Abstract
Objective:
Primary nitinol stenting (PNS) and drug coated balloon angioplasty (DCB) are two of the most common endovascular interventions for femoropopliteal atherosclerotic disease. While many prospective randomized controlled trials have compared PNS or DCB to plain balloon angioplasty (POBA), no studies have directly compared PNS against DCB therapy. The purpose of this network meta-analysis is to determine whether there is a significant difference in outcomes between PNS and DCB.
Methods:
The primary outcome measure was binary restenosis, the secondary outcome measures were target lesion revascularization (TLR) and change in ankle brachial index (ABI). Outcomes were evaluated at 6, 12, and 24 months. A literature review identified all randomized controlled trials published prior to March 2020 that compared DCB to POBA or PNS to POBA in the treatment of native atherosclerotic lesions of the femoropopliteal artery. Studies were excluded if they contained in-stent stenosis or tibial artery disease that could not be delineated out in a subgroup analysis. Network meta-analysis was performed using the network and mvmeta commands in STATA 14.
Results:
Twenty-seven publications covering 19 trials were identified, eight trials compared PNS to POBA and 11 trials compared DCB to POBA. The odds of freedom from binary restenosis for patients treated with DCB compared to PNS at 6 months was 1.19 (95% CI 0.63 – 2.22), at 12 months was 1.67 (95% CI 1.04 – 2.68), and at 24 months was 1.36 (95% CI 0.78 – 2.37). The odds of freedom from target lesion revascularization for patients treated with DCB compared to PNS at 6 months was 0.66 (95% CI 0.12 – 3.80), at 12 months was 1.89 (95% CI 1.04 – 3.45), and at 24 months was 1.68 (95% CI 0.82 – 3.44). The mean increase in ABI for patients treated with PNS compared to DCB at 6 months was 0.06 higher (95% CI −0.03 – 0.15), at 12 months was 0.05 higher (95% CI 0.00 – 0.09), and at 24 months was 0.07 higher (95% CI −0.01 – 0.14).
Conclusion:
Both DCB and PNS demonstrated a lower rate of binary restenosis compared to POBA at the 6-month, 12-month, and 24-month timepoints. When comparing DCB to PNS through network meta-analysis, DCB had a statistically lower rate of a binary restenosis and target lesion revascularization at the 12-month timepoint. This network meta-analysis demonstrates that both DCB and PNS are superior to POBA, and that PNS is a satisfactory substitute for DCB when paclitaxel is not desirable.
Keywords: Network Meta-analysis, Drug-coated balloon, Bare Metal stent, Nitinol stenting, Peripheral arterial disease
Introduction
Over the past 30 years, endovascular therapy for femoropopliteal arterial occlusive disease has evolved to be the first-line treatment for most patients. As the adoption of endovascular therapy for peripheral arterial disease (PAD) grows, so do its technical variations. The first widely adopted endovascular intervention for PAD was plain balloon angioplasty (POBA), which remains a common treatment. Today, clinicians have a wide range of endovascular modalities to choose from including balloon-expandable stainless-steel stents, primary nitinol stenting (PNS), drug-eluting stents (DES), drug-coated balloons (DCB), and atherectomy.
Multiple randomized-controlled trials (RCTs) have demonstrated that both PNS and DCB have superior patency rates to POBA for most native femoropopliteal atherosclerotic lesions.1,2 However, there are unique concerns about both PNS and DCB. PNS leaves behind a permanent foreign object which could potentially alter future surgical options.3 DCB exposes the patient to paclitaxel which has been associated with increased all-cause mortality.4
While both PNS and DCB have had RCTs demonstrating their superiority to POBA, the two therapies have not been directly compared to each other. Although there are other options such as DES and PNS combined with DCB, VQIP data demonstrates that DCB and PNS remain the two most frequently performed interventions for femoropopliteal disease at 37% and 26% respectively.5 In addition, with the controversy surrounding paclitaxel-based devices, surgeons are interested in alternatives to DCB. Since a trial directly comparing DCB to PNS is unlikely to occur, we performed a network meta-analysis to determine whether PNS remains a reasonable alternative to DCB.
Methods
Search Strategy
The Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, and PubMed were queried for publications or trials prior to March, 2020 with a title including the word “femoral”, “popliteal”, or “femoropopliteal” along with the word “endovascular”, “percutaneous”, “transluminal”, “angioplasty”, “stent”, or “balloon.”
Selection Criteria
Studies were included if they were an RCT that contained at least a POBA arm and either a PNS arm or a DCB arm. Studies had to be limited to, or contain sub-analysis for, native femoropopliteal atherosclerotic disease exclusive of in-stent stenosis and tibial disease. Studies had to contain one of the three timepoints of interest (6 months, 12 months, and 24 months) and one of the three outcome measures of interest (binary restenosis, target lesion revascularization, and change in mean ankle brachial index). Studies were excluded if they utilized balloon-expandable stents instead of self-expanding stents.
