Abstract
Background
Psittacosis is a systemic disease usually with respiratory involvement, caused by the obligate intracellular bacterium Chlamydia psittaci. Exposure to birds, the main zoonotic reservoir, is a major risk factor for infection. The spectrum of disease is highly variable, ranging from subclinical infection to severe pneumonia requiring mechanical ventilation. There is limited data on psittacosis progressing to organizing pneumonia and management of such cases.
Case presentation
A 63-year-old man was referred to a rural hospital with 11 days of fevers to 39 °C, myalgia, lethargy and several days of dry cough. After initial treatment with benzylpenicillin and doxycycline for left lower pneumonia found on CXR, the patient deteriorated with extensive bilateral consolidation on chest CT requiring mechanical ventilation. Atypical pneumonia screening was negative, however, exposure to a sick bird prior to symptom onset triggered testing for C. psittaci which was positive. Doxycycline was recommenced with minimal benefit, and organizing pneumonia was later suspected. The patient slowly improved with a weaning course of corticosteroids started after 19 days and was discharged from hospital. He unfortunately was re-admitted and died several months later.
Conclusion
Severe pneumonia is a rare, but potentially life-threatening complication of psittacosis. We present a case of psittacosis which progressed to suspected organizing pneumonia despite appropriate antibiotics, and subsequent treatment with corticosteroids. This case suggests it may be useful to consider corticosteroids early in therapy for patients with severe psittacosis. Our paper underlines the need for further research to determine the best management of severe psittacosis to improve patient outcomes.
Keywords: Chlamydia psittaci, Psittacosis, Atypical pneumonia, Cryptogenic organizing, Pneumonia, Organizing pneumonia, Corticosteroid
Abbreviations: ARDS, Acute respiratory distress syndrome; COP, Cryptogenic organizing pneumonia; CRP, C-reactive protein; CT, computed tomography; CXR, chest X-ray; ICU, intensive care unit; MV, mechanical ventilation; OP, Organizing pneumonia; PCR, polymerase chain reaction
1. Case presentation
A 63-year-old Caucasian man was referred to a rural hospital with 11 days of fevers, myalgia, lethargy and anorexia, and several days of dry cough. His medical history included chronic urinary tract infections secondary to hypospadias, asthma, recurrent sinusitis requiring surgery, and anxiety. He previously was a smoker with a minimal pack-year history, and had an excellent exercise tolerance. He had no history of recent travel or intravenous drug use.
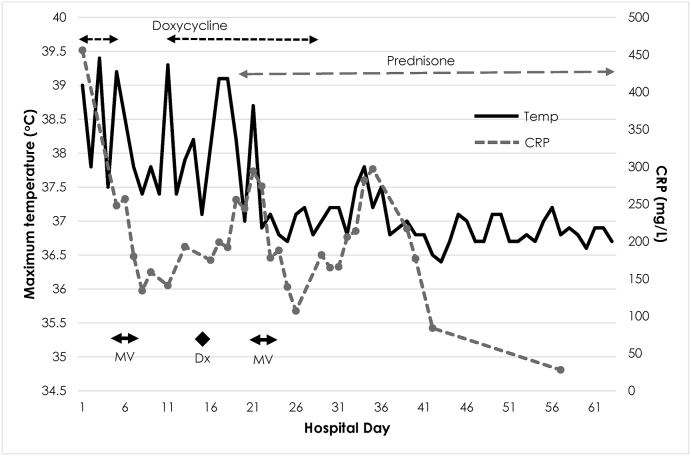
On presentation the patient was febrile at 39.0 °C (Fig. 1) with an oxygen saturation of 94 % on room air, but otherwise normotensive and neither tachypnoeic nor tachycardic. He had decreased breath sounds at the left base, associated with dullness to percussion. Chest x-ray (CXR) confirmed consolidation in the left lower zone with a moderate left-sided pleural effusion (Fig. 2). C-Reactive Protein (CRP) was markedly elevated at 456mg/dL, with a white cell count of 12.4 × 109/L (differential 11.4 × 109/L). Liver enzymes were also elevated with GGT 521 U/L, ALP 596 U/L, ALT 162 U/L and AST 82 U/L. Testing for SARS-CoV-2 was negative.
Fig. 1.
CRP and temperature charting and relationship to doxycycline and prednisone prescribing. MV, mechanical ventilation; Dx, diagnosis of psittacosis made via serology and PCR testing.
