Key Points
Question
In patients prescribed stable, long-term, high-dose opioid therapy, is dose tapering associated with an increased risk of overdose or mental health crisis?
Findings
In this retrospective cohort study that included 113 618 patients prescribed stable, high-dose opioid therapy, patients in periods following dose tapering, compared with patients before or without tapering, had an adjusted incidence rate ratio of 1.68 for overdose and 2.28 for mental health crisis; both risks were statistically significant.
Meaning
Opioid dose tapering was associated with increased risk for overdose and mental health crisis, but interpretation of these findings is limited by the study design.
Abstract
Importance
Opioid-related mortality and national prescribing guidelines have led to tapering of doses among patients prescribed long-term opioid therapy for chronic pain. There is limited information about risks related to tapering, including overdose and mental health crisis.
Objective
To assess whether there are associations between opioid dose tapering and rates of overdose and mental health crisis among patients prescribed stable, long-term, higher-dose opioids.
Design, Setting, and Participants
Retrospective cohort study using deidentified medical and pharmacy claims and enrollment data from the OptumLabs Data Warehouse from 2008 to 2019. Adults in the US prescribed stable higher doses (mean ≥50 morphine milligram equivalents/d) of opioids for a 12-month baseline period with at least 2 months of follow-up were eligible for inclusion.
Exposures
Opioid tapering, defined as at least 15% relative reduction in mean daily dose during any of 6 overlapping 60-day windows within a 7-month follow-up period. Maximum monthly dose reduction velocity was computed during the same period.
Main Outcomes and Measures
Emergency or hospital encounters for (1) drug overdose or withdrawal and (2) mental health crisis (depression, anxiety, suicide attempt) during up to 12 months of follow-up. Discrete time negative binomial regression models estimated adjusted incidence rate ratios (aIRRs) of outcomes as a function of tapering (vs no tapering) and dose reduction velocity.
Results
The final cohort included 113 618 patients after 203 920 stable baseline periods. Among the patients who underwent dose tapering, 54.3% were women (vs 53.2% among those who did not undergo dose tapering), the mean age was 57.7 years (vs 58.3 years), and 38.8% were commercially insured (vs 41.9%). Posttapering patient periods were associated with an adjusted incidence rate of 9.3 overdose events per 100 person-years compared with 5.5 events per 100 person-years in nontapered periods (adjusted incidence rate difference, 3.8 per 100 person-years [95% CI, 3.0-4.6]; aIRR, 1.68 [95% CI, 1.53-1.85]). Tapering was associated with an adjusted incidence rate of 7.6 mental health crisis events per 100 person-years compared with 3.3 events per 100 person-years among nontapered periods (adjusted incidence rate difference, 4.3 per 100 person-years [95% CI, 3.2-5.3]; aIRR, 2.28 [95% CI, 1.96-2.65]). Increasing maximum monthly dose reduction velocity by 10% was associated with an aIRR of 1.09 for overdose (95% CI, 1.07-1.11) and of 1.18 for mental health crisis (95% CI, 1.14-1.21).
Conclusions and Relevance
Among patients prescribed stable, long-term, higher-dose opioid therapy, tapering events were significantly associated with increased risk of overdose and mental health crisis. Although these findings raise questions about potential harms of tapering, interpretation is limited by the observational study design.
This study examines whether there are associations between opioid dose tapering and subsequent rates of overdose and mental health crisis among patients prescribed stable, long-term, high-dose opioids.
Introduction
Amidst the ongoing US national crisis of opioid-related mortality and morbidity, heightened scrutiny and shifts in opioid prescribing trends have occurred in the US.1,2,3 Key guidelines released in 2016 by the Centers for Disease Control and Prevention (CDC)4 recommended against higher doses of opioids in managing chronic pain and recommended dose tapering when harms of continued therapy outweigh perceived benefits for individual patients. These and other widely disseminated recommendations have led to increased opioid tapering among patients prescribed long-term opioid therapy.5,6 However, opioid-related mortality has continued to rise.7
Subsequent US recommendations have advised caution in opioid de-prescribing.8,9 Studies suggest risks of suicidal ideation, transition to illicit opioids, and overdose after opioid tapering and discontinuation. The US Food and Drug Administration issued a prescriber warning about potential hazards of rapid dose reduction in patients prescribed long-term opioids.9 However, studies assessing harms of opioid dose reduction have been limited to smaller samples,10 veteran populations,11 or specific regions12 or have focused on discontinuation and not included sensitive indicators for tapering initiation.11,13,14 As clinicians and patients face difficult decisions about whether and how to de-prescribe opioids,15 there is a need to elucidate the potential harms of stopping or decreasing these medications.
It was hypothesized that tapering the dose of patients receiving stable, long-term, high-dose opioid therapy would be associated with increased risk for specific adverse events. Dose disruption among this population might trigger unhealthy substance use, withdrawal, depression, or anxiety, leading to increased acute care events related to drug toxicity and mental health. The objective of this study was to assess whether there are associations between opioid dose tapering among patients prescribed stable, long-term, higher-dose opioids and subsequent rates of overdose and mental health crisis. The potential risks associated with faster rate of opioid dose reduction were also assessed.
