Abstract
Background
Individuals with schizophrenia have an increased risk of severe COVID-19 outcomes, nonetheless, no previous study has provided a year-long account of this risk, or assessed postvaccination trends in this population. This study assessed temporal trends in COVID-19 hospitalisation and mortality among people with schizophrenia during the first year of the pandemic, the predictors for COVID-19 vaccination, postvaccination infection, admission to hospital, and mortality.
Methods
In this longitudinal cohort study, people with schizophrenia (n=25 539) and controls (n=25 539) were assessed for COVID-19 outcomes before and after vaccination, up to April 30, 2021. Cox proportional hazard regression models and Kaplan-Meier analyses were done to assess longitudinal trends. The study used the databases of Clalit Health Services, the largest health-care organisation in Israel.
Findings
The sample included 51 078 participants, of which 31 141 (61·0%) male and 19 937 (39·0%) female participants, with a mean age of 51·94 years (SD 15·62). Most of the sample was from the general Jewish population (75·9%), followed by the Arab (19·1%) and Jewish Ultraorthodox population (5·1%). Overall of 51 078 individuals, 356 (0·7%) people had been hospitalised, 133 (0·3%) had died, and a total of 27 400 (53·6%) had been vaccinated. People with schizophrenia showed a higher risk for COVID-19 hospitalisation (HR 4·81, 95% CI 3·57–6·48, p<0·0001) and mortality (HR 2·52, 95% CI 1·64–3·85, p<0·0001), and showed a sharper decline in survival as time progressed. The control group showed a sharper incline in probability to vaccinate (log-rank=309·88, p<0·0001). Medical comorbidity of diabetes, hypertension, obesity, or ischaemic heart disease played a significant role in predicting vaccination rates in the schizophrenia group (all p<0·0001), but not in the control group. Hospitalisation and mortality disparities remained higher among people with schizophrenia who had not been vaccinated in comparison to controls (incidence rate difference of 6·2 and 3·2, respectively) but substantially declined in fully vaccinated groups (incidence rate difference of 1·1 and −0·9, respectively).
Interpretation
People with schizophrenia have higher hospitalisation and mortality risk, yet have lower rates of vaccination than in the general population. Disparities in COVID-19 severe outcomes can be substantially reduced by national vaccination plans aimed at actively reaching out to people with schizophrenia.
Funding
No funding.
Introduction
Since the onset of the COVID-19 pandemic caused by SARS-CoV-2, an accumulating body of research has indicated an increased risk for severe COVID-19 outcomes among individuals with mental illnesses.1, 2, 3, 4, 5, 6 Studies assessing different diagnostic clusters of mental illnesses found increased COVID-19 mortality among patients with psychotic, mood, substance use, and intellectual and developmental disorders, with no clear association with anxiety disorders.6, 7 COVID-19 hospitalisation was also found to be increased among patients with mood and substance use disorders.7 Several factors have been offered to explain the higher risk for COVID-19 hospitalisation and mortality among patients with psychiatric disorders compared with the people who do not have mental illness, with the most frequent ones being the prevalent comorbidity of physical diseases, altered immune function, higher rates of obesity and smoking, elevated stress, socioeconomic factors, exposure to antipsychotic and anxiolytics, and factors related to help-seeking behaviour.7, 8, 9 These findings have led to calls to give priority to these patients for COVID-19 vaccinations to minimise the effects of the pandemic on this vulnerable population of patients.10
Although several countries have already taken steps to ensure prioritisation of patients with psychiatric conditions,11, 12 scholars are raising questions regarding patients' ability to use these prioritisation efforts. Studies indicate that patients with mental illnesses are less likely to receive preventive vaccinations for illnesses such as influenza and pneumonia.13 Several predictive factors were found to be associated with the decision to be vaccinated among individuals with mental illness, such as obtainment of medical insurance, received recommendation from health-care providers, and perception of vaccine effectiveness.14 Initial findings showed that vulnerable populations such as people with severe mental illness have lower COVID-19 vaccination rates.15 These findings were recently supported by a study done in Israel that showed that people with schizophrenia are being under-vaccinated compared with the general population even when prioritised by their age group.16
Research in context.
Evidence before this study
To review the literature on COVID-19 outcomes of individuals with schizophrenia, we searched Web of Science and PubMed in May 15, 2021, for all studies reported in English, with the terms “COVID-19 and schizophrenia” and “mortality”. We found 14 papers discussing the association between schizophrenia and COVID-19 mortality, of which six were cohort studies (including cross-sectional, case control, population-based, and retrospective designs), four were commentaries and perspective papers, two studies were based on administrative and electronic record analyses, and two were reviews and meta-analyses. We did not find any papers assessing longitudinal trends in people with schizophrenia in the context of COVID-19 vaccination, or papers describing a full year of follow-up. We did not find any papers assessing hospitalisation and mortality before and after vaccination for COVID-19 among this group of patients.
Added value of this study
To the best of our knowledge, this study is the first to comprehensively provide a year-long estimation of differences in hospitalisation and mortality among patients with schizophrenia compared with controls. Furthermore, this report is the first to show postvaccination effects among individuals with schizophrenia. Consistent hospitalisation and mortality differences were noted between individuals with schizophrenia and controls, in addition to differences in vaccination rates. Results indicated that several comorbid medical factors were significant predictors of vaccination rates among patients with schizophrenia but not among controls (ie, diabetes, hypertension, obesity, and ischaemic heart disease). We also showed that differences in hospitalisation among individuals with schizophrenia and controls were reduced postvaccination.