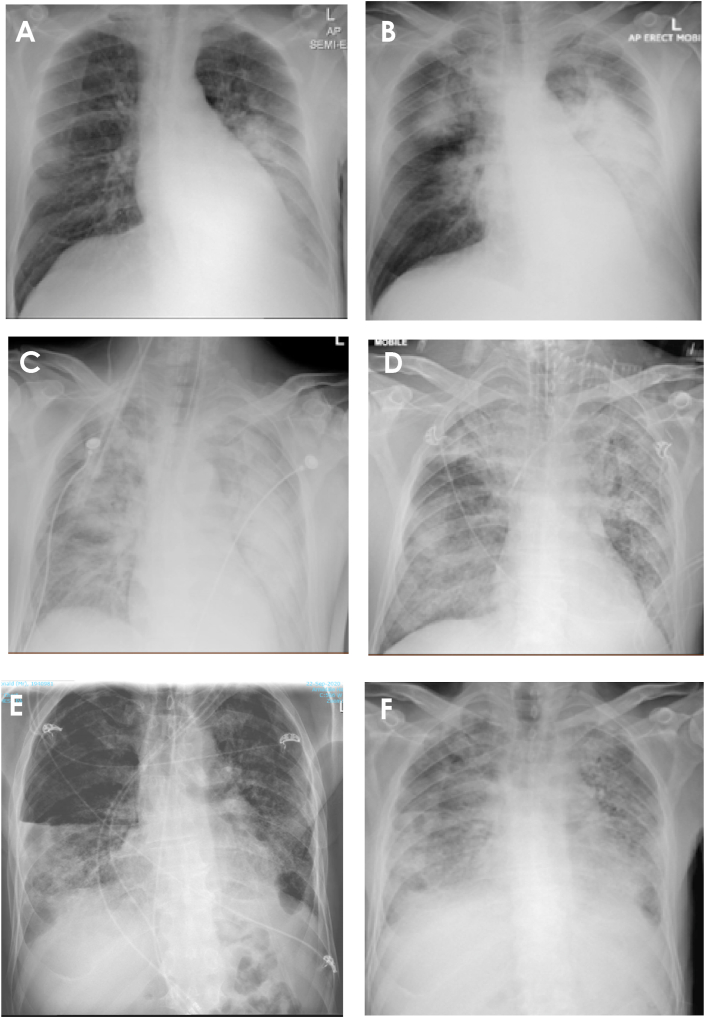
Fig. 2.
Serial chest x-rays; A – hospital day 1: consolidation in the left lower zone with a moderate left-sided pleural effusion, B – hospital day 5: near complete consolidation of the left lung with pleural effusion, new consolidation in the right upper lobe, C – hospital day 5 (after first intubation): rapidly worsening and severe bilateral consolidation, D – hospital day 21 (re-intubation): diffuse bilateral airspace opacification, E − 1 month follow up: diffuse bilateral reticulations, right sided hydropneumothorax, corticosteroids ceased, F – deterioration at 3 months post discharge: recurrence of bilateral airspace opacification and small left pleural effusion.
He was started on intravenous benzylpenicillin and oral doxycycline for community acquired pneumonia, with initial improvement. After five days the patient rapidly deteriorated with hypoxia (SpO2 91 % on 8L O2, RR 30/min), hypotension (BP 107/61 mmHg) and ongoing fever (39.2 °C). Arterial blood gas demonstrated type I respiratory failure with respiratory alkalosis and incomplete compensatory metabolic acidosis (pH 7.55, pCO2 19, pO2 52, bicarbonate 17.8 mmol/L). His CXR had progressed with near complete consolidation of the left lung with pleural effusion and new consolidation in the right upper lobe (Fig. 2); confirmed with computed tomography (CT) (Fig. 3). The patient was placed on high flow nasal oxygen and then intubated for retrieval to a larger rural intensive care unit (ICU). Antibiotic treatment was changed to piperacillin/tazobactam and azithromycin with a loading dose of vancomycin to cover hospital acquired pneumonia. He was extubated after 48 hours of mechanical ventilation, azithromycin was ceased (piperacillin/tazobactam continued for 5 days) and subsequently stepped down to the ward.
