Abstract
Background:
Insufficient or excessive gestational weight gain are associated with increased risks of adverse birth and childhood outcomes. Increasing evidence suggests that exposure to bisphenols and phthalates may disrupt hormonal pathways and thereby influence gestational weight gain.
Objective:
To examine the associations of early and mid-pregnancy bisphenol and phthalate urine concentrations with gestational weight gain.
Methods:
In a population-based prospective cohort study among 1,213 pregnant women, we measured early and mid-pregnancy bisphenol and phthalate urine concentrations. Maternal anthropometrics before pregnancy were obtained by questionnaire and repeatedly measured at our research center during pregnancy. We used linear and logistic regressions to evaluate the associations of bisphenols and phthalates with total and period-specific gestational weight gain.
Results:
Higher maternal total bisphenols and bisphenol S were associated with a lower total gestational weight gain at nominal level. Stratification by body mass index group showed that higher total bisphenols and bisphenol S were associated with lower total gestational weight gain specifically in normal weight women (respectively −509 g [95% CI −819, −198] and −398 g [95% CI −627, −169]). Each log unit increase in early pregnancy total bisphenol and bisphenol A urine concentrations were associated with lower mid- to late pregnancy gestational weight gain in the whole group (effect estimates −218 g/log unit increase [95% CI −334, −102] and −132 g/log unit increase [95% CI −231, −34], respectively). These associations were independent of mid-pregnancy compounds. Mid-pregnancy bisphenols and phthalates concentrations were not associated with gestational weight gain.
Discussion:
Higher maternal bisphenol urine concentrations in early pregnancy may lead to reduced gestational weight in second half of pregnancy. Further research is needed to assess the effects of maternal bisphenols and phthalates urine concentrations on placental and fetal growth and development.
Keywords: Bisphenol, Phthalate, Gestational weight gain, Cohort studies
1. Background
Insufficient or excessive gestational weight gain are associated with increased risks of adverse birth and childhood outcomes. The US Institute of Medicine and others have established criteria for excessive as well as insufficient gestational weight gain, recognizing a substantial literature documenting increases in adverse pregnancy, birth and offspring outcomes among women with excessive and insufficient gestational weight gain (Gaillard et al., 2013; Hrolfsdottir et al., 2015; Kaimura et al., 2017; Marchi et al., 2015; Santos et al., 2019).
Gestational weight gain is a multifactorial phenotype. Risk factors for excessive gestational weight gain include nulliparity, higher total energy intake and smoking during pregnancy (Gaillard et al., 2013; Nohr et al., 2008). Studies reporting associations of increased maternal progesterone and leptin levels with greater gestational weight gain suggest that hormonal responses may be important mechanisms contributing to insufficient or excessive gestational weight gain (Lacroix et al., 2016; Lof et al., 2009). A substantial literature has suggested that synthetic chemicals, such as bisphenols and phthalates, can disrupt hormones and thereby influence gestational weight gain (Buser et al., 2014; Cantonwine et al., 2014; Harley et al., 2013; Philips et al., 2017; Trasande et al., 2012; Valvi et al., 2015). For example, mono-ethyl phthalate (MEP) has been associated with lower maternal progesterone levels in the second trimester of pregnancy (Johns et al., 2015). Higher maternal progesterone levels have been associated with increased gestational weight gain (Lof et al., 2009). A study in mice reported increased leptin concentrations in pregnant mice exposed to bisphenol A (BPA) (Alonso-Magdalena et al., 2010). Exposure to bisphenols and phthalates can be modified through behavioral modifications as well as regulatory action (Carwile et al., 2009; Carwile et al., 2011; Harley et al., 2016; Rudel et al., 2011). To our knowledge, the associations of bisphenol and phthalate concentrations with maternal gestational weight gain have not been studied yet.
We examined among 1,213 women participating in a population-based prospective cohort study the associations of early and mid-pregnancy bisphenol and phthalate urine concentrations with total and period-specific gestational weight gain and the risks of insufficient or excessive gestational weight gain.
2. Methods
2.1. Study design and population for analysis
The present study was embedded in the Generation R Study, a population-based prospective cohort study from early pregnancy onwards (Kooijman et al., 2016). In total, 8,879 women were enrolled in pregnancy, of which 76% before a gestational age of 18 weeks. The study has been approved by the Medical Ethical Committee of the Erasmus Medical Center in Rotterdam. Written consent was obtained from all participating women (World Medical Association 2013). Bisphenol and phthalate urine concentrations were measured in a subgroup study among 1,406 mothers with an available early or mid-pregnancy urine sample and whose children participated in postnatal studies. This subgroup included singleton pregnancies only. We excluded women without an available urine sample at both time points, without information on gestational weight gain until late pregnancy or total gestational weight gain (n = 193), which led to 1,213 women included in the analysis. For analysis on total gestational weight gain and clinical gestational weight gain categories, we excluded women without information on total gestational weight gain (n = 397), leading to 823 women included in those analyses (Fig. 1).
Fig. 1.