Outcome measures
The primary outcome measure was freedom from binary restenosis. Binary restenosis was defined as a duplex ultrasound derived peak systolic velocity ratio of >2.5 or 2.4, or if duplex ultrasound was not available, a >50% stenosis as seen on arteriography. The secondary outcome measures were freedom from target lesion revascularization (TLR) and change in ankle-brachial index (ABI). Target lesion revascularization was defined as reintervention on the target lesion to maintain or restore patency. Change in ABI was defined as the change from the baseline pre-intervention ABI to the follow-up ABI.
Data Extraction
Intention-to-treat data were extracted. If a patient required an adjunct, such as stent placement due to dissection during dilation, this was not considered a loss of patency, restenosis, or target-lesion revascularization. Binary restenosis was assumed to be equal to primary patency if the protocol stated that target-lesion revascularization would only occur with ≥50% restenosis and if the authors defined primary patency as restenosis on imaging or a protocol-driven target lesion revascularization. A normal distribution was assumed for ABI thus allowing for median values and interquartile ranges to be interchanged to means and standard distribution using guidelines from the Cochrane Handbook.6 If standard deviations or interquartile ranges were missing, these were imputed by calculating a correlation coefficient from the other studies. Studies were reviewed for risk of bias using the Cochrane Collaboration’s risk of bias tool.7
Statistics
A network meta-analysis was performed using frequentist methods implemented in the network and mvmeta commands in STATA 15 by fitting a multivariate random-effects meta-analysis model using restricted maximum likelihood (REML). Between-studies variance τ2 was assumed to be common across comparisons. At each timepoint, direct and indirect comparisons were presented as odds ratio (OR) and 95% confidence intervals (CI) for the freedom from binary restenosis and freedom from target lesion revascularization outcomes and as mean differences (MD) and 95% CIs for ABI. Since our networks were simple star-shaped and contained no loops with direct PNS-DCB comparisons, we were not able to evaluate or test for inconsistency. Publication bias and small study effects were inspected by generating comparison-adjusted funnel plots for each outcome at each timepoint and by further visual inspection using the criterion of symmetry.
Results:
Study Characteristics
The search strategy and selection process identified 27 publications detailing 19 studies (Supplementary Figure 1.) Eight studies compared PNS to POBA and 11 studies compared DCB to POBA; these 19 studies had a combined enrollment of 3,287 patients (Table 1.) All studies included 12-month results, but 6-month and 24-month results were less frequently reported. All studies reported binary restenosis. Most studies excluded Rutherford class 1 and Rutherford class 6 patients. The shortest lesions studied ranged 1 cm to 10 cm and the longest ranged 5 cm to 22 cm. Among the 565 patients randomized to POBA in the studies comparing PNS to POBA, 145 patients were crossed over to stenting for an adjunctive stenting rate of 25.0%. Among the 2,111 patients in the studies comparing DCB to POBA, 253 underwent adjunctive stenting for a rate of 12.0%. Studies were similar in their risk of bias (Supplementary Figures 2–3.) All studies used random sequence generation and an independent core laboratory for interpretation of duplex results. No study was able to blind personnel to the treatment selected. The majority specified an allocation concealment method, provided complete outcome data, and avoided selective reporting. All DCB versus POBA studies and 5 of 8 PNS versus POBA studies were industry sponsored. The comparison-adjusted funnel plots appear symmetric, suggesting the absence of small-study effects in the network (Supplementary Figure 4.)
Table 1.
Characteristics of included studies
| PNS vs POBA | Publication year(s) | PNS n | POBA n | Time, months | Outcomes studied | Lesion location | RC | Lesion length | Industry sponsor | Adjunct stenting |
|---|---|---|---|---|---|---|---|---|---|---|
| Brancaccio1 | 2012 | 25 | 25 | 6, 12 | BR | SFA | 3-5 | Unknown | No | 14 POBA |
| Dick2 | 2009 | 34 | 39 | 6, 12 | BR, ABI | SFA | 3-5 | 3-20cm | No | 10 POBA |
| FAST3 | 2007 | 123 | 121 | 12 | BR, TLR, ABI | SFA | 2-5 | 1-10cm | Yes | 13 POBA |
| ETAP4,5 | 2013, 2015 | 119 | 127 | 6, 12, 24 | BR, TLR, ABI | Pop | 2-5 | 5-180mm | Yes | 32 POBA |
| RESILIENT67 | 2009, 2012 | 134 | 72 | 6, 12, | BR, TLR | SFA + Pop | 1-3 | < 15cm | Yes | 29 POBA |