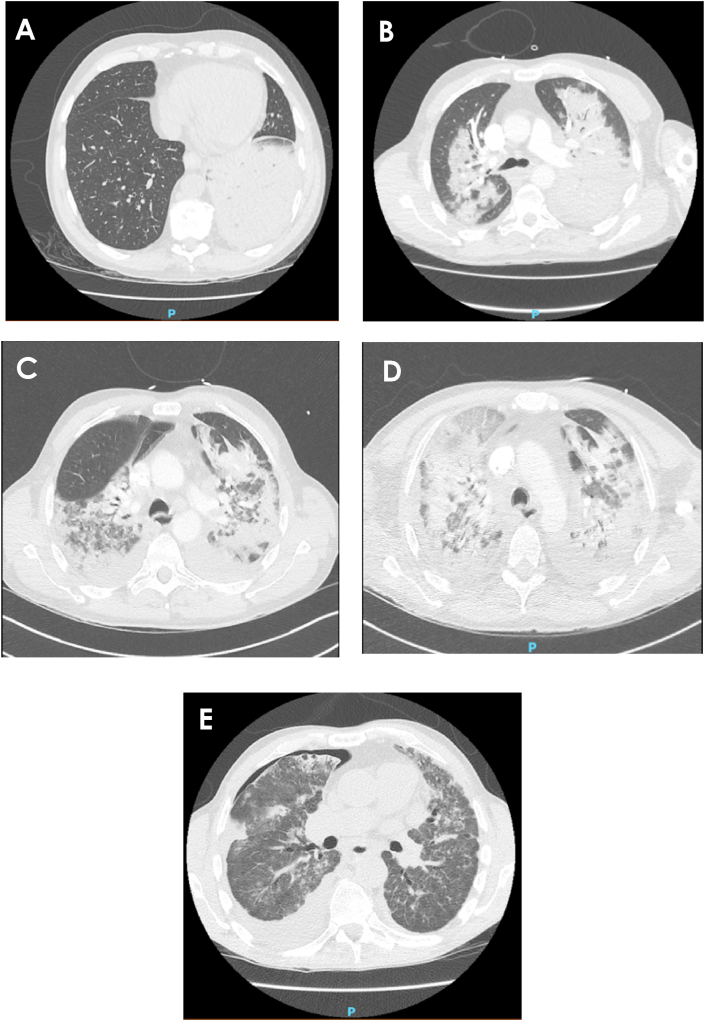
Fig. 3.
Serial chest computed tomography; A – hospital day 2: consolidation left lower lobe with moderate pleural effusion, B – hospital day 5: severe bilateral consolidation with bilateral pleural effusions, C – hospital day 17 and D – hospital day 19: both show worsening airspace consolidation with areas of ground-glass appearance, migratory infiltrates and pleural effusions, E − 1 month follow up: improvement in the extent of consolidation, moderately pronounced bilateral pulmonary infiltrates, new areas of subpleural opacity in both upper lobes, loculated right sided hydropneumothorax likely secondary to a bronchopleural fistula.
Over the following 4 days the patient had persistent fever and increasing oxygen requirement prompting additional investigation for atypical pneumonia aetiologies (Table 1). Once off the ventilator a history of exposure to a sick Eolophus roseicapilla “gallah” days prior to symptom onset triggered testing for C. psittaci and the patient was recommenced on doxycycline (day 11 admission, day 22 illness). Psittacosis was confirmed by serology (titre of 256) and PCR 4 days later. Despite another 6 days of doxycycline therapy the patient continued to deteriorate with extensive multi-lobar consolidation (Fig. 2). Meropenem and vancomycin were added and he was returned to ICU. Given the minimal response to extended antibiotics, a trial of corticosteroid therapy was commenced with prednisone 50mg daily (day 19 admission). However, re-intubation was required 2 days later for another 48 hours, during which a bronchoscopy was performed. Bronchoalveolar lavage recovered a small amount of fluid with neutrophilia and the presence of macrophages. This, in combination with the fluctuating patchy air-space consolidation on imaging and clinical features led to a suspected diagnosis of organizing pneumonia, though lung biopsy was not performed. Prednisone was continued at a weaning dose and an 18-day course of doxycycline was completed (total 23 days including initial 5 days of therapy).
Table 1.
