Abstract
Objectives
To compare lung function in a representative sample of World Trade Center (WTC)-exposed children with matched comparisons, and examine relationships with reported exposures.
Study design
Study population consisted of 402 participants. Oscillometry, spirometry, and plethysmography were performed on WTC Health Registry (WTCHR) respondents who were ≤8 years of age on September 11, 2001 (n = 180) and a sociodemographically matched group of New York City residents (n = 222). We compared lung function by study arm (WTCHR and comparison group) as well as dust cloud (acute); home dust (subchronic); and other traumatic, nondust exposures.
Results
In multivariable models, post-9/11 risk of incident asthma was higher in the WTCHR participants than in the comparison group (OR 1.109, 95% CI 1.021, 1.206; P = .015). Comparing by exposure rather than by group, dust cloud (OR 1.223, 95% CI 1.095, 1.365; P < .001) and home dust (OR 1.123, 95% CI 1.029, 1.226; P = .009) exposures were also associated with a greater risk of incidence of post-9/11 asthma. No differences were identified for lung function measures.
Conclusions
Although we cannot exclude an alternative explanation to the null findings, these results may provide some measure of reassurance to exposed children and their families regarding long-term consequences. Further study with bronchodilation and/or methacholine challenge may be needed to identify and further evaluate effects of WTC exposure. Biomarker studies may also be more informative in delineating exposure-outcome relationships.
Trial registration
The destruction of the World Trade Center (WTC) on September 11, 2001 resulted in exposure of large populations of individuals, including children, to numerous inhaled toxins. Defined health effects have been predominantly documented in the adult population,1 but the consequences of a disaster-related exposure during vulnerable developmental years has yet to be clearly defined.1 In the adult population, studies have shown that WTC dust exposure is associated with declines in spirometry measures including a reduced vital capacity along with a number of other physiological airway abnormalities often localized to the small airways.2,3 Forced oscillation testing (FOT) demonstrated that adults exposed to the dust had increased small airway resistance, positive response to bronchodilator, and an association with severity and frequency of wheeze.1–3
Three studies have examined asthma and/or lung function in children exposed to the disaster, but none examined an un-exposed comparison group. Among children participating in the World Trade Center Health Registry (WTCHR), the largest, most representative sample of exposed children, increased asthma prevalence has been documented among children exposed at <5 years of age, and new asthma diagnosis post-September 11 was associated with dust exposure for all age groups.4 Another study of students living in Chinatown after the attack documented 29% prevalence of abnormal forced expiratory volume in 1 second (FEV1, <80% predicted normal).5 Most recently, in a sample of children reporting to a clinic for WTC-related medical care, lower FEV1 and forced vital capacity (FVC) percent predicted were identified when compared with children who participated in the National Health and Nutrition Examination Survey (NHANES) 2007–2010. We also identified an association between dust cloud exposure and abnormal spirometry, low FVC, and obstructive patterns.6
Lung function tracks throughout childhood into adult life7 and that attained early in the third decade is one of the best predictors of chronic obstructive pulmonary disease.8 An insult during childhood, such as exposure to WTC dust, known to pathologically lead to alveolar destruction,9 could lead to a critical, yet subclinical loss in lung function and might confer a unique vulnerability to chronic obstructive pulmonary disease. If a subpopulation of WTC-exposed children has such a decrement in lung function, intensified vaccination efforts (for prevention of influenza and pneumococcal illnesses), counseling against smoke exposure, and initiating inhaled steroids might attenuate an accelerated lung function decline.10
Based on the above consideration, the present study recruited children from the WTCHR and compared measures of airway function and lung volume obtained by spirometry, plethysmography, and oscillometry with a matched comparison group. Our hypotheses were that lung function would be reduced among WTCHR participants compared with a matched comparison group and WTC-related dust exposures would independently predict lung function outcomes.
Methods
The present study is a comparison of WTCHR children who were ages 0–8 years on the date of the disaster compared with a sociodemographically matched group. We have previously described our study population and recruitment strategy11 (Appendix; available at www.jpeds.com). Briefly, eligibility for the WTCHR was based upon dates of birth on or between September 11, 1993 and September 10, 2001, residence or school attendance south of Canal Street (or presence south of Chambers Street) on September 11, 2001.
Comparisons were not eligible for the WTCHR, and had dates of birth on or between September 11, 1993 and September 10, 2001. In addition to online and web-based recruitment, participants for the comparison group were recruited from general primary care visits at clinics affiliated with the New York University School of Medicine and were excluded if they presented to these clinics for a clinical concern, especially previously diagnosed asthma.
Potential participants that indicated presence of serious lung or heart disease, heart or lung surgery, or an active upper respiratory infection, pregnancy, and inability to follow procedures at the time of their scheduled visit, were also excluded from participation. To maximize comparability between the 2 study populations, we developed a table of the desired frequencies of controls by age (0–2, 3–5, or 6–8 years old on September 11, 2001), sex, race (White, African-American, Asian, other), ethnicity (Hispanic, non-Hispanic), and income (<$25 000, ≥$25 000).
Pulmonary Evaluation Procedures
For comparison with the previous studies, we adjusted questions used in the NHANES pertaining to current and previous diagnosis of asthma12–14: (1) Do(es) you (your child) currently have asthma?; (2) Have (Has) you (your child) ever been diagnosed with asthma? If yes - were (was) you (your child) diagnosed before or after September 11, 2001?; (3) Can you write the month and year you were (your child was) first diagnosed with asthma?; (4) In the past 12 months, have you (has your child) had wheezing or whistling in your (his/her) chest? By wheezing or whistling, we mean a high-pitched whistling sound you (your child) makes during breathing. It happens when air moves through tight breathing tubes in your (child’s) chest.
We focused on asthma prevalence rather than current asthma prevalence in the present analysis. There were 5 participants in whom there was discordance. All 5 children reported pre-September 11, 2001 asthma, and the parents did not report asthma at all. We used parent response when the child’s age on September 11, 2001 was <5 years. Testing also included urine and saliva collection, blood draw, cardiovascular testing, psychosocial stress questionnaires, and medical history and diet questionnaires.
Spirometry, plethysmography, and FOT were performed according to standards outlined by the American Thoracic Society and the European Respiratory Society.15,16 Each test was performed a minimum of 3 times with the maximum number of trials limited to 5 to avoid participant fatigue. An average of these measures was used. Standard quality assurance procedures including monthly internal staff quality control tests were performed and maintained periodically throughout the study. All pulmonary data were evaluated separately by 2 investigators to ensure reproducibility and reliability of measurements, and exclude possible spurious data.
Spirometry
Spirometry measures included FVC, FEV1, FEV1/FVC, and forced expiratory flow over 25%−75% of the vital capacity (FEF25%−75%; Jaeger Masterscreen Impulse Oscillometry; Carefusion, Yorba Linda, California). NHANES III reference equations17 were used to determine ethnically/racially appropriate normative values, with statistical analysis applied to percent of predicted values. Because NHANES does not contain Asian norms, Caucasian/White norms were used for Asian participants.
Plethysmography
A Jaeger body box plethysmograph was used to measure functional residual capacity in all subjects. Subjects were instructed so that 4 stable tidal breaths were obtained, followed by 5 pant breaths and a return to normal breathing. Thereafter, subjects were asked to do a maximum exhalation from end tidal volume, a maximum inspiration, and finally return to normal tidal breathing. Total lung capacity and residual volume were derived from the functional residual capacity coupled with the measured inspiratory capacity and expiratory reserve volume.18 Measurement of residual volume in relation to total lung capacity permitted assessment of air trapping as a further measure of obstructive dysfunction.