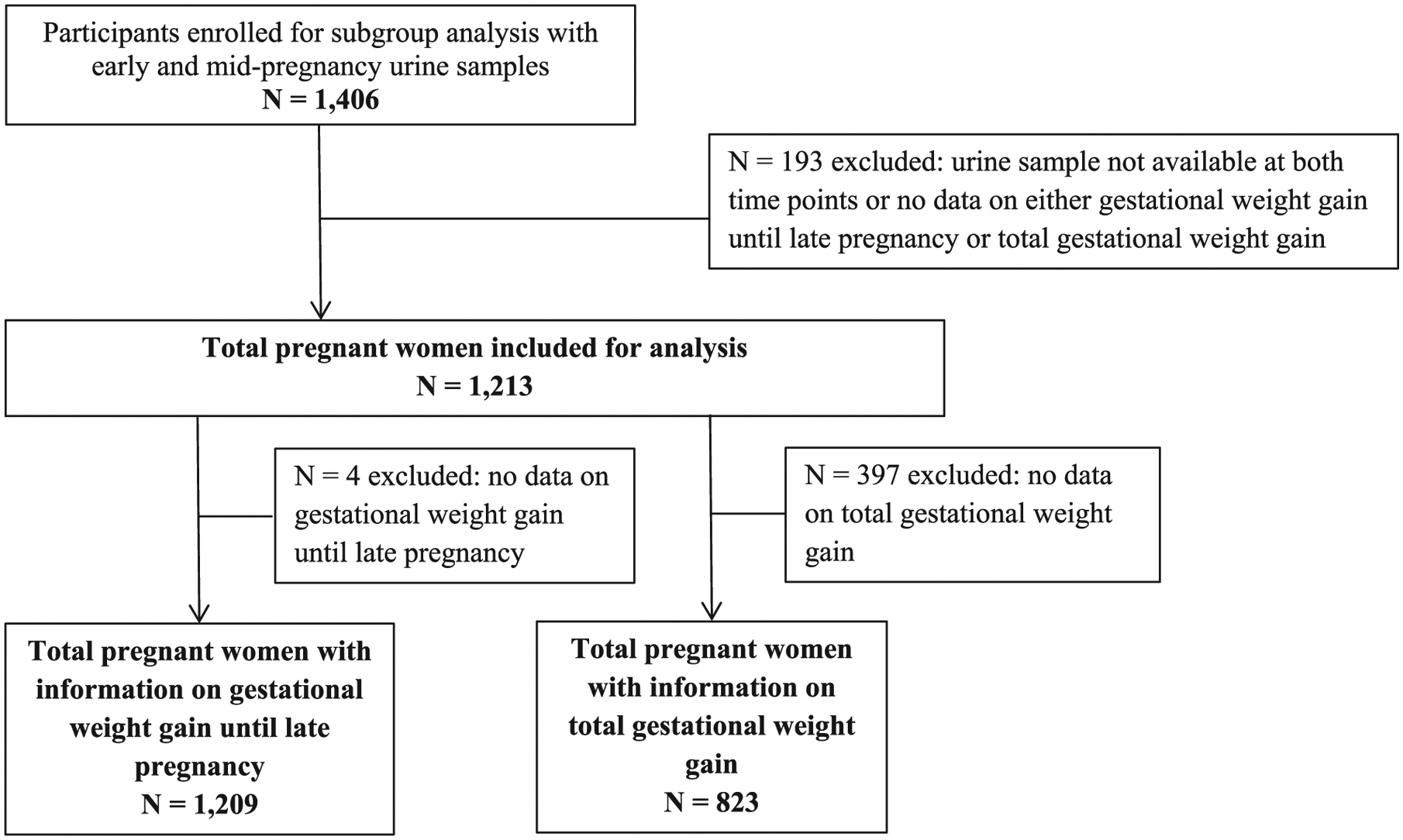
Flowchart.
2.2. Bisphenol and phthalate urine concentrations
As previously described, bisphenol and phthalate concentrations were measured in a spot urine sample obtained from each subject during the early and mid-pregnancy measurement (median gestational age 13.1 weeks [inter-quartile range (IQR) 12.1–14.5 weeks] and 20.4 weeks [IQR 19.9–20.9], respectively). All urine samples were collected between February 2004 and October 2005. Details on collection, transportation and analysis methodology are provided elsewhere (Philips et al., 2018).
We grouped urinary biomarkers for exposure to phthalates according to their use in product categories. These product categories were first personal care products, and second plasticizers to impart flexibility to plastics. Based on these categories, phthalates we grouped in low and high molecular weight phthalates. We calculated the weighted molar sums for low molecular weight (LMW) phthalate, high molecular weight (HMW) phthalate, di-2-ethylhexylphthalate (DEHP) metabolites, and di-n-octylphthalate metabolites. Phthalic acid (PA) was used separately as a proxy of total phthalate exposure. Among HMW phthalates, DEHP is of particular interest because of its widespread use in food packaging (Serrano et al., 2014). DNOP is also of concern because, although banned from use in the European Union since 2005, its primary metabolite, mono(3-carboxypropyl)phthalate (mCPP), is still detectable in biosamples (Casas et al., 2011; Philips et al., 2018). Individual compounds were included in if they were detected in ≥20% of the samples. Also, bisphenols that were detected in ≥50% of the samples were analyzed separately. For bisphenol and phthalate concentrations below the level of detection we substituted the level of detection divided by the square root of 2, as routinely performed in bisphenols and phthalates (Hornung and Reed 1990). Table 1 shows the metabolites that were included in all separate groups, their values and detection rates.
Table 1.
Bisphenol and phthalate urinary concentrations (n = 1,213).
| Early pregnancy (< 18 weeks) Median (IQR) (ng/mL) | Percentage of values below the limit of detection (LOD) | Mid-pregnancy (18–25 weeks) Median (IQR) (ng/mL) | Percentage of values below the limit of detection (LOD) | |
|---|---|---|---|---|
| Total bisphenols1 | 9.31 (3.61, 20.85) | 6.31 (3.04, 13.87) | ||
| Bisphenol A (BPA) | 1.67 (0.71, 3.61) | 21.2 | 1.46 (0.74, 3.19) | 6.7 |
| Bisphenol S (BPS) | 0.35 (0.17, 1.09) | 31.9 | 0.24 (0.12, 0.49) | 70.9 |
| Bisphenol F (BPF) | 0.58 (0.30, 1.31) | 59.6 | NA | 88.5 |
| Phthalic acid (PA) metabolites | 57.38 (31.03, 123.45) | 0.3 | 149.68 (61.74, 280.94) | 0.1 |
| Low molecular weight (LMW) metabolites1 | 1080.01 (425.05, 2940.32) | 586.77 (238.87, 1444.95) | ||
| Monomethylphthalate (mMP) | 5.59 (2.75, 9.85) | 0.2 | 3.47 (1.84, 6.21) | 0.2 |
| Monoethylphthalate (mEP) | 136.55 (41.15, 488.49) | 0.1 | 72.64 (25.05, 222.41) | - |
| Mono-isobutylphthalate (mIBP) | 20.93 (9.52, 45.65) | 0.2 | 8.88 (4.59, 17.80) | - |
| Mono-n-butylphthalate (mBP) | 16.08 (7.01, 30.94) | 0.7 | 9.68 (5.51, 18.91) | - |
| High molecular weight (HMW) metabolites1 | 219.09 (112.60, 403.22) | 131.83 (73.85, 242.94) | ||
| Di-2-ethylhexylphthalate (DEHP) metabolites1 | 171.59 (89.23, 323.30) | 96.82 (53.12, 183.72) | ||
| Mono-(2-ethyl-5-carboxypentyl)phthalate (mECPP) | 16.09 (8.25, 31.29) | 0.2 | 10.45 (5.77, 19.98) | 0.1 |
| Mono-(2-ethyl-5-hydroxyhexyl)phthalate (mEHHP) | 11.84 (5.76, 22.80) | 0.2 | 5.57 (2.96, 10.68) | 0.1 |
| Mono-(2-ethyl-5-oxohexyl)phthalate (mEOHP) | 7.75 (3.54, 15.34) | 0.1 | 7.44 (3.68, 16.30) | - |
| Mono-[(2-carboxymethyl)hexyl]phthalate (mCMHP) | 14.06 (7.60, 26.36) | 0.1 | 4.02 (2.27, 7.38) | 0.2 |
| Di-n-octylphthalate (DNOP) | 5.78 (3.17, 10.81) | 3.53 (2.06, 6.77) | ||
| Mono(3-carboxypropyl)phthalate (mCPP) | 1.45 (0.80, 2.71) | 0.2 | 0.89 (0.52, 1.70) | 0.1 |
| Other high molecular weight metabolites | ||||
| Monobenzylphthalate (mBzP) | 6.40 (3.06, 12.55) | 8.0 | 5.27 (2.29, 11.19) | 1.5 |
| Mono-hexylphthalate (mHxP) | 0.33 (0.16, 0.62) | 23.9 | NA | 98.7 |
| Mono-2-heptylphthalate (mHpP) | 1.09 (0.58, 2.33) | 35.4 | NA | 96.8 |
NA: not applicable; bisphenol or phthalate is not included in the group due to > 80% below the limit of detection.