Investigations performed for atypical pneumonia aetiologies.
| Investigation | Result |
|---|---|
| Polymerase chain reaction (PCR) | |
| Chlamydia pneumoniae DNA | Negative |
| Chlamydia psittaci DNA | Detected |
| Legionella longbeachae DNA | Negative |
| Legionella pneumophila DNA | Negative |
| Mycoplasma pneumoniae PCR | Negative |
| Serology | |
| Brucella abortus-V | <20 |
| Leptospirosis IgM-V | <1.0 |
| Q fever IgM index-V | <0.9 |
| Q fever ph2 IgG EIA | <0.9 |
| Mycoplasma IgG ind-V | Non-reactive |
| Mycoplasma IgM ind-V | Non-reactive |
| C.psittaciimmunofluorescence | |
| Initial titre (hospital day 11) | 256 |
| Repeat titre (hospital day 38) | 512 |
The patient remained very fragile with an extremely poor respiratory reserve, critical illness myopathy and deconditioning with 20-kg weight loss. His admission was further complicated by a positive sputum culture for Pseudomonas aeruginosa treated with a 5-day course of piperacillin/tazobactam; development of a hydropneumothorax managed conservatively; and a right arm deep venous thrombosis secondary to a long line managed with anticoagulation. His recovery was slow and the potential need for lung transplant was discussed, however, it was decided to reconsider when the patient's general condition improved. After a total of 63 days, including 44 days in ICU, the patient was discharged home with a supply of home oxygen and continued on a weaning dose of prednisone.
At follow up one month later, the patient was still desaturating on exertion and though repeat CT demonstrated improvement in the extent of consolidation, there were some new subpleural infiltrates, an area of associated traction bronchiectasis and a persistent right sided hydropneumothorax, likely secondary to a bronchopleural fistula (Fig. 3). Prednisone was ceased after 11 weeks. Unfortunately, the patient was re-admitted to hospital a month later for worsening dyspnoea. Despite initial discussion of potential lung transplantation, the patient made the decision for palliative measures. He died peacefully several weeks later.
2. Discussion
Psittacosis is caused by the obligate intracellular bacterium Chlamydia psittaci. The condition has been documented globally and is estimated to cause ~1 % of cases of community acquired pneumonia [1]. Exposure to birds, the main zoonotic reservoir, is a major risk factor with transmission occurring by inhalation of aerosolised bacteria from infected secretions, droppings or feathers [2].
The spectrum of disease is highly variable, ranging from subclinical infection in many, to rare severe cases associated with multi-organ failure. After an incubation period of usually 5–14 days, patients typically present with systemic symptoms including fevers, rigors, sweats, headache and myalgias, with respiratory symptoms such as cough and dypsnoea often developing late [[3], [4], [5], [6]]. Whilst the above may be seen with other respiratory infections, clues for suspecting psittacosis include a history of contact with birds, headache as an early symptom, which is often severe [4], hepatomegaly or splenomegaly on examination (which may occur in 10 % of patients [4]) and a lack of response to beta-lactam antibiotics. Severe cases can result in hypoxic respiratory failure requiring mechanical ventilation, even in patients who are previously healthy, and may also be associated with neurologic, renal, gastrointestinal and haematological complications [3,7]. In such cases, mortality can be as high as 66–70 % [4,7].
Common laboratory findings include left shift of neutrophils often with normal white cells, elevated ESR and CRP [4], and deranged liver enzymes [3]. Chest x-ray (CXR) is abnormal in 80 % of patients, usually with consolidation affecting a single, often lower, lobe [4,8], and less frequently, multilobar changes [8]. Diagnosis is usually by serology testing, with a fourfold rise between acute and convalescent sera at least 2 weeks apart considered significant [9]. PCR and monoclonal antibody techniques have also been developed [10]. Culture is not recommended due to the highly infectious nature of C. psittaci [10].
Tetracycline therapy is recognised as first line treatment for psittacosis [11], with a duration of generally 7–14 days [3,11]. Doxycycline (100mg BD) often produces a rapid response, with patients usually becoming afebrile and defervescing by 48 hours [4]. Even for severe cases, improvement is usually seen once tetracycline therapy is commenced. A recent case review of nine patients in China with severe psittacosis pneumonia found all patients’ fevers generally subsided within three days after commencing minocycline therapy, and their respiratory function gradually improved [12]. This is in contrast to our case, where the patient continued to deteriorate despite doxycycline treatment and progressed to suspected organizing pneumonia.
