Abstract
The Indian subcontinent has an origin geologically different from Eurasia, but many terrestrial animal and plant species on it have congeneric or sister species in other parts of Asia, especially in the Southeast. This faunal and floral similarity between India and Southeast Asia is explained by either of the two biogeographic scenarios, ‘into-India’ or ‘out-of-India’. Phylogenies based on complete mitochondrial genomes and five nuclear genes were undertaken for ricefishes (Adrianichthyidae) to examine which of these two biogeographic scenarios fits better. We found that Oryzias setnai, the only adrianichthyid distributed in and endemic to the Western Ghats, a mountain range running parallel to the western coast of the Indian subcontinent, is sister to all other adrianichthyids from eastern India and Southeast–East Asia. Divergence time estimates and ancestral area reconstructions reveal that this western Indian species diverged in the late Mesozoic during the northward drift of the Indian subcontinent. These findings indicate that adrianichthyids dispersed eastward ‘out-of-India’ after the collision of the Indian subcontinent with Eurasia, and subsequently diversified in Southeast–East Asia. A review of geographic distributions of ‘out-of-India’ taxa reveals that they may have largely fuelled or modified the biodiversity of Eurasia.
Keywords: biodiversity, Eurasia, Indian subcontinent, Oryzias setnai, Western Ghats
1. Introduction
The Indian subcontinent geologically originated from Gondwana, a Neoproterozoic supercontinent composed of the present-day Africa, South America, Australia, Antarctica, Arabian Peninsula, Madagascar, and the Indian subcontinent [1]. During the breakup of Gondwana, the Indian subcontinent became isolated from Africa around 130–160 Ma, drifted northwards, and eventually collided with Eurasia, which originated from Laurasia, around 35–55 Ma (figure 1a) [2–7]. Though the origin of the Indian subcontinent differs geologically from that of Eurasia, many terrestrial animal and plant species on it have congeneric or sister species in other parts of Asia, especially in the Southeast [8–15].
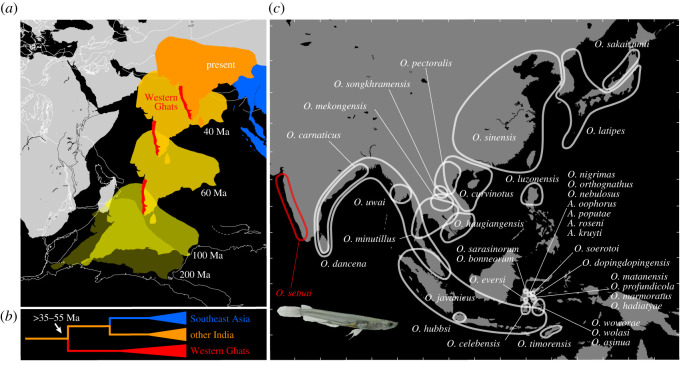
Figure 1.
(a) Time sequence of drifting continental blocks at each age from 200 Ma to present (map provided by and modified from [2] with permission). (b) Schematic phylogeny under the scenario ‘out-of-India’. (c) Map depicting the geographic distributions of all species in the family Adrianichthyidae.
The faunal and floral similarity between India and Southeast Asia has been attributed to dispersals from Eurasia to the Indian subcontinent [16,17]. One hypothesis on which this ‘into-India’ biogeographic scenario is based is the ‘Satpura hypothesis’ by S. L. Hora, an Indian ichthyologist [16,18], which considers that the westward dispersals of Southeast Asian fauna occurred through the central Indian Satpura hill ranges in the Pleistocene. In contrast, it is also theoretically possible that the Indian taxa originated on the Indian subcontinent, and that the dispersals occurred ‘out-of-India’, from India to Southeast Asia, after the collision of the Indian plate with Eurasia [19,20]. It is essential to evaluate the ‘into-India’ versus ‘out-of-India’ scenarios to understand how the biodiversity in these regions of different geological origins was formed [21–25].
Fauna and flora in the Western Ghats, a mountain range running parallel to the western coast of the Indian subcontinent, hold the key for the test of these two biogeographic scenarios. The Western Ghats harbour unique, evolutionarily distinct lineages of many taxa [26–30]. The unique fauna and flora in the Western Ghats suggest that they have long been isolated from other regions of the Indian subcontinent. If this isolation predates the collision of the Indian subcontinent, and if the Western Ghats clade is sister to all other clades, then the presence of their common ancestor in the Indian subcontinent is supported, consistent with the ‘out-of-India’ scenario (figure 1b). In contrast, if the Western Ghats taxa are nested within a larger phylogeny consisting of species from outside the region, and if the isolation of the Western Ghats postdates the collision of the Indian subcontinent, then the ‘into-India’ scenario is instead supported.
