Abstract
Background
Non-Whites are more likely to suffer from cognitive impairment and complications of atrial fibrillation (AF) than Whites, though Whites are more likely to be diagnosed with AF. We examined whether non-Whites with AF are biologically older than Whites with AF and whether accelerated biological aging is associated with cognitive functioning.
Methods
We used baseline data from the ongoing Systematic Assessment of Geriatric Elements in Atrial Fibrillation prospective cohort study, collected 2016–2020 across ambulatory care practices in Massachusetts and Georgia. Of 1244 enrolled, 974 participants with full biological data were included in the present analysis. Accelerated aging (AccA) was calculated based on a combination of biomarkers associated with age and physiological “wear and tear.”
Findings
The main outcome was score on Montreal Cognitive Assessment (MoCA). Non-Whites had 2.9 years more AccA than Whites and higher AccA was associated with a lower MoCA score among both Whites (-0.06, 95% CI: -0.10, -0.03) and non-Whites (-0.14, 95% CI: -0.27, 0.02). This association was significantly greater among non-whites (-0.11, 95% CI: -0.20, -0.01).
Interpretation
Non-White AF patients are functionally “older” than their White counterparts and experience a stronger deleterious association between AccA and cognition. These findings underscore the importance of taking functional age into account when treating patients with AF, particularly non-White patients, to enhance treatment and improve AF outcomes.
1. Introduction
Atrial fibrillation (AF) affects 9% of individuals age 65 years and older and about 2% of those younger than 65, with an estimated three to six million people in the US affected [1]. As a consequence of the aging population in the US, the number of those affected by AF is expected to rise. Dementia is also strongly associated with aging and currently affects about 14 million Americans over the age of 71 [2]. Dementia and AF share common risk factors including age, heart failure, hypertension, history of stroke, excessive alcohol intake, smoking, and diabetes and AF is consistently associated with higher rates of dementia [3,4].
Racial patterning of incidence is found in both AF and dementia. Risk factors for both AF and dementia, including hypertension, history of stroke, and diabetes, are significantly more prevalent among Blacks and other minorities (e.g. Hispanics, Native Americans) with Blacks twice as likely to be diagnosed with dementia [5], but with a 41% lower risk of developing AF as compared to Whites [6]. Although information on AF incidence in other non-White populations is limited [7] the data that does exist suggests that other non-White populations such as Hispanics and Asians also have a lower incidence of AF than Whites [8]. This seeming paradox might be explained by sampling bias (e.g. minorities may be less likely to be screened for AF), but it provides a useful framework for which to study racial patterns of cardiovascular and geriatric predictors of cognitive functioning.
Age is the strongest risk factor for stroke and heart failure, the two most serious complications of AF, and current management guidelines for healthcare providers responsible for advising older adults with AF suggest that therapeutic decision-making should take frailty and cognitive status into consideration [3]. Biological age, or functional age of the body, compared to chronological age, may better predict AF complications and be an additional risk factor to consider when making AF treatment decisions. In older populations, frailty indices defined by diseases, symptoms, physical and cognitive functioning deficiencies, and self-rated health have been found to predict mortality better than chronological age alone [9]. Such indices have not, however, been applied to populations with AF. The objective of this study is to create a biological age variable that includes markers that are relevant to older populations and to people with AF and measure the association between biological age and impaired cognitive function, a precursor of dementia, separately by race. We hypothesize that non-White participants will have higher biological age than White participants and that biological age will be associated with cognitive functioning.
2. Methods
2.1. Design, setting, and participants
The SAGE-AF (Systematic Assessment of Geriatric Elements in Atrial Fibrillation) study is an ongoing, prospective study of AF [10]. In 2016–2018, consenting participants completed a comprehensive baseline geriatric assessment, a structured interview and trained study staff performed a comprehensive medical record review. Participants were drawn from five ambulatory practices in Massachusetts and two such practices in Georgia. To participate, participants had to have AF (verified by electrocardiogram, Holter monitor, or AF diagnosis in clinic note or hospital record), be 65 years of age or older, have a CHA2DS2VASC risk score ≥ 2, not had a major contraindication to oral anticoagulants, be able to provide informed consent, and be able to speak English.
All participants had a physical examination in the context of their routine care, had a medical history obtained, and underwent a 60 min interviewer-administered, computer-assisted interview with standardized measures, including assessments of mood, frailty, and cognition. All SAGE-AF participants provided informed written consent, and all protocols were approved by the University of Massachusetts Medical School, Boston University, and Mercer University review boards. SAGE-AF had a total of 996 participants. Our study sample included those who had complete cognitive functioning data and complete data for selected biological variables (described below) for an analytical sample of 974. This manuscript adheres to the STROBE reporting guidelines for cross-sectional studies.
