Abstract
Decreasing exercise tolerance is one of the key features related to a poor prognosis in patients with chronic obstructive pulmonary disease (COPD). Cardiopulmonary exercise testing (CPET) is useful for evaluating exercise tolerance. The present study was performed to clarify the correlation between exercise tolerance and clinical parameters, focusing especially on the cross-sectional area (CSA) of skeletal muscle. The present study investigated 69 patients with COPD who underwent CPET. The correlations between oxygen uptake () at peak exercise and clinical parameters of COPD, including skeletal muscle area measured using single-section axial computed tomography (CT), were evaluated. The COPD assessment test score (ρ = − 0.35, p = 0.02) was weakly correlated with at peak exercise. In addition, forced expiratory volume in one second (FEV1) (ρ = 0.39, p = 0.0009), FEV1/forced vital capacity (ρ = 0.33, p = 0.006), and the CSA of the pectoralis muscles (PMs) (ρ = 0.36, p = 0.007) and erector spinae muscles (ECMs) (ρ = 0.39, p = 0.003) were correlated with at peak exercise. Multivariate analysis adjusted by age and FEV1 indicated that PMCSA was weakly correlated after adjustment (β value [95% confidence interval] 0.175 [0.03–0.319], p = 0.02). In addition, ECMCSA tended to be correlated, but not significantly after adjustment (0.192 [− 0.001–0.385] p = 0.052). The COPD assessment test, FEV1, FEV1/FVC, PMCSA, and ECMCSA were significantly correlated with at peak exercise.
Subject terms: Chronic obstructive pulmonary disease, Physiology, Respiration
Introduction
Chronic pulmonary obstructive disease (COPD) is a common respiratory disease, with a reported global prevalence of 251 million cases1, and it is considered a life-threatening disease with decreasing pulmonary function and airflow limitation2.
Recently, factors related to a poor prognosis of COPD patients, including mortality and exacerbations, are becoming understood as evidence increases. For example, low-level physical activity, percent predicted forced expiratory volume in one second (%FEV1), 6-min walk distance, body mass index (BMI), and a high frequency of exacerbations are significantly associated with mortality in COPD patients3,4. We and others have also reported that low-level pulmonary function, exercise tolerance (including 6-min walk distance and exercise-induced desaturation), and BMI are correlated with a high frequency of exacerbations5–8, indicating that evaluations of exercise tolerance and body composition, in addition to pulmonary function, are important for predicting the clinical course of COPD.
Decreasing exercise tolerance, normally measured by the 6-min walk test or cardiopulmonary exercise testing (CPET), is one of the important clinical features related to a poor prognosis in COPD patients3,9,10, and with CPET one can evaluate exercise tolerance with exertional ventilatory parameters precisely and safely11,12. For example, oxygen uptake () at peak exercise, which represents exercise tolerance, is significantly correlated with FEV1 and %FEV1 reflecting the severity of COPD13,14. Notably, with CPET, one can detect physical problems including cardiac dysfunction and functional skeletal muscle disorders during the test, which contributes to rapid initiation of treatment15.
Weight loss is a common systemic characteristic of patients with COPD16, and skeletal muscle loss has greater impact on the severity of COPD than decreased BMI17. Radiological analysis of skeletal muscles on computed tomography (CT) is a useful procedure for quantitation without onerous physical intervention18,19, and the cross-sectional area (CSA) of skeletal muscle on single-slice axial CT is significantly correlated with a poor prognosis in COPD patients20,21. In addition, the CSA of the erector spinae muscles (ECMs), which are anti-gravity muscles, but not of the pectoralis muscles (PMs), is significantly associated with mortality in Japanese patients with COPD21. Obviously, skeletal muscles are important for exercise tolerance, but the impact of exertional ventilatory parameters on CPET compared to clinical parameters in patients with COPD is not fully understood.
The aim of the present study was to identify the correlations between exertional ventilatory parameters, especially at peak exercise, and clinical parameters of COPD including skeletal muscle area. Our hypothesis was that skeletal muscle areas are correlated with at peak exercise, and the correlation coefficient of ECMCSA is higher than that of PMCSA.