| SM-018 | 2019 | 51 | 52 | 6, 12, 24 | BR, TLR | SFA + Pop | 1-3 | 4-15cm | Yes | 26 POBA |
| SUPER9 | 2012 | 74 | 76 | 12 | BR, TLR | SFA | 1-6 | 5-22cm | Yes | 4 POBA |
| Schillinger10,11 | 2006, 2007 | 51 | 53 | 6, 12, 24 | BR, ABI | SFA + Pop | 3-5 | >3cm | No | 17 POBA |
| DCB vs POBA | Publication year(s) | DCB n | POBA n | Time, months | Outcomes studied | Lesion location | RC | Lesion length | Industry sponsor | Adjunct stenting |
| BIOLUX12 | 2015 | 30 | 30 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-5 | 3-20cm | Yes | 2 DCB 8 POBA |
| BIOPAC13 | 2018 | 33 | 33 | 6, 12 | BR, TLR | SFA | 2-4 | 4-15cm | Yes | 13 DCB 13 POBA |
| CONSEQUENT14,15 | 2017, 2018 | 78 | 75 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-4 | 4-27cm | Yes | 11 DCB 14 POBA |
| EffPac16 | 2019 | 85 | 86 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-4 | <15cm | Yes | 13 DCB 16 POBA† |
| ILLUMENATE.EU17,18 | 2017, 2018 | 222 | 72 | 6, 12, 24 | BR, TLR, ABI | SFA + Pop | 2-4 | 3-20cm | Yes | 38 DCB 9 DCB† |
| ILLUMENATE PK19 | 2017 | 200 | 100 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-4 | 3-18cm | Yes | 12 DCB 6 POBA† |
| INPACT JP20,21 | 2018, 2018 | 68 | 32 | 6, 12, 24 | BR, TLR, ABI | SFA + Pop | 2-4 | 4-20 cm | Yes | 3 DCB 1 POBA† |
| INPACT SFA22,23 | 2014, 2015 | 220 | 111 | 6, 12, 24 | BR, TLR, ABI | SFA + Pop | 2-4 | 4-18 cm | Yes | 16 DCB 14 POBA† |
| LEVANT I24 | 2014 | 31 | 24 | 6, 12, 24 | BR, TLR, ABI | SFA + Pop | 2-5 | 4-15cm | Yes | 12 DCB 14 POBA |
| LEVANT II25 | 2015 | 316 | 160 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-4 | <15cm | Yes | 8 DCB 11 POBA† |
| RANGER26,27 | 2017, 2018 | 71 | 34 | 6, 12 | BR, TLR, ABI | SFA + Pop | 2-4 | 2-15cm | Yes | 15 DCB 4 POBA |
PNS = Primary Nitinol Stenting, POBA = Uncoated balloon angioplasty, BR = Binary Restenosis, TLR=Target Lesion Revascularization, ABI = Ankle Brachial Index, RC = Rutherford Classification, SFA = Superficial Femoral Artery, Pop = Popliteal Artery
= These studies were protocoled such that randomization would occur after successful predilation and that patients who dissected during predilation would be screened out of the study.
Brancaccio G, Lombardi R, Stefanini T, Torri P, Russo D, Gorji N, Cappelletti D, Celoria GM. Comparison of embolic load in femoropopliteal interventions: percutaneous transluminal angioplasty versus stenting. Vascular and endovascular surgery. 2012 Apr;46(3):229-35.
Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, Koppensteiner R, Minar E, Schillinger M. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheterization and Cardiovascular Interventions. 2009 Dec 1;74(7):1090-5.
Krankenberg H, Schlüter M, Steinkamp HJ. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Journal of Vascular Surgery. 2008 Jan 1;47(1):239.
Rastan A, Krankenberg H, Baumgartner I, Blessing E, Müller-Hülsbeck S, Pilger E, Scheinert D, Lammer J, Gißler M, Noory E, Neumann FJ. Stent placement versus balloon angioplasty for the treatment of obstructive lesions of the popliteal artery: a prospective, multicenter, randomized trial. Circulation. 2013 Jun 25;127(25):2535-41.
Rastan A, Krankenberg H, Baumgartner I, Blessing E, Müller-Hülsbeck S, Pilger E, Scheinert D, Lammer J, Beschorner U, Noory E, Neumann FJ. Stent placement vs. balloon angioplasty for popliteal artery treatment: two-year results of a prospective, multicenter, randomized trial. Journal of endovascular therapy. 2015 Feb;22(1):22-7.
Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circulation: Cardiovascular Interventions. 2010 Jun;3(3):267-76.
Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: three-year follow-up from the RESILIENT randomized trial. Journal of Endovascular Therapy. 2012 Feb;19(1):1-9.
Iida O, Urasawa K, Komura Y, Soga Y, Inoue N, Hara H, Yajima J, Nakamura S, Ohki T, Ando H, Hirano K. Self-expanding nitinol stent vs percutaneous transluminal angioplasty in the treatment of femoropopliteal lesions: 3-year data from the SM-01 trial. Journal of Endovascular Therapy. 2019 Apr;26(2):158-67.
Chalmers N, Walker PT, Belli AM, Thorpe AP, Sidhu PS, Robinson G, van Ransbeeck M, Fearn SA. Randomized trial of the SMART stent versus balloon angioplasty in long superficial femoral artery lesions: the SUPER study. Cardiovascular and interventional radiology. 2013 Apr 1;36(2):353-61.
Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. New England Journal of Medicine. 2006 May 4;354(18):1879-88.
Schillinger M, Sabeti S, Dick P, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115(21):2745-2749.
Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX PI randomized trial. Journal of Endovascular Therapy. 2015 Feb;22(1):14-21.