Oscillometry
FOT was used to assess respiratory resistance during tidal breathing (Jaeger Masterscreen Impulse Oscillometry; Carefusion), in accord with published recommendations.19–24 FOT is a noninvasive test that measures the relationship between pressure and airflow fluctuations applied externally to the respiratory system. The relationship between airflow and pressure is analyzed to derive the respiratory system resistance. Measurements were performed during tidal breathing in a seated position for 30 seconds with support of the cheeks. Resistance was measured at oscillating frequencies of 5 Hz (R5) and 20 Hz (R20). In addition, frequency dependence of resistance (FDR) was calculated as the difference between R5 and R20 (R5–20). FDR provides a measure of nonuniformity of airflow distribution that may reflect regional functional abnormalities in the distal airways.25–27 FDR correlates with frequency dependence of compliance measured by esophageal manometry, an established test of distal airway function.28–30
Exposure Variables
Acute (dust cloud) and subchronic (home dust) exposure information was collected from both study participants and parent/guardian, if applicable. Dust cloud exposure was assessed categorically as present or absent with the question: “Were you caught in the WTC dust or debris cloud in the morning after the buildings collapsed on 9/11?” Home dust exposure was assessed categorically as present or absent with the question: “In the year after 9/11/01, did you live in an apartment or home in which WTC dust was visible on surfaces at any time, even if only briefly?” A positive response from either child or parent/guardian was used to indicate exposure.
Psychologically traumatic exposures were measured with the 8-item questionnaire developed by Comer et al.31 Traumatic exposure-related was noted if either the adolescent or parent/guardian gave a positive response to the questionnaire items of sight of either tower collapse, sight of injured people, sight of dead bodies, sight of people falling out of buildings, physical injury to self, need to depart home/work for safety, and worry about safety of a loved one.
Key Covariates
Identified covariates included body mass index (BMI) category (normal, overweight, obese), race/ethnicity (White, African American, Asian, other, and Hispanic), sex (male or female), and cotinine for tobacco smoke exposure. BMI (kg/m2) was calculated from height and weight measurements using calibrated stadiometers and was controlled to adjust for the effect on pulmonary function. Salivary cotinine was analyzed using a highly reliable (r = .99 compared with serum) and sensitive (limit of detection 0.15 ng/mL) test from Salimetrics, Inc (State College, Pennsylvania).
Cotinine was measured as a continuous variable and categorized into low (<0.15 ng/mL), medium (<2 and ≥0.15 ng/mL), and high (≥2 ng/mL) categories, using established conventions.32,33 For those with missing data, self-reported smoking information (last 30 days exposure) was used for data analysis. For subjects without saliva cotinine concentration, we categorized nonsmokers and no secondhand smoke exposure into the low category, nonsmokers with secondhand smoke exposure into the medium category, and active smokers into the high category.
Anthropometric Measures
We derived BMI z scores from the Centers for Disease Control and Prevention norms, incorporating height, weight, and sex; overweight and obese were categorized as BMI z score ≥1.036 and ≥1.64.23
Institutional Review Board Approval
The study was reviewed and approved by the New York University (NYU) School of Medicine Institutional Review Board and, as well as research committees at Bellevue and Gouverneur Hospital Centers. The New York City Department of Health and Mental Hygiene Institutional Review Board identified this study not to involve human subject activity by New York City Department of Health and Mental Hygiene staff. Adolescents <18 years of age provided informed assent forms along with parental informed consent forms before undergoing study procedures. A Certificate of Confidentiality was obtained to protect participant privacy.
Statistical Analyses
Descriptive, univariate, and multivariable analyses were performed using R Statistical Software (v 3.3.1, R Development Core Team, Auckland, New Zealand). χ2 test was used to compare the 2 cohorts by sociodemographic and exposure variables. Dust cloud exposure was analyzed through the Fisher exact test. Sociodemographic variables of WTCHR children who participated in the study were compared with those in the WTCHR who did not participate using χ2 tests.
Primary analyses compared the WTCHR participants with matched comparisons. Because dust exposure was present in some individuals within the comparison group and a segment of the WTCHR was not exposed to dust or trauma exposures, secondary analyses compared individuals with either home dust or dust cloud exposure to those without these exposures, without regard to study arm. Analyses of asthma as an outcome excluded participants with pre-9/11 asthma.
Correlation coefficients for exposures were also calculated. We identified substantial correlation between reported exposures and measured exposures. To avoid multicollinearity in our statistical analyses, separate multivariable models examined dust cloud, home dust, and traumatic exposure, controlling for other covariates. All multivariable linear and logistic regressions for continuous and binary outcomes controlled for sex, race, BMI category, and tobacco smoke exposure. Except for tobacco smoke exposure, these variables were added to multivariable models because differences were identified between the 2 comparison groups at P < .1.
Recognizing that BMI can be highly influential on spirometry, oscillometry, and plethysmography,34,35 sensitivity analyses were performed to examine the observed associations in models stratified by BMI category.
Results
A flow diagram of participants into our study along with a detailed description of population data is presented (Figure 1). The WTCHR group (n = 180) was more likely to be male (P = .008) than comparison participants. Participants in the comparison group (n = 122) were more likely to be non-Hispanic White and Hispanic, and the WTCHR group was more likely to be Asian (Table I).Our analysis of BMI category showed that the comparison group was more overweight or obese compared with the WTCHR group (P = .045). We identified a significant difference in WTC exposures between groups (P < .0001; Table I), but even with exclusion criteria, WTC exposures were found in the comparison group: 0.5% for dust cloud, 7.7% for home dust, and 42.8% for traumatic exposure. WTCHR participants were different from their nonparticipant counterparts by income status (P < .001) and age (P = .004; Table II; available at www.jpeds.com). Self-reported WTC exposures were moderately correlated with each other and by study group, supporting separate treatment in multivariable models controlling for confounders (Table III; available at www.jpeds.com).
Figure 1.