Methods
Study Data, Setting, and Participants
This retrospective cohort study used administrative claims data from the OptumLabs Data Warehouse from 2007 to 2019. The database contains deidentified retrospective administrative data, including medical and pharmacy claims (with associated diagnosis codes) and eligibility information as well as electronic health data for commercial and Medicare Advantage enrollees. The database contains longitudinal health information on patients representing a diverse mix of ages, ethnicities, and US geographical regions (OptumLabs and OptumLabs Data Warehouse Descriptions and Citation. OptumLabs; 2020). The Institutional Review Board of the University of California determined this study to not be human subjects research because it involved analysis of preexisting, deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
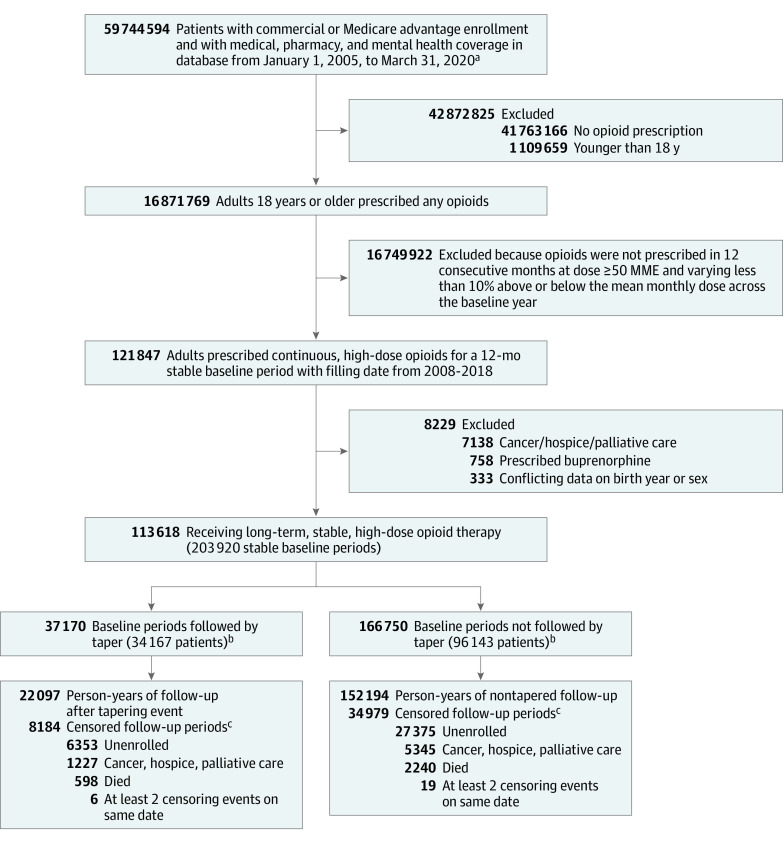
We identified a cohort of patients prescribed stable, high doses of opioids for at least 12 months. Patients 18 years or older at the time of receiving an opioid prescription between January 1, 2008, and December 31, 2019, were eligible for inclusion if they had at least 14 months of continuous enrollment in medical, pharmacy, and mental health coverage and 12 months of continuous opioid prescriptions (operationalized as ≥90% of days filled), with mean daily dose of at least 50 morphine milligram equivalents (MME) that varied by no more than 10% above or below the mean monthly dose across the baseline year. We required at least 14 months of enrollment to allow for establishment of the stable baseline period and at least 2 months of follow-up to observe for tapering. We excluded patients with cancer, those receiving hospice or palliative care, and those prescribed buprenorphine (Figure 1).
Figure 1. Selection and Inclusion of Patients in a Study of the Association of Dose Tapering With Overdose or Mental Health Crisis Among Patients Prescribed Long-term Opioids.
aInitial date range chosen to allow adequate buffering on either end of study period (January 1, 2007, through December 31, 2019).
bA total of 34 167 patients had at least 1 baseline period followed by a taper event, of whom 29 101 had a tapering event following their most recent stable baseline period. Similarly, 96 143 patients contributed a baseline period that was not followed by a tapering event, of whom 84 517 patients did not undergo tapering following their most recent stable baseline period, as reflected in Table 1. In analyses, patients who underwent tapering contributed pretaper follow-up time to the nontapered group.
cCensoring events occurred in 8184 of 37 170 (22.0%) follow-up periods after tapering events and 34 979 of 166 750 (21.0%) follow-up periods when no tapering event was identified.
Beginning the first day after the end of the baseline year of stable dosing, patients were followed up for up to 1 year for outcome events. Patients were censored if they died; their enrollment was disrupted; they developed a new diagnosis of cancer; or they entered hospice, palliative, or skilled nursing care for at least 90 days. We also censored patients during the first 7 months of follow-up if their mean daily dose increased by greater than or equal to 15% above their baseline dose and they had not previously initiated a taper; this censoring rule was adopted to allow comparison of outcomes among patients with continued opioid dose stability and those who underwent dose tapering. The study design allowed individual patients to contribute multiple baseline and follow-up periods during the study period, and the analysis plan accounted for time-varying covariates and variable follow-up duration.
Identifying Opioid Tapering
Following the 12-month baseline period, we computed 60-day moving mean daily doses during 6 overlapping windows spanning the 7 months following the baseline period. A taper was identified if the mean daily dose during one of these periods was greater than or equal to 15% below the mean daily dose in the baseline period. Tapering status was defined as a binary time-varying event history variable, specified as nontapered during all months prior to and during the 60-day period in which tapering was identified and tapered during all subsequent study months (eFigure 1 in the Supplement).5 The sensitivity and specificity of this measure has not been determined. However, the predictive validity of the measure was suggested by a longitudinal study in which 69.8% of patients identified as undergoing dose tapering showed sustained relative dose reduction of greater than or equal to 15% at least 9 months after tapering was identified.16
Dose Reduction Velocity
For all patients in the cohort, we used dosing data from the initial 6 overlapping 60-day periods of follow-up to identify the fastest monthly rate of dose reduction (velocity) occurring prior to the observation month. Detailed methods for specifying dose reduction velocities are included in the eMethods in the Supplement. For each follow-up month, we identified the maximum velocity of dose reduction that occurred in previous dosing intervals, and maximum velocity of dose reduction was a time-varying variable. Based on descriptive and graphical analyses of the distribution of tapering velocities, maximum velocity of dose reduction was then categorized as less than 10%, 10% to 19.9%, 20% to 49.9%, or at least 50%.
