Abstract
Rationale & Objective
Coronavirus disease 2019 (COVID-19) disproportionately affects people with chronic diseases such as chronic kidney disease (CKD). We assessed the incidence and outcomes of COVID-19 in people with CKD.
Study Design
Systematic review and meta-analysis by searching MEDLINE, EMBASE, and PubMed through February 2021.
Setting & Study Populations
People with CKD with or without COVID-19.
Selection Criteria for Studies
Cohort and case-control studies.
Data Extraction
Incidences of COVID-19, death, respiratory failure, dyspnea, recovery, intensive care admission, hospital admission, need for supplemental oxygen, hospital discharge, sepsis, short-term dialysis, acute kidney injury, and fatigue.
Analytical Approach
Random-effects meta-analysis and evidence certainty adjudicated using an adapted version of GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Results
348 studies (382,407 participants with COVID-19 and CKD; 1,139,979 total participants with CKD) were included. Based on low-certainty evidence, the incidence of COVID-19 was higher in people with CKD treated with dialysis (105 per 10,000 person-weeks; 95% CI, 91-120; 95% prediction interval [PrI], 25-235; 59 studies; 468,233 participants) than in those with CKD not requiring kidney replacement therapy (16 per 10,000 person-weeks; 95% CI, 4-33; 95% PrI, 0-92; 5 studies; 70,683 participants) or in kidney or pancreas/kidney transplant recipients (23 per 10,000 person-weeks; 95% CI, 18-30; 95% PrI, 2-67; 29 studies; 120,281 participants). Based on low-certainty evidence, the incidence of death in people with CKD and COVID-19 was 32 per 1,000 person-weeks (95% CI, 30-35; 95% PrI, 4-81; 229 studies; 70,922 participants), which may be higher than in people with CKD without COVID-19 (incidence rate ratio, 10.26; 95% CI, 6.78-15.53; 95% PrI, 2.62-40.15; 4 studies; 18,347 participants).
Limitations
Analyses were generally based on low-certainty evidence. Few studies reported outcomes in people with CKD without COVID-19 to calculate the excess risk attributable to COVID-19, and potential confounders were not adjusted for in most studies.
Conclusions
The incidence of COVID-19 may be higher in people receiving maintenance dialysis than in those with CKD not requiring kidney replacement therapy or those who are kidney or pancreas/kidney transplant recipients. People with CKD and COVID-19 may have a higher incidence of death than people with CKD without COVID-19.
Index Words: Coronavirus disease 2019 (COVID-19), chronic kidney disease (CKD), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), meta-analysis, systematic review, cohort studies, incidence, prognosis, mortality, respiratory failure, end-stage kidney disease (ESKD), dialysis patients
Plain-Language Summary.
Previous studies suggest that people with chronic kidney disease (CKD) may be severely affected by coronavirus disease 2019 (COVID-19). We searched for observational studies that investigated how many people with CKD were diagnosed with COVID-19 and experienced related health outcomes, including death, respiratory failure, and need for dialysis. Data were pooled from 348 studies that included a total of more than 1 million people with CKD. COVID-19 occurred more commonly in people who required long-term dialysis than in those with CKD not requiring dialysis (including kidney transplant recipients). People with CKD and COVID-19 may have a 10-fold higher risk of death than people with CKD without COVID-19.
Editorial, p. 777
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 as the cause of coronavirus disease 2019 (COVID-19) as defined by the World Health Organization (WHO). By May 2021, more than 151 million people were confirmed to have been infected with SARS-CoV-2, with more than 3.1 million deaths reported as a result of COVID-19 globally.1 Initial evidence suggested a higher incidence of severe COVID-19 in people with chronic diseases, including chronic kidney disease (CKD),2 and an association with acute kidney injury (AKI) due to SARS-CoV-2 infection of the tubular epithelium and podocytes,3 cytokine production, cardiac dysfunction, and hypoxemic tubular injury.4 Initial evidence suggested a poor prognosis of COVID-19 in kidney transplant recipients,5 with a 25% mortality rate, and further evidence highlighting CKD as a risk factor for severe COVID-19 has since been published.6
A systematic analysis of the incidence and prognosis of COVID-19 is necessary to understand the extent and severity of clinical outcomes associated with COVID-19 in people with CKD. This can inform patient and clinician knowledge, treatment and vaccination strategies, resource management, stratification according to risk of clinical outcomes in clinical guidelines, public health policy-making, and intervention trials. In this systematic review, we evaluated the incidence and outcomes of COVID-19 in adults and children with CKD, including those treated with kidney replacement therapy (KRT).
Methods
Data Sources and Searches
We searched MEDLINE, EMBASE, and PubMed (using LitCovid; https://www.ncbi.nlm.nih.gov/research/coronavirus/) between November 1, 2019, and February 22, 2021, using a highly sensitive search strategy designed by an information specialist (Item S1). We have reported this review in accordance with the PRISMA statement.7
Study Selection
We included retrospective and prospective cohort studies or case-control studies investigating the incidence of COVID-19 or outcomes in adults and children with any level of CKD (including CKD treated by dialysis [CKD G5D] and kidney or pancreas/kidney transplant recipients [KTRs]) with or without COVID-19. We defined CKD using the KDIGO CKD guideline8 and defined COVID-19 using the WHO criteria9 based on a positive reverse transcriptase–polymerase chain reaction assay for SARS-CoV-2.10
We included “gray literature,” studies in any language, and studies including people with or without CKD, from which we extracted data on people with CKD. We excluded case series, case reports, randomized and quasirandomized trials, participants without preexisting CKD, and diagnoses of COVID-19 based on serologic evaluation. We excluded studies that included only deceased patients, patients admitted into the intensive care unit (ICU), or hospitalized patients in the analyses of the incidences of death, ICU admission, and hospital admission, respectively.
Data Extraction
Using Cochrane COVID Rapid Review methods,11 11 review authors (EYMC, PN, AK, TEC, VMS, MR, EA, SJ, AL, DJJD, and JCC) screened the citations retrieved in the literature search, and 1 reviewer (EYMC) checked the study selection. Any differences in study selection were resolved by consensus by author VMS (GFMS was also an adjudicator but was not needed). For companion publications, we focused on the analysis of the publication describing the longest follow-up from baseline and extracted outcomes from the publications with shorter follow-up intervals that were not reported in the publication with the longest follow-up.
