Abstract
Background
Metastatic urothelial carcinoma (mUC) historically is treated with first‐line platinum‐based combination chemotherapy, preferably cisplatin plus gemcitabine whenever possible. In recent years, multiple classes of targeted therapy have demonstrated benefit, with some receiving approval in mUC. This review will summarize phase III efficacy and safety data for targeted agents, principally immune checkpoint inhibitors (ICIs), as either first‐line or first‐line switch‐maintenance therapy for mUC and interpret these findings in the context of the current treatment landscape.
Materials and Methods
Published and presented phase III data on targeted therapy for the first‐line or first‐line switch‐maintenance treatment of mUC were identified using the key search terms “targeted therapy” AND “urothelial carcinoma” AND “advanced” OR respective aliases according to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).
Results
Of the six eligible phase III targeted therapy trials, two assessing ICIs met their primary endpoints in platinum‐eligible patients. First‐line ICI plus chemotherapy combinations have not improved overall survival (OS), although final OS results of the IMVigor 130 trial are pending. Switch‐maintenance using an ICI in patients achieving at least stable disease following platinum‐based chemotherapy statistically significantly improved OS (21.4 vs. 14.3 months, hazard ratio, 0.69; 95% confidence interval, 0.56–0.86; p = .001). Current sequencing options for mUC include first‐line platinum‐based chemotherapy with a switch to ICI either immediately or upon disease progression.
Conclusion
Recent targeted therapy trials have expanded ICI sequencing options for mUC. The treatment landscape is likely to evolve rapidly, with results from multiple phase III trials expected in the next 5 years.
Implications for Practice
Multiple classes of targeted agents are approved for use in metastatic urothelial carcinoma (mUC). Six phase III trials have recently provided insight on the benefit of these agents in the first‐line setting. In platinum‐eligible patients, immune checkpoint inhibitors (ICIs) combined with first‐line platinum‐based chemotherapy failed to demonstrate improved survival, although ICI monotherapy as switch‐maintenance significantly improved overall survival in patients with mUC who had achieved at least stable disease following first‐line platinum‐based chemotherapy. In patients ineligible for any chemotherapy, pembrolizumab, atezolizumab, or pembrolizumab in combination with enfortumab vedotin may be options.
Keywords: Urothelial carcinoma, Targeted therapy, Immune checkpoint inhibitors, Antibody‐drug conjugates, Human fibroblast growth factor receptor inhibitors
Short abstract
Treatment options for metastatic urothelial carcinoma are evolving, with multiple targeted treatment options, including immune checkpoint inhibitors, showing benefit. It is unclear whether any of these agents will replace the long established first‐line standard of care, platinum‐based chemotherapy. This review summarizes efficacy and safety data from first‐line trials and considers these findings within the current treatment landscape to offer guidance on treatment selection and sequencing.
Introduction
Bladder cancer is one of the most commonly diagnosed malignancies. Approximately 550,000 new cases occurred worldwide in 2018, resulting in 200,000 deaths [1]. Moreover, an estimated 81,400 new cases and nearly 18,000 deaths are expected in the U.S. in 2020 [2]. Men are approximately three to four times more likely to be diagnosed with and die of bladder cancer compared with women [1, 2, 3] and approximately 75% of new cases in the U.S. are aged 65 or older [2]. The vast majority of bladder cancers (90%) are urothelial carcinomas [4, 5]. From these, approximately 25% of patients are diagnosed with muscle‐invasive urothelial carcinoma [6, 7], and many will develop local or distant disease progression defined here as metastatic urothelial carcinoma (mUC). Historically, 5‐year survival rates for unresectable, locally advanced disease are approximately 36%, which is reduced to approximately 5% for those with distant metastases [2, 3]. The first‐line standard of care for most patients has been platinum‐based combination chemotherapy, with preference given to cisplatin plus gemcitabine in the approximately 50% of patients who can tolerate this regimen [8, 9, 10, 11]. Carboplatin and gemcitabine is the preferred choice in those who are cisplatin ineligible (approximately 40% of patients) [12], with immunotherapy being a common choice in the remaining patients [9, 11].
From 1992 to 2017, platinum‐based chemotherapy was the only treatment to demonstrate a survival benefit in mUC [13, 14]. Since then, however, multiple classes of targeted agents have been evaluated in urothelial cancer. For the purpose of this review, targeted agents are defined per the National Cancer Institute Dictionary as “A type of treatment that uses drugs or other substances to identify and attack specific types of cancer cells with less harm to normal cells. Some targeted therapies block the action of certain enzymes, proteins, or other molecules involved in the growth and spread of cancer cells. Other types of targeted therapies help the immune system kill cancer cells or deliver toxic substances directly to cancer cells and kill them” [15]. Recently evaluated targeted therapies in mUC include agents designed to inhibit the vascular endothelial growth factor (VEGF), human fibroblast growth factor receptors (FGFRs), and HER1/2 [16, 17, 18, 19], and immune checkpoints cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4), the programmed cell death protein 1 (PD‐1), and the programmed death ligand 1 (PD‐L1) [20], as well as antibody‐drug conjugates (ADC) targeting cell adhesion molecules (nectin‐4 or Trop‐2 receptors) [21, 22]. Multiple immune checkpoint inhibitors (ICIs), the FGFR 1–4 inhibitor, erdafitinib, and the ADC, enfortumab vedotin, have been approved for second‐line use in patients with mUC by various agencies including the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and Health Canada (HC) [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. Availability of these agents has multiplied the number of therapeutic options available for patients with mUC.
The treatment paradigm for mUC is rapidly evolving, with multiple targeted treatment options showing benefit for mUC [9]. In the last year, several phase III trials assessing targeted therapy, specifically ICIs, as first‐line or first‐line switch‐maintenance have reported results. However, it remains unclear whether any of these agents will replace the long established first‐line standard of care, platinum‐based chemotherapy. This review will summarize efficacy and safety data from these first‐line trials and consider these findings within the current treatment landscape to produce thoughtful guidance on treatment selection and sequencing.
Materials and Methods
Targeted therapy was defined as small molecule drugs such as tyrosine kinase inhibitors (TKIs) or monoclonal antibodies (MoAbs) that selectively target specific molecules on tumor cells or their microenvironment, thereby blocking tumor growth and spread. A search of published and presented literature was conducted to identify phase III trials reporting first‐line or first‐line maintenance with outcomes on the use of targeted therapy in mUC. PubMed (all time to October 6, 2020) as well as the proceedings from the 2019 and 2020 American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology annual meetings and the ASCO Genitourinary Cancers Symposium were searched using the key search terms “targeted therapy” AND “urothelial carcinoma” AND “advanced” OR respective aliases. A supplemental bibliographic search of review articles and pooled/meta‐analyses was also conducted. In addition, directed searches were performed after the database search cutoff date to ensure that the most up‐to‐date reports of eligible studies were considered.
English language records were vetted at abstract level and confirmed at full text as needed. Excluded studies included those that were nonoriginal research, preclinical, correlative science, not specific to mUC, in early stages of disease, retrospective, prospective phase I, II, IV or undefined phase, studies not assessing targeted therapy agents as first‐line or first‐line maintenance therapy, and duplicate or prior reports. Studies without reported efficacy outcomes were also excluded.
Results
The literature search identified a total of 308 records, resulting in a total of six eligible phase III trials (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses, Fig. 1) [38, 39, 40, 41, 42, 43]. Four studies assessed targeted agents as first‐line systemic therapy [38, 39, 40, 41] and two assessed targeted therapy as first‐line maintenance [42, 43].
Figure 1.
