ABSTRACT
Immunotherapy shows promising therapeutic efficacy against various types of cancer, but most fail to respond. Preclinical studies have suggested that concomitant medications, such as statins, non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, metformin and beta-blockers, might affect clinical outcomes if used with immune checkpoint inhibitors (ICIs), but their clinical roles are conflicting. This meta-analysis investigates the effect of these concomitant medications on outcomes in patients treated with ICIs. A search was conducted for all reports published until 31 March 2021 in PubMed, Web of Science, Cochrane Library, EMBASE and conference proceedings. Studies were included if they investigated the association between the concomitant use of these medications and progression-free survival (PFS) or overall survival (OS) during ICI treatment. A total of 3331 patients from 13 eligible studies were included. Among them, five articles on statins, six studies evaluating NSAIDs, five studies employing low-dose aspirin, eight studies on metformin and four articles on beta-blockers were included. The concomitant use of statins during ICI treatment was correlated with improved OS and PFS. Low-dose aspirin was associated with better PFS instead of OS. No significant association was demonstrated between the concurrent use of NSAIDs, beta-blockers and metformin and OS or PFS. The concomitant use of statins and low-dose aspirin during ICI treatment showed a positive impact on treatment outcomes. The concurrent use of NSAIDs, beta-blockers and metformin is not significantly associated with clinical benefits. The effect of these medications in different cancer patients treated with ICI is needed to be further validated.
KEYWORDS: Immune checkpoint inhibitors, statin, aspirin, meta-analysis
Introduction
Immune checkpoint inhibitors (ICIs) targeting cytotoxic T-lymphocyte–associated-4 (CLTA-4) and programmed cell death protein-1 (PD-1) or its ligand (PD-L1) have revolutionized the treatment landscape of multiple solid tumors, such as melanoma, non-small-cell lung cancer (NSCLC) and elicited durable survival benefits.1 Nevertheless, a significant portion of patients do not benefit from ICIs creating an unmet need to identify determinants that impact the efficacy of ICIs and to develop combined treatment modalities to improve the clinical outcome of ICIs.2
Drug–drug interactions affect the efficacy and safety profile of medications. The expanded indications of ICIs pose new challenges in clinical practice due to interactions with medications used concomitantly. Several studies and meta-analyses on the use of simultaneous medications, including corticosteroids, proton pump inhibitors (PPIs) and antibiotics, have demonstrated that the concomitant use of these drugs influences survival outcomes in patients treated with ICIs compared with those who treated with ICIs alone.3–6 Cortellini et al. further demonstrated that negative impact of antibiotics on ICIs monotherapy but not chemotherapy might be as a result of their underlying immune-modulatory effect, while the effects of corticosteroids and PPIs on clinical outcomes might be driven by adverse disease features.7
Recently, great interest has been garnered in the anticancer properties of commonly prescribed drugs, such as statins, non-steroidal anti-inflammatory drugs (NSAIDs), low-dose aspirin that is different from non-aspirin NSAIDs in terms of indications and adverse effects, metformin and beta-blockers. Several studies have demonstrated the direct or indirect anticancer roles of these drugs in preclinical models. These medications have also been postulated to have positive roles in the reduced incidence and mortality of various cancer types (e.g., hepatocellular carcinoma, NSCLC, melanoma) in clinical settings.8–13
More recently, researchers have revealed that these medications exert immunomodulatory effects on components of the tumor microenvironment and can enhance the efficacy of ICIs in preclinical studies. However, the impact of these medications is conflicting in patients administered ICIs. We thus undertook a meta-analysis to examine the effect of concomitant use of these drugs on outcomes and their potential combined treatment in patients treated with ICIs.
Materials and methods
Search strategies
An electronic search was performed using PubMed, EMBASE, Cochrane Library, Web of Science database and the abstracts from conference proceedings from the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) of were also screened to identify more potentially relevant studies up to March 31, 2021. Studies were identified with MESH terms and free text including “immune check point inhibitor*”, “neoplasm”, “statin*”, “anti-inflammatory agents, Non-Steroidal”, “aspirin”, “metformin”, “adrenergic beta-antagonists” as well as specific drug names (details are seen in Supplement Table 1).
Table 1.
The main characteristics of studies included in this meta-analysis
| ID | Country | Cancer type | ICIs treatment | ICIs line of treatments | Concomitant medications | Sample size (Y/N) |
Outcome | Median PFS (Users vs non-users) (Month) |
Median OS (Users vs non-users) (Month) |
Analysis model | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Afzal2019 | US | NSCLC | Pembrolizumab Nivolumab Atezolizumab |
First-line Second-line Third-line | Metformin | 21/29 | PFS/OS | 4 vs 3 | 11.5 vs 7.6 | Univariate | 6 |
| Afzal2018 | US | Melanoma | Pembrolizumab Nivolumab Ipilimumab + Nivolumab Ipilimumab |
First-line | Metformin | 33/22 | PFS/OS | 19.8 vs 5 | 46.7 vs 28 | Univariate | 6 |
| Buti2021 | Italy | NSCLC Melanoma RCC Others |
PD-1 inhibitors PD-L1 inhibitors CTLA-4 inhibitors |
First-line Second-line Third-line |
Statin Low-dose aspirin Metformin |
56/161 43/174 17/200 |
PFS/OS | 3.6 vs 3.6 3.2 vs 3.6 3.8 vs 3.6 |
12.4 vs 8.4 8.9 vs 8 12.6 vs 8.7 |
Univariate | 6 |
| Cortellini2020 | Italy | NSCLC Melanoma RCC Others |
Pembrolizumab Nivolumab Atezolizumab Others |
First Non- first |
Statin NSAIDs Low-dose aspirin Metformin Beta blockers |
192/820 59/953 189/823 114/868 114/898 |
PFS/OS | NA | NA | Multivariate | 7 |
| Cantini 2021 | Italy | NSCLC MPM |
Nivolumab Pembrolizumab |
Second-line Third-line | Statin | 67/186 | PFS/OS | 7.8 vs 3.6 6.7 vs 2.3 |
13.1 vs 10.1 NR vs 6 |
Multivariate | 6 |
| Failing2016 | US | Melanoma | Ipilimumab | First-line | Statin NSAIDs Low-dose aspirin Metformin |
40/119 31/128 38/121 12/147 |
PFS/OS | NA | NA | Multivariate | 6 |
| Kanai2021 | Japan | NSCLC | Atezolizumab Nivolumab Pembrolizumab |
Second-line Third-line | NSAIDs | 65/133 | PFS/OS | 3.45 vs 3.94 | 7.85 vs 15.11 | Univariate | 5 |
| Gaucher2021 | France | Lung Melanoma Renal and urothelial Head and neck Others |
Nivolumab Pembrolizumab Ipilimumab Nivolumab + Ipilimumab |
First-line Second-line Third-line |
Metformin | 17/355 | OS | NA | NR vs 15.62 | Multivariate | 6 |
| Michael 2020 | US | NSCLC | Nivolumab Pembrolizumab Atezolizumab |
Second-line Third-line | Beta-blockers | 28/81 | PFS/OS | 10.3 vs 5 | 21.5 vs13.3 | Univariate | 5 |
| Nichetti 2020 | Italy | NSCLC | PD-1 inhibitors PD-L1 inhibitors PD-L1 + CTLA-4 inhibitors |
First-line Second-line Third-line |
Low-dose aspirin | 61/156 | PFS | 6.59 vs 3.18 | NA | Univariate | 5 |
| Svaton2020 | Czech | NSCLC | Nivolumab | First-line Second-line Third-line or higher |
Statin NSAIDs Metformin |
31/193 45/178 18/206 |
PFS/OS | 7.2 vs 5.4 6.9 vs 5.3 3.3 vs 6 |
16.8 vs 12.9 16.8 vs 12.8 10.6 vs 13.1 |
Multivariate | 6 |
| Wang D.Y 2020 | Mixed | Melanoma | Nivolumab Pembrolizumab | First-line | NSAIDs Low-dose aspirin Metformin Beta-blocker |
122/208 47/283 34/296 65/265 |
PFS/OS | 8.5 vs 5.2 NA 11.1 vs 5.6 11.2 vs 5.5 |
25.7 vs 27.3 NA 27.6 vs 26.0 27.8 vs 25.8 |
Multivariate/Univariate | 5 |
| Wang S.J 2020* | US | Melanoma NSCLC |
PD-1 inhibitors PD-L1 inhibitors CTLA-4 inhibitors |
NA | NSAIDs | 32/58 20/17 |
OS | NA | 25.44 vs22.08 37.68 vs 14.28 |
Multivariate | 6 |
*:Wang S.J 2020 study included 2 cohorts which are melanoma patients and NSCLC patients, respectively.