Implications of all the available evidence
The results of our study indicate that disparities in hospitalisation and mortality among patients with severe mental illness are stable and accumulating. The differences in vaccination rates highlight the importance of active outreach to individuals with severe mental illness, as these inequalities exist even when vaccinations are fully available. The predictive effect of medical comorbidities among patients with schizophrenia (but not among controls) might be suggestive of the potential effect of general physicians or family caretakers actively facilitating vaccination for patients at risk; nonetheless, such a hypothesis should be further examined. The reduced differences in hospitalisation postvaccination indicate the potential beneficial effect of vaccination in reducing the reported disparities and highlight the importance of preventive strategies in this population.
Even if patients adhere to the national recommendations to be vaccinated, scholars have suggested that vaccinations might be less effective in patients with severe mental illness. It has been suggested that individuals with severe mental illness might have a reduced immune response to vaccinations and therefore vaccinations might not reduce morbidity risk.9 Previous studies have shown a lower antibody response to the influenza vaccination among older adults with depressive symptoms,17 as well as a weaker immune response to the hepatitis B vaccination in people with schizophrenia, bipolar disorder, and depression.18 Furthermore, several studies have shown a greater risk of worse COVID-19 outcomes among patients with severe mental illness even after adjusting for medical comorbidities, thus leading to the question of whether these patients will be sufficiently protected by vaccine strategies.9 Nevertheless, to the best of our knowledge, no study has thus far investigated whether vaccination reduces the risk of severe COVID-19 outcomes among individuals with severe mental illness to the same extent as it does among individuals without severe mental illness.
In two cross-sectional studies published in 2021,5, 16 we reported an association between schizophrenia and COVID-19 hospitalisation and mortality, as well as an association between schizophrenia and lower odds of being vaccinated. Nonetheless, to the best of our knowledge, no study has previously assessed the longitudinal trends of these effects over time in this patient population. As such, this study aimed to provide a longitudinal evaluation of hospitalisation, mortality, and vaccination, as well as to assess outcomes before and after vaccination. The focus on a relatively homogeneous group of people with schizophrenia enables a clearer comparison between this study and previous cross-sectional studies, and the mapping of specific challenges for this group of patients. As the management of health-care services in Israel changed rapidly during the pandemic owing to accumulated experience in managing recurrent infection waves, our study aimed to assess whether the cumulative data obtained during the full year of the pandemic might lead to a differential pattern of outcomes, or whether disparities in hospitalisation and mortality in this population were sustained throughout the year.
Methods
Data source and definition of main variables
A mass vaccination plan in Israel was launched in Dec 19, 2020. Medical staff and individuals older than 60 years were prioritised first, along with patients suffering from chronic illnesses. During January, 2021, age of a priority gradually decreased, until all citizens of Israel aged 16 years and older had full access to COVID-19 vaccination.19 This study used the databases of Clalit Health Services (CHS), which is the largest health-care organisation in Israel. The CHS is one of four health-care organisations that provides health services for all citizens of Israel,20 and covers over 50% of the country's population.21 The CHS databases are regularly updated with real-time data collected in primary care units, hospitals, pharmacies, and administrative medical operating systems.
We used the datasets of 25 539 people with schizophrenia and their matched controls (overall n=51078) from the CHS registry. The database was originally mined at the end of 2017, and was updated on April 30, 2021 with current data relevant to the study objectives. Subsequently, all people with schizophrenia included in this study were those known and identified by 2017. An extended report of the ICD codes (from ICD-9 and ICD-10) used to extract the main study variables is provided elsewhere.5 In short, a schizophrenia diagnosis was determined based on a senior psychiatrist's documentation, or when listed in a discharge letter from a psychiatric hospital. The diagnosis underwent a manual validation across 10% of the cases and was found to be 94% accurate.22 A group of CHS-insured individuals was randomly sampled at a 1:1 ratio and matched by age and sex. Additional clinical variables extracted and updated included age, sex, socioeconomic status, population group (ie, Jewish, Arab, or Jewish Ultraorthodox), and marital status; clinical factors included asthma, diabetes, ischaemic heart disease, chronic obstructive pulmonary disease, hypertension, obesity, and smoking; and COVID-19-related factors included rates of SARS-CoV-2 infection, rates of hospitalisation, mortality, and rates of vaccination. COVID-19 hospitalisation was defined as admission to a COVID-19 ward of a general hospital. Mortality was defined as registry of COVID-19 being the cause of death as determined by the Ministry of Health registry. The study was approved by the CHS institutional review board. Informed consent was waived due to the anonymous nature of data extraction.
Statistical analysis
Data were analysed by April 30, 2021. Hazard ratios (HRs) were estimated with Cox proportional hazard regression models. Incidence rates and crude and adjusted models controlling for demographic and clinical factors were reported. The proportional hazard assumption was tested as the correlation between the Schoenfeld residuals and survival time, with significance level of p<0·05 indicating non-proportionality.23, 24 In cases of violation of the assumption, the validity of the models was assessed by stratifying time into two phases: the early (first 208 days) and late phases.
Estimated projections of the cumulative probability of hospitalisation, mortality, and vaccination were obtained by Kaplan-Meier analysis. The log-rank test was used to determine whether survival curves differed statistically between individuals with and without schizophrenia. Hazard ratios with 95% CIs were obtained to assess the differential rate of vaccination across the groups following the launch of the vaccination plan. Four-block hierarchical binary logistic regression was used to assess the predictive main effects of demographic (ie, age, sex, socioeconomic status, marital status, and population group) and clinical risk factors (ie, previous COVID-19 infection asthma, diabetes, hypertension, obesity, smoking, chronic obstructive pulmonary disease, hyperlipidaemia, and ischaemic heart disease) and their interaction by group. Univariate binary logistic regressions were used to assess simple effects.