2.2. Global cognitive functioning
Our primary outcome measure, cognitive function, is assessed by the Montreal Cognitive Assessment Battery [11], a 30-item screening tool validated to detect mild cognitive impairment with 23 used as the cut-point for impairment [12].
2.3. Frailty
Frailty was assessed using the Cardiovascular Health Survey frailty scale [13] which includes five components: weight loss/shrinking, exhaustion, low physical activity, slow gait speed, and weakness.
2.4. Race
Race was self-reported as Asian/Pacific Islander, African American, Alaskan Native, White, more than one race, other race, or unknown. Due to low percentages (< 2%) of all race categories except White and African American, we created a race variable that was dichotomized as White or non-white.
2.5. Accelerated aging
Our primary predictor, accelerated aging (AccA), was defined as biological age minus chronological age where a positive number indicates a biological age higher than chronological age and has harmful implications. Biological age was computed by the following steps. We ran correlations between candidate markers including markers of liver, kidney, metabolic, cardiovascular, frailty, and immune function and chronological age and retained those markers that had a correlation greater than 0.10 (the mean correlation). We then ran pairwise correlations of the remaining biomarkers with chronological age and eliminated those markers that had strong correlations (> 0.40) with multiple biomarkers (to avoid redundancy). We next eliminated the remaining markers that had missingness of > 30% in our sample and reran the pairwise correlation with the remaining biomarkers for strong correlations with all remaining biomarkers. The final biomarkers retained were Glomerular Filtration Rate (GFR), CHA2DS2VASC score, frailty, blood glucose, and BMI. We utilized the Klemera and Doubal equation (KDM) [14] which uses the intercept, slope, and root mean squared error to solve the regression equation of each biomarker on chronological age in standardized units of x (biomarker) and combines these estimates into biological age (BA) using chronological age (CA). Thus, BA is a weighted average of expected biomarker values minus the biomarker value at CA that is determined through regressing each biological marker on CA.
In order to calculate biological age so that it represents the approximate chronological age at which a person in the general population would have the combination of biomarkers corresponding to the biological age, we used a reference dataset from NHANES to calculate the biological age parameters and applied the parameters to the SAGE-AF dataset. We used NHANES participants from 1999 through 2006 (the only consecutive years in which all of our selected measures were available) that were non-pregnant and aged 65 years and older. We ran the series of regressions required by KDM and calculated the parameters and then used the parameters along with the SAGE-AF participant's chronological ages to create the final BA. We used 4-year weights provided by NHANES to ensure that our results are representative of the general population. The Klemera and Doubal equation has been validated in multiple large population studies including NHANES,[15] the Dunedin cohort [16], and CARDIA [17], [18], [19].
2.6. Covariates
All models were adjusted for self-reported sex, study site, education, and chronological age. We tested associations of the following possible confounders: depression score, anxiety score, amount of exercise, cost-related non-adherence to medications, glycemic meds, statins, antiplatelets/aspirin, anti-coagulants, hypertension meds, anti-arrhythmics, rate control meds, and NSAIDs. Education was categorical and defined as the level of education attained ranging from less than 8th grade to post graduate degree. Depression was measured with the Patient Health Questionnaire (PHQ-9) which asks about frequency of common depression symptoms in the last two weeks and was modeled as a continuous score. Anxiety was measured with the Generalized Anxiety Disorder −7 Scale (GAD-7) and was also measured as a continuous score. Amount of exercise in the last two weeks was ascertained with a modified version of the Minnesota Leisure Time Questionnaire which provides number of metabolic equivalents (METs) or energy expenditure in the last two weeks based on intensity and length of physical activity. Number of METs was modeled continuously. Cost-related non-adherence (CRN) was intended to be a proxy for socioeconomic status and was measured with the CRN questions from the Current Medicare Beneficiary Survey [20]. Medication use was a dichotomous yes/no variable and was abstracted from prescribed medications during medical chart abstraction. Burden of AF was measured by a question asking if the participant said that they had AF symptoms at the baseline interview.
2.7. Statistical analysis
All measurements were comparable in the non-White and White groups. Descriptive statistics were calculated for each study variable and covariate. The outcome for all main analyses was MoCA score. We first ran race-stratified unadjusted models to ascertain the association between AccA and cognitive functioning. Next, we ran race-stratified models adjusted for self-reported sex, study site, education, and chronological age. In order to include other important covariates, we used linear regression to measure the relationships between covariates and the main dependent and independent variables. We checked the assumptions of linear regression visually since our sample size was nearly 1000 making chi2 tests unreliable. We checked normality of residuals with a kernel density plot and standardized normal probability plot. We checked homogeneity of residuals with a residuals versus fitted (rvf) plot. Linear regression showed that depression score and anxiety score were significantly associated with both AccA and MoCA score, amount of exercise and taking an antiplatelets/aspirin was significantly associated with AccA only, and taking glycemic meds was significantly associated with MoCA score but not AccA. Thus, these variables were selected as covariates.