Abbreviations: OS, overall survival; PFS, progression-free survival; NSCLC, non-small cell lung cancer; NSAIDS, non-steroidal anti- inflammatory drugs; MPM, malignant pleural mesothelioma; RCC, renal cell carcinoma; NA, not available; NR, not reached.
Study selection criteria
The inclusion criteria for the study were as follows: (1) Studies focusing on patients with solid tumors or hematological malignancy treated with ICIs; (2) Studies involved the association between concurrent use of metformin, statin, NSAIDs, low-dose aspirin (considered for cardiovascular prevention), beta blocker and ICIs efficacy in patients with cancer reporting overall survival (OS) or progression-free survival (PFS); (3) Sufficient data were provided to calculate the hazard ratio (HR) and 95% confidence interval (CI). Studies with insufficient information to evaluate HRs and 95% CIs, and in languages other than English are excluded.
Data extraction and quality assessment
Two investigators selected the studies that fulfilled our inclusion criteria and extracted the relevant information independently. Disagreements were resolved by discussion with an independent expert. The following information was extracted: first author’s name, publication year, country, sample size, concomitant medications received, study design, type of cancer, ICIs agent, ICIs line of treatments, concomitant medications median PFS and OS, HRs for OS and PFS and 95% CIs between uses and non-users. The Quality Assessment of Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of studies. This scale consists of three parameters: selection, comparability and outcome assessment. NOS scores ≥6 are considered high-quality studies.
Statistical analysis
HRs with their 95% CIs from included studies were used to calculate pooled HR. Heterogeneity of pooled results was accessed by using Higgins I2 statistics. I2 > 50% was defined as significant heterogeneity. A fixed effect model or random effect model was employed according to the heterogeneity of the studies. The data were synthesized using a fixed effect model with I2 < 50%. Otherwise, a random effect model was utilized. The sources of heterogeneity were evaluated by sensitivity and subgroup analysis. Sensitivity analysis was used to appraise the stability of the outcome. Funnel plots and Egger’s test were constructed to evaluate publication bias. All statistical tests were two-sided, and statistical significance was defined as P < .05. The pooled data were analyzed with STATA 16.0.
Results
Selection and characteristics of studies
A flowchart showing our literature selection is shown as Figure 1. Initially, 2517 relevant records were retrieved from selected databases. A total of 2184 records were retained after duplicate removal. Of these, 2152 were excluded by screening the title and abstract, thereby leaving 32 potentially relevant full-text articles. Eventually, we selected 13 studies14–26 that evaluated the impact of related concomitant medications on the survival of patients with cancer treated with ICIs. All eligible studies were retrospective and involved 3331 patients, who were included in our meta-analysis. All studies were graded as “moderate” or “high” quality according to NOS criteria and qualified for a meta-analysis.
Figure 1.
Flow chart of literature search and study selection. A total of 2571 articles were initially retrieved. After carefully reviewed 13 studies reporting the impact of related concomitant medications on the survival of patients with cancer treated with ICI were included in the analysis
Among them, five articles16–19,24 evaluating statins, six studies18,19,21,24–26 employing NSAIDs, five studies16,18,19,22,25 evaluating low-dose aspirin, eight studies14–16,18–20,24,25 based on metformin and four articles18,19,23,25 on beta-blockers were included in the quantitative synthesis. The most common types of cancer investigated were NSCLC and melanoma. The characteristics of patients in the studies at baseline are summarized in Table 1.
Statins
Five studies reported the influence of concurrent use of statins on OS and PFS in cancer patients administered ICIs. The study by Cantini and colleagues in 202117 included two cohorts in which patients were diagnosed with NSCLC or malignant pleural mesothelioma (MPM) and showed the HR and 95% CI, respectively, so we termed them “Cantini 2021 cohort 1” and “Cantini 2021 cohort 2”. Overall, the concomitant use of statins was significantly associated with improved OS and PFS in patients receiving ICIs (statin users versus non-statin users: OS: HR = 0.76, 95% CI = 0.63–0.92, P = .005; PFS: HR = 0.86, 95% CI = 0.75–0.99, P = .036) (Figure 2a, b). Subgroup analyses stratified by ICIs showed that concomitant use of statins was significantly associated with favorable OS and PFS in patients receiving PD-1/PD-L1 inhibitors alone, whereas other ICIs regimens showed no significant difference. The results for subgroups based on cancer types showed that the concomitant use of statins was related to better OS and PFS in MPM patients. Interestingly, the concomitant use of statins was linked to improved PFS but not OS in NSCLC patients. No association between the concomitant use of statins and outcomes in patients with other types of cancer was shown in our meta-analysis. Moreover, we performed stratified analysis by analysis model, which showed that the concomitant use of statins led to better OS and PFS for patients in the multivariate analysis, but not in the univariate analysis (Table 2).
Figure 2.