Postvaccination infection, hospitalisation, and mortality were assessed during Feb 1 to April 30, 2021, among fully vaccinated and non-vaccinated individuals until the end of Jan 31, 2021. As the overall number of these events postvaccination were low, incidence rate difference was considered to assess the magnitude of difference between unvaccinated people with schizophrenia and controls, compared with the corresponding vaccinated groups. Fully vaccinated individuals were defined as those who received two doses of vaccination by Jan 31, 2021. Partially vaccinated individuals (ie, individuals administered one vaccination shot) were grouped together with the non-vaccinated. Statistical analysis was done using SPSS software (version 25) and R software (version 4.02).
Role of the funding source
There was no funding source for this study.
Results
General characteristics of the study sample are reported by Tzur Bitan and colleagues.5 In short, the cohort of 51 078 participants comprised individuals with schizophrenia and controls with a mean age of 51·94 (SD 15·62) years, of whom 31 141 (61·0%) were male and 19 937 (39·0%) were female participants. Most of the sample was from the general Jewish population (75·9%), followed by the Arab population (19·1%) and Jewish Ultraorthodox population (5·1%). Study size was determined based on all available patients with a verified diagnosis at the time of data extraction. Compared with the controls, individuals with schizophrenia were significantly less likely to be married and to belong to the Jewish Ultraorthodox population group, and were more likely to belong to the general Jewish or Arab population group, as well as to have a low or medium socioeconomic status (compared with high).5 As previously reported, people with schizophrenia were also found to have higher rates of obesity, smoking, diabetes, hyperlipidaemia, and chronic obstructive pulmonary disease.5
Overall of 51 078 individuals, throughout the first year of the pandemic, 356 (0·7%) individuals were hospitalised in a COVID-19 hospital unit, of whom 298 were people with schizophrenia and 58 controls (table 1 ). A total of 133 (0·3%) had died due to COVID-19, of whom 100 (75%) of these were people with schizophrenia and 33 (25%) controls (table 1). The overall incidence rate of hospitalisation was estimated at 11·79 (95% CI 10·52–13·20) cases per 1000 person-years and of mortality was 3·93 (3·21–4·76) cases per 1000 person-years, among the schizophrenia group, compared with 2·27 (1·74–2·92) for hospitalisation and 1·29 (0·90–1·79) cases per 1000 person-years for mortality among the control group (table 1).
Table 1.
Incidence rates, hazard ratio, and 95% CI of COVID-19 hospitalisation, mortality, and vaccination among schizophrenia patients and controls within 1 year follow-up
|
Hospitalisation |
Mortality |
Vaccination |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | Control | p value | Schizophrenia | Control | p value | Schizophrenia | Control | p value | |
| Follow-up time, person-years | 25 257 | 25 487 | .. | 25 443 | 25 511 | .. | 5305 | 4880 | .. |
| Number of events | 298 | 58 | .. | 100 | 33 | .. | 12 928 | 14 472 | .. |
| Incidence rate per 1000 person-years (95% CI) | 11·79 (10·52–13·20) | 2·27 (1·74–2·92) | .. | 3·93 (3·21–4·76) | 1·29 (0·90–1·79) | .. | 2436·96 (2395·82–2478·81) | 2965·57 (2918·37–3013·56) | .. |
| Unadjusted HR (95% CI) | 5·16 (3·89–6·83) | Ref | p<0·0001 | 3·03 (2·04–4·49) | Ref | p<0·0001 | 0·83 (0·81–0·85) | Ref | p<0·0001 |
| HR adjusted for demographic factors* (95% CI) | 4·58 (3·40–6·16) | Ref | p<0·0001 | 2·48 (1·62–3·77) | Ref | p<0·0002 | 0·85 (0·83–0·87) | Ref | p<0·0001 |
| HR adjusted for clinical factors† (95% CI) | 4·81 (3·57–6·48) | Ref | p<0·0001 | 2·52 (1·64–3·85) | Ref | p<0·0002 | 0·83 (0·81–0·85) | Ref | p<0·0001 |
For people with schizophrenia group, n=25 539 and for the control group, n=25 539.
Adjusted for age, sex, marital status, population group, and socioeconomic status.
Adjusted for diabetes, hypertension, asthma, hyperlipidaemia, chronic obstructive pulmonary disease, ischaemic heart disease, smoking, and obesity.
