Abstract
Absent in melanoma 2 (AIM2) is a novel member of interferon (IFN)‐inducible PYHIN proteins. In innate immune cells, AIM2 servers as a cytoplasmic double‐stranded DNA sensor, playing a crucial role in the initiation of the innate immune response as a component of the inflammasome. AIM2 expression is increased in patients with systemic lupus erythematosus (SLE), psoriasis, and primary Sjogren's syndrome, indicating that AIM2 might be involved in the pathogenesis of autoimmune diseases. Meanwhile, AIM2 also plays an antitumorigenesis role in an inflammasome independent‐manner. In melanoma, AIM2 is initially identified as a tumor suppressor factor. However, AIM2 is also found to contribute to lung tumorigenesis via the inflammasome‐dependent release of interleukin 1β and regulation of mitochondrial dynamics. Additionally, AIM2 reciprocally dampening the cGAS‐STING pathway causes immunosuppression of macrophages and evasion of antitumor immunity during antibody treatment. To summarize the complicated effect and role of AIM2 in autoimmune diseases and cancers, herein, we provide an overview of the emerging research progress on the function and regulatory pathway of AIM2 in innate and adaptive immune cells, as well as tumor cells, and discuss its pathogenic role in autoimmune diseases, such as SLE, psoriasis, primary Sjogren's syndrome, and cancers, such as melanomas, non‐small‐cell lung cancer, colon cancer, hepatocellular carcinoma, renal carcinoma, and so on, hopefully providing potential therapeutic and diagnostic strategies for clinical use.
Keywords: AIM2, autoimmune diseases, cancers, cGAS‐STING
Herein, we provide an overview of the emerging research progress on the function and regulatory pathway of absent in melanoma 2 (AIM2) in innate and adaptive immune cells, as well as tumor cells, and discuss its pathogenic role in autoimmune diseases, such as systemic lupus erythematosus, psoriasis, primary Sjogren's syndrome, and cancers, such as melanomas, non‐small‐cell lung cancer, colon cancer, hepatocellular carcinoma, renal carcinoma, and so on, hopefully providing potential therapeutic and diagnostic strategies for clinical use.
1. INTRODUCTION
Absent in melanoma 2 (AIM2), discovered in melanoma in 1997, 1 was originally described as a novel member of interferon (IFN)‐inducible PYHIN proteins, which contains four members in humans (AIM2, IFI16, IFIX, and MNDA) and 13 homologous proteins (e.g., Aim2, p202, p204, and p205) in mice. 2 In 2009, four groups independently found that AIM2 is capable of recognizing cytosolic double stranded DNA (dsDNA) of pathogens‐associated or host origin, recruiting apoptosis‐associated speck‐like protein containing a CARD (ASC) and pro‐caspase‐1, and inducing caspase‐dependent inflammasome formation, thereby triggering mature interleukin 18 (IL‐18) and IL‐1β production or leading to gasdermin‐D (GSDMD)‐mediated pyroptosis,3, 4, 5, 6 which eventually results in the initiation of an innate immune response against pathogens invasion. 5 However, abnormal activation of AIM2‐mediated immune response in response to cellular perturbations has been reported to cause immune‐linked disorders such as systemic lupus erythematosus (SLE), 7 psoriasis, 8 primary Sjogren's syndrome,9, 10 and polyarthritis. 11 However, the underlying mechanism is poorly understood.
AIM2 was originally described as a tumor suppressor for melanoma. 1 Recent developments have indicated that AIM2 contributes to the tumorigenesis of non‐small‐cell lung cancer (NSCLC) via regulation of mitochondrial dynamics and inflammasome activation. 12 An inflammasome‐independent pathway of AIM2 has also been discovered, controlling intestinal cell proliferation, apoptosis, and metastasis in the regulation of colon cancer through suppression of AKT activation. 13 Therefore, dysregulation of AIM2 plays a multifarious role in the pathogenesis of tumors. Indeed, tumor‐suppressive properties of AIM2 have been identified in hepatocellular carcinoma, 14 renal carcinoma, 15 breast cancer, 16 colon cancer, 13 HPV‐infected cervical carcinoma, 17 and prostate cancer. 18 The absence of AIM2 promotes hepatocellular carcinoma metastasis, while overexpression of AIM2 induces breast cancer cell apoptosis.14, 16 More recently, activation of AIM2 was also shown to exert a carcinogenic effect in NSCLC, 12 squamous cell carcinoma (SCC),19, 20 and Epstein‐Barr virus‐associated nasopharyngeal carcinoma (EBV‐associated NPC). 21
In this review, we systemically summarize the current research progress on the function of AIM2 and discuss its pathogenic role in autoimmune diseases and tumors, providing a better understanding of disease pathogenesis and potential diagnostic and therapeutic targets.
2. ASSEMBLY AND ACTIVATION OF THE AIM2 INFLAMMASOME
The AIM2 proteins consist of two main domains: a C‐terminal HIN domain and an N‐terminal pyrin domain (PYD) (Figure 1). 22 Upon sensing pathogen‐derived dsDNA or release of dsDNA secondary to disruption of the integrity of the nuclear or mitochondrial envelope, the HIN domain directly recognizes dsDNA through its two oligonucleotides/oligosaccharide‐binding folds in a sequence‐independent manner. The PYD domain forms the structural template via ligand binding and oligomerization and interacts with the PYD of a recruiting adapter protein ASC, resulting in ASC polymerization.4, 5, 6, 23, 24, 25 Inactive zymogen procaspases‐1 are subsequently recruited into these multimolecular complexes via the CARD–CARD interaction, and cleaved into heterodimers consisting of a small subunit p10 and a large subunit p20. 26 Active caspase‐1 eventually leads to proteolytic cleavage of IL‐1β, IL‐18, and caspase substrate GSDMD, whose N‐terminal polymerizes at the cell membrane forming the GSDMD pores and mediating pyroptosis.26, 27, 28, 29, 30 Activation of the AIM2 inflammasome is a double‐edged sword. It provides immunosurveillance for foreign pathogens by the initiation of the innate immune response, and it maintains central nervous system homeostasis during normal nerve development by GSDMD‐mediated pyroptosis. 31 However, abnormal activation can also lead to tissue injury and inflammation such as that induced by ionizing radiation. 32
Figure 1.
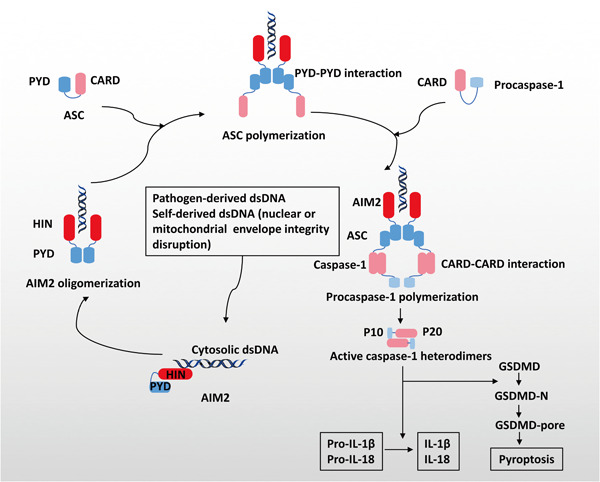
Assembly and activation of the AIM2 inflammasome. Sensing abnormal dsDNA in the cytoplasm triggers the assembly of the AIM2 inflammasome. The adapter protein ASC and the effector protein procaspase‐1 are recruited to participate in this activation process. AIM2, absent in melanoma 2; ASC, apoptosis‐associated speck‐like protein containing a CARD; dsDNA, double stranded DNA; GSDMD, gasdermin‐D; IL, interleukin
3. ROLE OF AIM2 IN IMMUNE CELLS
Since AIM2 is reported as a component of the inflammasome, the function and regulatory pathway of AIM2 in immune cells, especially in innate immune cells, has been intensively studied (Table 1). In the following paragraphs, we will summarize the role of AIM2 in innate and adaptive immune cells in detail.
Table 1.
Role of AIM2 in immune cells
| Cell types | Role of AIM2 | References |
|---|---|---|
| Macrophages | AIM2 inflammasome activation leads to antimicrobial host immune responses | 3, 4, 5, 27, 28, 33 |
| AIM2 inflammasome over‐activation leads to abnormal functional maturation of macrophages thereby facilitating the progression of SLE | ||
| AIM2 inflammasome activation leads to high levels of IL‐18 secretion from Kupffer cells thereby promoting hepatic NK cell activity and increasing NK cell‐dependent IFN‐γ | ||
| Induction caspase 1‐mediated cleavage of cGAS thereby upregulating PD‐L1 and IDO, which inhibit antibody‐dependent cellular cytotoxicity and T cell‐mediated cytotoxicity to evade the adaptive response | ||
| DCs | AIM2 inflammasome activation leads to antimicrobial host immune responses | 34, 35, 36, 37 |
| AIM2 inflammasome participates in the process of CD137L‐mediated monocyte to DC differentiation | ||
| AIM2 inflammasome activation leads to high levels of IL‐1α production thereby promoting lung cancer cell proliferation | ||
| Neutrophils | Expression of key components of AIM2 inflammasome | 38 |
| T cells | Induction antigen‐specific antibody response thereby enhancing adaptive immunity of CD8+ T cells | 39, 40, 41, 42, 43 |
| Upregulation of AIM2 in Treg restrain autoimmune diseases by reducing AKT–mTOR signaling and a T cell‐intrinsic role | ||
| B cells | Upregulation of AIM2 in gastric B cells inhibits CXCL16 production in the control of infiltration and retention of CD8+ T cells within chronic inflammatory tissues | 44, 45 |
| Upregulation of AIM2 preferential in mature memory CD27+ B cells of adults | ||
| Downregulation of AIM2 by FOXP1 in mature human B cells |
Abbreviations: AIM2, absent in melanoma 2; DCs, dendritic cells; IFN‐γ, interferon γ; IL, interleukin; SLE, systemic lupus erythematosus.
