Abstract
Objectives
This study assessed antimicrobial stewardship (AMS) and infection prevention (IP) interventions targeting healthcare-associated Clostridioides difficile and carbapenem-resistant Klebsiella pneumoniae (CRKP) infections, their key outcomes and the application of behaviour change principles in these interventions.
Design
This scoping review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews (PRISMA-ScR) guidelines while focusing on acute healthcare settings in both low-to-middle income and high-income countries.
Data sources
The databases searched were MEDLINE, PubMed, Web of Science and CINAHL between 22 April 2020 and 30 September 2020.
Eligibility
The review included peer-reviewed articles published in English language between 2010 and 2019. Studies that focussed on IP and/or AMS interventions primarily targeting C. difficile or CRKP were included. Studies that assessed effectiveness of diagnostic devices or treatment options were excluded from this review.
Data extraction and synthesis
An abstraction sheet calibrated for this study was used to extract data on the main study characteristics including the population, intervention and outcomes of interest (antimicrobial use, compliance with IP interventions and risk for C. difficile and CRKP). A narrative synthesis of the results is provided.
Results
The review included 34 studies. Analysis indicates that interventions targeting C. difficile and CRKP include Education, Surveillance/Screening, Consultations, Audits, Policies and Protocols, Environmental measures, Bundles, Isolation as well as Notifications or alerts (represented using the ESCAPE-BIN acronym). The identified outcomes include antimicrobial use, resistance rates, risk reduction, adherence to contact precautions, hospital stay and time savings. AMS and IP interventions tend to be more adhoc with limited application of behaviour change principles.
Conclusion
This scoping review identified the AMS and IP interventions targeting C. difficile and CRKP in healthcare settings and described their key outcomes. The application of behaviour change principles in AMS and IP interventions appears to be limited.
Keywords: public health, infection control, microbiology, preventive medicine, epidemiology
Strengths and limitations of this study.
This review considered the specific antimicrobial stewardship (AMS) and infection prevention (IP) interventions in line with the core elements of AMS as outlined by the Centres for Disease Control and Prevention.
The review only considered studies that primarily focussed on AMS and/or IP interventions targeting Clostridioides difficile and/or carbapenem-resistant Klebsiella pneumoniae.
The screening and selection of studies as well as data extraction were completed by two reviewers.
The COM-B (‘capability’, ‘opportunity’, ‘motivation’ and ‘behaviour’) model elements were used to assess the application of behaviour change principles in AMS and IP interventions.
Introduction
Infectious diseases have remained a leading cause of morbidity and mortality over the past centuries.1 The discovery of antimicrobial agents during the 19th and 20th centuries2 following observations by Alexander Fleming on the effect of Penicillium mold on bacteria cultures birthed the era of anti-infective agents3 and was a major breakthrough in the fight against infectious diseases. In 1947, Waksman, coined the term ‘antibiotic’ in reference to a chemical agent capable of destroying or inhibiting the growth of microorganisms.4 Subsequently, clinicians began to recognise and rely on antibiotics as an effective strategy for treating and eradicating pathogenic microorganisms. As the use of antibiotics gained popularity worldwide with noted successes including the treatment of gram positive cocci with penicillin,3 5 a new threat namely antimicrobial resistance (AMR), emerged due to the over-reliance on these life-saving therapeutic agents.6 More than 50% of antimicrobials used are either inappropriate or unnecessary and within the last two decades alone, the use of antimicrobial agents has risen by 65% significantly contributing to AMR.7 Coupled with the ongoing human-to-human transmission of pathogens,8 microorganisms continue to evolve adaptively rendering antibiotics ineffective9–11 and causing more potent infections as they acquire resistance. AMR represents a public health emergency with 10 million fatalities globally projected by 205012 coupled with increasing costs for treating multidrug-resistant organisms (MDROs).13
Today, the burden of infectious diseases remains high globally14 with a worrying increase of deaths attributable to MDROs. A modelling study reported 33 000 deaths associated with MDROs in Europe in the year 2015, representing a significant rise since the year 2007.15 Healthcare settings appear to have a higher risk of human-to-human transmission of MDROs. According to the European Center for Disease Prevention and Control (ECDC), the EU records an estimated 3.2 million healthcare-associated infections (HCAIs) and an associated 37 000 deaths annually.16 This translates to 2.5 million disability-adjusted life years, 16 million additional hospitalisation days and an annual economic burden of 7 billion euros.17 18 This burden is largely attributed to MDROs19 of which ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp) pathogens play a significant role.20–23 In recent years, scientists have suggested the inclusion of Clostridioides (Clostridium) difficile as a member of the ESKAPE pathogens and subsequently amending the acronym to ESCAPE pathogens.24 Significant efforts have been made to reduce the burden of HCAIs and AMR, but the problem persists. To aid the understanding of potential gaps in evidence, this scoping review explored the literature on interventions targeting C. difficile and carbapenem-resistant Klebsiella pneumoniae (CRKP) which are among the most common infections in healthcare settings and on the WHO’s pathogen priority list for research and development of new antibiotics.
Rationale
A preliminary exploration of literature retrieved three scoping reviews on antimicrobial misuse and AMS interventions. The first scoping review25 was limited to dentistry settings; the second26 examined literature on knowledge, attitudes and practices among community pharmacists and the third focussed on supply-related factors for reducing prescription of antibiotics in low-to-middle-income countries.27 In this scoping review, the focus is on healthcare-associated C. difficile and CRKP infections. Clostridioides difficile is the single most leading cause of nosocomial diarrhoea globally primarily linked with the use of antibiotics that disrupt the stability of gut microbiota allowing the pathogenic bacteria to flourish.28–30 Klebsiella pneumoniae ranks among the top three leading causes of neonatal sepsis in resource limited settings31 32 with some strains known to produce extended-spectrum B-lactamases associated with multidrug resistance to carbapenems and colistin.33 More often, cultures obtained from patient environments, stools, water and blood have been shown to contain CRKP33 and C. difficile. Studies show that approximately 25% of patients in England, Australia and the USA are colonised by CRKP during their hospitalisation period.33–35 Patient-to-patient transmission of CRKP accounts for an estimated 52% of the cases identified in healthcare settings.36 There appears to be evidence-based infection prevention (IP) and antimicrobial stewardship (AMS) interventions aimed at curbing the healthcare-associated transmission of C. difficile and CRKP. However, the prevalence of infections caused by these organisms remains high. The interventions broadly aim at changing the behaviours of healthcare workers with regard to antimicrobial prescribing and/or compliance with IP measures. As recently acknowledged by the WHO,37 it has become increasingly clear that application of evidence-based interventions is not a guarantee for success emphasising the need to focus more on the underlying psychosocial mechanisms that influence people’s behaviours.38 39 It therefore remains unclear whether there is sufficient application behaviour change principles in AMS and IP interventions for improved effectiveness and sustainability, hence, this scoping review.
Research objectives
To assess IP and AMS interventions targeting healthcare-associated C. difficile and CRKP.
To describe the key outcomes for IP and AMS interventions targeting healthcare-associated C. difficile and CRKP.
To assess the application of behaviour change principles in IP and AMS interventions targeting healthcare associated C. difficile and CRKP infections.
Methods
Research protocol
The protocol for this scoping review is available on Open Science Framework registries via https://osfio/nk7wf. This scoping review was undertaken and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis Extension for Scoping Reviews guidelines.40 These guidelines integrate the five-stages proposed by Arksey and O’Malley with regard to the conduct of scoping reviews.41
Eligibility criteria
Table 1 summarises the eligibility criteria that was used to screen the retrieved articles. The review included peer-reviewed studies involving human participants published in English over the previous 10 years. Studies on IP and/or AMS that did not primarily target C. difficile or CRKP were excluded as were studies that explored new diagnostic devices or therapeutic interventions in relation to the two organisms.
Table 1.