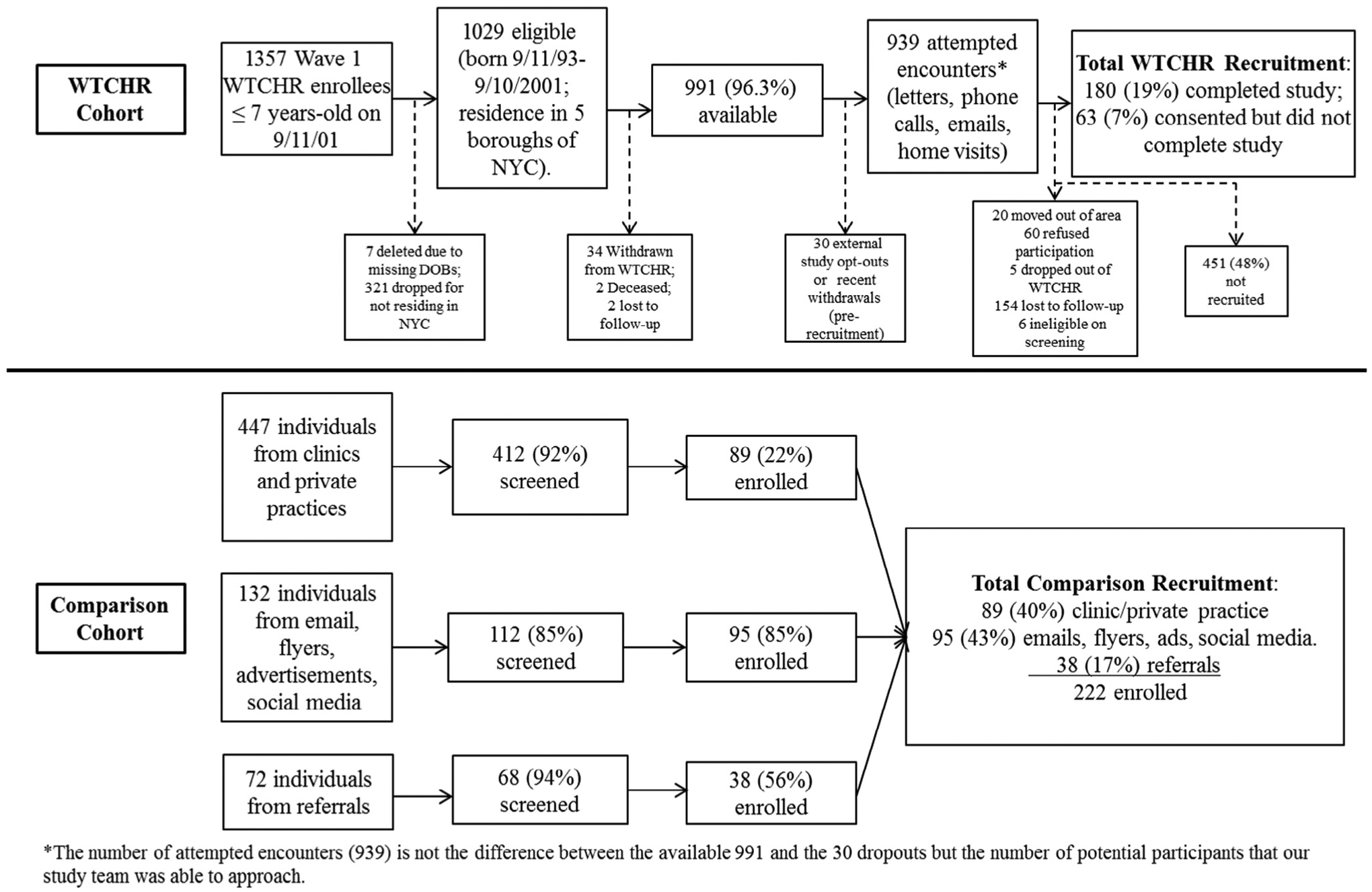
Flow chart of recruitment of WTCHR and comparison group participants into the present study. DOB, date of birth; NYC, New York City.
Table I.
Subject population characteristics
| Comparison | WTCHR | ||
|---|---|---|---|
| n = 222 | n = 180 | P value | |
| Sex | .008 | ||
| Male | 89 (40.1%) | 97 (53.9%) | |
| Female | 133 (59.9%) | 83 (46.1%) | |
| Date of birth | .159 | ||
| 9/11/93–9/10/95 | 45 (20.3%) | 47 (26.1%) | |
| 9/11/95–9/10/98 | 89 (40.1%) | 77 (42.8%) | |
| 9/11/98–9/10/01 | 88 (39.6%) | 56 (31.1%) | |
| Income < $25 000* | 49 (27.4%) | 28 (19.4%) | .126 |
| Race/ethnicity† | .053 | ||
| White, % | 89 (40.1%) | 66 (36.9%) | |
| Black, % | 19 (8.6%) | 16 (8.9%) | |
| Asian, % | 44 (19.8%) | 49 (27.4%) | |
| Other, % | 10 (4.5%) | 16 (8.9%) | |
| Hispanic | 60 (27.0%) | 32 (17.9%) | |
| Exposures‡ | |||
| Dust cloud exposure (%) | 1 (0.5) | 61 (38.6) | <.0001 |
| Home dust exposure (%) | 17 (7.7) | 98 (56) | <.0001 |
| Traumatic exposure (%) | 95 (42.8) | 150 (83.3) | <.0001 |
| BMI category | .045 | ||
| Normal | 162 (73.0%) | 150 (83.3%) | |
| Overweight | 36 (16.2%) | 19 (10.6%) | |
| Obese | 24 (10.8%) | 11 (6.1%) | |
| Tobacco smoke exposure§ | |||
| Cotinine, ng/mL (IQR) | 0.324 (0.106, 0.69) | 0.412 (0.106, 0.984) | .294 |
| Low | 102 (45.9%) | 73 (40.6%) | |
| Medium | 95 (42.8%) | 79 (43.9%) | .353 |
| High | 25 (11.3%) | 28 (15.6%) | |
| Asthma before September 11, 2001 | 15 (6.9%) | 11 (6.3%) | .842 |
All bold values indicate P < 0.05.
n = 43 missing for comparison group; n = 27 missing for WTCHR group.
n = 1 missing for WTCHR.
n = 18 missing for dust cloud exposure; n = 2 missing for home dust exposure.
Evaluated by saliva cotinine concentration and questionnaire. For subjects without saliva cotinine concentration, we categorized no smoker and no secondhand smoke exposure into low, no smoker but secondhand smoke exposure into medium, and smoker into high category.
Table II.
Comparison of WTCHR participants in the present study to other eligible participants who did not take part in the WTCHR
| WTCHR participants (n = 180) | Nonparticipants (n = 759) | P value | |
|---|---|---|---|
| Male, % | 53.9% | 50.5% | .457 |
| Date of birth | |||
| 9/11/93–9/10/95 | 26.1% | 35.6% | .004 |
| 9/11/95–9/10/98 | 42.8% | 43.9% | |
| 9/11/98–9/10/01 | 31.1% | 20.6% | |
| Income < $25 000* | 19.4% | 41.7% | <.001 |
| Race/ethnicity† | |||
| Non-Hispanic White | 36.7% | 46.9% | .092 |
| Non-Hispanic Black | 8.9% | 5.8% | |
| Non- Hispanic Asian | 27.2% | 21.6% | |
| Non-Hispanic other | 8.9% | 7.5% | |
| Hispanic or Latino | 17.8% | 18.3% |
All bold values indicate P < 0.05.
n = 1 missing for WTCHR study participants.
n = 36 missing for WTCHR study participants, n = 93 missing for WTCHR nonparticipants.
Table III.
Pearson correlation coefficients among exposure variables
| Home dust | Dust cloud | Traumatic exposures | WTCHR | |
|---|---|---|---|---|
| Home dust | 0.501 (<.001) | 0.298 (<.001) | 0.529 (<.001) | |
| Dust cloud | 0.324 (<.001) | 0.507 (<.001) | ||
| Traumatic Exposures | 0.413 (<.001) | |||
| WTCHR |
P values in parentheses.
Figure 2 shows the incidence of new onset post-9/11 asthma by cohort and exposure groups. Univariate analysis showed that post-9/11 risk of incident asthma was higher in the WTCHR participants (OR 1.102, 95% CI 1.016, 1.195) than in the comparison group (P = .019). Comparing by exposure rather than by group, dust cloud (OR 1.229, 95% CI 1.103, 1.371; P < .001) and home dust (OR 1.121, 95% CI 1.027, 1.223; P = .011) exposures were associated with a greater incidence of post-9/11 asthma (Table IV; available at www.jpeds.com). There were no differences in post-9/11 risk of incident asthma by psychologically traumatic exposures (P = .661). Multivariable models confirmed these findings: WTCHR (OR 1.109, 95% CI 1.021, 1.206; P = .015), dust cloud (OR 1.223, 95% CI 1.095, 1.365; P < .001), home dust (OR 1.123, 95% CI 1.029, 1.226; P = .009), and traumatic exposure (OR 1.023, 95% CI 0.941, 1.112; P = .597) (Table IV).
Figure 2.
