Abstract
Background
Residents of prisons have experienced disproportionate COVID-19-related health harms. To control outbreaks, many prisons in the USA restricted in-person activities, which are now resuming even as viral variants proliferate. This study aims to use mathematical modelling to assess the risks and harms of COVID-19 outbreaks in prisons under a range of policies, including resumption of activities.
Methods
We obtained daily resident-level data for all California state prisons from Jan 1, 2020, to May 15, 2021, describing prison layouts, housing status, sociodemographic and health characteristics, participation in activities, and COVID-19 testing, infection, and vaccination status. We developed a transmission-dynamic stochastic microsimulation parameterised by the California data and published literature. After an initial infection is introduced to a prison, the model evaluates the effect of various policy scenarios on infections and hospitalisations over 200 days. Scenarios vary by vaccine coverage, baseline immunity (0%, 25%, or 50%), resumption of activities, and use of non-pharmaceutical interventions (NPIs) that reduce transmission by 75%. We simulated five prison types that differ by residential layout and demographics, and estimated outcomes with and without repeated infection introductions over the 200 days.
Findings
If a viral variant is introduced into a prison that has resumed pre-2020 contact levels, has moderate vaccine coverage (ranging from 36% to 76% among residents, dependent on age, with 40% coverage for staff), and has no baseline immunity, 23–74% of residents are expected to be infected over 200 days. High vaccination coverage (90%) coupled with NPIs reduces cumulative infections to 2–54%. Even in prisons with low room occupancies (ie, no more than two occupants) and low levels of cumulative infections (ie, <10%), hospitalisation risks are substantial when these prisons house medically vulnerable populations. Risks of large outbreaks (>20% of residents infected) are substantially higher if infections are repeatedly introduced.
Interpretation
Balancing benefits of resuming activities against risks of outbreaks presents challenging trade-offs. After achieving high vaccine coverage, prisons with mostly one-to-two-person cells that have higher baseline immunity from previous outbreaks can resume in-person activities with low risk of a widespread new outbreak, provided they maintain widespread NPIs, continue testing, and take measures to protect the medically vulnerable.
Funding
Horowitz Family Foundation, National Institute on Drug Abuse, Centers for Disease Control and Prevention, National Science Foundation, Open Society Foundation, Advanced Micro Devices.
Introduction
In the COVID-19 pandemic's first year, US prison populations had infection rates five to six times higher than in free-living populations, with mortality rates two to three times higher.1, 2, 3 Overcrowded congregate living spaces, inadequate testing, lack of personal protective equipment and adequate sanitation, mistrust of medical personnel, and policies that disincentivise symptom reporting by people who are incarcerated all increase outbreak risks in US prisons.3, 4 Non-pharmaceutical interventions (NPIs) to reduce transmission, such as mask wearing and physical distancing, are less feasible in such settings, which often house populations that are more medically vulnerable than the general population. Individuals who are incarcerated and living in large dormitories are at especially high risk for infection.5, 6
Whether prisons already have sufficient natural immunity from previous outbreaks to safely resume pre-epidemic activities without first achieving high vaccination levels is an open question.7 Two epidemiological features suggest otherwise.
First, the threshold level of natural immunity required to prevent outbreaks depends on contacts.8 This threshold is likely to be higher in prisons than in the general population given the population density. Since 2020, many prisons have halted in-person activities, including group therapy and outside visitation, to reduce contacts. While resumption of these activities could yield tangible benefits, including health and wellbeing improvements and better outcomes after release,9, 10 it also increases contacts and transmission risks that could overwhelm the protection conferred by built-up immunity.
Research in context.
Evidence before this study
Prisons in the USA, a country that accounts for almost a quarter of the global incarcerated population, have experienced devastating COVID-19 outbreaks. There has been a lack of evidence on how to prevent and mitigate outbreaks in prisons, and prison systems have taken a range of approaches to preventing infections as well as severe outcomes such as hospitalisations and deaths, with mixed results. A common approach was halting in-person activities (eg, group therapy or educational classes) to reduce transmission, with many prisons now considering resumption of such activities. We searched the published literature (using PubMed) and preprints (using medRxiv) using the search terms “covid-19 OR sars-cov-2 OR coronavirus” AND “prison OR prisons OR jail OR correctional OR carceral OR carcel” for studies published between Jan 1, 2020, and April 23, 2021, that assessed the safety of resuming in-person activities in carceral settings given COVID-19 vaccination and the proliferation of viral variants of concern. We did not find any studies meeting these criteria. Some studies have analysed the increased risks of dense and overcrowded congregate settings, including prisons, and others have used mathematical models to assess depopulation and non-pharmaceutical interventions. Studies have also called for the prioritisation of people who are incarcerated in vaccination efforts. However, we could not find any studies that have evaluated the impact of vaccines, assessed reopening scenarios, or considered the effects of viral variants on populations in correctional settings.
Added value of this study
To our knowledge, this is the first study to assess the impact of vaccination, reopening, and non-pharmaceutical interventions on COVID-19 outcomes in correctional settings. Our individual-level model is backed by comprehensive, daily, resident-level data from the Californian state prison system, the second largest state prison system in the USA. To enable generalisability across settings within and potentially beyond the USA, we model a variety of prison types and varying levels of baseline immunity from previous outbreaks. We also consider both single and repeated infection introductions into the prisons and analyse introductions of both COVID-19 viral variants of concern similar to the alpha variant (B.1.1.7) and wild-type virus.
Implications of all the available evidence
Our findings suggest that prisons need to consider a multifaceted approach to preventing and managing COVID-19 outbreaks if they want to safely resume in-person activities that are beneficial to the health and wellbeing of people who are incarcerated. This approach should include widespread vaccination for people who are incarcerated and continuation of non-pharmaceutical interventions such as masking and physical distancing, as well as regular surveillance testing and additional protections for medically vulnerable populations. As COVID-19 case rates stabilise in the USA but variants of concern proliferate, these conclusions constitute important evidence for correctional settings in the USA and potentially beyond.
Second, SARS-CoV-2 viral variants have emerged that are more transmissible, are more likely to result in severe disease, and can evade immunity from previous infection.11, 12, 13 Studies indicate that more transmissible variants' share of total COVID-19 cases is growing exponentially.14 The paucity of genetic sequencing in most US prisons3 increases the risk that a new variant will trigger a large outbreak before systems are aware of the introduction, limiting the efficacy of reactive measures such as mass testing and quarantine.