Data extraction was performed by 11 review authors (EYMC, PN, AK, TC, VMS, MR, EA, SJ, AL, DJJD, and JCC) and checked by 1 reviewer (EYMC). Outcomes of interest were the incidence of COVID-19, COVID-19–attributable outcomes drawn from the global COVID-19 Core Outcomes Set (COS) initiative (death, respiratory failure defined as mechanical ventilation or acute respiratory distress syndrome), multiorgan failure, dyspnea, recovery from COVID-19 (as defined by study authors), ICU admission, hospital admission, hospital discharge, sepsis (defined as septicemia or bacteremia), financial impact, depression, lung function, physical function, and viral load/clearance,12 and kidney-specific outcomes drawn from the Standardised Outcomes in Nephrology CKD (SONG-CKD) initiative (kidney failure, acute dialysis, AKI, vascular access thrombosis, myocardial infarction, stroke, limb amputation, fatigue, and life participation).13
Risk of Bias Assessment
Eleven review authors (EYMC, PN, AK, TEC, VMS, MR, EA, SJ, AL, DJJD, and JCC) assessed the risk of bias of the included studies, which was checked by 2 authors (EYMC, VMS). We resolved disagreements through discussion with another author (EMH, GFMS). We used the Quality In Prognosis Studies (QUIPS) tool for risk of bias assessment (Item S2).14 , 15
Data Synthesis
We pooled the incidence of COVID-19 and outcomes in people with CKD using a random-effects model with the Freeman-Tukey double arcsine transformation for variance stabilization.16 Meta-analysis of single proportions was performed using metaprop in the R meta package.17 We used the Wilson approach for calculation of confidence intervals (CIs)18 and reported the range of the effects using prediction intervals (PrIs) for analyses that included at least 3 studies to improve clinical interpretation of the range of COVID-19 incidence and related outcomes in people with CKD because individual included studies may have large sample sizes that result in narrow CIs.19, 20, 21, 22 When data were available in people with and without COVID-19, we pooled incidence rates as an incidence rate ratio using a random-effects model to evaluate the prognostic association between COVID-19 and outcomes in people with CKD. For meta-analysis of incidence rates, we used the metainc function in the R meta package.17 We adjudicated evidence certainty using an adapted GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework for prognostic factor research, which involves evaluating 6 factors that can decrease evidence certainty (phase of investigation, study limitations, inconsistency, indirectness, imprecision, publication bias) and 2 factors that can increase it (moderate to large effect size, exposure-response gradient).23
Sensitivity Analysis
We conducted sensitivity analyses based on study sample size (≥1,000 vs ≤1,000 participants), studies with high risk of bias in any methodological domain, and studies reporting the incidences of both COVID-19 and death. When data were available, we performed subgroup analysis based on age, CKD stage, COVID-19 severity (defined by the study investigators), case definitions of COVID-19 (suspected, probable, and confirmed), WHO region (Africa, Americas, South-East Asia, European, Eastern Mediterranean, or Western Pacific), World Bank income group (low, low-middle, upper-middle, or high income), study location (hospital or community), diabetes, and obesity. Subgroup analysis was performed by testing the significance of the between-study variance with a χ2 Q-test using the R packages meta and metainc.17 Studies reporting the incidence of COVID-19 or outcomes across CKD categories (CKD without KRT, CKD G5D, and/or KTRs) but not in each subgroup were included in overall analyses but excluded from subgroup analyses.
Results
Study Selection and Population Characteristics
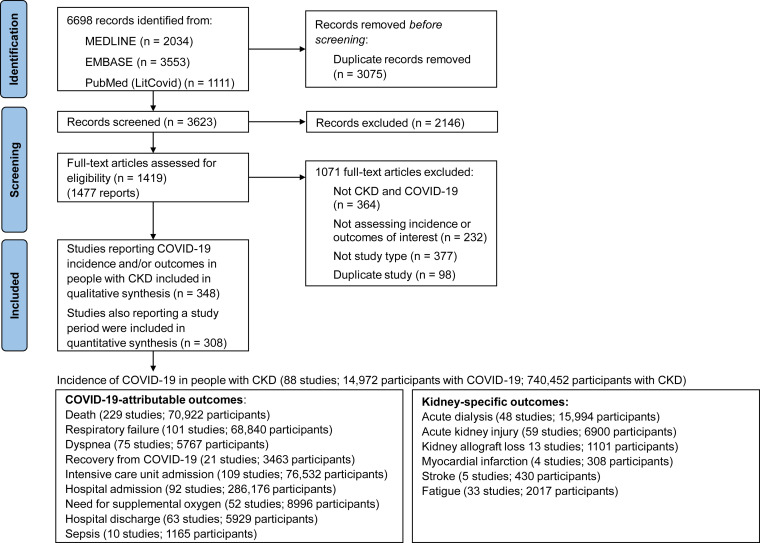
We identified 6,698 citations and included 348 studies (382,407 participants with CKD and COVID-19, 1,139,979 total participants with CKD; Fig 1 ), including 336 cohort studies (45 prospective, 245 retrospective, and 46 not reported as retrospective or prospective) and 12 case-control studies (references in Item S3). The case-control studies included all eligible participants with CKD without KRT, participants with CKD G5D, or KTRs during the study period, but CKD was not reported in the controls, and therefore only case participants with CKD were included. Gray literature included 29 conference abstracts, 2 government reports, and 4 preprints. The incidence of COVID-19 in people with CKD was reported in 110 studies (366,931 participants with CKD and COVID-19; 772,389 total participants with CKD), outcomes were reported in 330 studies (381,422 participants with COVID-19), and 309 studies (373,141 participants with CKD and COVID-19; 1,114,991 participants with CKD) reported on study duration for calculation of incidence rates. Outcomes were reported in 179 studies including hospitalized people (76,800 participants), 143 studies including people hospitalized or treated in the community (299,536 participants), and 22 studies including people treated in the community (6,009 participants). Four studies reported the incidence of death in people with CKD without COVID-19. Otherwise, none of the included studies reported the incidences of outcomes in people with CKD without COVID-19.
Figure 1.
Study identification and selection.
Participant age ranged from 11 to 79 years, and 5 studies included children with CKD (165 participants; Table 1 ; Table S1). Baseline estimated glomerular filtration rate was reported in 18 studies of KTRs (24-60 mL/min/1.73 m2) and 2 studies of participants with CKD without KRT (44-74 mL/min/1.73 m2). Study duration ranged from 7 to 274 days in 308 studies. Most studies were performed in Europe, the Americas, and the Western Pacific region and in high- to upper-middle–income countries (Item S4).