3.1. AIM2 in macrophages
Macrophages play an essential role in the innate immune response by engulfing pathogens and cellular debris, presenting antigens, and producing cytokines. 46 Numerous references in the literature confirm that phagocytosed pathogens‐ or self‐derived dsDNA can be recognized by AIM2 in macrophage cytosol, triggering caspase‐1‐dependent IL‐18 and IL‐1β release or leading to GSDMD‐mediated pyroptosis (Figure 2).3, 4, 5, 27, 28, 33 Additionally, the AIM2 inflammasome also drives apoptosis through caspase‐1‐mediated caspase‐3 activation in GSDMD−/− macrophages,47, 48 or caspase‐8‐mediated caspase‐3 activation in caspase‐1−/− macrophages.49, 50, 51 Recent studies show that caspase‐9 is also involved in this apoptosis pathway to induce caspase‐3 activation, which in turn cleaves GSDME inducing secondary necrosis or pyroptosis.47, 52, 53
Figure 2.
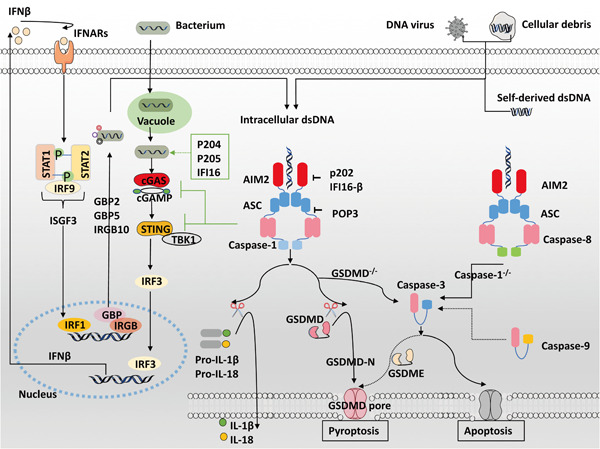
Regulation of the AIM2 inflammasome in macrophages. Cytosolic dsDNA from pathogens or host cells is sensed by AIM2, which attracts ASC and procaspase‐1 to trigger mature IL‐18 and IL‐1β production or GSDMD‐mediated pyroptosis. In GSDMD‐deficient macrophages, AIM2 induces caspase‐1‐dependent caspase‐3 activation whereas AIM2 drives caspase‐8‐dependent caspase‐3 activation without caspase‐1, resulting in apoptosis. The AIM2 inflammasome negatively regulates the cGAS/STING‐driven type I IFN response and can be inhibited by the IFN‐inducible proteins POP3 and IFI16‐β in humans or p202 in mice. AIM2, absent in melanoma 2; ASC, apoptosis‐associated speck‐like protein containing a CARD; dsDNA, double stranded DNA; GSDMD, gasdermin‐D; IFN, interferon; IL, interleukin
Copious evidence indicates the activation of AIM2 inflammasome involved in the elimination of macrophages infected with intracellular pathogens such as Francisella tularensis,34, 35, 36 Mycobacterial species,54, 55 Listeria monocytogenes,56, 57, 58 Brucella abortus, 59 Legionella pneumophila, 60 Streptococcus pneumonia, 61 vaccinia virus, 4 murine cytomegalovirus, 36 and Toxoplasma. 50 Aim2−/− mice are prone to infections. For example, Aim2−/− mice challenged with cytomegalovirus exhibit greater viral titers than wild‐type mice. 36 Aim2‐deficient mice are exceedingly susceptible to infections with Francisella tularensis or Mycobacterium tuberculosis and have a higher bacterial burden than wild‐type mice.34, 35 Bone marrow‐derived macrophages from these mice fail to generate an inflammasome.34, 35, 54, 62 This indicated in some cases AIM2 is indispensable for a complete antimicrobial host response.35, 36 Besides, macrophages lacking type I IFN response impair AIM2 inflammasome activation.34, 63, 64 This implies efficient activation of the AIM2 inflammasome requires the presence of type I IFN signaling during bacterial infections.35, 59, 61, 63, 64, 65
It has been recognized that bacterial DNA sensed by other cytosolic sensors, such as cGAS, leads to the binding and trafficking of STING and TANK‐binding kinase 1 (TBK1), 66 thus inducing transcription factor IRF3 phosphorylation and IFN‐β production.63, 67, 68, 69 IFNβ then combines with type I IFN receptors (IFNARs), 65 forming IFN‐stimulated gene factor 3 (ISGF3) complexes, inducing transcription factor IRF1 expression and further upregulating immunity‐related GTPase (IRG) and guanylate‐binding protein (GBP).63, 64, 70, 71 The combination of GBP2 and GBP5 with IRGB10 promotes bacteriolysis and subsequent complete activation of AIM2 inflammasome.63, 64, 71, 72, 73 However, type I IFN signaling also upregulates TNF‐related apoptosis‐inducing ligand (TRAIL) to activate apoptotic caspases and cause cell death, which is detrimental in vivo during Francisella infections.67, 70 Previous studies have shown that the AIM2 inflammasome inhibits the type I IFN pathway. 74 It is likely that the AIM2 inflammasome negatively regulates cGAS/STING‐driven type I IFN activity by depleting intracellular potassium, hampering STING and TBK1 binding, and inducing caspase 1‐dependent cleavage of cGAS.62, 74, 75, 76, 77
Macrophage phagocytosis contributes to tumor elimination. 78 However, the antitumor effects during antibody treatment also lead to a concomitant undesired effect. Macrophages can lead to immunosuppression of HER2+ breast cancers after antibody‐dependent cellular phagocytosis, in which AIM2 inflammasome plays a vital role. 77 Mechanistically, AIM2 dampens the cGAS–STING pathway by upregulating compensatory immunosuppressive checkpoints PD‐L1 and IDO, which inhibit antibody‐dependent cellular cytotoxicity and T cell‐mediated cytotoxicity to evade the adaptive response. 77 Thus, AIM2 inflammasome in macrophages can initiate the innate immune response, and participate in escape adaptive immune response for tumor cells.
3.1.1. The regulation of AIM2 in macrophages
AIM2 inflammasome pathway is tightly regulated in cells and affected by posttranslational modifications. The assembled inflammasomes in macrophages trigger the G protein RalB and undergo degradation by ubiquitinated TRIM11 via p62‐dependent selective autophagy.79, 80 Also, the mitochondrial serine protease HtrA2 regulates the duration and magnitude of AIM2 inflammasome activity in a protease activity‐dependent manner. 81
The upregulation of 25‐hydroxycholesterol in activated macrophages is required to restrain abnormal AIM2 inflammasome activation. 82 Human POP3 directly binds to AIM2 and dampens inflammasome activation. 83 Additionally, several other proteins within the IFN‐inducible PYHIN family have a close connection to AIM2 inflammasome activation signaling. IFI16‐β, a DNA sensor, inhibits AIM2‐mediated dsDNA sensing in human THP‐1 cells. 84 Similar to IFI16‐β, mouse p202, a lupus susceptibility factor, interacts with AIM2 and halts dsDNA‐dependent caspase activation. 3 Mouse p205 activates STING‐driven type I IFN signaling, whereas AIM2 dampens this pathway, likely by sequestering p205 from STING. 85 Mouse p204 or its human ortholog IFI16 are also involved in type I IFN production,67, 86 whereas the AIM2 inflammasome inhibits type I IFN signaling. Therefore, the relationship between AIM2 and the regulatory protein response to cytoplasmic DNA needs further understanding in the context of various diseases.
3.2. AIM2 in dendritic cells
Dendritic cells “swallow” pathogens by phagocytosis and migrate to lymphoid tissue, where they present processed antigens to T cells.87, 88 Similar to macrophages, activation of AIM2 inflammasome in dendritic cells (DCs) triggers an innate immune response against pathogen infection, including infection with Francisella novicida,36, 37 Mycobacterial species, 62 and Adenovirus. 89 However, interactions of the AIM2 inflammasome and type I IFN pathway within macrophages do not always mirror their crosstalk within DCs. For example, IFN‐β signaling is indispensable for inflammasome activation within macrophages, while it is partially required in DCs infected with Francisella novicida.34, 35, 36, 37 Additionally, AIM2 inflammasome activation and IL‐1β secretion are involved in the process of CD137L‐mediated monocyte to DC differentiation, which triggers a stronger T cell response against cancer‐associated viruses. 90 In contrast, AIM2 inflammasome activation and high levels of IL‐1α production in plasmacytoid dendritic cells (pDCs) can facilitate lung cancer cell proliferation. 91 Similarly, AIM2 in DCs may play an important role in host immune response and tumor microenvironment.
3.3. AIM2 in neutrophils
Neutrophils are swiftly attracted to areas of cell or tissue injury upon infection or inflammation and release multiple cytokines, including IL‐1β.92, 93, 94 Key components of the AIM2 inflammasome complexes are highly expressed, mainly in the cytoplasm of neutrophils. 38 However, the precise relationship between the AIM2 inflammasome and proinflammatory cytokine secretion in neutrophils is poorly understood.
