2081. doi: 10.1007/s10529-015-1892-3. [DOI] [PubMed] [Google Scholar]
- 11.van Nieuwenhuijzen EJ. Aureobasidium. In: Tortorello ML, editor. Encycl. Food Microbiol. Second Ed. Academic Press; Oxford: 2014. pp. 105–109. [Google Scholar]
- 12.Chi Z, Wang F, Chi Z, Yue L, Liu G, Zhang T. Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl Microbiol Biotechnol. 2009;82(5):793–804. doi: 10.1007/s00253-009-1882-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Feng J, Wang P, Xia J, Li X, Zou X. CRISPR/Cas9-mediated efficient genome editing via protoplast-based transformation in yeast-like fungus Aureobasidium pullulans. Gene. 2019;709:8–16. doi: 10.1016/j.gene.2019.04.079.. [DOI] [PubMed] [Google Scholar]
- 14.Gostincar C, Ohm RA, Kogej T, Sonjak S, Turk M, Zajc J, Zalar P, Grube M, Sun H, Han J, Sharma A, Chiniquy J, Ngan CY, Lipzen A, Barry K, Grigoriev IV, Gunde-Cimerman N. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014;15:549. doi: 10.1186/1471-2164-15-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bencheqroun SK, Bajji M, Massart S, Labhilili M, Jaafari SE, Jijakli MH. In vitro and in situ study of postharvest apple blue mold biocontrol by Aureobasidium pullulans: Evidence for the involvement of competition for nutrients. Postharvest Biol Technol. 2007;46(2):128–135. doi: 10.1016/j.postharvbio.2007.05.005. [DOI] [Google Scholar]
- 16.Wang W, Chi Z, Liu G, Buzdar MA, Chi Z, Gu Q. Chemical and biological characterization of siderophore produced by the marine-derived Aureobasidium pullulans HN6.2 and its antibacterial activity. Biometals. 2009;22(6):965–972. doi: 10.1007/s10534-009-9248-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang WL, Chi ZM, Chi Z, Li J, Wang XH. Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresour Technol. 2009;100(9):2639–2641. doi: 10.1016/j.biortech.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Banani H, Spadaro D, Zhang D, Matic S, Garibaldi A, Gullino ML. Biocontrol activity of an alkaline serine protease from Aureobasidium pullulans expressed in Pichia pastoris against four postharvest pathogens on apple. Int J Food Microbiol. 2014;182–183:1–8. doi: 10.1016/j.ijfoodmicro.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Spadaro D, Valente S, Garibaldi A, Gullino ML. Cloning, characterization, expression and antifungal activity of an alkaline serine protease of Aureobasidium pullulans PL5 involved in the biological control of postharvest pathogens. Int J Food Microbiol. 2012;153(3):453–464. doi: 10.1016/j.ijfoodmicro.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Di Francesco A, Ugolini L, Lazzeri L, Mari M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol Control. 2014;81:8–14. doi: 10.1016/j.biocontrol.2014.10.004. [DOI] [Google Scholar]
- 21.Di Francesco A, Ugolini L, Lazzeri L, Mari M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol Control. 2015;81:8–14. doi: 10.1016/j.biocontrol.2014.10.004. [DOI] [Google Scholar]
- 22.Di Francesco A, Zajc J, Gunde-Cimerman N, Aprea E, Gasperi F, Placì N, Caruso F, Baraldi E. Bioactivity of volatile organic compounds by Aureobasidium species against gray mold of tomato and table grape. World J Microbiol Biotechnol. 2020;36(11):171. doi: 10.1007/s11274-020-02947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Francesco A, Di Foggia M, Baraldi E. Aureobasidium pullulans volatile organic compounds as alternative postharvest method to control brown rot of stone fruits. Food Microbiol. 2020;87:103395. doi: 10.1016/j.fm.2019.103395. [DOI] [PubMed] [Google Scholar]
