Abstract
Adolescent alcohol drinking is widely recognized as a significant public health problem, and evidence is accumulating that sufficient levels of consumption during this critical period of brain development have an enduring impact on neural and behavioral function. Recent studies have indicated that adolescent intermittent ethanol (AIE) exposure alters astrocyte function, astrocyte-neuronal interactions, and related synaptic regulation and activity However, few of those studies have included female animals, and a broader assessment of AIE effects on the proteins mediating astrocyte-mediated glutamate dynamics and synaptic function is needed. We measured synaptic membrane expression of several such proteins in the dorsal and ventral regions of the hippocampal formation (DH, VH) from male and female rats exposed to AIE or adolescent intermittent water. In the DH, AIE caused elevated expression of glutamate transporter 1 (GLT-1) in both males and females, elevated postsynaptic density 95 expression in females only, and diminished NMDA receptor subunit 2A expression in males only. AIE and sex interactively altered ephrin receptor A4 (EphA4) expression in the DH. In the VH, AIE elevated expression of the cystine/glutamate antiporter and the glutamate aspartate transporter 1 (GLAST) in males only. Compared to males, female animals expressed lower levels of GLT-1 in the DH and greater levels of ephrin receptor B6 (EphB6) in the VH, in the absence of AIE effects. These results support the growing literature indicating that adolescent alcohol exposure produces long-lasting effects on astrocyte function and astrocyte-neuronal interactions. The sex and subregion specificity of these effects have mechanistic implications for our understanding of AIE effects generally.
Keywords: adolescence, alcohol, astrocyte, glutamate, hippocampus, RRID:AB_1658870, RRID:AB_2662758, RRID:AB_2864292, RRID:AB_304334, RRID:AB_304857, RRID:AB_444362, RRID:AB_90949, RRID:RGD_10395233, synapse
1 |. INTRODUCTION
Adolescence is a critical period for cognitive, emotional, and social maturation (Choudhury et al., 2006) that is accompanied by the pruning of synapses, the refinement of neural circuitry, and changes in receptor expression and sensitivity (Kilb, 2012). These processes contribute to neurobehavioral maturation that is crucial for successful adult function (Paus, 2005). Because alcohol affects these processes, it is not surprising that repeated adolescent ethanol exposure produces deficits in neural function and behavior that persist into adulthood and possibly throughout the life span (Crews et al., 2019; Spear & Swartzwelder, 2014).
Astrocytes are instrumental for synaptogenesis and the regulation of synaptic function through the secretion of regulatory signaling molecules as well as direct physical contact with dendritic spines (Allen & Eroglu, 2017). In addition, astrocytes participate in the regulation of innate and adaptive immune responses in the brain, including neuroinflammation (Colombo & Farina, 2016). They have an extensive network of filament processes, and each astrocyte ensheaths hundreds of neuronal dendritic processes and many thousands of synapses, including both pre-and postsynaptic elements. One central feature of astrocyte-neuronal interaction is the maintenance of glutamate homeostasis. In the synaptic region, astrocytic clearance of excess glutamate reduces neuronal excitotoxic liability. More broadly, astrocytes participate in the maintenance of extrasynaptic glutamate tone, which influences neuronal membrane excitability. Thus, the physical association of astroglial processes with synaptic markers and dendritic spines is a valuable index of astrocyte modulation of synaptic function and plasticity. Against this backdrop, the recent report that AIE exposure alters astrocyte-neuronal proximity in adulthood (Healey, Kibble, et al., 2020) indicates that astrocytes are a principal target of ethanol during adolescence and that those enduring changes may represent a mechanism underlying brain and behavioral effects of adolescent alcohol drinking. Those findings are particularly notable because AIE has been shown to alter the morphology of dendritic spines in both area CA1 (Risher, Fleming, et al., 2015) and the dentate gyrus (Mulholland et al., 2018) of the hippocampal formation. This suggests possible involvement of the ephrin system in the mediation of AIE effects on astrocytes and dendritic function because the neuronal ephrin receptor A4 (EphA4) and its astrocyte surface-associated ligand (EphA3) are known to regulate the structure of hippocampal dendritic spines (Carmona et al., 2009). Thus, one focus of this project is an assessment of AIE effects on ephrin expression in dorsal and ventral hippocampi in adulthood after AIE.
In the rat, both astrocyte morphology and astrocyte-neuronal proximity vary across juvenile, adolescent, and young adult development in the prefrontal cortex; however, in the hippocampal formation, astrocyte morphology remains unchanged while astrocyte-neuronal proximity drops during the early adolescent period (Testen et al., 2019). Thus, there is an adolescent developmental trajectory of astrocytes and astrocyte-neuronal proximity that may render those processes vulnerable to the effects of ethanol exposure. Indeed, previous studies have shown AIE to alter glia, specifically astrocytes, in adulthood. Adult medial prefrontal cortical glia were reduced in AIE-treated males, but not in females, though neuronal density remained unchanged (Koss et al., 2012). Further, Risher, Sexton, et a I. (2015) showed that AIE resulted in elevated immunohistochemical expression of glial fibrillary acidic protein in the CA1 region of the hippocampal formation, indicating an upregulation of astrocyte reactivity in adulthood. Thus, chronic elevation of astrocyte reactivity in adulthood after AIE may represent a companion mechanism, along with chronic neuroimmune activation (Crews et al., 2019; Swartzwelder et al., 2019). In addition to increasing astrocyte reactivity, AIE was also shown to elevate the levels of astrocyte-released thrombospondins (TSPs; TSP-2, TSP-4) in hippocampal area CA1 in adulthood (Risher, Sexton, et al., 2015). TSPs are known to be both synaptogenic and to regulate synaptic availability and function (see Allen & Eroglu, 2017; Walker et al., 2020), in part through their interaction with their neuronal receptor, the α2δ-l calcium channel subunit (Eroglu et al., 2009), which has also been shown to be upregulated in adult area CA1 after AIE (Risher, Sexton, et al., 2015). These findings suggest that AIE produces its enduring effects on hippocampal function (see Crews et al., 2019, for review), in part, by elevating astrocyte activity and astrocyte-neuronal interaction, perhaps through aberrant synaptogenesis. Importantly, gabapentin (Neurontin), which antagonizes the interaction of TSPs with the α2δ-l subunit receptor (Eroglu et al., 2009), has been shown to reverse both AIE-induced elevations of excitatory synaptic activity (Swartzwelder et al., 2017) and AIE-induced reductions of astrocyte-neuronal proximity (Healey, Kibble, et al., 2020) in the adult hippocampus. This indicates that some of the enduring effects of AIE on the hippocampus are specifically driven by changes in TSP/α2δ-l interaction. In addition, because gabapentin is a commonly used therapeutic agent, this finding suggests a possible therapeutic target for reversing the enduring effects of adolescent alcohol exposure on neurobehavioral function.
Previous studies have shown that AIE alters hippocampal glutamatergic signaling (Risher, Fleming, et al., 2015; Swartzwelder et al., 2016,2017), which is known to be regulated by astrocytes and affected by other drugs of abuse (Kalivas, 2009; Kim et al., 2018). Astrocytes are integral for glutamate homeostasis—the balance between synaptically released glutamate and extrasynaptic glutamatergic tone. Importantly, AIE has been found to increase basal glutamate release in the nucleus accumbens core (Carrara-Nascimento et al., 2011), though this has not been investigated in the hippocampus. Of note, gabapentin has been implicated in astroglial glutamate release via the predominantly astroglial glutamate transporter (GLT-1) (Suto et al., 2014). GLT-1 (EEAT2) and GLAST (EAAT1) are primarily expressed on astrocytes and are members of the high affinity glutamate transporters family; they uptake glutamate at the synapse and prevent excitotoxicity (Pajarillo et al., 2019). GLT-1 is responsible for removing approximately 90% of all vesicularly released glutamate at the synapse and, therefore, is integral for glutamate homeostasis (Bjørnsen et al., 2014; Takahashi et al., 2015). Because gabapentin has been shown to modulate AIE-induced changes in glutamatergic signaling (Swartzwelder et a I., 2017) and astrocyte-neuronal proximity (Healey, Kibble, et al., 2020), GLT-1 seems a likely target of AIE effects. Additionally, the cystine/glutamate antiporter (xCT), responsible for extrasynaptic glutamatergic tone, is altered by multiple drugs of abuse (Kim et al., 2018). The effect of AIE on astroglial high affinity glutamate transporters or glutamatergic signaling machinery in the hippocampus, such as GLT-1, GLAST, and xCT, remains to be investigated despite the importance of this system in hippocampal functions known to be compromised by AIE.
As compelling as these findings are with respect to identifying astroglial-neuronal interactions as a mechanism underlying the enduring effects of AIE on the brain, they remain incomplete in several ways. One limitation is that the work to date has been done in male animals only, thus there is a pivotal need for comparison studies in which sex is assessed as an independent variable. Additionally, the field would benefit from studies to identify AIE effects on endpoints that reflect astrocyte function, such as glutamate transporters and exchangers, and markers of astrocyte-neuronal interactions that influence synaptic regulation and dendritic spine morphology, such as synaptic marker proteins and the ephrin system. Therefore, the present studies were designed to assess sex as a biological variable and markers of astrocytic function and neuronal interactions in adulthood after AIE.
2 |. METHODS
All of the procedures used in this study were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care, the National Research Council’s Guide for Care and Use of Laboratory Animals, US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals and were approved by the Duke University Animal Care and Use Committees.
Sixteen male and sixteen female adolescent Sprague-Dawley rats (Charles River, USA, RRID:RGD_10395233) were double housed with ad libitum access to food and water. Animals were delivered to the laboratory at PND 25 and allowed to acclimate for 5 days during which they were handled twice for at least 5 min. The colony room was kept on a reverse 12:12-hr light:dark cycle at 20–25°C and did not exceed 65% humidity; temperature and humidity were recorded daily. Animal cages were changed twice a week. All animals received an AIE (8 male/8 female) or water (AIW; 8 male/8 female) exposure regimen beginning PND 30 with 10 doses over 16 days using 2 days on, 1 day off, 2 days on, and 2 days off schedule. A dosage of 5.0 g/kg ethanol (35% EtOH v/v in water at 18.12 ml/kg; VWR, Suwanee, GA) or isovolumetric water was administered by intragastric gavage (i.g.). EtOH doses were selected to produce blood EtOH concentrations consistent with our previous studies and with human adolescent binge drinking episodes (199.7 ± 19.9 to 172.8 ± 13.3 mg/dl, measured 60 min after dose 2, 4, 6, 8, and 10, (Healey, Landin, et al., 2020; Risher, Fleming, et al., 2015; Risher, Sexton, et al., 2015; Swartzwelder et al., 2015, 2019). Groups were assigned by cage numbers selected from a random number generator. After dosing, animals aged into adulthood (handled twice a week) and at PND 70 were sacrificed by rapid decapitation and brains harvested for dorsal and ventral hippocampi.