Results
Parameters of cardiopulmonary exercise testing
In the present study, 69 COPD patients (66 males, 3 females) who underwent CPET were enrolled. The clinical baseline characteristics of the COPD patients are shown in Table 1. , which is a marker that reflects exercise tolerance22, was 295.6 ml/min at rest and 926.0 ml/min at peak exercise. Body weight-adjusted was 5.3 ml/min/kg at rest and 16.2 ml/min/kg at peak exercise. VT and VE were 773.2 ml and 12.9 l/min at rest and 1245.7 ml and 36.6 l/min at peak exercise, respectively. ˙E/CO2, which reflects pulmonary clearance of CO222, was 49.3 at rest and 41.1 at peak exercise. VD/VT, which reflects the efficacy of pulmonary gas exchange, was 0.28 at rest and 0.26 at peak exercise. The respiratory rate was 17.7 breaths/min at rest and 30.5 breaths/min at peak exercise (Table 2).
Table 1.
Demographics of the study participants (n = 69).
| Clinical parameters | |
|---|---|
| Age (years) | 71.1 ± 9.0 |
| Gender (male/female) | 66/3 |
| BMI (kg/m2) | 21.4 ± 3.8 |
| Smoking history (pack-year) | 67.6 ± 33.0 |
| GOLD stage (I/II/III/IV, n) | 12/27/25/5 |
| mMRC dyspnea scale (0/1/2/3/4, n) | 5/18/27/16/3 |
| COPD assessment test (n = 41) | 16.8 ± 7.6 |
| 6-min walk distance (n = 48) (m) | 386.1 ± 115.8 |
| Medications | |
| No respiratory medication, n (%) | 5 (7.2%) |
| LAMA or LABA alone, n (%) | 17 (24.6%) |
| LABA-LAMA combo, n (%) | 19 (27.5%) |
| ICS-LABA combo, n (%) | 9 (13.0%) |
| Triple combo, n (%) | 19 (27.5%) |
| Pulmonary function | |
| %VC (%) | 100.4 ± 18.4 |
| %FVC (%) | 95.2 ± 17.5 |
| FEV1 (L) | 1.35 ± 0.59 |
| FEV1/FVC (%) | 43.8 ± 13.4 |
| %FEV1 (%) | 60.2 ± 24.2 |
| DLco (%) | 65.9 ± 24.3 |
| Evaluation of skeletal muscle on CT (n = 56) | |
| PMCSA (cm2) | 25.9 ± 7.9 |
| ECMCSA (cm2) | 27.8 ± 6.2 |
BMI body mass index, GOLD global initiative for chronic obstructive lung disease, mMRC modified medical research council, COPD chronic obstructive pulmonary disease, LAMA long-acting muscarinic antagonist, LABA long acting β2 adrenergic agonist, ICS inhaled corticosteroid, VC vital capacity, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, DLco diffusing capacity of lung for carbon monoxide, PMCSA cross-sectional area of pectoralis muscles, ECMCSA cross-sectional area of erector spinae muscles. Data are presented as mean ± standard deviation.
Table 2.
Results of cardiopulmonary exercise testing at rest and at peak exercise (n = 69).
| At rest | At peak exercise | |
|---|---|---|
| Incremental load testing | ||
| (ml/min) | 295.6 ± 68.2 | 926.0 ± 338.4 |
| (ml/min/kg) | 5.3 ± 1.2 | 16.2 ± 4.7 |
| VT (ml) | 773.2 ± 204.5 | 1245.7 ± 362.6 |
| VE (L/min) | 12.9 ± 2.6 | 36.6 ± 10.9 |
| E/CO2 | 49.3 ± 8.9 | 41.1 ± 8.4 |
| VD/VT | 0.28 ± 0.07 | 0.26 ± 0.07 |
| Breathing frequency (times/min) | 17.7 ± 4.2 | 30.5 ± 8.1 |
oxygen uptake, VT tidal volume, VE minute ventilation, E/CO2 ventilatory equivalent for carbon dioxide, VD/VT dead space to tidal volume ratio. Data are presented as mean ± standard deviation.
