Abstract
Background
COVID-19 prevention and control policies have entailed lockdowns and confinement. This study aimed to summarize the global research evidence describing the effect of COVID-19 isolation measures on the health of people living with dementia.
Methods
We searched Pubmed, PsycINFO and CINAHL up to 27th of February 2021 for peer-reviewed quantitative studies about the effects of isolation during COVID-19 on the cognitive, psychological and functional symptoms of people with dementia or mild cognitive impairment. The Joanna Briggs Institute critical appraisal tool was used to conduct the quality assessment. PROSPERO registration: CRD42021229259.
Findings
15 eligible papers were identified, examining a total of 6442 people with dementia. 13/15 studies investigated people living in the community and 2 in care homes. Out of 15 studies, 9 (60%) reported changes in cognition and 14 (93%) worsening or new onset of behavioral and psychological symptoms. Six studies (46%) reported a functional decline in daily activities in a variable proportion of the population analyzed.
Interpretation
COVID-19 isolation measures have damaged the cognitive and mental health of people with dementia across the world. It is urgent to issue guidance that balances infection control measures against the principles of non-maleficence to guarantee fair and appropriate care during pandemic times for this population.
Keywords: Dementia, COVID-19, Lockdown, Isolation, Neuropsychiatric symptoms
Research in context.
Evidence before this study
Concerns have been raised about the effects of some COVID-19 prevention and control policies on the health of people with dementia across the world. Lockdown and confinements, in particular, involve greater levels of isolation and, indirectly, suspension of therapeutic, medical and care services for people with dementia.
Added value of this study
We synthesize all available quantitative data on the effect of COVID-19 related isolation measures on the cognitive, psychological and functional health of people living with dementia. We report that lockdowns are associated with worsening of cognitive, behavioural and psychological symptoms and functional decline in people with dementia.
Implications of all the available evidence
Our findings highlight the need for infection and prevention control plans affecting people with dementia to be redesigned under non-maleficence and compassionate care principles.
Alt-text: Unlabelled box
1. Introduction
The first wave of the new coronavirus disease, COVID-19, swept across the world from March 2020 becoming a universal health challenge. In that month, many countries declared national lockdowns or imposed physical contact restrictions in an effort to reduce the exposure and spread of the virus. Such measures have evolved, coming and going as new waves of COVID-19 unfolded and receded. People with dementia have great vulnerability to COVID-19 infection, but also would be expected to be particularly susceptible to the indirect consequences of lockdown and confinement. They usually require day-to-day assistance and may not be able to understand or adapt to rapidly changing situations [1]. The challenge of living with dementia during COVID-19 was increased by the reduction in therapeutic and essential support services as a consequence of public health COVID-19 containment measures [2,3]. For instance, specialist dementia consultations were postponed or suspended and day centers, home services and therapeutic and support activities closed or curtailed in many countries [1,3]. These are all essential services to help people with dementia maintain their health, well-being and quality of life [4,5]. Restrictions in place during lockdown often limited time outdoors, meaning that even those people with dementia who lived with relatives were more isolated than before. For those living in care homes, the experience of going through lockdown was even harder since the measures in residential facilities were considerably more restrictive than for those living in the community and lasted longer. For instance, to reduce infection and death rates in care and nursing homes, residents who were in contact with possible COVID-19 cases were kept isolated in their own room for weeks and many care homes opted for imposing a total ban on visitors (including children and spouses) lasting many months. No exception to this rule was announced for people with dementia [6].
Since the early days of the pandemic, families, healthcare professionals and scientists raised concerns about the potentially adverse consequences of confinement on the health of people with dementia [7,8]. Isolation and stimulus deprivation are the opposite of what is therapeutically recommended for someone living with dementia [9]. Lack of social and sensory stimulation and pleasant meaningful activities make people with dementia more vulnerable to boredom, which in turn increases the chances of appearance of behavioural and psychological symptoms (BPSD) such as, anxiety, apathy, sleep disturbance, agitation, and hallucinations. The presence of significant BPSD is associated with worse quality of life, more rapid progression to severe dementia and death [10]. These symptoms result from a combination of factors: biological (product of the neuropathological changes of the brain caused by the disease), psychological, and social, as well as unmet needs (responsive behaviors), and are therefore susceptible to be modulated by the environment and get worse when such environment is not adequate. Lastly, dementia experts also warned about the noxious effect of reduced daily physical activity and increased time spent in sedentary activities, which may negatively impact the level of physical function and independence. As the first studies on the relationship between COVID-19 lockdowns and dementia emerged, all these concerns about the impact of isolation policies on this population began to crystalize [11], [12], [13].