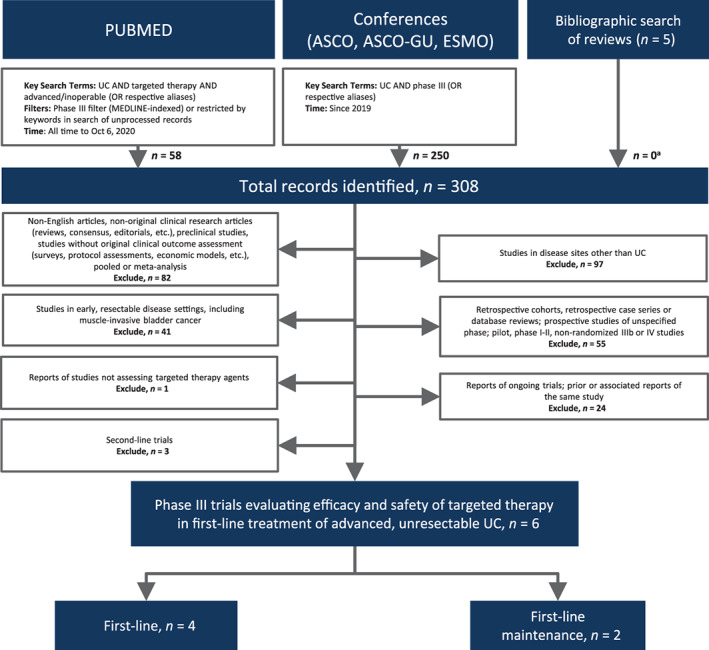
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses diagram. aPrimary or associated reports of eligible studies that were not identified through database search. Abbreviations: ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; GU, genitourinary; UC, urothelial carcinoma.
First‐Line Targeted Therapy Plus Chemotherapy Combinations
Four phase III trials assessed targeted therapy combinations as first‐line systemic treatment of locally advanced or mUC, resulting in median overall survival (OS) of 14.5–17.0 months (Table 1) [38, 39, 40, 41]. One added a MoAb against VEGFA to chemotherapy [41] and three investigated ICIs in this setting [38, 39, 40]. CALGB 90601 randomized 506 patients 1:1 to receive the anti‐VEGFA MoAb bevacizumab plus platinum‐based chemotherapy compared with placebo plus chemotherapy, with both arms receiving bevacizumab or placebo as maintenance until disease progression. With the median follow‐up not reported, the addition of bevacizumab did not statistically significantly improve the primary endpoint of median OS versus standard chemotherapy (14.5 vs. 14.3 months; hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.72–1.06; p = .17) [41]. A statistically significant increase in median progression‐free survival (PFS) was reported, which was not deemed clinically meaningful (7.7 vs. 6.6 months; HR, 0.79; 95% CI, 0.66–0.95; p = .013). Overall response rates (ORRs) were also not substantially improved and median duration of responses (DoRs) were not reported. Discontinuation because of adverse events (AEs) and overall grade ≥ 3 AEs were also not reported (Table 2). AE‐related deaths were more frequent with the addition of bevacizumab compared with placebo (3.4% vs. 0.4%).
Table 1.
Efficacy outcomes of phase III trials assessing targeted therapy in advanced UC
| Trial key eligibility criteria | Regimen(s) | n | Median follow‐up, months | Overall response rate | DoR | Progression‐free survival | Overall survival | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORR (95% CI) | p value | Median (95% CI), months | Median, months | HR (95% CI) | p value | Median, months | HR (95% CI) | p value | ||||
| First‐line | ||||||||||||
| CALGB 90601 [41] | Bevacizumab 15 mg/kg D1 + gemcitabine 1,000 mg/m2 D1,8 + cisplatin 70 mg/m2 D1, q3w up to 6 cycles then bevacizumab 15 mg/kg, q3w until progression | 252 | NR for patients still alive: 46.2 | 40.4 | p = .12 | NR | 7.7 | 0.79 (0.66–0.95) | p = .013 | 14.5 | 0.87 (0.72–1.06) | p = .17 |
| Placebo + gemcitabine 1,000 mg/m2 D1,8 + cisplatin 70 mg/m2 D1, q3w up to 6 cycles then placebo until progression | 254 | 33.0 | NR | 6.6 | 14.3 | |||||||
| IMvigor130 [38] | Atezolizumab 1,200 mg D1 + platinum agent a D1 + gemcitabine 1,000 mg/m2 D1, 8, q3w approximately 6 cycles then until atezolizumab q3w until progression | 451 | 11.8 | 47b (43–52) | 8.5 b (7.2–10.4) | 8.2 | 0.82 (0.70–0.96) | p = .007 c | 16.0 | 0.83 (0.69–1.00) | p = .027 c , d | |
| Atezolizumab 1,200 mg D1, q3w until progression | 362 | 23 e (19–28) | NE e (15.9–NE) | NR f | 15.7 | 1.02 (0.83–1.24) | p = NR g | |||||
| Placebo 1,200 mg D1 + platinum agent a D1 + gemcitabine 1,000 mg/m2 D1,8, q3w until progression | 400 h | 44 i (39–49) | 7.6 i (6.3–8.5) | 6.3 | 13.4 j | |||||||
| KEYNOTE‐361 [40] | Pembrolizumab 200 mg D1 + gemcitabine 1,000 mg/m2 D1 and D8 cisplatin 70 mg/m2 or carboplatin AUC 5 D1 q3w up to 6 cycles then pembrolizumab 200 mg q3w up to 29 cycles | 351 | 31.7 | 54.7 | 8.5 [2.0+ to 35.5+] | 8.3 | 0.78 (0.66–0.93) | p = .003 k | 17.0 | 0.86 (0.72–1.02) | p = .04 k | |
| Pembrolizumab 200 mg D1 q3w up to 35 cycles | 307 | 30.3 | 28.2 [2.1+ to 36.1+] | NR l | 15.6 l | 0.92 (0.77–1.11) | ||||||
| Cisplatin 70 mg/m2 or carboplatin AUC 5 D1 + gemcitabine 1,000 mg/m2 D1, D8 q3w up to 6 cycles | 352 | 44.9 | 6.2 [1.8+ to 36.3+] | 7.1 | 14.3 | |||||||
| DANUBE [39] | Durvalumab 1,500 mg + tremelimumab 75 mg q4w up to 4 cycles then durvalumab 1,500 mg q4w until progression | 342 | 41.2 | 36 | 11.1 (7.9–18.5) | 3.7 | (3.4–3.8) | 15.1 | 0.85 (0.72–1.02) | p = .075 | ||
| Durvalumab 1,500 mg q4w until progression | 346 | 26 | 9.3 (5.8–20.5) | 2.3 | (1.9–3.5) | 13.2 m | 0.99 (0.83–1.17) | |||||
| Gemcitabine + cisplatin or carboplatin up to 6 cycles | 344 | 49 | 5.7 (5.6–6.2) | 6.7 | (5.7–7.3) | 12.1 | ||||||
| First‐line switch‐maintenance | ||||||||||||
|
OCTG‐LaMB [42] HER1/2–positive Platinum‐eligible and ‐ineligible No PD on first‐line CT |
Lapatinib 1,500 mg qd until progression | 116 | NR | 14 n (NR) | p = .14 | NR | 4.5 | 1.07 (0.81–1.43) | p = .63 | 12.6 | 0.96 (0.70–1.31) | p = .80 |
| Placebo | 116 | 8o (NR) | NR | 5.1 | 12.0 | |||||||
|
JAVELIN Bladder 100 [43] Platinum‐eligible CR, PR, or SD on first‐line platinum CT |
Avelumab 10 mg/kg q2w until progression + BSC | 350 | >19 | 9.7 (6.8–13.3) OR = 7.5 | NR | 3.7 | 0.62 (0.52–0.75) | p < .001 | 21.4 | 0.69 (0.56–0.86) | p = .001 | |
| BSC | 350 | 1.4 (0.5–3.3) | NR | 2.0 | 14.3 | |||||||
Efficacy outcomes of phase III targeted therapy trials in UC ordered by line of therapy, platinum eligibility status (in first‐line) then publication date.
+ indicates an ongoing response at data cutoff.
Investigator choice of cisplatin 70 mg/m2 or carboplatin AUC 4.5.
Evaluable patients for ORR, n = 447; for DoR, n = 212.
One‐sided, vs. placebo+platinum/gemcitabine treatment arm.
Did not cross the interim efficacy boundary of 0.007 per the O'Brien‐Fleming function.
Evaluable patients for ORR, n = 359; for DoR, n = 82.
Will be reported elsewhere or as applicable with additional study follow‐up.
Statistical hypothesis testing not done due to lack of statistical significance for atezolizumab plus chemotherapy versus placebo plus chemotherapy arms as per protocol.
n = 359 for comparisons with atezolizumab monotherapy arm.
