Abstract
The dysregulation of K+ channels is a hallmark of pulmonary arterial hypertension (PAH). Herein, the channelome was analyzed in lungs of patients with PAH in a public transcriptomic database. Sixty six (46%) mRNA encoding cationic channels were dysregulated in PAH with most of them downregulated (83%). The principal component analysis indicated that dysregulated cationic channel expression is a signature of the disease. Changes were very similar in idiopathic, connective tissue disease and congenital heart disease associated PAH. This analysis 1) is in agreement with the widely recognized pathophysiological role of TASK1 and KV1.5, 2) supports previous preliminary reports pointing to the dysregulation of several K+ channels including the downregulation of KV1.1, KV1.4, KV1.6, KV7.1, KV7.4, KV9.3 and TWIK2 and the upregulation of KCa1.1 and 3) points to other cationic channels dysregulated such as Kv7.3, TALK2, CaV1 and TRPV4 which might play a pathophysiological role in PAH. The significance of other changes found in Na+ and TRP channels remains to be investigated.
Subject terms: Molecular biology, Molecular medicine
Introduction
Pulmonary arterial hypertension (PAH) is a severe disease characterized by an abnormally high mean pulmonary arterial pressure due to distal pulmonary vessel structural remodeling, altered pulmonary arterial tone and inflammation, causing right ventricular hypertrophy and right heart failure1,2. Some characteristic pathophysiological features of the disease include an imbalance between the TGF-β/BMPR2 pathways, endothelial dysfunction, mitochondrial dysfunction and dysregulation of several ion channels, leading to vasoconstriction, changes in vascular cell phenotype, proliferation, de-differentiation and inflammation2. The disease causes a significant burden in terms of quality of life and entails a poor prognosis.
Cationic channels include K+, Na+, Ca2+ and non-selective cationic channels3. The K+ channels superfamily comprises 4 main families: voltage-gated (KV), inward rectifier (KIR), Ca2+-activated (KCa) and two-pore-domain (K2P) K+ channels. Other cationic channel families include voltage-dependent Na+ and Ca2+ channels (NaV and CaV), transient receptor potential channels (TRP) and cyclic nucleotide gated channels (CNG and HCN). All these families have evolved from a common ancestor, share structural similarities and have been classified together as the "voltage-gated-like” ion channels3. The families, subfamilies, gene names and the corresponding encoded proteins of the channels studied herein are shown in Table 1.
Table 1.
Ion channel encoding genes and the corresponding cationic channel proteins.
| Fam | Subfam | Gene | Channel | Fam | Subfam | Gene | Channel | Fam | Subfam | Gene | Channel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KV | KV1.x | KCNA1 | KV1.1 | K2P | K2P | KCNK1 | TWIK | TRP | TRPA | TRPA1 | TRPA1 |
| KCNA2 | KV1.2 | KCNK2 | TREK1 | TRPC | TRPC1 | TRPC1 | |||||
| KCNA3 | KV1.3 | KCNK3 | TASK1 | TRPC2 | TRPC2 | ||||||
| KCNA4 | KV1.4 | KCNK4 | TRAAK1 | TRPC3 | TRPC3 | ||||||
| KCNA5 | KV1.5 | KCNK5 | TASK2 | TRPC4 | TRPC4 | ||||||
| KCNA6 | KV1.6 | KCNK6 | TWIK2 | TRPC5 | TRPC5 | ||||||
| KCNA7 | KV1.7 | KCNK7 | K2P7.1 | TRPC6 | TRPC6 | ||||||
| KCNA10 | KV1.8 | KCNK9 | TASK3 | TRPC7 | TRPC7 | ||||||
| KV2.x | KCNB1 | KV2.1 | KCNK10 | TREK2 | TRPM | TRPM1 | TRPM1 | ||||
| KCNB2 | KV2.2 | KCNK12 | THIK2 | TRPM2 | TRPM2 | ||||||
| KV3.x | KCNC1 | KV3.1 | KCNK13 | THIK1 | TRPM3 | TRPM3 | |||||
| KCNC2 | KV3.2 | KCNK15 | TASK5 | TRPM4 | TRPM4 | ||||||
| KCNC3 | KV3.3 | KCNK16 | TALK1 | TRPM5 | TRPM5 | ||||||
| KCNC4 | KV3.4 | KCNK17 | TALK2 | TRPM6 | TRPM6 | ||||||
| KV4.x | KCND1 | KV4.1 | KCNK18 | TRESK | TRPM7 | TRPM7 | |||||
| KCND2 | KV4.2 | TRPM8 | TRPM8 | ||||||||
| KCND3 | KV4.3 | KCa | KCa | KCNMA1 | KCa1.1 | TRPV | TRPV1 | TRPV1 | |||
| KV5.x | KCNF1 | KV5.1 | KCNN1 | KCa2.1 | TRPV2 | TRPV2 | |||||
| KV6.x | KCNG1 | KV6.1 | KCNN2 | KCa2.2 | TRPV3 | TRPV3 | |||||
| KCNG2 | KV6.2 | KCNN3 | KCa2.3 | TRPV4 | TRPV4 | ||||||
| KCNG3 | KV6.3 | KCNN4 | KCa3.1 | TRPV5 | TRPV5 | ||||||
| KCNG4 | KV6.4 | KNa | KCNT1 | KNa1.1 | TRPV6 | TRPV6 | |||||
| KV7.x | KCNQ1 | KV7.1 | KCNT2 | KNa1.2 | TRPML | MCOLN1 | TRPML1 | ||||
| KCNQ2 | KV7.2 | MCOLN2 | TRPML2 | ||||||||
| KCNQ3 | KV7.3 | CaV | CaV1.x | CACNA1S | CaV1.1 | MCOLN3 | TRPML3 | ||||
| KCNQ4 | KV7.4 | CACNA1C | CaV1.2 | TRPP | PKD2 | TRPP1 | |||||
| KCNQ5 | KV7.5 | CACNA1D | CaV1.3 | PKD2L1 | TRPP2 | ||||||
| KV8.x | KCNV1 | KV8.1 | CACNA1F | CaV1.4 | PKD2L2 | TRPP3 | |||||
| KCNV2 | KV8.2 | CaV2.x | CACNA1A | CaV2.1 | TPCN | TPCN1 | TPCN1 | ||||
| KV9.x | KCNS1 | KV9.1 | CACNA1B | CaV2.2 | TPCN2 | TPCN2 | |||||
| KCNS2 | KV9.2 | CaV3.x | CACNA1E | CaV2.3 | |||||||
| KCNS3 | KV9.3 | CACNA1G | CaV3.1 | CNG HCN | CNGA | CNGA1 | CNGA1 | ||||
| KV10 | KCNH1 | KV10.1 | CACNA1H | CaV3.2 | CNGA2 | CNGA2 | |||||
| KCNH5 | KV10.2 | CACNA1I | CaV3.3 | CNGA3 | CNGA3 | ||||||
| KV11.x | KCNH2 | KV11.1 | CNGA4 | CNGA4 | |||||||
| KCNH6 | KV11.2 | ORAI | ORAI | ORAI1 | ORAI1 | CNGB | CNGB1 | CNGB1 | |||
| KCNH7 | KV11.3 | ORAI2 | ORAI2 | CNGB3 | CNGB3 | ||||||
| KV12.x | KCNH8 | KV12.1 | ORAI3 | ORAI3 | HCN | HCN1 | HCN1 | ||||
| KCNH3 | KV12.2 | HCN2 | HCN2 | ||||||||
| KCNH4 | KV12.3 | NaV | NaV1.x | SCN1A | NaV1.1 | HCN3 | HCN3 | ||||
| SCN2A | NaV1.2 | HCN4 | HCN4 | ||||||||
| KIR | KIR1.x | KCNJ1 | KIR1.1 | SCN3A | NaV1.3 | ||||||
| KIR2.x | KCNJ2 | KIR2.1 | SCN4A | NaV1.4 | |||||||