In the USA, states have taken different approaches, but most did not prioritise people who are incarcerated for COVID-19 vaccination.15 As of May 18, 2021, the latest available data indicated that about half of people incarcerated in US prisons had received at least one dose of COVID-19 vaccine.16 However, vaccine coverage varied widely across prison systems, ranging from 7% (Utah) to 91% (North Dakota). In the three largest prison systems in the country, coverage varied from 43% (federal) and 49% (Texas) up to 75% (California).
Whether current vaccination levels in US carceral settings, which made up almost a quarter of the global incarcerated population before the COVID-19 pandemic,17 are sufficient to halt transmission is thus unclear.18 As prison systems scale up vaccination for incarcerated populations,15 acceptance will determine achievable coverage. Even in settings where people who are incarcerated have been prioritised for vaccination, hesitancy remains a concern.3, 19 Our study uses simulation modelling to assess how the size and consequences of COVID-19 outbreaks stemming from the introduction of a viral variant into a US prison depend on baseline immunity, vaccine coverage, and whether and how activities are resumed.
Methods
Overview
Using a mathematical model, we assessed the impact of vaccination on COVID-19 in US prisons. We examined how three factors influence risks of outbreaks and severe outcomes: resumption of in-person activities, use of NPIs, and vaccination of individuals who are incarcerated. We also assessed the effects of three exogeneous factors: the introduction of a highly transmissible viral variant, baseline natural immunity from previous outbreaks, and whether only one or multiple introductions occur. We used detailed data from California state prisons and facilitate generalisability to other systems by simulating a range of prisons that differ in room occupancies, layouts, demographics, security levels, and contact patterns.
Stanford University's Institutional Review Board approved the protocol for the use and analysis of primary California Department of Corrections and Rehabilitation (CDCR) data used in this study (IRB-55835). Our study follows relevant EQUATOR network reporting guidelines (appendix pp 27–28).
Model
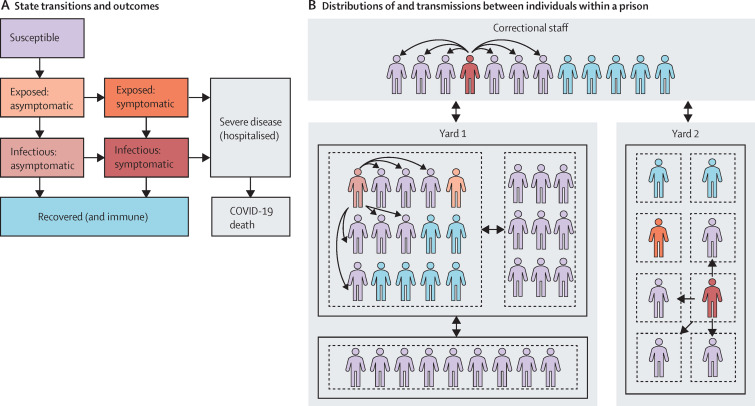
The transmission-dynamic stochastic microsimulation follows residents and correctional staff within a prison (figure 1 ; appendix pp 1–2). We simulated different prisons by instantiating the model with prison-specific characteristics. These include the prison's residential layout: composition of rooms (ie, cells or dormitories) and room occupancy (which has been identified as an important risk factor for prison outbreaks5, 6) and their organisation within buildings and yards. The characteristics also include the number of residents and their age, sex, comorbidities, security level, room assignment and type, and participation in prison labour or other out-of-room activities. Correctional staff are characterised by age; the staff population size varies by prison. We selected five specific prisons from California's 35 state prisons that vary across these characteristics and are comparable to other prison systems in the USA. Simulated prisons include a low-to-medium security men's prison consisting mostly of dormitories (rooms with at least three occupants), a low-to-medium security men's prison with a mix of dormitories and cells (rooms with no more than two occupants), a high-security men's prison with mostly cells, a women's prison with mixed security levels and mostly cells, and a medical prison that houses older residents and those with medical vulnerabilities mostly in cells (appendix pp 15–20). Notably, the medical prison simulated here is for male residents only; since California has no women's medical prison, medically vulnerable women are housed in two general population women's prisons, one of which is included in this study.
Figure 1.
Prison microsimulation model diagram
(A) Possible state transitions and outcomes for an individual who becomes infected. Exposed states are not infectious. (B) Example of distributions of and transmissions between individuals within a prison, with colours denoting infection states defined in panel A. Black boxes denote buildings within prison yards (in this illustrative example, yard 1 has two buildings and yard 2 has one building), while dashed boxes denote rooms within buildings, and arrows designate different possible routes of transmission from infectious individuals: within room, within building, within yard, staff to staff, staff to resident, and resident to staff. Staff are assumed to mix homogeneously with residents across all locations of the prison.
Daily transmission risk is based on the number of contacts each person has, the proportion of contacts that are infectious, and the probability of transmission by type of contact (appendix pp 3–10). Residents have contact with their roommates, other residents in the same building, and correctional staff across the prison. Residents who participate in out-of-room activities (labour, school, or other activities) also have contact with other residents in the same yard who participate in those activities. Transmission risks are highest for in-room contacts, followed by activity contacts, and then building and staff contacts. Because the model reflects differences in residents' housing and activity participation, it captures variation in individuals' exposures and thus replicates previous study findings on the increased risks associated with higher-occupancy housing and activity participation.5 Correctional staff are assumed to mix homogenously with each other and with residents. We explored lower levels of activity and staff transmission in sensitivity analysis.
The model is constructed in R (version 3.6.3).
COVID-19 epidemiology
Infected individuals start out exposed (ie, infected but not infectious) and become infectious over time (after 3 days on average; appendix pp 11–12). Around 40% of people infected never develop symptoms (transitioning from exposed asymptomatic to infectious asymptomatic to recovered), while 60% do develop symptoms, either after becoming infectious (from exposed asymptomatic to infectious asymptomatic to infectious symptomatic) or before (from exposed asymptomatic to exposed symptomatic to infectious symptomatic; figure 1).20, 21, 22, 23 Those with symptomatic infections have age-specific and comorbidity-specific risks of severe COVID-19 requiring hospitalisation and COVID-19-related mortality (appendix pp 23–24, 26). Individuals who survive recover and are immune to future infection. Due to the short analytical timeframe (200 days) and evidence on the durability of SARS-CoV-2 immunity, we did not consider waning immunity.24 Individuals also face background age-specific and sex-specific mortality risks (appendix pp 25–26). COVID-19 natural history is the same for residents and staff.