We examine which of these two biogeographic scenarios better fits a group of small-sized ricefishes (family Adrianichthyidae) comprising 37 species (figure 1c) distributed throughout Southeast and East Asia, and the Indian subcontinent [31], with one species, the Malabar ricefish, Oryzias setnai, (formerly Horaichthys setnai, named after S. L. Hora), endemic to the Western Ghats lowlands [32,33]. Though the endemism of O. setnai suggests long-term isolation, no study has investigated its phylogenetic position or evolutionary history. Using sequences of the complete mitochondrial genomes and five nuclear genes, we reconstruct a comprehensive phylogeny of the family Adrianichthyidae, including this Western Ghats endemic species, and estimate divergence times and ancestral areas of major adrianichthyid lineages. We demonstrate that this family originated in India and subsequently dispersed east as far as Wallacea, the biogeographical transition zone between Indomalaya and Australasia, where it there became one of the most important elements of the region's current freshwater ichthyofauna.
2. Materials and methods
(a) . Field collections
Twenty-two adrianichthyid species were collected from throughout the geographic range of this family (electronic supplementary material, table S1). Full details of field collections are provided in the electronic supplementary material.
(b) . Mitochondrial and nuclear sequencing of O. setnai
Total DNA was extracted from one O. setnai individual from an aquarium strain. The entire length of the mitogenome was de novo assembled using long PCR (electronic supplementary material, table S2) [34,35] and shotgun-sequencing (electronic supplementary material, figure S1). We also Sanger-sequenced five nuclear genes (electronic supplementary material, table S3). Full details of sequencing are provided in the electronic supplementary material.
(c) . Whole-genome sequencing of other adrianichthyids
Whole-genome sequencing was performed for wild or laboratory individuals of 10 adrianichthyid species (electronic supplementary material, table S1). Reads were mapped to a reference genome assembly of O. latipes (ASM223467v1) or O. javanicus (OJAV_1.1), and bases were called across each reference mitochondrial genome and across the five nuclear genes of each reference. Each mitogenome sequence was annotated using MitoAnnotator [36,37]. Full details of sequencing are provided in the electronic supplementary material.
(d) . Phylogenetic analysis
Sequences of the mitogenomes and the five nuclear genes were obtained for an additional 20 adrianichthyid taxa, using short read sequences of the whole genome retrieved from DDBJ-DRA (electronic supplementary material, table S1); reads were mapped to a reference genome assembly of O. celebensis (OryCel_1.0) or O. latipes, and bases were called. For two species (O. javanicus and O. dancena), mitogenome sequences retrieved from DDBJ were used (electronic supplementary material, table S1). Sequences of mitogenomes and nuclear genes of five beloniform, seven cyprinodontiform, five atheriniform and two cichlid (perciform) species were also retrieved from DDBJ (electronic supplementary material, table S1).
Alignments were performed separately for each gene. For mitochondrial genes, we excluded ambiguously aligned regions in the rRNA and tRNA genes, the third codon positions from the protein-coding genes, and the whole NADH dehydrogenase subunit 6, resulting in sequences of a total 11 233 bp. Alignments of nuclear genes resulted in sequences of a total 4204 bp. A maximum-likelihood phylogeny was estimated separately for the mitochondrial, nuclear and concatenated sequences using raxmlGUI v. 1.31 [38]. Full details of phylogenetic analyses are provided in the electronic supplementary material.
(e) . Divergence time estimation
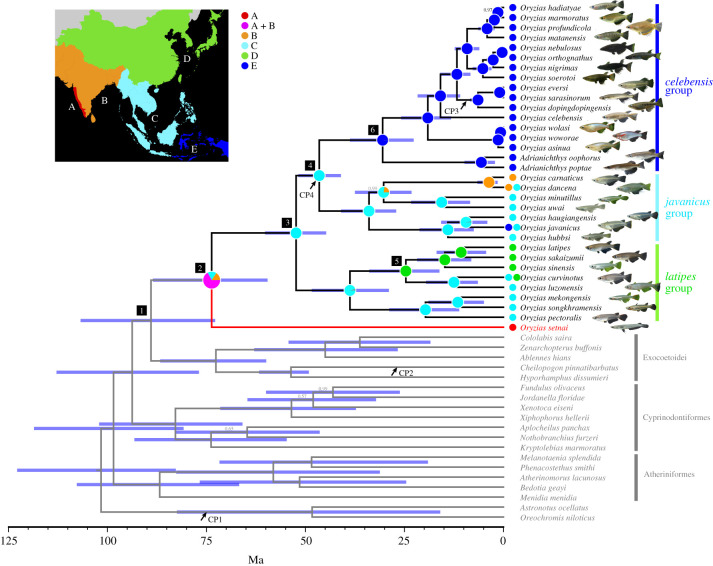
Lognormal relaxed clock analyses were performed separately on the 11 233 bp mitochondrial and 4204 bp nuclear sequences using BEAST v. 2.5.2 [39]. We employed three fossil records: (i) †Mahengechromis (the minimum age 45.46 Ma) [40] for the node between the two cichlids [41], (ii) †Rhamphexocoetus volans (the minimum age 49.11 Ma) [42] for the branch leading to the flyingfish [41], and (iii) †Lithopoecilus brouweri (the minimum age 5.33 Ma) [43] for the node between Oryzias sarasinorum and Oryzias eversi [44], and the opening of the Makassar Strait, ca 45 Ma [45–47], to time-calibrate the phylogenetic tree (figure 2). Appropriate substitution models were selected for each gene (for rRNA and tRNA genes) and codon (for coding genes). Full details on the divergence time estimation are provided in the electronic supplementary material.