3.4. AIM2 in adaptive immune cells
T cell reactivity against AIM2 has been found in patients with melanoma, suggesting that AIM2‐derived peptides are an ideal candidate for immunomonitoring. 95 Indeed, as a sensor of DNA, AIM2 induces an antigen‐specific antibody response and is used as an adjuvant to enhance therapeutic efficacy through CD8+ T cell adaptive immunity.39, 40, 41, 42 For example, AIM2 promotes the multifunctional CD8+ T cell activation elicited by the viral capsid protein 1 vaccine, 39 which favors long‐lasting protection against Coxsackievirus B3‐induced myocarditis. 40 Similarly, AIM2‐adjuvant vaccines exhibit antitumor therapeutic efficacy by heightening tumor‐specific CD8+ T cell immunity. 41 The involvement of AIM2 in vaccine‐induced immune responses requires type I IFN signaling via cGAS‐independent STING‐IRF7 signaling. 96 Interestingly, antigen‐specific adaptive immune responses are dramatically decreased in Aim2−/− mice after DNA vaccination, which is independent of IL‐1β and IL‐18. 42
A recently published paper in Nature shows that AIM2 is expressed at a much higher level in Treg cells than in innate immune cells, in both mice and humans, and can be induced by TGFβ. A high level of AIM2 can maintain the normal function of Treg cells and protect mice from developing autoimmune encephalomyelitis and inflammatory colitis by reducing AKT–mTOR signaling. 43 These findings indicating the inflammasome‐independent role of AIM2 in adaptive cells differ from AIM2's classic function in innate immunity.
Additionally, Aim2−/− mice have an increased frequency of gastric CD8+ TRM cells, whereas an elevated production of CXCL16 in B cells contributes to the suppression of homing receptors. 44 This result indicated that highly expressed AIM2 in gastric B cells may inhibit CXCL16 production in the control of infiltration and retention of CD8+ T cells within chronic inflammatory tissues, which is independent of inflammasome and IFN‐β signaling. 44 Furthermore, preferential expression of AIM2 is found in mature memory CD27+ B cells of adults. 45 However, AIM2 is directly suppressed by transcription factor FOXP1 in mature human B cells. 97 To date, the role of AIM2 in B cells is largely unknown.
3.5. AIM2 in autoimmune diseases
One of the hallmarks of SLE is dysregulation of type I IFN signaling. 98 Recent studies have revealed that mouse p202 and human IFI16‐β impede AIM2 inflammasome formation and stimulate IFN‐β production.84, 99, 100, 101 An altered AIM2 inflammasome system together with other IFN‐inducible protein‐mediated responses may trigger the pathogenesis of SLE. In line with this hypothesis, treatment of murine macrophages with IFN‐α differentially modulates the levels of AIM2 and p202. 99 Notably, reduced levels of AIM2 within immune cells as well as high production of p202 and IFN‐β have been described in lupus‐prone strains of mice.99, 102 Further studies have indicated that activation of the IRF5‐Blimp‐1‐p202 pathway increases SLE susceptibility, 103 possibly by affecting B cell differentiation. 104 B cell‐activating factor (BAFF), which is highly expressed in circulating CD3+ T cells and SLE patients' serum, decreased the expression of AIM2 but increased the levels of p202.105, 106 Therefore, the imbalance between AIM2 regulation and the type I IFN pathway leads to the progress of SLE.
Constitutive levels of AIM2 are higher in the livers, kidneys, and PBMCs of MRL/LPR mice than wild mice. 107 A reduction in AIM2 expression is found in macrophages isolated from female SLE patients versus sex‐matched healthy individuals. 108 A reduction in DNA methylation of AIM2 was identified in SLE patients in comparison with their healthy siblings. 109 All these findings suggest that organ‐, cell type‐, and sex‐dependent expression and epigenetic changes in AIM2 are related to the occurrence of SLE.99, 109, 110 Besides, defective clearance of apoptotic cell debris and aberrant activation of macrophages have been proposed to facilitate the progression of SLE. 98 Indeed, increased activation of AIM2 inflammasome has been found in unstimulated macrophages collected from male SLE patients, 108 leading to abnormal macrophage maturation and thereby contributing to the immune dysregulation of severe lupus nephritis. 7 Abnormal AIM2 activation in macrophages thereby contributes to disease severity of SLE.
Additionally, abundant cytosolic DNA can induce AIM2‐dependent release of IL‐18 and IL‐1β in keratinocytes and ductal salivary epithelia that contributes to the pathogenesis of psoriasis and primary Sjogren's syndrome, respectively.9, 111 Intriguingly, the antibacterial peptide LL‐37 interacts with DNA to prevent AIM2 inflammasome overactivation and IL‐1β oversecretion in psoriatic skin.111, 112 The silencing of AIM2 leads to inflammasome inactivation, inhibition of macrophage infiltration, and reduced signs of polyarthritis in self‐DNA‐driven chronic polyarthritis model DNase II−/− mice.11, 33, 113 Overall, dysregulation of the AIM2 response to self‐derived dsDNA plays a role as a key driver of autoimmune diseases.
4. ROLE OF AIM2 IN CANCERS
4.1. AIM2 in melanomas
The introduction of normal human chromosome 6 can inhibit human malignant melanoma growth rate, and restrain the tumorigenicity of nude mice. 114 Further studies showed that the expression of AIM2 was related to the inhibition of melanoma phenotype. 1 AIM2 is constitutively expressed in melanocytes from normal skin. 115 An increase in AIM2 expression occurs in common melanocytic nevi and most primary melanomas, whereas AIM2 expression is generally low or even nonexistent in melanoma metastases. 116 The upregulation of AIM2 can reverse malignant properties (Table 2). 1 AIM2 was initially demonstrated as a tumor‐suppressive factor in the control of tumorigenicity in melanoma.
Table 2.
Role of AIM2 in cancers
| Cancers | AIM2 expression | Role of AIM2 | Mechanism of AIM2 role | References |
|---|---|---|---|---|
| Melanoma | ↓ | Tumor‐suppressive | – | 1, 116 |
| Proliferation↓ Metastasis↓ | ||||
| NSCLC | ↑ | Tumor‐promoting | Regulation of inflammasome pathways (the IL‐1β/STAT3 pathway) | 12, 91, 117, 118, 119 |
| Proliferation↑ | ||||
| EMT↑ | Regulation of mitochondrial dynamics | |||
| Metastasis↑ | Induction of high levels of IL‐1α secretion from pDCs | |||
| Colon cancer | ↓ | Tumor‐suppressive | Regulation in an inflammasome‐independent manner (suppression of DNA‐PK‐mediated AKT activation and suppression of the PI3K/AKT pathway) | 13, 120, 121, 122, 123, 124, 125, 126 |
| Proliferation↓ Apoptosis↑ | ||||
| EMT↓ | ||||
| Intestinal stem cell proliferation | ||||
| Effects on the gut microbiota | Regulation of inflammasome pathways | |||
| Hepatocellular carcinoma | ↓ | Tumor‐suppressive | Regulation of inflammasome pathways (suppression of the mTOR‐S6K1 pathway and regulation of the HBx/AIM2/FN1 signaling axis) | 127, 128 |
| Proliferation↓ Metastasis↓ | ||||
| EMT↓ Liver inflammation | ||||
| Proliferative responses | ||||
| Renal carcinoma | ↓ | Tumor‐suppressive | Regulation of inflammasome pathways (induction of autophagy‐related gene expression) | 15, 129 |
| Proliferation↓ Apoptosis↑ | ||||
| Autophagy↑ Metastasis↓ | ||||
| Breast cancer | ↓ | Tumor‐suppressive | Antagonization of NF‐κB transcriptional activity (suppression of antiapoptotic protein expression) | 16, 77, 130, 131, 132 |
| Proliferation↓ Apoptosis↑ | ||||
| Metastasis↓ | Regulation of TRAIL‐expressing hMSCs through secretion of IFN‐β | |||
| EMT↓ | Immunosuppression of macrophages | |||
| Prostate cancer | ↓ | Tumor‐suppressive | Induction of loss of IFN signaling | 133, 134 |
| HPV‐infected cervical carcinoma | ↓ | Tumor‐suppression | Regulation of inflammasome pathways | 17, 135 |
| Pyroptosis↑ | (SIRT1–AIM2 axis) | |||
| SSC | ↑ | Tumor‐promoting | Regulation of inflammasome pathways | 19, 20 |
| Proliferation↑ Apoptosis↓ | ||||
| EMT↑ | Activation of NF‐κB signaling (induction of cell cycle regulatory genes expression) | |||
| Metastasis↑ | ||||
| EBV‐associated NPC | ↑ | Tumor‐promoting | Regulation of inflammasome pathways | 21 |
| Proliferation↑ | ||||
| Neutrophil recruitment |
Abbreviations: ↑, significantly higher; ↓, significantly lower; AIM2, absent in melanoma 2; EBV, Epstein–Barr virus; EMT, epithelial–mesenchymal transition; hMSCs, human mesenchymal stem/stromal cells; HPV, human papillomavirus; IFN, interferon; NF‐κB, nuclear factor κB; NPC, nasopharyngeal carcinoma; NSCLC, non‐small‐cell lung cancer; SCC, squamous cell carcinoma; TRAIL, TNF‐related apoptosis‐inducing ligand.