Table 1.
Characteristics of the Included Studies
| Variable | Value |
|---|---|
| No. of studies | 348 |
| No. of participants | |
| With COVID-19 | 382,407 |
| With CKD | 1,139,979 |
| Mean age | 11-79 y in 162 studies (47%) |
| Male sex | 0%-88% in 150 studies (43%) |
| Baseline eGFR | |
| CKD without KRT | 44-74 mL/min/1.73 m2 in 2 studies (0.6%) |
| KTR | 24-60 mL/min/1.73 m2 in 18 studies (5%) |
| Type of study | |
| Cohort study | 336 (97%) |
| Case-control study | 12 (3%) |
| Study duration | 7-274 d in 308 studies (89%) |
| CKD category included in study | |
| CKD without KRT | 153 (44%) |
| CKD G5D | 163 (47%) |
| KTR | 95 (27%) |
| Incidence of COVID-19 reported | 110 (32%) |
| Outcomes of interest reported | 330 (95%) |
| Inpatient | 178 (51%) |
| Inpatient and outpatient | 140 (40%) |
| Outpatient | 26 (8%) |
| Unclear location of treatment | 4 (1%) |
| WHO region | |
| Americas | 115 (33%) |
| Europe | 139 (40%) |
| Western Pacific | 54 (16%) |
| South East Asia | 8 (2%) |
| Eastern Mediterranean | 22 (6%) |
| Africa | 4 (1%) |
| Multiple regions | 6 (2%) |
| World Bank income group | |
| High income | 244 (70%) |
| Upper middle income | 86 (25%) |
| Lower middle income | 12 (3%) |
| Low income | 1 (0.3%) |
| Multiple income groups | 5 (1%) |
Values for continuous variables given as ranges, with number (%) of studies reporting the variable. Abbreviations: CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; KTR, kidney transplant recipient; WHO, World Health Organization.
Risk of Bias Assessment
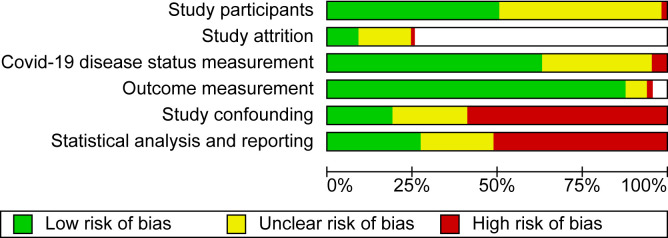
Study participation was adequate in 178 studies (51%), inadequate in 4 studies, and unclear in the remaining studies (Fig 2 ). Risk of bias from study attrition was low in 27 studies (30% of potential prospective studies), high in 5 studies, unclear in the remaining studies, and not applicable in 245 retrospective cohort studies and 12 case-control studies. COVID-19 diagnosis measurement was adequate in 218 studies (63%), inadequate in 15 studies, and unclear in the remaining studies. Outcome measurement was adequate in 310 studies (89%), inadequate in 6 studies, unclear in 13 studies, and not applicable in 19 studies that reported only the incidence of COVID-19. Risk of bias due to study confounding was low in 71 studies (20%), high in 201 studies (58%), and unclear in the remaining studies. Statistical analysis and reporting were adequate in 90 studies (26%), inadequate in 181 studies (52%), and unclear in the remaining studies.
