Abstract
Differentiation from asexual blood stages to mature sexual gametocytes is required for the transmission of malaria parasites. Here, we report that the ApiAP2 transcription factor, PfAP2-G2 (PF3D7_1408200) plays a critical role in the maturation of Plasmodium falciparum gametocytes. PfAP2-G2 binds to the promoters of a wide array of genes that are expressed at many stages of the parasite life cycle. Interestingly, we also find binding of PfAP2-G2 within the gene body of almost 3000 genes, which strongly correlates with the location of H3K36me3 and several other histone modifications as well as Heterochromatin Protein 1 (HP1), suggesting that occupancy of PfAP2-G2 in gene bodies may serve as an alternative regulatory mechanism. Disruption of pfap2-g2 does not impact asexual development, but the majority of sexual parasites are unable to mature beyond stage III gametocytes. The absence of pfap2-g2 leads to overexpression of 28% of the genes bound by PfAP2-G2 and none of the PfAP2-G2 bound genes are downregulated, suggesting that it is a repressor. We also find that PfAP2-G2 interacts with chromatin remodeling proteins, a microrchidia (MORC) protein, and another ApiAP2 protein (PF3D7_1139300). Overall our data demonstrate that PfAP2-G2 establishes an essential gametocyte maturation program in association with other chromatin-related proteins.
Introduction
Malaria is a life-threatening disease that continues to impact the lives of millions of people worldwide. According to the latest WHO estimates, there were 228 million cases of malaria in 2018, resulting in 405,000 deaths (World Malaria Report, WHO, 2019). Malaria is caused by unicellular protozoan parasites belonging to the genus Plasmodium. Of the six species that infect humans, Plasmodium falciparum has the highest mortality rate (Weiss et al. 2019; WHO 2019). P. falciparum exhibits a complex life cycle with asexual and sexual erythrocytic phases in the human host, followed by development in Anopheles mosquitoes which transmit the parasite back to a new host, initiating the pre-erythrocytic liver stage of infection. Although the cyclic 48-hour asexual blood-stage is responsible for symptomatic disease and severe malaria, this form of the parasite cannot be transmitted. Rather, in each round of replication, a fraction of the parasites (<10%) exit the asexual pathway and undergo sexual differentiation to form male and female gametocytes, which are transmission competent (Bruce et al. 1989). Inhibition of gametocyte development is of great interest, because it would prevent malaria parasite transmission, which is one of the major goals in the effort to achieve disease eradication (Rabinovich et al. 2017).
Gametocyte development in P. falciparum is a 9–12 day process, which is substantially longer than that of other human infecting Plasmodium species such as P. vivax and the rodent malaria parasites P. berghei (26–30 hours) and P. yoelii (36 hours) (Gautret and Motard, 1999; Liu, Miao, & Cui, 2011; Armistead et al., 2018). P. falciparum gametocyte development is divided into five (stage I to V) morphologically distinct stages (Sinden 1982). The earliest phase of gametocyte development, stage Ia, occurs around 24 to 30 hours post-invasion (hpi) and is morphologically indistinguishable from the young trophozoite stage. However, there are several well-defined markers of early stage I gametocytes such as Pfs16, Pfg27, and PfGEXP-5 (Alano, Premawansa, Bruce, & Carter, 1991; Marian C. Bruce, Carter, Nakamura, Aikawa, & Carter, 1994; Silvestrini et al., 2010; Poran et al., 2017; Josling et al., 2020; Llorà-Batlle et al., 2020). In the human host, stage Ib to stage IV sexually-developing parasites are sequestered in deep tissues like the bone marrow and only stage V gametocytes freely circulate in the blood and can be picked up by mosquitoes (Aguilar et al. 2014; Joice et al. 2014; Venugopal et al. 2020)
Regulation of Plasmodium development is driven by stage-specific transcription factors (TFs), such as the well-studied Apicomplexan AP2 (ApiAP2) family of DNA-binding proteins. ApiAP2 proteins are found among all members of the phylum, and each ApiAP2 protein contains between one to three Apetala2 (AP2) DNA binding domains (Balaji et al. 2005; Painter et al. 2011). In P. falciparum there are 27 members of the ApiAP2 protein family. ApiAP2 proteins have been shown to control all developmental transitions in Plasmodium (Jeninga, Quinn, and Petter 2019; Modrzynska et al. 2017; Zhang et al. 2017)
A master regulator of sexual (gametocyte) commitment, AP2-G, has been identified in both P. falciparum and Plasmodium berghei, a rodent malaria parasite (Kafsack et al. 2014; Sinha et al. 2014). Expression levels of PfAP2-G (PF3D7_1222600) strongly correlate with the formation of gametocytes, and targeted disruption of the pfap2-g locus results in a complete block in gametocytogenesis and downregulation of many gametocyte-associated genes (Kafsack et al. 2014). Another regulator of Plasmodium gametocyte development is AP2-G3. Disruption of pyap2-g3 (PY17X_1417400) in P. yoelii resulted in significant reduction in the numbers of male and female gametocytes, day 8 oocysts, and sporozoites, but it did not affect the asexual growth of the parasites (Zhang et al. 2017). The P. berghei orthologue, PBANKA_1415700, has also been disrupted and shown to be female specific and essential for female gametocyte development, and was thus renamed PbAP2-FG (Yuda et al. 2019). The P. falciparum orthologue, PF3D7_1317200, was also found to be associated with gametocytogenesis (Ikadai et al. 2013), although it has not been extensively characterized.
AP2-G2 has been shown to play a role in gametocytogenesis in both P. berghei and P. yoelii (Sinha et al., 2014; Yuda, Iwanaga, Kaneko, & Kato, 2015; Modrzynska et al., 2017). Deletion of pbap2-g2 does not inhibit sexual stage conversion but rather results in the nearly complete loss of gametocyte maturation and a block in transmission to mosquitoes (Yuda et al. 2015). Yuda et al. reported that this P. berghei transcription factor is bound to roughly 1,500 genes, or slightly over 1/3 of the genome during asexual development, and a number of these genes were up-regulated by more than two-fold in pbap2-g2 knockout lines. In another study, disruption of pbap2-g2 also caused premature expression of liver stage and sporozoite stage genes during asexual development in red blood cells (Modrzynska et al. 2017). Moreover, a P. berghei liver-stage (LS) transcriptome of P. berghei reported strong upregulation of pbap2-g2 in early LS that is negatively correlated with the expression of liver stage-specific transcripts (Derbyshire 2019). Together, these findings suggest that AP2-G2 acts as a repressor in both the asexual and sexual stages and during early liver-stage infection in P. berghei. Accordingly, P. yoelii parasites lacking the pyap2-g2 gene had greatly reduced numbers of gametocytes and oocysts (Zhang et al. 2017). A recent genome-wide knockout screen suggests that AP2-G2 is also not essential for blood-stage development in P. falciparum (Zhang et al. 2018), and may have a role in a later stage of development.
In this study we explore the function of AP2-G2 in P. falciparum during parasite blood-stage development. The period of gametocyte maturation for rodent malaria parasites is shorter than in P. falciparum, making it difficult to discern the developmental phenotypes associated with ap2-g2 knockouts. Therefore, disrupting this gene in P. falciparum is of interest due to the longer maturation period. Furthermore, two studies have reported differences in asexual blood-stage development resulting from pbap2-g2 deletion. On one hand, Modrzynska et al. observed reduced growth and premature expression of liver- and sporozoite-stage genes (Modrzynska et al. 2017). Conversely, Yuda et al. reported that pbap2-g2- parasites proliferate normally in blood (Yuda et al. 2015). Another discrepancy regards the impact of the gene deletion on the sex ratio. Sinha et al. reported a difference in the ratio of male to female gametocytes in pbap2-g2- lines (Sinha et al. 2014) while Yuda et al. observed no such differences (Yuda et al. 2015).
We find that the P. falciparum orthologue of AP2-G2 also plays a critical role in the maturation of gametocytes. Disruption of pfap2-g2 does not impact asexual development, parasite multiplication rate, or commitment to sexual development, but most parasites are unable to develop normally beyond stage III gametocytes. Using transcriptomic analysis and ChIP-seq we have identified a number of candidate genes that are bound and regulated by PfAP2-G2. We also identify interacting partners using protein immunoprecipitation followed by mass spectrometry. Overall our work suggests that PfAP2-G2 is a transcriptional repressor that likely recruits additional transcription factors and chromatin remodeling machinery to the genome to control gene expression. PfAP2-G2 plays a critical role in the regulation of the development of malaria parasites as they transition from asexual to sexual parasites, allowing for proper gametocyte maturation.
Results
PfAP2-G2 is expressed during the trophozoite and schizont stages of asexual development
The P. falciparum orthologue of AP2-G2, PF3D7_1408200, encodes a 189kDa protein that contains a single AP2 DNA-binding domain (Figure 1A). pfap2-g2 is maximally transcribed at the ring and early trophozoite stages (Bozdech et al., 2003; Painter et al., 2018) and proteomics data indicates protein expression occurs at the trophozoite and schizont stages (Oehring et al. 2012). To precisely determine the timing of PfAP2-G2 expression and its subcellular localization, we tagged the gene at the C-terminus with GFP (Supplementary Fig. 1A). Using live-cell fluorescence microscopy, we imaged a highly synchronized PfAP2-G2::GFP parasite population for one complete asexual replication cycle, every 7 h beginning with the newly invaded ring stage (2–5 hpi) revealed that PfAP2-G2 was localized to the nucleus, as expected, and was expressed from the early trophozoite to the late schizont stages (Figure 1B). Further confirming the nuclear localization of PfAP2-G2, nuclear fractionation of trophozoite stage parasites (~30 hpi) showed that full length PfAP2-G2::GFP (~250 kDa) was only detected in the nuclear fraction of the parasite lysates (Figure 1C). We also detected other smaller sized peptides indicating that PfAP2-G2 may be degraded during the preparation of parasite lysate or is proteolytically cleaved. No bands were detected in a WT 3D7 control (Supplementary Fig. 1B).
Figure 1. PfAP2-G2 is expressed from around 16–19 hpi in asexual stage parasite.
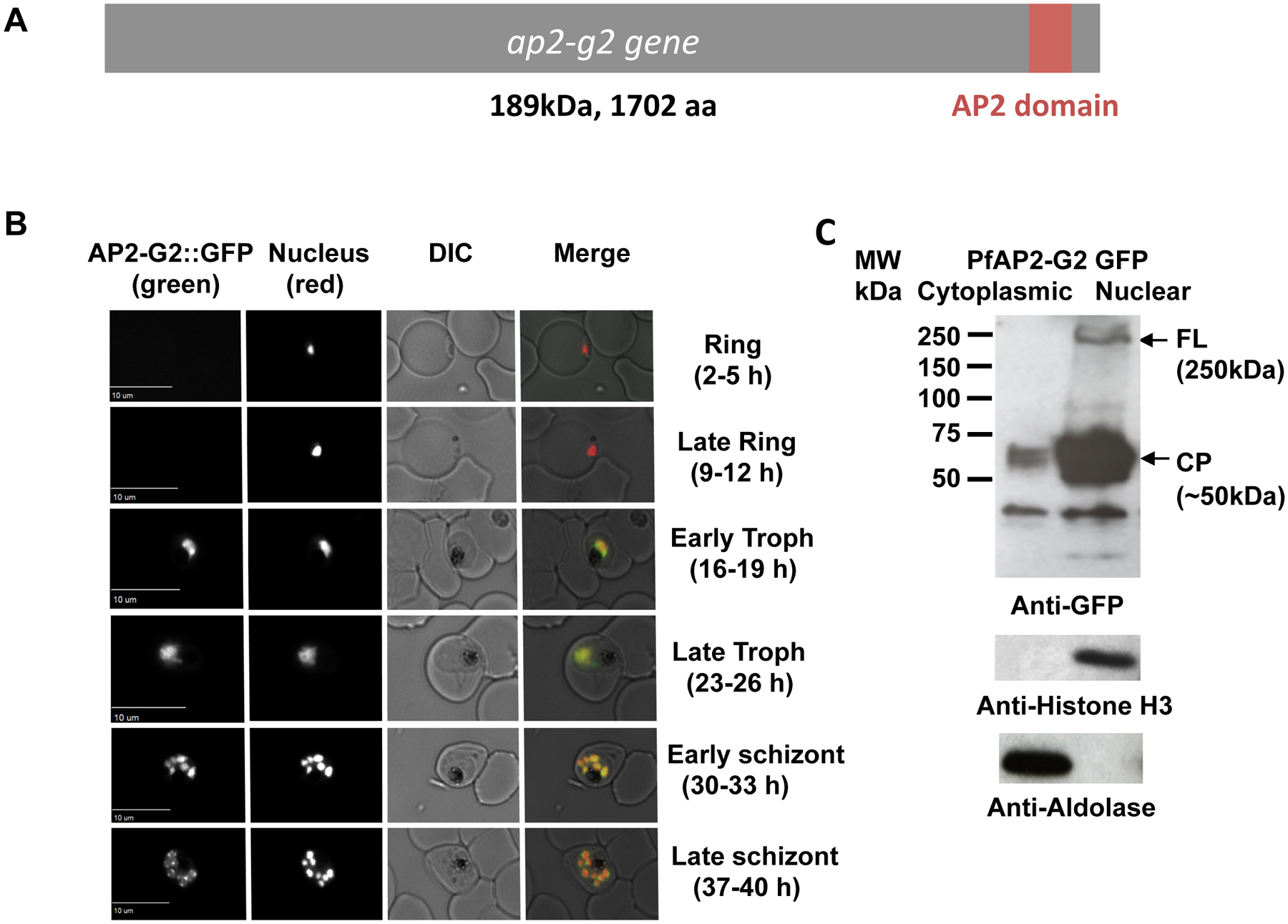
(A) Structure of the PfAP2-G2 protein which contains a single AP2 DNA-binding domain. (B) Live fluorescence microscopy performed on a highly synchronized population of AP2-G2::GFP parasites every 7 h of the asexual life cycle showed that PfAP2-G2::GFP colocalizes with the nucleus in both the trophozoite and schizont stages. DRAQ-5 was used as the nuclear stain. (C) Nuclear and cytoplasmic fractions from synchronized trophozoite stage parasites expressing GFP-tagged PfAP2-G2 were subjected to western blot analysis using anti-GFP antibodies (1:1000). Full length (FL) GFP-tagged PfAP2-G2 (~250 kDa) was detected only in the nuclear fraction of the parasite lysate whereas a cleavage product (CP, ~50kDa) was found in both fractions. Anti-histone H3 (1:3000) and anti-aldolase (1:1000) were used as loading controls for the nuclear and cytoplasmic fractions, respectively. WT parasites were used as a negative control (Supplementary Fig. 1B).
