Abstract
Methamphetamine (METH) is an illicit psychostimulant that is abused throughout the world. METH addiction is also a major public health concern and the abuse of large doses of the drug is often associated with serious neuropsychiatric consequences that may include agitation, anxiety, hallucinations, paranoia, and psychosis. Some human methamphetamine users can also suffer from attention, memory, and executive deficits. METH-associated neurological and psychiatric complications might be related, in part, to METH-induced neurotoxic effects. Those include altered dopaminergic and serotonergic functions, neuronal apoptosis, astrocytosis, and microgliosis. Here we have endeavored to discuss some of the main effects of the drug and have presented the evidence supporting certain of the molecular and cellular bases of METH neurotoxicity. The accumulated evidence suggests the involvement of transcription factors, activation of dealth pathways that emanate from mitochondria and endoplasmic reticulum (ER), and a role for neuroinflammatory mechanisms. Understanding the molecular processes involved in METH induced neurotoxicity should help in developing better therapeutic approaches that might also serve to attenuate or block the biological consequences of use of large doses of the drug by some humans who meet criteria for METH use disorder.
Keywords: autophagy, cell death, ER stress, mitochondrial cascade, Bcl2, neuroinflammation, transcription factors
1. General introduction
Methamphetamine (METH) is a psychostimulant that is abused worldwide (UNODC 2018; Yang et. al., 2018). METH was first synthesized from ephedrine by Japanese chemist, Nagayoshi Nagai in 1893. In 1919, another Japanese chemist, Akira Ogata streamlined the process and produced the first crystallized form of drug (Nagai and Kamiyama, 1988; Buxton and Dove, 2008; Panenka et. al., 2013). The use of METH, which is also a schedule II drug, has been restricted by USA law since 1971. METH is used as a second line of treatment for attention deficit hyperactivity disorder (ADHD), severe obesity, and narcolepsy (Moszczynska and Callan, 2017). Its repeated use, under uncontrolled conditions, can lead to the user meeting diagnostic criteria for METH use disorder which is characterized by compulsive use in the presence of adverse consequences and craving for the drug (DSM5). Some of the adverse consequences include neurological and psychiatric complications, cardiovascular problems, ulmonary arterial hypertension, periodontal (gum) disease, and renal failure (Ho et al., 2009; Schep et al., 2010; Moratalla et al., 2017; Yang et. al., 2018). Specific METH-induced neurological and psychiatric effects include cerebral stroke, seizures, schizophrenia, and psychotic illness (Cadet and Gold, 2017; Hsieh et. al., 2014; Yang et. al., 2018; Lappin and Sara, 2019; Wearne and Cornish, 2018).
In addition to the clinical signs and symptoms associated with the large doses of METH, several lines of evidence have documented its toxic effects on dopamine (DA) and serotonin (5-HT) systems (Cadet and Krasnova, 2009). Large METH doses can cause neuronal apoptosis and glial activation in the brain (Panenka et al., 2013; Moratalla et al., 2017; Sekine et al., 2008; Yang et. al., 2018). Moreover, acute and chronic injection of the drug have induced a diversity of toxic responses in animal models (Cadet et. al., 2003; 2005; 2007; Cadet and Bisagno, 2016). Despite knowing the potential toxic effects of the drug, little effort has been spent to develop pharmacological approaches in order to counter these effects in human users. Studies focusing biological mechanisms of METH induced neurotoxicity should provide the knowledge necessary to approach these problems more rationally (Ashok et.al., 2017; Moszczynska and Callan, 2017; Yang et. al., 2018; Xie et. al., 2018).
2. Epidemiology of METH use
METH is a member of the amphetamine-type stimulants (ATSs) that include amphetamine, methylene dioxy methamphetamine (MDMA), and other designer amphetamine (Chomchai and Chomchai, 2015; Yang et. al., 2018). It has been reported that approximately 27 million individuals use ATSs in 2019, a number that corresponds to 0.5 per cent of the world adult population (UNODC, 2020). North America with 2.3 per cent, Australia and New Zealand with 1.3 per cent, and Asia with 0.5 per cent of their populations have the highest prevalence of METH use between ages 15 and 64 (UNODC, 2020). METH is indeed the second most used illicit drug after cannabis (Stoneberg et. al., 2018). METH manufacturing contributes to 95% of illigally synthesized ATS, with the quantities of the drug having been seized between 2009 and 2017 increasing by sevenfold (UNODC, 2020). Seizures of illegal METH remain highly concentrated in the United States, Thailand, and Mexico, accounting for 80% of total global seizures (UNODC, 2020). The compounds utilized to synthesize METH, ephedrine and pseudoephedrine, are used in Asia, Oceania, Africa and in some European regions whereas phenyl-2-propanone (P-2-P), a pseudoephedrine precursor, is mostly used in North America and Western Europe (EU Drug Markets Report 2019: E/INCB/2019/1; UNODC, 2020).
In the USA, nearly 1.6 million individuals were reported to use METH in 2016, with an average age of 23.3 years old according to the National Survey on Drug Use and Health (2017). In 2017, METH-related overdose deaths in United states increased by 7.5 times compared to 2007. These cases occurred mostly in Washington, Colorado, Texas, Florida, and Georgia (National Survey on Drug Use and Health, 2017).
3. METH use and its clinical neuropsychiatric presentations
METH use is associated with several health complications secondary to the negative impact of the drug on the central nervous system (CNS). These include cognitive and psychomotor impairments users of large doses of the drug (Panenka et al., 2013; Yang et. al., 2018; Paulus and Stewart, 2020). Human METH users can suffer drug-induced agitation, anxiety, paranoia, and psychosis (Paulus and Stewart, 2020; Zhao et. al., 2020). METH users have presented to emergency rooms with strokes, seizures, renal and liver failure, cardiac arrythmias, extreme hyperthermia, or in comatose states (Perez et al., 1999; Turnipseed et al., 2003; McGee et al., 2004; Ho et al., 2009; Schep et al., 2010; Jones and Rayner, 2015). A meta-analysis has reported that METH users can suffer from neuropsychological impairments consisting of dysfunctions of decision making, information processing speed, language, and visuoconstructional abilities (Scott et al., 2007).
Importantly, some recent reports have documented a higher prevalence of Parkinosism in METH users (Callaghan et al., 2010; 2012; McNeely et al., 2012; Panenka et al., 2013; Curtin et al., 2015; Todd et al., 2016). For example, Callaghan et al. (2012) reported that METH abusers have a 75% higher risk of developing Parkinsonism than non-METH using individuals. Retrospective case-controlled studies have also found that prolonged use of METH was also associated with an increased risk for developping Parkinson’s disease (PD) (Garwood et. al., 2006; Curtin et al., 2015). Neurodegenerative changes consisting of loss of dopamine transporters (DAT), serotonin transporters (5-HTT), and decreased levels of dopamine (DA) and its metabolites have been detected in the brains of human METH users (Wilson et al., 1996; Worsley et al., 2000; Volkow et al., 2001; Sekine et al., 2003, 2006).
METH users also exhibit prominent gray matter reduction in the cortical (Berman et al., 2008) and hippocampal (Thompson et. al., 2004; Hall et al., 2015) brain regions. Other investigators have reported higher striatal volume was observed in METH abusers (Thompson et al., 2004). Moreover, Tobias et al., (2010) reported that METH users exhibited decreased fractional anisotropy in the prefrontal white matter, the midline genu of the corpus callosum, and in the midcaudal superior corona radiata bilaterally. It is possible that some of the neuropathological changes observed in the brains of METH users might be secondary to the activation of microglial cells observed in the brains of some of these patients (Sekine et. al., 2008).
4. Animal models of METH neurotoxicity
Starting from the 1970’s, various studies have been published to show that both acute and chronic injections of METH can cause damage to monoaminergic terminals and neuronal apoptosis (Seiden et. al., 1976; Ando et. al., 1985; Woolverton et al., 1989; Fukumura et. al., 1998; Villemagne et. al., 1998; Harvey et. al., 2000a, 2000b; Ladenheim et al., 2000; Armstrong and Noguchi, 2004; Jayanthi et al., 2001, 2005; Truong et al., 2005; Deng et. al., 2001, 2007; Melega et. al., 1997, 2008; Ares-Santos et. al., 2013; Schweppe et. al., 2020).
4.1. Studies in rodents
Injections of large METH doses induce degeneration of monoaminergic terminals in rodents (Fukumura et. al., 1998; Ladenheim et al., 2000; Armstrong and Noguchi, 2004; Jayanthi et al., 2001, 2005; Truong et al., 2005; Deng et. al., 2001, 2007; Ares-Santos et. al., 2013). These are characterized by longterm decreases in vesicular DA uptake and vesicular monoamine transporters (VMAT2) (Guilarte et al., 2003; Truong et al., 2005), striatal DA transporters (Fukumura et. al., 1998; Truong et al., 2005; Krasnova et. al., 2011), levels of tyrosine hydroxylase (TH) protein and activity (Hotchkiss and Gibb, 1980; Fukumura et. al., 1998; Cappon et al., 2000; Krasnova et. al., 2011). Large doses of METH also negatively impact serotonerigc systems in the dorsal striatum where striatal serotonin (5-HT) levels (Fukumura et. al., 1998: Armstrong and Noguchi, 2004) and tryptophan hydroxylase (TPH) activity (Bakhit et al., 1981; Bakhit and Gibb, 1981) are reduced after injections of the drug. METH-induced abnormalities in 5-HT have also been reported in the nucleus accumbens (Nac), cortex, hippocampus, and hypothalamus (Hotchkiss and Gibb, 1980; Bakhit et al., 1981; Bakhit and Gibb, 1981; Baldwin et al., 1993; Armstrong and Noguchi, 2004). A very recent paper by Schweppe et. al. (2020) reported that rats challenged with toxic doses of METH showed reduction in striatal and hippocampal DA, 5-HT, brain derived neurotrophic factor (BDNF), and TrkB even as long as 75 days after the drug injections. METH neurotoxicity is also associated with astrocytic and microglial activation (Fukumura et. al., 1998; Guilarte et al., 2003).
Consistent with data obtained from rats, mice also suffer from METH-induced decreased levels of DA and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), as well as reduced levels of VMAT2, DAT and TH activity in various brain regions including the dorsal striatum, cortex, hippocampus and the olfactory bulb (Ladenheim et al., 2000; Achat-Mendes et. al., 2005; Deng et. al., 2007; Fantegrossi et al., 2008; Granado et. al., 2010; 2011a; 2011b; Ares-Santos et. al. 2012; Ares-Santos et. al., 2013).
The neurotoxic effects of METH have also been assessed in rodent model of METH self-administration (SA). Specifically, rats given long access to METH SA in order to mimic patterns of METH use in humans (Perez et al., 1999; Darke et al., 2008) exhibited persistent decreases in DA, DAT and TH but increased glial fibrillary acidic protein (GFAP) expression in the cortex and dorsal striatum and cortex (Krasnova et al., 2010). Similar to the report by Krasnova et al. (2010), McFadden et al. (2012) also observed persistent deficits in dopaminergic neuronal function consistent of decreased striatal DAT uptake, DAT concentrations, and increased GFAP using a SA paradigm of 8 h/day for 7 days (0.06 mg/infusion). Together, these two SA studies provide further evidence for the toxic effects of this drug.