Reflecting California prison policies, modelled infected individuals can be detected via symptom screening, admission to hospital for COVID-19-like symptoms, surveillance testing, or reactive testing, triggered by other cases detected nearby (appendix pp 13–14). Once detected, an individual is isolated and all others residing in the building of the detected individual are quarantined. Those quarantined continue to mix with each other but have no contact with other buildings. Quarantine lasts for 14 days after the last case detected in a building. Test sensitivity varies by day since infection, with sensitivity increasing substantially from day 5 (appendix p 21).25
New admissions, releases, and transfers between prisons were not explicitly modelled. Instead, we focused on two main scenarios for infection introduction. In the first, a single infected resident is seeded on the first day of analysis and no further infections are imported. In the second, in addition to seeding an infection on the first day, we also allowed for repeated importations by modelling a 0·1% daily risk of infection per susceptible staff member. Staff importations have been identified as an important root cause of prison outbreaks.6 In a prison with 200 susceptible staff, 0·1% translates to approximately one infected staff member per week. 0·1% was selected on the basis of an analysis of cumulative correctional officer infections from CDCR data. Lower risks were assessed in sensitivity analysis.
Resumption of activities and NPIs
We modelled reopening as a return to pre-COVID-19 out-of-room activity levels and doubling of building and staff contacts, the latter based on average differences between pre-COVID-19 (January, 2020) and COVID-19 (November, 2020) activity levels from CDCR data. We assessed reopening with or without NPIs (eg, facial coverings and physical distancing during in-person activities), where NPIs reduce transmission risks from contacts resulting from reopening by 75%. We selected 75% as an illustrative effectiveness for a hypothetical set of NPIs used with realistic compliance, but it is broadly consistent with evidence on the impact of facial coverings and physical distancing.26 Lower NPI effectiveness was explored in sensitivity analysis. These NPIs are assumed to already be in place in scenarios that keep activities at current levels.
COVID-19 variants and immunity
We considered scenarios in which either a new, more transmissible and severe SARS-CoV-2 variant such as the alpha variant (B.1.1.7)12, 13, 27 or non-variant (wild-type) infection is introduced into a prison. Scenarios were also differentiated by the size of previous outbreaks and consequent baseline immunity levels (0%, 25%, and 50% immunity to wild type) in the incarcerated and staff populations. Immunity was modelled as moderately less protective against the new variant than wild type (appendix pp 3–4).12
Vaccination
We analysed two levels of vaccine acceptance among residents: a best-case scenario of 90% acceptance, and a more realistic lower and age-varying acceptance (18–29 years: 36% acceptance; 30–39 years: 46%; 40–49 years: 57%; 50–59 years: 66%; 60–69 years: 71%; and ≥70 years: 76%), based on CDCR data. We modelled 40% vaccination among staff, also based on CDCR data (appendix p 22). Given limited available data on correctional staff and flatlining of staff coverage in California prisons, we did not focus on the effects of increasing staff coverage, but explored the effects of 80% vaccine acceptance among correctional staff in a sensitivity analysis. We assumed that residents and staff accepting a vaccine are fully vaccinated before an infection is imported into the prison. In sensitivity analysis, we assessed an alternative scenario in which vaccination begins on the same day that an infection is introduced to the prison.
Vaccine efficacy was based on the Pfizer-BioNTech and Moderna vaccine trials and observational studies that indicate lower efficacy against transmissibility than against clinical infection, and higher efficacy after complete vaccination than after the first dose (appendix pp 3–4).28, 29 We assumed the vaccine is moderately less effective against new variants than wild type (appendix pp 3–4).
Data
The CDCR provided daily individual-level data on all residents in its custody. Data used in this study span Jan 1, 2020, to May 15, 2021. Data describe prison layouts, sociodemographic characteristics, housing status, chronic conditions and health status, participation in in-person activities (all based on data from the week of Nov 22–28, 2020), and COVID-19 testing, infection, and vaccination status (based on data covering the entire period). We supplemented these data with literature-based estimates related to COVID-19 epidemiology, testing, and vaccination and public data describing correctional staff (appendix pp 3–4).
Outcomes
We compared total resident infections (regardless of detection and symptoms) and severe cases (requiring hospitalisation and possibly resulting in death) across scenarios. We computed cumulative risks of these outcomes over 200 days. 200 days was selected because it is an appropriate timespan for capturing measures of cumulative risk: most modelled outbreaks ended well before 200 days of simulation time (appendix pp 9–10).
We compared mean outcomes from 500 simulations across each scenario and prison, employing variance reduction techniques.
Role of the funding source
The funders of the study had no role in study design, study conduct, data collection, data analysis, data interpretation, writing of the report, or decision to publish.
Results
The characteristics of each prison modelled are shown in the table . Risks of COVID-19 outbreaks are highest when a viral variant is introduced to a prison with little-to-no baseline immunity where only moderate but realistic vaccination coverage has been achieved and activities have been reopened without NPIs (figure 2A ). In these settings, the percentage of residents infected over 200 days ranges from 23% in a medical prison with mostly cells and protected medically vulnerable populations, to 54–55% in celled prisons with greater activity participation than medical settings (including the women's prison), and up to 73–74% in prisons with some or almost all dormitories. Even when half of residents have immunity to wild-type SARS-CoV-2 from previous infection (translating to 40% of residents protected against infection with a new variant), 25–32% of residents are expected to be infected in prisons with dormitories.
Table.