PfAP2-G2 is not required for proliferation during the asexual stages of the life cycle
To determine the role of pfap2-g2, we generated a genetic disruption (KO) line using selection linked integration (SLI) (Birnbaum et al. 2017) to truncate the pfap2-g2 coding sequence and isolated several clones (Supplementary Fig. 2A). As expected, pfap2-g2 KO parasites were readily obtained. Live-cell fluorescence imaging of the transgenic parasites showed diffuse cytoplasmic staining suggesting that the remaining non AP2 domain-containing protein fragment is largely outside the nucleus (Supplementary Fig. 2B). We next determined if truncation of PfAP2-G2 impacted asexual stage parasite growth or multiplication rate. Using a SYBR green growth assay (Vossen et al. 2010), we find that pfap2-g2 KO parasites develop similarly to that of WT (Figure 2A), and have similar multiplication rates (Figure 2B). Therefore, although PfAP2-G2 is expressed in the asexual blood stages, it is not required for normal asexual development.
Figure 2. Absence of PfAP2-G2 has no effect on the growth of asexual blood stage parasite.

(A) Growth profiles of the WT and pfap2-g2 KO parasites measured using an SYBR green assay every 24 hours over a period of 10 days starting with highly synchronous ring-stage parasites. The graph represents the plotted average +/−S.D. of biological triplicates. Fluorescence units are plotted along the Y-axis and the number of days was plotted along the X-axis (ANOVA P=0.65). (B) Measurement of the ring parasitemia (multiplication rate) of the WT and pfap2-g2 KO parasites. The values are the average of three biological replicates, and the error bars represent the standard deviation (P=0.5, not significant (N.S.)).
PfAP2-G2 is expressed in the gametocyte stages
Recent transcriptomic data has shown that the mRNA abundance of pfap2-g2 in gametocytes is low relative to the asexual stages, with a broad peak seen only in the early gametocyte stages followed by low expression during the later stages (Supplementary Fig. 3) (Van Biljon et al. 2019). To characterize protein expression of PfAP2-G2 during gametocyte development we imaged PfAP2-G2::GFP parasites (see methods). PfAP2-G2 was expressed in all stages of gametocyte development, but it was not confined to the nucleus in later stages (Figure 3A). Indeed nuclear fractionation of stage III gametocytes detected expression of full-length protein in both fractions, unlike in asexual parasites (Figure 3B). It also seems that the nuclear protein undergoes different proteolytic processing in gametocytes versus the asexual stages.
Figure 3. PfAP2-G2 is expressed throughout the gametocyte stages and is essential for maturation of gametocytes.

(A) Images obtained by live fluorescence microscopy performed on Stage I through Stage V gametocytes. The DIC image was merged with the DRAQ5 nuclear stain imaging to confirm the nuclear localization of the signal. The results indicate that PfAP2-G2 was expressed at all stages of gametocyte development and was localized to the nucleus. (B) Nuclear and cytoplasmic fractions from stage III gametocytes were subjected to western blot analysis using anti-GFP antibodies (1:1000). The GFP-tagged AP2-G2 of expected size (~250 kDa) was detected in both the nuclear and cytoplasmic fractions of the parasite lysate. Anti-histone H3 (1:3000) and anti-aldolase (1:1000) were used as the loading controls for the nuclear and cytosolic fractions, respectively. (C) Giemsa-stained smears of gametocytes from the WT and PfAP2-G2 KO lines showed that the morphology of the KO gametocytes appeared normal until Stage III. Most pfap2-g2 KO parasites committed to sexual development did not progress beyond stage III. Morphological staging was quantified by counting ≥ 100 parasites for each time point during gametocyte development from Giemsa-stained smears. The reported number of counts are based on the single replicate.
PfAP2-G2 is essential for gametocyte maturation
To determine if sexually committed pfap2-g2 KO gametocytes mature fully to stage V, we induced PfAP2-G2 WT and KO parasite lines to undergo gametocytogenesis and followed their development for 14 days, monitoring their maturation via morphological analysis of Giemsa-stained thin-blood smears. Whereas WT parasites matured into stage V gametocytes by day 9–11, most pfap2-g2 KO parasites committed to sexual development did not progress beyond stage III (Figure 3C). We also noticed that a larger proportion of dead or morphologically abnormal gametocytes were present in the pfap2-g2 KO compared to the WT in later time points, and only 2% of the pf2ap2-g2 KO resembled mature stage V gametocytes at day 11, compared to 30% in the parental line (Figure 3C). PfAP2-G2 is thus a critical regulator of gametocyte maturation and development.
PfAP2-G2 makes extensive genome-wide interactions
Because PfAP2-G2 is predicted to be a DNA-binding protein (Campbell, de Silva, Olszewski, Elemento, & Llinás, 2010; Yuda et al., 2015) we sought to determine the genome-wide localization of PfAP2-G2 in both asexual and gametocyte life cycle stages. To do this we first performed ChIP-seq at the trophozoite stage using the PfAP2-G2::GFP parasites. Peak calling identified ~5000 peaks in at least 2 of the 3 biological replicates (FDR 0.05) (Supplementary Fig. 4A). All replicates showed a high correlation with each other with respect to the overall peaks identified (Figure 4A). To our surprise only 401 of the predicted binding sites, corresponding to 94 genes, were found in the non-coding upstream regions of genes (Supplementary Table 1), while 4,600 peaks, corresponding to 2,932 genes, were located in exonic gene bodies (Supplementary Table 2). This differs from what has been previously observed for other characterized P. falciparum ApiAP2 proteins, such as SIP2 (Flueck et al. 2010), AP2-I (Santos et al. 2017) and AP2-G (Josling et al. 2020) which were found to predominantly bind to non-coding 5’ upstream regions. Although wide-scale binding to gene bodies was not previously reported for PbAP2-G2 (Yuda et al. 2015), a re-analysis of the ChIP-seq data from this study using the same pipeline that we used for our ChIP-seq data analysis, revealed that PbAP2-G2 is also broadly distributed across gene bodies in the P. berghei genome with 4,345 binding sites (Supplementary Table 3).
Figure 4. Genome-wide predictions of PfAP2-G2 targets and binding regions by ChIP seq.

(A) Pearson correlation analysis on three biological replicates of ChIP-seq including the Input (In) and AP2-G2 GFP (GFP) (B) DREME logos for the motifs enriched in the genomic locations bound by PfAP2-G2 in the gene bodies and non-coding regions in the asexual stage. Shown is the enriched PF3D7_1139300 motif AGAA and PfAP2-G2 motif ACCA obtained by DREME along with the PBM motif. The P-values are reported by DREME. (C) Example PfAP2-G2 peaks in asexual stages for all three ChIP-seq replicates. Peaks are represented as red tracks below with the motifs present highlighted in blue. (D) Example PfAP2-G2 peaks in gametocytes for both replicates. (E) Venn diagram showing common genes with PfAP2-G2 peaks in trophozoite and gametocyte stages (F) Representative peaks for Pf2AP2-G2 binding upstream of the msp2 gene in both trophozoite and gametocyte stages. (G) DREME logo for the motifs enriched in the genomic locations bound by PfAP2-G2 in the gene bodies and non-coding regions in gametocytes. The P-values are reported by DREME.
An analysis of all the regions (both upstream regions and ORFs) bound by PfAP2-G2, using DREME (Bailey 2011) identified two significantly enriched DNA sequence motifs. The first is an AGAA sequence motif which is related to a previously reported DNA motif found to associate with one (of the three) AP2 domains of the ApiAP2 protein PF3D7_1139300 (Campbell et al. 2010). We obtained the ACCA core motif as the second most enriched motif (Figure 4B), which closely resembles the previously identified PfAP2-G2 motif obtained by protein binding microarrays (PBM) (Campbell et al. 2010).
In order to characterize possible regulatory targets of PfAP2-G2, we first focused on the 94 genes that contained PfAP2-G2 peaks in their upstream regions. Nearly 87% of these 94 putative target genes are bound within 2.5kb upstream of the start codon (Supplementary Fig. 4B). 278 of the remaining intergenic peaks were localized to the subtelomeric ends of each chromosome (Supplementary Fig. 4C). For further analysis we only considered genes with upstream binding interactions less than 2.5 kb from the start codon, leaving 82 candidate genes (Supplementary Table 1). Interestingly, PfAP2-G2 at the asexual trophozoite stage binds to the promoters of genes known to be expressed later in the gametocyte and mosquito life cycle stages. These include 6-cysteine protein (p36 PF3D7_0404400), cysteine repeat modular protein 2 (crmp2 PF3D7_0718300), sporozoite protein essential for cell traversal (spect PF3D7_1342500), circumsporozoite protein (csp PF3D7_0304600), cell traversal protein for ookinetes and sporozoites (celtos PF3D7_1216600), secreted protein altered thrombospondin repeat protein (spatr PF3D7_0212600), stearoyl-CoA desaturase (scd PF3D7_0511200), and cap380 oocyst capsule protein (PF3D7_0320400) among others (Supplementary Table 1) (Chattopadhyay et al. 2003; van Dijk et al. 2010; Espinosa et al. 2017; Gratraud et al. 2009; Ishino et al. 2004; Itsara et al. 2018; Singh et al. 2007; Thompson et al. 2007; Zhao et al. 2016). Representative PfAP2-G2 peaks upstream of two target genes are shown in (Figure 4C). We also found PfAP2-G2 associated with the upstream regions of genes that are highly transcribed in the asexual ring stage (as analyzed in (López-Barragán et al. 2011)) including the knob-associated histidine rich protein (kahrp PF3D7_0202000), Plasmodium helical interspersed subtelomeric proteins (phista PF3D7_0115100), several variant family genes including vars and rifins and genes encoding proteins involved in egress and invasion such as the Rh5 interacting protein (ripr PF3D7_0323400) and serine repeat antigen 7 (sera7 PF3D7_0207400) (Miller et al. 2002; Pei et al. 2005; Sargeant et al. 2006; Volz et al. 2016). Therefore, PfAP2-G2 binds to the promoters and ORFs of genes that are expressed at a different developmental stage than that in which pfap2-g2 is expressed. This suggests that, as in the rodent malaria species, PfAP2-G2 is a transcriptional repressor protein that prevents the premature expression of later stage genes..
Since the developmental arrest we observed in pfap2-g2 KO parasites occurred at state III gametocytes, we next examined the genome-wide occupancy of PfAP2-G2 in stage III gametocytes by ChIP-seq. We identified 947 peaks in common across two replicates, corresponding to 674 genes at an FDR of 0.05 (Figure 4D) (Supplementary Table 4, Supplementary Table 5). PfAP2-G2 binding, once again, was found in both upstream regions and within gene bodies. Although PfAP2-G2 bound many fewer regions in gametocytes compared to trophozoites, which may be associated with the difficulty in obtaining high amounts of chromatin in gametocytes, virtually all stage III PfAP2-G2 target genes were also targets during the trophozoite stages (Figure 4E). This suggests that there are a large number of genes that are always bound by PfAP2-G2 during both asexual and sexual parasite development (Figure 4F). DNA motif analysis yet again identified the ACCA motif enriched in the genomic regions bound by PfAP2-G2 in gametocytes (Figure 4G).
Genetic disruption of PfAP2-G2 leads to aberrant gene expression in both asexual and sexual stages
Using synchronized parasites, we collected seven total RNA samples from WT or KO parasites throughout the 48-hour asexual life cycle for transcriptome analysis (parasite counts are in (Supplementary Fig 5). In a separate experiment, total RNA samples were collected during sexual differentiation every 12 hours for 7 continuous days starting at 2 days post-gametocyte induction (Supplementary Fig. 6A & 6B). Gene expression analysis of the asexual blood stage timecourse using Significance Analysis of Microarray (SAM) (Tusher, Tibshirani, and Chu 2001) identified 327 differentially expressed transcripts (>1.5 log2FC, 0.30 local FDR) in the pfap2-g2 KO line. Out of these, 237 showed enhanced transcript abundance in the pfap2-g2 KO line and 90 showed decreased abundance (Supplementary Fig. 7, Supplementary Table 6). This result is quite surprising given the lack of any asexual growth phenotype between the pfap2-g2 KO and the WT (Figure 2), implying that these changes in gene expression do not impact asexual parasite fitness or gametocyte commitment. A comparison of transcript abundance in the KO and WT sexual stages identified increased abundance for 274 transcripts and reduced abundance for 169 transcripts in the pfap2-g2 KO line (>1.5 log2FC, 0.30 local FDR) (Supplementary Fig. 7, Supplementary Table 7). These differential transcription patterns presumably lead to the stall in development at Stage III gametocytes in parasites lacking PfAP2-G2.
To determine at which stage the significantly differential mRNAs (by SAM) are maximally transcribed in wild-type parasites, we used a published RNA-seq dataset covering four different asexual blood stages (rings, early trophozoite, late trophozoite, and schizonts), two gametocyte stages (stage II and stage V), and the ookinete stage using the 3D7 P. falciparum strain (López-Barragán et al. 2011). Using this dataset, we identified the 3D7 parasite lifecycle stage at which each gene has the highest fragments per kilobase of transcript, per million mapped sequencing reads (FPKM). For the asexual transcriptome, most of the differentially expressed genes we measured as differentially regulated in the pfap2-g2 KO are normally transcribed maximally in stage V gametocyte (and not in asexual stages), followed by the ookinete and ring stages (Figure 5A). This suggests that loss of PfAP2-G2 impacts the expression of genes from late-stage gametocytes, ookinetes, and asexual stages. However the number of genes showing enhanced transcript abundance is strikingly high for genes that are normally expressed in stage V gametocytes (Figure 5A). Therefore, PfAP2-G2 may prevent the premature expression of late-stage gametocyte genes during asexual development.
