Abstract
Objective:
Amygdala-ventrolateral prefrontal cortex (VLPFC) circuitry is disrupted in pediatric anxiety disorders, yet how selective serotonin reuptake inhibitors (SSRIs), impact this circuitry is unknown. We examined the impact of SSRI on functional connectivity (FC) within this circuit, and whether early FC changes predict treatment response in adolescents with generalized anxiety disorder (GAD).
Method:
Resting-state functional MR images were acquired before and after 2-weeks of treatment in 41 adolescents with GAD (age: 12–17) who received double-blind escitalopram or placebo over 8 weeks. Change in amygdala-based whole-brain FC and anxiety severity were analyzed.
Results:
Controlling for age, sex and pretreatment anxiety, escitalopram increased amygdala-VLPFC connectivity compared to placebo (F=17.79, p=0.002 FWE-corrected). This early FC change predicted 76.7% of the variability in improvement trajectory in patients who received escitalopram (p<0.001) but not placebo (p=0.169); the predictive power of early amygdala-VLPFC FC change significantly differed between placebo and escitalopram (p=0.013). Further, this FC change predicted improvement better than baseline FC or demographics. Exploratory analyses of amygdala subfields’ FC revealed connectivity of left basolateral amygdala (BLA)-VLPFC (F=19.64, p<0.001 FWE-corrected) and superficial amygdala-posterior cingulate cortex (F=22.92, p=0.001 FWE-corrected) were also increased by escitalopram, but only BLA-VLPFC FC predicted improvement in anxiety over 8 weeks of treatment.
Conclusions:
In adolescents with GAD, escitalopram increases amygdala-prefrontal connectivity within the first 2 weeks of treatment, and the magnitude of this change predicts subsequent clinical improvement. Early normalization of amygdala-VLPFC circuitry might represent a useful tool for identifying future treatment responders as well as a promising biomarker for drug development.
Trial Registration:
ClinicalTrials.gov Identifier: NCT02818751.
Keywords: selective serotonin reuptake inhibitors (SSRIs), antidepressant, clinical trial, anxiety disorders, MRI
Lay summary:
Selective serotonin reuptake inhibitors (SSRIs) are the most commonly used medications to treat teens with anxiety. In this study, the SSRI, escitalopram increased the strength of the connection between two brain regions—the amygdala and the ventrolateral prefrontal cortex—that are overactive in teens with anxiety disorders. Compared to placebo, escitalopram increased the strength of these connections by the second week of treatment and predicted which patients would improve most with treatment.
INTRODUCTION
Selective serotonin reuptake inhibitors (SSRIs) represent an effective treatment for many children and adolescents with anxiety disorders.1,2 Improvement varies considerably from patient to patient and 6–8 weeks of treatment is often needed to evaluate response to an SSRI.1,3,4 Early prediction of limited treatment response to this first line treatment could indicate the need for clinicians to consider alternative or adjunctive treatments and thus improve outcomes. Further, because the effects of SSRIs on functional neurocircuitry in youth with anxiety disorders are poorly understood,5,6 this knowledge would also be important for understanding circuit-level mechanisms of treatment efficacy.7
Several magnetic resonance imaging (MRI) studies have examined the treatment effects of SSRIs on brain structure and activity. In open-label trials, SSRIs attenuated insula and amygdala activity, and enhanced posterior cingulate cortex (PCC) activity during emotion perception tasks in adults with anxiety and/or depression. Changes in functional activation in these regions correspond to improvement in anxiety.8–11 Compared with placebo, SSRIs reduce insula and amygdala reactivity during emotion processing tasks in healthy adults.12,13 Only two open-label studies have examined the effects of SSRIs on functional brain activity in youth with anxiety disorders. In the first, fluoxetine increased ventrolateral prefrontal cortex (VLPFC) activity in response to angry faces in adolescents with GAD.6 In the second, effective treatment increased rostral anterior cingulate cortex (ACC) activation, and greater activation was associated with more improvement in anxiety and avoidance symptoms.14 In addition, SSRI-exposed infants compared with both healthy controls and non SSRI-exposed infants, had gray matter volume expansion in the amygdala and insula, and increased white matter connectivity between amygdala and insula.15 Taken together, these studies indicate that SSRIs change functional reactivity, structure and connectivity in the amygdala and other regions that subserve emotion processing and these effects might mechanistically relate to SSRI-driven improvement in anxiety. Lower animal studies reveal that SSRIs induce plasticity in fear circuitry and reorganize inhibitory circuits—at the level of the amygdala—through expression of the plasticity-related molecules (e.g., polysialylated form of the neural cell adhesion molecule, [PAS-NCAM]). At the molecular level, these changes enhance interneuronal connectivity as a result of changes in dendritic spines and axonal architecture in the amygdala.16 Based on these recent findings and the established role of the amygdala in pediatric anxiety,17 we focused on the amygdala as the seed for connectivity analyses.
Clinical and demographic characteristics predict treatment response in pediatric anxiety disorders.1 In the largest trial of an SSRI in youth with anxiety disorders, the Child and Adolescent Anxiety Multimodal Study (CAMS), patients who were younger, were non-minority, had less severe anxiety and fewer co-morbidities were more likely to remit.18 Additionally in this sample, anxiety severity and caregiver strain were significantly related with treatment response to sertraline.19 To date, studies have failed to predict response to SSRIs with pharmacokinetic or pharmacogenetic parameters in youth with mixed anxiety and depressive disorders treated with sertraline or escitalopram.20,21 Neuroimaging studies in youth with GAD have reported pretreatment amygdala activation being correlated with improvement following fluoxetine treatment.5 Increased activation of the dorsolateral prefrontal cortex, VLPFC and precentral/postcentral gyri before treatment have also been associated with greater treatment-related improvement in anxiety in children and adolescents with anxiety disorders.22 In adults with social anxiety disorder, pre-treatment dorsal ACC reactivity separated treatment responders from non-responders (83% accuracy) who received an SSRI.23,24 Studying the connectome of these regions is a promising next step, as the amygdala and prefrontal regions are widely considered to influence the intensity and modulation of emotions respectively.25
To date, several studies have examined SSRI-related changes in brain function in open-label trials, as well as baseline neurofunctional predictors of SSRI response in anxiety disorders. However, acute SSRI effects on functional connectivity (FC) in youth with anxiety and the potential utility of early treatment-related FC changes (vs clinical characteristics) to predict subsequent clinical outcomes have never been examined in a double-blind, placebo-controlled trial. With these considerations in mind, the primary aim of this study is to examine early treatment effects of escitalopram on amygdala-based FC, and then to explore the relationship of these neurofunctional treatment effects and improvement in adolescents with GAD. Since the amygdala comprises several subfields that might have specific connectivities and are differentially involved in anxiety,26 we further investigated the effect of escitalopram on amygdala subfields’ connectivity. As an exploratory analysis, we also examined whether the SSRI-related FC change at week 2 predicted improvement of anxiety better than demographic/clinical characteristics and baseline (i.e., pretreatment) FC. Based on previous findings, we hypothesized that treatment would increase amygdala-prefrontal FC and that early, treatment-related increases in amygdala-prefrontal FC would predict subsequent improvement in anxiety over the course of the trial.