Buszman PP, Nowakowski P, Milewski K, Orlik B, Żurakowski A, Ludyga T, Polczyk F, Dębiński M, Jelonek M, Kachel M, Gąsior M. Clinical Randomized Trial Evaluating Novel, Microcrystalline, and Biocompatible Polymer Paclitaxel-Coated Balloon for the Treatment of Femoropopliteal Occlusive Disease: The BIOPAC Trial. JACC: Cardiovascular Interventions. 2018 Dec 10;11(23):2436-8.
Tepe G, Gögebakan Ö, Redlich U, Tautenhahn J, Ricke J, Halloul Z, Meyer DR, Waliszewski M, Schnorr B, Zeller T, Müller-Hülsbeck S. Angiographic and clinical outcomes after treatment of femoro-popliteal lesions with a novel paclitaxel-matrix-coated balloon catheter. CardioVascular and Interventional Radiology. 2017 Oct 1;40(10):1535-44.
Albrecht T, Waliszewski M, Roca C, Redlich U, Tautenhahn J, Pech M, Halloul Z, Gögebakan Ö, Meyer DR, Gemeinhardt I, Zeller T. Two-year clinical outcomes of the CONSEQUENT trial: can femoropopliteal lesions be treated with sustainable clinical results that are economically sound?. Cardiovascular and interventional radiology. 2018 Jul 1;41(7):1008-14.
Teichgräber U, Lehmann T, Aschenbach R, Scheinert D, Zeller T, Brechtel K, Blessing E, Lichtenberg M, Sixt S, Brucks S, Beschorner U. Efficacy and safety of a novel paclitaxel-nano-coated balloon for femoropopliteal angioplasty: one-year results of the EffPac trial. Eurointervention: Journal of Europcr in Collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2020 Apr 3;15(18):e1633-40.
Schroeder H, Werner M, Meyer DR, Reimer P, Krüger K, Jaff MR, Brodmann M. Low-Dose Paclitaxel–Coated Versus Uncoated Percutaneous Transluminal Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease: One-Year Results of the ILLUMENATE European Randomized Clinical Trial (Randomized Trial of a Novel Paclitaxel-Coated Percutaneous Angioplasty Balloon). Circulation. 2017 Jun 6;135(23):2227-36.
Brodmann M, Werner M, Meyer DR, Reimer P, Krüger K, Granada JF, Jaff MR, Schroeder H, ILLUMENATE EU RCT Investigators. Sustainable antirestenosis effect with a low-dose drug-coated balloon: the ILLUMENATE European randomized clinical trial 2-year results. JACC: Cardiovascular Interventions. 2018 Dec 10;11(23):2357-64.
Krishnan P, Faries P, Niazi K, Jain A, Sachar R, Bachinsky WB, Cardenas J, Werner M, Brodmann M, Mustapha JA, Mena-Hurtado C. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation. 2017 Sep 19;136(12):1102-13.
Iida O, Soga Y, Urasawa K, Saito S, Jaff MR, Wang H, Ookubo H, Yokoi H. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. Journal of Endovascular Therapy. 2018 Feb;25(1):109-17.
Iida O, Soga Y, Urasawa K, Saito S, Jaff MR, Wang H, Ookubo H, Yokoi H, MDT-2113 SFA Japan Investigators. Drug-coated balloon versus uncoated percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal artery: 2-year results of the MDT-2113 SFA Japan randomized trial. Catheterization and Cardiovascular Interventions. 2019 Mar 1;93(4):664-72.
Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN. PACT SFA randomized trial. Circulation. 2015 Feb 3;131(5):495-502.
Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN. PACT SFA. Journal of the American College of Cardiology. 2015 Dec 1;66(21):2329-38.
Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC: Cardiovascular Interventions. 2014 Jan 1;7(1):10-9.
Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. New England Journal of Medicine. 2015 Jul 9;373(2):145-53.
Bausback Y, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, Sauguet A, Diaz-Cartelle J, Marx C, Stroebel A, Schult I. Six-month results from the initial randomized study of the Ranger paclitaxel-coated balloon in the femoropopliteal segment. Journal of Endovascular Therapy. 2017 Aug;24(4):459-67.
Steiner S, Willfort-Ehringer A, Sievert H, Geist V, Lichtenberg M, Del Giudice C, Sauguet A, Diaz-Cartelle J, Marx C, Ströbel A, Schult I. 12-month results from the first-in-human randomized study of the ranger paclitaxel-coated balloon for femoropopliteal treatment. JACC: Cardiovascular Interventions. 2018 May 21;11(10):934-41.
Binary Restenosis
The odds of freedom from binary restenosis for patients treated with PNS compared to POBA at 6 months was 2.65 (95% CI 1.59 – 4.42), at 12 months was 1.89 (95% CI 1.31 – 2.71), and at 24 months was 1.98 (95% CI 1.27 – 3.10) (Figure 1.) The odds of freedom from binary restenosis for patients treated with DCB compared to POBA at 6 months was 3.14 (95% CI 2.12 – 4.67), at 12 months was 3.16 (95% CI 2.31 – 4.32), and at 24 months was 2.69 (95% CI 1.96 – 3.68). The odds of freedom from binary restenosis for patients treated with DCB compared to PNS at 6 months was 1.19 (95% CI 0.63 – 2.22), at 12 months was 1.67 (95% CI 1.04 – 2.68), and at 24 months was 1.36 (95% CI 0.78 – 2.37).