Outcome Variables
After each stable baseline period, we examined medical claims during the following 12 months to identify monthly (30-day) counts of 2 coprimary end points.
Overdose or withdrawal events were defined as emergency department visits or inpatient hospital admissions for any drug overdose, alcohol intoxication, or drug withdrawal. We identified International Classification of Diseases, Clinical Modification, Ninth Revision and International Classification of Diseases, Clinical Modification, Tenth Revision codes for this outcome by augmenting the definition for “all-drug overdose” specified in CDC drug overdose surveillance guidelines,17 with additional codes for alcohol intoxication and alcohol or drug withdrawal. In sensitivity analyses, we also assessed the “all-drug overdose,” as specified in the CDC guidance (without additional alcohol or withdrawal codes), and “opioid overdose.” In validation studies based on medical record reviews, diagnostic codes for opioid overdoses identified emergency or hospital events for opioid overdose with positive predictive values ranging from 67% to 84%.18,19
Mental health crisis events were defined as emergency department or inpatient hospital admissions with depression or anxiety diagnosis codes in the primary diagnosis position (to make the outcome more specific to acute episodes of depression or anxiety rather than prevalent depressive/anxiety disorders coded during an episode for a different medical issue) or suicide attempt or intentional self-harm in any diagnosis position. In addition to the main analysis using the composite outcome, we also examined each mental health crisis outcome separately (depression, anxiety, and suicide attempt). In a systematic review and validation study, diagnostic codes for depression had sensitivities of 29% to 36%, specificities of approximately 99%, and positive predictive values of approximately 90%.20 In a systematic review, diagnostic codes for suicide attempts or intention had positive predictive values between 55% and 100%.21 The list of diagnostic codes used to identify study outcomes is included in eTable 1 in the Supplement.
Covariates
We included covariates that might contribute to differences in rates of the outcomes of interest in populations prescribed opioids. Sociodemographic information included age, sex, education status, rurality of home address (dichotomized as metropolitan/micropolitan vs small town/rural using rural-urban commuting area codes 1-6 vs 7-10),22 and insurance status (commercial vs Medicare Advantage). Age was categorized as 18 to 34, 35 to 49, 50 to 64, and 65 years or older. Education was categorized based on median household education level for the patient’s zip code derived from US census data. A valid race variable was not available in the data set.
We included key clinical factors as covariates. Baseline opioid dose was calculated using pharmacy claims for all opioids during the baseline period and categorized as 50 to 89, 90 to 149, 150 to 299, and greater than or equal to 300 MME per day. To account for the increased risk of adverse outcomes conferred by co-prescribing benzodiazepines and opioids, we included a variable for whether the patient was concurrently prescribed a benzodiazepine (identified by National Drug Codes) on the final day of the baseline period. We included a count variable for the number of overdose events in the baseline year, specified by the same criteria as the overdose outcome variable. Baseline depression or anxiety was identified by either claims diagnoses23 or 1 or more pharmacy claim for a selective serotonin reuptake inhibitor prescription during the baseline year. Comorbidities were accounted for using 27 discrete indicator variables for noncancer conditions in the Elixhauser comorbidity index, which includes variables labeled drug abuse and alcohol abuse (hereafter referred to as drug use disorder and alcohol use disorder), as well as psychosis.24 Year of cohort entry was also included.
Statistical Analysis
Analyses were conducted using Stata MP, version 15.1 (StataCorp). We performed descriptive analyses to characterize the study population at baseline and identify bivariate differences between the tapered and nontapered samples.
We performed 2 key sets of analyses. First, we compared tapered and nontapered patient months and their associated risk of overdose and mental health crisis. To account for potential overdispersion of study outcomes, we used negative binomial regression to estimate adjusted incidence rates and incidence rate ratios (IRRs) for the 2 outcomes. We accounted for variable follow-up time by using a discrete time regression framework, with follow-up months nested in patients with eligible baseline periods. In adjusted analyses, models included the time-varying taper history variable and fixed patient-level covariates, including age, sex, education status, rurality, insurance status, Elixhauser comorbidities, baseline opioid dose, co-prescription of benzodiazepines, baseline overdose events, baseline depression/anxiety, and study year. In main analyses, a missing category was included for education (missing for 5.6% of patients), while the small percentage of patients with missing rurality data (0.2%) were grouped with the “small town/rural” category. We used postestimation commands to estimate adjusted incidence rates by tapering status. We used the same analytic approach in modeling associations between tapering and alternative specifications of the count outcomes (ie, all drug overdose; opioid-only overdose; or depression, anxiety, or suicide events). All analyses used cluster robust standard errors to account for clustering of multiple eligible baseline periods in individual patients. All tests were 2-sided with significance level of α = .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
To examine whether tapering associations varied by covariates that we expected to be associated with overdose or mental health crisis, we assessed for meaningful 2-way interactions between tapering status and key covariates using Akaike’s information criterion and χ2 tests for the significance of interaction terms. Statistically significant interaction effects were displayed graphically.
In the second set of analyses, we used negative binomial regression to examine associations between maximum dose reduction velocity and overdose and mental health outcomes. We included the time-varying independent variable maximum velocity of dose reduction first as a continuous measure and then as categorized above. These models adjusted for the same covariates described above. Regression analyses of maximum velocity of dose reduction only included patients who accrued follow-up time in month 3 of follow-up or later, which was the first month when maximum velocity of dose reduction could be defined based entirely on prior dosing. Thus, 4395 patients were not included in maximum velocity of dose reduction analyses due to early censoring events (3.9% of total).