Evaluable patients for ORR, n = 397; for DoR, n = 174.
n = 359 and OS = 13.1 months for the comparison with atezolizumab monotherapy.
Not significant by prespecified criteria; p value boundary .0019 for PFS and .014 for OS.
Not formally tested because OS for pembrolizumab plus chemotherapy not significantly improved over chemotherapy.
Secondary endpoint; co‐primary endpoint was OS for durvalumab versus chemotherapy in the PD‐L1 high population.
Best response rate.
Abbreviations: AUC, area under the curve; BSC, best supportive care; CI, confidence interval; CR, complete response; CT, chemotherapy; D, day; DoR, duration of response; HR, hazard ratio; NE, not estimable; NR, not reported; NYR, not yet reached; OR, odds ratio; ORR; overall response rate; OS, overall survival; qd, once per day; PD, progressive disease; PD‐L1, programmed death ligand 1; PFS, progression‐free survival; PR, partial response; qXw, every X weeks; SD, stable disease; UC, urothelial carcinoma.
Table 2.
Safety outcomes of select phase III trials assessing targeted therapy in advanced urothelial carcinoma
| Trial | Regimen(s) | Safety population, n | Treatment discontinuation due to TRAEs, % | Overall grade 3/4 TRAEs, % | Deaths due to TRAEs, % |
|---|---|---|---|---|---|
| First‐line | |||||
| IMvigor130 [38] | Atezolizumab 1,200 mg D1 + platinum agent a D1 + gemcitabine 1,000 mg/m2 D1, 8, q3w approximately 6 cycles then until atezolizumab q3w until progression | 453 | 34.4 b | 81.0 | 2.0 |
| Atezolizumab 1,200 mg D1, q3w until progression | 354 | 6.2 b | 15.3 | 0.8 | |
| Placebo 1,200 mg D1 + platinum agent a D1 + gemcitabine 1,000 mg/m2 D1,8, q3w until progression | 390 | 33.8 b | 80.8 | 1.0 | |
| KEYNOTE‐361 [40] | Pembrolizumab 200 mg D1 + gemcitabine 1,000 mg/m2 D1 and D8 cisplatin 70 mg/m2 or carboplatin AUC 5 D1 q3w up to 6 cycles then pembrolizumab 200 mg q3w up to 29 cycles | 351 c | 30.9 | 87.4 b , d |
9.2 (Deaths due to any AE) |
| Pembrolizumab 200 mg D1 q3w up to 35 cycles | 307 c | 15.9 | 62.9 b , d | 8.6 c | |
| Cisplatin 70 mg/m2 or carboplatin AUC 5 D1 + gemcitabine 1,000 mg/m2 D1, D8 q3w up to 6 cycles | 352 c | 18.1 | 81.9 b , d | 2.6 b | |
| DANUBE [39] | Durvalumab 1,500 mg + tremelimumab 75 mg q4w up to 4 cycles then durvalumab 1,500 mg q4 until progression | 340 | 23.5 b | 27.4 | 0.6 |
| Durvalumab 1,500 mg q4w until progression | 345 | 11.9 b | 13.6 | 0.6 | |
| Gemcitabine + cisplatin or carboplatin up to 6 cycles | 313 | 16.9 b | 60.1 | 0.3 | |
| First‐line switch‐maintenance | |||||
| JAVELIN Bladder 100 [43] | Avelumab 10 mg/kg q2w until progression + BSC | 344 | 9.6 e | 16.6 d , e | 0.3 e |
| BSC | 345 | 0 e | 0 e | 0 e | |
Safety outcomes of phase III targeted therapy trials in urothelial carcinoma ordered by line of therapy, platinum eligibility status (in first‐line), and then publication date.
Investigator choice of cisplatin 70 mg/m2 or carboplatin AUC 4.5.
Any AE.
Intention‐to‐treat population.
Grade ≥3.
Treatment‐related treatment‐emergent AEs.
Abbreviations: AE, adverse event; AUC, area under the curve; BSC, best supportive care; D, day; qXw, every X weeks; TRAEs, treatment‐related AEs.
Two trials, IMvigor 130 and KEYNOTE‐361, compared ICIs in combination with platinum‐based chemotherapy and ICI monotherapy with standard platinum‐based chemotherapy [38, 40]. Following a protocol amendment, IMvigor 130 randomized patients 1:1:1 to receive atezolizumab plus platinum‐based chemotherapy (n = 451) or atezolizumab monotherapy (n = 362) compared with placebo plus platinum‐based chemotherapy (n = 400). At a median follow‐up of 11.8 months, the atezolizumab plus chemotherapy combination demonstrated a statistically significant improvement in the coprimary endpoint of investigator‐assessed median PFS (8.2 vs. 6.3 months; HR, 0.82; 95% CI, 0.70–0.96; p = .007) and a numerical increase in the coprimary endpoint of median OS that was not statistically significant (16.0 vs. 13.4 months; HR, 0.83; 95% CI, 0.69–1.00; p = .027, interim boundary p = .007) compared with placebo plus chemotherapy (Table 1) [38]. There was also a numerical increase in the primary endpoint of median OS for atezolizumab monotherapy compared with placebo plus chemotherapy (15.7 vs. 13.1 months; HR, 1.02; 95% CI, 0.83–1.24), although this benefit emerged late, and the hierarchical study design precluded a formal analysis at this time. Time to deterioration of quality of life (QoL) was 24.8 months in the combination arm and 16.6 months in the placebo plus chemotherapy arm [44]. Confirmed ORRs were similar in the atezolizumab combination and chemotherapy arms, although lower for atezolizumab monotherapy, and median DoR favored atezolizumab plus chemotherapy versus chemotherapy (Table 1). Atezolizumab monotherapy was better tolerated than both atezolizumab plus chemotherapy and chemotherapy alone, with treatment‐related adverse events (TRAEs) leading to treatment discontinuation occurring in 6.2% versus 34.4% and 33.8% of patients and grade 3/4 TRAEs occurring in 15.3% versus 81.0% and 80.8% of patients, respectively (Table 2). Deaths because of TRAEs occurred in 2.0%, 0.8%, and 1.0% of patients who received atezolizumab plus chemotherapy, atezolizumab monotherapy, and placebo plus chemotherapy, respectively.
KEYNOTE‐361 compared the PD‐1 inhibitor pembrolizumab plus platinum‐based chemotherapy (n = 351) with pembrolizumab monotherapy (n = 307) and chemotherapy alone (n = 352). With a median time from randomization to cutoff of 31.7 months, no statistically significant improvement was seen in the coprimary endpoints of median OS or PFS in the intent‐to‐treat (ITT) population for the pembrolizumab combination compared with chemotherapy alone (Table 1) [40]. Median OS was 17.0 versus 14.3 months (HR, 0.86; 95% CI, 0.72–1.02; p = .041, p‐threshold for significance = .0142), median PFS was 8.3 versus 7.1 months (HR, 0.78; 95% CI, 0.65–0.93; p = .003, p‐threshold for significance = .0019) and ORRs were marginally higher for pembrolizumab plus chemotherapy versus controls (Table 1). Although not formally tested according to the hierarchal design, the median OS for pembrolizumab monotherapy versus chemotherapy was 15.6 versus 14.3 months (HR, 0.92; 95% CI, 0.77–1.11) with reduced ORRs for pembrolizumab. Treatment discontinuation because of any AEs occurred in 30.9%, 15.9%, and 18.1% of patients. Deaths because of any AEs occurred in 9.2%, 8.6%, and 2.6% of patients who received pembrolizumab plus chemotherapy, pembrolizumab monotherapy, and chemotherapy, respectively.