Figure 2.
Bayesian chronogram of adrianichthyid taxa based on mitochondrial and nuclear sequences. Bars represent 95% high posterior density (HPD). Ancestral areas reconstructed using a DEC + J model are shown as pies at each node. Numbers on branches are Bayesian posterior probabilities (BPPs); branches with no number represent BPP = 1. Arrows indicate calibration points; CP1: †Mahengechromis for the node between the two cichlids, CP2: †Rhamphexocoetus volans for the branch leading to the flyingfish, CP3: †Lithopoecilus brouweri for the node between Oryzias sarasinorum and Oryzias eversi; and CP4: the opening of the Makassar Strait (see text for details).
(f) . Ancestral area reconstruction
The geographic range of Adrianichthyidae was divided into five geological areas: (A) Western Ghats, (B) Indian subcontinent (excluding the Western Ghats), (C) Southeast Asia (excluding Wallacea and New Guinea), (D) East Asia, and (E) Wallacea and New Guinea. Using the tree obtained from the BEAST analysis above, ancestral areas at each node of the tree were reconstructed under different biogeographical models with RASP v. 4.2 [48]. Likelihood under each model was estimated, and the fit of each model to the data was compared by consulting Akaike information criteria corrected for a small sample size. Full details on the ancestral area reconstruction are provided in the electronic supplementary material.
3. Results
(a) . Phylogeny of Adrianichthyidae
All phylogenies revealed O. setnai to be a sister to all other members of the family Adrianichthyidae (figure 2 and electronic supplementary material, figure S2). The branch of O. setnai in these phylogenies was disproportionally longer compared with other adrianichthyids. The latter were composed of three main clades—the ‘latipes’, ‘javanicus’, and ‘celebensis’ species groups. The latipes species group comprises species distributed mainly in the inland areas of the Indochinese Peninsula, Philippines and East Asia; the javanicus species group occurs in the eastern part of India and throughout Southeast Asia; and the celebensis species group is endemic to Sulawesi Island (figure 2). Among the three species groups, the latipes species group is sister to the other two.
(b) . Divergence time estimates
Adrianichthyidae was estimated to have separated from other members of the order Beloniformes around 89 Ma (73–107 Ma in 95% HPD; figure 2, node 1). The divergence time of O. setnai was estimated at around 74 (60–88) Ma (node 2) in the late Mesozoic (see also electronic supplementary material, figure S3). Thereafter, the latipes species group split off around 52 (45–60) Ma (node 3), and the subsequent split between the javanicus and celebensis species groups occurred around 47 (41–52) Ma (node 4).
(c) . Reconstruction of ancestral areas
The best biogeographical model (Dispersal-Extinction Cladogenesis with founder event speciation model: DEC + J; electronic supplementary material, table S4) estimated that the most probable distribution area for the common ancestor of adrianichthyids was on the Indian subcontinent (i.e. the Western Ghats and other parts of India) (figure 2, node 2). The common ancestor of the latipes, javanicus and celebensis species groups was estimated to be distributed in Southeast Asia (node 3). Thereafter, dispersals from Southeast Asia to East Asia and to Wallacea occurred within the latipes species group (node 5), and in the most recent common ancestor of the celebensis group (node 6), respectively.
4. Discussion
(a) . Origin and dispersal history of the Adrianichthyidae
We found that O. setnai, endemic to the Western Ghats western plains, is sister to all other Adrianichthyidae taxa, which comprise three major groups (the latipes, javanicus and celebensis species groups) [49,50]. Our results also reveal that the divergence time of this species (60–88 Ma) predates the collision of the Indian subcontinent with Eurasia (35–55 Ma [2–7]), and that the common ancestor of the Adrianichthyidae was estimated to have been distributed on this ancient subcontinent. This lends credibility to the hypothesis that the split of O. setnai occurred on the Indian subcontinental ‘raft’, supporting the ‘out-of-India’ hypothesis.
The divergence of O. setnai and its endemism to western India may be related to the formation of the Western Ghats mountain ranges. The western coast of India could have appeared as an abrupt cliff some 1000 m in elevation ca 65–90 Ma after the Indian subcontinent broke away from Madagascar [51], becoming the present-day Western Ghats. This long (1600 km) and high mountain range running parallel to the southwestern coast of the Indian subcontinent may have acted as a physical barrier preventing the migration of species between the western and eastern coasts. We think that the common ancestor of Adrianichthyidae was divided into the west and east by this mountain range, and that the western population would have evolved in isolation as O. setnai. The common ancestor of the Eurasian clade probably diverged from the eastern population.