4.2. AIM2 in NSCLC
Dysregulation of inflammatory cytokines triggered by activation of inflammasomes in the lung is reported to promote lung tumorigenesis.136, 137 Considerable evidence has identified the carcinogenic role of highly expressed AIM2 in NSCLC (Figure 3).12, 138 Strong expression of AIM2 significantly increases cell viability, migration, and invasion in an inflammasome‐dependent way, whereas the silencing of AIM2 can suppress cell proliferation and result in G2/M phase accumulation. 12 IL‐1β upregulates hypoxia‐induced factor‐1α (HIF‐1α) that promotes tumor growth and metastasis through the NF‐kB‐dependent cyclooxygenases‐2 (COX‐2) pathway, whereas depleting IL‐1β reverses the malignant phenotype via dephosphorylation of STAT3.117, 138, 139 Also, AIM2 colocalizes with mitochondria, promoting tumor growth by regulating mitochondrial dynamics. 118 Increased mitochondrial fission induced by AIM2 causes downregulation of fusion‐related protein MFN2 and increases cellular reactive oxygen species 140 responses, thereby contributing to the MAPK/ERK pathway activation. 118 Thus, AIM2 in NSCLC exerts tumor‐promoting effects in an inflammasome‐dependent manner and regulation of mitochondrial dynamics, presumably triggering an IL‐1β/STAT3 response by the nuclear factor‐κB (NF‐κB)/COX‐2/HIF‐1α pathway and contributing to the MAPK/ERK pathway activation.
Figure 3.
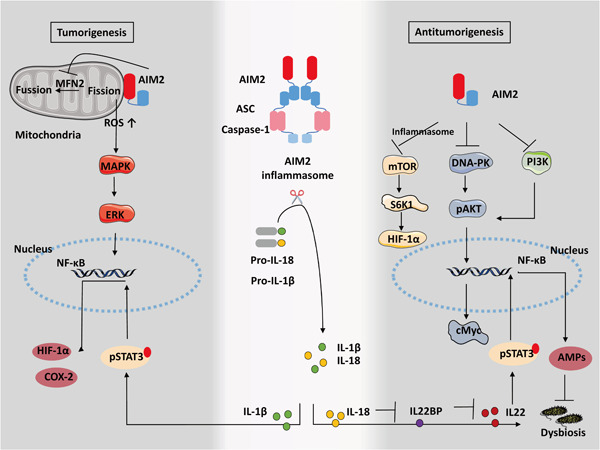
Tumorigenesis and antitumorigenesis of AIM2 in mammary tumors. The mature IL‐1β driven by AIM2 inflammasome promotes HIF‐1α expression through the NF‐κB/COX‐2 pathway. The association between mitochondria and AIM2 contributes to the MAPK/ERK signaling response that leads to tumorigenesis in NSCLC. In contrast, in colon cancer, DNA‐PK, as well as PI3K, are inhibited by AIM2, triggering the inactivation of AKT and c‐Myc, thereby preventing tumorigenesis. Additionally, the mTOR‐S6K1‐HIF‐1α pathway is inhibited by the AIM2 inflammasome, which plays an antitumorigenic role in hepatocellular carcinoma. AIM2, absent in melanoma 2; ASC, apoptosis‐associated speck‐like protein containing a CARD; dsDNA, double stranded DNA; GSDMD, gasdermin‐D; IFN, interferon; IL, interleukin
A higher percentage of cancer‐derived pDCs, which do not manifest cytotoxic activity but instead facilitate cancer cell growth, 119 are detected in NSCLC tumors than in control tissues. 91 Surprisingly, the AIM2 inflammasome is highly expressed in pDCs, which enhances calcium efflux and reactive oxygen species (ROS) release from mitochondria, resulting in calpain activation and IL‐1α release, thereby promoting tumor proliferation. 91 Therefore, inhibiting the expression of AIM2 in NSCLC may pave a new way for the treatment of NSCLC.
Epithelial–mesenchymal transition (EMT) is considered one of the essential steps in the progression of malignant tumors. 141 Activation of the AIM2 inflammasome promotes EMT in lung cancer. 119 The antitumor drug luteolin downregulates the expression of AIM2 and inhibits EMT of NSCLC. 142
4.3. AIM2 in colon cancer
Increased expression of AIM2 is detected in inflammatory bowel diseases, 143 but many of the AIM2 alterations that have been reported in patients with colon cancer involve a lack or even complete loss of AIM2 expression.144, 145 Several previous studies have analyzed the link between AIM2 expression and colon cancer progression. Surprisingly, the absence of AIM2 is closely related to poor clinicopathological features and prognosis.120, 121, 146 Similarly, many tumors are found in AIM2‐deficient mice. 13 This suggests that AIM2 is required to restrain the progression of colon cancer.
It has been revealed the AIM2 inflammasome in colonic epithelial cells is activated and contributes to the maintenance of intestinal integrity against dysbiosis. The AIM2 inflammasome executes its function by regulating the IL‐18/IL‐22BP/IL‐22 pathway and the levels of specific antimicrobial peptides (AMPs). 122 Genomic deletion of AIM2 in colon cancer contributes to DNA‐dependent protein kinase (DNA‐PK)‐induced AKT overactivation, thereby enhancing cell survival, 13 whereas AIM2 restoration promotes cell cycle G2/M arrest and prevents tumor cell proliferation and viability through suppression of DNA‐PK‐mediated AKT activation independent of the inflammasome.13, 123, 124, 125 Also, AIM2 contributes to tumor apoptosis by inhibiting the PI3K/AKT pathway. 126 Noticeably, AIM2 upregulation blocks EMT‐mediated cell migration and invasion in a manner that is dependent on AKT and inflammasome pathways.124, 147 Interestingly, AIM2 also restricts intestinal stem cell proliferation and expansion by dampening phosphorylation of AKT and cMyc activation, as evidenced by the great number of proliferating tumor‐initiating stem cells seen without AIM2. 125 Surprisingly, the decrease in AIM2 in intestinal epithelial cells is accompanied by overexpression of AIM2 in infiltrating immune cells that possibly leads to deleterious protumorigenic responses or angiogenesis. 121 Additionally, AIM2−/− mice that are hyper susceptible to tumorigenesis of colon cancer are aggravated by dysbiotic gut microbiota, but an exchange of the dysbiotic gut microbiota with that of healthy mice ameliorates this increased susceptibility.122, 148 Therefore, AIM2 plays a protective role in colon cancer. It exerts function by inflammasome‐independent manner, control of intestinal stem cell proliferation, and regulation of gut microbiota.
4.4. AIM2 in hepatocellular carcinoma
AIM2 expression is noticeably reduced in hepatocellular carcinoma patients and significantly associated with poor overall survival.14, 127 Activation of the AIM2 inflammasome triggers pyroptosis and suppresses cell proliferation and invasion, thereby inhibiting tumorigenicity in nude mice through antagonism of the mTOR‐S6K1‐HIF‐1α signaling axis. 14 Additionally, hepatitis B virus X protein (HBx)‐mediated loss of AIM2 is correlated with a high tendency for metastasis and activation of the EMT process in HBV‐related hepatocellular carcinoma tissues, which is mediated by fibronectin 1 (FN1) expression. 127 Consequently, DHA, as an autophagy promoter that triggers ROS‐induced nuclear and mitochondrial DNA damage, suppresses cell proliferation through the AIM2/caspase‐1 inflammasome complex. 149 In contrast, another study showed that AIM2 responds by promoting inflammation and proliferative responses during tumor initiation in an inflammasome‐dependent manner. 128 Elevated AIM2 in Kupffer cells promotes inflammation during carcinogenic liver injury. 128 Accordingly, the genetic deletion of AIM2 protected against tissue damage and cancer progression in the diethylnitrosamine‐induced hepatocellular carcinoma model. 128 This implies two contrasting roles of AIM2, which may be caused by different models or different disease stages.
4.5. AIM2 in renal carcinoma
Necrotic cell DNA can induce AIM2 inflammasome activation in macrophages that contributes to the progression of chronic kidney disease. 150 In contrast, AIM2 expression is dramatically decreased in renal carcinoma patients.15, 129 Restoration of AIM2 inhibits tumor proliferation, migration, and invasion while enhancing cell apoptosis by inducing autophagy‐related gene expression. 15 Additionally, the DNA vaccine‐containing renal carcinoma specific antigen carbonic anhydrase IX and AIM2 adjuvant, which promotes cells overexpressing inflammasome components and proinflammatory cytokines, can prevent tumor growth.41, 129 Thus, AIM2 is regarded as a tumor‐impressive factor and used as an adjuvant to attenuate renal carcinoma.