4.2. Studies in primates
Rhesus monkeys injected with METH showed 70% loss of DA levels in the caudate, 33% loss of NE in the midbrain and 55% loss of NE in the frontal cortex (Seiden et. al., 1976). There were signficant decreases in DA and 5-HT level in various brain regions of nonhuman primates even 4 years after the injections of METH (Woolverton et al., 1989). Other groups of investigators have confirmed the effects of METH on the nonhuman primate brain. For example, Ando et al. (1985) reported a 32% loss of DA levels in the caudate nucleus and 71% loss of 5-HT levels in the frontal cortex after METH. In addition, Harvey et al. (2000a, 2000b) have provided evidence that METH injections can cause decreased levels TH, DAT, and VMAT2 in the nigrostriatal dopaminergic system. In vivo positron emission tomography (PET) studies in vervet monkeys also reported METH-induced reduced DA synthesis (Melega et. al., 1997, 2008). Furthermore, administration of METH to baboons exhibited reductions in striatal DAT which is associated with decreased level of DA (Villemagne et. al., 1998).
5. METH neurotoxicity, reactive oxygen species, and neuroinflammation
The pathways involved in causing METH neurotoxicity are varied and complex. They include the formation of reactive oxygen species including hydrogen peroxide, superoxide radicals, and hydroxyl radicals. The levels of some of these appear to be increased consequent to microglial cell activation and associated changes in proinflammatory factors in brain regions of interest.
5.1. Production of reactive oxygen species (ROS)
Oxidative stress plays an integral role in METH neurotoxicity. This occurs because METH administration leads to release of DA from vesicular pools followed by DA accumulation within monoaminergic terminals and DA release via DAT into the synaptic cleft (Chu et al, 2008; Hedges et al., 2018). Increased DA levels lead to DA auto-oxidation in intraneuronal and extracellular spaces, quinone production, superoxide radicals, hydrogen peroxide, and hydroxyl radicals (Graham, 1978; Cadet and Brannock, 1998; Yamamoto and Zhu, 1998; LaVoie and Hastings, 1999). The role of superoxide radicals in METH neurotoxicity was documented in a series of studies that showed that transgenic mice that over-express superoxide dismutase, the enzyme that breaks down superoxide radicals (Lewandowski et al., 2019), were protected against injections of large doses of METH (Cadet et al., 1994; Hirata et al., 1996; Jayanthi et al., 1998). Reactive nitrogen species also participate in generating METH neurotoxicity. This occurs through the production of nitric oxide secondary to METH-induced increases in nitric oxide synthase (NOS) activity (Imam et al., 2001). METH-induced ROS and RNS lead to lipid peroxidation and protein carbonyl formation in various brain regions and secondary damage to neuronal cell membrane (Jayanthi et al., 1998; Yamamoto and Zhu, 1998; Gluck et al., 2001). METH-induced impairment of blood-brain barrier (BBB) permeability may also occur via its pro-oxidant effects via activation of NADPH oxidase (Ramirez et al., 2009; Park et al., 2012; Jumnongprakhon et al., 2016).
Moreover, human chronic METH users have been reported with increased levels of oxidative stress markers (4-hydroxynonenal and malondialdehyde) (Fitzmaurice et. al., 2006) along with decreased activity of phospholipid metabolic enzymes (Ross et. al., 2002) and antioxidant systems in their brain (Mirecki et al., 2004).
5.2. Participation of microglial cells in METH-induced neurotoxic events
METH exposure causes both microglial and astrocyte activation in the brain (Sekine et. al., 2008, Krasnova et. al., 2010; McFadden et. al., 2012). This is associated with increased production and secretion of pro-inflammatory cytokines that can cause neurodegeneration (Xu et al., 2017; Tahmasebinia and Pourgholaminejad, 2017; Temmingh et al., 2020; Fukumura et al., 1998). Release of pro-inflammatory cytokines can then activate apoptotic signaling cascades in several model systems (Allagnat et al., 2012; Park et al., 2017; Butovsky and Weiner, 2018).
METH administration increases the expression of glial fibrillary acidic protein (GFAP) in various brain regions (Fukumura et. al., 1998; Guilarte et al., 2003; Krasnova et. al., 2010; McFadden et. al., 2012). METH also causes microglial activation in various brain regions (Goncalves et al., 2017; Gou et al., 2020; Sekine et al., 2008; Thomas et al., 2004; 2009). A role for microglial in the appearance of METH neurotoxicity is supported by reports that drugs such as MK-801 and dextromethorphan that block microglial activation can protect against the toxic effects of the drug (Asanuma et. al., 2003; Thomas and Kuhn, 2005). The sigma-1 receptor may also participate in mediating METH neurotoxicity via their effects on glial activation. Specifically, sigma receptor-1 antagonists such as BD1047 and SN79 that block glial activation and expression of cytokines have been shown to protect against METH-induced neurotoxicity (Kaushal et al., 2013; Robson et. al., 2013, 2014; Zhang et. al., 2015).
6. METH neurotoxicity and cell death mechanisms
Biochemical studies using a diversity of animal models have provided evidence for the participation of multiple mechanisms in METH-induced neuronal cell death. These include pathways regulated by mitochondrial and endoplasmic reticulum (ER) proteins, and involvement of some transcription factors.
6.1. Mitochondrial stress and METH neurotoxicity
Mitochondrial dysfunction plays a critical role in METH-induced neurotoxicity (Cadet et al., 2005, 2007). Auto-oxidation of excessive cytosolic and extracellular DA produces DA quinone and other reactive oxygen species (ROS) (Cadet, 1988; Cadet and Brannock, 1998; Cadet and Lohr, 1987). Similar mechanisms appear to be involved in the clinical manifestations and basic neuropathology of other neurological and psychiatric disorders including schizophrenia and Parkinson’s disease (Cadet and Lohr, 1987; Evans, 1993; Perfeito et al., 2012). DA oxidation products can cause mitochondrial dysfunctions which include mitochondrial swelling, opening the permeability transition pore, and inhibition of enzyme complex I (Berman and Hastings 1999; Jana et al. 2011; Khan et al. 2005). Evidence for a role for mitochondrial dysfunctions in METH neurotoxicity was provided in a series of studies that showed that METH injections can cause increased expression of pro-death proteins, BAX and BID, concurrent with decreases in anti-apoptotic proteins, Bcl-2 and Bcl-XL in the brains of rodents (Jayanthi et al., 2001; 2005; Deng et al., 2002). These changes are associated with release of cytochrome c, apoptosis inducing factor (AIF) and Smac/DIABLO from intra-mitochondrial spaces into the cytosol followed by induction of neuronal apoptosis (Jayanthi et al., 2001; 2004; Deng et al., 2002). Treatment with the antioxidant, melatonin, was able to attenuate these degenerative effects of METH (Wisessmith et al. 2009). A role for superoxide radicals in METH-induced cell death is also supported by the demonstration that METH-induced pathological changes were suppressed in copper-zinc superoxide dismutase transgenic mice (Deng and Cadet, 2000).
Of related interest, Brown et al. (2005) have reported that administration of high doses of METH can inhibit the enzymatic activity of mitochondrial complexes in the dorsal striatum via glutamate receptor- and peroxynitrite-mediated mechanisms. Sepehr et al. (2020) has also reported that METH can cause dysfunctions in the mitochondrial respiratory chain via a BDNF-TrkB-PGC-1α signaling pathway. This cascade is initiated when BDNF binds to its receptor TrkB and activates CREB signaling followed by increased expression of proliferator-activated gamma receptor coactivator 1-alpha (PGC-1α). PGC-1α, which is a key regulator of mitochondrial biogenesis (Fanibunda et., 2019) and of the mitochondrial uncoupling protein-2 (UCP-2) (Sepehr et al., 2020). Additionally, several in vivo and in vitro studies have documented disturbances in mitochondrial biogenesis consequent to METH injections; those include decreased mRNA expression of mitochondrial biogenesis-involved factors, PGC1α, NRF1 and TFAM (Beirami et al., 2018; Valian et al., 2017, 2019; Seyedhosseini et al., 2019). METH-induced mitochondrial dysfunctions might also occur via activation of protein kinase C-delta (PKCδ) (Dang et al., 2016; 2018; Nam et al., 2015; Nguyen et al., 2015; Shin et al., 2014; 2019) and its phosphorylation (Dang et al., 2018). Interactions between phosphorylated PKCδ and microsomal epoxide hydrolase (mEH) and between cleaved-PKCδ and mEH appear to also be involved in METH-induced cell death (Shin et al., 2019). It is also possible that METH-induced mitochondrial apoptotic signaling pathway might involve activation of the CCAAT-enhancer binding protein (C/EBPβ) (Qiao et al., 2014; Chen et al., 2016; Xu et al., 2018). For example, C/EBPβ can up-regulate the expression levels of insulin-like growth factor-binding protein 5 (IGFBP5) and p53-up-regulated modulator of apoptosis (PUMA) (Qiao et al., 2014; Chen et al., 2016; Xu et al., 2018) that eventually leads to downstream activation caspase cascade.
6.2. ER stress and METH neurotoxicity
The accumulated evidence suggests that METH-induced cell death can also occur via the activation of the endoplasmic reticulum (ER) stress (Krasnova and Cadet, 2009; Yu et al., 2015; Yang et al., 2018). ER stress is mediated by three pathways initiated by protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring transmembrane kinase/endonuclease 1 (IRE1), and activating transcription factor (ATF) 6 (Khanna et al., 2021; Shacham et al., 2021; Siwecka et al., 2021; van Anken et al., 2021). ER stress occurs consequent to accumulation of unfolded proteins within the ER lumen followed by disruption of the ER-associated protein degradation (ERAD) pathway, altered ER homeostasis, and neuronal apoptosis (Kim et. al., 2008; Hacker, 2017). Similar occurrences have been observed in the case of METH-induced neuronal death. Specifically, METH injections are accompanied by increased expression of several ER stress genes, including those that encode the 78-kDa glucose-regulated protein (GRP-78)/BiP, CCAAT/enhancer-binding protein homologous protein (CHOP), and ATF4 (Jayanthi et al., 2005; Jayanthi et al., 2009; Hayashi et al., 2010; Beauvais et al., 2011; Takeichi et al., 2012; Cai et. al., 2016; Wen et al., 2019; Chen et. al., 2021). ER stress is accompanied by activation of the upstream ER-specific caspase, caspase-12 (Nakagawa et al. 2000; Szegezdi et al. 2003) followed by cleavage of the executioner caspase, caspase-3 (Jayanthi et al., 2004). Recent in vitro studies by Wongprayoon and Govitrapong (2017) have also documented the involvement of the ER pathway in METH-induced death of SH-SY5Y neuronal cells treated with toxic doses of METH. The death mechanism includes increased CHOP expression, spliced X-box binding protein 1 (XBP1), caspase-12, and caspase-3 (Wongprayoon and Govitrapong, 2017). It is important to note that METH-mediated ER stress has been shown to be dependent on the activation of the DA D1 receptor in the rat brain (Jayanthi et al. 2009; Cadet et al., 2010; Beauvais et al. 2011), thus suggesting the possibility of using similar agents to counteract the toxic effects of the drug in humans. Xiao et al. (2018) have also reported that toxic doses of METH can significantly up-regulate the expression of phosphorylated PERK and caspase-12 and these effects can be suppressed by silencing of cyclin-dependent kinase (CDK) 5, a kinase that specifically phosphorylates Tau protein (Hashiguchi et al., 2002). In addition, Liu et al. (2020) documented METH induced time and dose-dependent activation of the three ER stress cascades, PERK, IRE1α and ATF6 signaling pathways, in hippocampal neuronal cells (HT-22). METH-induced disruptions of ER functions are accompanied by altered ER calcium homeostasis (Chen et al., 2019). They found that secreted ER calcium-monitoring proteins (SERCaMPs), a marker of ER stress that is triggered by depletion of ER calcium (Henderson et al., 2014) was significantly increased by METH (Chen et al., 2019).