2.1 |. Western blot analysis
2.1.1 |. Tissue preparation
Hippocampi were Dounce homogenized in sucrose-HEPES buffer: 320 mM sucrose, 10 mM HEPES, 1 mM Na Fluoride, 1 mM Na Orthovanadate activated, 1:100 Protease Inhibitors (“Complete” PI tablets, cat#1697489, Roche, Sigma-Aldrich, St. Louis, MO) by hand homogenizing (10–15 strokes) and spun at 23,000× g, 4°C, for 30 min. The resulting pellet was resuspended in 500 μl lysis buffer (50 mM Tris-HCL, 1 mM EGTA, 1 mM EDTA, 1 mM Na Fluoride, Na Orthovanadate activated, protease inhibitors, 0.5% Triton X-100) and rotated for 15 min, 4°C. Then spun at 12,000× g, 4°C for 20 min. Supernatant, triton-soluble membrane fraction (non-synaptic), was collected. The triton-insoluble pellet was gently washed with 200 μl lysis buffer and removed without disrupting pellet. Pellet was resuspended in 300 μl of 2% LDS and sonicated, postsynaptic density 95 (PSD-95) enriched membrane fraction (synaptic).
2.1.2 |. Immunoblot analysis
The protein concentration of homogenized samples was determined using Pierce BCA Protein Assay Kit (cat#23227, ThermoFisher Scientific, Waltham) and read on an EPOCH spectrophotometer (BioTek Instruments, Winoski, VT). The proteins (10 or 20 μg) were resolved by SDS-PAGE (12% Tris-HCL gels, Bio-Rad, Hercules, CA) and transferred to PVDF membranes. Membranes were stained with REVERT Total Protein Stain (LI-COR, Lincoln, NE) and reversed. PVDF membranes were then blocked in Intercept Blocking Buffer (TBS, LI-COR, Lincoln, NE) for 1 hr. All primary antibodies were probed overnight at 4°C (see Table 1 for dilutions) and followed by IRDye secondary antibodies (1:15,000, LI-COR). Blots were imaged using Odyssey Fc (700 and 800 nm, LI-COR). Optical densities of all bands and total protein stain were quantified using Image Studio software (LI-COR) and adjusted to background subtraction using the standard LI-COR Image Studio-defined parameters. Bands of interest (1 lane per independent brain sample) were normalized to REVERT Total Protein Stain using the lane normalization factor (LNF) recommended by LI-COR (Signal/LNF). Signal intensity deviates by channel (700 or 800 nm) and blot conditions, resulting in a range of values.
TABLE 1.
Antibody table
| Antibody | Immunogen | Manufacture | Concentration | |||
|---|---|---|---|---|---|---|
| Host | Clonality | Source | RRID | |||
| Anti-Glutamate Transporter (GLT1) | Synthetic peptide from the carboxy-terminus of rat GLT-1 | Guinea Pig | Polyclonal | Millipore Sigma, Inc. (AB1783) | AB_90949 | 1:5,000 |
| Cystine-Gluramate Antiporter (xCT) | Synthetic peptide made to N-terminal region of human x-CT protein (between residues 1–50) | Rabbit | Polyclonal | Novus Biologicals (NB300–318) | AB_10000581 | 1:5,000 |
| Anti-EAATl (GLAST) | Synthetic peptide, corresponding to 20 residues from the C-terminus of rat EAAT1 | Rabbit | Polyclonal | Abcam Inc. (ab416) | AB_304334 | 1:3,000 |
| Phospho-EphA3 | Synthetic peptide derived from human EPHA3 | Rabbit | Polyclonal | Invitrogren, Inc. (PA5–64785) | AB_2662758 | 1:1,000 |
| Anti-EphA4/Sek | Synthetic peptide corresponding to Human Eph receptor A4/SEK aa 875–904 (C terminal) conjugated at key hole limpet haemocyanin | Mouse | Polyclonal | Abcam Inc. (ab5396) | AB_304857 | 1:4,000 |
| Anti-Eph receptor B6 | Synthetic peptide within Human Eph receptor B6 aa 544–594 conjugated to keyhole limpet haemocyanin | Rabbit | Polyclonal | Abcam Inc. (ab217542) | AB_2864292 | 1:500 |
| Anti-PSD-95 | Synthetic peptide within Human PSD95 (N terminal) | Rabbit | Polyclonal | Abcam Inc. (abl8258) | AB_444362 | 1:1,000 |
| Anti-NMDAR2A (GluN2A) | Recombinant protein from Rat NMDAR2A | Mouse | Polyclonal | Milipore Sigma, Inc (MAB5530) | AB_570634 | 1:1,000 |
| Anti-NMDAR2B(GluN2B) | Synthetic peptide conjugated to KLH derived from within residues 1,450 to the C-terminus of Rat NMDAR2B | Rabbit | Polyclonal | Abcam Inc. (ab65783) | AB_1658870 | 1:1,000 |
2.2 |. Statistical analyses
All analyses were performed using GraphPad Prism 8.2.0. For the effect of AIE exposure, sex, and their interaction on expression (Signal/LNF values), a two-way Analysis of Variance (ANOVA) was used to test for statistical significance, followed by Fisher’s LSD post hoc analysis where appropriate to assess simple main effects. Factors were AIE treatment (AIE/AIW) and sex. Post hoc analyses compared AIE to AIW and male to female. Alpha was set at p ≤ 0.05 for all simple main effects analyses, and at p ≤ 0.10 for interactions due to the low power of interactions at a given sample size relative to main effects (Snedecor & Cochran, 1989). Outliers were assessed by ROUT outlier test (Q = 1%). Ail data are presented in figures as mean + SEM.
3 |. RESULTS
3.1 |. Effect of AIE and sex on dorsal and ventral hippocampal glial glutamate transporter protein membrane expression
Dorsal hippocampal (DH) protein levels of synaptic GLT-1 were significantly increased by AIE in both males and females (Main effect [ME] of AIE F(1, 25) = 5.49, n = 29, p < 0.03; Figure 1b). There was no effect of AIE on VH synaptic GLT-1 expression; however, males exhibited significantly greater levels of GLT-1 compared to females (ME of sex F(1, 27) = 5.67, n = 31, p < 0.03; Figure 1c). These effects on GLT-1 expression were specific to the synaptic membrane fraction of the homogenate, as there was no effect of AIE or sex on GLT-1 expression in the non-synaptic membrane fraction of dorsal or ventral hippocampus (p > 0.05; data not shown).
FIGURE 1.
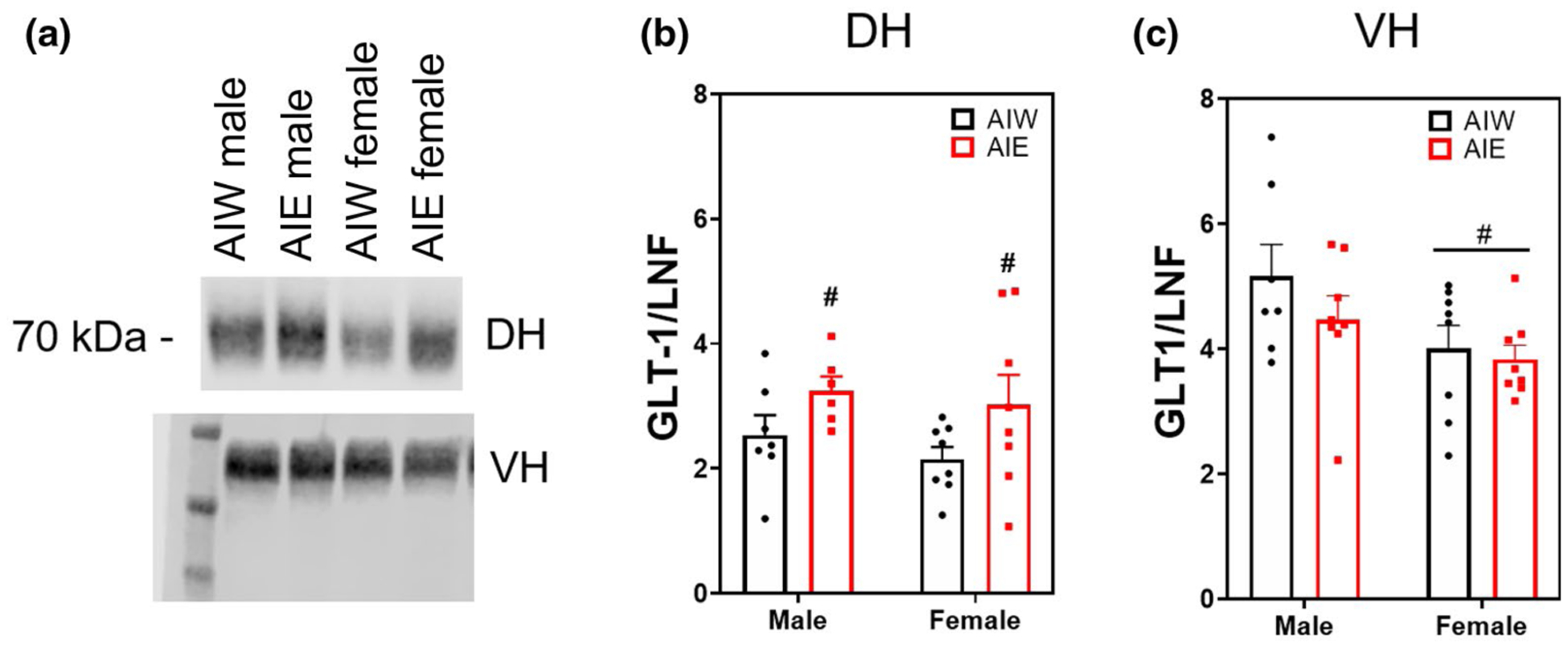
Adolescent intermittent ethanol (AIE) increases dorsal hippocampal (DH) glutamate transporter 1 (GLT-1) synaptic expression (mean + SEM). Western blot analysis of DH and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) representative GLT-1 bands from DH (top) and VH (bottom) samples, (b) Mean (+SEM) DH GLT-1 expression was upregulated after AIE (main effect [ME] of AIE, F(1, 25) = 5.49, n = 29, p < 0.03). (c) VH GLT-1 females exhibited reduced expression compared to males (ME of sex, F(1, 27) = 5.67, n = 31, p < 0.03). #p < 0.05 ME
There was a significant sex by AIE interaction effect on expression of the xCT in the VH (Interaction effect F(1, 26) = 3.60, n = 30, p < 0.07; Figure 2c). Post hoc analysis revealed male AIE-treated animals had significantly greater protein expression of xCT relative to controls (Fishers LSD, p < 0.04). There was no effect of AIE or sex on xCT in the DH (Figure 2b) or in the VH non-synaptic fraction (p > 0.05; data not shown).
