Abstract
The exopolysaccharide Psl contributes to biofilm structure and antibiotic tolerance and may play a role in the failure to eradicate Pseudomonas aeruginosa from cystic fibrosis (CF) airways. The study objective was to determine whether there were any differences in Psl in P. aeruginosa isolates that were successfully eradicated compared to those that persisted, despite inhaled tobramycin treatment, in children with CF. Initial P. aeruginosa isolates were collected from children with CF undergoing eradication treatment, grown as biofilms and labeled with 3 anti-Psl monoclonal antibodies (Cam003/Psl0096, WapR001, WapR016) before confocal microscopy visualization. When grown as biofilms, P. aeruginosa isolates from children who failed antibiotic eradication therapy, had significantly increased Psl0096 binding compared to isolates from those who cleared P. aeruginosa. This was confirmed in P. aeruginosa isolates from the SickKids Eradication Cohort as well as the Early Pseudomonas Infection Control (EPIC) trial. Increased anti-Psl antibody binding was associated with bacterial aggregation and tobramycin tolerance. The biofilm matrix represents a potential therapeutic target to improve P. aeruginosa eradication treatment.
Subject terms: Biofilms, Clinical microbiology
Introduction
Cystic fibrosis (CF) is a genetic disease characterized by Pseudomonas aeruginosa pulmonary infection1. To prevent the detrimental outcomes associated with chronic infection, antimicrobial treatment is used to eradicate initial P. aeruginosa infection2–5. However, in 10–40% of cases, eradication therapy fails, with no clear superiority of one antibiotic regimen over another, and the reasons for this are not entirely understood6.
In addition to antibiotic treatment, clearance of the organism from the airways depends on mucociliary action and immune-mediated mechanisms such as phagocytosis by neutrophils7,8. Studies that have examined outcomes of eradication therapy have not identified any host factors, such as gender or lung function, that are associated with failure to clear P. aeruginosa9,10. P. aeruginosa phenotypes characteristic of chronic pulmonary infection, such as mucoidy status, decreased motility and wrinkly colony morphology, have occasionally been identified as risk factors for failure of antibiotic eradication therapy11,12.
Using a collection of new onset P. aeruginosa isolates from children with CF undergoing antibiotic eradication treatment, we showed that Staphylococcal protein A (SpA) bound to the exopolysaccharide Psl in P. aeruginosa isolates that failed eradication therapy but bound much less in isolates successfully cleared13. This Psl-SpA interaction led to P. aeruginosa aggregation within biofilms and tolerance to high concentrations of tobramycin. These data suggest that, although the reasons for the failure of eradication therapy are likely multifactorial, Psl may be playing a role. Psl is a neutral repeating pentasaccharide that contributes to cell–cell and cell–substrate attachment adhesion, aggregation and biofilm formation in vitro14–19. Patients with invasive P. aeruginosa infections have serum antibodies against Psl, however, we do not know whether this occurs in CF patients20. Psl also protects P. aeruginosa from antimicrobials, including tobramycin and ciprofloxacin21, by forming a barrier matrix, and from activities of the innate immune system such as phagocytosis by neutrophils22–24. However, its contribution to the persistence of P. aeruginosa in the CF airways following inhaled antibiotic treatment is not known.
Therefore, the goals of this study were to examine Psl production and function in P. aeruginosa isolates that were successfully eradicated compared to those that persisted, despite inhaled tobramycin treatment, in the airways of children with CF. To do so, we used two sets of P. aeruginosa isolates, from the SickKids Eradication Cohort and the Early Pseudomonas Infection Control (EPIC) trial11,25. In addition, we used three separate anti-Psl antibodies (Cam003/Psl0096, WapR001 and WapR016), which recognize distinct epitopes and vary in their characteristics for promoting opsonization and phagocytosis and preventing epithelial cell binding20,26. We identified differences in Psl0096 binding between persistent and eradicated P. aeruginosa isolates with corresponding differences in bacterial aggregation and tobramycin tolerance.
Results
Quantification of Psl production in eradicated and persistent P. aeruginosa isolates
Initial investigations focused on determining whether there were any differences in the quantity of secreted and cell-associated Psl produced by eradicated and persistent isolates from the complete SickKids collection (29 persistent, 63 eradicated isolates). Biofilms were grown and sonicated to disrupt cell aggregation and lyse the bacteria. The supernatant and lysate were then incubated with three separate anti-Psl monoclonal antibodies recognizing distinct epitopes (Cam003/Psl0096, WapR001 and WapR016). Figure 1 illustrates that there was no difference in the amount of Psl detected via densitometric analysis of signal intensity between the eradicated (N = 63 isolates) and persistent (N = 29 isolates) isolates, either in the supernatant or lysate components.
Fig. 1. Dot blot analysis of Psl levels in persistent (N = 29, black triangles) versus eradicated (N = 63, white triangles) P.aeruginosa isolates from SickKids cohort.