We believe the commencement of corticosteroid therapy was significant in improving our patient's clinical recovery. There is limited data on the role of corticosteroids in severe psittacosis. Compared to other Chlamydialies species, C. psittaci is more pathogenic and causes a more severe inflammatory reaction [13]. Given the role of inflammation, it is reasonable corticosteroid therapy may offer some benefit. Price & Harrison [14] described a case of severe psittacosis pneumonia complicated by severe restrictive lung function, which subsequently resolved with a weaning course of prednisone (Table 2). Two Japanese case reports documented the successful treatment of fulminant psittacosis with simultaneous administration of minocycline and corticosteroids [15,16]. Interestingly, another Japanese report of severe psittacosis found despite starting corticosteroids early for suspicion of cryptogenic organizing pneumonia (COP), significant improvement was not seen until minocycline was commenced [17]; whilst in another case despite antibiotics, corticosteroids were required for clinical improvement [18] (Table 2).
Table 2.
Case reports of severe psittacosis and use of corticosteroids.
| Age/Sex | Risk factors | Clinical features | Diagnosis | Treatment | MV | Year/Ref |
|---|---|---|---|---|---|---|
| 53 F | Eviscerated 4 ducks 10 days before admission | Dyspnea | Serology | Initially ampicillin, cloxacillin, gentamicin | Yes | 1982 [14] |
| Night sweats | 4 weeks after admission | Oxytetracycline started day 26 of admission as not improving | ||||
| Prednisone started day 33 for suspicion of post-infective alveolitis – tapered over 4.5 months | ||||||
| 65 M | No contact history with birds | Shock | Positive sputum PCR and serology day 6 of admission | Initially flomoxef, followed by imipenem/cilastatin | Yes | 2004 [18] |
| Altered consciousness | After diagnosis, IV erythromycin | |||||
| Hypothermia | High-dose methylprednisolone started day 39 of admission for ARDS | |||||
| Multiple organ dysfunction | ||||||
| 40 F | Cared for 2 budgerigars which died 1 week before admission | Severe dyspnea | Serology day 10 of admission | IV minocycline day 1 admission due to high clinical suspicion of psittacosis and corticosteroids for ARDS – weaned after 2 weeks | Yes | 1989 [15] |
| Cough | Confirmed with isolation of C. psittaci from throat swab | |||||
| Fever | ||||||
| 52 F | Parakeet recent died at patient's home | High fever | Serology, diagnosed after bird history known | Initially cefpirom, followed by methylprednisolone and then prednisolone for suspicion of COP | Yes | 2007 [17] |
| Non-productive cough | Minocycline commenced later after bird history obtained | |||||
| General fatigue | ||||||
| 47 F | Hundreds of parrots and budgerigars at home | Fever | Serology day 17 of admission | Commenced on methylprednisolone and minocycline day 2 of admission | Yes | 1988 [16] |
| Non-productive cough |
F: female, M: male, PCR: polymerase chain reaction, ARDS: acute respiratory distress syndrome, COP: cryptogenic organizing pneumonia, MV: mechanical ventilation.
In our case, the response to prednisone was gradual and raises the question whether earlier and more aggressive treatment may have been beneficial. Corticosteroid use is associated with reduced mortality for critically ill patients with COVID-19 [19], though the optimal dose and duration is not known, with a recent meta-analysis finding similar benefits with both dexamethasone and hydrocortisone, and no evidence suggesting higher doses are associated with greater benefit compared with lower doses [19]. For patients with persistent COP, oral corticosteroids usually produce a rapid improvement, with 12 studies (total of 160 patients) showing a complete response to prednisone in 60 % and a partial response in 27 % [20]. Additionally, intravenous therapy with methylprednisolone may be appropriate in cases of rapidly progressive and extensive COP [20], though this is recommended after infection is excluded. Whether the use IV therapy or the commencement of corticosteroids earlier in admission may have avoided further deterioration in our case is not clear. Prednisone was continued for 11 weeks and at follow up the patient was found to have ongoing lung infiltrates, though with marked improvement to previous. Overall, given the severity of our patient's disease, we believe the use of prednisone was significant in his initial discharge from hospital, giving him time with family prior to his re-admission and unfortunate death.
3. Conclusion
In conclusion, severe pneumonia is a rare complication of psittacosis, and in cases with limited response to tetracyclines, it may be useful to consider the use of corticosteroids early in therapy. This is an area which requires further research to determine the best therapy for severe psittacosis to optimise patient outcomes.
4. Consent to publish
Written informed consent was obtained from the patient's partner for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Co-Editors-in-Chief of this journal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no competing interests, financial or otherwise.