FIGURE 2.
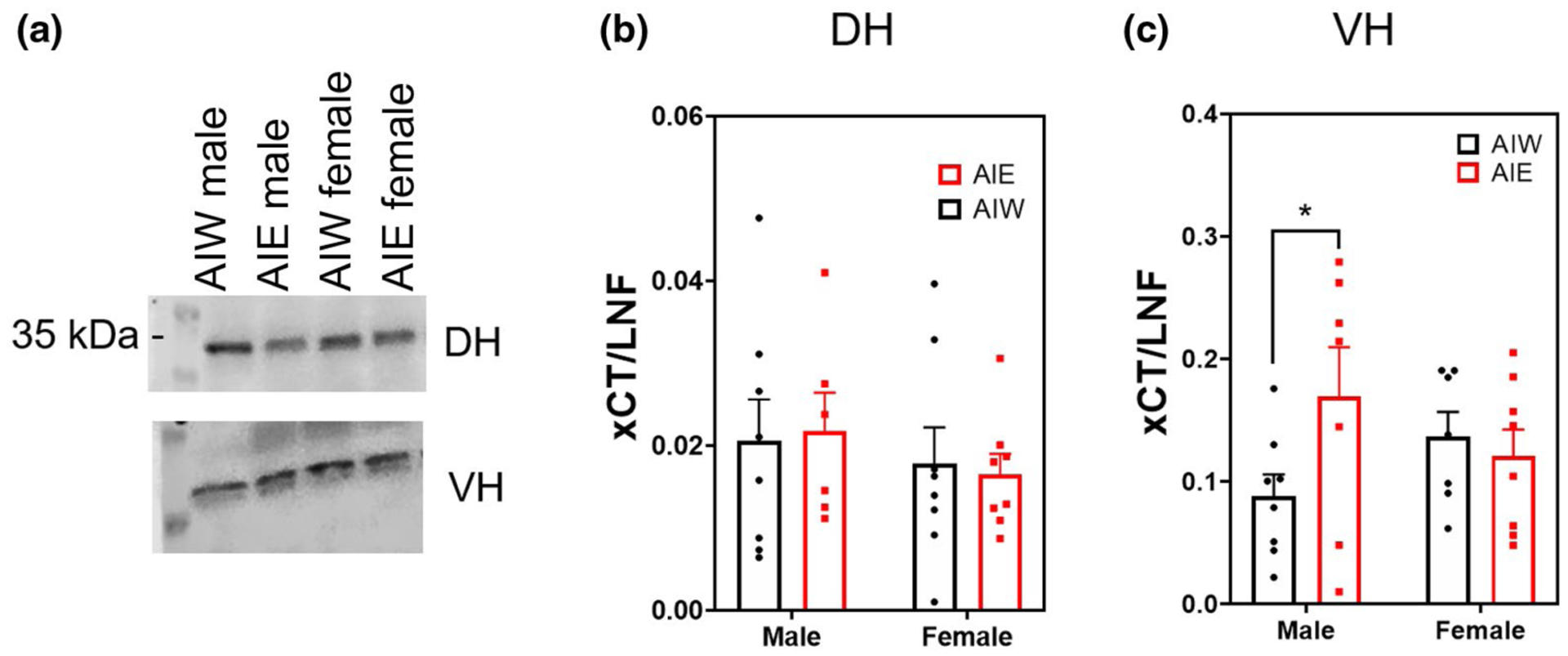
Adolescent intermittent ethanol (AIE) increases ventral hippocampal (VH) cystine-glutamate antiporter (xCT) synaptic expression (mean + SEM) in males but not in females. Western blot analysis of dorsal hippocampal (DH) and VH adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative xCT bands from DH (top) and VH (bottom) samples, (b) No effect of AIE or Sex on DH xCT expression, (c) AIE increased VH xCT expression in males, but not in females (Interaction effect F(1,26) = 3.60, n = 30, p < 0.07; AIW male < AIE male, p < 0.05). Y axis units in (b) and (c) differ due to channel signal intensity. *p < 0.05 in post hoc analysis
There was no overall main effect of AIE or sex on DH synaptic fraction expression of GLAST (F(1, 27) = 0.22, n = 31, p > 0.05, Figure 3b). There was a trend for AIE to increase GLAST expression in the VH (ME of AIE F(1, 25) = 3.62, n = 29, p < 0.07; Figure 3c). This trend was driven by a simple main effect of AIE in males, indicating an AIE-induced increase of GLAST expression in the VH of male animals but not in females (Male AIW < Male AIE, p < 0.04).
FIGURE 3.
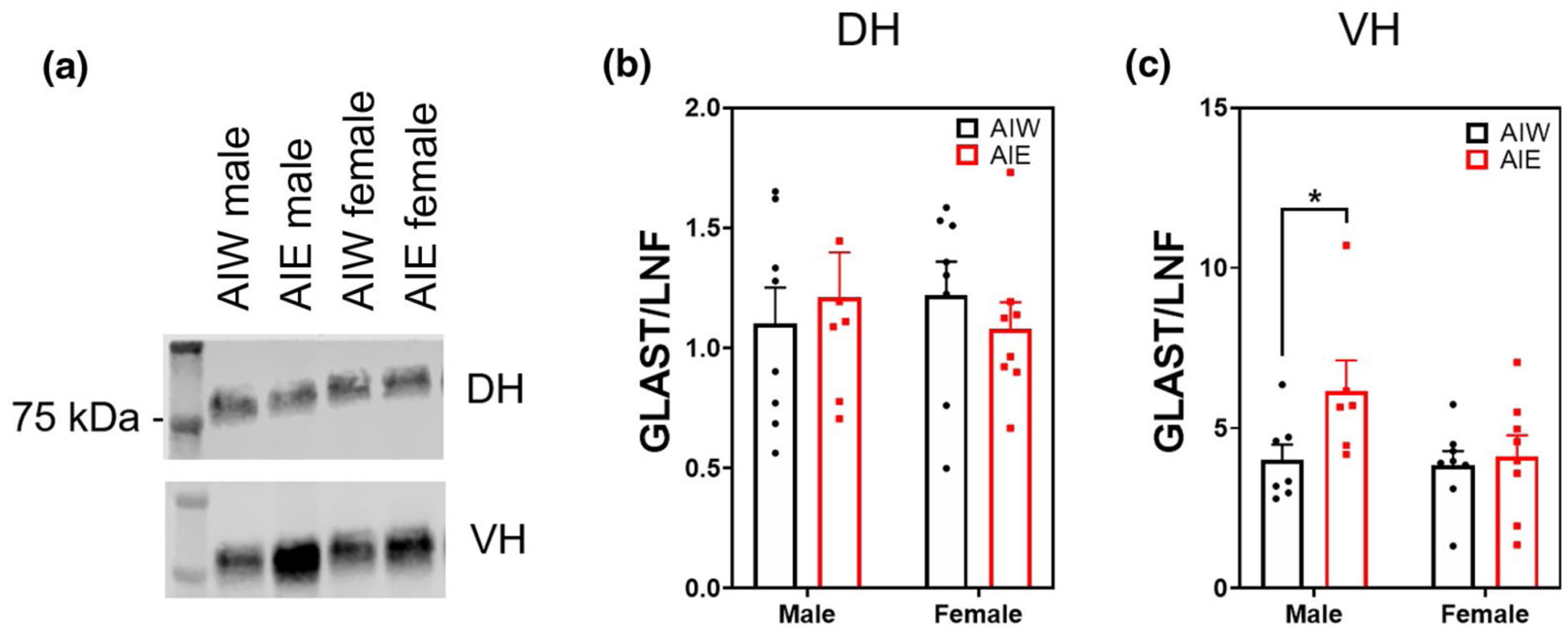
Adolescent intermittent ethanol (AIE) increases ventral hippocampal (VH) glutamate aspartate transporter (GLAST) synaptic expression (mean + SEM) in males but not in females. Western blot analysis of dorsal hippocampal (DH) and VH adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative GLAST bands from DH (top) and VH (bottom) samples, (b) No effect of AIE or Sex on DH GLAST expression, (c) AIE caused a trend toward increased GLAST in VH (main effect of AIE, F(1, 25) = 3.62, n = 29, p < 0.07). Analysis of simple main effect of AIE male VH showed a significant increase in GLAST expression (p < 0.05). Y axis units in (b) and (c) differ due to channel signal intensities (800 vs. 700 nm). *p < 0.05 in post hoc analysis
3.2 |. Effect of AIE and sex on dorsal and ventral hippocampal glial and neuronal ephrin membrane levels
EphA3 is the astrocytic membrane bound ligand for the neuronal receptor EphA4, which regulates dendritic spine morphology (known to be affected by AIE (Mulholland et al., 2018; Risher, Fleming, et al., 2015)). Western blot analysis of the synaptic fractionation of the DH and VH revealed no effect of AIE or sex on astrocytic EphA3 expression (p > 0.05; Figure 4b,c). However, sex and AIE interactively affected synaptic expression of EphA4 in the DH (Interaction effect (F(1, 27) = 5.42, n = 31, p < 0.03; Figure 5b), indicating that AIE influences Eph4A expression in adulthood in a sex-dependent manner. Post hoc analyses revealed nonsignificant trends for AIE to decrease EphA4 expression in females (p = 0.08) and increase it in males (p = 0.10). EphA4 expression in the synaptic fraction of the VH was not altered by AIE, sex, or their interaction (p > 0.05; Figure 5c). DH EphA4 AIE alterations were specific to the synaptic fraction, as there was no effect of AIE on EphA4 expression in the non-synaptic fraction (p > 0.05; data not shown). However, there was a nearly significant trend for females to have higher levels of non-synaptic EphA4 (ME of sex (F(1, 27) = 5.42, n = 31, p = 0.06; data not shown).