We then ran a race-stratified model where we added psychosocial and lifestyle variables (depression, anxiety, amount of exercise) to the aforementioned covariates. Finally, we ran race-stratified fully adjusted models where we added covariates that indicated whether or not the participant was taking glycemic, antiplatelet, cognitive, anti-arrhythmic, rate control, and anti-coagulation medications, type of atrial fibrillation, and burden of atrial fibrillation to the standard covariates and psychosocial/lifestyle covariates. Our race-stratified models took the form: MoCA Scorei = β0 + β1AccAi + β2sexi + β3sitei + β4agei + β5educi +β6 PHQ9i + β7GADi + β8Metsi + β9 glycemici + β10 anti-plateleti + β11anti-arrhythmici + β12rate controli + β13anti-coagulantsi + β14AF typei + β15AF burden i. The next set of models were overall (not race-stratified) and were run in a similar order as the aforementioned models (unadjusted, adjusted for standard covariates, adjusted for standard covariates and psychosocial/lifestyle covariates, fully adjusted including medication use), but each set included a term that modeled an interaction between race and weathering. Our overall models took the form: MoCA Scorei = β0 + β1AccAi + βsracei + β3Acca*racei + β4sexi + β5sitei + β6agei + β7educi +β8PHQ9i + β9GADi + β10Metsi + β11 glycemici + β12 anti-plateleti + β13anti-arrhythmici + β14rate controli + β15anti-coagulantsi + β16AF typei + β17AF burden i. We also ran an additional fully adjusted model with the interaction term that adjusted for presence cognitive impairment at baseline. All analyses were done using Stata 16 [21]. There was no missing data since we used a sample with only complete data.
2.8. Role of the funding source
The funding source had no role in study concept and design, acquisition of subjects and/or data, collection, analysis, and interpretation of the data, or preparation of the manuscript.
3. Results
3.1. Sample description
Descriptive statistics, by race, for the sample can be found in Table 1. Non-Whites were 2 years younger chronologically than Whites and nearly 1 year older biologically. AccA was 9.1 years on average for non-Whites and 6.2 years for Whites. The average Montreal Cognitive Assessment score for both non-Whites and Whites was in the range of Mild Cognitive Impairment (18–25) though the score for non-Whites (20.4, SD = 4.7) was lower (worse) than the score for Whites (24.2, SD = 3.6). Of the biomarkers included in the biological age equation, CHADS2VASC score, blood glucose, and frailty were significantly worse among non-Whites than among Whites. Histograms of the distribution of MoCA score and AccA can be found in supplementary Figs. 1 and 2.
Table 1.
Psychosocial, biological, cognitive, and geriatric variables by race: SAGE-AF, 2016–2018.
| Non-White* (n = 127) |
White (n = 847) |
p-value# | ||||||||||||
| n | mean or% | sd | n | mean or% | sd | |||||||||
| Chronological Age | 127 | 73.7 | 6.7 | 847 | 75.8 | 7.2 | 0.002 | |||||||
| Biological Age | 127 | 82.8 | 15.0 | 847 | 81.9 | 13.6 | 0.029 | |||||||
| Accelerated Aging | 127 | 9.1 | 12.6 | 847 | 6.2 | 11.5 | < 0.001 | |||||||
| Female | 56 | 44% | 415 | 49% | 0.303 | |||||||||
| MoCA† Score | 127 | 20.4 | 4.7 | 1069 | 24.2 | 3.6 | < 0.001 | |||||||
| Type of AF | ||||||||||||||
| Paroxysmal | 90 | 70% | 495 | 58% | 0.042 | |||||||||
| Persistent | 17 | 13% | 106 | 12% | ||||||||||
| Long-Standing Persistent | 6 | 5% | 98 | 12% | ||||||||||
| New Onset | – | – | 13 | 1% | ||||||||||
| Permanent | 5 | 4% | 53 | 6% | ||||||||||
| Left Atrium Diameter (mm) | 33 | 40.3 | 10.0 | 253 | 41.8 | 8.8 | 0.364 | |||||||