Eligibility criteria
| Proposed criteria | Refined criteria | |
| Population/setting | Healthcare facilities | Healthcare facilities and healthcare workers |
| Intervention/ exposure | AMS interventions for C. diff or CRKP | Infection prevention and antimicrobial stewardship interventions primarily targeting C. difficile and/or CRKP |
| Comparison | No intervention | No intervention |
| Outcome | Control of C. diff and/or CRKP | Changes in use of antimicrobial agents associated with C. difficile or CRKP. Compliance with infection prevention (IP) interventions Risk of C. difficile and CRKP |
| Study designs | All study designs | Observational studies, quasi-experimental studies, randomised controlled trials |
AMS, antimicrobial stewardship; C. diff, Clostridioides difficile; CRKP, carbapenem-resistant Klebsiella pneumoniae.
Information sources
The search for literature was conducted across electronic databases accessible through the Bangor University library search engine, bibliographies, key journals and websites for relevant organisations. The specific databases searched were MEDLINE via EBSCOhost, PubMed Open Access via NCBI, Web of Science Core Collection and CINAHL Plus via EBSCOhost (see search strategy in online supplemental file 1). The search for sources was undertaken with the assistance of the Bangor University librarian between 22 April 2020 and 30 September 2020. To ensure that the search was comprehensive and inclusive, a search of additional sources including unpublished and grey literature, general searches on Google Scholar as well as PhD theses and dissertations was conducted.
bmjopen-2021-051983supp001.pdf (38.4KB, pdf)
Study selection
Two reviewers independently applied the inclusion and exclusion criteria on the retrieved articles for inclusion in this review and resolved any disagreements through discussions with the third reviewer acting as an arbitrator.
Data charting
The data items extracted (see online supplemental file 2) included the reference, the study type, the study objectives, population or setting, country, the intervention, intervention duration, healthcare workers involved, outcome measures or findings and the conclusions of the study. Twenty percent of the extracted data was checked for completeness and accuracy by two reviewers who exchanged their extracted data for checking. See online supplemental file 2 for presentation of the extracted study characteristics.
bmjopen-2021-051983supp002.pdf (110.9KB, pdf)
Results collation, summary, and report compilation
The extracted data were organised into themes and a narrative synthesis was conducted. The subsequent sections provide a narrative synthesis of the existing literature on IP and AMS interventions targeting C. difficile and CRKP as well as the identified gaps in line with the study objectives.
Patient and public involvement
There were no patients involved in the conduct of this scoping review.
Results
Selection of studies
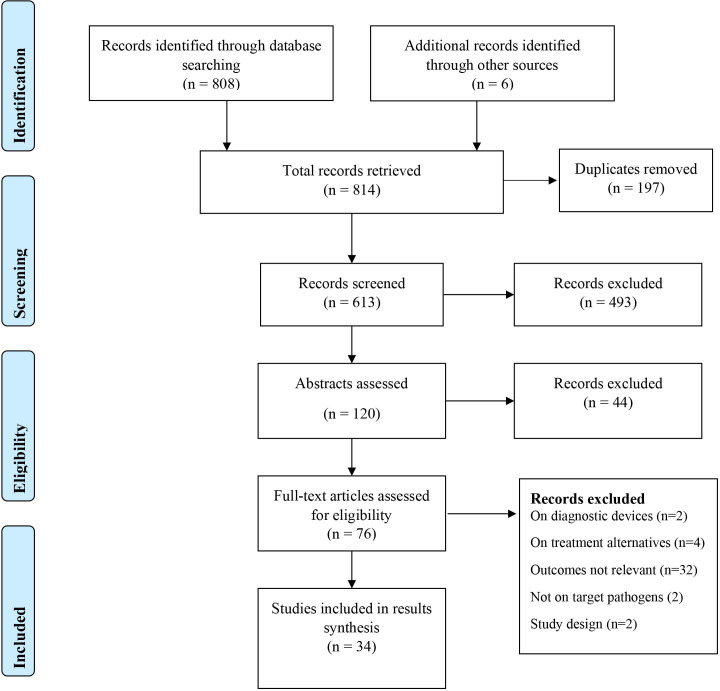
The PRISMA diagram in figure 1 summarises the study screening and selection process. Thirty-four studies were ultimately included in the current review.
Figure 1.
PRISMA flow diagram summarising the study screening and selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Characteristics of selected studies
Sixteen studies (see table 2) focussed on C. difficile42–57 and 18 studies (see table 3) focussed on CRKP.58–75 The studies varied in their designs with the majority (n=31) being quasi-experiments. Other study designs included cohort studies (n=2) and one secondary analysis of a randomised controlled trial. Twenty-seven studies were undertaken prospectively, whereas seven studies followed a retrospective approach. 32.4% (n=11) of the studies were conducted in the USA,45 48–52 54 56 57 66 71 whereas two studies each are based in Canada42 44 and Greece.65 67 Four of the retrieved studies were conducted in Italy,43 47 64 72 while Israel55 60 63 and China59 69 70 had three studies each. Finally, the selected articles included one study each from Japan,53 UK,46 South Africa, Denmark,61 Brazil,62 France,68 South Korea,73 Hungary74 and the Netherlands.75 There were variations in the study populations with three studies on K. pneumoniae involving neonates in the neonatal intensive care unit,70 71 74 whereas 31 studies involved adults admitted for care within the hospital settings. All the studies on C. difficile involved adult populations.70 71 74
Table 2.
IP and AMS interventions targeting C. difficile
| References | ||||||||||||||||
| 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | |
| Interventions | ||||||||||||||||
| Surveillance/screening | – | – | – | – | ✓ | – | ✓ | – | – | – | – | ✓ | – | – | – | – |
| Alerts and notifications | – | – | – | – | – | – | ✓ | – | – | – | – | ✓ | – | – | ✓ | – |
| Isolation precautions | – | – | – | – | – | – | ✓ | – | – | – | – | ✓ | – | ✓ | ✓ | ✓ |
| Environmental disinfection | – | – | – | – | – | – | – | – | – | ✓ | – | – | – | ✓ | – | ✓ |
| Audits and feedback | ✓ | – | ✓ | ✓ | – | ✓ | – | – | – | – | ✓ | ✓ | ✓ | – | – | – |
| Consultations | – | ✓ | ✓ | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Antimicrobial policies and/protocols | – | ✓ | – | – | ✓ | – | – | – | – | – | ✓ | – | – | – | ✓ | – |
| Care bundles | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ✓ |
| Staff education | – | ✓ | ✓ | – | ✓ | – | – | – | – | – | ✓ | – | – | ✓ | – | – |
| Biocidal (Cu2O) linen | – | – | – | – | – | – | – | ✓ | ✓ | – | – | – | – | – | – | – |
| Intervention duration (months) | 24 | 18 | 18 | 12 | 16 | 18 | – | 8 | 27 | 27 | 16 | 12 | 13 | 24 | – | 22 |
| Behaviour change elements addressed | ||||||||||||||||
| Capability | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Opportunity | – | ✓ | ✓ | – | – | ✓ | – | – | – | – | – | – | – | – | – | – |
| Motivation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
AMS, antimicrobial stewardship; C. difficile, Clostridioides difficile; IP, infection prevention.
Table 3.