Knowledge of the impact of isolation and confinement on the health of people with dementia during the pandemic is crucial to balance the risks and benefits of current public health policies and to plan for the provision of care in post-emergency scenarios and future pandemics. This rapid review seeks therefore to answer the following question: What is the relationship between COVID-19 isolation measures and the cognitive and psychological symptoms and level of independence of people with dementia living in the community and in care homes?
2. Methods
The protocol of this review was registered in PROSPERO (CRD42021229259; https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=229259 [14] PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) were followed to conduct the review and prepare the report [15]. We systematically reviewed the literature about COVID-19, isolation measures and the cognitive, mental health and functional level of people with dementia using the recommended general framework for rapid systematic reviews [16,17].
2.1. Information sources and search terms
The following databases were searched: Pubmed, PsycINFO and CINAHL. The literature included was indexed, published and peer-reviewed, with unlimited date range up until 27th February 2021. The syntax was customized for individual databases according to each database specific conventions. To streamline the review process according to frameworks recommended for rapid reviews [16,17], reference lists from key articles and reviews were not checked, neither grey literature included. The following search terms were developed in consultation with experienced researchers and librarians and piloted before being used in the current review:
(dementia OR neurocognitive disorders OR cognitive impairment) AND (Covid* OR coronavirus* OR coronavirus) AND (pandemic OR outbreak OR lockdown OR confinement OR isolation OR quarantine) AND (behavioral symptoms OR behavioral symptom* OR behavioral symptom* OR psychological symptom* OR psychological well-being OR psychological wellbeing OR loneliness OR loneliness OR neuropsychiatric symptom* OR mental health OR physical health OR mood* OR communication OR communication OR depression OR depressi* OR anxiety OR anxiety OR apathy OR apathy OR agitation OR irritab* OR wandering OR insomnia OR sleep* OR functional decline OR cognitive decline OR activities of daily living OR hallucinations OR hallucinat* OR delusions OR delusion*OR facing OR coping).
The search strategy for the three databases is fully reported in Appendix 1. Results were exported into Endnote Ⓡ software version X9 and automatically deduplicated. A multi-level title-first method was conducted to screen and select the candidate articles, screen titles first and abstracts after [18]. In accordance with rapid review standard practice, the main reviewer (ASG) conducted the item's screening and selection and applied inclusion and exclusion criteria [16]. A 10% sample of the full-text articles selected and 10% of the excluded were independently double screened (JR).
2.2. Selection of studies
We included studies of any kind of design that reported quantitative data (e.g., percentages; mean and standard deviation) at any level (individual patient-level or summary estimates) about the effects of isolation measures (i.e., lockdowns) on at least one of the three outcome measures (cognitive function, psychological symptoms, and activities of daily living) in people with any kind of dementia or mild cognitive impairment with no age limits.
We excluded studies that were qualitative only papers, those providing information about the variables of interest during the pandemic without referring to any change due to isolation, studies in languages other than English, conference abstracts, grey literature and non-peer-reviewed material (pre-prints were excluded).
2.3. Data extraction and quality assessment
Data extraction from each article was completed by one reviewer, verified by a second one independently and recorded in a standard extraction form covering: sample size, measure used, time of data collection, decline in cognition, appearance or worsening of BPSD, decline in activities of daily living, increase or addition of pharmacological therapy and quality assessment score. We used the Joanna Briggs Institute (JBI) critical appraisal tools https://joannabriggs.org/critical-appraisal-tools for quality assessment to describe the characteristics of the studies included. The assessment was done on study design (e.g., criteria for inclusion, description of study subjects and setting, reliability of measures used and identification of confounding factors), by a reviewer (ASG) and 20% of scores verified by a second one (JR) following guidance and recommendations for rapid reviews [16]). Discrepancies were resolved between raters without the need for a third reviewer's involvement.
In line with JBI manual for evidence synthesis recommendations, we considered as good quality those studies achieving a cut-off score of ≥ 70% of items assessed with the critical appraisal tool (e.g. ≥ 8/ 11 “yes” answers for cohort studies and 5 /f 8 for cross-sectional studies) [19].
2.4. Synthesis of results
Studies were grouped according to whether they addressed people with dementia living in the community or in care homes and by study design.