| Left Atrium Volume± | ||||||||||||||
| Normal | 12 | 9% | 82 | 10% | 0.337 | |||||||||
| Mildly Enlarged | 3 | 2% | 52 | 6% | ||||||||||
| Moderately Abnormal | 6 | 5% | 50 | 6% | ||||||||||
| Severely Abnormal | 105 | 83% | 661 | 78% | ||||||||||
| Psychosocial/Lifestyle Variables | ||||||||||||||
| Education | ||||||||||||||
| 8th Grade or less | 9 | 7% | 12 | 1% | < 0.001 | |||||||||
| HS Graduate | 36 | 28% | 185 | 22% | ||||||||||
| College Graduate | 14 | 11% | 132 | 16% | ||||||||||
| PHQ9 Score‡ | 127 | 4.7 | 5.4 | 847 | 3.4 | 3.9 | 0.001 | |||||||
| GAD-7 Score | 127 | 3.52 | 4.6 | 847 | 2.6 | 0.007 | ||||||||
| Exercise (METs) § | 127 | 1536.3 | 6762.7 | 847 | 1371 | 2393.5 | 0.600 | |||||||
| Physiological Variables | ||||||||||||||
| BMI | 127 | 31.6 | 6.2 | 847 | 31.1 | 6.7 | 0.461 | |||||||
| CHADS2VASC Score | 127 | 4.9 | 1.7 | 847 | 4.4 | 1.6 | 0.003 | |||||||
| Glomerular Filtration Rate | 127 | 59.0 | 19.7 | 847 | 60.6 | 15.9 | 0.319 | |||||||
| Blood Glucose | 127 | 119.3 | 41.1 | 847 | 111.9 | 36.8 | 0.039 | |||||||
| Geriatric Elements | ||||||||||||||
| Frailty|| | 127 | 1.7 | 1.2 | 847 | 1.12 | 1.1 | < 0.001 | |||||||
| Medications | ||||||||||||||
| Glycemic-yes | 53 | 42% | 188 | 22% | < 0.001 | |||||||||
| Anti-platelet-yes | 60 | 47% | 326 | 38% | 0.060 | |||||||||
| Oral anti-coagulation-yes | 110 | 87% | 729 | 86% | 0.868 | |||||||||
| Anti-arrhythmic-yes | 45 | 35% | 291 | 35% | 0.812 | |||||||||
| Rate Control-yes | 111 | 87% | 660 | 78% | 0.014 | |||||||||
| Comorbidities | ||||||||||||||
| Prior stroke-yes | 21 | 16% | 77 | 9% | 0.009 | |||||||||
| Hypertension-yes | 123 | 97% | 763 | 90% | 0.013 | |||||||||
| Heart Failure-yes | 68 | 53% | 305 | 36% | < 0.001 | |||||||||
| Diabetes-yes | 71 | 56% | 248 | 29% | < 0.001 | |||||||||
| Sleep Apnea-yes | 18 | 14% | 138 | 16% | 0.544 | |||||||||
Body Surface Area Adjusted; Normal (≤ 34 ml/mg2), Mildly enlarged (35 ml/mg2 – 41 ml/mg2), Moderately abnormal (42 ml/mg2 – 48 ml/mg2), Severely abnormal (> 48 ml/mg2).
Non-White includes Black/African American, Asian/Pacific Islander, Native American/Alaskan Native, More than one race, other race.
MoCA = Montreal Cognitive Assessment (range = 6–31).
Score of 5 + on PHQ9 indicates mild to severe depression.
METs = Metabolic Equivalent measured by Modified Minnesota Leisure Time Activities Questionnaire.
Cardiovascular Health Survey Frailty Scale (range = 0–5).
p-value of t-test (continuous variables) or chi square (categorical variables).
3.2. Race-stratified models
For both Whites and non-Whites there was a significant inverse association between AccA and MoCA score. Among White participants there no change in the inverse association between AccA and MoCA score for the unadjusted model (β = −0.06, 95% CI: −0.10, −0.03) and the model adjusted for sex, study site, education, and age (β = −0.03, 95% CI: −0.05, −0.01) and the addition of further covariates did not change the association appreciably (Table 2).
Table 2.
Linear regression of unadjusted and adjusted associations of change in mountreal cognitive assessment score (MoCA) with each 1-year increase in accelerated aging SAGE-AF, 2016–2018.
| Non-White β (SE) |
White β (SE) |
||
| Model 1-Unadjusted | −0.17 (0.06) § | −0.06 (0.02) § | |
| Model 2-Adjusted for sex, study site, education, age | −0.02 (0.04) ‡ | −0.06 (0.02) § | |
| Model 3-Adjusted model 2 covariates plus psychosocial and lifestyle variables* | −0.09 (0.06) | −0.06 (0.02) § | |
| Model 4-Adjusted for model 3 covariates plus medications and AF variables† | −0.14 (0.06) ‡ | −0.06 (0.02) § | |
Psychosocial and Lifestyle variables: CES-D score, GAD-7 score, amount of exercise.