6.3. METH and the ubiquitin/proteasome proteolytic pathway
METH neurotoxicity appears to also involve dysfunctions of the ubiquitin/proteasome system (UPS), a system that degrades intracellular proteins is involved in regulation of a broad array of cellular processes that include regulation of transcription factors and intracellular quality control (Glickman and Ciechanover, 2002, Ciechanover, 2013; Tai and Schuman, 2008). Maintenance of UPS function is by release of ubiquitin from the polyubiquitin tail and is catalyzed by ubiquitin C-terminal hydrolase L1/PGP 9.5 (UCH-L1) (Glickman and Ciechanover 2002). In the nervous system, this system is important in the modulation of synaptic plasticity (Tai and Schuman, 2008) and in controlling mechanisms involved in neurodegenerative processes (Schmidt et al., 2021). These facts are consistent with the demonstration that toxic doses of METH alter (UCH-L1) protein levels (Liao et al., 2005) accompanied by incomplete degradation of target proteins and accumulation of prion protein aggregates in DA-containing cells (Ferrucci et al., 2017). A proteomic analysis has also revealed that METH injections caused increased ubiquitin-conjugating enzyme E2N in various brain regions of rats (Li et al., 2008). Involvement of this system in METH neurotoxicity needs to be investigated further.
6.4. Transcription factors and their involvement in METH neurotoxicity
Injections of toxic of METH have been shown to alter the expression of several transcription factors including immediate-early genes (IEGs) in various brain regions (Bisagno and Cadet, 2019; Cadet et al., 2002; 2010) where METH-induced terminal degeneration and/or neuronal cell death have been observed (Deng et al., 2001; 2002; 2007; Jayanthi et al., 2004; 2005). Some of these transcription factors include c-fos, fosB, Fra-2, Egr-1, Egr-2, and Egr3 (Hirata et. al., 1998; Cadet et al., 2001; Thiriet et al., 2001; Jayanthi et al., 2005; Beauvais et. al., 2010; Cadet et al., 2010; McCoy et. al., 2011; Martin et. al., 2012). A role for c-fos in the METH-induced cell death was provided by Deng et al., (1999) who reported that METH-neurotoxicity was significantly exacerbated in heterozygous and homozygous c-fos knock-out mice, with the homozygous showing greater loss of striatal dopaminergic markers. The authors also showed c-fos knock-out mice exhibited more DNA fragmentation in nondopaminergic cells in the and dorsal striatum (Deng et al., 1999). Together, these observations suggest that c-fos induction after injections of toxic METH doses might occur to promote the induction of protective mechanisms such as the production of antioxidant enzymes or BDNF to attenuate METH neurotoxicity.
Further support for a role of IEGs in METH-induced cell death was provide by Jayanthi et al. (2005) who found that increased expression of expression of members of the Jun, Egr, and Nur77 subfamilies of transcription factors (TFs) occurred concurrently with increased markers of cell death in the rodent brain. They found, in addition, that these increases were accompanied increased expression of Fas ligand (FasL) mRNA which is known to be regulated by several IEG transcription factors. Moreover, METH neurotoxicity was accompanied by increased FasL protein expression in striatal GABAergic neurons that express enkephalin. There was also METH-induced cleavage of caspase-3 in FasL- and Fas-containing neurons. Importantly, pre-injections of the dopamine D1 receptor antagonist, SCH23390, that block the METH-induced IEG and FasL responses also attenuated METH-induced neuronal apoptosis.
7. Meth neurotoxicity and autophagy
Autophagy plays an important role in the maintenance of neuronal function (Yamamoto and Yue, 2014; Ariosa and Klionsky, 2016). Autophagic processes are highly conserved with multiple ordered sequences of events that are tightly regulated by autophagy-related proteins (Klionsky, 2000; Mizushima, 2007; Meijer and Codogno, 2004). The sequences are initiated via the formation of a phagophore that can be triggered by a diversity of cellular stressors that include food or energy deprivation and hyperthermia (Klionsky and Emr. 2000, Cuervo et al., 2004; Chu, 2008). This initial step is regulated by the phosphoinositide 3-kinase (PI3K)-Beclin-1-Atg14-Vps15 complex (Klionsky and Emr, 2000; Klionsky, 2007). A subsequent step involves the formation of the autophagosome, a process by which the phagophore expands and engulfs the cytosolic component controlled by autophagy-associated genes (Atg) genes through Atg12-Atg5 and LC3 complexes (Sanchez-Martin and Komatsu, 2020). Ultimately, the fusion of autophagosomes and lysosomes leads to degradation of cytosolic contents (Gatica et al., 2018; Nakamura and Yoshimori, 2018). Autophagic mechanisms are thought to participate in molecular and biochemical pathways that modulate neurodegenerative processes that constitute the substrates of diseases such as Alzheimer’s and Parkinson’s disease (Giorgi et al., 2021; Lu et al., 2020). It was therefore of interest to investigate the effects of METH on autophagic mechanisms.
METH-induced autophagic changes were initially reported by Larsen et al. (2002) who document the formation of autophagic granules upon exposure to the drug. Castino et al. (2008) also observed that METH can also cause autophagosome formation in a cell culture system. Of note, genetic inhibition of autophagy in rat dopaminergic has been reported to exacerbate METH-induced apoptosis (Lin et al. 2012), thus implicating autophagic mechanisms as protective factors against METH neurotoxicity. Overexpression of LC3-II (microtubule-associated light chain 3), an autophagy regulatory protein, was also shown to protect against METH-induced cell death (Lin et al. 2012). Those results are not consistent with those of other investigators who have shown that inhibition of autophagy via mTOR (negative regulator of autophagy) attenuated METH-induced cell death (Kongsuphol et al. 2009; Li et al. 2012). Moreover, Xu et al. (2018) have intimated that autophagy may constitute an early response in a METH-induced cell death cascade. Xu et al (2018) injected high doses of METH and reported increased protein expression of autophagy markers, Beclin-1 and LC3-II, in the rat striatum (Xu et al., 2018). A recent study by Subu et al (2020) had also reported the observation that rats that self-administered large quantities of METH during a self-administration experiment exhibited significant alterations in markers of autophagy and neuronal apoptosis in their dorsal striatum.
Other investigators have also provided evidence for METH-induced autophagy. For example, Li et al (2016) also reported that METH exposure increased the expression of DNA damage-inducible transcript 4 (DDIT4) and upregulation of Beclin-1 and LC-II. Yang et al (2019) showed that large doses of METH can increase Beclin-1 expression in human neuroblastoma cells via the AKT- mTOR signaling pathway. Both AKT and DDIT4 are negative mTOR regulators that promote the formation of autophagosomes (Moore et al. 2016; Miao et al., 2020; Wang et al., 2012). A recent study by Huang et al. (2019) also documented METH-induced increased expression of tribbles homolog 3 (Trib3), an inducible ER stress protein (Ohoka et al., 2005), that participates in autophagic mechanisms (Ord and Ord, 2017). Trib3 was shown to decrease p-Akt/p-mTOR interaction that resulted in increased expression of Beclin-1 and LC3-II (Huang et al., 2019).
Chaperone-mediated autophagy (CMA) (Dice, 2007) has also been reported after toxic METH doses (Sun et al. 2019). CMA is different from micro- or macroautophagy in that degradation can occur with vesicle formation (Dice, 2007). CMA degrades thirty percent of cytosolic proteins during prolonged nutrient deprivation (Dice, 2007). Molecular chaperones in the cytoplasm and within lysosomes are responsible for this degradation pathway. One of the critical CMA components is the lysosome-associated membrane protein (LAMP) type 2A, a receptor located on the lysosomal membrane (Cuervo and Dice, 1996). LAMP-2A is the rate limiting step for CMA (Cuervo and Dice, 1996). Another protein of interest is the heat shock protein of 70kd (hsc70) which can form a complex with LAMP-2A (Dice, 2007). METH exposure was accompanied by time- and dose-dependent increases in LAMP-2A expression in human neuroblastoma cell lines, PC12 cells, and primary mice neurons (Sun et al., 2019). Interestingly, LAMP-2A silencing exacerbated METH-induced cell death (Sun et al., 2019), suggesting that CMA might work as a protective mechanism in those in vitro models. Much more remains to be done to understand the potential role of CMA in METH-induced neurotoxicity using in vivo models.
Interestingly, C/EBPβ, appears to be a critical effector of METH-induced autophagy via the activation of DDIT4/TSC2/mTOR signaling or Trib3/Parkin/alpha-synuclein mechanisms (Huang et al., 2019; Xu et al., 2018). A report by Li et al. (2017) has also suggested a role for glycogen synthase kinase3β (GSK3β) in METH-induced autophagy and neurodegeneration via the promotion of Tau and α-syn phosphorylation, α-syn accumulation, inhibition of lysosomal degradation, and consequent apoptotic cell death.
8. METH neurotoxicity and potential relevance to therapeutic approaches
METH users have been reported to suffer from cognitive impairments that can impact their activities of daily living and course of treatment when they seek treatment (Cadet and Bisagno, 2016). Importantly, there is a suggestion that recovery of cognitive functions can occur in conjunction with improvement in impulsivity and self-regulation after a psychological intervention with working memory training during in-patient treatment for methamphetamine use disorder (Brooks et. al., 2016). These observations are supported by pre-clinical studies that have reported improvement in cognitive functions after administration of pharmacological compounds such as ZSET1446, a T-type calcium channel activator (Ito et. al., 2007), silibinin, a natural polyphenolic flavonoid (Lu et. al., 2010), 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide (CDPPB) a mGluR5 allosteric modulator (Reichel et. al., 2011) and modafinil, a dopamine uptake blocker (Kalechstein et. al., 2010; González et. al., 2014; Reichel et. al., 2014). The ameliorating effect of ZSET1446 (azaindolizinone derivative, a T-type calcium channel activator) and modafinil (2-[(diphenylmethyl) sulfinyl] acetamide) on METH-induced impairment of recognition memory is mediated via activation of ERK cascade (Ito et. al., 2007; González et. al., 2014). In addition to cognitive improvement, modafinil can also provide protection against METH-induced cell death and neuroinflammation in the rodent models (Raineri et. al., 2012), it is a drug that might have substantial anti-neurotoxic effects in humans who are treated with it. Silibinin mediates its ameliorating effects by reducing dopamine and serotonin levels in the prefrontal cortex and hippocampus, respectively (Lu et. al., 2010). CDPPB, a positive allosteric modulator of mGluR5 receptors, improved METH-induced cognitive impairments via interactions with these receptors (Reichel et. al., 2011).
Pharmacological interventions that target ROS and RNS signaling cascades also hold great potential in alleviating METH-induced neurotoxicity. Specifically, selenium, a dietary antioxidant is known to reduce METH-induced ROS production (Kim et al., 1999; Imam et al., 1999). Another antioxidant, N-acetyl-L-cysteine, can also suppress METH-induced oxidative stress in both rodent (Zhang et al., 2012) and primate (Hashimoto et al., 2004) models. Moreover, inhibitors of nitric oxide synthase, (7-nitroindazole and AR-R17477AR), and a selective peroxynitrite scavenger, (5,10,15,20-tetrakis [2,4,6-trimethyl-3,5-sulfonatophenyl] porphyrinato iron III (FeTPPS)), have been shown to reduce METH-induced hyperthermia, peroxynitrite production, and METH-induced dopaminergic depletion (Imam et al., 2000; Sanchez et al., 2003). Although much remains to be done to test whether these agents could improve cognitive functions in METH-treated animals, when taken together, these results suggest that the addition of anti-oxidative compounds may improve cognitive by reducing the neurotoxic effects of METH.