IP and AMS interventions targeting CRKP
| References | ||||||||||||||||||
| 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | |
| Interventions | ||||||||||||||||||
| Surveillance/screening | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | ✓ | ✓ |
| Alerts and notifications | ✓ | – | ✓ | ✓ | – | ✓ | – | ✓ | ✓ | – | – | – | – | – | – | – | – | – |
| Isolation precautions | ✓ | – | ✓ | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – |
| Environmental disinfection | – | – | ✓ | – | – | – | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | – | – | – |
| Audits and feedback | – | – | – | ✓ | – | ✓ | – | – | – | ✓ | – | – | – | ✓ | – | ✓ | – | – |
| Consultations | – | – | – | – | – | – | ✓ | – | – | – | – | – | – | – | – | – | – | – |
| Antimicrobial policies and protocols | ✓ | – | – | ✓ | ✓ | – | – | – | – | – | – | – | – | – | – | – | ✓ | – |
| Care bundles | – | – | – | – | – | – | – | – | – | – | – | ✓ | – | – | – | – | – | – |
| Staff education and/patient education | – | – | ✓ | ✓ | – | – | – | – | – | ✓ | – | – | – | ✓ | – | ✓ | – | – |
| Intervention duration (months) | 36 | 14 | 48 | 36 | 24 | 14 | 2 | 36 | 6 | 17 | 4 | 24 | 8 | <1 | 2 | 72 | 3 | 6 |
| Behaviour change elements addressed | ||||||||||||||||||
| Capability | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Opportunity | – | – | – | ✓ | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Motivation | – | – | – | ✓ | ✓ | – | – | ✓ | – | – | – | – | – | – | – | – | – | – |
AMS, antimicrobial stewardship; CRKP, carbapenem-resistant Klebsiella pneumoniae; IP, infection prevention.
Synthesis of results
Interventions
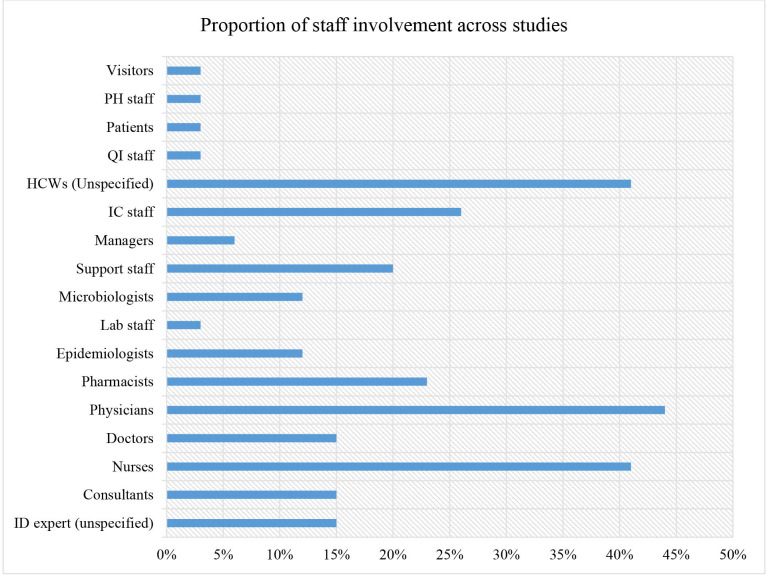
Broadly, the interventions entailed components of AMS and/or IP measures targeting C. difficile and CRKP. Tables 2 and 3 provide an outline of the specific AMS or IP components included across the included studies. The duration of interventions varied across the studies from 3 weeks up to 6 years.73 The interventions involved various cadres of professionals namely infectious disease experts, consultants, nurses, doctors, physicians, pharmacists, epidemiologists, laboratory personnel, microbiologists and support staff (cleaners, caregivers, housekeepers, paramedics, porters and environmental officers). Additional cadres involved include managers, infection control staff, unspecified clinicians/medical personnel, quality improvement staff, patients, public health staff and patient visitors. Figure 2 summarises the proportions while online supplemental file 2) highlights the specific cadres of health professionals included across study interventions.
Figure 2.
Proportion of staff involvement in infection prevention interventions targeting C. difficile and K. pneumoniae in healthcare settings per staff cadre. C. difficile, Clostridioides difficile; HCW, healthcare workers; IC, infection control; ID, infectious diseases; K. pneumoniae, Klebsiella pneumoniae; PH, public health; QI, quality improvement.
The interventions tended to be multifaceted involving the implementation of at least two strategies to achieve the intended outcomes as highlighted in tables 2 and 3. The strategies employed in interventions targeting C. difficile and how they were combined across studies are also summarised in table 2.
The most common strategy targeting C. difficile reported across seven studies involved the use of audits and feedback.42 44 45 47 52–54 This entailed reviewing the prescribed antibiotics by an antimicrobial pharmacist42 44 45 47 52 54 or the infection control team53 and feedbacking to the prescriber. In some instances, the audits were undertaken offsite using electronic records systems44 45 and teleconferences. Audits were also combined with staff education sessions organised on identified gaps aimed at optimising the use of antimicrobials.44 52 Some interventions combined the audits with formulary restrictions and treatment protocols occasionally requiring approval prior to issuing a prescription.52 Another intervention combined audits with screening patients and notifying physicians on detection of C. difficile, promptly isolating infected patients and monitoring appropriate use of antibiotics with prompt feedback to the responsible prescribers.53 Additional interventions with a component of staff education included bedside infectious diseases consultation,43 restricting the use of broad spectrum antibiotics43 46 55 and contact precautions.55 Bedside consultations involved a part-time infectious diseases expert reviewing antibiotic prescriptions three times a week and discussing these with attending physicians.43 This was coupled with revising antimicrobial treatment protocols and educating staff on reducing the appropriate use of antimicrobials.43 Finally, an intervention undertaken in a hospital caring for older adults involved educating all healthcare workers on isolation precautions and environmental disinfection as well as restricting the use of broad spectrum antibiotics.55
A multisite collaborative intervention involving an IP bundle also promoted adherence to isolation precautions and an environmental cleaning protocol.57 The isolation precautions included nursing patients in a single room, hand washing at recommended times and the use of appropriate personal protective equipment namely gloves, and disposable aprons. Environmental cleaning entailed the use of appropriate decontamination agents to clean the patient environment and reduce the presence of C. difficile. A single-centre study56 combined isolation precautions with a computer-generated real-time notification system for toxigenic C. difficile results and a treatment protocol using vancomycin only or vancomycin with metronidazole. The final study on isolation precautions48 also incorporated an automated system that tracked C. difficile results and triggered alerts on the patient’s electronic records as well as automatically ordering for the appropriate isolation precautions thus aiding the healthcare personnel’s actions. Three standalone interventions49–51 aimed at reducing the bioavailability of C. difficile in the hospital environment. One multisite randomised controlled trial employed four disinfection strategies for environmental cleaning following the discharge of C. difficile patients.51 These strategies included standard disinfection with an ammonium solution or 10% hypochlorite (bleach), standard disinfection with ultraviolet (UV) light or bleach with UV light, bleach only or UV light with bleach.51 Finally, two quasi-experiments involved replacing hospital linen with biocidal copper oxide impregnated bedsheets, pillow cases, washcloths and towels.49 50
Interventions targeting CRKP included surveillance and/or active screening through the use cultures,58 60 63–66 68–72 74–76 alerts and notifications on detection of CRKP,58 60 61 63 65 66 isolation precautions,58 60 63–66 68–72 74 76 environmental decontamination,60 64 66 67 69–71 antimicrobial audits and feedback,61 63 67 71 specialist consultations,64 antimicrobial policies and/or protocols,58 61 62 74 care bundles69 and staff and/or patient education.60 61 67 71 72 The most common strategy targeting CRKP appears to be surveillance or active screening through cultures to detect the presence of CRKP. One surveillance intervention58 involved the use of a flagging system for suspected patients at the emergency department, cohorting active cases, sampling cultures from hands of healthcare personnel and the environment and a policy restricting the use of carbapenems. Another multisite intervention63 combined routine screening of patients with mandatory isolation of confirmed cases with dedicated staff looking after the patients and mandatory notification of all carbapenem-resistant cases to public health authorities. Similarly, a surveillance intervention65 in a 250-bed general hospital required adherence to isolation precautions and compulsory notification of public health authorities on identified cases.