The branch leading to O. setnai is, notably, disproportionally long, indicating an acceleration in evolutionary rate (electronic supplementary material, figure S2). This acceleration might be related to the geological history of the Western Ghats. It is well known that large-scale, long-term volcanic eruptions occurred along the western coast of the Indian subcontinent during its northward drift, forming the Deccan Traps [52]. It is, therefore, no wonder that O. setnai has repeatedly experienced strong bottlenecks caused by recurring eruptions. According to the nearly neutral theory of molecular evolution [53], evolutionary rates of protein-coding genes increase with decreasing population size, which may explain this long branch. A detailed demographic history of O. setnai using the nuclear genome is required to test this hypothesis.
The split between the Adrianichthyidae and other members of the Beloniformes, that is, Exocoetoidei, estimated at 73–107 Ma (figure 2, node 1), perhaps occurred in conjunction with the separation of the Indian subcontinent from Africa and Madagascar. Since most extant species in the Exocoetoidei: Belonidae (needlefishes), Execoetidae (flyingfishes), Hemiramphidae (halfbeaks) and Zenarchopteridae (viviparous halfbeaks) are marine [54], the expansion of coastal areas following the breakup of the Indian subcontinent may have increased opportunities for a common ancestor of Exocoetoidei to pioneer new habitat. In contrast, Adrianichthyidae pioneered the inland areas of the Indian subcontinent, and subsequently those in Eurasia, probably spreading via coastal areas.
(b) . Conclusion: Eurasian biodiversity fuelled by ‘out-of-India’ dispersals
Contrary to Hora's hypothesis [16,18], Hora's fish and its relatives originated on the Indian subcontinent and subsequently dispersed east into Southeast and East Asia, where they have greatly diversified. Our review of geographic distributions (electronic supplementary material, table S5) reveals that other ‘out-of-India’ taxa have also diversified more or less in Southeast and East Asia (table 1). This probably reflects that ‘out-of-India’ taxa were newcomers to Eurasia, where they may have found empty niches and/or competitively excluded native Laurasian taxa. The biodiversity of Eurasia may have been largely fuelled or modified by these taxa which came on the subcontinental raft.
Table 1.
Geographic distributions of taxa demonstrated to be ‘out-of-India’ by molecular studies (i.e. ‘out-of-India’ taxa demonstrated solely by taxonomic and/or palaeontological studies are not included). Numbers represent the numbers of species in each geographic area. Bold numbers represent modes. See electronic supplementary material, table S5 for the list of species and genera used, and their geographic ranges.
| taxon [reference] | geographic area |
||||
|---|---|---|---|---|---|
| Western Ghats (A) | Indian subcontinent (B) | Southeastern Asia (C) | Eastern Asia (D) | Wallacea and New Guinea (E) | |
| plants | |||||
| Allioideaea [55] | 0 | 0 | 0 | 3 | 0 |
| Paliurus [56] | 0 | 0 | 0 | 4 | 0 |
| Crypteroniaceae [20] | 0 | 2 | 11 | 1 | 2 |
| Dipterocarpaceaea [57] | 3 | 7 | 10 | 3 | 4 |
| invertebrates | |||||
| Theotiminae (spiders) [58] | 0 | 1 | 49 | 7 | 7 |
| Heterometrinae (scorpions) [59] | 5 | 31 | 9 | 0 | 0 |
| oriental Rhysida (centipedes) [60] | 8 | 6 | 2 | 0 | 0 |
| tribe Dacini (fruit flies) [61] | 44 | 78 | 238 | 75 | 384 |
| freshwater fishes | |||||
| Scleropages [62] | 0 | 0 | 5 | 0 | 0 |
| Notopteridae [63] | 0 | 2 | 6 | 0 | 0 |
| Channoidei [64] | 5 | 23 | 22 | 6 | 0 |
| Aplocheilidae [65] | 4 | 6 | 1 | 0 | 1 |
| Adrianichthyidae [this study] | 1 | 2 | 11 | 4 | 22 |
| amphibians | |||||
| Ichthyopiidae [66] | 13 | 11 | 31 | 1 | 0 |
| Microhylidae [14] | 16 | 22 | 58 | 13 | 2 |
| Ranoideaa [67] | 19 | 23 | 43 | 25 | 15 |
| reptiles | |||||
| Agamidaea [68] | 8 | 15 | 26 | 10 | 10 |
aThe number of genera was used for the taxon.
Acknowledgements
The World Medaka Aquarium and the National BioResource Project ‘Medaka’ kindly provided the laboratory strains. We also thank Steve O'Shea, PhD, from the Edanz Group for editing a draft of this manuscript.
Ethics
Field collections were conducted based on the Memorandum of Agreement between the Kerala University of Fisheries and Ocean Studies (KUFOS) and the University of the Ryukyus, the Research Permits issued from the Ministry of Research, Technology, and Higher Education, Republic of Indonesia (394/SIP/FRP/SM/XI/2014 and 106/SIP/FRP/E5/Dit.KI/IV/2018), and the Memorandum of Agreement between the Hanoi National University of Education and the Okinawa Institute of Science and Technology Graduate University. Field collections in Laos and Myanmar were supported by the Living Aquatic Resources Research Center and the Ministry of Natural Resources and Environmental Conservation, respectively. We followed the Regulations for Animal Experiments at the University of the Ryukyus for handling the fish, and all experiments were approved by the Animal Care Committee of the University of the Ryukyus (2018099 and 2019084).