9. Conclusions
This review has discussed the evidence that has documented the toxic effects of METH in the central nervous system. These include degeneration of monoaminergic terminals and neuronal apoptosis. There is now evidence that METH administration is also accompanied by autophagic changes in the brain. It remains to be clearly determined if these autophagic changes are precursors of neuronal apoptosis or serve as attempts to protect the neurotoxicity of the drug. We have provided a schema (Figure 1) that illustrates the various biochemical cascades that have been shown to work as an ensemble to cause terminal damage and neuronal apoptosis in the mammalian brain. Although much more remains to be done to document if autophagy occurs in the brains of human METH users, a recent paper has reported that METH users exhibit increased Atg5 and LC3 in the pre-frontal cortical region (Khoshsirat et al., 2020). Nevertheless, because there is evidence that there might be pathobiological events in the brains of METH users who consume large quantities of the drug, it is essential that developers of pharmacological agents to treat METH use disorder take these toxic consequences into consideration.
Figure 1. Methamphetamine neurotoxicity- ROS and neuroinflammation.
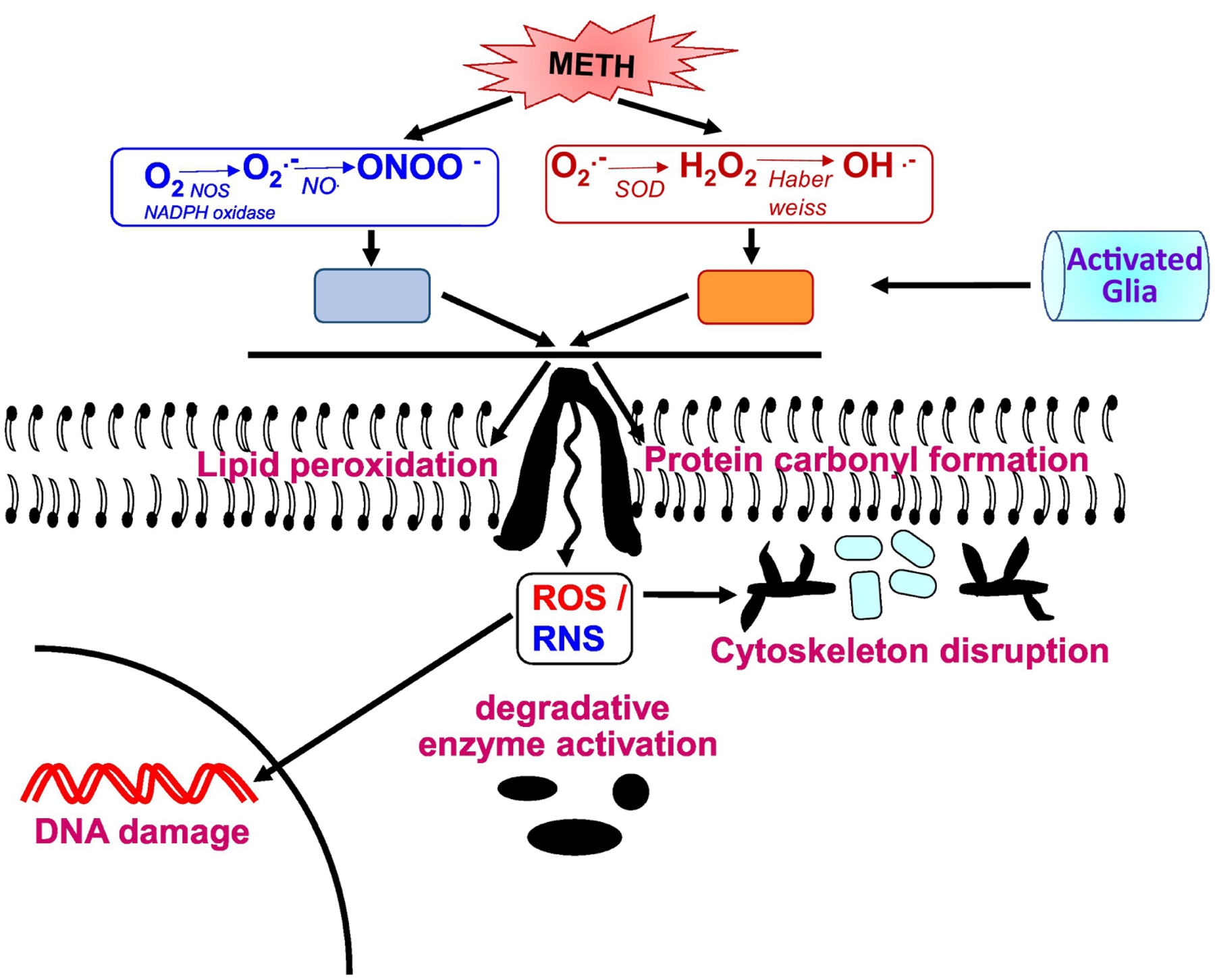
METH augments ROS and RNS levels that eventually lead to oxidative stress state. ROS in excessive concentrations can cause cellular damage to DNA, lipid membranes, proteins and other macromolecules. The end products of lipid peroxidation and protein carbonyl oxidation cause cytoskeleton disruption and DNA damage, such as double-strand DNA breaks. Moreover, the reactive species (ROS and RNS) triggers signaling pathways that lead to the over-activation of the major glial inflammatory characters: microglia and astrocytes. These glial cells are the mediators of neuroinflammatory response and ultimately leads to neurodegeneration.
Figure 2. Methamphetamine neurotoxicity-cell death mechanisms.
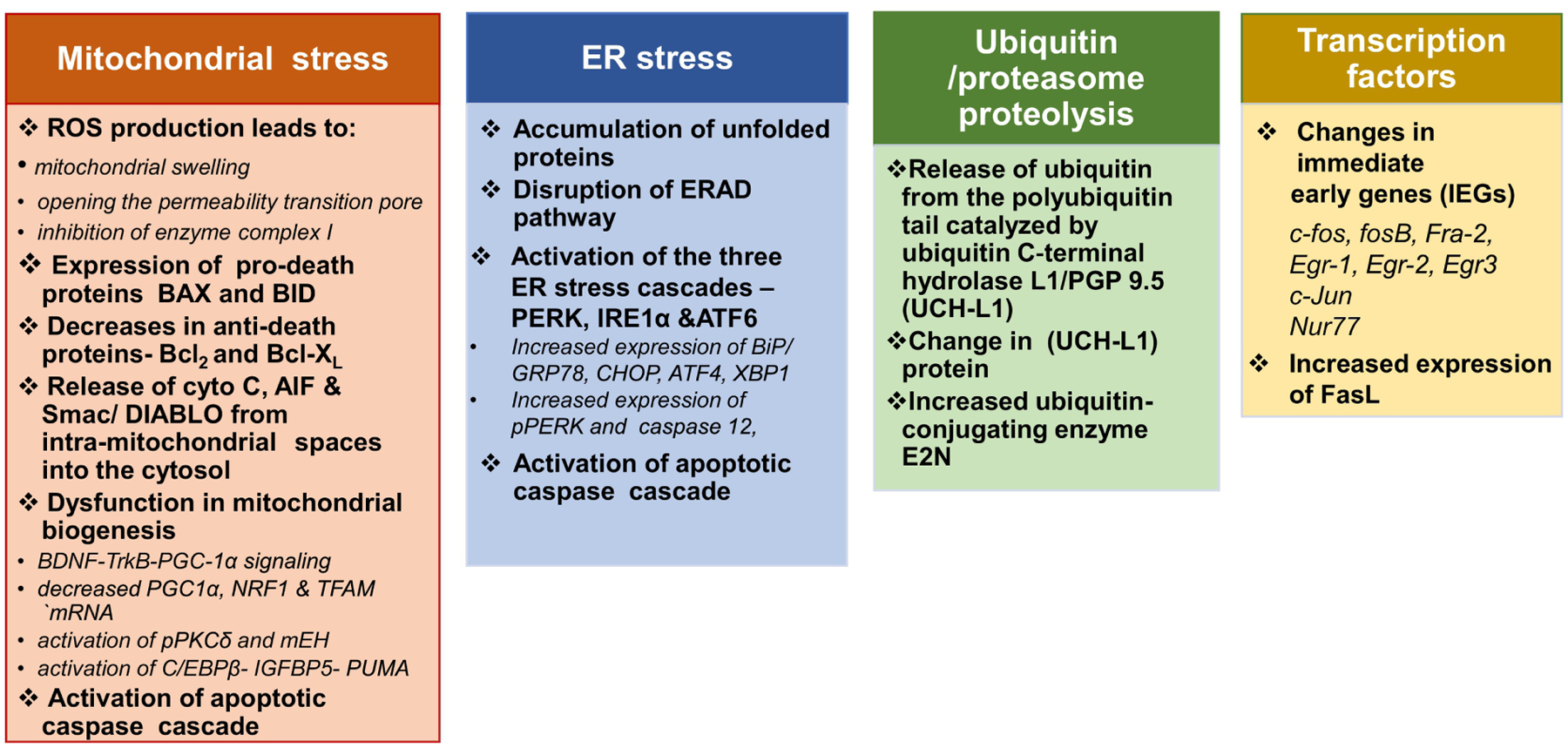
Scheme summarizes insights into the various molecular and functional connections between the different METH-induced neuronal cell death mechanisms.
Figure 3. Methamphetamine neurotoxicity-autophagy.