Groups are molar concentrations in nmol/L with non-detectable levels of separate metabolites imputed as LOD/sqr(2). Separate metabolites are included only if < 80% of values was below the LOD.
2.3. Maternal anthropometrics
Maternal height (cm) and weight (kg) were measured at enrollment without shoes and heavy clothing and body mass index (kg/m2) was calculated. Weight was measured repeatedly during subsequent visits at the research center (early pregnancy median gestational age 13.1 weeks [IQR 12.1, 14.5], mid pregnancy median 20.4 weeks [IQR 19.9, 20.9], and late pregnancy median 30.2 weeks [IQR 29.9, 30.8]). Information on maternal weight just before pregnancy was obtained by questionnaire. In our population for analysis, 68.2% of all women were enrolled before a gestational of 14 weeks. Information on total weight during pregnancy was assessed by questionnaire 2 months after delivery (median gestational age at delivery 40.3 [IQR 39.3, 41.0]). Total gestational weight gain was calculated as the difference between the highest weight before birth and pre-pregnancy weight and was available in a subgroup of 823 mothers. For sensitivity analysis, gestational weight gain until the late pregnancy visit was calculated as the difference between late pregnancy weight and pre-pregnancy weight and was available for 1,209 mothers. Correlation of late pregnancy weight and total weight was 0.96 (P-value < 0.001).
According to the IOM guidelines, we classified total gestational weight gain as insufficient, sufficient and excessive in relation to maternal pre-pregnancy BMI (Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines 2009). Weight gain was further analyzed in specific periods of pregnancy (weight gain between the measured weight at the early and mid-pregnancy visit; weight gain between the measured weight at the mid- and late pregnancy visit; and weight gain between the measured weight at the late pregnancy visit and reported total pregnancy weight).
2.4. Covariates
Covariates were selected based on previous analyses of potential determinants of first trimester bisphenol and phthalate concentrations (Philips et al., 2018). Information on maternal age at enrollment, educational level, ethnicity, parity, pre-pregnancy weight, and folic acid supplementation use was obtained from the first questionnaire at enrollment. Information on smoking and alcohol consumption was assessed by questionnaires in each trimester (Jaddoe et al., 2010). Maternal daily dietary intake was assessed at enrollment using a modified version of the validated semi-quantitative food-frequency questionnaire (FFQ) of Klipstein-Grobusch et al. (Klipstein-Grobusch et al., 1998). The FFQ covered the average dietary intake over the previous three months, covering the dietary intake in the first trimester of pregnancy (Tielemans et al., 2016). We used caloric intake derived from the FFQ as a covariate in statistical analyses.
2.5. Statistical analysis
Differences in subject characteristics between groups of gestational weight gain were assessed using one-way ANOVA tests for continuous variables and chi-square tests for proportions. Non-response analysis was performed to assess distributions of maternal characteristics and investigated outcomes. For the main analyses, all bisphenol and phthalate urinary metabolite concentrations were log-transformed to account for right skewness in the distribution.
We performed multivariable linear and multinomial logistic regressions to evaluate associations of early and mid-pregnancy urinary concentrations with total gestational weight gain continuously, gestational weight gain per pregnancy period and clinical categories of gestational weight gain. To investigate total gestational weight gain continuously and in clinical categories, early and mid-pregnancy bisphenol and phthalate groupings were used simultaneously to examine the relative influence of early versus mid-pregnancy urinary concentrations. When testing associations of gestational weight gain in specific pregnancy periods, metabolite concentrations of all earlier time points were added simultaneously to the model to adjust for measures at other visits. Therefore, models for early-to-mid-pregnancy gestational weight gain included metabolite concentrations in early pregnancy only. Because detection rates of bisphenol S (BPS) dropped below 50% in mid-pregnancy, early pregnancy BPS concentrations were adjusted for total bisphenol concentrations in mid-pregnancy.
For all significant models, subanalyses of individual bisphenol compounds or phthalate metabolites were performed to determine which metabolites were driving the association. Subanalysis of significant models with early and mid-pregnancy concentrations of bisphenols and phthalates used simultaneously were performed with the separate compounds of the significant group together with the total group of the other pregnancy period, to keep models comparable. As a sensitivity analysis, we used multivariable linear regression models to examine the associations between the logs of molar concentrations of the metabolite groups with gestational weight gain until late pregnancy.
In all models, urinary concentrations of each bisphenol or phthalate compound or grouping were converted to μg/g or μmol/g creatinine to adjust for dilution (Barr et al., 2005). All models were adjusted for maternal age, educational level, ethnicity, parity, daily dietary caloric intake, folic acid supplement use, smoking, and alcohol consumption. Higher pre-pregnancy BMI has been associated with a lower gestational weight gain (Santos et al., 2018). Our previous studies showed that higher pre-pregnancy BMI was associated with higher bisphenol and phthalate concentrations in early pregnancy (Philips et al., 2018). Therefore, models with gestational weight gain as outcome were additionally adjusted for pre-pregnancy BMI. To investigate potential effect modification by pre-pregnancy BMI of the associations of bisphenol and phthalate concentrations with gestational weight gain, we have tested interaction terms with categories of pre-pregnancy BMI. Additionally stratified analyses have been performed for significant interactions. Non-linear effects of early and mid-pregnancy metabolite concentrations on total gestational weight gain were assessed using quartiles.
Missing data of the covariates were imputed using multiple imputation. Five imputed data sets were created. Effect estimates were pooled to obtain the overall result, taking into account the within and between imputation variance according to Rubin’s Rules (Rubin 1987). The percentage of missing values within the population for analysis were lower than or equal to 10%, except for maternal folic acid supplementation use (17.0%) and daily dietary caloric intake (23.8%). To correct for multiple hypothesis testing, each p-value was compared with a threshold defined as 0.05 divided by the effective number of independent tests estimated based on the correlation between the exposures (p-value threshold of 0.011) (Li et al., 2012). All analyses were performed using the Statistical Package of Social Sciences version 21.0 for Windows (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Subject characteristics
Mid-pregnancy urine concentrations of bisphenols and phthalates were generally lower than in early pregnancy. Also, detection rates of BPS, bisphenol F (BPF), mono-hexylphthalate (mHxP) and mono-2-heptylphthalate (mHpP) urine concentrations were considerably lower in mid-pregnancy (Table 1). Characteristics of the included mothers are given in Table 2. Of all women, 19.1%, 30.0%, and 50.9% had insufficient, sufficient, and excessive gestational weight gain, respectively. Women with excessive gestational weight gain had a higher pre-pregnancy BMI and were more often younger, smokers, and nulliparous. As shown in Supplementary Table S1, nonresponse analysis showed similar distributions of sociodemographic factors and other risk factors for gestational weight gain in the subgroup study population as in the entire study cohort. However, the subgroup of mothers with information on total gestational weight gain tended to be slightly higher educated and healthier.