Figure 1.
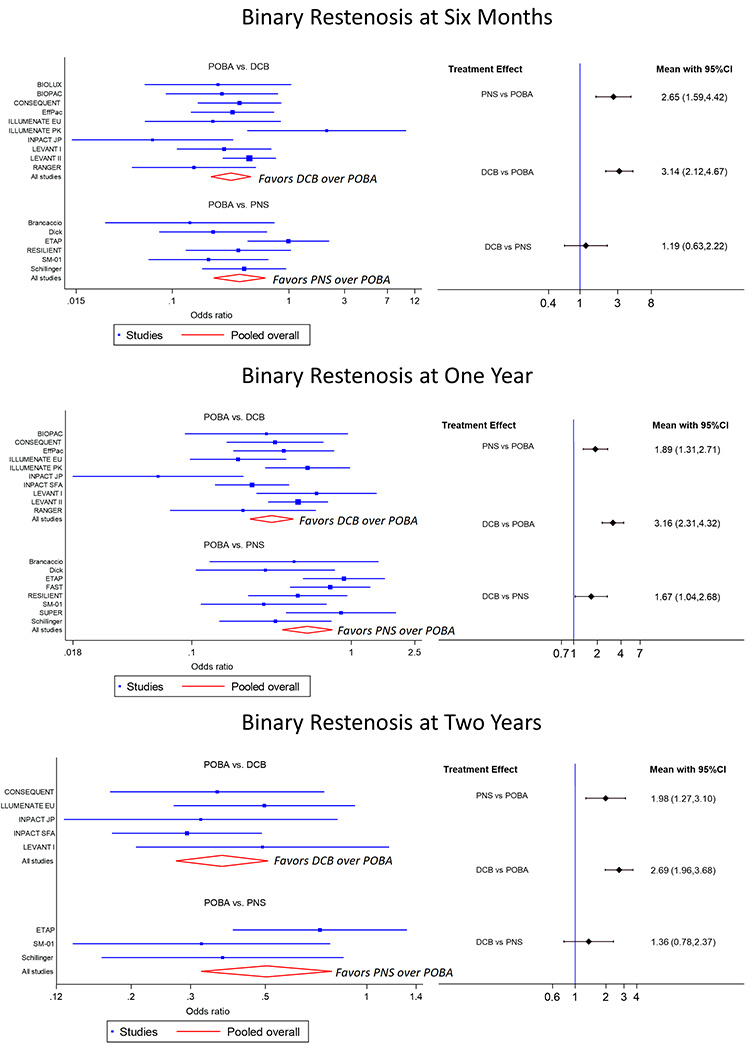
The left side shows forest plots for freedom from binary restenosis at 6 months (top), 12 months (middle), and 24 months (bottom). The right side shows interval plots for freedom from binary restenosis at 6 months (top), 12 months (middle), and 24 months (bottom). Black horizonal lines represent confidence intervals (CI).
Target Lesion Revascularization
The odds of freedom from target lesion revascularization for patients treated with PNS compared to POBA at 6 months was 3.66 (95% CI 0.72 – 18.53), at 12 months was 1.61 (95% CI 1.01 – 2.58), and at 24 months was 1.64 (95% CI 0.87 – 3.08) (Figure 2.) The odds of freedom from target lesion revascularization for patients treated with DCB compared to POBA at 6 months was 2.42 (95% CI 1.28 – 4.56), at 12 months was 3.05 (95% CI 2.10 – 4.43), and at 24 months was 2.75 (95% CI 1.95 – 3.88). The odds of freedom from target lesion revascularization for patients treated with DCB compared to PNS at 6 months was 0.66 (95% CI 0.12 – 3.80), at 12 months was 1.89 (95% CI 1.04 – 3.45), and at 24 months was 1.68 (95% CI 0.82 – 3.44).
Figure 2.