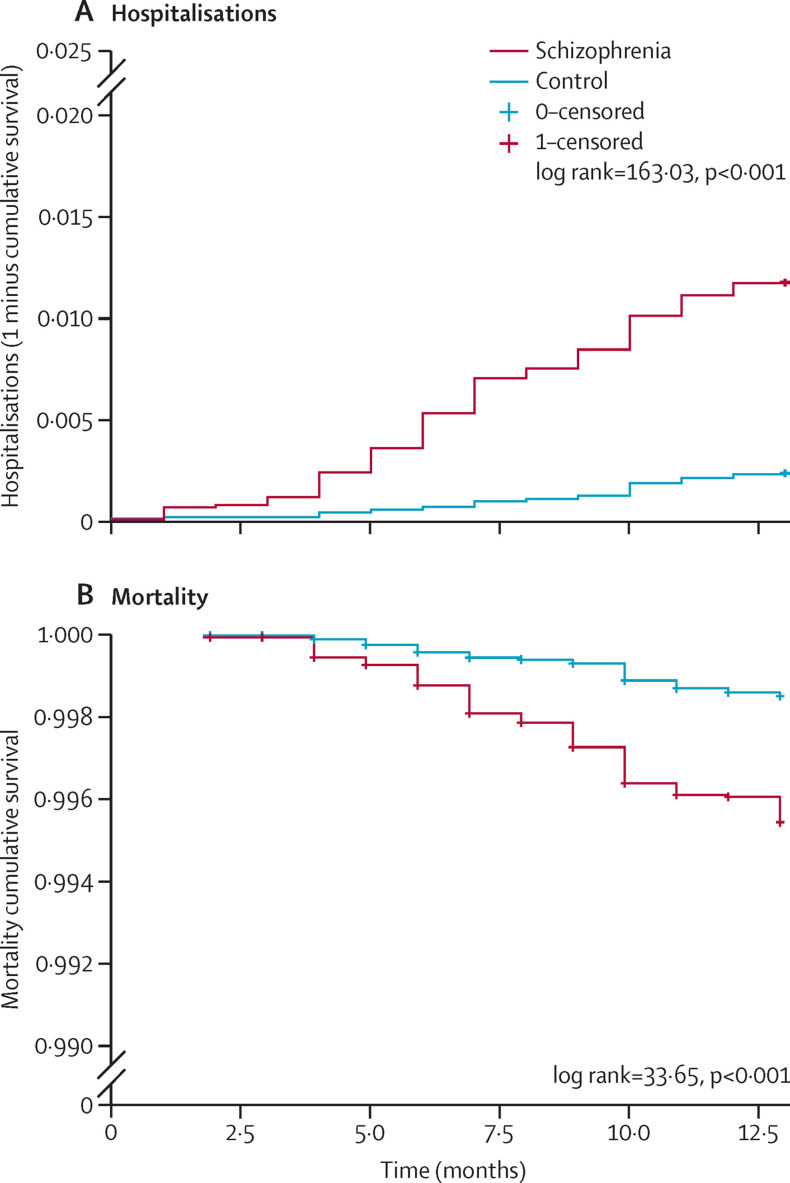
Individuals with schizophrenia presented a significantly sharper incline in probability of COVID-19 hospitalisation as time progressed compared with controls (log-rank test 163·03, p<0·0001); figure 1A , and showed a significantly higher risk after adjusting for demographic and clinical confounders (HR 4·81, 95% CI 3·57–6·48, p<0·0001). The proportional hazard assumption was valid for all factors except for group, marital status, and smoking. Therefore, the adjusted model was reanalysed while stratifying time into two phases: early and late. These analyses indicated an elevated HR at early stage (6·44) and lowered at late stage (3·40). Nonetheless, both were greater than 1 and within the range of CIs of the examined models, and both produced significant effects (p<0·0001; appendix p 1).
Figure 1.
Kaplan-Meier survival curves of hospitalisations and mortality among individuals with schizophrenia and controls
Mortality rates also differed significantly between the two groups throughout the pandemic year (figure 1B), with individuals with schizophrenia presenting a sharper decline in survival rates compared with controls (log-rank test=33·65, p<0·0001). Cox regression analysis indicated significantly higher mortality risk among the schizophrenia group after adjustment for confounders (HR 2·52, 95% CI 1·64–3·85, p<0·0001). Proportional hazard assumption was found valid for all variables except smoking; therefore, the adjusted model was stratified into early and late phase. The early-stage HR was non-significant with the estimate of 2·15 owing to lower number of cases in the early phase (n=24); nonetheless, HR estimates of early and late phases were 2·15 and 2·72, which are within the range of the HR in the full sample (2·61; appendix p 1).
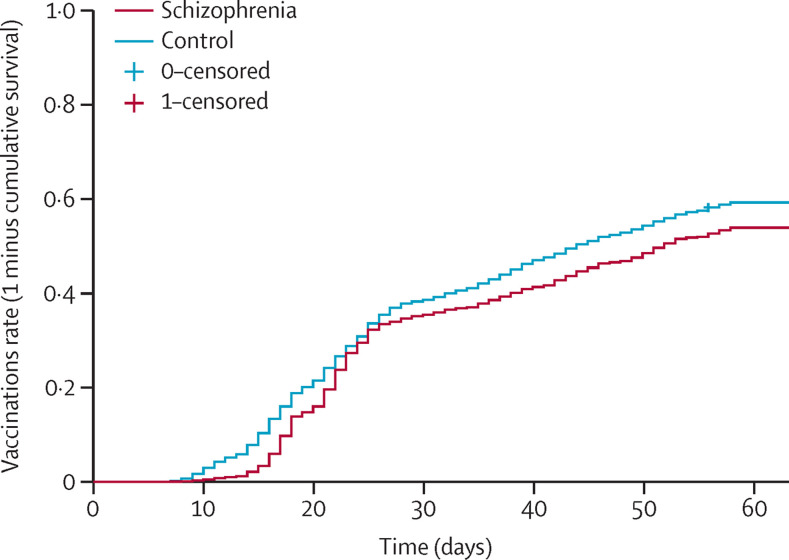
Overall, since the launch of the national vaccination plan in Israel and up until the beginning of March 2021, a total of 27 400 (53·6%) of the 51 078 in the cohort were fully vaccinated, with 14 472 (52·8%) fully vaccinated among the control group compared with 12 928 (50·6%) among the schizophrenia group, resulting in a 2·2% difference in vaccination proportion. For the cumulative probability of vaccination among the two groups during the first 3 months of the vaccination plan, individuals with schizophrenia presented a milder incline for vaccination as time progressed, compared with the control group (log-rank test 309·88, p<0·0001; figure 2 ).
Figure 2.
Kaplan-Meier survival curves of vaccinations among individuals with schizophrenia and controls
The incidence rate of vaccination was estimated at 2·43 cases per 1000 person-years for the schizophrenia group (95% CI 2·39–2·47) compared with 2·96 cases per 1000 person-years for the control group (2·91–3·01; table 1). After adjusting for demographic and clinical variables, the HR of vaccination was significantly lower among people with schizophrenia compared to controls (HR 0·83, 95% CI 0·81–0·86, p<0·0001). Non-proportionality was found across all variables except for asthma; therefore, these models were also assessed for early (first month of vaccination plan) and late phases (second month of vaccination plan; appendix p 2). These analyses indicated HR estimates ranging from 0·84 to 0·88, which are within the range of the HR in the full sample (0·83), and with both models producing a significant effect (p<0·0001).