An outbreak containment intervention58 in a tertiary hospital employed active screening of patients, disinfection of the environment and respiratory equipment and isolation precautions. One standalone intervention investigated the effectiveness of active screening on detection of CRKP cases in an ICU setting,59 while another study tracked sporadic hospital outbreaks using whole genome sequencing.75 An observational study used rectal swabs for the active surveillance of CRKP in a cancer centre and a tertiary hospital.66 Subsequently, the confirmed cases were promptly isolated requiring healthcare personnel’s adherence to contact precautions and environmental cleaning protocols.66 Other surveillance interventions similarly effected isolation precautions for confirmed cases68 combined with either environmental cleaning protocols, staff education, adherence audits or a bathing protocol.67 68 70–72 74 An intervention based in an Israeli medical centre rolled out isolation guidelines in combination with staff education and environmental cleaning protocols supported with a computerised system for flagging CRKP cases.60 A multidisciplinary intervention in a 510-bed Danish university hospital employed Kotter’s eight stages of change61 by delivering staff training and notification systems to enhance isolation precautions, and appropriate use of antimicrobial agents. An AMS intervention in a Brazilian tertiary care hospital examined the effectiveness of a restrictive antimicrobial policy on the use of carbapenems.62 Finally, a south-Korean based study in a 900-bed tertiary university hospital examined the effectiveness of enhanced contact isolation precautions on CRKP incidence. This was delivered through staff education, auditing prescriptions, discontinuing inappropriate antibiotics within 72 hours and strict adherence to contact precautions including hand hygiene, single use gowns and gloves.
Outcomes reported from IP and AMS interventions targeting C. difficile and CRKP
The key outcomes reported across the studies included consumption of antimicrobial agents42–44 46 47 52 53 and/or associated costs,42–47 52 53 58 61 62 incidence of C. difficile42–47 49–55 57 or incidence and/resistance rates of CRKP,58–64 67 69 73 74 as well as risk of other HCAIs,44 51 53 57 60 67 69 outbreak containment,65 66 68 70–72 75 adherence to IP precautions,44 50 57 64–66 70 73 74 time savings,48 56 hospital stay74 and associated mortality rates.43 Tables 4 and 5 summarise the reported outcomes.
Table 4.
Summary of outcomes for interventions targeting C. difficile
| Key outcomes | References | |||||||||||||||
| 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | |
| Antimicrobials use (DDD/1000 PDs) | ↓310 | ↓200 | ↓6.58 | ↓124 | ↓141 | ↓34 | ↓10.7 | |||||||||
| Antimicrobials use (% reduction) | 11 | 47 | 46 | 72.5–95 | 22 | 12 | 37 | |||||||||
| Antibiotics cost (↓%) | ↓54 | ↓24 | ↓51 | |||||||||||||
| Antimicrobials streamlining (%/week) | ↑52 | |||||||||||||||
| Resistance rates | ||||||||||||||||
| CD risk/100 000 or/10 000 PDs (postintervention) | ⇔ | 12 | 14 | 55 | 60 | 2.8 | 170 | 2.8 | 11 | 16 | ⇔ | 85 | ||||
| CD absolute risk (%) | ⇔ | ↓67 | ↓46 | ↓83 | ↓77 | ↓31 | ↓51 | ↑87 | ↓5 | ↓71 | ↓36 | ↓71 | ⇔ | ↓37 | ||
| Risk of HCAIs (AR) |
|
↓25 | 17–25 | ⇔ | ↓ | ↓ | ||||||||||
| % reduction in time for start of treatment | 64 | |||||||||||||||
| Time savings (h/1000 admissions) | ↓43 | |||||||||||||||
| Hospital stay | ||||||||||||||||
| Adherence to infection prevention precautions (%) | ↓6 | ↑95 | ||||||||||||||
| Mortality | ⇔ | |||||||||||||||
↓, significant reduction; ↑, significant increase; ⇔, no significant changes (remained the same); ●, outbreak was contained.
AR, absolute risk; CD, Clostridioides difficile; C. difficile, Clostridioides difficile; DDD, daily defined doses; HCAIs, healthcare-associated infections; PD, patient days.
Table 5.
Summary of outcomes for interventions targeting CRKP
| Key outcomes | References | |||||||||||||||||
| 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | |
| Antimicrobial use (DDD/1000 PDs) | ↓ | 13 | ||||||||||||||||
| Antimicrobials use (% reduction) | ↓75 | ↓21 | ||||||||||||||||
| Antibiotics cost (↓%) | ||||||||||||||||||
| Antimicrobials streamlining (%/week) | ||||||||||||||||||
| Resistance rates | ↓ | ⇔ | ● | ↓ | ||||||||||||||
| CRKP risk/100 000 or/10 000 PDs | 18 | 0.5 | 23% | ↓ | 56 | ↓ | ↓ | ● | 28 | 0.9 | ↓ | |||||||
| CRKP absolute risk (%) | ↓97 | ↓92 | ↓17 | 12 | 12 | ● | ● | 10 | ● | ● | ● | ● | 46 | ↓ | ● | |||
| Risk of HCAIs (AR) | ↓55 | ↑59 | ↓84 | |||||||||||||||
| % reduction in time for start of treatment | ||||||||||||||||||
| Time savings (h/1000 admissions) | ||||||||||||||||||
| Hospital stay (%PDs) | ↓15 | |||||||||||||||||
| Adherence to IP precautions (%) | ↑ | ↑ | ↑ | ↑ | ↑35 | ↑29 | ||||||||||||
| Mortality | ||||||||||||||||||
↓, significant reduction; ↑, significant increase; ⇔, no significant changes (remained the same); ●, outbreak was contained.
AR, absolute risk; CRKP, carbapenem-resistant Klebsiella pneumoniae Ca; DDD, daily defined doses; HCAIs, healthcare-associated infections; IP, infection prevention; PD, patient days.
Interventions targeting C. difficile
Antimicrobial use
Seven studies reported variations in the consumption of antimicrobial agents following the stewardship interventions.42 44 45 47 52–54 The changes in antimicrobial use were reported in daily defined doses per 1000 patient days (DDD/1000 PDs). Reduction in the use of antimicrobials ranged between 6.58 DDDs/1000 PDs and 310 DDDs/1000 PDs. The least (11%) reduction in antimicrobial use was reported from an intervention that involved audits for prescribed antibiotics and providing feedback to the prescribers.42 The largest (79%) reduction in antimicrobials use was reported following an intervention involving restrictive antimicrobial policies and staff education.46 A 54% reduction in antimicrobial costs was reported from an intervention involving half-hour monthly staff education sessions on AMS and audits of prescribed antibiotics using a structured electronic checklist.44 679 patients from two internal medicine units in a tertiary care hospital were observed over 18 months in the study.44 One study reported a 52% improvement in antimicrobial streamlining following weekly reviews of prescribed antibiotics combined with remote consultations with an infectious diseases pharmacist through teleconferencing.54 The latter study was conducted in a 141-bed community hospital over 13 months.54 None of the C. difficile targeting interventions reported on the resistance rates for specific antimicrobial agents following their implementation.