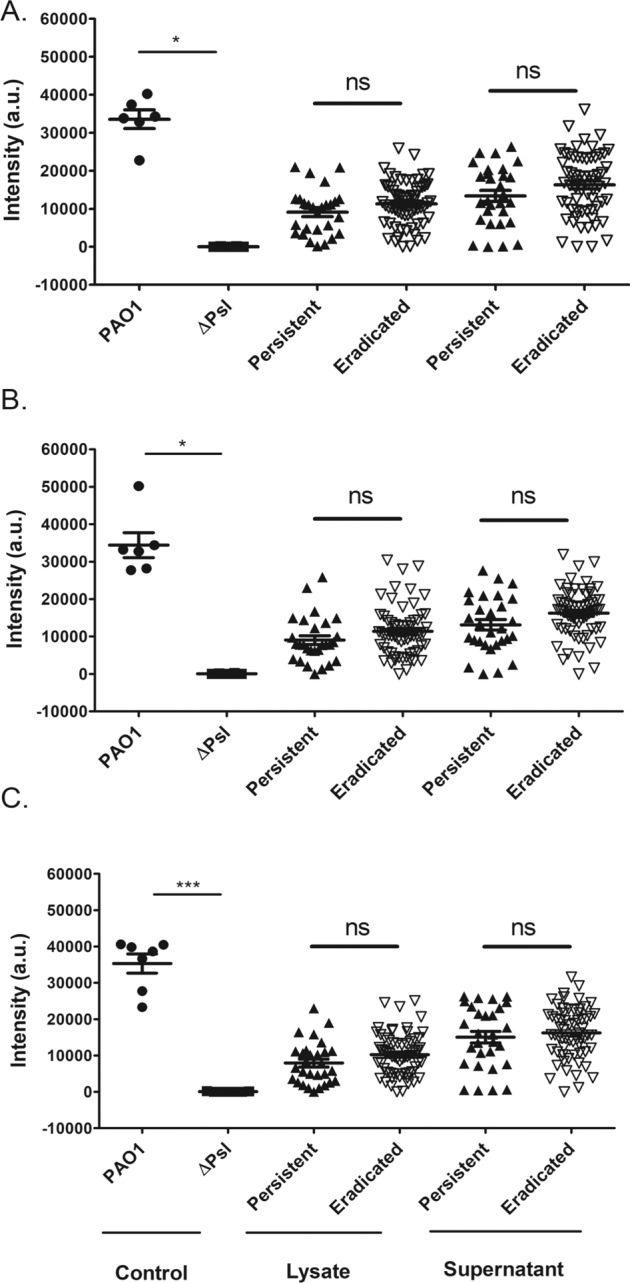
SickKids P. aeruginosa isolates, PAO1 and ΔPsl (Psl deficient P. aeruginosa) were grown as biofilms. The supernatant and lysate were spotted on nitrocellulose membrane and incubated with Cam003 (A), WapR001 (B) or WapR016 (C) anti-Psl antibodies. Bound antibody was detected with a chemiluminescent substrate after secondary antibody incubation, and signal intensities were analyzed using the ChemiDoc Imaging System. The mean intensity values for each isolate (done in triplicate) were plotted as arbitrary units (a.u.) with standard error of the mean (SEM). Statistics were performed using non-parametric Mann–Whitney test ***p < 0.001, *p < 0.05, ns not significant.
Comparative anti-Psl antibody binding to in vitro grown biofilms of eradicated and persistent isolates
In order to visualize Psl within intact biofilms (without sonication), P. aeruginosa were grown as biofilms and then stained with fluorescently labeled anti-Psl antibodies. For these detailed experiments, seven eradicated and seven persistent isolates from the SickKids collection were used. These isolates were chosen to represent 1 isolate per patient and to have similarities in other phenotypic characteristics which may influence eradication success, such as motility, mucoidy status, and planktonic tobramycin minimum inhibitory concentrations (MICs) between the eradicated and persistent groups of isolates, as previously published13.
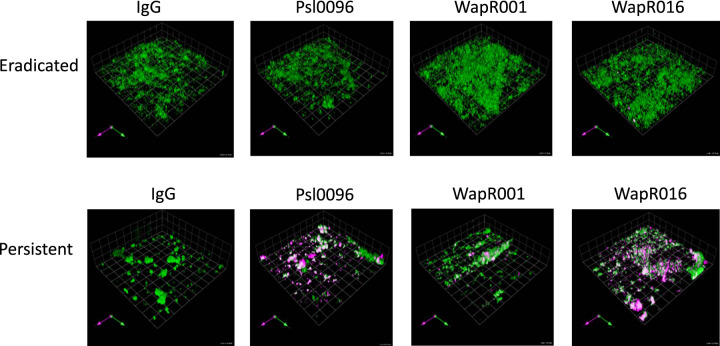
Representative images of an eradicated and a persistent isolate are shown in Fig. 2, demonstrating increased anti-Psl antibody binding in the persistent P. aeruginosa biofilm. Figure 3 depicts the volume of anti-Psl antibody staining (per 100,000 µm3 of biofilm) in all persistent versus eradicated isolates. In the SickKids collection, there was significantly more anti-Psl antibody binding of Psl0096 (Fig. 3A) and WapR001 (Fig. 3B) antibodies in persistent compared to eradicated P. aeruginosa.
Fig. 2. P. aeruginosa isolates were grown as biofilms for 48 h then incubated for 90 min with fluorescently labeled anti-Psl antibodies (IgG control, Psl0096, WapR01, and WapR016).
Representative images are shown for a SickKids eradicated (Pa50) and persistent (Pa565) isolate, where biofilms are stained green and the antibody binding is magenta.
Fig. 3. Anti-Psl antibody binding.
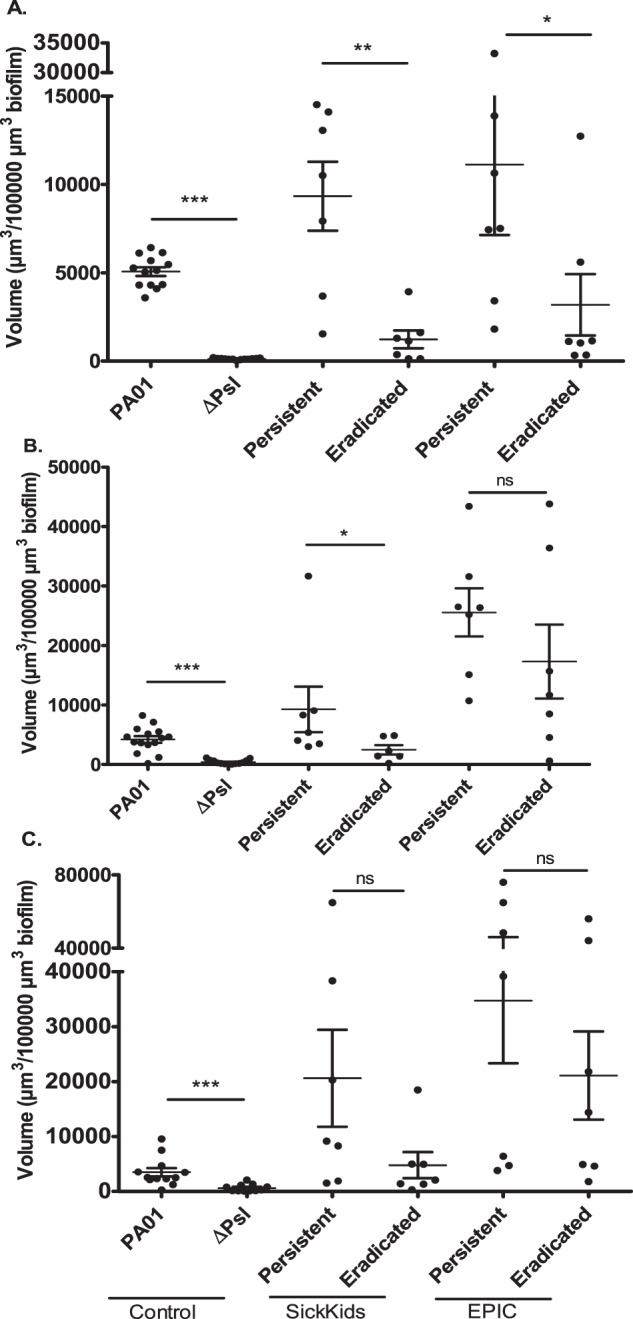
SickKids P. aeruginosa isolates (N = 7 Persistent; N = 7 eradicated), EPIC P. aeruginosa isolates (N = 7 Persistent; N = 7 eradicated), laboratory P. aeruginosa strain PAO1 and Δpsl (Psl deficient PAO1) were grown as biofilms for 48 h then incubated for 90 min with fluorescently labeled monoclonal anti-Psl antibodies, A Psl0096, B WapR001, and C WapR016. The mean antibody fluorescence volume (µm3/100,000 µm3 biofilm) for each isolate (done in triplicate) was calculated and the mean of all isolates was plotted with standard error of the mean (SEM). Statistics were performed using non-parametric Mann–Whitney test ***p < 0.001, **p < 0.01, *p < 0.05, ns not significant.