4.6. AIM2 in breast cancer
Activation of AIM2 drives apoptosis and suppresses proliferation through antagonizing NF‐κB transcriptional activity and inhibiting antiapoptotic protein expression, thereby restraining mammary tumor growth in vivo. 16 Besides, elevated levels of AIM2 in the tumor stroma can also suppress tumor cells in TRAIL‐sensitive triple‐negative breast cancers.130, 151 TRAIL‐expressing human mesenchymal stem/stromal cells (hMSCs) promote apoptosis and inhibit metastasis of breast cancer cells (MDA cells) after TNF‐α treatment by secreting IFN‐β. This occurs in an AIM2‐dependent manner triggered by DNA fragments from apoptotic cells.152, 153 Apoptotic cell‐derived DNA fragments sensed by AIM2 further increase TRAIL levels in hMSCs, resulting in feed‐forward stimulation and increased apoptosis of MDA cells. 153 Considering that TRAIL is modulated by type I IFNs and induces apoptosis during microbial infections, 154 both AIM2 and type I IFNs likely participate in the tumor‐suppressive microenvironment. Additionally, cancer‐associated fibroblasts (CAFs) exhibit functional similarities to hMSCs, indicating that hMSCs transition into CAFs.151, 153 A further understanding of how AIM2 mediates TRAIL upregulation upon exposure to cytosolic DNA fragments will provide a novel therapeutic approach for TRAIL‐sensitive cancer.
Cancerous cell‐free DNA (cfDNA) molecules promote tumor progression and resistance to anticancer therapies. 131 Ribosomal cfDNA (cf‐rDNA) exists in circulating cfDNA in breast cancer patients. 155 Exposure to extracellular cf‐rDNA molecules stimulates the survival of tumor cells, represses AIM2 expression, and reduces apoptosis, thereby facilitating tumor malignancy by triggering TLR9‐MyD88‐NF‐kB signaling. 132 Taken together, these findings show that AIM2 exerts tumor suppression in breast cancer.
4.7. AIM2 in prostate cancer
Increased AIM2 expression and IL‐1β production in senescent prostate epithelial cells induce benign prostatic hyperplasia,156, 157 whereas decreased AIM2 expression is accompanied by the tumorigenesis of prostate cancer, which is characterized by the loss of IFN signaling.133, 134 IFN stimulation robustly induces the AIM2 inflammasome in prostate tumors. 18 Additionally, NLRP12 is significantly increased, which may promote tumor growth by triggering NF‐κB and IL‐1β signaling. 157 Further work is needed to investigate the potential function of these sensors in the control of prostate diseases.
4.8. AIM2 in HPV‐infected cervical cancer
The AIM2 inflammasome is known to trigger pyroptosis in response to DNA viruses4, 135; for example, AIM2 is activated against HPV16 infection in keratinocytes. 135 However, AIM2 expression is inhibited by the deacetylase Sirtuin 1 (SIRT1) through the destabilization of RELB messenger RNA in HPV‐infected cervical cancer, assisting HPV‐infected tumors in escaping antiviral immunity. 17 Pyroptosis triggered in SIRT1‐knockdown cells can be transmitted to naïve tumor cells via the intercellular transmission of the AIM2 inflammasome,4, 135 whereas pyroptotic death signaling can be prevented by SIRT1 restoration. 17 This implies AIM2 inflammasome plays the tumor‐suppressive role in HPV‐infected cervical cancer.
4.9. AIM2 in SCC
The high levels of AIM2 expression in intestinal epithelial cells in response to pathogenic infections indirectly inhibit AKT activation, reducing stem cell proliferation in colonic tumors. 125 In the epidermis, the AIM2 inflammasome promotes wound repair in the skin recovering from inflammation, which is accompanied by a high level of pAKT and proliferating epithelial stem cells. 158 However, this imbalance may lead to uncontrolled proliferative disease. Indeed, the AIM2 gene is overexpressed in both cutaneous and oral SCC.20, 159 Increased activation of the AIM2 inflammasome promotes cell viability and restrains apoptosis by inducing cell cycle regulatory gene expression in cutaneous SCC 20 or by activating NF‐κB signaling in p53‐deficient oral SCC. 159 Additionally, high levels of AIM2 are associated with strong tumor invasion through upregulation of the production of the invasion‐related proteinases MMP1 and MMP13 in cutaneous SCC. 20 Likewise, a high metastatic capacity of oral SCC cells is associated with increased EMT. 19 Depletion of AIM2 inhibits tumor growth and vascularization of SCC in vivo 20 and therefore AIM2 is a potential oncogenic driver in SCC.
4.10. AIM2 in EBV‐associated NPC
In EBV‐associated NPC, EBV and irradiation‐induced AIM2 inflammasome activation lead to mature IL‐1β release that promotes tumor proliferation. 21 Interestingly, when the IL‐1β level reaches a certain threshold, this effect can be reversed by the recruitment of immunostimulatory tumor‐associated neutrophils.21, 160 This implies that immune cells can be recruited by tumor cells to control the host response through activation of the AIM2 inflammasome. 21
5. AIM2 SERVES AS A THERAPEUTIC TARGET?
In the past decade, several groups have attempted to identify and develop treatment strategies by targeting AIM2. A series of animal experiments have been reported, especially in cancers (Table 3).
Table 3.
Animal data targeting AIM2
| Disease | Approach | References |
|---|---|---|
| Polyarthritis | Silence of AIM2 reduced signs of polyarthritis in self‐DNA driven chronic polyarthritis model DNase II−/−mice | 11 |
| NSCLC | Downregulation of AIM2 mediated by luteolin reduced tumorigenicity in the A549 and H460 xenograft mouse models | 142 |
| Colon cancer | Reciprocal exchange of gut microbiota with wild‐type mice reduced colorectal tumorigenesis in Aim2‐deficient mice | 125, 126, 147 |
| Hepatocellular carcinoma | Knockdown of AIM2 reduced tissue damage and cancer progression in the diethylnitrosamine‐induced hepatocellular carcinoma mode | 128 |
| Renal carcinoma | H1/pAIM2 nanoparticles attenuated tumor growth in 786‐O‐xenograft mice | 41, 161 |
Abbreviations: AIM2, absent in melanoma 2; NSCLC, non‐small‐cell lung cancer.
The traditional drug luteolin, which was developed as a treatment for NSCLC, was found to decrease the AIM2 inflammasome to therapeutic effect. 149 Recently, promoting an adaptive response with an AIM2‐adjuvanted vaccine has demonstrated therapeutic efficacy, relieving the symptoms of coxsackievirus B3‐induced myocarditis and renal carcinoma.40, 129 Thus, AIM2 is a potential therapeutic target for cancer treatment. However, the utility of this therapeutic strategy will vary depending on the function of AIM2 in different tumors. For example, gene‐based restoration of AIM2 in colon cancer may be a novel approach to cure AIM2‐deficient cancers by restraining DNA‐PK and PI3K, 13 whereas downregulation of AIM2 leads to inhibition of AIM2‐activated tumors. Furthermore, the tumor‐promoting effects of the AIM2 inflammasome in EBV‐associated NPC can be reversed by neutrophil recruitment upon irradiation. 21 However, irradiation‐induced nuclear gene damage can increase the potential risks of pneumonitis and chronic fibrosis via AIM2 inflammasome‐mediated cell death. 32 Similarly, chemotherapy‐induced intestinal toxicity is caused by AIM2 inflammasome activation. 162 Additionally, macrophages can be transformed into an immunosuppressive phenotype in a process mediated by the AIM2 inflammasome during antibody treatment.77, 163 The role of the AIM2 inflammasome in autoimmune disease and cancers is complex and multifaceted and more research is needed to clarify these relationships and interactions.
6. CONCLUSIONS AND PERSPECTIVES
In recent years, the underlying mechanism by which AIM2 inflammasome affects the innate immune response has been extensively studied. However, the effect of AIM2 in T and B cells remains unclear, which be a shortage if AIM2 is used as a therapeutic target but the current study cannot provide an overall knowledge of AIM2 in the immune system. Therefore, future study is needed to address this issue. Also, AIM2 displays either tumor‐suppressive function or tumorigenesis effects in different cancers. The contrasting roles of AIM2 in cancer are dependent on the model, the disease, and even the stage of the disease. However, AIM2 exerts an important role in the regulation of tumor local microenvironment. Further studies are needed to elucidate the exact mechanism by which self‐derived DNA triggers AIM2 activation and the interactions of AIM2 with other sensor pathways.
AUTHOR CONTRIBUTIONS
Huan Zhu wrote the manuscript, Christopher Chang, Vera Chan, and Ming Zhao did the editing, Haijing Wu and Qianjin Lu revised the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests.
Zhu H, Zhao M, Chang C, Chan V, Lu Q, Wu H. The complex role of AIM2 in autoimmune diseases and cancers. Immun Inflamm Dis. 2021;9:649‐665. 10.1002/iid3.443
Contributor Information
Qianjin Lu, Email: qianlu5860@csu.edu.cn.
Haijing Wu, Email: chriswu1010@csu.edu.cn.