Risk of CDIs, other HCAIs and associated mortality
Fourteen studies reported on the impact of the interventions on the risk of C. difficile infections (CDIs) or other HCAIs.42–47 49–55 57 The highest overall reduction of 83% in absolute risk of CDIs was reported from a 12-month antimicrobial audits and feedback intervention involving physicians and pharmacists in a 212-bed Massachusetts hospital.45 On the other hand, a 24-month multisite intervention among leukaemia patients involving antimicrobial audits and feedbacks42 reported no significant change on the risk of CDIs and associated mortality. Similarly, a second 24-month cross-sectional study involving older adults from two Israeli hospitals that entailed staff education, environmental disinfection and isolation precautions had no impact on the risk of CDIs.55 Regarding the effect of CDI interventions on other HCAIs, an AMS intervention in a 150-bed spinal injury hospital involving bedside infectious diseases consultation, staff education and antimicrobial policies reported a 25% absolute risk reduction for other HCAIs43 but no differences on mortality between the experimental and control groups.43 A multisite RCT investigating the effectiveness of four environmental disinfection strategies reported no effect on the risk of other HCAIs.51 A 12-month intervention assessing the impact of intensified IP precautions on MDROs in a 409-bed Japanese tertiary hospital reported a reduction in the risk of other HCAIs but it is not clear whether this change was significant.53 Two studies involving the use of biocidal linen impregnated with copper oxide reported contradictory findings which could be partly due to the differences in study settings and how the interventions were delivered. The first study involved six hospitals in both urban and rural settings with a total of 1019 beds implemented over 8 months (568 397 PDs) and reported a 51% reduction in the risk of CDIs.49 The second study was conducted in one long-term care hospital over 27 months (29 342 PDs) reported an 87% increase in the risk of CDIs.50 In the latter study, the researchers acknowledged that study participants were never blinded possibly leading to the deterioration of contact precautions specifically hand hygiene that reduced by 6%.50
Adherence to isolation precautions
The highest (95%) improvement in adherence to isolation precautions was reported by a 22-month multisite (35 hospitals) intervention57 involving the use of an IP bundle with isolation precautions and an environmental cleaning protocol.57 On the other hand, an intervention involving the use of biocidal linen impregnated with copper oxide reported a 6% reduction in adherence to isolation precautions50 as discussed above.
Time savings
Two studies reported outcomes related to time savings.48 56 The first intervention involved treatment protocols for C. difficile, real-time computerised notifications of toxigenic C. difficile results and isolation precautions. This was undertaken in a 433-bed adults medical centre and recorded a 64% reduction in time prior to the initiation of appropriate antibiotics treatment.56 The second study involving active surveillance, an alert system and isolation precautions in a 410-bed hospital treating trauma, burns and cancer patients reported a 43% reduction in care hours per 1000 admissions.48 There were no studies on C. difficile that reported on the effect of interventions on the length of hospital stay.
Carbapenem-resistant K. pneumoniae
Antimicrobials use
Three studies58 69 70 reported on antimicrobial use as a key outcome of CRKP interventions. One study involving a flagging system for confirmed cases, isolation precautions and a carbapenems restriction policy in a 1000-bed tertiary university hospital reported a reduction in the use of meropenem.58 The second study employed Kotter’s stages of change61 in a multidisciplinary intervention involving staff education on isolation precautions and appropriate prescribing, notifications on prescription of restricted antibiotics and antimicrobial protocols in a 510-bed Danish hospital recorded a 75% reduction in antibiotics consumption.61 The last study involving restrictive antimicrobial policies reported a 21% (12.9 DDDs/1000 PDs) reduction in antibiotics use.62 Two interventions involving active surveillance through screening59 and staff education combined with isolation precautions73 reported a reduction of the resistance rates for K. pneumoniae. The first intervention was conducted over 14 months in an ICU setting in China,59 while the second intervention was undertaken in a 900-bed tertiary hospital in South Korea.73 A 24-month intervention in a tertiary hospital (200 beds) involving restriction of group two carbapenems recorded no changes in the resistance rates for K. pneumoniae.62
Risk of CRKP, other HCAIs, and associated mortality
The largest risk reduction (97%) for CRKP was reported from a 36-month hospital wide intervention that involved physicians, epidemiologists, nurses and the infection control team.58 The lowest reported reduction in the absolute risk of CRKP was from a 17-month multifaceted intervention that entailed active surveillance, isolation precautions, audits and feedback, environmental cleaning and staff education.67 Seven outbreak investigations did not have outcomes on the relative risk CRKP.65 66 68 70–72 75 An intervention involving staff education, isolation, environmental cleaning and computerised flagging of cases reported a 55% reduction in other HCAIs,60 while another intervention involving screening, isolation, environmental disinfection and care bundles reported an 84% reduction in other HCAIs over a 48-month period.69 On the other hand, one study reported a 59% rise in the risk of other HCAIs following an intervention that involved screening, isolation, environmental decontamination, audits and education over a 17-month duration.67 The intervention involved 601 patients retrospectively and 250 patients prospectively in the solid organ transplant (SOT) department. The increase in the incidence of other carbapenem-resistant organisms was attributed to the intrahospital transfer of carriers to the SOT department and the subsequent transfer of postsurgical patients to the ICU where they were allegedly colonised by the bacteria.67 There are no studies that reported on mortality associated with CRKP.
Hospital stay and adherence to contact precautions
A 3-month intervention involving 355 patients in a 17-bed neonatal intensive care unit in Hungary reported a 15% reduction in the hospitalisation duration with an associated 29% increase in adherence to contact precautions.74 Another 6 years intervention involving staff education reported a 35% improvement in adherence to contact precautions.73 Finally, four additional studies also reported an improvement in adherence to contact precautions.64–66 70
Application of behaviour change theory
There was only one study that explicitly stated the application of a behaviour change theory (Kotter’s stages of change theory),61 while the remaining 33 studies did not indicate whether they applied behaviour change principles or strategies in their interventions. However, 62.5% of the C. difficile interventions had a component that targeted modifying antibiotics prescription behaviours, and 31.3% of the interventions targeted improving compliance with IP bundles, screening, isolation, hand hygiene and environmental cleaning protocols (as summarised in table 2). However, 18.8% of the interventions lacked a behavioural component as they focussed on either replacing patient linen with biocidal copper oxide or tested the effectiveness of cleaning strategies on reducing the burden of C. difficile in hospital settings. On the other hand, 22.2% of CRKP interventions had a component targeting antibiotics prescription behaviours, whereas 94.4% of the interventions focussed on improving compliance with IP bundles, screening, isolation, hand hygiene and environmental cleaning protocols (as summarised in table 3).
A mapping of the interventions using the COM-B (capability, opportunity, motivation and behaviour) elements77 revealed that 81.3% of the studies on C. difficile focussed on improving the competence/capacity of healthcare workers to adopt the desired behaviour, and, 18.8% of the studies focussed on creating opportunities for healthcare workers to express the desired behaviour.43 44 47 None of the interventions targeting C. difficile had a component aimed at motivating healthcare workers to adopt desired behaviours as recommended in the COM-B framework. However, all the interventions targeting CRKP had a component aimed at improving the competence of healthcare workers regarding the desired behaviour, 5.6% of the interventions had a component focusing on opportunities for behaviour change and 16.7% of the studies addressed the motivation element for behaviour change.61 62 65
The strategies used to enhance the capability component of behaviour change included staff education on appropriate prescribing and/or IP precautions, trainee led audits and providing feedback;44–47 54 63 65 69 70 use of checklists, protocols and guidelines for antibiotics prescription, screening, isolation, hand hygiene and environmental cleaning44 48 52 58 63–67 69 71–74 and the use of alerts, notifications, information leaflets, signposts and stickers on the targeted behaviours.47 58 69 70 The strategies used to address the opportunity element of behaviour change included audits undertaken by trainee prescribers, opportunities to issue new prescriptions following review of the prescribed antibiotics during the patients’ hospitalisation period53 and bedside consultations with microbiologists, pharmacists and infectious diseases consultants.43 44 47 61
Discussion
Summary of evidence
This scoping review mapped studies on IP and AMS interventions targeting healthcare associated C. difficile and CRKP published between 2010 and 2019. Interventions on AMS included restrictive antimicrobial policies and treatment protocols, specialists’ consultations, notifications and alert systems, as well as audits and feedback (also referred to as academic detailing). Interventions on IP precautions aimed at curbing the healthcare-associated transmission of C. difficile and CRKP included surveillance through active screening and cultures, isolation precautions, environmental measures (disinfection and biocidal linen), use of care bundles and education of staff and or patients. Interventions targeting C. difficile appeared to focus more on AMS, while interventions targeting CRKP appeared to focus more on screening, isolation precautions or environmental disinfection as core strategies. Clostridioides difficile and CRKP belong to the wider group of ESKAPE pathogens that significantly contribute to the burden of HCAIs. The findings above also show that interventions targeting either C. difficile or CRKP have a significant impact on the healthcare associated risk of other ESKAPE pathogens. The interventions could also be applicable to interventions targeting other members of the ESKAPE pathogens in healthcare settings.