Medications: glycemic, antiplatelet, antiarrhythmic, rate control, anti-coagulation; AF variables: type of AF, burden of AF.
p < 0.05.
p < 0.01.
Among White participants, for each additional year of AccA, performance on the MoCA was lower by, on average, 0.06 points. The unadjusted inverse association between AccA and MoCA in Non-Whites was higher than in Whites (β = −0.17, 95% CI: −0.29, −0.05) and remained when adjusting for sex, study site, education, and age (β = −0.13, 95% CI: −0.25, −0.01) so that among non-White participants, for each additional year of AccA performance on the MoCA is lower, on average, by 0.13 points. Adding psychosocial and lifestyle variables attenuated the association among non-Whites (β = −0.09, 95% CI: −0.21, 0.03) or 0.09 points per year. After adding medication and AF covariates to the model, the association among non-Whites was similar to original adjusted association (β = −0.14, 95% CI: −0.27, −0.02). Among non-Whites the average AccA was 9.1 years, this would correspond to a MoCA score 1.3 points lower than someone who is the same age biologically as chronologically (9.1*–0.14) compared to a MoCA score 0.37 points lower for Whites (6.2*–0.06).
3.3. Interactions
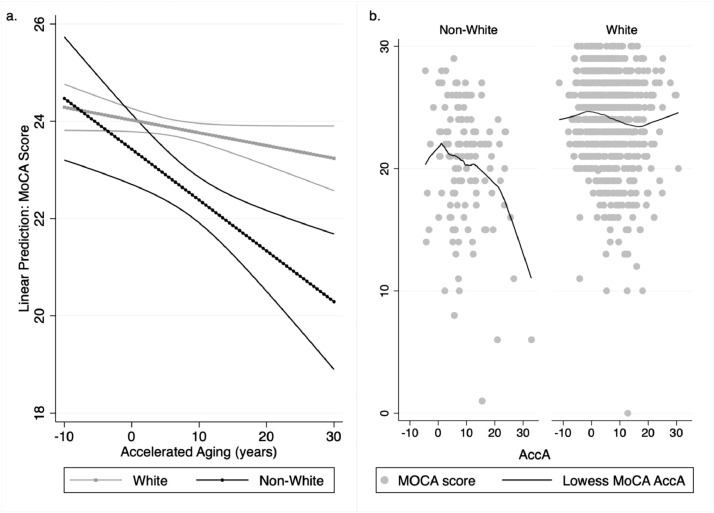
Each model with an interaction term between race and AccA showed similar results. The unadjusted model revealed a significant interaction term (β = −0.11, 95% CI: −0.21, −0.01) and the interaction did not change very much with the addition of standard covariates (sex, study site, education and age) (β = −0.10, 95% CI: −0.19, −0.003), psychosocial and lifestyle covariates (β = −0.09, 95% CI: −0.19, 0.002), or medication and AF covariates (β = −0.11, 95% CI: −0.20, −0.01). In the fully adjusted model, change for non-Whites was −0.14 points per year of AccA (95% CI: −0.27, -0.02). The significant interaction term indicates that the amount of change (decline) in MoCA score per additional year of AccA for non-Whites of about −0.26 points per year of AccA (−0.14 + −0.11 = −0.26) is significantly higher than the change for Whites suggesting that AccA has a significantly greater negative impact on cognitive scores in non-whites than in whites. Fig. 1 panel a illustrates the linear prediction of MoCA score at each level of AccA by race based on the model and panel b illustrates the actual data by race for comparison. The effect size for the fully adjusted model was 0.27 (95% CI: 0.21, 0.30).
Fig. 1.
a. Predicted change in montreal cognitive assessment score for each 5-year increment in accelerated aging by race with 95% confidence intervals: SAGE-AF, 2016–2018; b. Original data points of accelerated aging and montreal cognitive assessment score with lowess smoothing.
Fig. 1 shows that at AccA of −8.1 (biological age about 8 years less than chronological age non-Whites on average have a MoCA score of 23.6 and Whites on average have a MoCA score of 24.42. At an AccA of 7.31 (biological age about 7 years higher than chronological age) non-Whites on average have a MoCA score of 20.22, and Whites, on average, have a MoCA score of 24.8. Although non-Whites start with lower scores their scores are expected to decrease more per year than Whites with a decrease of 1.41 points for non-Whites, compared to 0.54 for Whites. An additional adjustment that accounted for presence of cognitive impairment at baseline only slightly changed the change for non-Whites but it did render the difference no longer significant in the stratified models (β = −0.08, 95% CI: −0.18, 0.03). For the interaction term it attenuated the association, but it remained significant (β = −0.08, 95% CI: −0.15, −0.02).