Figure 5. Transcriptional changes in parasites lacking PfAP2-G2.

(A) Classification of significantly changing genes in asexual and gametocyte stages based on their maximum transcription in either asexual, gametocyte or mosquito stages, using RNA-seq data (López-Barragán et al., 2011). (B) Overlap between the genes with decreased transcript abundance in gametocytes and established late gametocyte markers (Figure 2, cluster 9 from Van Biljon et al., 2019). (C) Overlap between genes with decreased transcript abundance in gametocytes and asexual stages. (D) Overlap of genes with PfAP2-G2 enrichment in the upstream regions and increased transcript abundance in the pfap2-g2 KO (analyzed by SAM, >1.5 log2FC, 0.30 local FDR). (E) List of genes with PfAP2-G2 enrichment in the upstream regions and increased transcript abundance in the pfap2-g2 KO (F) Expression fold change (log2 KO versus WT) for genes bound by PfAP2-G2 in the upstream regions during the asexual blood stages. The genes are clustered based on Pearson’s correlation. Boxed is the timepoint at which ChIP-seq was performed.
In the gametocyte transcriptome, we observed the opposite trend when comparing to the López-Barragán data, where 70% of the transcripts that showed increased abundance in the KO line were maximally abundant in the asexual stages, especially at the ring stage (Figure 5A). Examples of protein products whose mRNA transcript abundance was higher in gametocytes include the knob-associated histidine-rich protein (KAHRP, PF3D7_0202000), 15 Plasmodium helical interspersed subtelomeric proteins (PHISTs), 7 members of the FIKK serine/threonine protein kinase family proteins, 12 Plasmodium exported hypothetical gene family proteins (HYPs), the merozoite surface proteins MSP1, MSP2, MSP6, MSP7, MSP9, and MSP11, the ApiAP2 transcription factor AP2-L (PF3D7_0730300), and serine repeat antigen 5 (SERA5, PF3D7_0207600) among others (Supplementary Table 7) (Pei et al., 2005; Sargeant et al., 2006; Nunes, Goldring, Doerig, & Scherf, 2007; Beeson et al., 2016). These results indicate that PfAP2-G2 represses a subset of genes throughout both asexual and sexual development, and the absence of PfAP2-G2 leads to aberrant timing of gene expression.
To identify enriched DNA sequence motifs associated with gene expression changes, we analyzed the 5’ upstream region (1000bp upstream of the start codon) of the genes showing significant change in transcript abundance in the asexual and sexual stages using the Finding Informative Regulatory Elements (FIRE) algorithm (Elemento, Slonim, and Tavazoie 2007). The most significant enriched sequence motif was the known DNA-interacting motif for PfAP2-G2, TGCAACCA (P-value 1.24 e-21) in genes with enhanced transcript abundance in the asexual stage (Supplementary Fig. 7). Interestingly, we also found an AGAACAA (P-value 3.36 e-15) DNA-binding motif that is recognized by the ApiAP2 protein, PF3D7_1139300 (Supplementary Fig. 7). Similar motifs were found for genes with increased transcript abundance in gametocytes (Supplementary Fig. 7). Intriguingly both pfap2-g2 and pf3d7_1139300 (pf11_0404) are expressed at similar times throughout asexual development based on transcriptomic data, suggesting that the two proteins may be acting together in some manner to regulate transcription (Supplementary Fig. 8). Based on earlier evidence, genetic disruption of the pf3d7_1139300 orthologue in P. berghei (pbanka_0909600) and P. yoelii (py17x_0911000) was refractory, indicating its essentiality (Modrzynska et al., 2017; Zhang et al., 2017). Single-cell RNA-seq has shown a sharp upregulation of the gene encoding the ApiAP2 protein PF3D7_1139300 when ap2-g expression peaks just before egress in committed schizonts (Poran et al. 2017). Although we couldn’t detect the ACCA PfAP2-G2 binding motif associated with genes showing decreased transcript abundance (Supplementary Fig. 9), we found enrichment of the AGACA motif, which has been associated with gametocyte commitment and development (Supplementary Fig. 9) (Young et al., 2005; Bischoff & Vaquero, 2010; Painter, Carrasquilla, & Llinás, 2017), in genes showing decreased transcript abundance in gametocytes.
Genes with decreased mRNA abundance in the gametocyte transcriptome from the pfap2-g2 KO line (170 genes) overlap significantly with genes previously reported as late gametocyte markers (Van Biljon et al. 2019) (Figure 5B). Some example genes are alpha tubulin 2 (PF3D7_0422300), TRAP-like protein (tlp PF3D7_0616500), secreted ookinete protein (psop13 PF3D7_0518800, psop20 PF3D7_0715400), dynein heavy chain (PF3D7_0729900), ookinete surface protein P28 (PF3D7_1031000), and ookinete surface protein P25 (PF3D7_1031000) (Rawlings et al., 1992; Heiss et al., 2008; Ecker et al. 2008; Villard et al., 2007; Duffy & Kaslow, 1997). Therefore, one reason that pfap2-g2 KO parasites may fail to progress beyond stage III may be due to the aberrant expression of mRNAs required during this and subsequent stages of gametocyte development. The inhibition of gametocyte maturation may also be due to the decreased expression of a few critical genes required for gametocyte development, such as male development gene 1 (mdv1, PF3D7_1216500), gametocyte erythrocyte cytosolic protein (geco, PF3D7_1253000), gametocyte exported protein (gexp06 PF3D7_0114000), and gametocyte specific protein (pf11-1, PF3D7_1038400) during the asexual stage. Interestingly, we also see overlap between the genes with decreased transcript abundance in both the asexual and gametocyte stages. These overlapping genes are enriched mostly for members of the var, stevor, and rifin variant gene families, Pfmc-2tm among others (Figure 5C). An early gametocyte proteome revealed that exported and erythrocyte remodeling proteins are the most overrepresented proteins in gametocytes (Silvestrini et al. 2010). Although members of the PfEMP1, STEVOR and RIFIN protein repertoire are expressed in gametocyte stages, their role is not well established (Sharp et al., 2006; Petter, Bonow, & Klinkert, 2008; Tibúrcio et al., 2012, Mwakalinga et al., 2012; Tibúrcio et al., 2013; Neveu et al., 2018; Neveu & Lavazec, 2019;), and decreased abundance of these genes may contribute to the observed phenotypic effect.
PfAP2-G2 binding in the promoter and in gene bodies is associated with differential regulation of transcription
Out of 82 genes with upstream PfAP2-G2 peaks at the trophozoite stage, only 23 displayed significantly elevated mRNA abundance in the pfap2-g2 KO (as analyzed by SAM, >1.5 log2FC, 0.30 local FDR) (Figure 5D, 5E). This suggests that the transcription of other genes may be impacted indirectly in the PfAP2-G2 knockout. On the other hand, none of the genes with significantly reduced mRNA abundance were bound by PfAP2-G2 in the upstream regions. However, considering the stage at which ChIP was performed we see that the mRNA abundance of many of the PfAP2-G2 ChIP-seq targets do not change in the absence of PfAP2-G2 indicating that binding of PfAP2-G2 alone might not be sufficient to affect transcription (Figure 5F). PfAP2-G2 binding to gene bodies results in both repression and activation of gene expression in the pfap2-g2 KO. Open reading frames bound by PfAP2-G2 in the gene body in gametocytes showed similar effects (Supplementary Fig. 10). Overall these results indicate that PfAP2-G2 binding to the upstream promoter region is largely associated with repression, but PfAP2-G2 alone is not sufficient to repress transcription.
PfAP2-G2 shares occupancy with exonic epigenetic marks during asexual development
To understand the role of the pervasive genome-wide PfAP2-G2 binding in exonic gene bodies detected by ChIP-seq (Supplementary Table 2), we investigated whether PfAP2-G2 is associated with any known epigenetic marks. We calculated a pairwise Pearson correlation coefficient by comparing the read counts per 1000bp bins of the PfAP2-G2 ChIP-seq results against an array of histone marks for which ChIP data is available (H3K9me3, H4ac, H4K20me3, H3K26ac, H3K9ac, H3K4me3 (Karmodiya et al., 2015), H3K36me2 and H3K36me3 (Jiang et al., 2013), as well as heterochromatin protein 1 (PfHP1) (Brancucci et al., 2014)). Interestingly, we found that PfAP2-G2 occupancy has the strongest correlation with H3K36me3 (R=0.933), followed by H4K20me3 (R=0.911), H3K27ac (R=0.826), and H4ac (R=0.765) (Figure 6A), which are all histone marks that show moderate positive correlation with P. falciparum transcription and are associated with transcriptionally poised genes (Karmodiya et al. 2015). In total, 3,633 (80%) PfAP2-G2 binding sites co-occur with H3K36me3 marks (Figure 6B, 6C). The functional role of this association between PfAP2-G2 and H3K36me3 and what the potential role of PfAP2-G2 in H3K36me3 recruitment remain to be determined.
Figure 6. PfAP2-G2 shows high correlation with repressive histone modifications.
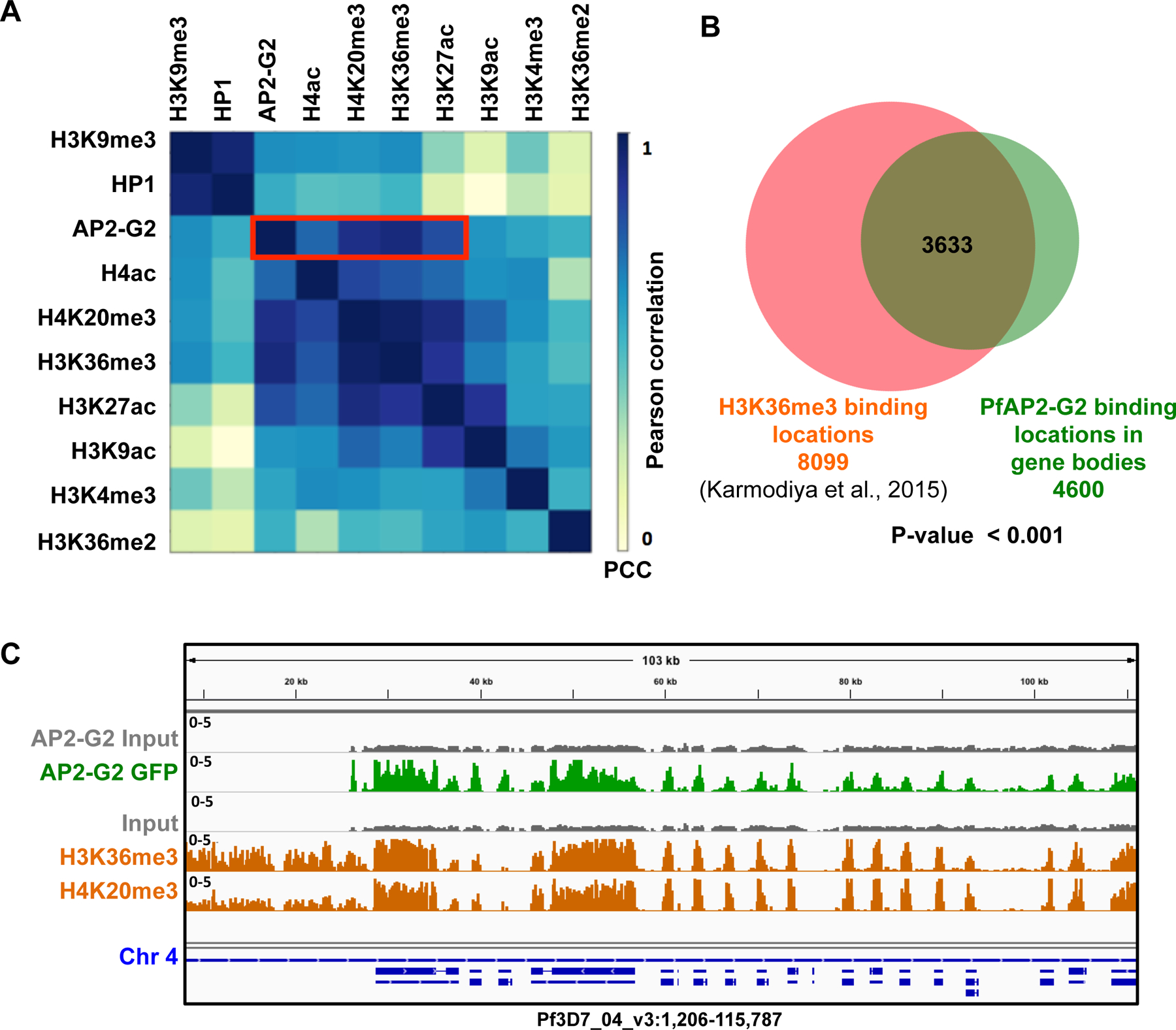
(A) Heatmap showing correlation between occupancy of PfAP2-G2 and nine histone modifications in the gene body of all P. falciparum genes (n=5735). PfAP2-G2 strongly correlates with H3K36me3 and H4K20me3 (B) Overlap of the peaks obtained by ChIP-seq on H3K36me3 marks (orange) (Jiang et al., 2013) and PfAP2-G2-GFP (green) shows 80% co-occupancy between PfAP2-G2 and H3K36me3 modified histones. (C) IGV screenshot showing co-occupancy of PfAP2-G2 with H3K36me3 and H4K20me3. First gray track represents the input for PfAP2-G2 and the second green track shows the regions bound by PfAP2-G2. The third gray track represents input for H3K36me3 and H4K20me3 and the last two orange tracks represent the regions bound by H3K36me3 and H4K20me3 as indicated in the figure. Blue track shows genes in this region.