FIGURE 4.
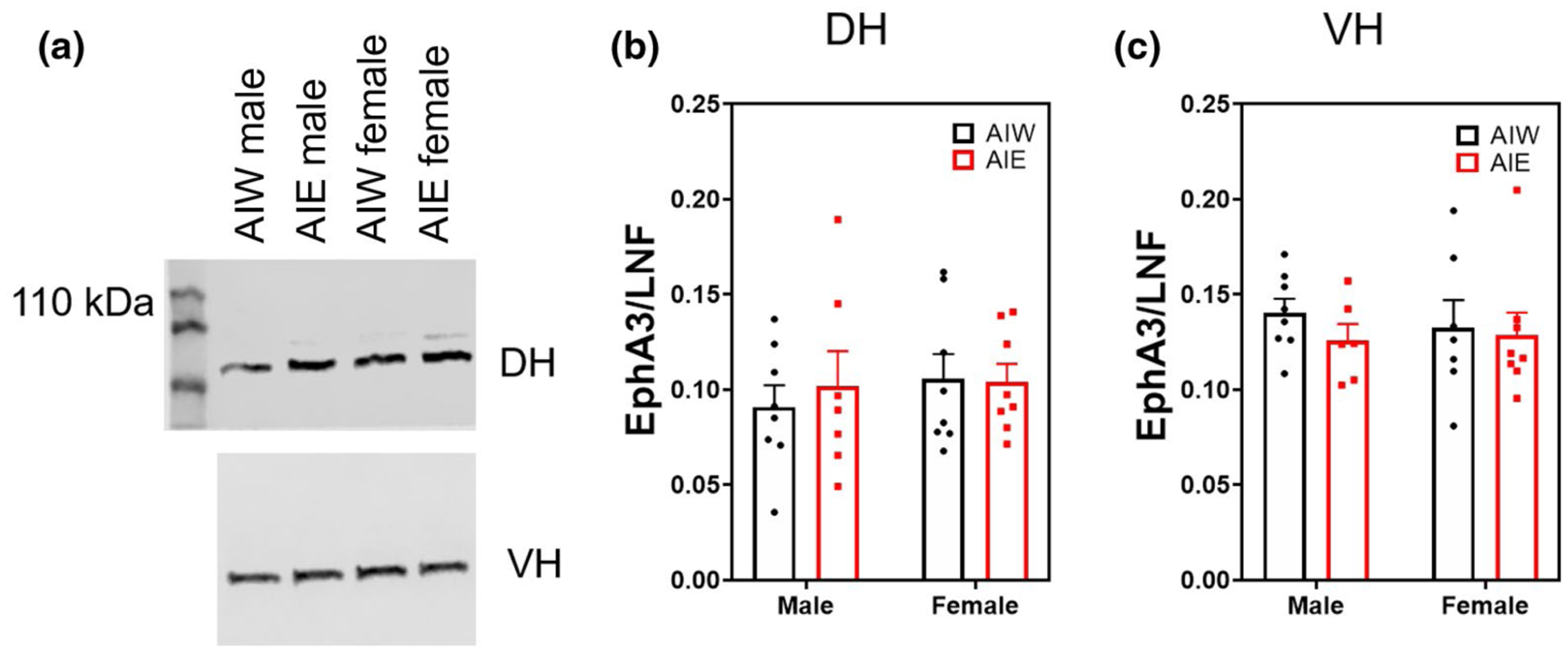
Ephrin receptor A3 (EphA3) synaptic expression (mean + SEM) unchanged by AIE or Sex Western blot analysis of dorsal hippocampal (DH) and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative EphA3 bands from DH (top) and VH (bottom) samples. (b,c) No effect of AIE or sex on DH or VH EphA3 expression
FIGURE 5.
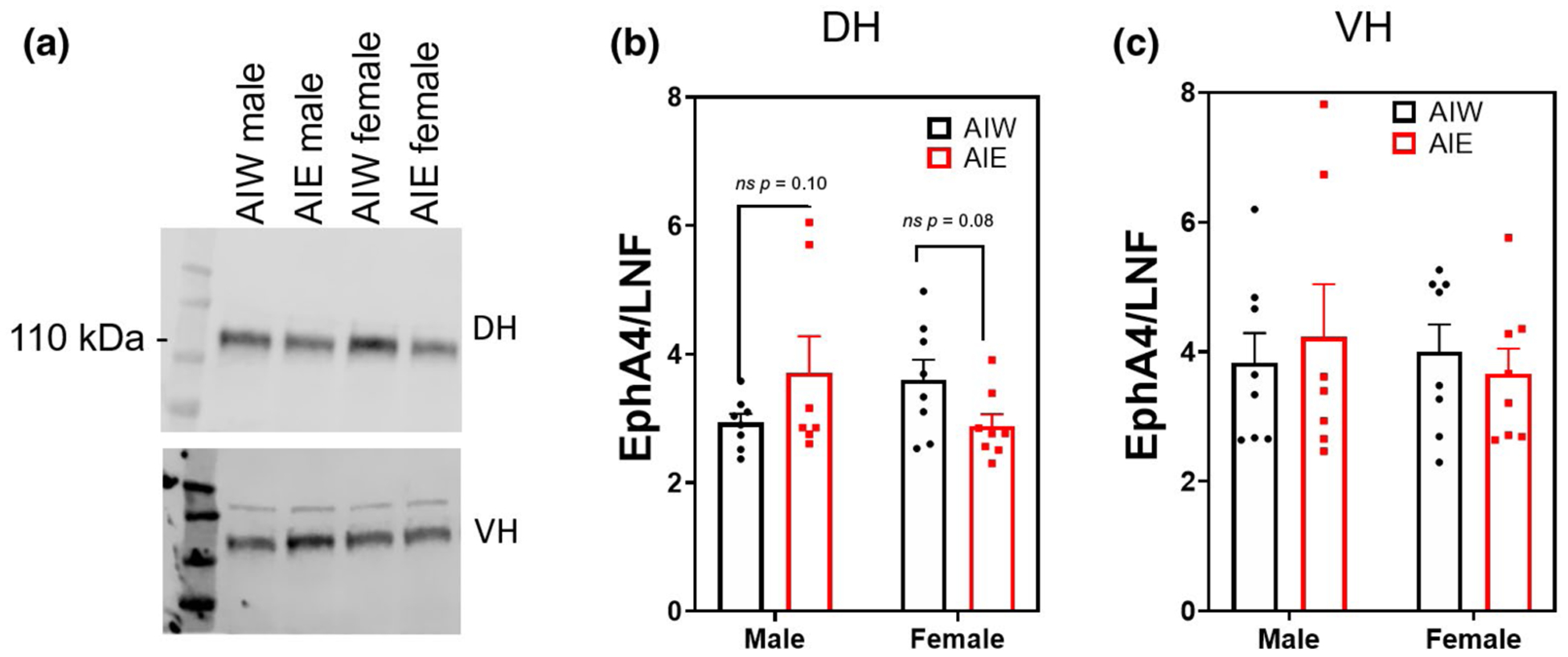
Region-specific differences in adolescent intermittent ethanol (AIE) and sex effects on ephrin receptor A4 (EphA4) synaptic expression (mean + SEM). Western blot analysis of dorsal hippocampal (DH) and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative EphA4 bands from DH (top) and VH (bottom) samples, (b) Significant interaction of AIE and sex on EphA4 synaptic expression in DH (interaction effect, F(1, 27) = 5.42, n = 31, p < 0.03). (c) No effect of AIE or sex on VH EphA4 expression
AlE had no effect on DH synaptic expression of the neuronally expressed Eph B6. However, there was a sex effect as females exhibited higher synaptic EphB6 expression compared to males (ME sex F(1, 27) = 6.00, n = 31, p < 0.03; Figure 6b). There was no effect of AIE or sex on VH EphB6 expression (p > 0.05; Figure 6c). EphB6 did not exhibit quantifiable levels in the extrasynaptic fractionations.
FIGURE 6.
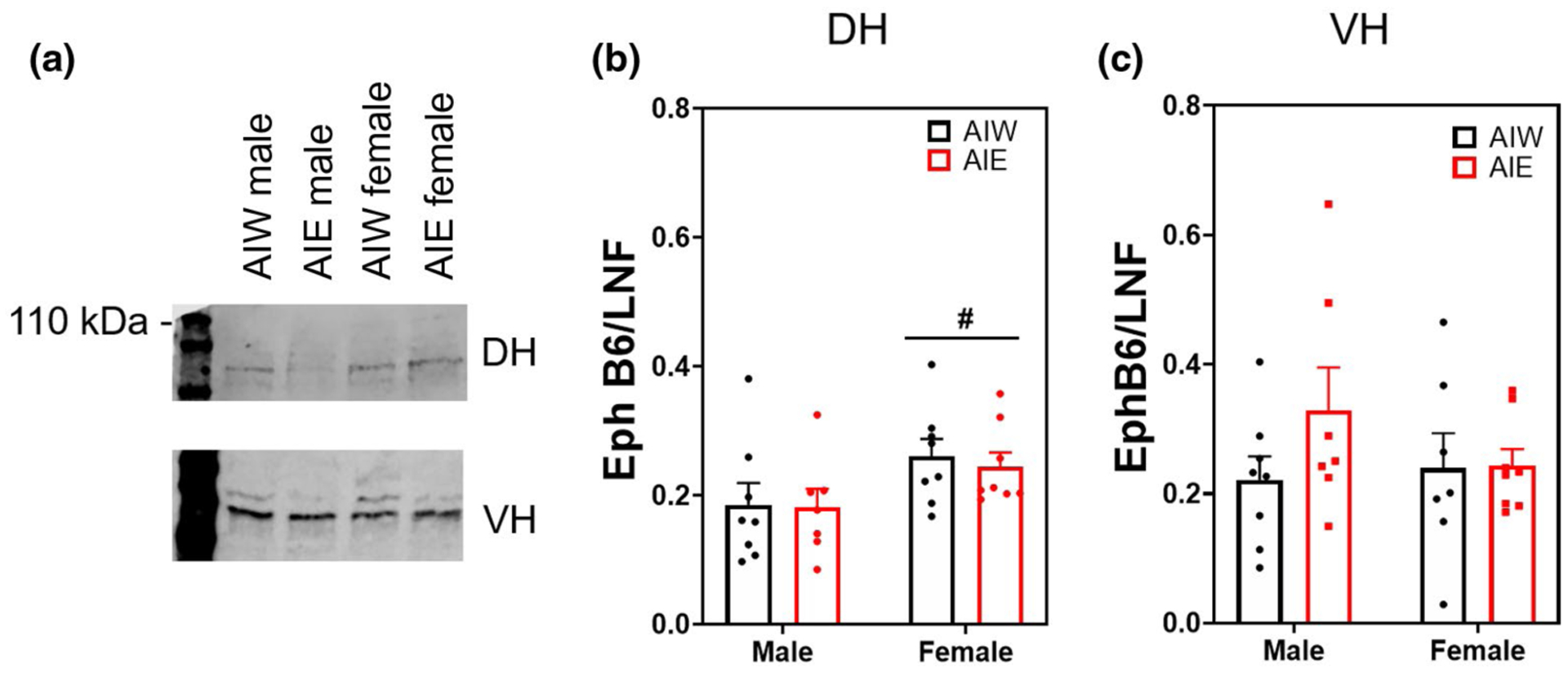
Ephrin receptor B6 synaptic expression (mean ± SEM) elevated in females in the dorsal hippocampus. Western blot analysis of dorsal hippocampal (DH) and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative EphB6 bands from DH (top) and VH (bottom) samples, (b) Female rats had significantly elevated EphB6 compared to males (main effect [ME] of Sex, F(1, 27) = 6.00, n = 31, p < 0.03) in the absence of any adolescent intermittent ethanol (AIE) effect, (c) No effect of AIE or sex on DH or VH EphA3 expression. #p < 0.05 ME
3.3 |. Effect of AIE on dorsal and ventral hippocampal neuronal synaptic membrane protein levels
Protein levels of PSD-95 in the DH were significantly increased relative to controls in female animals after AIE, but not in males (Interaction effect F(1, 25) = 5.90, n = 29, p < 0.03; ME of Sex F(1, 25) = 4.3, p < 0.05; post hoc Female AIW < Female AIE, p < 0.02; Figure 7b). There was no effect of AIE, sex, or their interaction on VH synaptic PSD-95 (p > 0.05; Figure 7c), and there was no quantifiable representation of PSD-95 in non-synaptic membrane fractions.