Correlations between (ml/min/kg) at peak exercise and other parameters on CPET and the 6-min walk distance
Because (ml/min) is affected by body weight differences, adjusted by body weight (ml/min/kg) at peak exercise is considered a precise marker for exercise tolerance22. Therefore, the evaluation focused on that and its correlations with other CPET parameters and the 6-min walk distance. at peak exercise was significantly correlated with E/CO2 at rest (ρ = − 0.46, p < 0.0001) and at peak exercise (ρ = − 0.45, p < 0.0001), VD/VT at rest (ρ = − 0.36, p = 0.002) and at peak exercise (ρ = − 0.53, p < 0.0001), respiratory rate at rest (ρ = − 0.35, p = 0.003) and at peak exercise (ρ = − 0.33, p = 0.006), and the 6-min walk distance (ρ = 0.74, p < 0.0001) (Table 3, Supplementary Fig. S2a online). These data showed that (ml/min/kg) at peak exercise reflected exercise tolerance in COPD patients.
Table 3.
Correlation coefficients between at peak exercise and other CPET parameters and the 6-min walk distance.
| O2 (ml/min/kg) at peak exercise | ||
|---|---|---|
| ρ | p value | |
| E/CO2 at rest | − 0.46 | < 0.0001 |
| E/CO2 at peak exercise | − 0.45 | < 0.0001 |
| VD/VT at rest | − 0.36 | 0.002 |
| VD/VT at peak exercise | − 0.53 | < 0.0001 |
| Breathing frequency at rest | − 0.35 | 0.003 |
| Breathing frequency at peak exercise | − 0.33 | 0.006 |
| 6-min walk distance (n = 48) | 0.74 | < 0.0001 |
oxygen uptake, VT tidal volume, VE minute ventilation, E/CO2 ventilatory equivalent for carbon dioxide, VD/VT dead space to tidal volume ratio.
Correlations between (ml/min/kg) at peak exercise and clinical parameters of COPD including skeletal muscle area
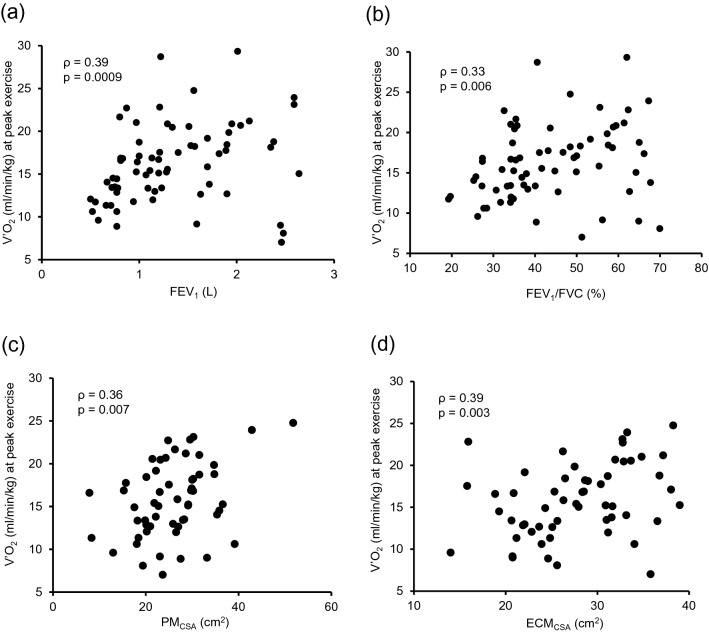
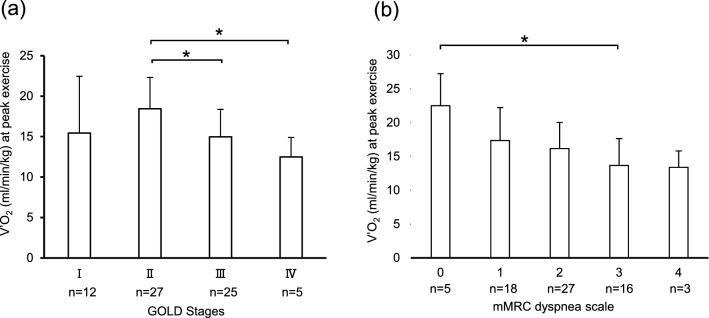
To clarify the factors correlated with exercise tolerance as reflected by (ml/min/kg) at peak exercise, correlation analysis between (ml/min/kg) at peak exercise and clinical parameters of COPD including skeletal muscle area was performed. Age, BMI, %VC, %FVC, %FEV1, and diffusing capacity of the lung for carbon monoxide (DLco) were not significantly correlated with at peak exercise. The COPD assessment test score (ρ = − 0.35, p = 0.02, Supplementary Fig. S2b online) was weakly correlated with at peak exercise. FEV1 (ρ = 0.39, p = 0.0009, Fig. 1a), FEV1/FVC (ρ = 0.33, p = 0.006, Fig. 1b), PMCSA (ρ = 0.36, p = 0.007, Fig. 1c), and ECMCSA (ρ = 0.39, p = 0.003, Fig. 1d) were significantly correlated with at peak exercise (Table 4). Examining the difference in at peak exercise by COPD stage, COPD stage III and IV patients had significantly lower levels of at peak exercise than stage II patients (Fig. 2a). In addition, examining the difference in at peak exercise by the mMRC dyspnea scale score, patients with