PfAP2-G2 interacts with the chromatin remodeling machinery
To identify proteins potentially interacting in a complex with PfAP2-G2, we performed immunoprecipitations (IP) from PfAP2-G2::GFP parasites at the trophozoite stage without crosslinking using an anti-GFP antibody. Western blot analysis confirmed that the full length PfAP2-G2 protein was purified and eluted (Figure 7A), although smaller GFP-positive band(s) were prevalent. The resulting IP was separated by SDS PAGE and two regions of the gel (upper and lower band, Figure 7A) were subjected to protein analysis by mass spectrometry. The purpose of analyzing these bands separately was to ensure that we could detect all of the proteins including the low abundance protein which otherwise would be masked when analyzing all of the bands together (Figure 7B).
Figure 7. Identification of potential interacting partners of PfAP2-G2.

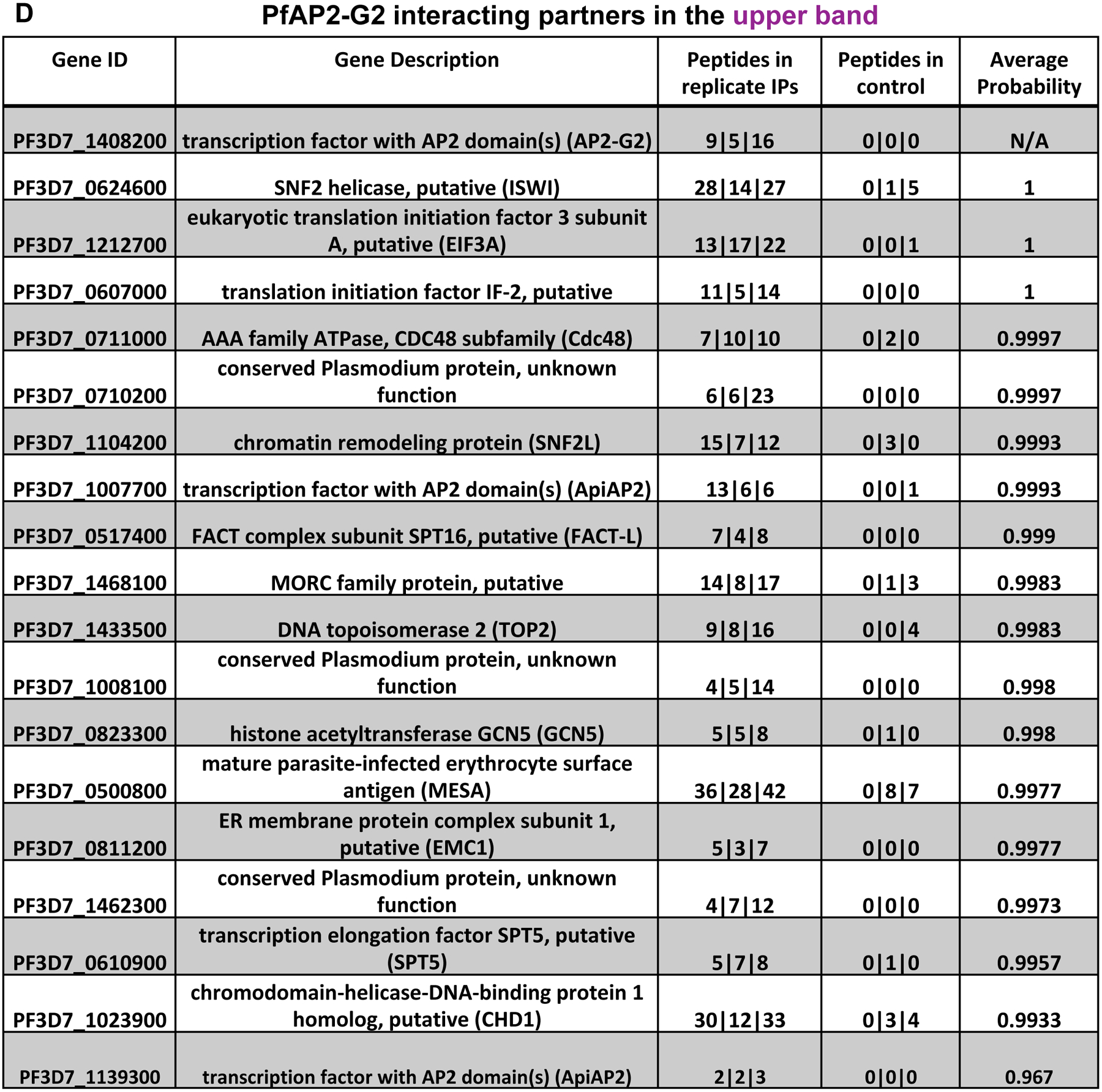
(A) Western blot showing immunoprecipitation (without crosslinking) of the PfAP2-G2 GFP parasite line using antibodies against GFP. Full length PfAP2-G2 and the lower molecular weight cleaved product were analyzed separately by mass spectrometry to identify interacting partners of PfAP2-G2. (B) Schematic showing the number and location of PfAP2-G2 peptides recovered from the upper and lower bands. (C) List of PfAP2-G2-associated proteins in the lower band with high probability of interaction measured by SAINT (pSAINT 0.9 and 1%FDR). (D) List of PfAP2-G2-associated proteins in the upper band with high probability of interaction measured by SAINT (pSAINT 0.9 and 1%FDR).
The proteomic IP data was analyzed using SAINT (Choi et al. 2011) with a pSAINT score of 0.9 or higher and 1% FDR across all three replicates (Supplementary Table 8). Although several high abundance cytoplasmic proteins (HSP70, RPS15) were detected, the majority are known to be localized to the nucleus. Reassuringly, both the upper and lower bands contained the PfAP2-G2 DNA binding domain fused to GFP (Figure 7B). In the lower band we also identified histone protein 1 (HP1), the FACT complex proteins (FACT-S and FACT-L), and several histones (Figure 7C). The upper band contained larger chromatin remodeling proteins such as ISWI, SNF2L, GCN5, FACT-L, SPT5, and a MORC family protein (Figure 7D). We also found an association with two other ApiAP2 family proteins, including PF3D7_1139300 (PF11_0404) whose motif was found to be enriched in both the transcription and ChIP-seq data suggesting that this interaction may be required for the regulation of a subset of target genes. Overall, these results demonstrate that PfAP2-G2 does not function alone, but rather interacts with a number of chromatin remodeling proteins as well as established transcription associated factors, although the presence of different complexes is likely.
Discussion
Gametocytogenesis in Plasmodium falciparum parasites is a long, 10–12 day developmental progression resulting in the formation of fertile male and female gametes that will form a zygote only upon transmission to the mosquito host. Recent studies have shed light on the commitment and differentiation of asexual parasites into sexual parasites, (reviewed in Rea, Le Roch, and Tewari 2018 and Josling, Williamson, and Llinás 2018) which is driven by the AP2-G master regulator (Kafsack et al. 2014; Sinha et al. 2014; Josling et al. 2020). However, how asexual cells are reprogrammed for gametocyte commitment, and the downstream post-commitment regulation of sexual development is not well characterized. There are known examples of gametocyte stage genes such as pfsegxp, mdv1, gexp02 and pfs16 that are expressed very early on in the asexual stages suggesting that programming for gametocytogenesis may begin early in asexual development, and disruption of this program could result in profound effects post-commitment during sexual development (Furuya et al., 2005; Joice et al., 2014; Painter et al., 2017; Nixon et al., 2018; Portugaliza et al. 2020)
In this study we show that the ApiAP2 protein PfAP2-G2 is essential for the development and maturation of P. falciparum gametocytes. PfAP2-G2 is expressed from the very early trophozoite stage through to stage V gametocytes. This is in contrast to P. berghei, where the protein is expressed from 16hpi, during the schizont stage of development (Yuda et al. 2015). Live fluorescence microscopy and nuclear fractionation show that PfAP2-G2 is localized to the nucleus of the trophozoite, schizont, and early gametocyte stages (Figure 1B). In later stage gametocytes PfAP2-G2 protein levels are reduced and expressed throughout the cytoplasm (Figure 3A). This is consistent with the mRNA abundance profile, demonstrating peak pfap2-g2 expression in the early asexual stages, whereas in gametocytes its expression is broad and weak (Van Biljon et al. 2019). The nuclear localization and strength of protein expression indicate that PfAP2-G2 is functionally important during the asexual stages and in early gametocytes.
By performing genome-wide ChIP-seq analysis at the asexual trophozoite stage and stage III gametocytes, we show that PfAP2-G2 binds extensively to both intergenic and genic regions. We note that the ChIP-seq results likely arise from full-length PfAP2-G2::GFP as well as smaller protein fragments (Figure 1C, 3B and 7B) that also contain the C-terminal AP2-domain fused to GFP. Genes bound by PfAP2-G2 in the upstream regions are largely expressed later in the gametocyte and mosquito stages. However, PfAP2-G2 also binds the promoters of genes expressed in the asexual ring stage and genes that are transcribed in schizont stages like msps encoding the merozoite surface proteins, which are regulated by PfAP2-I (Santos et al. 2017). Of course MSPs are unlikely to be required in committed gametocytes, as they no longer egress from the red blood cell for subsequent re-invasion. Interestingly, genes bound by PfAP2-G2 in stage III gametocytes are essentially the same as those bound in trophozoites. An analysis of the DNA motifs enriched in the regions bound by PfAP2-G2 identified two motifs: an AGAA motif and an ACCA motif (Figure 4B and 4G). The latter resembles the PBM-based predicted motif for PfAP2-G2, but it is different from that identified in P. berghei (GTTGT), even though the AP2 DNA-binding domain is highly conserved (97% identical). We also found enrichment for a second motif in the PfAP2-G2-bound regions, which resembles the in vitro DNA sequence motif bound by the ApiAP2 protein PF3D7_1139300 (PF11_0404) (Campbell et al. 2010). Supporting the hypothesis that these ApiAP2 proteins may function together in a complex, our immunoprecipitation experiments against PfAP2-G2::GFP detected AP2 PF3D7_1139300 (PF11_0404) as an interaction partner (Figure 7D, Supplementary Table 8).
Parasites expressing a truncated PfAP2-G2 (pfap2-g2 KO) proliferate normally in the asexual blood stages. These results are surprising given the many global transcriptional (327 transcripts, >1.5 log2FC, 0.30 local FDR) changes occurring in the asexual stages, and suggests that the parasite is tolerant to large fluctuations in gene expression. Compared with wild-type parasites, the expression levels of 90 genes (>1.5 log2FC, 0.30 local FDR) were significantly decreased during asexual development in pfap2-g2 KO parasites, including genes that have been reported to have functional roles in gametocytes, perhaps leading to the developmental stall observed in stage III gametocytes (Painter, Carrasquilla, & Llinás, 2017; Van Biljon et al., 2019). This suggests that the program for sexual progression is established very early, in the asexual stage. KO of PbAP2-G2 also resulted in the downregulation of gametocyte genes (Yuda et al. 2015). Genes upregulated in the pfap2-g2 KO were mostly those normally expressed at other later developmental stages including the gametocyte and ookinete stages (Figure 5A). In the upstream promoter regions of genes showing increased transcript abundance in the pfap2-g2 KO, we found an enrichment of the PfAP2-G2 ACCA motif. PfAP2-G2 therefore is involved in the repression of a number of genes that are not required for asexual development.
A previous study that examined the genome-wide binding of AP2-G2 from P. berghei gametocytes using ChIP-seq reported PbAP2-G2 binding at over 1,500 genes that were mostly associated with roles in asexual-stage proliferation (Yuda et al. 2015). Transcriptome analysis in gametocytes revealed the upregulation of 927 genes by more than twofold in pbap2-g2 KO lines, suggesting that PbAP2-G2 acts as a repressor for asexual-stage genes during sexual development. However, only 397 (26.5%) of the PbAP2-G2 bound genes were upregulated in the pbap2-g2 KO, leading Yuda et al. to hypothesize that relieving repression was not sufficient for the upregulation of the rest of the genes. Similarly, in our pfap2-g2 KO transcriptomic data, not all genes that are bound by PfAP2-G2 show enhanced transcript abundance; only 23/82 genes with upstream PfAP2-G2 peaks (28%) displayed significantly elevated mRNA abundance. In fact, the PfAP2-G2 motif is only present in the promoter of genes upregulated in both developmental stages indicating that, as in P. berghei, PfAP2-G2 occupancy is not always predictive of changes in gene expression, perhaps because it requires interaction with other proteins. None of the genes bound by PfAP2-G2 in the upstream region are downregulated in the pfap2-g2 KO, again highlighting the repressive action of PfAP2-G2.
The effect of PfAP2-G2 truncation upon transcript abundance is more pronounced in gametocytes. Unfortunately, due to the massive dysregulation of mRNA transcripts, it is difficult to ascertain the specific genes that cause the stall in development observed at stage III gametocytes. The significantly upregulated genes in gametocytes are mostly thought to be important for asexual blood stage development, similar to what was shown in P. berghei (Yuda et al. 2015). Interestingly, the ApiAP2 protein AP2-L, which plays a critical role in liver-stage development, is upregulated in the gametocyte stage in both the P. falciparum and P. berghei ap2-g2 knockouts, and parasites lacking PbAP2-G2 are unable to cause liver infection (Yuda et al. 2015).
We observed a moderate to strong correlation between PfAP2-G2 occupancy and that of H3K9me3 (R=0.933), followed by H4K20me3 (R=0.911), H3K27ac (R=0.826), H4ac (R=0.765) and PfHP1 (R=0.6) (Figure 6A). H3K36 methylation is a well-studied histone modification in model organisms, and carries out several roles. Studies in metazoans have shown that H3K36me3 is enriched at gene exons and plays a role in the regulation of alternative splicing (Kolasinska-Zwierz et al. 2009). Although H3K36me3 correlates with active transcription, another study found an association of this modification with facultative heterochromatin (Chantalat et al. 2011). Thus, H3K36me3 is involved with both actively transcribed as well as silenced regions and may contribute to the formation of heterochromatin in combination with other histone modifications. At a subset of heterochromatic genes, binding to HP1a is required for enzyme-mediated H3K36me3 demethylation suggesting that there are different ways in which H3K36me3 regulates chromatin (Lin et al. 2012). In P. falciparum, H3K36me3 is present along the entire gene body of silent var genes including the transcription start site (TSS), and it is deposited by the P. falciparum variant-silencing methyltransferase containing a SET domain (PfSETvs) (Jiang et al. 2013). However, enrichment of H3K36me3 was also found at the 3’ end of other ring-stage active genes besides the variable antigen var, rifin and stevor genes in PfSET KO parasites. This result indicates there is an alternative methyltransferase associated with H3K36 methylation.