| KCNJ12 | KIR2.2 | SCN5A | NaV1.5 | ||||||||
| KCNJ4 | KIR2.3 | SCN8A | NaV1.6 | ||||||||
| KCNJ14 | KIR2.4 | SCN9A | NaV1.7 | ||||||||
| KIR3.x | KCNJ3 | KIR3.1 | SCN10A | NaV1.8 | |||||||
| KCNJ6 | KIR3.2 | SCN11A | NaV1.9 | ||||||||
| KCNJ9 | KIR3.3 | NaV2.x | SCN7A | NaV2.1 | |||||||
| KCNJ5 | KIR3.4 | ||||||||||
| KIR4.x | KCNJ10 | KIR4.1 | NaVI | NaVI2.x | NALCN | Navi2.1 | |||||
| KCNJ15 | KIR4.2 | ||||||||||
| KIR5.x | KCNJ16 | KIR5.1 | |||||||||
| KIR6.x | KCNJ8 | KIR6.1 | |||||||||
| KCNJ11 | KIR6.2 | ||||||||||
| KIR7.x | KCNJ13 | KIR7.1 |
A wide variety of cationic channels are functionally expressed in pulmonary artery smooth muscle (PASMC) and endothelial (PAEC) cells4–6. K+ channel conductance plays a fundamental role in the control of their membrane potential (Em), which regulates the cell excitability and the activity of other voltage-gated channels including Ca2+ channels6. Ca2+ conductance in the cell membrane is the main determinant of cytosolic Ca2+ which in turn regulates the contractility of pulmonary arteries, the release of endothelial factors and neurotransmitters and the cell proliferation/apoptotic balance. Therefore, an adequate vascular K+ and Ca2+ channel function ensures an optimal blood flow distribution within the lungs, safeguarding the heart from excessive vascular afterload. NaV channels are essential elements of action potential generation and propagation in neurons and in skeletal and cardiac muscles. Na+ currents have also been measured in pulmonary and systemic vascular smooth muscle cells and systemic endothelial cells4. The functional role of Na+ channels in vascular cells is unclear but some studies have suggested that they may play a role in both regulation of Em and cell proliferation and apoptosis.
Channelopathies are an emerging group of disorders affecting ion channel function. Some of these disorders may be corrected by drugs and therefore, ion channels represent the second largest group of drug targets after G protein-coupled receptors7. Alterations in the K+ and Ca2+ channel density and activity at the cell surface strongly impact in the pulmonary circulation4–6. These diseases may result from a mutation in the encoding genes, from dysregulated gene expression, from the blockade of the ion channel protein by toxins or drugs or from other forms of impaired regulation of channel activity. Deleterious genetic variants in the KCNK3, KCNJ8, ABCC8 and ABCC9 genes (which encode the TASK1 and KIR6.1 channels and the auxiliary subunits SUR1 and SUR2, respectively) and possibly in KCNA5 (encodes for KV1.5 channels) have been associated to PAH, representing paradigmatic examples of channelopathies8. Downregulation of K+ channel expression is also a typical feature of the disease. Several K+ channels have been reported to be downregulated in animal models of PAH including KV1.1, KV1.5, KV1.6, KV2.1, KV3.1b, KV7.1, KV7.4 and TASK1 while others were upregulated such as KV11.1, KCa1.1, KCa3.1 and KIR6.19,10. Some studies have also been carried out to analyze the alteration of specific cationic channels in patients with PAH. Notably, Yuan et al. demonstrated attenuated expression of Kv1.5 mRNA in lungs from PAH patients9,10 and reduced Kv1.1 and Kv4.3 channel mRNA have also been shown4. Downregulation of TASK1 expression and activity has also recently been demonstrated in PASMC from PAH patients11.
In the present study, the expression of the cationic channel encoding genes in lungs from patients with PAH was analyzed using a transcriptomic approach.
Methods
Data was downloaded from the publically available Gene Expression Omnibus (GEO) dataset GSE113439 (https://www.ncbi.nlm.nih.gov/gds/). This dataset contained the gene expression profile of samples from explanted lungs of fifteen patients with pulmonary hypertension who underwent lung transplantation. Specimens were obtained from the peripheral area at the base of each organ. Lung samples obtained from the region of normal healthy tissue flanking lung cancer resections from eleven patients without pulmonary hypertension or left-side heart disease were used as controls. The PAH group (class 1 pulmonary hypertension) included six patients with idiopathic PAH (IPAH), four patients with PAH secondary to connective tissue disease (CTDPAH) and four patients with PAH secondary to congenital heart disease (CHDPAH). There was only one patient with chronic thromboembolic pulmonary hypertension (class 4 pulmonary hypertension) in the dataset which was not included for the present study. The sample preparation, RNA extraction, cDNA synthesis, microarray analysis, gene annotation, preprocessing and data curation and normalization followed standard procedures and are described in detail in the original paper and its supplement12. Briefly, microarrays were processed with Genechip® Human Gene Array and scanned on Affymetrix Genechip Scanners. The expression in the control and the PAH samples was compared for the whole transcriptome by the GEO2R analysis tool (https://www.ncbi.nlm.nih.gov/gds/) using the Benjamini & Hochberg approach for false discovery rate to calculate the adjusted p value. The principal component analysis was performed using the Past software (ver3.21, Oslo, Norway). The changes in channel expression between the different PAH subgroups (log[fold change] versus controls) were compared by regression analysis using Prism (Ver 7.04). P < 0.05 was considered statistically significant.