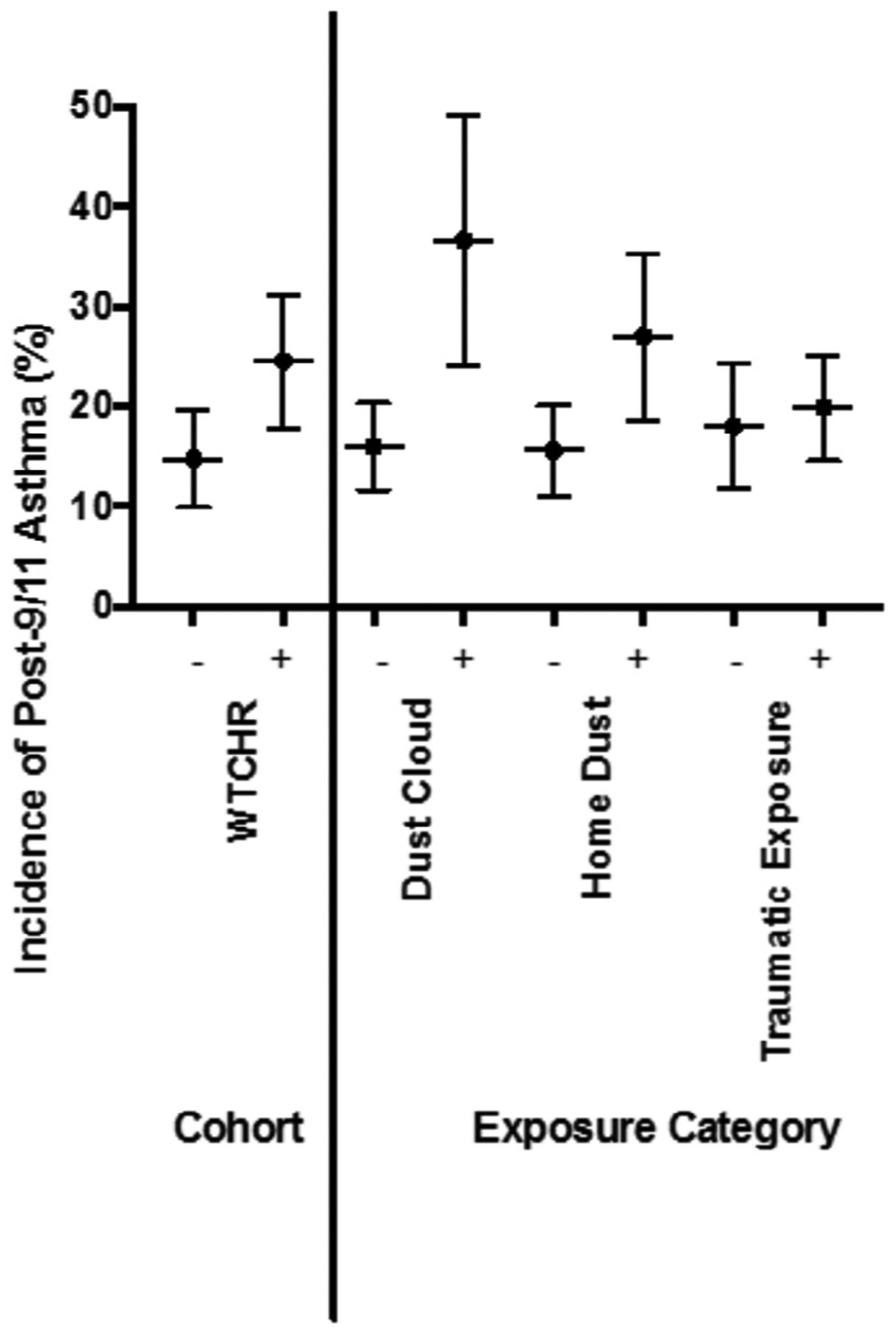
Incidence of post-9/11/01 asthma by WTCHR, dust cloud, home dust, and other traumatic exposures.
Table IV.
Logistic regression analysis of development of incident post-9/11 asthma by cohort and exposure category
| WTCHR | Dust cloud | Home dust | Traumatic exposure | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Univariate | 1.102 (1.016, 1.195) | .019 | 1.229 (1.103, 1.371) | <.001 | 1.121 (1.027, 1.223) | .011 | 1.019 (0.938, 1.107) | .661 |
| Multivariable | 1.109 (1.021, 1.206) | .015 | 1.223 (1.095, 1.365) | <.001 | 1.123 (1.029, 1.226) | .009 | 1.023 (0.941, 1.112) | .597 |
Each column represents an examination of a single exposure variable or study arm. Multivariable analyses controlled for sex, race/ethnicity, BMI category, and tobacco smoke exposure.
In contrast to the significant difference in asthma, univariate analyses failed to yield any significant differences in spirometry, plethysmography, or oscillometry by any of the exposure variables (Figures 3–6; available at www.jpeds.com). Multivariable models confirmed absence of differences in lung function between exposure groups (Table V). In addition, subgroup analysis of individuals with new diagnosis of asthma post-September 11 showed no deficits in lung function using spirometry, plethysmography, or oscillometry (data not shown).
Figure 3.
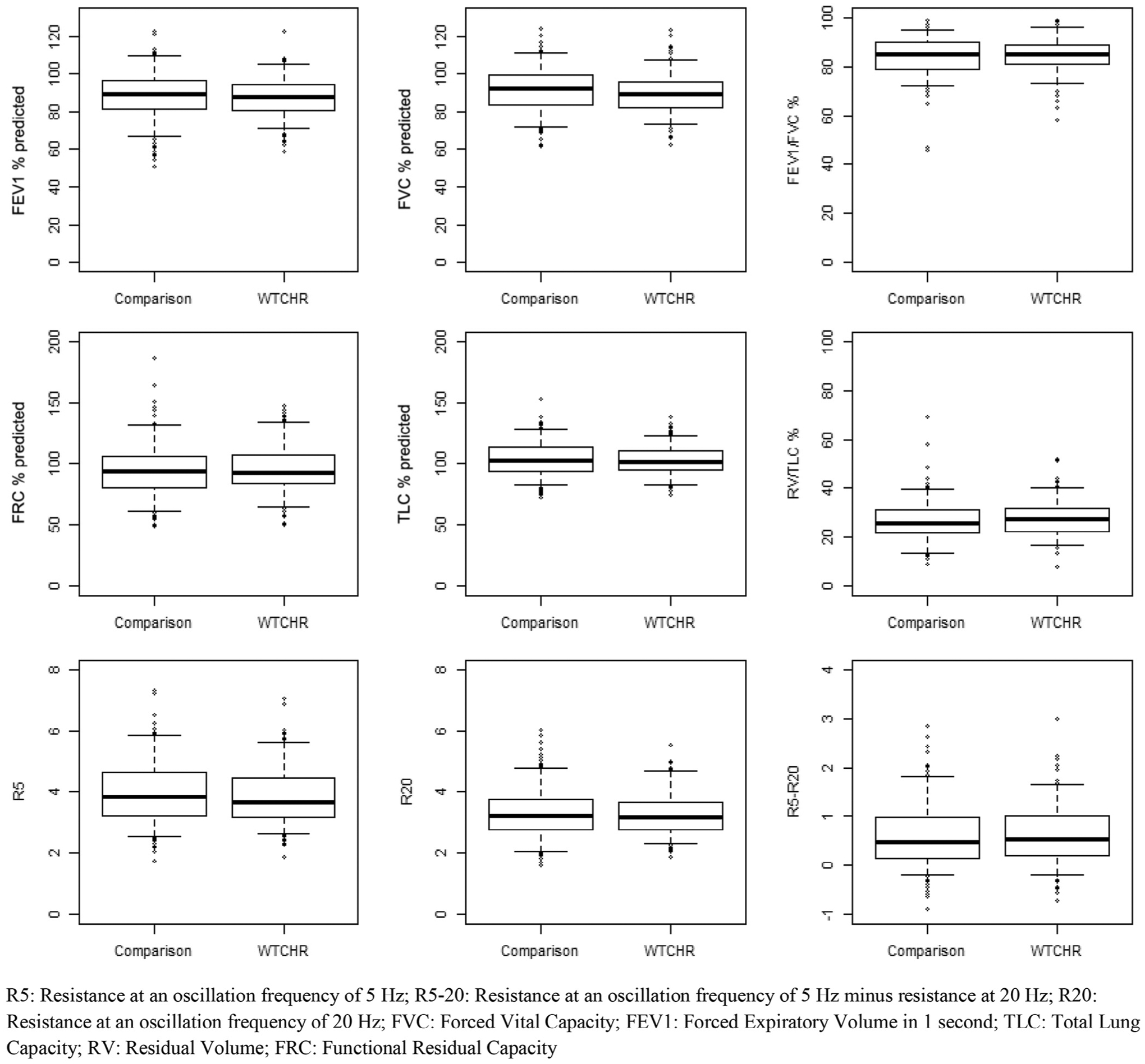
Pulmonary function outcome comparison by WTCHR.