We also found that PfHP1 localized with PfAP2-G2 towards the end of the chromosomes and is associated with both sub-telomeric and chromosome-internal var genes and Plasmodium-exported proteins (Supplementary Fig. 11A, B). A direct interaction between PfHP1 and PfAP2-G2 was supported by IP-MS (Figure 7C). PfAP2-G2 also shows perinuclear localization as previously described for PfHP1 and repressed var genes (Supplementary Fig. 12) (Flueck et al. 2009; Guizetti et al. 2013). However future experiments are needed to determine a direct functional link between PfAP2-G2 and HP1. We also found a significant association between PfAP2-G2 and the MORC protein PF3D7_1468100 (Figure 7D). MORC proteins have been identified to play important roles in chromatin compaction in plants and animals and was recently established as the upstream transcriptional repressor of sexual commitment in Toxoplasma gondii (Farhat et al. 2020). This study found that MORC forms a complex with T. gondii ApiAP2 transcription factors at sexual stage genes. Two of the T. gondii ApiAP2 proteins found to associate with MORC by IP-MS are TgAP2IV-2 and TgAP2IX-9, whose AP2 DNA-binding domains are most identical to the PfAP2-G2 AP2 domain (Farhat et al. 2020). PfAP2-G2 also interacts with the P. falciparum ISWI chromatin-remodeling protein (Figure 7D), which was recently identified in a complex with the MORC protein at var gene promoters (Bryant et al. 2020). We speculate that PfAP2-G2 plays a role in regulating regions of the genome which need to be silenced to prevent spurious transcription.
Previous studies have shown that expression of the master gametocyte regulator pfap2-g peaks at two distinct points during asexual blood stage development (Poran et al. 2017). The initial pfap2-g expression peak in trophozoites is associated with the activation of genes encoding ISWI, SNF2L, and the ApiAP2 protein P3D7_1222400. The second expression peak occurs just before merozoite egress from committed schizonts and leads to the expression of genes encoding LSD2, HDA1 and the AP2-G2 associated ApiAP2 protein PF3D7_1139300 (Poran et al. 2017). Therefore, we propose that PF3D7_1139300 may act in a complex with PfAP2-G2 to compress chromatin structure. In summary, we have demonstrated that PfAP2-G2 plays an important role in gene repression in concert with other chromatin-related proteins during the maturation of the P. falciparum parasite sexual stages.
Materials & Methods:
Construction of plasmids for tagging the 3’end of pfap2-g2 for pfap2-g2 gene disruption
The pDC2-cam-CRT-GFP (Fidock et al. 2000) plasmid was used to tag the 3’ end of the gene pfap2-g2 with gfp. A 948 bp homologous fragment from the 3’ end of the gene was amplified from 3D7 genomic DNA using the oligonucleotide pairs (Forward Primer P7 and Reverse Primer P8, see primer list), which also encoded restriction sites BglII and XhoI for cloning. This fragment was ligated into the BglII-and XhoI-digested pDC2-cam-CRT-GFP resulting in the final vector pDC2-cam-AP2-G2-GFP.
To create the pfap2-g2 disrupted line, the ap2-g2 gene locus was targeted using the selection linked integration (SLI) method (Birnbaum et al. 2017). To do this a 500 bp fragment of the 5’ end of pfap2-g2 was amplified using a forward primer with a NotI restriction site as well as a single point mutation in the homologous sequence to introduce a stop codon (Forward Primer P9) and a reverse primer with an MluI site (Reverse Primer P10) to be ligated into the final vector. This fragment was cloned (in frame) into pSLI-TGD using NotI and MluI restriction sites resulting in the final vector, pSLI-TGD-AP2-G2. A list of all oligonucleotide primers used is available in Supplementary Table 9.
Culturing, synchronization, transfection, and cloning of Plasmodium falciparum parasites
Plasmodium falciparum parasites were cultured as previously described (Trager & Jensen, 1976) and maintained in 6% O2 and 5% CO2 and grown in sterile filtered RPMI 1640 media (Gibco) supplemented with 2 M HEPES, NaHCO3 (2g/L), 0.1 M hypoxanthine, 0.25%(w/v) Albumax II (Gibco), and gentamycin (50 ug/ml). The parasites were maintained at a hematocrit of 3% and were kept between 2–5% parasitemia or greater based on the needs of the individual experiments. Parasite synchronization was performed using 5% sorbitol (Lambros and Vandenberg 1979).
To generate transgenic AP2-G2::GFP and AP2-G2 KO lines, transfections were carried out according to a standard protocol (Deitsch, Driskill, and Wellems 2001). The plasmid pDC2-cam-AP2-G2-GFP was transfected into the 3D7 strain of P. falciparum whereas pSLI-TGD-AP2-G2 was transfected into the 3D7 clone, E5 (Rovira-Graells et al. 2012). In brief, 100 μg of plasmid DNA (maxi prepped using Qiagen kit) was preloaded into O+ RBCs at 50% hematocrit in cytomix prior to culturing with parasite-infected RBCs. After reinvasion, media containing 2.5 nM WR99210 was added to select for transgenic parasites. Stable integrants were maintained under constant drug pressure and were PCR-verified using specific primers (P1, P2, P4, P5, P6) outside the homology region after the isolation of gDNA (DNeasy Blood and Tissue kit, Qiagen). The resulting PfAP2G2::GFP and pfap2-g2 KO parasites were cloned by limiting dilution (Rosario 2008) and verified by diagnostic PCR (Supplementary Fig. 1 & 2A) and whole genome sequencing. Sequencing analysis was done by creating a reference genome with GFP and rest of the plasmid at the expected location and reads were aligned to it.
Plasmodium falciparum gametocyte production
Gametocytes were generated using the nutrient deprivation induction method as described (Miao et al. 2013). Briefly, synchronized trophozoites were cultured at ~2% parasitemia using 50% fresh RBC at 4% hematocrit. When the parasitemia reached 7–10%, gametocyte production was induced by nutrient deprivation. The following day, stressed schizonts were split 50% into two flasks by adding fresh blood and fresh media. The following day, ring-stage committed gametocytes were counted as Day 1 of gametocytogenesis. To prevent the reinvasion and propagation of asexual parasites, parasites were treated with media with heparin (20 U/ml) from Day 1 post induction through Day 4. The media was changed daily to ensure the proper development and growth of the gametocytes.
Nuclear and cytoplasmic protein extraction from parasites for western blotting
Nuclear and cytoplasmic extracts from parasites were prepared as previously described (Voss et al. 2002) using 5–7% of the synchronized parasites. The proteins from the nuclear and cytoplasmic fractions were run on a gel (mini PROTEAN precast TGX gels) and then transferred to a nitrocellulose membrane. The membrane was then blocked using 5% w/v non-fat milk powder in 1X PBS-0.05% v/v Tween for 45 minutes at room temperature. The membrane was then incubated overnight at 4°C with an anti-GFP (ROCHE Anti-GFP, from mouse IgG1K, Millipore Sigma) (1:1000), anti-histone (Anti-Histone H3 antibody, AbCam) (1:3000), or anti-aldolase (Anti-Plasmodium aldolase antibody (HRP), AbCam) (1:1000) primary antibody in the blocking buffer. After incubation, the membrane was washed three times with 1X PBS-0.05% v/v Tween and was incubated with the appropriate secondary antibodies, then washed. The secondary antibodies used were as follows: peroxidase-conjugated goat anti-rat HRP conjugate (Millipore) / goat anti-rabbit HRP conjugate (Millipore) / goat anti-mouse HRP conjugate (Pierce) was used (1:3000). Bound antibodies were then visualized on a film after enhancing the signal with ECL (Pierce) as the substrate.
Parasite growth assays and multiplication rate
To compare the growth rates of WT and pfap2-g2 KO parasites we used a SYBR green growth assay (Vossen et al., 2010). Parasites were sampled every 24 hours for 10 days, starting at 0.1% parasitemia at the trophozoite stage. Both the WT and pfap2-g2 KO strains were synchronized using 5% w/v sorbitol (described above) 3x before the start of the timecourse. Parasites were seeded in a 25 cm2 culture flask at 0.1% parasitemia and 3% hematocrit. To evaluate growth differences every 24 hours for 10 days, 100 μl of parasite culture was collected in triplicate, transferred to a 96-well plate, and stored at −80°C. Following the completion of the timecourse, the parasites were thawed at room temperature, 100 μl of SYBR green I (Molecular Probes, Eugene Oregon) was diluted into parasite lysis buffer (20mM Tris-HCl pH 7.5, 5mM EDTA, 0.08% Triton X-100, 0.008% saponin in PBS) (0.2 μl of SYBR Green I/ml of lysis buffer) and added to each well and mixed. The plates were incubated at 37°C for 2 hours. Cell growth was measured as quantification of DNA by SYBR green I using a Tecan GENios microplate detection device at the excitation wavelength and emission wavelength of 485 nm and 535 nm, respectively. All values were plotted as an average of three biological replicates (±S.D.) using GraphPad Prism (version 7). Linear model construction and ANOVA statistical analysis were performed in R (https://www.r-project.org). To calculate the multiplication rate, three independent biological experiments were performed in which synchronized WT and pfap2-g2 KO parasites were seeded at 1% parasitemia and monitored for subsequent reinvasion of red blood cells. After reinvasion the number of ring stage parasites were counted from Giemsa-stained smears by microscopy.
RNA purification and cDNA synthesis for DNA microarrays
Total RNA was extracted from tightly synchronized PfAP2-G2::KO and WT parasites every 6 hours, starting at 3 hpi, throughout the 48 hour asexual life cycle. A separate timecourse was designed to collect RNA from gametocytes starting at Day 1 and every 12 hours for the following 7 days. RNA was extracted from parasite-infected RBCs collected at each time point using TRIzol (ThermoFisher Scientific), following the manufacturer’s protocol. cDNA synthesis and DNA microarray analysis was carried out as previously described (Painter et al. 2013). Two-channel Agilent DNA microarrays (AMADID 037237) were hybridized, washed, and scanned on an Axon 4200A scanner. Signal intensities for each gene were extracted from the scanned image using Agilent Feature Extraction Software version 9.5. Detailed microarray protocols can be found at http://llinaslab.psu.edu/protocols/. The data were analyzed by Significance Analysis of Microarrays (SAM) (Tusher et al. 2001) for the significantly (>1.5 log2FC, 0.30 local FDR) changing genes between the WT and ap2-g2 KO lines using two-class paired t-test throughout the timecourse. No data was available for TP6 due to technical reasons. All heat maps were clustered using Cluster 4.0 and was visualized using Java Tree View.
Chromatin immunoprecipitation and Library preparation for sequencing (ChIP-Seq)
ChIP was performed as described (Josling et al. 2020) using synchronized trophozoite and gametocyte stage PfA2-G2-GFP parasites (around 7% parasitemia). Extracted chromatin was sheared in SDS lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA pH 8.0, 0.1 mM EGTA pH 8.0, 1 mM DTT and protease inhibitors) (200 μl 1× 109 trophozoites and 5×108 gametocytes) to obtain a fragment size of 100–150bp using an M220 focused-ultrasonicator (Covaris Inc.) using the following settings: peak power 75W, 2% duty factor, 200 cycles per burst and total treatment time of 600 s. Sonication time was optimized for PfAP2-G2 using the crosslinked DNA and shearing it for 0, 4, 8, 10, 12 and 15 minutes and then running it on agarose gel for the size estimate. For immunoprecipitations, sheared chromatin was pre-cleared using 20 μl/ml of magnetic beads A/G (Millipore 16–663) for 2 hours at 4°C with gentle agitation. The supernatant was collected and 50μl of the aliquot was removed as input. The rest of the chromatin was incubated overnight at 4°C with 1 μg of polyclonal anti-GFP antibody (Abcam ChIP grade, Abcam 290) or, as control, the same amount of IgG. The immunoprecipitated antibody/chromatin complex was collected using Protein A/G magnetic beads (Millipore 16–663). The input and ChIP samples were reverse cross-linked overnight at 45°C using 0.2M final concentration of NaCl. The samples were then treated with RNAase (30min at 37°C) and proteinase K (3 μL of 20 mg/mL with 2 hours incubation at 45°C) and purified by using the QIAGEN MinElute PCR purification kit. Library was prepared as described earlier (Santos et al. 2017). The final library was quantified using a Qubit fluorometer HD DNA kit and analyzed using an Agilent DNA 1000 Bioanalyzer to assess the quality, size distribution, and detection of any artifacts. Sequencing was performed using an Illumina Hiseq 2500 to obtain 150 bp single-end reads.
ChIP-seq data analysis
ChIP-seq data analysis was done using tools in Galaxy (usegalaxy.org). Before starting the analysis, the quality of the sequencing reads were assessed using FastQC and were trimmed to remove adapter and low quality bases using Trimmomatic (Bolger, Lohse, and Usadel 2014). The resulting reads were mapped to the P. falciparum genome (Pf 3D7 v28, obtained from PlasmoDB) using BWA-MEM (Li and Durbin 2009) and duplicates reads were removed using SAMtools (Li et al. 2009). Mapped sequences were converted into bigwig files using bamCoverage (Ramírez et al. 2016) and were viewed in using the Integrative Genomics Viewer (IGV) (Thorvaldsdóttir, Robinson, and Mesirov 2013) for each input and treatment. MACS2 (Feng et al. 2012) was used to call peaks with q-value cutoff of 0.05. The common overlapping intervals between replicates were established using Multiple Intersect function of BEDtools, and the overlapping intervals were combined into a single file using MergeBED (Quinlan and Hall 2010). The genes closest to the peak summits were identified using closestBED. Peaks that were farther than 2.5Kb were removed from the analysis. When the peak was present between two genes then only the gene closest to the peak was considered for the analysis.