Characteristics of selected prisons
| Dormitories (n=3413) | Mixed (n=3231) | Cells (n=2243) | Women's (n=1093) | Medical (n=2399) | Total (n=12 379) | |||
|---|---|---|---|---|---|---|---|---|
| Number of yards | 7 | 5 | 7 | 5 | 5 | 29 | ||
| Number of buildings | 26 | 26 | 37 | 24 | 61 | 174 | ||
| Sex of residents | ||||||||
| Female | 0 | 0 | 0 | 1093 (100%) | 0 | 1093 (9%) | ||
| Male | 3413 (100%) | 3231 (100%) | 2243 (100%) | 0 | 2399 (100%) | 11 286 (91%) | ||
| Age of residents, years | ||||||||
| 18–39 | 1285 (38%) | 1459 (45%) | 1458 (66%) | 546 (50%) | 468 (20%) | 5216 (42%) | ||
| 40–64 | 2080 (61%) | 1374 (43%) | 748 (34%) | 476 (43%) | 1299 (54%) | 5977 (48%) | ||
| ≥65 | 48 (1%) | 398 (12%) | 37 (2%) | 71 (6%) | 632 (26%) | 1186 (10%) | ||
| Room occupancies* | ||||||||
| Single cell | 34 (1%) | 387 (12%) | 909 (41%) | 497 (45%) | 1259 (52%) | 3086 (25%) | ||
| Double cell | 8 (<1%) | 1324 (41%) | 1326 (60%) | 558 (51%) | 352 (15%) | 3568 (29%) | ||
| Small-to-medium dormitory | 2698 (79%) | 1110 (34%) | 8 (<1%) | 38 (3%) | 592 (25%) | 4446 (36%) | ||
| Large dormitory | 673 (20%) | 410 (13%) | 0 | 0 | 195 (8%) | 1278 (10%) | ||
| Missing | 0 | 0 | 0 | 0 | 1 (<1%) | 1 (<1%) | ||
| Security level† | ||||||||
| 1 (lowest) | 161 (5%) | 125 (4%) | 52 (2%) | 251 (23%) | 123 (5%) | 712 (6%) | ||
| 2 | 3221 (94%) | 1771 (55%) | 409 (19%) | 555 (51%) | 1673 (70%) | 7629 (62%) | ||
| 3 | 30 (1%) | 1021 (32%) | 218 (10%) | 101 (9%) | 122 (5%) | 1492 (12%) | ||
| 4 (highest) | 1 (<1%) | 314 (10%) | 1564 (71%) | 178 (16%) | 477 (20%) | 2534 (20%) | ||
| Missing | 0 | 0 | 0 | 8 (1%) | 4 (<1%) | 12 (<1%) | ||
| Comorbidities‡ | ||||||||
| None | 2441 (72%) | 1668 (52%) | 1634 (74%) | 583 (53%) | 658 (27%) | 6984 (56%) | ||
| ≥1 | 972 (28%) | 1563 (48%) | 609 (28%) | 510 (46%) | 1741 (73%) | 5395 (44%) | ||
| Participation in activities§ | ||||||||
| Before closures | ||||||||
| Labour | 1985/4026 (49%) | 1452/4310 (34%) | 978/2647 (37%) | 697/1641 (42%) | 568/2816 (20%) | 3695/15 440 (32%) | ||
| School | 1467/4026 (36%) | 669/4310 (16%) | 248/2647 (9%) | 495/1641 (30%) | 81/2816 (3%) | 1493/15 440 (13%) | ||
| Other | 1516/4026 (38%) | 1730/4310 (40%) | 1108/2647 (42%) | 967/1641 (59%) | 1543/2816 (55%) | 5348/15 440 (47%) | ||
| With closures | ||||||||
| Labour | 1387 (41%) | 755 (23%) | 757 (34%) | 350 (32%) | 333 (14%) | 2195 (25%) | ||
| School | .. | .. | .. | .. | .. | .. | ||
| Other | 119 (3%) | 148 (5%) | 129 (6%) | 310 (29%) | 489 (21%) | 1075 (12%) | ||
Data are number of residents (%), unless stated otherwise. Dormitories are rooms with at least three occupants, and cells are rooms with no more than two occupants, with the mixed prison composed of a mix of dormitories and cells. CDCR=California Department of Corrections and Rehabilitation.
Small-to-medium dormitories have three to 30 occupants, and large dormitories have at least 31 occupants.
Based on the CDCR's security level system.
Comorbidities include advanced liver disease, asthma, cancer, chronic obstructive pulmonary disease, chronic lung disease, cardiovascular disease, diabetes, HIV, immunocompromised, kidney disease (eg, on dialysis).
Residents' participation in activities out of their rooms (but still in the prison) in the past week with at least one other resident. Before closures indicates January, 2020 (before closures due to COVID-19), and with closures indicates November, 2020 (with closures due to COVID-19 implemented). Before COVID-19-related closures, population sizes were larger than the column total because COVID-19 led to population reductions. Data on activities are only displayed for residents who were in custody the entire week. Labour includes both jobs that support the upkeep of the prison (resident workers at medical prisons, laundry, kitchen duty, etc) and industries. School includes any educational activities; these are all set to 0 during closures because the CDCR has had residents participate in educational activities in their rooms to minimise transmission. Other captures additional activities such as medical appointments, group therapy, and worship. Additional model parameters are shown in the appendix (pp 3–4).
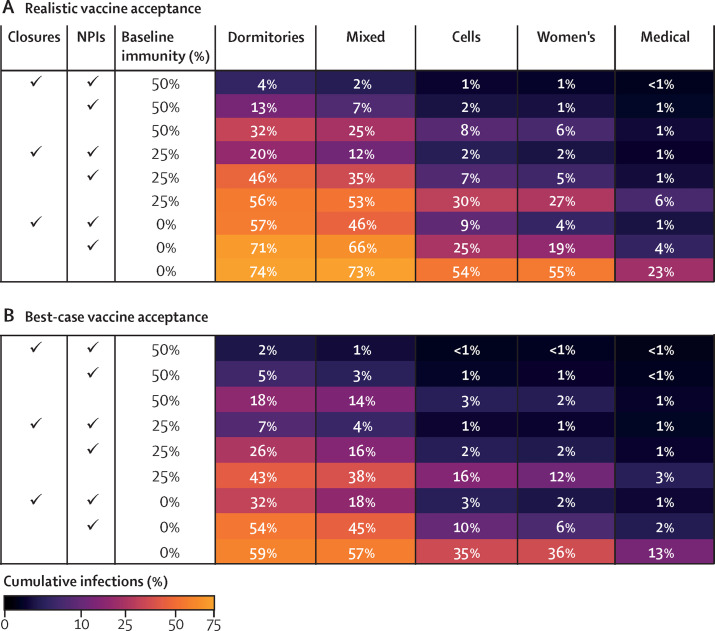
Figure 2.
Cumulative resident infections over 200 days after introduction of a single variant infection, by in-person activity status, use of NPIs, and baseline immunity
Figure shows average cumulative infections among residents across 500 model simulations over 200 days for each scenario shown. In the best-case vaccine coverage scenario (B), 90% of residents are vaccinated. In the realistic scenario (A), vaccine coverage is lower and varies by age. NPIs are assumed to be 75% effective. Closures indicates continued cessation of in-person activities. Dormitories are rooms with at least three occupants, and cells are rooms with no more than two occupants, with the mixed prison composed of a mix of dormitories and cells. NPI=non-pharmaceutical intervention.
NPIs that are 75% effective in reducing contact transmissibility can reduce infections in settings with realistic vaccine coverage but alone are insufficient to counteract risks from resumption of activities. In prisons with little-to-no baseline immunity, even when NPIs are in place and in-person activities have not been resumed, infection levels of 4–9% are expected in the men's and women's prisons with mostly cells (figure 2A). Under these same conditions, infection levels of 46–57% are expected in prisons with dormitories. With reopening of activities with NPIs in place, these levels increase to 19–25% for prisons consisting mostly of cells and 66–71% for those composed mostly of dormitories (figure 2A).