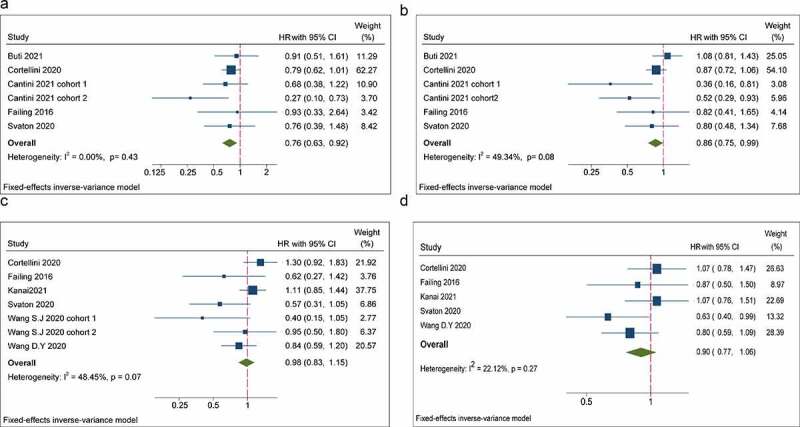
Forest plots of the hazard ratios and 95% CIs for overall survival (a) and progression-free survival (b) in patients with the concomitant use of statins compared patients without use of these drugs. Forest plots of the hazard ratios and 95% CIs for overall survival (c) and progression-free survival (d) in patients with the concomitant use of NSAIDs compared patients without use of these drugs
Abbreviation: NSAIDs, non-steroidal anti-inflammatory drugs; HR, hazard ratio; CI, confidence Interval.
Table 2.
Results of subgroup analysis
| Analysis | N | OS |
N | PFS |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Association | Heterogeneity |
Association |
Heterogeneity | |||||||
| HR (95% CI) | P | I2 | HR (95% CI) | P | I2 | |||||
| Statin | ||||||||||
| Total | 6a | 0.76(0.63–0.92) | 0.005 | 48.4% | 6a | 0.86(0.75–0.99) | 0.036 | 22.1% | ||
| Agent | ||||||||||
| PD-1/PD-L1 | 4 | 0.74 (0.60–0.91) | 0.004 | 29.6% | 4 | 0.79(0.67–0.94) | 0.008 | 54.1% | ||
| CTLA-4 | 1 | 0.93(0.33–2.64) | 0.891 | 0% | 1 | 0.82(0.41–1.65) | 0.578 | 0% | ||
| Mixed | 1 | 0.91(0.51–1.61) | 0.747 | 0% | 1 | 1.08(0.81–1.43) | 0.596 | 0% | ||
| Cancer type | ||||||||||
| NSCLC | 2 | 0.71(0.46–1.11) | 0.131 | 0% | 2 | 0.64(0.41–0.98) | 0.041 | 62.4% | ||
| Melanoma | 1 | 0.93(0.33–2.64) | 0.891 | 0% | 2 | 0.82(0.62–1.07) | 0.578 | 0% | ||
| MPM | 1 | 0.27(0.1–0.73) | 0.010 | 0% | 1 | 0.52(0.29–0.93) | 0.028 | 0% | ||
| Multiple | 2 | 0.81(0.65–1.01) | 0.062 | 0% | 1 | 0.93(0.79–1.09) | 0.385 | 34.2% | ||
| Analysis model | ||||||||||
| Multivariate | 5 | 0.74(0.61–0.91) | 0.004 | 10.0% | 5 | 0.80(0.75–0.99) | 0.006 | 38.9% | ||
| Univariate | 1 | 0.91(0.51–1.61) | 0.747 | 0% | 1 | 1.08(0.81–1.43) | 0.596 | 0% | ||
| NSAIDs | ||||||||||
| Total | 7b | 0.98(0.83–1.15) | 0.769 | 48.4% | 5 | 0.90(0.77–1.06) | 0.213 | 22.1% | ||
| Agent | ||||||||||
| PD-1/PD-L1 | 5 | 1.03 (0.86–1.22) | 0.769 | 56.5% | 4 | 0.90(0.76–1.07) | 0.250 | 41.4% | ||
| CTLA-4 | 1 | 0.62(0.27–1.42) | 0.259 | 0% | 1 | 0.87(0.50–1.50) | 0.617 | - | ||
| Mixed | 2 | 0.73(0.43–1.25) | 0.249 | 53.2% | - | - | - | - | ||
| Cancer type | ||||||||||
| NSCLC | 4 | 0.95(0.76–1.18) | 0.645 | 64.3% | 2 | 0.88(0.67–1.16) | 0.356 | 70.4% | ||
| Melanoma | 2 | 0.80(0.58–1.11) | 0.184 | 12.2% | 2 | 0.82(0.62–1.07) | 0.137 | 0% | ||
| Multiple | 1 | 1.30(0.92–1.83) | 0.135 | 56.1% | 1 | 1.07(0.78–1.47) | 0.676 | 0% | ||
| Analysis model | ||||||||||
| Multivariate | 6 | 0.90(0.74–1.11) | 0.327 | 50.8% | 4 | 0.86(0.71–1.03) | 0.104 | 22.9% | ||
| Univariate | 1 | 1.11(0.85–1.44) | 0.435 | 0% | 1 | 1.07(0.76–1.51) | 0.699 | 0% | ||
| Low-dose aspirin | ||||||||||
| Total | 4 | 0.93(0.76–1.15) | 0.514 | 9.2% | 5 | 0.84(0.72–0.98) | 0.024 | 38.3% | ||
| Agent | ||||||||||
| PD-1/PD-L1 | 2 | 0.86(0.68–1.08) | 0.192 | 0% | 2 | 0.80(0.65–0.99) | 0.043 | 34.6% | ||
| CTLA-4 | 1 | 1.56(0.79–13.10) | 0.202 | 0% | 1 | 0.93(0.55–1.57) | 0.786 | - | ||
| Mixed | 1 | 1.10(0.62–1.95) | 0.744 | 0% | 2 | 0.87(0.69–1.10) | 0.2436 | 38.3% | ||
| Cancer type | ||||||||||
| NSCLC | 0 | - | - | - | 1 | 0.67(0.48–0.94) | 0.020 | 0% | ||
| Melanoma | 2 | 1.55(0.81–2.94) | 0.184 | 0% | 2 | 1.01(0.61–1.66) | 0.968 | 0% | ||
| Multiple | 2 | 0.88(0.71–1.10) | 0.256 | 0% | 2 | 0.88(0.73–1.05) | 0.144 | 66.9% | ||
| Analysis model | ||||||||||
| Multivariate | 3 | 0.91(0.73–1.14) | 0.41 | 32.1% | 3 | 0.82(0.67–1.00) | 0.048 | 0% | ||
| Univariate | 1 | 1.10(0.62–1.95) | 0.744 | 0% | 2 | 0.87(0.69–1.10) | 0.246 | 38.3% | ||
| Metformin | ||||||||||
| Total | 8 | 1.07(0.89–1.30) | 0.462 | 40.3% | 7 c | 1.08(0.92–1.27) | 0.346 | 29.2% | ||
| Agent | ||||||||||
| PD-1/PD-L1 | 5 | 1.12 (0.91–1.38) | 0.204 | 53.6% | 5 | 1.02(0.85–1.23) | 0.799 | 29.6% | ||
| CTLA-4 | 1 | 1.37(0.48–3.90) | 0.555 | 0% | 1 | 1.83(0.87–3.85) | 0.238 | - | ||
| Mixed | 2 | 0.85(0.52–1.37) | 0.266 | 62.6% | 1 | 1.33(0.83–2.14) | 0.112 | - | ||
| Cancer type | ||||||||||
| NSCLC | 2 | 1.22(0.73–2.04) | 0.438 | 64.3% | 2 | 1.09(0.71–1.69) | 0.691 | 16.3% | ||
| Melanoma | 3 | 0.82(0.54–1.24) | 0.347 | 12.2% | 3 | 0.89(0.64–1.24) | 0.501 | 60.9% | ||
| Multiple | 3 | 1.13(0.90–1.43) | 0.284 | 56.1% | 2 | 1.17(0.95–1.44) | 0.149 | 0% | ||
| Analysis model | ||||||||||
| Multivariate | 6 | 1.21(0.96–1.53) | 0.103 | 52.5% | 3 | 1.68(0.71–1.19) | 0.084 | 0% | ||
| Univariate | 4 | 0.85(0.62–1.17) | 0.322 | 0% | 4 | 1.20(0.98–1.48) | 0.516 | 29.4% | ||
| Beta-blockers | ||||||||||
| Total | 4 | 0.87(0.71–1.08) | 0.588 | 53.6% | 4 | 0.91(0.66–1.26) | 0.207 | 0% | ||
| Agent | ||||||||||
| PD-1/PD-L1 | 3 | 0.86 (0.69–1.06) | 0.159 | 0% | 3 | 0.88(0.73–1.06) | 0.183 | 32.8% | ||