2.5. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
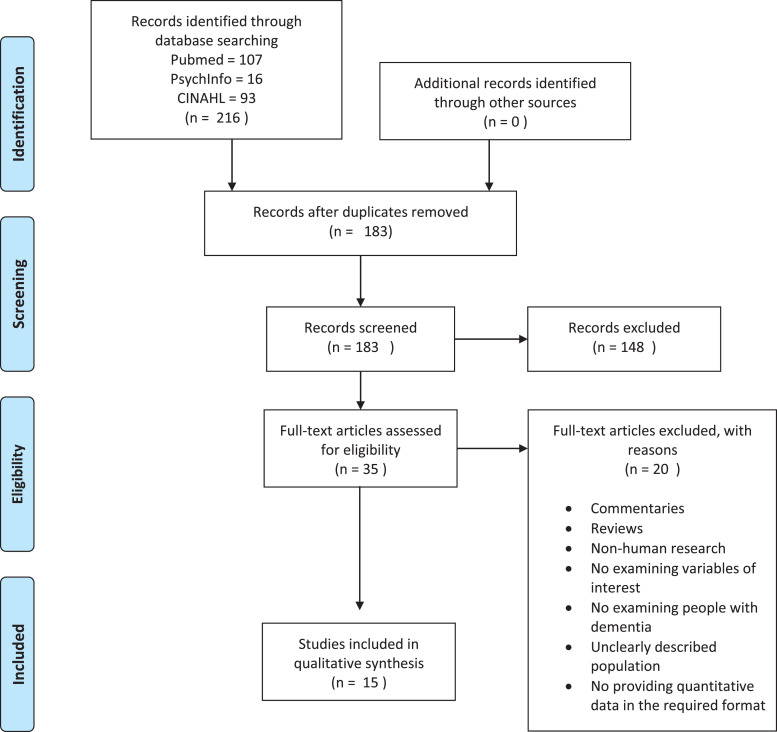
Fig. 1 presents the PRISMA flow diagram of the inclusion process. The studies’ characteristics are summarized in Table 1 and Table 2 by order of their quality assessment score. Table 1 describes studies reporting data on people with dementia living in the community, while Table 2 shows studies in care homes. We identified 15 studies examining the effects of lockdown, comprising a total of 6442 people with dementia. 13 studies included people living in the community (2 of them collecting data pre- and post-pandemic and 11 cross-sectionally) and 2 in care homes. All studies report data collected during the first COVID-19 lockdown.
Fig. 1.
Prisma flow diagram.
Table 1.
Summary of studies examining the effect of lockdown in people living with dementia in the community.
| Studies with longitudinal data collection (pre and post lockdown). | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Setting | N† | Measure used | Time of data collection | Decline in Cognition | Appearance or worsening of behavioral/psychological symptoms | Decline in ADL | Increase/addition pharmacological therapy | Quality Score (max 11) |
| Lara et la., 2020 12 | Spain | Outpatient clinic. | 40 MCI = 20 AD = 20 |
Neuropsychiatric Inventory (NPI) and EuroQol-5D question- naire (EQ-5D) NPI |
A month before lockdown and 5 weeks after. | NR | Total% NR NPI:33.75 (baseline)– 39.05 (post-lockdown) * (apathy most commonly reported) |
NR | NR | 6/8 § |
| Borges-Machado et al. [20] | Portugal | Community dwelling. | 36 | NPI Barthel Index . |
November 2019 and June 2020 (3 months after home confinement) | 80% | 44% NPI: 5.72 (pre-confinement) 10.25, (post-confinement) * |
Barthel Index: (pre-confinement) 92.92-(post-confinement) 88.33* | 11% (type of change not reported) |
7/9 § |
| Studies with one time point data collection (during or after lockdown) | ||||||||||
| Study | Country | Setting | N† | Measure used | Time of data collection | Decline in Cognition | Appearance or worsening of behavioural/psychological symptoms | ADL | Increase/addition pharmacological therapy | Quality score (max 8) |
| Baschi et al. [21] | Italy | Outpatient clinic. | PD-MCI: 31 MCI: 31 |
Semi-structured questionnaire NPI |
2 months before lockdown and immediately after end of lockdown | 45% (PD-MCI) 41% (MCI) |
Worsening of existing symptoms: 42% (PD-MCI) 38% (MCI) New symptoms: 42% (PD-MCI), 22% (MCI) |
Number of IADL lost (1.6 ± 1.8 (PD-MCI) Number of IADL lost 0.8 ± 1.2 (MCI) |
NR | 7 |
| Palermo et al. [22] |
Italy | Outpatient clinic. | PD–MCI: 10†† PDD: 8†† |
Structured questionnaire | After lockdown (June-July 2020) | PD-MCI: 80% PDD: 12% (difficulties with concentration) |
Total% NR Anxiety: 70% (PD–MCI) 50% (PDD) Sleep quality:20% (PD–MCI) 12% (PDD) |
5% | NR | 6 |
| Borelli et al. [23] | Brazil | Outpatient clinic. | 58 | NPI |
May to July 2020 | 53% | 48% (particularly apathy and depression) |
34% | 22% (mostly starting antipsychotics and benzodiazepine) |
6 |
| Boutoleau-Bretonniere et al. [24] | France | Outpatient clinic. |
38 (AD) |
NPI | Three months into lockdown | NR | 26% | NR | NR | 6 |
| Cagnin et al. [25] | Italy | Outpatient memory clinic. | 4913 | Structured interview |
1 month after beginning of quarantine |
NR | 59% Worsening of existing symptoms: 51% New onset of symptoms: 26% (Irritability, apathy, agitation most commonly reported) |
NR | 27% (type of change no reported) |
6 |
| Pogan et al. 26] | France | Community. | 389 | Survey | During the first lockdown (April to June 2020) | NR | 43% | NR | NR |
6 |
| Cohen et al. [27] | Argentina | Outpatient clinic. | 119 | Survey | First 8 weeks of lockdown. | NR | 60% (anxiety, depression and insomnia most common) |
NR | Antipsychotics: 20% Benzodiazepines: 15% Hypnotics: 6% Antidepressants: 10%. |
6 |
| Van Maurik et al. [28] | The Netherlands | Outpatient clinic. |