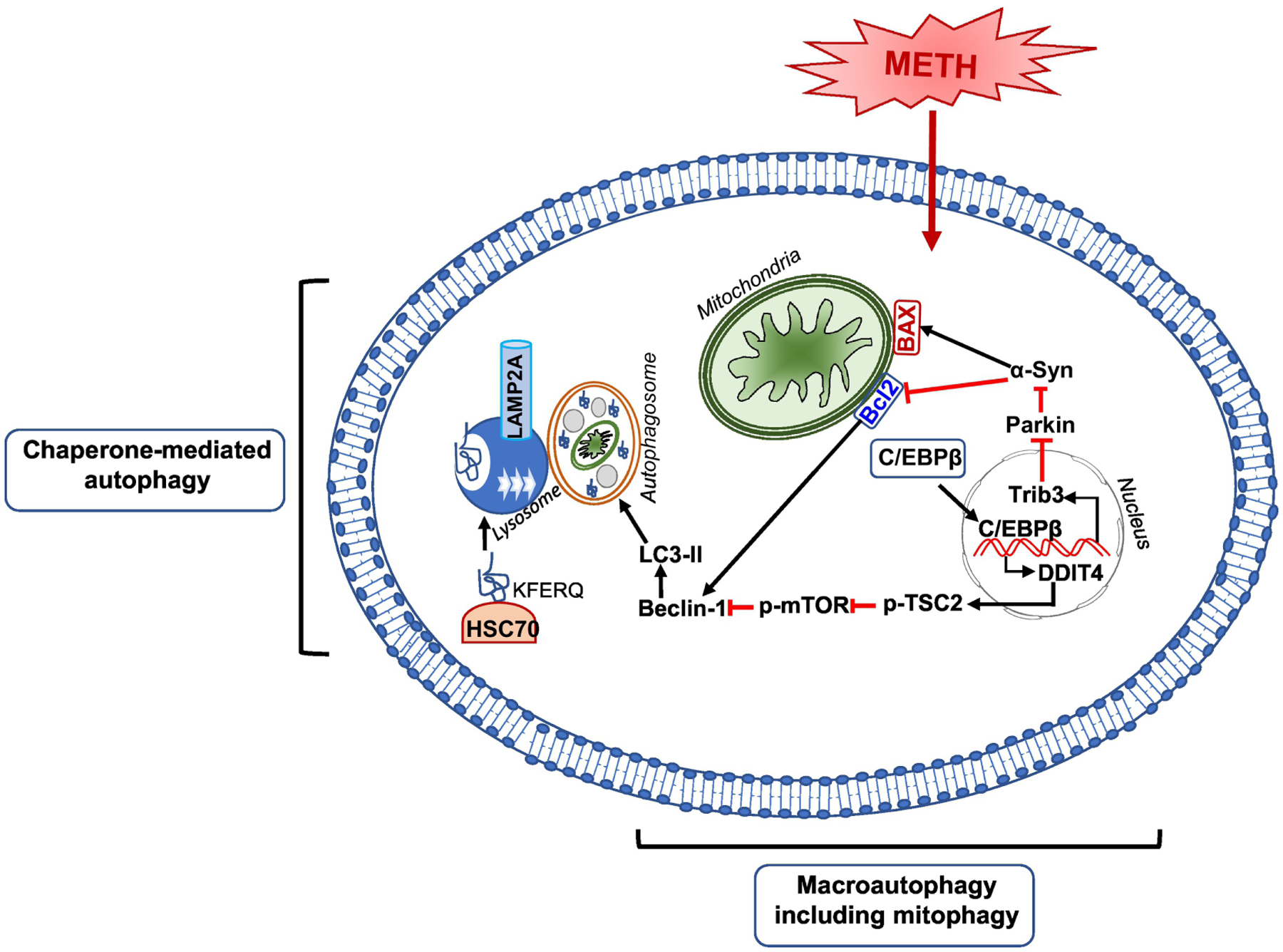
Schematic figure shows the signaling pathways that may be involved in METH-induced autophagy. METH is shown to facilitate both chaperone-mediated autophagy (CMA) and macro-autophagy. In both autophagic mechanisms, Beclin1 is the vital protein that associates with Bcl2 and promotes autophagy. METH-induced macro-autophagy is mediated via C/EBPβ by induction of C/EBPβ/DDIT4 /TSC2/mTOR signal axis or induction of C/EBPβ/Trib3 /Parkin/α-Syn signal axis. For the METH-induced CMA, The KFERQ-like motif of a cargo protein is detected by the chaperone, HSC70. This complex binds to the lysosomal membrane protein lysosome-associated membrane protein type 2A (LAMP2A). Then, the assembled LAMP2A is translocated through the lysosomal membrane. Once inside the lysosomal lumen, the substrate protein is rapidly degraded by lysosomal proteases and the hsc70 chaperone complex is released from the lysosome and ready to bind to another substrate protein for CMA.
Figure 4. Possible mechanisms of neurotoxicity produced by methamphetamine.
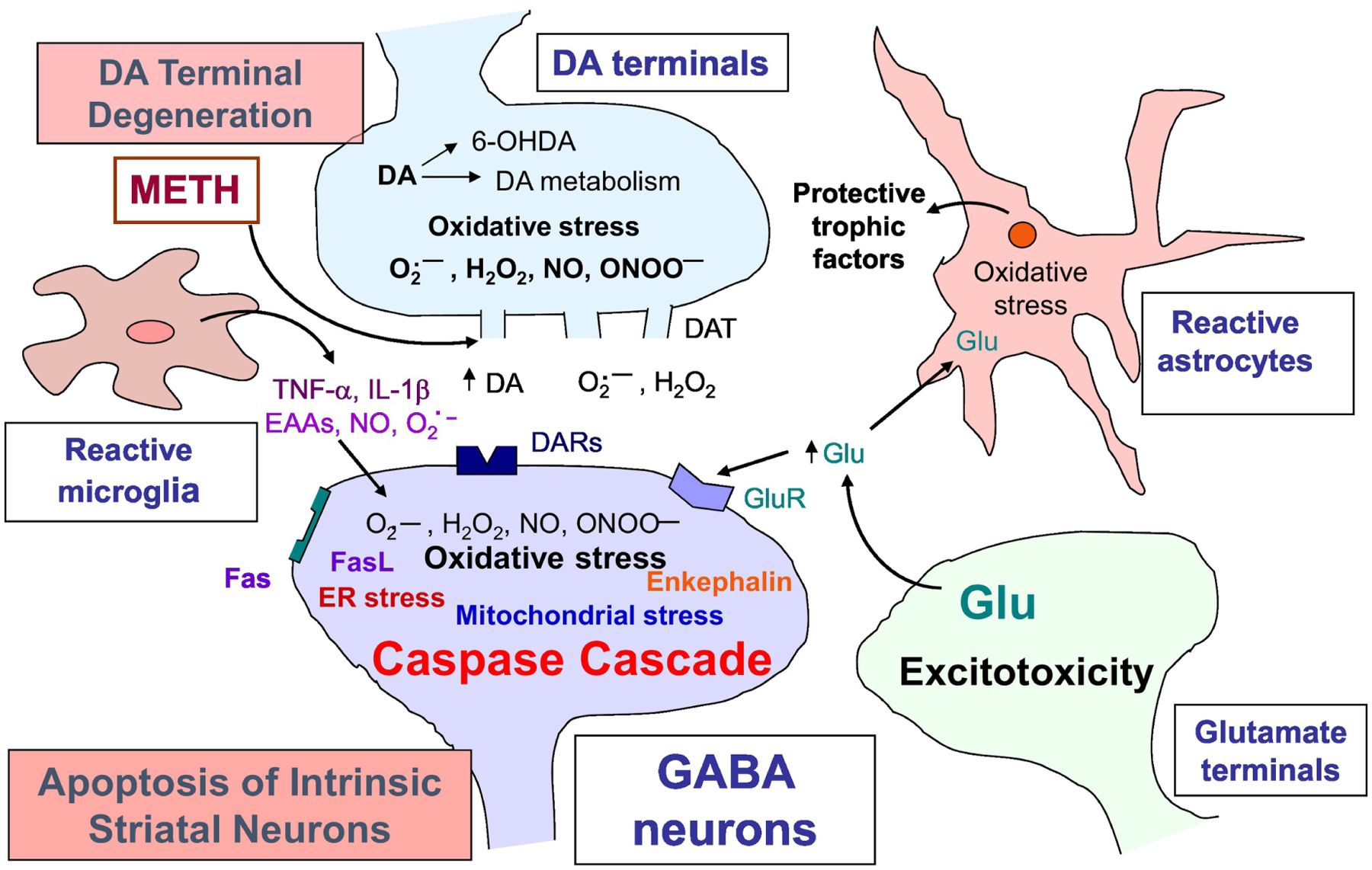
The depicted scheme provides a well- studied mechanistic outline in METH neurotoxicity; however, it mainly refers to the striatal synapses. Large doses of METH causes degeneration of DA terminals characterized by excessive DA release from the storage vesicles and causes perturbation in DA metabolism. Extracellular DA auto-oxidizes to produce reactive oxygen (O2.-, H2O2) and nitrogen (NO, ONOO-) species (ROS/RNS). METH exposure also causes increased activity of microglia and astrocytes. Reactive gliosis is associated with increased production of pro-inflammatory cytokines (TNFα; IL-1β) and ROS/RNS. This process initiates oxidative-stress mediated neuronal apoptotic cascade that includes mitochondrial stress, endoplasmic reticulum (ER) stress, ubiquitin-proteasome proteolysis, and neuroinflammation. In addition to affecting DA transmission, METH also exerts excitotoxicity via glutamate release that binds to glutamate receptors (GluR), triggers calcium influx, produces RNS and further oxidative stress-induced damage.
Table 1.