REFERENCES
- 1. DeYoung KL, Ray ME, Su YA, et al. Cloning a novel member of the human interferon‐inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997;15(4):453‐457. [DOI] [PubMed] [Google Scholar]
- 2. Cridland JA, Curley EZ, Wykes MN, et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol Biol. 2012;12:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts TL, Idris A, Dunn JA, et al. HIN‐200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057‐1060. [DOI] [PubMed] [Google Scholar]
- 4. Hornung V, Ablasser A, Charrel‐Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase‐1‐activating inflammasome with ASC. Nature. 2009;458(7237):514‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandes‐Alnemri T, Yu JW, Datta P, Wu JH, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bürckstümmer T, Baumann C, Blüml S, et al. An orthogonal proteomic‐genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266‐272. [DOI] [PubMed] [Google Scholar]
- 7. Zhang W, Cai Y, Xu W, Yin Z, Gao X, Xiong S. AIM2 facilitates the apoptotic DNA‐induced systemic lupus erythematosus via arbitrating macrophage functional maturation. J Clin Immunol. 2013;33(5):925‐937. [DOI] [PubMed] [Google Scholar]
- 8. Yun M, Seo G, Lee JY, Chae GT, Lee SB. Epigallocatechin‐3‐gallate attenuates the AIM2‐induced secretion of IL‐1beta in human epidermal keratinocytes. Biochem Biophys Res Commun. 2015;467(4):723‐729. [DOI] [PubMed] [Google Scholar]
- 9. Vakrakou AG, Svolaki IP, Evangelou K, Gorgoulis VG, Manoussakis MN. Cell‐autonomous epithelial activation of AIM2 (absent in melanoma‐2) inflammasome by cytoplasmic DNA accumulations in primary Sjogren's syndrome. J Autoimmun. 2020;108:102381. [DOI] [PubMed] [Google Scholar]
- 10. Vakrakou AG, Boiu S, Ziakas PD, Xingi E, Boleti H, Manoussakis MN. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjogren's syndrome fueled by inflammagenic DNA accumulations. J Autoimmun. 2018;91:23‐33. [DOI] [PubMed] [Google Scholar]
- 11. Baum R, Sharma S, Carpenter S, et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II‐deficient mice. J Immunol. 2015;194(3):873‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M, Jin C, Yang Y, et al. AIM2 promotes non‐small‐cell lung cancer cell growth through inflammasome‐dependent pathway. J Cell Physiol. 2019;234(11):20161‐20173. [DOI] [PubMed] [Google Scholar]
- 13. Wilson JE, Petrucelli AS, Chen L, et al. Inflammasome‐independent role of AIM2 in suppressing colon tumorigenesis via DNA‐PK and Akt. Nat Med. 2015;21(8):906‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma X, Guo P, Qiu Y, et al. Loss of AIM2 expression promotes hepatocarcinoma progression through activation of mTOR‐S6K1 pathway. Oncotarget. 2016;7(24):36185‐36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chai D, Shan H, Wang G, et al. AIM2 is a potential therapeutic target in human renal carcinoma and suppresses its invasion and metastasis via overexpression of AIM2 enhances the hepatocellular carcinoma metastasis. Exp Cell Res. 2018;370(2):561‐570. [DOI] [PubMed] [Google Scholar]
- 16. Chen IF, Ou‐Yang F, Hung JY, et al. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 17. So D, Shin H‐W, Kim J, et al. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37(38):5191‐5204. [DOI] [PubMed] [Google Scholar]
- 18. Ponomareva L, Liu H, Duan X, et al. AIM2, an IFN‐inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11(10):1193‐1202. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura Y, Nakahata S, Kondo Y, et al. Overexpression of absent in melanoma 2 in oral squamous cell carcinoma contributes to tumor progression. Biochem Biophys Res Commun. 2019;509(1):82‐88. [DOI] [PubMed] [Google Scholar]
- 20. Farshchian M, Nissinen L, Siljamäki E, et al. Tumor cell‐specific AIM2 regulates growth and invasion of cutaneous squamous cell carcinoma. Oncotarget. 2017;8(28):45825‐45836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen LC, Wang LJ, Tsang NM, et al. Tumour inflammasome‐derived IL‐1beta recruits neutrophils and improves local recurrence‐free survival in EBV‐induced nasopharyngeal carcinoma. EMBO Mol Med. 2012;4(12):1276‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu A, Kabaleeswaran V, Fu T, Magupalli VG, Wu H. Crystal structure of the F27G AIM2 PYD mutant and similarities of its self‐association to DED/DED interactions. J Mol Biol. 2014;426(7):1420‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morrone SR, Matyszewski M, Yu X, Delannoy M, Egelman EH, Sohn J. Assembly‐driven activation of the AIM2 foreign‐dsDNA sensor provides a polymerization template for downstream ASC. Nat Commun. 2015;6:7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem. 2013;288(19):13225‐13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Micco A, Frera G, Lugrin J, et al. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci USA. 2016;113(32):E4671‐E4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180(5):941‐55. [DOI] [PubMed] [Google Scholar]
- 27. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660‐665. [DOI] [PubMed] [Google Scholar]
- 28. Kayagaki N, Stowe IB, Lee BL, et al. Caspase‐11 cleaves gasdermin D for non‐canonical inflammasome signalling. Nature. 2015;526(7575):666‐671. [DOI] [PubMed] [Google Scholar]
- 29. Wang B, Yin Q. AIM2 inflammasome activation and regulation: a structural perspective. J Struct Biol. 2017;200(3):279‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lugrin J, Martinon F The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol Rev. 2018;281(1):99‐114. [DOI] [PubMed] [Google Scholar]
- 31. Lammert CR, Frost EL, Bellinger CE, et al. AIM2 inflammasome surveillance of DNA damage shapes neurodevelopment. Nature. 2020;580(7805):647‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu B, Jin C, Li HB, et al. The DNA‐sensing AIM2 inflammasome controls radiation‐induced cell death and tissue injury. Science. 2016;354(6313):765‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jakobs C, Perner S, Hornung V. AIM2 drives joint inflammation in a self‐DNA triggered model of chronic polyarthritis. PLOS One. 2015;10(6):e0131702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jones JW, Kayagaki N, Broz P, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci USA. 2010;107(21):9771‐9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandes‐Alnemri T, Yu JW, Juliana C, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11(5):385‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rathinam VA, Jiang Z, Waggoner SN, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belhocine K, Monack DM. Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol. 2012;14(1):71‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bakele M, Joos M, Burdi S, et al. Localization and functionality of the inflammasome in neutrophils. J Biol Chem. 2014;289(8):5320‐5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chai D, Yue Y, Xu W, Dong C, Xiong S. AIM2 co‐immunization favors specific multifunctional CD8(+) T cell induction and ameliorates coxsackievirus B3‐induced chronic myocarditis. Antiviral Res. 2015;119:68‐77. [DOI] [PubMed] [Google Scholar]
- 40. Yin L, Chai D, Yue Y, Dong C, Xiong S. AIM2 co‐immunization with VP1 Is associated with increased memory CD8 T cells and mounts long lasting protection against coxsackievirus B3 challenge. Front Cell Infect Microbiol. 2017;7:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chai D, Shan H, Wang G, et al. Combining DNA vaccine and AIM2 in H1 nanoparticles exert anti‐renal carcinoma effects via enhancing tumor‐specific multi‐functional CD8(+) T‐cell responses. Mol Cancer Ther. 2019;18(2):323‐334. [DOI] [PubMed] [Google Scholar]
- 42. Suschak JJ, Wang S, Fitzgerald KA, Lu S. Identification of AIM2 as a sensor for DNA vaccines. J Immunol. 2015;194(2):630‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chou WC, Guo Z, Guo H, et al. AIM2 in regulatory T cells restrains autoimmune diseases. Nature. 2021;591:300‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El‐Zaatari M, Bishu S, Zhang M, et al. AIM2‐mediated/IFN‐beta‐independent regulation of gastric metaplastic lesions via CD8+ T cells. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Svensson A, Patzi Churqui M, Schluter K, Lind L, Eriksson K. Maturation‐dependent expression of AIM2 in human B‐cells. PLOS One. 2017;12(8):e0183268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643‐675. [DOI] [PubMed] [Google Scholar]
- 47. Tsuchiya K, Nakajima S, Hosojima S, et al. Caspase‐1 initiates apoptosis in the absence of gasdermin D. Nat Commun. 2019;10(1):2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sagulenko V, Vitak N, Vajjhala PR, Vince JE, Stacey KJ. Caspase‐1 Is an apical caspase leading to caspase‐3 cleavage in the AIM2 inflammasome response, independent of caspase‐8. J Mol Biol. 2018;430(2):238‐247. [DOI] [PubMed] [Google Scholar]
- 49. Pierini R, Juruj C, Perret M, et al. AIM2/ASC triggers caspase‐8‐dependent apoptosis in Francisella‐infected caspase‐1‐deficient macrophages. Cell Death Differ. 2012;19(10):1709‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fisch D, Bando H, Clough B, et al. Human GBP1 is a microbe‐specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J. 2019;38(13):e100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sagulenko V, Thygesen SJ, Sester DP, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20(9):1149‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase‐3 cleavage of a gasdermin. Nature. 2017;547(7661):99‐103. [DOI] [PubMed] [Google Scholar]
- 53. Rogers C, Fernandes‐Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase‐3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Saiga H, Kitada S, Shimada Y, et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol. 2012;24(10):637‐644. [DOI] [PubMed] [Google Scholar]
- 55. Yang Y, Zhou X, Kouadir M, et al. The AIM2 inflammasome is involved in macrophage activation during infection with virulent Mycobacterium bovis strain. J Infect Dis. 2013;208(11):1849‐1858. [DOI] [PubMed] [Google Scholar]
- 56. Meixenberger K, Pache F, Eitel J, et al. Listeria monocytogenes‐infected human peripheral blood mononuclear cells produce IL‐1beta, depending on listeriolysin O and NLRP3. J Immunol. 2010;184(2):922‐930. [DOI] [PubMed] [Google Scholar]