Results and discussion
A total of 22,195 mRNA products were identified and 12,408 (56%) of them were significantly dysregulated in PAH compared to control lungs (adjusted p < 0.05). This large transcriptomic change is consistent with the advanced stage of the disease in these PAH patients undergoing transplant, which show complex lesions and accumulation of different PASMC and endothelial cells (PAECs), fibroblasts, myofibroblasts and inflammatory cells2. Therefore, the hypertensive lung transcriptome must reflect not only changes in the regulation of gene transcription in the different specific cell types but also changes in cell phenotypes and a different proportion of cell types within the lung. In fact, PAH cells show a hyperproliferative phenotype and share several characteristics with cancer cells13. The Vulcano plot shows an apparently symmetrical distribution of the changes in expression (blue dots in Fig. 1A) with 40.2% of these mRNA products significantly upregulated and 59.8% downregulated.
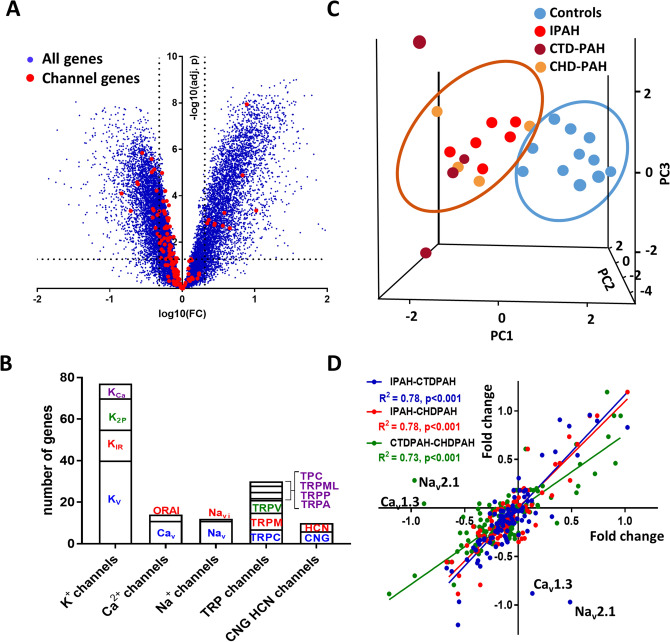
Figure 1.
Channel gene expression profile analysis in pulmonary arterial hypertension. (A) Vulcano plot [log fold change against − log (adjusted p value)] showing the changes in mRNA transcripts for all identified genes (blue) and for cationic channel encoding genes (red). The vertical lines correspond to twofold up and down, respectively, and dots above the horizontal dotted line indicate adjusted p < 0.05. (B) Families and subfamilies of mRNAs encoding the pore forming alpha subunits of cationic channels identified in the control samples, (C) 3D principal component analysis performed with the 143 channel encoding mRNAs. (D) Regression analysis of the changes (fold change vs control) in the different subgroups of PAH: idiopathic PAH (IPAH), connective tissue disease associated PAH (CTD-PAH) and congenital heart disease PAH (CHD-PAH).
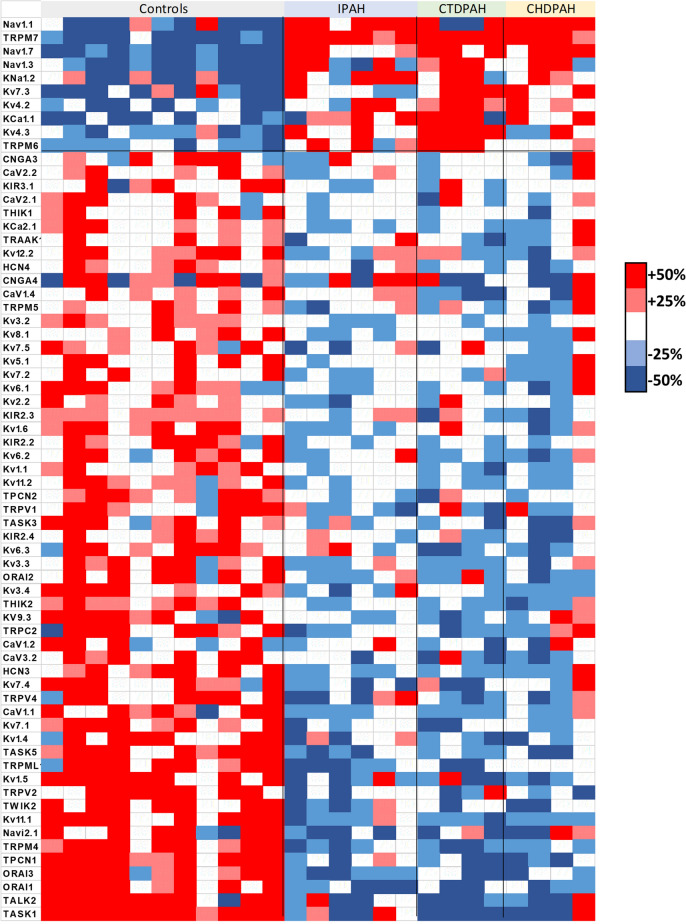
The different families and subfamilies of mRNAs encoding the pore forming alpha subunits of cationic channels identified are shown in Fig. 1B. K+ channels comprise the family of cationic channels with the highest relative abundance, followed by TRP channels; and to a lesser extent Ca2+, Na+ and CNG/HCN channels (Figs. 1B, 2). As expected, there were large differences in the relative expression of the different cationic channel genes in control lungs (Fig. 2), which gives a rough estimate of the importance of each channel. Among the 143 mRNA products identified as cationic channels, 66 of them (46.2%) were dysregulated in PAH with a strongly asymmetrical distribution in the Vulcano plot (red dots in Fig. 1A), with most of them downregulated (83.1%) and only 16.9% of them upregulated. This broad decrease in channel expression might be partly attributed to a relative loss or a phenotypic change in the electrically excitable cells in the lung in which voltage-gated cation channels are abundant, including smooth muscle, neurons and endocrine cells. The principal component analysis (PCA) for the whole transcriptome previously published12 indicated a complete separation between PAH subjects and normal controls. Herein, the unsupervised PCA applied to the 143 channel transcripts also clearly separated controls from PAH patients (Fig. 1C). This indicates that dysregulated cationic channel expression is a signature of the disease. However, the three different forms of PAH analyzed, i.e. IPAH, CTDPAH and CHDPAH, were indistinguishable in the PCA analysis. Two samples from the CTDPAH group were clearly separated from the rest of the PAH patients and further away from the controls. Moreover, the plots of the changes in channel gene expression in IPAH vs CTDPAH, IPAH vs CHDPAH and CTDPAH vs CHDPAH, shows an excellent correlation (Fig. 1D, P < 0.001 for the three pairs of comparisons). That confirms that the changes in channel gene expression were very similar in the three forms of PAH. The data was very internally consistent. The exceptions to this rule were only two genes, CaV1.3 and NaV2.1, which were downregulated specifically in CTDPAH (supplementary Fig. 1). If confirmed, these might represent biomarkers of this specific subtype of PAH. The heat map for the genes encoding cationic channel that displayed significant difference (adjusted p < 0.05) in expression is shown in Fig. 3.