Markers of Methamphetamine Toxicity
| Alterations in the expression and balance of proinflammatory factors |
| Decreased levels of TH, DA, and DAT |
| Decreased levels of TPH, 5-HT, and 5-HTT |
| DA and 5-HT terminal degeneration |
| Neuronal Apoptosis |
| Neuronal Autophagy |
| Astrocytosis |
| Microgliosis |
Acknowledgments
This work is supported by the Department of Health and Human Services/National Institutes of Health/National Institute on Drug Abuse/Intramural Research Program, Baltimore, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Achat-Mendes C, Ali SF, Itzhak Y (2005) Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive pavlovian conditioning in mice. Neuropsychopharmacology 30:1128–1137. [DOI] [PubMed] [Google Scholar]
- Allagnat F, Fukaya M, Nogueira TC, Delaroche D, Welsh N, Marselli L, Marchetti P, Haefliger JA, Eizirik DL, Cardozo AK (2012) C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ 19:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Johanson CE, Seiden LS, Schuster CR (1985) Sensitivity changes to dopaminergic agents in fine moto control of rhesus monkeys after repeated methamphetamine administration. Pharmacology Biochemistry and Behavior 22:737–743. [DOI] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Moratalla R (2013) The role of dopamine receptors in the neurotoxicity of methamphetamine. J Intern Med 273:437–453. [DOI] [PubMed] [Google Scholar]
- Ares-Santos S, Granado N, Oliva I, Martin ED, Colado MI, Moratalla R (2012) Dopamine D (1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis 45:810–820. [DOI] [PubMed] [Google Scholar]
- Ariosa AR, Klionsky DJ (2016) Autophagy core machinery: overcoming spatial barriers in neurons. J Mol Med (Berl) 94:1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BD, Noguchi KK (2004) The neurotoxic effects of 3,4 – methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABAergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 25:905–914. [DOI] [PubMed] [Google Scholar]
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N (2003) Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett 352:13–16. [DOI] [PubMed] [Google Scholar]
- Ashok AH, Mizuno Y, Volkow ND, Howes OD (2017) Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine a systematic review and meta-analysis. JAMA Psychiatry 74:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhit C, Gibb JW (1981) Methamphetamine-induced depression of tryptophan hydroxylase: Recovery following acute treatment. European Journal of Pharmacology 76:229–233. [DOI] [PubMed] [Google Scholar]
- Bakhit C, Morgan ME, Peat MA, Gibb JW (1981) Long-term effects of methamphetamine on the synthesis and metabolism of 5-hydroxytryptamine in various regions of the rat brain. Neuropharmacology 20:1135–1140. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Colado MI, Murray TK, DeSouza RJ, Green AR (1993) Striatal dopamine release in vivo following neurotoxic doses of methamphetamine and effect of the neuroprotective drugs, chlormethiazole and dizocilpine. Br J Pharmacol 108:590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais G, Atwell K, Jayanthi S, Ladenheim B, Cadet JL (2011) Involvement of dopamine receptors in binge methamphetamine-Induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One 6:e28946, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais G, Jayanthi S, McCoy MT, Ladenheim B, Cadet JL (2010) Differential effects of methamphetamine and SCH23390 on the expression of members of IEG families of transcription factors in the rat striatum. 1318:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirami E, Oryan S, Seyedhosseini Tamijani SM, Ahmadiani A, Dargahi L (2018) Intranasal insulin treatment restores cognitive deficits and insulin signaling impairment induced by repeated methamphetamine exposure. J Cell Biochem 119:2345–2355. [DOI] [PubMed] [Google Scholar]
- Berman SB, Hastings TG (1999) Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson’s disease. J Neurochem 73:1127–1137. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Cadet JL (2019) Expression of immediate early genes in brain reward circuitries: Differential regulation by psychostimulant and opioid drugs. Neurochem Int 124:10–18. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Burch KH, Maiorana SA, Cocolas E, Schioth HB, Nilsson EK, Kamaloodien K, Stein DJ (2016) Psychological intervention with working memory training increases basal ganglia volume: A VBM study of inpatient treatment for methamphetamine use. Neuroimage Clin 12:478–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Quinton MS, Yamamoto BK (2005) Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. Journal of Neurochemistry 95:429–436. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Weiner HL (2018) Microglial signatures and their role in health and disease. Nat Rev Neurosci 19:622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton JA, Dove NA (2008) The burden and management of crystal meth use. CMAJ 178:1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL (1988) Free radical mechanisms in the central nervous system: an overview. Int J Neurosci 40:13–18. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V (2015) Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry 6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32:117–131. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Gold M (2017) Methamphetamine-induced psychosis: who says all drug use is reversible. Current Psychiatry 11:14–20. [Google Scholar]
- Cadet JL, Jayanthi S, Deng X (2003) Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J 17:1775–1788. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X (2005) Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Neurotox Res 8:199–206. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Beauvais G, Cai NS (2010) Dopamine D1 receptors, regulation of gene expression in the brain, and neurodegeneration. CNS Neurol Disord Drug Targets 9:526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B (2001) Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: evidence from cDNA array. Synapse 41:40–48. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN (2009) Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol 88:101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J (2007). Neurotoxicity of substituted amphetamines: Molecular and cellular mechanisms. Neurotox Res.11:183–202. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Lohr JB (1987) Free Radicals and the Developmental Pathobiology of Schizophrenic Burnout. Integrative Psychiatry 5:40–43. [Google Scholar]
- Cadet JL, McCoy MT, Ladenheim B (2002) Distinct gene expression signatures in the striata of wild-type and heterozygous c-fos knockout mice following methamphetamine administration: evidence from cDNA array analyses. Synapse 44:211–226. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C (1994). Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem 62:380–383. [DOI] [PubMed] [Google Scholar]
- Cai D, Huang E, Luo B, Yang Y, Zhang F, Liu C, Lin Z, Xie WB, Wang H (2016). Nupr1/Chop signal axis is involved in mitochondrion-related endothelial cell apoptosis induced by methamphetamine. Cell Death Dis 7:e2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ (2010) Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord 25:2333–2339. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ (2012) Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend 120:35–40. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV (2000) Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 863:106–111. [DOI] [PubMed] [Google Scholar]
- Castino R, Lazzeri G, Lenzi P, Bellio N, Follo C, Ferrucci M, Fornai F, Isidoro C (2008) Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. J Neurochem 106:1426–1439. [DOI] [PubMed] [Google Scholar]
- Chen C, Qincao L, Xu J, Du S, Huang E, Liu C, Lin Z, Xie WB, Wang H (2016) Role of PUMA in methamphetamine-induced neuronal apoptosis. Toxicol Lett 240:149–160. [DOI] [PubMed] [Google Scholar]
- Chen G, Wei X, Xu X, Yu G, Yong Z, Su R, Tao L (2021) Methamphetamine Inhibits Long-Term Memory Acquisition and Synaptic Plasticity by Evoking Endoplasmic Reticulum Stress. Front Neurosci 14:630713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Bae E, Chen H, Yu SJ, Harvey BK, Greig NH, Wang Y (2019) Pifithrin-alpha reduces methamphetamine neurotoxicity in cultured dopaminergic neurons. Neurotox Res 36:347–356. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Chomchai S, (2015) Global patterns of methamphetamine use. Curr. Opin. Psychiatry 28:269–274. [DOI] [PubMed] [Google Scholar]
- Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS, Hanson GR, Fleckenstein AE (2008) Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol 590:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT (2008) Eaten alive: autophagy and neuronal cell death after hypoxia-ischemia. Am J Pathol 172:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A (2013) Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem 21:3400–3410. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF (1996) A receptor for the selective uptake and degradation of proteins by lysosomes. Science 273:501–503. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305:1292–1295. [DOI] [PubMed] [Google Scholar]
- Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR (2015) Methamphetamine /amphetamine abuse and risk of Parkinson’s disease in Utah: a population-based assessment. Drug Alcohol Depend 146:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DK, Shin EJ, Kim DJ, Tran HQ, Jeong JH, Jang CG, Ottersen OP, Nah SY, Hong JS, Nabeshima T, Kim HC (2018) PKCδ-dependent p47phox activation mediates methamphetamine-induced dopaminergic neurotoxicity. Free Radic Biol Med 115:318–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DK, Shin EJ, Nam Y, Ryoo S, Jeong JH, Jang CG, Nabeshima T, Hong JS, Kim HC (2016) Apocynin prevents mitochondrial burdens, microglial activation, and pro-apoptosis induced by a toxic dose of methamphetamine in the striatum of mice via inhibition of p47phox activation by ERK. J Neuroinflammation 13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J (2008) Major physical and psychological harms of methamphetamine use Drug Alcohol Rev 27:253–262. [DOI] [PubMed] [Google Scholar]
- Deng X, Cadet JL (2000) Methamphetamine-induced apoptosis is attenuated in the striata of copper-zinc superoxide dismutase transgenic mice. Brain Res Mol Brain Res 83:121–124. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL (2002) Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology 42:837–845. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL (2007) Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry 61:1235–1243. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL (1999) Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci 19:10107–10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL (2001) Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res 93:64–69. [DOI] [PubMed] [Google Scholar]
- Dice JF (2007) Chaperone-mediated autophagy. Autophagy 3:295–299. [DOI] [PubMed] [Google Scholar]
- DSM–5 (2013) Diagnostic and statistical manual of mental disorders, Fifth Edition ed. American Psychiatric Association. [Google Scholar]
- EU Drug Markets Report 2019: E/INCB/2019/1. The Report of the International Narcotics Control Board for 2019 (E/INCB/2019/1), United Nations. [Google Scholar]
- Evans PH (1993) Free radicals in brain metabolism and pathology. Br Med Bull 49:577–587. [DOI] [PubMed] [Google Scholar]
- Fanibunda SE, Deb S, Maniyadath B, Tiwari P, Ghai U, Gupta S, Figueiredo D, Weisstaub N, Gingrich JA, Vaidya ADB, Kolthur-Seetharam U, Vaidya VA (2019) Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT2A receptor and SIRT1-PGC-1α axis. PNAS 116:11028–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R 2nd, Traynor JR, Woods JH (2008) A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience 151:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci M, Ryskalin L, Biagioni F, Gambardella S, Busceti CL, Falleni A, Lazzeri G, Fornai F. (2017) Methamphetamine increases Prion Protein and induces dopamine-dependent expression of protease resistant PrPsc. Arch Ital Biol. Jul 1;155(1–2):81–97. doi: 10.12871/000398292017129. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice PS, Tong J, Yazdanpanah M, Liu PP, Kalasinsky KS, Kish SJ (2006) Levels of 4-hydroxynonenal and malondialdehyde are increased in brain of human chronic users of methamphetamine. J Pharmacol Exp Ther 319:703–709. [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV (1998) A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res 806:1–7. [DOI] [PubMed] [Google Scholar]
- Garwood ER, Bekele W, McCulloch CE, Christine CW (2006) Amphetamine Exposure Is Elevated in Parkinson’s Disease. Neurotoxicology 27:1003–1006. [DOI] [PubMed] [Google Scholar]
- Gatica D, Lahiri V, Klionsky DJ (2018) Cargo recognition and degradation by selective autophagy. Nat Cell Biol 20:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Bouhamida E, Danese A, Previati M, Pinton P, Patergnani S (2021) Relevance of Autophagy and Mitophagy Dynamics and Markers in Neurodegenerative Diseases. Biomedicines 9:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK (2001) Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem 79:152–160. [DOI] [PubMed] [Google Scholar]
- Gonçalves J, Leitão RA, Higuera-Matas A, Assis MA, Coria SM, Fontes-Ribeiro C, Ambrosio E, Silva AP (2017). Extended-access methamphetamine self-administration elicits neuroinflammatory response along with blood-brain barrier breakdown. Brain Behav Immun 62:306–317. [DOI] [PubMed] [Google Scholar]
- González B, Raineri M, Cadet JL, García-Rill E, Urbano FJ, Bisagno V (2014) Modafinil improves methamphetamine-induced object recognition deficits and restores prefrontal cortex ERK signaling in mice. Neuropharmacology 87:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou H, Sun D, Hao L, An M, Xie B, Cong B, Ma C, Wen D (2020) Cholecystokinin-8 attenuates methamphetamine-induced inflammatory activation of microglial cells through CCK2 receptor. Neurotoxicology 81:70–79. [DOI] [PubMed] [Google Scholar]
