Abstract
Background: The efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in elderly patients with heart failure with preserved ejection fraction (HFpEF) remains unclear.
Methods and Results: In a multicenter, controlled trial, the CANONICAL study, we enrolled 82 HFpEF (left ventricular ejection fraction [LVEF] ≥50%) patients with type 2 diabetes (T2D) aged ≥65 years, with plasma B-type natriuretic peptide (BNP) ≥100 pg/mL or plasma N-terminal pro BNP (NT-proBNP) ≥400 pg/mL or history of HF. Patients were randomly assigned to 2 groups and were administered either the SGLT2 inhibitor canagliflozin (100 mg/day) for 24 weeks or standard therapy. The primary endpoints were changes in body weight (BW) and BNP concentrations. Mean (±SD) patient age, body mass index, and LVEF were 75.7±6.5 years, 25.0±3.6 kg/m2 and 61.5±7.6%, respectively. At 24 weeks, BW was significantly lower in the canagliflozin than standard therapy group. The extent of BNP reductions at 4 weeks was significantly greater in the canagliflozin than standard therapy group (P<0.05), but at 24 weeks there was no significant difference between the 2 groups.
Conclusions: In this study, canagliflozin treatment reduced BW, but did not significantly reduce plasma BNP concentrations compared with standard therapy after 24 weeks treatment in T2D patients with HFpEF. Further large-scale randomized studies are needed to conclude the beneficial effects of canagliflozin in T2D patients with HFpEF.
Key Words: B-type natriuretic peptide, Elderly, Heart failure with preserved ejection fraction, Sodium-glucose cotransporter 2 inhibitors, Type 2 diabetes
The incidence of type 2 diabetes (T2D) and heart failure (HF) has increased worldwide with population aging.1,2 T2D is a well-known major risk factor for developing HF with both reduced and preserved ejection fraction (HFrEF and HFpEF, respectively). Except for clinically overt HF, which includes both HFrEF and HFpEF, 30–40% of patients with HF also have T2D and thus have higher risk of mortality.3,4 Therefore, better treatment strategies for patients with T2D and HF are of major interest.
Over the past 3 decades, renin-angiotensin system blockers and β-adrenergic receptor antagonists have been established as treatments for patients with HFrEF with and without T2D.5–9 Recently, new drugs for HFrEF, including sodium-glucose cotransporter 2 (SGLT2) inhibitors, have emerged,10–13 and another 2 classes of drugs, soluble guanylyl cyclase stimulators and myosin stimulators, will be approved in the near future because of the results of recent randomized control trials (RCTs).14,15 Thus, the prognosis of HFrEF continues to improve.
However, with the exception of SGLT2 inhibitors, the drugs mentioned above have failed to reduce cardiovascular (CV) death and hospitalization for HF (HHF) in large RCTs on HFpEF.16 Consequently, none of the recommended treatment guidelines reduce the long-term CV outcomes of HFpEF. Although the prognosis of HFrEF has improved over the past 3 decades, it remains poor. Furthermore, the prognosis of HFpEF is as poor as that of HFrEF and has not improved.17,18
SGLT2 inhibitors were originally developed to lower serum glucose concentrations by blocking SGLT2-mediated glucose reabsorption in the renal proximal tubules.19 In addition to their blood glucose-lowering effects, SGLT2 inhibitors also decrease body weight (BW) and blood pressure without increasing heart rate.20 Empagliflozin, canagliflozin, and dapagliflozin have demonstrated favorable CV effects in patients with T2D, and can also slow reductions in estimated glomerular filtration rate (eGFR).20–23 Moreover, 2 recent large-scale RCTs demonstrated that dapagliflozin and empagliflozin could significantly reduce CV mortality and worsening HF in patients with HFrEF, regardless of the presence of T2D.12,13 Therefore, whether SGLT2 inhibitors are effective against HFpEF is currently a topic of great interest. A few large outcome trials examining this are ongoing, but require more time to complete. We conducted a clinical trial, the CANagliflOziN heart faIlure with preserved ejection fraCtion study for type 2 diAbetes meLlitus (CANONICAL) study, to investigate whether canagliflozin decreases fluid retention and plasma B-type natriuretic peptide (BNP) concentrations in elderly patients with T2D and HFpEF.
Methods
Design
The study design and the characteristics of the patients in the CANONICAL study have been published elsewhere.24 Briefly, the CANONICAL study is a multicenter open-label randomized parallel-group study comparing the effect of canagliflozin on cardiac function to that of standard diabetes treatment in elderly patients with T2D and HFpEF. Eligible participants were patients aged ≥65 years who had inadequate glycemic controlled with diet and exercise or with an oral hypoglycemic drug other than an SGLT2 inhibitor.