an mMRC scale score of 3 had a significantly lower at peak exercise than those with an mMRC scale score of 0 (Fig. 2b). For other parameters on CPET, E/CO2 at peak exercise was significantly correlated with BMI (ρ = − 0.33, p = 0.007), the COPD assessment test score (ρ = 0.58, p < 0.0001), DLco (ρ = − 0.42, p = 0.001), PMCSA (ρ = − 0.32, p = 0.02), and ECMCSA (ρ = − 0.34, p = 0.01). In addition, VD/VT at peak exercise was significantly correlated with age (ρ = 0.34, p = 0.005), BMI (ρ = − 0.28, p = 0.02), the COPD assessment test score (ρ = 0.41, p = 0.009), %VC (ρ = − 0.28, p = 0.02), FEV1 (ρ = -0.42, p = 0.004), FEV1/FVC (ρ = − 0.36, p = 0.003), %FEV1 (ρ = − 0.27, p = 0.03), PMCSA (ρ = − 0.35, p = 0.008), and ECMCSA (ρ = − 0.38, p = 0.004) (Supplementary Table S1 online).
Figure 1.
Correlations between at peak exercise and clinical parameters of COPD. Correlations between at peak exercise and (a) FEV1, (b) FEV1/FEV, (c) PMCSA, and (d) ECMCSA. oxygen uptake, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, PMCSA cross-sectional area of the pectoralis muscles, ECMCSA cross-sectional area of the erector spinae muscles.
Table 4.
Correlation coefficients between at peak exercise and clinical parameters of COPD including skeletal muscle area.
| (ml/min/kg) at peak exercise | ||
|---|---|---|
| ρ | p value | |
| Age (years) | − 0.22 | 0.08 |
| BMI (kg/m2) | 0.08 | 0.54 |
| COPD assessment test | − 0.35 | 0.02 |
| %VC (%) | 0.19 | 0.11 |
| %FVC (%) | 0.16 | 0.2 |
| FEV1 (L) | 0.39 | 0.0009 |
| FEV1/FVC (%) | 0.33 | 0.006 |
| %FEV1 (%) | 0.24 | 0.05 |
| DLco (%) | 0.26 | 0.05 |
| PMCSA (cm2) | 0.36 | 0.007 |
| ECMCSA (cm2) | 0.39 | 0.003 |
CPET Cardiopulmonary exercise testing, oxygen uptake, BMI; body mass index, COPD chronic obstructive pulmonary disease, VC vital capacity, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, DLco diffusing capacity of lung for carbon monoxide, PMCSA cross-sectional area of pectoralis muscles, ECMCSA cross-sectional area of erector spinae muscles.
Figure 2.
Results of by (a) GOLD stage and (b) mMRC dyspnea scale score. *p < 0.05. oxygen uptake, GOLD Global Initiative for Chronic Obstructive Lung Disease, mMRC modified Medical Research Council.
Multivariate analysis of the correlation between at peak exercise and predictive variables including age, FEV1, and skeletal muscle areas
To evaluate the impact of skeletal muscle areas on exercise tolerance, multivariate analysis was performed using variables of age, FEV1 and skeletal muscle areas. PMCSA (β value [95% confidence interval] 0.175 [0.03–0.319], p = 0.02) was weakly correlated after adjustment (Table 5). In addition, ECMCSA (0.192 [− 0.001–0.385] p = 0.052) tended to be correlated, but not significantly after adjustment (Table 6).