METHOD
Participants
This randomized clinical trial aimed to identify neurofunctional predictors of treatment-response in adolescents with GAD (ClinicalTrials.gov Identifier: NCT02818751). The protocol was approved by the Institutional Review Board of the University of Cincinnati and conducted in accordance with Good Clinical Practice guidelines. This study was conducted at a single academic site in the United States from February 2015 to November 2018. Outpatients aged 12–17 years who met DSM-IV-TR criteria for GAD, assessed using the Anxiety Disorders Interview Schedule (ADIS), had a Pediatric Anxiety Rating Scale (PARS) score ≥15 at screening and baseline visits; and had a Clinical Global Impression of Severity (CGI-Severity) score ≥4 were eligible. Patients with secondary diagnoses of separation or social anxiety disorder or panic disorder and/or agoraphobia were enrolled, provided that GAD was the primary diagnosis; however, patients with current MDD or any history of bipolar disorder, psychotic disorder, obsessive compulsive disorder or post-traumatic stress disorder were excluded. Concomitant psychotherapy was allowed during the study provided the psychotherapy was stable prior to study entry and remained stable, no new psychotherapy was allowed during the trial. Other exclusion criteria included: a contraindication to MRI, pregnancy, a history of alcohol and drug abuse or any major medical or neurological disorder. Additionally, the original proposal, as reflected in clinicialtrials.gov, proposed a sample size of 64 patients with GAD, although only 51 were randomized. Further, in the original conception of the study, 20 healthy controls were to have been enrolled (not part of this analysis).
Treatment and Assessments
Patients were randomized to an 8-week double-blind placebo-controlled clinical trial with escitalopram, the most serotonergically selective SSRI, or placebo (1:1) which was delivered in identically-appearing purple capsules. Treatment was assigned by investigational pharmacists using a random number generator and randomization was stratified by sex. Patients, caregivers, and investigational staff were blind to treatment assignment. As previously described,27 escitalopram was initiated at 5 mg daily for 2 days and then 10 mg daily for 7 days and then 15 mg daily. At week 4 and 6 visits, escitalopram could be titrated to 20 mg daily.
The PARS was used to measure the anxiety symptom severity and was administered at each study visit (week 0, 1, 2, 4, 6, and 8) to provide a trajectory of symptom change. The PARS was selected as it has been utilized as the primary outcome measure in the majority of psychopharmacologic treatment studies in youth with anxiety disorders.17 The PARS includes a 50-item symptom checklist which encompasses social anxiety/performance anxiety (9 items), separation anxiety (10 items), generalized anxiety (8 items), specific phobia (4 items), physical/somatic symptoms (13 items) as well as “other” items (6 items). However, the PARS score is determined from 7 severity and impairment items that are rated on a 6-point scale, with higher scores representing more severe symptoms and impairment. The PARS has established, acceptable, convergent validity with 3 anxiety rating scales.28 For patients who discontinued treatment prior to week 8 (endpoint), a last observation carried forward (LOCF) approach was utilized for missing PARS scores (3 participants in each group discontinued, p=1.0).
Image Acquisition
Resting-state functional MR images (rsfMRI) and high-resolution anatomical images were acquired on a 3-T scanner (Achieva; Philips, USA) with a 32-channel phased-array head coil at baseline and 2 weeks after beginning treatment. Scanner noise was attenuated with earplugs and headphones; head motion was restricted with foam padding. Functional images were obtained using a single shot, fast Fourier echo, echo planar (FFE-EPI) sequence with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; number of axial slices = 40; resolution = 2.8 mm × 2.8 mm; slice thickness = 3 mm; flip angle = 75°; matrix = 80 × 80; field of view = 224 mm × 224 mm. High-resolution anatomical images were obtained using a three dimensional T1-weighted Turbo field echo (T1-TFE) sequence with the following parameters: TR = 6.8 ms; TE = 2.9 ms; number of sagittal slices = 160; resolution = 1 mm × 1 mm; slice thickness = 1 mm; flip angle = 9°; matrix = 256 × 256; field of view = 256 mm × 256 mm. Images were reviewed by a pediatric neuroradiologist to exclude cases with gross structural abnormalities and image artifacts.
Functional Connectivity of Amygdala and Amygdala-subfields
Functional images were preprocessed with the SPM12 package (www.fl.ion.ucl.ac.uk/spm) and DPABI toolbox.29 For each patient, the first 10 volumes were discarded to ensure signal stabilization. The remaining images were corrected for slice time. Head motion was corrected by regression of 24 head motion parameters, mean framewise displacement (FD) of every subject was <0.5 mm and did not different between groups (pretreatment, t=0.70, p=0.49; week 2, t=0.48, p=0.63). Functional images were spatially normalized to standard Montreal Neurological Institute space using unified segmentation on individual T1 images, and each voxel was resampled to 3×3×3 mm3. The normalized images were then smoothed with a 6-mm full-width at half-maximum Gaussian kernel. Linear trends and nuisance signals (six motion parameters, white matter signal, and cerebrospinal fluid signal) were removed with linear regression and a temporal band pass filter (0.01–0.08 Hz) was utilized to exclude high and low frequency signals. Four amygdala subfields (amygdalostriatal transition [AStr], basolateral [BLA], centralmedial [CMA] and superficial amygdala [SFA]) were identified using a cytoarchitectonically-defined probabilistic map of the amygdala.30 Seed-based resting-state FC analyses were conducted for the left and right amygdala and their subfields separately (10 seeds in total including bilateral amygdala, AStr, BLA, CMA, and SFA) using the Resting-State fMRI Data Analysis Toolkit (http://resting-fmri.sourceforge.net). Specifically, the time series of voxels within each region of interest were extracted and averaged, then voxel-wise correlation analyses were performed with the rest of the brain to obtain FC maps. Correlation coefficients were transformed to z-value images using the Fisher r-to-z transformation for statistical analyses.