147 |
Survey | During April-July 2020 | 53% |
75% (Apathy, sleeping behaviour and repetitive behaviour were the most common) |
NR | NR | 5 |
| Barguilla et al. [29] |
Spain | Outpatient clinic. |
60 |
NPI CDR |
Between three and four months after lockdown | 60% |
65% (agitation, depression and anxiety were the most common) |
No changes in CDR | 21% (type of change no reported) |
5 |
| Canevelli et al. | Italy | Outpatient clinic. | 139 Dementia: 96 MCI: 43†† |
Survey |
During first month of lockdown. |
31% (memory and orientation) |
54% (agitation, apathy and depression were the most common) |
18% (dependence in personal care and housekeeping) |
7% (mostly introduction of antipsychotics) |
3/7§ |
| Tsapanou et al. [30] | Greece | Day centres and private neurological/psychiatric practices. | 204 |
Self-reported questionnaire | During lockdown (February to June 2020) | 61% (decline in communication) |
Total% NR (Mood, apathy and appetite most commonly reported) |
NR | NR | 2/7§ |
†N refers to people with dementia recruited but informants are caregivers unless specified otherwise, ††informants are patients/people living with cognitive impairment.
§Some of the items from the critical appraisal checklist not applicable, *Significant p value, NR: Not reported, NPI: Neuropsychiatric inventory, EQ-5D: EuroQol-5D, HADS: Hospital Anxiety and Depression Scale, MCI: mild cognitive impairment, AD: Alzheimer's disease, PD-MCI: Parkinson disease with mild cognitive impairment, PDD: Parkinson disease dementia.
Table 2.
Summary of studies examining the effect of lockdown in people living with dementia in care homes.
| Study | Country | N | Measure used | Time of data collection | Worsening cognition | Worsening psychological symptoms | Worsening ADL | Changes in pharmacological therapy | Quality Score (max 8) |
|---|---|---|---|---|---|---|---|---|---|
| El Haj et al. [31] | France | AD: 58†† | HADS |
During the Covid pandemic |
NR | Total% NR Depression: 0.005* Anxiety: 0.004* |
NR | NR | 6 |
| O'Caoimh et al. [32] | Ireland | 162 | Online survey |
2 weeks up until end of June 2020 | 54% (memory) |
51% (mood) |
43% | NR | 6 |
††informants are patients/people living with cognitive impairment.
*p-value.
13 studies met the criteria for good quality standard, whereas 2 studies did not. Common sources of bias were the incorrect report of standard diagnostic criteria, failure to identify and deal with confounding factors and the absence of valid and reliable outcome measure tools.
4. Studies involving people with dementia living in the community
4.1. Decline in cognition
61% (8/13) of studies reported decline in cognition in a proportion of the sample examined. The most commonly used measurement tools were tailored questionnaires and surveys. Reported worsening in cognition ranged 12–80% across studies, with 75% (6/8) of them describing decline in > 50% of respondents. Concentration, memory, orientation and communication were the cognitive domains most named as affected.
4.2. Appearance or worsening of behavioral/psychological symptoms
92% (12/13) of studies reported worsening or new onset of behavioral and psychological symptoms in a variable proportion of the sample examined, as measured by validated questionnaires such as the Neuropsychiatric Inventory (NPI) and the Hospital Anxiety and Depression Scale (HADS). Reported changes ranged from 22% to 75%. In 75% (9/12) of the studies, more than 40% of respondents confirmed worsening or onset of new symptoms. An increase of anxiety, apathy, depression and agitation were the most common changes reported.
4.3. Decline in activities of daily living (ADL)
46% (6/13) studies reported changes in ADL, as measured by tailored questionnaires and Barthel Index. The reported worsening in ADL ranges from 5% to 34%, with one study reporting no changes. When the type of ADL decline was stated, this related to the level of independence in personal care and housekeeping.