Table 5.
Multivariate analysis of correlations between at peak exercise and age, FEV1, and PMCSA as predictive variables.
| Multivariate analysis | |||
|---|---|---|---|
| β | 95% CI | p value | |
| Age (years) | − 0.059 | − 0.185–0.068 | 0.36 |
| FEV1 (L) | 0.826 | − 1.01–2.662 | 0.37 |
| PMCSA (cm2) | 0.175 | 0.03–0.319 | 0.02 |
FEV1 forced expiratory volume in 1 s, ECMCSA cross-sectional area of erector spinae muscles, β standardized β value, CI confidence interval.
Table 6.
Multivariate analysis of correlations between at peak exercise and age, FEV1, and ECMCSA as predictive variables.
| Multivariate analysis | |||
|---|---|---|---|
| β | 95% CI | p value | |
| Age (years) | − 0.058 | − 0.187–0.072 | 0.37 |
| FEV1 (L) | 0.785 | − 1.099–2.668 | 0.41 |
| ECMCSA (cm2) | 0.192 | − 0.001–0.385 | 0.052 |
FEV1 forced expiratory volume in 1 s, ECMCSA cross-sectional area of erector spinae muscles, β standardized β value, CI confidence interval.
Discussion
In the present cross-sectional study, correlations between exercise tolerance indicated by at peak exercise and clinical parameters including skeletal muscle area were examined in Japanese patients with COPD. It was confirmed that at peak exercise was significantly correlated with 6-min walk distance and other CPET parameters, such as E/CO2, VD/VT, and respiratory rate, which suggested that at peak exercise is a useful marker of exercise tolerance for COPD patients. The analysis of correlation coefficients showed that the COPD assessment test, FEV1, FEV1/FVC, PMCSA, and ECMCSA were significantly correlated with at peak exercise, even though the correlations were weak. Additionally, the correlation coefficient between at peak exercise and ECMCSA are comparable to that between at peak exercise and PMCSA.
Loss of exercise tolerance is an important and widely recognized clinical manifestation of COPD15,22. With respect to the mechanisms, exercise-induced dyspnea with dynamic pulmonary hyperinflation and desaturation of oxygen, which is a representative manifestation of COPD, contributes to a low threshold of exhaustion with the early appearance of anaerobic metabolites in skeletal muscles during exercise22. Thus, at peak exercise on CPET, which is determined by cellular O2 demand and the maximal rate of O2 transport, is considered a useful marker of exercise tolerance in COPD patients15. Diaz et al. analyzed 52 patients with mild to severe COPD, and air-flow limitation, which reflects the presence of dynamic hyperinflation, was found to be significantly associated with at peak exercise12. Moreover, Kagawa et al. analyzed 294 patients with COPD who underwent CPET, and they found that decreased FEV1 was associated with a low at peak exercise13. These reports showed that limitation of exercise tolerance predicted by decreased at peak exercise is an important phenotype of COPD, as shown in the present study (Table 4, Fig. 1a,b). The severity of COPD predicted by %FEV1 is also related to the decrease of exercise tolerance, and Yamamoto et al. reported that at peak exercise was significantly higher in COPD patients in GOLD stages I and II than in those in GOLD stages III and IV14. The current results also showed that the level of at peak exercise tended to be decreased depending on the GOLD stage, except for stage I (Fig. 2a), although the correlation between at peak exercise and %FEV1 was weak (Table 4). Notably, at peak exercise in GOLD stage II patients was higher than that in GOLD stage I patients, as shown in Fig. 2a, although the difference was not significant. As indicated in Fig. 1a, the level of at peak exercise has various values in patients who showed a high FEV1, which might indicate that exercise tolerance in the early stage of COPD involves factors except for airway limitation such as skeletal muscle mass.
Loss of skeletal muscles with bodyweight reduction, called sarcopenia, is also an important characteristic of COPD patients20,23,24. Reduction of fat-free mass containing skeletal muscle is associated with mortality in patients with COPD25. In addition, a previous report showed that COPD patients with decreased skeletal muscles, calculated by bioelectrical impedance analysis, walked a significantly shorter distance on the incremental shuttle walk test, which is another index of exercise tolerance, than those with preserved skeletal muscles26. With respect to the mechanisms, loss of skeletal muscles causes increased O2 demand as exercise intensity increases and earlier reaching of the anaerobic threshold with metabolic acidosis and increased lactate, which limits exercise tolerance in patients with COPD27,28. The present study showed that skeletal muscle area, including PMCSA and ECMCSA, was significantly correlated with at peak exercise, which is consistent with these data (Table 4, Fig. 1c,d).