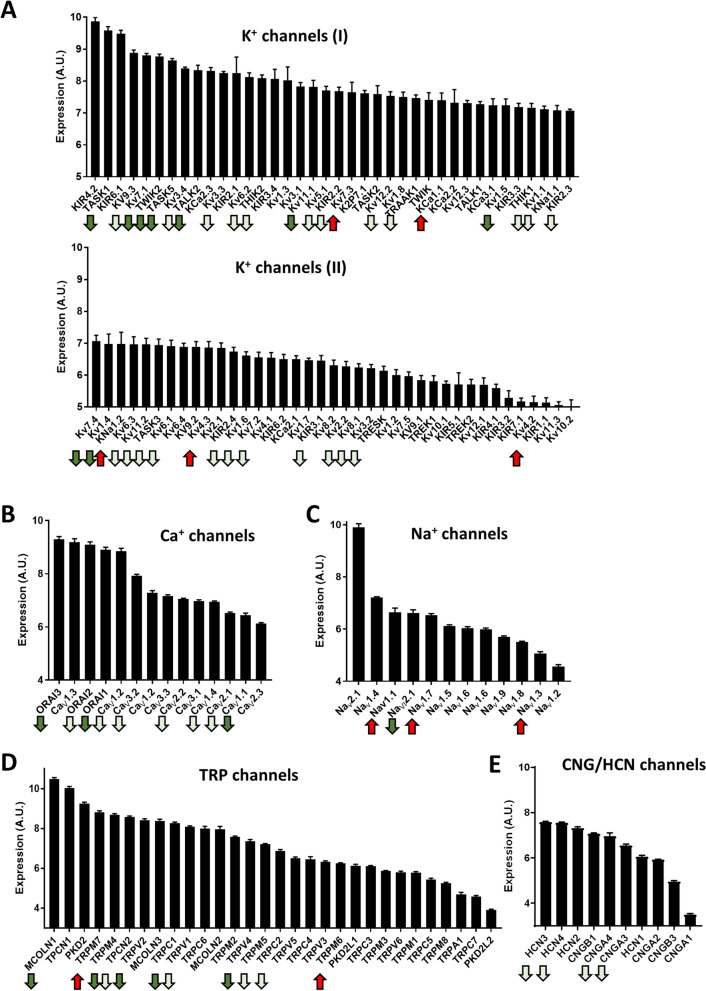
Figure 2.
Relative abundance of ion channel encoding genes in the control lungs. Bars represent means ± SEM (n = 10). Arrows indicate significantly (adjusted p < 0.05) dysregulated channels in PAH: dark green for > twofold downregulation, light green for < twofold downregulation and red for > twofold upregulation.
Figure 3.
Heat map of genes encoding cationic channel that displayed significant difference (adjusted p < 0.05) in expression.
The group of K+ channels is the largest family of cationic channels (Fig. 2A). The differential expression of K+ channels in PAH with 33 (44%) mRNAs encoding K+ channels significantly downregulated and 5 (7%) mRNAs upregulated is shown in Fig. 4A. Because K+ conductance is essential to maintain a normally polarized cell membrane, this reduced expression of K+ channels explains the depolarized Em observed in PASMC in PAH leading to increased contractility and cell proliferation9.
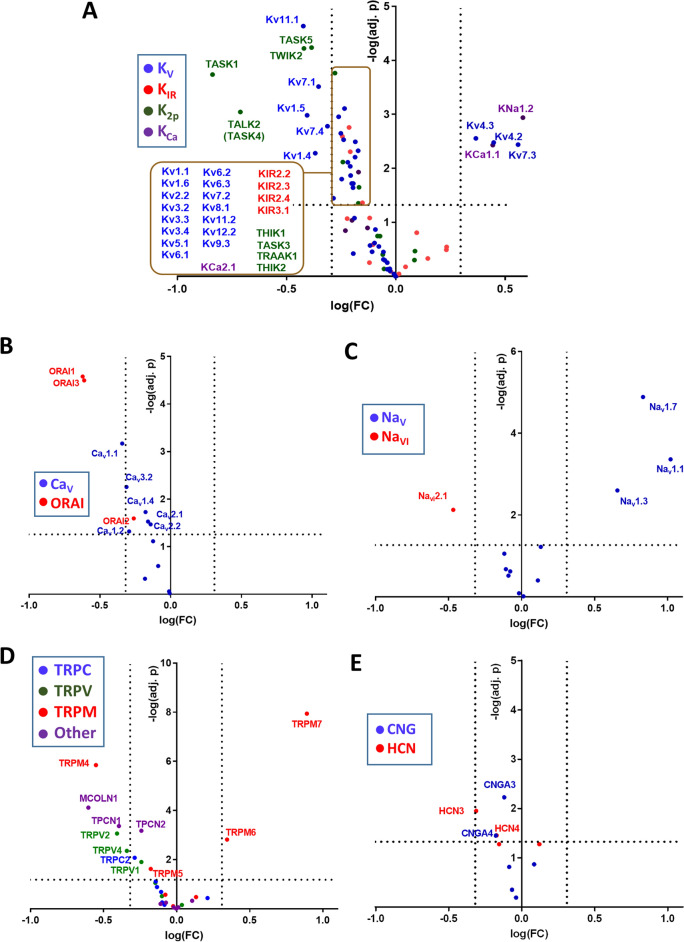
Figure 4.
Vulcano plot [log fold change against − log (adjusted p value)] showing the changes in mRNA transcripts for cationic channel encoding genes (A) K+, B) Ca2+, C) Na+, D) TRP and E) CNG/HCN superfamilies. The vertical lines correspond to twofold up and down, respectively, and dots above the horizontal dotted line indicate adjusted p < 0.05.
The analysis of the expression of each channel family (Fig. 4A–E) shows that the K+, Ca2+, TRP and CNG/HCN superfamilies of cationic channels present an asymmetrical distribution of their expression in PAH in the Vulcano plots with a majority of their members downregulated. The Na+ channel family with a smaller number of members shows a nearly symmetrical plot. The data with significant changes are shown in supplementary Figs. 2–5.
The KV channel family contributed the largest number of dysregulated K+ channel genes (3 up- and 20 downregulated, Fig. 4A). Some of them have been previously reported to be downregulated in PAH including KV1.5 channels (KCNA5) which are key regulators of Em and are inhibited by vasoactive factors involved in PAH such as 5-HT, endothelin-1, thromboxane A2 and hypoxia14–16. In fact, KV1.5 downregulation is widely recognized as a hallmark of the disease in both humans and animal models10,17,18. Consistent with previous reports, other downregulated channels from this family are KV1.1, KV1.4 and KV1.6 channels. KV1.2 and KV2.1 channels, which were previously shown to be reduced in vitro by hypoxia in animal models of PAH9,18 were unchanged. However, the electrically-silent KV9.3 channels, which form a functional heteromer with KV2.1, were downregulated in the present and in the vitro study18. Several other KV channels such as KV2.2, KV3.2, KV3.3, KV3.4, KV5.1, KV6.1, KV6.2, KV6.3, KV8.1 and KV12.2, that have been previously reported to be expressed in the pulmonary arteries19 but whose functional importance is unclear, were also modestly downregulated. In contrast, KV4.3 previously reported to be downregulated in vitro by hypoxia, as well as KV4.2, both of which generate transient currents, were found to be upregulated in PAH patients. On the other hand, the expression of KV11.1 channels, also known as hERG channels and involved in heart repolarization, was increased as measured by immunohistochemistry in the pulmonary arteries of humans affected by chronic obstructive pulmonary disease-associated pulmonary hypertension (class 3)20 but were significantly downregulated in the three forms of PAH in the present study.