A full description of the results of the hierarchical logistic regression is specified in the appendix, p 3. Among the overall sample, older age, male sex, medium-high socioeconomic status, being married, being from the general Jewish (rather than the Arab or Jewish Ultraorthodox) population, and having obesity and hyperlipidaemia were significantly associated with vaccination, whereas smoking, ischaemic heart disease, and schizophrenia were negatively associated with vaccination. Significant interactions by group were observed in age, socioeconomic status, marital status, asthma, diabetes, hypertension, obesity, and chronic obstructive pulmonary disease. The predictive value of age and socioeconomic status were slightly lower in the schizophrenia group, suggesting that older age and high socioeconomic status were slightly more predictive of vaccination in the control group (table 2 ). Significant interaction with marital status indicated that being married was associated with higher odds of being vaccinated in the control group, but lower odds of being vaccinated in the schizophrenia group. As expected, previous COVID-19 infection was associated with significantly lower odds for vaccination. Diabetes, hypertension, obesity, and ischaemic heart disease were all positively and significantly associated with vaccination in the schizophrenia group but not in the control group. Chronic obstructive pulmonary disease and hyperlipidaemia were positively and significantly associated with vaccination across both groups.
Table 2.
Predictors of COVID–19 vaccination in the schizophrenia and control groups
|
Schizophrenia (n=25 539) |
Controls (n=25 539) |
||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Demographic factors | |||||||
| Age | 1·03 | 1·03–1·03 | <0·0001 | 1·05 | 1·04–1·05 | <0·0001 | |
| Sex | 1·11 | 1·04–1·17 | <0·0003 | 1·23 | 1·16–1·31 | <0·0001 | |
| Socioeconomic status | |||||||
| Medium | 1·64 | 1·55–1·73 | <0·0001 | 1·98 | 1·86–2·11 | <0·0001 | |
| High | 2·17 | 2·00–2·36 | <0·0001 | 3·44 | 3·18–3·72 | <0·0001 | |
| Marital status | 0·80 | 0·74–0·85 | <0·0001 | 1·42 | 1·34–1·51 | <0·0001 | |
| Population group | |||||||
| Jewish Ultraorthodox | 0·40 | 0·36–0·44 | <0·0001 | 0·34 | 0·30–0·39 | <0·0001 | |
| Arab | 0·34 | 0·31–0·36 | <0·0001 | 0·27 | 0·25–0·28 | <0·0001 | |
| Clinical factors | |||||||
| Previous COVID-19 infection | 0·31 | 0·28–0·35 | <0·0001 | 0·04 | 0·04–0·04 | <0·0001 | |
| Asthma | 0·97 | 0·87–1·10 | 0·72 | 1·11 | 0·98–1·26 | 0·10 | |
| Diabetes | 1·18 | 1·09–1·26 | <0·0001 | 0·95 | 0·87–1·05 | 0·39 | |
| Hypertension | 1·10 | 1·02–1·19 | <0·0001 | 0·95 | 0·86–1·00 | 0·26 | |
| Obesity | 1·20 | 1·13–1·27 | <0·0001 | 1·04 | 0·97–1·13 | 0·20 | |
| Smoking | 0·93 | 0·88–0·98 | 0·012 | 0·87 | 0·82–0·93 | <0·0001 | |
| Chronic obstructive pulmonary disease | 1·69 | 1·50–1·91 | <0·0001 | 1·68 | 1·39–2·03 | <0·0001 | |
| Hyperlipidaemia | 2·10 | 2·00–2·21 | <0·0001 | 2·54 | 2·41–2·68 | <0·0001 | |
| Ischaemic heart disease | 1·66 | 1·47–1·87 | <0·0001 | 2·21 | 1·98–2·47 | 2·47 | |
Reference group for sex is female; for socioeconomic status is low; for marital status is not being married; for population group is general; for COVID-19 infection is being infected; and not having the condition in all clinical factors. OR=odds ratio.