| CTLA-4 | 1 | 1.37(0.48–3.90) | 0.555 | 0% | 1 | 1.83(0.87–3.85) | 0.112 | 0% | ||
| Cancer type | ||||||||||
| NSCLC | 1 | 0.66(0.38–1.16) | 0.148 | 0% | 1 | 0.48(0.23–1.01) | 0.052 | 0% | ||
| Melanoma | 2 | 0.94 (0.65–1.36) | 0.739 | 0% | 2 | 0.97 (0.72–1.32) | 0.685 | 69.7% | ||
| Multiple | 1 | 0.90(0.68–1.20) | 0.467 | 0% | 1 | 0.95(0.74–1.21) | 0.679 | 0% | ||
| Analysis model | ||||||||||
| Multivariate | 2 | 0.93(0.70–1.22) | 0.585 | 0% | 3 | 0.94(0.53–1.68) | 0.839 | 68.0% | ||
| Univariate | 2 | 0.81(0.71–1.08) | 0.192 | 0% | 1 | 0.86(0.62–1.20) | 0.371 | 0% | ||
Abbreviations: OS, overall survival; PFS, progression-free survival; NSCLC, non-small cell lung cancer; NSAIDS, non-steroidal anti- inflammatory drugs; MPM, malignant pleural mesothelioma.
Annotation
a. The study by Cantini et al. included two cohorts and showed the HR and 95% CI respectively, and the total number refers to cohorts rather than studies.
b. The study by Wang et al. 2020 included two cohorts and the HR and 95% CI were reported, respectively, and the total number refers to cohorts rather than studies.
c. The study by Gaucher et al. only reported the HR and 95%CI for OS, and the total number for PFS is 7.
A funnel plot of the included studies showed no obvious asymmetry for HR of OS and PFS (Supplementary Figure S1, S2), suggesting that no publication bias existed. Egger’s test further confirmed these results (OS: P = .416; PFS: P = .153, respectively). Sensitivity analyses for OS demonstrated that the pooled HRs and 95% CIs were not changed significantly, but PFS was influenced significantly if the studies by Cortellini et al. 2020,18 Cantini et al. 2021 cohort 1, cohort 217 or Svaton et al. 202024 were excluded, suggesting that pooled results were unstable (Supplementary Figure S3, S4).
NSAIDs
Six studies and five studies exhibited the effect of NSAIDs on OS and PFS in patients treated with ICIs, respectively. The study by Wang et al. 202026 included two cohorts in which patients had melanoma or NSCLC and the HR and 95% CI were reported, respectively, so we termed them “Wang et al. 2020 cohort 1” and “Wang et al. 2020 cohort 2”. Pooled data of HRs showed that the concurrent use of NSAIDs was not significantly related to OS or PFS in patients receiving ICIs (NSAIDs users versus non-NSAIDs users: OS: HR = 0.98, 95% CI = 0.83–1.15, P = .769; PFS: HR = 0.90, 95% CI = 0.77–1.06, P = .213) (Figure 2c, d). In subgroup analyses of ICI agents, cancer type and analytical model, no significant association between the concomitant use of NSAIDs and OS and PFS, respectively, was observed (Table 2).
A funnel plot demonstrated slight asymmetry for HR of OS but not for PFS (Supplementary Figure S5, S6). Egger’s test was done to further confirm these results (OS: P = .037; PFS: P = .504), and indicated a low risk of a potential publication bias in OS but not PFS. Sensitivity analyses of OS and PFS showed that the pooled HRs and their 95% CIs were not changed significantly if a single study was removed, suggesting that pooled results were robust and stable (Supplementary Figure S7, S8).
Low-dose aspirin
Low-dose aspirin revealed its combined effect on OS and PFS in four studies and five studies, respectively. Pooled data of HRs showed that the concurrent use of low-dose aspirin was not significantly relevant to OS, whereas PFS was improved significantly in patients receiving ICIs concomitantly (low-dose aspirin users versus non-aspirin users: OS: HR = 0.93, 95% CI = 0.76–1.15, P = .514; PFS: HR = 0.84, 95% CI = 0.72–0.98, P = .024) (Figure 3a, b). Then, in subgroup analyses according to ICI agents, cancer type and analytical model, a significant association between the concomitant use of aspirin and OS was not observed. However, the concomitant use of aspirin and ICIs was found to be associated with better PFS in patients treated with PD-1/PD-L1 inhibitors, NSCLC patients and in a multivariate subgroup (PD-1/PD-L1: HR = 0.80, 95% CI = 0.65–0.99, P = .043; NSCLC patients: HR = 0.67, 95% CI = 0.48–0.94, P = .02; multivariate subgroup: HR = 0.82, 95% CI = 0.67–1.00, P = .048, respectively) (Table 2).
Figure 3.
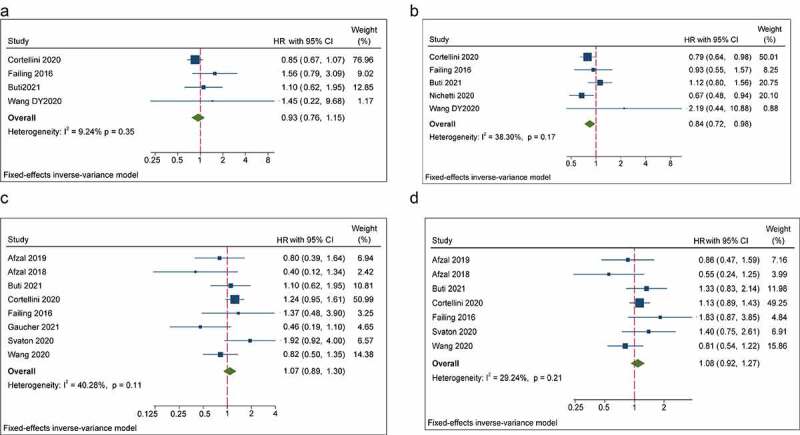
Forest plots of the hazard ratios and 95% CIs for overall survival (a) and progression-free survival (b) in patients with the concomitant use of aspirins compared patients without use of these drugs. Forest plots of the hazard ratios and 95% CIs for overall survival (c) and progression-free survival (d) in patients with the concomitant use of metformin compared patients without use of these drugs
Abbreviation: HR, hazard ratio; CI, confidence Interval.