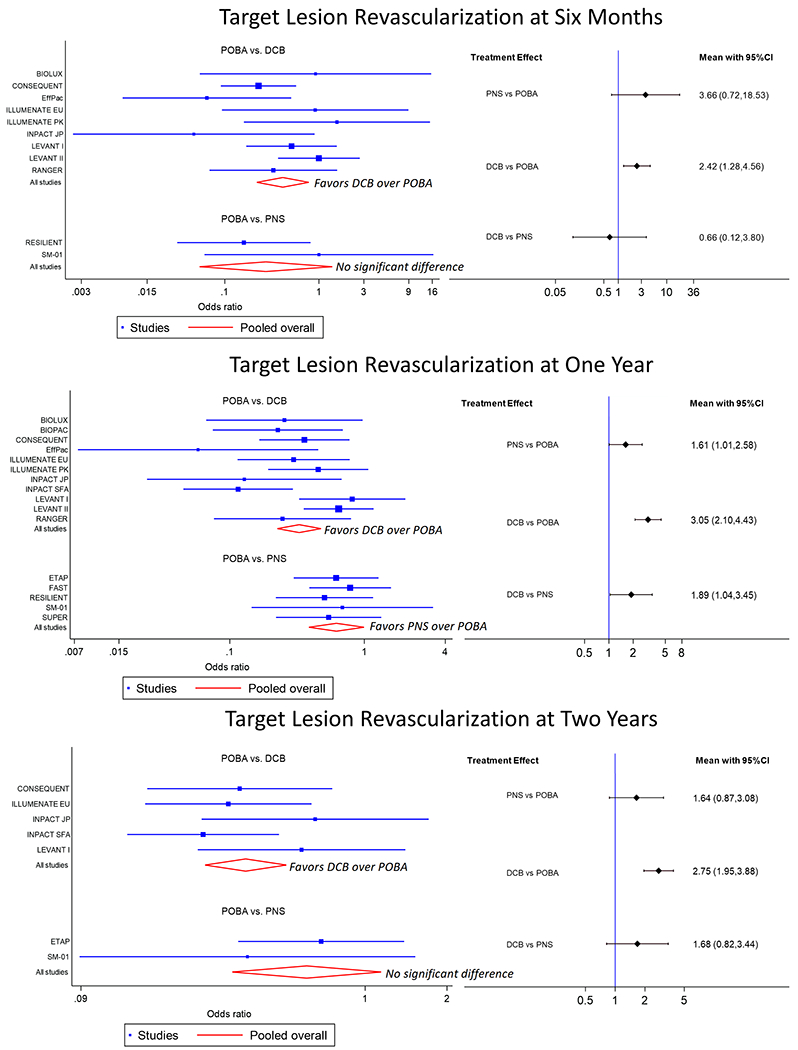
The left side shows forest plots for freedom from target lesion restenosis at 6 months (top), 12 months (middle), and 24 months (bottom). The right side shows interval plots for freedom from target lesion revascularization at 6 months (top), 12 months (middle), and 24 months (bottom). Black horizonal lines represent confidence intervals (CI).
Ankle-Brachial Index
The mean increase in ABI for patients treated with PNS compared to POBA at 6 months was 0.07 higher (95% CI 0.00 – 0.13), at 12 months was 0.04 higher (95% CI 0.00 – 0.08), and at 24 months was 0.03 higher (95% CI −0.03 – 0.09) (Figure 3.) The mean increase in ABI for patients treated with DCB compared to POBA at 6 months was 0.01 higher (95% CI −0.06 – 0.07), at 12 months was equal (95% CI −0.03 – 0.02), and at 24 months was 0.04 lower (95% CI −0.08 – 0.01). The mean increase in ABI for patients treated with PNS compared to DCB at 6 months was 0.06 higher (95% CI −0.03 – 0.15), at 12 months was 0.05 higher (95% CI 0.00 – 0.09), and at 24 months was 0.07 higher (95% CI −0.01 – 0.14).
Figure 3.
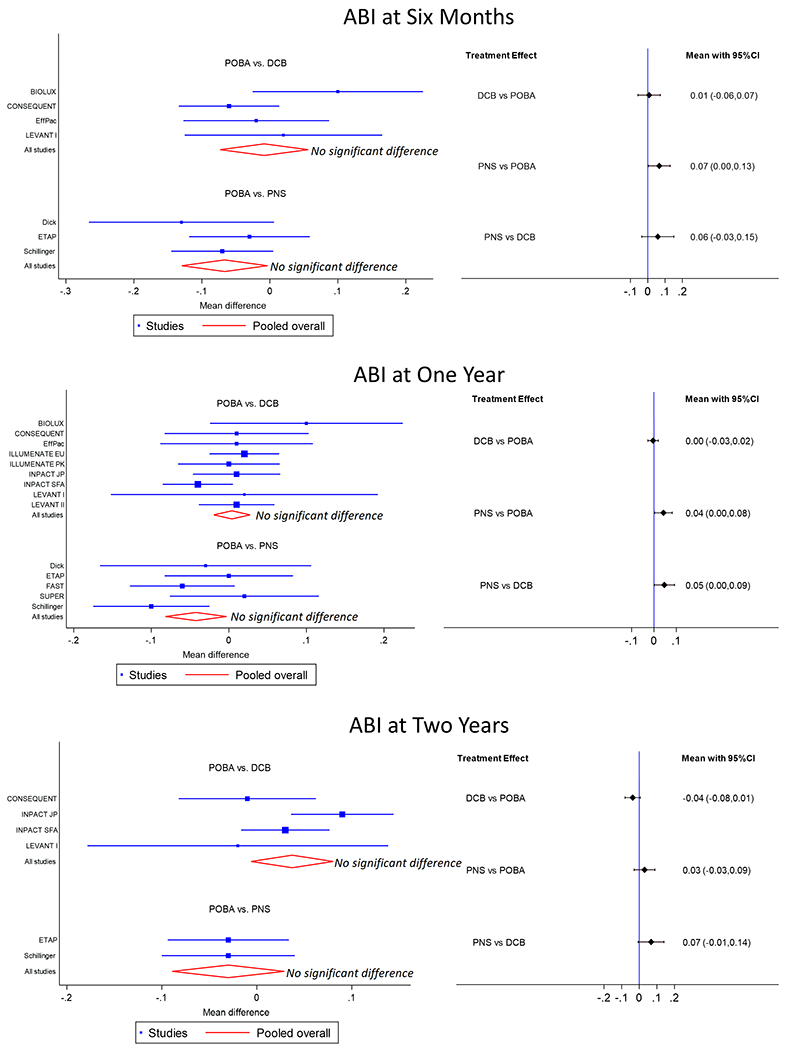
The left side shows forest plots for mean increase in ABI at 6 months (top), 12 months (middle), and 24 months (bottom). The right side shows interval plots for mean increase in ABI at 6 months (top), 12 months (middle), and 24 months (bottom). Black horizonal lines represent confidence intervals (CI).