4. Discussion
In a large cohort of older patients with AF we found considerable AccA in non-White participants. Our objectives were to understand the association between biological aging in a population with AF and measure the association between biological aging and cognitive function. We found that on average, non-White participants had a biological age that was more than 4 years older than their chronological age while White participants had a biological age that was approximately a year younger than their chronological age. We also found that higher AccA is associated with lower global cognitive functioning and that this association is significantly stronger in non-Whites than in Whites.
Although global cognitive function has been found to be lower among non-Whites than Whites, it is especially significant in this population with AF. Because AccA is inversely associated with cognitive functioning, we posit that those factors that are known to be associated with AccA, in minorities especially, may affect cognitive functioning. Thus, non-While patients, though reported elsewhere to have a lower prevalence of AF, who have AF may be at especially high risk of cognitive decline: both because they suffer more AccA and because the association of AccA and cognitive function is even stronger in non-Whites than in Whites.
Another possible mechanism lies in the paradox of the lower prevalence AF in non-Whites compared to Whites despite the higher prevalence of AF risk factors among non-Whites. Research has shown that Whites may have a genetic predisposition to AF [22] and that European ancestry predicts risk of AF in African Americans [23]. If the genetic predisposition is true, then Whites would presumably have a lower threshold for AF, and therefore may not require as intense or sustained exposures to risk factors to develop AF as non-White patients. Stronger or longer exposure to risk factors for AF may provide an explanation for higher biological age in non-Whites with AF and the subsequent risk of global cognitive decline. Another theory that is worth considering relies on a framework from the health disparities literature known as weathering.
The prevailing theory of the association between AccA and health outcomes is the weathering hypothesis, which posits that stressors such as discrimination, racism, segregation, and poor economic circumstances “weathers” the health of minorities so that they show age-related declines in health earlier than Whites [24]. These minority specific stressors are also associated with the risk factors for AF such as diabetes, hypertension, and heart attack [25,26]. Although we were unable to fully test this theory here, previous research has shown that higher levels of perceived stress were associated with the prevalence of AF in the REGARDS study [27]. Consistently worse outcomes among minorities with AF compared to Whites with AF, even though Whites are more likely to be diagnosed with AF, are also consistent with this theory. For example, minorities with AF have a higher risk of developing an ischemic stroke [28], have more severe strokes [29], and are at a higher risk of death [30] than Whites. But one study using Medicare administrative data showed that controlling for pre-existing morbidities eliminated the higher hazard for death and attenuated the higher hazard of stroke. The biomarkers that make up our biological age variable (GFR, CHA2DS2VASC score, frailty, blood glucose, and BMI) are significantly associated with survival and stroke risk in AF patients [31,32] so it could be that our accelerated aging variable is a convenient predictor that captures duration and intensity of exposure to risk factors for poor outcomes in AF. Either theory seems to point to longer duration and intensity of exposure to risk factors for AF. The American Heart Association's “Life's Simple 7″ which focuses on improving seven key cardiac risk factors -smoking, diet, physical activity, BMI, blood pressure, total cholesterol, and fasting glucose is related to both cognitive impairment [33] and risk of AF [34]. Since these risk factors are also impacted by biological age, the benefits of understanding AccA in this population may be clinically useful for other treatment targets.
The association between AccA and global cognitive function may have broader clinical implications for the management and treatment of older non-White patients with AF. If non-White patients are “older” due to AccA, clinicians may need to take this into account and consider screening younger non-White AF patients for cognitive impairment. Cognitive impairment has implications for treatment and medication adherence [35], which can significantly complicate the treatment of AF since AF requires long-term use of high-risk medications, including oral anticoagulants. Future research should investigate the usefulness of AccA to better inform treatment decisions (i.e., type of anticoagulant used) in non-White AF patients. There are also implications for lifestyle and risk factor management.
Our results should be considered in light of some limitations. Although the SAGE-AF study specifically recruited minorities into the study, the proportion of minorities was still relatively low and required us to collapse all minority categories into one “non-White” variable. We recognize that stress and minority status interact differently for different non-White groups and thus limits our ability to draw conclusions regarding stress related to minority status in this sample. Our analysis needs to be replicated in a cohort with a larger proportion of non-White persons where there is enough power to look at specific minority groups separately. We only had cross-sectional data available, so we are unable to assess the association between AccA and cognitive decline, although this will be examined using follow-up data in our cohort. It should also be noted, however, that we had medical record data as well as data collected at visits, making our AccA variable more convenient and thus more likely to be able to be reproduced and tested on other AF outcomes. Finally, we don't have data on underuse and lack of early use of anti-coagulation medications which is directly related to stroke and thus cognitive decline. Future research should explore this accelerated aging framework in light of disparities in anti-coagulation treatment.