Based on the findings of this scoping review, we propose that the acronym ESCAPE-BIN (Education, Surveillance/Screening, Consultations, Audits, Policies and Protocols, Environmental measures, Bundles of care, Isolation and Notifications or alerts) is used to denote the common AMS and IP interventions targeting C. difficile and CRKP in healthcare settings. The proposed acronym provides a useful categorisation of the specific actions applicable to AMS programmes as broadly outlined in the core elements for AMS by the Centres for Disease Control and Prevention.78 This acronym could potentially improve the understanding of the core elements by AMS teams as it highlights the specific interventions that address the requirements of the core elements. These include educating clinicians on appropriate use of antibiotics, specialist consultations to provide required expertise in antimicrobial prescribing, as well as audits, feedback and surveillance to track and report on appropriate use of antimicrobials as outlined in the core elements.37 78 Furthermore, the acronym provides a quick reference for AMS teams that could be useful in identifying gaps in AMS programmes or mapping intervention priorities.
This study also set out to describe the key outcomes for IP and AMS interventions targeting healthcare associated C. difficile and CRKP. The identified outcomes included antimicrobial use, resistance rates of the targeted pathogens, risk reduction, adherence to IP precautions, hospital stay and time savings. The majority (56%) of the interventions targeting C. difficile appeared to focus more on the use of antimicrobial agents as a key outcome. This is consistent with available evidence on the inappropriate use of antimicrobial agents as a key risk factor for CDIs. Recent studies have shown that reducing the prescription of antimicrobials can potentially reduce the incidence of CDIs in both healthcare and community settings.79 80 Comparatively, only 16% of the interventions targeting CRKP reported an impact on the use of antimicrobial agents as summarised in the findings above.
This scoping review also sought to assess whether AMS and IP interventions targeting C. difficile and K. pneumoniae incorporated existing evidence on behaviour change. A systematic review on behaviour change frameworks identified three key components namely capability, opportunity and motivation (COM-B) as being critical for interventions targeting behaviour change.77 Capability refers to one’s capacity/ability (perceived or actual) to engage in a behaviour, while motivation comprises the cognitive and emotional processes that energise or directs a person’s behaviour. Finally, opportunity refers to factors extrinsic to an individual that make a desired behaviour possible, such as time, equipment and space.77 Broadly, the interventions assessed in this scoping review focussed on antimicrobial prescription behaviours and IP behaviours from a ‘capability’ or ‘opportunity’ perspective. However, it was not possible to ascertain whether a specific behaviour change framework was applied across the included studies except for one intervention that applied Kotter’s eight-steps model for organisational change61 and recorded the second largest (75%) sustained reduction in antimicrobials use over a 3-year period. Although Kotter’s model provides detailed guidance on organisational change, it s been criticised for being too top-down with more focus on the management staff as opposed to junior employees.81 Due to the limited information provided about the interventions, this review could not establish whether the interventions considered all the critical elements necessary for successful behaviour change namely capability, opportunity and motivation. Comparatively, interventions targeting CRKP generally appeared to impact more on the risk of other HCAIs when compared with interventions targeting C. difficile. This could be because CRKP interventions appeared to broadly target IP behaviours of healthcare personnel which cut across most pathogens while C. difficile interventions broadly targeted prescription behaviours which tend to be specific to the targeted organism.
Generally, IP and AMS interventions targeting C. difficile and CRKP in healthcare settings tend not to be based on behaviour change principles but are rather more adhoc and building interventions around behaviour change techniques (BCT) and their principles could potentially lead to greater success. There was limited evidence from the included studies on how the interventions influenced compliance with either IP or AMS interventions targeting C. difficile and CRKP. This scoping review also established that physicians tend to be involved more in IP and AMS interventions targeting C. difficile and CRKP in comparison to other cadres of healthcare professionals. Almost half of the interventions in the present study involved physicians which was slightly higher than nurses (44%), whereas support staff including care workers participated in nearly one-third of the interventions. In healthcare settings, physicians are among the least proportionate healthcare workers and their contact with patients may be less frequent compared with nurses and carers looking after patients round the clock. Consequently, it is also worth exploring whether proportionate variations in the cadres involved in IP and AMS interventions could have an influence on the key outcomes.
Conclusions
AMR represents a global threat requiring urgent measures to protect lives. Reducing the burden of AMR entails a host of multilevel approaches on IP and AMS. This review mapped out IP and AMS interventions targeting C. difficile and CRKP. These interventions include ESCAPE-BIN. The review also described the key outcomes for these interventions including antimicrobial use, cost reductions, resistance rates and risk of infection, time savings, hospital stay, as well as adherence to contact/IP precautions and protocols. Finally, the review established evidence gaps on the application of current evidence on behaviour change interventions and adherence to IP and AMS interventions.
Supplementary Material
Footnotes
Twitter: @ValMorrisonProf
Contributors: BOO conceived the idea, designed the study protocol and was the first reviewer; both JCH and VM reviewed the study protocol, methods and the final report. All the three authors discussed the findings of this study and contributed to the final report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data sets generated during and/or analysed during the current study are also available from the corresponding author on reasonable request through brk18vjr@bangor.ac.uk or bernardokeah@gmail.com.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Mohr KI. History of antibiotics research. Curr Top Microbiol Immunol 2016;398:237–72. 10.1007/82_2016_499 [DOI] [PubMed] [Google Scholar]
- 2.Clardy J, Fischbach MA, Currie CR. The natural history of antibiotics. Curr Biol 2009;19:R437–41. 10.1016/j.cub.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 1929;10:226–36. [Google Scholar]
- 4.Waksman SA. What is an antibiotic or an antibiotic substance? Mycologia 1947;39:565–9. 10.1080/00275514.1947.12017635 [DOI] [PubMed] [Google Scholar]
- 5.Houbraken J, Frisvad JC, Samson RA. Fleming’s penicillin producing strain is not penicillium chrysogenum but P. rubens. IMA Fungus 2011;2:87–95. 10.5598/imafungus.2011.02.01.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaffiri L, Gardner J, Toledo-Pereyra LH. History of antibiotics. from Salvarsan to cephalosporins. J Invest Surg 2012;25:67–77. 10.3109/08941939.2012.664099 [DOI] [PubMed] [Google Scholar]
- 7.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A 2018;115:E3463–70. 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huttner A, Harbarth S, Carlet J, et al. Antimicrobial resistance: a global view from the 2013 world healthcare-associated infections forum. Antimicrob Resist Infect Control 2013;2:31. 10.1186/2047-2994-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Légaré F, Labrecque M, Cauchon M, et al. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ 2012;184:E726–34. 10.1503/cmaj.120568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alumran A, Hou X-Y, Hurst C. Assessing the overuse of antibiotics in children in Saudi Arabia: validation of the parental perception on antibiotics scale (PAPA scale). Health Qual Life Outcomes 2013;11:39. 10.1186/1477-7525-11-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin MJ, Thottathil SE, Newman TB. Antibiotics overuse in animal agriculture: a call to action for health care providers. Am J Public Health 2015;105:2409–10. 10.2105/AJPH.2015.302870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim JM, Singh SR, Duong MC. Impact of national interventions to promote responsible antibiotic use: a systematic review. J Antimicrob Chemother 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorpe KE, Joski P, Johnston KJ. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 Billion annually. Health Aff 2018;37:662–9. 10.1377/hlthaff.2017.1153 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . The top 10 causes of death, 2018. Available: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed 25 Jan 2020].