Statistical Analysis
The treatment-by-time interaction was examined using a full factorial analysis in SPM12, with FC maps as dependent variables, treatment (i.e., placebo or escitalopram) and time (baseline or week 2) as independent variables, and age, sex and baseline PARS score as covariates. Family-wise error (FWE) was applied to correct for multiple comparisons, with thresholds of p<0.001 at the voxel level and p<0.05 at the cluster level. Bonferroni correction was utilized to control type 1 error given that 10 seeds we used in current study (corrected p<0.005=0.05/10). FC strength was extracted from clusters with significant treatment-by-time interactions, and a post-hoc analysis was performed to examine the change of FC from baseline to week 2 within the escitalopram and placebo groups respectively.
Using a mixed effects model (with log trend specification), we examined the relationship between change in FC at week 2 and the trajectory of anxiety improvement (i.e., PARS score) using week 0, 1, 2, 4, 6 and 8 ratings in both escitalopram and placebo group. Each model was estimated with covariates (e.g., age, sex) and refined as previously described, using the Bayesian Information Criterion (BIC) and the Akaike Information Criterion (AIC), to obtain the most parsimonious response prediction model.3 Predicted variation in response models was measured by R2; adjusted R2 (R2adj), a model selection criterion, was not used as it is superseded by AIC and BIC and does not directly measure the proportion of variation explained by the model. As an exploratory analysis of potential superiority of the change in FC as a predictor of outcome, models that included baseline FC and demographic characteristics were also examined and compared. Non-imaging statistical analyses were performed using R (version 3.1.2) and SPSS (version 22), p-values <0.05 were considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
Fifty-one participants were randomized to treatment and detailed demographic and clinical characteristics have been reported previously.31 Ten participants (5 from escitalopram and 5 from placebo group) were excluded from this fMRI analysis, 8 of them did not complete baseline and week 2 scans, 1 had orthodontia artifacts, and 1 was scanned with a different MR sequence. Thus, forty-one participants were included in the analysis (escitalopram: n=21; placebo: n=20, Table 1 and Figure 1). There were no significant differences between groups in age, sex, IQ, comorbidity and baseline anxiety severity (i.e., PARS score). Seventy six percent of escitalopram-treated patients and 81% of patients who received placebo were antidepressant naïve (p=0.73). Moreover, no pretreatment amygdala/amygdala-subfield based FC difference between the two groups was found. Escitalopram-treated patients had significantly greater improvement (PARS score), at endpoint and over time, compared to those who received placebo.
Table 1.
Demographic and Clinical Characteristics of Patients Receiving Escitalopram and Placebo
| Baseline Characteristics | Escitalopram (n=21) | Placebo (n=20) | Summary statistic | p-value |
|---|---|---|---|---|
| Age, mean (SD), year | 14.9±1.7 | 15.0±1.6 | −0.18 | 0.86 |
| Female, n (%) | 16 (76) | 14 (70) | 0.20 | 0.66 |
| Full scale IQ, mean (SD) | 106±10 | 104±11 | 0.48 | 0.64 |
| Race | 1.51 | 0.87 | ||
| Asian | 0 (17) | 1 (6) | ||
| Black and African American | 1 (0) | 1 (6) | ||
| Caucasian | 19 (83) | 17 (81) | ||
| Other | 1 (0) | 1 (6) | ||
| Hispanic or Latino | 2 (0) | 0 (0) | 0.49 | |
|
| ||||
| PARS score, baseline | 17±2 | 17±3 | 0.17 | 0.86 |
| PARS score, week 8/ET | 7±6 | 15±3 | −2.37 | 0.02 |
| CGI-Severity score, median | 4 | 4 | 0.22 | |
|
| ||||
| Secondary diagnoses | ||||
| Separation anxiety disorder | 3 (14) | 4 (20) | 0.70 | |
| Panic disorder | 10 (48) | 12 (60) | 0.54 | |
| Agoraphobia | 6 (29) | 6 (30) | 1.00 | |
| ADHD | 4 (19) | 4 (20) | 1.00 | |
| Specific phobia | 7 (33) | 2 (10) | 0.13 | |
|
| ||||
| Prior SSRI/SNRI treatment, n (%) | 5 (24) | 6 (19) | 0.20 | 0.73 |
CGI-S, Clinical Global Impression Scale-Severity; CDRS-R, Children’s Depression Rating Scale-Revised; PARS, Pediatric Anxiety Rating Scale; ADHD, Attention/Deficit-Hyperactivity Disorder; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin-norepinephrine reuptake inhibitor.
FIGURE 1.