To validate these findings in a second dataset, we repeated the same experiments using seven persistent and seven eradicated isolates (from separate patients) from the EPIC cohort. Although there were no differences between groups using the WapR001 and WapR016 antibodies, Psl0096 bound significantly more in the persistent P. aeruginosa isolates compared to the eradicated ones, as seen in the SickKids cohort (Fig. 3).
Effects of anti-Psl antibody binding in the presence of tobramycin in P. aeruginosa biofilms
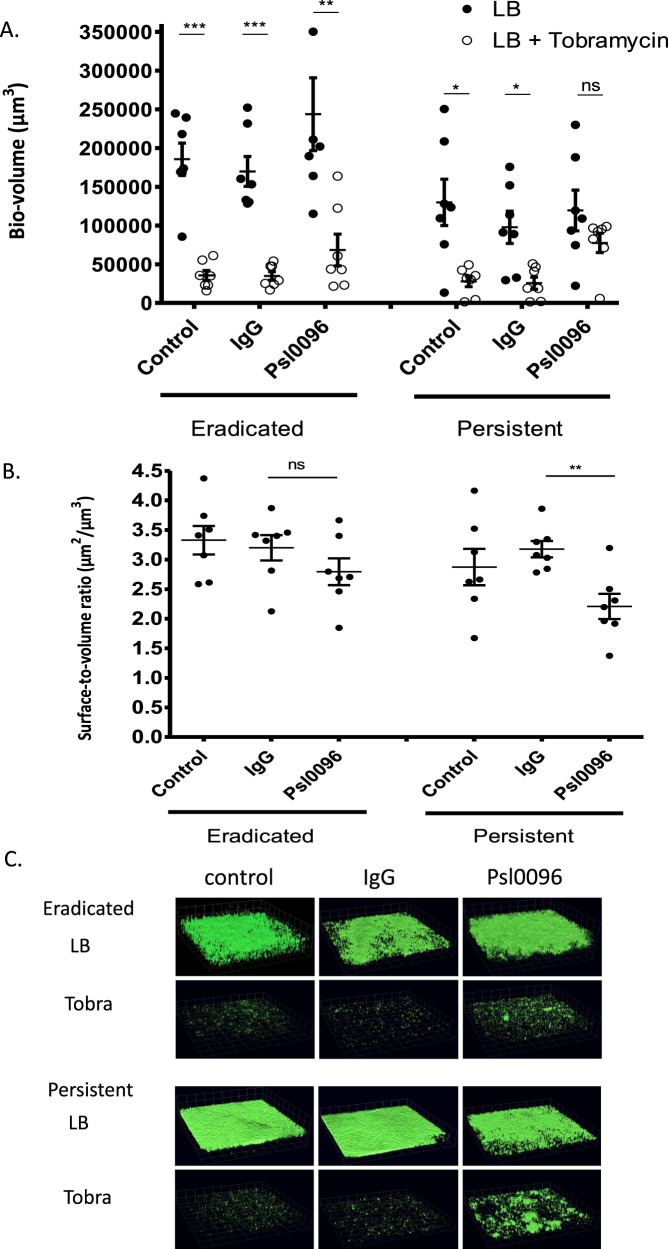
We previously noted that Spa-Psl binding led to bacterial aggregation and tobramycin tolerance in persistent isolates13. To determine whether this finding was a Psl specific phenomenon and would occur with the increased binding of Psl0096 to persistent isolates, we investigated the effects of anti-Psl antibody binding on tobramycin resistance in P. aeruginosa biofilms. Biofilms of the SickKids seven eradicated and seven persistent isolates were grown for 24 h and then exposed to Psl0096 followed by tobramycin for an additional 24 h. Tobramycin significantly reduced the amount of biofilm volume for the eradicated isolates in media alone as well as in the presence of IgG and Psl0096 (Fig. 4A). However, for the persistent isolates, tobramycin significantly reduced biofilm volume only in the media and IgG control conditions; in the presence of Psl0096, there was no significant reduction in P. aeruginosa biovolume. Furthermore, in comparison to the IgG controls (to account for the non-specific effects of antibody binding), persistent isolates also had significant reductions in biofilm surface to volume ratio in the presence of Psl0096, indicating increased aggregation; increased aggregation was not observed in the eradicated group (Fig. 4B). Figure 4C shows representative images of a persistent and an eradicated isolate.
Fig. 4. SickKids P. aeruginosa isolates (N = 7 persistent; N = 7 eradicated) were grown as biofilms for 24 h after which antibodies (LB control, IgG or anti-Psl 0096) and tobramycin 1000 µg/ml (or LB alone) were added for the following 24 h before imaging was performed.