We conducted additional sensitivity analyses, as described in the eMethods in the Supplement. Briefly, these analyses assessed the effect of alternative specifications of the study outcomes or the tapering measure. For example, to account for the potential bias that could have arisen from censoring due to death, we repeated main analyses wherein death was counted as an overdose or a mental health crisis event in the respective models. In addition, to account for the potential endogeneity arising from a patient’s propensity to undergo dose tapering, we conducted analyses with inverse probability weighting by a propensity score for the likelihood of undergoing tapering.
Results
The study cohort consisted of 113 618 patients prescribed stable, long-term, higher-dose opioid therapy for at least 12 months who contributed a total of 203 920 baseline periods (mean per patient, 1.8; median per patient, 1.0). Among the patients who underwent dose tapering, 54.3% were women (vs 53.2% among those who did not undergo dose tapering), the mean age was 57.7 years (vs 58.3 years), and 38.8% were commercially insured (vs 41.9%). A total of 18.2% of baseline periods were followed by tapering (37 170 tapering events), of which 7620 (20.5%) were discontinued at some point during follow-up (eg, prescribed 0 MME during a 60-day dosing period). The median (interquartile range) maximum velocity of dose reduction was 22.7% (15.0%-41.3%) per month for tapered periods and 3.2% (1.7%-5.9%) per month without tapering.
Table 1 compares the baseline characteristics of patients by tapering status during their most recent baseline period. Patients who underwent tapering had significantly higher baseline opioid doses; were more likely to be co-prescribed benzodiazepines; and had significantly higher baseline rates of overdose, drug use disorder, depression, and anxiety.
Table 1. Baseline Characteristics of Patients in a Study of the Association of Dose Tapering With Overdose or Mental Health Crisis Among Patients Prescribed Long-term Opioidsa.
| Characteristic | No. (%) | |
|---|---|---|
| Taper | No taper | |
| Total patients | 29 101 (25.6) | 84 517 (74.4) |
| Age category, y | ||
| 18-<35 | 873 (3.0) | 2292 (2.7) |
| 35-<50 | 5687 (19.5) | 16 268 (19.2) |
| 50-<65 | 14 704 (50.5) | 41 530 (49.1) |
| ≥65 | 7837 (26.9) | 24 427 (28.9) |
| Women | 15 805 (54.3) | 44 967 (53.2) |
| Men | 13 296 (45.7) | 39 550 (46.8) |
| Educationb | 27 281 | 79 970 |
| <12th grade | 130 (0.5) | 399 (0.5) |
| High school diploma | 12 540 (46.0) | 36 687 (45.9) |
| <Bachelor’s degree | 12 982 (47.6) | 37 881 (47.4) |
| ≥Bachelor’s degree | 1629 (6.0) | 5003 (6.3) |
| Rural vs urbanc | 29 030 | 84 411 |
| Metropolitian | 24 096 (83.0) | 70 601 (83.6) |
| Micropolitian | 2856 (9.8) | 7970 (9.4) |
| Small town | 1361 (4.7) | 3846 (4.6) |
| Rural | 717 (2.5) | 1994 (2.4) |
| Commercial insurance (vs Medicare Advantage) | 11 288 (38.8) | 35 372 (41.9) |
| Elixhauser comorbiditiesd | ||
| Alcohol use disorder | 798 (2.7) | 2009 (2.4) |
| Drug use disorder | 4856 (16.7) | 11 500 (13.6) |
| Psychosis | 2784 (9.6) | 7717 (9.1) |
| Baseline opioid dose, MME/d | ||
| 50-<90 | 8015 (27.5) | 33 684 (39.9) |
| 90-<150 | 7503 (25.8) | 22 121 (26.2) |
| 150-<300 | 8561 (29.4) | 19 345 (22.9) |
| ≥300 | 5022 (17.3) | 9367 (11.1) |
| Co-prescribed benzodiazepinee | 8613 (29.6) | 23 208 (27.5) |
| Baseline year overdose eventsf | ||
| 0 | 27 908 (95.9) | 81 945 (97.0) |
| 1 | 780 (2.7) | 1776 (2.1) |
| 2 | 168 (0.6) | 344 (0.4) |
| ≥3 | 245 (0.8) | 452 (0.5) |
| Baseline depression/anxietyg | 16 141 (55.5) | 44 464 (52.6) |
Abbreviation: MME, morphine milligram equivalents.
For patients contributing multiple baseline periods, information was captured at the end of their most recent baseline period.
Education is an estimate based on median household education level for the patient’s zip code derived from US census data; variable missing for 6367 (5.6%) patients, including 1820 (6.3%) who underwent tapering and 4547 (5.4%) who did not taper after their most recent baseline periods.
Rurality derived from rural-urban commuting area codes; variable missing for 177 patients (0.2% of total), including 71 (0.2%) and 106 (0.1%) patients who did and did not taper after their most recent baseline periods.
Elixhauser comorbidities included 27 noncancer conditions, including alcohol use disorder, drug use disorder, and psychoses. The depression Elixhauser indicator was not included due to redundancy with the “preexisting depression/anxiety” variable.
Co-prescribed benzodiazepine was defined as a concurrent benzodiazepine prescription on the date of cohort entry.
Baseline overdose events defined by specified diagnosis codes identified on emergency department or hospital claims in baseline year.