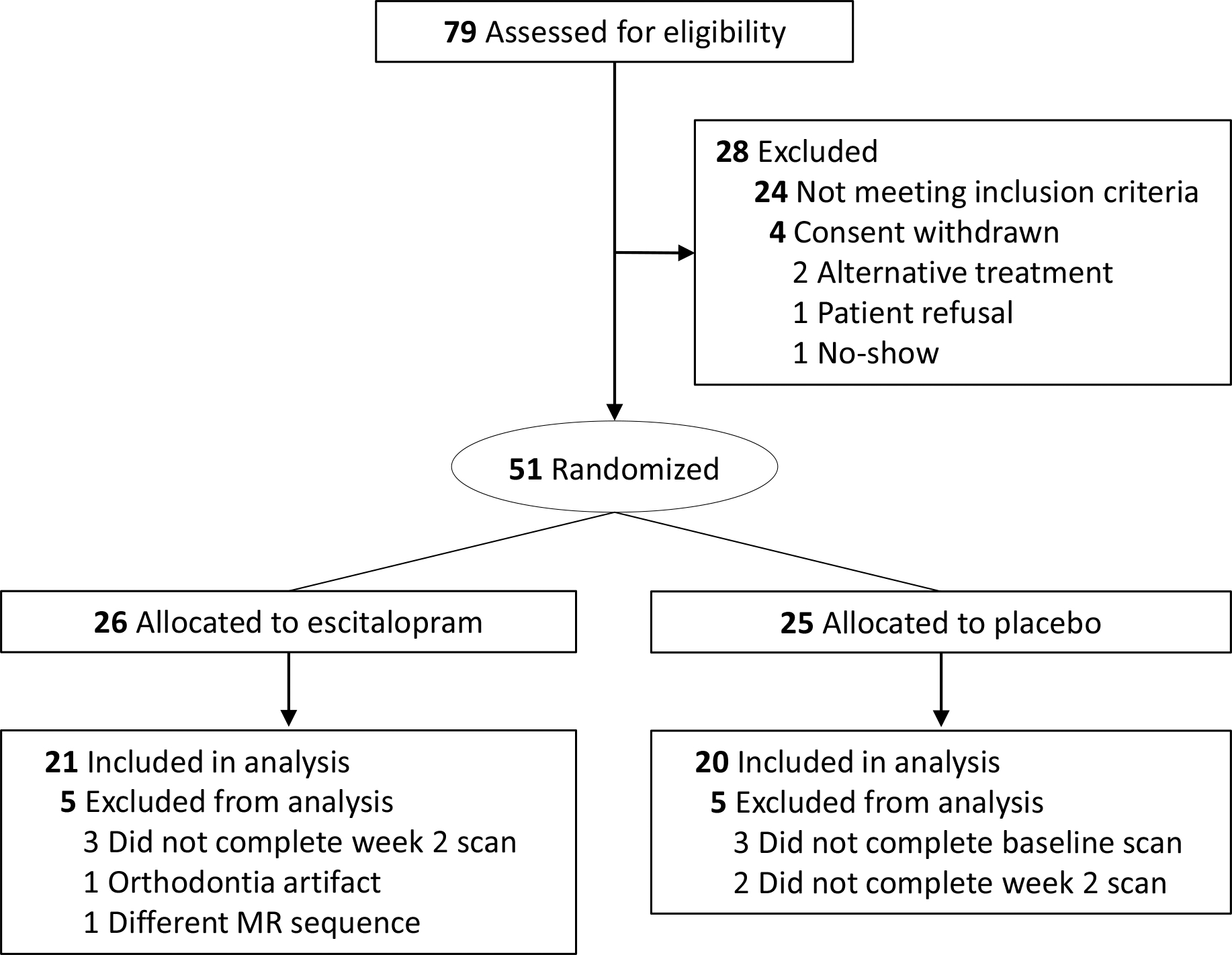
CONSORT Diagram
Escitalopram Increases Amygdala Functional Connectivity
Adolescents with GAD showed a significant treatment-by-time interaction in the FC between left amygdala and left VLPFC (cluster size: 144 voxels; peak coordinate: x=−45, y=20, z=24; F=17.79, p=0.002 FEW- and Bonferroni-corrected). Post-hoc analysis revealed that escitalopram significantly increased functional amygdala-VLPFC connectivity compared to placebo. Specifically, the amygdala-VLPFC connectivity increased after 2 weeks of escitalopram treatment (F=3.62, p=0.064) (Figure 2).
FIGURE 2. Treatment-by-Time Interaction in Amygdala- and Amygdala Subdivision-based Functional Connectivity (FC) in Adolescents with Generalized Anxiety Disorder.
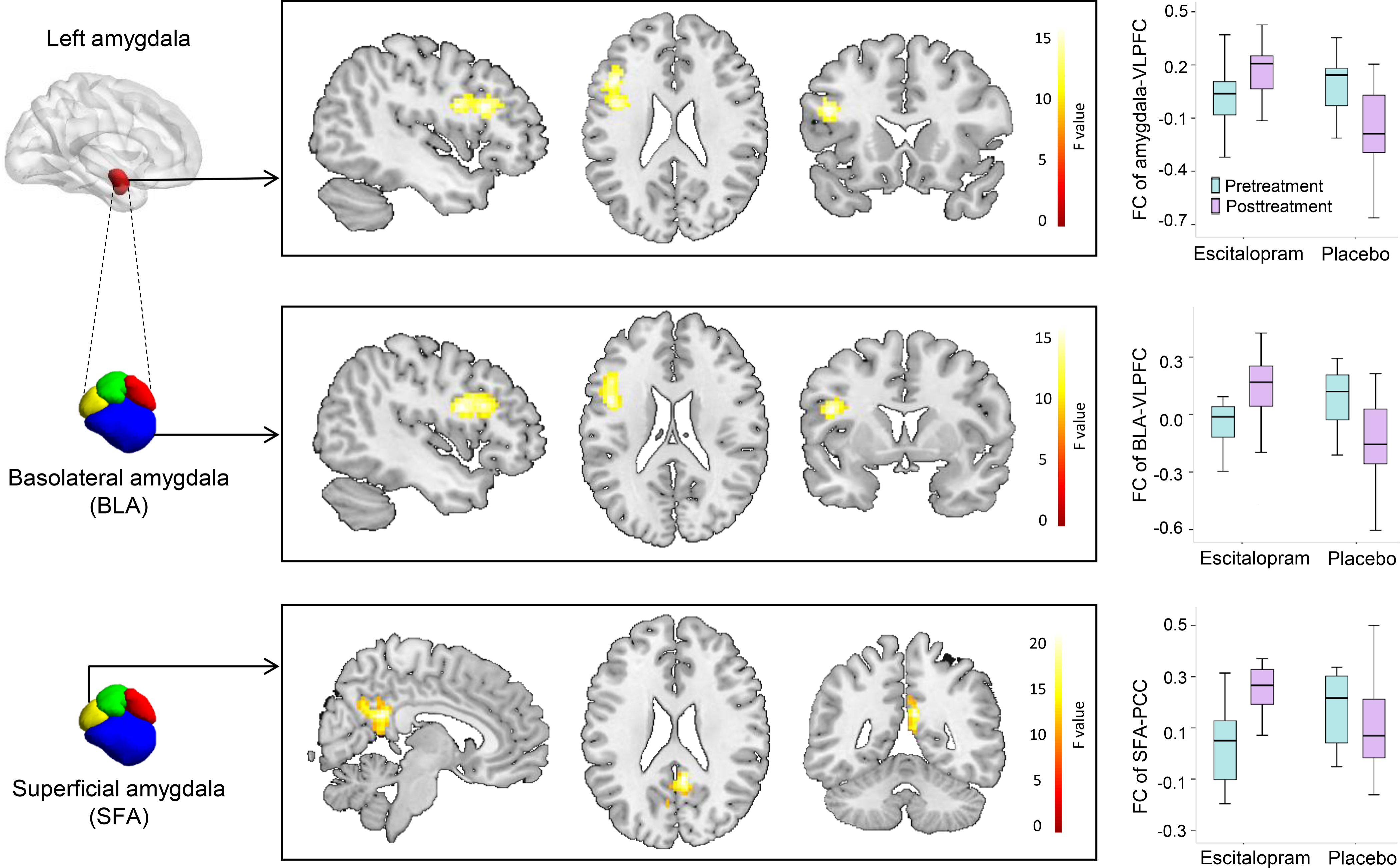
Note: In the box and whisker plots, the horizontal line inside the box represents the median FC strength, the bottom and top edges reflect interquartile range (25th and 75th percentiles, respectively) and the whiskers extend to the furthest datum within 1.5 times the interquartile range. BLA, basolateral amygdala; FC, functional connectivity; L, left; PCC, posterior cingulate cortex; SFA, superficial amygdala; VLPFC, ventrolateral prefrontal cortex. *, p<0.05; **, p<0.001.