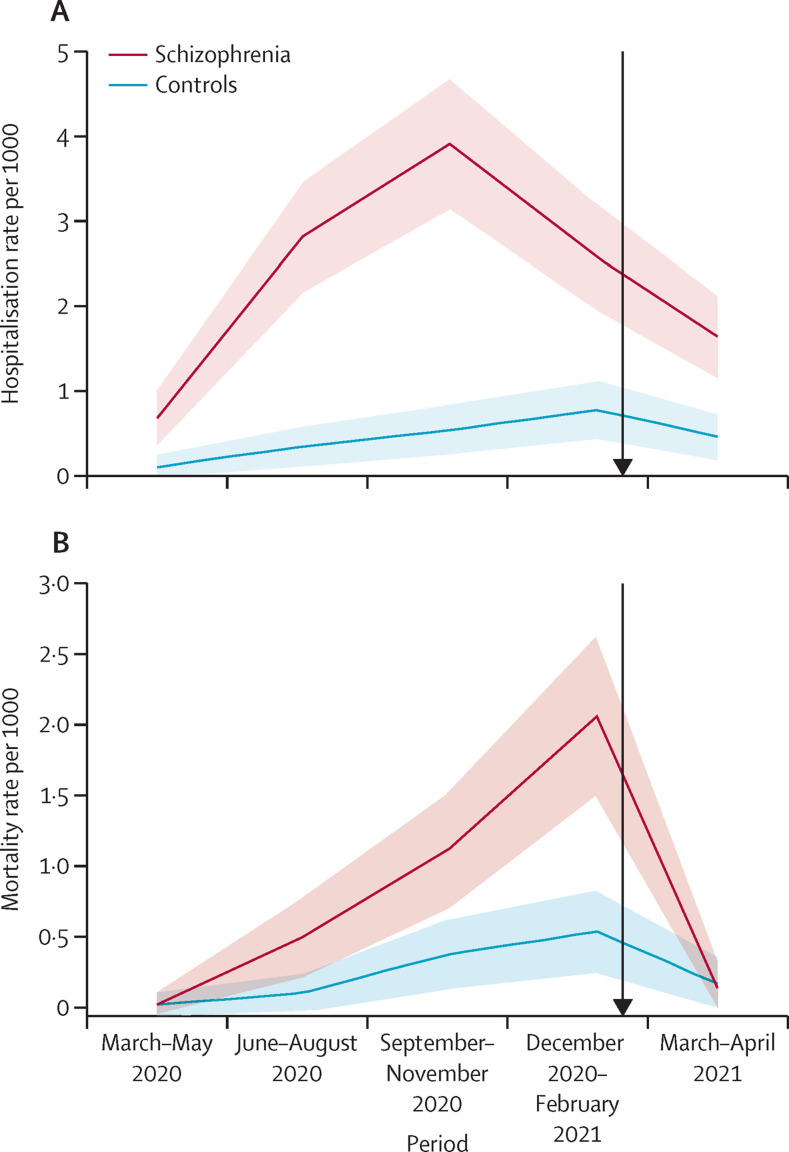
Changes in hospitalisation and mortality patterns since the onset of the vaccination plan in the schizophrenia and control group were assessed among individuals who were fully vaccinated by Feb 1, 2021 (figure 3 ). Overall, prevalence of infection decreased from Feb 1, to April 30, 2021 from 1411 (from Nov 1, 2020 to Jan 1, 2021) to 643. People hospitalised with COVID-19 decreased from 86 to 54, and mortality decreased from 40 to 36. Non-vaccination during February–April of 2021 resulted in an infection incidence rate difference of −16·9 between people with schizophrenia and controls, which was reduced to −10·9 among vaccinated individuals (table 3 ). Incidence rate difference of hospitalisation between people with schizophrenia and controls was 6·2 among non-vaccinated individuals, and decreased to 1·1 among vaccinated individuals. Mortality rate differences were 3·2 among non-vaccinated individuals with schizophrenia, and decreased to −0·9 among vaccinated people with schizophrenia.
Figure 3.
Hospitalisation and mortality across the entire year of the pandemic for both the schizophrenia and cohort group (n=51 078)
Shaded areas are 95% CI. Black arrow represents the start of the vaccination plan.
Table 3.
Differences in infection, hospitalization, and mortality rates across the two groups for fully vaccinated and non-vaccinated individuals.
|
Non-vaccinated by Feb 1, 2021 (n=41 102)* |
Fully vaccinated by Feb 1, 2021 (n=9976) |
|||
|---|---|---|---|---|
| Schizophrenia (n=20 879) | Controls (n=20 223) | Schizophrenia (n=4660) | Control (n=5316) | |
| COVID-19 infection (n=643) | ||||
| N of events | 279 (1·33%) | 343 (1·69%) | 4 (0·08%) | 17 (0·31%) |
| Follow-up, PY | 4540·8 | 4381·1 | 1017·4 | 1148·1 |
| Incidence rate, /1000 PY (95% CI) | 61·4 (54·5–69·0) | 78·3 (70·3–86·9) | 3·9 (1·3-9·5) | 14·8 (8·9–23·2) |
| Incidence rate difference, 1000 PY | −16·9 | Reference | −10·9 | Reference |
| COVID-19 hospitalization (n=54) | ||||
| N of events | 40 (0·19%) | 11 (0·05%) | 2 (0·04%) | 1 (0·01%) |
| Follow-up, PY | 4572·0 | 4430·0 | 1020·8 | 1164·1 |
| Incidence rate (95% CI) | 8·7 (6·3–11·8) | 2·5 (1·3–4·3) | 2·0 (0·3-6·5) | 0·9 (0·4–4·2) |
| Incidence rate difference, 1000 PY | 6·2 | Reference | 1·1 | Reference |
| COVID-19 mortality (n=36) | ||||
| N of events | 25 (0·11%) | 10 (0·04%) | 0 (0·0%) | 1 (0·01%) |
| Follow-up, PY | 4576·2 | 4426·4 | 1021·3 | 1165·2 |
| Incidence rate (95% CI) | 5·5 (3·6–7·9) | 2·3 (1·1–4·0) | 0·0 | 0·9 (0·0–4·2) |
| Incidence rate difference, 1000 PY | 3·2 | Reference | −0·9 | Reference |
Outcomes in this table were collected from Feb 1, 2021 to end of April 2021. PY=person-year.
Includes individuals who were only partially vaccinated as of Jan 31, 2021.
Discussion
We investigated hospitalisation and mortality rates among people with schizophrenia throughout the first year of the COVID-19 pandemic, and assessed vaccination predictors and postvaccination effects. Overall, people with schizophrenia showed consistently higher hospitalisation and mortality rates and showed a sharper decline in survival rates across time than did people in the control group. These findings are consistent with previous reports1, 2, 3, 4, 5, 6, 7 and indicated the robustness of hospitalisation and mortality disparities among patients with severe mental illness. As individuals with severe mental illness have more frequently diagnosed medical comorbidity and reduced life expectancy,25 the emergence of COVID-19, along with other barriers to medical help,26, 27, 28 might have resulted in stable and long-lasting inequalities with the general population that require action.