Achieving 90% vaccination of residents substantially reduces expected outbreak sizes (figure 2B). The largest reductions from high vaccine coverage (relative to realistic) are in settings that already have substantial baseline immunity (eg, 25–50%) or do not resume in-person activities. In these settings, high vaccination coverage approximately halves (or more) the percentage of infected residents. In settings with lower immunity that reopen in-person activities, with or without NPIs, achieving high vaccine coverage is still beneficial but has less impact. Additionally, NPIs that are 75% effective are most impactful in settings where vaccine coverage and baseline immunity are already high (figure 2). Thus, NPIs and vaccination are complementary interventions, not substitutes.
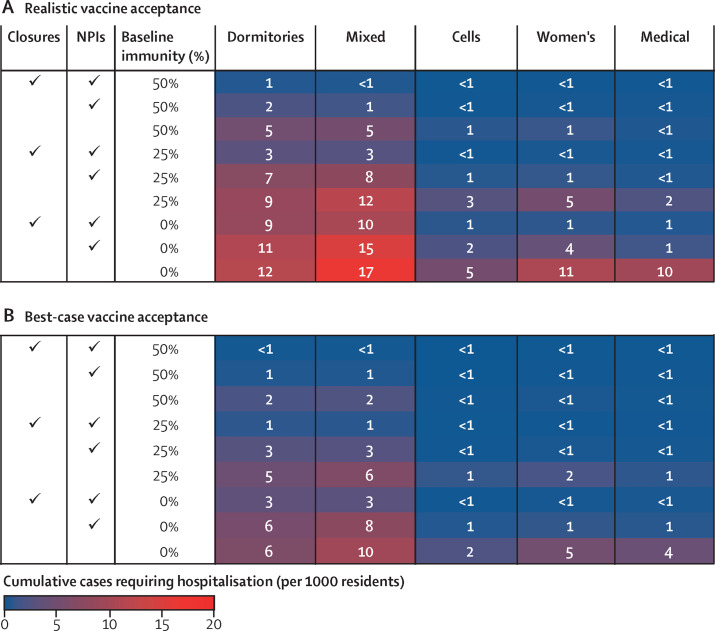
Severe outcomes follow similar patterns to infections, with several notable exceptions (figure 3 ). The highest hospitalisation levels are expected when a new variant is introduced to a prison with dormitories and low or no baseline immunity that has resumed activities without NPI use (figure 3A). In these settings when there is no baseline immunity, with realistic vaccine acceptance, between 12 and 17 hospitalisations per 1000 residents are expected. Achieving high vaccine coverage approximately halves these hospitalisations to six to ten per 1000 (figure 3B). High vaccine coverage reduces hospitalisation to an even greater degree when these prisons with dormitories do not resume in-person activities (nine to ten per 1000 with realistic vaccine acceptance vs three per 1000 with best-case vaccine acceptance). Because vaccines yield greater protection against symptomatic infections than transmissibility, they have a larger impact on incidence of severe outcomes than on infections.
Figure 3.
Cumulative resident cases requiring hospitalisation over 200 days after introduction of a single variant infection, by in-person activity status, use of NPIs, and baseline immunity
Figure shows average cumulative severe cases (requiring hospitalisation) per 1000 residents across 500 model simulations over 200 days for each scenario shown. In the best-case vaccine coverage scenario (B), 90% of residents are vaccinated. In the realistic scenario (A), vaccine coverage is lower and varies by age. NPIs are assumed to be 75% effective. Closures indicates continued cessation of in-person activities. Dormitories are rooms with at least three occupants, and cells are rooms with no more than two occupants, with the mixed prison composed of a mix of dormitories and cells. NPI=non-pharmaceutical intervention.
In each scenario, medical prisons are expected to have the lowest levels of cumulative infections among the five prison types. In addition to having more protections in place than other prisons, residents of the medical prison we modelled mostly live in cells within many small buildings, reducing opportunities for rapid spread across the prison (table). However, residents of medical prisons also tend to be older and have more comorbidities, increasing their risk of severe outcomes from infection (table). Those vulnerabilities manifest in disproportionately high rates of hospitalisation, ranging from 29 to 54 per 1000 infected residents across the various scenarios, compared with six to 30 per 1000 infected residents in other prison types (appendix p 31). While fewer infections are expected in medical prisons, hospitalisations are, in some scenarios, double those in the high-security prison with mostly cells (figure 3). With resumption of activities, no NPI use, little-to-no baseline immunity, and realistic vaccination levels, hospitalisations in medical prisons could be as high as 23 per 1000 residents, translating to almost 20 COVID-related deaths in a prison of 3000 people (appendix p 32).
The women's prison we model is similar to the medical prison in this respect: while the women's prison has mostly cells and similar infection levels to the high-security men's prison with mostly cells, it experiences higher rates of hospitalisations, reflecting the greater proportion of older residents and residents with comorbidities (figure 3; table).
Vaccination reduces the proportion of infections that require hospitalisation and hence, compared with no vaccination, conveys additional benefits to the older and medically vulnerable residents, both because they are at greater risk of adverse outcomes and because they have observably higher levels of vaccine acceptance (appendix pp 31, 33–34).
Cumulative infections and severe cases are expected to be substantially lower if a wild-type infection is introduced to a prison, compared with a new variant introduction (appendix pp 35–36). With realistic vaccine coverage, prisons with little-to-no baseline immunity, especially those with dormitories, are still at risk of large outbreaks (19–23% of residents infected) if activities are resumed without NPIs in place. With high vaccine coverage, even settings with no baseline immunity and high levels of contacts are anticipated to have few infections (≤1% of residents infected). However, as variants predominate globally, the likelihood that infection introductions will be new variants of concern grows as well.