In the exploratory analyses, a significant treatment-by-time interaction was observed in the FC between left BLA and left VLPFC (cluster size: 198 voxels; peak coordinate: x=−45, y=6, z=21; F=19.64, p<0.001 FEW- and Bonferroni-corrected). BLA-VLPFC connectivity increased significantly after 2 weeks of escitalopram treatment (F=4.84, p=0.034). Specifically, the BLA-VLPFC connectivity increased significantly after 2 weeks’ escitalopram treatment (F=4.84, p=0.034) and decreased significantly in youth who received placebo (F=12.33, p=0.001) (Figure 2). A significant treatment-by-time interaction was also found in the FC between left SFA and PCC (cluster size: 161 voxels; peak coordinates: x=6, y=−48, z=24; F=22.92, p=0.001 FEW- and Bonferroni-corrected). SFA-PCC connectivity increased significantly after 2 weeks of escitalopram treatment (F=45.97, p<0.001) and marginally decreased in youth receiving placebo (F=4.12, p=0.049) (Figure 2). No connectivity was changed by escitalopram with right amygdala and its subfields as seeds.
Increased Amygdala Functional Connectivity Predicts Improvement in Anxiety
The change in left amygdala-VLPFC connectivity from baseline to week 2 predicted the trajectory of improvement in PARS scores from baseline to week 8 in escitalopram-treated patients (β=3.706, p<0.001) but not those who received placebo (β=1.179, p=0.169). For escitalopram treated patients, this response model explained >76% of the variation in improvement (i.e, R2=0.767) compared to just 41% in patients who received placebo. Likewise, the change in left BLA-VLPFC (β=−4.340, p<0.001) also predicted the trajectory of PARS score in adolescents taking escitalopram, while the change of SFA-PCC FC did not (β=−1.356, p=0.087). The predictive power of early amygdala-VLPFC FC change significantly differed between patients who received placebo and those who received escitalopram (β=2.685, p=0.013). This difference in predictive power of the two interventions (i.e., placebo, escitalopram) also significantly differed for the BLA-VLPFC (β=−3.672, p=0.005) and the SFA-PCC (β=−2.709, p=0.023) (Table 2).
Table 2.
Regression Models of Improvement in Pediatric Anxiety Rating Scale (PARS) Score over 8-weeks in Adolescents Receiving Escitalopram and Placebo
|
Trajectory of anxiety symptoms, escitalopram (n=21)
| ||||
| Estimate | Std. Error | t value | p-value | |
|
| ||||
| Week (log) | −1.862 | 1.214 | −1.534 | 0.128 |
| Amygdala-VLPFC FC change | 3.706 | 0.747 | 4.961 | <0.001 |
| BLA-VLPFC FC change | −4.340 | 1.046 | −4.150 | <0.001 |
| SFA-PCC FC change | −1.356 | 0.785 | −1.724 | 0.087 |
| Age | −0.043 | 0.086 | −0.502 | 0.617 |
| Sex, female | 0.969 | 0.318 | 3.046 | 0.003 |
|
Trajectory of anxiety symptoms, placebo (n=20) | ||||
| Estimate | Std. Error | t value | p-value | |
|
| ||||
| Week (log) | −3.476 | 1.213 | −2.866 | 0.005 |
| Amygdala-VLPFC FC change | 1.179 | 0.850 | 1.387 | 0.169 |
| BLA-VLPFC FC change | −0.920 | 0.864 | −1.064 | 0.290 |
| SFA-PCC FC change | 1.191 | 0.867 | 1.373 | 0.173 |
| Age | 0.161 | 0.081 | 1.994 | 0.049 |
| Sex, female | 0.493 | 0.273 | 1.806 | 0.074 |
|
Trajectory of anxiety symptoms, all patients (n=41) | ||||
| Estimate | Std. Error | t value | p-value | |
|
| ||||
| Week (log) | −2.162 | 0.880 | −2.457 | 0.015 |
| Tx x week | −1.008 | 0.303 | −3.331 | 0.001 |
|
| ||||
| amygdala-VLPFC change | 0.982 | 0.855 | 1.149 | 0.252 |
| BLA-VLPFC FC change | −0.778 | 0.886 | −0.879 | 0.381 |
| SFA-PCC FC change | 1.231 | 0.905 | 1.360 | 0.175 |
|
| ||||
| Tx by amygdala-VLPFC change | 2.685 | 1.075 | 2.498 | 0.013 |
| Tx by BLA-VLPFC FC change | −3.672 | 1.283 | −2.861 | 0.005 |
| Tx by SFA-PCC FC change | −2.709 | 1.183 | −2.290 | 0.023 |
|
| ||||
| age | 0.064 | 0.059 | 1.081 | 0.281 |
| sex | 0.676 | 0.209 | 3.243 | 0.001 |
VLPFC, ventrolateral prefrontal cortex; FC, functional connectivity; BLA, basolateral amygdala; SFA, superficial amygdala; PCC, posterior cingulate cortex
Escitalopram-related Change in Functional Connectivity Predicts Treatment Response Better than Clinical Features and Pre-treatment Functional Connectivity
Consistent with previous studies, sex predicted the trajectory of anxiety symptoms in escitalopram-treated patients (Table 2). Including baseline FCs with sex and age did not improve model fit (F=0.974, p=0.408). However, including change in FC in the demographic model significantly improved model fit (F=4.08, p=0.020) and provided the best prediction of improvement in anxiety symptoms based on model fit statistics (BIC and AIC).