The main inclusion criteria were: (1) age ≥65 years; (2) echocardiographic left ventricular ejection fraction (LVEF) ≥50% and mean E/e′ >14 (septal E/e′ >15 or lateral E/e′ >13) or septal e′ <7 cm/s or lateral e′ <10 cm/s; (3) 6.5%≤HbA1c<10.0%; (4) New York Heart Association functional class II–III; and (5) either plasma BNP ≥100 pg/mL or plasma N-terminal pro BNP (NT-proBNP) ≥400 pg/mL during the screening period, or plasma BNP ≥40 pg/mL or NT-proBNP ≥125 pg/mL during the screening period with a history of plasma BNP ≥100 pg/mL or plasma NT-proBNP ≥400 pg/mL at any time before enrollment. The main exclusion criteria were: type 1 diabetes, a body mass index (BMI) <18.5 kg/m2, previous treatment with glucagon-like peptide-1 receptor agonists, a need for insulin therapy for blood glucose management, severe renal dysfunction or treatment with hemodialysis for end-stage renal disease, a history of acute coronary syndrome, cerebrovascular disease, myocarditis, or contractile pericarditis, and severe valvular disease in the 12 weeks prior to consenting to take part in the study. Details of the inclusion and exclusion criteria can be found in the protocol paper.24
Patients meeting the inclusion criteria were randomly assigned to either the canagliflozin (CAN) or standard diabetic therapy (STDT) group. Patients in the CAN group were administered 100 mg canagliflozin (CANAGLU® tablets 100 mg; Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) orally once a day, before or after breakfast, for 24 weeks in addition to ongoing diabetic treatment. In the STDT group, antihyperglycemic drugs other than SGLT2 inhibitors were administered for 24 weeks in addition to ongoing diabetes treatment if needed. A glycemic (HbA1c) goal was set for each patient according to the Japanese Diabetes Society guidelines,25 and patients were provided HF treatment in accordance with the Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure of the Japanese Circulation Society and Japanese Heart Failure Society.26
This study was performed in compliance with the Declaration of Helsinki (as revised in October 2013) and according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labour, and Welfare of Japan. Each patient was provided with an explanation of the study and provided written informed consent. This study was approved by the Nara Medical University Certified Review Board and has been registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN 000028668) and Japan Registry of Clinical Trials (ID: 051180030). The research period was from October 1, 2017 to March 31, 2021.
Randomization
Registration and allocation of the study participants was performed using the central registration modality in an electronic data capturing system. Participants were dynamically assigned to the STDT and CAN groups using the following assignment factors: plasma BNP, BW, presence or absence of chronic atrial fibrillation, age, eGFR, and sex.
Endpoints
The primary endpoints were reductions in BW and plasma BNP concentrations after 24 weeks of treatment compared with baseline values. Secondary endpoints were HHF, CV death and all-cause death, BW changes from baseline at each time point, changes in the use of diuretics, HbA1c changes from baseline, changes in echocardiography parameters and nutritional status (based on the Controlling Nutritional Status score and Geriatric Nutritional Risk Index). For safety analysis, adverse events (AEs) were collected. An AE was defined as any unfavorable or unintended sign, symptom, or disease, including abnormal laboratory values.
For efficacy evaluation, the full analysis set (FAS) and the per-protocol set (PPS) were used. The FAS was defined as the patient population with plasma BNP concentrations and BW values at baseline and at ≥1 subsequent time point. The PPS was defined as the patient population that was excluded from the FAS for any of the following reasons: violation of safety-related exclusion criteria, meeting the criteria for study discontinuation, non-compliance with allocated drugs, violation of effectiveness-related inclusion criteria, the use of a prohibited treatment, a study drug compliance rate <70%, or a treatment period <16 weeks. The safety analysis set (SAS) included patients with safety evaluation data after the start of the study treatment.
Sample Size
Based on previous reports,27–30 the difference in BW from baseline to 24 weeks between the 2 groups should have been 2.0±2.0 kg, and the sample size required to detect this difference by 2-sided t-test (1−β=0.98, α=0.05) was estimated to be 34 patients per group. Considering typical dropout rates, the target number of patients was set to 40 in each group and 80 in total.
Statistical Analysis
For changes in BW and log-transformed plasma BNP concentrations from baseline, the least-squares mean (LSM) difference and 95% confidence intervals (CIs) were calculated for each group, which were compared by analysis of covariance using the baseline value as a covariate. The significance level for statistical tests was 0.05 on both sides, and the confidence coefficient for statistical estimation was 95% on both sides. Missing values in the efficacy analysis were supplemented with the immediately preceding value (last observation carried forward). In addition, an observed case analysis was performed. Unless indicated otherwise, data are presented as the mean±SD.