Discussion
Both DCB and PNS demonstrated a statistically lower rate of binary restenosis compared with POBA at all timepoints. Indirect comparisons via network meta-analysis demonstrated that DCB had a smaller, but still significant, advantage over PNS at preventing binary restenosis and target lesion revascularization at the 12-month timepoint but not at the 6-month or 24-month timepoints. The clinical significance of a difference in binary restenosis and TLR at 12 months, but not at six months or 24 months, is open to debate. The reader should note, however, that there was less data reported at 6 months and 24 months resulting in wider confidence intervals at these timepoints. For example, there were only two studies reporting TLR at 6 months when comparing POBA and PNS resulting in a 95% confidence interval ranging from 0.72 to 18.53.
In designing this network meta-analysis, we chose binary restenosis as our primary outcome measure because it was ubiquitous, operator-independent, and uniformly defined. During our initial review of the literature, we found many papers which used primary patency as an outcome measure. Unfortunately, the definitions of primary patency were inconsistent. However, we were able to infer binary restenosis rates from primary patency rates with supporting data. Target lesion revascularization, though commonly included and clearly defined, is often dependent on the clinical decisions made by an unblinded interventionist and thus prone to bias. This problem is magnified in studies where routine follow-up angiography is performed thus forcing an unblinded operator to quickly decide the need for target lesion revascularization. Ankle-brachial index was clearly defined, but imprecise, and studies had inconsistent methods for addressing patients who had undergone interim target-lesion revascularization.
This study was subject to the usual limitations of network meta-analyses such as inconsistency in reporting standards, incomplete data, the transitivity assumption, and industry bias. Additionally, we were not able to evaluate inconsistency between direct and indirect PNS-DCB comparisons due to our star-shaped network geometry. A limitation unique to this network meta-analysis was the high rate of adjunctive stenting. Among the 565 patients randomized to POBA in the studies comparing PNS against POBA, 145 patients were crossed over to stenting for an adjunctive stenting rate of 25.0%. In the studies comparing POBA against DCB, the adjunctive stenting rates were lower as many trials were designed such that randomization would occur after a successful pre-dilation. Among the 2,111 patients in the studies comparing DCB against POBA, 253 underwent adjunctive stenting for a rate of 12.0%. Although most PNS versus POBA studies provided adequate as-treated subgroup analysis, similar data was not available from the DCB versus POBA studies. Therefore, we are unable to perform valuable as-treated analysis.
As of 2020, paclitaxel-based devices remain controversial. In 2020, the consortium led by the VIVA Physicians produced a patient-level meta-analysis using data from 2185 patients in eight paclitaxel-containing device trials with a median follow-up of four years; at five years mortality for patients receiving paclitaxel-based devices was 18.3% versus 13.7% for controls.8 Multiple publications have investigated the concern that stenting may eliminate future therapeutic options, such as open bypass, due to the presence of a permanent foreign body in the artery. Conway examined 621 patients who underwent femoropopliteal stenting of whom 30 had subsequent stent occlusion. Within this group they identified 7 patients whose theoretical bypass target would become more distal. Joels et al examined 276 patients who underwent femoral stenting and noted that 9% had early failure (<200 day) and that early failure altered the distal bypass target in 28% of those cases.9 Gur et al identified 239 patients who underwent femoropopliteal stenting, 69 lost patency, and 2 ultimately had bypasses which, if not for the presence of a stent, would have had a more proximal target.10 Thus, while a metallic stent can impede a future open bypass, such scenarios are uncommon in practice.
There are concerns that reintervention following stent failure is complicated. Unfortunately, there is limited prospective randomized data regarding secondary patency following primary nitinol stenting. Among the eight studies comparing POBA to PNS, only two studies reported secondary patency. The ETAP study reported a two-year secondary patency rate of 78.3% for PNS and 77.8% for POBA. The RESILIENT study reported a one-year secondary patency rate of 100% for PNS and 98.3% for PTA. The Schillinger study did not report secondary patency but did mention that three of the thirteen PNS patients who required reintervention received bypass surgery compared to none among the sixteen reinterventions in the POBA cohort. Therefore, while concerns regarding reintervention following PNS are valid, there is limited data regarding outcomes following reintervention.