DISCUSSION
This is the first prospective, double-blind, placebo-controlled examination of early SSRI-related changes in FC in anxiety disorders. Within two weeks, escitalopram increased amygdala FC relative to placebo (amygdala-VLPFC, BLA-VLPFC and SFA-PCC). Further, in escitalopram-treated (but not placebo-treated) patients, the magnitude of increases in amygdala-VLPFC and BLA-VLPFC FC predicted the trajectory of anxiety symptom reduction over 8 weeks, and did so better than baseline amygdala FC and demographic characteristics. Taken together, these findings demonstrate acute, neurofunctional effects of escitalopram on amygdala FC and the promising role of these FC changes in predicting treatment response in adolescents with anxiety disorders.
Hyperactivity in the amygdala and its attenuated FC with VLPFC have been observed in task-based fMRI studies of anxious youth.32,33 Specifically, the VLPFC known to be involved in emotion regulation has decreased connectivity with amygdala compared to healthy youth in cross-sectional studies of adolescents with GAD.34,35 Our findings indicate that escitalopram increases this impaired connectivity preceding full treatment response, suggesting that normalizing this FC produces early restorative effects on the neuropathophysiology of deficient emotion regulation in GAD. Further, prior fMRI studies using emotional processing tasks in adolescents with GAD revealed increased amygdala and VLPFC activation when viewing angry faces, and the increased VLPFC activation was associated with attenuated amygdala activation and less anxiety.36,37 By increasing amygdala-VLPFC FC, escitalopram may enhance VLPFC’s regulation of amygdala activity and thus reduce anxiety in youth with GAD. This interpretation is consistent with previous findings showing that both fluoxetine and cognitive behavior therapy increase VLPFC activation in response to angry faces in adolescents with GAD.6 Further, in adults with high state anxiety, real-time FC-informed neurofeedback training during threat exposure increases amygdala-VLPFC connectivity and decreases anxiety.38 Thus, our findings are consistent with the view that reduced functional integration of amygdala-VLPFC circuitry may weaken regulation of amygdala-driven emotional responses to threatening stimuli and thus to manifest anxiety. Our findings indicate that increasing amygdala-VLPFC connectivity may improve this top-down emotion regulation and relieve anxiety symptoms, and represent a target for interventions aiming to reduce anxiety.
In our study, significant effects of escitalopram on amygdala-VLPFC FC were identified in the BLA amygdala subfield. Importantly, the BLA receives information about the external environment from the sensory thalamus and neocortices, and reciprocally projects to cortical regions implicated in the pathophysiology of anxiety disorders.39 This study extends previous findings by providing evidence that amygdala FC can be successfully modified by treatment to reduce anxiety and that greater BLA-VLPFC FC predicts better treatment response. Additionally, the importance of the BLA subfield is consistent with lower animal models showing the importance of this region for anxiety and the role of 5-HT in regulating BLA neuronal response to aversive sensory cues.40–42
In addition to escitalopram-related changes in amygdala-VLPFC FC, treatment-related changes in SFA-PCC were identified. The effects of SSRI treatment are not limited to the amygdala-prefrontal circuitry but involve its connections to the default mode network (DMN). Decreased DMN activity and decreased FC between DMN hubs and amygdala have been observed in anxiety disorders and in individuals with high state anxiety.26,43–47 Recently, an examination of individual-specific FC of the amygdala subdivisions revealed connectivity between SFA and PCC.48 The SFA processes socially-relevant information and modulates approach-avoidance behavior.49,50 The PCC is involved in evaluating the affective valence of external stimuli.51 In healthy adults, escitalopram has been shown to decrease PCC activity and anxiety during a self-evaluation task.52 Thus, our findings suggest that SSRIs may impact amygdala-PPC FC to modulate the evaluation of emotional stimuli by decreasing the affective valence of threatening stimuli and reducing avoidance.
Since about 50% of adolescents with anxiety disorders fail to respond to SSRIs,1 there is an urgent need to identify biomarkers that predict treatment response prior to or early in the course of a treatment so that long trials of treatments that are unlikely to be effective can be avoided. Previous studies reveal that demographic factors and baseline activation in the amygdala, PFC, and ACC during emotional processing are associated with improvement in anxiety symptoms.5,22,53 Our findings extend this work by revealing that SSRI-related normalization of FC of the amygdala could better predict treatment response than pretreatment neuroimaging biomarkers and demographic factors, and could do so early after treatment initiation. Further, the early FC changes observed herein relate to recent data suggesting that—early in the course of treatment—SSRIs attenuate the negative bias in processing emotionally salient information in patients with anxiety. It has been hypothesized that this change facilitates the clinical improvement that occurs later.54 Finally, it is also possible that these early FC changes could relate to tolerability, including “activation,” a common side effect of SSRIs in youth with anxiety and affective disorders.55,56 Importantly, activation which consists of transient increases in anxiety, restlessness and insomnia emerges early in the course of treatment is related to plasma blood levels of SSRIs and may relate to effects of SSRIs on amygdala-prefrontal circuits.55,57
While this is the first investigation of acute effect of escitalopram on FC in adolescents, several limitations warrant additional discussion. First, the sample size is relatively small and limits our ability to characterize the effects of sex on the neurocircuitry of treatment response. Specifically, the sample size is smaller than our planned sample size and 6 patients discontinued treatment prior to endpoint, although for trajectory analyses, a last observation carried forward approaches was used.57 Second, the stability of FC changes cannot be determined based on our study design; the acute changes in FC may represent transient, persistent or progressively increasing treatment-related changes. Third, the dependent variable in the prediction models—PARS score—which reflects general anxiety burden could relate to a clinician’s decision to titrate escitalopram from 15 to 20 mg at week 4 or 6. However, the clinician’s decision to titrate escitalopram at week 4 or 6 is unlikely to correlate with the change in FC during the first two weeks of treatment. In other words, if Y represents the change in PARS score from baseline to endpoint, X represents the change in FC during the first two weeks of treatment and Z represents a clinician’s decision to titrate at week 4 or 6, then if X and Z do not correlate, the statistical relationship between Y and X is unaffected by the presence or exclusion of Z in the model. Thus, omitted variable bias related to a decision to titrate dose at week 4 or 6 is unlikely. Fourth, the stereotactic positions of amygdala subfields vary across patients and template-based approaches to measure amygdala sub-regional connectivity may lack precision and affect generalizability, although we would point out that amygdala sub-regional FC patterns match known subfield anatomic connectivity patterns. Finally, the resolution of functional images, compared to anatomical images, may limit precision of the segmentation of amygdala subfields and then result in imprecise connectivity assessment; however, this approach has been used in prior pediatric studies.58 The significant result in our study comes from the left BLA, the largest subfield of amygdala, which has 85 voxels with voxel size of 3×3×3 mm3. Moreover, our BLA-VLPFC connectivity map overlapped with our amygdala-VLPFC connectivity map.