Whereas IMvigor 130 and KEYNOTE‐361 combined ICI with platinum‐based chemotherapy [38, 40], DANUBE compared a dual ICI combination as well as ICI monotherapy with platinum‐based chemotherapy. DANUBE randomized patients 1:1:1 to receive the PD‐L1 inhibitor durvalumab plus the CTLA‐4 inhibitor tremelimumab (n = 342) or durvalumab monotherapy (n = 346) compared with platinum‐based chemotherapy (n = 344). At a median follow‐up of 41.2 months, no statistically significant improvement was seen in the coprimary endpoint of median OS in the ITT population for durvalumab plus tremelimumab compared with standard chemotherapy (15.1 vs. 12.1 months; HR, 0.85; 95% CI, 0.72–1.02; p = .075; Table 1) [39]. There was also no statistically significant improvement in the coprimary endpoint of median OS for durvalumab monotherapy versus chemotherapy among patients with high PD‐L1 expression (14.4 vs. 12.1 months; HR, 0.89; 95% CI, 0.71–1.11; p = .30). ORRs were not improved in the experimental arms compared with controls. Treatment discontinuation because of any AEs occurred in 23.5%, 11.9%, and 16.9% of patients and deaths because of TRAEs occurred in 0.6%, 0.6%, and 0.3% of patients who received pembrolizumab plus chemotherapy, pembrolizumab monotherapy, and chemotherapy, respectively.
First‐Line Targeted Therapy Switch‐Maintenance
Two phase III trials have assessed targeted therapy switch‐maintenance following first‐line platinum‐based chemotherapy in mUC [42, 43]. The small OCTG‐LaMB double‐blind study randomized 232 patients with HER1‐ or HER2‐positive disease to receive lapatinib, a pan HER TKI, or placebo following four to eight cycles of chemotherapy for mUC. Lapatinib did not improve the primary endpoint of median PFS (HR, 1.07; 95% CI, 0.81–1.43; p = .63) or the secondary endpoints of median OS (HR, 0.96; 95% CI, 0.70–1.31; p = .80) and best response rates compared with placebo (Table 1) [42]. Discontinuation because of toxicity and grade 3/4 AEs were similar between arms, and deaths because of AEs were not reported (Table 2).
The phase III JAVELIN Bladder 100 trial included patients with locally advanced or mUC. Patients who achieved at least stable disease following four to six cycles of standard first‐line chemotherapy were randomized 1:1 to receive switch‐maintenance therapy with the ICI avelumab plus best supportive care (BSC; n = 350) or BSC alone (n = 350). At a median follow‐up of greater than 19 months, a statistically significant improvement in the primary endpoint of median OS was demonstrated with the addition of avelumab to BSC compared with BSC alone (21.4 vs. 14.3 months; HR, 0.69; 95% CI, 0.56–0.86; p = .001; Table 1) [43]. Median PFS was longer for avelumab (3.7 vs. 2.0 months; HR, 0.62; 95% CI, 0.52–0.75; p = not reported). Mean changes from baseline for the Functional Assessment of Cancer Therapy ‐ Bladder Symptom Index (FBISI‐18) and EuroQol‐5 Dimension‐5 Level (EQ‐5D‐5L) QoL scores were similar between treatment arms [45]. ORRs were higher for avelumab compared with the control arm, although DoRs were not reported [43]. Discontinuation of avelumab because of treatment‐related treatment‐emergent AEs (TRTEAEs) occurred in 9.6% of patients, and grade 3 or higher TRTEAEs occurred in 16.6% in the avelumab arm (Table 2). The most common grade ≥ 3 TRAEs in the avelumab arm were increased lipase (2.9%), increased amylase (2.0%), infusion‐related reactions (0.9%), and pruritus, hypothyroidism, fatigue, rash, nausea, and arthralgia (0.3% for each). Death attributed to TRTEAEs was reported in one patient in the avelumab arm (0.3%).
Discussion
What Is the Clinical Benefit of Targeted Therapy in the First‐Line Treatment of mUC?
Chemotherapy‐Eligible mUC
Platinum‐based chemotherapy has been standard of care for first‐line patients with mUC for decades, with cisplatin and gemcitabine preferred in cisplatin‐eligible and carboplatin and gemcitabine used in cisplatin‐ineligible patients [9, 10, 11]. The phase III IMvigor 130, KEYNOTE‐361, and DANUBE trials compared ICI combinations with platinum‐based chemotherapy [38, 39, 40]. Although numerical increases in the primary endpoints of PFS and/or OS were seen in KEYNOTE‐361 and DANUBE, they did not reach statistical significance [39, 40]. IMvigor 130 demonstrated a statistically significant 18% reduction in the risk of disease progression (p = .007) and an 8.2‐month numerical increase in the time to deterioration of QoL with the addition of atezolizumab to chemotherapy [38, 44]. At the interim OS analysis, the 2.6‐month increase in median OS had not reached statistical significance and results from the final analysis are awaited [38]. It is unclear why first‐line ICI combinations did not improve median OS compared with platinum‐based chemotherapy. The lack of substantial differences in ORRs for ICI combination compared with chemotherapy arms in the KEYNOTE‐361 (54.7% vs. 44.9%), IMvigor 130 (47% vs. 44%), and DANUBE (36% vs. 49%) would suggest a lack of synergy [38, 39, 40]. However, outcomes could also be explained by the fact that ICI combination had to overcome both the strong initial efficacy of their chemotherapy comparator, an antagonistic effect between concurrent chemotherapy and ICI therapy, or a dilution effect related to patient cross‐over from the chemotherapy‐only arm to the chemotherapy and ICI arms. For example, 48%, 31%, and 20% of patients in the chemotherapy arms of KEYNOTE‐361, DANUBE, and IMvigor 130, respectively, received subsequent immunotherapy. No FDA, EMA, or HC approvals have been granted for first‐line ICI combinations at this time and the final OS results of IMvigor 130 as well as the results of other trials evaluating additional ICI chemotherapy combinations (CheckMate901 [NCT03036098], NILE [NCT03682068], and BGB‐Q317‐310 [NCT03967977]) and dual ICI combinations (CheckMate901 [NCT03036098] and NILE [NCT03682068]) are awaited. Outcomes from these trials will help shed light on the role of first‐line ICI and chemotherapy combinations and the optimal sequencing of ICIs following up‐front chemotherapy.
The JAVELIN Bladder 100 study reported a statistically significant OS benefit for avelumab switch‐maintenance and BSC compared with BSC alone in patients who achieved at least stable disease following four to six cycles of platinum‐based chemotherapy, resulting in a 31% reduction in the risk of death in the overall study population (p = .001) as well as a 44% reduction in the risk of death among patients with tumors overexpressing PD‐L1 (p < .001) [43]. These benefits remained, despite more than half of the patients on the BSC alone arm receiving an ICI upon progression. Avelumab maintenance was approved by the FDA on June 30, 2020 [46], by HC on January 11, 2021 [47], and by the EMA on January 21, 2021 [48], although this option still may be unavailable in some jurisdictions. No further phase III switch‐maintenance trials are underway. Given first‐line findings to date, it is clear that the standard of care for platinum‐naive patients involves chemotherapy first, followed by ICIs delivered immediately as switch‐maintenance or upon progression.
Chemotherapy‐Ineligible mUC
A proportion of first‐line patients with mUC cannot tolerate platinum‐based chemotherapy and are in need of alternate treatment options. The ICIs pembrolizumab and atezolizumab have been approved as first‐line monotherapy in patients who are chemotherapy ineligible, regardless of PD‐L1 status [49], based on results of the phase II IMvigor 210 [50] and the phase II KEYNOTE‐052 [51] studies (Table 3). Three of the large first‐line ICI trials (IMvigor 130, KEYNOTE‐361, and DANUBE) assessed ICI monotherapy compared with platinum‐based chemotherapy in chemotherapy‐eligible patients [38, 39, 40]. None of them demonstrated a benefit for ICI monotherapy compared with chemotherapy among platinum‐eligible patients overall (IMvigor 130 [HR, 1.02], KEYNOTE‐361 [HR, 0.92], or DANUBE [HR, 0.99]). ICIs therefore remain appropriate options for patients who cannot tolerate any platinum‐based chemotherapy. Recent results from the phase II EV‐103 trial also reported an ORR of 73.3% and a 1‐year OS of 81.6% for a combination of the anti‐nectin ADC enfortumab vedotin combined with pembrolizumab among 45 treatment‐naive patients who were cisplatin ineligible [52]. These findings led to the FDA granting breakthrough therapy designation for this combination among first‐line cisplatin‐ineligible patients in February 2020 [53]. Although carboplatin‐based chemotherapy followed by ICI switch‐maintenance is the best option for first‐line patients who are cisplatin ineligible, ICI monotherapy or eventually pembrolizumab combined with enfortumab vedotin are good options for patients who cannot tolerate chemotherapy.