Results
Baseline Characteristics
To investigate the effects of canagliflozin on BW and BNP in patients aged ≥65 years with HFpEF and T2D, 82 patients were randomly assigned to either the CAN (n=42) or STDT group (n=40). As indicated in Table 1, the mean age of the 82 patients was 75.7±6.5 years, and 67.1% were men. The mean BW and BMI were 63.36±11.04 kg and 25.0±3.6 kg/m2, respectively. The mean HbA1c was 7.01±0.66%. The median BNP value was 133 pg/mL, with an interquartile range (IQR) of 79–219 pg/mL.
Table 1.
Patient Characteristics
| Total (n=82) |
CAN (n=42) |
STDT (n=40) |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 75.7±6.5 | 76.5±6.4 | 75.9±5.8 | 0.6691 |
| Male sex (%) | 67.1 | 66.7 | 67.5 | 0.9360 |
| Body weight (kg) | 63.36±11.04 | 62.89±10.96 | 63.90±11.38 | 0.9077 |
| BMI (kg/m2) | 25.0±3.6 | 24.7±3.6 | 25.2±3.7 | 0.4923 |
| Duration of T2D (years) | 6.0 [2.0–14.3] | 7.0 [4.0–11.0] | 5.0 [2.0–15.9] | 0.5367 |
| Duration of HF (years) | 4.0 [1.8–9.0] | 4.3 [1.6–8.0] | 4.5 [2.6–10.0] | 0.4630 |
| Medical history (%) | ||||
| Hypertension | 90.2 | 95.2 | 85.0 | 0.1116 |
| Chronic atrial fibrillation | 34.1 | 35.7 | 32.5 | 0.8184 |
| NYHA class (%) | 0.2704 | |||
| II | 91.5 | 88.1 | 95.0 | |
| III | 8.5 | 11.9 | 5.0 | |
| Vital signs | ||||
| SBP (mmHg) | 131.3±15.3 | 131.1±14.6 | 131.5±16.2 | 0.8672 |
| DBP (mmHg) | 70.3±11.4 | 71.6±12.1 | 69.0±10.5 | 0.2942 |
| Heart rate (beats/min) | 69.0±11.0 | 70.5±11.0 | 67.4±10.9 | 0.2633 |
| Echocardiographic parameters | ||||
| LVEF (%) | 61.5±7.6 | 61.1±7.8 | 61.9±7.6 | 0.7051 |
| E/e′ | 15.0 [11.4–18.0] | 14.4 [11.2–17.8] | 15.7 [12.7–18.3] | 0.2112 |
| Laboratory data | ||||
| HbA1c (%) | 7.01±0.66 | 7.13±0.74 | 6.90±0.55 | 0.2233 |
| eGFR (mL/min/1.73 m2) | 57.0±14.0 | 57.8±14.2 | 56.0±13.8 | 0.5786 |
| Plasma BNP (pg/mL) | 133 [79–219] | 134 [75–213] | 130 [93–219] | 0.5186 |
| Nutritional status | ||||
| CNS score | 1.2±1.0 | 1.2±0.9 | 1.2±1.0 | 0.9457 |
| GNRI | 103.0±5.0 | 102.9±5.4 | 103.0±4.6 | 0.9360 |
| Medications (%) | ||||
| ACE inhibitors | 25.6 | 28.6 | 22.5 | 0.7065 |
| ARBs | 58.5 | 59.5 | 57.5 | 0.8525 |
| MR blockers | 17.1 | 14.3 | 20.0 | 0.6937 |
| β-blockers | 70.7 | 69.0 | 72.5 | 0.9198 |
| Loop diuretics | 43.9 | 42.9 | 45.0 | 0.8450 |
| DPP-4 inhibitors | 45.1 | 42.9 | 47.5 | 0.8412 |
| Biguanides | 18.3 | 16.7 | 20.0 | 0.9167 |
| α-glucosidase inhibitors | 20.7 | 19.0 | 22.5 | 0.9100 |
| Sulfonylureas | 11.0 | 7.1 | 15.0 | 0.4328 |
Unless indicated otherwise, data are shown as the mean±SD or as the median [interquartile range]. P values were calculated using t-tests for differences in mean values between groups of metric values, and using Fisher’s exact test or the Wilcoxon rank-sum test for differences in proportions between groups. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CAN, canagliflozin; CNS, Controlling Nutrition Status; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase-4; E/e′, ratio of early mitral inflow velocity to mitral annular early diastolic velocity; eGFR, estimated glomerular filtration rate; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; LVEF, left ventricular ejection fraction; MR, mineralocorticoid receptor; NYHA, New York Heart Association; SBP, systolic blood pressure; STDT, standard diabetic therapy; T2D, type 2 diabetes.