In summary, escitalopram increases amygdala-VLPFC connectivity during the first 2 weeks of treatment, which is driven by changes in FC with the basolateral amygdala. The magnitude of these changes predict subsequent reduction of anxiety in adolescents with GAD. These findings reveal a potential neural systems mechanism of escitalopram efficacy in GAD, providing a target engagement model for novel medications and a potential early means to identify SSRI responders. Finally, this study provides a preliminary rationale for future studies of acute neurofunctional changes to guide treatment selection in youth with anxiety disorders, and perhaps, those with other internalizing disorders.
Acknowledgements:
The authors thank the patients and their families for participating in this study and the Data Safety Monitoring Board for their oversight of the study. Additionally, we thank the MR technologists and pediatric radiologists from the Imaging Research Center at Cincinnati Children’s Hospital Medical Center.
Funding: This work was supported by the National Institute of Mental Health (K23 MH106037, JRS), the National Institute of Child Health and Development (R01 HD098757, JRS/AL), the National Institute of Environmental Health Sciences (R01 ES027224, KMC), and the National Natural Science Foundation (Grant No. 81621003 and No. 81820108018, JAS/QG/LL; Grant No. 8202780056, XH/QG/LL)
Disclosures: Dr. Lu received the Chinese Government Scholarship. Dr. Mills has received research support from the Yung Family Foundation. Drs. Huang and Gong received research support from the Functional and Molecular Imaging Key Laboratory of Sichuan Province (FMIKLSP) and Psychoradiology Research Unit of Chinese Academy of Science, China. Dr. Ramsey has received research support from BTG, International Inc. Dr. DelBello receives research support from NIH, PCORI, Acadia, Allergan, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, and Sunovion. She is also a consultant, on the advisory board, or has received honoraria for speaking for Alkermes, Allergan, Assurex, CMEology, Janssen, Johnson and Johnson, Lundbeck, Myriad, Neuronetics, Otsuka, Pfizer, Sunovion, and Supernus. Dr. Strawn has received research support from Allergan, Neuronetics, Lundbeck, Otsuka and the National Institutes of Health. He receives royalties from Springer Publishing for two texts and has received material support from Myriad. The remaining authors have nothing to disclose.
Footnotes
Experts: Dr. Mills served as the statistical expert for this research.
Contributor Information
Lu Lu, West China Hospital of Sichuan University, Chengdu, China.; University of Cincinnati, Cincinnati, Ohio.
Jeffrey A. Mills, University of Cincinnati, Cincinnati, Ohio..
Hailong Li, West China Hospital of Sichuan University, Chengdu, China..
Heidi K. Schroeder, University of Cincinnati, Cincinnati, Ohio..
Sarah A. Mossman, University of Cincinnati, Cincinnati, Ohio..
Sara T. Varney, University of Cincinnati, Cincinnati, Ohio..
Kim M. Cecil, University of Cincinnati, Cincinnati, Ohio..
Xiaoqi Huang, West China Hospital of Sichuan University, Chengdu, China..
Qiyong Gong, West China Hospital of Sichuan University, Chengdu, China..
Laura B. Ramsey, University of Cincinnati, Cincinnati, Ohio..
Melissa P. DelBello, University of Cincinnati, Cincinnati, Ohio..
John A. Sweeney, West China Hospital of Sichuan University, Chengdu, China.; University of Cincinnati, Cincinnati, Ohio.
Jeffrey R. Strawn, University of Cincinnati, Cincinnati, Ohio..
REFERENCES
- 1.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA. Efficacy and Tolerability of Antidepressants in Pediatric Anxiety Disorders: A Systematic Review and Meta-Analysis. Depress Anxiety. 2015;32(3):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strawn JR, Dobson ET, Mills JA, et al. Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. J Child Adolesc Psychopharmacol. 2017;27(6):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med. 2001;344(17):1279–1285. [DOI] [PubMed] [Google Scholar]
- 5.McClure EB, Adler A, Monk CS, et al. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl). 2007;191(1):97–105. [DOI] [PubMed] [Google Scholar]
- 6.Maslowsky J, Mogg K, Bradley BP, et al. A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adolesc Psychopharmacol. 2010;20(2):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlop K, Rizvi SJ, Kennedy SH, et al. Clinical, behavioral, and neural measures of reward processing correlate with escitalopram response in depression: a Canadian Biomarker Integration Network in Depression (CAN-BIND-1) Report. Neuropsychopharmacology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorka SM, Young CB, Klumpp H, et al. Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: a randomized trial. Neuropsychopharmacology. 2019;44(9):1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gingnell M, Frick A, Engman J, et al. Combining escitalopram and cognitive-behavioural therapy for social anxiety disorder: randomised controlled fMRI trial. Br J Psychiatry. 2016;209(3):229–235. [DOI] [PubMed] [Google Scholar]
- 10.Faria V, Gingnell M, Hoppe JM, et al. Do You Believe It? Verbal Suggestions Influence the Clinical and Neural Effects of Escitalopram in Social Anxiety Disorder: A Randomized Trial. Ebiomedicine. 2017;24:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whalen PJ, Johnstone T, Somerville LH, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63(9):858–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP. Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. Int J Neuropsychopharmacol. 2009;12(8):1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windischberger C, Lanzenberger R, Holik A, et al. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2010;49(2):1161–1170. [DOI] [PubMed] [Google Scholar]
- 14.Burkhouse KL, Kujawa A, Hosseini B, et al. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Prog Neuro-Psychoph. 2018;84:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugo-Candelas C, Cha J, Hong SS, et al. Associations Between Brain Structure and Connectivity in Infants and Exposure to Selective Serotonin Reuptake Inhibitors During Pregnancy. Jama Pediatr. 2018;172(6):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carceller H, Perez-Rando M, Castren E, Nacher J, Guirado R. Effects of the Antidepressant Fluoxetine on the Somatostatin Interneurons in the Basolateral Amygdala. Neuroscience. 2018;386:205–213. [DOI] [PubMed] [Google Scholar]