Using data from an ongoing contemporary cohort study of older AF patients, we observed considerable acceleration of aging among non-White participants and that AccA was higher amongst non-Whites and was associated with lower global cognitive functioning. We observed the deleterious association of AccA with cognitive function in both Whites and non-Whites, but the association was even more pronounced among non-White AF patients. Thus, non-Whites may suffer the impact of AF on cognition both because they undergo more AccA and because AccA is more strongly associated with worse cognitive functioning in non-Whites placing them at “double jeopardy.” These findings may underscore the importance of taking functional age into account when treating patients with AF, particularly non-White patients, to enhance treatment and improve AF outcomes.
Contributors: Sarah N. Forrester affirms that she has listed everyone who contributed to significantly to the work. All authors meet the criteria for authors in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. All authors had substantial contributions in conception and design, acquisition of data, or analysis and interpretation of data; in drafting the article or revising it critically for important intellectual content; and in final approval of the version to be published. All authors have accessed the underlying data. Dr. Forrester handled and is responsible for the veracity of the raw data associated with the study. Dr. Forrester conceptualized the study, completed the analysis, and wrote the manuscript; Dr. McManus provided the data and assisted in study design, analysis and interpretation of data. Dr. Saczynski provided data and assisted in interpretation of data and analysis. Ms. Pierre-Louis assisted with writing of the manuscript. Dr. Bamgbade assisted in interpretation of the data and revision of the manuscript. Dr. Kiefe conceptualized the study and assisted in data analysis and interpretation.
Declaration of Interests: Dr. Forrester reports grants from NIH, grants from UMASS CCTS, outside the submitted work. Ms. Pierre-Louis has nothing to disclose. Dr. Bamgbade reports grants from NHLBI, during the conduct of the study. Dr. Saczynski has nothing to disclose. Dr. Kiefe reports grants from NIH, during the conduct of the study. Dr. McManus reports grants from NIH, during the conduct of the study; grants and personal fees from Bristol Myers Squibb/Pfizer, grants from Boerhinger Ingelheim, grants and personal fees from Flexcon, personal fees and non-financial support from Fitbit, non-financial support from Apple, personal fees from Avania, grants and personal fees from HRS, outside the submitted work.
Data Sharing Statement: The authors do not have permission to share the study data. Please contact the Systematic Assessment of Geriatric Elements in Atrial Fibrillation (SAGE-AF) study to obtain data.
4.1. Research in context
Evidence before this study: Atrial fibrillation (AF) and dementia affect a growing amount of the older population. Racial patterning is found in both AF and dementia. White persons are more likely to be diagnosed with AF despite most risk factors for AF being disproportionately higher among non-White populations. Non-White persons are more likely to be diagnosed with dementia and risk factors are similar as in AF. Biological age predictors have been created and found to predict mortality and other outcomes better than chronological age alone. This work has not been undertaken in a sample with AF.
Added value of this study: Our study provides evidence that specific markers may be associated with a decline in cognitive functioning in non-White populations compared to White populations with AF. The results indicate that some patients, particularly non-White patients, may be functionally older than they are chronologically and that this it could be associated with a decline in cognitive function.
4.2. Implications of all the available evidence
If non-White patients are “older” due to accelerated aging, clinicians may need to take this into account and consider screening younger non-White AF patients for cognitive impairment. Cognitive impairment has implications for treatment and medication adherence, which can significantly complicate the treatment of AF since AF requires long-term use of high-risk medications, including oral anticoagulants
Declaration of Competing Interest
None.
Funding
The SAGE-AF study is supported by Grant R01HL126911 from the National Heart, Lung, and Blood Institute (NHLBI).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101060.