Figure 6.
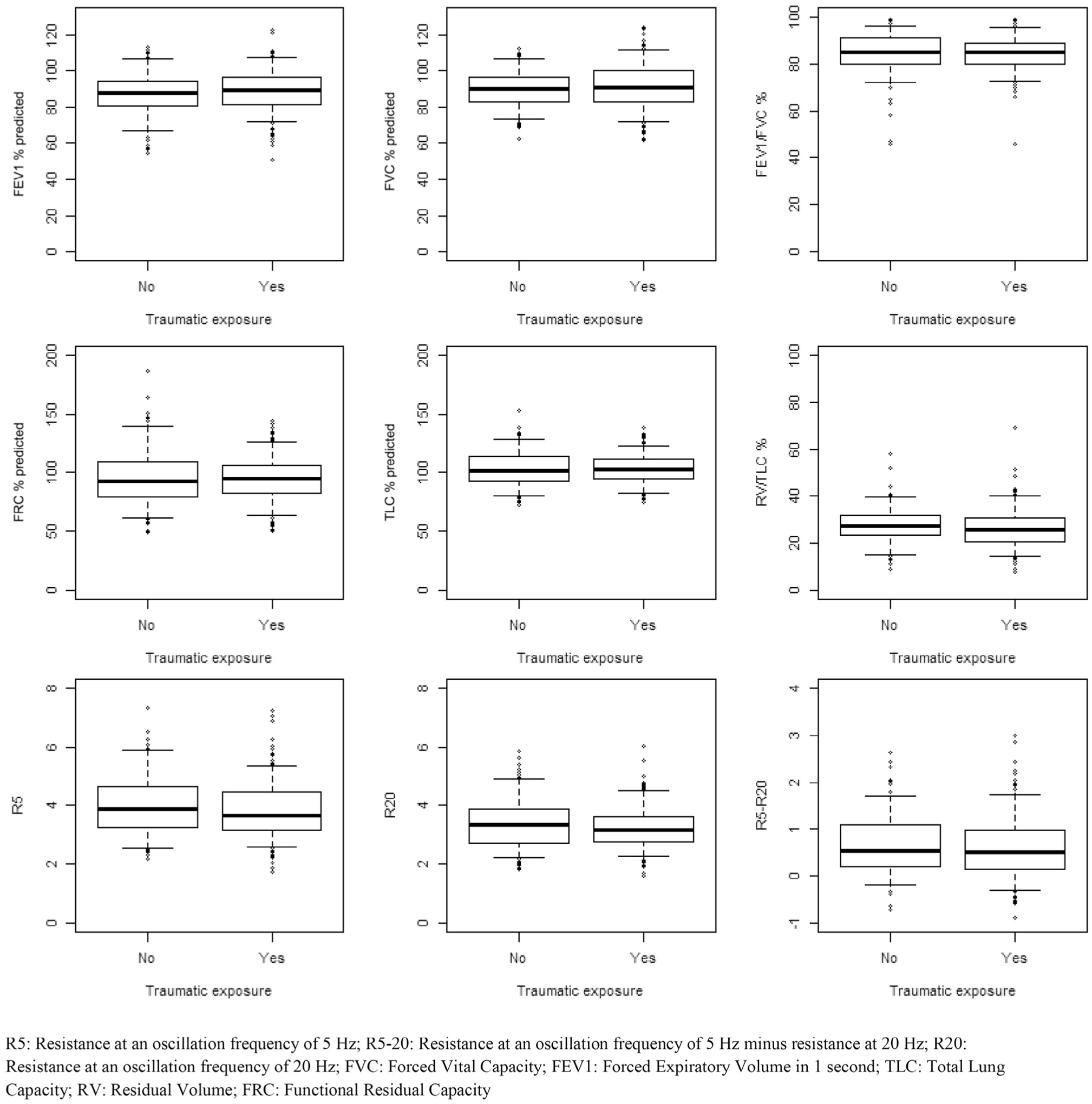
Pulmonary function comparison by traumatic exposure.
Table V.
Multivariable logistic regression analysis of relevant pulmonary function outcomes by WTCHR, dust cloud, home dust, and other traumatic exposures
| WTCHR | Dust cloud | Home dust | Traumatic exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Unit increase (95% CI) | P value | Unit increase (95% CI) | P value | Unit increase (95% CI) | P value | Unit increase (95% CI) | P value | |
| R5 | .06 (−0.141, 0.261) | .559 | 0.14 (−0.145, 0.425) | .334 | .078 (−0.139, 0.296) | .479 | −0.158 (−0.358, .042) | .122 |
| R5–20 | .073 (−.067, 0.212) | .308 | .067 (−0.122, 0.255) | .488 | −.022 (−0.171, 0.126) | .768 | −.012 (−0.153, 0.13) | .873 |
| R20 | .035 (−0.118, 0.187) | .655 | 0.134 (−.08, 0.348) | .219 | .061 (−0.103, 0.226) | .465 | −0.124 (−0.275, .028) | .11 |
| FVC | −0.942 (−3.31, 1.427) | .435 | −0.403 (−3.591, 2.785) | .804 | −0.349 (−2.915, 2.217) | .789 | 1.508 (−0.818, 3.833) | .203 |
| FEV1 | −0.337 (−2.913, 2.239) | .797 | 0.803 (−2.699, 4.304) | .652 | 0.465 (−2.333, 3.263) | .744 | 2.249 (−0.273, 4.77) | .08 |
| FEV1/FVC | 0.827 (−1.213, 2.867) | .426 | 1.375 (−1.407, 4.157) | .332 | 0.895 (−1.326, 3.115) | .429 | 1.222 (−0.782, 3.225) | .231 |
| TLC | −0.179 (−0.48, 0.121) | .241 | −0.304 (−0.722, 0.114) | .153 | −0.184 (−0.506, 0.138) | .262 | .061 (−0.244, 0.366) | .693 |
| RV | −0.139 (−0.521, 0.243) | .473 | −0.272 (−0.806, 0.262) | .317 | −0.234 (−0.644, 0.176) | .263 | −0.221 (−0.608, 0.166) | .262 |
| FRC | −0.168 (−0.543, 0.206) | .377 | −0.202 (−0.721, 0.316) | .443 | −0.18 (−0.585, 0.224) | .381 | .032 (−0.347, 0.412) | .867 |
FRC, functional residual capacity; R20, resistance at an oscillation frequency of 20 Hz; R5, resistance at an oscillation frequency of 5 Hz; R5–20, resistance at an oscillation frequency of 5 Hz minus resistance at 20 Hz; RV, residual volume; TLC, total lung capacity.
Each column represents an examination of exposure variable or study arm controlled for sex, race/ethnicity, BMI category, and tobacco smoke exposure.
Models stratified by BMI category failed to reveal differences in spirometry, oscillometry, or plethysmography in relationship to reported exposure (data not shown).