In recent years, a key role of KV7 channels (KCNQ1-5) in the control of pulmonary vascular tone has been demonstrated21–24. KV7.1, KV7.3, KV7.4 and KV7.5 mRNAs are known to be expressed in rat pulmonary arteries24,25 but KV7.3 was not detected in vascular smooth muscle cells. Based on the effects of KV7 activators flupirtine and retigabine, these channels have been proposed as therapeutic targets for PAH23,24. In control human lungs, the five KV7 were expressed, with KV7.5 showing the lowest levels (Fig. 2A). Downregulation of KV7.1, KV7.4 and KV7.5 have been shown previously in animal models of PAH21,23,24,26–28. Herein KV7.1, KV7.2 and KV7.4 were downregulated in human PAH (Fig. 4A). Interestingly, KV7.3 channels were upregulated in the three forms of human PAH seemingly compensating for the loss of the other KV7 channels. This may also help to support the preserved Kv7 function in PAH24. Because KV7.3 are sensitive to flupirtine and retigabine, it would be worth analyzing the role of these channels in the context of PAH.
KIR are expressed in vascular smooth muscle and endothelial cells9,10. The only change reported in PAH so far is an increase in KIR6.1, which forms the vascular KATP channel in the monocrotaline rat model of PAH26. The present results indicate that KIR6.1 was strongly expressed in the lungs (Fig. 2A) but unchanged by PAH and that most other KIR are unchanged as well, except KIR2.2-KIR2.4 and KIR3.1 that were modestly downregulated (Fig. 4A).
KCa channels are activated by cytosolic Ca2+. The KCa1.1 channel which is responsible of the large conductance KCa current (BKCa), was strongly upregulated in lungs from PAH patients (Fig. 4A). Similarly, these channels were upregulated in laser microdissected pulmonary arteries from IPAH patients29, suggesting the utility of BKCa openers for pharmacological interventions in PAH. On the contrary, KCa2.1 which carries a small conductance Ca2+-activated K+ channel (SKCa) was modestly downregulated.
K2P channels, also referred to as leak or background conductance channels, comprise a large family of voltage-independent K+ channels that control resting Em in smooth muscle cells. These K2P channels are classified into six subfamilies according to their sequence and functional properties: the weak rectifying group (TWIK1, TWIK2, and K2P7.1), the mechano-gated group (TREK1, TREK2, and TRAAK), the acid-sensing group (TASK1, TASK3, and TASK5), the alkaline-sensitive group (TALK1, TALK2, and TASK2), the halothane-sensitive group (THIK1 and THIK2), and the spinal cord-expressed TRESK channel. Among this family, the mRNA of TASK1, TASK2, THIK1, TREK2 and TWIK2 are reported to be expressed in pulmonary arteries, playing a role in pulmonary vascular tone30. The present data also indicate a high expression of the mRNA of TALK2 (also named as TASK4), TASK5 and THIK2 in the whole lung (Fig. 2A). Remarkably, TASK1 has gained a reputation as an important player in PAH. In fact, loss of function mutations in KCNK3 (TASK1) were the first channelopathy described in PAH8,31. Herein, the present results confirm the downregulation of TASK1 reported in both human and animal models of PAH9–11. In fact, TASK1 was the second most highly expressed K+ channel and the strongest downregulated channel in the channelome. Interestingly, other K2P channels were also markedly downregulated, including TALK2, TWIK2 and TASK5. TALK2 heterodimerizes with TASK132 and, thus, it might play a role together with TASK1 in controlling Em and tone in pulmonary arteries. Based on experiments carried in TWIK2 knockout mice, Pandit et al.33 suggested that these channels are involved in PAH. This concept was challenged by Lambert et al.4, which showed that mRNA expression of TWIK2 was unchanged in pulmonary arteries from IPAH patients. The present data, however, would argue in favor of the former possibility. The physiological role of TASK5 is unclear as it produces a weak current in transfected mammalian cell lines34. Other members of the family that were modestly downregulated include THIK1, THIK2, TRAAK1 and TASK3 and none of the channels of this family were upregulated.
CaV channels are activated by depolarization and the subsequent elevation in cytosolic Ca2+ constitutes the main mechanism for the activation of electrically excitable cells, including PASMC, and some non-excitable cells35. Pulmonary artery contraction depends on Ca2+-dependent pathways (mediated mainly by L-type Ca2+ channels) and Ca2+-independent pathways (mediated mainly by Rho kinase activation), and the relative role of each one may vary with age, the vasoconstrictor agent involved and the hypertensive state36,37. The downregulation of K+ channels in PAH as described above and the subsequent depolarization increases the activation of these voltage-gated CaV channels promoting contraction and proliferation. The CaV1 subfamily (CaV1.1–CaV1.4) corresponds with the voltage-dependent channels known as L-type channels which are sensitive to classic Ca2+ channel blockers (verapamil, diltiazem and dihydropyridines such as nifedipine or amlodipine)7. These drugs constitute a first line therapy for PAH, but unfortunately only 10% of the patients respond to these drugs at clinically tolerable doses. CaV1.2 and CaV1.3 showed the highest expression in the lungs (Fig. 2B). Downregulation of CaV1.2 has been previously reported in arteries from a model of persistent pulmonary hypertension of the newborn38. However, hypoxia upregulated these CaV1.2 channels in mouse lungs39. The Vulcano plot shows that in PAH most CaV channels are modestly downregulated including the L-type Ca2+ channels CaV1.1, CaV1.2 and CaV1.4 (Fig. 4B). The downregulation of CaV1.x and the upregulation of Rho kinase in these patients12 might account for a higher role of Ca2+-independent pathways for pulmonary artery contraction37. It may also account for the low proportion of responders to L-type Ca2+ channel blockers. We also analyzed the relationship of the expression of the different CaV1.x with the expression of KV channels; the significant associations are shown in supplementary Fig. 6. It is interesting that in control patients there is an inverse association for certain KV and the different CaV1.x channels. Curiously, each KV channel is only associated with a single CaV1.x channel: KV1.3, KV4.2 and KV6.3 with CaV1.1; KV12.1 with CaV1.2; KV1.2, KV3.1, KV4.1, KV5.1 and KV6.2 with CaV1.3 and finally KV4.3 with CaV1.4 (black symbols in supplementary Fig. 6). On the contrary, in the lung from PAH patients these inverse associations were lost and all significant associations were direct (supplementary Fig. 6, blue symbols). The cause-effect relationships and their meaning are unclear. It is possible that KV activity negatively regulates CaV1.x expression and that this relationship is lost with the missing excitable phenotype in PAH cells. CaV2.1, CaV2.2 and CaV2.3 correspond to the P/Q, N and R type, respectively, while the CaV3 subfamily (CaV3.1-CaV3.3) correspond to the T-type channels. Hypoxia upregulated CaV3.2 channels in mouse lungs39 while these channels were decreased in patients with PAH (Fig. 4B).