Table 2.
Subject characteristics by gestational weight gain classification1
| Total | Insufficient gestational weight gain | Sufficient gestational weight gain | Excessive gestational weight gain | p-value2 | |
|---|---|---|---|---|---|
| n = 1,213 | n = 157 | n = 247 | n = 419 | ||
| Maternal age (years) | 30.6 (4.8) | 31.2 (4.5) | 31.8 (4.1) | 30.6 (4.6) | 0.005 |
| Pre-pregnancy BMI (kg/m2)* | 22.7 (20.8, 25.3) | 22.1 (20.7, 23.9) | 21.9 (20.2, 23.7) | 23.2 (21.0, 25.7) | 0.000 |
| Educational level | 0.020 | ||||
| Low | 583 (48.1) | 70 (45.2) | 85 (34.7) | 187 (45.3) | |
| High | 596 (49.1) | 85 (54.8) | 160 (65.3) | 226 (54.7) | |
| Missings | 35 (2.8) | – | – | – | |
| Ethnicity | 0.751 | ||||
| Dutch/European | 756 (62.3) | 107 (69.0) | 176 (71.3) | 302 (72.2) | |
| Non-European | 452 (37.3) | 48 (31.0) | 71 (28.7) | 116 (27.8) | |
| Missings | 5 (0.4) | – | – | – | |
| Parity | 0.001 | ||||
| Nulliparous | 742 (61.2) | 85 (54.1) | 152 (61.5) | 293 (69.9) | |
| Multiparous | 471 (38.8) | 72 (45.9) | 95 (38.5) | 126 (30.1) | |
| Missings | – | – | – | – | |
| Daily dietary caloric intake (kcal) | 2080 (5 0 8) | 2035 (5 4 8) | 2145 (4 9 0) | 2113 (4 8 5) | 0.163 |
| Creatinine early pregnancy (< 18 weeks) μg/mL)* | 1030 (491, 1661) | 958 (522, 1582) | 1026 (450, 1705) | 1032 (472, 1642) | 0.647 |
| Creatinine mid-pregnancy (18–25 weeks) μg/mL)* | 1164 (740, 1818) | 1291 (814, 2025) | 1222 (676, 1861) | 1106 (709, 1654) | 0.375 |
| Smoking | 0.000 | ||||
| Never | 867 (71.5) | 136 (86.6) | 192 (77.7) | 286 (68.3) | |
| Until pregnancy was known | 108 (8.9) | 7 (4.5) | 19 (7.7) | 50 (11.9) | |
| Continued | 160 (13.2) | 7 (4.5) | 21 (8.5) | 57 (13.6) | |
| Missings | 78 (6.4) | 7 (4.5) | 15 (6.1) | 26 (6.2) | |
| Alcohol consumption | 0.566 | ||||
| Never | 491 (40.5) | 64 (40.8) | 84 (34.0) | 157 (37.5) | |
| Until pregnancy was known | 193 (15.9) | 24 (15.3) | 47 (19.0) | 62 (14.8) | |
| Continued | 451 (37.2) | 63 (40.1) | 101 (40.9) | 172 (41.1) | |
| Missings | 78 (6.4) | 6 (3.8) | 15 (6.1) | 28 (6.7) | |
| Folic acid supplementation | 0.893 | ||||
| No | 191 (15.7) | 18 (11.5) | 28 (11.3) | 51 (12.2) | |
| Start first 10 weeks | 330 (27.2) | 40 (25.5) | 62 (25.1) | 122 (29.1) | |
| Start periconceptional | 484 (39.9) | 72 (45.9) | 116 (47.0) | 189 (45.1) | |
| Missings | 208 (17.1) | 27 (17.2) | 41 (16.6) | 57 (13.6) | |
| Early to-mid pregnancy weight gain (kg)* | 3.0 (2.0, 5.0) | – | – | – | |
| Mid- to late pregnancy weight gain (kg)* | 5.0 (3.5, 7.0) | – | – | – | |
| Late to total pregnancy weight gain (kg)* | 4.5 (3.0, 7.0) | – | – | – | |
| Total gestational weight gain(kg)* | 15.0 (12.0, 18.0) | – | – | – | |
| Gestational weight gain until late pregnancy (kg)* | 10.0 (8.0, 13.0) | – | – | – |
Values are means (standard deviation) or numbers of subjects (percentage). Only women with available information on total gestational weight gain were classified in a total gestational weight gain category (n = 823).
Differences between groups of insufficient, sufficient and excessive gestational weight gain were assessed using one-way ANOVA tests for continuous variables and chi-square tests for proportions.
Median (IQR range)
3.2. Bisphenol and phthalate urine concentrations and gestational weight gain
Early and mid-pregnancy phthalates were not associated with total gestational weight gain (Table 3). For total bisphenols and BPS, associations with a decreased total gestational weight gain were observed at nominal level.
Table 3.
Associations of early and mid-pregnancy bisphenol and phthalate urine concentrations with gestational weight gain (n = 1,213).