A The mean biofilm volume (measured in µm3) for each isolate (done in triplicate) was calculated and the mean of all isolates was plotted with standard error of the mean (SEM). Comparisons with and without tobramycin treatment were performed for each condition using non-parametric Mann–Whitney test ***p < 0.001, **p < 0.01, *p < 0.05, ns not significant. B In the presence of tobramycin, aggregation was measured using biofilm surface to volume ratio. The mean biofilm surface to volume ratio (µm2/µm3) was calculated for each isolate (done in triplicate) and the mean of all isolates was plotted with standard error of the mean (SEM). Comparisons to IgG control were performed for each condition using non-parametric Mann–Whitney test ***p < 0.001, **p < 0.01, *p < 0.05, ns: not significant. C Representative images are shown for a persistent (Pa375) and eradicated (Pa558) isolate.
We then used an ATP assay to investigate the metabolic activity of the remaining P. aeruginosa in the presence of tobramycin. For the eradicated isolates, tobramycin again significantly reduced ATP production under all conditions (Fig. 5). In the persistent group, tobramycin significantly reduced ATP production only in the control conditions and not in the presence of Psl0096. When colony-forming units (CFUs) were measured under the same conditions, however, tobramycin was able to significantly reduce the colony count in both the persistent and eradicated groups (Supplemental Fig. 1).
Fig. 5. SickKids P. aeruginosa isolates (N = 7 persistent; N = 7 eradicated) were grown as biofilms for 24 h after which antibodies (LB control, IgG or anti-Psl 0096) and tobramycin (or LB alone) were added for the following 24 h before ATP was measured.
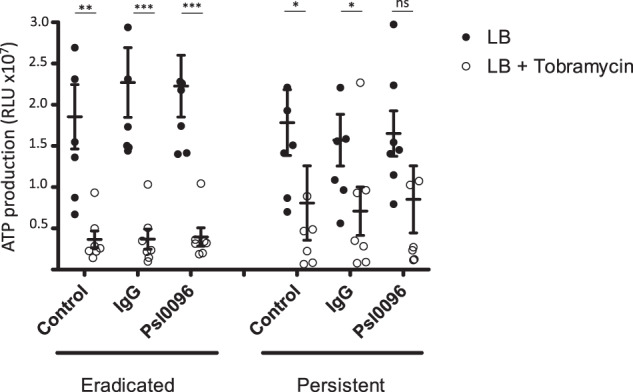
The mean ATP activity (measured in Relative Light Units (RLU) ×107) for each isolate (done in triplicate) was calculated and the mean of all isolates was plotted with standard error of the mean (SEM). Comparisons with and without tobramycin treatment were performed for each condition using non-parametric Mann–Whitney test ***p < 0.001, **p < 0.01, *p < 0.05, ns not significant.
Psl polymorphism among eradicated and persistent P. aeruginosa isolates
To determine whether there were any underlying genetic differences among the seven persistent and seven eradicated SickKids isolates, we examined the 14 locus Psl region from pslA – pslN and identified all non-synonymous (missense) mutations. These genes total to 17,657 bp and span the region from 2,453,667 to 2,472,076 on the PAO1 genome (note that some psl genes overlap). There was an average of 0.81 ± 0.549 (stddev) non-synonymous polymorphisms per strain across all 14 loci, with no variation found in pslD, and only one polymorphism found in both pslA and pslG. The most polymorphic locus was pslB with an average of 1.57 non-synonymous polymorphisms per strain. There were no statistically significant differences in the number of polymorphisms per gene (or when all genes were considered together) between eradicated and persistent strains (Supplemental Fig. 2).
CdrA expression in eradicated and persistent P. aeruginosa isolates
Given that CdrA is a Psl associated matrix protein that plays a significant role in aggregation, we investigated the expression of CdrA in the 21 persistent and 25 eradicated SickKids isolates grown as biofilms. Supplemental Fig. 3 shows that CdrA expression was detected (particularly in the supernatant fraction) in a similar number of persistent compared to eradicated isolates. Size differences in CdrA were observed between isolates in the western blot analysis. This is consistent with past observations of P. aeruginosa isolates27.
Discussion
This study demonstrated that there was increased binding of anti-Psl antibodies in P. aeruginosa isolates (when grown as biofilms) from CF patients, from both the SickKids and EPIC cohort, who failed antibiotic eradication therapy compared to those who did not; anti-Psl antibody binding led to the aggregate formation in biofilms and tobramycin tolerance.
To our knowledge, this is the largest study to examine the specific contribution of Psl in the failure to eradicate P. aeruginosa with antibiotic therapy in children with CF. Psl is involved in early bacterial surface attachment and is an important structural component of the extracellular matrix of biofilms28,29. Psl production can also confer resistance to antibiotics such as colistin, tobramycin, and ciprofloxacin, likely through a protective barrier effect21. Furthermore, Psl prevents complement deposition and opsonization, inhibiting bacterial killing by phagocytes23,30. In vitro studies of Psl have included P. aeruginosa strains from CF patients with chronic infection but not from those with early infection31,32. It is important to understand the significance of Psl in initial P. aeruginosa colonization which is more amenable to antibiotic treatment and clearance than chronic infection6.
In our study, we noted differential binding of anti-Psl antibodies to biofilm cells of eradicated versus persistent isolates. Monoclonal antibodies to the Class I epitope (Cam003 or its affinity optimized derivative, Psl0096) were previously found to be the most active in promoting opsonophagocytic killing, in preventing P. aeruginosa from binding to epithelial cells and preferentially bind to the surface of in vitro grown biofilms20,26. We also noted that mAb Psl0096 was the most discriminatory mAb in terms of binding between persistent and eradicated isolates, both for the SickKids and EPIC cohort. There were no significant differences in the number of polymorphisms in Psl genes between the groups to explain our finding. Furthermore, the amount of Psl as measured by dot blot analysis (using Psl0096) was similar between both groups. However, when P. aeruginosa was allowed to form a mature biofilm, undisturbed by sonication or processing, the increased affinity to Psl0096 was demonstrated. It is possible that persistent isolates grown as biofilms produce more surface Psl with a conformation that allows Psl0096 to bind more avidly than do eradicated isolates. In a screening study using synthetic oligosaccharides, Class I mAbs (Cam003/Psl0096) did not bind any of the oligosaccharides, suggesting the presence of a Psl-associated functional group or modification that is sensitive to experimental conditions such as alkaline exposure33.