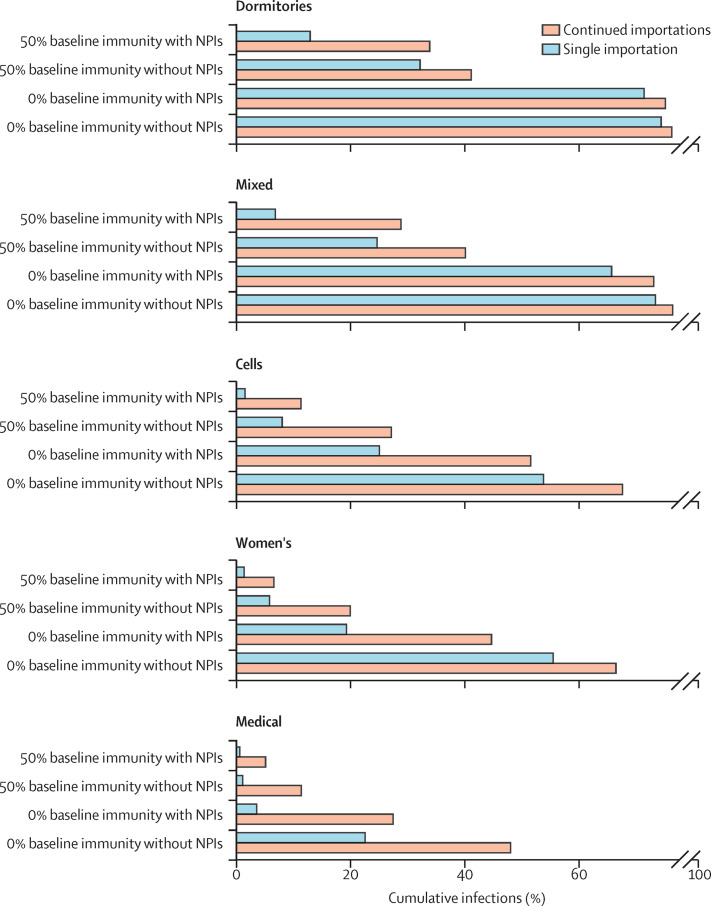
Many prisons have experienced repeated importations of infection, in which case outbreaks are far less likely to die out. In an analysis that allowed for repeated importation, which could arise from staff, new arrivals, or transfers, increases in resident infections from resumption of in-person activities are substantially larger than in the analysis of a single importation (figure 4 ; appendix p 37). The biggest differences occur in settings that are at overall lower risk: those with high baseline immunity and NPIs in place and celled, women's, and medical prisons. With realistic vaccine coverage, resumption of activities in these three prison types is expected to result in 5–27% of residents infected if there is high baseline immunity and 27–68% of residents infected if there is no baseline immunity (figure 4), translating to increased expected numbers of hospitalisations, with the greatest increase seen for the medical prison (21 per 1000 residents; appendix p 38). Average resident infections by scenario over time are available in the appendix (pp 39–42).
Figure 4.
Cumulative resident infections over 200 days with resumption of in-person activities and realistic vaccine coverage, by baseline immunity, use of NPIs, and frequency of infection importation
Figure shows average cumulative infections among residents across 500 model simulations over 200 days with no importations after day 1 (single importation) or 0·1% daily incidence among susceptible staff members (continued importations) for each scenario shown. Results showing the full set of scenarios are available in the appendix (p 37). Vaccine coverage varies by age. NPIs are assumed to be 75% effective. Dormitories are rooms with at least three occupants, and cells are rooms with no more than two occupants, with the mixed prison composed of a mix of dormitories and cells. NPI=non-pharmaceutical intervention.
Our main analyses assumed that vaccination of both residents and correctional staff occurred before the introduction of a new infection. Vaccination of residents has substantially less impact when scaled up concurrently with infection introduction, and outbreaks in these settings are expected to be far worse. In settings that are currently at lower risk, such as prisons with cells whose residents have moderate-to-high baseline immunity, outbreaks are generally around twice as large with concurrent vaccination scale-up compared with completed vaccination at baseline, even if 90% vaccine coverage is eventually reached (appendix pp 43–44).
In sensitivity analyses, doubling staff vaccination coverage to be 80% from the day an infection is introduced to a prison was a relatively minor determinant of resident infections and hospitalisations (appendix pp 45–46). Similar top-line conclusions were also drawn when considering NPIs that were only 50% effective, although outbreak sizes were somewhat larger (appendix pp 47–48). Results did not change substantially when the daily risk of repeated infection importation was halved compared with the main repeated importations analysis shown in figure 4 (appendix pp 49–50).
With reduced activity and staff transmission, cumulative infections are expected to be lower in high-immunity settings and prisons with mostly cells when a single infection is introduced (appendix p 51). Reopening remains risky in settings with dormitories and low or no baseline immunity, and the benefits of vaccination and NPIs are evident. Results are less sensitive to lower activity and staff transmission parameters when repeated importations are modelled (appendix p 52).
In addition to parameter uncertainty, we also assessed stochastic variation in results by examining the distributions of cumulative infections across the 500 model simulations for the main analysis of single importation (appendix pp 53–56).
Discussion
As highly transmissible SARS-CoV-2 variants proliferate, their introduction into the USA's 110 federal and 1833 state prisons is inevitable. Resumption of in-person activities, undoubtedly important for the welfare of people who are incarcerated, can greatly increase outbreak risks. Achieving high levels of vaccination before resumption of in-person activities lowers risks, as does continuing widespread use of effective NPIs. These findings hold even in prisons that predominantly house residents in lower-occupancy rooms or have accrued substantial natural immunity from previous outbreaks.
Resuming in-person activities safely requires a multifaceted approach. In addition to vaccination and NPIs to reduce transmission, other measures might prevent infection introductions, limit spread, or mitigate harms. We found a substantial increase in expected infections and hospitalisations when the likelihood of repeated importation of infections is high, showing the importance of preventing infections from being introduced. Staff vaccination is crucial for choking one of the main avenues of introduction.6 Maintaining screening and testing of residents, staff, and visitors could also prevent introductions and limit outbreak sizes. Furthermore, analysis of hospitalisations reveals that older and medically vulnerable residents should receive additional protections beyond vaccine priority, such as lower occupancy housing and additional NPIs. Our study shows the benefits of lower occupancy housing and points to the potential impact of depopulation,18 which other studies have also highlighted.30, 31, 32 Future work could include an assessment of other prevention measures, such as holding activities outdoors or limiting contact across yards or buildings within a prison.
Our analysis reveals that immediate efforts to achieve widespread vaccination among people who are incarcerated are crucial. Sensitivity analysis showed that if vaccination scale-up is delayed and an infection is introduced in the meantime, the rate of spread is likely to outpace feasible vaccine scale-up, especially in prisons that have resumed in-person activities. Currently, vaccine coverage and acceptance among people who live and work in correctional settings is highly variable across systems;15, 19 at the time of writing this Article, around half of residents in the USA were vaccinated and many states did not prioritise incarcerated populations,15, 16 and vaccination of incarcerated populations was at an early stage in many other countries.33, 34 Employing the best-available vaccination offer and re-offer strategies might be required to increase acceptance, especially among those with low perceived risk and distrust of prison authorities or health-care providers.19, 35
Regular surveillance testing equips prison health officials with early knowledge of the presence and spread of infections, but, to our knowledge, most correctional systems are not using genetic sequencing to monitor for the presence of variants. Hence, detection of an outbreak cannot indicate whether additional precautions are needed to curb the spread of a variant of concern. Alternatively, the seeding of wild-type virus might not warrant complete shutdown but still the reintroduction of some control measures. Until prisons can rapidly distinguish between wild-type infections and variants of concern, detecting new infections could trigger policies that either under-protect or over-protect residents and staff.