- 24.Yalage Don SM, Schmidtke LM, Gambetta JM, Steel CC. Volatile organic compounds produced by Aureobasidium pullulans induce electrolyte loss and oxidative stress in Botrytis cinerea and Alternaria alternata. Res Microbiol. 2021;172(1):103788. doi: 10.1016/j.resmic.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Yalage Don SM, Schmidtke LM, Gambetta JM, Steel CC. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci Rep. 2020;10(1):4498. doi: 10.1038/s41598-020-61471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madhupani YDS, Adikaram NKB. Delayed incidence of stem-end rot and enhanced defences in Aureobasidium pullulans-treated avocado (Persea americana Mill.) fruit. J Plant Dis Prot. 2017;124(3):227–234. doi: 10.1007/s41348-017-0086-8. [DOI] [Google Scholar]
- 27.Ippolito A, El Ghaouth A, Wilson CL, Wisniewski M. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol Technol. 2000;19(3):265–272. doi: 10.1016/s0925-5214(00)00104-6. [DOI] [Google Scholar]
- 28.Di Francesco A, Mari M, Ugolini L, Baraldi E. Effect of Aureobasidium pullulans strains against Botrytis cinerea on kiwifruit during storage and on fruit nutritional composition. Food Microbiol. 2018;72:67–72. doi: 10.1016/j.fm.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Di Francesco A, Ugolini L, D'Aquino S, Pagnotta E, Mari M. Biocontrol of Monilinia laxa by Aureobasidium pullulans strains: Insights on competition for nutrients and space. Int J Food Microbiol. 2017;248:32–38. doi: 10.1016/j.ijfoodmicro.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Zajc J, Černoša A, Francesco AD, Castoria R, Curtis FD, Lima G, Badri H, Jijakli H, Ippolito A, GostinČar C, Zalar P, Gunde-Cimerman N, Janisiewicz WJ. Characterization of Aureobasidium pullulans Isolates Selected as Biocontrol Agents Against Fruit Decay Pathogens. Fungal Genom Biol. 2020;10(163):13. doi: 10.35248/2165-8056.20.10.163.. [DOI] [Google Scholar]
- 31.Mari M, Martini C, Spadoni A, Rouissi W, Bertolini P. Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol Technol. 2012;73:56–62. doi: 10.1016/j.postharvbio.2012.05.014. [DOI] [Google Scholar]
- 32.Mari M, Martini C, Guidarelli M, Neri F. Postharvest biocontrol of Monilinia laxa, Monilinia fructicola and Monilinia fructigena on stone fruit by two Aureobasidium pullulans strains. Biol Control. 2012;60(2):132–140. doi: 10.1016/j.biocontrol.2011.10.013. [DOI] [Google Scholar]
- 33.Kunz S, Schmitt A, Haug P. Field testing of strategies for fire blight control in organic fruit growing. Acta Horti. 2011;896:431–436. doi: 10.17660/ActaHortic.2011.896.62. [DOI] [Google Scholar]
- 34.Kunz S. Weinsberg; Germany: 2004. Development of “Blossom-Protect” - a yeast preparation for the reduction of blossom infections by fire blight. [Google Scholar]
- 35.Kunz S, Haug P. Fördergemeinschaft ökologischer Obstbau; Weinsberg: 2006. Development of a strategy for fire blight control in organic fruit growing. pp. 113–117. [Google Scholar]
- 36.Ni X, Yue L, Chi Z, Li J, Wang X, Madzak C. Alkaline protease gene cloning from the marine yeast Aureobasidium pullulans HN2-3 and the protease surface display on Yarrowia lipolytica for bioactive peptide production. Mar Biotechnol. 2009;11(1):81–89. doi: 10.1007/s10126-008-9122-9. [DOI] [PubMed] [Google Scholar]
- 37.Hilber-Bodmer M, Schmid M, Ahrens CH, Freimoser FM. Competition assays and physiological experiments of soil and phyllosphere yeasts identify Candida subhashii as a novel antagonist of filamentous fungi. BMC Microbiol. 2017;17(1):4. doi: 10.1186/s12866-016-0908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross S, Kunz L, Muller DC, Santos Kron A, Freimoser FM. Characterization of antagonistic yeasts for biocontrol applications on apples or in soil by quantitative analyses of synthetic yeast communities. Yeast. 2018;35(10):559–566. doi: 10.1002/yea.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy CGP, Fitzpatrick DA. Pan-genome analyses of model fungal species. Microb Genom. 2019;5(2):e000243. doi: 10.1099/mgen.0.000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy CGP, Fitzpatrick DA. Pangloss: a tool for pan-genome analysis of microbial eukaryotes. Genes. 2019;10(7):521. doi: 10.3390/genes10070521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39:W339–W346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izumi Y, Ohtani K, Miyamoto Y, Masunaka A, Fukumoto T, Gomi K, Tada Y, Ichimura K, Peever TL, Akimitsu K. A polyketide synthase gene, ACRTS2, is responsible for biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Mol Plant-Microbe Interact MPMI. 2012;25(11):1419–1429. doi: 10.1094/mpmi-06-12-0155-r. [DOI] [PubMed] [Google Scholar]