We also observed that anti-Psl antibody binding was associated with aggregation, particularly in the persistent isolates. Earlier investigations actually noted a decrease in aggregation when PAO1 was exposed to Psl mAbs, however, such studies were done with planktonic growth of P. aeruginosa in culture medium34. The aggregation we noted in our study was Psl specific rather than simply an effect of adding antibodies as the aggregation seen with anti-Psl antibodies was significantly greater than that seen with equal concentrations of IgG. The bacterial aggregation may have been caused by the cross-linking of anti-Psl antibodies between P. aeruginosa cells. The mechanism did not appear to be due to an increase in the expression of CdrA, a Psl-associated matrix protein which enhances bacterial aggregation35,36, in persistent versus eradicated isolates. Bacterial aggregation is known to be a mechanism of antibiotic resistance;37 the aggregation noted in our persistent isolates was associated with tolerance (persistence under confocal imaging and greater metabolic activity as measured by ATP assay) to high levels of tobramycin, concentrations of 1000 µg/ml, achievable only with inhalation therapy38. There is also the possibility of non-Psl-mediated mechanism to account for the increased survival of the persistent isolates.
The presence of P. aeruginosa aggregates expressing Psl in the airways of a CF patient with chronic infection has been demonstrated by our collaborators in a study using immunohistochemical techniques to visualize CF sputum39. It is not known what the specific trigger for this aggregation may be in vivo; simply growing the persistent isolates as biofilms did not reveal persistence in the presence of tobramycin, suggesting that the ability to form biofilms alone may not fully explain persistence in the CF airways40. We previously showed that Staphylococcal protein A (SpA) can bind to Psl in our persistent P. aeruginosa strains leading to aggregation and tobramycin tolerance13. However, this aggregative phenotype is not dependent on SpA, as many of these CF patients who failed eradication therapy were not co-infected with Staphylococcus aureus and this aggregation phenotype can be replicated in the persistent isolates with the addition of anti-Psl antibodies. Patients with invasive P. aeruginosa infections are known to have serum antibodies against Psl, however, it is not known whether anti-Psl antibodies can be detected in the sputum of CF patients colonized with P. aeruginosa20. There are many factors that can contribute to the formation of bacterial aggregates in CF airways, which occur by two general mechanisms: bridging and depletion aggregation41–45. Host-derived polymers at the site of chronic infections may physically connect bacterial cells via a bridging mechanism leading to Psl mediated aggregation39.
This study had several limitations. The SickKids Eradication cohort was limited to children with CF who could produce sputum and the study defined eradication based on an early time point (1 week post treatment)11,46. To make our findings more generalizable, we validated our results using a second isolate collection, from the EPIC cohort, that was obtained from oropharyngeal swabs, and for which eradication was defined at the 3 month post treatment follow up visit25. Thus, our data suggest that differences in Psl are important in the persistence of P. aeruginosa infection in most children with CF. In addition, we do not have the data in either cohort to confirm that failure of eradication treatment was due to the persistence of the same strain of P. aeruginosa, as these were clinical isolates and genotyping is not done routinely in the clinical microbiology laboratory. We focused our study on the role of Psl solely within P. aeruginosa biofilms but have not included the potential contribution of host factors such as neutrophils, which clearly interact with Psl34,47. Finally, we visualized P. aeruginosa biofilms in vitro but have not yet confirmed our study findings in vivo, directly in the sputum of children with CF; this is the focus of a currently ongoing observational study.
In conclusion, we demonstrated that P. aeruginosa isolates that fail to be cleared from the airways of children with CF following inhaled tobramycin therapy, have increased binding to anti-Psl antibodies when grown as biofilms, leading to aggregation and tobramycin tolerance. These components of the biofilm matrix represent possible therapeutic targets to improve the success of early P. aeruginosa eradication treatment.
Methods
P. aeruginosa isolate collections
The SickKids cohort consisted of clinical P. aeruginosa isolates (N = 92) from sputum samples obtained from 67 pediatric CF patients undergoing eradication treatment with inhaled tobramycin for new-onset P. aeruginosa infection at SickKids (Toronto, Canada) from 2011 to 2018 (ongoing)11,46. New-onset infection was defined as a sputum culture positive for P. aeruginosa with at least 3 P. aeruginosa negative respiratory tract cultures in the prior 12 months. Eradication was defined based on a P. aeruginosa negative culture of the first respiratory tract specimen taken after the end of inhaled tobramycin therapy (1 week post end of treatment). The initial P. aeruginosa isolates were cultured, before antibiotic treatment, from patients in whom P. aeruginosa was successfully eradicated with inhaled tobramycin (eradicated isolates) and from patients in whom eradication therapy failed (persistent isolates).
In addition, we also used the larger P. aeruginosa isolate collection (N = 194) from the EPIC trial of early eradication (2004–2009)25. In this randomized trial, four anti-pseudomonal treatment regimens were compared in 304 children with CF with new-onset P. aeruginosa infection (at least 2 years of P. aeruginosa negative respiratory tract cultures). The subset of subjects who most closely resembled the SickKids population were the 37 children with CF who were P. aeruginosa positive at baseline (week 0), had not received prior antibiotic treatment and subsequently received 28 days of inhaled tobramycin (within 1 month of the initial screen). In contrast to the SickKids cohort, in the EPIC cohort, P. aeruginosa isolates were cultured from oropharyngeal swabs rather than sputum and eradication was defined as a P. aeruginosa negative culture 10 weeks following the end of treatment. Experiments were repeated in this secondary P. aeruginosa collection, comparing baseline P. aeruginosa isolates in a blinded fashion, to determine the generalizability of results.