Figure 2.
Risk of bias in the included studies.
Incidence of COVID-19 in People With CKD
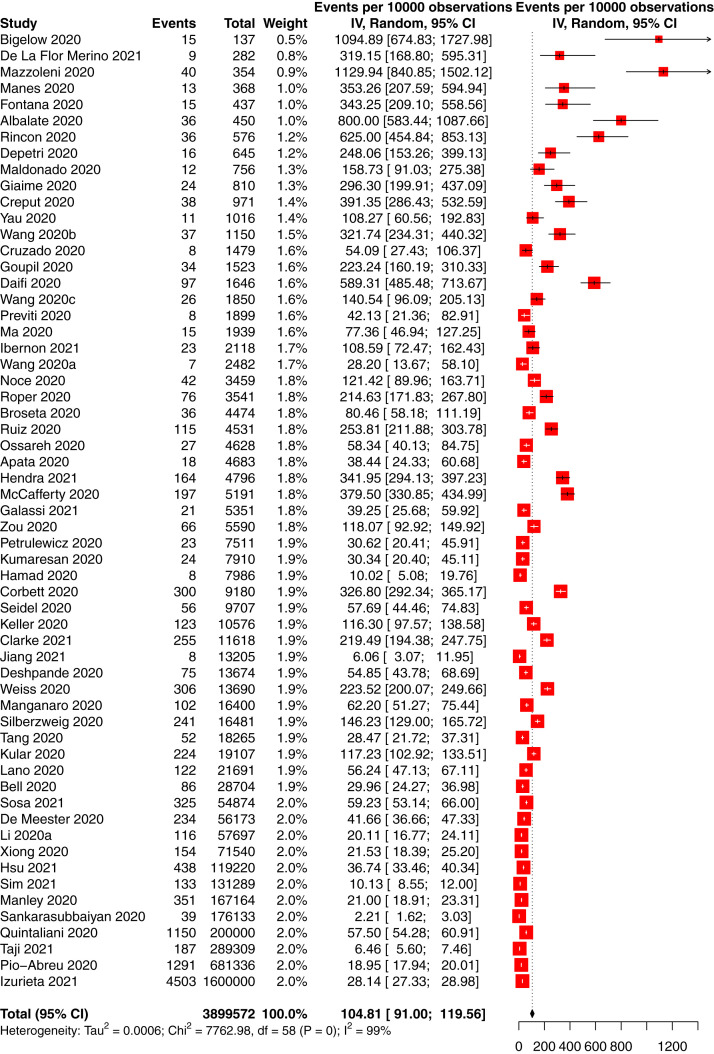
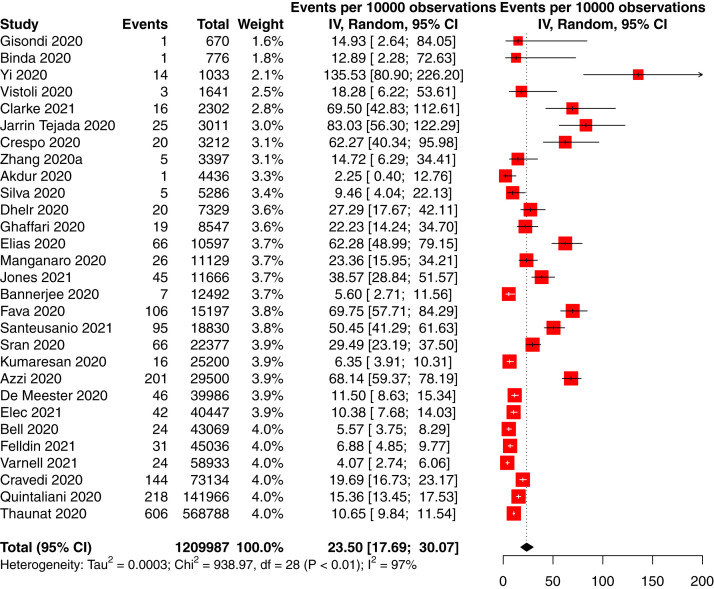
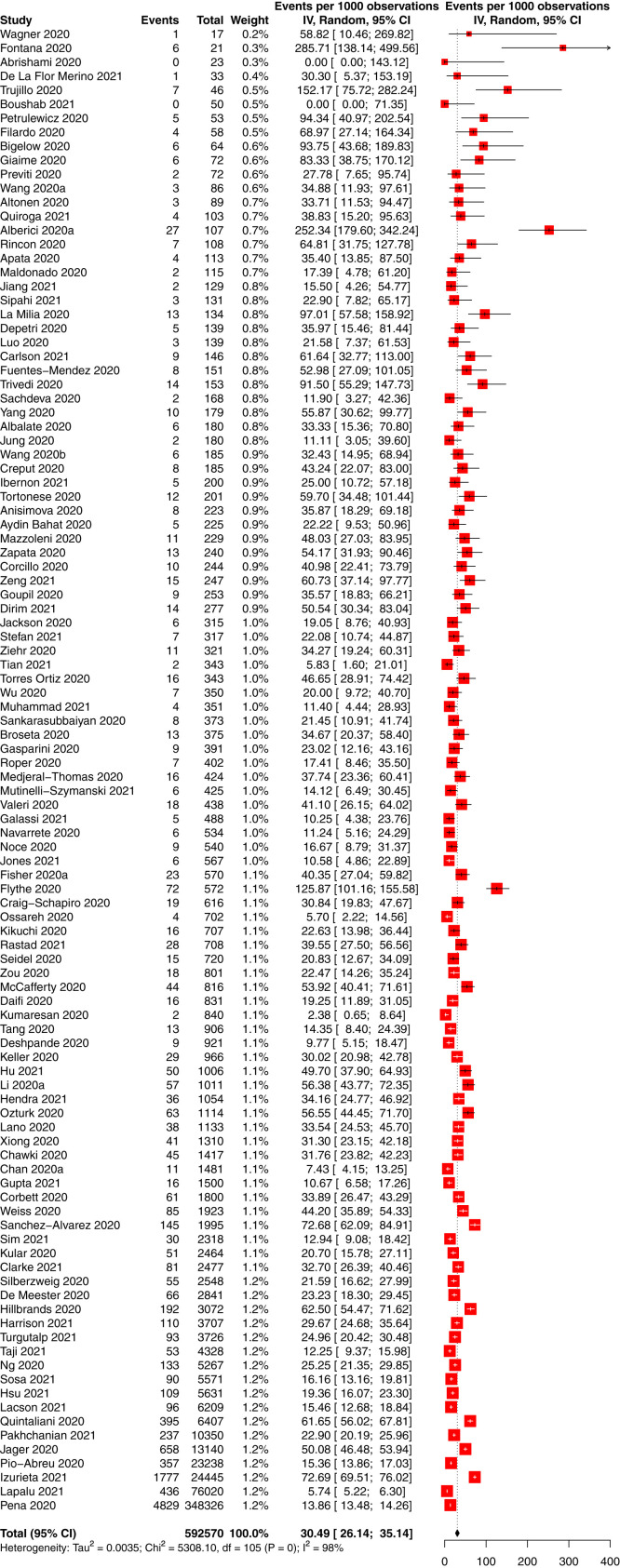
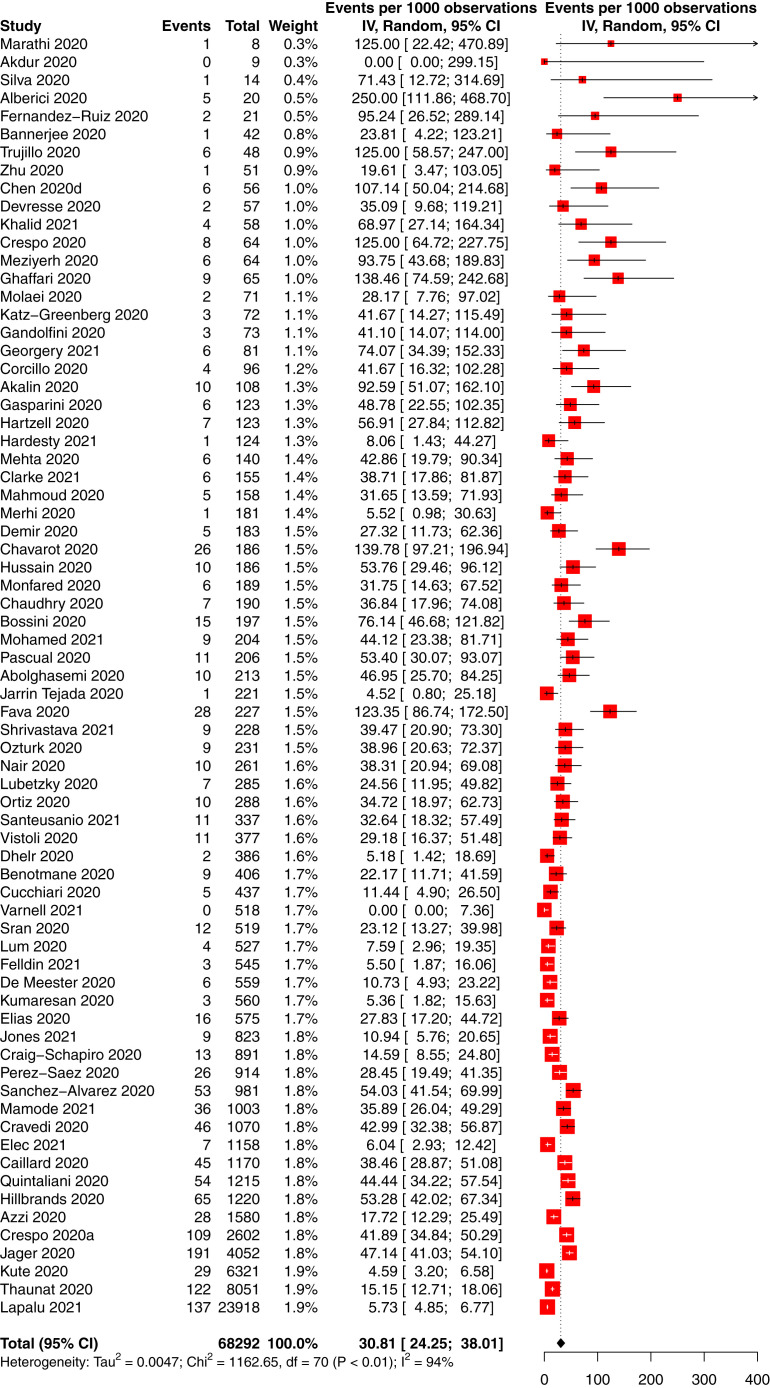
The incidence of COVID-19 in people with CKD was 66 per 10,000 person-weeks (95% CI, 58-75; 95% PrI, 10-169) in 88 studies of 14,972 participants with CKD and COVID-19 and 740,452 total participants with CKD (low-certainty evidence; Table 2 ). The incidence of COVID-19 in people with CKD without KRT was 16 per 10,000 person-weeks (95% CI, 4-33; 95% PrI, 0-92) in 5 studies of 701 participants with CKD and COVID-19 and 70,683 total participants with CKD (low-certainty evidence; Fig S1); the incidence in CKD G5D was 105 per 10,000 person-weeks (95% CI, 91-120; 95% PrI, 25-235) in 59 studies of 12,208 participants with CKD and COVID-19 and 468,233 total participants with CKD (low-certainty evidence; Fig 3 ), and the incidence in KTRs was 23 per 10,000 person-weeks (95% CI, 18-30; 95% PrI, 2-67) in 29 studies of 1,893 participants with CKD and COVID-19 and 120,281 total participants with CKD (low-certainty evidence; P < 0.001 between CKD subgroups; Fig 4 ).
Table 2.
Summary of Findings: The Incidence of COVID-19 and Outcomes in People With CKD
| Incidence of Outcome | Effect |
Incidencea | No. of Studies | Evidence Certaintyb | |
|---|---|---|---|---|---|
| No. of Events | No. of Individuals | ||||
| COVID-19 in people with CKD (per 10,000 person-weeks) | |||||
| CKD | 14,972 | 740,452 | 66 (58-75) [10-169] | 88 | Lowc |
| CKD without KRT | 701 | 70,683 | 16 (4-33) [0-92] | 5 | Lowd |
| CKD G5D | 12,208 | 468,233 | 105 (91-120) [25-235] | 59 | Lowc |
| KTR | 1,893 | 120,281 | 23 (18-30) [2-67] | 29 | Lowc |
| COVID-19–attributable outcomes in people with COVID-19 and CKD (per 1,000 person-weeks) | |||||
| Death | 19,938 | 70,922 | 32 (30-35) [4-81] | 229 | Lowc |
| Respiratory failure | 14,635 | 68,840 | 31 (27-35) [3-81] | 101 | Lowc |
| Dyspnea | 2,587 | 5,767 | 80 (66-95) [2-234] | 75 | Lowc |
| COVID-19 recovery | 1,473 | 3,463 | 83 (52-120) [0-304] | 21 | Very lowe |