- 57. Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2‐mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7(5):412‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsuchiya K, Hara H, Kawamura I, et al. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol. 2010;185(2):1186‐1195. [DOI] [PubMed] [Google Scholar]
- 59. Costa Franco MM, Marim F, Guimarães ES, et al. Brucella abortus triggers a CGAS‐independent sting pathway to induce host protection that involves guanylate‐binding proteins and inflammasome activation. J Immunol. 2018;200(2):607‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cunha LD, Silva ALN, Ribeiro JM, et al. AIM2 engages active but unprocessed caspase‐1 to induce noncanonical activation of the NLRP3 inflammasome. Cell Rep. 2017;20(4):794‐805. [DOI] [PubMed] [Google Scholar]
- 61. Fang R, Hara H, Sakai S, et al. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun. 2014;82(6):2310‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan S, Shen H, Lian Q, et al. Deficiency of the AIM2‐ASC signal uncovers the STING‐driven overreactive response of type I IFN and reciprocal depression of protective IFN‐gamma immunity in mycobacterial infection. J Immunol. 2018;200(3):1016‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Man SM, Karki R, Malireddi RK, et al. The transcription factor IRF1 and guanylate‐binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16(5):467‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meunier E, Wallet P, Dreier RF, et al. Guanylate‐binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida . Nat Immunol. 2015;16(5):476‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204(5):987‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA‐mediated, type I interferon‐dependent innate immunity. Nature. 2009;461(7265):788‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol. 2015;194(7):3236‐3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang X, Shi H, Wu J, et al. Cyclic GMP‐AMP containing mixed phosphodiester linkages is an endogenous high‐affinity ligand for STING. Mol Cell. 2013;51(2):226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signaling. 2012;5(214):ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu Q, Man SM, Karki R, Malireddi RKS, Kanneganti TD. Detrimental type I interferon signaling dominates protective AIM2 inflammasome responses during francisella novicida infection. Cell Rep. 2018;22(12):3168‐3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Man SM, Karki R, Sasai M, et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase‐11‐NLRP3 inflammasomes. Cell. 2016;167(2):382‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sharma BR, Karki R, Kanneganti TD. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 2019;49(11):1998‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Man SM, Karki R, Kanneganti TD. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46(2):269‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Corrales L, Woo SR, Williams JB, McWhirter SM, Dubensky TW Jr., Gajewski TF. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J Immunol. 2016;196(7):3191‐3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhu Q, Zheng M, Balakrishnan A, Karki R, Kanneganti TD. Gasdermin D promotes AIM2 inflammasome activation and is required for host protection against Francisella novicida. J Immunol. 2018;201(12):3662‐3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Banerjee I, Behl B, Mendonca M, et al. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49(3):413‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Su S, Zhao J, Xing Y, et al. Immune checkpoint inhibition overcomes ADCP‐induced immunosuppression by macrophages. Cell. 2018;175(2):442‐457. [DOI] [PubMed] [Google Scholar]
- 78. Chao MP, Alizadeh AA, Tang C, et al. Anti‐CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non‐Hodgkin lymphoma. Cell. 2010;142(5):699‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shi CS, Shenderov K, Huang NN, et al. Activation of autophagy by inflammatory signals limits IL‐1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13(3):255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu T, Tang Q, Liu K, et al. TRIM11 suppresses AIM2 inflammasome by degrading AIM2 via p62‐dependent selective autophagy. Cell Rep. 2016;16(7):1988‐2002. [DOI] [PubMed] [Google Scholar]
- 81. Rodrigue‐Gervais IG, Doiron K, Champagne C, et al. The mitochondrial protease HtrA2 restricts the NLRP3 and AIM2 inflammasomes. Sci Rep. 2018;8(1):8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dang EV, McDonald JG, Russell DW, Cyster JG. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell. 2017;171(5):1057‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Khare S, Ratsimandresy RA, de Almeida L, et al. The PYRIN domain‐only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol. 2014;15(4):343‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang PH, Ye ZW, Deng JJ, et al. Inhibition of AIM2 inflammasome activation by a novel transcript isoform of IFI16. EMBO Rep. 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nakaya Y, Lilue J, Stavrou S, Moran EA, Ross SR. AIM2‐like receptors positively and negatively regulate the interferon response induced by cytosolic DNA. mBio. 2017;8(4):e00944‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Unterholzner L, Keating SE, Baran M, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Worbs T, Hammerschmidt SI, Forster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17(1):30‐48. [DOI] [PubMed] [Google Scholar]
- 88. Morell M, Varela N, Maranon C. Myeloid populations in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2017;53(2):198‐218. [DOI] [PubMed] [Google Scholar]
- 89. Eichholz K, Bru T, Tran TT, et al. Immune‐complexed adenovirus induce AIM2‐mediated pyroptosis in human dendritic cells. PLOS Pathog. 2016;12(9):e1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dharmadhikari B, Nickles E, Harfuddin Z, et al. CD137L dendritic cells induce potent response against cancer‐associated viruses and polarize human CD8(+) T cells to Tc1 phenotype. Cancer Immunol Immunother. 2018;67(6):893‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sorrentino R, Terlizzi M, Di Crescenzo VG, et al. Human lung cancer‐derived immunosuppressive plasmacytoid dendritic cells release IL‐1alpha in an AIM2 inflammasome‐dependent manner. Am J Pathol. 2015;185(11):3115‐3124. [DOI] [PubMed] [Google Scholar]
- 92. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159‐175. [DOI] [PubMed] [Google Scholar]
- 93. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173‐182. [DOI] [PubMed] [Google Scholar]
- 94. Mutua V, Gershwin LJ. A review of neutrophil extracellular traps (NETs) in disease: potential anti‐NETs therapeutics. Clin Rev Allergy Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Andersen RS, Andersen SR, Hjortsø MD, et al. High frequency of T cells specific for cryptic epitopes in melanoma patients. Oncoimmunology. 2013;2(7):e25374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Suschak JJ, Wang S, Fitzgerald KA, Lu S. A cGAS‐independent STING/IRF7 pathway mediates the immunogenicity of DNA vaccines. J Immunol. 2016;196(1):310‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van Keimpema M, Grüneberg LJ, Mokry M, et al. FOXP1 directly represses transcription of proapoptotic genes and cooperates with NF‐kappaB to promote survival of human B cells. Blood. 2014;124(23):3431‐3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Katsiari CG, Liossis SN, Sfikakis PP. The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal. Semin Arthritis Rheum. 2010;39(6):491‐503. [DOI] [PubMed] [Google Scholar]
- 99. Panchanathan R, Duan X, Arumugam M, Shen H, Liu H, Choubey D. Cell type and gender‐dependent differential regulation of the p202 and Aim2 proteins: implications for the regulation of innate immune responses in SLE. Mol Immunol. 2011;49(1‐2):273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Thygesen SJ, Takizawa KE, Robertson AAB, Sester DP, Stacey KJ. Compromised NLRP3 and AIM2 inflammasome function in autoimmune NZB/W F1 mouse macrophages. Immunol Cell Biol. 2019;97(1):17‐28. [DOI] [PubMed] [Google Scholar]
- 101. Yang CA, Chiang BL. Inflammasomes and human autoimmunity: a comprehensive review. J Autoimmun. 2015;61:1‐8. [DOI] [PubMed] [Google Scholar]
- 102. Panchanathan R, Duan X, Shen H, et al. AIM2 deficiency stimulates the expression of IFN‐inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J Immunol. 2010;185(12):7385‐7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Panchanathan R, Liu H, Liu H, et al. Distinct regulation of murine lupus susceptibility genes by the IRF5/Blimp‐1 axis. J Immunol. 2012;188(1):270‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Martins G, Calame K. Regulation and functions of Blimp‐1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133‐169. [DOI] [PubMed] [Google Scholar]
- 105. Chen Y, Yang M, Long D, et al. Abnormal expression of BAFF and its receptors in peripheral blood and skin lesions from systemic lupus erythematosus patients. Autoimmunity. 2020;53:1‐9. [DOI] [PubMed] [Google Scholar]
- 106. Panchanathan R, Choubey D. Murine BAFF expression is up‐regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity. Mol Immunol. 2013;53(1‐2):15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ding L, Dong G, Zhang D, Ni Y, Hou Y. The regional function of cGAS/STING signal in multiple organs: one of culprit behind systemic lupus erythematosus? Med Hypotheses. 2015;85(6):846‐849. [DOI] [PubMed] [Google Scholar]
- 108. Yang CA, Huang ST, Chiang BL. Sex‐dependent differential activation of NLRP3 and AIM2 inflammasomes in SLE macrophages. Rheumatology. 2015;54(2):324‐331. [DOI] [PubMed] [Google Scholar]
- 109. Javierre BM, Fernandez AF, Richter J, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20(2):170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shin JI, Lee KH, Joo YH, et al. Inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J Autoimmun. 2019;103:102299. [DOI] [PubMed] [Google Scholar]