Discussion
In the present study, we identified increases in post-September 11 asthma persisting 13–15 years after the WTC disaster. We did not identify a significant difference in lung function. The frequent diagnosis of post-September 11 asthma (~25%) is a serious concern, consistent with other previous reports,4 and further supports the need for ongoing care in young adults exposed to the disaster in early life. Yet, the absence of differences in spirometry, oscillometry, and plethysmography may provide some reassurance to exposed children and their families regarding long-term consequences for their pulmonary health.
Strengths to this study include inclusion of a sociodemographically matched group, as well as careful and rigorously standardized measurement of pulmonary function, which was performed for the first time in a population exposed to the disaster in early life. Like other studies of disaster-exposed populations, residual confounding limits our interpretation. During the 13- to 15-year period after the disaster, participants in both arms of the study may have experienced adverse and beneficial environmental exposures that could have modified the effects of dust and stress related exposures, and thereby affected measurements of pulmonary function. Participation rates within the WTCHR were modest, limiting generalizability. However, this is a disaster-related recruitment sample and there is no other population well-poised to inform our research question. There were also differences in sociodemographic characteristics and adiposity (as evaluated with anthropometric measurements) between the WTCHR participants and matched comparisons that could contribute to explaining our results.
We also evaluated participants when they were asymptomatic. This approach to evaluation could have attenuated real differences that may otherwise have been detected. Although other studies have used bronchodilators to unmask deficits in lung function or even bronchoprovocation with mannitol or methacholine,36 the NYU School of Medicine Institutional Review Board judged such methods to be significantly more than minimal risk, rather than a minor increase above minimal risk. We, therefore, decided to exclude bronchodilation or bronchoprovocation from the present study, recognizing that null findings might leave open the possibility that we may have missed a significant decrement in pulmonary function masked (or compensated) in an otherwise healthy adolescent.
The use of questionnaire data for evaluating exposure also creates difficulties with exposure imprecision, specifically with regard to dust cloud and home dust exposure. Typically, exposure imprecision biases associations toward the null for categorical outcomes,37 though the direction of bias for continuous outcomes such as those measured in our study cannot be ascertained. Biomarkers of exposure may be better predictors of exposure this far out after the disaster. We have identified increases in perfluoroalkylchemicals (a chemical known to be in WTC dust) in the serum of children in the WTCHR and in relationship to self-reported exposure.11 Insofar as a biomarker can be used as a better proxy for exposure to the disaster, especially in comparison to questionnaire-based recall 13–15 years later, associations of biomarkers of exposure with pulmonary function may be more revealing. That said, there will be interesting challenges, in part because perfluoroalkyl substances (PFAS) exposure can be both related to the WTC disaster but also due to dietary and other non-WTC-related exposures. Careful control for confounding will be needed because PFAS themselves may be immune disruptors and affect pulmonary development.38,39 We anticipate examining PFAS biomarkers both separately and in addition to WTC dust and stress-related exposures.
We appreciate that we only evaluated asthma prevalence rather than current asthma prevalence. Our methodological approach was predicated on the concept that medical care under the World Trade Center Health Program is based upon diagnosis rather than current asthma. In addition, our reliance on self-reported indications of asthma in NHANES is a potential methodological error, a concern that other studies have previously raised.40,41 In contrast, lung function measures can be more specific in their diagnostic value. However, we were limited by the inability to preform bronchoprovocation, which would have further resolved these issues.
The present study confirms differences in incident asthma, but did not identify presence of chronic lung function abnormalities among asymptomatic children exposed to the WTC disaster when studied together with a comparison group. Further study with bronchodilation and/or methacholine challenge may be needed to identify and further evaluate effects of WTC exposure. Biomarker studies may also be more informative in delineating exposure-outcome relationships.
Figure 4.
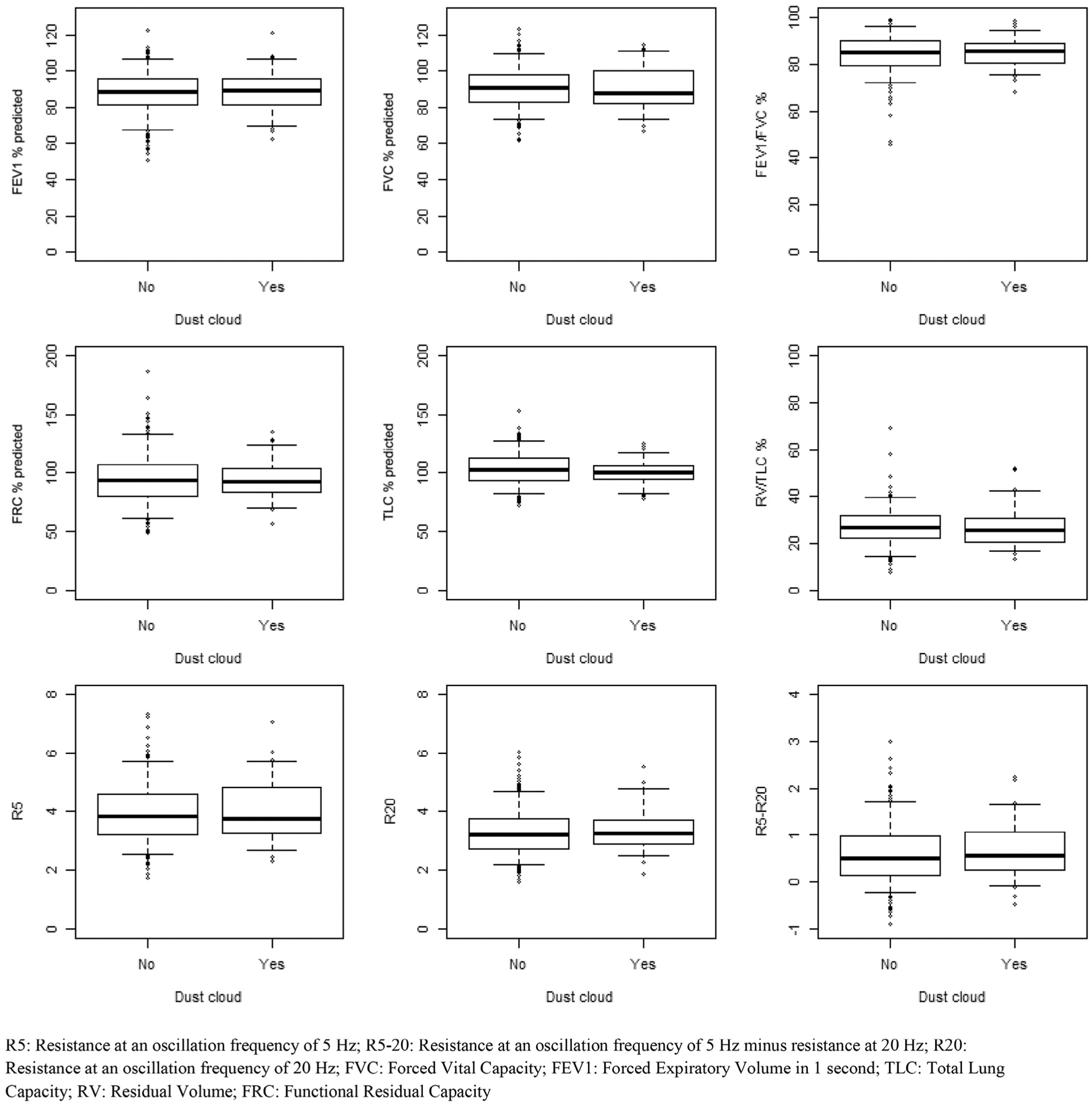
Pulmonary function outcomes comparison by dust cloud exposure.