| Gestational weight gain (grams) Early to mid-pregnancy, (95% Confidence Interval) (n = 1,205) | Mid- to late pregnancy, (95% Confidence Interval) (n = 1,207)1 | Late pregnancy to total, (95% Confidence Interval) (n = 819)1 | Total (95% Confidence Interval) (n = 823)1 | |
|---|---|---|---|---|
| Early pregnancy (< 18 weeks) | ||||
| Total bisphenols | 0 (−98, 98) | −218 (−334, −102) * † | −82 (−261, 98) | −354 (−641, −68)* |
| Bisphenol A | 17 (−66, 100) | −132 (−231, −34)* † | −54 (−205, 98) | −125 (−367, 117) |
| Bisphenol S2 | −26 (−97, 44) | −76 (−160, 7) | −41 (−169, 87) | −261 (−466, −56)* |
| Phthalic acid | 32 (−84, 147) | −139 (−277, 0) | −131 (−334, 71) | −50 (−375, 274) |
| LMW phthalate metabolites | 63 (−33, 159) | −110 (−230, 9) | −196 (−375, −17)* | −191 (−478, 96) |
| HMW phthalate metabolites | 13 (−113, 140) | −133 (−285, 18) | −175 (−411, 61) | −268 (−646, 111) |
| DEHP metabolites | 24 (−100, 147) | −122 (−270, 27) | −183 (−413, 47) | −259 (−627, 109) |
| DNOP metabolites | 40 (−83, 162) | −176 (−324, −29)* | −218 (−436, −1)* | −319 (−666, 29) |
| Mid-pregnancy (18-25 weeks) | ||||
| Total bisphenols | – | −119 (−251, 14) | 161 (−35, 356) | 143 (−168, 453) |
| Bisphenol A | – | −112 (−238, 14) | 151 (−36, 338) | 147 (−150, 444) |
| Bisphenol S | – | – | – | – |
| Phthalic acid | – | −125 (−271, 21) | 217 (−6, 440) | 33 (−323, 389) |
| LMW phthalate metabolites | – | −86 (−221, 49) | 145 (−56, 346) | 60 (−262, 381) |
| HMW phthalate metabolites | – | −149 (−304, 5) | 112 (−129, 353) | −30 (−415, 354) |
| DEHP metabolites | – | −140 (−292, 11) | 156 (−81, 393) | 64 (−315, 444) |
| DNOP metabolites | – | −68 (−235, 99) | 96 (−149, 342) | −112 (−505, 280) |
Estimates are based on multivariate regression analyses. Increases are per log unit increase in early and mid-pregnancy urinary Total bisphenols/BPA/BPS/Phthalic acid/LMW/HMW/DEHP/DNOP metabolite concentrations per gram creatinine. All models are adjusted for maternal age, maternal pre-pregnancy BMI, daily dietary caloric intake, parity, ethnicity, education, maternal smoking, maternal alcohol, and folic acid supplementation. In total, 1,213 women are included in the analyses in this table. Due to random nonresponse, not all women had available information about all the weights.
Early and mid-pregnancy compounds have been used in the model simultaneously, yielding estimates adjusted for compounds at the other time point.
For models of early pregnancy BPS, the total group of mid-pregnancy bisphenols has been used in the model simultaneously, if applicable. Estimates for mid-pregnancy total bisphenols in these models are not presented.
p-value < 0.05
significant after multiple testing correction
We observed effect modification by pre-pregnancy BMI of the associations of bisphenol and phthalate concentrations with total gestational weight gain (statistical interaction p-value < 0.1) for early pregnancy total bisphenols, BPA, BPS, PA, LMW phthalate metabolites and DEHP metabolites (data not shown). Further stratification yielded significant results for total bisphenols and BPS in the normal weight group with a decreased total gestational weight gain (respectively −509 g (95% CI −819, −198) and −398 g (95% CI −627, −169), both p-value = 0.001). To illustrate, an interquartile range increase in total bisphenols was associated with −864 g (95% CI −1391, −336) decrease in total gestational weight gain among normal weight women. Because the numbers per stratum were low for underweight and obese women these analyses were not presented as main analyses. Assessment of potential non-linear association of early and mid-pregnancy bisphenol and phthalate concentrations using quartiles did not reveal any indications of non-linearity (data not shown).
Each log unit increase in early pregnancy total bisphenol urine concentrations was associated with −218 g (95% CI −334, −102) gestational weight gain in mid- to late pregnancy. Analysis of individual bisphenol compounds in early pregnancy showed that maternal BPA concentrations were driving this association with a −132 g (95% CI −231, −34) lower mid- to late pregnancy weight gain/log unit increase. The associations of early pregnancy BPS and BPF urine concentrations with gestational weight gain in mid-to-late pregnancy tended toward nominal significance (Table 3 and Supplementary Table S2). Early pregnancy DNOP metabolite concentrations were associated with mid- to late pregnancy weight gain at nominal level. Bisphenol and phthalate concentrations in early and mid-pregnancy were not associated with early-to-mid-pregnancy weight gain or late pregnancy-to-total gestational weight gain. We did not observe effect modification by pre-pregnancy BMI for the analyses on gestational weight gain during specific periods of pregnancy (data not shown).
3.3. Bisphenol and phthalate levels and clinical categories of gestational weight gain
Table 4 shows that bisphenol and phthalate urine concentrations in early and mid-pregnancy were not associated with insufficient or excessive gestational weight gain. Early pregnancy LMW phthalate metabolites were associated with higher odds of insufficient gestational weight gain, However, this associations attenuated into non-significance correction for multiple testing.
Table 4.
Associations of early and mid-pregnancy bisphenol and phthalate urine concentrations with clinical categories of gestational weight gain (n = 823).
| Insufficient weight gain, Odds Ratio (95% Confidence Interval) (n = 157) | Excessive weight gain, Odds Ratio (95% Confidence Interval) (n = 419) | |
|---|---|---|
| Early pregnancy (< 18 weeks) | ||
| Total bisphenols | 0.96 (0.82, 1.13) | 0.91 (0.80, 1.03) |
| Bisphenol A | 0.97 (0.84, 1.11) | 0.96 (0.86, 1.07) |
| Bisphenol S1 | 1.03 (0.92, 1.15) | 0.96 (0.88, 1.06) |
| Phthalic acid | 1.18 (0.98, 1.41) | 1.11 (0.96, 1.28) |
| LMW phthalate metabolites | 1.18 (1.01, 1.39)* | 1.03 (0.91, 1.17) |
| HMW phthalate metabolites | 1.16 (0.94, 1.43) | 1.00 (0.84, 1.18) |
| DEHP metabolites | 1.16 (0.94, 1.42) | 1.00 (0.85, 1.18) |
| DNOP metabolites | 1.15 (0.95, 1.40) | 0.97 (0.83, 1.13) |
| Mid pregnancy (18-25 weeks) | ||
| Total bisphenols | 0.94 (0.79, 1.12) | 1.02 (0.89, 1.17) |
| Bisphenol A | 0.94 (0.79, 1.11) | 1.03 (0.90, 1.17) |
| Bisphenol S | – | – |
| Phthalic acid | 0.97 (0.80, 1.19) | 1.00 (0.86, 1.17) |
| LMW phthalate metabolites | 0.97 (0.81, 1.16) | 0.97 (0.84, 1.12) |
| HMW phthalate metabolites | 0.92 (0.75, 1.14) | 0.97 (0.82, 1.14) |
| DEHP metabolites | 0.91 (0.74, 1.12) | 0.98 (0.83, 1.15) |
| DNOP metabolites | 0.96 (0.78, 1.19) | 0.88 (0.74, 1.04) |
Estimates are based on multivariate regression analyses. Reference category is sufficient weight gain. Only women with available information on total gestational weight gain were classified in a total gestational weight gain category. Increases are per log unit increase in early and mid-pregnancy urinary total bisphenols/BPA/BPS/Phthalic acid/LMW/HMW/DEHP/DNOP metabolite concentrations per gram creatinine. Models are adjusted for maternal age, daily dietary caloric intake, parity, ethnicity, education, maternal smoking, maternal alcohol, and folic acid supplementation. Early and mid-pregnancy compounds have been used in the model simultaneously, yielding estimates adjusted for compounds at the other time point.