A funnel plot of all eligible studies (Supplementary Figure S9, S10) and Egger’s test demonstrated no evidence of a publication bias (Egger’s test OS: P = .664; PFS: P = .239, respectively). Sensitivity analyses revealed that no individual study could substantially affect the pooled HRs of OS, whereas the pooled HRs of PFS were influenced significantly by removal of the studies by Cortellini et al. 202018 or Nichetti et al. 2020,22 thereby suggesting that pooled results were not robust or stable (Supplementary Figure S11, S12).
Metformin
Eight studies and seven studies reported the impact of metformin on OS and PFS, respectively. Overall, the concomitant use of metformin was not significantly associated with OS in patients receiving ICIs (metformin users versus non-metformin users: HR = 1.07, 95% CI = 0.89–1.30, P = .46). Likewise, there was no significant association between the concomitant use of metformin and PFS for patients undergoing ICI therapy (metformin users versus non-metformin users: HR = 1.08, 95% CI = 0.92–1.27, P = .51) (Figure 3c, d). In subgroup analyses stratified by ICI agents, cancer type and analytical model, the concomitant use of metformin showed no significant association with OS or PFS, respectively (Table 2).
A funnel plot of all eligible studies (Supplementary Figure S13, S14) and Egger’s test demonstrated no evidence of a publication bias (Egger’s test OS: P = .227; PFS: P = .765, respectively). Sensitivity analyses for OS and PFS showed that the combined HRs and their 95% CIs were not altered significantly if a study was excluded, which suggested that no single study had a significant impact on the pooled results (Supplementary Figure S15, S16).
Beta-blockers
Four studies reported the impact of beta-blockers on OS and PFS in patients treated with ICIs. Our meta-analysis demonstrated that the concomitant use of beta-blockers was not significantly related to OS and PFS in patients receiving ICIs (beta-blocker users versus non-beta-blocker users: OS: HR = 0.87, 95% CI = 0.71–1.08, P = .207; PFS: HR = 0.91, 95% CI = 0.66–1.22, P = .486) (Figure 4a, b). In subgroup analyses stratified by cancer type showed that the concomitant use of beta-blockers and ICIs had a trend toward improved PFS in NSCLC patients (P = .052), but a significant association in patients with melanoma or other cancer types was not observed. Furthermore, no statistical association between the concomitant use of beta-blockers and improved OS and PFS was found in subgroup analyses based on ICIs regimen and analysis models (Table 2).
Figure 4.
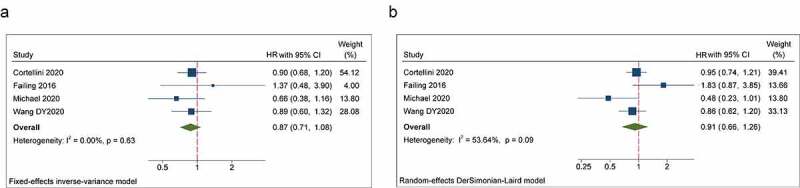
Forest plots of the hazard ratios and 95% CIs for overall survival (a) and progression-free survival (b) in patients with the concomitant use of beta-blockers compared patients without use of these drugs
Abbreviation: HR, hazard ratio; CI, confidence Interval.
A funnel plot of included studies demonstrated no obvious asymmetry for HRs of OS and PFS (Supplementary Figure S17, S18). Egger’s test further validated these results (OS: P = .851; PFS: P = .982, respectively). Sensitivity analyses for OS and PFS revealed that the pooled HRs and their 95% CIs were not changed significantly if a single study was omitted, thereby suggesting that these results were robust and stable (Supplementary Figure S19, S20).
Discussion
Recently, the anticancer effects of commonly used drugs, including statins, NSAIDs, low-dose aspirin, beta-blockers and metformin, have attracted considerable attention. Numerous studies have demonstrated that these medications can directly or indirectly inhibit the proliferation and genesis of tumors in vitro and in vivo and decrease the incidence and mortality rate of various cancers compared with patients who do not use such medications.6,8,9,11,13
This is the first meta-analyses to assess the impact of the concomitant use of statins, NSAIDs, low-dose aspirin, beta-blockers and metformin on the survival of patients with various types of cancer treated with ICIs. We revealed that the concomitant use of statins during ICI treatment was correlated with improved OS and PFS of patients. Moreover, the concurrent use of low-dose aspirin was associated with better PFS instead of OS in patients treated with ICIs. In addition, we found no significant association between the concurrent use of NSAIDs, beta-blockers and metformin and OS or PFS for patients receiving ICI treatment.
We demonstrated that the concurrent use of statins was correlated with improved OS and PFS in cancer patients receiving ICIs, particularly in the anti-PD-1/PD-L1 subgroup, NSCLC patients and multivariate subgroup. Sensitivity analysis showed robustness of pooled results of PFS was poor, primarily due to limited studies and heterogeneity of populations. Studies have been inconsistent with regard to the association between statin use and improved outcomes, so study of the concomitant use of statins in different cancer types and types of ICIs is needed to provide further evidence. The potential reason why subgroup analysis differed by analytical model (univariate vs multivariate) is partly due to limited studies in univariate analysis and the fact if variables have no statistical association in the univariate analysis, they will not be included in the multivariate analysis. Although no statistical association in univariate analysis, statin users have better median PFS and OS than non-statin users.17,24,27 Statins, inhibitors of 3‑hydroxy‑3‑methylglutaryl coenzyme A reductase, are commonly used cholesterol-lowering medications with an excellent safety profile.28 Cholesterol metabolism has an important role in tumor growth and regulation of the immunological landscape.29 Preclinical studies have shown that statin arrest cells in G1 or S phases by affecting cell‑cycle regulatory proteins, which results in the apoptosis of cancer cells and inhibition of intracellular signaling pathways in cancer cells. Furthermore, studies have reported that statins (particularly lipophilic statins) downregulate expression of PD-1 and CTLA-4 in T cells,30 reduce T-cell exhaustion in patients infected with human immunodeficiency virus-1,31 increase antigen occupation in dendritic cells and synergize with PD-1 inhibitors in murine models.32 Those features explain the biological rationale of our meta-analysis. Notably, intensity of statins, the duration of concurrent therapy using statins and immunotherapy, and lipophilicity of statins probably affect immune response. Cantini et al.17 reported that statin intensity might be essential in the response to ICIs, particularly high-intensity statins boosting the activity of PD-1 inhibitors. If statins work as immune adjuvants, temporary accumulation of statins in combination with antigens is probably not sufficient to induce an adaptive immune response. Long-term duration of concurrent therapy using statins and immunotherapy is probably indispensable to enhance immune responses.33 The lipophilicity of statins has been shown to have an effect on response in one preclinical study,32 whereas only one study in our meta-analysis analyzed this association.