| Intensive care admission | 17,590 | 76,532 | 27 (24-30) [4-63] | 109 | Lowc |
| Hospital admission | 120,953 | 286,176 | 93 (82-104) [15-223] | 92 | Lowc |
| Hospital discharge | 3,134 | 5,929 | 106 (90-123) [13-262] | 63 | Lowc |
| Need for oxygen supplementation | 3,014 | 8,996 | 96 (78-116) [4-272] | 52 | Lowc |
| Sepsis | 47 | 1,165 | 3 (0-8) [0-22] | 10 | Lowf |
| Kidneydisease–specificoutcomes in people withCOVID-19and CKD (per 1,000 person-weeks) | |||||
| Short-term dialysis | 1,017 | 15,994 | 17 (11-24) [0-82] | 48 | Lowc |
| Acute kidney injury | 3,418 | 6,900 | 73 (60-87) [5-199] | 59 | Lowc |
| Kidney allograft loss (death-censored) | 47 | 1,101 | 3 (1-6) [0-18] | 13 | Lowf |
| Myocardial infarction | 21 | 308 | 9 (0-31) [0-101] | 4 | Very lowg |
| Stroke | 16 | 430 | 4 (0-9) [0-28] | 5 | Lowf |
| Fatigue | 731 | 2,017 | 57 (41-75) [0-180] | 33 | Lowc |
Abbreviations: CKD, chronic kidney disease; CKD G5D, CKD treated by dialysis; COVID-19, coronavirus disease 2019; KRT, kidney replacement therapy; PrI, prediction interval; KTR, kidney transplant recipient.
Values in parentheses are 95% CIs; values in brackets are 95% prediction intervals.
“High” indicates that further research is very unlikely to change our confidence in the estimate of effect. “Moderate” indicates that further research is likely to have an important effect on our confidence in the estimate of effect and may change the estimate. “Low” indicates that further research is very likely to have an important effect on our confidence in the estimate of effect and is likely to change the estimate.
Evidence certainty downgraded for study methodological limitations and inconsistency with significant heterogeneity between studies.
Evidence certainty downgraded for study methodological limitations and imprecision with excessively wide confidence intervals.
Evidence downgraded for study methodological limitations, inconsistency with significant heterogeneity between studies, and imprecision with excessively wide confidence intervals.
Evidence certainty downgraded for study methodological limitations and imprecision with inadequate study sample size.
Evidence certainty downgraded for serious study methodological limitations and imprecision with inadequate study sample size.
Figure 3.