There were no significant differences in age, sex, BW, or the duration of T2D and HF between the 2 groups, and the proportion of comorbidities was equal between them. Moreover, vital signs and laboratory findings were similar in the 2 groups. With regard to echocardiography parameters, LVEF values and early mitral inflow velocity to mitral annular early diastolic velocity (E/e′) ratios were equal between the 2 groups. The proportion of patients treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, β-blockers, mineralocorticoid receptor blockers, or diuretics was similar between the 2 groups.
Outcomes
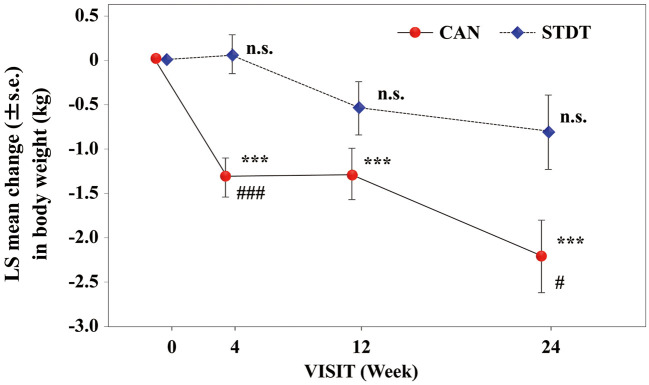
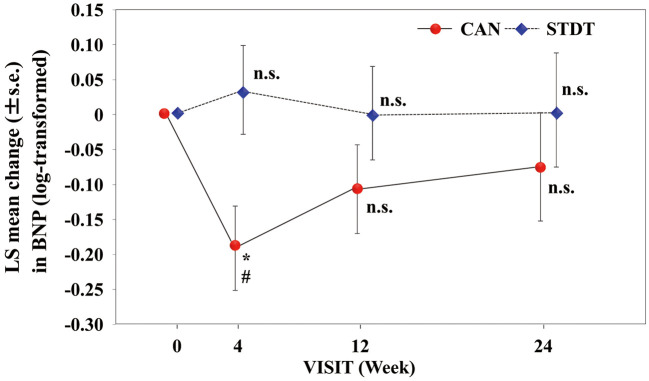
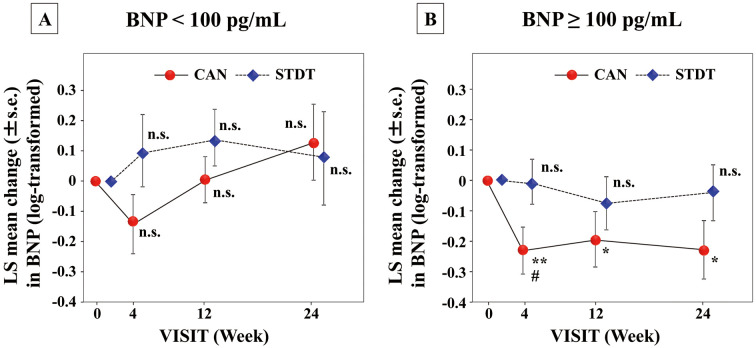
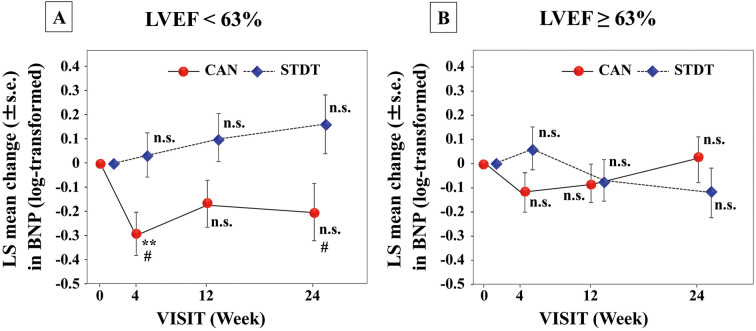
BW and BNP concentrations were measured after 4, 12, and 24 weeks of treatment (Supplementary Table 1). The LSM BW in the CAN group was significantly decreased from the baseline (P<0.001 at 4, 12, and 24 weeks), and the reduction in BW was significantly greater than in the STDT group after 24 weeks of treatment (P=0.019; Figure 1). The LSM log-transformed plasma BNP concentration in the CAN group was significantly decreased at 4 weeks compared with baseline, and the extent of change in BNP at this time point was greater in the CAN than STDT group (P<0.05); however, at 12 and 24 weeks, neither group exhibited a significant decline in the log-transformed plasma BNP concentrations compared with baseline (Figure 2). In patients with baseline BNP ≥100 pg/mL, the LSM plasma BNP concentration was significantly lower after 24 weeks compared with baseline in the CAN group (P<0.05). However, in patients with baseline BNP <100 pg/mL, these changes were not observed at any time point (Figure 3). Moreover, in patients with LVEF values lower than the median (63%), the LSM log-transformed plasma BNP concentration was significantly lower in the CAN than STDT group after 24 weeks (P=0.0395; Figure 4).