- 44.Kihara J, Moriwaki A, Tanaka N, Tanaka C, Ueno M, Arase S. Characterization of the BMR1 gene encoding a transcription factor for melanin biosynthesis genes in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett. 2008;281(2):221–227. doi: 10.1111/j.1574-6968.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 45.Westermann AJ, Gorski SA, Vogel J. Dual RNA-seq of pathogen and host. Nat Rev Microbiol. 2012;10(9):618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- 46.DeIulio GA, Guo L, Zhang Y, Goldberg JM, Kistler HC, Ma L-J. Kinome expansion in the Fusarium oxysporum species complex driven by accessory chromosomes. mSphere. 2018;3(3):e00231. doi: 10.1128/msphere.00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li T, Wu Y, Wang Y, Gao H, Gupta VK, Duan X, Qu H, Jiang Y. Secretome profiling reveals virulence-associated proteins of Fusarium proliferatum during interaction with banana fruit. Biomolecules. 2019;9(6):246. doi: 10.3390/biom9060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai Y, Wang S, Zhong H, Yang Q, Zhang F, Zhuang Z, Yuan J, Nie X, Wang S. Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci Rep. 2015;5(1):14582. doi: 10.1038/srep14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunawardana Y, Niranjan M. Bridging the gap between transcriptome and proteome measurements identifies post-translationally regulated genes. Bioinformatics. 2013;29(23):3060–3066. doi: 10.1093/bioinformatics/btt537. [DOI] [PubMed] [Google Scholar]
- 50.Weeks ME, Sinclair J, Butt A, Chung Y-L, Worthington JL, Wilkinson CRM, Griffiths J, Jones N, Waterfield MD, Timms JF. A parallel proteomic and metabolomic analysis of the hydrogen peroxide- and Sty1p-dependent stress response in Schizosaccharomyces pombe. PROTEOMICS. 2006;6(9):2772–2796. doi: 10.1002/pmic.200500741. [DOI] [PubMed] [Google Scholar]
- 51.Griffin TJ, Gygi SP, Ideker T, Rist B, Eng J, Hood L, Aebersold R. Complementary profiling of gene expression at the transcriptome and proteome levels in Saccharomyces cerevisiae. Mol Cell Proteomics. 2002;1(4):323–333. doi: 10.1074/mcp.m200001-mcp200. [DOI] [PubMed] [Google Scholar]
- 52.Nobel H de, Lawrie L, Brul S, Klis F, Davis M, Alloush H, Coote P. Parallel and comparative analysis of the proteome and transcriptome of sorbic acid-stressed Saccharomyces cerevisiae. Yeast. 2001;18(15):1413–1428. doi: 10.1002/yea.793. [DOI] [PubMed] [Google Scholar]
- 53.Muers M. Transcriptome to proteome and back to genome. Nat Rev Genet. 2011;12(8):518–518. doi: 10.1038/nrg3037. [DOI] [PubMed] [Google Scholar]
- 54.Varadarajan AR, Allan RN, Valentin JDP, Castañeda Ocampo OE, Somerville V, Pietsch F, Buhmann MT, West J, Skipp PJ, van der Mei HC, Ren Q, Schreiber F, Webb JS, Ahrens CH. An integrated model system to gain mechanistic insights into biofilm-associated antimicrobial resistance in Pseudomonas aeruginosa MPAO1. Npj Biofilms Microbiomes. 2020;6(1):1–17. doi: 10.1038/s41522-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niu X, Thaochan N, Hu Q. Diversity of linear non-ribosomal peptide in biocontrol fungi. J Fungi. 2020;6(2):61. doi: 10.3390/jof6020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segal E, Yehuda H, Droby S, Wisniewski M, Goldway M. Cloning and analysis of CoEXG1, a secreted 1,3-β-glucanase of the yeast biocontrol agent Candida oleophila. Yeast. 2002;19(13):1171–1182. doi: 10.1002/yea.910. [DOI] [PubMed] [Google Scholar]