Table 3.
Efficacy outcomes of select phase II first‐line trials in cisplatin‐ineligible metastatic urothelial carcinoma
| Trial ID Phase | Regimen(s) | n | Median follow‐up, months | Overall response rate, (95% CI), % | Median DoR (95% CI), months | Median progression‐free survival, (95% CI), months | Median overall survival (95% CI), months |
|---|---|---|---|---|---|---|---|
|
KEYNOTE‐052 |
Pembrolizumab 200 mg D1, q3w (up to 24 months) | 370 | 24 (minimum) | 28.6 (24.1–33.5) | 30.1(18.1–NYR) | 2.2 (2.1–3.4) | 11.3 (9.7–13.1) |
| PD‐L1 CPS ≥10: 110 | 47.3 (37.7–57.0) | NYR (18.1–NE) | NR | 18.5 (12.2–28.5) | |||
|
IMvigor 210 multicohort phase II cohort 1 [50] |
Atezolizumab 1,200 mg D1, q3w | 119 | 17.2 | 23 (16–31) | NYR | 2.7 (2.1–4.2) | 15.9 (10.4–NE) |
| PD‐L1 IC2/3: 32 | 28 (14–47) | 4.1 (2.3–11.8) | 12.3 (6.0–NE) | ||||
|
EV‐103 multicohort phase I/II cohort A [51] |
Enfortumab vedotin 1.25 mg/kg D1,8 + pembrolizumab 200 mg D1, q3w | 45 | 11.5 | 73.3 (58.1–85.4) | NYR [1.2–12.9+] | 12.3 (8.0–NE) | NYR (range, 0.66–19.2+) |
| PD‐L1 CPS ≥10: 14 | CPS ≥10: 78.6 (Primary endpoint safety) |
Efficacy outcomes of select phase I–II targeted therapy trials in urothelial carcinoma ordered by size of trial.
Abbreviations: CI, confidence interval; CPS, combined positive score; D, day; DoR, duration of response; IC, immune cells; ID, identifier; NE, not estimable; NR, not reported; NYR, not yet reached; PD‐L1, programmed death ligand; qXw, every X weeks.
How Can Biomarkers and Clinical Features Be Used to Guide Therapy?
Results from first‐line trials to date show that patients with mUC derive some benefit from ICIs [38, 40], although data across trials suggest that not all patients benefit equally and that biomarkers may be helpful for patient selection. The most common biomarkers assessed in mUC include PD‐L1 expression and tumor mutation burden (TMB), although predictive benefit seems to depend on the treatment approach. For ICI monotherapy, DANUBE failed to show a statistically significant survival benefit for this approach compared with chemotherapy among patients overall [39]. Although this trial was both stratified and prospectively designed to assess OS in patients with tumors overexpressing PD‐L1, a statistically significant improvement in survival compared with chemotherapy was not seen in these patients (HR, 0.89; 95% CI, 0.71–1.11; p = .30). However, an exploratory analysis from IMvigor 130 suggested an OS benefit for atezolizumab monotherapy compared with platinum‐based chemotherapy for patients who had a PD‐L1 immune cell (IC) 2/3 tumor status (p = .015) [38]. For ICI combination therapy, a secondary analysis of DANUBE (HR, 0.74; 95% CI, 0.59–0.93) [39] and exploratory analyses of IMvigor 130 confirmed that PD‐L1 status did not statistically significantly predict OS benefit from ICI combination therapy [54, 55]. For ICI switch‐maintenance therapy, JAVELIN Bladder 100 was prospectively designed and stratified to assess OS benefit in patients with PD‐L1‐positive tumors. The study reported a benefit among all comers (HR, 0.69; 95% CI, 0.56–0.86; p = .001) with a slightly greater benefit among patients with tumors overexpressing PD‐L1 (HR, 0.56; 95% CI, 0.40–0.79; p < .001) [43]. Findings to date suggest that PD‐L1 overexpression may have a role in predicting benefit from ICI monotherapy in platinum‐eligible patients, has a low predictive capacity for ICI combination therapy, and may have a role in predicting benefit for ICI switch‐maintenance therapy.
In addition to PD‐L1, both IMvigor 130 and DANUBE conducted additional exploratory biomarker analyses. IMvigor 130 found that high TMB (p = .007), high apolipoprotein B mRNA editing enzyme, catalytic polypeptide‐like cytidine deaminases (p = .01), and high PD‐L1 plus high TMB (p = .009) predicted benefit for atezolizumab monotherapy, whereas high tubuloglomerular feedback‐response signatures of fibroblasts was negatively predictive (p = .018) [54]. For JAVELIN Bladder 100, high TMB (HR, 0.48; 95% CI, 0.33–0.71), PD‐L1 overexpression (HR, 0.56; 95% CI, 0.40–0.79), and a combination of high TMB plus PD‐L1 overexpression (HR, 0.51; 95% CI, 0.31–0.87) appeared predictive of OS benefit compared with BSC [56]. There is some controversy as to whether FGFR alterations such as those found in the luminal papillary subtype may be negatively predictive for ICI benefit [50, 57, 58, 59]. The ongoing phase III THOR trial of erdafitinib compared with pembrolizumab second‐line (NCT03390504) may help further elucidate the role of this biomarker.
In addition to biomarkers, use of clinical and disease characteristics can help identify patients who might preferentially benefit from ICI combination or ICI switch‐maintenance therapy. It should be noted that both the IMvigor 130 [38] and JAVELIN Bladder 100 [43] trials restricted inclusion of patients with central nervous system (CNS) metastases; the former excluded patients with active CNS metastases and the latter excluded those with symptomatic metastases requiring treatment with steroids. Moreover, the IMvigor 130 trial excluded patients with an Eastern Cooperative Oncology Group performance status (PS) >2 and PFS subgroup analyses performed in IMvigor 130 [38] and OS subgroup analyses performed in JAVELIN Bladder 100 [43] showed that patients with good PS (0) benefited more from ICI combination (HR, 0.69) or switch‐maintenance therapy (HR, 0.64) than those with a poor PS (2 and ≥ 1; HR, 0.94 and HR, 0.74, respectively). Additionally, patients in JAVELIN Bladder 100 without liver metastases at baseline (HR, 0.65) benefited more from avelumab switch‐maintenance than those with liver metastases (HR, 0.92) [60]. Importantly, the best response to first‐line chemotherapy (stable disease vs. complete/partial response) was not a variable influencing OS benefit from avelumab switch‐maintenance (HR, 0.92).
What Is the Optimal First‐ and Second‐Line Sequence for Targeted Therapy in the Treatment of mUC?
Treatment selection for mUC should be guided by a multidisciplinary team of specialists and may involve multiple lines of systemic therapy. Results from first‐line and second‐line trials to date present two viable sequencing options for patients with mUC who have a PS ≤2 and are platinum eligible. The first option is platinum‐based chemotherapy followed by switch‐maintenance in those who have achieved at least stable disease [43]. Another option is the use of standard platinum‐based chemotherapy followed by pembrolizumab upon progression [31, 33, 34]. If the final OS results of IMvigor 130 are positive, first‐line atezolizumab in combination with platinum‐based chemotherapy would be another option. Regardless of the initial option, selection of subsequent targeted therapy may include enfortumab vedotin based on recent results from the phase III EV‐301 trial (n = 608) showing an ORR of 40.6% versus 17.9% (p < .001) compared with chemotherapy and a statistically significant OS improvement (HR, 0.70; p = .001) [61] and a phase II study of erdafitinib (n = 101) with an ORR of 40% in FGFR‐selected patients [62]. For first‐line patients who are chemotherapy ineligible, a standard of care has not yet been definitively established, but pembrolizumab, atezolizumab, or pembrolizumab in combination with enfortumab vedotin may be options [27, 28, 29, 31, 33, 34, 53]. For patients progressing on the aforementioned options, who are cisplatin ineligible, enfortumab vedotin is an option based on results of the EV‐201 cohort 2 (ORR, 52%) [63]. Other therapies such as sacituzumab govitecan (ORR, 27%; median DoR, 5.9 months) [64] or eganelisib plus nivolumab (ORR, 30.3%) [65] may eventually represent yet another option for pretreated patients with mUC.