Figure 1.
Least-squares (LS) mean (±SEM) changes in body weight in the canagliflozin (CAN) and standard diabetic therapy (STDT) groups. ***P<0.001 compared with baseline (ANCOVA); #P<0.05, ###P<0.001 compared with the STDT group (ANCOVA).
Figure 2.
Least-squares (LS) mean (±SEM) changes in plasma B-type natriuretic peptide (BNP) concentrations in the canagliflozin (CAN) and standard diabetic therapy (STDT) groups. *P<0.05 compared with baseline (ANCOVA); #P<0.05 compared with the STDT group (ANCOVA).
Figure 3.
Least-squares (LS) mean (±SEM) changes in plasma B-type natriuretic peptide (BNP) concentrations in patients with baseline BNP (A) <100 pg/mL (n=30) and (B) ≥100 pg/mL (n=48) in the canagliflozin (CAN) and standard diabetic therapy (STDT) groups. *P<0.05, **P<0.01 compared with baseline (ANCOVA); #P<0.05 compared with the STDT group (ANCOVA).
Figure 4.
Least-squares (LS) mean (±SEM) changes in plasma B-type natriuretic peptide (BNP) in patients with baseline left ventricular ejection fraction (LVEF) (A) <63% (n=39) and (B) ≥63pg/mL (n=39) in the canagliflozin (CAN) and standard diabetic therapy (STDT) groups. **P<0.01 compared with baseline (ANCOVA); #P<0.05 compared with the STDT group (ANCOVA).
Regarding secondary endpoints, 1 HHF occurred in each group, but no CV death or all-cause death was observed. There was also no difference in the use of diuretics from baseline between the 2 groups. Changes from baseline in all echocardiography parameters were equal between the 2 groups. Moreover, changes in HbA1c and nutritional status after 24 weeks were similar between the 2 groups (Table 2).
Table 2.
Secondary Endpoints
| CAN (n=42) |
STDT (n=40) |
P value | |
|---|---|---|---|
| All-cause death | 0 (0.0) | 0 (0.0) | |
| Cardiovascular death | 0 (0.0) | 0 (0.0) | |
| Hospitalization for HF | 1 (2.4) | 1 (2.5) | 1.000 |
| LS mean change from baseline (%) | |||
| HbA1c | −0.29 | 0.11 | 0.0929 |
| Echocardiography parameters | |||
| LVEF | 0.97 | −1.18 | 0.0795 |
| E/e′ | −0.66 | −0.65 | 0.9851 |
| Nutritional status | |||
| CNS score | 0.1 | 0.4 | 0.2786 |
| GNRI | −0.4 | −0.8 | 0.7703 |
Unless indicated otherwise, data are given as n (%). P values were calculated by analysis of covariance with the baseline value as a covariate for differences in mean values between groups for metric values, and continuity-corrected Chi-squared statistics for differences in proportions between groups. LS mean, least-squares mean. Other abbreviations as in Table 1.
In the STDT group, the antihyperglycemic drugs added during the observation period were biguanides (in 3 patients), sulfonylureas, dipeptidyl peptidase-4 inhibitors, and α-glucosidase inhibitors (in 2 patients each), and glinide (in 1 patient). Moreover, diuretics were added in 4 patients in the STDT group, but not in the CAN group, during the observation period.
Regarding vital signs, there were no significant differences in systolic blood pressure, diastolic blood pressure, or heart rate between the 2 groups at any time point (Supplementary Table 2).
Safety
The incidence of major AEs was similar in the 2 groups. The rates of hyperkalemia, diabetic ketoacidosis, and HF did not differ significantly between the 2 groups. Furthermore, the frequency of fractures was similar in the 2 groups. However, the incidence of infection was higher in the STDT than CAN group (P=0.0146; Table 3).
Table 3.