Forest plot of the incidence of COVID-19 in people with chronic kidney disease treated by dialysis. Total and observations shown in person-weeks.
Figure 4.
Forest plot of the incidence of COVID-19 in kidney and pancreas-kidney transplant recipients. Total and observations shown in person-weeks.
COVID-19–Attributable Outcomes in People With COVID-19 and CKD
Death
The incidence of death may be higher in people with CKD and COVID-19 than in people with CKD without COVID-19 (incidence rate ratio, 10.26; 95% CI, 6.78-15.53; 95% PrI, 2.62-40.15; 4 studies, 18,347 participants; low-certainty evidence; Fig S2). Compared with people without COVID-19, the incidence of death was higher in people with CKD G5D and COVID-19 (incidence rate ratio, 8.10; 95% CI, 6.29-10.42; 95% PrI, 3.38-19.37; 3 studies, 23,239 participants) and in KTRs with COVID-19 (incidence rate ratio, 42.32; 95% CI, 15.38-116.44; 1 study, 3,293 participants; P = 0.002 between CKD subgroups). No studies reported death in people with non-KRT CKD without COVID-19.
The overall incidence of death in people with CKD and COVID-19 was 32 per 1,000 person-weeks (95% CI, 30-35; 95% PrI, 4-81; 229 studies, 70,922 participants; low-certainty evidence). The incidence of death in people with COVID-19 and CKD without KRT was 40 per 1,000 person-weeks (95% CI, 35-45; 95% PrI, 9-88; 75 studies, 28,459 participants; Fig S3), the incidence in CKD G5D was 30 per 1,000 person-weeks (95% CI, 26-35; 95% PrI, 2-85; 107 studies, 34,639 participants; Fig 5 ), and the incidence in KTRs was 31 per 1,000 person-weeks (95% CI, 24-38; 95% PrI, 0-100; 71 studies, 7,287 participants; P = 0.02 between CKD subgroups; Fig 6 ).
Figure 5.
Forest plot of the incidence of death in people with dialysis-treated chronic kidney disease and COVID-19. Total and observations shown in person-weeks.
Figure 6.
Forest plot of the incidence of death in kidney and pancreas-kidney transplant recipients with COVID-19. Total and observations shown in person-weeks.
Respiratory Failure
The overall incidence of respiratory failure in people with CKD and COVID-19 was 31 per 1,000 person-weeks (95% CI, 27-35; 95% PrI, 3-81; 101 studies, 68,840 participants; low-certainty evidence). The incidence of respiratory failure in people with COVID-19 and non-KRT CKD was 28 per 1,000 person-weeks (95% CI, 20-38; 95% PrI, 2-74; 17 studies, 57,077 participants), the incidence in CKD G5D was 30 per 1,000 person-weeks (95% CI, 22-38; 95% PrI, 0-100; 48 studies, 8,134 participants), and the incidence in KTRs was 40 per 1,000 person-weeks (95% CI, 30-52; 95% PrI, 0-136; 48 studies, 3,210 participants; P = 0.1 between CKD subgroups).
Other
The incidences of dyspnea, recovery from COVID-19, ICU admission, hospital admission, hospital discharge, need for supplemental oxygen, and sepsis are reported in Table 2 and Item S5. None of the included studies reported on multiorgan failure, financial impact, depression, lung function, physical function, or viral load/clearance.
Kidney Disease–Specific Outcomes in People With COVID-19 and CKD
The overall incidence of short-term dialysis in people with CKD and COVID-19 was 17 per 1,000 person-weeks (95% CI, 11-24; 95% PrI, 0-82; 48 studies, 15,994 participants; low-certainty evidence). The incidence of short-term dialysis in people with COVID-19 and non-KRT CKD was 19 per 1,000 person-weeks (95% CI, 5-42; 95% PrI, 0-145; 10 studies, 10,723 participants), and the incidence in KTRs was 15 per 1,000 person-weeks (95% CI, 10-21; 95% PrI, 0-57; 39 studies, 5,271 participants; P = 0.9 between CKD subgroups).
The incidences of AKI, death-censored kidney allograft loss, myocardial infarction, stroke, and fatigue are reported in Table 2 and Item S6. A single study reported on vascular access thrombosis, and none reported on kidney failure, life participation, or limb amputation.
Sensitivity and Subgroup Analyses
Sensitivity analysis including only studies that reported incidence of COVID-19 and death revealed a higher incidence of death in patients with CKD G5D and KTRs than in those with CKD without KRT. The incidences of COVID-19, death, and respiratory failure in people with CKD were higher in studies with a low or unclear risk of bias, small sample size, or from the Americas or Europe compared with studies with a high risk of bias, large sample size, or from other WHO regions. Studies from high-income countries reported higher incidences of COVID-19 in people with CKD, respiratory failure, hospital admission, and short-term dialysis compared with upper- and lower-middle–income countries. High- and low-income countries reported a higher incidence of death than upper- and lower-middle–income countries. Children with CKD were reported to have a lower incidence of COVID-19 and associated outcomes than adults with CKD. The was no association between diabetes or obesity and death in people with CKD and COVID-19 (Items S7 and S8).
Discussion
Three hundred forty-eight studies reported the incidence or prognosis of COVID-19 in people with CKD. The certainty of the evidence was generally low as a result of study limitations, inconsistency in the findings between studies, and/or imprecision in the calculated estimates. Study participants were mostly hospitalized adults; from Europe, the United States, or China; and from high- or upper-middle income countries, which may limit the generalizability of our findings. With low-certainty evidence, we found a COVID-19 incidence in people with CKD of 66 per 10,000 person-weeks, which is higher than the global COVID-19 incidence of 5 per 100,000 person-weeks.24 This may be in part due to ascertainment bias because people with CKD are more likely to receive close health care monitoring than the general population. Also with low-certainty evidence, we found a higher incidence of COVID-19 in people with CKD G5D than in people with CKD without KRT or in KTRs, which may be attributable to greater exposure to SARS-CoV-2 from greater use of health facilities in people undergoing maintenance hemodialysis.25 This hypothesis is supported by the single included study that reported a COVID-19 incidence in people receiving home-based dialysis of 6 per 10,000 person-weeks,26 which is similar to the reported incidence in the general populations in Italy and the United States of 2-6 per 10,000 person-weeks.27 , 28 Based on low-certainty evidence, people with CKD and COVID-19 may have a tenfold higher incidence of death than those without COVID-19. The incidence of death from COVID-19 of 16 per 1,000 person-weeks in the general population is lower than our findings in people with CKD,1 which may be attributed to a dysfunctional immune system in CKD.29, 30, 31 We found a higher incidence of COVID-19 and associated death in people with CKD from the Americas and Europe compared with other regions, and in adults compared with children, which is similar to the general population.1 , 32 , 33 Therefore, the heterogeneity observed in most of our analyses could be partially explained by similar variations in the general population based on geographic location and age. However, such significant heterogeneity lowers our confidence in the summary estimates, which need to be interpreted in the context of the 95% PrIs. Data were absent on outcomes other than death in people with CKD without COVID-19 and on COVID-19 severity stratified by CKD subgroup, preventing comprehensive evaluation of COVID-19 as a prognostic factor in people with CKD.