What Is the Direction of Ongoing Targeted‐Therapy Research in Urothelial Carcinoma?
The role of targeted therapy in bladder cancer is rapidly evolving with multiple phase III trials underway in all lines of therapy, including first‐line combinations of ICIs plus chemotherapy (BGB‐A317‐310, NCT03967977; NILE, NCT03682068; CheckMate 901, NCT03036098; JS001‐038‐III‐UBC, NCT04568304), a TKI (LEAP‐011, NCT03898180), another ICI (NILE, NCT03682068; CheckMate 901, NCT03036098), or enfortumab vedotin (EV‐302, NCT04223856) (Table 4). In earlier disease stages, recent results from CheckMate‐274 trial have demonstrated improved disease‐free survival (HR, 0.70; p < .001) for the ICI nivolumab as adjuvant therapy for muscle‐invasive urothelial carcinoma [66]. Additional ICI (AMBASSADOR, NCT03244384) and FGFR inhibitor studies (PROOF 302, NCT04197986) are underway as both neoadjuvant and adjuvant therapy.
Table 4.
Ongoing phase III clinical trials of targeted therapy in urothelial carcinoma
| Experimental agent(s) | Trial ID (NCT#) | Key eligibility criteria | Experimental regimen | Comparator | Primary endpoint(s) | Estimated PCD |
|---|---|---|---|---|---|---|
| MIUC | ||||||
| Nivolumab Bempegaldesleukin |
CA045‐009 |
Cisplatin‐ineligible | Perioperative nivolumab ± Bempegaldesleukin | Observation | pCR/EFS | August 28, 2023 |
| Atezolizumab |
IMvigor‐011 |
High‐risk, ctDNA‐positive after cystectomy | Adjuvant Atezolizumab | Adjuvant Placebo | DFS | November 1, 2023 |
| Nivolumab, BMS‐986205 |
ENERGIZE |
Cisplatin‐eligible |
Neoadjuvant nivolumab + CT + BMS‐986205 or Placebo followed by adjuvant nivolumab + BMS‐986205 or placebo |
Neoadjuvant CT | pCR/EFS | November 28, 2023 |
| Infigratinib |
PROOF 302 |
Invasive UC (UTUC or BC) FGFR3‐positive |
Adjuvant Infigratinib | Adjuvant Placebo | DFS | January 31, 2024 |
| Pembrolizumab Enfortumab Vedotin |
KEYNOTE‐905 |
Cisplatin‐ineligible | Perioperative pembrolizumab +/‐Enfortumab vedotin + SR + PLND | SR + PLND | pCR/EFS | June 26, 2026 |
| Atezolizumab |
SWOG 1806 |
Localized MIBC | Atezolizumab + concurrent CRT | Concurrent CRT | BI‐EFS | June 1, 2025 |
| Pembrolizumab |
AMBASSADOR |
MIBC or locally advanced cisplatin‐ineligible |
Adjuvant pembrolizumab | Observation | DFS/OS | June 1, 2025 |
| Pembrolizumab |
KEYNOTE‐866 |
Cisplatin‐eligible | Perioperative pembrolizumab + neoadjuvant CT | Perioperative placebo + neoadjuvant CT | pCR/EFS | June 15, 2025 |
| Durvalumab |
NIAGARA |
Cisplatin‐eligible | Perioperative durvalumab + CT | Perioperative + CT | pCR/EFS | December 18, 2025 |
| Pembrolizumab |
KEYNOTE‐992 |
Localized MIBC, opting for bladder preservation | Pembrolizumab + CRT | Placebo + CRT | BI‐EFS | October 10, 2026 |
|
Cetrelimab TAR‐200 |
Not eligible for SR or opting for bladder preservation | Cetrelimab + TAR‐200 | CRT | BI‐EFS | December 30, 2026 | |
| Unresectable or metastatic UC | ||||||
| First‐line | ||||||
| Tislelizumab |
BGB‐A317‐310 |
Platinum‐eligible | Tislelizumab + CT | CT | OS | July 2022 |
| Pembrolizumab, Lenvatinib |
LEAP‐011 |
Cisplatin‐ineligible PD‐L1‐positive or platinum‐ineligible | Pembrolizumab + Lenvatinib | Pembrolizumab + Placebo | PFS/OS | December 30, 2022 |
| Durvalumab, Tremelimumab |
NILE |
Platinum‐eligible | Durvalumab + CT +/‐Tremelimumab | CT | OS | April 28, 2023 |
| Nivolumab, Ipilimumab |
CheckMate 901 |
Platinum‐eligible | Nivolumab + Ipilimumab or CT | CT | PFS/OS | October 16, 2023 |
| Enfortumab vedotin, Pembrolizumab |
EV‐302 |
Platinum‐eligible | Enfortumab vedotin + Pembrolizumab | CT | PFS/OS | November 2023 |
| Toripalimab | JS001‐038‐III‐UBC (NCT04568304) | Platinum‐eligible or ineligible PD‐L1‐positive | Toripalimab + CT | CT | PFS | November 30, 2023 |
| Second‐line and beyond | ||||||
| Rogaratinib |
FORT‐1 |
Platinum‐treated FGFR1/3‐Positive |
Rogaratinib | CT | OS (phase III stage) | October 27, 2020 |
| Erdafitinib |
THOR |
≤2 prior lines of systemic treatment PD‐(L)1i‐treated (cohort 1) and ‐naïve (cohort 2) FGFR‐positive |
Erdafitinib | CT (cohort 1) or Pembrolizumab (cohort 2) | OS | November 24, 2020 |
| Sacituzumab Govitecan |
TROPiCS‐04 |
Platinum‐treated ICI‐treated |
Sacituzumab Govitecan | CT | OS | April 2023 |
Ongoing (trials that are actively recruiting for which efficacy outcomes are not yet available) phase III trials of targeted therapy for treatment of muscle‐invasive and advanced (including locally advanced, unresectable, and metastatic) UC listed at clinicaltrials.gov on December 12, 2020, ordered by line of treatment and estimated primary completion date.
Abbreviations: BC, bladder cancer; BI‐EFS, bladder intact event‐free survival; CRT, chemoradiotherapy; CT, chemotherapy; DFS, disease‐free survival; EFS, event‐free survival; FGFR, fibroblast growth factor receptor; ICI, immune checkpoint inhibitor; ID, identifier; MIBC, muscle‐invasive bladder cancer; MIUC, muscle‐invasive urothelial carcinoma; OS, overall survival; PCD, primary completion date; pCR, pathological complete response; PFS, progression‐free survival; PLND, pelvic lymph node dissection; SR, surgical resection; UC, urothelial carcinoma; UTUC, upper urinary tract urothelial carcinoma.
Conclusion
Recent outcomes from first‐line targeted therapy trials have reported benefit for ICI therapy in mUC. The use of platinum‐based chemotherapy followed by avelumab administered as switch‐maintenance among patients who have achieved at least stable disease has now become a clear standard of care, while pembrolizumab monotherapy remains a standard second‐line option for those who progress following first‐line chemotherapy. Research further clarifying the role of ICI therapy, antibody‐drug conjugates, and FGFR inhibitors is ongoing and will help refine targeted sequencing options in this rapidly evolving treatment landscape.