Peak sequences were extracted using extract genomic DNA tool using genomic coordinates, and motifs associated with called peaks were identified using DREME (Bailey 2011) and compared to identified ApiAP2 motifs using TOMTOM (Gupta et al. 2007). The correlation heatmap of the replicates performed on GFP tagged PfAP2-G2 ChIP-seq was made using multiBigwigSummary and plotCorrelation in the deepTools suite (Ramírez et al. 2016).
Correlation analysis of PfAP2-G2 with other histone marks
Correlations were calculated between the occupancy of PfAP2-G2 and that of various histone marks previously reported in the literature, including H3K9me3, H4ac, H4K20me3, H3K26ac, H3K9ac, H3K4me3 (Karmodiya et al. 2015), H3K36me2 and H3K36me3 (Jiang et al. 2013) and heterochromatin protein 1 (Brancucci et al. 2014) First, read counts were obtained from PfAP2-G2 and nine histone marks in the gene body of all P. falciparum genes (n=5735; PlasmoDB annotations, release 28.0). NCIS (Liang and Keleş 2012) was used to scale a control input experiment to each analyzed signal ChIP-seq experiment. Pairwise Pearson correlation coefficient of normalized read counts across 5735 regions for PfAP2-G2 and nine histone marks. Then, proteins were ordered using complete-linkage hierarchical clustering with Euclidean distance.
PfAP2-G2-GFP Immunoprecipitation and Mass Spectrometry
Nuclear extraction was performed from the PfAP2-G2::GFP parasites using lysis buffer (20 mM HEPES pH 7.8, 10 mM KCl, 1 mM EDTA, 1 mM DTT, 1x protease inhibitors) and a high salt extraction buffer (20 mM HEPES pH 7.8, 800 mM KCl, 1 mM EDTA, 1 mM DTT, 1x protease inhibitors) as previously described (Voss et al. 2002). A day before the immunoprecipitation, ~1mg of M-270 Epoxy Dynabeads (Invitrogen) per 100 ml of culture were conjugated with polyclonal anti-GFP antibodies at concentration of 5ug/mg (Abcam ab290) or with IgG (Abcam ab46540) overnight at 30°C as previously described (Joshi et al. 2013). Immunoprecipitation was carried out after washing the conjugated beads three times with dilution buffer (20 mM HEPES pH 7.8, 1 mM EDTA, 1 mM DTT, 1x protease inhibitors) and incubating with diluted nuclear extracts for 90 minutes at 4°C. The beads were again washed five times with dilution buffer and three times with cold 1X PBS. Protein was eluted using 20 μl loading buffer by shaking for 10 minutes at 70°C at 1000 rpm and protein was stored at −20°C. Samples were quality controlled by western blotting and subjected to in-gel tryptic digest.
For tryptic digest the samples in polyacrylamide gel slices were destained with 50mM AmBic/50% ACN (Acetonitrile) and dehydrated using 100% ACN. Disulfide bonds in protein were reduced with 10mM DTT and reduced cysteine residues were then alkylated with 50mM iodoacetamide. Finally, the processed samples were digested with trypsin (Trypsin gold Promega, 6ng/μl) overnight at 37°C on a thermomixer, and peptides were extracted from the gels. The dried peptide pellets were analyzed LC-MS/MS using a Q Exactive mass spectrometer (Indiana University Core Proteomics).
The data was processed using Trans-Proteomic Pipeline (TPP) as previously described with a few modifications (Deutsch et al. 2010). Spectra were searched against the P. falciparum proteome and contaminant repository database (CRAPome) using tandem and comet searches (Mellacheruvu et al. 2013). This search was combined in InterProphet and the proteins were searched with ProteinProphet. Only proteins with error rate less than 0.01 were reported. The immunopurification assay was performed 3 times and the data was combined to identify the significantly enriched proteins between the IP and control using SAINT (Choi et al. 2011). Proteins with probability score of 0.99 or greater were considered for analysis.
Supplementary Material
Acknowledgements
This project was largely funded by NIH/NIAID 1R01 AI125565 (ML). NY and SM were supported by R01 GM121613 (SM). We thank Kelly Rios for assistance with proteomic data analysis and Scott Lindner for critical feedback.
Data availability
All ChIP-seq sequencing reads are deposited at the NCBI Sequence Read Archive (SRA) in the Gene Expression Omnibus (GEO) under the accession number GSE157753. All DNA microarray data are also deposited at GEO under accession number GSE160923 (asexual) and GSE160924 (sexual gametocyte). Whole genome sequencing reads for ap2-g2 gfp and ap2-g2 KO parasites are directly submitted to SRA under accession number PRJNA673876.
References
- Aguilar Ruth, Magallon-Tejada Ariel, Achtman Ariel H., Moraleda Cinta, Joice Regina, Cisteró Pau, Li Connie S. N. Suen Wai, Nhabomba Augusto, Macete Eusebio, Mueller Ivo, Marti Matthias, Alonso Pedro L., Menéndez Clara, Schofield Louis, and Mayor Alfredo. 2014. “Molecular Evidence for the Localization of Plasmodium Falciparum Immature Gametocytes in Bone Marrow.” Blood 123(7):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano Pietro, Premawansa Sunil, Bruce Marian C., and Carter Richard. 1991. “A Stage Specific Gene Expressed at the Onset of Gametocytogenesis in Plasmodium Falciparum.” Molecular and Biochemical Parasitology 46(1):81–88. [DOI] [PubMed] [Google Scholar]
- Armistead Jennifer S., Moraes Barros Roberto R., Gibson Tyler J., Kite Whitney A., Mershon J. Patrick, Lambert Lynn E., Orr-Gonzalez Sachy E., Sá Juliana M., Adams John H., and Wellems Thomas E.. 2018. “Infection of Mosquitoes from in Vitro Cultivated Plasmodium Knowlesi H Strain.” International Journal for Parasitology 48(8):601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey Timothy L. 2011. “DREME: Motif Discovery in Transcription Factor ChIP-Seq Data.” Bioinformatics 27(12):1653–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Babu M. Madan, Lakshminarayan M. Iyer, and Aravind L. 2005. “Discovery of the Principal Specific Transcription Factors of Apicomplexa and Their Implication for the Evolution of the AP2-Integrase DNA Binding Domains.” Nucleic Acids Research 33(13):3994–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson James G., Drew Damien R., Boyle Michelle J., Feng Gaoqian, Fowkes Freya J. I., and Richards Jack S.. 2016. “Merozoite Surface Proteins in Red Blood Cell Invasion, Immunity and Vaccines against Malaria.” FEMS Microbiology Reviews 40(3):343–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biljon Van, Riëtte Roelof Van Wyk, Painter Heather J., Orchard Lindsey, Reader Janette, Niemand Jandeli, Llinás Manuel, and Birkholtz Lyn Marie. 2019. “Hierarchical Transcriptional Control Regulates Plasmodium Falciparum Sexual Differentiation.” BMC Genomics 20(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum Jakob, Flemming Sven, Reichard Nick, Soares Alexandra Blancke, Mesén-Ramírez Paolo, Jonscher Ernst, Bergmann Bärbel, and Spielmann Tobias. 2017. “A Genetic System to Study Plasmodium Falciparum Protein Function.” Nature Methods 14(4):450–56. [DOI] [PubMed] [Google Scholar]
- Bischoff Emmanuel and Vaquero Catherine. 2010. “In Silico and Biological Survey of Transcription-Associated Proteins Implicated in the Transcriptional Machinery during the Erythrocytic Development of Plasmodium Falciparum.” BMC Genomics 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger Anthony M., Lohse Marc, and Usadel Bjoern. 2014. “Trimmomatic: A Flexible Trimmer for Illumina Sequence Data.” Bioinformatics 30(15):2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdech Zbynek, Llinás Manuel, Pulliam Brian Lee, Wong Edith D., Zhu Jingchun, and DeRisi Joseph L.. 2003. “The Transcriptome of the Intraerythrocytic Developmental Cycle of Plasmodium Falciparum.” PLoS Biology 1(1):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancucci Nicolas M.B., Bertschi Nicole L., Zhu Lei, Niederwieser Igor, Chin Wai Hoe, Wampfler Rahel, Freymond Céline, Rottmann Matthias, Felger Ingrid, Bozdech Zbynek, and Voss Till S.. 2014. “Heterochromatin Protein 1 Secures Survival and Transmission of Malaria Parasites.” Cell Host and Microbe 16(2):165–76. [DOI] [PubMed] [Google Scholar]
- Brancucci Nicolas M B, Bertschi Nicole L., Zhu Lei, Niederwieser Igor, Chin Wai Hoe, Wampfler Rahel, Rottmann Matthias, Felger Ingrid, Bozdech Zbynek, and Voss Till S.. 2014. “Article Heterochromatin Protein 1 Secures Survival and Transmission of Malaria Parasites.” 165–76. [DOI] [PubMed]
- Bruce MC, Alano P, Duthie S, and Carter R. 1989. “Commitment of the Malaria Parasite Plasmodium Falciparum to Sexual and Asexual Development.” Parasitology 100(2):191–200. [DOI] [PubMed] [Google Scholar]
- Bruce Marian C., Carter Roderick N., Nakamura Kei ichiro, Aikawa Masamichi, and Carter Richard. 1994. “Cellular Location and Temporal Expression of the Plasmodium Falciparum Sexual Stage Antigen Pfs16.” Molecular and Biochemical Parasitology 65(1):11–22. [DOI] [PubMed] [Google Scholar]
- Bryant Jessica M., Baumgarten Sebastian, Dingli Florent, Loew Damarys, Sinha Ameya, Claës Aurélie, Preiser Peter R., Dedon Peter C., and Scherf Artur. 2020. “ Exploring the Virulence Gene Interactome with CRISPR / DC As9 in the Human Malaria Parasite.” Molecular Systems Biology 16(8):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell Tracey L., de Silva Erandi K., Olszewski Kellen L., Elemento Olivier, and Llinás Manuel. 2010. “Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite.” PLoS Pathogens 6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantalat Sophie, Depaux Arnaud, Héry Patrick, Barral Sophie, Thuret Jean Yves, Dimitrov Stefan, and Gérard Matthieu. 2011. “Histone H3 Trimethylation at Lysine 36 Is Associated with Constitutive and Facultative Heterochromatin.” Genome Research 21(9):1426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay Rana, Rathore Dharmendar, Fujioka Hishasi, Kumar Sanjai, De la Vega Patricia, Haynes David, Moch Kathleen, Fryauff David, Wang Ruobing, Carucci Daniel J., and Hoffman Stephen L.. 2003. “PfSPATR, a Plasmodium Falciparum Protein Containing an Altered Thrombospondin Type I Repeat Domain Is Expressed at Several Stages of the Parasite Life Cycle and Is the Target of Inhibitory Antibodies.” Journal of Biological Chemistry 278(28):25977–81. [DOI] [PubMed] [Google Scholar]
- Choi Hyungwon, Larsen Brett, Lin Zhen Yuan, Breitkreutz Ashton, Mellacheruvu Dattatreya, Fermin Damian, Qin Zhaohui S., Tyers Mike, Gingras Anne Claude, and Nesvizhskii Alexey I.. 2011. “SAINT: Probabilistic Scoring of Affinity Purificationg-Mass Spectrometry Data.” Nature Methods 8(1):70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitsch Kirk W., Driskill Casey L., and Wellems Thomas E.. 2001. “Transformation of Malaria Parasites by the Spontaneous Uptake and Expression of DNA from Human Erythrocytes.” Nucleic Acids Research 29(3):850–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire Emily. 2019. “RNA-Seq Analysis Illuminates the Early Stages of Plasmodium Liver Infection.” [DOI] [PMC free article] [PubMed]
- Deutsch Eric W., Mendoza Luis, Shteynberg David, Farrah Terry, Lam Henry, Tasman Natalie, Sun Zhi, Nilsson Erik, Pratt Brian, Prazen Bryan, Eng Jimmy K., Martin Daniel B., Nesvizhskii Alexey I., and Aebersold Ruedi. 2010. “A Guided Tour of the Trans-Proteomic Pipeline.” Proteomics 10(6):1150–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk Melissa R., van Schaijk Ben C. L., Khan Shahid M., van Dooren Maaike W., Ramesar Jai, Kaczanowski Szymon, van van Gemert Geert Jan, Kroeze Hans, Stunnenberg Hendrik G., Eling Wijnand M., Sauerwein Robert W., Waters Andrew P., and Janse Chris J.. 2010. “Three Members of the 6-Cys Protein Family of Plasmodium Play a Role in Gamete Fertility.” PLoS Pathogens 6(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy PE and Kaslow DC. 1997. “A Novel Malaria Protein, Pfs28, and Pfs25 Are Genetically Linked and Synergistic as Falciparum Malaria Transmission-Blocking Vaccines.” Infection and Immunity 65(3):1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker Andrea, Bushell Ellen S. C., Tewari Rita, and Sinden Robert E.. 2008. “Reverse Genetics Screen Identifies Six Proteins Important for Malaria Development in the Mosquito.” Molecular Microbiology 70(1):209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemento Olivier, Slonim Noam, and Tavazoie Saeed. 2007. “A Universal Framework for Regulatory Element Discovery across All Genomes and Data Types.” Molecular Cell 28(2):337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa Diego A., Vega-Rodriguez Joel, Flores-Garcia Yevel, Noe Amy R., Muñoz Christian, Coleman Russell, Bruck Torben, Haney Keith, Stevens Alex, Retallack Diane, Allen Jeff, Vedvick Thomas S., Fox Christopher B., Reed Steven G., Howard Randall F., Salman Ahmed M., Janse Chris J., Khan Shahid M., Zavala Fidel, and Gutierrez Gabriel M.. 2017. “The Plasmodium Falciparum Cell-Traversal Protein for Ookinetes and Sporozoites as a Candidate for Preerythrocytic and Transmission-Blocking Vaccines.” Infection and Immunity 85(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat Dayana C., Swale Christopher, Dard Céline, Cannella Dominique, Ortet Philippe, Barakat Mohamed, Sindikubwabo Fabien, Belmudes Lucid, De Bock Pieter Jan, Couté Yohann, Bougdour Alexandre, and Hakimi Mohamed Ali. 2020. “A MORC-Driven Transcriptional Switch Controls Toxoplasma Developmental Trajectories and Sexual Commitment.” Nature Microbiology 5(4):570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Jianxing, Liu Tao, Qin Bo, Zhang Yong, and Liu Xiaole Shirley. 