Psl dot blot analysis
P. aeruginosa isolates, PAO1 and Δpsl (Psl deletion strain (PAO1ΔpslBCD) were grown overnight in lysogeny broth (LB) with shaking (225 rpm). The overnight culture was diluted 1:100 and grown for 3 h at 37 °C to obtain an OD of 0.1 at 600 nm (early log phase). Of this culture, 1 mL was transferred to a Sarstedt 96-well Tissue Culture plate (Thermo Fisher Scientific, Mississauga, ON) and grown for 48 h at 37 °C with LB replacement after the first 24 h. The wells were washed 1× with phosphate-buffered saline (PBS) and 200 µL of PBS was added back into the wells. The cell-attached fraction of the wells was disrupted and the optical density (OD) of the cell suspension in PBS was normalized to 0.2 at 600 nm, followed by centrifugation (3000 × g, 4 oC, 15 min).
The centrifuged product consisted of a cell pellet and supernatant. The cell pellet was re-suspended in 500 µL of 0.9 % NaCl and lysed by bath sonication (VWR Ultrasonic 50D, 5 × 2 min)16 with 2 min cooling on ice after each interval. The supernatant (pre-lysis) and lysate (post-lysis) were loaded into the Minifold II Slot-Blot Manifold system (50 µL each) and spotted on a nitrocellulose membrane. The blots were air dried and blocked with 2% bovine serum albumin (BSA), followed by 24 h incubation at 4 °C. The blots were rinsed 3× with 1× tris buffered saline with Tween 20 (TBST) and incubated with 1:5000 primary anti-Psl antibody in 1× TBST for 1 h at room temperature (RT). The primary antibody was decanted and the blots were rinsed 3× with 1× TBST, then incubated with 1:3000 secondary goat anti-rabbit IgG antibody (Bio-Rad, Mississauga, ON) for 1 h at RT. The secondary antibody was decanted and the blots were rinsed 3× with 1× TBST, after which 50 µL of 1× TBST was added back onto the blots to prevent drying. Bound antibody was detected using a SuperSignal West Atto chemiluminescent substrate (Thermo Fisher Scientific, Mississauga, ON). Blots were imaged using the ChemiDoc Imaging System and variations in signal intensities were quantified with signal accumulation mode. The mean intensity value for each isolate (done in triplicate) was calculated as arbitrary units (a.u).
Confocal imaging of P. aeruginosa biofilms
P. aeruginosa isolates were grown in Nunc Lab-Tek II, 8-chambered cover glass slides (Thermo Fisher Scientific, Mississauga, ON) as previously described13. P. aeruginosa was grown in LB overnight at 37 °C on a shaker (225 rpm). The overnight culture was diluted 1:100 and grown to an OD of 0.1 at 600 nm (early log phase), after which 220 µL of the culture was seeded into an 8-chambered cover glass slide (Thermo Fisher Scientific, Mississauga, ON) and incubated at 37 °C.
To measure antibody binding alone, after 48 h of growth, media was removed and fluorescently labeled anti-Psl antibody (56 µg/mL) was added for 90 min. Media was then removed from the wells and 200 µL of Syto-9TM live-cell fluorescent stain (Thermo Fisher Scientific) was added and incubated for 45 min at RT. The wells were then gently washed 2× with LB and 200 µL of LB was added back into the wells prior to confocal laser scanning microscopy (CLSM). This procedure was repeated in triplicates for each P. aeruginosa isolate.
To measure antibody binding in the presence of tobramycin, after 24 h of growth, media was removed and replaced with fluorescently labeled anti-Psl antibody (56 µg/mL) for 1 h at RT. Then, either 100 µL of LB alone or LB with tobramycin (final concentration 1000 µg/mL based on achievable aerosolized concentrations in CF patients38) was added to designated wells and incubated for a total of 24 h at 37 °C. Media was then removed from the wells and 200 µL of Syto-9TM live-cell fluorescent stain (Thermo Fisher Scientific) was added and incubated for 45 min at RT. The wells were then gently washed 2× with LB and 200 µL of LB was added back into the wells prior to CLSM. This procedure was repeated in triplicates for each P. aeruginosa isolate.
Images were acquired using a Quorum Wave FX Borealis Spinning Disk Confocal Microscope, based on a Yokohama CSU-10 scan head. Fluorescently labeled (red) anti-Psl antibody and live cell (green) stains were excited using 561 and 491 nm excitation lines, respectively, and visualized with a 25×/0.80 Zeiss water immersion lens, coupled to a 1.6× magnification coupler. Image acquisition was performed using the Quorum Volocity 6.3 software. A total of 6 z-stack images were acquired per well in increments of 0.3 µm. Anti-Psl binding within P. aeruginosa biofilms was calculated using Volocity software to quantify total volume of voxels in the green and red channels. Quantification of P. aeruginosa biovolume intensity was performed using Volocity software. Surface-to-biovolume ratio, as a measure of P. aeruginosa aggregation, was quantified as previously described, using Comstat2 software as a plugin to ImageJ48. All images are included in the Supplemental materials as Supplemental Fig. 4.
Bacterial counts of P. aeruginosa biofilms
Colony-forming units (CFU) counts were determined by growing P. aeruginosa biofilms in cation-adjusted Mueller Hinton broth (CAMHB) in 8-chambered cover glass slides (Thermo Fisher Scientific, Mississauga, ON), under the same conditions previously described herein for confocal imaging. After 24 h growth with subsequent 24 h growth with exposure to antibodies and tobramycin, media was removed, washed 2× with PBS, and 500 µL of fresh PBS was added into the wells. The cell-attached fraction of the wells were disrupted and cell suspensions were serial diluted, then plated on blood agar. Plates were grown for 24 h at 37 °C and CFUs were counted. This procedure was repeated in triplicates for each P. aeruginosa isolate, reporting mean CFU counts (log transformed) as previously described49.
ATP assays of P. aeruginosa biofilms
Cell metabolic activity was assessed using a modified ATP assay method for biofilms50,51. In brief, P. aeruginosa was grown overnight in CAMHB (Sigma-Aldrich, Oakville, ON) at 37 °C with shaking (225 rpm). The overnight culture was diluted 1:100 and grown to an OD of 0.1 at 600 nm. Of this log phase culture, 220 µL was added to 8 wells of a white Greiner Medium Binding 96-well plate (Sigma-Aldrich, Oakville, ON) and incubated for 24 h at 37 °C. After 24 h, media was removed and the wells were washed 2× with CAMHB. Subsequently, 100 µL of anti-Psl antibody (56 µg/mL) was added to designated wells and incubated for 1 h at RT. Either 100 µL of CAMHB alone or CAMHB with tobramycin (final concentration 1000 µg/mL) was added to designated wells and incubated for 24 h at 37 °C. Media was then removed and the wells were washed 3× with CAMHB. Finally, to measure ATP in the cell-attached fraction of the wells, 100 µL of Bac Titer-GloTM reagent (Promega, Madison, WI) was added. The cell-attached fraction of the wells was disrupted and the plate was gently mixed on an orbital shaker for 10 min prior to luminescence reading as per the manufacturer’s protocol. This procedure was repeated in triplicates for each P. aeruginosa isolate.