Although multiple systematic reviews have evaluated the prognostic impact of preexisting CKD in people with COVID-19,34, 35, 36 the incidence rates of COVID-19 and associated outcomes in people with CKD have not been comprehensively assessed. A systematic review of people receiving maintenance hemodialysis that included 29 studies reported an incidence of COVID-19 of 7.7%, death in 22.4%, acute respiratory distress syndrome in 18.5%, and ICU admission in 6.6%.37 Another systematic review of KTRs with COVID-19 that included 15 studies reported a mortality rate of 24% and AKI in 50%.38 Two systematic reviews also found CKD to be associated with severe COVID-19.39 , 40 However, time periods were not reported in these reviews, preventing calculation of an incidence rate and comparison with our results. These systematic reviews also included substantially fewer studies than our review, and none adjudicated evidence certainty using GRADE or reported other COVID-19 COS outcomes. A large systematic review found increased risks of death in people with CKD G5D and organ transplant recipients with COVID-19 compared with people with CKD without KRT,41 which is not consistent with our findings. These discrepant findings could be due to a falsely high incidence of death in people with non-KRT CKD in our study because of an inaccurate denominator of all people with non-KRT CKD or reporting of mostly hospitalized people.41 , 42
There are several strengths and limitations to this review. We performed a systematic search designed by an information specialist for studies evaluating COVID-19 in people with any level of CKD. We evaluated COVID-19 incidence and prognosis using the COVID-19 COS and SONG core outcomes.
Limitations of our review included the limited measurement of known confounding factors impacting the incidence and outcomes of COVID-19 in people with CKD, such as old age, male sex, Black or South Asian ethnicity, lower socioeconomic status, obesity, diabetes, malignancy, or respiratory, cardiovascular, liver, neurologic, or autoimmune diseases.41 Second, the lack of reporting of prognostic outcomes in people with CKD but without COVID-19 limited the evaluation of COVID-19 as a prognostic factor in people with CKD. Third, for those not receiving KRT, different stages of CKD were not reported in most studies, preventing investigation of the differing risk of COVID-19 and prognostic outcomes with worsening kidney function. Fourth, more than half of the included studies reported outcomes in only hospitalized people with COVID-19, which may not reflect the risk of prognostic outcomes in the community. Obtaining an accurate denominator for the calculation of the incidence of COVID-19 and prognostic outcomes is especially difficult with people with CKD without KRT, which is often underreported,43 lowering the generalizability of our results for people with CKD without KRT. Furthermore, detection of CKD in our review is limited by the lack of reporting of albuminuria and a high proportion of studies (49%) in which there was a high or unclear risk of inadequate participation by all eligible participants with CKD. Fifth, only a subset of the prespecified outcomes were reported in each study. Although this may be understandable for kidney-specific outcomes, selective reporting of the COVID-19 COS represents a significant risk of selective reporting.44 This was highlighted by our finding of a higher incidence of COVID-19 in people with CKD G5D but no difference in the incidence of death as a result of different studies reporting each outcome. Indeed, sensitivity analysis including only studies that reported the incidences of COVID-19 and death found that CKD G5D was also associated with a higher incidence of death. Sixth, the median study duration varied from 7 to 274 days, which was inadequate in a significant proportion of studies for the detection of patient-level outcomes. Seventh, variability in study definitions of COVID-19 and prognostic outcomes may have affected the accuracy of our results. The method of COVID-19 diagnosis was inadequate or unclear in 37% of included studies, the definition of recovery was not defined in most studies, and there was heterogeneity in the definition of AKI. Most studies were retrospective in nature, which may lead to higher risks of selection bias, misclassification bias, and confounding compared with prospective studies. Last, we reported the incidence rate of COVID-19 in people with CKD, which assumes a constant risk of COVID-19 regardless of the time interval, even though this assumption is unlikely to be true because the incidence of COVID-19 in the general population varies from month to month.1 In particular, our search of results up to February 2021 does not account for the impact of COVID-19 vaccination implementation strategies in many countries or the impact of recent surges of COVID-19 in countries such as India.
Our systematic review found that people with CKD may be at a higher risk of COVID-19 than the general population and may be at a higher risk of death than people with CKD without COVID-19. Decision-making by clinicians and policy makers should focus on preventive measures for people with CKD, particularly people receiving maintenance dialysis. Future studies that measure and adjust for confounders, and that are adequately powered to report the COVID-19 COS and SONG CKD core outcomes in people with CKD with and without COVID-19, are needed to better evaluate the prognostic effect of COVID-19 in people with CKD.
Article Information
Authors’ Full Names and Academic Degrees
Edmund Y.M. Chung, MD, Suetonia C. Palmer, PhD, Patrizia Natale, PhD, Anoushka Krishnan, MSc, Tess E. Cooper, MPH, Valeria M. Saglimbene, PhD, Marinella Ruospo, PhD, Eric Au, MBBS, Sumedh Jayanti, MD, Amy Liang, MD, Danny Jia Jie Deng, BCom, Juanita Chui, MD, Gail Y. Higgins, BA, Allison Tong, PhD, Germaine Wong, PhD, Armando Teixeira-Pinto, PhD, Elisabeth M. Hodson, MBBS, Jonathan C. Craig, PhD, and Giovanni F.M. Strippoli, PhD.