- 57.Yehuda H, Droby S, Bar-Shimon M, Wisniewski M, Goldway M. The effect of under- and overexpressed CoEXG1-encoded exoglucanase secreted by Candida oleophila on the biocontrol of Penicillium digitatum. Yeast. 2003;20(9):771–780. doi: 10.1002/yea.1006. [DOI] [PubMed] [Google Scholar]
- 58.Bar-Shimon M, Yehuda H, Cohen L, Weiss B, Kobeshnikov A, Daus A, Goldway M, Wisniewski M, Droby S. Characterization of extracellular lytic enzymes produced by the yeast biocontrol agent Candida oleophila. Curr Genet. 2004;45(3):140–148. doi: 10.1007/s00294-003-0471-7. [DOI] [PubMed] [Google Scholar]
- 59.Muszewska A, Stepniewska-Dziubinska MM, Steczkiewicz K, Pawlowska J, Dziedzic A, Ginalski K. Fungal lifestyle reflected in serine protease repertoire. Sci Rep. 2017;7(1):9147. doi: 10.1038/s41598-017-09644-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67(3):400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao B-J, Mou Y-N, Tong S-M, Ying S-H, Feng M-G. Subtilisin-like Pr1 proteases marking the evolution of pathogenicity in a wide-spectrum insect-pathogenic fungus. Virulence. 2020;11(1):365–380. doi: 10.1080/21505594.2020.1749487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yike I. Fungal proteases and their pathophysiological effects. Mycopathologia. 2011;171(5):299–323. doi: 10.1007/s11046-010-9386-2. [DOI] [PubMed] [Google Scholar]
- 63.Umemura M. Peptides derived from Kex2-processed repeat proteins are widely distributed and highly diverse in the Fungi kingdom. Fungal Biol Biotechnol. 2020;7:11. doi: 10.1186/s40694-020-00100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wendler J, Schroeder BO, Ehmann D, Koeninger L, Mailänder-Sánchez D, Lemberg C, Wanner S, Schaller M, Stange EF, Malek NP, Weidenmaier C, LeibundGut-Landmann S, Wehkamp J. Proteolytic degradation of reduced human β defensin 1 generates a novel antibiotic octapeptide. Sci Rep. 2019;9(1):3640. doi: 10.1038/s41598-019-40216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouertani A, Chaabouni I, Mosbah A, Long J, Barakat M, Mansuelle P, Mghirbi O, Najjari A, Ouzari H-I, Masmoudi AS, Maresca M, Ortet P, Gigmes D, Mabrouk K, Cherif A. Two new secreted proteases generate a casein-derived antimicrobial peptide in Bacillus cereus food born isolate leading to bacterial competition in milk. Front Microbiol. 2018;9:1148. doi: 10.3389/fmicb.2018.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bradshaw RA. Aminopeptidases. In: Lennarz WJ, Lane MD, editors. Encycl. Biol. Chem. Second Ed. Academic Press; Waltham: 2013. pp. 97–99. [Google Scholar]
- 67.Gore-Lloyd D, Sumann I, Brachmann AO, Schneeberger K, Ortiz-Merino RA, Moreno-Beltrán M, Schläfli M, Kirner P, Santos Kron A, Rueda-Mejia MP, Somerville V, Wolfe KH, Piel J, Ahrens CH, Henk D, Freimoser FM. Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol Microbiol. 2019;112(1):317–332. doi: 10.1111/mmi.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Augusto Corrêa dos Santos R, Goldman GH, Riaño-Pachón DM. ploidyNGS: visually exploring ploidy with Next Generation Sequencing data. Bioinformatics. 2017;33(16):2575–2576. doi: 10.1093/bioinformatics/btx204. [DOI] [PubMed] [Google Scholar]
- 70.Nersisyan L, Arakelyan A. Computel: computation of mean telomere length from whole-genome next-generation sequencing data. PloS One. 2015;10(4):e0125201. doi: 10.1371/journal.pone.0125201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolmogorov M, Yuan J, Lin Y, Pevzner PA. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 2019;37(5):540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- 73.Sović I, Šikić M, Wilm A, Fenlon SN, Chen S, Nagarajan N. Fast and sensitive mapping of nanopore sequencing reads with GraphMap. Nat Commun. 2016;7:11307. doi: 10.1038/ncomms11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high–performance genomics data visualization and exploration. Brief Bioinform. 2013;14(2):178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. ArXiv12073907 Q-Bio. 2012.