Psl gene polymorphisms
To determine whether there were mutational differences in the Psl genes of persistent versus eradicated isolates, we analyzed the gene region from 2453667-2472076 spanned by the 14 psl genes, pslA, pslB, pslC, pslD, pslE, pslF, pslG, pslH, pslI, pslJ, pslK, pslL, pslM, pslN for polymorphisms in each of the seven persistent and seven eradicated SickKids P. aeruginosa strains. Whole genome sequence data for each of the strains was previously collected via DNA extraction and sequencing13. The data for each strain was then mapped to PAO1 as a reference, and single nucleotide polymorphisms called via Snippy52. We identified non-synonymous (missense) mutations found in each psl locus and compared polymorphism levels between persistent and eradicated strains on a per gene (and across all genes) basis and for the entire Psl region. All data are available through Bioproject PRJNA556419 with the following Genbank accessions: JAGHMW000000000 (Pa50), JAGHMV000000000 (Pa263), JAGHMU000000000 (Pa288), JAGHMT000000000 (Pa325), JAGHMS000000000 (Pa342), JAGHMR000000000 (Pa375), JAGHMQ000000000 (Pa380), JAGHMP000000000 (Pa404), JAGHMO000000000 (Pa505), JAGHMN000000000 (Pa551), JAGHMM000000000 (Pa558), JAGHML000000000 (Pa565), JAGHMK000000000 (Pa580), JAGHMI000000000 (Pa549).
Anti-CdrA western blot analysis of static biofilms
Sample preparation for western blot analysis was performed as previously described27. Briefly, static biofilms were cultured in 6-well plates using TSB for 24 h at 30 °C. Biofilms were then harvested by passage through an 18-gauge syringe. Biofilms were normalized by OD 600 nm. Biofilms were separated by centrifugation into supernatant and cellular fractions. Samples were then analyzed by western blot analysis as previously described36 using an anti-CdrA antibody raised against CADGKTKVYGDADPS (Genscript). This amino acid sequence is a conserved stretch of amino acids in the C-terminal region of the protein.
Statistical analysis and institutional approvals
All statistical analyses were done using GraphPad 5.0. Continuous variables were compared using non-parametric Mann–Whitney test; p-value of <0.05 was considered significant. This study was approved by the SickKids Research Ethics Board (REB#1000065841) with waiver of consent due to de-identified nature of bacterial isolates.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Funded by a grant from the US Cystic Fibrosis Foundation (WATERS17G0). C.R. was supported by a K99 “Pathway to Independence” Award (5K99GM134121-02) and a Postdoc-to-Faculty Transition Award from the Cystic Fibrosis Foundation (REICHH19F5).
Author contributions
A.J.M., L.J., C.R., T.B., S.U. and K.M.G. performed the experiments. Y.C.W.Y., P.L.H., M.R.P., L.R.H., D.N., D.S.G., D.J.W. and V.W. contributed to the study design, data interpretation and analysis, and writing of the manuscript. A.D. contributed experimental materials and contributed to the writing of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data are available through Bioproject PRJNA556419 for the above mentioned Genbank accessions.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-021-00234-3.
References
- 1.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 3.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr. Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J. Pediatr. 2007;151:134–139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Ratjen F, Doring G, Nikolaizik WH. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet. 2001;358:983–984. doi: 10.1016/S0140-6736(01)06124-4. [DOI] [PubMed] [Google Scholar]
- 6.Schelstraete P, Haerynck F, Van daele S, Deseyne S, De Baets F. Eradication therapy for Pseudomonas aeruginosa colonization episodes in cystic fibrosis patients not chronically colonized by P. aeruginosa. J. Cyst. Fibros. 2013;12:1–8. doi: 10.1016/j.jcf.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI0215217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology. 2010;15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 9.Stanojevic S, Waters V, Mathew JL, Taylor L, Ratjen F. Effectiveness of inhaled tobramycin in eradicating Pseudomonas aeruginosa in children with cystic fibrosis. J. Cyst. Fibros. 2014;13:172–178. doi: 10.1016/j.jcf.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Mayer-Hamblett N, et al. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr. Pulmonol. 2012;47:125–134. doi: 10.1002/ppul.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidya P, et al. Chronic infection phenotypes of Pseudomonas aeruginosa are associated with failure of eradication in children with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:67–74. doi: 10.1007/s10096-015-2509-4. [DOI] [PubMed] [Google Scholar]
- 12.Mayer-Hamblett N, et al. Pseudomonas aeruginosa phenotypes associated with eradication failure in children with cystic fibrosis. Clin. Infect. Dis. 2014;59:624–631. doi: 10.1093/cid/ciu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaudoin T, et al. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes. 2017;3:25. doi: 10.1038/s41522-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson KD, Starkey M, Kremer S, Parsek MR, Wozniak DJ. Identification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formation. J. Bacteriol. 2004;186:4466–4475. doi: 10.1128/JB.186.14.4466-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma L, Lu H, Sprinkle A, Parsek MR, Wozniak DJ. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. J. Bacteriol. 2007;189:8353–8356. doi: 10.1128/JB.00620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd MS, et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 2009;73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, et al. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011;13:1705–1717. doi: 10.1111/j.1462-2920.2011.02503.x. [DOI] [PubMed] [Google Scholar]
- 18.Chew SC, et al. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. MBio. 2014;5:e01536–01514. doi: 10.1128/mBio.01536-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, et al. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009;5:e1000354. doi: 10.1371/journal.ppat.1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGiandomenico A, et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012;209:1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billings N, et al. The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2013;9:e1003526. doi: 10.1371/journal.ppat.1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thanabalasuriar A, et al. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J. Clin. Invest. 2017;127:2249–2261. doi: 10.1172/JCI89652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra M, et al. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol. 2012;14:95–106. doi: 10.1111/j.1462-5822.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybtke, M., Jensen, P. O., Nielsen, C. H. & Tolker-Nielsen, T. The extracellular polysaccharide matrix of Pseudomonas aeruginosa biofilms is a determinant of polymorphonuclear leukocyte responses. Infect. Immun. 10.1128/IAI.00631-20 (2020). [DOI] [PMC free article] [PubMed]