Baseline depression/anxiety defined by specified diagnoses identified on emergency department, hospital, or outpatient claims, or pharmacy claims for selective serotonin reuptake inhibitor, during baseline year. All differences were statistically significant with P value <.05.
Table 2 shows the adjusted incidence rates for the study outcomes by tapering status. Posttapering patient periods were associated with an adjusted incidence rate of 9.3 overdose events per 100 person-years compared with 5.5 events per 100 person-years in nontapered periods (adjusted incidence rate difference [aIRD], 3.8 per 100 person-years [95% CI, 3.0-4.6]; adjusted incidence rate ratio [aIRR], 1.68 [95% CI, 1.53-1.85]). Tapering was associated with an adjusted incidence rate of 7.6 mental health crisis events per 100 person-years compared with 3.3 events per 100 person-years among nontapered periods (aIRD, 4.3 per 100 person-years [95% CI, 3.2-5.3]; aIRR, 2.28 [95% CI, 1.96-2.65]).
Table 2. Primary and Secondary Outcomes in a Study of the Association of Dose Tapering With Overdose or Mental Health Crisis Among Patients Prescribed Long-term Opioidsa.
| Outcome | Taperedb | Not taperedb | Adjusted incidence rate difference per 100 person-years by tapering status (95% CI)d,e | Adjusted incidence rate ratio (95% CI)d | P valuec | ||
|---|---|---|---|---|---|---|---|
| No. of events/total person-years | Adjusted incidence rate per 100 person-years (95% CI)d | No. of events/total person-years | Adjusted incidence rate per 100 person-years (95% CI)d | ||||
| Overdosef | |||||||
| Entire cohort | 3241/22 097 | 9.3 (8.5-10.1) | 11 433/152 194 | 5.5 (5.3-5.8) | 3.8 (3.0-4.6) | 1.68 (1.53-1.85) | <.001 |
| Baseline opioid dose, MME/dg | .008 | ||||||
| 50-89 | 530/5321 | 6.6 (5.3-7.9) | 3277/53 260 | 4.6 (4.2-5.0) | 2.0 (0.3-3.3) | 1.43 (1.15-1.77) | |
| 90-149 | 676/5524 | 7.7 (6.4-9.1) | 2607/38 994 | 5.1 (4.6-5.5) | 2.6 (1.2-4.1) | 1.67 (1.34-2.00) | |
| 150-299 | 1047/6864 | 10.9 (9.2-12.7) | 3462/38 782 | 6.5 (6.0-7.0) | 4.4 (2.6-6.2) | 2.36 (1.93-2.79) | |
| ≥300 | 988/4388 | 16.2 (13.9-18.4) | 2087/21 159 | 7.4 (6.6-8.2) | 8.8 (6.4-11.1) | 3.51 (2.94-4.09) | |
| Mental health crisisi | |||||||
| Entire cohort | 3117/22 097 | 7.6 (6.5-8.6) | 8258/152 194 | 3.3 (3.0-3.6) | 4.3 (3.2-5.3) | 2.28 (1.96-2.65) | <.001 |
| Baseline opioid dose, MME/dg | .003 | ||||||
| 50-89 | 525/5321 | 5.2 (3.8-6.5) | 2801/53 260 | 3.4 (3.0-3.9 | 1.8 (0.4-3.2) | 1.51 (1.14-2.01) | |
| 90-149 | 615/5524 | 5.8 (4.3-7.4) | 2045/38 994 | 3.2 (2.7-3.7) | 2.6 (1.0-4.2) | 1.70 (1.21-2.19) | |
| 150-299 | 1080/6864 | 8.7(6.6-10.9) | 2051/38 782 | 3.0 (26-3.5) | 5.7 (3.5-7.8) | 2.54 (1.84-3.24) | |
| ≥300 | 897/4388 | 11.9 (8.8-15.0) | 1361/21 159 | 3.8 (3.0-4.7) | 8.1 (4.9-11.3) | 3.47 (2.45-4.49) | |
| Secondary mental health end pointsh | |||||||
| Depression | 2485/22 097 | 5.5 (4.6-6.4) | 6174/152 194 | 2.2 (2.0-5) | 3.3 (2.4-4.2) | 2.46 (2.05-2.96) | <.001 |
| Anxiety | 505/22 097 | 1.4 (12-1.7) | 1737/152 194 | 0.8 (0.7-0.9) | 0.6 (0.4-0.9) | 1.79 (1.48-2.15) | <.001 |
| Suicide attempt | 127/22 097 | 0.4 (0.2-0.5) | 347/152 194 | 0.1 (0.08-0.13) | 0.3 (0.1-0.4) | 3.30 (2.19-4.98) | <.001 |
A total of 113 618 patients were observed for 174 291 person-years.
Tapering status at the time of an event was defined as tapered for all months after the first of 6 overlapping 60-day period in which a person’s mean daily opioid dose decreased by 15% or more from their baseline period and nontapered for all other periods.
P value refers to the adjusted incidence rate ratio in the whole cohort and the χ2 for the likelihood ratio test for the interaction by baseline dose.
Analyses adjusted for age; sex; education status; rurality of home address; insurance status; comorbidities; baseline opioid dose; co-prescription for benzodiazepines; number of overdose events in the baseline period; baseline depression, anxiety, or suicide attempt; and study year.
Adjusted rate differences and 95% CIs are within-row group rate differences by tapering status.
Overdose refers to the composite outcome for emergency department visits or hospital admissions for drug overdose or withdrawal.
Baseline dose is the average daily opioid dose, in morphine milliequivalents (MME), across the 12-month stable baseline period.
Secondary end points were specified for each of the 3 components.
Mental health crisis refers to the composite outcome for emergency department visits or hospital admissions for depression, anxiety, or suicide attempt.