For models of early pregnancy BPS, the total group of mid-pregnancy bisphenols has been used in the model simultaneously, if applicable. Estimates for second trimester total bisphenols in these models are not presented.
p-value < 0.05
3.4. Sensitivity analysis
Sensitivity analysis shows that the associations of early pregnancy bisphenols with gestational weight gain until late pregnancy somewhat attenuated but had the same directionality (Supplementary Table S3). Early pregnancy BPS concentrations were associated with gestational weight gain until late pregnancy at nominal level, adjusted for total bisphenol concentrations in mid-pregnancy. However, this associations attenuated into non-significance after correction for multiple testing.
4. Discussion
Results from this prospective population-based cohort study showed that among normal weight women total gestational weight gain was lower for women with higher total bisphenols or BPS concentrations in early pregnancy, independent of bisphenol concentrations in mid-pregnancy. Early pregnancy total bisphenols and BPA were associated with a lower gestational weight gain in mid- to late pregnancy in the whole group.
4.1. Interpretation of main findings
To the best of our knowledge, this is the first prospective study that examined the associations of maternal bisphenols and phthalates concentrations with gestational weight gain. Our findings suggest that maternal bisphenol concentrations in early pregnancy are associated with a lower gestational weight gain, mainly in second half of pregnancy. Additionally, the findings suggest that women with a normal weight are most vulnerable for effects of early pregnancy bisphenols on gestational weight gain. We did not observe associations of bisphenol and phthalate concentrations with clinical categories of gestational weight gain. An additional analysis suggests that among women with insufficient weight gain each log unit increase in total bisphenols was associated with a stronger reduction in weight gain than in women with sufficient and excessive weight gain (data not shown). Since we have only used early and mid-pregnancy bisphenol and phthalate urine concentrations, we cannot rule out that also late pregnancy bisphenol and phthalate concentrations have a certain effect on gestational weight gain. However, this seems unlikely, since the associations of early pregnancy exposures were independent of mid pregnancy exposure concentrations.
Previous cross-sectional studies investigating determinants of bisphenols and phthalates reported associations of higher concentrations of BPA and phthalates in pregnant women with a higher BMI (Arbuckle et al., 2014; Cantonwine et al., 2014; Lewin et al., 2017; Philips et al., 2018; Valvi et al., 2015). A recent prospective study of pregnant women reported a negative association between DEHP metabolites in early pregnancy and early gestational weight gain (Bellavia et al., 2017). Persistent organic pollutants (POPs) have also been examined for associations with gestational weight gain. Similar to bisphenols and phthalates, the majority of POPs are lipophilic chemicals, except for perfluoroalkyl substances (PFASs) (DeWitt 2015; Stockholm Convention). The results from studies investigating effects of POPs on gestational weight gain show different associations with gestational weight gain for the PFASs and other POPs. Higher perfluorooctanesulfonate (PFOS) levels – a perfluoroalkyl substance - before and in early pregnancy have been associated with a higher gestational weight gain in normal and underweight women, while in overweight women this effect was not observed (Ashley-Martin et al., 2016; Jaacks et al., 2016). Other POPs, including dichlorodiphenyl dichloroethene (DDE), polychlorinated bisphenyls (PCBs) in early pregnancy and neonatal DDE, hexachlorocyclohexanes (HCHs), PCBs and polybrominated diphenyl ethers (PBDEs), have been associated with lower or even insufficient gestational weight gain (Herbstman et al., 2007; Vafeiadi et al., 2014; Vizcaino et al., 2014). Thus our study results add to previous studies suggesting that various environmental exposures in specifically early pregnancy may influence gestational weight gain.
Gestational weight gain is a complex phenotype (Gaillard et al., 2013; Nohr et al., 2008). Besides increased maternal fat storage, several compartments could be responsible for the observed change in gestational weight gain. Information about maternal fat storage, measurements of body composition during pregnancy would be informative. However, measurements of body composition during pregnancy were not available in the current study. In our previous study, we did not observe associations of early pregnancy bisphenol and phthalate concentrations with placental weight at birth (Philips et al., 2019). A previous study within the same cohort suggested lower fetal growth in association with maternal BPA concentrations (Snijder et al., 2013). Gestational weight gain, in particular in mid- and late pregnancy, is associated with birth weight (Ay et al., 2009; Gaillard et al. 2013). In a recent rodent study, early pregnancy BPA exposure was associated with impaired remodeling of the uterine spiral arteries and intrauterine growth restriction (Muller et al., 2018). Altogether, previous studies and our results suggest that higher maternal bisphenol urine concentrations in early pregnancy may lead to reduced gestational weight in second half of pregnancy. Further research is needed to assess the effects of maternal bisphenol and phthalate urine concentrations on different aspects of gestational weight gain, such as placental and fetal growth and development.
4.2. Strengths and limitations
Strengths of this study were the prospective data collection from early pregnancy onwards, large sample size of 1,213 participants with a urine sample in early and mid-pregnancy, and information on gestational weight gain. The subgroup of women with information on total gestational weight gain tended to a slightly higher educated, healthier population, which might have influenced results. However, sensitivity analysis of gestational weight gain until late pregnancy and period-specific gestational weight gain argue against biased estimates. The response rate at baseline was 61% (Kooijman et al., 2016). Although we cannot rule out selection towards a relatively healthy population, selection bias in cohort studies is more likely to arise from loss to follow up rather than from non-response at baseline (Nohr et al., 2006). Additionally, models have been adjusted for several potential proxies for health, reducing the odds of biased estimates due to selection bias. Less variation in our study population than in the general population may have led to underestimation of effect estimates. Repeated exposures were analyzed using multiple regression analysis, enabling investigation of potential windows of vulnerability (Chen et al., 2015). In our analysis, collinearity was not an issue (Supplementary Table S4). Bisphenol and phthalate metabolites were measured in spot urine samples in early and mid-pregnancy and typically have half-lives of less than 24 h (Braun et al., 2013; Mattison et al., 2014). A single spot urine sample for phthalates could reasonably reflect exposure for up to three months (Hauser et al. 2004), but bisphenols have a high temporal variability, even over the day (Vernet et al., 2019). This non-differential misclassification is expected to lead to attenuation bias in dose-response relationships.
A common method to account for dilution of urinary chemical concentrations is via creatinine adjustment (O’Brien et al., 2016). Endogenous creatinine clearance, measured by 24-hr urine collection, remains the most precise estimation of the glomerular filtration rate in pregnant women (Ahmed et al., 2009). A recent study suggested that specific gravity adjustment is a better correction method in pregnant women (MacPherson et al., 2018). Unfortunately, specific gravity measurements were not available. Additional analysis of models without creatinine adjustment yielded similar results (data not shown).