- 25.Treggiari MM, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch. Pediatr. Adolesc. Med. 2011;165:847–856. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray VA, et al. Anti-Psl targeting of Pseudomonas aeruginosa biofilms for neutrophil-mediated disruption. Sci. Rep. 2017;7:16065. doi: 10.1038/s41598-017-16215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichhardt, C. et al. The versatile Pseudomonas aeruginosa biofilm matrix protein CdrA promotes aggregation through different extracellular exopolysaccharide interactions. J. Bacteriol. 10.1128/JB.00216-20 (2020). [DOI] [PMC free article] [PubMed]
- 28.Byrd, M. S., Pang, B., Mishra, M., Swords, W. E. & Wozniak, D. J. The Pseudomonas aeruginosa exopolysaccharide Psl facilitates surface adherence and NF-kappaB activation in A549 cells. mBio10.1128/mBio.00140-10 (2010). [DOI] [PMC free article] [PubMed]
- 29.Zhao K, et al. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature. 2013;497:388–391. doi: 10.1038/nature12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones, C. J. & Wozniak, D. J. Psl produced by mucoid Pseudomonas aeruginosa contributes to the establishment of biofilms and immune evasion. MBio10.1128/mBio.00864-17 (2017). [DOI] [PMC free article] [PubMed]
- 31.Colvin KM, et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012;14:1913–1928. doi: 10.1111/j.1462-2920.2011.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse HK, et al. Pseudomonas aeruginosa enhances production of a non-alginate exopolysaccharide during long-term colonization of the cystic fibrosis lung. PLoS ONE. 2013;8:e82621. doi: 10.1371/journal.pone.0082621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, et al. Epitope mapping of monoclonal antibodies using synthetic oligosaccharides uncovers novel aspects of immune recognition of the Psl exopolysaccharide of Pseudomonas aeruginosa. Chemistry. 2013;19:17425–17431. doi: 10.1002/chem.201302916. [DOI] [PubMed] [Google Scholar]
- 34.Ray VA, et al. Anti-Psl targeting of Pseudomonas aeruginosa biofilms for neutrophil-mediated disruption. Sci. Rep. 2018;8:9637. doi: 10.1038/s41598-018-26660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borlee BR, et al. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010;75:827–842. doi: 10.1111/j.1365-2958.2009.06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichhardt, C., Wong, C., Passos da Silva, D., Wozniak, D. J. & Parsek, M. R. CdrA Interactions within the Pseudomonas aeruginosa biofilm matrix safeguard it from proteolysis and promote cellular packing. MBio10.1128/mBio.01376-18 (2018). [DOI] [PMC free article] [PubMed]
- 37.Staudinger BJ, et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014;189:812–824. doi: 10.1164/rccm.201312-2142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chmiel JF, et al. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann. Am. Thorac. Soc. 2014;11:1120–1129. doi: 10.1513/AnnalsATS.201402-050AS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennings, L. K. et al. Pseudomonas aeruginosa aggregates in cystic-fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 34, 108782 (2021). [DOI] [PMC free article] [PubMed]
- 40.Lee B, et al. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J. Clin. Microbiol. 2005;43:5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caceres SM, et al. Enhanced in vitro formation and antibiotic resistance of nonattached Pseudomonas aeruginosa aggregates through incorporation of neutrophil products. Antimicrob. Agents Chemother. 2014;58:6851–6860. doi: 10.1128/AAC.03514-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phan, J., Gallagher, T., Oliver, A., England, W. E. & Whiteson, K. Fermentation products in the cystic fibrosis airways induce aggregation and dormancy-associated expression profiles in a CF clinical isolate of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 10.1093/femsle/fny082 (2018). [DOI] [PMC free article] [PubMed]
- 43.Secor PR, Michaels LA, Ratjen A, Jennings LK, Singh PK. Entropically driven aggregation of bacteria by host polymers promotes antibiotic tolerance in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA. 2018;115:10780–10785. doi: 10.1073/pnas.1806005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strand SP, Vårum KM, Østgaard K. Interactions between chitosans and bacterial suspensions: adsorption and flocculation. Colloids Surf. B Biointerfaces. 2003;27:71–81. doi: 10.1016/S0927-7765(02)00043-7. [DOI] [Google Scholar]
- 45.Schwarz-Linek, J. et al Polymer-induced phase separation in suspensions of bacteria. Europhys. Lett. 89, 68003 (2010).
- 46.Blanchard, A. C. et al. Effectiveness of a stepwise Pseudomonas aeruginosa eradication protocol in children with cystic fibrosis. J. Cyst. Fibros. 16, 395–400 (2017). [DOI] [PubMed]
- 47.Kelly, K. A., Benedetti, Y., Yau, V., Waters, D. N. The Journal of Infectious Diseases. 10.1093/infdis/jiab102 (2021).
- 48.Morris, A. J., Li, A., Jackson, L., Yau, Y. C. W. & Waters, V. Quantifying the effects of antimicrobials on in vitro biofilm architecture using COMSTAT Software. JoVE, e61759, 10.3791/61759 (2020). [DOI] [PubMed]
- 49.Yau YC, et al. Randomized controlled trial of biofilm antimicrobial susceptibility testing in cystic fibrosis patients. J. Cyst. Fibros. 2015;14:262–266. doi: 10.1016/j.jcf.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Beaudoin T, et al. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci. Rep. 2018;8:14728. doi: 10.1038/s41598-018-33016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stiefel P, et al. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 2016;100:4135–4145. doi: 10.1007/s00253-016-7396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seemann, T. Snippy. https://github.com/tseemann/snippy (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data are available through Bioproject PRJNA556419 for the above mentioned Genbank accessions.