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.931zcrjkg [69].
Authors' contributions
All authors substantially contributed to this study. K.Y., Y.Take. and R.R. conceived the study; K.Y., H.K., R.T., K.M., H.D.T., N.K., S.M., V.B., K.W., P.M., S.T., L.K.C.Y., K.W.A.M., V.K.A. and R.R. conducted fieldwork; S.A., H.Y., T.K., J.M., H.K. and J.K. performed laboratory work; and K.Y., S.A., H.Y., J.M., R.Ka., S.F., R.Ki., Y.Take., D.H.E.S. and Y.Taka. conducted analyses. K.Y., S.A., D.H.E.S., K.W., R.R. and J.K. drafted the manuscript, and all authors were engaged in editing and/or revising the manuscript. All authors are accountable for the work performed and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the University of the Ryukyus Research Project Promotion Grant, the Spatiotemporal Genomics Project promoted by the University of the Ryukyus, NIG collaborative grant (B), JSPS KAKENHI (grant no. 17H01675) and JST CREST (grant no. JPMJCR20S2).
References
- 1.Torsvik TH, Cocks LRM. 2013. Gondwana from top to base in space and time. Gondwana Res. 24, 999-1030. ( 10.1016/j.gr.2013.06.012) [DOI] [Google Scholar]
- 2.Yoshida M, Hamano Y. 2015. Pangea breakup and northward drift of the Indian subcontinent reproduced by a numerical model of mantle convection. Scient. Rep. 5, 8407. ( 10.1038/srep08407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storey BC. 1995. The role of mantle plumes in continental breakup: case histories from Gondwanaland. Nature 377, 301-308. ( 10.1038/377301a0) [DOI] [Google Scholar]
- 4.Collins WJ. 2003. Slab pull, mantle convection, and Pangaean assembly and dispersal. Earth Planet. Sci. Lett. 205, 225-237. ( 10.1016/S0012-821X(02)01043-9) [DOI] [Google Scholar]
- 5.Aitchison JC, Ali JR, Davis AM. 2007. When and where did India and Asia collide? J. Geophys. Res. 112, B05423. ( 10.1029/2006jb004706) [DOI] [Google Scholar]
- 6.Kumar P, Yuan X, Kumar MR, Kind R, Li X, Chadha RK. 2007. The rapid drift of the Indian tectonic plate. Nature 449, 894-897. ( 10.1038/nature06214) [DOI] [PubMed] [Google Scholar]
- 7.van Hinsbergen, DJJ, Steinberger B, Doubrovine PV, Gassmöller R.. 2011. Acceleration and deceleration of India-Asia convergence since the Cretaceous: roles of mantle plumes and continental collision. J. Geophys. Res. 116, B06101. ( 10.1029/2010jb008051) [DOI] [Google Scholar]
- 8.Blanford WT. 1901. Distribution of vertebrate animals in India, Ceylon and Burma. Phil. Trans. R. Soc. Lond. B 194, 335-436. ( 10.1098/rstb.1901.0008) [DOI] [Google Scholar]
- 9.Hora SL. 1937. Distribution of Himalayan fishes and its bearing on certain palaeogeographical problems. Rec. Indian Mus. 39, 251-259. [Google Scholar]
- 10.Hora SL. 1944. On the Malayan affinities of the freshwater fish fauna of Peninsular India, and its bearing on the probable age of the Garo-Rajmahal gap. Proc. Natl Inst. Sci. India 10, 423-439. [Google Scholar]
- 11.Ali S, Ripley SD. 1983. Handbook of the birds of India and Pakistan, compact edn. New Delhi, India: Oxford University Press. [Google Scholar]
- 12.Daniels RJR. 2001. Endemic fishes of the Western Ghats and the Satpura hypothesis. Curr. Sci. India 81, 240-244. [Google Scholar]
- 13.Karanth KP. 2015. An island called India: phylogenetic patterns across multiple taxonomic groups reveal endemic radiations. Curr. Sci. India 108, 1847-1851. [Google Scholar]
- 14.Garg S, Biju SD. 2019. New microhylid frog genus from Peninsular India with Southeast Asian affinity suggests multiple Cenozoic biotic exchanges between India and Eurasia. Scient. Rep. 9, 1906. ( 10.1038/s41598-018-38133-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidharthan A, Raghavan R, Anoop VK, Keskar A, Dahanukar N. 2021. Phylogenetic position and relationships of mountain loaches (Teleostei: Balitoridae) of the Western Ghats as revealed by CO1 sequences. Zootaxa 4926, 79-92. ( 10.11646/zootaxa.4926.1.5) [DOI] [PubMed] [Google Scholar]
- 16.Hora SL. 1949. Satpura hypothesis of the distribution of the Malayan fauna and flora to Peninsular India. Proc. Natl Inst. Sci. India 15, 309-422. [Google Scholar]