Major Adverse Events
| Total (n=82) |
CAN (n=42) |
STDT (n=40) |
P value | |
|---|---|---|---|---|
| HF | 4 (4.9) | 1 (2.4) | 3 (7.5) | 0.2691 |
| Infection | 4 (4.9) | 0 (0.0) | 4 (10.0) | 0.0146 |
| Bone fracture | 3 (3.7) | 2 (4.8) | 1 (2.5) | 0.5816 |
| Diabetic ketoacidosis | 1 (1.2) | 0 (0.0) | 1 (2.5) | 0.2310 |
| Hyperkalemia | 1 (1.2) | 0 (0.0) | 1 (2.5) | 0.2310 |
| Liver dysfunction | 1 (1.2) | 1 (2.4) | 0 (0.0) | 0.2417 |
| Gout | 1 (1.2) | 1 (2.4) | 0 (0.0) | 0.2417 |
| Kidney stone | 1 (1.2) | 0 (0.0) | 1 (2.5) | 0.2310 |
Unless indicated otherwise, data are given as n (%). P values were calculated a test with a continuity-corrected Chi-squared statistics for differences in proportions between groups. CAN, canagliflozin; HF, heart failure; STDT, standard diabetic therapy.
Discussion
This is the first report focusing on the effect of canagliflozin on BW and plasma BNP concentrations in patients with T2D and HFpEF. Treatment with canagliflozin for 24 weeks significantly reduced BW in the CAN group, but not in the STDT group. Between-group differences in BW were evident throughout the study period. One of the reasons for BW loss may be a decrease in fluid volume due to the effect of canagliflozin. Moreover, in patients with T2D and obesity, two-thirds of the BW loss due to the effects of SGLT2 inhibitors is attributed to a decrease in fat mass.31,32 In the present study, the mean BMI was 25.0 kg/m2, and patients tended to be obese. Therefore, it cannot be ruled out that the BW loss observed in this study may be due to calorie loss due to increased urinary sugar excretion.
After 4 weeks, canagliflozin treatment reduced plasma BNP concentrations to a greater extent than the standard treatment. This difference was no longer significant after 12 and 24 weeks of treatment, although the BNP concentration tended to be lower in the CAN than STDT group. However, post hoc analyses revealed greater reductions in plasma BNP concentrations after 24 weeks compared with baseline in the CAN group in the subgroup with BNP ≥100 pg/mL, although there was no significant difference in plasma BNP concentrations after 24 weeks between the CAN and STDT groups. Conversely, in patients with LVEF <63%, plasma BNP concentrations after 24 weeks were not significantly reduced from baseline in the CAN group, but were lower in the CAN than STDT group after 24 weeks.
Regarding secondary endpoints, there was no difference in the magnitude of the decrease in HbA1c between the 2 groups. One of the reasons for this is thought to be that the therapeutic goals for glycemic control were set to be at the same in both groups. Furthermore, the relatively low baseline HbA1c may have limited hypoglycemic effects in all treatment groups. The increase in specific AEs in the CAN group was similar to that in the STDT group.
The CANONICAL study should be considered hypothesis generating because we enrolled only 82 patients with T2D and HFpEF and used the plasma BNP concentrations as a surrogate marker of HF instead of hard endpoints. In this study, canagliflozin treatment lowered BW as expected; however, a significant and long-lasting decline in plasma BNP was not observed. The reason for this is not clear, but there are a few possible reasons. First, the sample size may have been insufficient. Because there were no data available on canagliflozin-induced BNP changes in patients with T2D and HFpEF, we used the known BW change induced by canagliflozin to calculate the sample size for this study. Therefore, the present study may have been underpowered to detect reductions in BNP. Second, the lack of a long-lasting decrease in BNP may be due to the fact that the patients in this study had mild HF. Patients in the CANONICAL study had similar or older ages but lower natriuretic peptide levels at enrollment than patients in other RCTs. NT-proBNP levels in these other RCTs were approximately 900 pg/mL, roughly corresponding to a BNP concentration of 200 pg/mL.33,34 The mean LVEF was slightly higher in the CANONICAL study than in other RCTs, which reported mean LVEF ranging from 54% to 60%.4,16,35–37 Therefore, HF was less severe in the CANONICAL study than in other RCTs.