FIGURE 7.
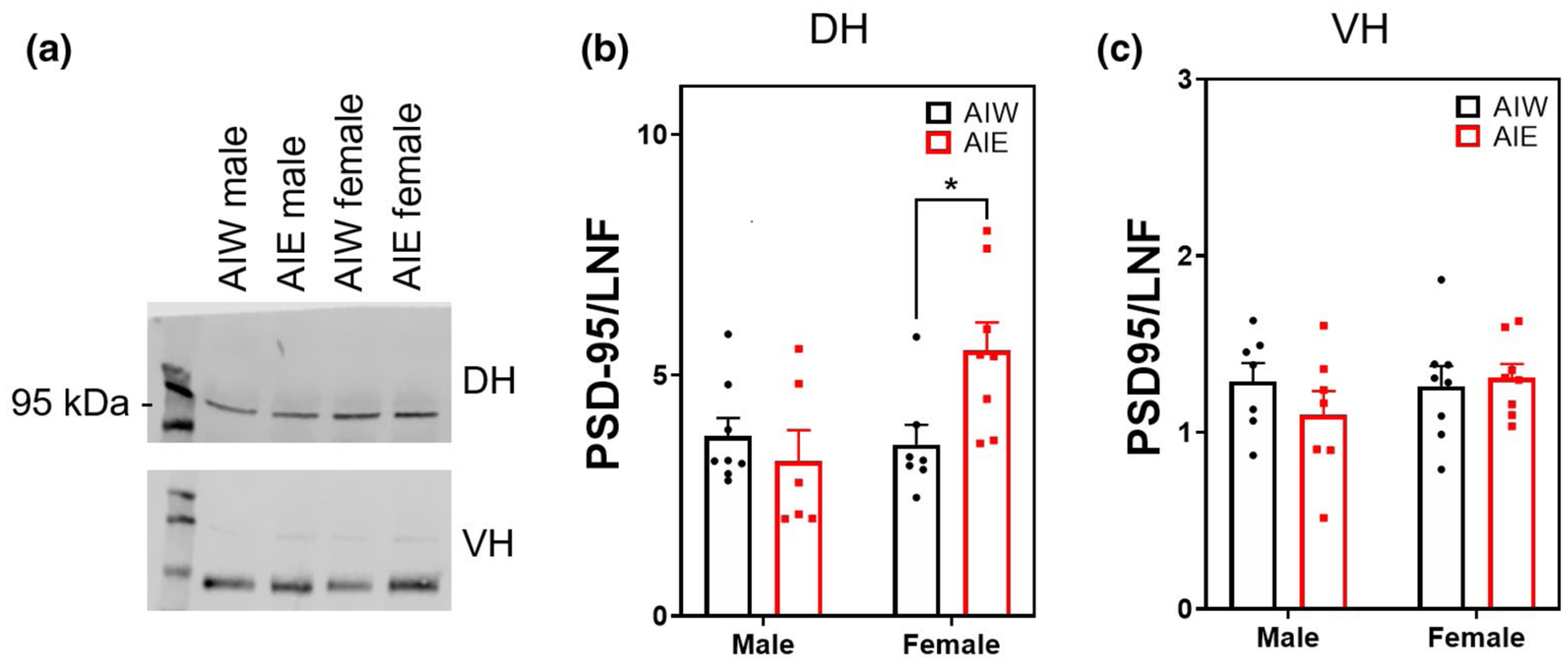
Adolescent intermittent ethanol (AIE)-induced elevation of postsynaptic density 95 (PSD-95) (mean + SEM) in the dorsal hippocampus of females but not in males. Western blot analysis of dorsal hippocampal (DH) and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative PSD-95 bands from DH (top) and VH (bottom) samples. (b) AIE-treated female rats exhibit elevated PSD-95 in DH compared to AIE controls (Interaction effect F(1, 25) = 5.90, n = 29, p < 0.03; main effect of Sex F(1, 25) = 4.3, p < 0.05; adolescent intermittent water (AIW) female < AIE female, p < 0.05). (c) No effect of AIE or sex on PSD-95 expression in the VH. Y axis units in (b) and (c) differ due to channel signal intensity (800 vs. 700). *p < 0.05 in post hoc analysis
Both AIE and sex affected DH GluN2A expression (ME of AIE F(1, 26) = 5.68, n = 30, p < 0.03; ME of sex F(1, 26) = 5.05, p < 0.04; Figure 8b). Post hoc analysis revealed that AIE significantly reduced GluN2A expression in males (p < 0.03) but not in females. There was no effect of AIE or sex on VH GluN2A expression (p > 0.05; Figure 8c). There was no effect of AIE or sex on synaptic GluN2B expression in the DH or VH (p > 0.05; Figure 9b,c).
FIGURE 8.
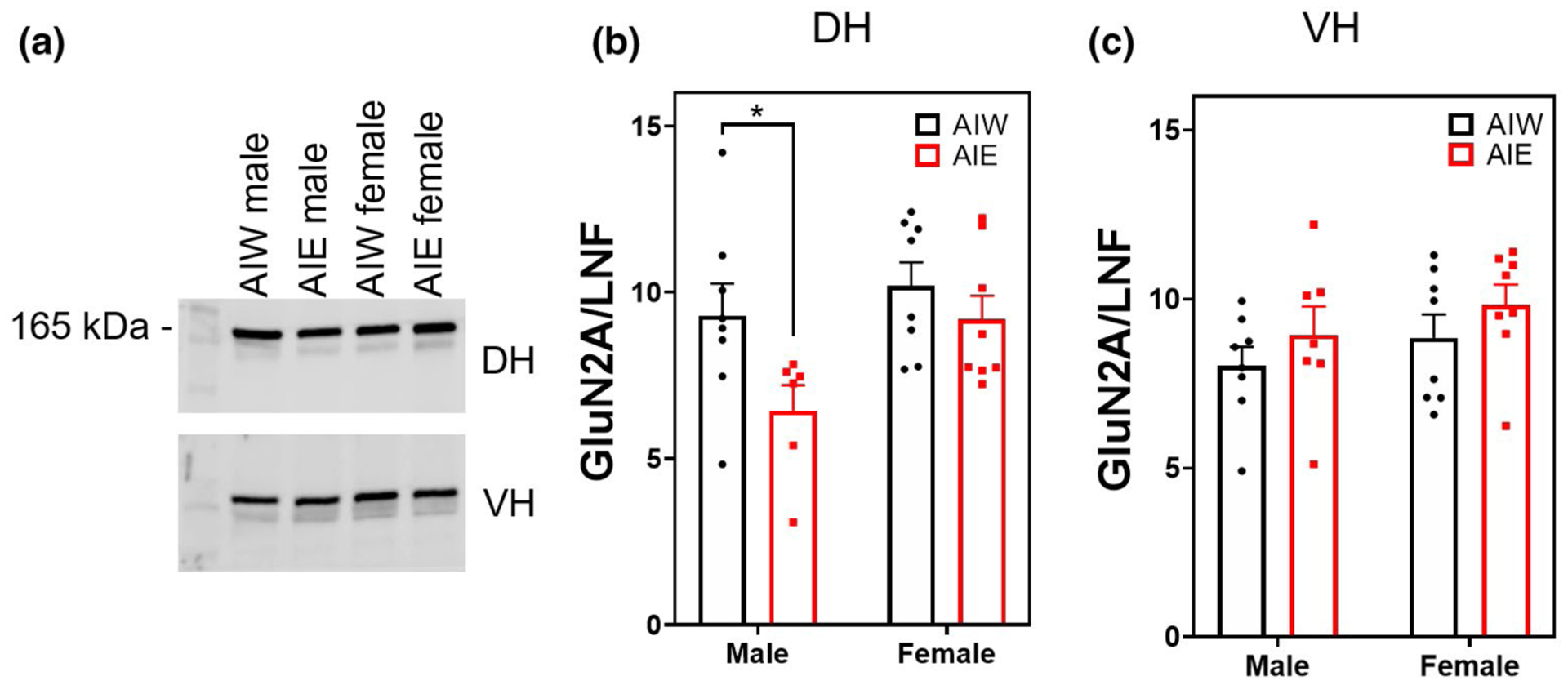
Adolescent intermittent ethanol (AIE) reduces dorsal hippocampal (DH) NMDA receptor subunit 2A (GluN2A) expression (mean + SEM). Western blot analysis of DH and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative GluN2A bands from DH (top) and VH (bottom) samples, (b) AIE significantly reduced GluN2A expression in the DH (main effect [ME] of AIE, F(1,26) = 5.68, n = 30, p < 0.03) and females had greater expression of GluN2A than males (ME of Sex, F(1, 26) = 5.05, n = 30, p < 0.04). (c) No effect of AIE or sex on VH GluN2A expression. *p < 0.05 in post hoc analysis
FIGURE 9.