Appendix. Supplementary materials
References
- 1.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2014;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Plassman B.L., Langa K.M., Fisher G.G. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zathar Z., Karunatilleke A., Fawzy A.M., Lip G.Y.H. Atrial fibrillation in older people: concepts and controversies. Front Med. 2019;6:175. doi: 10.3389/fmed.2019.00175. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bruijn R.F., Heeringa J., Wolters F.J. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72(11):1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 5.Gaugler J., James B., Johnson T., Marin A., Weuve J. 2019 Alzheimer's disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. [Google Scholar]
- 6.Alonso A., Agarwal S.K., Soliman E.Z. Incidence of atrial fibrillation in whites and African-Americans: the atherosclerosis risk in communities (ARIC) study. Am Heart J. 2009;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman E.Z., Alonso A., Goff D.C. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5(6):547–556. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 8.Shen A.Y.J., Contreras R., Sobnosky S. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults-a cross-sectional study. J Natl Med Assoc. 2010;102(10):906–914. doi: 10.1016/s0027-9684(15)30709-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim S., Welsh D.A., Cherry K.E., Myers L., Jazwinski S.M. Association of healthy aging with parental longevity. Age. 2013;35(5):1975–1982. doi: 10.1007/s11357-012-9472-0. (Omaha) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saczynski J.S., Sanghai S.R., Kiefe C.I. Geriatric elements and oral anticoagulant prescribing in older atrial fibrillation patients: SAGE-AF. J Am Geriatr Soc. 2020;68(1):147–154. doi: 10.1111/jgs.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasreddine Z.S., Phillips N.A., Bédirian V. MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Saczynski J.S., Inouye S.K., Guess J. The montreal cognitive assessment: creating a crosswalk with the mini-mental state examination. J Am Geriatr Soc. 2015;63(11):2370–2374. doi: 10.1111/jgs.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried L.P., Tangen C.M., Walston J. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Klemera P., Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Levine M.E., Crimmins E.M. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi: 10.1016/j.socscimed.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belsky D.W., Caspi A., Houts R. Quantification of biological aging in young adults. Proc Natl Acad Sci. 2015;112(30):E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrester S., Jacobs D., Zmora R., Schreiner P., Roger V., Kiefe C.I. Racial differences in weathering and its associations with psychosocial stress: the CARDIA study. SSM Popul Health. 2019;7 doi: 10.1016/j.ssmph.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester S.N., Zmora R., Schreiner P.J. Racial differences in the association of accelerated aging with future cardiovascular events and all-cause mortality: the coronary artery risk development in young adults study, 2007–2018. Ethn Health. 2020:1–13. doi: 10.1080/13557858.2020.1839021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrester S.N., Zmora R., Schreiner P.J. Accelerated aging: a marker for social factors resulting in cardiovascular events? SSM Popul Health. 2021;13 doi: 10.1016/j.ssmph.2021.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler G.S. A profile of the medicare current beneficiary survey. Health Care Financ Rev. 1994;15(4):153. [PMC free article] [PubMed] [Google Scholar]
- 21.StataCorp LP; College Station, TX: 2019. Stata statistical software: release 16 [Computer Program] [Google Scholar]
- 22.Christensen M.A., Nguyen K.T., Stein P.K. Atrial ectopy as a mediator of the association between race and atrial fibrillation. Heart Rhythm. 2017;14(12):1856–1861. doi: 10.1016/j.hrthm.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcus G.M., Alonso A., Peralta C.A. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122(20):2009–2015. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geronimus A.T. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 25.Cuevas A.G., Ho T., Rodgers J. Developmental timing of initial racial discrimination exposure is associated with cardiovascular health conditions in adulthood. Ethn Health. 2019:1–14. doi: 10.1080/13557858.2019.1613517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barajas C.B., Jones S.C., Milam A.J. Coping, discrimination, and physical health conditions among predominantly poor, urban african americans: implications for community-level health services. J Community Health. 2019;44(5):954–962. doi: 10.1007/s10900-019-00650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Neal W.T., Efird J.T., Judd S.E. Impact of awareness and patterns of nonhospitalized atrial fibrillation on the risk of mortality: the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol. 2016;39(2):103–110. doi: 10.1002/clc.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel P.J., Katz R., Borovskiy Y. Race and stroke in an atrial fibrillation inception cohort: findings from the penn atrial fibrillation free study. Heart Rhythm. 2018;15(4):487–493. doi: 10.1016/j.hrthm.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amponsah M.K., Benjamin E.J., Magnani J.W. Atrial fibrillation and race–a contemporary review. Curr Cardiovasc Risk Rep. 2013;7(5):336–345. doi: 10.1007/s12170-013-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabra R., Cram P., Girotra S., Sarrazin M.V. Effect of race on outcomes (stroke and death) in patients>65 years with atrial fibrillation. Am J Cardiol. 2015;116(2):230–235. doi: 10.1016/j.amjcard.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Velde M., Matsushita K., Coresh J. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 32.Lee M., Saver J.L., Chang K.H., Liao H.W., Chang S.C., Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. Bmj. 2010;341:c4249. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thacker E.L., Gillett S.R., Wadley V.G. The American heart association life's simple 7 and incident cognitive impairment: the re asons for geographic and racial differences in stroke (REGARDS) study. J Am Heart Assoc. 2014;3(3) doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogunmoroti O., Michos E.D., Aronis K.N. Life's simple 7 and the risk of atrial fibrillation: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2018;275:174–181. doi: 10.1016/j.atherosclerosis.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins L.A., Kilian S., Firek A., Kashner T.M., Firek C.J., Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung J Acute Critical Care. 2012;41(6):572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.