The CANDLE trial, a prospective randomized trial to investigate the effect of 24 weeks of canagliflozin treatment in patients with T2D and chronic HF, was recently reported.38 In that trial, HF was diagnosed by a skilled cardiologist according to clinical symptoms and related laboratory findings, and approximately 70% of enrolled patients had HFpEF. NT-proBNP levels were 245 pg/mL at baseline and 229 pg/mL at the end of treatment. NT-proBNP changes were similar between the canagliflozin and comparator (glimepiride) groups. HFrEF or HFpEF subgroups showed no changes in NT-proBNP concentrations before and after canagliflozin treatment.38 Thus, the present findings are not in conflict with the CANDLE results. Moreover, given that NT-proBNP concentrations of 200–240 pg/mL correspond to BNP concentrations of 50–60 pg/mL, it is plausible that canagliflozin treatment fails to produce favorable results in patients with BNP <100 pg/mL.
In another post hoc analysis, patients with EF values lower than the median (63%) showed a greater decline in plasma BNP concentrations. A recent large RCT on HFpEF, the PARAGON trial, reported that sacubitril/valsartan significantly reduced the risk of CV death and HF hospitalization in patients with EF <57%.16 Of course, the pharmacological actions of canagliflozin are different from those of sacubitril/valsartan; however, HFpEF may respond to these drugs in similar ways. Although a threshold EF value of 50% has been used empirically without rationale, an EF-dependent strategy should be considered for medical intervention for HFpEF. A more precise investigation to understand the mechanism underlying the development of HFpEF is necessary. Some studies recently found that the subgroup of patients with HFpEF and the night-time riser pattern of blood pressure, as determined ambulatory blood pressure monitoring, was at higher risk for CV events and HHF.39,40 The use of SGLT2 inhibitors may be more promising in these patients, because these drugs likely reduce night-time blood pressure in patients with T2D and uncontrolled nocturnal hypertension.41
Because this study is hypothesis generating by nature, solid evidence could not be generated regarding the effects of SGLT2 inhibitors on CV death and HHF risk reduction in patients with T2D and HFpEF. Similarly, post hoc analysis of the DECLARE-TIMI58 study provided uncertain findings as to whether dapagliflozin reduced HHF in the subgroup of patients with HFpEF.42 Conversely, the SOLOIST-WHF trial suggested that the SGLT2 inhibitor effectively prevented CV events in patients with T2D and a history of HHF, even in patients with EF ≥50%.43 Therefore, upcoming results from ongoing large RCTs should help confirm the effects of SGLT2 inhibitors on HFpEF.44
In this study, compared with other studies of HFpEF patients, there was a lower complication rate of hypertension. We do not know the exact reason for this. However, we think that renin-angiotensin system blockers given for renal protection may have had an effect. In the present study, renin-angiotensin system blockers were administered in >80% of the patients, which may have controlled the increase in blood pressure. Therefore, the rate of hypertension may have been lower in the present study than in other HFpEF studies.
This study has several limitations, including a short observation period, a small number of patients, and an open-label modality. Moreover, there were not enough data related to fluid volume, such as the cardiothoracic ratio on chest X-ray and inferior vena cava and left ventricle dimension on the echocardiography.
Conclusions
The present study demonstrated that treatment with canagliflozin reduced BW, but did not significantly reduce plasma BNP concentrations, after 24 weeks of treatment in patients with T2D and HFpEF. Further large-scale randomized studies are needed to conclude the beneficial effects of canagliflozin in patient with T2D and HFpEF.
Grant
None.
Sources of Funding
This study was funded by Mitsubishi Tanabe Pharma Corporation, a collaborative research institution.
Disclosures
K.T. is a member of Circulation Reports’ Editorial Team and has received scholarship funds from Mitsubishi Tanabe Pharma Corporation. Y. Saito has received research funds, research expenses, and speakers’ bureau fees/honoraria from Mitsubishi Tanabe Pharma Corporation. Y.O. and Y. Susuta are employees of Mitsubishi Tanabe Pharma Corporation. H.T. is an employee of Mitsubishi Tanabe Pharma Corporation and profits from stocks of Mitsubishi Tanabe Pharma Corporation. The remaining authors have no conflicts of interest to disclose.
IRB Information
This study was approved by the Nara Medical University Certified Review Board and has been registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN 000028668) and Japan Registry of Clinical Trials (ID: 051180030).
Supplementary Files
Supplementary Table 1. Body Weight and Plasma BNP Levels at 4, 12, and 24 Weeks After Treatment Supplementary Table 2. Systolic Blood Pressure, Diastolic Blood Pressure and Heart Rate at 4, 12, and 24 Weeks After Treatment CANONICAL Study Group Member
Acknowledgments
The authors thank Makoto Kato, a former employee of Mitsubishi Tanabe Pharma Corporation, and the staff at the Clinical Research Center at Nara Medical University Hospital for their support.