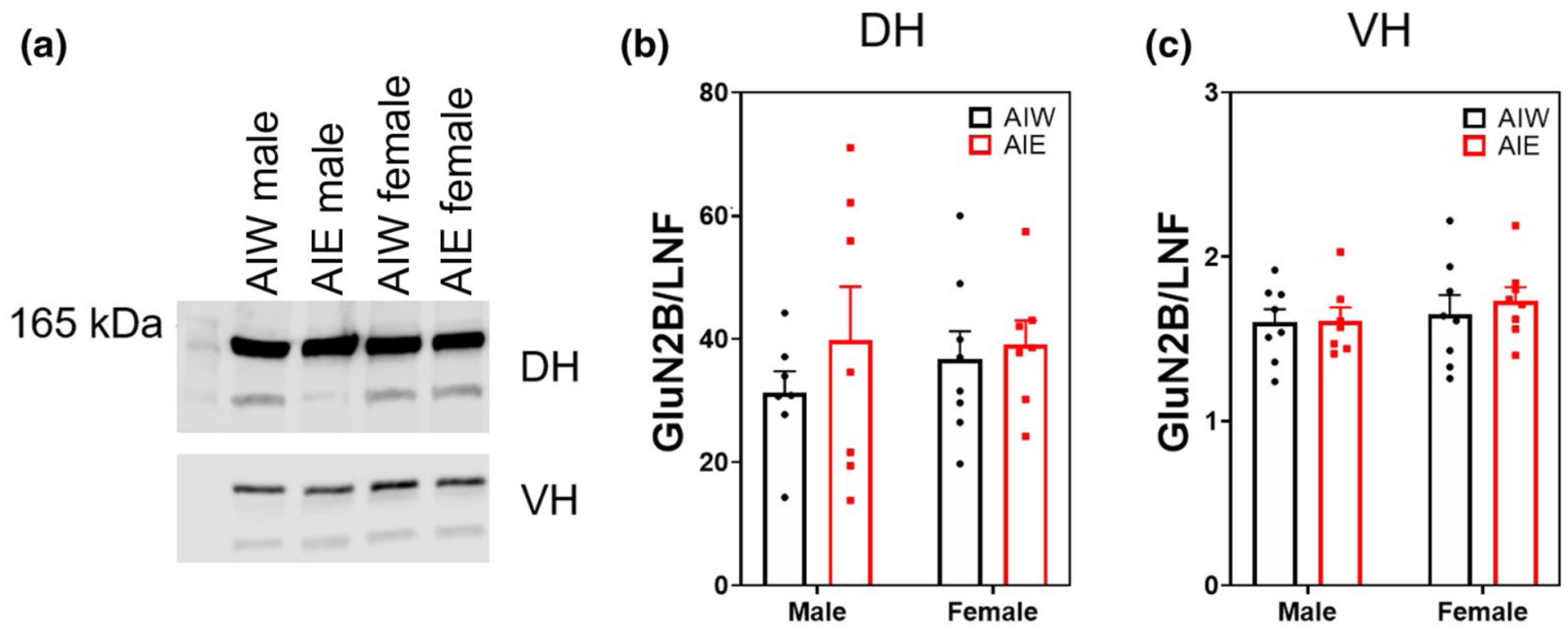
No effect of adolescent intermittent ethanol (AIE) or sex on hippocampal NMDA receptor subunit 2B (GluN2B) expression (mean + SEM). Western blot analysis of dorsal hippocampal (DH) and ventral hippocampal (VH) adolescent intermittent ethanol- or water-exposed male and female rats, (a) Representative GluN2B bands from DH (top) and VH (bottom) samples. (b,c) No effect of AIE or sex on DH or VH GluN2B expression. Y axis units differ due to channel signal intensities (800 vs. 700 nm)
For comparison, the above results are represented in Table 2 and statistical analyses are presented in full in Table 3.
TABLE 2.
The effects of AIE and sex on glutamate homeostasis and astrocyte-neuronal tethering proteins in male and female rats
| Antibody | Dorsal hippocampus | Ventral hippocampus | ||
|---|---|---|---|---|
| Synaptic | Non-synaptic | Synaptic | Non-synaptic | |
| GLT-1 | ↑ AIE male and female | – | Male > female | – |
| xCT | – | ↑ AIE male | – | |
| GLAST | – | ↑ AIE male | ||
| EphA3 | – | – | ||
| EphA4 | ↑ AIE male, ↓AIE female, AIW male < AIW female | Male < female | – | – |
| EphB6 | Male < female | No quantifiable expression | – | No quantifiable expression |
| PSD-95 | ↑ AIE female | No quantifiable expression | – | No quantifiable expression |
| GluN2A | ↓AIE male | – | ||
| GluN2B | – | – | ||
Note: Main effect p < 0.05, –no effect, all other listed findings are significant post hoc comparisons of p < 0.05.
TABLE 3.
Two-way ANOVA statistics of dorsal and ventral hippocampus protein expression
| F(interaction) | n | p | F(Sex) | n | p | F(AIE) | n | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Glial glutamate transporter protein membrane expression | ||||||||||
| GLT-1 | ||||||||||
| DH | Synaptic | F(1, 25) = 0.06 | n = 29 | p = 0.81 | F(1, 25) = 0.08 | n = 7–8 | p = 0.38 | F(1, 25) = 5.49 | n = 7–8 | p = 0.03 |
| Extrasynaptic | F(1, 27) = 0.03 | n = 31 | p = 0.87 | F(1, 27) = 0.08 | n = 7–8 | p = 0.78 | F(1, 27) = 0.30 | n = 7–8 | p = 0.59 | |
| VH | Synaptic | F(1, 27) = 0.48 | n = 31 | p = 0.50 | F(1, 27) = 5.67 | n = 7–8 | p = 0.03 | F(1, 27) = 1–30 | n = 7–8 | p = 0.27 |
| Extrasynaptic | F(1, 26) = 0.64 | n = 30 | p = 0.43 | F(1, 26) = 0.23 | n = 7–8 | p = 0.64 | F(1, 26) = 0.13 | n = 7–8 | p = 0.72 | |
| xCT | ||||||||||
| DH | Synaptic | F(1, 27) = 0.32 | n = 30 | p = 0.58 | F(1, 27) = 2.57 | n = 7–8 | p = 0.12 | F(1, 27) = 0.19 | n = 7–8 | p = 0.67 |
| Extrasynaptic | F(1, 26) = 0.08 | n = 31 | p = 0.78 | F(1, 26) = 0.88 | n = 6–8 | p = 0.36 | F(1, 26) = 0.0001 | n = 6–8 | p = 0.99 | |
| VH | Synaptic | F(1, 26) = 3.60 | n = 30 | p = 0.07 | F(1, 26) = 0.000078 | n = 7–8 | p = 0.99 | F(1, 26) = 1.65 | n = 7–8 | p = 0.21 |
| Extrasynaptic | F(1, 27) = 0.0009 | n = 31 | p = 0.98 | F(1, 27) = 2.79 | n = 7–8 | p = 0.11 | F(1, 27) = 0.01 | n = 7–8 | p = 0.92 | |
| GLAST | ||||||||||
| DH | Synaptic | F(1, 27) = 0.75 | n = 31 | p = 0.40 | F(1, 27) = 0.002 | n = 7–8 | p = 0.97 | F(1, 27) = 0.01 | n = 7–8 | p = 0.92 |
| VH | Synaptic | F(1, 25) = 2.12 | n = 29 | p = 0.16 | F(1, 25) = 2.93 | n = 7–8 | p = 0.01 | F(1, 25) = 3.62 | n = 7–8 | p = 0.07 |
| Glial and neuronal ephrin protein membrane expression | ||||||||||
| EphA3 | ||||||||||
| DH | Synaptic | F(1, 27) = 0.22 | n = 31 | p = 0.65 | F(1, 27) = 0.41 | n = 7–8 | p = 0.53 | F(1, 27) = 0.13 | n = 7–8 | p = 0.73 |
| VH | Synaptic | F(1, 25) = 2.12 | n = 29 | p = 0.16 | F(1, 25) = 2.93 | n = 6–8 | p = 0.10 | F(1, 25) = 3.62 | n = 6–8 | p = 0.07 |
| EphA4 | ||||||||||
| DH | Synaptic | F(1, 27) = 5.42 | n = 31 | p = 0.03 | F(1, 27) = 0.06 | n = 7–8 | p = 0.81 | F(1, 27) = 0.008 | n = 7–8 | p = 0.93 |
| Extrasynaptic | F(1, 26) = 0.55 | n = 30 | p = 0.47 | F(1, 26) = 4.05 | n = 7–8 | p = 0.06 | F(1, 26) = 0.32 | n = 7–8 | p = 0.58 | |
| VH | Synaptic | F(1, 27) = 0.49 | n = 31 | p = 0.49 | F(1, 27) = 0.15 | n = 7–8 | p = 0.71 | F(1, 27) = 0.004 | n = 7–8 | p = 0.95 |
| Extrasynaptic | F(1, 26) = 0.17 | n = 30 | p = 0.69 | F(1, 26) = 0.01 | n = 7–8 | p = 0.92 | F(1, 26) = 0.08 | n = 7–8 | p = 0.78 | |
| EphB6 | ||||||||||
| DH | Synaptic | F(1, 27) = 0.04 | n = 31 | p = 0.84 | F(1, 27) = 6.01 | n = 7–8 | p = 0.02 | F(1, 27) = 0.14 | n = 7–8 | p = 0.72 |
| VH | Synaptic | F(1, 26) = 1.23 | n = 30 | p = 0.28 | F(1, 26) = 0.52 | n = 7–8 | p = 0.48 | F(1, 26) = 1.42 | n = 7–8 | p = 0.24 |
| Neuronal synaptic protein membrane expression | ||||||||||
| PSD-95 | ||||||||||
| DH | Synaptic | F(1, 25) = 5.91 | n = 29 | p = 0.02 | F(1, 25) = 4.35 | n = 6–8 | p = 0.05 | F(1, 25) = 1.98 | n = 6–8 | p = 0.17 |
| VH | Synaptic | F(1, 26) = 1.22 | n = 30 | p = 0.28 | F(1, 26) = 0.72 | n = 7–8 | p = 0.40 | F(1, 26) = 0.44 | n = 7–8 | p = 0.51 |
| GluN2A | ||||||||||
| DH | Synaptic | F(1, 26) = 1.30 | n = 30 | p = 0.26 | F(1, 26) = 5.05 | n = 6–8 | p = 0.03 | F(1, 26) = 5.68 | n = 6–8 | p = 0.03 |
| VH | Synaptic | F(1, 25) = 0.74 | n = 29 | p = 0.40 | F(1, 25) = 3.30 | n = 6–8 | p = 0.08 | F(1, 25) = 1.00 | n = 6–8 | p = 0.33 |
| GluN2B | ||||||||||
| DH | Synaptic | F(1, 25) = 0.31 | n = 29 | p = 0.58 | F(1, 25) = 0.18 | n = 7–8 | p = 0.68 | F(1, 25) = 0.97 | n = 7–8 | p = 0.34 |
| VH | Synaptic | F(1, 27) = 0.15 | n = 31 | p = 0.71 | F(1, 27) = 0.91 | n = 7–8 | p = 0.35 | F(1, 27) = 0.24 | n = 7–8 | p = 0.63 |
4 |. DISCUSSION
One of the principal findings of this study is that AIE elevated the levels of glial glutamate transporters and exchangers in the hippocampal formation in adulthood. Specifically, the astrocytic glutamate transporter GLT-1 was elevated by AIE in the DH formation in both male and female rats. This suggests an elevation of glutamate turnover in the DH that could contribute to the excitability changes observed in DH circuits after AIE (Risher, Fleming, et al., 2015; Swartzwelder et al., 2017). It is interesting to consider the possibility that this increase in GLT-1 expression may reflect a compensatory response to increased giutamatergic signaling after AIE (Swartzwelder et al., 2016, 2017) and suggests possible elevated levels of hippocampal glutamate, as has been found in the nucleus accumbens core (Carrara-Nascimento et al., 2011). Additionally, AIE-induced decrease of astrocyte-neuronal synaptic proximity (Healey, Kibble, et al., 2020) potentially limits the ability of astrocytes to uptake synapticaliy released glutamate and likely increases glutamate spillover. An increase in GLT-1 at the synapse could be a compensatory mechanism to combat the astrocytes’ reduced ability to uptake synapticaliy released glutamate.