Notably, other gas exchange parameters on CPET such as E/CO2 and VD/VT at peak exercise were associated with the clinical data of COPD, including skeletal muscle area (Supplementary Table S1 online). These parameters were reported to be significantly higher in patients with COPD than in healthy individuals29, and E/CO2, which reflects decreased pulmonary clearance of CO2 during exercise, was correlated with BMI, %FEV1, and DLco, in addition to skeletal muscle area. Moreover, VD/VT, which reflects worse pulmonary gas exchange efficacy, was correlated with age, BMI, %VC, FEV1, FEV1/FVC, and %FEV1, in addition to skeletal muscle area. Interestingly, the COPD assessment test score was strongly correlated with these parameters, suggesting that E/CO2 and VD/VT might reflect COPD-related symptoms (Supplementary Table S1 online).
The present study has several limitations. First, correlations with physical activity were not evaluated. Second, correlations were evaluated using clinical parameters of COPD and skeletal muscle area, which acted as confounding factors. Third, study participants were selected by physicians’ suggestions and patients’ acceptance, which might have caused selection bias. Fourth, it is unclear that the current results for the correlation between exercise tolerance and skeletal muscle area is specific for patients with COPD, because healthy controls were not included. Fifth, the present study involved patients at a single hospital with limited ethnic diversity and a small sample size. Additionally, the percentage of females was extremely low in the present study, consistent with the general population of COPD, which might affect generalizability. To confirm the validity of the present results, multicenter, prospective studies with a larger number of patients should be performed.
Conclusions
The present cross-sectional study showed that in FEV1, FEV1/FVC, and skeletal muscle areas including PMCSA and ECMCSA are significantly correlated with exercise tolerance, even though the correlations are weak. These data suggest that pulmonary function and skeletal muscles contribute to exercise tolerance in patients with COPD.
Methods
Study design
The cross-sectional study was designed following the recommendations of the STROBE statement and approved by the ethics committee of Saga University Hospital (approval number: 2020-11-R-03, approval date: Jan 27, 2021) in accordance with the 1964 Declaration of Helsinki. Informed consent of the participants was obtained in the form of opt-out on the website. Those who rejected were excluded.
Patients and setting
The medical records of 69 patients diagnosed with COPD who underwent CPET at the Saga University Hospital between 2009 and 2020 were included in the present study. All patients satisfied the definition criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Briefly, patients were confirmed to have FEV1/FEV < 0.7 after using a bronchodilator, a smoking index > 10 pack years, and symptoms including chronic cough, sputum, and dyspnea. Patients with either a current or a previous diagnosis of asthma were excluded. For patient information, age at the time CPET was performed was used, and clinical parameters including BMI, modified Medical Research Council (mMRC) dyspnea scale, COPD assessment test, 6-min walk test, medication record, and pulmonary function at the time closest to when CPET was performed (within ± 3 months) were evaluated. Thus, 41 patients who underwent the COPD assessment test and 48 patients who underwent the 6-min walk test were analyzed. Medications were selected at each physician’s discretion. For handling of missing values, the participant data record was excluded for waves of data collection with missing values. The primary outcome was set as a significant correlation between at peak exercise and skeletal muscle areas including PMCSA and ECMCSA. For sample size calculation, the correlation between at peak exercise and skeletal muscle area have not been assessed, to the best of our knowledge, which suggests that the accurate calculation was not feasible. However, previous studies reported that FEV1 was significantly correlated with at peak exercise 14 and skeletal muscle area20. We hypothesized a significant correlation between at peak exercise and skeletal muscle area as with FEV1 (r = 0.4) and performed test of no correlation with two-sided 0.05 of significant level and 0.8 of statistical power, which estimated a sample size of 47 patients. Thus, we considered the current sample size of 69 patients was sufficient to achieve this primary outcome.