Authors’ Contributions
Drafted study protocol: SCP, GW, GFMS; performed electronic database search: GYH, EYMC; screened the citations retrieved from electronic searches: EYMC, PN, AK, TEC, VMS, MR, EA, SJ, AL, DJJD, JC; consulted on discrepancies during screening and data extraction: EMH, GFMS; performed data extraction: EYMC, PN, AK, TEC, VMS, MR, EA, SJ, AL, DJJD, JC; performed data synthesis: EYMC, SCP, GW, AT-P; data interpretation: JCC; supervision: SCP, AT, GW, GFMS. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This research was investigator-initiated with no funding.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank Alexander Li for translation of non-English papers.
Peer Review
Received June 11, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 29, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1: Forest plot of the incidence of COVID-19 in people with CKD without KRT.
Figure S2: Forest plot of the incidence rate ratio of death in people with CKD and COVID-19 compared with people with CKD without COVID-19.
Figure S3: Forest plot of the incidence of death in people with CKD without KRT and COVID-19.
Item S1: Electronic database search strategies.
Item S2: QUIPS tool for assessing the risk of bias in studies of the prognostic factor.
Item S3: References for the included studies.
Item S4: Studies classified by WHO regions and World Bank income Group.
Item S5: Other COVID-19–attributable outcomes in people with COVID-19 and CKD.
Item S6: Other kidney disease–specific outcomes in people with COVID-19 and CKD.
Item S7: Sensitivity analysis by study sample size, risk of bias, and studies reporting the incidences of COVID-19 and death.
Item S8: Subgroup analysis by WHO region, World Bank income Group, age, study location, diabetes. and obesity.
Table S1: Characteristics of each included study
Supplementary Material
Figures S1-S3; Items S1-S8; Table S1.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19) Weekly epidemiological update - 4 May 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---4-may-2021 Accessed July 5, 2021.
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naicker S., Yang C., Hwang S., Liu B., Chen J., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee D., Popoola J., Shah S., Ster I.C., Quan V., Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberici F., Delbarba E., Manenti C., et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization WHO COVID-19: case definitions, 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2
- 10.Cheng M.P., Yansouni C.P., Basta N.E., et al. Serodiagnostics for severe acute respiratory syndrome-related coronavirus 2 : a narrative review. Ann Intern Med. 2020;173(6):450–460. doi: 10.7326/M20-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane Rapid Reviews. COVID Rapid Reviews. https://covidrapidreviews.cochrane.org/resources
- 12.COVID-19-COS Core outcome measures for trials in people with COVID-19: respiratory failure, multiorgan failure, shortness of breath and recovery. Crit Care Med. 2021;49(3):503–516. doi: 10.1097/CCM.0000000000004817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standardised Outcomes in Nephrology The SONG Handbook version 1.0. https://songinitiative.org/reports-and-publications/
- 14.Dretzke J., Ensor J., Bayliss S., et al. Methodological issues and recommendations for systematic reviews of prognostic studies: an example from cardiovascular disease. Syst Rev. 2014;3 doi: 10.1186/2046-4053-3-140. 140-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden J.A., Windt D.A., Cartwright J.L., Cote P., Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 16.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 17.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M. Prediction intervals. https://www.meta-analysis.com/prediction
- 20.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J R Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riley R.D., Elia E.G., Malin G., Hemming K., Price M.P. Multivariate meta-analysis of prognostic factor studies with multiple cut-points and/or methods of measurement. Stat Med. 2015;34:2481–2496. doi: 10.1002/sim.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. B Med J. 2011;342 doi: 10.1136/bmj.d549. d549-d549. [DOI] [PubMed] [Google Scholar]
- 23.Huguet A., Hayden J.A., Stinson J., et al. Judging the quality evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst Rev. 2013;2 doi: 10.1186/2046-4053-2-71. 71-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchie H., Ortiz-Ospina E., Beltekian D., et al. Coronavirus pandemic (COVID-19) https://ourworldindata.org/coronavirus
- 25.Gagliardi I., Patella G., Michael A., Serra R., Provenzano M., Andreucci M. Covid-19 and the kidney: from epidemiology to clinical practice. J Clin Med. 2020;9(8) doi: 10.3390/jcm9082506. 2506-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H.J., Tang H., Xiong F., et al. COVID-19 in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2020;16(1):121–123. doi: 10.2215/CJN.07200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martellucci C.A., Sah R., Rabaan A.A., et al. Changes in the spatial distribution of COVID-19 incidence in Italy using GIS-based maps. Ann Clin Microbiol Antimicrob. 2020;19(1):1–4. doi: 10.1186/s12941-020-00373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Geographic differences in COVID-19 cases, deaths, and incidence — United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:465–471. doi: 10.15585/mmwr.mm6915e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callender L.A., Curran M., Bates S.M., Mairesse M., Weigandt J., Betts C.J. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01991. 1991-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Marco L., Puchades M.J., Romero-Parra M., et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J. 2020;13(3):297–306. doi: 10.1093/ckj/sfaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K.D., Zhao J., Auh S., et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13(10):1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Götzinger F., Santiago-García B., Noguera-Julián A., et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang X., Li S., Yu H., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Fang X., Cai Z., et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash DC) 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C.Y., Shao S.C., Chen Y.T., et al. Incidence and clinical impacts of COVID-19 infection in patients with hemodialysis: systematic review and meta-analysis of 396,062 hemodialysis patients. Healthcare (Basel) 2021;9(1):47. doi: 10.3390/healthcare9010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phanish M., Ster I.C., Ghazanfar A., et al. Systematic review and meta-analysis of Covid-19 and kidney transplant recipients, the South West London Kidney Transplant Network experience. Kidney Int Rep. 2021;6(3):574–585. doi: 10.1016/j.ekir.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menon T., Gandhi S.A.Q., Tariq W., et al. Impact of chronic kidney disease on severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Cureus. 2021;13(4) doi: 10.7759/cureus.14279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh J., Malik P., Patel N., et al. Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin Exp Med. 2021:1–11. doi: 10.1007/s10238-021-00715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gansevoort R.T., Hilbrands L.B. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;26:1–2. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qaseem A., Wilt T.J., Cooke M., Denberg T.D. The paucity of evidence supporting screening for stages 1–3 CKD in asymptomatic patients with or without risk factors. Clin J Am Soc Nephrol. 2014;9(11):1993–1995. doi: 10.2215/CJN.02940314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid C.H. Outcome reporting bias: a pervasive problem in published meta-analyses. Am J Kidney Dis. 2017;69(2):172–174. doi: 10.1053/j.ajkd.2016.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S3; Items S1-S8; Table S1.