Conclusion
This network meta-analysis demonstrates that both DCB and PNS are superior to POBA. When comparing DCB to PNS across multiple timepoints and outcome measures, we observed DCB narrowly outperform PNS in binary restenosis and target lesion revascularization at the 12-month timepoint. However, these differences are much smaller than those observed when comparing DCB or PNS to POBA. This study demonstrates that for femoropopliteal atherosclerotic lesions, PNS remains a reasonable alternative to DCB when the use of paclitaxel is not desirable.
Supplementary Material
Supplementary Figure 1. Diagram detailing the study selection process according to the Preferred Reporting for Systematic Reviews and Meta-Analysis (PRISMA) statement.1
Supplementary Figure 2. Weighted summary plot. The risk of each bias item is presented as percentages across all included studies.
Supplementary Figure 3. Traffic light plot. Risk of bias per item per individual study. The items were scored as adequate (+), unclear (−), or inadequate (X). Other bias refers to the fact that the study was supported (X) or not supported (+) by industry sponsors.
Supplementary Figure 4. Left column: funnel plot for binary restenosis for 6-month (top), 12-month (middle), and 24 month (bottom). Middle column: funnel plot for target lesion revascularization for 6-month (top), 12-month (middle), and 24 month (bottom). Right column: funnel plot for change in ABI restenosis for 6-month (top), 12-month (middle), and 24 month (bottom).
Acknowledgements:
This work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Meeting Presentation: Western Vascular Society Annual Meeting, September 2020
Disclosures: None
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med. 2009 Jul 21;6(7):e1000097.
References
- 1.Giacoppo D, Cassese S, Harada Y, Colleran R, Michel J, Fusaro M, Kastrati A, Byrne RA. Drug-coated balloon versus plain balloon angioplasty for the treatment of femoropopliteal artery disease: an updated systematic review and meta-analysis of randomized clinical trials. JACC: Cardiovascular Interventions. 2016. August 22;9(16):1731–42. [DOI] [PubMed] [Google Scholar]
- 2.Chowdhury MM, McLain AD, Twine CP. Angioplasty versus bare metal stenting for superficial femoral artery lesions. Cochrane Database of Systematic Reviews. 2014(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway AM, Qato K, Bottalico D, Lugo J, Giangola G, Carroccio A. Occluded superficial femoral and popliteal artery stents can have a negative impact on bypass target. Journal of Endovascular Therapy. 2015. December;22(6):868–73. [DOI] [PubMed] [Google Scholar]
- 4.Rocha-Singh KJ, Duval S, Jaff MR, Schneider PA, Ansel GM, Lyden SP, Mullin CM, Ioannidis JP, Misra S, Tzafriri AR, Edelman ER. Mortality and paclitaxel-coated devices: an individual patient data meta-analysis. Circulation. 2020. June 9;141(23):1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohapatra A, Saadeddin Z, Bertges DJ, Madigan MC, Al-Khoury GE, Makaroun MS, Eslami MH. Nationwide trends in drug-coated balloon and drug-eluting stent utilization in the femoropopliteal arteries. Journal of Vascular Surgery. 2020. February 1;71(2):560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. September 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011. October 18;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha-Singh KJ, Duval S, Jaff MR, Schneider PA, Ansel GM, Lyden SP, Mullin CM, Ioannidis JP, Misra S, Tzafriri AR, Edelman ER. Mortality and paclitaxel-coated devices: an individual patient data meta-analysis. Circulation. 2020. June 9;141(23):1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joels CS, York JW, Kalbaugh CA, Cull DL, Langan EM III, Taylor SM. Surgical implications of early failed endovascular intervention of the superficial femoral artery. Journal of vascular surgery. 2008. March 1;47(3):562–5. [DOI] [PubMed] [Google Scholar]
- 10.Gur I, Lee W, Akopian G, Rowe VL, Weaver FA, Katz SG. Clinical outcomes and implications of failed infrainguinal endovascular stents. Journal of vascular surgery. 2011. March 1;53(3):658–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Diagram detailing the study selection process according to the Preferred Reporting for Systematic Reviews and Meta-Analysis (PRISMA) statement.1
Supplementary Figure 2. Weighted summary plot. The risk of each bias item is presented as percentages across all included studies.
Supplementary Figure 3. Traffic light plot. Risk of bias per item per individual study. The items were scored as adequate (+), unclear (−), or inadequate (X). Other bias refers to the fact that the study was supported (X) or not supported (+) by industry sponsors.
Supplementary Figure 4. Left column: funnel plot for binary restenosis for 6-month (top), 12-month (middle), and 24 month (bottom). Middle column: funnel plot for target lesion revascularization for 6-month (top), 12-month (middle), and 24 month (bottom). Right column: funnel plot for change in ABI restenosis for 6-month (top), 12-month (middle), and 24 month (bottom).