Among patients who underwent tapering and had 1 or more outcome events during follow-up, the median time to first event was 6 months for both outcomes. In secondary analyses of the individual components of the mental health crisis outcome (depression, anxiety, and suicide attempt), tapering was associated with depression events (aIRR, 2.46 [95% CI, 2.05-2.96]), anxiety events (aIRR, 1.79 [95% CI, 1.48-2.15]), and suicide attempts (aIRR, 3.30 [95% CI, 2.19-4.98]). The full regression model for the analysis of main effects is provided in eTable 2 in the Supplement.
In analyses with interaction terms between tapering status and other key covariates, only interactions between baseline dose category and tapering status were found to be both informative (based on reductions in Akaike’s information criterion) and statistically significant, based on χ2 tests of significance of the dose category × tapering interaction terms (P < .01). For both outcomes, patients prescribed higher baseline doses had greater risks associated with tapering than patients prescribed lower baseline doses (Table 2; eFigure 2 in the Supplement).
In analyses of maximum monthly dose reduction velocity (n = 109 599 patients followed up for 139 941 person-years), an incremental increase in maximum monthly dose reduction velocity of 10% was associated with an increased aIRR for overdose (1.09 [95% CI, 1.07-1.11]) and mental health crisis (1.18 [95% CI, 1.14-1.21]) (eTable 3 in the Supplement). Higher maximum monthly dose reduction velocity categories (compared with maximum monthly dose reduction velocity <10%) were associated with higher event rates for overdose and mental health crisis (Figure 2; eTable 4 in the Supplement). The associations observed in the main analyses were robust to a series of sensitivity analyses (eTables 5-6 in the Supplement).
Figure 2. Adjusted Event Rates for Overdose and Mental Health Crisis Events .
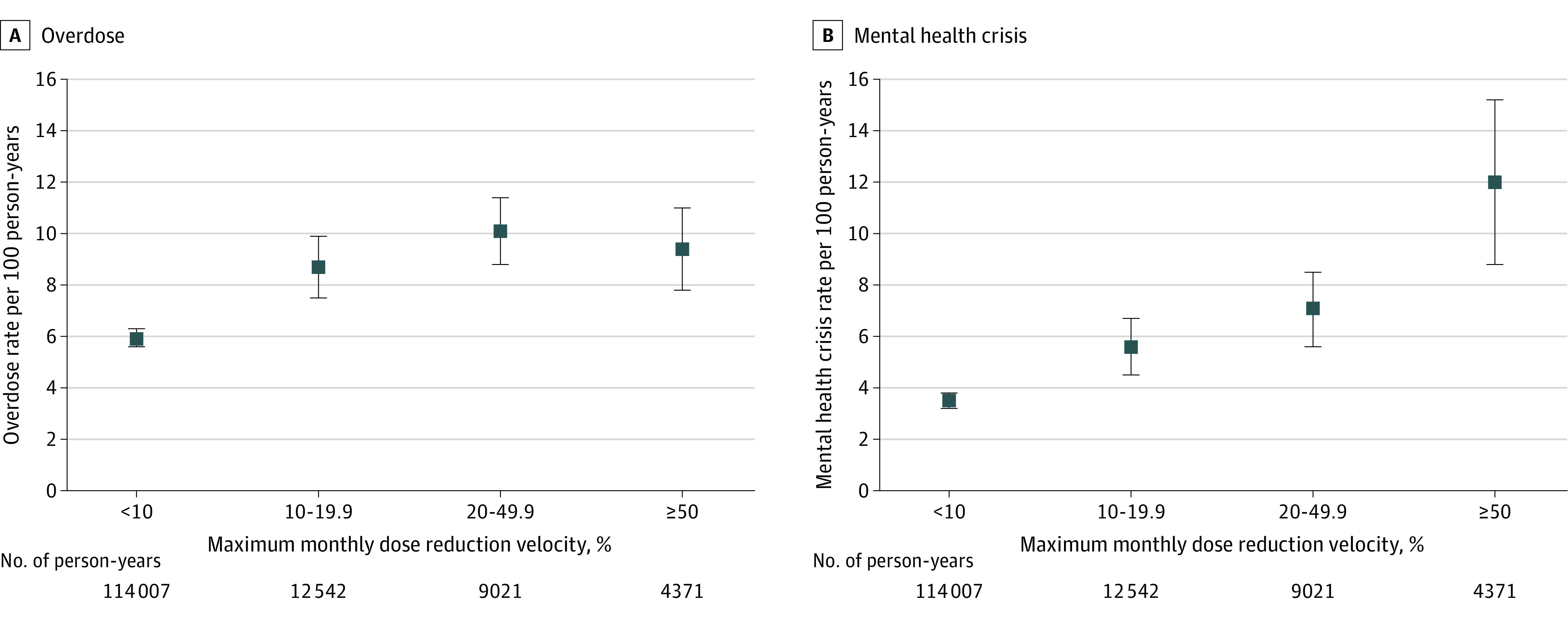
A total of 109 223 patients and 139 941 person-years of follow-up are included in the analyses. Monthly outcome counts during follow-up were modeled as a function of the maximum monthly rate of dose reduction during any previous 60-day period. Thus, the less than 10% category may include patient periods prior to tapering, patients with gradual dose reductions, or patients with no dose change. Of the total cohort of 113 618 patients, 4395 (3.9%) were not included in these analyses due to an absence of follow-up time beyond the initial 60-day period or a dose increase of greater than or equal to 15% relative to baseline in the first 60 days. Plotted estimates are adjusted for patient age, sex, education, rurality of home address, commercial vs Medicare Advantage insurance, baseline opioid dose, co-prescription of benzodiazepines, study year, number of overdose events in the baseline period, baseline depression or anxiety, alcohol use disorder, drug use disorder, psychosis, and 24 other noncancer comorbidities included in the Elixhauser comorbidity index. Error bars show 95% CIs.