Our study has several limitations. Our results might not be fully generalisable to other carceral settings. The impacts of infection introduction on incarcerated populations depend in part on how likely prisons are to detect outbreaks and take measures to isolate and quarantine. We model widespread testing as practised in California's prisons during 2020–21. However, in settings where screening and testing are less intensive, the risk and size of outbreaks are likely to be larger than our estimates. Although we analysed a range of prison types, incarcerated populations, and levels of existing immunity, outcomes in individual prisons could differ if their housing configurations or demographics are distinctive. The extent to which our findings are generalisable to settings beyond the USA depends on how similar a setting's prison system is to the US system. For example, many prison systems in other high-income countries had lower population sizes and room occupancies before the pandemic than many US prisons, and some may have reduced them relatively more than prisons in the USA as a response to COVID-19. In these settings, the analysis of the women's prison, which had the lowest population and was predominantly made up of single or double cells, might be most applicable.
The frequency of infection importations is also setting-specific. Our analysis did not explicitly consider new admissions or transfers, but this was proxied via the analysis of continued importations. Inflows could be an important source of introductions, as was documented for a large COVID-19 outbreak in California.36 In CDCR prisons, admissions volumes have dropped substantially (to <1% of resident populations monthly), and new admissions are isolated for at least 14 days, conditional on testing negative for SARS-CoV-2.37 However, admissions volumes and protocols might differ in other prison systems and could change as cases decline and vaccination levels increase. Furthermore, in settings with high rates of community COVID-19 infections or high inflow volumes, such as reception centres, jails (which hold people before trial or sentencing in the USA), and countries that do not have separate jail and prison systems, continual importation of infections is more likely. Our analysis of repeated importations is therefore more applicable to these settings.
Finally, while we considered scenarios that broadly represent variants such as the alpha variant, other variants are circulating globally, and new variants are emerging even as the scientific community works to keep pace in characterising them.12, 14 Some variants might be able to completely evade natural immunity from previous wild-type infection; although we did not model these explicitly, we did model equivalent scenarios with 0% baseline immunity. However, variants that are simultaneously able to evade vaccine-induced immunity and are more likely to cause severe outcomes, although not yet detected, would require further evaluation.
Prison health authorities will need to determine to what extent the risks of infection and serious COVID-19 outcomes must be reduced before the benefits of resumption of in-person activities outweigh the risks. Numerous studies have documented the disruptive effects of COVID-19 on important activities such as education programmes and visitation,38, 39 which are linked to reduced recidivism, improved employment rates after release, and better health outcomes.9, 10 Conversely, while vaccination reduces risks of serious COVID-19 outcomes, the longer-term implications of even mild infections are not yet well understood. Studies have detailed long-term sequelae from both mild and severe infections, including respiratory conditions, cardiovascular disease, and mental health disorders.40 Furthermore, outbreaks in prisons can have consequences for the broader community and uncontrolled spread of the virus can lead to further mutations that produce even harder-to-combat variants, as has been observed for other infectious diseases.41 Decision making regarding pandemic control should be integrated between public health authorities in incarcerated and free-living settings. Neglecting prison health and deprioritising vaccination is both unethical and bad policy.
Our analysis yields important conclusions based on models that incorporate detailed primary data from the second-largest state prison system in the USA; explicitly reflect demography, residential structure, and mixing over a range of prison types; and can reproduce the size and heterogeneity of viral spread reported in the literature. To enable resumption of in-person activities so important for the wellbeing of people who are incarcerated, prisons should prioritise widespread vaccination as soon as possible, remain dedicated to NPIs and regular surveillance testing including genetic sequencing, and continue to protect medically vulnerable populations.
Data sharing
The modelling presented in this study is parameterised by data from public sources and by non-public data shared by the CDCR via a data use agreement with Stanford University. Please contact the CDCR regarding requests for data and data use agreements, and the corresponding author for analytical code.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This research was supported by Stanford's COVID-19 Emergency Response Fund, established with a gift from the Horowitz Family Foundation; the National Institute on Drug Abuse (R37-DA15612); the Centers for Disease Control and Prevention (through the Council of State and Territorial Epidemiologists, NU38OT000297-02); the National Science Foundation's Graduate Research Fellowship (DGE-1656518); the Stanford Graduate Fellowship in Science and Engineering, and the Open Society Foundations (OR2020-69521). Advanced Micro Devices (Santa Clara, CA, USA) provided a donation of servers. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program (DGE-1656518). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We thank staff members at the CDCR for providing data and assistance with interpretation of study results. We would also like to thank Liesl Hagan (US Centers for Disease Control and Prevention, Atlanta, GA, USA) and Robert Canning (CDCR, Sacramento, CA, USA) for sharing their expertise to help to inform our selection of prisons for this analysis. We also acknowledge assistance from other members of the Stanford-CIDE Coronavirus Simulation Modelling consortium.
Contributors
JDG-F, DMS, TR, ETC, FA-E, JRA, and DL were involved in the study conceptualisation. TR and ETC coded the microsimulation model and TR was responsible for running all model analyses. LP, EL, ETC, and TR analysed the primary data. DL, LP, EL, ETC, and TR validated the data used in the analysis. JDG-F, JAS, JRA, FA-E, ETC, and TR supported the choice of and validated the model assumptions, structural decisions, and parameters. TR and JDG-F led the initial drafting of the manuscript. All authors contributed to ideas for analysis and manuscript edits and subsequent drafts. All authors had access to the underlying data, have seen and approved of the final text, and were responsible for the decision to submit.