As previously reported,16 people with schizophrenia showed a lower cumulative probability to be vaccinated throughout the period of investigation. Several barriers to vaccination have been suggested among patients with severe mental illness, such as accessibility issues, costs, potential fears, and absence of medical recommendation.29 These factors might have contributed to the observed differences between the groups. Several explanations might account for the predictive effects of clinical factors among the schizophrenia group, but not among the controls. One potential explanation is that health-care providers encouraged their patients to be vaccinated considering their medical comorbidities. Previous studies have suggested that patients with anxiety and depression show higher rates of influenza vaccination uptake because of their frequent visits to medical facilities.9, 30 An alternative reasoning might be that people with schizophrenia and metabolic comorbidities represent a more chronic subset of patients who received their vaccinations in collective facilities. Such an explanation could also account for the lower odds of vaccination among married patients, who are less likely to be living in collective facilities. These competing explanatory routes should be the subject of future research.
Assessment of differences in infection, hospitalisation, and mortality rates among individuals who are not vaccinated indicated lower infection and higher hospitalisation and mortality rates among people with schizophrenia compared with controls. These differences correspond to the pattern of differences previously reported.5 Nonetheless, when evaluating the magnitude of difference between the groups among only vaccinated individuals, these differences were substantially attenuated. Although the overall number of events after vaccination precluded the ability to do testing for significance, the observation of fewer differences in rates of hospitalisation and mortality postvaccination might indicate that vaccinations are effective in minimising the observed disparities between people with severe mental illnesses and the general population, and that efforts should be made to continue closing this gap.
The results reported in this study have several important clinical and empirical implications. The health-care system in Israel is based on the National Health Insurance Law, which allows all citizens to receive health-care services through one of four official health insurance organisations. Thus, people with schizophrenia in Israel have full access to medical treatment as part of their basic rights. The evidence of morbidity and treatment disparities among people with schizophrenia in a country with full health-care access raises concerns about the magnitude of gaps in countries with less accessible health systems, and warrants public health policy makers taking proactive actions to narrow them. The finding indicating that medical comorbidities predict higher vaccination rates provides some evidence for the possibility that medical caretakers might facilitate such outreach when medically imperative. Future studies are needed to assess whether family members or mental health workers might be suitable providers for such facilitation.
Several limitations should be acknowledged. As the vaccination plan in Israel was based on gradual inclusion of groups at risk of severe COVID-19 outcomes, vaccination rates might be associated with these changes in policy. Future studies should examine the presence of disparities when vaccinations are fully available without prioritisation. Israel is considered an outlier with respect to both health system coverage and vaccine coverage; therefore, additional studies are needed to assess the generalisability of the reported findings in countries with different health-care organisations. As the sample comprised mainly individuals from the general Jewish population group, the generalisability of our findings should be assessed in other populations and ethnicity groups. The current sample only included patients identified with schizophrenia by 2017, and did not include new-onset cases. Future studies should examine whether similar outcomes are produced among patients with new-onset schizophrenia. As this study focused on a relatively homogeneous group of people with schizophrenia, future studies should assess whether the observed patterns are replicable in wider groups of patients such as individuals with psychotic disorders and other severe mental illnesses. Although diagnoses in the CHS datasets have been previously validated, the use of ICD codes rather than clinical assessment might result in an insufficient level of accuracy. Future studies should assess the replicability of our findings while using other measures of diagnosis, such as clinical or laboratory examinations. This study assessed the effect of clinical factors on COVID-19 outcomes. Additional studies are needed to explore the influence of emotional factors, such as fear, on the observed outcomes. Vaccination-related anxiety might have affected the lower rates of vaccination among people with schizophrenia and should be assessed in future studies. Incidence rate differences between the groups were calculated among those who were fully vaccinated, or non-vaccinated, by Feb 1, 2021, in order to allow for longer follow-up time and more stable estimates of the incidence rate differences. As the vaccination plan continued during the follow-up period, with vaccination rates varying on a daily basis, fully vaccinated individuals during this period were not excluded for the sake of simplicity of reporting. Finally, this study did not control for psychiatric clinical factors such as medication exposure, duration of illness, or inpatient status. Future studies should assess the predictive effect of these factors on vaccination rates. Taken together, the results stress the importance of facilitating better prevention strategies for individuals with severe mental illness, to provide better care for patients suffering from severe mental illnesses.
Data sharing
Due to ethical restrictions, data for the current study will not be shared.
Declaration of interests
Other financial relations are as follows: ADC received research grants from Janssen, Novartis, AbbVie, Janssen and Sanofi. ADC served as a consultant, advisor, or speaker to AbbVie, Amgen, Boehringer Ingelheim, Dexcel pharma, Janssen, Kamedis, Lilly, Neopharm, Novartis, Perrigo, Pfizer, Rafa, Samsung Bioepis, Sanofi, Sirbal, and Taro. DTB received a research grant from Pfizer and from the American Psychological Foundation. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Noga Givon-Lavi from the Faculty of Health Sciences of Ben-Gurion University, for providing statistical and epidemiological consultation, and Eve Horowitz Leibowitz for the professional English editing.