- 17.Grismer JL, Schulte JA, Alexander A, Wagner P, Travers SL, Buehler MD, Welton LJ, Brown RM. 2016. The Eurasian invasion: phylogenomic data reveal multiple Southeast Asian origins for Indian Dragon Lizards. BMC Evol. Biol. 16, 43. ( 10.1186/s12862-016-0611-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hora SL. 1953. The Satpura hypothesis. Sci. Prog. 162, 245-255. [Google Scholar]
- 19.Bossuyt F, Milinkovitch MC. 2001. Amphibians as indicators of early tertiary ‘Out-of-India’ dispersal of vertebrates Science 292, 93-95. ( 10.1126/science.1058875) [DOI] [PubMed] [Google Scholar]
- 20.Conti E, Eriksson T, Schonenberger J, Sytsma KJ, Baum DA. 2002. Early tertiary out-of-India dispersal of Crypteroniaceae: evidence from phylogeny and molecular dating. Evolution 56, 1931-1942. ( 10.1111/j.0014-3820.2002.tb00119.x) [DOI] [PubMed] [Google Scholar]
- 21.Datta-Roy A, Karanth KP. 2009. The Out-of-India hypothesis: what do molecules suggest? J. Biosci. 34, 687-697. ( 10.1007/s12038-009-0057-8) [DOI] [PubMed] [Google Scholar]
- 22.Joshi J, Karanth KP. 2011. Cretaceous–Tertiary diversification among select Scolopendrid centipedes of South India. Mol. Phylogenet. Evol. 60, 287-294. ( 10.1016/j.ympev.2011.04.024) [DOI] [PubMed] [Google Scholar]
- 23.Sil M, Aravind NA, Karanth KP. 2019. Role of geography and climatic oscillations in governing into-India dispersal of freshwater snails of the family: Viviparidae. Mol. Phylogenet. Evol. 138, 174-181. ( 10.1016/j.ympev.2019.05.027) [DOI] [PubMed] [Google Scholar]
- 24.Loria SF, Prendini L. 2020. Out of India, thrice: diversification of Asian forest scorpions reveals three colonizations of Southeast Asia. Scient. Rep. 10, 22301. ( 10.1038/s41598-020-78183-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sil M, Aravind NA, Karanth KP. 2020. Into-India or out-of-India? Historical biogeography of the freshwater gastropod genus Pila (Caenogastropoda: Ampullariidae). Biol. J. Linn. Soc. 129, 752-764. ( 10.1093/biolinnean/blz171) [DOI] [Google Scholar]
- 26.Praschag P, Schmidt C, Fritzsch G, Müller A, Gemel R, Fritz U. 2006. Geoemyda silvatica, an enigmatic turtle of the Geoemydidae (Reptilia: Testudines), represents a distinct genus. Org. Divers. Evol. 6, 151-162. ( 10.1016/j.ode.2005.10.001) [DOI] [Google Scholar]
- 27.Biju SD, Bossuyt F. 2003. New frog family from India reveals an ancient biogeographical link with the Seychelles. Nature 425, 711-714. ( 10.1038/nature02019) [DOI] [PubMed] [Google Scholar]
- 28.Anoop VK, Dahanukar N, Philip S, Thomas L, Raghavan R. 2018. Phylogeny of the hillstream loach genus Mesonoemacheilus reveals widespread diversification through ancient drainage connections in the Western Ghats Biodiversity Hotspot. Mol. Phylogenet. Evol. 129, 77-84. ( 10.1016/j.ympev.2018.08.013) [DOI] [PubMed] [Google Scholar]
- 29.Chaitanya R, Khandekar A, Caleb DG, Mukherjee N, Ghosh A, Giri V. 2019. Herpetofauna of the Meghamalai Wildlife Sanctuary, southern Western Ghats, India: an updated checklist with annotations on taxonomy and nomenclature. J. Bombay Nat. Hist. Soc. 115, 21-37. [Google Scholar]
- 30.Sidharthan A, Raghavan R, Anoop KV, Philip S, Dahanukar N. 2020. Riddle on the riffle: Miocene diversification and biogeography of endemic mountain loaches in the Western Ghats Biodiversity Hotspot. J. Biogeogr. 47, 2741-2754. ( 10.1111/jbi.13972) [DOI] [Google Scholar]
- 31.Parenti LR. 2008. A phylogenetic analyses and taxonomic revision of ricefishes, Oryzias and relatives (Beloniformes, Adrianichthyidae). Zool. J. Linn. Soc. 154, 494-610. ( 10.1111/j.1096-3642.2008.00417.x) [DOI] [Google Scholar]
- 32.Silas EG. 1959. On the natural distribution of the Indian cyprinodont fish Horaichthys setnai Kulkarni. J. Mar. Biol. Assoc. India 1, 256. [Google Scholar]
- 33.Talwar PK, Jhingran AG. 1991. Inland fishes of India and adjacent countries. New Delhi, India: Oxford and IBH Publishing Company. [Google Scholar]
- 34.Cheng S, Higuchi R, Stoneking M. 1994. Complete mitochondrial genome amplification. Nat. Genet. 7, 350-351. ( 10.1038/ng0794-350) [DOI] [PubMed] [Google Scholar]