Overall, our study confirmed that the concurrent use of NSAIDs was not associated significantly with improved OS or PFS in cancer patients receiving ICIs. However, the concomitant use of low-dose aspirin was related to significantly improved PFS rather than OS, especially in patients receiving PD-1/PD-L1 blockade, in patients with NSCLC, and HR from multivariate subgroup. Preclinical studies and clinical studies have demonstrated that cyclo-oxygenase-2 (COX2), whose overexpression has been observed to be associated with poor prognoses in multiple cancer types, promotes immune evasion and, by increasing the production of prostaglandin E2 (PGE2), it weakens the ability and reduces the number of immune cells in the tumor microenvironment.34 Moreover, studies have reported that NSAIDs and aspirin can enhance the efficacy of PD-1/PD-L1 blockade by inhibiting COX-2 activity in animal models.35–37 The potential explanation of different results between aspirin and NSAIDs is that aspirin non-selectively and irreversibly blocks expression of COX-1 and COX-2, whereas other NSAIDs reversibly inhibit COX enzymes or selectively block COX-2. Zelenay et al. reported that melanoma mice administrated with celecoxib, a COX-2-specific inhibitor, significantly regressed combined with anti-PD-1 treatment, but the combined efficacy is inferior to combined aspirin and PD-1 inhibitors, possibly owing to suboptimal COX-2 inhibition and/or an underlying contribution of COX-1-derived PGE2.35 The different results for the concomitant use of aspirin between OS and PFS may be explained (at least in part) by the fact that OS, unlike PFS, may not reflect the positive effect of combined treatment because of the influence of post-treatment and poor systemic conditions in NSAIDs users and low-dose aspirin users. For low-dose aspirin, the different results based on analytical model are partly due to similar reasons to statins. However, in contrast to statins, improved median PFS was not consistent for low-dose aspirin users in two studies, which might be explained by patient heterogeneity.16,22
We found no significant association between the concomitant use of metformin and OS or PFS in patients undergoing ICI treatment. Metformin is the most widely used agent for type-2 diabetes mellitus. It has received considerable publicity over its potential anticancer function. Metformin appears to arrest the cell cycle and cell proliferation by regulating adenosine monophosphate-activated protein kinase (AMPK)/liver kinase B1, thereby suppressing cancer cells and inducing apoptosis.38 Metformin also: (i) activates the immune response by targeting cancer cells; (ii) inhibiting expression of CD39/73 on MDSCs to prevent the development of immune tolerance of cancer cells by targeting CD8+ tumor-infiltrating leukocytes; (iii) enhancing the antitumor activity of PD-1 blockers by regulating the oxygen tension in the tumor microenvironment in murine models.13,39 However, all eligible studies in a clinical setting reported no significant association between the use of metformin and improved outcomes. Notably, Afzal et al.14,15 reported that the objective response rate (melanoma: 68.2% vs. 54.5%, P = .31; NSCLC: 41.1% vs. 30.7%, P = .4), disease control rate (77.3% vs. 60.6%, P = .19; NSCLC: 70.5 vs. 61.6%, P = .5), median PFS (46.7 vs. 28 months, P = .15; 4.0 vs. 3.0 months, P = .6) and median OS (melanoma: 46.7 vs. 28 months, P = .12; NSCLC: 11.5 vs. 7.6 months P = .5) was higher in melanoma patients and NSCLC patients receiving concurrent use of metformin and ICI treatment, but did not reach significance.14,15 A phase-II trial (NCT03800602) of nivolumab and metformin in patients with treatment-refractory microsatellite-stable (MSS) metastatic colorectal cancer (mCRC) was reported in the 2021 Gastrointestinal Cancers Symposium.40 Briefly, stage-IV metastatic treatment-refractory MSS mCRC patients were administered nivolumab (480 mg, i.v.) every 4 weeks and metformin (1000 mg, p.o., b.d.) in 28-d cycles following a 14-d metformin-only lead-in phase. Eighteen patients could tolerate this regimen and two patients achieved stable disease, but an objective response rate was not seen, so the study did not proceed. Hence, recent studies have, like our meta-analysis, shown that metformin may be unable to potentiate PD-1/PD-L1 inhibitors in the clinical setting.
Our meta-analysis revealed no significant association between the concomitant use of beta-blockers and OS or PFS in patients given ICIs. Increasing evidence suggests that beta-adrenergic signaling has been shown to influence the genesis and progression of multiple tumors and to intertwine with immune cells (e.g., CD8 + T cells, regulatory T cells, myeloid-derived suppressor cells).41,42 Nevertheless, all eligible studies reported no significant association between the concomitant use of beta-blockers and OS and PFS in the multivariate analysis. Cortellini et al.18 reported that beta-blockers are significantly associated with a favorable objective response rate in the multivariate analysis. Michael et al.23 showed that NSCLC patients were associated with improved PFS in the univariate analysis, but the effect was offset by other factors in the multivariate analysis, thereby indicating that beta-blockers may have no impact on clinical outcomes in patients receiving ICIs.
Most of the studies included in our meta-analysis failed to report the doses and duration of medications used concomitantly. Preclinical studies demonstrated that the anticancer effect of these drugs was dependent on the dose and time.19 Interestingly, Afzal et al.14,15 reported that the overall duration of metformin therapy and metformin dose had no impact on OS and PFS in the multivariate analysis, whereas the duration of concurrent therapy using metformin and ICIs had a significant impact on OS and PFS in NSCLC patients in univariate and multivariate analyses. Those results are partly contrary to data from preclinical studies and this phenomenon warrants further investigation. It seems that, to be more efficacious, medications should be employed long term and at high doses, but there is no consensus concerning optimal doses and treatment duration in patients without indications for these drugs. Although exact time frame of these baseline medications, which are generally indefinite prescriptions, might be unretrievable in prospective clinical trials, collection of the duration and dose of concurrent medications and ICIs might be feasible, which have been reported by Afzal et al.14 Basic-science studies and phase-I/II trials are also needed to explore these issues.
Of note, these medications are often taken by patients with high body mass index (BMI) or metabolic syndrome, who have been confirmed to be better outcomes when treated with ICIs instead of chemotherapy.43 Cortellini et al18reported that the significant association between statins, low-dose aspirin, β-blockers and higher baseline BMI in a large cohort. Interestingly, multivariate analysis revealed that BMI was not significantly associated with improved ORR, PFS and OS, while statins, low-dose aspirin and β-blockers were independently related to an increased ORR and low-dose aspirin were significantly associated with PFS. These results might be due to the distinct study population compared to previous studies. An alternative explanation might be that BMI was a confounder, and the positive roles of these baseline medications were revealed when BMI was adjusted in multivariate analysis. It is these baseline medications taken by obese patients that exert immune-modulatory effect, which enhanced efficacy of ICIs and improved clinical outcomes in high BMI patients. Since most studies43,46,47,48,49 44,45that reported better clinical outcomes in high BMI patients fail to consider the roles of these concurrent medications, those complex interaction between high BMI and the immune-modulating effect of these drugs on survival of patients receiving ICIs deserves to be further elucidated.