Author Contributions
Conception/design: Jean‐Michel Lavoie, Deanna McLeod, Bernhard J. Eigl
Collection and/or assembly of data: Jean‐Michel Lavoie, Deanna McLeod, Bernhard J. Eigl
Data analysis and interpretation: Jean‐Michel Lavoie, Srikala S. Sridhar, Michael Ong, Scott North, Nimira Alimohamed, Deanna McLeod, Bernhard J. Eigl
Manuscript writing: Jean‐Michel Lavoie, Srikala S. Sridhar, Michael Ong, Scott North, Nimira Alimohamed, Deanna McLeod, Bernhard J. Eigl
Final approval of manuscript: Jean‐Michel Lavoie, Srikala S. Sridhar, Michael Ong, Scott North, Nimira Alimohamed, Deanna McLeod, Bernhard J. Eigl
Disclosures
Jean‐Michel Lavoie: Astellas Roche Canada (H), Pfizer (SAB); Srikala S. Sridhar: Roche, Pfizer, Bristol‐Myers Squibb, AstraZeneca, Seagen, Immunomedex, Astellas, Janssen, Bayer (C/A); Michael Ong: AstraZeneca (RF), AstraZeneca, Merck, Bristol‐Myers Squibb, Roche, Bayer, Janssen, Sun Pharma (H); Nimira Alimohamed: Merck, Pfizer, AstraZeneca, Janssen, Bristol‐Myers Squibb, Astellas (C/A); Bernhard J. Eigl: Janssen, AstraZeneca, Roche, Merck, Astellas, Pfizer (C/A), Janssen, Astellas, Bayer, Sanofi, Tokai, Eli Lilly & Co., Bristol‐Myers Squibb, Merck, Roche (RF), Janssen (Other—travel compensation). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Ilidio Martins and Paul Card from Kaleidoscope Strategic Inc. for their research and editorial support, as well as Hoffmann‐La Roche Limited, Bayer, Janssen Pharmaceuticals, Inc., Merck Canada Inc., EMD Serono Canada, and Pfizer Canada Inc. for funding this review.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program . Cancer stat facts: Bladder cancer. National Cancer Institute: Suveillance, Epidemiology, and End Results Program Web site. Available at: https://seer.cancer.gov/statfacts/html/urinb.html. Accessed August 26, 2020.
- 3. Saginala K, Barsouk A, Aluru JS et al. Epidemiology of bladder cancer. Med Sci (Basel) 2020;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaig TW, Spiess PE, Agarwal N et al. Bladder cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:329–354. [DOI] [PubMed] [Google Scholar]
- 5. Pasin E, Josephson DY, Mitra AP et al. Superficial bladder cancer: An update on etiology, molecular development, classification, and natural history. Rev Urol 2008;10:31–43. [PMC free article] [PubMed] [Google Scholar]
- 6. Ghandour R, Singla N, Lotan Y. Treatment options and outcomes in nonmetastatic muscle invasive bladder cancer. Trends Cancer 2019;5:426–439. [DOI] [PubMed] [Google Scholar]
- 7. Aragon‐Ching JB, Werntz RP, Zietman AL et al. Multidisciplinary management of muscle‐invasive bladder cancer: Current challenges and future directions. Am Soc Clin Oncol Educ Book 2018;38:307–318. [DOI] [PubMed] [Google Scholar]
- 8. Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl 2016;14:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Black PC, Alimohamed NS, Berman D et al. Optimizing management of advanced urothelial carcinoma: A review of emerging therapies and biomarker‐driven patient selection. Can Urol Assoc J 2020;14:E373–E382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bladder cancer: Diagnosis and management of bladder cancer. BJU Int 2017;120:755–765. [DOI] [PubMed] [Google Scholar]
- 11. Witjes JA, Bruins HM, Cathomas R et al. European Association of Urology guidelines on muscle‐invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur Urol 2021;79:82–104. [DOI] [PubMed] [Google Scholar]
- 12. De Vos F, de Wit R. Choosing chemotherapy in patients with advanced urothelial cell cancer who are unfit to receive cisplatin‐based chemotherapy. Ther Adv Med Oncol 2010;2:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loehrer PJ Sr, Einhorn LH, Elson PJ et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol 1992;10:1066–1073. [DOI] [PubMed] [Google Scholar]
- 14. Bellmunt J, De Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Cancer Institute . Targeted therapy. National Cancer Institute Web site. Available at https://www.cancer.gov/publications/dictionaries/cancer-terms/def/targeted-therapy. Accessed January 28, 2021.
- 16. Dai S, Zhou Z, Chen Z et al. Fibroblast growth factor receptors (FGFRs): Structures and small molecule inhibitors. Cells 2019;8:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katayama Y, Uchino J, Chihara Y et al. Tumor neovascularization and developments in therapeutics. Cancers (Basel) 2019;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch S, Tugues S, Li X et al. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011;437:169–183. [DOI] [PubMed] [Google Scholar]
- 19. Ichikawa K, Miyano SW, Minoshima Y et al. Activated FGF2 signaling pathway in tumor vasculature is essential for acquired resistance to anti‐VEGF therapy. Sci Rep 2020;10:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dietrich B, Srinivas S. Urothelial carcinoma: The evolving landscape of immunotherapy for patients with advanced disease. Res Rep Urol 2018;10:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaman S. Targeting Trop‐2 in solid tumors: Future prospects. Onco Targets Ther 2019;12: 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenberg JE, O'Donnell PH, Balar AV et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti‐programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019;37:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bavencio (avelumab) . Prescribing information. EMD Serono; 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761049s006lbl.Pdf. Accessed August 26, 2020.
- 24. Bavencio (avelumab) . Product monograph. EMD Serono; 2021. Available at https://www.pfizer.ca/sites/default/files/202101/BAVENCIO_PM_E_235471_13-Jan-2021.pdf. Accessed April 19, 2021.
- 25. Opdivo (nivolumab) . Prescribing information. Bristol‐Myers Squibb; 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s070lbl.pdf. Accessed January 11, 2021.
- 26. European Medicines Agency . Product Information: Opdivo (nivolumab). Available at: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf. Accessed April 19, 2021.
- 27. Tecentriq (atezolizumab) . Prescribign information. Genentech; 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761034s018lbl.Pdf. Accessed August 26, 2020.
- 28.Tecentriq (atezolizumab). Product monograph. Roche Canada Ltd. Available at https://www.rochecanada.com/PMs/Tecentriq/Tecentriq_PM_E.pdf. Accessed May 26, 2021.
- 29.European Medicines Agency. Product Information: Tecentriq (atezolizumab). Available at https://www.ema.europa.eu/en/documents/product-information/tecentriq-epar-product-information_en.pdf. Accessed August 26, 2020.
- 30.Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2018. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf. Accessed August 26, 2020.
- 31.Keytruda (pembrolizumab). Prescribing information. Merck; 2020. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125514s066lbl.pdf. Accessed August 26, 2020.
- 32.Imfinzi (durvalumab). Product monograph. AstraZeneca Canada Inc. Available at https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/imfinzi-product-monograph-en.pdf. Accessed May 26, 2021.
- 33.Keytruda (pembrolizumab). Product monograph. Merck Canada Inc. Available at https://www.merck.ca/static/pdf/KEYTRUDA-PM_E.pdf. Accessed May 26, 2021.
- 34.European Medicines Agency. Keytruda (pembrolizumab). Available at https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed May 26, 2021.
- 35.Balversa (erdafitinib). Prescribing information. Janssen; 2019. Available at Https://www.Accessdata.Fda.Gov/drugsatfda_docs/label/2019/212018s000lbl.Pdf. Accessed August 26, 2020.
- 36.Balversa (erdafitinib). Product monograph. Janssen. Available at https://www.janssen.com/canada/sites/www_janssen_com_canada/files/prod_files/live/balversa_cpm.pdf. Accessed August 26, 2020.
- 37.Padcevtm (enfortumab vedotin‐ejfv). Prescribing information. Agensys; 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761137s000lbl.pdf. Accessed January 11, 2021.