Cardiopulmonary exercise testing
A symptom-limited cycle ergometer (Strength Ergo 8, Mitsubishi Electric Engineering, Japan) was used for CPET. Each patient wore a mask, and breath was analyzed using a gas analyzer (Cpex-1, Inter Reha; Japan); , expiratory tidal volume (VT), minute ventilation (VE), ventilatory equivalent for carbon dioxide (E/CO2), dead space to tidal volume ratio (VD/VT), and breathing frequency at rest and at peak exercise were evaluated. Oxygen saturation, blood pressure, and the electrocardiogram were measured during the test. In the exercise protocol, pre-exercise resting measurements were obtained within the steady state period for more than 3 min. Incremental testing was then started by increasing the load by 10 W per minute with a ramp-exercise protocol. The examination was continued until exhaustion or the predicted maximum heart rate or blood pressure was surpassed, and showing electrocardiographic changes such as ST segment depression of greater than 2 mm and a short run of premature ventricular contractions. Dyspnea intensity was evaluated by a 10-point modified Borg category-ratio scale at rest and every 1 min after initiation of the incremental load test. The data generated were measured breath-by-breath and as 30-s averages at rest and during exercise.
CT scan acquisition and analysis
Chest CT for analysis of the pectoralis and erector spinae muscles that was performed most closely to the time of CPET (within ± 3 years) was also selected; the average time between CPET and chest CT was 198 days. Consequently, 56 patients were examined. For quantitative analysis, the CSAs of the pectoralis muscles (PMCSA) and the erector spinae muscles (ESMCSA) were evaluated referring to the previously described method20,24,30. Briefly, left and right areas of the PMCSA identified by the superior aspect of the aortic arch and the ESMCSA identified by the lower aspect of the 12th thoracic vertebrae on CT imaging reconstructed using the mediastinal setting were identified and shaded manually. Finally, the sum of the left and right muscle areas was examined. The measurements were performed by two pulmonary physicians independently referring to the representative images (Supplemental Fig. S1a,b online), and average values were used.
Statistical analysis
For correlation analysis, Spearman’s rank correlation coefficients between exercise tolerance parameters such as , E/CO2, VD/VT, breathing frequency, and 6-min walk distance, and clinical parameters including age, BMI, COPD assessment test score, %VC, %FVC, FEV1, FEV1/FVC, %FEV1, DLco, PMCSA, and ECMCSA were calculated to determine whether they were zero. Differences of at peak exercise depending on GOLD stages and the mMRC dyspnea scale were analyzed by the Steel–Dwass method. Multivariate analysis with linear regression analysis was performed for continuous variables, and β coefficient values were calculated. Quantitative data are presented as means ± standard deviation (SD); significance was considered a p value less than 0.05. Statistical analysis was performed with JMP Pro version 14.2.0 software (SAS Institute Inc., Cary, NC, USA).
Supplementary Information
Author contributions
H.T., K.T., and M.T. conceived and designed the project. H.T., Y.K., H.N., and H.I. analyzed and interpreted the data. R.T. and A.T. advised on the statistical analysis. H.T., K.T., and H.S. prepared the manuscript with input from all other authors. S.K. and N.A. checked the prepared manuscript. All authors reviewed the manuscript.
Data availability
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95413-9.