ORAI channels are store-operated Ca2+ entry channels which are activated by depletion of Ca2+ in the endoplasmic reticulum and do not share a common structure with voltage-gated like channels. It has been suggested that ORAI1 channels play a role in PAH40 but evidences are weak so far. The expression of ORAI2 was found increased in PASMC in culture from patients with iPAH40. ORAI2 was also upregulated in endothelial cells from PAH patients in a single cell transcriptomic analysis41. ORAI1 is involved in the transition of PASMC from a contractile to a proliferative phenotype42. However, the three ORAI genes were downregulated in the whole lungs from PAH patients in the present study (Fig. 4B) suggesting that its inhibition is not an appealing therapeutic strategy.
NaV channels play a major role in regulating neuron excitability. Nonetheless, human pulmonary arteries expresses multiple NaV. channels4,43 although their functional role is unclear. The present study shows that the mRNA expression of NaV2.1 predominates in the lung (Fig. 2C). In lungs from PAH patients there was a strong upregulation of NaV1.1, NaV1.3 and NaV1.7 (Fig. 4C). In contrast, the sodium leak channel, NaVi2.1, a voltage-insensitive member of the NaV family, was downregulated in PAH lungs. The significance of these findings deserves further investigation.
The TRP channel family comprises seven subfamilies of cationic channels with diverse biophysical properties and physiological functions: the TRPC (“canonical”), the TRPV (“vanilloid”), the TRPM (“melastatin”), the TRPP (“polycystin”), the TRPML (“mucolipin”), the TRPA (“ankyrin”) and the TRPN (“nompC”, no mechanoreceptor potential C) families. TRP channels contribute to the modulation of cytosolic Ca2+ directly by carrying Ca2+ entry or indirectly by modulating the resting Em4,44. TRPC1, TRPC4 and TRPC6 are the major TRPC channels expressed in rat, mouse, and human pulmonary arteries3,45 and in the human lung (present results). TRPV channels, except TRPV2, are involved in thermic sensitivity. However, TRPV1, TRPV2, TRPV3 and TRPV4 mRNA were also detected in pulmonary arteries44 and in whole lung (present results). In contrast to other TRPs, TRPM4 and TRPM5 are impermeable to Ca2+ and its role in the lung is unknown. TRPM6 and TRPM7 are closely related; together they form a heterotetrameric channel permeable to divalent (Ca2+, Mg2+, Zn2+) and monovalent cations and play a role in Mg2+ homeostasis44. The mRNA of TRPM2, TRPM3, TRPM4, TRPM7 and TRPM8 were detected in pulmonary arteries44. Similarly, in the whole lungs analyzed in the present study, the highest expression was found in TRPM7, TRPM4, TRPM2 and TRMP5 channels. Among other TRP channels belonging to the TRPML, TRPP, TRPA and TPCN families, little is known about their expression and function in the lungs. The present study, however, shows a strong expression of the TRPP channel PKD2, the TRPML channels MCOLN1, MCOLN2, and MCOLN3 and the TPCN channels TPCN1 and TPCN2.
A number of changes were found in the literature about the expression of TRPs in pulmonary arteries of PAH patients44. TRPC1, TRPC4 and TRPC6 isoforms are upregulated in rat BMP2 knockdown PASMC, leading to increased Ca2+ entry and increased human PASMC proliferation and migration46. TRPC6 and TRPC3 mRNA and protein were also upregulated in lungs from IPAH patients47. Moreover, TRPC6 gene deletion suppresses chronic hypoxia-induced PH in mice48. All these findings strongly suggest a role for TRPC6 in PAH. However, the present results do not reproduce the upregulation of any TRPC mRNAs. The only significant change in this subfamily was a modest decrease in TRPC2 expression, probably irrelevant because TRPC2 is a pseudogene in humans. Both TRPV1 and TRPV4 were found to be upregulated in cultured PASMC from PAH patients49. In contrast, in the present PAH cohort there was a downregulation of lung TRPV1, TRPV2 and TRPV4. Interestingly, TRPV4 channels mediate a rise in endothelial intracellular Ca2+ concentration, leading to the activation of KCa3.1 (IKCa) and KCa2.3 (SKCa) currents and activating endothelial-dependent hyperpolarization (EDH) and relaxation in pulmonary arteries. The downregulation of TRPV4 is consistent with reduced EDH in pulmonary arteries from hypoxic mice50. In the TRPM family, TRPM7 was downregulated in cultured PASMCs from PAH patients and in rats with hypoxia-induced PAH and its inhibition exacerbates PAH40. Similarly, mice with a deletion of the kinase domain in TRPM7 channels in heterozygosis, show a severe systemic hypertensive phenotype induced by angiotensin II51. However, herein, TRPM7 and, to a minor extent, TRPM6 were upregulated in PAH. Conversely, TRPM5 was modestly reduced and TRPM4 was strongly downregulated. Other TRP channels downregulated include MCOLN1, TPCN1 and TPC2. Taken together, there was little correlation of the present findings regarding changes of TRP channel expression in PAH between the present data compared to isolated PASMC in animal models. This may reflect that TRP expression is quantitatively important in lung cells different from PASMC.
The CNG channels are permeable to Ca2+ and/or Na+ and are gated by cyclic nucleotides. They are involved in fluid absorption and CNGA2 has been reported to regulate Ca2+ in bovine PAECs52. CNGA3, CNGA4, CNGB2 and to a minor extent CNGA2 were expressed in the control lungs (Fig. 2E) and the CNGA3 and CNGA4 were downregulated in PAH lungs (Fig. 4E). HCN channels are permeable to Na+ and/or K+ and are activated by membrane hyperpolarization and by cyclic nucleotides53. They regulate neuronal excitability, pain and sinoatrial and atrioventricular node activities but their physiological role in the lung is unknown. Downregulation of the HCN4 channel in the atrioventricular node was observed in the monocrotaline rat model of PAH54. All isoforms of HCN (Fig. 2E) were present in the lung and HCN3 and HCN4 were moderately downregulated in PAH lungs (Fig. 4E).