- 77.Kuo A, Bushnell B, Grigoriev IV. Chapter one - fungal genomics: sequencing and annotation. In: Martin FM, editor. Adv. Bot. Res. Academic Press; 2014. pp. 1–52. [Google Scholar]
- 78.Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, Riley R, Salamov A, Zhao X, Korzeniewski F, Smirnova T, Nordberg H, Dubchak I, Shabalov I. MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014;42(D1):D699–D704. doi: 10.1093/nar/gkt1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haridas S, Salamov A, Grigoriev IV. Fungal Genome Annotation. Methods Mol Biol Clifton NJ. 2018;1775:171–184. doi: 10.1007/978-1-4939-7804-5_15. [DOI] [PubMed] [Google Scholar]
- 80.Enright AJ, Van Dongen S, Ouzounis CA. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 2002;30(7):1575–1584. doi: 10.1093/nar/30.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wickham H. Springer-Verlag; New York: 2009. ggplot2: elegant graphics for data analysis. [Google Scholar]
- 82.Gohel D, Fazilleau Q, Nazarov M, Robert T, Barrowman M, Yasumoto A. Flextable: functions for tabular reporting. 2020 Available at: https://cran.r-project.org/web/packages/flextable/index.html [Accessed 30.06.2020]
- 83.Neuwirth E. RColorBrewer: ColorBrewer palettes. 2014 Available at: https://cran.r-project.org/web/packages/RColorBrewer/index.html [Accessed 30.06.2020]
- 84.RStudio. Wickham H, Seidel D. scales: scale functions for visualization. 2020 https://cran.r-project.org/web/packages/scales/index.html [Accessed 30.06.2020]
- 85.Chen H. VennDiagram: generate high-resolution venn and euler plots. 2018 Available at: https://cran.r-project.org/web/packages/VennDiagram/index.html [Accessed 30.06.2020]
- 86.Essig A, Hofmann D, Munch D, Gayathri S, Kunzler M, Kallio PT, Sahl HG, Wider G, Schneider T, Aebi M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J Biol Chem. 2014;289(50):34953–34964. doi: 10.1074/jbc.M114.599878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kopylova E, Noé L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinforma Oxf Engl. 2012;28(24):3211–3217. doi: 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- 88.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37(8):907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinforma Oxf Engl. 2014;30(7):923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 90.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PloS One. 2011;6(7):e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Omasits U, Quebatte M, Stekhoven DJ, Fortes C, Roschitzki B, Robinson MD, Dehio C, Ahrens CH. Directed shotgun proteomics guided by saturated RNA-seq identifies a complete expressed prokaryotic proteome. Genome Res. 2013;23(11):1916–1927. doi: 10.1101/gr.151035.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hulstaert N, Shofstahl J, Sachsenberg T, Walzer M, Barsnes H, Martens L, Perez-Riverol Y. ThermoRawFileParser: modular, scalable, and cross-platform RAW file conversion. J Proteome Res. 2020;19(1):537–542. doi: 10.1021/acs.jproteome.9b00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trachsel C, Panse C, Kockmann T, Wolski WE, Grossmann J, Schlapbach R. rawDiag: an R package supporting rational LC-MS method optimization for bottom-up proteomics. J Proteome Res. 2018;17(8):2908–2914. doi: 10.1021/acs.jproteome.8b00173. [DOI] [PubMed] [Google Scholar]
- 95.Qeli E, Ahrens CH. PeptideClassifier for protein inference and targeted quantitative proteomics. Nat Biotechnol. 2010;28(7):647–650. doi: 10.1038/nbt0710-647. [DOI] [PubMed] [Google Scholar]
- 96.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of Intersecting Sets. IEEE Trans Vis Comput Graph. 2014;20(12):1983–1992. doi: 10.1109/tvcg.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.