4.4. Increase/addition pharmacological therapy
Six studies (46%) reported pharmacological treatment adjustments as a result of the worsening of BPSD during confinement. Changes in medication ranged from 7 to 27%, most commonly associated to the introduction of antipsychotics and benzodiazepines.
5. Studies involving people with dementia living in care homes
5.1. Decline in cognition
One of the two studies examining the effects of lockdown in people with dementia in care homes reported changes in cognition, with 54% of residents experiencing worsening of memory, according to reports from an online survey administered to caregivers.
5.2. Appearance or worsening of behavioral/psychological symptoms
Mean depression and anxiety scores increased in one study and mood deteriorated in 51% of residents examined in another.
5.3. Decline in ADL
One study reported worsening of independence in ADL in 43% of the residents whose caregivers responded to the survey.
5.4. Increase/addition pharmacological therapy
This was not reported in studies conducted in care homes.
6. Discussion
This rapid review summarizes evidence about the impact of COVID-19 lockdown on people with dementia living at both private homes and care homes in Europe and Latin America. Our results suggest a worsening of cognition, behavioral and psychological symptoms and level of function for ADL in people with dementia during this period, and an increase in the prescription of antipsychotics and benzodiazepines. Changes in behavioral and psychological symptoms (BPSD) was the most frequently investigated variable (14/15 studies), followed by changes in cognition (9/15) and ADL (7/15). This is particularly concerning since BPSD are known to be highly disruptive and directly associated with increased risk of care home admission and worse cognitive and functional outcomes [33].
The majority of the studies in this review reported large percentages of people living with dementia that experienced exacerbation or new onset of BPSD. Nine studies reported more than 40% of participants experiencing a worsening of exisiting symptoms or onset of new ones (ranging 42–75%) [11,[21], [22], [23],[25], [26], [27], [28], [29]]. The majority of studies named depression, anxiety and apathy as the symptoms aggravated the most during lockdowns. This is highly relevant since depression apathy, and anxiety are strongly associated with increased caregiver burden and lower quality of life in people living with dementia [34], [35], [36], [37], which may have contributed towards making the experience of living in lockdown particularly stressful and difficult.
This rapid review also found a large proportion of people with dementia experiencing decline in their cognitive abilities during the lockdown [11,20,21,23,[28], [29], [30],32] The majority of studies report cognitive decline in more than 50% of the sample examined. However, one study reported figures of 41–45% [21] and another 12% [22]. While people with dementia deteriorate gradually over time the decline reported by these studies occurred in an unusual short window of time (3,4 months). Such accelerated rate of decline does not seem attributable to the typical natural course of dementia.
Data regarding the impact of lockdown on the ADL of people with dementia is scarce and more difficult to interpret due to variation in the outcomes reported (from no affectation at all to 34%) and also measurement tools.
Very little information is available about the impact of COVID-19 on people residing in care homes with only two studies identified up to the date of conduction of this review [31,32]. This population has been more difficult to reach than those living in the community due to additional restrictions imposed in the social care systems [6]. One of the studies reports a decline in cognition and ADL in around half the residents [32] in keeping with other emerging evidence on the topic [38], and the other one reports significant increase in levels of anxiety and depression [31]. Decisions such as not referring care homes residents to hospitals and the blanket ban on visitors, conflicted with individual human rights and have likely contributed to both mortality and accelerated deterioration [39]. Although speculative without more data available, it is reasonable to expect that the deleterious effect of confinement is amplified in this population compared to those living in the community. The reason is that not only isolation has been greater in this group (in most care homes all activities, outings and visits stopped) but, in addition, those living in care homes tend to be older, at more severe stages of the dementia progression and therefore more fragile and vulnerable to the effects of isolation.
The one previous study on the prescription of antipsychotics for people with dementia in care homes during the pandemic have already suggested that their use increased significantly during the first months of 2020 [40]. Our review is in line with this notion since increases in dosage or initiation of medication was found in up to 27% of people with dementia studied (most commonly antipsychotics and benzodiazepines). This may be indicative of generalized aggravation of dementia symptoms due to lockdown and impossibility of resorting to non-pharmacological management strategies due to the limitations imposed by anti-Covid measures.
The heterogeneity of tools and in the choice of the kind of data reported are the main limitation to synthesise and interpret the data from the studies identified in this review. This applies particularly to the variability in the percentage of people with dementia experiencing worsening of symptoms during lockdown. For instance, whether a total percentage was given, or whether the percentage was broken down by symptoms (with no overall figures) or whether the method used to measure the variables allowed the extraction of percentages or mean and standard deviation. In addition, some measurement instruments, such as the HADS, are not validated to be used in dementia. Lastly, it was difficult to conduct these studies during the first COVID-19 wave, in particular in care homes, which usually banned visitors, and went through many strains to continue care throughout the pandemic.