- 15.Gasser M, Zingg W, Cassini A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in Switzerland. Lancet Infect Dis 2019;19:17–18. 10.1016/S1473-3099(18)30708-4 [DOI] [PubMed] [Google Scholar]
- 16.Zarb P, Coignard B, Griskeviciene J. Surveillance report - point prevalence survey of healthcareassociated infections and antimicrobial use in European acute care hospitals 2012. 10.2900/86011 [DOI]
- 17.Antonioli P, Bolognesi N, Valpiani G, et al. A 2-year point-prevalence surveillance of healthcare-associated infections and antimicrobial use in Ferrara university Hospital, Italy. BMC Infect Dis 2020;20:75. 10.1186/s12879-020-4791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med 2016;13:e1002150. 10.1371/journal.pmed.1002150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner-Lastinger LM, Abner S, Benin AL, et al. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: summary of data reported to the National healthcare safety network, 2015-2017. Infect Control Hosp Epidemiol 2020;41:19–30. 10.1017/ice.2019.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baptista AB, Ramos JMM, das Neves RR. Diversity of environmental and patients bacteria in the hospital Geral de Palmas-TO. J Bioenergy Food Sci 2015;2:160–4. [Google Scholar]
- 21.Singh NP, Rani M, Gupta K, et al. Changing trends in antimicrobial susceptibility pattern of bacterial isolates in a burn unit. Burns 2017;43:1083–7. 10.1016/j.burns.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 22.Gundogdu A, Kilic H, Ulu-Kilic A, et al. Epidemiological features of nosocomial bloodstream infections in pediatric patients. Klimik Dergisi 2016;29:29–35. 10.5152/kd.2016.07 [DOI] [Google Scholar]
- 23.Liu S, Wang M, Zheng L, et al. Antimicrobial resistance profiles of nosocomial pathogens in regional China: a brief report from two tertiary hospitals in China. Med Sci Monit 2018;24:8602–7. 10.12659/MSM.911229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores-Treviño S, Garza-González E, Mendoza-Olazarán S, et al. Screening of biomarkers of drug resistance or virulence in escape pathogens by MALDI-TOF mass spectrometry. Sci Rep 2019;9:18945. 10.1038/s41598-019-55430-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein K, Farmer J, Singhal S, et al. The use and misuse of antibiotics in dentistry: a scoping review. J Am Dent Assoc 2018;149:869–84. 10.1016/j.adaj.2018.05.034 [DOI] [PubMed] [Google Scholar]
- 26.Saha SK, Barton C, Promite S, et al. Knowledge, perceptions and practices of community pharmacists towards antimicrobial stewardship: a systematic scoping review. Antibiotics 2019;8:263. 10.3390/antibiotics8040263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson A, Ebata A, Macgregor H. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutton ML, Pehlivanoglu H, Vidor CJ, et al. Repurposing auranofin as a Clostridioides difficile therapeutic. J Antimicrob Chemother 2020;75:409–17. 10.1093/jac/dkz430 [DOI] [PubMed] [Google Scholar]
- 29.Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017;376:305–17. 10.1056/NEJMoa1602615 [DOI] [PubMed] [Google Scholar]
- 30.Dingle KE, Didelot X, Quan TP, et al. Effects of control interventions on Clostridium difficile infection in England: an observational study. Lancet Infect Dis 2017;17:411–21. 10.1016/S1473-3099(16)30514-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaidi AKM, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365:1175–88. 10.1016/S0140-6736(05)71881-X [DOI] [PubMed] [Google Scholar]
- 32.Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis 2019;19:1219–34. 10.1016/S1473-3099(19)30414-1 [DOI] [PubMed] [Google Scholar]
- 33.Ludden C, Moradigaravand D, Jamrozy D, et al. A one health study of the genetic relatedness of Klebsiella pneumoniae and their mobile elements in the East of England. Clin Infect Dis 2020;70:219–26. 10.1093/cid/ciz174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorrie CL, Mirceta M, Wick RR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 2017;65:208–15. 10.1093/cid/cix270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin RM, Cao J, Brisse S, et al. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 2016;1:261–77. 10.1128/mSphere.00261-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AD, Perencevich EN, Johnson JK, et al. Patient-to-patient transmission is important in extended-spectrum beta-lactamase-producing Klebsiella pneumoniae acquisition. Clin Infect Dis 2007;45:1347–50. 10.1086/522657 [DOI] [PubMed] [Google Scholar]
- 37.WHO . Antimicrobial stewardship programmes in healthcare facilities in low-and-middle-income countries: a WHO practical toolkit antimicrobial stewardship, France, 2019. Available: https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf?sequence=1&isAllowed=y [Accessed 21 Mar 2021]. [DOI] [PMC free article] [PubMed]
- 38.Teixeira Rodrigues A, Roque F, Falcão A, et al. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013;41:203–12. 10.1016/j.ijantimicag.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 39.Hulscher MEJL, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect 2017;23:799–805. 10.1016/j.cmi.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 40.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 41.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 42.So M, Mamdani MM, Morris AM, et al. Effect of an antimicrobial stewardship programme on antimicrobial utilisation and costs in patients with leukaemia: a retrospective controlled study. Clin Microbiol Infect 2018;24:882–8. 10.1016/j.cmi.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 43.Tedeschi S, Trapani F, Giannella M, et al. An antimicrobial stewardship program based on systematic infectious disease consultation in a rehabilitation facility. Infect Control Hosp Epidemiol 2017;38:76–82. 10.1017/ice.2016.233 [DOI] [PubMed] [Google Scholar]
- 44.Lee TC, Frenette C, Jayaraman D, et al. Antibiotic self-stewardship: trainee-led structured antibiotic time-outs to improve antimicrobial use. Ann Intern Med 2014;161:S53–8. 10.7326/M13-3016 [DOI] [PubMed] [Google Scholar]
- 45.Beaulac K, Corcione S, Epstein L, et al. Antimicrobial stewardship in a long-term acute care hospital using Offsite electronic medical record audit. Infect Control Hosp Epidemiol 2016;37:433–9. 10.1017/ice.2015.319 [DOI] [PubMed] [Google Scholar]
- 46.Dancer SJ, Kirkpatrick P, Corcoran DS, et al. Approaching zero: temporal effects of a restrictive antibiotic policy on hospital-acquired Clostridium difficile, extended-spectrum β-lactamase-producing coliforms and meticillin-resistant Staphylococcus aureus. Int J Antimicrob Agents 2013;41:137–42. 10.1016/j.ijantimicag.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 47.Elligsen M, Walker SAN, Pinto R, et al. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: a controlled interrupted time series analysis. Infect Control Hosp Epidemiol 2012;33:354–61. 10.1086/664757 [DOI] [PubMed] [Google Scholar]
- 48.Quan KA, Cousins SM, Porter DD, et al. Automated tracking and ordering of precautions for multidrug-resistant organisms. Am J Infect Control 2015;43:577–80. 10.1016/j.ajic.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 49.Butler JP. Effect of copper-impregnated composite bed linens and patient gowns on healthcare-associated infection rates in six hospitals. J Hosp Infect 2018;100:e130–4. 10.1016/j.jhin.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 50.Madden GR, Heon BE, Sifri CD. Effect of copper-impregnated linens on multidrug-resistant organism acquisition and Clostridium difficile infection at a long-term acute-care hospital. Infect Control Hosp Epidemiol 2018;39:1384–6. 10.1017/ice.2018.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson DJ, Moehring RW, Weber DJ, et al. Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR disinfection). Lancet Infect Dis 2018;18:845–53. 10.1016/S1473-3099(18)30278-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moffa MA, Walsh TL, Tang A, et al. Impact of an antimicrobial stewardship program on healthcare-associated Clostridium difficile rates at a community-based teaching hospital. J Infect Prev 2018;19:191–4. 10.1177/1757177418767760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki H, Senda J, Yamashita K, et al. Impact of intensive infection control team activities on the acquisition of methicillin-resistant Staphylococcus aureus, drug-resistant Pseudomonas aeruginosa and the incidence of Clostridium difficile-associated disease. J Infect Chemother 2013;19:1047–52. 10.1007/s10156-013-0621-x [DOI] [PubMed] [Google Scholar]