Maternal weight was measured during the visits at our research center. Information on maternal pre-pregnancy weight and total weight during pregnancy was self-reported. Self-reported weight tends to be underestimated, leading to misclassification. Consequently, this might have led to biased estimates. In the period-specific analysis, early-to-mid and mid-to-late pregnancy analyses were based on measured weights only and provide therefore the most reliable estimates. Detailed information on a large number of potential confounding factors was available. Nonetheless, due to the observational design of the study, residual confounding due to unmeasured environmental exposures, socio-demographic or lifestyle factors still might still be an issue.
4.3. Conclusion
Higher maternal bisphenol urine concentrations in early pregnancy may lead to reduced gestational weight in second half of pregnancy. Further research is needed to assess the effects of maternal bisphenols urine concentrations on placental and fetal growth and development.
Supplementary Material
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaborations with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Funding
The general design of the Generation R Study is made possible by financial support from the Erasmus MC, University Medical Center, Rotterdam, the Netherlands, the Organization for Health Research and Development (ZonMw) and the Ministry of Health, Welfare and Sport. This study was supported by grant RO1ES022972 and RO1ES029779-01 from the National Institutes of Health, USA. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. VWVJ received additional grant the European Research Council (ERC Consolidator Grant, ERC-2014-CoG-64916).
Abbreviations:
- BPA
bisphenol A
- BPF
bisphenol F
- BPS
bisphenol S
- DDE
dichlorodiphenyl dichloroethene
- DEHP
Di-2-ethylhexylphthalate
- DNOP
di-n-octylphthalate
- FFQ
food-frequency questionnaire
- GWG
gestational weight gain
- HCHs
hexachlorocyclohexanes
- HMW
high molecular weight
- IQR
inter-quartile range
- LMW
low molecular weight
- mCPP
mono)3-carboxypropyl) phthalate
- MEP
mono-ethyl phthalate
- mHpP
mono-2-heptylphthalate
- mHxP
mono-hexylphthalate
- PA
phthalic acid
- PBDEs
polybrominated diphenyl ethers
- PCBs
polychlorinated bisphenyls
- PFASs
perfluoroalkyl substances
- PFOS
perfluorooctanesulfonate
- POPs
persistant organic pollutions
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.105342.
References
- Ahmed SB, Bentley-Lewis R, Hollenberg NK, Graves SW, Seely EW, 2009. A comparison of prediction equations for estimating glomerular filtration rate in pregnancy. Hypertens Pregnancy 28, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A, 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ. Health Perspect 118, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD, Group MS, 2014. Phthalate and bisphenol A exposure among pregnant women in Canada–results from the MIREC study. Environ. Int 68, 55–65. [DOI] [PubMed] [Google Scholar]
- Ashley-Martin J, Dodds L, Arbuckle TE, Morisset AS, Fisher M, Bouchard MF, Shapiro GD, Ettinger AS, Monnier P, Dallaire R, Taback S, Fraser W, 2016. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int. J. Environ. Res. Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay L, Kruithof CJ, Bakker R, Steegers EA, Witteman JC, Moll HA, Hofman A, Mackenbach JP, Hokken-Koelega AC, Jaddoe VW, 2009. Maternal anthropometrics are associated with fetal size in different periods of pregnancy and at birth. Generation R Study. BJOG 116, 953–963. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavia A, Hauser R, Seely EW, Meeker JD, Ferguson KK, McElrath TF, James-Todd T, 2017. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int. J. Hyg. Environ. Health 220, 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Sathyanarayana S, Hauser R, 2013. Phthalate exposure and children’s health. Curr. Opin. Pediatr 25, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser MC, Murray HE, Scinicariello F, 2014. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int. J. Hyg. Environ. Health 217, 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY, Alshawabkeh AN, Meeker JD, 2014. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ. Int 62, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB, 2009. Polycarbonate bottle use and urinary Bisphenol A concentrations. Env. Health Persp 9, 1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB, 2011. Canned soup consumption and urinary Bisphenol A: A randomized crossover Trial. JAMA 306, 2218–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J, 2011. Project, I. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ. Int 37, 858–866. [DOI] [PubMed] [Google Scholar]
- Chen YH, Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, 2015. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ. Health 14, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, 2015. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances edêds. Humana Press, Springer. [Google Scholar]
- Gaillard R, Durmus B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW, 2013. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 21, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B, 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect 121, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Kogut K, Madrigal DS, Cardenas M, Vera IA, Meza-Alfaro G, She J, Gavin Q, Zahedi R, Bradman A, Eskenazi B, Parra KL, 2016. Reducing phthalate, paraben, and phenol exposure from personal care products in adolescent girls: findings from the HERMOSA intervention study. Environ. Health Perspect [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM, 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect 112, 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR, 2007. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ. Health Perspect 115, 1794–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Hrolfsdottir L, Rytter D, Olsen SF, Bech BH, Maslova E, Henriksen TB, Halldorsson TI, 2015. Gestational weight gain in normal weight women and offspring cardio-metabolic risk factors at 20 years of age. Int. J. Obes (Lond) 39, 671–676. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight gain during pregnancy: reexamining the guidelines. 2009. [Google Scholar]
- Jaacks LM, Boyd Barr D, Sundaram R, Grewal J, Zhang C, Buck Louis GM, 2016. Pre-pregnancy maternal exposure to persistent organic pollutants and gestational weight gain: A prospective cohort study. Int. J. Environ. Res. Public Health 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, van Duijn CM, van der Heijden AJ, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Hofman A, 2010. The Generation R Study: design and cohort update 2010. Eur. J. Epidemiol 25, 823–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD, 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod. Biol. Endocrinol 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaimura M, Oda M, Mitsubuchi H, Ohba T, Katoh T, 2017. Participant Characteristics in the Kumamoto University Regional Center of Japan Environment and Children’s Study (JECS): Association of pregnancy outcomes with pregestational maternal body mass index and maternal weight gain during pregnancy. Nihon Eiseigaku Zasshi 72, 128–134. [DOI] [PubMed] [Google Scholar]
- Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC, 1998. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur. J. Clin. Nutr 52, 588–596. [DOI] [PubMed] [Google Scholar]
- Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, Van IMH, de Jongste JC, Klaver CC, Van der Lugt A, Mackenbach JP, Moll HA, Peeters RP, Raat H, Rings EH, Rivadeneira F, van der Schroeff MP, Steegers EA, Tiemeier H, Uitterlinden AG, Verhulst FC, Wolvius E, Felix JF, Jaddoe VW, 2016. The Generation R Study: design and cohort update 2017. Eur. J. Epidemiol 31, 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M, Battista MC, Doyon M, Moreau J, Patenaude J, Guillemette L, Menard J, Ardilouze JL, Perron P, Hivert MF, 2016. Higher maternal leptin levels at second trimester are associated with subsequent greater gestational weight gain in late pregnancy. BMC Pregnancy Childbirth 16, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A, Arbuckle TE, Fisher M, Liang CL, Marro L, Davis K, Abdelouahab N, Fraser WD, 2017. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int. J. Hyg. Environ. Health 220, 77–85. [DOI] [PubMed] [Google Scholar]
- Li MX, Yeung JM, Cherny SS, Sham PC, 2012. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet 131, 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof M, Hilakivi-Clarke L, Sandin SS, de Assis S, Yu W, Weiderpass E, 2009. Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women. BMC Womens Health 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson S, Arbuckle TE, Fisher M, 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J. Expo. Sci. Environ. Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi J, Berg M, Dencker A, Olander EK, Begley C, 2015. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes. Rev 16, 621–638. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Karyakina N, Goodman M, LaKind JS, 2014. Pharmaco- and toxicokinetics of selected exogenous and endogenous estrogens: a review of the data and identification of knowledge gaps. Crit. Rev. Toxicol 44, 696–724. [DOI] [PubMed] [Google Scholar]
- Muller JE, Meyer N, Santamaria CG, Schumacher A, Luque EH, Zenclussen ML, Rodriguez HA, Zenclussen AC, 2018. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci. Rep 8, 9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr EA, Frydenberg M, Henriksen TB, Olsen J, 2006. Does low participation in cohort studies induce bias? Epidemiology 17, 413–418. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM, 2008. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am. J. Clin. Nutr 87, 1750–1759. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR, 2016. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health. Perspect 124, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VW, Trasande L, 2017. Effects of early exposure to phthalates and bisphenols on cardiometabolic outcomes in pregnancy and childhood. Reprod. Toxicol 68, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, Trasande L, 2017. Bisphenol analogue exposures are widely prevalent in pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ. Int 161, 562–572 [submitted for publication]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Jaddoe VWV, Asimakopoulos AG, Kannan K, Steegers EAP, Santos S, Trasande L, 2018. Bisphenol and phthalate concentrations and its determinants among pregnant women in a population-based cohort in the Netherlands, 2004–5. Environ. Res 161, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips EM, Trasande L, Kahn LG, Gaillard R, Steegers EAP, Jaddoe VWV, 2019. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum. Reprod 34, 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, 1987. Multiple Imputation for Nonresponse in Surveys edêds. John Wiley and Sons, Inc., New York. [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG, 2011. Food Packaging and Bisphenol A and Bis (2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, Chatzi L, Chevrier C, Chrousos GP, Corpeleijn E, Costet N, Crozier S, Doyon M, Eggesbo M, Fantini MP, Farchi S, Forastiere F, Gagliardi L, Georgiu V, Godfrey KM, Gori D, Grote V, Hanke W, Hertz-Picciotto I, Heude B, Hivert MF, Hryhorczuk D, Huang RC, Inskip H, Jusko TA, Karvonen AM, Koletzko B, Kupers LK, Lagstrom H, Lawlor DA, Lehmann I, Lopez-Espinosa MJ, Magnus P, Majewska R, Makela J, Manios Y, McDonald SW, Mommers M, Morgen CS, Moschonis G, Murinova L, Newnham J, Nohr EA, Andersen AN, Oken E, Oostvogels A, Pac A, Papadopoulou E, Pekkanen J, Pizzi C, Polanska K, Porta D, Richiardi L, Rifas-Shiman SL, Roeleveld N, Santa-Marina L, Santos AC, Smit HA, Sorensen TIA, Standl M, Stanislawski M, Stoltenberg C, Thiering E, Thijs C, Torrent M, Tough SC, Trnovec T, van Gelder M, van Rossem L, von Berg A, Vrijheid M, Vrijkotte TGM, Zvinchuk O, van Buuren S, Jaddoe VWV, 2018. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med 16, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergstrom A, Charles MA, Chatzi L, Chevrier C, Chrousos GP, Corpeleijn E, Costa O, Costet N, Crozier S, Devereux G, Doyon M, Eggesbo M, Fantini MP, Farchi S, Forastiere F, Georgiu V, Godfrey KM, Gori D, Grote V, Hanke W, Hertz-Picciotto I, Heude B, Hivert MF, Hryhorczuk D, Huang RC, Inskip H, Karvonen AM, Kenny LC, Koletzko B, Kupers LK, Lagstrom H, Lehmann I, Magnus P, Majewska R, Makela J, Manios Y, McAuliffe FM, McDonald SW, Mehegan J, Melen E, Mommers M, Morgen CS, Moschonis G, Murray D, Ni Chaoimh C, Nohr EA, Nybo Andersen AM, Oken E, Oostvogels A, Pac A, Papadopoulou E, Pekkanen J, Pizzi C, Polanska K, Porta D, Richiardi L, Rifas-Shiman SL, Roeleveld N, Ronfani L, Santos AC, Standl M, Stigum H, Stoltenberg C, Thiering E, Thijs C, Torrent M, Tough SC, Trnovec T, Turner S, van Gelder M, van Rossem L, von Berg A, Vrijheid M, Vrijkotte T, West J, Wijga AH, Wright J, Zvinchuk O, Sorensen T, Lawlor DA, Gaillard R, Jaddoe V, 2019. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European North American and Australian cohorts. BJOG 126, 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S, 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder CA, Heederik D, Pierik FH, Hofman A, Jaddoe VW, Koch HM, Longnecker MP, Burdorf A, 2013. Fetal growth and prenatal exposure to bisphenol A: the generation R study. Environ. Health Perspect 121, 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockholm Convention. Protecting human health and the environment from persistent organic pollutants. United Nations Environment Programme. [Google Scholar]
- Tielemans MJ, Steegers EA, Voortman T, Jaddoe VW, Rivadeneira F, Franco OH, Kiefte-de Jong JC, 2016. Protein intake during pregnancy and offspring body composition at 6 years: the Generation R Study. Eur J Nutr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J, 2012. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 308, 1113–1121. [DOI] [PubMed] [Google Scholar]
- Vafeiadi M, Vrijheid M, Fthenou E, Chalkiadaki G, Rantakokko P, Kiviranta H, Kyrtopoulos SA, Chatzi L, Kogevinas M, 2014. Persistent organic pollutants exposure during pregnancy, maternal gestational weight gain, and birth outcomes in the mother-child cohort in Crete, Greece (RHEA study). Environ. Int 64, 116–123. [DOI] [PubMed] [Google Scholar]
- Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M, 2015. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int. J. Hyg. Environ. Health 218, 220–231. [DOI] [PubMed] [Google Scholar]
- Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, Hainaut P, Siroux V, Schisterman EF, Slama R, 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30, 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Glomstad B, Fernandez-Somoano A, Tardon A, 2014. Gestational weight gain and exposure of newborns to persistent organic pollutants. Environ. Health Perspect 122, 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association, 2013. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