This rapid review describes the extent and nature of the research evidence regarding the effect of isolation measures on the health of people living with dementia during the first year of the COVID-19 pandemic. Public infection control and prevention protocols affecting people with dementia have become a source of harm and they need, as a matter of urgency, to be redesigned under principles of non-maleficence and compassionate care. From this evidence, three calls for action emerge: First, family caregivers and paid carers should be prioritized for vaccines, and work-life balance policies for family caregivers implemented until the pandemic is over and extended to more than one family member; Second, we now know that the risk of infection is low outdoors, and the correct use of appropriate PPE can allow safe physical contact and this knowledge should be used to restore routines, support and therapeutic activities in the community for people with dementia. Third, care homes in many countries are progressing in the immunisation of both residents and workers but even in the cases where this is not happening, safe visits can and should take place (see Storr et al. [41] Low et al. [42] and https://enablesafecare.org/ for a list of specific measures to enable safe human interaction in care homes).
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Data sharing statement
Data sharing in not applicable to this article as no new data were created or analyzed in this study.
Funding
ASG receives funding from a grant jointly funded by the Economic and Social Research Council (UK) and the National Institute for Health Research (UK) (ES/S010467/1). GL is supported by UCLH National Institute for Health Research (NIHR) Biomedical Research Centre, and by NIHR North Thames ARC as a NIHR senior investigator. JR is supported by a UK Research and Innovation ‘s Global Challenges Research Fund (UKRI GCRF) (ES/P0109381).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101047.
Appendix. Supplementary materials
References
- 1.Liu K., Howard R., Banerjee S., Comas-Herrera A., Goddard J., Knapp M. Dementia wellbeing and COVID-19: review and expert consensus on current research and knowledge gaps. Int J Geriatr Psychiatry. 2021 doi: 10.1002/gps.5567. 2021 May 1610.1002/gps.5567. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna K., Giebel C., Tetlow H., Ward K., Shenton J., Cannon J. Emotional and mental wellbeing following COVID-19 public health measures on people living with dementia and carers. J Geriatr Psychiatry Neurol. 2021 doi: 10.1177/0891988721996816. Feb 25Epub ahead of print. PMID: 33626977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giebel C., Cannon J., Hanna K., Butchard S., Eley R., Gaughan A. Impact of COVID-19 related social support service closures on people with dementia and unpaid carers: a qualitative study. Aging Ment Health. 2020:1–8. doi: 10.1080/13607863.2020.1822292. Sep 21Epub ahead of printPMID: 32954794. [DOI] [PubMed] [Google Scholar]
- 4.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. Aug 8doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulos C.J., Bayer A., Beaupre L., Clare L., Poulos R.G., Wang R.H. A comprehensive approach to reablement in dementia. Alzheimers Dement. 2017;3(3):450–458. doi: 10.1016/j.trci.2017.06.005. N YJul 27doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A. Comas-Herrera, M. Salcher-Konrad, J. Baumbusch, N. Farina, C. Goodman, K. Lorenz-Dant, L.F. Low. Rapid Review of the Evidence on Impacts of Visiting Policies in Care Homes During the COVID-19 Pandemic. (https://ltccovid.org/wp-content/uploads/2020/11/Rapid-review-of-evidence-on-impacts-of-visiting-policies-in-care-homes-during-the-COVID-pandemic-LSE068110.pdf
- 7.Suarez-Gonzalez A. 2020. Detrimental Effects of Confinement and Isolation in the Cognitive and Psychological Health of People Living with Dementia During COVID-19: Emerging Evidence. LTC COVID, International Long-Term Care Policy Network, Cpec-Lse.https://ltccovid.org/wp-content/uploads/2020/07/LTCcovid-1-July-Detrimental-effects-confinement-on-people-with-dementia.pdf 23 June 2020. [Google Scholar]
- 8.Suarez-Gonzalez A., Comas-Herrera A., Livingston G. Rapid Response BMJ. 2020. Impact of COVID-19 on people living with dementia: emerging international evidence.https://www.bmj.com/content/369/bmj.m2463/rr-0 a, 29 June. [Google Scholar]
- 9.National Institute for Health and Care Excellence. (2018). Dementia: assessment, management and support for people living with dementia and their carers. Retrieved from: https://www.nice.org.uk/guidance/ng97/chapter/recommendations [PubMed]