Supplementary Material
References
- 1.Saloner B, Parish K, Ward JA, DiLaura G, Dolovich S. COVID-19 cases and deaths in federal and state prisons. JAMA. 2020;324:602–603. doi: 10.1001/jama.2020.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toblin RL, Hagan LM. COVID-19 case and mortality rates in the Federal Bureau of Prisons. Am J Prev Med. 2021;61:120–123. doi: 10.1016/j.amepre.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhalter E, Colón I, Derr B. Incarcerated and infected: how the virus tore through the US prison system. The New York Times. April 10, 2021. https://www.nytimes.com/interactive/2021/04/10/us/covid-prison-outbreak.html
- 4.National Academies of Sciences, Engineering, and Medicine . The National Academies Press; Washington, DC: 2020. Decarcerating correctional facilities during COVID-19: advancing health, equity, and safety. [PubMed] [Google Scholar]
- 5.Chin ET, Ryckman T, Prince L. COVID-19 in the California State Prison System: an observational study of decarceration, ongoing risks, and risk factors. J Gen Intern Med. 2021 doi: 10.1007/s11606-021-07022-x. published online July 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan LM, Williams SP, Spaulding AC. Mass testing for SARS-CoV-2 in 16 prisons and jails—six jurisdictions, United States, April–May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1139–1143. doi: 10.15585/mmwr.mm6933a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aschwanden C. The false promise of herd immunity for COVID-19. Nature. 2020;587:26–28. doi: 10.1038/d41586-020-02948-4. [DOI] [PubMed] [Google Scholar]
- 8.Keeling MJ, Rohani P. Princeton University Press; Princeton, NJ: 2007. Modeling infectious diseases in humans and animals. [Google Scholar]
- 9.Davis LM, Bozick R, Steele JL, Saunders J, Miles JNV. RAND Corporation; Santa Monica, CA: 2013. Evaluating the effectiveness of correctional education: a meta-analysis of programs that provide education to incarcerated adults. [Google Scholar]
- 10.De Claire K, Dixon L. The effects of prison visits from family members on prisoners' well-being, prison rule breaking, and recidivism: a review of research since 1991. Trauma Violence Abuse. 2017;18:185–199. doi: 10.1177/1524838015603209. [DOI] [PubMed] [Google Scholar]
- 11.Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA. 2021;325:821–822. doi: 10.1001/jama.2021.1114. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions. March 16, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance/variant-info.html
- 13.Graham MS, Sudre CH, May A. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: an ecological study. Lancet Public Health. 2021;6:e335–e345. doi: 10.1016/S2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullen JL, Tsueng G, Latif AA. Outbreak.info: a standardized, open-source database of COVID-19 resources and epidemiology data. outbreak.info. https://outbreak.info
- 15.Quandt KR. Incarcerated people and corrections staff should be prioritized in COVID-19 vaccination plans. March 2, 2021. https://www.prisonpolicy.org/blog/2020/12/08/covid-vaccination-plans
- 16.Herring T, Widra E. Just over half of incarcerated people are vaccinated, despite being locked in COVID-19 epicenters. May 18, 2021. https://www.prisonpolicy.org/blog/2021/05/18/vaccinationrates
- 17.National Research Council . The National Academies Press; Washington, DC: 2014. The growth of incarceration in the United States: exploring causes and consequences. [Google Scholar]
- 18.Barsky BA, Reinhart E, Farmer P, Keshavjee S. Vaccination plus decarceration—stopping Covid-19 in jails and prisons. N Engl J Med. 2021;384:1583–1585. doi: 10.1056/NEJMp2100609. [DOI] [PubMed] [Google Scholar]
- 19.Chin ET, Leidner D, Ryckman T. Covid-19 vaccine acceptance in California state prisons. N Engl J Med. 2021 doi: 10.1056/NEJMc2105282. published online May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He X, Lau EHY, Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 21.Lauer SA, Grantz KH, Bi Q. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashcroft P, Huisman JS, Lehtinen S. COVID-19 infectivity profile correction. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20336. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner JS, Kim W, Kalaidina E. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021 doi: 10.1038/s41586-021-03647-4. published online May 24. [DOI] [PubMed] [Google Scholar]
- 25.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu DK, Akl EA, Duda S. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuite AR, Fisman DN, Odutayo A. COVID-19 hospitalizations, ICU admissions and deaths associated with the new variants of concern. Science Briefs of the Ontario COVID-19 Science Advisory Table. March 29, 2021. [DOI]
- 28.Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polack FP, Thomas SJ, Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vest N, Johnson O, Nowotny K, Brinkley-Rubinstein L. Prison population reductions and COVID-19: a latent profile analysis synthesizing recent evidence from the Texas state prison system. J Urban Health. 2021;98:53–58. doi: 10.1007/s11524-020-00504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malloy GSP, Puglisi L, Brandeau ML, Harvey TD, Wang EA. Effectiveness of interventions to reduce COVID-19 transmission in a large urban jail: a model-based analysis. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry BF. Social distancing and incarceration: policy and management strategies to reduce COVID-19 transmission and promote health equity through decarceration. Health Educ Behav. 2020;47:536–539. doi: 10.1177/1090198120927318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Union Agency for Fundamental Rights Coronavirus pandemic in the EU—fundamental rights implications: vaccine rollout and equality of access in the EU. June 16, 2021. https://s3.eu-central-1.amazonaws.com/euobs-media/9e963f6a0c43b7d92add444b4c30565f.pdf
- 34.Gallagher C. Inmates being vaccinated on a prison-by-prison basis. The Irish Times. June 17, 2021. https://www.irishtimes.com/news/crime-and-law/inmates-being-vaccinated-on-a-prison-by-prison-basis-1.4595211
- 35.Stern MF, Piasecki AM, Strick LB. Willingness to receive a COVID-19 vaccination among incarcerated or detained persons in correctional and detention facilities—four states, September–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:473–477. doi: 10.15585/mmwr.mm7013a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griesbach R, Williams T. San Quentin ordered to reduce prison population by half over virus fears. The New York Times. Oct 21, 2020. https://www.nytimes.com/2020/10/21/us/san-quentin-prison-coronavirus.html
- 37.California Department of Corrections and Rehabilitation COVID-19 information. COVID-19 response efforts. https://www.cdcr.ca.gov/covid19/covid-19-response-efforts
- 38.Pettus-Davis C, Kennedy SC, Veeh CA. Incarcerated individuals' experiences of COVID-19 in the United States. Int J Prison Health. 2021 doi: 10.1108/IJPH-11-2020-0094. published online March 24. [DOI] [PubMed] [Google Scholar]
- 39.Hewson T, Shepherd A, Hard J, Shaw J. Effects of the COVID-19 pandemic on the mental health of prisoners. Lancet Psychiatry. 2020;7:568–570. doi: 10.1016/S2215-0366(20)30241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 41.Mabud TS, de Lourdes Delgado Alves M, Ko AI. Evaluating strategies for control of tuberculosis in prisons and prevention of spillover into communities: an observational and modeling study from Brazil. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The modelling presented in this study is parameterised by data from public sources and by non-public data shared by the CDCR via a data use agreement with Stanford University. Please contact the CDCR regarding requests for data and data use agreements, and the corresponding author for analytical code.