2012. “Identifying ChIP-Seq Enrichment Using MACS.” Nature Protocols 7(9):1728–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D. 2000. “Mutations in the P. Falciparum Digestive Vacuole Transmembrane Protein PfCRT and Evidence for Their Role in Chloroquine Resistance.” Molecular Cell 6(4):861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flueck Christian, Bartfai Richard, Niederwieser Igor, Witmer Kathrin, Alako Blaise T. F., Moes Suzette, Bozdech Zbynek, Jenoe Paul, Stunnenberg Hendrik G., and Voss Till S.. 2010. “A Major Role for the Plasmodium Falciparum ApiAP2 Protein PfSIP2 in Chromosome End Biology.” PLoS Pathogens 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flueck Christian, Bartfai Richard, Volz Jennifer, Niederwieser Igor, Salcedo-Amaya Adriana M., Alako Blaise T. F., Ehlgen Florian, Ralph Stuart A., Cowman Alan F., Bozdech Zbynek, Stunnenberg Hendrik G., and Voss Till S.. 2009. “Plasmodium Falciparum Heterochromatin Protein 1 Marks Genomic Loci Linked to Phenotypic Variation of Exported Virulence Factors.” PLoS Pathogens 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya Tetsuya, Mu Jianbing, Hayton Karen, Liu Anna, Duan Junhui, Nkrumah Louis, Joy Deirdre A., Fidock David A., Fujioka Hisashi, Vaidya Akhil B., Wellems Thomas E., and Su Xin Zhuan. 2005. “Disruption of a Plasmodium Falciparum Gene Linked to Male Sexual Development Causes Early Arrest in Gametocytogenesis.” Proceedings of the National Academy of Sciences of the United States of America 102(46):16813–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratraud Paul, Huws Enlli, Falkard Brie, Adjalley Sophie, Fidock David A., Berry Laurence, Jacobs William R., Baird Mark S., Vial Henri, and Kremer Laurent. 2009. “Oleic Acid Biosynthesis in Plasmodium Falciparum: Characterization of the Stearoyl-CoA Desaturase and Investigation as a Potential Therapeutic Target.” PLoS ONE 4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizetti Julien, Martins Rafael Miyazawa, Guadagnini Stéphanie, Claes Aurélie, and Scherfa Artur. 2013. “Nuclear Pores and Perinuclear Expression Sites of Var and Ribosomal DNA Genes Correspond to Physically Distinct Regions in Plasmodium Falciparum.” Eukaryotic Cell 12(5):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Shobhit, Stamatoyannopoulos John A., Bailey Timothy L., and Noble William Stafford. 2007. “Quantifying Similarity between Motifs.” Genome Biology 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss Kirsten, Nie Hui, Kumar Sumit, Daly Thomas M., Bergman Lawrence W., and Matuschewski Kai. 2008. “Functional Characterization of a Redundant Plasmodium TRAP Family Invasin, TRAP-like Protein, by Aldolase Binding and a Genetic Complementation Test.” Eukaryotic Cell 7(6):1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikadai Hiromi, Saliba Kathryn Shaw, Kanzok Stefan M., McLean Kyle J., Tanaka Takeshi Q., Cao Jun, Williamson Kim C., and Jacobs-Lorena Marcelo. 2013. “Transposon Mutagenesis Identifies Genes Essential for Plasmodium Falciparum Gametocytogenesis.” Proceedings of the National Academy of Sciences of the United States of America 110(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino Tomoko, Yano Kazuhiko, Chinzei Yasuo, and Yuda Masao. 2004. “Cell-Passage Activity Is Required for the Malarial Parasite to Cross the Liver Sinusoidal Cell Layer.” PLoS Biology 2(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsara Leslie S., Zhou Yaxian, Do Julie, Dungel Samrita, Fishbaugher Matthew E., Betz Will W., Nguyen Thao, Navarro Mary Jane, Flannery Erika L., Vaughan Ashley M., Kappe Stefan H. I., and Ghosh Anil K.. 2018. “PfCap380 as a Marker for Plasmodium Falciparum Oocyst Development in Vivo and in Vitro.” Malaria Journal 17(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeninga Myriam, Quinn Jennifer, and Petter Michaela. 2019. “ApiAP2 Transcription Factors in Apicomplexan Parasites.” Pathogens 8(2):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Lubin, Mu Jianbing, Zhang Qingfeng, Ni Ting, Srinivasan Prakash, Rayavara Kempaiah, Yang Wenjing, Turner Louise, Lavstsen Thomas, Theander Thor G., Peng Weiqun, Wei Guiying, Jing Qingqing, Wakabayashi Yoshiyuki, Bansal Abhisheka, Luo Yan, Ribeiro José M. C., Scherf Artur, Aravind L, Zhu Jun, Zhao Keji, and Miller Louis H.. 2013. “PfSETvs Methylation of Histone H3K36 Represses Virulence Genes in Plasmodium Falciparum.” Nature 499(7457):223–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joice Regina, Nilsson Sandra K., Montgomery Jacqui, Dankwa Selasi, Egan Elizabeth, Morahan Belinda, Seydel Karl B., Bertuccini Lucia, Alano Pietro, Williamson Kim C., Duraisingh Manoj T., Taylor Terrie E., Milner Danny A., and Marti Matthias. 2014. “Plasmodium Falciparum Transmission Stages Accumulate in the Human Bone Marrow.” Science Translational Medicine 6(244):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi Preeti, Greco Todd M., Guise Amanda J., Luo Yang, Yu Fang, Nesvizhskii Alexey I., and Cristea Ileana M.. 2013. “The Functional Interactome Landscape of the Human Histone Deacetylase Family.” Molecular Systems Biology 9(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josling Gabrielle A., Russell Timothy J., Venezia Jarrett, Orchard Lindsey, van Biljon Riëtte, Painter Heather J., and Llinás Manuel. 2020. “Dissecting the Role of PfAP2-G in Malaria Gametocytogenesis.” Nature Communications 11(1):1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josling Gabrielle A., Williamson Kim C., and Llinás Manuel. 2018. “Regulation of Sexual Commitment and Gametocytogenesis in Malaria Parasites.” Annual Review of Microbiology 72(1):501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack Björn F. C., Rovira-Graells Núria, Clark Taane G., Bancells Cristina, Crowley Valerie M., Campino Susana G., Williams April E., Drought Laura G., Kwiatkowski Dominic P., Baker David A., Cortés Alfred, and Llinás Manuel. 2014. “A Transcriptional Switch Underlies Commitment to Sexual Development in Malaria Parasites.” Nature 507(7491):248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmodiya Krishanpal, Pradhan Saurabh J., Joshi Bhagyashree, Jangid Rahul, Reddy Puli Chandramouli, and Galande Sanjeev. 2015. “A Comprehensive Epigenome Map of Plasmodium Falciparum Reveals Unique Mechanisms of Transcriptional Regulation and Identifies H3K36me2 as a Global Mark of Gene Suppression.” Epigenetics and Chromatin 8(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinska-Zwierz Paulina, Down Thomas, Latorre Isabel, Liu Tao, Liu X. Shirley, and Ahringer Julie. 2009. “Differential Chromatin Marking of Introns and Expressed Exons by H3K36me3.” Nature Genetics 41(3):376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros Chris and Vandenberg Jerome P.. 1979. “Synchronization of Plasmodium Falciparum Erythrocytic Stages in Culture Author (s): Chris Lambros and Jerome P. Vanderberg Published by : Allen Press on Behalf of The American Society of Parasitologists Stable URL : Https://Www.Jstor.Org/Stable/3280287.” Journal Parasitology 65(3):418–20. [PubMed] [Google Scholar]
- Li Heng and Durbin Richard. 2009. “Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform.” Bioinformatics 25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Heng, Handsaker Bob, Wysoker Alec, Fennell Tim, Ruan Jue, Homer Nils, Marth Gabor, Abecasis Goncalo, and Durbin Richard. 2009. “The Sequence Alignment/Map Format and SAMtools.” Bioinformatics 25(16):2078–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Kun and Keleş Sündüz. 2012. “Normalization of ChIP-Seq Data with Control.” BMC Bioinformatics 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Chia Hui, Paulson Ariel, Abmayr Susan M., and Workman Jerry L.. 2012. “HP1a Targets the Drosophila KDM4A Demethylase to a Subset of Heterochromatic Genes to Regulate H3K36me3 Levels.” PLoS ONE 7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Zhenyu, Miao Jun, and Cui Liwang. 2011. “Gametocytogenesis in Malaria Parasite: Commitment, Development and Regulation.” Future Microbiology 6(11):1351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorà-Batlle Oriol, Michel-Todó Lucas, Witmer Kathrin, Toda Haruka, Fernández-Becerra Carmen, Baum Jake, and Cortés Alfred. 2020. “Conditional Expression of PfAP2-G for Controlled Massive Sexual Conversion in Plasmodium Falciparum.” Science Advances 6(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barragán María J., Lemieux Jacob, Quiñones Mariam, Williamson Kim C., Molina-Cruz Alvaro, Cui Kairong, Barillas-Mury Carolina, Zhao Keji, and Su Xin zhuan. 2011. “Directional Gene Expression and Antisense Transcripts in Sexual and Asexual Stages of Plasmodium Falciparum.” BMC Genomics 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellacheruvu Dattatreya, Wright Zachary, Couzens Amber L., Lambert Jean Philippe, St-Denis Nicole A., Li Tuo, Miteva Yana V., Hauri Simon, Sardiu Mihaela E., Low Teck Yew, Halim Vincentius A., Bagshaw Richard D., Hubner Nina C., Al-Hakim Abdallah, Bouchard Annie, Faubert Denis, Fermin Damian, Dunham Wade H., Goudreault Marilyn, Lin Zhen Yuan, Badillo Beatriz Gonzalez, Pawson Tony, Durocher Daniel, Coulombe Benoit, Aebersold Ruedi, Giulio Superti-Furga, Colinge Jacques, Heck Albert J. R., Choi Hyungwon, Gstaiger Matthias, Mohammed Shabaz, Cristea Ileana M., Bennett Keiryn L., Washburn Mike P., Raught Brian, Ewing Rob M., Gingras Anne Claude, and Nesvizhskii Alexey I.. 2013. “The CRAPome: A Contaminant Repository for Affinity Purification-Mass Spectrometry Data.” Nature Methods 10(8):730–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Jun, Wang Zenglei, Liu Min, Parker Daniel, Li Xiaolian, Chen Xiaoguang, and Cui Liwang. 2013. “Plasmodium Falciparum: Generation of Pure Gametocyte Culture by Heparin Treatment.” Experimental Parasitology 135(3):541–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Susanne K., Good Robert T., Drew Damien R., Delorenzi Mauro, Sanders Paul R., Hodder Anthony N., Speed Terence P., Cowman Alan F., De Koning-Ward Tania F., and Crabb Brendan S.. 2002. “A Subset of Plasmodium Falciparum SERA Genes Are Expressed and Appear to Play an Important Role in the Erythrocytic Cycle.” Journal of Biological Chemistry 277(49):47524–32. [DOI] [PubMed] [Google Scholar]
- Modrzynska Katarzyna, Pfander Claudia, Chappell Lia, Yu Lu, Suarez Catherine, Dundas Kirsten, Gomes Ana Rita, Goulding David, Rayner Julian C., Choudhary Jyoti, and Billker Oliver. 2017. “A Knockout Screen of ApiAP2 Genes Reveals Networks of Interacting Transcriptional Regulators Controlling the Plasmodium Life Cycle.” Cell Host and Microbe 21(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu Gaëlle and Lavazec Catherine. 2019. “Erythrocyte Membrane Makeover by Plasmodium Falciparum Gametocytes.” Frontiers in Microbiology 10(November):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon Christian P., Nixon Christina E., Michelow Ian C., Silva-Viera Rayna A., Colantuono Bonnie, Obeidallah Aisha S., Jha Ambrish, Dockery Dominique, Raj Dipak, Park Sangshin, Duffy Patrick E., and Kurtis Jonathan D.. 2018. “Antibodies to PfsEGXP, an Early Gametocyte-Enriched Phosphoprotein, Predict Decreased Plasmodium Falciparum Gametocyte Density in Humans.” Journal of Infectious Diseases 218(11):1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Marta C., Goldring J. P. Dea, Doerig Christian, and Scherf Artur. 2007. “A Novel Protein Kinase Family in Plasmodium Falciparum Is Differentially Transcribed and Secreted to Various Cellular Compartments of the Host Cell.” Molecular Microbiology 63(2):391–403. [DOI] [PubMed] [Google Scholar]
- Oehring Sophie C., Woodcroft Ben J., Moes Suzette, Wetzel Johanna, Dietz Olivier, Pulfer Andreas, Dekiwadia Chaitali, Maeser Pascal, Flueck Christian, Witmer Kathrin, Brancucci Nicolas M. B., Niederwieser Igor, Jenoe Paul, Ralph Stuart A., and Voss Till S.. 2012. “Organellar Proteomics Reveals Hundreds of Novel Nuclear Proteins in the Malaria Parasite Plasmodium Falciparum.” Genome Biology 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter Heather J., Altenhofen Lindsey M., Kafsack Björn F. C., and Llinás Manuel. 2013. Whole-Genome Analysis of Plasmodium Spp. Utilizing a New Agilent Technologies DNA Microarray Platform. Vol. 923. [DOI] [PubMed] [Google Scholar]