The glial cystine/glutamate antiporter subunit xCT was similarly elevated after AIE, but in the ventral, not dorsal, hippocampal formation and in male animals only. Consistent with these specific effects of AIE on xCT, the astrocytic glutamate aspartate transporter GLAST was also elevated after AIE in the ventral hippocampal formation in male rats only. Together these findings provide support for a chronic (perhaps permanent) AIE-induced dysregulation of the glutamatergic system and possible elevation of glutamate release by astrocytes that persists into adulthood. This is consistent with previous studies indicating that AIE elevates the release of other astrocyte-released synaptically regulatory factors (i.e., TSPs) in the adult hippocampus (Risher, Sexton, et al., 2015) and with studies demonstrating AIE-induced elevation of glutamatergic neurotransmission and aberrant excitatory synaptic plasticity in hippocampal slices from adult animals (Risher, Fleming, et al., 2015; Swartzwelder et al., 2017). With respect to males, specifically, the AIE-induced elevation of xCT suggests the possibility that males may be at elevated risk for VH glutamate-mediated excitotoxicity. If that is the case, it would indicate that males are at greater risk than females for affective dysregulation in adulthood after AIE, either at baseline or in response to environmental stressors.
The hippocampal subregion differences in these AIE effects is informative due to the overlapping but distinct influences of the dorsal and ventral hippocampi on behavior. Although longitudinal circuitry throughout the hippocampal formation promotes interaction between dorsal and ventral regions, it is generally understood that the dorsal region is principally involved with the consolidation of memory while the ventral region additionally drives anxiety-like behavior (Fanselow & Dong, 2010; Jimenez et al., 2018; Strange et al., 2014). It has also been reported that the VH region manifests greater general excitability than the dorsal region (Bragdon et al., 1986). Thus, the presently reported elevation of xCT and GLAST by AIE in the male ventral, but not dorsal hippocampus, suggests an enduring, glially mediated upreguiation of glutamate turnover in this region that may underlie elevations of anxiety-like behavior reported after AIE (Pandey et al., 2015; see Crews et al., 2019). Moreover, that those elevations were observed in male animals, and not in females, indicates that the AIE effects on hippocampal xCT and GLAST expression are sex specific as well as region specific. This is particularly interesting in light of a recent report that male mice, but not females, with a history of AIE exhibited elevated contextual freezing behavior (Kasten et al., 2020). Thus, it is possible that AIE induces a hyperexcitabie state in the ventral hippocampus of male animals, which leads to dysregulated affective reactivity. Considering these subregional AIE-induced changes more broadly, it is notable that while AIE-induced effects on DH were observed in both sexes, effects on VH were only observed in males. In the context of the behavioral studies cited above, this suggests the possibility that male adolescents have a distinctive vulnerability to enduring hyperexcitability of the VH and a propensity toward anxiety in adulthood after AIE.
Our hypothesis regarding AIE effects on the ephrin system was not directly supported by the present experiments. We observed no significant main effect of AIE on either astrocyte-released EphA3 or its neuronal EphA4 receptor. Thus it appears unlikely that an alteration in ephrin levels by AIE would account for the changes we have previously observed in astrocyte reactivity (Risher, Sexton, et al., 2015) or astrocyte-neuronal proximity (Healey, Kibble, et al., 2020). However, the sex by AIE interaction that we observed on EphA4 expression levels in the dorsal hippocampus suggest that AIE does impact EphA4, though in a sex-dependent manner. Further studies will be required to explore the functional significance of this interaction, but the finding does support the general hypothesis that AIE can produce an enduring influence on hippocampal EphA4.
Previous studies have shown evidence of AIE-induced alterations in glutamatergic function, specifically NMDA receptor-mediated processes, in male rats (Risher, Fleming, et al., 2015; Swartzwelder et al., 2016, 2017). The present findings are generally consistent with such changes, indicating an AIE-induced reduction of GIuN2A expression in the dorsal hippocampus of male rats, though there were no effects in the ventral hippocampus or in females. We did not observe any effects of AIE on GIuN2B expression. The decrease in synaptic GluN2A expression among males after AIE may, in part, underlie our previous observation that AIE induces a shift toward GluN2B drive of NMDA receptor-mediated synaptic currents (Swartzwelder et al., 2017). That is, the increase in GluN2B drive could reflect a compensatory shift in physiological current modulation due to a decrease in GluN2A receptor expression.
Because of the known effects of AIE on hippocampal synaptic function (see Crews et al., 2019; Spear & Swartzwelder, 2014), its effects on the synaptic marker PSD-95 have been of interest. Earlier studies from our laboratory used male rats and immunohistochemical techniques to assess PSD-95 expression specifically in the CA1 region of the dorsal hippocampus (Risher, Fleming, et al., 2015) and found decreased PSD-95 immunoreactivity in that region. In contrast, the present findings indicate no change in PSD-95 expression in synaptic tissue homogenates from dorsal hippocampus of male rats, using Western blot techniques. It is likely that this inconsistency is related to the fact that the present data included all hippocampal subregions, rather than focusing on area CA1 alone. Regardless, the present data do show a significant increase in PSD-95 expression in the dorsal hippocampus of female rats. This sex difference could be quite informative because spatial memory function has not been shown to be altered markedly by AIE in male rats (White et al., 2000), but female rats have not been tested. Thus, AIE-induced elevations of DH PSD-95 in females could reflect aberrantly high excitatory synaptogenesis, which could compromise or enhance hippocampally mediated spatial learning. Clearly, memory studies must be conducted in female animals to test this hypothesis.
The data presented in this report address a range of issues that are emerging in the literature on the long-term consequences of adolescent alcohol exposure. The principal limitations of this study are that it does not directly address the physiological or behavioral consequences of AIE that may relate to the astrocytic changes previously reported or the presently reported changes in synaptically expressed proteins related to glutamate homeostasis or synaptic structure and function. The membrane subfractionation methods used were crude membrane fractions, and likely the synaptic membrane fraction included the terminals of perisynaptic astrocytic processes that tightly hug the synapse, allowing for the measurement of astrocytic targets. This is supported by previous research detailing the many purification steps necessary to get a pure synaptosome fractionation (Huttner et al., 1983); however, this was not directly measured in this study. Still, the present findings provide new potential mechanisms that may underlie the physiological and behavioral effects of AIE and, thereby, will help to guide future studies.
Overall, the present findings support the hypothesis that AIE produces enduring alterations in glially mediated glutamate homeostasis in the hippocampal formation. Moreover, the data indicate that certain of those alterations are mediated by sex and are also subregion specific within the hippocampal formation. These findings add to an emerging literature indicating that adolescent alcohol exposure alters glial function and glial-neuronal interactions in adulthood, a set of effects that may identify both mechanisms underlying the effects of adolescent alcohol exposure and potential therapeutic targets for the reversal or amelioration of those effects.
Supplementary Material
FIGURE S1 GLT-1 and PSD-95 synaptic membrane fraction Western biot. Representative image of blots for GLT-1 (~70 kDA) and PSD-95 (~95 kDA) in dorsal (a) and ventral (b) hippocampus
FIGURE S2 Cystine-glutamate antiporter synaptic membrane fraction Western blot. Representative image of blots for cystine-glutamate antiporter (xCT, ~27 kDA) in dorsal (a) and ventral (b) hippocampus. Dorsal hippocampus representative image also contains GluN2A; ventral hippocampus image contains EphA4
FIGURE S3 GLAST synaptic membrane fraction Western blot. Representative image of blots for GLAST (~75 kDa) in dorsal (a) and ventral (b) hippocampus. Membranes were cut above 75 kDa before blotting
FIGURE S4 Ephrin A3 synaptic membrane fraction Western blot. Representative image of blots for EphA3 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. Membranes were cut below 100 kDa before blotting
FIGURE S5 Ephrin A4 synaptic membrane fraction Western blot Representative image of blots for EphA4 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 75 kDa before blotting
FIGURE S6 Ephrin B6 synaptic membrane fraction Western blot Representative image of blots for EphB6 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. Membranes were cut below 75 kDa before blotting
FIGURE S7 GluN2A synaptic membrane fraction Western blot Representative image of blots for GluN2A (~165 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 100 kDa before blotting
FIGURE S8 GluN2B synaptic membrane fraction Western blot Representative image of blots for GluN2B (~165 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 100 kDa before blotting
Significance.