A limitation of this study is that control samples were from neoplasia-free areas of lungs from patients with lung cancer, which might obviously affect the results. K+ channels, including Kv1.5, have been implicated in the abnormal growth of lung cancer cells55. KV1.5 expression negatively correlated with tumor grade; the higher the histologic tumor grade the lower the KV1.5 expression. It is unknown whether healthy cells from cancer patients also show dysregulated K+ channels. However, if anything these issues should hide the differences observed herein. Moreover, PAH samples were obtained from advanced-stage, severe ill patients undergoing transplantation. Then, some changes found may be the consequence of a critical health condition rather than a pathophysiological causative factor of the disease. An additional important limitation of the present study is that mRNA expression is not always translated into protein and even when protein is present it might not traffic to the membrane or may not form a functional channel. However, the study has the strength of using clinically relevant samples in contrast with much of the previous work done on cell culture from PAH patients or controls with or without exposure to hypoxia, which can be problematic because channel expression can be altered by culture passage. Some studies have analyzed specific channels genes in PASMC or PAECS or in pulmonary arteries. These include cells from manually dissected medium and small pulmonary arteries, illustrative of vascular changes but which may not be very representative of the complex lesions present in the microcirculation in PAH lungs, such as onion-skin lesions, plexiform core lesions and dilation lesions2.
In conclusion, the dysregulation of cationic channel expression is a signature of PAH with multiple channels being downregulated and a few of them upregulated. Changes were very similar in IPAH, CTDPAH and CHDPAH. When compared to changes reported in the literature, this analysis of the cationic channel transcriptome in PAH: 1) is in agreement with previously published changes in the expression of channels which play an important and widely recognized pathophysiological role in the disease such as the downregulation of TASK1 and KV1.5 in humans and animal models; 2) supports previous preliminary reports pointing to the dysregulation of several K+ channels including the downregulation of KV1.1, KV1.4, KV1.6, KV7.1, KV7.4, KV9.3 and TWIK2 and the upregulation of KCa1.1; 3) argues against the proposed role of other K+ channels including KV1.2, KV2.1, KV4.2, KV4.3, KV11.1, KIR6.1 and ORAIs and 4) points to other novel cationic channels dysregulated such as Kv7.3, TALK2, CaV1 and TRPV4 which might play a pathophysiological role in PAH. The significance of other changes found in Na+ and other TRPs remains to be investigated.
Supplementary Information
Acknowledgements
We thank Dr. Mura and colleagues for sharing publically the data in NCBI GEOdatasets.
Author contributions
F.P.V. designed the study, analyzed the data and wrote the manuscript, A.C. and G.M.P. made important contributions to the discussion and manuscript edition.
Funding
This study is supported by grants from Ministerio de Economía y Competitividad (PID2019-107363RB-I00 to FPV and SAF2016-77222R to FP-V and AC) with funds from the European Union (Fondo Europeo de Desarrollo Regional FEDER) and Fundación Contra la Hipertensión Pulmonar (Empathy grant).
Data availability
The data that support the findings of this study are available in Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/gds/, reference number GSE113439.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95196-z.
References
- 1.Simonneau G, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019 doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur. Respir. J. 2019 doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 4.Lambert M, et al. Ion channels in pulmonary hypertension: a therapeutic interest? Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucherat O, et al. Potassium channels in pulmonary arterial hypertension. Eur. Respir. J. 2015;46:1167–1177. doi: 10.1183/13993003.00798-2015. [DOI] [PubMed] [Google Scholar]
- 6.Mondejar-Parreno G, Cogolludo A, Perez-Vizcaino F. Potassium (K(+)) channels in the pulmonary vasculature: Implications in pulmonary hypertension physiological, pathophysiological and pharmacological regulation. Pharmacol. Ther. 2021;225:107835. doi: 10.1016/j.pharmthera.2021.107835. [DOI] [PubMed] [Google Scholar]
- 7.IUPHAR/BPS. IUPHAR/BPS Guide to PHARMACOLOGY, <http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=689> (2020).
- 8.Southgate L, Machado RD, Graf S, Morrell NW. Molecular genetic framework underlying pulmonary arterial hypertension. Nat. Rev. Cardiol. 2020;17:85–95. doi: 10.1038/s41569-019-0242-x. [DOI] [PubMed] [Google Scholar]
- 9.Yuan JX, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 10.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998;351:726–727. doi: 10.1016/s0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- 11.Antigny F, et al. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation. 2016;133:1371–1385. doi: 10.1161/circulationaha.115.020951. [DOI] [PubMed] [Google Scholar]
- 12.Mura M, Cecchini MJ, Joseph M, Granton JT. Osteopontin lung gene expression is a marker of disease severity in pulmonary arterial hypertension. Respirology. 2019;24:1104–1110. doi: 10.1111/resp.13557. [DOI] [PubMed] [Google Scholar]
- 13.Boucherat O, et al. The cancer theory of pulmonary arterial hypertension. Pulm Circ. 2017;7:285–299. doi: 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cogolludo A, et al. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ. Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 15.Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ. Res. 2003;93:656–663. doi: 10.1161/01.Res.0000095245.97945.Fe. [DOI] [PubMed] [Google Scholar]
- 16.Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv. Exp. Med. Biol. 2000;475:219–240. doi: 10.1007/0-306-46825-5_21. [DOI] [PubMed] [Google Scholar]
- 17.Mondejar-Parreno G, et al. miR-1 is increased in pulmonary hypertension and downregulates Kv1.5 channels in rat pulmonary arteries. J. Physiol. 2019;597:1185–1197. doi: 10.1113/JP276054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J. Clin. Invest. 1997;100:2347–2353. doi: 10.1172/jci119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platoshyn O, et al. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L226–238. doi: 10.1152/ajplung.00438.2003. [DOI] [PubMed] [Google Scholar]
- 20.Shults NV, Rybka V, Suzuki YJ, Brelidze TI. Increased smooth muscle Kv11.1 channel expression in pulmonary hypertension and protective role of Kv11.1 channel blocker dofetilide. Am. J. Pathol. 2020;190:48–56. doi: 10.1016/j.ajpath.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedivy V, et al. Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L48–57. doi: 10.1152/ajplung.00362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondejar-Parreno G, et al. Activation of Kv 7 channels as a novel mechanism for NO/cGMP-induced pulmonary vasodilation. Br. J. Pharmacol. 2019;176:2131–2145. doi: 10.1111/bph.14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morecroft I, Murray A, Nilsen M, Gurney AM, MacLean MR. Treatment with the Kv7 potassium channel activator flupirtine is beneficial in two independent mouse models of pulmonary hypertension. Br. J. Pharmacol. 2009;157:1241–1249. doi: 10.1111/j.1476-5381.2009.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondejar-Parreno G, et al. Uncovered contribution of Kv7 channels to pulmonary vascular tone in pulmonary arterial hypertension. Hypertension. 2020;76:1134–1146. doi: 10.1161/HYPERTENSIONAHA.120.15221. [DOI] [PubMed] [Google Scholar]