- Graham DG (1978) Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol 14:633–643. [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R (2010) Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration: early loss of TH in striosomes after methamphetamine. Neurotox Res 18:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, O’Shea E, Martin ED, Colado MI, Moratalla R (2011a) Dopamine D2- receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Dis 42:391–403. [DOI] [PubMed] [Google Scholar]
- Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, Cuadrado A, Moratalla R (2011b) Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia 59:1850–1863. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience 122:499–513. [DOI] [PubMed] [Google Scholar]
- Hacker G (2014) ER-stress and apoptosis: molecular mechanisms and potential relevance in infection. Microbes Infect 16:805–810. [DOI] [PubMed] [Google Scholar]
- Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, Radua J (2015) Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse 41:290–299. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Laćan G, Melegan WP (2000a) Regional heterogeneity of dopaminergic deficits in vervet monkey striatum and substantia nigra after methamphetamine exposure. Exp Brain Res 133:349–358. [DOI] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP (2000b) Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 871:259–270. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Saito T, Hisanaga S, Hashiguchi T (2002) Truncation of CDK5 activator p35 induces intensive phosphorylation of Ser202/Thr205 of human tau. J Biol Chem 277:44525–44530. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M (2004) Effects of N-acetyl-L-cysteine on the reduction of brain dopamine transporters in monkey treated with methamphetamine. Ann. NY Acad. Sci 1025:231–235. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, Barnes C, Goldberg SR, Su TP (2010) Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther 332:1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DM, Obray JD, Yorgason JT, Jang EY, Weerasekara VK, Uys JD, Bellinger FP, Steffensen SC (2018) Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway. Neuropsychopharmacology 43:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MJ, Wires ES, Trychta KA, Richie CT, Harvey BK (2014) SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell 25:2828–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Asanuma M, Cadet JL (1998) Superoxide radicals are mediators of the effects of methamphetamine on Zif268 (Egr-1, NGFI-A) in the brain: evidence from using CuZn superoxide dismutase transgenic mice. Brain Res Mol Brain Res 58:209–216. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ladenheim B, Carlson E, Epstein C, Cadet JL (1996) Autoradiographic evidence for methamphetamine-induced striatal dopaminergic loss in mouse brain: attenuation in CuZn-superoxide dismutase transgenic mice. Brain Res 714:95–103. [DOI] [PubMed] [Google Scholar]
- Ho EL, Josephson SA, Lee HS, Smith WS (2009) Cerebrovascular complications of methamphetamine abuse. Neurocrit. Care 10:295–305. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW (1980) Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther 214:257–262. [PubMed] [Google Scholar]
- Hsieh JH, Stein DJ, Howells FM (2014) The neurobiology of methamphetamine induced psychosis. Front Hum Neurosci 8:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E, Huang H, Guan T, Liu C, Qu D, Xu Y, Yang J, Yan L, Xiong Y, Liang T, Wang Q, Chen L (2019) Involvement of C/EBPβ-related signaling pathway in methamphetamine-induced neuronal autophagy and apoptosis. Toxicol Lett 312:11–21. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W, Ali SF (2000) Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann. N. Y. Acad. Sci 914:157–171. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Islam F, Slikker W Jr, Ali SF (1999) Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res. 818:575–578. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W Jr, Ali SF (2001) Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem 76:745–749. [DOI] [PubMed] [Google Scholar]
- Ito Y, Takuma K, Mizoguchi H, Nagai T, Yamada K (2007) A novel azaindolizinone derivative ZSET1446 (spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one) improves methamphetamine-induced impairment of recognition memory in mice by activating extracellular signal-regulated kinase 1/2. J Pharmacol Exp Ther 320:819–827. [DOI] [PubMed] [Google Scholar]
- Jana S, Sinha M, Chanda D, Roy T, Banerjee K, Munshi S, Patro BS, Chakrabarti S (2011) Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim Biophys Acta 1812:663–73. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL (2001) Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J 15:1745–1752. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL (2005) Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. PNAS 18:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Cadet JL (1998) Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann N Y Acad Sci 844:92–102. [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W 3rd, Becker K, Cadet JL (2009) Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS One 4:e6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ES, Rayner BL (2015) Hypertension, end-stage renal disease and mesangiocapillary glomerulonephritis in methamphetamine users. S Afr Med J 105:199–201. [DOI] [PubMed] [Google Scholar]
- Jumnongprakhon P, Govitrapong P, Tocharus C, Tocharus J (2016) Inhibitory effect of melatonin on cerebral endothelial cells dysfunction induced by methamphetamine via NADPH oxidase-2. Brain Res 1650:84–92. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R 2nd, Newton TF (2010) Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict 19:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal N, Seminerio MJ, Robson MJ, McCurdy CR, Matsumoto RR (2013) Pharmacological evaluation of SN79, a sigma (σ) receptor ligand, against methamphetamine-induced neurotoxicity in vivo. Eur Neuropsychopharmacol 23:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan FH, Sen T, Maiti AK, Jana S, Chatterjee U, Chakrabarti S (2005) Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implications for Parkinson’s disease. Biochim Biophys Acta 1741:65–74. [DOI] [PubMed] [Google Scholar]
- Khanna M, Agrawal N, Chandra R, Dhawan G (2021) Targeting unfolded protein response: a new horizon for disease control. Expert Rev Mol Med 23:e1. [DOI] [PubMed] [Google Scholar]
- Khoshsirat S, Khoramgah MS, Mahmoudiasl GR, Rezaei-Tavirani M, Abdollahifar MA, Tahmasebinia F, Darabi S, Niknazar S, Abbaszadeh HA (2020) LC3 and ATG5 overexpression and neuronal cell death in the prefrontal cortex of postmortem chronic methamphetamine users. J Chem Neuroanat 107:101802. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Choi DY, Im DH, Shin EJ, Suh JH, Floyd RA, Bing G (1999) Protection of methamphetamine nigrostriatal toxicity by dietary selenium. Brain Res. 851:76–86. [DOI] [PubMed] [Google Scholar]
- Kim I, Xu W, Reed J (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7:1013–1030. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL (2010) Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One 5:e8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Hodges AB, Volkow ND, Cadet JL (2011) Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS One 25:e19179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL (2000) Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol 58:1247–1256. [DOI] [PubMed] [Google Scholar]
- Lappin JM, Sara GE (2019) Psychostimulant use and the brain. Addiction 114:2065–2077. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG (1999) Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19:1484–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D (2002) Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci 22(20):8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski Ł, Kepinska M, Milnerowicz H (2019) The copper-zinc superoxide dismutase activity in selected diseases. Eur J Clin Invest 49:e13036. [DOI] [PubMed] [Google Scholar]
- Li B, Chen R, Chen L, Qiu P, Ai X, Huang E, Huang W, Chen C, Liu C, Lin Z, Xie WB, Wang H (2016) Effects of DDIT4 in methamphetamine-induced autophagy and apoptosis in dopaminergic neurons. Mol Neurobiol 54:1642–1660. [DOI] [PubMed] [Google Scholar]
- Li L, Chen S, Wang Y, Yue X, Xu J, Xie W, Qiu P, Liu C, Wang A, Wang H (2017) Role of GSK3β/α-synuclein axis in methamphetamine-induced neurotoxicity in PC12 cells. Toxicol Res (Camb) 7:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang H, Qiu P, Luo H (2008) Proteomic profiling of proteins associated with methamphetamine-induced neurotoxicity in different regions of rat brain. Neurochem Int 52:256–264. [DOI] [PubMed] [Google Scholar]
- Liao PC, Kuo YM, Hsu HC, Cherng CG, Yu L (2005) Local proteins associated with methamphetamine-induced nigrostriatal dopaminergic neurotoxicity. J Neurochem 95:160–168. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wen D, Gao J, Xie B, Yu H, Shen Q, Zhang J, Jing W, Cong B, Ma C (2020) Methamphetamine induces GSDME-dependent cell death in hippocampal neuronal cells through the endoplasmic reticulum stress pathway. Brain Res Bull 162:73–83. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu M, Yue Z (2020) Autophagy and Parkinson’s Disease. Adv Exp Med Biol 1207:21–51. [DOI] [PubMed] [Google Scholar]
- Lu P, Mamiya T, Lu L, Mouri A, Niwa M, Kim HC, Zou LB, Nagai T, Yamada K, Ikejima T, Nabeshima T (2010) Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res 207:387–393. [DOI] [PubMed] [Google Scholar]
- Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL (2012) Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS One 7:e34236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MT, Jayanthi S, Wulu JA, Beauvais G, Ladenheim B, Martin TA, Krasnova IN, Hodges AB, Cadet JL (2011) Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat: normalization by an acute methamphetamine injection. Psychopharmacology (Berl) 215:353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE (2012) Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther 340:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee SM, McGee DN, McGee MB (2004) Spontaneous intracerebral hemorrhage related to methamphetamine abuse: autopsy findings and clinical correlation. Am J Forensic Med Pathol 25:334–337. [DOI] [PubMed] [Google Scholar]
- McNeely ME, Duncan RP, Farhart GM (2012) Medication improves balance and complex gait performance in Parkinson’s disease. Gait Posture 36:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P (2004) Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol 36:2445–2462. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Laćan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA (2008) Long-Term Methamphetamine Administration in the Vervet Monkey Models Aspects of a Human Exposure: Brain Neurotoxicity and Behavioral Profiles. Neuropsychopharmacology 33:1441–1452. [DOI] [PubMed] [Google Scholar]
- Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME (1997) Recovery of striatal dopamine function after acute amphetamine- and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res 766:113–120. [DOI] [PubMed] [Google Scholar]
- Miao ZF, Cho CJ, Wang ZN, Mills JC (2020). Autophagy repurposes cells during paligenosis. Autophagy, Ahead-of-print:1–2. doi: 10.1080/15548627.2020.1857080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirecki A, Fitzmaurice P, Ang L, Kalasinsky K, Peretti FK, Aiken SS, Wickham DJ, Sherwin A, Nobrega JN, Forman HJ, Kish SJ (2004) Brain antioxidant systems in human methamphetamine users. J Neurochem 89:1396–1408. [DOI] [PubMed] [Google Scholar]
- Mizushima N (2007). Autophagy: process and function. Genes Dev 21:2861–2873. [DOI] [PubMed] [Google Scholar]
- Moore J, Megaly M, MacNeil AJ, Klentrou P, Tsiani E (2016) Rosemary extract reduces Akt/mTOR/p70S6K activation and inhibits proliferation and survival of A549 human lung cancer cells. Biomed Pharmacother 83:725–732. [DOI] [PubMed] [Google Scholar]
- Nagai T, Kamiyama S (1988) Forensic toxicologic analysis of methamphetamine and amphetamine optical isomers by high performance liquid chromatography. Z Rechtsmed 101:151–159. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Yoshimori T (2017). New insights into autophagosome-lysosome fusion. J Cell Sci 130:1209–1216. [DOI] [PubMed] [Google Scholar]
- Nam Y, Wie MB, Shin EJ, Nguyen TT, Nah SY, Ko SK, Jeong JH, Jang CG, Kim HC (2015) Ginsenoside Re protects methamphetamine-induced mitochondrial burdens and proapoptosis via genetic inhibition of protein kinase C δ in human neuroblastoma dopaminergic SH-SY5Y cell lines. J Appl Toxicol 35:927–44. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health (2017) Substance abuse and mental health services administration (2018) key substance use and mental health indicators in the united states: Results from the 2017 national survey on drug use and health (HHS Publication No. SMA 18–5068, NSDUH Series H-53) Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Nguyen XK, Lee J, Shin EJ, Dang DK, Jeong JH, Nguyen TT, Nam Y, Cho HJ, Lee JC, Park DH, Jang CG, Hong JS, Nabeshima T, Kim HC (2015) Liposomal melatonin rescues methamphetamine-elicited mitochondrial burdens, pro-apoptosis, and dopaminergic degeneration through the inhibition PKCδ gene. J Pineal Res 58:86–106. [DOI] [PubMed] [Google Scholar]
- Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord T, Ord T (2017) Mammalian Pseudokinase TRIB3 in Normal Physiology and Disease: Charting the Progress in Old and New Avenues. Curr Protein Pept Sci 18:819–842. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM (2013). Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend 129:167–179. [DOI] [PubMed] [Google Scholar]