Adolescent alcohol drinking is a significant public health problem, and there is increasing evidence that binge drinking during this critical period of brain development has an enduring impact on neural and behavioral function. Early initiation of drinking is a risk factor for the development of alcohol use disorders. Therefore, studies aimed at identifying mechanisms of these chronic effects, and developing potential therapies to combat them are much needed. The present report identifies several such potential mechanisms and may facilitate the development of therapeutic treatments.
ACKNOWLEDGMENTS
We thank the National Institute on Alcohol and Alcohol Abuse for funding this work (NIAAA U01AA019925). We thank Dr. Rebecca Klein, Duke University, for her contribution during various stages of data acquisition. This work was completed as part of the Neurobiology of Adolescent Drinking in Adulthood Consortium (NADIA).
Funding information
National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: 3U01AA019925 (NADIA)
Footnotes
CONFLICT OF INTEREST
None of the authors have any competing financial or non-financial interests that could directly undermine, or be perceived to undermine, the objectivity, integrity, and value of this work or its publication.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Allen NJ, & Eroglu C (2017). Cell biology of astrocyte-synapse interactions. Neuron, 96(3), 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnsen LP, Hadera MG, Zhou Y, Danbolt NC, & Sonnewald U (2014). The GLT-1 (EAAT2; slcla2) glutamate transporter is essential for glutamate homeostasis in the neocortex of the mouse. Journal of Neurochemistry, 128(5), 641–649. 10.1111/jnc.12509 [DOI] [PubMed] [Google Scholar]
- Bragdon AC, Taylor DM, & Wilson WA (1986). Potassium-induced epileptiform activity in area CAS varies markedly along the septo-temporal axis of the rat hippocampus. Brain Research, 378(1), 169–173. 10.1016/0006-8993(86)90300-8 [DOI] [PubMed] [Google Scholar]
- Carmona MA, Murai KK, Wang L, Roberts AJ, & Pasquale EB (2009). Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proceedings of the National Academy of Sciences of the United States of America, 106(30), 12524–12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Griffin WC, Pastrello DM, Olive MF, & Camarini R (2011). Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol, 45(5), 451–460. 10.1016/j.alcohol.2011.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury S, Blakemore SJ, & Charman T (2006). Social cognitive development during adolescence. Social Cognitive and Affective Neuroscience, 1(3), 165–174. 10.1093/scan/nsl024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, & Farina C (2016). Astrocytes: Key regulators of neuroinflammation. Trends in Immunology, 37(9), 608–620. 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, Rodd ZA, Spear LP, Swartzwelder HS, & Vetreno RP (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcoholism, Clinical and Experimental Research, 43(9),1806–1822. 10.1111/acer.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW,O’Rourke NA, Park CY, Özkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, & Green EM (2009). Gabapentin receptor alpha2delta-l is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell, 139(2), 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron, 65(1), 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey KL, Landin JD, Dubester K, Kibble S, Marquardt K, Brutman JN, Davis JF, Swartzwelder HS, & Chandler LJ (2020). Effects of ethanol on plasma ghrelin levels in the rat during early and late adolescence. Alcohol, 85, 111–118. 10.1016/j.alcohol.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey KL, Kibble S, Hodges S, Reissner KJ, Testen A, Wills TA, Acheson SK, Siemsen BM, McFaddin JA, Scofield MD, & Swartzwelder HS (2020). Enduring alterations in hippocampal astrocytesynaptic proximity following adolescent alcohol exposure: Reversal by gabapentin. Neural Regeneration Research, 15(8), 1496–1501. 10.4103/1673-5374.274339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, & De Camilli P (1983). Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. Journal of Cell Biology, 96(5), 1374–1388. 10.1083/jcb.96.5.1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, & Kheirbek MA (2018). Anxiety cells in a hippocampai-hypotha-lamic circuit. Neuron, 97(3), 670–683.e6. 10.1016/j.neuron.2018.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nature Reviews Neuroscience, 10(8), 561–572. [DOI] [PubMed] [Google Scholar]
- Kasten CR, Carzoli KL, Sharfman NM, Henderson T, Holmgren EB, Lerner MR, Miller MC, & Wills TA (2020). Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity. Neuropsychopharmacology, 45(8), 1306–1315. 10.1038/s41386-020-0670-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W (2012). Development of the GABAergic system from birth to adolescence. Neuroscientist, 18(6), 613–630. 10.1177/1073858411422114 [DOI] [PubMed] [Google Scholar]
- Kim R, Healey KL, Sepulveda-Orengo MT, & Reissner KJ (2018). Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 87(Pt A), 126–146. 10.1016/j.pnpbp.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, & Juraska JM (2012). Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Research, 1466, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Teppen TL, Miller KM, Sexton HG, Pandey SC, & Swartzwelder HS (2018). Donepezil reverses dendritic spine morphology adaptations and Fmrl epigenetic modifications in hippocampus of adult rats after adolescent alcohol exposure. Alcoholism, Clinical and Experimental Research, 42(4), 706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajarillo E, Rizor A, Lee J, Aschner M, & Lee E (2019). The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology, 161,107559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, & Zhang H (2015). Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiology of Diseases, 82, 607–619. 10.10l6/j.nbd.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2005). Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences, 9(2), 60–68. 10.1016/j.tics.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Risher M-L, Fleming RL, Risher WC, Miller KM, Klein RC, Wills T, Acheson SK, Moore SD, Wilson WA, Eroglu C, & Swartzwelder HS (2015). Adolescent intermittent alcohol exposure: Persistence of structural and functional hippocampal abnormalities into adulthood. Alcoholism, Clinical and Experimental Research, 39(6), 989–997. 10.llll/acer.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher ML, Sexton HG, Risher WC, Wilson WA, Fleming RL, Madison RD, Moore SD, Eroglu C, & Swartzwelder HS (2015). Adolescent intermittent alcohol exposure: Dysregulation of thrombospondins and synapse formation are associated with decreased neuronal density in the adult hippocampus. Alcoholism, Clinical and Experimental Research, 39(12), 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, & Cochran WG (1989). Statistical methods (8th ed.). lowa State University Press. [Google Scholar]
- Spear LP, & Swartzwelder HS (2014). Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: A mini-review. Neuroscience and Biobehavioral Reviews, 45,1–8. 10.1016/j.neubiorev.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, & Moser E. l. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655–669. 10.1038/nrn3785 [DOI] [PubMed] [Google Scholar]
- Suto T, Severino AL, Eisenach JC, & Hayashida K (2014). Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology, 81, 95–100. 10.1016/j.neuropharm.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Acheson SK, Miller KM, Sexton HG, Liu W, Crews FT, & Risher M-L (2015). Adolescent intermittent alcohol exposure: Deficits in object recognition memory and forebrain cholinergic markers. PLoS ONE, 20(11), e0140042. 10.1371/journal.pone.0140042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Risher ML, Miller KM, Colbran RJ, Winder DG, & Wills TA (2016). Changes in the adult GluN2B associated proteome following adolescent intermittent ethanol exposure. PLoS ONE, 11(5), e0155951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Healey KL, Liu W, Dubester K, Miller KM, & Crews FT (2019). Changes in neuroimmune and neuronal death markers after adolescent alcohol exposure in rats are reversed by donepezil. Scientific Reports, 9(1), 12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Park MH, & Acheson S (2017). Adolescent ethanol exposure enhances NMDA receptor-mediated currents in hippocampal neurons: Reversal by gabapentin. Scientific Reports, 7(1), 13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Foster JB, & Lin C-L-G (2015). Glutamate transporter EAAT2: Regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cellular and Molecular Life Sciences, 72(18), 3489–3506. 10.1007/s00018-015-1937-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testen A, Ali M, Sexton HG, Hodges S, Dubester K, Reissner KJ, Swartzwelder HS, & Risher ML (2019). Region-specific differences in morphometric features and synaptic colocalization of astrocytes during development. Neuroscience, 400,98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Risher WC, & Risher ML (2020). Regulation of synaptic development by astrocyte signaling factors and their emerging roles in substance abuse. Cells, 9(2), 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, & Swartzwelder HS (2000). Binge pattern ethanol exposure in adolescent and adult rats: Differential impact on subsequent responsiveness to ethanol. Alcoholism, Clinical and Experimental Research, 24(8), 1251–1256. 10.1111/j.1530-0277.2000.tb02091.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 GLT-1 and PSD-95 synaptic membrane fraction Western biot. Representative image of blots for GLT-1 (~70 kDA) and PSD-95 (~95 kDA) in dorsal (a) and ventral (b) hippocampus
FIGURE S2 Cystine-glutamate antiporter synaptic membrane fraction Western blot. Representative image of blots for cystine-glutamate antiporter (xCT, ~27 kDA) in dorsal (a) and ventral (b) hippocampus. Dorsal hippocampus representative image also contains GluN2A; ventral hippocampus image contains EphA4
FIGURE S3 GLAST synaptic membrane fraction Western blot. Representative image of blots for GLAST (~75 kDa) in dorsal (a) and ventral (b) hippocampus. Membranes were cut above 75 kDa before blotting
FIGURE S4 Ephrin A3 synaptic membrane fraction Western blot. Representative image of blots for EphA3 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. Membranes were cut below 100 kDa before blotting
FIGURE S5 Ephrin A4 synaptic membrane fraction Western blot Representative image of blots for EphA4 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 75 kDa before blotting
FIGURE S6 Ephrin B6 synaptic membrane fraction Western blot Representative image of blots for EphB6 (~118 kDA) in dorsal (a) and ventral (b) hippocampus. Membranes were cut below 75 kDa before blotting
FIGURE S7 GluN2A synaptic membrane fraction Western blot Representative image of blots for GluN2A (~165 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 100 kDa before blotting
FIGURE S8 GluN2B synaptic membrane fraction Western blot Representative image of blots for GluN2B (~165 kDA) in dorsal (a) and ventral (b) hippocampus. VH membrane was cut below 100 kDa before blotting
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