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al.. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Sakata Y, Shimokawa H.. Epidemiology of heart failure in Asia. Circ J 2013; 77: 2209–2217. [DOI] [PubMed] [Google Scholar]
- 3. Goodlin SJ.. Palliative care in congestive heart failure. J Am Coll Cardiol 2009; 54: 386–396. [DOI] [PubMed] [Google Scholar]
- 4. Nagai T, Yoshikawa T, Saito Y, Takeishi Y, Yamamoto K, Ogawa H, et al.. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction: A report from the Japanese heart failure syndrome with preserved ejection fraction (JASPER) registry. Circ J 2018; 82: 1534–1545. [DOI] [PubMed] [Google Scholar]
- 5. CONSENSUS Trial Study Group.. Effects of enalapril on mortality in severe congestive heart failure: Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al.. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet 2003; 362: 772–776. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al.. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure: Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 9. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al.. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21.21073363 [Google Scholar]
- 10. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al.. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 12. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 13. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 14. Pieske B, Patel MJ, Westerhout CM, Anstrom KJ, Butler J, Ezekowitz J, et al.. Baseline features of the VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial. Eur J Heart Fail 2019; 21: 1596–1604. [DOI] [PubMed] [Google Scholar]
- 15. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, et al.. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021; 384: 105–116. [DOI] [PubMed] [Google Scholar]
- 16. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al.. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019; 381: 1609–1620. [DOI] [PubMed] [Google Scholar]
- 17. Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, et al.. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction: Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1893–1900. [DOI] [PubMed] [Google Scholar]
- 18. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC).. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur Heart J 2012; 33: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 19. Ferrannini E, Solini A.. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat Rev Endocrinol 2012; 8: 495–502. [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 21. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al.. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 22. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 23. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al.. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 24. Kasama S, Masuyama T, Uemura S, Sato Y, Hiramitsu S, Masuda I, et al.. Rationale and design of the CANONICAL study: Randomized, open-label study to evaluate the efficacy and safety of canagliflozin for heart failure with preserved ejection fraction with type 2 diabetes mellitus. Circ Rep 2019; 1: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Japan Diabetes Society.. Treatment guide for diabetes 2018–2019. Tokyo: Bunkodo, 2018.
- 26. Japanese Circulation Society and Japanese Heart Failure Society.. Guidelines for diagnosis and treatment of acute and chronic heart failure (JCS 2017/JHFS 2017) [in Japanese]. http://www.j-circ.or.jp/guideline/pdf/JCS2017_tsutsui_h.pdf (accessed December 10, 2018).
- 27. Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H.. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: A 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother 2014; 15: 1501–1515. [DOI] [PubMed] [Google Scholar]
- 28. Kadowaki T, Kondo K.. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab 2013; 15: 810–818. [DOI] [PubMed] [Google Scholar]
- 29. Kadowaki T, Kondo K.. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H.. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: A pooled analysis of two Phase III clinical studies. Expert Opin Pharmacother 2015; 16: 971–981. [DOI] [PubMed] [Google Scholar]
- 31. Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, et al.. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 32. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al.. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 33.http://www.asas.or.jp/jhfs/topics/bnp201300403.html (accessed February 12, 2021).
- 34. Horii M, Matsumoto T, Uemura S, Sugawara Y, Takitsume A, Ueda T, et al.. Prognostic value of B-type natriuretic peptide and its amino-terminal proBNP fragment for cardiovascular events with stratification by renal function. J Cardiol 2013; 61: 410–416. [DOI] [PubMed] [Google Scholar]
- 35. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al.. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 36. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al.. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 37. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al.. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, et al.. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: A randomized trial (CANDLE). ESC Heart Fail 2020; 7: 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ueda T, Kawakami R, Nakada Y, Nakano T, Nakagawa H, Matsui M, et al.. Differences in blood pressure riser pattern in patients with acute heart failure with reduced mid-range and preserved ejection fraction. ESC Heart Fail 2019; 6: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komori T, Eguchi K, Saito T, Hoshide S, Kario K.. Riser pattern is a novel predictor of adverse events in heart failure patients with preserved ejection fraction. Circ J 2017; 81: 220–226. [DOI] [PubMed] [Google Scholar]
- 41. Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, et al.. 24-Hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: Results from the randomized, placebo-controlled SACRA study. Circulation 2018; 139: 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al.. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 43. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al.. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 44. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al.. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: Rationale for and design of the EMPEROR-Preserved trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Body Weight and Plasma BNP Levels at 4, 12, and 24 Weeks After Treatment Supplementary Table 2. Systolic Blood Pressure, Diastolic Blood Pressure and Heart Rate at 4, 12, and 24 Weeks After Treatment CANONICAL Study Group Member