- 111. Dombrowski Y, Peric M, Koglin S, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3(82):82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dombrowski Y, Schauber J. Cathelicidin LL‐37: a defense molecule with a potential role in psoriasis pathogenesis. Exp Dermatol. 2012;21(5):327‐330. [DOI] [PubMed] [Google Scholar]
- 113. Kawane K, Ohtani M, Miwa K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443(7114):998‐1002. [DOI] [PubMed] [Google Scholar]
- 114. DeRisi J, Penland L, Brown PO, et al. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996;14(4):457‐460. [DOI] [PubMed] [Google Scholar]
- 115. de Koning HD, Bergboer JG, van den Bogaard EH, et al. Strong induction of AIM2 expression in human epidermis in acute and chronic inflammatory skin conditions. Exp Dermatol. 2012;21(12):961‐964. [DOI] [PubMed] [Google Scholar]
- 116. de Koning HD, van Vlijmen‐Willems IM, Zeeuwen PL, Blokx WA, Schalkwijk J. Absent in melanoma 2 is predominantly present in primary melanoma and primary squamous cell carcinoma, but largely absent in metastases of both tumors. J Am Acad Dermatol. 2014;71(5):1012‐1015. [DOI] [PubMed] [Google Scholar]
- 117. Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL‐1beta‐mediated up‐regulation of HIF‐1alpha via an NFkappaB/COX‐2 pathway identifies HIF‐1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17(14):2115‐2117. [DOI] [PubMed] [Google Scholar]
- 118. Qi M, Dai D, Liu J, et al. AIM2 promotes the development of non‐small cell lung cancer by modulating mitochondrial dynamics. Oncogene. 2020;39:2707‐2723. [DOI] [PubMed] [Google Scholar]
- 119. Otsuki Y, Saya H, Arima Y. Prospects for new lung cancer treatments that target EMT signaling. Dev Dyn. 2018;247(3):462‐472. [DOI] [PubMed] [Google Scholar]
- 120. Zhang Z, Dong X, Yang X, et al. Expression and clinical significance of absent in melanoma 2 in colorectal cancer. Biomed Pharmacother. 2017;94:843‐849. [DOI] [PubMed] [Google Scholar]
- 121. Dihlmann S, Tao S, Echterdiek F, et al. Lack of absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int J Cancer. 2014;135(10):2387‐2396. [DOI] [PubMed] [Google Scholar]
- 122. Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL‐18/IL‐22/STAT3 pathway. Cell Mol Immunol. 2017;14(1):127‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Patsos G, Germann A, Gebert J, Dihlmann S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int J Cancer. 2010;126(8):1838‐1849. [DOI] [PubMed] [Google Scholar]
- 124. Griemert EV, Schwarzmaier SM, Hummel R, et al. Absent in melanoma 2 suppresses epithelial‐mesenchymal transition via Akt and inflammasome pathways in human colorectal cancer cells. J Cell Biochem. 2019;85:667‐680. [DOI] [PubMed] [Google Scholar]
- 125. Man SM, Zhu Q, Zhu L, et al. Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer. Cell. 2015;162(1):45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen J, Wang Z, Yu S AIM2 regulates viability and apoptosis in human colorectal cancer cells via the PI3K/Akt pathway. Onco Targets Ther. 2017;10:811‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen SL, Liu LL, Lu SX, et al. HBx‐mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11(9):1225‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128. Martínez‐Cardona C, Lozano‐Ruiz B, Bachiller V, et al. AIM2 deficiency reduces the development of hepatocellular carcinoma in mice. Int J Cancer. 2018;143(11):2997‐3007. [DOI] [PubMed] [Google Scholar]
- 129. Chai D, Liu N, Li H, et al. H1/pAIM2 nanoparticles exert anti‐tumour effects that is associated with the inflammasome activation in renal carcinoma. J Cell Mol Med. 2018;22(11):5670‐5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Rahman M, Davis SR, Pumphrey JG, et al. TRAIL induces apoptosis in triple‐negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113(2):217‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Niu Z, Tang W, Liu T, et al. Cell‐free DNA derived from cancer cells facilitates tumor malignancy through Toll‐like receptor 9 signaling‐triggered interleukin‐8 secretion in colorectal cancer. Acta Biochim Biophys Sin. 2018;50(10):1007‐1017. [DOI] [PubMed] [Google Scholar]
- 132. Malinovskaya EM, Ershova ES, Okorokova NA, et al. Ribosomal DNA as DAMPs signal for MCF7 Cancer Cells. Front Oncol. 2019;9:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Untergasser G, Koch HB, Menssen A, Hermeking H. Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer. Cancer Res. 2002;62(21):6255‐6262. [PubMed] [Google Scholar]
- 134. Xin H, Curry J, Johnstone RW, Nickoloff BJ, Choubey D. Role of IFI 16, a member of the interferon‐inducible p200‐protein family, in prostate epithelial cellular senescence. Oncogene. 2003;22(31):4831‐4840. [DOI] [PubMed] [Google Scholar]
- 135. Reinholz M, Kawakami Y, Salzer S, et al. HPV16 activates the AIM2 inflammasome in keratinocytes. Arch Dermatol Res. 2013;305(8):723‐732. [DOI] [PubMed] [Google Scholar]
- 136. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000‐14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391(10125):1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cho WC, Kwan CK, Yau S, So PP, Poon PC, Au JS. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets. 2011;15(9):1127‐1137. [DOI] [PubMed] [Google Scholar]
- 138. Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36(10):7501‐7513. [DOI] [PubMed] [Google Scholar]
- 139. Dauer DJ, Ferraro B, Song L, et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene. 2005;24(21):3397‐3408. [DOI] [PubMed] [Google Scholar]
- 140. Gao J, Shi LZ, Zhao H, et al. Loss of IFN‐γ pathway genes in tumor cells as a mechanism of resistance to anti‐CTLA‐4 therapy. Cell. 2016;167(2):397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Shi ZM, Wang L, Shen H, et al. Downregulation of miR‐218 contributes to epithelial‐mesenchymal transition and tumor metastasis in lung cancer by targeting Slug/ZEB2 signaling. Oncogene. 2017;36(18):2577‐2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yu Q, Zhang M, Ying Q, et al. Decrease of AIM2 mediated by luteolin contributes to non‐small cell lung cancer treatment. Cell Death Dis. 2019;10(3):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vanhove W, Peeters PM, Staelens D, et al. Strong upregulation of AIM2 and IFI16 inflammasomes in the mucosa of patients with active inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(11):2673‐2682. [DOI] [PubMed] [Google Scholar]
- 144. Woerner SM, Kloor M, Schwitalle Y, et al. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer. 2007;46(12):1080‐1089. [DOI] [PubMed] [Google Scholar]
- 145. Schulmann K, Brasch FE, Kunstmann E, et al. HNPCC‐associated small bowel cancer: clinical and molecular characteristics. Gastroenterology. 2005;128(3):590‐599. [DOI] [PubMed] [Google Scholar]
- 146. Liu R, Truax AD, Chen L, et al. Expression profile of innate immune receptors, NLRs and AIM2, in human colorectal cancer: correlation with cancer stages and inflammasome components. Oncotarget. 2015;6(32):33456‐33469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE. Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res. 2010;70(10):4151‐4162. [DOI] [PubMed] [Google Scholar]
- 148. Hu GQ, Song PX, Li N, et al. AIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infection. Mucosal Immunol. 2016;9(5):1330‐1339. [DOI] [PubMed] [Google Scholar]
- 149. Shi X, Wang L, Ren L, et al. Dihydroartemisinin, an antimalarial drug, induces absent in melanoma 2 inflammasome activation and autophagy in human hepatocellular carcinoma HepG2215 cells. Phytother Res. 2019;33(5):1413‐1425. [DOI] [PubMed] [Google Scholar]
- 150. Komada T, Chung H, Lau A, et al. Macrophage uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol. 2018;29(4):1165‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yoon N, Park MS, Shigemoto T, Peltier G, Lee RH. Activated human mesenchymal stem/stromal cells suppress metastatic features of MDA‐MB‐231 cells by secreting IFN‐beta. Cell Death Dis. 2016;7:e2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lee RH, Yoon N, Reneau JC, Prockop DJ. Preactivation of human MSCs with TNF‐alpha enhances tumor‐suppressive activity. Cell Stem Cell. 2012;11(6):825‐835. [DOI] [PubMed] [Google Scholar]
- 153. Yoon N, Park MS, Peltier GC, Lee RH. Pre‐activated human mesenchymal stromal cells in combination with doxorubicin synergistically enhance tumor‐suppressive activity in mice. Cytotherapy. 2015;17(10):1332‐1341. [DOI] [PubMed] [Google Scholar]
- 154. McNab F, Mayer‐Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Veĭko NN, Bulycheva NV, Roginko OA, et al. Ribosomal repeat in the cell free DNA as a marker for cell death. Biomed Khim. 2008;54(1):78‐93. [PubMed] [Google Scholar]
- 156. Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 157. Choi J, Shendrik I, Peacocke M, et al. Expression of senescence‐associated beta‐galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56(1):160‐166. [DOI] [PubMed] [Google Scholar]
- 158. Naik S, Larsen SB, Gomez NC, et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017;550(7677):475‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kondo Y, Nagai K, Nakahata S, et al. Overexpression of the DNA sensor proteins, absent in melanoma 2 and interferon‐inducible 16, contributes to tumorigenesis of oral squamous cell carcinoma with p53 inactivation. Cancer Sci. 2012;103(4):782‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double‐edged sword. Protein Cell. 2014;5(1):12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Chai D, Shan H, Wang G, et al. AIM2 is a potential therapeutic target in human renal carcinoma and suppresses its invasion and metastasis via enhancing autophagy induction. Exp Cell Res. 2018;370(2):561‐570. [DOI] [PubMed] [Google Scholar]
- 162. Lian Q, Xu J, Yan S, et al. Chemotherapy‐induced intestinal inflammatory responses are mediated by exosome secretion of double‐strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27(6):784‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Huang C, Zhu HX, Yao Y, et al. Immune checkpoint molecules. Possible future therapeutic implications in autoimmune diseases. J Autoimmun. 2019;104:102333. [DOI] [PubMed] [Google Scholar]