- 35.Miya M, Nishida M. 1999. Organization of the mitochondrial genome of a deep-sea fish, Gonostoma gracile (Teleostei: Stomiiformes): first example of transfer RNA gene rearrangements in bony fishes. Mar. Biotechnol. 1, 416-426. ( 10.1007/PL00011798) [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki W, et al. 2013. MitoFish and MitoAnnotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 30, 2531-2540. ( 10.1093/molbev/mst141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato Y, Miya M, Fukunaga T, Sado T, Iwasaki W. 2018. MitoFish and MiFish pipeline: a mitochondrial genome database of fish with an analysis pipeline for environmental DNA metabarcoding. Mol. Biol. Evol. 35, 1553-1555. ( 10.1093/molbev/msy074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silvestro D, Michalak I. 2012. RaxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12, 335-337. ( 10.1007/s13127-011-0056-0) [DOI] [Google Scholar]
- 39.Bouckaert R, et al. 2019. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15, e1006650. ( 10.1371/journal.pcbi.1006650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray AM. 2000. Eocene cichlid fishes from Tanzania, East Africa. J. Vertebr. Paleontol. 20, 651-664. ( 10.1671/0272-4634(2000)020[0651:ECFFTE]2.0.CO;2) [DOI] [Google Scholar]
- 41.Benton MJ, Donoghue PCJ, Asher RJ, Friedman M, Near TJ, Vinther J. 2015. Constraints on the timescale of animal evolutionary history. Palaeontol. Electron., 18.1.1FC. [Google Scholar]
- 42.Bannikov A, Parin NV, Pinna G. 1985. Rhamphexocetus volans, gen. et sp. nov.: a new beloniform fish (Beloniformes, Exocetoidei) from the Lower Eocene of Italy. J. Ichthyol. 25, 150-155. [Google Scholar]
- 43.Frickhinger KA. 1991. Fossilian atlas. Fische. Melle, Germany: Hans A. Bensch. [Google Scholar]
- 44.Horoiwa M, et al. 2021. Mitochondrial introgression by ancient admixture between two distant lacustrine fishes in Sulawesi Island. PLoS ONE 16, e0245316. ( 10.1371/journal.pone.0245316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss SJ, Wilson EJ. 1998. Biogeographic implications of the tertiary palaeogeographic evolution of Sulawesi and Borneo. In Biogeography and geological evolution of SE Asia (eds Hall R, Holloway JD), pp. 133-163. Leiden, The Netherlands: Backhuys Publishers. [Google Scholar]
- 46.Hall R. 2009. Continental growth at the Indonesian margins of southeast Asia. Arizona Geol. Soc. Digest. 22, 245-258. [Google Scholar]
- 47.Spakman W, Hall R. 2010. Surface deformation and slab-mantle interaction during Banda Arc subduction rollback. Nat. Geosci. 3, 562-566. ( 10.1038/ngeo917) [DOI] [Google Scholar]
- 48.Yu Y, Blair C, He XJ. 2020. RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 37, 604-606. ( 10.1093/molbev/msz257) [DOI] [PubMed] [Google Scholar]
- 49.Takehana Y, Naruse K, Sakaizumi M. 2005. Molecular phylogeny of the medaka fishes genus Oryzias (Beloniformes: Adrianichthyidae) based on nuclear and mitochondrial DNA sequences. Mol. Phylogenet. Evol. 36, 417-428. ( 10.1016/j.ympev.2005.01.016) [DOI] [PubMed] [Google Scholar]
- 50.Naruse K, Yamahira K, Takehana Y. 2019. Medaka and Oryzias species as model organisms and the current status of medaka biological resources. In Medaka. Biology, management, and experimental protocols, vol. 2 (eds Murata K, Kinoshita M, Naruse K, Tanaka M, Kamei Y), pp. 31-48. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- 51.Radhakrishna T, Mohamed AR, Venkateshwarlu M, Soumya GS, Prachiti PK. 2019. Mechanism of rift flank uplift and escarpment formation evidenced by Western Ghats, India. Scient. Rep. 9, 10511. ( 10.1038/s41598-019-46564-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoene B, Eddy MP, Samperton KM, Keller CB, Keller G, Adatte T, Khadri SFR. 2019. U-Pb constraints on pulsed eruption of the Deccan Traps across the end-Cretaceous mass extinction. Science 363, 862-866. ( 10.1126/science.aau2422) [DOI] [PubMed] [Google Scholar]
- 53.Ohta T. 1973. Slightly deleterious mutant substitutions in evolution. Nature 246, 96-98. ( 10.1038/246096a0) [DOI] [PubMed] [Google Scholar]