Discussion
In a large cohort of patients in the US prescribed stable, long-term, higher-dose opioids, undergoing opioid dose tapering was associated with statistically significant risk of subsequent overdose and mental health crisis, including suicidality.
Guidelines for opioid tapering published in 2019 by the US Department of Health and Human Services (HHS) cautioned about the potential hazards of rapid dose reduction, including withdrawal, transition to illicit opioids, and psychological distress.9 Qualitative studies suggest that many patients experience the tapering process as emotionally challenging,25,26 and both the HHS and CDC guidelines advise clinicians to monitor patients carefully during tapering and to provide psychosocial support. In the current study, tapering was associated with absolute differences in rates of overdose or mental health crisis events of approximately 3 to 4 events per 100 person-years compared with nontapering. These findings suggest that adverse events associated with tapering may be relatively common and support HHS recommendations for more gradual dose reductions when feasible and careful monitoring for withdrawal, substance use, and psychological distress.9
Previous research has examined adverse outcomes associated with discontinuing long-term opioids.10,11,12,13,14 This analysis demonstrated associations between adverse outcomes and a more sensitive indicator of opioid dose reduction (≥15% from baseline). The associations persisted in sensitivity analyses that excluded patients who discontinued opioids during follow-up, suggesting that the observed associations between tapering and overdose and mental health crisis are not entirely explained by events occurring in patients discontinuing opioids. Additionally, all categories of maximum dose reduction velocity demonstrated higher relative rates of outcomes compared with the lowest (<10% per month), suggesting that risks were not confined to patients undergoing rapid tapering.
Patients undergoing tapering from higher baseline opioid doses had higher associated risk for the study outcomes compared with patients undergoing tapering from lower baseline doses. Due to physiologic opioid tolerance,27 patients receiving higher doses may have heightened intolerance of opioid dose disruption, potentially warranting additional caution in patients tapering from higher doses.
The risks of long-term opioids are well-documented, particularly at higher doses and in the presence of other risk factors for opioid toxicity,4 and clinicians and patients must carefully weigh risks and benefits of both opioid continuation and tapering in decisions regarding ongoing opioid therapy.28 The risks associated with opioid tapering warrant further exploration to inform clinical guidelines regarding patient selection for tapering, optimal rates of dose reduction, and how best to monitor and support patients during periods of dose transition.
Limitations
This study has several limitations. First, although it included a number of key covariates, unmeasured factors may have contributed to increased risk for adverse events in the population who underwent tapering. Nevertheless, the findings are consistent with recent studies of opioid discontinuation10,11,12,13,14,15,29,30 and were robust to adjustment for baseline overdose, mental health conditions, and a range of sensitivity analyses. Second, the analyses could not assess tapering circumstances. Recent evidence has shown that the majority of opioid tapering and discontinuation is clinician-initiated,31 and risks may differ with voluntary vs involuntary tapering.32,33 Third, the study design considered any dose reduction of greater than or equal to 15% of the baseline dose as a taper initiation but did not account for subsequent dose trajectory. Fourth, the data set lacked an accurate measure of race, limiting the ability to account for the potential differential opioid prescribing and tapering trends between racial and ethnic groups. Fifth, the data set does not measure illicit opioid use or account for methadone administered in certified treatment programs. Sixth, administrative claims data have inherent measurement error. Seventh, these data were claims from commercially-insured and Medicare Advantage patients in the US, and the generalizability of these findings is uncertain.
Conclusions
Among patients prescribed stable, long-term, higher-dose opioid therapy, tapering events were significantly associated with increased risk of overdose and mental health crisis. Although these findings raise questions about potential harms of tapering, interpretation is limited by the observational study design.