Figure 5.
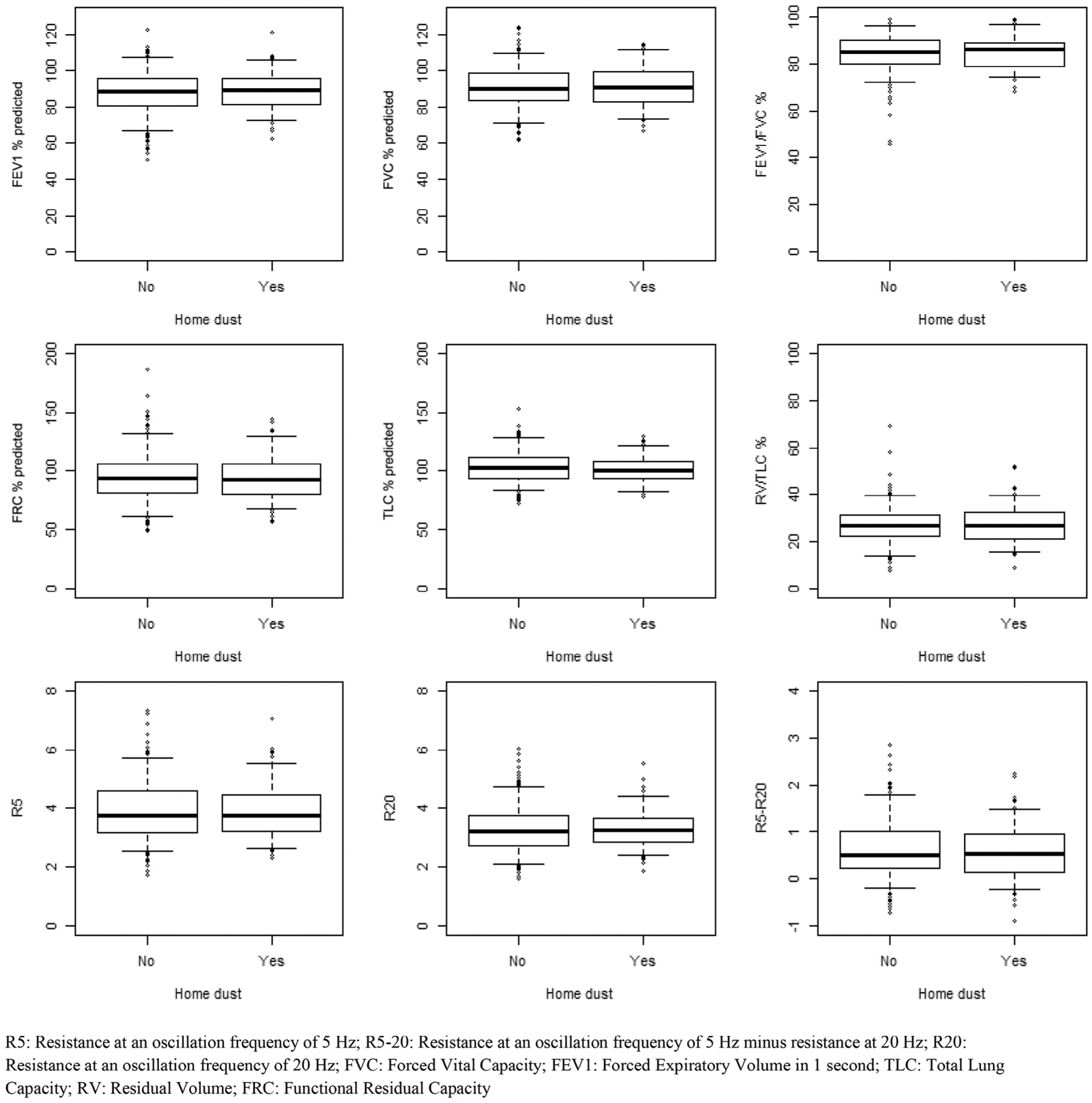
Pulmonary function outcomes comparison by home dust exposure.
Acknowledgments
We thank the staff, coinvestigators, participants, and volunteers for their time and support.
Supported by the Centers for Disease Control and Prevention/National Institute of Occupational Safety and Health, through cooperative agreements U01OH01394 and U01OH01714. The funding organizations had no roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Glossary
- BMI
Body mass index
- FEV1
Forced expiratory volume in 1 second
- FOT
Forced oscillation technique
- FVC
Forced vital capacity
- FDR
Frequency dependence of resistance
- NHANES
National Health and Nutrition Examination Survey
- NYU
New York University
- PFAS
Perfluoroalkyl substances
- WTC
World Trade Center
- WTCHR
World Trade Center Health Registry
Appendix
Study Population Data
Population.
The study population consisted of (1) a cohort of New York City residents enrolled in the World Trade Center Health Registry (WTCHR) with dates of birth on or between September 11, 1993 and September 10, 2001 and (2) a comparison cohort of individuals born during the same time period, who were ineligible for enrollment in the WTCHR because they either did not reside south of Canal Street, did not attend school south of Canal Street, and were not present south of Chambers Street on the morning of 9/11.
To enroll members of the WTCHR cohort in the present study, WTCHR staff of the New York City Department of Health (NYC DOHMH) who were fluent in English, Spanish, Mandarin, or Cantonese attempted contacts by mail, email, phone, and in-person visits. Both a hard-copy letter and brochure describing the study were mailed to each potential participant. Two weeks after the mailing, phone calls were initiated to individuals who had not responded to the mailed invitation to participate. Calls were made to all known telephone numbers, and calls were attempted at different hours of the day and evening, and on different days of the week. Emails were sent to potential participants who did not respond to mail or telephone contacts. If there was no response to emails, then Lexis-Nexis (RELX Group: New York City, New York) search tools were used to identify new contact information. If new contact information was identified, then telephone and/or email contact were reinitiated. If no new contact information was obtained from tracing, 2 WTCHR staff members attempted a home visit to the last known place of residence. In all methods of contact, WTCHR staff described the study and invited individuals to call the WTCHR or New York University (NYU) School of Medicine staff to further discuss study details and make an appointment. For participants less than 18 years of age, a parent or guardian was required to schedule an appointment and be available and present to authorize participation on the scheduled visit date.
For our comparison group, we recruited individuals who were not eligible for enrollment in the WTCHR. To maximize comparability between the 2 study populations, we developed a table of the desired frequencies of controls by age (0–2, 3–5, or 6–8 years-old on 9/11/2001), sex, race (White, African American, Asian, other), ethnicity (Hispanic, non-Hispanic), and income (<$25 000, ≥$25 000), assuming that the enrolled group of WTCHR participants would reflect participants in the WTCHR’s most recent (2011–2012) survey cycle. Three modes of recruitment were employed to recruit the frequency-matched comparison group: (1) well visits at pediatric clinics affiliated with NYU School of Medicine; (2) contact through health fairs, youth organizations, and postings in areas where youth congregate, posting and advertisements at local colleges; and (3) social media outreach by West Coast Clinical Trials Global, a contract research organization. Participants’ eligibility and ability to fill slots in the frequency-matching table were assessed using a screening questionnaire, which staff conducted over the phone or in person. Individuals were excluded from the present study as matched comparisons if they would have qualified for WTCHR enrollment because of place of residence or school, or having been in the vicinity of the WTC towers on 9/11/2001.