- 54.Nelson JS, Grande TC, Wilson MVH. 2016. Fishes of the world, 5th edn. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 55.Costa L, Jimenez H, Carvalho R, Carvalho-Sobrinho J, Escobar I, Souza G. 2020. Divide to conquer: evolutionary history of Allioideae tribes (Amaryllidaceae) is linked to distinct trends of karyotype evolution. Front. Plant Sci. 11, 320. ( 10.3389/fpls.2020.00320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen YS, et al. 2017. Out-of-India dispersal of Paliurus (Rhamnaceae) indicated by combined molecular phylogenetic and fossil evidence. Taxon 66, 78-90. ( 10.12705/661.4) [DOI] [Google Scholar]
- 57.Dayanandan S, Ashton PS, Williams SM, Primack RB. 1999. Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast RBCL gene. Am. J. Bot. 86, 1182-1190. ( 10.2307/2656982) [DOI] [PubMed] [Google Scholar]
- 58.Li F, Shao L, Li S. 2020. Tropical niche conservatism explains the Eocene migration from India to Southeast Asia in ochyroceratid spiders. Syst. Biol. 69, 987-998. ( 10.1093/sysbio/syaa006) [DOI] [PubMed] [Google Scholar]
- 59.Prendini L, Loria SF. 2020. Systematic revision of the Asian forest scorpions (Heterometrinae Simon, 1879), revised suprageneric classification of Scorpionidae Latreille, 1802, and revalidation of Rugodentidae Bastawade et al., 2005. Bull. Am. Mus. Nat. Hist. 442, 1-480. ( 10.1206/0003-0090.442.1.1) [DOI] [Google Scholar]
- 60.Joshi J, Karanth PK, Edgecombe GD. 2020. The out-of-India hypothesis: evidence from an ancient centipede genus, Rhysida (Chilopoda: Scolopendromorpha) from the Oriental Region, and systematics of Indian species. Zool. J. Linn. Soc. 189, 828-861. ( 10.1093/zoolinnean/zlz138) [DOI] [Google Scholar]
- 61.Krosch M, Schutze M, Armstrong K, Graham G, Yeates D, Clarke A. 2012. A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): systematic and biogeographic implications. Mol. Phylogenet. Evol. 64, 513-523. ( 10.1016/j.ympev.2012.05.006) [DOI] [PubMed] [Google Scholar]
- 62.Kumazawa Y, Nishida M. 2000. Molecular phylogeny of osteoglossoids: a new model for Gondwanian origin and plate tectonic transportation of the Asian arowana. Mol. Biol. Evol. 17, 1869-1878. ( 10.1093/oxfordjournals.molbev.a026288) [DOI] [PubMed] [Google Scholar]
- 63.Barby FF, et al. 2018. From chromosomes to genome: insights into the evolutionary relationships and biogeography of Old World knifefishes (Notopteridae: Osteoglossiformes). Genes 9, e306. ( 10.3390/genes9060306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Musikasinthorn P, Kumazawa Y. 2006. Molecular phylogenetic analyses of snakeheads (Perciformes: Channidae) using mitochondrial DNA sequences. Ichthyol. Res. 53, 148-159. ( 10.1007/s10228-005-0321-3) [DOI] [Google Scholar]
- 65.Murphy WJ, Collier GE. 1997. A molecular phylogeny for aplocheiloid fishes (Atherinomorpha, Cyprinodontiformes): the role of vicariance and the origins of annualism. Mol. Biol. Evol. 14, 790-799. ( 10.1093/oxfordjournals.molbev.a025819) [DOI] [PubMed] [Google Scholar]
- 66.Gower DJ, et al. 2002. A molecular phylogeny of ichthyophiid caecilians (Amphibia: Gymnophiona: Ichthyophiidae): out of India or out of South East Asia? Proc. R. Soc. Lond. B 269, 1563-1569. ( 10.1098/rspb.2002.2050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bossuyt F, Brown RM, Hillis DM, Cannatella DC, Milinkovitch MC. 2006. Phylogeny and biogeography of a cosmopolitan frog radiation: late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Syst. Biol. 55, 579-594. ( 10.1080/10635150600812551) [DOI] [PubMed] [Google Scholar]
- 68.Macey JR, Schulte JA II, Larson A, Ananjeva NB, Wang Y, Pethiyagoda R, Rastegar-Pouyani N, Papenfuss TJ. 2000. Evaluating trans-Tethys migration: an example using acrodont lizard phylogenetics. Syst. Biol. 49, 233-256. ( 10.1093/sysbio/49.2.233) [DOI] [PubMed] [Google Scholar]
- 69.Yamahira K, et al. 2021. Data from: Mesozoic origin and ‘out-of-India’ radiation of ricefishes (Adrianichthyidae). Dryad Digital Repository. ( 10.5061/dryad.931zcrjkg) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Yamahira K, et al. 2021. Data from: Mesozoic origin and ‘out-of-India’ radiation of ricefishes (Adrianichthyidae). Dryad Digital Repository. ( 10.5061/dryad.931zcrjkg) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.931zcrjkg [69].