- 10.Peters M.E., Schwartz S., Han D., V Rabins P., Steinberg M., Tschanz J.T. Neuropsychiatric symptoms as predictors of progression to severe alzheimer's dementia and death: the cache county dementia progression. study. Am J Psychiatry. 2015;172(5):460–465. doi: 10.1176/appi.ajp.2014.14040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canevelli M., Valletta M., Blasi M.T., Remoli G., Sarti G., Nuti F. Facing dementia during the COVID-19 outbreak. J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16644. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara B., Carnes A., Dakterzada F., Benitez I., Piñol-Ripoll G. Neuropsychiatric symptoms and quality of life in Spanish patients with alzheimer's disease during the COVID-19 lockdown. Eur J Neurol. 2020;27(9):1744–1747. doi: 10.1111/ene.14339. SepEpub 2020 Jun 24. PMID: 32449791; PMCID: PMC7283827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M., Hotta M., Nagase A., Yamamoto Y., Hirakawa N., Satake Y. The behavioral pattern of patients with frontotemporal dementia during the COVID-19 pandemic. Int Psychogeriatr. 2020:1–6. doi: 10.1017/S104161022000109X. Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez-Gonzalez A., Rajagopalan J., Alladi S. 2021. A Rapid Review Of The Effect Of Covid-19 Isolation Measures On The Cognitive And Mental Health Of People Living With Dementia.https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021229259 PROSPERO 2021 CRD42021229259 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tricco A.C., Langlois E.V., Straus S.E. Rapid reviews to strengthen health policy and systems: a practical guide. Geneva: world health organization. Licence. 2017 CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 17.Garritty C., Gartlehner G., Nussbaumer-Streit B., King V.J., Hamel C., Kamel C. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2021;130:13–22. doi: 10.1016/j.jclinepi.2020.10.007. FebEpub 2020 Oct 15. PMID: 33068715; PMCID: PMC7557165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateen F.J., Oh J., Tergas A.I., Bhayani N.H., Kamdar B.B. Titles versus titles and abstracts for initial screening of articles for systematic reviews. Clin Epidemiol. 2013;5:89–95. doi: 10.2147/CLEP.S43118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tufanaru C., Munn Z., Aromataris E., Campbell J., Hopp L., Aromataris E., Munn Z. Joanna Briggs Institute Reviewer's Manual. 2017. Systematic reviews of effectiveness. Chapter 3In. editors. [Internet]. Adelaide: Joanna Briggs Institute[cited 2017 Jan. [Google Scholar]
- 20.Borges-Machado F., Barros D., Ribeiro Ó., Carvalho J. The effects of COVID-19 home confinement in dementia care: physical and cognitive decline, severe neuropsychiatric symptoms and increased caregiving burden. Am. J Alzheimer's Dis. Other Dement®. 2020;35 doi: 10.1177/1533317520976720. Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baschi R., Luca A., Nicoletti A., Caccamo M., Cicero C.E., D'Agate C. Changes in motor, cognitive, and behavioral symptoms in parkinson's disease and mild cognitive impairment during the COVID-19 lockdown. Front Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.590134. Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palermo G., Tommasini L., Baldacci F., Del Prete E., Siciliano G., Ceravolo R. Impact of coronavirus disease 2019 pandemic on cognition in parkinson's disease. Mov Disord. 2020;35(10):1717–1718. doi: 10.1002/mds.28254. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borelli W.V., Augustin M.C., de Oliveira P.B.F., Reggiani L.C., Bandeira-de-Mello R.G., Schumacher-Schuh A.F. Neuropsychiatric symptoms in patients with dementia associated with increased psychological distress in caregivers during the COVID-19 pandemic. J Alzheimers Dis. 2021;80(4):1705–1712. doi: 10.3233/JAD-201513. [DOI] [PubMed] [Google Scholar]
- 24.Boutoleau-Bretonnière C., Pouclet-Courtemanche H., Gillet A., Bernard A., Laure Deruet A., Gouraud I. The effects of confinement on neuropsychiatric symptoms in Alzheimer's disease during the COVID-19 crisis. J Alzheimer's Dis. 2020:1–7. doi: 10.3233/JAD-200604. Jan 1 (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cagnin A., Di Lorenzo R., Marra C., Bonanni L., Cupidi C., Laganà V. Vol. 11. 2020. Behavioral and psychological effects of coronavirus disease-19 quarantine in patients with dementia; p. 916. (Front Psychiatry). Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pongan E., Dorey J.M., Borg C., Getenet J.C., Bachelet R., Lourioux C. COVID-19: association between increase of behavioral and psychological symptoms of dementia during lockdown and caregivers' poor mental health. J Alzheimers Dis. 2021;80(4):1713–1721. doi: 10.3233/JAD-201396. [DOI] [PubMed] [Google Scholar]