The study was reviewed and approved by the NYU School of Medicine Institutional Review Board, as well as research committees at Bellevue and Gouverneur Hospital Centers. The NYC DOHMH Institutional Review Board identified this study not to involve human subject activity by NYC DOHMH staff. In addition to parental consent on behalf of minors, assent was obtained from adolescents prior to initiation of the study procedures. A Certificate of Confidentiality was obtained to protect participant privacy. The study was approved by New York State Department of Health for the analysis of serum samples.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Friedman SM, Maslow CB, Reibman J, Pillai PS, Goldring RM, Farfel MR, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med 2011;184:582–9. [DOI] [PubMed] [Google Scholar]
- 2.Kazeros A, Zhang E, Cheng X, Shao Y, Liu M, Qian M, et al. Systemic inflammation associated with world trade center dust exposures and airway abnormalities in the local community. J Occup Environ Med 2015;57:610–6. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheimer BW, Goldring RM, Herberg ME, Hofer IS, Reyfman PA, Liautaud S, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007;132:1275–82. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PA, Brackbill R, Thalji L, DiGrande L, Campolucci S, Thorpe L, et al. Respiratory and other health effects reported in children exposed to the World Trade Center disaster of 11 September 2001. Environ Health Perspect 2008;116:1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szema AM, Savary KW, Ying BL, Lai K. Post 9/11: high asthma rates among children in Chinatown, New York. Allergy Asthma Proc 2009;30:605–11. [DOI] [PubMed] [Google Scholar]
- 6.Trasande L, Fiorino EK, Attina T, Berger KI, Goldring RM, Chemtob C, et al. Associations of World Trade Center exposures with pulmonary and cardiometabolic outcomes among children seeking care for health concerns. Sci Total Environ 2013;444:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007;370:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the busselton health study. Am J Respir Crit Care Med 2005;171:109–14. [DOI] [PubMed] [Google Scholar]
- 9.Caplan-Shaw CE, Yee H, Rogers L, Abraham JL, Parsia SS, Naidich DP, et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med 2011;53:981–91. [DOI] [PubMed] [Google Scholar]
- 10.The Childhood Asthma Management Program Research Group. Long term effects of budesonide or nedocromil in children with asthma. NEJM 2000;343:1054–63. [DOI] [PubMed] [Google Scholar]
- 11.Trasande L, Koshy TT, Gilbert J, Burdine LK, Attina TM, Ghassabian A, et al. Serum perfluoroalkyl substances in children exposed to the world trade center disaster. Environ Res 2017;154:212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinbami LJ, Schoendorf KC, Parker JJ. US childhood asthma prevalence estimates: the Impact of the National Health Interview Survey redesign. Am J Epidemiol 2003;158:99–104. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Psychological and emotional effects of the September 11 attacks on the World Trade Center—Connecticut, New Jersey, and New York, 2001. MMWR Morb Mortal Wkly Rep 2002;51:784–6. [PubMed] [Google Scholar]
- 14.Koru-Sengul T, Clark JD, Ocasio MA, Wanner A, Fleming LE, Lee DJ. Utilization of the National Health and Nutrition Examination (NHANES) Survey for symptoms, tests, and diagnosis of chronic respiratory diseases and assessment of second hand smoke exposure. Epidemiology (Sunnyvale) 2011;1:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson J, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U. S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrino R, Viegi G, Brusasco V. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 17.Beydon N Assessment of bronchial responsiveness in preschool children. Paediatr Respir Rev 2006;7:S23–5. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal M, Cramer D, Bain SH, Denison D, Bush A, Warner JO. Lung function in white children aged 4 to 19 years: II-Single breath analysis and plethysmography. Thorax 1993;48:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducharme FM, Davis GM, Ducharme GR. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest 1998;113:1322–8. [DOI] [PubMed] [Google Scholar]
- 20.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest 2005;128:1266–73. [DOI] [PubMed] [Google Scholar]
- 21.Malmberg L, Mieskonen S, Pelkonen A, Kari A, Sovijarvi A, Turpeinen M. Lung function measured by the oscillometric method in prematurely born children with chronic lung disease. Eur Respir J 2000;16:598–603. [DOI] [PubMed] [Google Scholar]
- 22.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol 2008;43:3–18. [DOI] [PubMed] [Google Scholar]
- 23.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60. [DOI] [PubMed] [Google Scholar]
- 24.Solymar L, Landser FJ, Duiverman E. Measurement of resistance with the forced oscillation technique. Eur Respir J Suppl 1989;4:150S–3S. [PubMed] [Google Scholar]
- 25.Bates JH, Lutchen KR. The interface between measurement and modeling of peripheral lung mechanics. Respir Physiol Neurobiol 2005;148:153–64. [DOI] [PubMed] [Google Scholar]
- 26.Fredberg JJ, Mead J. Impedance of intrathoracic airway models during low-frequency periodic flow. J Appl Physiol Respir Environ Exerc Physiol 1979;47:347–51. [DOI] [PubMed] [Google Scholar]
- 27.Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol 2005;148:179–94. [DOI] [PubMed] [Google Scholar]
- 28.Kjeldgaard JM, Hyde RW, Speers DM, Reichert WW. Frequency dependence of total respiratory resistance in early airway disease. Am Rev Respir Dis 1976;114:501–8. [DOI] [PubMed] [Google Scholar]
- 29.Oppenheimer BW, Goldring RM, Berger KI. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. COPD 2009;6:162–70. [DOI] [PubMed] [Google Scholar]
- 30.van den Elshout FJ, van Herwaarden CL, Folgering HT. Oscillatory respiratory impedance and lung tissue compliance. Respir Med 1994;88:343–7. [DOI] [PubMed] [Google Scholar]
- 31.Comer JS, Fan B, Duarte CS. Attack-related life disruption and child psychopathology in New York City public schoolchildren 6-months post-9/11. J Clin Child Adolesc Psychol 2010;39:460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss RS. Environmental tobacco smoke and serum vitamin C levels in children. Pediatrics 2001;107:540–2. [DOI] [PubMed] [Google Scholar]
- 33.Wilson KM, Finkelstein JN, Blumkin AK, Best D, Klein JD. Micronutrient levels in children exposed to secondhand tobacco smoke. Nicotine Tob Res 2011;13:800–8. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheimer BW, Berger KI, Segal LN, Stabile A, Coles KD, Parikh M, et al. Airway dysfunction in obesity: response to voluntary restoration of end expiratory lung volume. PLoS ONE 2014;9:e88015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Kant KD, Paredi P, Meah S, Kalsi HS, Barnes PJ, Usmani OS. The effect of body weight on distal airway function and airway inflammation. Obes Res Clin Pract 2016;10:564–73. [DOI] [PubMed] [Google Scholar]
- 36.Berger KI, Kalish S, Shao Y, Marmor M, Kazeros A, Oppenheimer BW, et al. Isolated small airway reactivity during bronchoprovocation as a mechanism for respiratory symptoms in WTC dust-exposed community members. Am J Ind Med 2016;59:767–76. [DOI] [PubMed] [Google Scholar]
- 37.Fleiss JL, Shrout PE. The effects of measurement errors on some multivariate procedures. Am J Public Health 1977;67:1188–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liew Z, Goudarzi H, Oulhote Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 2018;5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye L, Zhao B, Cai XH, Chu Y, Li C, Ge RS. The inhibitory effects of perfluoroalkyl substances on human and rat 11beta-hydroxysteroid dehydrogenase 1. Chem Biol Interact 2012;195:114–8. [DOI] [PubMed] [Google Scholar]
- 40.Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Biomarkers of insecticide exposure and asthma in children: a National Health and Nutrition Examination Survey (NHANES) 1999–2008 Analysis. Archiv Environ Occup Health 2015;70:309–22. [DOI] [PubMed] [Google Scholar]
- 41.Humblet O, Diaz-Ramirez LG, Balmes JR, Pinney SM, Hiatt RA. Perfluoroalkyl chemicals and asthma among children 12–19 years of age: NHANES (1999–2008). Environ Health Perspect 2014;122:1129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