- 25.Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J. Pharmacol. Exp. Ther. 2009;329:368–376. doi: 10.1124/jpet.108.147785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Kim KC, Hong YM. Change of voltage-gated potassium channel 1.7 expressions in monocrotaline-induced pulmonary arterial hypertension rat model. Korean J. Pediatr. 2018;61:271–278. doi: 10.3345/kjp.2018.06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondejar-Parreno G, et al. HIV transgene expression impairs K(+) channel function in the pulmonary vasculature. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;315:711–723. doi: 10.1152/ajplung.00045.2018. [DOI] [PubMed] [Google Scholar]
- 28.Li SS, Ran YJ, Zhang DD, Li SZ, Zhu D. MicroRNA-190 regulates hypoxic pulmonary vasoconstriction by targeting a voltage-gated K(+) channel in arterial smooth muscle cells. J. Cell Biochem. 2014;115:1196–1205. doi: 10.1002/jcb.24771. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraj C, et al. Docosahexaenoic acid causes rapid pulmonary arterial relaxation via KCa channel-mediated hyperpolarisation in pulmonary hypertension. Eur. Respir. J. 2016;48:1127–1136. doi: 10.1183/13993003.01814-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardener MJ, et al. Functional evidence of a role for two-pore domain potassium channels in rat mesenteric and pulmonary arteries. Br. J. Pharmacol. 2004;142:192–202. doi: 10.1038/sj.bjp.0705691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olschewski A, et al. TASK-1 (KCNK3) channels in the lung: from cell biology to clinical implications. Eur. Respir. J. 2017 doi: 10.1183/13993003.00754-2017. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki Y, Tsutsumi K, Miyamoto T, Yamamura H, Imaizumi Y. Heterodimerization of two pore domain K+ channel TASK1 and TALK2 in living heterologous expression systems. PLoS ONE. 2017;12:e0186252. doi: 10.1371/journal.pone.0186252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandit LM, et al. TWIK-2 channel deficiency leads to pulmonary hypertension through a rho-kinase-mediated process. Hypertension. 2014;64:1260–1265. doi: 10.1161/hypertensionaha.114.03406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bichet D, et al. Silent but not dumb: how cellular trafficking and pore gating modulate expression of TWIK1 and THIK2. Pflugers Arch. 2015;467:1121–1131. doi: 10.1007/s00424-014-1631-y. [DOI] [PubMed] [Google Scholar]
- 35.Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol. Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- 36.Cogolludo A, Moreno L, Lodi F, Tamargo J, Perez-Vizcaino F. Postnatal maturational shift from PKCzeta and voltage-gated K+ channels to RhoA/Rho kinase in pulmonary vasoconstriction. Cardiovasc. Res. 2005;66:84–93. doi: 10.1016/j.cardiores.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Jernigan NL, Resta TC. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation. 2014;21:259–271. doi: 10.1111/micc.12096. [DOI] [PubMed] [Google Scholar]
- 38.Resnik E, et al. Chronic intrauterine pulmonary hypertension selectively modifies pulmonary artery smooth muscle cell gene expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L426–433. doi: 10.1152/ajplung.00281.2005. [DOI] [PubMed] [Google Scholar]
- 39.Wan J, et al. Chronic hypoxia selectively enhances L- and T-type voltage-dependent Ca2+ channel activity in pulmonary artery by upregulating Cav12 and Cav32. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013;305:L154–164. doi: 10.1152/ajplung.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rode B, Bailey MA, Marthan R, Beech DJ, Guibert C. ORAI channels as potential therapeutic targets in pulmonary hypertension. Physiology (Bethesda) 2018;33:261–268. doi: 10.1152/physiol.00016.2018. [DOI] [PubMed] [Google Scholar]
- 41.Saygin D, et al. Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension. Pulm Circ. 2020 doi: 10.1177/2045894020908782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez RA, et al. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am. J. Physiol. Cell Physiol. 2015;308:C581–593. doi: 10.1152/ajpcell.00202.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Platoshyn O, Remillard CV, Fantozzi I, Sison T, Yuan JX. Identification of functional voltage-gated Na(+) channels in cultured human pulmonary artery smooth muscle cells. Pflugers Arch. 2005;451:380–387. doi: 10.1007/s00424-005-1478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guibert C, Ducret T, Savineau JP. Expression and physiological roles of TRP channels in smooth muscle cells. Adv. Exp. Med. Biol. 2011;704:687–706. doi: 10.1007/978-94-007-0265-3_36. [DOI] [PubMed] [Google Scholar]
- 45.Yang XR, Lin MJ, Sham JS. Physiological functions of transient receptor potential channels in pulmonary arterial smooth muscle cells. Adv. Exp. Med. Biol. 2010;661:109–122. doi: 10.1007/978-1-60761-500-2_7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, et al. Bone morphogenetic protein 2 decreases TRPC expression, store-operated Ca(2+) entry, and basal [Ca(2+)]i in rat distal pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2013;304:C833–843. doi: 10.1152/ajpcell.00036.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, et al. Classical transient receptor potential 1 and 6 contribute to hypoxic pulmonary hypertension through differential regulation of pulmonary vascular functions. Hypertension. 2014;63:173–180. doi: 10.1161/hypertensionaha.113.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song S, et al. Capsaicin-induced Ca(2+) signaling is enhanced via upregulated TRPV1 channels in pulmonary artery smooth muscle cells from patients with idiopathic PAH. Am. J. Physiol. Lung Cellular Mol. Physiol. 2017;312:L309–l325. doi: 10.1152/ajplung.00357.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Si R, et al. Chronic hypoxia decreases endothelial connexin 40, attenuates endothelium-dependent hyperpolarization-mediated relaxation in small distal pulmonary arteries, and leads to pulmonary hypertension. J. Am. Heart Assoc. 2020;9:e018327. doi: 10.1161/JAHA.120.018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antunes TT, et al. Transient receptor potential melastatin 7 cation channel kinase: new player in angiotensin ii-induced hypertension. Hypertension. 2016;67:763–773. doi: 10.1161/hypertensionaha.115.07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Xia SL, Block ER, Patel JM. NO upregulation of a cyclic nucleotide-gated channel contributes to calcium elevation in endothelial cells. Am. J. Physiol. Cell Physiol. 2002;283:C1080–1089. doi: 10.1152/ajpcell.00048.2002. [DOI] [PubMed] [Google Scholar]
- 53.Benarroch EE. HCN channels: function and clinical implications. Neurology. 2013;80:304–310. doi: 10.1212/WNL.0b013e31827dec42. [DOI] [PubMed] [Google Scholar]
- 54.Temple IP, et al. Atrioventricular node dysfunction and ion channel transcriptome in pulmonary hypertension. Circ. Arrhythm. Electrophysiol. 2016 doi: 10.1161/circep.115.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in Gene Expression Omnibus (GEO) at https://www.ncbi.nlm.nih.gov/gds/, reference number GSE113439.