References
- 1.Hogerwerf L., De Gier B., Baan B. Van Der Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired Pneumonia: a systematic review and meta-analysis. Epidemiol. Infect. 2017;145(15):3096–3105. doi: 10.1017/S0950268817002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mair-Jenkins J., Lamming T., Dziadosz A., Flecknoe D., Stubington T., Mentasti M. A psittacosis outbreak among English office workers with little or no contact with birds, PLoS Curr. August 2015;2018(10):646. doi: 10.1371/currents.outbreaks.b646c3bb2b4f0e3397183f31823bbca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewardson A.J., Grayson M.L. Psittacosis. Infect Dis Clin North Am. 2010;24(1):7–25. doi: 10.1016/j.idc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Yung A.P., Grayson M.L. Psittacosis - a review of 135 cases. Med. J. Aust. 1988;148(5):228–233. doi: 10.5694/j.1326-5377.1988.tb99430.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmahmann J. Psittacosis centenary – “pneumotyphus” reviewed. S. Afr. Med. J. 1982;62(24):898–901. [PubMed] [Google Scholar]
- 6.Moroney J., Guevara R., Iverson C., Chen F., Skelton S., Messmer T. Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clin. Infect. Dis. 1998;26(6):1425–1429. doi: 10.1086/516368. [DOI] [PubMed] [Google Scholar]
- 7.Verweij P., Meis J., Eijk R., Melchers W., Galama J. Severe human psittacosis requiring artificial ventilation: case report and review. Clin. Infect. Dis. 1995;20(2):440–442. doi: 10.1093/clinids/20.2.440. [DOI] [PubMed] [Google Scholar]
- 8.Sahn S. Pleural effusions in the atypical pneumonias. Semin. Respir. Infect. 1988;3(4):322–334. [PubMed] [Google Scholar]
- 9.Smith K., Bradley K., Stobierski M., Tengelsen L. Committee NA of SPHVPC. Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds. J Am Vet Med Assoc. 2005. 2005;226(4):532–539. doi: 10.2460/javma.2005.226.532. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen A., Dijkstra F., Notermans D., Van Der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect. Dis. 2018;18(1):442. doi: 10.1186/s12879-018-3317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeckman D., Vanrompay D. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 2009;15(1):11–17. doi: 10.1111/j.1469-0691.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Cao K., Wei Y., Qian Y., Liang J., Dong D. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi: 10.1007/s15010-020-01429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knittler M., Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73(1):1–15. doi: 10.1093/femspd/ftu007. [DOI] [PubMed] [Google Scholar]
- 14.Price M.E., Harrison B.D.W. Restrictive pattern of lung function following psittacosis treated with corticosteroids. Br. J. Dis. Chest. 1982;76(C):199–201. [PubMed] [Google Scholar]
- 15.Chonabayashi N., Nakatani T., Otani M., Noguchi M., Yoshimura Y., Nakamori Y. [Successful treatment of a patient with fulminant psittacosis] Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27(3):357–366. [PubMed] [Google Scholar]
- 16.Hirata M., Noto M., Oda K., Tofuku Y., Takeda R., Kitagawa S. [A case of psittacosis presenting as adult respiratory distress syndrome and successfully treated with steroid pulse therapy] Kokyu Junkan. 1988;36(8):898. 897. [PubMed] [Google Scholar]
- 17.Okubo T., Miyazaki E., Ueo M., Okubo F., Ando M., Fukami T. [A case of psittacosis with wandering infiltrates developing to acute respiratory distress syndrome] Nihon Kyobu Shikkan Gakkai Zasshi. 2007;45(5):419–423. [PubMed] [Google Scholar]
- 18.Toyokawa M., Kishimoto T., Cai Y., Ogawa M., Shiga S., Nishi I. Severe Chlamydophila psittaci pneumonia rapidly diagnosed by detection of antigen in sputum with an immunochromatography assay. J. Infect. Chemother. 2004;10(4):245–249. doi: 10.1007/s10156-004-0324-4. [DOI] [PubMed] [Google Scholar]
- 19.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne J., Murphy S., Diaz J. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA, J. Am. Med. Assoc. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley B., Branley H., Egan J., Greaves M., Hansell D., Harrison N. Interstitial lung disease guideline: the British thoracic society in collaboration with the thoracic society of Australia and New Zealand and the Irish thoracic society. Thorax. 2008;63(11):1029. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]