Our meta-analysis had fifth main limitations. First, all of the eligible studies were retrospective, whose results have an inevitable bias in terms of selection and reporting. Second, although we have performed subgroup based on types of cancers and treatment modality (including ICI alone or in combination), different lines of treatment might have affected the stability and reliability of our results. Third, the small number of studies and small sample size did not permit comprehensive subgroup analyses according to confounders, which may have influenced our results. Fourth, poor systemic status may have been present in patients using these medications concomitantly. Fifth, patients in eligible studies often assume several concomitant medications to treat comorbidities or cancer-related symptoms, and the effect of concomitant use of these drugs on patients receiving ICIs has not been evaluated, which might affect our results. Drug-based prognostic score, which includes the comorbidities and the possible concomitant baseline medications for patients, is likely to become promising tools in clinical practice.16
Conclusions
The concomitant use of statins during ICI treatment was correlated with improved OS and PFS. The concurrent use of low-dose aspirin was associated with better PFS in patients treated with ICIs. The ICI regimen, cancer type and analytical model may have affected these outcomes. We found no significant association between the concurrent use of NSAIDs, beta-blockers and metformin and clinical outcomes. These findings need to be confirmed with larger and perspective studies.
Supplementary Material
Acknowledgments
This work was supported by the Capital’s Funds for Health Improvement and Research [grant number 2020-2475 2175] and Beijing Talents Project.
Funding Statement
This study is supported by Capital’s Funds for Health Improvement and Research (grant number 2020-2475 2175) and Beijing Talents Project.
Availability of data and material
The data used in the present study are available from the corresponding author on reasonable request.
Authors’ contributions
Conception/design: Yongchao Zhang and Wei Li. Collection and/or assembly of data: Hualei Chen, Shanshan Chen and Zhen li. Wei Li Data analysis and interpretation: Yongchao Zhang, Jinglong Chen and Wei Li. Manuscript writing: Yongchao Zhang and Wei Li. Final approval of manuscript: Yongchao Zhang, Hualei Chen, Shanshan Chen, Zhen li, Jinglong Chen, Wei Li
Ethics approval and consent to participate
Not applicable.
Abbreviations
ICIs, Immune checkpoint inhibitors; CTLA-4, cytotoxic T-lymphocyte–associated-4; PD-1/PD-L1, programmed cell death protein-1/programmed cell death protein-1 ligand; NSCLC, non-small cell lung cancer; NSAIDS, non-steroidal anti- inflammatory drugs; MPM, malignant pleural mesothelioma; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; COX2: cyclo-oxygenase-2; PGE2, prostaglandin E2; AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian target of rapamycin; ASCO, Society of Clinical Oncology; ESMO, European Society for Medical Oncology; MSS, microsatellite-stable; mCRC, metastatic colorectal cancer
Disclosure statement
Authors have no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
References
- 1.Bagchi S, Yuan R, Engleman EG.. Immune Checkpoint Inhibitors for the Treatment of Cancer: clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16(1):223–11. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SA, Sznol M. Resistance mechanisms to checkpoint inhibitors. Curr Opin Immunol. 2021;69:47–55. doi: 10.1016/j.coi.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Lurienne L, Cervesi J, Duhalde L, de Gunzburg J, Andremont A, Zalcman G, Buffet R, Bandinelli PA. NSCLC Immunotherapy Efficacy and Antibiotic Use: a Systematic Review and Meta-Analysis. J Thorac Oncol. 2020;15(7):1147–1159. doi: 10.1016/j.jtho.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Tsikala-Vafea M, Belani N, Vieira K, Khan H, Farmakiotis D. Antibiotic use is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int J Infect Dis. 2021;106:142–154. doi: 10.1016/j.ijid.2021.03.063. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Zeng C, Yao J, Ge Y, An G. The association between proton pump inhibitors use and clinical outcome of patients receiving immune checkpoint inhibitors therapy. Int Immunopharmacol. 2020;88:106972. doi: 10.1016/j.intimp.2020.106972. [DOI] [PubMed] [Google Scholar]
- 6.Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother. 2021;17(1):55–61. doi: 10.1080/21645515.2020.1769398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortellini A, Di Maio M, Nigro O, Leonetti A, Cortinovis DL, Aerts JG, Guaitoli G, Barbieri F, Giusti R, Ferrara MG, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J Immunother Cancer. 2021;9(4):e002421. doi: 10.1136/jitc-2021-002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41(6):554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Zappavigna S, Cossu AM, Grimaldi A, Bocchetti M, Ferraro GA, Nicoletti GF, Filosa R, Caraglia M. Anti-Inflammatory Drugs as Anticancer Agents. Int J Mol Sci. 2020;21(7):2605. doi: 10.3390/ijms21072605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi G, Pezzuto A, Sini C, Tuzi A, Citarella F, McCusker MG, Nigro O, Tanda E, Russo A. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol. 2019;142:26–34. doi: 10.1016/j.critrevonc.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhong S, Yu D, Zhang X, Chen X, Yang S, Tang J, Zhao J, Wang S. beta-Blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cancer Prev. 2016;25(5):440–448. doi: 10.1097/CEJ.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 12.Weberpals J, Jansen L, Carr PR, Hoffmeister M, Brenner H. Beta blockers and cancer prognosis - The role of immortal time bias: a systematic review and meta-analysis. Cancer Treat Rev. 2016;47:1–11. doi: 10.1016/j.ctrv.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Wang Y, Luo J, Liu M, Luo Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front Immunol. 2020;11:586760. doi: 10.3389/fimmu.2020.586760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzal MZ, Dragnev K, Sarwar T, Shirai K. Clinical outcomes in non-small-cell lung cancer patients receiving concurrent metformin and immune checkpoint inhibitors. Lung Cancer Manag. 2019;8(2):LMT11. doi: 10.2217/lmt-2018-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. Journal for ImmunoTherapy of Cancer. 2018;6(1). doi: 10.1186/s40425-018-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Cantini L, Pecci F, Hurkmans DP, Belderbos RA, Lanese A, Copparoni C, Aerts S, Cornelissen R, Dumoulin DW, Fiordoliva I, et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur J Cancer. 2021;144:41–48. (Oxford, England: 1990). doi: 10.1016/j.ejca.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 18.Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, Rastelli F, Bisonni R, Santini D, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. Journal for ImmunoTherapy of Cancer. 2020;8(2):e001361. doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Failing JJ, Finnes HD, Kottschade LA, Allred JB, Markovic SN; Failing JJ, Finnes HD, Kottschade LA, Allred JB, Markovic SN . Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. Melanoma Res. 2016;26(6):609–615. doi: 10.1097/CMR.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 20.Gaucher L, Adda L, Séjourné A, Joachim C, Guillaume C, Poulet C, Liabeuf S, Gras-Champel V, Masmoudi K, Houessinon A, et al. Associations between dysbiosis-inducing drugs, overall survival and tumor response in patients treated with immune checkpoint inhibitors. Ther Adv Med Oncol. 2021;13:175883592110005. doi: 10.1177/17588359211000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai O, Ito T, Saito Z, Yamamoto Y, Fujita K, Okamura M, Hashimoto M, Nakatani K, Sawai S, Mio T, et al. Effect of cyclooxygenase inhibitor use on immunotherapy efficacy in non-small cell lung cancer. Thoracic Cancer. 2021;12(6):949–957. doi: 10.1111/1759-7714.13845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichetti F, Ligorio F, Zattarin E, Signorelli D, Prelaj A, Proto C, Galli G, Marra A, Apollonio G, Porcu L, de Braud F, Lo Russo G, Ferrara R, Garassino MC. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers (Basel). 2019. Dec 25;12(1):67. doi: 10.3390/cancers12010067. PMID: 31881699; PMCID: PMC7016680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh MS, Guzner A, Wainwright DA, Mohindra NA, Chae YK, Behdad A, Villaflor VM. The Impact of Beta Blockers on Survival Outcomes in Patients With Non–small-cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin Lung Cancer. 2021;22(1):e57–e62. doi: 10.1016/j.cllc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svaton M, Zemanova M, Zemanova P, KULTAN J, Fischer O, SKRICKOVA J, JAKUBIKOVA L, CERNOVSKA M, HRNCIARIK M, JIROUSEK M, et al. Impact of concomitant medication administered at the time of initiation of nivolumab therapy on outcome in non-small cell lung cancer. Anticancer Res. 2020;40(4):2209–2217. doi: 10.21873/anticanres.14182. [DOI] [PubMed] [Google Scholar]
- 25.Wang DY, McQuade JL, Rai RR, Park JJ, Zhao S, Ye F, Beckermann KE, Rubinstein SM, Johnpulle R, Long GV, Carlino MS, Menzies AM, Davies MA, Johnson DB. The Impact of Nonsteroidal Anti-Inflammatory Drugs, Beta Blockers, and Metformin on the Efficacy of Anti-PD-1 Therapy in Advanced Melanoma. Oncologist. 2020;25(3):e602–e605. doi: 10.1634/theoncologist.2019-0518. Epub 2019 Nov 29. PMID: 32162820; PMCID: PMC7066699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S-J, Khullar K, Kim S, Yegya-Raman N, Malhotra J, Groisberg R, Crayton SH, Silk AW, Nosher JL, Gentile MA, et al. Effect of cyclo-oxygenase inhibitor use during checkpoint blockade immunotherapy in patients with metastatic melanoma and non-small cell lung cancer. Journal for Immunotherapy of Cancer. 2020;8(2):e000889. doi: 10.1136/jitc-2020-000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi A, Filetti M, Taurelli Salimbeni B, Piras M, Rizzo F, Giusti R, Marchetti P. Statins and immunotherapy: togetherness makes strength The potential effect of statins on immunotherapy for NSCLC. Cancer Rep (Hoboken). 2021:e1368. doi: 10.1002/cnr2.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8):52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2(2):132–141. doi: 10.1038/s42255-020-0174-0. [DOI] [PubMed] [Google Scholar]
- 30.Okoye I, Namdar A, Xu L, Crux N, Elahi S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget. 2017;8(58):98215–98232. doi: 10.18632/oncotarget.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elahi S, Weiss RH, Merani S. Atorvastatin restricts HIV replication in CD4+ T cells by upregulation of p21. AIDS. 2016;30(2):171–183. doi: 10.1097/QAD.0000000000000917. [DOI] [PubMed] [Google Scholar]
- 32.Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X, Yang Y, Li X, Zhao M, Li L, et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell. 2018;175(4):1059–73.e21. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 33.Wu TY. Strategies for designing synthetic immune agonists. Immunology. 2016;148(4):315–325. doi: 10.1111/imm.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 2019;234(5):5683–5699. doi: 10.1002/jcp.27411. [DOI] [PubMed] [Google Scholar]
- 35.Zelenay S, van der Veen AG, Bottcher JP, Snelgrove K, Rogers N, Acton S, Chakravarty P, Girotti M, Marais R, Quezada S, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162(6):1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botti G, Fratangelo F, Cerrone M, Liguori G, Cantile M, Anniciello AM, Scala S, D’Alterio C, Trimarco C, Ianaro A, et al. COX-2 expression positively correlates with PD-L1 expression in human melanoma cells. J Transl Med. 2017;15(1):46. doi: 10.1186/s12967-017-1150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar D, Rahman H, Tyagi E, Liu T, Li C, Lu R, Lum D, Holmen SL, Maschek JA, Cox JE, et al. Aspirin Suppresses PGE 2 and Activates AMP Kinase to Inhibit Melanoma Cell Motility, Pigmentation, and Selective Tumor Growth In Vivo. Cancer Prev Res (Phila). 2018;11(10):629–642. doi: 10.1158/1940-6207.CAPR-18-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–419. doi: 10.1016/j.diabres.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunology Research. 2017;5(1):9–16. doi: 10.1158/2326-6066.Cir-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akce M. 2021. Phase II trial of nivolumab and metformin in patients with treatment refractory microsatellite stable metastatic colorectal cancer. Mehmet Akce MRJMSWLSCWOBAMDGBLBFE-R, Department of H, Medical Oncology WCIEUAGA, Winship Cancer Institute EUAGA, Emory University DoB, Bioinformatics AGA, Department of H, Medical Oncology WCIoEUAGA , editor. American Society of Clinical Oncology, Gastrointestinal Cancers Symposium. https://meetinglibrary.asco.org/record/194148/abstract [Google Scholar]
- 41.Jensen AWP, Carnaz Simoes AM, Thor Straten P, Holmen Olofsson G. Adrenergic Signaling in Immunotherapy of Cancer: friend or Foe? Cancers (Basel). 2021:13. doi: 10.3390/cancers13030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, Schell TD. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7(3):e1405205. doi: 10.1080/2162402x.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortellini A, Ricciuti B, Tiseo M, Bria E, Banna GL, Aerts JG, Barbieri F, Giusti R, Cortinovis DL, Migliorino MR, et al. Baseline BMI and BMI variation during first line pembrolizumab in NSCLC patients with a PD-L1 expression >/= 50%: a multicenter study with external validation. J Immunother Cancer. 2020;8(2):e001403. doi: 10.1136/jitc-2020-001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. Journal for Immunotherapy of Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Giorgi U, Procopio G, Giannarelli D, Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli P, et al. Association of Systemic Inflammation Index and Body Mass Index with Survival in Patients with Renal Cell Cancer Treated with Nivolumab. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2019;25(13):3839–3846. doi: 10.1158/1078-0432.CCR-18-3661. [DOI] [PubMed] [Google Scholar]
- 47.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncology. 2020;6(4):512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the present study are available from the corresponding author on reasonable request.