Contributors
DTB conceptualised the study design and methodology; did the literature search; wrote the original draft; contributed to validation, curation, analysis, and interpretation of the data; and reviewed, edited, and finalised the manuscript. KK conceptualised the study design and methodology; contributed to validation, curation, analysis, and interpretation of the data; and writing, reviewing, and editing. ADC conceptualised the study design and methodology; contributed to project administration, investigation, and data acquisition, curation, and validation; and writing, reviewing, and editing. OW conceptualised the study design and methodology; contributed to project administration, investigation, and data acquisition, curation, and validation; and writing, reviewing, and editing. The database for the study has been accessed and verified by DTB and KK. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7:1025–1031. doi: 10.1016/S2215-0366(20)30421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20:124–130. doi: 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Chen W, Hu Y, et al. Pre-pandemic psychiatric disorders and risk of COVID-19: a UK Biobank cohort analysis. Lancet Healthy Longev. 2020;1:e69–e79. doi: 10.1016/S2666-7568(20)30013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maripuu M, Bendix M, Öhlund L, Widerström M, Werneke U. Death associated with coronavirus (COVID-19) infection in individuals with severe mental disorders in Sweden during the early months of the outbreak-an exploratory cross-sectional analysis of a population-based register study. Front Psychiatry. 2021;11 doi: 10.3389/fpsyt.2020.609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzur Bitan D, Krieger I, Kridin K, et al. COVID-19 prevalence and mortality among schizophrenia patients: a large-scale retrospective cohort study. Schizophr Bull. 2021 doi: 10.1093/schbul/sbab012. published online Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78:380–386. doi: 10.1001/jamapsychiatry.2020.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vai B, Mazza MG, Delli Colli C, et al. Mental disorders and risk of COVID-19 related mortality, hospitalization and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021 doi: 10.1016/S2215-0366(21)00232-7. published online July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno C, Wykes T, Galderisi S, et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry. 2020;7:813–824. doi: 10.1016/S2215-0366(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazereel V, Van Assche K, Detraux J, De Hert M. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8:444–450. doi: 10.1016/S2215-0366(20)30564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Hert M, Mazereel V, Detraux J, Van Assche K. Prioritizing COVID-19 vaccination for people with severe mental illness. World Psychiatry. 2021;20:54–55. doi: 10.1002/wps.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Picker LJ, Dias MC, Benros ME, et al. Severe mental illness and European COVID-19 vaccination strategies. Lancet Psychiatry. 2021;8:356–359. doi: 10.1016/S2215-0366(21)00046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stip E, Javaid S, Amiri L. People with mental illness should be included in COVID-19 vaccination. Lancet Psychiatry. 2021;8:275–276. doi: 10.1016/S2215-0366(21)00068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32:519–543. doi: 10.1016/j.genhosppsych.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz RA, Norris MM, Norton LC, Westrick SC. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46:1–13. doi: 10.2190/PM.46.1.a. [DOI] [PubMed] [Google Scholar]
- 15.MacKenna B, Curtis HJ, Morton CE, et al. Trends, regional variation, and clinical characteristics of COVID-19 vaccine recipients: a retrospective cohort study in 23.4 million patients using OpenSAFELY. medRxiv. 2021 doi: 10.1101/2021.01.25.21250356. published online Jan 26. (preprint). [DOI] [Google Scholar]
- 16.Tzur Bitan D. Patients with schizophrenia are under-vaccinated for COVID-19: a report from Israel. World Psychiatry. 2021;20:300–301. doi: 10.1002/wps.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo R, Ciminale M, Ditommaso S, Siliquini R, Zotti C, Ruggenini AM. Hepatitis B vaccination in psychiatric patients. Lancet. 1994;343:356. doi: 10.1016/s0140-6736(94)91193-2. 56. [DOI] [PubMed] [Google Scholar]
- 19.Ministry of Health COVID-19 vaccine efficacy safety follow up committee. https://govextra.gov.il/ministry-of-health/covid19-vaccine/covid-19-vaccine-efficacy-safety-follow-up-committee
- 20.Ministry of Health Annual report of healthcare-providing companies for 2017. https://www.health.gov.il/PublicationsFiles/dochHashvaatui2017.pdf
- 21.Ministry of Health Annual report of healthcare-providing companies for 2018. https://www.health.gov.il/PublicationsFiles/dochHashvaatui2018.pdf
- 22.Tzur Bitan D, Krieger I, Berkovitch A, Comaneshter D, Cohen A. Chronic kidney disease in adults with schizophrenia: a nationwide population-based study. Gen Hosp Psychiatry. 2019;58:1–6. doi: 10.1016/j.genhosppsych.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE. In: SUGI supplemental library user's guide. Hastings RP, editor. SAS Institute; Cary, NC: 1986. The PHGLM procedure. [Google Scholar]
- 24.Hosmer D, Lemeshow S, May S. 2nd edn. Wiley series in probability and statistics; Hoboken: 2008. Applied survival analysis. [DOI] [Google Scholar]
- 25.Das-Munshi J, Chang CK, Dutta R, et al. Ethnicity and excess mortality in severe mental illness: a cohort study. Lancet Psychiatry. 2017;4:389–399. doi: 10.1016/S2215-0366(17)30097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muruganandam P, Neelamegam S, Menon V, Alexander J, Chaturvedi SK. COVID-19 and severe mental illness: impact on patients and its relation with their awareness about COVID-19. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villegas C, Ibabe I, Arnoso A. People at risk of social exclusion: mental health, structural-economic factors and sociocultural factors. Int J Soc Psychiatry. 2021;36:122–148. [Google Scholar]
- 28.Richter D, Hoffmann H. Social exclusion of people with severe mental illness in Switzerland: results from the Swiss Health Survey. Epidemiol Psychiatr Sci. 2019;28:427–435. doi: 10.1017/S2045796017000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles LW, Williams N, Luthy KE, Eden L. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26:172–180. doi: 10.1177/1078390319831763. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence T, Zubatsky M, Meyer D. The association between mental health diagnoses and influenza vaccine receipt among older primary care patients. Psychol Health Med. 2020;25:1083–1093. doi: 10.1080/13548506.2020.1717557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to ethical restrictions, data for the current study will not be shared.