References
- 1.Halpin DMG, Celli BR, Criner GJ, et al. The GOLD Summit on chronic obstructive pulmonary disease in low- and middle-income countries. Int. J. Tuberc. Lung. Dis. 2019;23:1131–1141. doi: 10.5588/ijtld.19.0397. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa W, Yamauchi Y, Yasunaga H, et al. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm. Med. 2014;14:151. doi: 10.1186/1471-2466-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 4.Prudente R, Franco EAT, Mesquita CB, et al. Predictors of mortality in patients with COPD after 9 years. Int. J. Chron. Obstruct. Pulmon. Dis. 2018;13:3389–3398. doi: 10.2147/COPD.S174665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tashiro H, Kurihara Y, Takahashi K, et al. Clinical features of Japanese patients with exacerbations of chronic obstructive pulmonary disease. BMC Pulm. Med. 2020;20:318. doi: 10.1186/s12890-020-01362-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EK, Singh D, Park JH, et al. Impact of body mass index change on the prognosis of chronic obstructive pulmonary disease. Respiration. 2020;11:943–953. doi: 10.1159/000511022. [DOI] [PubMed] [Google Scholar]
- 7.Chang CH, Lin HC, Yang CH, et al. Factors associated with exercise-induced desaturation in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2020;15:2643–2652. doi: 10.2147/COPD.S272511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herer B, Chinet T. Acute exacerbation of COPD during pulmonary rehabilitation: Outcomes and risk prediction. Int. J. Chron. Obstruct. Pulmon. Dis. 2018;13:1767–1774. doi: 10.2147/COPD.S163472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laboratories ATSCoPSfCPF ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.Horie J, Takahashi K, Shiranita S, et al. Validation of Clinical characteristics and effectiveness of pulmonary rehabilitation in a COPD population with discrepancy between exercise tolerance and FEV1. Healthcare. 2021;9:1–53. doi: 10.3390/healthcare9010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: An official ERS statement. Eur. Respir. J. 2016;47:429–460. doi: 10.1183/13993003.00745-2015. [DOI] [PubMed] [Google Scholar]
- 12.Diaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur. Respir. J. 2000;16:269–275. doi: 10.1034/j.1399-3003.2000.16b14.x. [DOI] [PubMed] [Google Scholar]
- 13.Kagawa H, Miki K, Kitada S, et al. Dyspnea and the varying pathophysiologic manifestations of chronic obstructive pulmonary disease evaluated by cardiopulmonary exercise testing with arterial blood analysis. Front. Physiol. 2018;9:1293. doi: 10.3389/fphys.2018.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Miki K, Matsuki T, et al. Evaluation of exertional ventilatory parameters using oscillometry in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2020;15:1697–1711. doi: 10.2147/COPD.S260735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATS, ACCP ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 16.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 17.Ischaki E, Papatheodorou G, Gaki E, et al. Body mass and fat-free mass indices in COPD: Relation with variables expressing disease severity. Chest. 2007;132:164–169. doi: 10.1378/chest.06-2789. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani HK, Gerstner GR, Mota JA, et al. Influence of demographic characteristics and muscle strength on the occupational fatigue exhaustion recovery scale in career firefighters. J. Occup. Environ. Med. 2020;62:223–226. doi: 10.1097/JOM.0000000000001806. [DOI] [PubMed] [Google Scholar]
- 19.Bak SH, Kwon SO, Han SS, et al. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: A case control study. Respir. Res. 2019;20:226. doi: 10.1186/s12931-019-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanimura K, Sato S, Fuseya Y, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease: Novel chest computed tomography-derived index for prognosis. Ann. Am. Thorac. Soc. 2016;13:334–341. doi: 10.1513/AnnalsATS.201507-446OC. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe N, Sato S, Tanimura K, et al. Associations of CT evaluations of antigravity muscles, emphysema and airway disease with longitudinal outcomes in patients with COPD. Thorax. 2020;76:295–297. doi: 10.1136/thoraxjnl-2020-215085. [DOI] [PubMed] [Google Scholar]
- 22.Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680. doi: 10.1161/CIRCULATIONAHA.109.914788. [DOI] [PubMed] [Google Scholar]
- 23.Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014;189:e15–62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: A cross-sectional study. Ann. Am. Thorac. Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: Findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 26.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: Prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 27.Patessio A, Casaburi R, Carone M, et al. Comparison of gas exchange, lactate, and lactic acidosis thresholds in patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1993;148:622–626. doi: 10.1164/ajrccm/148.3.622. [DOI] [PubMed] [Google Scholar]
- 28.Hughson RL, Weisiger KH, Swanson GD. Blood lactate concentration increases as a continuous function in progressive exercise. J. Appl. Physiol. 1985;1987(62):1975–1981. doi: 10.1152/jappl.1987.62.5.1975. [DOI] [PubMed] [Google Scholar]
- 29.Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease: Implications for dyspnea and exercise intolerance. Am. J. Respir. Crit. Care Med. 2015;191:1384–1394. doi: 10.1164/rccm.201501-0157OC. [DOI] [PubMed] [Google Scholar]
- 30.Lee CS, Cron DC, Terjimanian MN, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin. Transplant. 2014;28:1092–1098. doi: 10.1111/ctr.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.