- 54.Yam P, Fales D, Jemison J, et al. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Heal Pharm 2012;69:1142–8. 10.2146/ajhp110512 [DOI] [PubMed] [Google Scholar]
- 55.Goltsman G, Gal G, Mizrahi EH, et al. The impact of intensive staff education on rate of Clostridium difficile-associated disease in hospitalized geriatric patients. Aging Clin Exp Res 2020;32:2393–8. 10.1007/s40520-019-01424-y [DOI] [PubMed] [Google Scholar]
- 56.Polen CB, Judd WR, Ratliff PD, et al. Impact of real-time notification of Clostridium difficile test results and early initiation of effective antimicrobial therapy. Am J Infect Control 2018;46:538–41. 10.1016/j.ajic.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 57.Koll BS, Ruiz RE, Calfee DP, et al. Prevention of hospital-onset Clostridium difficile infection in the new York metropolitan region using a collaborative intervention model. J Healthc Qual 2014;36:35–45. 10.1111/jhq.12002 [DOI] [PubMed] [Google Scholar]
- 58.Borer A, Eskira S, Nativ R, et al. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in southern Israel. Infect Control Hosp Epidemiol 2011;32:1158–65 https://www.researchgate.net/publication/51796179_A_Multifaceted_Intervention_Strategy_for_Eradication_of_a_Hospital-Wide_Outbreak_Caused_by_Carbapenem-Resistant_Klebsiella_pneumoniae_in_Southern_Israel 10.1086/662620 [DOI] [PubMed] [Google Scholar]
- 59.Liu P, Fang J, Gu X, et al. Active screening diminishes antibiotic resistance to main pathogenic bacteria in the ICU. Int J Clin Exp Med 2016;9:4685–9 https://pdfs.semanticscholar.org/e065/f92881251de8f61234f72795f2a1e35ad4a7.pdf [Google Scholar]
- 60.Ciobotaro P, Oved M, Nadir E, et al. An effective intervention to limit the spread of an epidemic carbapenem-resistant Klebsiella pneumoniae strain in an acute care setting: from theory to practice. Am J Infect Control 2011;39:671–7. 10.1016/j.ajic.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 61.Andersen SE, Knudsen JD, Bispebjerg Intervention Group . A managed multidisciplinary programme on multi-resistant Klebsiella pneumoniae in a Danish university hospital. BMJ Qual Saf 2013;22:907–15. 10.1136/bmjqs-2012-001791 [DOI] [PubMed] [Google Scholar]
- 62.Lima ALLM, Oliveira PRDde, Paula APde, et al. Carbapenem stewardship: positive impact on hospital ecology. Braz J Infect Dis 2011;15:1–5 https://www.sciencedirect.com/science/article/pii/S1413867011701313 10.1016/s1413-8670(11)70131-3 [DOI] [PubMed] [Google Scholar]
- 63.Schwaber MJ, Lev B, Israeli A, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011;52:848–55. 10.1093/cid/cir025 [DOI] [PubMed] [Google Scholar]
- 64.Agodi A, Voulgari E, Barchitta M, et al. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J Clin Microbiol 2011;49:3986–9. 10.1128/JCM.01242-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meletis G, Oustas E, Botziori C, et al. Containment of carbapenem resistance rates of Klebsiella pneumoniae and Acinetobacter baumannii in a Greek hospital with a concomitant increase in colistin, gentamicin and tigecycline resistance. New Microbiol 2015;38:417–21 http://ezproxy.bangor.ac.uk/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=26147150&site=ehost-live [PubMed] [Google Scholar]
- 66.Alrabaa SF, Nguyen P, Sanderson R, et al. Early identification and control of carbapenemase-producing Klebsiella pneumoniae, originating from contaminated endoscopic equipment. Am J Infect Control 2013;41:562–4. 10.1016/j.ajic.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 67.Geladari A, Karampatakis T, Antachopoulos C, et al. Epidemiological surveillance of multidrug-resistant gram-negative bacteria in a solid organ transplantation department. Transpl Infect Dis 2017;19:e12686. 10.1111/tid.12686 [DOI] [PubMed] [Google Scholar]
- 68.Kassis-Chikhani N, Saliba F, Carbonne A, et al. Extended measures for controlling an outbreak of VIM-1 producing imipenem-resistant Klebsiella pneumoniae in a liver transplant centre in France, 2003–2004. Euro Surveill 2010;15. 10.2807/ese.15.46.19713-en [DOI] [PubMed] [Google Scholar]
- 69.Li M, Wang X, Wang J, et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: a 4-year quasi-experimental before-and-after study. Antimicrob Resist Infect Control 2019;8:8. 10.1186/s13756-018-0453-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou J, Li G, Ma X, et al. Outbreak of colonization by carbapenemase-producing Klebsiella pneumoniae in a neonatal intensive care unit: Investigation, control measures and assessment. Am J Infect Control 2015;43:1122–4. 10.1016/j.ajic.2015.05.038 [DOI] [PubMed] [Google Scholar]
- 71.Cantey JB, Sreeramoju P, Jaleel M, et al. Prompt control of an outbreak caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J Pediatr 2013;163:672–9. 10.1016/j.jpeds.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 72.Gaibani P, et al. Successful containment and infection control of a carbapenem-resistant Klebsiella pneumoniae outbreak in an Italian hospital. New Microbiol 014;37:87–90 http://ezproxy.bangor.ac.uk/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=24531175&site=ehost-live [PubMed] [Google Scholar]
- 73.Kim N-H, Han W-D, Song K-H, et al. Successful containment of carbapenem-resistant Enterobacteriaceae by strict contact precautions without active surveillance. Am J Infect Control 2014;42:1270–3. 10.1016/j.ajic.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 74.Szél B, Reiger Z, Urbán E, et al. Successful elimination of extended-spectrum beta-lactamase (ESBL)-producing nosocomial bacteria at a neonatal intensive care unit. World J Pediatr 2017;13:210–6. 10.1007/s12519-016-0069-z [DOI] [PubMed] [Google Scholar]
- 75.Snitkin ES, Zelazny AM, Thomas PJ. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 2012;148:ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geladari A, Karampatakis T, Antachopoulos C, et al. Epidemiological surveillance of multidrug-resistant gram-negative bacteria in a solid organ transplantation department. Transpl Infect Dis 2017;19:e12686. 10.1111/tid.12686 [DOI] [PubMed] [Google Scholar]
- 77.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.CDC . Core elements of hospital antibiotic stewardship programs, Georgia, 2019. Available: https://www.cdc.gov/antibiotic-use/core-elements/hspital.html [Accessed 21 Mar 2021].
- 79.Dantes R, Mu Y, Hicks LA, et al. Association between outpatient antibiotic prescribing practices and community-associated Clostridium difficile infection. Open Forum Infect Dis 2015;2. 10.1093/ofid/ofv113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown K, Valenta K, Fisman D, et al. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 2015;175:626–33. 10.1001/jamainternmed.2014.8273 [DOI] [PubMed] [Google Scholar]
- 81.Joseph Galli B, Galli BJ. Change management models: a comparative analysis and concerns. IEEE Eng Manag Rev 2018;46:124–32. 10.1109/EMR.2018.2866860 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051983supp001.pdf (38.4KB, pdf)
bmjopen-2021-051983supp002.pdf (110.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information. The data sets generated during and/or analysed during the current study are also available from the corresponding author on reasonable request through brk18vjr@bangor.ac.uk or bernardokeah@gmail.com.