- 38. Galsky MD, Arija JÁA, Bamias A et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2020;395:1547–1557. [DOI] [PubMed] [Google Scholar]
- 39. Powles T, van der Heijden MS, Castellano D et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open‐label, multicentre, phase 3 trial. Lancet Oncol 2020;21:1574–1588. [DOI] [PubMed] [Google Scholar]
- 40. Alva A, Csőszi T, Ozguroglu M et al. LBA23 pembrolizumab (P) combined with chemotherapy (C) vs C alone as first‐line (1L) therapy for advanced urothelial carcinoma (UC): KEYNOTE‐361. Ann Oncol 2020;31(suppl 4):S1142–S1215. [Google Scholar]
- 41. Rosenberg JE, Ballman KV, Halabi S et al. CALGB 90601 (Alliance): Randomized, double‐blind, placebo‐controlled phase iii trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. J Clin Oncol 2019;37(suppl 15):4503a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powles T, Huddart RA, Elliott T et al. Phase III, double‐blind, randomized trial that compared maintenance lapatinib versus placebo after first‐line chemotherapy in patients with human epidermal growth factor receptor 1/2‐positive metastatic bladder cancer. J Clin Oncol 2017;35:48–55. [DOI] [PubMed] [Google Scholar]
- 43. Powles T, Park SH, Voog E et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 44. Bamias A, De Santis M, Arranz J et al. 698O Patient‐reported outcomes (PROs) from IMvigor130: A global, randomised, partially blinded phase III study of atezolizumab (atezo)+ platinum‐based chemotherapy (PBC) vs placebo (PBO) + PBC in previously untreated locally advanced or metastatic urothelial carcinoma (mUC). Ann Oncol 2020;31(suppl 4):S551–S552. [Google Scholar]
- 45. Powles T, Kopyltsov E, Su P et al. 745P Patient‐reported outcomes (PROs) from JAVELIN Bladder 100: Avelumab first‐line (1L) maintenance+ best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC). Ann Oncol 2020;31(suppl 4):S578‐S579. [Google Scholar]
- 46. U.S. Food and Drug Administration . FDA approves avelumab for urothelial carcinoma maintenance treatment. U.S. Food and Drug Administration Web site, July 1, 2020. Available at https://www.fda.gov/drugs/drug‐approvals‐and‐databases/fda‐approves‐avelumab‐urothelial‐carcinoma‐maintenance‐treatment. Accessed October 19, 2020.
- 47.Health Canada approves BAVENCIO® for the maintenance treatment of patients with advanced bladder cancer. News Wire Canada Web site, January 11, 2021. Available at https://www.newswire.ca/news‐releases/health‐canada‐approves‐bavencio‐r‐for‐the‐maintenance‐treatment‐of‐patients‐with‐advanced‐bladder‐cancer‐817398194.html. Accessed January 14, 2021.
- 48. Pfizer . European Commission approves BAVENCIO® (avelumab) for first‐line maintenance treatment of locally advanced or metastatic urothelial carcinoma. Pfizer Web site, January 25, 2021. Available at https://www.pfizer.com/news/press‐release/press‐release‐detail/european‐commission‐approves‐bavencior‐avelumab‐first‐line#:~:text=(nyse%3a%20pfe)%20today%20announced,free%20following%20platinum%2dbased%20chemotherapy. Accessed April 19, 2021.
- 49. U.S. Food and Drug Administration . FDA limits the use of Tecentriq and Keytruda for some urothelial cancer patients. U.S. Food and Drug Administration Web site, July 5, 2018. Available at https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐limits‐use‐tecentriq‐and‐keytruda‐some‐urothelial‐cancer‐patients. Accessed October 19, 2020.
- 50. Balar AV, Galsky MD, Rosenberg JE et al. Atezolizumab as first‐line treatment in cisplatin‐ineligible patients with locally advanced and metastatic urothelial carcinoma: A single‐arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Balar AV, Castellano D, O'Donnell PH et al. First‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE‐052): A multicentre, single‐arm, phase 2 study. Lancet Oncol 2017;18:1483–1492. [DOI] [PubMed] [Google Scholar]
- 52. Rosenberg JE, Flaig TW, Friedlander TW et al. Study EV‐103: Preliminary durability results of enfortumab vedotin plus pembrolizumab for locally advanced or metastatic urothelial carcinoma. J Clin Oncol 2020;38(suppl 6):441a. [Google Scholar]
- 53. Slater H. FDA grants breakthrough therapy designation to enfortumab vedotin. Cancer Network. February 19, 2020. Available at https://www.cancernetwork.com/view/fda-grants-breakthrough-therapy-designation-enfortumab-vedotin. Accessed October 19, 2020. [Google Scholar]
- 54. Galsky MD, Banchereau R, Hamidi HR et al. Tumor, immune, and stromal characteristics associated with clinical outcomes with atezolizumab (atezo) + platinum‐based chemotherapy (PBC) or atezo monotherapy (mono) versus PBC in metastatic urothelial cancer (mUC) from the phase III IMvigor130 study. J Clin Oncol 2020;38(suppl 15):5011a. [Google Scholar]
- 55. Galsky MD, Arija JÁA, Bamias A et al. Atezolizumab (atezo) monotherapy versus chemotherapy in previously untreated locally advanced or metastatic urothelial carcinoma (mUC): Clinical outcomes by PD‐L1 status in cisplatin (cis)‐ineligible pts from the phase III IMvigor130 study. Presented at: 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium; February 11–14, 2021; virtual. Abstract 434.
- 56. Powles TB, Loriot Y, Bellmunt J et al. 699O ‐ Avelumab first‐line (1L) maintenance + best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma (UC): Association between clinical outcomes and exploratory biomarkers. Ann Oncol 2020;31(suppl 4):S552–S553. [Google Scholar]
- 57. Joerger M, Cassier P, Penel N et al. Rogaratinib treatment of patients with advanced urothelial carcinomas prescreened for tumor FGFR mRNA expression. J Clin Oncol 2018;36(suppl 15):4513a. [Google Scholar]
- 58. Siefker‐Radtke AO, Necchi A, Rosenbaum E et al. Efficacy of programmed death 1 (PD‐1) and programmed death 1 ligand (PD‐L1) inhibitors in patients with FGFR mutations and gene fusions: Results from a data analysis of an ongoing phase 2 study of erdafitinib (JNJ‐42756493) in patients (pts) with advanced urothelial cancer (UC). Presented at: 2018 American Society of Clinical Oncology Genitourinary Cancers Symposium; February 8–10. San Francisco, California. Abstract 450.
- 59. Wang L, Gong Y, Saci A et al. Fibroblast growth factor receptor 3 alterations and response to PD‐1/PD‐L1 blockade in patients with metastatic urothelial cancer. Eur Urol 2019;76:599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grivas P, Park S, Voog E. Avelumab 1L maintenance + best supportive care (BSC) vs BSC alone with 1L chemotherapy for advanced urothelial carcinoma: Subgroup analyses from JAVELIN bladder 100. Presented at: European Society for Medical Oncology Virtual Congress; September 18, 2020; virtual.
- 61. Powles T, Rosenberg JE, Sonpavde GP et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021;384:1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Siefker‐Radtke AO, Necchi A, Park SH et al. ERDAFITINIB in locally advanced or metastatic urothelial carcinoma (mUC): Long‐term outcomes in BLC2001. J Clin Oncol 2020;38(suppl 15):5015a. [Google Scholar]
- 63. Balar AV, McGregor BA, Rosenberg JE et al. EV‐201 Cohort 2: Enfortumab vedotin in cisplatin‐ineligible patients with locally advanced or metastatic urothelial cancer who received prior PD‐1/PD‐L1 inhibitors. J Clin Oncol 2021;39(suppl 6):394. [Google Scholar]
- 64. Loriot Y, Balar A, Petrylak D et al. LBA24 TROPHY‐U‐01 cohort 1 final results: A phase II study of sacituzumab govitecan (SG) in metastatic urothelial cancer (mUC) that has progressed after platinum (PLT) and checkpoint inhibitors (CPI). Ann Oncol 2020;31(suppl 4):S1156. [Google Scholar]
- 65. Tomczak T, Popovic L, Barthelemy P et al. Preliminary analysis of a phase II, multicenter, randomized, active‐control study to evaluate the efficacy and safety of eganelisib (IPI 549) in combination with nivolumab compared to nivolumab monotherapy in patients with advanced urothelial carcinoma. Presented at: 2021 American Society of Clinical Oncology Genitourinary Cancers Symposium; February 11–13, 2021; Virtual Abstract 436.
- 66. Bajorin DF, Witjes JA, Gschwend J et al. First results from the phase 3 CheckMate 274 trial of adjuvant nivolumab vs placebo in patients who underwent radical surgery for high‐risk muscle‐invasive urothelial carcinoma (MIUC). Presented at: 2021 American Society of Clinical Oncology Genitourinary Cancer Symposium; February 11–13, 2021; Virtual Abstract 391.
- 67. Vuky J, Balar AV, Castellano D et al. Long‐term outcomes in KEYNOTE‐052: Phase II study investigating first‐line pembrolizumab in cisplatin‐ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol 2020;38:2658–2666 [DOI] [PubMed] [Google Scholar]