eMethods
eFigure 1.Temporal relationships between periods for determining tapering status relative to the baseline period and follow-up months
eTable 1. Outcome definitions by ICD-9-CM or ICD-10-CM codes
eTable 2. Full Negative Binomial Regression Results for Main Analysis of Associations between Tapering and Overdose and Mental Health Crisis Outcomes
eFigure 2. Adjusted event rates for overdose and mental health crisis by baseline opioid dose and taper status
eTable 3. Negative Binomial Regression Analysis of the Association between Maximum Dose Reduction Velocity and Overdose and Mental Health Crisis Outcomes
eTable 4. Multivariate Analysis of Association between Maximum Dose Reduction Velocity and Overdose and Mental Health Crisis Outcomes
eTable 5. Results of Sensitivity Analyses Evaluating Impact on Main Results of Alternative Specifications of Adverse Outcomes, Tapering Exposure, or with Exclusions of Selected Subjects
eTable 6. Propensity Score Analysis of Association between Tapering and Overdose and Mental Health Crisis Outcomes
References
References
- 1.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi: 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- 2.Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007-16: retrospective cohort study. BMJ. 2018;362:k2833. doi: 10.1136/bmj.k2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276-286. doi: 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenton JJ, Agnoli AL, Xing G, et al. Trends and rapidity of dose tapering among patients prescribed long-term opioid therapy, 2008-2017. JAMA Netw Open. 2019;2(11):e1916271. doi: 10.1001/jamanetworkopen.2019.16271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnert ASB, Guy GPJ Jr, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169(6):367-375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson N, Kariisa M, Seth P, Smith H IV, Davis NL. Drug and opioid-involved overdose deaths—United States, 2017-2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290-297. doi: 10.15585/mmwr.mm6911a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. US Food and Drug Administration . Published April 12, 2019. Accessed July 6, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes
- 9.HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics. US Dept of Health and Human Services . Published October 2019. Accessed November 1, 2019. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf
- 10.Sullivan MD, Turner JA, DiLodovico C, D’Appollonio A, Stephens K, Chan YF. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. 2017;18(3):308-318. doi: 10.1016/j.jpain.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliva EM, Bowe T, Manhapra A, et al. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ. 2020;368:m283. doi: 10.1136/bmj.m283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binswanger IA, Glanz JM, Faul M, et al. The association between opioid discontinuation and heroin use: a nested case-control study. Drug Alcohol Depend. 2020;217:108248. doi: 10.1016/j.drugalcdep.2020.108248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mark TL, Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. 2019;103:58-63. doi: 10.1016/j.jsat.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 14.James JR, Scott JM, Klein JW, et al. Mortality after discontinuation of primary care-based chronic opioid therapy for pain: a retrospective cohort study. J Gen Intern Med. 2019;34(12):2749-2755. doi: 10.1007/s11606-019-05301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health. 2018;108(10):1394-1400. doi: 10.2105/AJPH.2018.304590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton JJ, Magnan EM, Agnoli AL, Henry SG, Xing G, Tancredi DJ. Longitudinal dose trajectory among patients tapering long-term opioids. Pain Med. Published online March 19, 2021. doi: 10.1093/pm/pnaa470 [DOI] [PubMed] [Google Scholar]
- 17.Vivolo-Kantor A, Pasalic E, Liu S, Martinez PD, Gladden RM; Overdose Morbidity Team . Defining indicators for drug overdose emergency department visits and hospitalisations in ICD-10-CM coded discharge data. Inj Prev. 2021;27(S1):i56-i61. doi: 10.1136/injuryprev-2019-043521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol Drug Saf. 2017;26(5):509-517. doi: 10.1002/pds.4157 [DOI] [PubMed] [Google Scholar]
- 19.Slavova S, Quesinberry D, Costich JF, et al. ICD-10-CM-based definitions for emergency department opioid poisoning surveillance: electronic health record case confirmation study. Public Health Rep. 2020;135(2):262-269. doi: 10.1177/0033354920904087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:289. doi: 10.1186/s12888-014-0289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swain RS, Taylor LG, Braver ER, Liu W, Pinheiro SP, Mosholder AD. A systematic review of validated suicide outcome classification in observational studies. Int J Epidemiol. 2019;48(5):1636-1649. doi: 10.1093/ije/dyz038 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Agriculture . Documentation: 2010 Rural-Urban Commuting Area (RUCA) Codes. Economic Research Service; 2016. [Google Scholar]
- 23.Chronic conditions data warehouse: condition categories. Centers for Medicare & Medicaid Services; 2019. Accessed January 28, 2019. https://www.ccwdata.org/web/guest/condition-categories
- 24.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care. 2017;55(7):698-705. doi: 10.1097/MLR.0000000000000735 [DOI] [PubMed] [Google Scholar]
- 25.Henry SG, Paterniti DA, Feng B, et al. Patients’ experience with opioid tapering: a conceptual model with recommendations for clinicians. J Pain. 2019;20(2):181-191. doi: 10.1016/j.jpain.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank JW, Levy C, Matlock DD, et al. Patients’ perspectives on tapering of chronic opioid therapy: a qualitative study. Pain Med. 2016;17(10):1838-1847. doi: 10.1093/pm/pnw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164(4):1322-1334. doi: 10.1111/j.1476-5381.2011.01335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285-2287. doi: 10.1056/NEJMp1904190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kertesz SG, Manhapra A. The drive to taper opioids: mind the evidence, and the ethics. Spinal Cord Ser Cases. 2018;4:64. doi: 10.1038/s41394-018-0092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manhapra A, Arias AJ, Ballantyne JC. The conundrum of opioid tapering in long-term opioid therapy for chronic pain: A commentary. Subst Abus. 2018;39(2):152-161. doi: 10.1080/08897077.2017.1381663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lovejoy TI, Morasco BJ, Demidenko MI, Meath THA, Frank JW, Dobscha SK. Reasons for discontinuation of long-term opioid therapy in patients with and without substance use disorders. Pain. 2017;158(3):526-534. doi: 10.1097/j.pain.0000000000000796 [DOI] [PubMed] [Google Scholar]
- 32.Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167(3):181-191. doi: 10.7326/M17-0598 [DOI] [PubMed] [Google Scholar]
- 33.Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35. doi: 10.1016/j.genhosppsych.2017.04.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1.Temporal relationships between periods for determining tapering status relative to the baseline period and follow-up months
eTable 1. Outcome definitions by ICD-9-CM or ICD-10-CM codes
eTable 2. Full Negative Binomial Regression Results for Main Analysis of Associations between Tapering and Overdose and Mental Health Crisis Outcomes
eFigure 2. Adjusted event rates for overdose and mental health crisis by baseline opioid dose and taper status
eTable 3. Negative Binomial Regression Analysis of the Association between Maximum Dose Reduction Velocity and Overdose and Mental Health Crisis Outcomes
eTable 4. Multivariate Analysis of Association between Maximum Dose Reduction Velocity and Overdose and Mental Health Crisis Outcomes
eTable 5. Results of Sensitivity Analyses Evaluating Impact on Main Results of Alternative Specifications of Adverse Outcomes, Tapering Exposure, or with Exclusions of Selected Subjects
eTable 6. Propensity Score Analysis of Association between Tapering and Overdose and Mental Health Crisis Outcomes
References