- Painter Heather J., Campbell Tracey L., and Llinás Manuel. 2011. “The Apicomplexan AP2 Family: Integral Factors Regulating Plasmodium Development.” Molecular and Biochemical Parasitology 176(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter Heather J., Carrasquilla Manuela, and Llinás Manuel. 2017. “Capturing in Vivo RNA Transcriptional Dynamics from the Malaria Parasite Plasmodium Falciparum.” Genome Research 27(6):1074–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter Heather J., Chung Neo Christopher, Sebastian Aswathy, Albert Istvan, Storey John D., and Llinás Manuel. 2018. “Genome-Wide Real-Time in Vivo Transcriptional Dynamics during Plasmodium Falciparum Blood-Stage Development.” Nature Communications 9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Xinhong, An Xiuli, Guo Xinhua, Tarnawski Michal, Coppel Ross, and Mohandas Narla. 2005. “Structural and Functional Studies of Interaction between Plasmodium Falciparum Knob-Associated Histidine-Rich Protein (KAHRP) and Erythrocyte Spectrin.” Journal of Biological Chemistry 280(35):31166–71. [DOI] [PubMed] [Google Scholar]
- Petter Michaela, Bonow Insa, and Klinkert Mo Quen. 2008. “Diverse Expression Patterns of Subgroups of the Rif Multigene Family during Plasmodium Falciparum Gametocytogenesis.” PLoS ONE 3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poran Asaf, Nötzel Christopher, Aly Omar, Mencia-Trinchant Nuria, Harris Chantal T., Guzman Monica L., Hassane Duane C., Elemento Olivier, and Kafsack Björn F. C.. 2017. “Single-Cell RNA Sequencing Reveals a Signature of Sexual Commitment in Malaria Parasites.” Nature 551(7678):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugaliza Harvie P., Miyazaki Shinya, Geurten Fiona J. A., Pell Christopher, Rosanas-Urgell Anna, Janse Chris J., and Cortés Alfred. 2020. “Artemisinin Exposure at the Ring or Trophozoite Stage Impacts Plasmodium Falciparum Sexual Conversion Differently.” ELife 9(stage V):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan Aaron R. and Hall Ira M.. 2010. “BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features.” Bioinformatics 26(6):841–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich Regina N., Drakeley Chris, Djimde Abdoulaye A., Hall B. Fenton, Hay Simon I., Hemingway Janet, Kaslow David C., Noor Abdisalan, Okumu Fredros, Steketee Richard, Tanner Marcel, Wells Timothy N. C., Whittaker Maxine A., Winzeler Elizabeth A., Wirth Dyann F., Whitfield Kate, and Alonso Pedro L.. 2017. “MalERA: An Updated Research Agenda for Malaria Elimination and Eradication.” PLoS Medicine 14(11):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez Fidel, Ryan Devon P., Grüning Björn, Bhardwaj Vivek, Kilpert Fabian, Richter Andreas S., Heyne Steffen, Dündar Friederike, and Manke Thomas. 2016. “DeepTools2: A next Generation Web Server for Deep-Sequencing Data Analysis.” Nucleic Acids Research 44(W1):W160–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings David J., Fujioka Hisashi, Fried Michal, Keister David B., Aikawa Masamichi, and Kaslow David C.. 1992. “α-Tubulin II Is a Male-Specific Protein in Plasmodium Falciparum.” Molecular and Biochemical Parasitology 56(2):239–50. [DOI] [PubMed] [Google Scholar]
- Rea Edward, Le Roch Karine G., and Tewari Rita. 2018. “Sex in Plasmodium Falciparum: Silence Play between GDV1 and HP1.” Trends in Parasitology 34(6):450–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario Virgilio. 2008. “Cloning of Naturally Occurring Mixed Infections of Malaria Parasites Author (s): Virgilio Rosario Published by : American Association for the Advancement of Science Stable URL : Http://Www.Jstor.Org/Stable/1685487 Cloning of Naturally Occurring Mixed In.” Advancement Of Science 212(4498):1037–38. [DOI] [PubMed] [Google Scholar]
- Rovira-Graells Núria, Gupta Archna P., Planet Evarist, Crowley Valerie M., Mok Sachel, De Pouplana Lluís Ribas, Preiser Peter R., Bozdech Zbynek, and Cortés Alfred. 2012. “Transcriptional Variation in the Malaria Parasite Plasmodium Falciparum.” Genome Research 22(5):925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Joana Mendonca, Josling Gabrielle, Ross Philipp, Joshi Preeti, Orchard Lindsey, Campbell Tracey, Schieler Ariel, Cristea Ileana M., and Llinás Manuel. 2017. “Red Blood Cell Invasion by the Malaria Parasite Is Coordinated by the PfAP2-I Transcription Factor.” Cell Host and Microbe 21(6):731–741.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant Tobias J., Marti Matthias, Caler Elisabet, Carlton Jane M., Simpson Ken, Speed Terence P., and Cowman Alan F.. 2006. “Lineage-Specific Expansion of Proteins Exported to Erythrocytes in Malaria Parasites.” Genome Biology 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini Francesco, Lasonder Edwin, Olivieri Anna, Camarda Grazia, Van Schaijk Ben, Sanchez Massimo, Younis Sumera Younis, Sauerwein Robert, and Alano Pietro. 2010. “Protein Export Marks the Early Phase of Gametocytogenesis of the Human Malaria Parasite Plasmodium Falciparum.” Molecular and Cellular Proteomics 9(7):1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE 1982. “Gametocytogenesis of Plasmodium Falciparum in Vitro: An Electron Microscopic Study.” Parasitology 84(1):1–11. [DOI] [PubMed] [Google Scholar]
- Singh Agam Prasad, Buscaglia Carlos A., Wang Qian, Levay Agata, Nussenzweig Daniel R., Walker John R., Winzeler Elizabeth A., Fujii Hodaka, Fontoura Beatriz M. A., and Nussenzweig Victor. 2007. “Plasmodium Circumsporozoite Protein Promotes the Development of the Liver Stages of the Parasite.” Cell 131(3):492–504. [DOI] [PubMed] [Google Scholar]
- Sinha Abhinav, Hughes Katie R., Modrzynska Katarzyna K., Otto Thomas D., Pfander Claudia, Dickens Nicholas J., Agnieszka a Religa Ellen Bushell, Graham Anne L., Cameron Rachael, Kafsack Bjorn F. C., Williams April E., Manuel Llinás Matthew Berriman, Billker Oliver, and Waters Andrew P.. 2014. “A Cascade of DNA-Binding Proteins for Sexual Commitment and Development in Plasmodium.” Nature 507(7491):253–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Joanne, Fernandez-Reyes Delmiro, Sharling Lisa, Moore Sally G., Eling Wijnand M., Kyes Sue A., Newbold Christopher I., Kafatos Fotis C., Janse Chris J., and Waters Andrew P.. 2007. “Plasmodium Cysteine Repeat Modular Proteins 1–4: Complex Proteins with Roles throughout the Malaria Parasite Life Cycle.” Cellular Microbiology 9(6):1466–80. [DOI] [PubMed] [Google Scholar]
- Thorvaldsdóttir Helga, Robinson James T., and Mesirov Jill P.. 2013. “Integrative Genomics Viewer (IGV): High-Performance Genomics Data Visualization and Exploration.” Briefings in Bioinformatics 14(2):178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibúrcio Marta, Niang Makhtar, Deplaine Guillaume, Perrot Sylvie, Bischoff Emmanuel, Ndour Papa Alioune, Silvestrini Francesco, Khattab Ayman, Milon Geneviève, David Peter H., Hardeman Max, Vernick Kenneth D., Sauerwein Robert W., Preiser Peter R., Odile Mercereau-Puijalon Pierre Buffet, Alano Pietro, and Lavazec Catherine. 2012. “A Switch in Infected Erythrocyte Deformability at the Maturation and Blood Circulation of Plasmodium Falciparum Transmission Stages.” Blood 119(24):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibúrcio Marta, Silvestrini Francesco, Bertuccini Lucia, Sander Adam Frederik, Turner Louise, Lavstsen Thomas, and Alano Pietro. 2013. “Early Gametocytes of the Malaria Parasite Plasmodium Falciparum Specifically Remodel the Adhesive Properties of Infected Erythrocyte Surface.” Cellular Microbiology 15(4):647–59. [DOI] [PubMed] [Google Scholar]
- Trager William and Jensen James B.. 2005. “Human Malaria Parasites in Continuous Culture.” Journal of Parasitology 91(3):484–86. [DOI] [PubMed] [Google Scholar]
- Tusher Virginia Goss, Tibshirani Robert, and Chu Gilbert. 2001. “Significance Analysis of Microarrays Applied to the Ionizing Radiation Response.” Proceedings of the National Academy of Sciences of the United States of America 98(9):5116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal Kannan, Hentzschel Franziska, Valkiūnas Gediminas, and Marti Matthias. 2020. “Plasmodium Asexual Growth and Sexual Development in the Haematopoietic Niche of the Host.” Nature Reviews Microbiology 18(3):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard Viviane, Agak George W., Frank Géraldine, Jafarshad Ali, Servis Catherine, Nébié Issa, Sirima Sodlomon B., Felger Ingrid, Arevalo-Herrera Myriam, Herrera Socrates, Heitz Frederic, Bäcker Volker, Druilhe Pierre, Kajava Andrey V., and Corradin Giampietro. 2007. “Rapid Identification of Malaria Vaccine Candidates Based on α-Helical Coiled Coil Protein Motif.” PLoS ONE 2(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz Jennifer C., Yap Alan, Sisquella Xavier, Thompson Jenn K., Lim Nicholas T. Y., Whitehead Lachlan W., Chen Lin, Lampe Marko, Tham Wai Hong, Wilson Danny, Nebl Thomas, Marapana Danushka, Triglia Tony, Wong Wilson, Rogers Kelly L., and Cowman Alan F.. 2016. “Essential Role of the PfRh5/PfRipr/CyRPA Complex during Plasmodium Falciparum Invasion of Erythrocytes.” Cell Host and Microbe 20(1):60–71. [DOI] [PubMed] [Google Scholar]
- Voss Till S., Mini Thierry, Jenoe Paul, and Beck Hans Peter. 2002. “Plasmodium Falciparum Possesses a Cell Cycle-Regulated Short Type Replication Protein A Large Subunit Encoded by an Unusual Transcript.” Journal of Biological Chemistry 277(20):17493–501. [DOI] [PubMed] [Google Scholar]
- Vossen Matthias G., Pferschy Sandra, Chiba Peter, and Noedl Harald. 2010. “The SYBR Green I Malaria Drug Sensitivity Assay: Performance in Low Parasitemia Samples.” American Journal of Tropical Medicine and Hygiene 82(3):398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Daniel J., Lucas Tim C. D., Nguyen Michele, Nandi Anita K., Bisanzio D, Battle Katherine E., Cameron Ewan, Twohig Katherine A., Pfeffer Daniel A., Rozier Jennifer A., Gibson Harry S., Rao Puja C., Casey Daniel, Bertozzi-Villa Amelia, Collins Emma L., Dalrymple Ursula, Gray N, Harris Joseph R., Howes Rosalind E., Kang Sun Yun, Keddie Suzanne H., May Daniel, Rumisha S, Thorn Michael P., Barber Ryan, Fullman N, Huynh Chantal K., Kulikoff Xie, Kutz Michael J., Lopez Alan D., Mokdad AH, Naghavi Mohsen, Nguyen G, Shackelford Katya Anne, Vos Theo, Wang Haidong, Smith David L., Lim Stephen S., Murray Christopher J. L., Bhatt S, Hay Simon I., and Gething Peter W.. 2019. “Mapping the Global Prevalence, Incidence, and Mortality of Plasmodium Falciparum, 2000–17: A Spatial and Temporal Modelling Study.” The Lancet 394(10195):322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2018. Malaria Report 2018.
- WHO. 2019. World Malaria Report.
- Young Jason A., Fivelman Quinton L., Blair Peter L., De La Vega Patricia, Le Roch Karine G., Zhou Yingyao, Carucci Daniel J., Baker David A., and Winzeler Elizabeth A.. 2005. “The Plasmodium Falciparum Sexual Development Transcriptome: A Microarray Analysis Using Ontology-Based Pattern Identification.” Molecular and Biochemical Parasitology 143(1):67–79. [DOI] [PubMed] [Google Scholar]
- Yuda Masao, Iwanaga Shiroh, Kaneko Izumi, and Kato Tomomi. 2015. “Global Transcriptional Repression: An Initial and Essential Step for Plasmodium Sexual Development.” Proceedings of the National Academy of Sciences of the United States of America 112(41):12824–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuda Masao, Kaneko Izumi, Iwanaga Shiroh, Murata Yuho, and Kato Tomomi. 2019. “Female‐specific Gene Regulation in Malaria Parasites by an AP2‐family Transcription Factor.” Molecular Microbiology 0–2. [DOI] [PubMed] [Google Scholar]
- Zhang Cui, Li Zhenkui, Cui Huiting, Jiang Yuanyuan, Yang Zhenke, and Wang Xu. 2017. “Systematic CRISPR-Cas9-Mediated ApiAP2 Genes Reveal Functional Insights into Parasite Development.” MBio 8(6):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Min, Wang Chengqi, Otto Thomas D., Oberstaller Jenna, Liao Xiangyun, Adapa Swamy R., Udenze Kenneth, Bronner Iraad F., Casandra Deborah, Mayho Matthew, Brown Jacqueline, Li Suzanne, Swanson Justin, Rayner Julian C., Jiang Rays H. Y., and Adams John H.. 2018. “Uncovering the Essential Genes of the Human Malaria Parasite Plasmodium Falciparum by Saturation Mutagenesis.” Science 360(6388). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Jinghua, Bhanot Purnima, Hu Junjie, and Wang Qian. 2016. “A Comprehensive Analysis of Plasmodium Circumsporozoite Protein Binding to Hepatocytes.” PLoS ONE 11(8):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All ChIP-seq sequencing reads are deposited at the NCBI Sequence Read Archive (SRA) in the Gene Expression Omnibus (GEO) under the accession number GSE157753. All DNA microarray data are also deposited at GEO under accession number GSE160923 (asexual) and GSE160924 (sexual gametocyte). Whole genome sequencing reads for ap2-g2 gfp and ap2-g2 KO parasites are directly submitted to SRA under accession number PRJNA673876.