- Park JH, Seo YH, Jang JH, Jeong CH, Lee S, Park B (2017) Asiatic acid attenuates methamphetamine-induced neuroinflammation and neurotoxicity through blocking of NF-kB/STAT3/ERK and mitochondria-mediated apoptosis pathway. J Neuroinflammation 14:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Hennig B, Toborek M (2012) Methamphetamine alters occludin expression via NADPH oxidase-induced oxidative insult and intact caveolae. J Cell Mol Med 16:362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL (2020) Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 77:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA Jr, Arsura EL, Strategos S (1999) Methamphetamine-related stroke: four cases. J Emerg Med 17:469–471. [DOI] [PubMed] [Google Scholar]
- Perfeito R, Cunha-Oliveira T, Rego AC (2012). Revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease--resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 53:1791–806. [DOI] [PubMed] [Google Scholar]
- Qiao D, Xu J, Le C, Huang E, Liu C, Qiu P, Lin Z, Xie WB, Wang H (2014) Insulin-like growth factor binding protein 5 (IGFBP5) mediates methamphetamine-induced dopaminergic neuron apoptosis. Toxicol Lett 230:444–53. [DOI] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V (2012) Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One 7:e46599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y (2009) Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab 29:1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Gilstrap MG, Ramsey LA, See RE (2014) Modafinil restores methamphetamine induced object-in-place memory deficits in rats independent of glutamate N-methyl-D-aspartate receptor expression. Drug Alcohol Depend 134:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36:782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Z. J. Naser ZJ, McCurdy CR, Huber JD, Matsumoto RR(2013) SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Experimental Neurology 247:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Z. J. Naser ZJ, McCurdy CR, O’Callaghan JP, Huber JD, Matsumoto RR (2014) SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation. Experimental Neurology 254:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Peretti FJ, Adams V, Schmunk GA, Kalasinsky KS, Ang L, Mamalias N, Turenne SD, Kish SJ (2002) Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug Alcohol Depend 67:73–79. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín P, Komatsu M (2020) Physiological Stress Response by Selective Autophagy. J Mol Biol 432:53–62. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Zeini M, Camarero J, O’Shea E, Bosca L, Green AR, Colado MI (2003) The nNOS inhibitor, AR-R17477AR, prevents the loss of NF68 immunoreactivity induced by methamphetamine in the mouse striatum. J Neurochem. 85:515–524. [DOI] [PubMed] [Google Scholar]
- Schep LJ, Slaughter RJ, Beasley DM (2010) The clinical toxicology of metamfetamine. Clin. Toxicol 48:675–694. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Gan ZY, Komander D, Dewson G (2021) Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ 28:570–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe CA, Burzynski C, Jayanthi S, Ladenheim B, Cadet JL, Gardner EL, Xi Z, Praag H, Newman AH, Keck TM (2020) Neurochemical and behavioral comparisons of contingent and non-contingent methamphetamine exposure following binge or yoked long-access self-administration paradigms. Comparative Study Psychopharmacology 237:1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I (2007) Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275–297. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR (1976) Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug and Alcohol Dependence 1:215–219. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K, Suzuki K, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Mori N (2003) Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine related psychiatric symptoms. Am. J. Psychiatry 160:1699–1701. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya K, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL (2008) Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H (2006) Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch. Gen. Psychiatry 63:90–100. [DOI] [PubMed] [Google Scholar]
- Sepehr A, Taheri F, Heidarian S, Motaghinejad M, Safari S (2020) Neuroprotective and neurosurvival properties of safinamide against methamphetamine-induced neurodegeneration: Hypothetic possible role of BDNF/TrkB/PGC-1α signaling pathway and mitochondrial uncoupling protein −2(UCP-2). Med Hypotheses 143:110094. [DOI] [PubMed] [Google Scholar]
- Seyedhosseini Tamijani SM, Beirami E, Ahmadiani A, Dargahi L (2019) Thyroid hormone treatment alleviates the impairments of neurogenesis, mitochondrial biogenesis and memory performance induced by methamphetamine. Neurotoxicology 74:7–18. [DOI] [PubMed] [Google Scholar]
- Shacham T, Patel C, Lederkremer GZ (2021) PERK Pathway and Neurodegenerative Disease: To Inhibit or to Activate? Biomolecules 11:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin EJ, Jeong JH, Sharma G, Sharma N, Kim DJ, Pham DT, Trinh QD, Dang DK, Nah SY, Bing G, Kim HC (2019) Protein kinase Cδ mediates methamphetamine-induced dopaminergic neurotoxicity in mice via activation of microsomal epoxide hydrolase. Food Chem Toxicol 133:110761. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Shin SW, Nguyen TT, Park DH, Wie MB, Jang CG, Nah SY, Yang BW, Ko SK, Nabeshima T, Kim HC (2014) Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cδ gene. Mol Neurobiol 49:1400–1421. [DOI] [PubMed] [Google Scholar]
- Siwecka N, Rozpędek-Kamińska W, Wawrzynkiewicz A, Pytel D, Diehl JA, Majsterek I (2021) The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneberg DM, Shukla RK, Magness MB (2018) Global Methamphetamine Trends: An Evolving Problem. International Criminal Justice Review 28:136–161. [Google Scholar]
- Subu R, Jayanthi S, Cadet JL (2020) Compulsive methamphetamine taking induces autophagic and apoptotic markers in the rat dorsal striatum. Arch Toxicol 94:3515–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Lian Y, Ding J, Meng Y, Li C, Chen L, Qiu P (2019) The role of chaperone-mediated autophagy in neurotoxicity induced by alpha-synuclein after methamphetamine exposure. Brain Behav 9:e01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A (2003) Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci 1010:186–194. [DOI] [PubMed] [Google Scholar]
- Tahmasebinia F, Pourgholaminejad A (2017) The role of Th17 cells in auto-inflammatory neurological disorders. Prog Neuropsychopharmacol Biol Psychiatry 79:408–416. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9:826–838. [DOI] [PubMed] [Google Scholar]
- Takeichi T, Wang EL, Kitamura O (2012) The effects of low-dose methamphetamine pretreatment on endoplasmic reticulum stress and methamphetamine neurotoxicity in the rat midbrain. Leg Med (Tokyo) 14:69–77. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Jayanthi S, McCoy M, Ladenheim B, Cadet JL (2001) Methamphetamine increases expression of the apoptotic c-myc and L-myc genes in the mouse brain. Brain Res Mol Brain Res 90:202–204. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2009) Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem 109:1745–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM (2005) MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res 1050:190–198. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED (2004) Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias MC, O’Neill L, Hudkins M, Bartzokis G, Dean AC, London ED (2010) White-matter abnormalities in brain during early abstinence from methamphetamine abuse. Psychopharmacology (Berl) 209:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Pearson-Dennett V, Wilcox RA, Chau MT, Thoirs K, Thewlis D, Vogel AP, White JM (2016) Adults with a history of illicit amphetamine use exhibit abnormal substantia nigra morphology and parkinsonism. Parkinsonism Relat Disord 25:27–32. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Annette E. Fleckenstein AE (2005) Age-Dependent Methamphetamine-Induced Alterations in Vesicular Monoamine Transporter-2 Function: Implications for Neurotoxicity. J Pharmacol Exp Ther 314:1087–1092. [DOI] [PubMed] [Google Scholar]
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, Amsterdam EA (2003) Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. J Emerg Med 24:369–73. [DOI] [PubMed] [Google Scholar]
- UNODC (2018) Global smart update: methamphetamine continues to dominate synthetic drug markets. United Nations Publication 20(UNIS/CP/648). [Google Scholar]
- UNODC (2020) World drug report 2020 (ISBN: 978-92-1-148291-1, eISBN: 978-92-1-060623-3, United Nations publication, Sales No. E.17.XI.6)
- Valian N, Ahmadiani A, Dargahi L (2017) Escalating methamphetamine regimen induces compensatory mechanisms, mitochondrial biogenesis, and GDNF expression, in substantia nigra. J Cell Biochem 118:1369–1378. [DOI] [PubMed] [Google Scholar]
- Valian N, Heravi M, Ahmadiani A, Dargahi L (2019) Effect of methamphetamine on rat primary midbrain cells; mitochondrial biogenesis as a compensatory response. Neuroscience 413:317–318. [DOI] [PubMed] [Google Scholar]
- van Anken E, Bakunts A, Hu CA, Janssens S, Sitia R (2021) Molecular Evaluation of Endoplasmic Reticulum Homeostasis Meets Humoral Immunity. Trends Cell Biol S0962–8924(21)00029–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V, Yuan J, Wong DF, Dannals RF, Hatzidimitriou G, Mathews WB, Ravert HT, Musachio J, McCann UD, Ricaurte GA (1998) Brain dopamine neurotoxicity in baboons treated with doses of methamphetamine comparable to those recreationally abused by humans: evidence from [11C] WIN-35,428 positron emission tomography studies and direct in vitro determinations. J Neurosci 18:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382. [DOI] [PubMed] [Google Scholar]
- Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, White M, Reichelt J, Levine B (2012) Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 338:956–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne TA, Cornish JL (2018) A Comparison of Methamphetamine-Induced Psychosis and Schizophrenia: A Review of Positive, Negative, and Cognitive Symptomatology. Front Psychiatry 9:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Hui R, Wang J, Shen X, Xie B, Gong M, Yu F, Cong B and Ma C (2019) Effects of Molecular Hydrogen on Methamphetamine-Induced Neurotoxicity and Spatial Memory Impairment. Front Pharmacol 10:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703. [DOI] [PubMed] [Google Scholar]
- Wisessmith W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B (2009) Melatonin reduces induction of Bax, caspase and cell death in methamphetamine-treated human neuroblastoma SH-SY5Y cultured cells. J Pineal Res 46:433–440. [DOI] [PubMed] [Google Scholar]
- Wongprayoon P, Govitrapong P (2017) Melatonin Protects SH-SY5Y Neuronal Cells Against Methamphetamine-Induced Endoplasmic Reticulum Stress and Apoptotic Cell Death. Neurotox Res 31:1–10. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS (1989) Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Research 486:73–78. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, Furukawa Y, Ang L, Adams V, Reiber G, Anthony RA, Wickham D, Kish SJ (2000) Dopamine D1 receptor protein is elevated in nucleus accumbens of human chronic methamphetamine users. Mol Psychiatry 5:664–672. [DOI] [PubMed] [Google Scholar]
- Xiao N, Zhang F, Zhu B, Liu C, Lin Z, Wang H, Xie WB (2018) CDK5-mediated tau accumulation triggers methamphetamine-induced neuronal apoptosis via endoplasmic reticulum-associated degradation pathway. Toxicol Lett 292:97–107. [DOI] [PubMed] [Google Scholar]
- Xie XL, He JT, Wang ZT, Xiao HQ, Zhou WT, Du SH, Xue Y, Wang Q (2018) Lactulose attenuates METH-induced neurotoxicity by alleviating the impaired autophagy, stabilizing the perturbed antioxidant system and suppressing apoptosis in rat striatum. Toxicol Lett 289:107–113. [DOI] [PubMed] [Google Scholar]
- Xu E, Liu J, Liu H, Wang X, Xiong H (2017) Role of microglia in methamphetamine-induced neurotoxicity. Int. J. Physiol. Pathophysiol Pharmacol 9:84–100. [PMC free article] [PubMed] [Google Scholar]
- Xu X, Huang E, Luo B, Cai D, Zhao X, Luo Q, Jin Y, Chen L, Wang Q, Liu C, Lin Z, Xie WB, Wang H (2018) Methamphetamine exposure triggers apoptosis and autophagy in neuronal cells by activating the C/EBPβ-related signaling pathway. FASEB J 25:fj201701460RRR. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Yue Z (2014) Autophagy and its normal and pathogenic states in the brain. Annu Rev Neurosci 37:55–78. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W (1998) The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther 287:107–14. [PubMed] [Google Scholar]
- Yang G, Zeng X, Li J, Leung CK, Zhang D, Hong S, He Y, Huang J, Li L, Li Z (2019) Protective effect of gastrodin against methamphetamine-induced autophagy in human dopaminergic neuroblastoma SH-SY5Y cells via the AKT/mTOR signaling pathway. Neurosci Lett 707:134287. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang Y, Li Q, Zhong Y, Chen L, Du Y, He J, Liao L, Xiong K, Yi C, Yan J (2018) The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front Mol Neurosci 11:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tobwala S, Ercal N (2012) N-acetylcysteine amide protects against methamphetamine-induced tissue damage in CD-1 mice. Hum. Exp. Toxicol 31:931–944. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lv X, Bai Y, Zhu X, Wu X, Chao J, Duan M, Buch S, Chen L, Yao H (2015) Involvement of sigma-1 receptor in astrocyte activation induced by methamphetamine via up-regulation of its own expression. Journal of Neuroinflammation 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhao W, Liu M, Liu L, Wang Y (2020) TBHQ-Overview of Multiple Mechanisms against Oxidative Stress for Attenuating Methamphetamine-Induced Neurotoxicity. Oxid Med Cell Longev 2020:8874304. [DOI] [PMC free article] [PubMed] [Google Scholar]