- 27.Cohen G., Russo M.J., Campos J.A., Allegri R.F. COVID-19 epidemic in Argentina: worsening of behavioral symptoms in elderly subjects with dementia living in the community. Front Psychiatry. 2020:11. doi: 10.3389/fpsyt.2020.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Maurik I.S., Bakker E.D., van den Buuse S., Gillissen F., Van De Beek M., Lemstra E. Psychosocial effects of corona measures on patients with dementia, mild cognitive impairment and subjective cognitive decline. Front Psychiatry. 2020:11. doi: 10.3389/fpsyt.2020.585686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barguilla A., Fernández-Lebrero A., Estragués-Gázquez I., García-Escobar G., Navalpotro-Gómez I., Manero R.M. Effects of COVID-19 pandemic confinement in patients with cognitive impairment. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.589901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsapanou A., Papatriantafyllou J.D., Yiannopoulou K., Sali D., Kalligerou F., Ntanasi E. The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int J Geriatr Psychiatry. 2020 doi: 10.1002/gps.5457. Nov 9. [DOI] [PubMed] [Google Scholar]
- 31.El Haj M., Altintas E., Chapelet G., Kapogiannis D., Gallouj K. High depression and anxiety in people with Alzheimer's disease living in retirement homes during the COVID-19 crisis. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113294. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Caoimh R., O'Donovan M.R., Monahan M.P., Dalton O'Connor C., Buckley C., Kilty C. Psychosocial Impact of COVID-19 nursing home restrictions on visitors of residents with cognitive impairment: a cross-sectional study as part of the engaging remotely in care (ERiC) project. Front Psychiatry. 2020;11:1115. doi: 10.3389/fpsyt.2020.585373. Oct 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kales H.C., Gitlin L.N., Lyketsos C.G. Detroit expert panel on assessment and management of neuropsychiatric symptoms of dementia. management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Gallego M., Gomez-Garcia J., Ato-Lozano E. Addressing the bias problem in the assessment of the quality of life of patients with dementia: determinants of the accuracy and precision of the proxy ratings. J Nutr Health Aging. 2015;19:365–372. doi: 10.1007/s12603-014-0564-7. [DOI] [PubMed] [Google Scholar]
- 35.Hoe J., Hancock G., Livingston G., Orrell M. Quality of life of people with dementia in residential care homes. Br J Psychiatry. 2006;188:460–464. doi: 10.1192/bjp.bp.104.007658. [DOI] [PubMed] [Google Scholar]
- 36.Seignourel P.J., Kunik M.E., Snow L., Wilson N., Stanley M. Anxiety in dementia: a critical review. Clin Psychol Rev. 2008;28:1071–1082. doi: 10.1016/j.cpr.2008.02.008. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Springate B.A., Tremont G. Dimensions of caregiver burden in dementia: impact of demographic, mood, and care recipient variables. Am J Geriatr Psychiatry. 2014;22:294–300. doi: 10.1016/j.jagp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leontjevas R., Knippenberg I.A.H., Smalbrugge M., Plouvier A.O.A., Teunisse S., Bakker C., Koopmans R.T.C.M., Gerritsen D.L. Challenging behavior of nursing home residents during COVID-19 measures in the Netherlands. Aging Ment Health. 2020:1–6. doi: 10.1080/13607863.2020.1857695. Dec 9Epub ahead of printPMID: 33291991. [DOI] [PubMed] [Google Scholar]
- 39.A. Suárez-González, G. Livingston, L.F. Low, S. Cahill, N. Hennelly, W.D. Dawson, W. Weidner, M. Bocchetta, C.P. Ferri, J.A. Matias-Guiu, S. Alladi, C.W. Musyimi, A. Comas-Herrera (2020 b) Impact and mortality of COVID-19 on people living with dementia: cross-country report. LTCcovid.org, international long-term care policy network, cpeclse, 19 August 2020. https://ltccovid.org/wp-content/uploads/2020/08/International-report-on-the-impact-of-COVID-19-on-people-living-with-dementia-19-August-2020.pdf
- 40.Howard R., Burns A., Schneider L. Antipsychotic prescribing to people with dementia during COVID-19. Lancet Neurol. 2020 Nov;19(11):892. doi: 10.1016/S1474-4422(20)30370-7. PMID: 33098796; PMCID: PMC7577650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Storr J., Kilpatrick C., Vassallo A. Safe infection prevention and control practices with compassion-a positive legacy of COVID-19. Am J Infect Control. 2021 Mar;49(3):407–408. doi: 10.1016/j.ajic.2020.12.016. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Low L.F., Hinsliff-Smith K., Sinha S., Stall N., Verbeek H., Siette J., Dow B., Backhaus R., Devi R., Spilsbury K., Brown J., Griffiths A., Bergman C., Comas- Herrera A. CPEC-LSE; 2021. Safe Visiting at Care Homes During COVID-19: a Review of International Guidelines and Emerging Practices During the COVID-19 Pandemic. LTC COVID.org, International Long-Term Care Policy Network. 19th January 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.