- 17.Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research Review: Pediatric anxiety disorders - what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginsburg GS, Kendall PC, Sakolsky D, et al. Remission After Acute Treatment in Children and Adolescents With Anxiety Disorders: Findings From the CAMS. J Consult Clin Psych. 2011;79(6):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compton SN, Peris TS, Almirall D, et al. Predictors and Moderators of Treatment Response in Childhood Anxiety Disorders: Results From the CAMS Trial. J Consult Clin Psych. 2014;82(2):212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poweleit EA, Aldrich SL, Martin LJ, Hahn D, Strawn JR, Ramsey LB. Pharmacogenetics of Sertraline Tolerability and Response in Pediatric Anxiety and Depressive Disorders. J Child Adol Psychop. 2019;29(5):348–361. [DOI] [PubMed] [Google Scholar]
- 21.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders. Front Pharmacol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujawa A, Swain JE, Hanna GL, et al. Prefrontal Reactivity to Social Signals of Threat as a Predictor of Treatment Response in Anxious Youth. Neuropsychopharmacology. 2016;41(8):1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick A, Engman J, Wahistedt K, Gingnell M, Fredrikson M, Furmark T. Anterior cingulate cortex activity as a candidate biomarker for treatment selection in social anxiety disorder. Bjpsych Open. 2018;4(3):157–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frick A, Engman J, Alaie I, et al. Neuroimaging, genetic, clinical, and demographic predictors of treatment response in patients with social anxiety disorder. J Affect Disorders. 2020;261:230–237. [DOI] [PubMed] [Google Scholar]
- 25.Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy AK, Fudge JL, Kelly C, et al. Intrinsic Functional Connectivity of Amygdala-Based Networks in Adolescent Generalized Anxiety Disorder. J Am Acad Child Psy. 2013;52(3):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research Review: Pediatric anxiety disorders - what have we learnt in the last 10 years? J Child Psychol Psyc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossman SA, Luft MJ, Schroeder HK, et al. The Generalized Anxiety Disorder 7-item scale in adolescents with generalized anxiety disorder: Signal detection and validation. Ann Clin Psychiatry. 2017;29(4):227–234A. [PMC free article] [PubMed] [Google Scholar]
- 29.Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339–351. [DOI] [PubMed] [Google Scholar]
- 30.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210(5–6):343–352. [DOI] [PubMed] [Google Scholar]
- 31.Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in Adolescents With Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J Clin Psychiatry. 2020;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiat. 2007;164(10):1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiat. 2008;65(5):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu WJ, Yin DZ, Cheng WH, et al. Abnormal Functional Connectivity of the Amygdala-Based Network in Resting-State fMRI in Adolescents with Generalized Anxiety Disorder. Med Sci Monitor. 2015;21:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiat. 2006;163(6):1091–1097. [DOI] [PubMed] [Google Scholar]
- 37.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiat. 2003;53(6):494–501. [DOI] [PubMed] [Google Scholar]
- 38.Zhao ZY, Yao SX, Li KS, et al. Real-Time Functional Connectivity-Informed Neurofeedback of Amygdala-Frontal Pathways Reduces Anxiety. Psychother Psychosom. 2019;88(1):5–15. [DOI] [PubMed] [Google Scholar]
- 39.Pitkänen A Connectivity of the rat amygdaloid complex. New York: Oxford University Press; 2000. [Google Scholar]
- 40.Bocchio M, McHugh SB, Bannerman DM, Sharp T, Capogna M. Serotonin, Amygdala and Fear: Assembling the Puzzle. Front Neural Circuits. 2016;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo JD, O’Flaherty BM, Rainnie DG. Serotonin gating of cortical and thalamic glutamate inputs onto principal neurons of the basolateral amygdala. Neuropharmacology. 2017;126:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Liu Y, Xiong J, et al. Reduced serotonin impairs long-term depression in basolateral amygdala complex and causes anxiety-like behaviors in a mouse model of perimenopause. Exp Neurol. 2019;321:113030. [DOI] [PubMed] [Google Scholar]
- 43.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167(5):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. [DOI] [PubMed] [Google Scholar]
- 45.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb Cortex. 2011;21(7):1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toazza R, Franco AR, Buchweitz A, et al. Amygdala-based intrinsic functional connectivity and anxiety disorders in adolescents and young adults. Psychiat Res-Neuroim. 2016;257:11–16. [DOI] [PubMed] [Google Scholar]
- 47.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted Amygdalar Subregion Functional Connectivity and Evidence of a Compensatory Network in Generalized Anxiety Disorder. Arch Gen Psychiat. 2009;66(12):1361–1372. [DOI] [PubMed] [Google Scholar]
- 48.Sylvester CM, Yu Q, Srivastava AB, et al. Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proc Natl Acad Sci U S A. 2020;117(7):3808–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An Investigation of the Structural, Connectional, and Functional Subspecialization in the Human Amygdala. Hum Brain Mapp. 2013;34(12):3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grijalva CV, Levin ED, Morgan M, Roland B, Martin FC. Contrasting Effects of Centromedial and Basolateral Amygdaloid-Lesions on Stress-Related Responses in the Rat. Physiol Behav. 1990;48(4):495–500. [DOI] [PubMed] [Google Scholar]
- 51.Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews SC, Simmons AN, Strigo IA, Arce E, Stein MB, Paulus MP. Escitalopram attenuates posterior cingulate activity during self-evaluation in healthy volunteers. Psychiatry Res. 2010;182(2):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. J Child Psychol Psyc. 2017;58(5):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godlewska BR, Harmer CJ. Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment. Psychopharmacology (Berl). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luft MJ, Lamy M, DelBello MP, McNamara RK, Strawn JR. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills JA, Strawn JR. Antidepressant Tolerability in Pediatric Anxiety and Obsessive-Compulsive Disorders: A Bayesian Hierarchical Modeling Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in Adolescents With Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J Clin Psychiatry. 2020;81(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A. 2012;109(20):7941–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]


